ABSTRACT
The book “Peixes da planície de inundação do alto rio Paraná e áreas adjacentes” represents the most cohesive data compilation for the rio Paraná floodplain. However, considering the dynamicity of the taxonomy of freshwater fishes, several new records and taxonomic changes occurred along the past years. Therefore, the results of that publication were revisited, providing an update of the species list, their taxonomic status, records and geographic distribution, and also new keys for genera and species. The species included were those recorded in the rio Paraná basin, from the mouth of the rio Paranapanema to the Itaipu Reservoir, following the general methodology presented in the book. A total of 10 orders, 41 families, 126 genera, and 211 species were registered, with an increase of one order, six families, 14 genera, and 29 species when compared to the book. Additionally, four new genera recently described, five synonymization proposals, 14 new identifications, four new combinations, 12 new species recently described, 34 new records, and nine misidentified species were recorded. These results are associated with the redirection of human and financial resources to that area, which enabled monitoring and intensive exploration of its watercourses; as well as training of taxonomists, and new taxonomic resolutions.
Keywords:
Geographical distribution; Ichthyological diversity; Key of identification; Non-native species; Taxonomy
RESUMO
O livro “Peixes da planície de inundação do alto rio Paraná e áreas adjacentes” representa a compilação de dados mais coesa para esta área. No entanto, considerando a dinamicidade da taxonomia de peixes de água doce, vários novos registros e alterações taxonômicas ocorreram ao longo desses dez anos. Assim, os resultados daquela publicação foram revisitados, fornecendo uma atualização da lista, status taxonômico, registros e distribuição geográfica das espécies, além de novas chaves de identificação para espécies e gêneros. Foram incluídas as espécies registradas na bacia do rio Paraná, entre a foz do rio Paranapanema e o reservatório de Itaipu, seguindo a metodologia geral apresentada no livro. Foi registrado um total de 10 ordens, 41 famílias, 126 gêneros e 211 espécies, com um aumento de uma ordem, seis famílias, 13 gêneros e 29 espécies quando comparado à primeira versão. Além disso, quatro gêneros novos descritos recentemente, cinco sinonimizações, 14 novas propostas de identificação, quatro novas combinações, 12 espécies novas descritas recentemente, 34 novos registros e nove espécies identificadas erroneamente foram registradas. Estes resultados estão associados ao redirecionamento de recursos humanos e financeiros para esta área, o que permitiu o monitoramento e exploração intensiva de seus corpos d’água; bem como a formação de taxonomistas e novas resoluções taxonômicas.
Palavras-chave:
Chave de identificação; Distribução geográfica; Diversidade ictiológica; Espécies não nativas; Taxonomia
Introduction
The rio Paraná is formed by the junction of the rio Grande and rio Paranaíba, and extends across southern Brazil, Paraguay and Argentina (Agostinho et al., 2004aAgostinho AA, Gomes LC, Thomaz SM, Hahn NS. The upper Paraná River and its floodplain: main characteristics and perspectives for management and conservation. In: Thomaz SM, Agostinho AA, Hahn NS, editors. The upper Paraná River and its floodplain: physical aspects, ecology and conservation. Leiden: Backhuys Publishers; 2004a. p.381-393.; Brea, Zucol, 2011Brea M, Zucol AF. The Paraná-Paraguay basin: geology and paleoenvironments. In: Albert JS, Reis RE, editors. Historical biogeography of Neotropical freshwater fishes. Berkeley; Los Angeles; London: University of California Press; 2011. p.69-87.). There is an extensive floodplain in the west side of its upper portion representing a very dynamic ecosystem, both biotically and abiotically, with high habitat heterogeneity, which is important for the maintenance of the biological diversity (Agostinho et al., 2007bAgostinho AA, Pelicice FM, Petry AC, Gomes LC, Júlio Júnior HF. Fish diversity in the upper Paraná River basin: habitats, fisheries, management and conservation. Aquat Ecosyst Health Manag. 2007b; 10(2):174-86., 2009Agostinho AA, Bonecker CC, Gomes LC. Effects of water quantity on connectivity: the case of the upper Paraná River floodplain. Ecohydrol Hidrobiol. 2009; 9(1):99-113.; Thomaz et al., 2007Thomaz SM, Bini LM, Bozelli RL. Floods increase similarity among aquatic habitats in river-floodplain systems. Hydrobiologia . 2007; 579(1):1-13.). In those systems, the hydrological regime, with alternating drought and flood periods, is the main force driving the ecological functioning and patterns of biological diversity, by influencing feeding, reproduction, and species distribution (Wootton, 1990Wootton RJ. Ecology of teleost fishes. London; New York: Chapman and Hall; 1990. (Fish and Fisheries; 1).; Petry et al., 2003Petry AC, Agostinho AA, Gomes LC. Fish assemblages of tropical floodplain lagoons: exploring the role of connectivity in a dry year. Neotrop Ichthyol . 2003; 1(2):111-19.; Agostinho et al., 2004bAgostinho AA, Gomes LC, Veríssimo S, Okada EK. Flood regime, dam regulation and fish in the upper Paraná River: effects on assemblage attributes, reproduction and recruitment. Rev Fish Biol Fish. 2004b; 14(1):11-19.; Fernandes et al., 2009Fernandes R, Agostinho AA, Ferreira EA, Pavanelli CS, Suzuki HI, Lima DP, Gomes LC. Effects of the hydrological regime on the ichthyofauna of riverine environments of the upper Paraná River floodplain. Braz J Biol . 2009; 69(2, Suppl.):669-80.; Oliveira et al., 2014Oliveira AG, Suzuki HI, Gomes LC, Agostinho AA. Interspecific variation in migratory fish recruitment in the upper Paraná River: effects of the duration and timing of floods. Environ Biol Fish. 2014; 98(5):1327-37.; Message et al., 2016Message HJ, Santos DA, Baumgartner MT. Planícies de inundação: a biodiversidade do rio Paraná ameaçada. Ciência Hoje. 2016; 56(334):36-39.), as well as limnological variables (Roberto et al., 2009Roberto MC, Santana NF, Thomaz SM. Limnology in the upper Paraná River floodplain: large-scale spatial and temporal patterns, and the influence of reservoirs. Braz J Biol . 2009; 69(2, Suppl.):717-25.).
Despite its ecological importance, the upper rio Paraná has been suffering drastic alterations in its annual flood regime (Agostinho et al., 2004bAgostinho AA, Gomes LC, Veríssimo S, Okada EK. Flood regime, dam regulation and fish in the upper Paraná River: effects on assemblage attributes, reproduction and recruitment. Rev Fish Biol Fish. 2004b; 14(1):11-19.) and flux characteristics of its main channel and landscape, mainly due to the construction of dams over the years (Agostinho et al., 2008Agostinho AA, Pelicice FM, Gomes LC. Dams and the fish fauna of the Neotropical region: impacts and management related to diversity and fisheries. Braz J Biol. 2008; 68(Suppl.4):1119-32.). The lotic stretch of the rio Paraná comprised within its floodplain (between the Porto Primavera Dam and the beginning of the Itaipu Reservoir) is the only that remains undammed in Brazilian territory (Agostinho et al., 2004aAgostinho AA, Gomes LC, Thomaz SM, Hahn NS. The upper Paraná River and its floodplain: main characteristics and perspectives for management and conservation. In: Thomaz SM, Agostinho AA, Hahn NS, editors. The upper Paraná River and its floodplain: physical aspects, ecology and conservation. Leiden: Backhuys Publishers; 2004a. p.381-393.). Additionally to those impacts, fish species from the lower reaches of the basin have invaded the upper rio Paraná floodplain after the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls, a previous natural and effective barrier (Júlio Júnior et al., 2009Júlio Júnior HF, Dei Tos C, Agostinho AA, Pavanelli CS. A massive invasion of fish species after eliminating a natural barrier in the upper rio Paraná basin. Neotrop Ichthyol . 2009; 7(4):709-18.). The mixing of the two distinct ichthyofaunistic provinces (lower and upper rio Paraná) has also been facilitated by the Canal da Piracema, a channel for spawning migration opened in December 2002 in the Itaipu Dam (Makrakis et al., 2007Makrakis S, Gomes LC, Makrakis MC, Fernandez DR, Pavanelli CS. The Canal da Piracema at Itaipu Dam as a fish pass system. Neotrop Ichthyol . 2007; 5(2):185-95.).
Therefore, in order to study the upper rio Paraná floodplain ichthyofauna, Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.) provided the most cohesive identification manual of this area, the book “Peixes da planície de inundação do alto rio Paraná e áreas adjacentes”. It represents a major effort for the data compilation and is an essential tool often used by the scientific community from different areas, including fieldwork and data survey about regional distribution or functional traits in species level. However, considering the dynamicity of the taxonomy of freshwater fishes, several new records and taxonomic changes occurred along the past ten years, their results were revisited, providing an Updated species list, their taxonomic status, records, and geographic distribution, and also new keys to species and genera.
Material and Methods
Species recorded were included from the mouth of the rio Paranapanema to the Itaipu Reservoir (Fig. 1), mainly focusing at its floodplain. Differently from Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.), who included the superior stretches, here the rivers and streams of the right and left banks of the rio Paraná only were considered in the region under the influence of its flood. Counts, measurements and other data followed the methodology presented by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.), with the addition of the counts of longitudinal line scales for Characidae species with incomplete lateral line, which includes all the pored scales and the remaining scales of the same series.
Map of the upper rio Paraná floodplain area (shaded) (from the mouth of the rio Paranapanema to the Itaipu Reservoir) and its main tributaries.
Because of the many changes in the current classifications, species were classified according to studies that used plenty of taxa for group analyses, as described below; and because the recent morphological and molecular phylogenetic analyses are not congruent or lack resolution regarding the relationships between some families and sub-families, they were presented in alphabetical order. The nomenclatural arrangement and classification of groupings in levels higher than family followed Betancur-R. et al. (2017Betancur-R R, Wiley EO, Arratia G, Acero A, Bailly N, Miya M, Lecointre G, Ortí G. Phylogenetic classification of bony fishes. BMC Evol Biol [serial on the Internet]. 2017 July 6. Available from: https://doi.org/10.1186/s12862-017-0958-3.
https://doi.org/10.1186/s12862-017-0958-...
) for bony fishes, and McEachran, Aschliman (2004McEachran JD, Aschliman N. Phylogeny of Batoidea. In: Carrier JC, Musick JA, Heithaus MR, editors. Biology of sharks and their relatives. London: CRC Press; 2004. p.79-113. (CRC Marine Biology Series).) for Myliobatiformes. Family names followed Betancur-R. et al. (2017Betancur-R R, Wiley EO, Arratia G, Acero A, Bailly N, Miya M, Lecointre G, Ortí G. Phylogenetic classification of bony fishes. BMC Evol Biol [serial on the Internet]. 2017 July 6. Available from: https://doi.org/10.1186/s12862-017-0958-3.
https://doi.org/10.1186/s12862-017-0958-...
), except for Cynolebiidae, which followed van der Laan (2016van der Laan R, editor. Freshwater fish list: an alphabetic scientific name list of the world’s freshwater fishes and an overview of the scientific names used in the aquarium literature. 18th ed. Almere: The Netherlands; 2016.). The classification of Cheirodontinae and Aphyocharacinae followed Tagliacollo et al. (2012Tagliacollo VA, Souza-Lima R, Benine RC, Oliveira C. Molecular phylogeny of Aphyocharacinae (Characiformes, Characidae) with morphological diagnoses for the subfamily and recognized genera. Mol Phylogenet Evol . 2012; 64(2):297-307.), Characinae followed Oliveira et al. (2011Oliveira C, Avelino GS, Abe KT, Mariguela TC, Benine RC, Ortí G, Vari RP, Castro RMC. Phylogenetic relationships within the speciose family Characidae (Teleostei: Ostariophysi: Characiformes) based on multilocus analysis and extensive ingroup sampling. BMC Evol Biol [serial on the internet]. 2011 Sep 26 [cited 2016 Dec 08]; 11:275. Available from: Available from: http://dx.doi.org/10.1186/1471-2148-11-275
http://dx.doi.org/10.1186/1471-2148-11-2...
), Stevardiinae followed Thomaz et al. (2015Thomaz AT, Arcila D, Orti G, Malabarba LR. Molecular phylogeny of the subfamily Stevardiinae Gill, 1858 (Characiformes: Characidae): classification and the evolution of reproductive traits. BMC Evol Biol [serial on the internt]. 2015 Jan 13 [cited 2016 Dec 08]; 15:146. Available from: Available from: http://dx.doi.org/10.1186/s12862-015-0403-4
http://dx.doi.org/10.1186/s12862-015-040...
), Otothyrinae followed Roxo et al. (2014Roxo FF, Albert JS, Silva GSC, Zawadzki CH, Foresti F, Oliveira C. Molecular phylogeny and biogeographic history of the armored Neotropical catfish subfamilies Hypoptopomatinae, Neoplecostominae and Otothyrinae (Siluriformes: Loricariidae). PloS ONE [serial on the internet]. 2014 Aug 22 [cited 2016 Dec 08]; 9(8):e105564. Available from: Available from: http://dx.doi.org/10.1371/journal.pone.0105564
http://dx.doi.org/10.1371/journal.pone.0...
), and Rhinelepinae is according to Lujan et al. (2015Lujan NK, Armbruster JW, Lovejoy NR, López-Fernández H. Multilocus molecular phylogeny of the suckermouth armored catfishes (Siluriformes: Loricariidae) with a focus on subfamily Hypostominae. Mol Phylogenet Evol . 2015; 82(pt. A):269-88.). The keys for genera and non monospecific genera were updated, and pictures of all species were provided.
The following standardized terms were used to describe color pattern: a spot is any small, rounded mark; a blotch is any large, rounded mark; a stripe is a thin, either longitudinal or transverse mark; a band is a broad, longitudinal mark; and a bar is a broad, transverse mark. Voucher specimens are hosted in the Coleção Ictiológica do Núcleo de Pesquisas em Limnologia, Ictiologia e Aquicultura (Nupélia), under the acronym NUP, and are listed in the Material Examined.
Results
Ten orders, 41 families, 126 genera, and 211 species were recorded in the upper rio Paraná, between the Porto Primavera and Itaipu reservoirs, including the floodplain and adjacent areas (Tab. 1), with the addition of one order, six families, 12 genera, and 29 species relative to Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
List of fish species from the upper rio Paraná, between Porto Primavera and Itaipu reservoirs, including both unmodified species from Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.) and changes and updates proposed herein, which are discriminated by different colors, as follows: new genera described (orange); synonym proposals (pink); new identification proposals (blue); new combinations (green); new species described (grey); new records (yellow); and misidentified species (lilac). One asterisk (*) represents non-native species from the upper rio Paraná basin. Two asterisks (**) represent possible non-native species. The references for the classification used are listed in the Material and Methods section.
Four new genera recently described were recorded [i.e. Ossancora Birindelli, Sabaj Pérez, 2011 - O. eigenmanni (Boulenger, 1895); Curculionichthys Roxo, Silva, Ochoa, Oliveira, 2015 - C. insperatus (Britski, Garavello, 2003); Megaleporinus Ramirez, Birindelli, Galetti Júnior, 2016 - M. macrocephalus (Garavello, Britski, 1988), M. obtusidens (Valenciennes, 1836) and M. piavussu (Britski, Birindelli, Garavello, 2012); and Rhyacoglanis Shibatta, Vari, 2017 - R. paranensis Shibatta, Vari, 2017]; five synonym proposals [i.e. Astyanax altiparanae Garutti, Britski, 2000 as junior synonym of A. lacustris (Lütken, 1875), Galeocharax knerii (Steindachner, 1879) as G. gulo (Cope, 1870), Pimelodus heraldoi Azpelicueta, 2001 as P. microstoma Steindachner, 1877, Pterygoplichthys anisitsi Eigenmann, Kennedy, 1903 as P. ambrosettii (Holmberg, 1893), and Roeboides paranensis Pignalberi, 1975 as R. descalvadensis Fowler, 1932]; 14 new identification proposals [i.e. Crenicichla niederleinii (Holmberg, 1891) is now identified as Crenicichla sp., Farlowella aff. amazona (Günther, 1864) as F. hahni Meinken, 1937, Hoplias sp. as H. intermedius (Günther, 1864), Hypophthalmus edentatus Spix, Agassiz, 1829 as H. oremaculatus Nani, Fuster, 1947, Hypostomus sp. as H. iheringii (Regan, 1908), Leporinus elongatus Valenciennes, 1850 as Megaleporinus obtusidens, L. macrocephalus Garavello, Britski, 1988 as M. macrocephalus, Hisonotus sp. as Otothyropsis polyodon Calegari, Lehmann A., Reis, 2013, Pamphorichthys sp. as P. hollandi (Henn, 1916), Pseudoplatystoma fasciatum (Linnaeus, 1766) as P. reticulatum Eigenmann, Eigenmann, 1889, Rivulus apiamici Costa, 1989 as Melanorivulus sp., Satanoperca pappaterra (Heckel, 1840) as Satanoperca sp., Trichomycterus sp. as T. davisi (Haseman, 1911), and Zungaro zungaro (Humboldt, 1821) as Z. jahu (Ihering, 1898)]; four new combinations [i.e. Apteronotus ellisi (Arámburu, 1957), Diapoma guarani (Mahnert, Géry 1987), Coptodon rendalli (Boulenger, 1897), and Piabarchus stramineus (Eigenmann, 1908)]; 12 new species recently described [i.e. Amaralia oviraptor Friel, Carvalho, 2016, Apteronotus acidops Triques, 2011, Brachyhypopomus gauderio Giora, Malabarba, 2009, Geophagus sveni Lucinda, Lucena, Assis, 2010, Gymnorhamphichthys britskii Carvalho, Ramos, Albert, 2011, Hoplias mbigua Azpelicueta, Benítez, Aichino, Mendez, 2015, Laetacara araguaiae Ottoni, Costa, 2009, Megaleporinus piavussu, Moenkhausia forestii Benine, Mariguela, Oliveira, 2009, Odontostilbe avanhandava Chuctaya, Bührnheim, Malabarba, 2018, Phalloceros harpagos Lucinda, 2008, and Potamotrygon amandae Loboda, Carvalho, 2013]; 34 new records [i.e. Aequidens plagiozonatus Kullander, 1984, Aphyocheirodon hemigrammus Eigenmann, 1915, Apistogramma commbrae (Regan, 1906), Astyanax biotae Castro, Vari, 2004, Bryconamericus turiuba Langeani, Lucena, Pedrini, Tarelho-Pereira, 2005, Chaetobranchopsis australis Eigenmann, Ward, 1907, Characidium gomesi Travassos, 1956, Clarias gariepinus (Burchell, 1822), Corydoras sp., Crenicichla jaguarensis Haseman, 1911, Curculionichthys insperatus, Eigenmannia guairaca Peixoto, Dutra, Wosiacki, 2015, Hemigrammus ora Zarske, Le Bail, Géry, 2006, Hoplias misionera Rosso, Mabragaña, González-Castro, Delpiani, Avigliano, Schenone, Días de Astarloa, 2016, Hyphessobrycon moniliger Moreira, Lima, Costa, 2002, Hypostomus hermanni (Ihering, 1905), Ictalurus punctatus (Rafinesque, 1818), Imparfinis borodini Mees, Cala, 1989, Leporinus tigrinus Borodin, 1929, Microglanis garavelloi Shibatta, Benine, 2005, Moenkhausia australe (Eigenmman, 1908), M. bonita Benine, Castro, Sabino, 2004, M. forestii, M. cf. gracilima Eigenmann, 1908, Otothyropsis marapoama Ribeiro, Carvalho, Melo, 2005, O. polyodon, Phallotorynus pankalos Lucinda, Rosa, Reis, 2005, P. victoriae Oliveros, 1983, Pimelodus mysteriosus Azpelicueta, 1998, Platanichthys platana (Regan, 1917), Rhyacoglanis paranensis, Serrapinnus calliurus (Boulenger, 1900), S. heterodon (Eigenmann, 1915), and Trichomycterus diabolus Bockmann, Casatti, de Pinna, 2004]; and nine misidentified species [i.e. Aphyocheirodon hemigrammus and S. calliurus misidentified as Serrapinnus sp. 1, Crenicichla jaguarensis as C. haroldoi Luengo, Britski, 1974, M. bonita and M. cf. gracilima as Hemigrammus marginatus Ellis, 1911, M. australe and M. forestii as M. aff. sanctaefilomenae (Steindachner, 1907), S. heterodon as Odontostilbe sp. (now identified as O. avanhandava), and P. mysteriosus as P. maculatus Lacépède, 1803].
Some species were also considered to be new records (new occurrence or not recorded before) and misidentified species, such as A. hemigrammus, C. jaguarensis, M. australe, M. bonita, M. cf. gracilima, P. mysteriosus, S. calliurus, S. heterodon; also a new record and a new identification proposal, such as O. polyodon; also a new record and new genus described, such as C. insperatus and R. paranensis; also a new record, new species described and misidentified, such as M. forestii; also a new genus and new species described, such as M. piavussu; or also a a new genus described and new identification proposal, such as M. obtusidens. For details, see section of remarks of each record.
Key for genera of fish from the upper rio Paraná basin, between the mouth of the rio Paranapanema and the Itaipu Reservoir, mainly focusing at its floodplain
1. Five pairs of gill openings in ventral region of head, not covered by opercle; dorsal region of tail with pointed, strong stingers (MYLIOBATIFORMES) .................... Potamotrygon (Potamotrygonidae)
1’. One single or a pair of gill openings; caudal peduncle lacking stingers .................... 2
2. Both eyes on same side of head, in adults (PLEURONECTIFORMES) .................... Catathyridium (Achiridae)
2’. Eyes bilaterally situated in head .................... 3
3. One single, small gill opening, behind head, in midventral region; body snake-shaped (SYNBRANCHIFORMES) .................... Synbranchus (Synbranchidae)
3’. One pair of gill openings; body not snake-shaped .................... 4
4. Anterior portion of dorsal and anal fins with one or more spines .................... 5
4’. Spine occasionally present on dorsal fin, always absent on anal fin .................... 19
5. Two pairs of maxillary barbels; pelvic fin in abdominal position, without spine (CYPRINIFORMES) .................... Cyprinus (Cyprinidae)
5’. Maxillary barbels absent; pelvic fin in thoracic position, usually below pectoral fin, with spine .................... 6
6. Nostril with two openings; lateral line with one sinuous branch, from above gill opening to end of caudal fin (Incertae sedis) .................... Plagioscion (Sciaenidae)
6’. Nostril with one opening; lateral line interrupted (with one superior branch, anterior, and another median branch, posterior), or occasionally continuous in some specimens of Cichla (in which case the two branches meet at right angles) (CICHLIFORMES) .................... 7
7. Teeth bicuspid; dark-brown blotch present on postero-dorsal margin of opercle .................... 8
7’. Teeth conical; dark-brown blotch absent on postero-dorsal margin of opercle .................... 9
8. Inferior branch of first gill arch with more than 20 long, thin gill rakers .................... Oreochromis (Cichlidae)
8’. Inferior branch of first gill arch with up to 15 short, thick gill rakers .................... Coptodon (Cichlidae)
9. Superior branch of first gill arch with a well-developed lobe .................... 10
9’. Superior branch of first gill arch without a lobe .................... 13
10. Dark-brown blotch present on flank .................... 11
10’. Dark-brown blotch absent on flank .................... 12
11. Dorsal fin with 15 to 18 spines .................... Geophagus (Cichlidae)
11’. Dorsal fin with 12 to 14 spines .................... Gymnogeophagus (Cichlidae)
12. Three or four dark-brown longitudinal and conspicuous stripes present on lower half of flank; dark-brown suborbital stripe present, from inferior margin of orbit to contact of subopercle and interopercle .................... Apistogramma (Cichlidae)
12’. Dark-brown longitudinal stripes absent on flank; suborbital stripe absent (a dark-brown suborbital stripe is present in juveniles, but it extends only from ventral margin of orbit to preopercle) .................... Satanoperca (Cichlidae)
13. First gill arch with more than 70 long, thin gill rakers (almost as long as gill filaments) .................... Chaetobranchopsis (Cichlidae)
13’. First gill arch with less than 40 short, thick gill rakers .................... 14
14. Notch between dorsal-fin spines and soft rays present .................... Cichla (Cichlidae)
14’. Notch between dorsal-fin spines and soft rays absent .................... 15
15. Body elongated, greatest depth contained 2.8 to 5.0 times in standard lengh .................... Crenicichla (Cichlidae)
15’. Body deep, greatest depth contained 1.7 to 2.5 times in standard lengh .................... 16
16. Dorsal fin with 19 to 21 soft rays and anal fin with 15 to 17 soft rays; more than 20 circumpeduncular scale rows .................... Astronotus (Cichlidae)
16’. Dorsal fin with 8 to 15 soft rays and anal fin with 8 to 10 soft rays; up to 16 circumpeduncular scale rows .................... 17
17. Two scale rows on cheek; three or four dark-brown longitudinal stripes on lower half of flank .................... Laetacara (Cichlidae)
17’. More than two scale rows on cheek; dark-brown longitudinal stripes absent on flank .................... 18
18. Interradial membranes of dorsal and anal fins with scales .................... Cichlasoma (Cichlidae)
18’. Interradial membranes of dorsal and anal fins without scales .................... Aequidens (Cichlidae)
19. Body knife-shaped; anal fin with more than 100 rays; dorsal and pelvic fins absent (GYMNOTIFORMES) .................... 20
19’. Body not knife-shaped; anal fin with up to 62 rays; dorsal and pelvic fins present .................... 27
20. Caudal fin present .................... 21
20’. Caudal fin absent .................... 22
21. Snout prolonged into a thin, long and tubular rostrum .................... Sternarchorhynchus (Apteronotidae)
21’. Snout, when prolonged into a rostrum, not tubular .................... Apteronotus (Apteronotidae)
22. Mouth superior; dentary prognathous, longer than premaxilla .................... Gymnotus (Gymnotidae)
22’. Mouth terminal; dentary slightly shorter than premaxilla, or both dentary and premaxilla of same size .................... 23
23. Snout long and tubular .................... 24
23’. Snout short, not tubular .................... 25
24. Body pale yellow with dark-brown transverse stripes; anal fin hyaline and all anal-fin rays unbranched .................... Gymnorhamphichthys (Rhamphichthyidae)
24’. Body brown with dark-brown vermiculate pattern, including anal fin; most anal-fin rays branched .................... Rhamphichthys (Rhamphichthyidae)
25. Anterior nostril on superior lip .................... Brachyhypopomus (Hypopomidae)
25’. Anterior nostril on dorsal region of snout .................... 26
26. Orbital margin free, eye not covered by skin and surrounded by groove; most of anal-fin rays unbranched .................... Sternopygus (Sternopygidae)
26’. Orbital margin covered by skin, continuous with skin of head; most of anal-fin rays branched .................... Eigenmannia (Sternopygidae)
27. Top of head flat and covered by scales (CYPRINODONTIFORMES) .................... 28
27’. Top of head not flat and without scales .................... 32
28. Adult males without gonopodium; dorsal fin much closer to caudal fin than to vertical through half of body .................... Melanorivulus (Rivulidae)
28’. Adult males with gonopodium; dorsal fin on vertical through half of body or slightly posterior to it .................... 29
29. Dark-brown blotches on lower half of flank .................... Phallotorynus (Poeciliidae)
29’. Dark-brown blotches absent or, when present, on midline of flank .................... 30
30. Body with dark-grey reticulate coloration pattern, except on ventral region; dorsal fin with dark-brown blotch .................... Pamphorichthys (Poeciliidae)
30’. Body entirely with dark-grey reticulate coloration pattern; dorsal fin hyaline .................... 31
31. Males and females with dark-brown, vertically elongated, blotch on flank, below dorsal fin; adult males with long gonopodium, its length contained 2.6 to 3.1 times in standard lengh .................... Phalloceros (Poeciliidae)
31’. Males irregularly multicolored, and females without dark-brown blotch on flank; adult males with short gonopodium, its length contained 3.2 to 3.6 times in standard lengh .................... Poecilia (Poeciliidae)
32. Body naked or covered by plates (SILURIFORMES) .................... 33
32’. Body covered by scales .................... 84
33. Body entirely naked, without plates .................... 34
33’. Body partially or entirely covered by plates (even when visible only on snout, such as in Scoloplax) .................... 64
34. Opercle with spines .................... 35
34’. Opercle without spines .................... 36
35. Mouth inferior; nasal barbel absent .................... Paravandellia (Trichomycteridae)
35’. Mouth terminal; nasal barbel present .................... Trichomycterus (Trichomycteridae)
36. Adipose fin absent .................... 37
36’. Adipose fin present .................... 39
37. Head strongly depressed; opercular opening in ventral position; caudal peduncle keeled in dorsal region .................... Amaralia (Aspredinidae)
37’. Head not depressed; opercular opening in lateral position; caudal peduncle rounded dorsally .................... 38
38. Dorsal and pectoral fins without spines .................... Cetopsis (Cetopsidae)
38’. Dorsal and pectoral fins with strong spines .................... Trachelyopterus (Auchenipteridae)
39. Eye covered by head skin, orbit without free margin .................... 40
39’. Eye not covered by head skin, orbit with free margin .................... 45
40. One pair of barbels, most of times encapsulated .................... Ageneiosus (Auchenipteridae)
40’. More than one pair of barbels, not encapsulated .................... 41
41. Eyes laterally on head (visible in ventral view); adipose fin very small, much shorter than head .................... 42
41’. Eyes dorsally on head (not visible in ventral view); adipose fin large, as long as or longer than head .................... 44
42. Posterior nare anteromedial to eye; anal-fin base about twice as long as head .................... Auchenipterus (Auchenipteridae)
42’. Posterior nares between eyes; anal-fin base about as long as long as head or shorter .................... 43
43. Caudal fin bifurcate; anal-fin base about as long as dorsal-fin base .................... Tatia (Auchenipteridae)
43’. Caudal fin truncate or slightly rounded; anal-fin base more than three times as long as dorsal-fin base .................... Parauchenipterus (Auchenipteridae)
44. Adipose fin fused to caudal fin; caudal fin rounded .................... Heptapterus (Heptapteridae)
44’. Adipose fin not fused to caudal fin; caudal fin bifurcate .................... Phenacorhamdia (Heptapteridae)
45. Nasal-barbel present .................... 46
45’. Nasal-barbel absent .................... 47
46. Dorsal fin with seven (I,6) and anal fin with 29 or 30 rays; caudal fin bifurcated .................... Ictalurus (Ictaluridae)
46’. Dorsal fin with 61-79 and anal fin with 45-60 rays; caudal fin rounded .................... Clarias (Clariidae)
47. Body with dark-brown transverse bars, from dorsal to ventral region, occasionally visible only on distal portion of caudal fin .................... 48
47’. Body without dark-brown transverse bars (only dark-brown blotches on dorsal region, not extending ventrally, in Imparfinis) .................... 50
48. Lateral line incompletely pored; caudal fin emarginate (caudal-fin lobes rounded, with no projecting tips); tip of tubular anterior nostril reaching past border of upper lip .................... Microglanis (Pseudopimelodidae)
48’. Lateral line completely pored; caudal fin bifurcate (caudal-fin lobes slightly pointed); tip of tubular anterior nostril distant from border of upper lip .................... 49
49. Dark-brown transverse bar on caudal-fin lobes united with dark-brown transverse bar on posterior portion of caudal peduncle; dorsal and lateral surfaces of head grey, with light-beige blotch on cheek .................... Rhyacoglanis (Pseudopimelodidae)
49’. Dark-brown transverse bar on caudal-fin lobes not united with dark-brown transverse bar on posterior portion of caudal peduncle; dorsal and lateral surfaces of head completely grey .................... Pseudopimelodus (Pseudopimelodidae)
50. Anal fin long, with 55 to 62 rays .................... Hypophthalmus (Pimelodidae)
50’. Anal fin short, with up to 25 rays .................... 51
51. Barbels flattened .................... Pinirampus (Pimelodidae)
51’. Barbels round .................... 52
52. Premaxilla much longer than dentary, its dentigerous plate entirely exposed, even with closed mouth .................... Sorubim (Pimelodidae)
52’. Premaxilla slightly shorter, or of same size, or slighlty longer than dentary, with its dentigerous plate entirely hidden with closed mouth .................... 53
53. Lips thick, dorsal and ventral portions folded outwards .................... Iheringichthys (Pimelodidae)
53’. Lips thin, not folded .................... 54
54. Premaxilla slightly shorter than dentary .................... Hemisorubim (Pimelodidae)
54’. Premaxilla slighlty longer than dentary, or of same size .................... 55
55. Dark-brown blotches on dorsal region of body present .................... Imparfinis (Heptapteridae)
55’. Dark-brown blotches on dorsal region of body absent .................... 56
56. First dorsal and pectoral-fin rays hardened on its base, but not pungent .................... 57
56’. First dorsal and pectoral-fin rays developed into pungent spines .................... 60
57. Posterior nostril much closer to orbit than to anterior nostril .................... Cetopsorhamdia (Heptapteridae)
57’. Posterior nostril equidistant or much closer to anterior nostril than to orbit .................... 58
58. First dorsal-fin ray much longer than the others; pectoral fin with 14 or 15 rays .................... Megalonema (Pimelodidae)
58’. Dorsal-fin rays approximately of same size; pectoral fin with up to 10 rays .................... 59
59. Head long, 2.4 to 3.2 times in standard length; tooth plate present on vomer .................... Steindachneridion (Pimelodidae)
59’. Head short, 4.1 to 4.2 times in standard length; tooth plate absent on vomer .................... Rhamdia (Heptapteridae)
60. Orbit small, its diameter contained 9.0 to 11.0 times in head length .................... 61
60’. Orbit large, its diameter contained 3.0 to 6.5 times in head length .................... 62
61. Orbital diameter contained 1.5 to 2.5 times in interorbital distance .................... Pseudoplatystoma (Pimelodidae)
61’. Orbital diameter 3.5 to 4.5 times in interorbital distance .................... Zungaro (Pimelodidae)
62. Dark-brown longitudinal stripe present on flank; supraoccipital process uniformly narrow .................... Pimelodella (Heptapteridae)
62’. Dark-brown longitudinal stripe absent, or, when present, alternating with light-beige stripes (only in P. ornatus); supraoccipital process wider basally, narrowing towards tip .................... 63
63. Supraoccipital process reaching nuchal plate; branched dorsal-fin rays of different size, first approximately twice as long as last .................... Pimelodus (Pimelodidae)
63’. Supraoccipital process not reaching nuchal plate; branched dorsal-fin rays of approximately same size, except the last .................... Rhamdia (Heptapteridae)
64. Bony plates hardly visible on body sides, but well developed on snout .................... Scoloplax (Scoloplacidae)
64’. Bony plates fully developed on body sides, absent or less developed on snout .................... 65
65. One series of plates along lateral line, each one with a posteriad spine-shaped process .................... 66
65’. More than one series of plates on flank, without a spine-shaped process .................... 70
66. Barbels branched; mental barbels united by membrane at their bases; coracoid process not covered by skin .................... 67
66’. Barbels unbranched; mental barbels not united by membrane at their bases; coracoid process covered by skin .................... 68
67. Opercle and preopercle not covered by skin; orbit large, its diameter contained 2.5 to 3.1 times in head length; maxillary-barbel not reaching pectoral-fin base .................... Trachydoras (Doradidae)
67’. Opercle and preopercle covered by skin; orbit small, its diameter contained 3.6 to 4.9 times in head length; maxillary-barbel reaching or surpassing pectoral-fin base .................... Ossancora (Doradidae)
68. Lateral plates very elongated vertically, almost meeting dorsal and adipose fins .................... Platydoras (Doradidae)
68’. Lateral plates slightly elongated vertically, distant from dorsal and adipose fins .................... 69
69. Dorsal and ventral region of caudal peduncle covered by plates .................... Rhinodoras (Doradidae)
69’. Dorsal and ventral region of caudal peduncle without plates .................... Pterodoras (Doradidae)
70. Two series of deep plates on flank; mouth terminal or subterminal .................... 71
70’. Several longitudinal series of plates on flank; mouth inferior, sucker-shaped .................... 74
71. Coracoid bones, between pectoral fins, covered by skin; orbit small, its diameter contained more than 9.0 times in head length .................... Callichthys (Callichthyidae)
71’. Coracoid bones exposed; orbit large, its diameter contained less than 7.5 times in head length .................... 72
72. Maxillary barbel short, not reaching pectoral-fin base; dorsal-fin spine approximately the same size as its first unbranched ray .................... Corydoras (Callichthyidae)
72’. Maxillary barbel long, surpassing gill opening; dorsal-fin spine short, smaller than half of its first unbranched ray .................... 73
73. Caudal fin bifurcated; dark-brown spots absent over body; maxillary barbel not reaching pelvic-fin base .................... Hoplosternum (Callichthyidae)
73’. Caudal fin emarginated; dark-brown spots present all over body; maxillary barbel surpassing end of pelvic fin .................... Lepthoplosternum (Callichthyidae)
74. Caudal peduncle very depressed (dorsoventrally compressed) .................... 75
74’. Caudal peduncle deeper than broad (laterolaterally compressed) .................... 78
75. Snout very long, prolonged into rostrum; dorsal-fin origin much posterior to vertical through pelvic-fin origin .................... Farlowella (Loricariidae)
75’. Snout short, not prolonged into rostrum; dorsal-fin origin anterior to slightly posterior to vertical through pelvic-fin origin .................... 76
76. Inferior lip with several filaments; premaxillary teeth at least twice as large as dentary teeth .................... Loricaria (Loricariidae)
76’. Inferior lip without filaments; premaxillary and dentary teeth of approximately same size .................... 77
77. Inferior lip cushioned, with few papillae; dentary with 10 to 20 teeth .................... Loricariichthys (Loricariidae)
77’. Inferior lip not cushioned, with several papillae; dentary with 5 to 8 teeth .................... Rineloricaria (Loricariidae)
78. Dorsal-fin with I,7-9 rays .................... 79
78’. Dorsal fin with I,10-15 rays .................... 83
79. Adipose fin absent .................... 80
79’. Adipose fin present .................... 82
80. Gill opening much larger than orbital diameter; least distance between posterior nostrils much larger than orbital diameter; large body size, reaching 440.0 mm standard lengh .................... Rhinelepis (Loricariidae)
80’. Gill opening approximately same size of orbital diameter; least distance between posterior nostrils smaller or equal to orbital diameter; small body size, reaching up to 40.0 mm standard lengh .................... 81
81. Caudal-fin lobes simetrically colored; both with an oblique dark-brown band .................... Curculionichthys (Loricariidae)
81’. Caudal-fin lobes asimetrically colored; dorsal caudal-fin lobe overall clearer than ventral, both without oblique bands .................... Otothyropsis (Loricariidae)
82. Tentacles present on snout; interopercular region mobile, with long and strong spines .................... Ancistrus (Loricariidae)
82’. Tentacles absent; interopercular region slightly mobile, without spines .................... Hypostomus (Loricariidae)
83. Ground color of body pale yellow with brown blotches .................... Megalancistrus (Loricariidae)
83’. Ground color of body black with white spots and vermiculate pattern .................... Pterygoplichthys (Loricariidae)
84. Lateral line canal system absent on body (CLUPEIFORMES) .................... Platanichthys (Clupeidae)
84’. Lateral line canal system present on body (CHARACIFORMES) .................... 85
85. Premaxilla, maxilla and dentary entirely without teeth .................... 86
85’. Premaxilla, maxilla or dentary at least partially with teeth .................... 87
86. Palate with two parallel grooves, forming three longitudinal folds; dark-brown longitudinal band on flank from vertical through dorsal-fin base to end of caudal peduncle, or restricted to caudal peduncle .................... Cyphocharax (Curimatidae)
86’. Palate with irregular glomerular projections; dark-brown longitudinal band on flank from opercle to caudal-fin base .................... Steindachnerina (Curimatidae)
87. Dentary without teeth, at least in its anterior edge .................... 88
87’. Dentary with teeth, even anteriorly .................... 90
88. Dentary with rounded edge, in ventral view .................... Hemiodus (Hemiodontidae)
88’. Dentary with straight edge, in ventral view .................... 89
89. Dentary without lateral teeth .................... Apareiodon (Parodontidae)
89’. Dentary with one to four lateral teeth (visualized in opened mouth) .................... Parodon (Parodontidae)
90. Teeth depressible, small and numerous, implanted in lips .................... Prochilodus (Prochilodontidae)
90’. Teeth well developed, not depressible, implanted in dentary, premaxilla and occasionally in maxilla .................... 91
91. Adipose fin absent .................... 92
91’. Adipose fin present .................... 95
92. Teeth in palate and lateral line absent; dark-brown blotch on dorsal fin present, occupying almost the entire fin .................... Pyrrhulina (Lebiasinidae)
92’. Teeth in palate and lateral line present; dark-brown blotch on dorsal fin absent .................... 93
93. Dorsal profile of head straight; pectoral, pelvic and anal fins with dark-brown stripes; dark-brown rounded blotch present on posterodorsal region of caudal peduncle .................... Hoplias (Erythrinidae)
93’. Dorsal profile of head convex; pectoral, pelvic and anal fins without dark-brown stripes; dark-brown blotch absent on caudal peduncle .................... 94
94. Dark-brown rounded blotch on opercle; caudal fin evenly darkened .................... Hoplerythrinus (Erythrinidae)
94’. Dark-brown rounded blotch, when present, on humeral region; caudal fin with dark-brown spots .................... Erythrinus (Erythrinidae)
95. Abdomen keeled .................... 96
95’. Abdomen rounded .................... 102
96. Abdominal keel without spines .................... 97
96’. Abdominal keel with spines .................... 98
97. Mouth very large, with well developed canine teeth; lateral line with 142 to 145 pored scales .................... Rhaphiodon (Cynodontidae)
97’. Mouth and teeth small; lateral line with 33 to 37 pored scales .................... Triportheus (Triportheidae)
98. Teeth tricuspid (lateral cusps very small, most of times immersed in gum), arranged in a single row both in premaxilla and in dentary .................... Serrasalmus (Serrasalmidae)
98’. Teeth not tricuspid, arranged in two rows both in premaxilla and in dentary (the second dentary row represented by one minute conical teeth, next to the symphysis) .................... 99
99. Adipose fin longer than deep .................... Metynnis (Serrasalmidae)
99’. Adipose fin as long as deep .................... 100
100. Dorsal fin with 24 to 27 rays .................... Myloplus (Serrasalmidae)
100’. Dorsal fin with 14 to 16 rays .................... 101
101. Adipose fin with rays .................... Colossoma (Serrasalmidae)
101’. Adipose fin without rays .................... Piaractus (Serrasalmidae)
102. Teeth mammiliform, arranged outside mouth and turned forward, in adults .................... Roeboides (Characidae)
102’. Teeth not mammiliform, arranged inside mouth .................... 103
103. Spinoid scales on body .................... Galeocharax (Characidae)
103’. Cycloid scales on body .................... 104
104. Palate with teeth .................... 105
104’. Palate without teeth absent .................... 106
105. Teeth conical and/or tricuspid in both premaxilla and dentary; gill rakers almost as long as gill filaments and close to each other, without spines .................... Oligosarcus (Characidae)
105’. Teeth conical and canine in both premaxilla and dentary; gill rakers short and spaced apart, with spines .................... Acestrorhyncus (Acestrorhynchidae)
106. One teeth row in premaxilla .................... 107
106’. Two or three teeth rows in premaxilla .................... 116
107. Pectoral fin with three unbranched rays .................... Characidium (Crenuchidae)
107’. Pectoral fin with one unbranched rays .................... 108
108. Pseudotympanum absent .................... 109
108’. Pseudotympanum present .................... 113
109. Lateral line incomplete; anal-fin origin on vertical through dorsal-fin base or slightly posterior to dorsal fin; small size, reaching 60.0 mm standard lengh .................... Aphyocharax (Characidae)
109’. Lateral line complete; anal-fin origin much posterior to end of dorsal fin; large size, reaching 500.0 mm standard lengh .................... 110
110. Caudal-fin lobes covered by small scales; dark-grey blotch present on dorsal fin .................... Leporellus (Anostomidae)
110’. Caudal-fin lobes naked, with scales covering only the base of its rays; dark-grey blotch absent on dorsal fin .................... 111
111. Teeth cuspidated in both premaxilla and dentary, in adults; teeth aligned to form an arch .................... Schizodon (Anostomidae)
111’. Teeth incisiform in both premaxilla and dentary, in adults; teeth not aligned, formig a stair pattern when seen in ventral view .................... 112
112. Premaxilla and dentary with three teeth .................... Megaleporinus (Anostomidae)
112’. Premaxilla with three or four, and dentary with four teeth, or, when premaxilla and dentary with three teeth, flank with dark-brown longitunal stripes (in L. amblyrhynchus) .................... Leporinus (Anostomidae)
113. Dentary teeth with three central cusps of the similar size, forming a somewhat straight edge .................... 114
113’. Dentary teeth cusps gradually decreasing in size from central cusp, or central cusp much longer than lateral cusps .................... 115
114. Lateral line complete; premaxilla with five or six teeth; anal fin of mature males with hooks .................... Serrapinnus (Characidae)
114’. Lateral line incomplete; premaxilla with eight to 11 teeth; anal fin of mature males without hooks .................... Aphyocheirodon (Characidae)
115. Lateral line complete; anal fin of mature males without bony hooks and caudal peduncle without arching .................... Odontostilbe (Characidae)
115’. Lateral line incomplete; anal fin of mature males with bony hooks and caudal peduncle arched ventrally .................... Serrapinnus (Characidae)
116. Maxilla with 11 to 33 teeth .................... 117
116’. Maxilla without teeth, or with up to 5 teeth .................... 118
117. Premaxilla with three teeth rows; body scales without black spots .................... Brycon (Characidae)
117’. Premaxilla with two teeth rows; body scales with black spots .................... Salminus (Characidae)
118. Predorsal region very convex; anal fin with 39 or more rays .................... Psellogrammus (Characidae)
118’. Predorsal region straight or slightly convex; anal fin with up to 35 rays .................... 119
119. Premaxilla projecting much more anteriorly than dentary; premaxilla with three rows of heavy teeth .................... Piabina
119’. Premaxilla and dentary aligned; premaxilla with two teeth rows (even when outer row is not aligned, a third row is never present) .................... 120
120. Lateral line complete .................... 121
120’. Lateral line incomplete .................... 126
121. Inner row of premaxilla with five teeth .................... 122
121’. Inner row of premaxilla with four teeth .................... 123
122. Caudal-fin lobes covered by small scales .................... Moenkhausia (Characidae)
122’. Caudal-fin lobes without scales, except on its base .................... Astyanax (Characidae)
123. Humeral blotch absent; anal-fin origin slightly posterior to dorsal-fin origin .................... Planaltina (Characidae)
123’. Humeral blotch present, sometimes inconspicuous; anal-fin origin on vertical through end of dorsal-fin base .................... 124
124. Caudal-fin lobes covered by small scales .................... Knodus (Characidae)
124’. Caudal-fin lobes without scales, except on the base .................... 125
125. Mouth terminal; greatest body depth contained 4.0 to 4.2 times in standard lengh; middle portion of dorsal and anal fins not dusky; distal portion of caudal-fin lobes hyaline .................... Piabarchus (Characidae)
125’. Any feature other than the combination of characters above (mouth subterminal or terminal; greatest body depth contained 2.7 to 4.2 times in standard lengh; middle portion of dorsal and anal fins dusky or not; distal portion of caudal-fin lobes dusky or hyaline) .................... Bryconamericus (Characidae)
126. Dark-brown blotch on caudal-fin base occupying its whole depth; or distal portion of caudal-fin lobes black .................... Moenkhausia (Characidae)
126’. Dark-brown blotch on caudal-fin base absent; or, when present, not occupying its whole depth, and distal portion of caudal-fin lobes hyaline .................... 127
127. Black blotch present on dorsal fin, or 7 or 8 scales in transverse series above lateral line .................... Hyphessobrycon (Characidae)
127’. Black blotch absent on dorsal fin, and transverse series of scales with 5 or 6 above lateral line .................... 128
128. Inner row of premaxilla with three or four teeth .................... Diapoma (Characidae)
128’. Inner row of premaxilla with five teeth .................... Hemigrammus (Characidae)
Comments and corrections on Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Crenicichla niederleinii (Holmberg, 1891), Hemigrammus marginatus, and Pimelodus cf. argenteus Perugia, 1891 do not occur in the upper rio Paraná floodplain and these records are considered misidentifications. The record of Mylossoma duriventre (Cuvier, 1918) in the upper rio Paraná basin is incorrect, and this species only occurs in the río Paraguay and lower río Paraná basins (Mateussi et al., 2018Mateussi NTB, Oliveira C, Pavanelli CS. Taxonomic revision of the Cis-Andean species of Mylossoma Eigenmann & Kennedy, 1903 (Teleostei: Characiformes: Serrasalmidae). Zootaxa . 2018; 4387(2):275-309.)).
The specimens identified as Corydoras flaveolus Ihering, 1911 by Graça, Pavanelli (2007)Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007. correspond to C. lacrimostigmata Tencatt, Britto, Pavanelli, 2014, species described from the rio Ivaí basin. Because of this species does not occur in the region under the influence of the upper rio Paraná floodplain, it was not not redescribed herein.
The pictures assigned as Hemigrammus marginatus (p. 63), Moenkhausia aff. sanctaefilomenae (p. 68), Serrapinnus sp. 1 (p. 94) and both Pimelodus cf. argenteus (p. 154) and Pimelodus maculatus (p. 156), represent, in fact, Moenkausia bonita, M. australe, Aphyocheirodon hemigrammus, and Pimelodus mysteriosus, respectively.
Hemiodus orthonops has no dentary teeth, contrary to the stated in the description by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). In their key to Cichla Bloch, Schneider, 1801, the number of scales refers to the longitudinal series, not the upper lateral line. The correct authorship is Astyanax aff. fasciatus (Cuvier, 1819), not (Cuvier, 1829).
Moenkhausia sanctaefilomenae was identified with the particle “aff.” because Benine (2004Benine RC. Análise filogenética do gênero Moenkhausia Eigenmann, 1903 (Characiformes: Characidae) com uma revisão dos táxons do alto rio Paraná. [PhD Thesis]. Botucatu, SP: Universidade Estadual Paulista; 2004.) stated that the specimes from the upper rio Paraná basin belonged to a new species. Posteriorly, Benine et al. (2009Benine RC, Mariguela TC, Oliveira C. New species of Moenkhausia Eigenmann, 1903 (Characiformes: Characidae) with comments on the Moenkhausia oligolepis species complex. Neotrop Ichthyol. 2009; 7(2):161-68.) described M. forestii from the Paraná-Paraguay system, but recorded the occurrence of M. sanctaefilomenae in the upper rio Paraná basin, and revalidated M. australe, which now is found in the upper rio Paraná floodplain as well. Therefore, the particle “aff.” to M. sanctaefilomenae is no longer used.
Accounts of fishes
MYLIOBATIFORMES
Potamotrygonidae
Potamotrygon
1. Dorsal surface of the disc with yellow or orange ocelli, larger than diameter of the eye, surrounded by a black ring, which do not extend distally over the tail .................... P. amandae
1’. Dorsal surface of the disc entirely covered by white or yellow spots, oval or reniform, smaller than diameter of the eye, which extend distally over the tail .................... P. cf. falkneri
Potamotrygon amandae Loboda, Carvalho, 2013Loboda TS, Carvalho MR. Systematic revision of the Potamotrygon motoro (Müller & Henle, 1841) species complex in the Paraná-Paraguay basin, with description of two new ocellated species (Chondrichthyes: Myliobatiformes: Potamotrygonidae). Neotrop Ichthyol . 2013; 11(4):693-737.
Body depressed; disc length contained 0.8 to 1.2 times in disc width (DW); distance from mouth to cloaca 1.3 to 1.7, distance from cloaca to caudal sting 1.8 to 2.6, tail length 0.8 to 1.0, and caudal sting length 3.1 to 9.3 in DW; mouth width 7.6 to 14.3, tail width 7.2 to 16.4 in DW; tail length 1.4 in disc length (DL); horizontal orbital diameter 1.0 to 5.3 in least interorbital width. Mouth inferior; upper jaw with 18-39 teeth and lower jaw with 20-39 teeth. Pectoral fin with 92-103 rays; mid-dorsal spines 11-70 (Loboda, Carvalho, 2013Loboda TS, Carvalho MR. Systematic revision of the Potamotrygon motoro (Müller & Henle, 1841) species complex in the Paraná-Paraguay basin, with description of two new ocellated species (Chondrichthyes: Myliobatiformes: Potamotrygonidae). Neotrop Ichthyol . 2013; 11(4):693-737.). Dorsal disc coloration variable, from brown-olive to dark-grey, with bicolor ocelli distributed throughout entire disc (Loboda, Carvalho, 2013Loboda TS, Carvalho MR. Systematic revision of the Potamotrygon motoro (Müller & Henle, 1841) species complex in the Paraná-Paraguay basin, with description of two new ocellated species (Chondrichthyes: Myliobatiformes: Potamotrygonidae). Neotrop Ichthyol . 2013; 11(4):693-737.).
Maximum disc length. 341.0 mm.
Distribution. Paraná-Paraguay system.
Remarks. Potamotrygon amandae was identified as P. cf. motoro by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Loboda, Carvalho (2013Loboda TS, Carvalho MR. Systematic revision of the Potamotrygon motoro (Müller & Henle, 1841) species complex in the Paraná-Paraguay basin, with description of two new ocellated species (Chondrichthyes: Myliobatiformes: Potamotrygonidae). Neotrop Ichthyol . 2013; 11(4):693-737.) revised the P. motoro species complex from the Paraguay-Paraná basin and assigned all specimens from the rio Paraná, upstream from the Itaipu dam, to P. amandae (which otherwise was found only in the Pantanal region). Additionally, the same authors recorded P. motoro from the rio Guaporé, upper rio Paraguai basin and lower reaches of the rio Paraná in Argentina. On the other hand, the only other Potamotrygon species found in the upper rio Paraná floodplain is P. cf. falkneri that, as P. amandae, is a non-native species in the region. Both occurrences are associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls (Langeani et al., 2007Langeani F, Castro RMC, Oyakawa OT, Shibatta OA, Pavanelli CS, Casatti L. Diversidade da ictiofauna do alto rio Paraná: composição atual e perspectivas futuras. Biota Neotropica , 2007; 7(3):181-97.; Júlio Júnior et al., 2009Júlio Júnior HF, Dei Tos C, Agostinho AA, Pavanelli CS. A massive invasion of fish species after eliminating a natural barrier in the upper rio Paraná basin. Neotrop Ichthyol . 2009; 7(4):709-18.; Loboda, Carvalho, 2013Loboda TS, Carvalho MR. Systematic revision of the Potamotrygon motoro (Müller & Henle, 1841) species complex in the Paraná-Paraguay basin, with description of two new ocellated species (Chondrichthyes: Myliobatiformes: Potamotrygonidae). Neotrop Ichthyol . 2013; 11(4):693-737.).
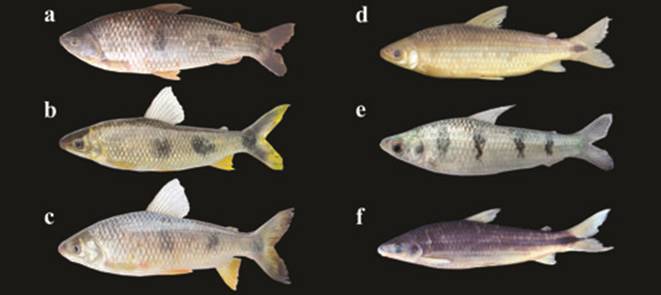
a. Potamotrygon amandae, 420.0 mm TL, fresh specimen, uncat. b. Potamotrygon cf. falkneri, 780.0 mm TL, fresh specimen, uncat. c. Platanichthys platana, NUP 16904, 28.9 mm SL, lagoa das Garças, tributary of the rio Paraná, Batayporã, State of Mato Grosso do Sul. d. Cyprinus carpio, NUP 1414, 203.3 mm SL, estação de piscicultura (CODAPAR-UEM), Maringá, State of Paraná.
Potamotrygon cf. falkneri Castex, Maciel, 1963
Body depressed; disc length contained 1.0 to 1.1 times in disc width (DW); distance from mouth to cloaca 1.4 to 1.6, distance from cloaca to caudal sting 1.7 to 2.1, tail length 1.0, and caudal sting length 4.2 to 6.0 in DW; mouth width 9.9 to 12.9, tail width 7.6 to 9.8 in DW; tail length 1.0 in disc length (DL); horizontal orbital diameter 2.3 to 3.9 in least interorbital width. Mouth inferior; upper jaw with 22-50 teeth and lower jaw with 26 to 44 teeth. Pectoral fin with 95-99 rays; mid-dorsal spines 18-56 (Rosa, 1985Rosa RS. A systematic revision of the South American freshwater stingrays (Chondrichthyes: Potamotrygonidae). [PhD Thesis]. Virginia, The Faculty of the School of Marine Science; 1985.). Dorsal disc coloration brown, with numerous oval-shaped to reniform, yellow (occasionally black-margined) ocelli, usually wider than eye diameter (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum disc length. 780.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system.
Remark. Potamotrygon cf. falkneri is a non-native species in the upper rio Paraná and its occurrence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
CLUPEIFORMES
Clupeidae
Platanichthys
Platanichthys platana (Regan, 1917)
Body elongated, laterally compressed; greatest body depth contained 3.8 to 4.5 and caudal peduncle depth 9.0 to 11.5 times in SL; head length 3.5 to 3.8, predorsal distance 2.0 to 2.1 and caudal peduncle length 8.5 to 12.3 in SL; snout length 4.2 to 5.6, horizontal orbital diameter 2.5 to 2.9 and least interorbital width 5.1 to 7.3 in HL. Mouth superior; anterior supra-maxilla small or absent; posterior frontal fontanel retained in adults. Dorsal fin with 14 rays, pectoral fin with 11 or 12 rays, pelvic fin with 7 rays, anal fin with 18-20 rays, and caudal fin with 19 rays. Ground color whitish.
Maximum standard length. 32.6 mm.
Biological data. Feeds on filamentous algae, debris, eggs, larvae of chironomids and bivalves, and zooplankton (Aguiaro et al., 2003Aguiaro T, Branco CWC, Verani JR, Caramaschi EP. Diet of the Clupeid fish Platanichthys platana (Regan, 1917) in two different Brazilian coastal lagoons. Braz Arch Biol Technol. 2003; 46(2):215-22.).
Distribution. Lagoons, estuaries and lower parts of rivers in Argentina, Brazil and Uruguay.
Remarks. Platanichthys platana, described from the río de la Plata, was already reported in the upper rio Paraná basin by Langeani et al. (2007Langeani F, Castro RMC, Oyakawa OT, Shibatta OA, Pavanelli CS, Casatti L. Diversidade da ictiofauna do alto rio Paraná: composição atual e perspectivas futuras. Biota Neotropica , 2007; 7(3):181-97.), but this is the first record in the upper rio Paraná floodplain, where it has been captured since 2013. Therefore, P. platana is considered a non-native species from the upper rio Paraná basin, and its occurrence can be associated with the functioning of the Canal da Piracema, a fish ladder that connects the river downstream from the Itaipu Dam to the lake upstream from the dam. The maximum size observed herein was 32.6 mm SL, much less than the 70.0 mm SL reported by Whitehead (1968Whitehead PJP. FAO species catalogue. Rome: Food and Agriculture Organization of the United Nations; 1985. vol. 7, Clupeoid fishes of the world (suborder Clupeoidei): An annotated and illustrated catalogue of the herrings, pilchards, sprats, shads, anchovies and wolf-herrings, pt. 1, Chirocentridae, Clupeidae and Pristigasteridae. (FAO Fisheries Synopsis; no. 125).).
CYPRINIFORMES
Cyprinidae
Cyprinus
Cyprinus carpio Linnaeus, 1758
Body deep; greatest depth contained 2.2 to 2.8 and caudal peduncle depth 6.4 to 7.6 times in SL; head length 3.8 to 4.9, predorsal distance 1.9 to 2.2 and caudal peduncle length 7.0 to 12.1 in SL; snout length 2.3 to 2.8, horizontal orbital diameter 4.5 to 6.4 and least interorbital width 2.3 to 2.9 in HL. Mouth terminal, toothless. Lateral line complete, with 35-38 pored scales; transverse series above lateral line with 5-6 scale rows and below with 6-8 scale rows. Dorsal fin with 21, pectoral fin with 16 or 18, pelvic fin with 8, anal fin with 7 to 9 rays. Ground color silvery to pale yellow; darker dorsally. Yellowish fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 480.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Drainages of Eurasia.
Remarks. Cyprinus carpio is an Eurasian species and its occurrence in the upper rio Paraná can be associated with fish-farming.
CHARACIFORMES
Acestrorhynchidae
Acestrorhynchus
1. Transverse series above the lateral line with 22 to 25 scale rows .................... A. lacustris
1’. Transverse series above the lateral line with 26 to 30 scale rows .................... A. pantaneiro
Acestrorhynchus lacustris (Lütken, 1875)
Body elongated; greatest depth contained 4.2 to 5.3 and caudal peduncle depth 13.2 to 15.6 times in SL; head length 3.1 to 3.6, predorsal distance 1.5 to 1.7 and caudal peduncle length 12.5 to 14.0 in SL; snout length 2.5 to 2.8, horizontal orbital diameter 2.8 to 5.0 and least interorbital width 4.0 to 5.4 in HL. Mouth terminal; outer row of premaxilla with 13-16 and inner row with 10-20, and maxilla with 16-39 teeth. Lateral line complete, with 86-102 pored scales; transverse series above lateral line with 22-25 and below with 13-15 scale rows. Dorsal fin with 11, pectoral fin with 14-18, pelvic fin with 8, anal fin with 23-27, and caudal fin with 19 rays (Menezes, 1992Menezes NA. Redefinição taxonômica das espécies de Acestrorhynchus do grupo lacustris com a descrição de uma nova espécie (Osteichthyes, Characiformes, Characidae). Com Mus Cien PUCRS . 1992; 5(5):39-54.). Ground color silvery to yellowish; black rounded humeral blotch; black oval, horizontally elongated, blotch on caudal-fin base, extending to median caudal-fin rays. Yellowish fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 280.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná and rio São Francisco basins.
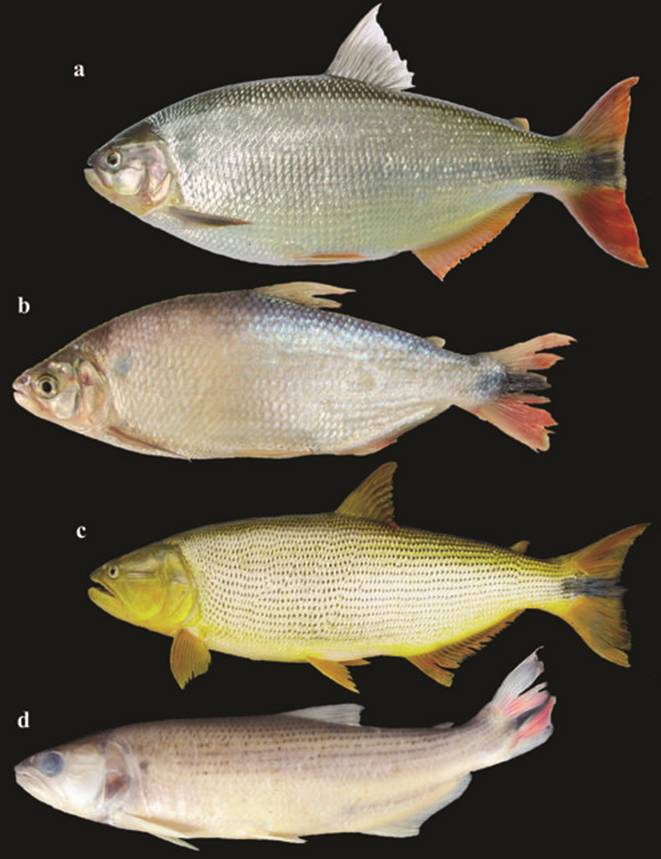
a. Acestrorhynchus lacustris, 150.0 mm SL, fresh specimen, uncat. b. Acestrorhynchus pantaneiro, 147.0 mm SL, fresh specimen, uncat. c. Leporellus vittatus, NUP 1902, 200.0 mm SL, Itaipu Reservoir, Foz do Iguaçu, State of Paraná.
Acestrorhynchus pantaneiro Menezes, 1992Menezes NA. Redefinição taxonômica das espécies de Acestrorhynchus do grupo lacustris com a descrição de uma nova espécie (Osteichthyes, Characiformes, Characidae). Com Mus Cien PUCRS . 1992; 5(5):39-54.
Body elongated; greatest depth contained 3.6 to 4.8 and caudal peduncle depth 12.1 to 14.9 times in SL; head length 3.0 to 3.6, predorsal distance 1.5 to 1.8 and caudal peduncle length 9.8 to 13.1 in SL; snout length 2.8 to 3.3, horizontal orbital diameter 3.5 to 5.0 and least interorbital width 3.5 to 4.6 in HL. Mouth terminal; outer row of premaxilla with 13-16 and inner row with 10-20, and maxilla with 16-39 teeth. Lateral line complete, with 93-108 pored scales; transverse series above lateral line with 26-30 scale rows and below with 15-17 scale rows. Dorsal fin with 11, pectoral fin with 14-18, pelvic fin with 8, anal fin with 23-27, and caudal fin with 19 rays. Ground color silvery to yellowish; black rounded humeral blotch; black oval, horizontally elongated, blotch on caudal-fin base, extending to median caudal-fin rays. Yellowish fins; anal and pectoral fin with distal margins black (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 180.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río de la Plata and rio Mamoré basins.
Remarks. Acestrorhynchus pantaneiro is a non-native species from the upper rio Paraná basin, and its recent occurrence can be associated with the functioning of the Canal da Piracema, a fish ladder that connects the region downstream from the Itaipu Dam to the region upstream from the dam (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Anostomidae
Leporellus
Leporellus vittatus (Valenciennes, 1850)
Description. Body elongated; greatest depth contained 3.7 to 4.5, and caudal peduncle depth 10.3 to 10.7 times in SL; head length 3.8 to 4.2, predorsal distance 2.2 to 2.4, caudal peduncle length 5.2 to 5.5 in SL; snout length 2.2 to 2.6, horizontal orbital diameter 3.8 to 5.7 and least interorbital width 2.5 to 2.7. Mouth terminal; premaxilla and dentary with 4 teeth, no maxillary teeth. Lateral line with 41-43 pored scales; transverse series above lateral line with 5½ scale rows and below with 4 scale rows. Dorsal fin with 10 or 11, pectoral fin with 14-16, pelvic fin with 9 or 10, anal fin with 10 and caudal fin with 19 rays. Ground color silvery to yellowish; dark-brown spots on dorsal and lateral portion of head; several longitudinal series of dark-brown spots on flank scales; dark-brown longitudinal band along lateral line, extending to median caudal-fin rays. Yellowish fins; black blotch on dorsal fin and two black oblique stripes on each caudal-fin lobe (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 220.0 mm SL (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Amazon, Paraná-Paraguay system and rio São Francisco basin.
Leporinus
1. Twelve circumpeduncular scale rows .................... L. amblyrhynchus
1’. Sixteen circumpeduncular scale rows .................... 2
2. Flank with four dark-brown longitudinal bands .................... L. striatus
2’. Flank with no dark-brown longitudinal bands .................... 3
3. Flank with dark-brown transverse bars .................... 4
3’. Flank with no dark-brown transverse bars, usually with black or dark-brown rounded blotches .................... 5
4. All dark-brown bars simple; pelvic fin with nine rays .................... L. octofaciatus
4’. At least some of the dark-brown bars Y-shaped; pelvic fin with 10 rays .................... L. tigrinus
5. Lateral line with 33 to 35 pored scales; three black or dark-brown rounded blotches on flank, the first larger and more conspicuous than the others .................... L. lacustris
5’. Lateral line with 37 to 44 pored scales; three black or dark-brown horizontally elongated blotches on flank, equally pigmented .................... L. friderici
Leporinus amblyrhynchus Garavello, Britski, 1987Garavello JC, Britski HA. Duas novas espécies do gênero Leporinus Spix, 1829, da bacia o alto rio Paraná (Teleostei, Anostomidae). Com Mus Cien PUCRS. 1987; 44:153-65.
Body elongated; greatest depth contained 3.8 to 4.3, and caudal peduncle depth 10.0 to 10.2 times in SL; head length 3.9 to 4.0, predorsal distance 2.0, caudal peduncle length 7.0 to 7.9 in SL; snout length 2.0 to 2.5, horizontal orbital diameter 2.9 to 3.8 and least interorbital width 6.7 to 8.4 in HL. Mouth subterminal; premaxilla and dentary with 3 teeth, no maxillary teeth. Lateral line with 37-40 pored scales; transverse series above lateral line with 5 scale rows and below with 4 scale rows. Dorsal fin with 12, pectoral fin with 18, pelvic fin with 9, anal fin with 10 and caudal fin with 19 rays (Garavello, Britski, 1987Garavello JC, Britski HA. Duas novas espécies do gênero Leporinus Spix, 1829, da bacia o alto rio Paraná (Teleostei, Anostomidae). Com Mus Cien PUCRS. 1987; 44:153-65.). Ground color pale yellow; dark-brown longitudinal band along lateral line, from posterior margin of opercle to caudal-fin base; 10-12 dark-brown transverse bars on dorsal surface, extending downward, not reaching longitudal band. Hyaline fins; distal margin of dorsal fin black (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 100.5 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río Paraná basin.
a. Leporinus amblyrhynchus , 97.5 mm SL, uncat. b. Leporinus friderici, 189.2 mm SL, fresh specimen, uncat. c. Leporinus lacustris, NUP 3308, 210.0 mm SL, ressaco do Pau Veio, tributary of the rio Paraná, Porto Rico, State of Paraná. d. Leporinus octofasciatus, NUP 281, 148.9 mm SL, rio São Francisco Falso, Santa Helena, State of Paraná. e. Leporinus striatus, 69.2 mm SL, uncat. f. Leporinus tigrinus, NUP 17488, 180.1 mm SL, rio Paraná, Querência do Norte, State of Paraná.
Leporinus friderici (Bloch, 1794)
Body elongated; greatest depth contained 3.2 to 4.1, and caudal peduncle depth 9.3 to 10.5 times in SL; head length 4.0 to 4.3, predorsal distance 2.1 to 2.3, caudal peduncle length 8.6 to 10.5 in SL; snout length 2.4 to 2.6, horizontal orbital diameter 3.2 to 3.9 and least interorbital width 2.1 to 2.8. Mouth terminal; premaxilla and dentary with 4 teeth, no maxillary teeth. Lateral line with 37-41 pored scales; transverse series above lateral line with 4-5½ scale rows and below with 4-5½ scale rows. Dorsal fin with 11 or 12, pectoral fin with 15-17, pelvic fin with 9, anal fin with 10 and caudal fin with 19 rays. Ground color silvery to yellowish; superior region of orbit red; three dark-brown rounded or oval, horizontaly elongated, blotches on flank; region of contact between flank scales below lateral line with orange or red spots, forming longitudinal series. Fins yellowish (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 370.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Drainages of Surinam, Amazon basin and Paraná-Paraguay system.
Leporinus lacustris Campos, 1945Campos AA. Contribuição ao estudo das espécies brasileiras do gênero Leporinus. Pap Avulsos Zool . 1945; 5(16):141-58.
Body deep; greatest depth contained 2.8 to 3.4 and caudal peduncle depth 6.4 to 8.0 times in SL; head length 3.2 to 3.8, predorsal distance 1.9 to 2.1, caudal peduncle length 13.3 to 13.5 in SL; snout length 1.3 to 2.5, horizontal orbital diameter 3.4 to 4.5 and least interorbital width 1.7 to 2.0. Mouth terminal; premaxilla and dentary with 4 teeth, no maxillary teeth. Lateral line with 32-36 pored scales; transverse series above and below lateral line with 4 or 4½ scale rows. Dorsal fin with 12, pectoral fin with 14-16, pelvic fin with 8 or 9, anal fin with 10 and caudal fin with 19 rays (Campos, 1945Campos AA. Contribuição ao estudo das espécies brasileiras do gênero Leporinus. Pap Avulsos Zool . 1945; 5(16):141-58.; Garavello, 1979Garavello JC. Revisão taxonômica do gênero Leporinus Spix, 1829 (Ostariophysi, Anostomidae). [PhD Thesis]. São Paulo, SP: Universidade de São Paulo; 1979.). Ground color greyish to pale yellow; three grey to black rounded blotches along lateral line, first and most conspicuous below dorsal fin. Yellowish fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 230.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system.
Leporinus octofasciatus Steindachner, 1915
Body elongated; greatest depth contained 3.6 to 3.8, and caudal peduncle depth 9.1 to 9.3 times in SL; head length 3.1 to 4.3, predorsal distance 1.9 to 2.1, caudal peduncle length 9.2 to 9.3 in SL; snout length 2.2 to 2.7, horizontal orbital diameter 4.0 to 4.2 and least interorbital width 2.2 to 2.6. Mouth terminal; premaxilla with 3 and dentary with 4 teeth, no maxillary teeth. Lateral line with 35-39 pored scales; transverse series above lateral line with 5 scale rows and below with 4-5 scale rows. Dorsal fin with 12, pectoral fin with 16 or 17, pelvic fin with 9, anal fin with 9 or 10 and caudal fin with 19 rays (Britski, Garavello, 1978Britski HA, Garavello JC. Sobre Leporinus octofasciatus Steindachner da bacia do rio Paraná (Pisces, Anostomidae). Pap Avulsos Zool . 1978; 31(16):237-50.; Garavello, 1979Garavello JC. Revisão taxonômica do gênero Leporinus Spix, 1829 (Ostariophysi, Anostomidae). [PhD Thesis]. São Paulo, SP: Universidade de São Paulo; 1979.). Ground color pale yellow; eight black transverse bars on body (posterior to head). Reddish fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 210.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Rio Cubatão (State of Santa Catarina) and río Paraná basin.
Leporinus striatus Kner, 1858
Body elongated; greatest depth contained 3.9 to 4.0, and caudal peduncle depth 9.6 to 10.6 times in SL; head length 3.7 tp 4.5, predorsal distance 2.1 to 2.2, caudal peduncle length 9.4 to 10.4 in SL; snout length 2.2 to 2.8, horizontal orbital diameter 3.1 to 3.5 and least interorbital width 2.5 to 2.7 in HL. Mouth terminal; premaxilla with 3, dentary with 4 teeth, no maxillary teeth. Lateral line with 34-36 pored scales; transverse series above lateral line with 4 or 4½ scale rows and below with 4 scale rows. Dorsal fin with 12, pectoral fin with 16 or 17, pelvic fin with 9 or 10 rays, anal fin with 10 and caudal fin with 19 rays (Garavello, 1979Garavello JC. Revisão taxonômica do gênero Leporinus Spix, 1829 (Ostariophysi, Anostomidae). [PhD Thesis]. São Paulo, SP: Universidade de São Paulo; 1979.). Ground color pale yellow; four dark-brown longitudinal bands on body, the superior one sometimes fused. Yellowish fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 83.2 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system.
Leporinus tigrinus Borodin, 1929
Body elongated; greatest depth contained 3.2 to 3.3, and caudal peduncle depth 9.4 to 9.5 times in SL; head length 4.0, predorsal distance 2.0, caudal peduncle length 10.2 to 11.0 in SL; snout length 2.1 to 2.2, horizontal orbital diameter 4.9 to 5.1 and least interorbital width 2.3 in HL. Mouth terminal; premaxilla with three and dentary with four teeth, no maxillary teeth. Lateral line with 39-41 pored scales; transverse series above lateral line with 7 scale rows and below with 5 or 5½ scale rows. Dorsal fin with 12, pectoral fin with 16 or 17, pelvic fin with 10, anal fin with 10 or 11 and caudal fin with 19 rays. Ground color yellowish; upper lip dark-brown; nine dark-brown transverse bars on body, one between orbits, one on nape, five on flank (usually Y-shaped), and two on caudal peduncle. Yellowish fins.
Maximum standard length. 410.0 mm.
Biological data. Lives in littoral and bathypelagic zones (Freitas et al., 2009Freitas IS, Lucinda PHF, Soares AB, Pelicice FM, Akama A. Variações espaciais na estrutura da ictiofauna entre os ambientes do reservatório de Peixe Angical. In: Agostinho CS, Pelicice FM, Marques EE, organizers. Reservatório de Peixe Angical: bases ecológicas para o manejo da ictiofauna. São Carlos: RiMa; 2009. p.41-57.); it is a short-distance migratory species, with external fertilization, and does not display parental care (Neuberger et al., 2009Neuberger AL, Marques EE, Agostinho CS, Pelicice FM. Variações espaciais na atividade reprodutiva de peixes na área de influência do reservatório de Peixe Angical. In: Agostinho CS, Pelicice FM, Marques EE, organizers. Reservatório de Peixe Angical: bases ecológicas para o manejo da ictiofauna. São Carlos: RiMa ; 2009. p.59-68.).
Distribution. Rio Tocantins and upper rio Paraná basins.
Remarks. Leporinus tigrinus has been captured in the upper rio Paraná floodplain since 2015 by Nupélia staff. Records of L. tigrinus were already reported to the upper rio Paraná basin by Langeani et al. (2007Langeani F, Castro RMC, Oyakawa OT, Shibatta OA, Pavanelli CS, Casatti L. Diversidade da ictiofauna do alto rio Paraná: composição atual e perspectivas futuras. Biota Neotropica , 2007; 7(3):181-97.), Pavanelli et al. (2007Pavanelli CS, Graça WJ, Zawadzki CH, Britski HA, Vidotti AP, Avelino GS, Veríssimo S. Fishes from the Corumbá Reservoir, Paranaíba River drainage, upper rio Paraná basin, State of Goiás, Brazil. Check List. 2007; 3(1):58-64.) and Santos et al. (2013Santos CJ, Tencatt LFC, Ota RR, Graça WJ. Second record of Leporinus tigrinus Borodin, 1929 (Characiformes: Anostomidae) in the upper Paraná River basin, Brasil. Check List . 2013; 9(6):1543-44.). Because museum specimens have not been captured in the basin before that, L. tigrinus is a possible non-native species in the region, probably introduced from the rio Tocantins basin (Santos et al., 2013Santos CJ, Tencatt LFC, Ota RR, Graça WJ. Second record of Leporinus tigrinus Borodin, 1929 (Characiformes: Anostomidae) in the upper Paraná River basin, Brasil. Check List . 2013; 9(6):1543-44.).
Megaleporinus
1. Lateral line with 39 to 40 (rarely 41) pored scales .................... M. piavussu
1’. Lateral line with 41 to 44 pored scales .................... 2
2. Mouth always subterminal, its cleft at horizontal through ventral margin of orbit or slightly below; three black blotches on flank, equally rounded; dark-brown transverse bars on flank usually persistent in large specimens; longest anal-fn ray more than twice the length of the last ray .................... M. obtusidens
2’. Mouth usually terminal, its cleft above horizontal through ventral margin of orbit; three black blotches on flank, first blotch vertically elongated, and remaining rounded; dark-brown transverse bars on flank usually not persistent in large specimens; longest anal-fn ray less than twice the length of the last ray .................... M. macrocephalus
Megaleporinus macrocephalus (Garavello, Britski, 1988Garavello JC, Britski HA. Leporinus macrocephalus sp. n. da bacia do rio Paraguai (Ostariophysi, Anostomidae). Naturalia. 1988; 13:67-74. )
Body deep; greatest depth contained 3.0 to 3.5, caudal peduncle depth 8.1 to 8.9 times in SL; head length 2.5 to 3.0, predorsal distance 2.0, caudal peduncle length 4.9 to 6.6 in SL; snout length 2.2 to 2.5, horizontal orbital diameter 2.9 to 3.7 and least interorbital width 1.7 to 1.9 in HL. Mouth terminal; premaxilla and dentary with 3 teeth, no maxillary teeth. Lateral line with 42 or 43 pored scales; transverse series above lateral line with 5½ or 6 scale rows and below with 5 or 5½ scale rows. Dorsal fin with 12, pectoral fin with 16 or 17, pelvic fin with 9, anal fin with 10 and caudal fin with 19 rays (Garavello, Britski, 1988Garavello JC, Britski HA. Leporinus macrocephalus sp. n. da bacia do rio Paraguai (Ostariophysi, Anostomidae). Naturalia. 1988; 13:67-74.). Ground color dark-grey; isthmus and abdomen yellowish; scales with dark-brown border, conferring reticulated pattern to body; three dark-brown blotches over lateral line (first vertically enlongated, second and third rounded), more visible in young specimens, inconspicuous or absent in large specimens. Dorsal, anal and caudal fin darker, pectoral and pelvic fins lighter (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 500.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río Paraguay and upper rio Paraná basin.
Remarks. Megaleporinus macrocephalus was identified as Leporinus macrocephalus by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Ramirez et al. (2016Ramirez JL, Birindelli JLO, Galetti Júnior PM. A new genus of Anostomidae (Ostariophysi: Characiformes): Diversity, phylogeny and biogeography based on cytogenetic, molecular and morphological data. Mol Phylogenet Evol . 2017; 107:308-23.) described the new genus, Megaleporinus, and proposed the new combination. Megaleporinus macrocephalus is a non-native species from the upper rio Paraná and its occurrence in the region can be associated with fish-farming and escapes from recreational angling ponds.
a. Megaleporinus macrocephalus , NUP 1838, 470.0 mm SL, rio Chopim, Cruzeiro do Iguaçu, State of Paraná. b. Megaleporinus obtusidens , 190.8 mm SL, fresh specimen, uncat. c. Megaleporinus piavussu, 148.9 mm SL, fresh specimen, uncat. d. Schizodon altoparanae, MZUSP 41102, holotype, 282.2 mm SL, rio Paraná, in front of Jupiá, Três Lagoas, State of Mato Grosso do Sul. e. Schizodon borellii, 207.5 mm SL, fresh specimen, uncat. f. Schizodon nasutus, NUP 2495, 160.0 mm SL, Itaipu Reservoir, Santa Helena, State of Paraná.
Megaleporinus obtusidens (Valenciennes, 1836)
Body elongated; greatest depth contained 3.2 to 3.5, caudal peduncle depth 8.6 to 8.9 times in SL; head length 3.2 to 3.8, predorsal distance 2.0 to 2.1, caudal peduncle length 7.0 to 7.9 in SL; snout length 2.0 to 2.2, horizontal orbital diameter 2.9 to 3.8 and least interorbital width 6.7 to 8.4 in HL. Mouth subterminal; premaxilla and dentary with 3 teeth, no maxillary teeth. Lateral line with 41-44 pored scales; transverse series above lateral line with 6 or 7 scale rows and below with 6 scale rows. Dorsal fin with 12, pectoral fin with 17, pelvic fin with 9, anal fin with 9 or 10 and caudal fin with 19 rays (Britski et al., 2012Britski HA, Birindelli JLO, Garavello JC. A new species of Leporinus Agassiz, 1829 from the upper rio Paraná basin (Characiformes, Anostomidae) with redescription of L. elongatus Valenciennes, 1850 and L. obtusidens (Valenciennes, 1837). Pap Avulsos Zool . 2012; 52(37):441-75.). Ground color silvery; three dark-brown rounded blotches over lateral line. Dorsal fin whitish, pectoral, pelvic, anal and caudal fins yellowish (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 350 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río de la Plata basin, Laguna dos Patos drainage, rio Parnaíba and rio São Francisco basins.
Remarks. Megaleporinus obtusidens was identified as Leporinus elongatus by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Ramirez et al. (2016Ramirez JL, Birindelli JLO, Galetti Júnior PM. A new genus of Anostomidae (Ostariophysi: Characiformes): Diversity, phylogeny and biogeography based on cytogenetic, molecular and morphological data. Mol Phylogenet Evol . 2017; 107:308-23.) described the new genus, Megaleporinus, and proposed the new combination. Britski et al. (2012Britski HA, Birindelli JLO, Garavello JC. A new species of Leporinus Agassiz, 1829 from the upper rio Paraná basin (Characiformes, Anostomidae) with redescription of L. elongatus Valenciennes, 1850 and L. obtusidens (Valenciennes, 1837). Pap Avulsos Zool . 2012; 52(37):441-75.) redescribed Megaleporinus elongatus and M. obtusidens (under Leporinus), distinguishing them by the number of circumpeduncular scale rows (12 in M. elongatus; 16 in M. obtusidens), and restricting the distribution of M. elongatus to the rio Jequitinhonha and rio Pardo. In turn, the specimens identified as Leporinus obtusidens by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.) belong to Megaleporinus piavussu, a species endemic to the upper rio Paraná basin.
Megaleporinus piavussu ( Britski, Birindelli, Garavello, 2012Britski HA, Birindelli JLO, Garavello JC. A new species of Leporinus Agassiz, 1829 from the upper rio Paraná basin (Characiformes, Anostomidae) with redescription of L. elongatus Valenciennes, 1850 and L. obtusidens (Valenciennes, 1837). Pap Avulsos Zool . 2012; 52(37):441-75. )
Body elongated; greatest depth contained 2.8 to 3.5, caudal peduncle depth 7.9 to 9.1 times in SL; head length 3.5 to 4.7, predorsal distance 2.0 to 2.2, caudal peduncle length 7.5 to 8.7 in SL; snout length 2.1 to 2.7, horizontal orbital diameter 3.7 to 5.1 and least interorbital width 1.9 to 2.4. Mouth subterminal; premaxilla and dentary with 3 teeth, no maxillary teeth. Lateral line with 41-44 pored scales; transverse series above lateral line with 6 or 7 scale rows and below with 5 or 6 scale rows. Dorsal fin with 11 or 12, pectoral fin with 15-19, pelvic fin with 9 and caudal fin with 19 rays (Britski et al., 2012Britski HA, Birindelli JLO, Garavello JC. A new species of Leporinus Agassiz, 1829 from the upper rio Paraná basin (Characiformes, Anostomidae) with redescription of L. elongatus Valenciennes, 1850 and L. obtusidens (Valenciennes, 1837). Pap Avulsos Zool . 2012; 52(37):441-75.). Ground color silvery; three dark-brown rounded blotches over lateral line. Dorsal fin whitish; pectoral, pelvic, anal and caudal fins yellowish.
Maximum standard length. 410.0 mm.
Distribution. Upper rio Paraná basin.
Remarks. Megaleporinus piavussu was identified as L. obtusidens by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Ramirez et al. (2016Ramirez JL, Birindelli JLO, Galetti Júnior PM. A new genus of Anostomidae (Ostariophysi: Characiformes): Diversity, phylogeny and biogeography based on cytogenetic, molecular and morphological data. Mol Phylogenet Evol . 2017; 107:308-23.) described the new genus, Megaleporinus, and proposed the new combination. Britski et al. (2012Britski HA, Birindelli JLO, Garavello JC. A new species of Leporinus Agassiz, 1829 from the upper rio Paraná basin (Characiformes, Anostomidae) with redescription of L. elongatus Valenciennes, 1850 and L. obtusidens (Valenciennes, 1837). Pap Avulsos Zool . 2012; 52(37):441-75.) redescribed M. obtusidens and described the new species, M. piavussu (under Leporinus) from the upper rio Paraná basin. Megaleporinus piavussu can be distinguished by having 39 or 40, rarely 41, pored scales in the lateral line, and mouth terminal, except in some large adults with somewhat subterminal mouth (vs. 41 to 43, rarely 44 in M. obtusidens, and mouth directed somewhat or entirely downward in M. obtusidens). The epithet piavussu is similar to the common name of M. macrocephalus “piavuçu, piaussu”, but these are different species and should not be confused.
Schizodon
1. Mouth subterminal; snout prominent .................... S. nasutus
1’. Mouth terminal; snout non-prominent .................... 2
2. Body without dark-brown transverse bars; dark-brown oval, horizontaly elongated, blotch on the caudal peduncle .................... S. altoparanae
2’. Body with dark-brown transverse bars; no dark-brown blotch on the caudal peduncle .................... S. borellii
Schizodon altoparanae Garavello, Britski, 1990Garavello JC, Britski HA. Duas novas espécies do gênero Schizodon Agassiz da bacia do alto Paraná, Brasil, América do Sul (Ostariophysi, Anostomidae). Naturalia . 1990; 15:153-70.
Body elongated; greatest depth contained 3.6 to 3.8, caudal peduncle depth 9.0 times in SL; head length 4.0 to 4.5, predorsal distance 2.0 to 2.5, caudal peduncle length 7.7 to 8.0 in SL; snout length 2.0 to 2.5, horizontal orbital diameter 2.0 to 2.5, least interorbital width 2.0 to 2.5 and dentary width 5.0 to 5.5 in HL. Mouth terminal; premaxilla and dentary with 4 teeth, no maxillary teeth. Lateral line with 42 or 43 pored scales; transverse series above lateral line with 5 scale rows and below with 4 or 4½ scale rows. Dorsal fin with 14 or 15, pectoral fin with 13-17, pelvic fin with 9, anal fin with 12 or 13 and caudal fin with 19 rays (Garavello, Britski, 1990Garavello JC, Britski HA. Duas novas espécies do gênero Schizodon Agassiz da bacia do alto Paraná, Brasil, América do Sul (Ostariophysi, Anostomidae). Naturalia . 1990; 15:153-70.). Ground color silvery to yellowish; black oval, horizontally elongated, blotch on posterior half of caudal peduncle and caudal-fin base, extending to median caudal-fin rays. Hyaline fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 300.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná basin.
Schizodon borellii (Boulenger, 1900)
Body elongated; greatest depth contained 3.0 to 5.0, caudal peduncle depth 9.7 to 10.0 times in SL; head length 3.6 to 4.6, predorsal distance 2.1 to 2.5, caudal peduncle length 8.2 to 9.7 in SL; snout length 2.3 to 2.4, horizontal orbital diameter 3.6 to 5.0, least interorbital width 1.8 to 2.0 and dentary width 4.0 to 4.7 in HL. Mouth terminal; premaxilla and dentary with 4 teeth, no maxillary teeth. Lateral line with 40-42 pored scales; transverse series above and below lateral line with 4½ scale rows. Dorsal and pectoral fin with 14 or 15, pelvic fin with 9, anal fin with 10 and caudal fin with 19 rays (Britski et al., 2007Britski HA, Silimon KZS, Lopes BS. Peixes do Pantanal: manual de identificação. Brasília, DF: Embrapa Pantanal; 2007.). Ground color silvery; four black transverse bars on flank. Hyaline fins, except caudal fin, with distal margins black (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 315.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system.
Remarks. Schizodon borellii is a non-native species from the upper rio Paraná and its occurrence can be associated with the releasing of specimens in several reservoirs in the region for restocking (CESP, 1996Companhia Energética de São Paulo (CESP). Aspectos limnológicos, ictiológicos e pesqueiros de reservatórios da CESP no Período de 1986 a 1994. São Paulo: CESP; 1996.; Júlio Júnior et al., 2009Júlio Júnior HF, Dei Tos C, Agostinho AA, Pavanelli CS. A massive invasion of fish species after eliminating a natural barrier in the upper rio Paraná basin. Neotrop Ichthyol . 2009; 7(4):709-18.).
Schizodon nasutus Kner, 1858
Body elongated; greatest depth contained 3.0 to 4.3, caudal peduncle depth 10.2 to 10.4 times in SL; head length 4.0 to 4.5, predorsal distance 2.0 to 2.2, caudal peduncle length 8.3 to 8.5 in SL; snout length 2.2 to 2.8, horizontal orbital diameter 3.6 to 4.6, least interorbital width 2.0 to 2.6 and dentary width 5.0 to 5.5 in HL. Mouth subterminal, snout prominent; premaxilla and dentary with 4 teeth, no maxillary teeth. Lateral line with 42 or 43 pored scales; transverse series above lateral line with 5 or 5½ scale rows and below with 4-5 scale rows. Dorsal fin with 14, pectoral fin with 15, pelvic fin with 9, anal fin with 9 or 10 and caudal fin with 19 rays (Garavello, Britski, 1990Garavello JC, Britski HA. Duas novas espécies do gênero Schizodon Agassiz da bacia do alto Paraná, Brasil, América do Sul (Ostariophysi, Anostomidae). Naturalia . 1990; 15:153-70.). Ground color silvery to yellowish; black horizontally elongated blotch on caudal peduncle, extending to median caudal-fin rays. Yellowish fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 390.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río de la Plata basin.
Bryconidae
Brycon
1. Lateral line with 65 to 82 pored scales .................... B. hilarii
1’. Lateral line with 52 to 63 pored scales .................... B. orbignyanus
Brycon hilarii (Valenciennes, 1850)
Body deep; greatest depth contained 2.8 to 3.9, caudal peduncle depth 8.6 to 11.8 times in SL; head length 2.9 to 4.7, predorsal distance 2.2 to 2.8, caudal peduncle length 5.5 to 8.1 in SL; snout length 2.9 to 3.8, horizontal orbital diameter 3.3 to 5.1 and least interorbital width 1.9 to 2.7 in HL. Mouth terminal; median row of premaxilla with 3-5, inner row with 4-6, outer row with 7-13, inner row of dentary with 4, outer row 7-14 and maxilla with 11-21 teeth. Lateral line with 67-82 pored scales; transverse series above lateral line with 13-16 scale rows and below with 6-9 scale rows. Dorsal fin with 11, pectoral fin with 12-16, pelvic fin with 8, anal fin with 22-31 and caudal fin with 19 rays (Lima, 2017Lima FCT. A revision of the cis-andean species of the genus Brycon Müller & Troschel (Characiformes: Characidae). Zootaxa . 2017; 4222(1):1-189.). Ground color silvery; dark-brown transverse band on caudal peduncle, extending to distal margin of median caudal-fin rays; black transverse humeral blotch, sometimes inconspicuous. Pelvic, anal and caudal fins reddish; dorsal and pectoral hyaline or grey (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 302.9 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río Paraguay and upper rio Amazonas basins (Eschmeyer et al., 2017Eschmeyer WN, Fricke R, van der Laan R, editors. Catalog of fishes: genera, species, references [Internet]. San Francisco: California Academy of Science; 2017 [updated 2017 Jan 31; cited 2017 Feb 24]. Available from: Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
http://researcharchive.calacademy.org/re...
).
Remarks. Brycon hilarii is a non-native species from the upper rio Paraná and its occurrence can be associated with the releasing of specimens in the Itaipu Reservoir for restocking (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.; Lima, 2017Lima FCT. A revision of the cis-andean species of the genus Brycon Müller & Troschel (Characiformes: Characidae). Zootaxa . 2017; 4222(1):1-189.).
a. Brycon hilarii , 288.9 mm SL, fresh specimen, uncat. b. Brycon orbignyanus, 270.0 mm SL, fresh specimen, uncat. c. Salminus brasiliensis, 500.0 mm SL, fresh specimen, uncat. d. Salminus hilarii, NUP 1893, 170.0 mm SL, rio Paraná, Porto Rico.
Brycon orbignyanus (Valenciennes, 1850)
Body deep; greatest depth contained 2.8 to 3.4, caudal peduncle depth 9.2 to 11.7 times in SL; head length 3.3 to 5.1, predorsal distance 1.8 to 2.1, caudal peduncle length 5.3 to 7.8 in SL; snout length 2.7 to 3.6, horizontal orbital diameter 3.1 to 5.0 and least interorbital width 2.1 to 3.0 in HL. Mouth terminal; median row of premaxilla with 2-4, inner row with 5-9, outer row with 9-13, inner row of dentary with 4, outer row 8-14 and maxilla with 11-20 teeth. Lateral line with 52-63 pored scales; transverse series above lateral line with 10-13 scale rows and below with 5-9 scale rows. Dorsal fin with 11, pectoral fin with 13-16, pelvic fin with 7 or 8, anal fin with 24-30 and caudal fin with 19 rays (Lima, 2017Lima FCT. A revision of the cis-andean species of the genus Brycon Müller & Troschel (Characiformes: Characidae). Zootaxa . 2017; 4222(1):1-189.). Ground color silvery; dark-brown transverse band on caudal peduncle, extending to distal margin of median caudal-fin rays; black transverse humeral blotch, sometimes inconspicuous. Pelvic, anal and caudal fins reddish; dorsal and pectoral hyaline or grey (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 400.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río Paraná and Uruguay basins.
Salminus
1. Lateral line with 93 to 96 pored scales; transverse series above lateral line with 16 to 18 scale rows and below with 8 to 9 scale rows .................... S. brasiliensis
1’. Lateral line with 68 to 71 pored scale; transverse series above lateral line with 10 scale rows and below with 6 scale rows .................... S. hilarii
Salminus brasiliensis (Cuvier, 1816)
Body deep; greatest depth contained 3.6 to 3.7, caudal peduncle depth 11.3 to 11.5 times in SL; head length 3.1 to 3.3, predorsal distance 1.8 to 1.9, caudal peduncle length 9.3 to 9.6 in SL; snout length 3.4 to 4.0, horizontal orbital diameter 5.7 to 5.9 and least interorbital width 2.7 to 3.2 in HL. Mouth terminal; inner row of premaxilla with 9-11, outer row with 8, inner row of dentary with 40-50, outer row with 28 or 29 and maxilla with 30-33 teeth. Lateral line with 93-96 pored scales; transverse series above lateral line with 16-18 scale rows and below with 8-9 scale rows. Dorsal fin with 11 or 12, pectoral fin with 15, pelvic fin with 9, anal fin with 26-29 and caudal fin with 19 rays. Ground color yellowish; longitudinal series of dark-brown spots on flank scales; dark-brown transverse band on posterior portion of caudal peduncle, extending to distal margin of median caudal-fin rays. Yellowish fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 780.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río de la Plata and río Mamoré basins, and Laguna dos Patos drainage.
Salminus hilarii Valenciennes, 1850
Body elongated; greatest depth contained 3.6 to 4.1, caudal peduncle depth 9.9 to 11.8 times in SL; head length 3.6 to 3.8, predorsal distance 1.8 to 1.9, caudal peduncle length 8.6 to 8.8 in SL; snout length 3.5 to 3.8, horizontal orbital diameter 4.2 to 4.7 and least interorbital width 3.5 to 3.7 in HL. Mouth terminal; inner row of premaxilla with 9-11, outer row with 7 or 8, inner row of dentary with 30-32, outer row with 18-20 and maxilla with 30-32 teeth. Lateral line with 68-71 pored scales; transverse series above lateral line with 10 scale rows and below with 6 scale rows. Dorsal fin with 11 or 12, pectoral fin with 15-17, pelvic fin with 9 or 10, anal fin with 25-29 and caudal fin with 19 rays. Ground color silvery; dark-brown transverse band on posterior portion of caudal peduncle, extending to distal margin of median caudal-fin rays. Reddish fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 340.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río Paraná, rio São Francisco, rio Tocantins, Amazon and río Orinoco basins.
Characidae
Incertae sedis
Astyanax
1. A well-defined black, horizontally elongate humeral spot, overlapped by a more diffuse, vertically elongate grey stripe and followed by a second diffuse, vertically elongate grey stripe .................... A. lacustris
1’. One or two relatively diffuse, grey to brown vertically elongate humeral spots .................... 2
2. One humeral spot .................... 3
2’. Two humeral spots .................... 4
3. Anal fin with 29 to 35 branched rays .................... A. schubarti
3’. Anal fin with 24 to 28 branched rays .................... A. aff. fasciatus
4. Anal-fin origin anterior to vertical through the end of dorsal fin .................... A. biotae
4’. Anal-fin origin posterior to vertical through the end of dorsal fin .................... 5
5. Greatest body depth on vertical through the middle of pectoral fin .................... A. aff. paranae
5’. Greatest body depth two scales anterior to the dorsal-fin origin .................... A. bockmanni
Astyanax biotae Castro, Vari, 2004
Body deep; greatest depth contained 2.4 to 2.9 and caudal peduncle depth 8.0 to 9.8 times in SL; head length 3.2 to 3.6, predorsal distance 1.8 to 1.9 and caudal peduncle length 10.6 to 11.2 in SL; snout length 4.1 to 4.9, horizontal orbital diameter 2.4 to 3.0 and least interorbital width 2.6 to 3.0 in HL. Mouth terminal; inner row of premaxilla with 5 teeth, outer with 4 or 5, dentary with 10-12, and maxilla with one tooth. Lateral line complete, with 33-35 pored scales; transverse series above lateral line with 6 or 7 scale rows and below with 4 or 5 scale rows. Dorsal fin with 11 rays, pectoral fin with 12 or 13, pelvic fin with 7 or 8, anal fin with 27-30, and caudal fin with 19 rays. Ground color silvery; silvery longitudinal band on flank (brown or grey in fixed specimens), from humeral blotch to median caudal-fin rays, larger on caudal peduncle, forming oval-shaped, horizontally elongated, blotch; flank scales with dark-brown border.
Maximum standard length. 41.3 mm.
Biological data. Feeds on arthropods, debris and seeds of vascular plants and filamentous algae (Castro, Vari, 2004aCastro RMC, Vari RP. Astyanax biotae, a new species of stream fish from the rio Paranapanema basin, upper rio Parana system, southeastern Brazil (Ostariophysi: Characiformes: Characidae). Proc Biol Soc Wash. 2004a; 117(3):330-38.).
Distribution. Upper rio Paraná basin.
a. Astyanax biotae, NUP 15137, 44.1 mm SL, córrego Peroba, tributary of the rio Ivinheima, Jateí, State of Mato Grosso do Sul. b. Astyanax bockmanni, 67.2 mm SL, fresh specimen, uncat. c. Astyanax aff. fasciatus, NUP 32, 65.0 mm SL, rio Paraná, Porto Rico; d. Astyanax lacustris, 79.9 mm SL, fresh specimen, uncat. e. Astyanax aff. paranae, NUP 3045, 72.9 mm SL, ribeirão Lageado, Marechal Cândido Rondon, State of Paraná. f. Astyanax schubarti, MZUSP 17101, 68.4 mm SL, rio Jaguara-mirim, São Paulo.
Astyanax bockmanni Vari, Castro, 2007
Body deep; greatest depth contained 2.4 to 2.7 and caudal peduncle depth 8.7 to 9.2 times in SL; head length 4.0 to 4.1, predorsal distance 1.9 to 2.0 and caudal peduncle length 8.0 to 12.4 in SL; snout length 3.7 to 4.4, horizontal orbital diameter 2.4 to 2.5 and least interorbital width 2.8 to 2.9 in HL. Mouth terminal; inner row of premaxilla with 5 teeth, outer with 4 or 5, dentary with 9 or 10, and maxilla with 2 teeth. Lateral line complete, with 37 or 38 pored scales; transverse series above lateral line with 5½ or 6 scale rows and below with 5 or 6 scale rows. Dorsal fin with 11 or 12, pectoral fin with 15 or 16, pelvic fin with 9, anal fin with 22 or 23, and caudal fin with 19 rays. Ground color silvery; one black humeral blotch, transversely elongated, followed by another similar black humeral blotch, smaller than the first; silvery longitudinal band on flank (brown or grey in fixed specimens), from humeral blotch to median caudal-fin rays, larger on caudal peduncle, forming oval-shaped, horizontally elongated, blotch. Yellowish or reddish fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 74.2 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná basin.
Astyanax aff.fasciatus (Cuvier, 1819)
Body elongated; greatest depth contained 2.9 to 3.4 and caudal peduncle depth 10.4 to 11.1 times in SL; head length 4.5 to 4.6, predorsal distance 2.0 to 2.1 and caudal peduncle length 9.5 to 13.3 in SL; snout length 3.4 to 3.6, horizontal orbital diameter 2.2 to 2.3 and least interorbital width 3.3 to 3.5 in HL. Mouth terminal; inner row of premaxilla with 5 teeth, outer with 4 or 5, dentary with 10-12, and maxilla with one tooth. Lateral line complete, with 34-36 pored scales; transverse series above lateral line with 5 or 5½ scale rows and below with 5 scale rows. Dorsal fin with 11, pectoral fin with 15, pelvic fin with 9, anal fin with 24-28, and caudal fin with 19 rays. Ground color silvery; black vertically elongated humeral blotch; silvery longitudinal band on flank (black in fixed specimens), from humeral blotch to median caudal-fin rays, larger on caudal peduncle, forming oval-shaped, horizontally elongated, blotch. Unpaired fins reddish, paired fins hyaline (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 102.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Drainages from Mexico to Argentina.
Astyanax lacustris (Lütken, 1875)
Body deep; greatest depth contained 1.8 to 2.9 and caudal peduncle depth 6.6 to 10.1 times in SL; head length 3.0 to 4.4, predorsal distance 1.6 to 2.4 and caudal peduncle length 7.8 to 8.4 in SL; snout length 3.2 to 4.1, horizontal orbital diameter 2.3 to 4.7 and least interorbital width 1.9 to 3.3 in HL. Mouth terminal; inner row of premaxilla with 5 teeth, outer with 4 or 5, dentary with 8-16, and no maxillary teeth. Lateral line complete, with 33-41 pored scales; transverse series above lateral line with 6 to 8 scale rows and below with 4-8 scale rows. Dorsal fin with 12, pectoral fin with 12 or 13, pelvic fin with 8 or 9, anal fin with 22-34, and caudal fin with 19. Ground color silvery; one black rounded humeral blotch followed by another vertically elongated humeral blotch; silvery longitudinal band on flank (brown or grey in fixed specimens), from humeral blotch to median caudal-fin rays, larger on caudal peduncle, forming oval-shaped, horizontally elongated, blotch. Yellowish fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 129.2 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río de la Plata basin, Laguna dos Patos and rio Tramandaí drainages, rio São Francisco (Lucena, Soares, 2016Lucena CAS, Soares HG. Review of species of the Astyanax bimaculatus “caudal peduncle spot” subgroup sensu Garutti & Langeani (Characiformes, Characidae) from the rio La Plata and rio São Francisco drainages and coastal systems of southern Brazil and Uruguay. Zootaxa . 2016; 4072(1):101-25.) and, according to Garutti, Langeani (2009Garutti V, Langeani F. Redescription of Astyanax goyacensis Eigenmann, 1908 (Ostariophysi: Characiformes: Characidae). Neotrop Ichthyol . 2009; 7(3):371-76.), rio Tocantins-Araguaia basins.
Remarks. Astyanax lacustris was identified as A. altiparanae by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Lucena, Soares (2016Lucena CAS, Soares HG. Review of species of the Astyanax bimaculatus “caudal peduncle spot” subgroup sensu Garutti & Langeani (Characiformes, Characidae) from the rio La Plata and rio São Francisco drainages and coastal systems of southern Brazil and Uruguay. Zootaxa . 2016; 4072(1):101-25.), in a revisionary study of some species in the Astyanax bimaculatus (Linnaeus, 1758) group from Southern Neotropical river basins, proposed the new synonym.
Astyanax aff. paranae Eigenmann, 1914
Body elongated; greatest depth contained 3.5 to 3.6 and caudal peduncle depth 10.9 to 11.6 times in SL; head length 3.9 to 4.1, predorsal distance 1.9 to 2.0 and caudal peduncle length 7.2 to 9.6 in SL; snout length 3.6 to 4.5, horizontal orbital diameter 2.4 to 2.8 and least interorbital width 2.9 to 3.1 in HL. Mouth terminal; inner row of premaxilla with 5 teeth, outer with 4 or 5, dentary with 8-13, and maxilla with one tooth. Lateral line complete, with 38 or 39 pored scales; transverse series above lateral line with 5 or 5½ scale rows and below with 4 or 4½ scale rows. Dorsal fin with 11, pectoral fin with 15 or 16, pelvic fin with 9, anal fin with 17-23, and caudal fin with 19 rays. Ground color silvery; black vertically elongated humeral blotch followed by another similar black humeral blotch, smaller than the first; silvery longitudinal band on flank (brown or grey in fixed specimens), from humeral blotch to median caudal-fin rays. Reddish fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 90.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná basin.
Astyanax schubarti Britski, 1964Britski HA. Sôbre uma nova espécie de Astyanax do rio Mogi-Guassu (Pisces, Characidae). Pap Avulsos Zool. 1964; 16(21):213-15.
Body deep; greatest depth contained 2.4 to 2.5 and caudal peduncle depth 10.7 to 11.2 times in SL; head length 4.1 to 4.6, predorsal distance 2.0 to 2.1 and caudal peduncle length 10.1 to 11.6 in SL; snout length 3.7 to 3.8, horizontal orbital diameter 2.1 to 2.2 and least interorbital width 3.0 to 3.1 in HL. Mouth terminal; inner row of premaxilla with 5, outer with 3-5, dentary with 10-13, and maxilla with one tooth. Lateral line complete, with 37-38 pored scales; transverse series above lateral line with 6-7 scale rows and below with 6 scale rows. Dorsal fin with 10-11, pectoral fin with 14-16, pelvic fin with 9, anal fin with 29-35 and caudal fin with 19 rays (Britski, 1964Britski HA. Sôbre uma nova espécie de Astyanax do rio Mogi-Guassu (Pisces, Characidae). Pap Avulsos Zool. 1964; 16(21):213-15.). Ground color pale yellow; dark-brown longiudinal stripe, along middle of body, from humeral spot to median caudal-fin rays; dark-brown humeral spot, transversely elongate. Dorsal and caudal fins yellowish, other fins hyaline (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 89.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná basin.
Hemigrammus
Hemigrammus ora Zarske, Le Bail, Géry, 2006Zarske A, Le Bail P-Y, Géry J. New and poorly known Characiform fishes from French Guiana. 1. Two new Tetras of the genera Hemigrammus and Hyphessobrycon (Teleostei: Characiformes: Characidae). Zool Abh. 2006; 55:17-30.
Body elongated; greatest depth contained 2.9 to 3.4 and caudal peduncle depth 8.3 to 11.2 times in SL; head length 3.3 to 4.0, predorsal distance 1.8 to 2.0 and caudal peduncle length 6.3 to 9.2 in SL; snout length 3.6 to 6.5, horizontal orbital diameter 2.1 to 3.3 and least interorbital width 2.3 to 3.3 in HL. Mouth terminal; inner row of premaxilla with 5 teeth, outer row with 3-6, dentary with 3 or 4 maxillary teeth. Lateral line incomplete, with 9-15 pored scales; longitudinal series with 31-35 scales; transverse series above lateral line with 5 or 6 scale rows and below with 4 or 5 scale rows. Dorsal fin with 10 or 11 rays, pectoral fin with 10-14 rays, pelvic fin with 7-10 rays, anal fin with 24-27 rays and caudal fin with 19 rays (Zarske et al., 2006Zarske A, Le Bail P-Y, Géry J. New and poorly known Characiform fishes from French Guiana. 1. Two new Tetras of the genera Hemigrammus and Hyphessobrycon (Teleostei: Characiformes: Characidae). Zool Abh. 2006; 55:17-30.; Jerep et al., 2011Jerep FC, Carvalho FR, Bertaco VA. Geographic distribution of Hemigrammus ora (Ostariophysi: Characiformes: Characidae) in the Amazon basin, Brazil. Zoologia. 2011; 28(4):545-50.). Ground color whitish to pale; silvery to dark-brown longitudinal stripe on flank; black rounded humeral spot; dark-brown rounded blotch on last portion of caudal peduncle and caudal-fin base, reaching only base of median caudal fin rays.
Maximum standard length. 38.4 mm.
Distribution. Coastal drainages of French Guiana, upper rio Paraná, rio Tocantins-Araguaia and rio Xingu basins.
Remarks. Hemigrammus ora has been captured in the upper rio Paraná floodplain since 2011 by Nupélia staff, and this is the first record to the upper rio Paraná basin. Zarske et al. (2006Zarske A, Le Bail P-Y, Géry J. New and poorly known Characiform fishes from French Guiana. 1. Two new Tetras of the genera Hemigrammus and Hyphessobrycon (Teleostei: Characiformes: Characidae). Zool Abh. 2006; 55:17-30.) described this species from the costal drainages of French Guiana, and Jerep et al. (2011Jerep FC, Carvalho FR, Bertaco VA. Geographic distribution of Hemigrammus ora (Ostariophysi: Characiformes: Characidae) in the Amazon basin, Brazil. Zoologia. 2011; 28(4):545-50.) widened its geographic distribution to the upper rio Tocantins-Araguaia and rio Xingu basins, hypothesizing the continuous distribution of this species from its type-locality to the lower Amazon tributaries. Additionally, Jerep et al. (2011Jerep FC, Carvalho FR, Bertaco VA. Geographic distribution of Hemigrammus ora (Ostariophysi: Characiformes: Characidae) in the Amazon basin, Brazil. Zoologia. 2011; 28(4):545-50.) found two morphological differences: three small dentary teeth and longer snout length 7.7-10.6% SL (vs. 8-10 small dentary teeth and snout length 3.9-7.0% SL from the type specimens). The specimens from the upper rio Paraná floodplain present three or four small dentary teeth (in accordance with Jerep et al., 2011Jerep FC, Carvalho FR, Bertaco VA. Geographic distribution of Hemigrammus ora (Ostariophysi: Characiformes: Characidae) in the Amazon basin, Brazil. Zoologia. 2011; 28(4):545-50.) and snout length 6.4-7.2% SL (23.3-28.0% HL; in accordance with Zarske et al., 2006Zarske A, Le Bail P-Y, Géry J. New and poorly known Characiform fishes from French Guiana. 1. Two new Tetras of the genera Hemigrammus and Hyphessobrycon (Teleostei: Characiformes: Characidae). Zool Abh. 2006; 55:17-30.). Hemigrammus ora is a non-native species from the upper rio Paraná basin, probably introduced from the rio Tocantins-Araguaia basin.
a. Hemigrammus ora, NUP 18973, 26.4 mm SL, ressaco do Pau Veio, tributary of the rio Paraná, State of Paraná. b. Hyphessobrycon eques, 32.0 mm SL, fresh specimen, uncat. c. Hyphessobrycon moniliger (top, female, NUP 1248, 29.9 mm SL, ribeirão Bocaina, tributary of the rio Meia Ponte, Piracanjuba, State of Goiás; bottom, male, NUP 16949, 26.2 mm SL, lagoa da Onça, tributary of the rio Baía, Taquarussu, State of Mato Grosso do Sul). d. Moenkhausia australe, NUP 11115, 45.0 mm SL, riacho Caracu, tributary of the rio Paraná, Porto Rico, State of Paraná. e. Moenkhausia bonita, NUP 11700, 30.1 mm SL, lagoa das Pombas, tributary of the rio Paraná, Porto Rico, State of Paraná. f. Moenkhausia forestii, NUP 16583, 36.0 mm SL, rio Baía, tributary of the rio Paraná, Taquarussu, State of Mato Grosso do Sul. g. Moenkhausia cf. gracilima, NUP 11099, 33.1 mm SL, ribeirão São Pedro, tributary of the rio Paraná, São Pedro do Paraná, State of Paraná. h. Moenkhausia aff. intermedia, NUP 2389, 68.4 mm SL, Segredo Reservoir, Mangueirinha, State of Paraná. i. Moenkhausia sanctaefilomenae, 24.1 mm SL, uncat.
Hyphessobrycon
1. Dorsal fin with black blotch and distal edge of the rays hyaline; black humeral blotch large and conspicuous .................... H. eques
1’. Dorsal fin hyaline; black humeral spot small and less conspicuous .................... H. moniliger
Hyphessobrycon eques (Steindachner, 1882)
Body moderately deep; greatest depth contained 2.7 to 2.9 and caudal peduncle depth 9.7 to 10.6 times in SL; head length 3.6 to 3.9, predorsal distance 1.9 to 2.0 and caudal peduncle length 8.6 to 11.1 in SL; snout length 4.0 to 4.5, horizontal orbital diameter 2.0 to 2.4 and least interorbital width 3.2 to 3.5 in HL. Mouth terminal; inner row of premaxilla with 3-5, outer row with 3-4, dentary with 8-11 and maxilla with 2-3 teeth. Lateral line incomplete, with 4-5 pored scales; longitudinal series with 29-33 scales; transverse series above lateral line with 5-6 scale rows and below with 3½-4 scale rows. Dorsal fin with 10-12, pectoral fin with 12-14, pelvic fin with 7 rays, anal fin with 28-32 and caudal fin with 19 rays. Ground color reddish in life; large, transversely elogate, black humeral blotch. Pelvic, caudal and anal fins red (latter with black margin); pectoral fin hyaline; dorsal fin with black blotch (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 58.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Amazon basin and Paraná-Paraguay system.
Hyphessobrycon moniliger Moreira, Lima, Costa, 2002Moreira CR, Lima FCT, Costa WJEM. Hyphessobrycon moniliger, a new characid fish from rio Tocantins basin, Central Brazil (Ostariophysi: Characiformes). Ichthyol Explor Freshw . 2002; 13(1):73-80.
Body moderately deep; greatest depth contained 2.6 to 3.2 and caudal peduncle depth 8.2 to 10.9 times in SL; head length 3.0 to 3.6, predorsal distance 1.7 to 2.0 and caudal peduncle length 6.1 to 10.3 in SL; snout length 3.4 to 4.9, horizontal orbital diameter 2.1 to 2.8 and least interorbital width 3.0 to 3.7 in HL. Mouth terminal; inner row of premaxilla with 5, rarely 6 teeth, outer row with 2-5, dentary with up to 12 and maxilla with 3 teeth. Lateral line incomplete, with 6-8 pored scales; longitudinal series with 27-35; transverse series above lateral line with 7 or 8 scale rows and below with 4-5 scale rows. Dorsal fin with 11 rays, pectoral fin with 11-14 rays, pelvic fin with 8 rays, anal fin with 26-30 rays and caudal fin with 19 rays (Moreira et al., 2002Moreira CR, Lima FCT, Costa WJEM. Hyphessobrycon moniliger, a new characid fish from rio Tocantins basin, Central Brazil (Ostariophysi: Characiformes). Ichthyol Explor Freshw . 2002; 13(1):73-80.). Ground color pale brown; one humeral spot (Teixeira et al., 2015Teixeira TF, Netto-Ferreira AL, Birindelli JLO, Sousa LM. Two new species of Hyphessobrycon (Characiformes: Characidae) from the headwaters of the Tapajós and Xingu River basins, Pará, Brazil. J Fish Biol. 2015; 88(2):459-76.); dark-brown longitudinal stripe on flank, continuous with caudal peduncle blotch; dark-brown caudal peduncle blotch triangle-shaped in females and juveniles, rectangular in males; hyaline fins.
Maximum standard length. 27.0 mm.
Biological data. Anal fin of mature males with an anterior lobe and convex outline and proximal portion of the lobed rays with a projection, anterodorsally oriented; females with concave outline (Moreira et al., 2002Moreira CR, Lima FCT, Costa WJEM. Hyphessobrycon moniliger, a new characid fish from rio Tocantins basin, Central Brazil (Ostariophysi: Characiformes). Ichthyol Explor Freshw . 2002; 13(1):73-80.).
Distribution. Middle rio Araguaia, rio Tocantins, rio Tapajós and upper rio Paraná basins.
Remarks. Hyphessobrycon moniliger has been captured in upper rio Paraná floodplain since 2013 by the Nupélia staff. Hyphessobrycon moniliger is a non-native species from the upper rio Paraná basin, probably introduced from the rio Tocantins-Araguaia basin.
Moenkhausia
1. Dark-brown blotch on posterior portion of caudal peduncle and proximal third of caudal-fin base, occupying their whole depth; caudal-fin lobes hyaline .................... 2
1’. Dark-brown blotch on caudal peduncle and caudal-fin base absent or, if present, restricted to the midlateral portion of the caudal peduncle (not covering its whole depth) .................... 4
2. Lateral line disrupted or completely pored .................... M. australe
2’. Lateral line incompletely pored .................... 3
3. Transversal series above lateral line with 4 scale rows, and below lateral line with 3 scale rows .................... M. sanctaefilomenae
3’. Transversal series above lateral line with 5 scale rows, and below lateral line with 4 scale rows .................... M. forestii
4. Distal portion of upper caudal-fin lobe darker than the lower .................... M. cf. gracilima
4’. Distal portion of caudal-fin lobes equally pigmented .................... 5
5. Distal portion and distal margin of caudal-fin lobes dark-brown; silvery longitudinal stripe (brown or grey on preserved specimens), from vertical through dorsal-fin origin to median caudal-fin rays, widened on the posterior portion of caudal peduncle to form a blotch; first gill arch with seven gill rakers on upper limb and 12 on lower limb .................... M. bonita
5’. Distal portion of caudal-fin lobes dark-brown and distal margin hyaline; silvery longitudinal stripe (brown or grey on fixed specimens), from opercle to caudal peduncle, not forming a blotch on the posterior portion of caudal peduncle; first gill arch with 10 gill rakers on the upper limb and 18 on lower limb .................... M. aff. intermedia
Moenkhausia australe (Eigenmann, 1908)
Body elongated; greatest depth contained 2.1 to 2.5 and caudal peduncle depth 7.0 to 8.0 times in SL; head length 3.1 to 3.5, predorsal distance 1.7 to 1.9 and caudal peduncle length 8.3 to 12.4 in SL; snout length 3.5 to 4.7, horizontal orbital diameter 2.2 to 2.6 and least interorbital width 2.5 to 2.7 in HL. Mouth terminal; inner row of premaxilla with 5 teeth, outer with 3 or 4, dentary with 9-12 and maxilla with 1 or 2 teeth. Lateral line complete, or occasionally discontinuous, with 19-26 pored scales; longitudinal series with 24-26 scales; transverse series above lateral line with 4, 4 ½ or 5 scale rows and below with 3, 3 ½ or 4 scale rows. Dorsal fin with 11 rays, pectoral fin with 11-13 rays, pelvic fin with 8 rays, anal fin with 24-27 rays and caudal fin with 19 rays. Ground color silvery; scales with dark-brown border, conferring reticulated pattern to body; one dark-brown humeral spot; anterior half of caudal peduncle with light-beige area. Hyaline fins, except caudal fin; posterior portion of caudal peduncle and proximal third of caudal fin with conspicuous black transverse bar occupying their whole depth.
Maximum standard length. 45.0 mm.
Distribution. Upper rio Paraná and río Paraguay basins.
Remarks. Some specimens of M. australe were identified as M. aff. sanctaefilomenae by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Both species occur in the upper rio Paraná floodplain, where M. australe has been captured since 1989 by the Nupélia staff. Moenkhausia australe can be distinguished by having lateral line completely pored, rarely disrupted (vs. lateral line incompletely pored, in M. sanctaefilomenae). Despite the general morphological similarity between M. australe, M. forestii and M. sanctaefilomenae, Benine et al. (2009Benine RC, Mariguela TC, Oliveira C. New species of Moenkhausia Eigenmann, 1903 (Characiformes: Characidae) with comments on the Moenkhausia oligolepis species complex. Neotrop Ichthyol. 2009; 7(2):161-68.) and Mariguela et al. (2013Mariguela TC, Benine RC, Abe KT, Avelino GS, Oliveira C. Molecular phylogeny of Moenkhausia (Characidae) inferred from mitochondrial and nuclear DNA evidence. J Zoolog Syst Evol Res. 2013; 51(4):327-32.), in molecular analyses, considered them as different species. Moenkhausia australe was described from the lower río Paraguay basin, and it was considered a junior-synonym of M. sanctaefilomenae by Eigenmann (1917Eigenmann CH. The American Characidae. Mem Mus Comp Zool . 1917; 43:1-102. pt. 1.), until Benine et al. (2009Benine RC, Mariguela TC, Oliveira C. New species of Moenkhausia Eigenmann, 1903 (Characiformes: Characidae) with comments on the Moenkhausia oligolepis species complex. Neotrop Ichthyol. 2009; 7(2):161-68.) revalidated it. Re-analyzing the material hosted at Coleção Ictiológica do Nupélia, the older occurence from 1989 (NUP 10677, NUP 10678, NUP 10680, previously identified as M. sanctaefilomenae) was found, few years after the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls. Because of the earliest record occurred after the Itaipu Dam building, M. australe is considered a possible non-native species from the upper rio Paraná.
Moenkhausia bonita Benine, Castro, Sabino, 2004Benine RC, Castro RMC, Sabino J. Moenkhausia bonita: a new small Characin fish from the rio Paraguay Basin, southwestern Brazil (Characiformes: Characidae). Copeia . 2004; (1):68-73.
Body elongated; greatest depth contained 2.9 to 3.4 and caudal peduncle depth 8.5 to 9.5 times in SL; head length 3.5 to 3.9, predorsal distance 1.9 to 2.0 and caudal peduncle length 7.4 to 9.0 in SL; snout length 3.4 to 4.2, horizontal orbital diameter 2.0 to 2.4 and least interorbital width 3.0 to 3.5 in HL. Mouth terminal; inner row of premaxilla with 5 teeth, outer with 2-5, dentary with 4 and maxilla with 2 or 3 teeth. Lateral line complete with 29-31 pored scales, sometimes disrupted or incomplete 6 to 13 pored scales; longitudinal series with 29-31 scales; transverse series above lateral line with 5 scale rows and below with 3 scale rows. Dorsal fin with 11 rays, pectoral fin with 12-13 rays, pelvic fin with 8 rays, anal fin with 24-26 rays and caudal fin with 19 rays. Ground color silvery to pale yellow; dark-brown longitudinal stripe from opercle (conspicuously from vertical through dorsal-fin origin) to median caudal fin rays; distal portion of caudal fin lobes equally dark-brown.
Maximum standard length. 43.8 mm.
Biological data. Lives in marginal backwaters with low current speeds and feeds mainly on terrestrial insects (Diptera and Coleoptera) (Benine et al., 2004Benine RC, Castro RMC, Sabino J. Moenkhausia bonita: a new small Characin fish from the rio Paraguay Basin, southwestern Brazil (Characiformes: Characidae). Copeia . 2004; (1):68-73.).
Distribution. Upper rio Paraná and río Paraguay basins.
Remarks. Some specimens of Moenkhausia bonita were identified as Hemigrammus marginatus by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.), who reported some specimens with lateral line completely pored, and others with lateral line disrupted or incompletely pored. R. P. Ota and A. G. Bifi (oral communication, 2009) were the first to identify M. bonita in the upper rio Paraná floodplain, formelly restricted to the type-locality (Baía Bonita) in its original description (Benine et al., 2004Benine RC, Castro RMC, Sabino J. Moenkhausia bonita: a new small Characin fish from the rio Paraguay Basin, southwestern Brazil (Characiformes: Characidae). Copeia . 2004; (1):68-73.). Hemigrammus marginatus was restricted the distribution of H. marginatus to the rio São Francisco basin and rivers of northeastern Brazil by Ota, RP et al. (2015Ota RP. Revisão taxonômica e análise filogenética das espécies do gênero Metynnis Cope, 1878 (Characiformes: Serrasalmidae). [PhD Thesis]. Manaus, AM: Instituto Nacional de Pesquisas da Amazônia; 2015.). Mota et al. (2018, in pressMota TFM, Fabrin TMC, Deprá GC, Gasques LS, Oliveira AV, Pavanelli CS, Prioli SMAP, Prioli A. Molecular characterization of Moenkhausia (Pisces: Characiformes) populations with different lateral line developmental levels. An Acad Bras Cienc. 2018, in press.), in a molecular analysis, concluded that the specimens from the upper rio Paraná floodplain, including those with variation in lateral line (complete, disrupted and incomplete), belong to M. bonita.
Moenkhausia forestii Benine, Mariguela, Oliveira, 2009Benine RC, Mariguela TC, Oliveira C. New species of Moenkhausia Eigenmann, 1903 (Characiformes: Characidae) with comments on the Moenkhausia oligolepis species complex. Neotrop Ichthyol. 2009; 7(2):161-68.
Body deep; greatest depth contained 2.2 to 2.6 and caudal peduncle depth 7.4 to 8.7 times in SL; head length 3.2 to 3.7, predorsal distance 1.7 to 1.9 and caudal peduncle length 9.4 to 13.9 in SL; snout length 3.0 to 4.0, horizontal orbital diameter 2.3 to 2.9 and least interorbital width 2.3 to 2.7 in HL. Mouth terminal; inner row of premaxilla with 5 teeth, outer with 3-5, dentary with 11 and maxilla with 1 or 2. Lateral line incomplete, with 7-11 pored scales; longitudinal series with 23-26 scales; transverse series above lateral line with 5 scale rows and below with 4 scale rows. Dorsal fin with 11 rays, pectoral fin with 12 or 13 rays, pelvic fin with 8 rays, anal fin with 21-27 rays and caudal fin with 19 rays. Ground color silvery; superior portion of eye red; scales with dark-brown border, conferring reticulated pattern to body; one dark-brown humeral spot; dark-brown longitudinal stripe along posterior half of flank; anterior half of caudal peduncle with light-beige area. Hyaline fins, except caudal fin; posterior portion of caudal peduncle and proximal third of caudal fin with conspicuous black transverse bar occupying their whole depth.
Maximum standard length. 36.4 mm.
Biological data. Males present small hooks on the segments of each anal-fin ray (Benine et al., 2009Benine RC, Mariguela TC, Oliveira C. New species of Moenkhausia Eigenmann, 1903 (Characiformes: Characidae) with comments on the Moenkhausia oligolepis species complex. Neotrop Ichthyol. 2009; 7(2):161-68.).
Distribution. Upper rio Paraná and río Paraguay basins.
Remarks. Some specimens of Moenkhausia forestii were identified as M. aff. sanctaefilomenae by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Both species occur in the upper rio Paraná floodplain, where M. forestii has been captured since 2009 by Nupélia staff. Moenkhausia forestii can be distinguished by having transverse series above lateral line with 5 and below with 4 scale rows (vs. 4 scale rows above and 3 below, in M. sanctaefilomenae). Additionally, M. forestii can be distinguished from M. australe, another similar species, by having lateral line incompletely pored, transverse series above lateral line with 5 and below with 4 scale rows, and upper jaw length 39.9-44.0% HL (vs. lateral line completely pored, 4 scale rows above and 3 below, and upper jaw length 46.4-52.6% HL). Despite the general morphological similarity between M. australe, M. forestii and M. sanctaefilomenae, Benine et al. (2009Benine RC, Mariguela TC, Oliveira C. New species of Moenkhausia Eigenmann, 1903 (Characiformes: Characidae) with comments on the Moenkhausia oligolepis species complex. Neotrop Ichthyol. 2009; 7(2):161-68.) and Mariguela et al. (2013Mariguela TC, Benine RC, Abe KT, Avelino GS, Oliveira C. Molecular phylogeny of Moenkhausia (Characidae) inferred from mitochondrial and nuclear DNA evidence. J Zoolog Syst Evol Res. 2013; 51(4):327-32.), in molecular analyses, considered them as different species. Moenkhausia forestii is a non-native species from the upper rio Paraná basin, and its recent occurrence can be associated with the functioning of the Canal da Piracema, a fish ladder that connects the region downstream from the Itaipu Dam to the region upstream from the dam (Benine et al., 2009Benine RC, Mariguela TC, Oliveira C. New species of Moenkhausia Eigenmann, 1903 (Characiformes: Characidae) with comments on the Moenkhausia oligolepis species complex. Neotrop Ichthyol. 2009; 7(2):161-68.).
Moenkhausia cf. gracilima Eigenmann, 1908
Body elongated; greatest depth contained 3.3 to 3.5 and caudal peduncle depth 8.1 to 9.7 times in SL; head length 4.0 to 4.6, predorsal distance 1.9 to 2.1 and caudal peduncle length 6.8 to 9.5 in SL; snout length 3.0 to 3.7, horizontal orbital diameter 2.2 to 2.3 and least interorbital width 2.8 to 3.2 in HL. Mouth terminal; inner row of premaxilla with 4 or 5 teeth, outer with 3-5, dentary with 4 and maxilla with 0 to 2 teeth. Lateral line complete, with 32-34 pored scales; longitudinal series with 32-34 scales; transverse series above lateral line with 5 scale rows and below with 3 scale rows. Dorsal fin with 11 rays, pectoral fin with 12-13 rays, pelvic fin with 8 rays, anal fin with 20-24 rays and caudal fin with 19 rays. Ground color whitish; silver longitudinal stripe (brown or grey in fixed specimens), from humeral spot to caudal peduncle; distal portion of upper caudal-fin lobe darker than lower.
Maximum standard length. 37.0 mm.
Distribution. Upper rio Paraná basin.
Remarks. Some specimens of Moenkhausia cf. gracilima were identified as Hemigrammus marginatus by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Ota, RP et al. (2015Ota RR, Message HJ, Graça WJ, Pavanelli CS. Neotropical Siluriformes as a model for insights on determining biodiversity of animal groups. PLoS ONE [serial on the internet]. 2015 Jul 13 [cited 2016 Dec 08]; 10(7):e0132913. Available from: Available from: http://dx.doi.org/10.1371/journal.pone.0132913
http://dx.doi.org/10.1371/journal.pone.0...
) restricted the distribution of H. marginatus to the rio São Francisco basin and rivers of northeastern Brazil. Moenkhausia cf. gracilima can be distinguished by having a dark-brown humeral spot, lateral line completely pored, median caudal-fin rays hyaline, and the distal portion of upper caudal-fin lobe darker than the lower (vs. dark-brown humeral spot absent, lateral line incompletely pored, median caudal-fin rays dark-brown, continuous with longitudinal stripe, and caudal-fin lobes equally pigmented, in M. bonita, also identified as H. marginatus by Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Marinho (2009Marinho MMF. Análise filogenética e revisão taxonômica das espécies de Moenkhausia Eigenmann, 1903 do grupo M. lepidura (Ostariophysi: Characiformes: Characidae). [Master Dissertation]. São José do Rio Preto, SP: Universidade Estadual Paulista; 2009 Apr 24 [cited 2016 Dec 08]. Available from: Repositório Institucional Universidade Estadual Paulista. Available from: Repositório Institucional Universidade Estadual Paulista. http://repositorio.unesp.br/handle/11449/87587
http://repositorio.unesp.br/handle/11449...
) and Marinho, Langeani (2016Marinho MMF, Langeani F. Reconciling more than 150 years of taxonomic confusion: the true identity of Moenkhausia lepidura, with a key to the species of the M. lepidura group (Characiformes: Characidae). Zootaxa . 2016; 4107(3):338-52.) noted a species very similar to M. gracilima, from the Amazon basin, in the influence area of the Ilha Solteira Reservoir, speculating the possibility to be new. The specimens from the upper rio Paraná floodplain, captured there since 2009 by the Nupélia staff, match the description of the specimens from the Ilha Solteira Reservoir. However, as some characters of the specimens from the upper rio Paraná and the Amazon basin are overlapped, M. M. F. Marinho (in an e-mail, manoela.marinho@gmail.com, June 2017), suggested the identification as M. cf. gracilima.
Moenkhausia aff. intermedia Eigenmann, 1908
Body elongated; greatest depth contained 3.0 to 4.6 and caudal peduncle depth 9.0 to 12.2 times in SL; head length 4.0 to 4.6, predorsal distance 1.9 to 2.1 and caudal peduncle length 7.7 to 11.9 in SL; snout length 3.4 to 4.2, horizontal orbital diameter 1.9 to 2.6 and least interorbital width 2.8 to 3.3 in HL. Mouth terminal; inner row of premaxilla with 5 teeth, outer with 4, dentary with 12-14 and maxilla with 1-2 teeth. Lateral line complete, with 33-36 pored scales; transverse series above lateral line with 5-6 scale rows and below with 34scale rows. Dorsal fin with 10, pectoral fin with 13-15, pelvic fin with 8-10, anal fin with 26-28 and caudal fin with 19 rays. Ground color whitish to silvery; silvery longitudinal band (brown or grey in preserved specimens), from posterior portion of opercle to caudal-fin base. Yellowish fins; caudal fin with black rounded blotch on each lobe and distal margins hyaline (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 104.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná basin.
Remarks. According to R. C. Benine (personal communication), the specimens from the upper rio Paraná are different from those of the rio Amazonas (type-locality of Moenkhausia intermedia). Therefore, the particle “aff.” was used.
Moenkhausia sanctaefilomenae (Steindachner, 1907)
Body deep; greatest depth contained 2.4 to 2.8 and caudal peduncle depth 8.0 to 9.2 times in SL; head length 3.7 to 3.9, predorsal distance 1.7 to 1.9 and caudal peduncle length 11.8 to 13.0 in SL; snout length 2.9 to 3.7, horizontal orbital diameter 2.4 to 3.0 and least interorbital width 2.3 to 2.9 in HL. Mouth terminal; inner row of premaxilla with 5 teeth, outer with 4-5, dentary with 9-11 and maxilla with 1-2 teeth. Lateral line incomplete, with 7-11 pored scales; longitudinal series with 25-29 scales; transverse series above lateral line with 4 scale rows and below with 3 scale rows. Dorsal fin with 10-11, pectoral fin with 14-15, pelvic fin with 9, anal fin with 24 to 26 and caudal fin with 19 rays (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Ground color silvery; superior portion of eye red; dark-brown chromatophores concentrated on distal margin of scales conferring reticulated pattern to body; one dark-brown humeral spot; anterior half of caudal peduncle with light-beige area. Yellowish fins; posterior portion of caudal peduncle and proximal third of caudal fin with conspicuous black transverse bar occupying their whole depth.
Maximum standard length. 54.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Rio Parnaíba, rio São Francisco and río de la Plata basins.
Oligosarcus
1. Lateral line with 47 to 54 pored scales; head length contained 2.9 to 3.5 times in standard length; dentary the same size or shorter than upper jaw .................... O. paranensis
1’. Lateral line with 36 to 40 pored scales; head length contained 3.7 to 3.8 in standard length; dentary much longer than upper jaw .................... O. pintoi
Oligosarcus paranensis Menezes, Géry, 1983Menezes NA, Géry J. Seven new Acestrorhynchin characid species (Osteichthyes, Ostariophysi, Characiformes) with comments on the systematics of the group. Rev Suisse Zool . 1983; 90(3):563-92.
Body elongate; greatest depth contained 2.9 to 3.7 and caudal peduncle depth 7.8 to 8.6 times in SL; head length 2.9 to 3.5, predorsal distance 1.7 to 1.9 and caudal peduncle length 8.7 to 9.4 in SL; snout length 3.5 to 4.5, horizontal orbital diameter 2.6 to 4.3 and least interorbital width 3.8 to 5.6 in HL. Mouth terminal; premaxilla with 4-7 teeth, dentary with 11-21, and maxilla with 18-35 teeth. Lateral line complete, with 47-54 pored scales; transverse series above lateral line with 9-10 scale rows and below with 6-8 scale rows. Dorsal fin with 12, pectoral fin with 12-16, pelvic fin with 8-9, anal fin with 20-27, and caudal fin with 19 rays (Menezes, Géry, 1983Menezes NA, Géry J. Seven new Acestrorhynchin characid species (Osteichthyes, Ostariophysi, Characiformes) with comments on the systematics of the group. Rev Suisse Zool . 1983; 90(3):563-92.). Ground color silvery; black humeral spot, vertically elongate; silvery longitudinal stripe on flank (brown or grey in preserved specimens), from humeral spot to median caudal-fin rays. Yellowish fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 100 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná basin.
a. Oligosarcus paranensis, NUP 303, 82.0 mm SL, Fiú Reservoir (rio Apucaraninha), Tamarana, State of Paraná. b. Oligosarcus pintoi, NUP 1772, 68.4 mm SL, rio Piquiri, Mariluz, State of Paraná. c. Psellogrammus kennedyi, 30.5 mm SL, fresh specimen, uncat. d. Aphyocharax anisitsi, 38.6 mm SL, fresh specimen, uncat. e. Aphyocharax dentatus, 48.0 mm SL, fresh specimen, uncat. f. Aphyocharax sp., NUP 3225, 33.4 mm SL, lagoa do Bilunga, Taquarussu, State of Mato Grosso do Sul. g. Galeocharax gulo, NUP 263, 200.0 mm SL, rio Paraná, Porto Rico, State of Paraná. h. Roeboides descalvadensis, NUP 3286, 88.8 mm SL, lagoa do Bilunga, Taquarussu, State of Mato Grosso do Sul.
Oligosarcus pintoi Campos, 1945Campos AA. Contribuição ao estudo das espécies brasileiras do gênero Leporinus. Pap Avulsos Zool . 1945; 5(16):141-58.
Body deep; greatest depth contained 2.5 to 3.1 and caudal peduncle depth 8.4 to 9.6 times in SL; head length 3.7 to 3.8, predorsal distance 1.7 to 1.9 and caudal peduncle length 9.5 to 9.6 in SL; snout length 3,5 to 4.2, horizontal orbital diameter 2.4 to 2.5 and least interorbital width 3.5 to 3.6 in HL. Mouth terminal; premaxilla with 10-12 teeth, dentary with 10-18, and maxilla with 15-23 teeth. Lateral line complete, with 36-40 pored scales; transverse series above lateral line with 7-9 scale rows and below with 6-8 scale rows. Dorsal fin with 12, pectoral fin with 14-17, pelvic fin with 9, anal fin with 28-33 and caudal fin with 19 rays (Menezes, 1987Menezes NA. Três espécies novas de Oligosarcus Günther, 1864 e redefinição taxonômica das demais espécies do gênero (Osteichthyes, Teleostei, Characidae). Bolm Zool, Univ S Paulo. 1987; 11(11):1-39.). Ground color yellowish to silvery; black rounded humeral spot with diffuse dorsal and ventral extensions, followed by second, inconspicuous humeral spot; silvery longitudinal stripe on flank (brown or grey in preserved specimens), from humeral spot to median caudal-fin rays, widest along caudal peduncle, forming horizontally elongate blotch. Yellowish fins, reddish in reproductive season (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 76 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná basin.
Psellogrammus
Psellogrammus kennedyi (Eigenmann, 1903)
Body deep and compressed; greatest depth contained 2.1 to 2.5 and caudal peduncle depth 9.7 to 10.8 times in SL; head length 3.4 to 4.0, predorsal distance 1.9 to 2.1; snout length 3.5 to 4.0, horizontal orbital diameter 2.3 to 2.8 and least interorbital width 2.6 to 3.2 in HL. Mouth terminal; inner row of premaxilla with 5 teeth, outer with 3-5, dentary with 8-11 and maxilla with 1 tooth. Lateral line irregular (incomplete, rarely complete); longitudinal series with 40-45 scales. Dorsal fin with 10, pectoral fin with 12-13, pelvic fin with 8, anal fin with 39-46, and caudal fin with 19 rays. Ground color whitish; diffuse black humeral spot; dark-brown rounded blotch on distal portion of caudal peduncle and caudal-fin base. Hyaline fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 45.5 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system and rio São Francisco basins.
Remarks. Psellogrammus kennedyi is a non-native species from the upper rio Paraná and its occurrence can be associated with the functioning of the Canal da Piracema (a fish ladder that connects the region downstream from the Itaipu Dam to the region upstream from the dam).
Aphyocharacinae
Aphyocharax
1. Fins, except pectoral and adipose, and ventral region of the body reddish; no humeral spot; 1 to 4 teeth in maxilla .................... A. anisitsi
1’. Just the caudal fin red; a black, diffuse, and oval, vertically elongated, humeral spot; 8 to 17 teeth in maxilla .................... 2
2. Orbital diameter contained less than 2.5 times in head length; short maxillary bone, its posterior tip not reaching the vertical through center of the eye .................... Aphyocharax sp.
2’. Orbital diameter contained 3.5 to 4.5 times in head length; long maxillary bone, its posterior tip reaching the vertical through center of the eye .................... A. dentatus
Aphyocharax anisitsi Eigenmann, Kennedy, 1903
Body elongate; greatest depth contained 3.1 to 4.4 and caudal peduncle depth 7.6 to 11.9 times in SL; head length 3.1 to 4.0, predorsal distance 1.7 to 2.0 and caudal peduncle length 7.3 to 7.5 in SL; snout length 3 to 4.7, horizontal orbital diameter 2.5 to 3.2 and least interorbital width 2.6 to 4.5 in HL. Mouth terminal; premaxilla with 4-8 teeth, dentary with 7 to 14, and maxilla with 1 to 4 teeth. Lateral line incomplete, with 7-10 pored scales; longitudinal series with 30-34 scales; transverse series above lateral line with 4-5 scale rows and below with 3½-5 scale rows. Dorsal fin with 9-11, pectoral fin with 9-12, pelvic fin with 6-8, anal fin with 18-23, and caudal fin with 19 rays (Lima, 2003Lima RS. Revisão taxonômica do gênero Aphyocharax Günther, 1868 (Aphyocharacinae, Characidae, Ostariophysi). [PhD Thesis]. São Paulo, SP: Universidade de São Paulo; 2003.). Ground color silvery; no black marks on body and fins. Reddish fins, except dorsal and adipose (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 49.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río Paraná basin.
Aphyocharax dentatus (Eigenmann, Kennedy, 1903)
Body elongate; greatest depth contained 3.8 to 4.7 and caudal peduncle depth 8.9 to 10.9 times in SL; head length 3.5 to 4.5, predorsal distance 1.8 to 2.0 and caudal peduncle length 7.2 to 7.6 in SL; snout length 3.2 to 4.6, horizontal orbital diameter 3.5 to 4.5 and least interorbital width 2.8 to 3.5 in HL. Mouth terminal, slightly prognathous; premaxilla with 4-8, dentary with 9-23 and maxilla with 8-17 teeth. Lateral line incomplete, with 8-13 pored scales; longitudinal series with 36-42 scales; transverse series above lateral line with 5-7 scale rows and below with 3-4 scale rows. Dorsal fin with 9-11, pectoral fin with 10-14, pelvic fin with 7-8, anal fin with 16-22, and caudal fin with 19 rays (Lima, 2003Lima RS. Revisão taxonômica do gênero Aphyocharax Günther, 1868 (Aphyocharacinae, Characidae, Ostariophysi). [PhD Thesis]. São Paulo, SP: Universidade de São Paulo; 2003.). Ground color silvery; diffuse, oval transversely elongated, humeral spot; no other black marks on body and fin. Caudal fin red, other fins hyaline to yellowish (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 60.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system.
Remarks. Aphyocharax dentatus is a non-native species from the upper rio Paraná and its occurence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Aphyocharax sp.
Body elongate; greatest depth contained 4.1 and caudal peduncle depth 9.8 times in SL; head length 4.4, predorsal distance 1.8 and caudal peduncle length 7.4 in SL; snout length 3.8, horizontal orbital diameter 2.5 and least interorbital width 2.7 in HL. Mouth terminal; premaxilla with 8 teeth, dentary with 16 and maxilla with 9 teeth. Lateral line incomplete, with 10 pored scales; longitudinal series with 37 scales; transverse series above lateral line with 6 scale rows and below with 4 scale rows. Dorsal fin with 10, pectoral fin with 13, pelvic fin with 9, anal fin with 17, and caudal fin with 19 rays. Ground color silvery; diffuse, oval transversely elongated, humeral spot; very thin, dark-brown longitudinal strip. Caudal fin red, remaining fins hyaline (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 53.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system.
Characinae
Galeocharax
Galeocharax gulo (Cope, 1870)
Body deep; greatest depth contained 3.0 to 3.7 and caudal peduncle depth 11.5 to 13.9 times in SL; head length 3.0 to 3.5, predorsal distance 1.8 to 2.2 and caudal peduncle length 9.4 to 11.3 in SL; snout length 8 to 9.3, horizontal orbital diameter 3.9 to 4.6 and least interorbital width 3.6 to 4.8 in HL. Mouth slightly subterminal; premaxilla with 6-10 teeth, inner dentary row with 7-11, outer with 4 large plus 33-35 small and maxilla with 36-51 teeth. Lateral line complete, with 81-86 pored scales; transverse series above lateral line with 16-18 scale rows and below with 15-17 scale rows. Dorsal fin with 11 or 12, pectoral fin with 15 or 16, pelvic fin with 8, anal fin with 43-50, and caudal fin with 19 rays. Ground color silvery to yellowish; silvery longitudinal band on flank (brown or grey in preserved specimens), from humeral spot to caudal peduncle; black oval, vertically elongated, humeral spot. Hyaline fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 257.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río Orinoco, rio Oyapok, rio Araguaia-Tocantins, upper rio Paraná and Amazon (except the rio Negro and rio Xingu) basins (Giovannetti et al., 2017Giovannetti V, Toledo-Piza M, Menezes NA. Taxonomic revision of Galeocharax Fowler, 1910 (Characiformes: Characidae: Characinae). Neotrop Ichthyol [serial in the internet]. 2017 Mar 23 [cited 2018 Jan 15]; 15(1):e160040. Available from: Available from: http://dx.doi.org/10.1590/1982-0224-20160040
.
http://dx.doi.org/10.1590/1982-0224-2016...
).
Remarks. Galeocharax gulo was identified as G. knerii by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Giovannetti et al. (2017Giovannetti V, Toledo-Piza M, Menezes NA. Taxonomic revision of Galeocharax Fowler, 1910 (Characiformes: Characidae: Characinae). Neotrop Ichthyol [serial in the internet]. 2017 Mar 23 [cited 2018 Jan 15]; 15(1):e160040. Available from: Available from: http://dx.doi.org/10.1590/1982-0224-20160040
.
http://dx.doi.org/10.1590/1982-0224-2016...
), in a revisionary study of Galeocharax Fowler, 1910, proposed the new synonym.
Roeboides
Roeboides descalvadensis Fowler, 1932
Body deep; greatest depth contained 2.2 to 2.7 and caudal peduncle depth 12.3 to 13.1 times in SL; head length 4.0 to 4.2, predorsal distance 1.9 to 2.0 and caudal peduncle length 12.8 to 13.5 in SL; snout length 3.6 to 4.4, horizontal orbital diameter 2.6 to 3.7 and least interorbital width 3.1 to 4.0 in HL. Mouth terminal; inner row of premaxilla with 6 teeth, outer with 4-6, inner row of dentary with 12-14, outer with 4-6 and maxilla with 9-11 teeth. Lateral line complete, with 66-71 pored scales; transverse series above lateral line with 13-16 scale rows and below with 8-10 scale rows. Dorsal fin with 10 or 11, pectoral fin with 11 or 12, pelvic fin with 9 or 10, anal fin with 50-55, and caudal fin with 19 rays. Ground color whitish; silvery longitudinal band on flank (brown or grey in preserved specimens), from humeral spot to caudal peduncle; black diffuse and oval, vertically elongated, humeral spot; one black spot on caudal peduncle, slightly forwarded prolonged. Yellowish fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 100.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system and Amazon basin.
Remarks. Roeboides descalvadensis was identified as R. paranensis by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Lucena (2007Lucena CAS. Revisão taxonômica das espécies do gênero Roeboides grupo-affinis (Ostariophysi, Characiformes, Characidae). Iheringia Ser Zool. 2007; 97(2):117-36.) in a revisionary study of the Roeboides affinis (Günther, 1868) species-group, proposed the new synonym. Roeboides descalvadensis is a non-native species from the upper rio Paraná, and its occurrence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls (Júlio Júnior et al., 2009Júlio Júnior HF, Dei Tos C, Agostinho AA, Pavanelli CS. A massive invasion of fish species after eliminating a natural barrier in the upper rio Paraná basin. Neotrop Ichthyol . 2009; 7(4):709-18.).
Cheirodontinae
Aphyocheirodon
Aphyocheirodon hemigrammus Eigenmann, 1915Eigenmann CH. The Cheirodontinae, a subfamily of minute characid fishes of South America. Mem Mus Comp Zool. 1915; 7(1):1-100.
Body elongated; greatest body depth contained 2.3 to 3.1 and caudal peduncle depth 9.2 to 10.0 times in SL; head length 3.5 to 4.2, predorsal distance 1.8 to 2.0 and caudal peduncle length 6.6 to 8.8 in SL; snout length 3.7 to 4.4, horizontal orbital diameter 2.8 to 3.2 and least interorbital width 3.0 to 3.4 in HL. Mouth terminal; premaxilla with 8-11 teeth, dentary with 20 to 26 teeth and expanded tips, and maxilla with 3-5 teeth. Lateral line incomplete, with 6-9 pored scales; longitudinal series with 34-36 scales; transverse series above lateral line with 5 or 6 scale rows and below with 3 or 4 scale rows. Dorsal fin with 10 or 11 rays, pectoral fin with 11 or 12 rays, pelvic fin with 8 or 9 rays, anal fin with 25-27 rays and caudal fin with 19 rays. Ground color pale yellow; dark-brown longitudinal stripe on flank, from pseudotympanum to caudal peduncle; dark-brown blotch occupying whole caudal peduncle depth and caudal-fin base; dark-brown stripe along anal-fin base.
Maximum standard length. 37.7 mm.
Biological data. Lives in creeks and lagoons (Agostinho et al., 1995Agostinho AA, Vazzoler AEAM, Thomaz SM. The high river Paraná basin: limnological and ichthyological aspects. In: Tundisi JG, Bicudo CEM, Matsumura-Tundisi T, editors. Limnology in Brazil. Rio de Janeiro: Brazilian Academy of Science and Brazilian Limnological Society; 1995. p.59-103.).
Distribution. Upper rio Paraná basin.
Remarks. Some specimens of A. hemigrammus were identified as Serrapinnus sp. 1 by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Both species occur in the upper rio Paraná floodplain, where A. hemigrammus has been captured since 2004. Aphyocheirodon hemigrammus can be distinguished by having the central cusp of dentary teeth of same size and with straight edge, and the anal fin of mature males without bony hooks (vs. central cusp of dentary teeth much longer than lateral ones, and anal fin of mature males with bony hooks, in Serrapinnus sp. 1). Some specimens of A. hemigrammus do not present predorsal series of scales, whereas other specimens only present scales next to dorsal fin. Eigenmann (1915Eigenmann CH. The Cheirodontinae, a subfamily of minute characid fishes of South America. Mem Mus Comp Zool. 1915; 7(1):1-100.) described that species from the rio Grande, upper rio Paraná basin in Minas Gerais State, Brazil, and stated that the species has “predorsal area rounded, with a median series of about twelve scales”. As no other distinguishing characters were observed, the specimens analyzed herein was considered as belonging to the same species, despite the apparently disrupted distribution of the species.
a. Aphyocheirodon hemigrammus, NUP 13774, 36.3 mm SL, lagoa do Bilunga, tributary of the rio Paraná, Taquarussu, State of Mato Grosso do Sul. b. Odontostilbe avanhandava, NUP 1517, 40.0 mm SL, canal do Meio (ilha Porto Rico), Porto Rico, State of Paraná. c. Serrapinus calliurus, NUP 17494, lagoa do Guaraná, tributary of the rio Baía, Taquarussu (top, female, 24.1 mm SL; bottom, male, 21.4 mm SL). d. Serrapinnus heterodon, NUP 17495, lagoa do Guaraná, tributary of the rio Baía, Taquarussu, State of Mato Grosso do Sul (top, female, 32.5 mm SL; bottom, male, 30.1 mm SL). e. Serrapinnus notomelas, 31.8 mm SL, fresh specimen, uncat. f. Serrapinnus sp. 1, NUP 3283, lagoa do Bilunga, Taquarussu, State of Mato Grosso do Sul (top, female, 28.7 mm SL; bottom, male, 28.2 mm SL). g. Serrapinnus sp. 2, NUP 17045, 33.5 mm SL, córrego Itauna, Alto Paraíso, State of Paraná.
Odontostilbe
Odontostilbe avanhandava
Chuctaya, Bührnheim, Malabarba, 2018Chuctaya J, Bührnheim CM, Malabarba LR. Two new species of Odontostilbe historically hidden under O. microcephala (Characiformes: Cheirodontinae). Neotrop Ichthyol [serial on the internet]. 2018 Mar 26 [cited 03 Apr 2018]; 16(1):e170047. Available from: Available from: http://dx.doi.org/10.1590/1982-0224-20170047
http://dx.doi.org/10.1590/1982-0224-2017...
Body elongated; greatest body depth contained 3.5 to 4.1 and caudal peduncle depth 8.7 to 11.7 times in SL; head length 3.4 to 4.4, predorsal distance 1.8 to 2.1 and caudal peduncle length 9.0 to 12.0 in SL; snout length 3.3 to 4.3, horizontal orbital diameter 2.2 to 2.6 and least interorbital width 2.7 to 3.8 in HL. Mouth terminal; premaxilla with 5, dentary with 7-10 teeth (narrow, with central cusp much longer than lateral ones), and maxilla with 2 teeth. Lateral line complete, with 26-30 pored scales; transverse series above lateral line with 4½-5 and below with 3-3½ scale rows. Dorsal fin with 10-11, pectoral fin with 11-12, pelvic fin with 8-9, anal fin with 20-22 and caudal fin with 19 rays. Ground color whitish; silvery longitudinal band (dark-brown in preserved specimens), from pseduotympanum to caudal peduncle; black rounded blotch on posterior portion of caudal peduncle and caudal-fin base. Fins hyaline to yellowish (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 50.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná basin.
Remarks. Odontostilbe avanhandava was identified as Odontostilbe sp. by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Chuctaya et al. (2018Chuctaya J, Bührnheim CM, Malabarba LR. Two new species of Odontostilbe historically hidden under O. microcephala (Characiformes: Cheirodontinae). Neotrop Ichthyol [serial on the internet]. 2018 Mar 26 [cited 03 Apr 2018]; 16(1):e170047. Available from: Available from: http://dx.doi.org/10.1590/1982-0224-20170047
http://dx.doi.org/10.1590/1982-0224-2017...
) described the new species.
Serrapinnus
1. Lateral line completely pored .................... S. heterodon
1’. Lateral line incompletely pored .................... 2
2. Dorsal and anal fins with black pigmentation .................... 3
2’. Dorsal and anal fins hyaline .................... 4
3. Longest unbranched ray and proximal portion of remaining dorsal-fin rays black; unbranched anal-fin rays dusky .................... S. notomelas
3’. Distal portion of dorsal fin and anal-fin lobe with a round, black blotch .................... Serrapinnus sp. 2
4. Dentary teeth wide, with 7-9 cusps gradually decreasing in size .................... S. calliurus
4’. Dentary teeth slender, with 3-5 cusps of different size and central cusp longer than the lateral ones .................... Serrapinnus sp. 1
Serrapinus calliurus (Boulenger, 1900)
Body elongated; greatest body depth contained 2.5 to 2.8 and caudal peduncle depth 6.7 to 8.8 times in SL; head length 3.1 to 3.6, predorsal distance 1.8 to 1.9 and caudal peduncle length 7.3 to 9.3 in SL; snout length 4.2 to 5.1, horizontal orbital diameter 2.5 to 3.0 and least interorbital width 2.8 to 3.6 in HL. Mouth terminal; premaxilla with 4-6, dentary with 6-8, and maxilla with 1 or 2 teeth. Lateral line incomplete, with 5-7 pored scales; longitudinal series with 32 or 33 scales; transverse series above lateral line with 5-6 scale rows and below with 4 or 4 ½ scale rows. Dorsal fin with 11, pectoral fin with 10 or 11, pelvic fin with 8, anal fin with 22-25 and caudal fin with 19 rays. Ground color pale yellow; dark-brown longitudinal stripe on flank, from pseudotympanum to caudal peduncle; black rounded blotch on posterior portion of caudal peduncle and caudal-fin base, extending to median caudal-fin rays.
Maximum standard length. 22.7 mm.
Biological data. Feeds on benthic organisms, detritus, unicellular and filamentous algae (Fiori et al., 2016Fiori LF, Alvez GHZ, Hahn NS, Benedito E. Influence of feeding plasticity on the fitness of small Neotropical characids. Iheringia Ser Zool [serial on the internet]. 2016 May 2 [cited 2016 Dec 08]; 106:e2016006. Available from: Available from: http://dx.doi.org/10.1590/1678-4766e2016006
http://dx.doi.org/10.1590/1678-4766e2016...
). Presents absolute average fecundity estimated in 406 oocytes, and relative fecundity in 0.6 oocytes per mg (Gelain et al., 1999Gelain D, Fialho CB, Malabarba LR. Biologia reprodutiva de Serrapinnus calliurus (Characidae, Cheirodontinae) do arroio Ribeiro, Barra do Ribeiro, Rio Grande do Sul, Brasil. Com Mus Cien PUCRS . 1999; 12:71-82.).
Distribution. Río de la Plata basin and Laguna dos Patos drainage.
Remarks. Some specimens of Serrapinnus calliurus were identified as Serrapinnus sp. 1 by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Both species occur in the upper rio Paraná floodplain, where S. calliurus has been captured since 2009 by the Nupélia staff. Serrapinnus calliurus can be distinguished by having dentary teeth wide, with 7-9 cusps gradually decreasing in size, and the black blotch on caudal peduncle reaching the base of median caudal fin rays (vs. dentary teeth slender, with 3-5 cusps of different size and central cusp longer than lateral ones, and the black blotch on caudal peduncle with no extension, in Serrapinnus sp. 1). Serrapinnus calliurus is a non-native species from the upper rio Paraná and its occurence can be associated with the functioning of the Canal da Piracema (a fish ladder that connects the region downstream from the Itaipu Dam to the region upstream from the dam), or with the aquarium trade.
Serrapinnus heterodon ( Eigenmann, 1915Eigenmann CH. The Cheirodontinae, a subfamily of minute characid fishes of South America. Mem Mus Comp Zool. 1915; 7(1):1-100. )
Body elongated; greatest body depth contained 3.3 to 4.0 and caudal peduncle depth 8.6 to 10.5 times in SL; head length 4.1 to 4.4, predorsal distance 1.9 to 2.0 and caudal peduncle length 5.0 to 6.4 in SL; snout length 2.8 to 3.2, horizontal orbital diameter 2.8 to 3.2 and least interorbital width 3.0 to 3.5 in HL. Mouth terminal; premaxilla with 5 or 6, dentary with 4-8 teeth with expanded tips, and maxilla with 1 to 3 teeth. Lateral line completely pored, with 32-36 pored scales; longitudinal series with 32-36 scales; transverse series above lateral line with 6 scale rows and below with 3-4 scale rows. Dorsal fin with 11, pectoral fin with 10-12, pelvic fin with 8, anal fin with 19-22 and caudal fin with 19 rays. Ground color whitish; dark-brown longitudinal stripe on flank, from pseudotympanum to caudal peduncle; black oval, horizontally elongated, blotch on posterior portion of caudal peduncle and caudal-fin base, not extended to median caudal-fin rays.
Maximum standard length. 30.9 mm.
Biological data. Feeding habit omnivorous; total spawning, and spawning period during rainy season (Gonçalves et al., 2011Gonçalves CS, Souza UP, Braga FMS. Population structure, feeding and reproductive aspects of Serrapinnus heterodon (Characidae, Cheirodontinae) in a Mogi Guaçu reservoir (SP), upper Paraná River basin. Acta Limnol Bras. 2011; 23(1):13-22.).
Distribution. Upper rio Paraná and rio São Francisco basins, and coastal drainages of the Rio Grande do Norte State, Northeastern Brazil.
Remarks. Some specimens of Serrapinnus heterodon were identified as Odontostilbe sp. (now as O. avanhandava) by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Both species occur in the upper rio Paraná floodplain, where S. heterodon has been captured since 2008 by the Nupélia staff. Serrapinnus heterodon can be distinguished by having the dentary teeth with three wide central cusps of same size and two small lateral ones, caudal peduncle blotch oval-shaped, reaching median caudal-fin rays, and mature males with a ventrally arched caudal peduncle (one of the synapomorphies for the genus, according to Malabarba, 1998Malabarba LR. Monophyly of the Cheirodontinae, characters and major clades (Ostariophysi: Characidae). In: Malabarba LR, Reis RE, Vari RP, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of Neotropical fishes. Porto Alegre: Edipucrs; 1998. p.193-233.) (vs. dentary teeth slender and pointed, with one central cusp longer than the four lateral ones, caudal peduncle blotch rounded, not reaching the median caudal-fin rays, and mature males not having the caudal peduncle arched, in Odontostilbe avanhandava). The confusion between the specimens of the upper rio Paraná floodplain can be associated with the completely pored lateral line. This character is common in Odontostilbe, whereas the number of pored scales is reduced in Serrapinnus. The only exception in Serrapinnus is found in S. heterodon, which has a completely pored lateral line.
Serrapinnus notomelas ( Eigenmann, 1915Eigenmann CH. The Cheirodontinae, a subfamily of minute characid fishes of South America. Mem Mus Comp Zool. 1915; 7(1):1-100. )
Body elongated; greatest body depth contained 2.4 to 2.8 and caudal peduncle depth 8.2 to 8.6 times in SL; head length 3.5 to 4.0, predorsal distance 1.8 to 2.0 and caudal peduncle length 9.5 to 10.3 in SL; snout length 3.5 to 3.8, horizontal orbital diameter 2.3 to 3.2 and least interorbital width 2.6 to 3.0 in HL. Mouth terminal; premaxilla with 4 or 5, dentary with 6 or 7 and maxilla with 1 or 2 teeth. Lateral line incompletely pored, with 5-7 pored scales; longitudinal series with 30-34 scales; transverse series above lateral line with 3½ or 4 scale rows and below with 3 or 3½ scale rows. Dorsal fin with 10 or 11, pectoral fin with 12 or 13, pelvic fin with 8 or 9, anal fin with 19-24 and caudal fin with 19 rays. Ground color whitish; dark-brown diffuse longitudinal stripe on flank, from pseudotympanum to caudal peduncle; black rounded blotch on posterior portion of caudal peduncle and caudal-fin base, not extended to median caudal-fin rays. Dorsal fin with black chromatophores along two unbranched and first branched rays and proximal half of remaining; pectoral, pelvic, anal and caudal fins yellowish (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 40.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná basin.
Serrapinnus sp. 1
Body elongated; greatest body depth contained 2.2 to 2.5 and caudal peduncle depth 7.9 to 9.8 times in SL; head length 4.3 to 4.5, predorsal distance 1.9 to 2.0 and caudal peduncle length 9.0 to 10.7 in SL; snout length 3.3 to 3.6, horizontal orbital diameter 2.3 to 3.2 and least interorbital width 2.8 to 3.0 in HL. Mouth terminal; premaxilla with 5 or 6, dentary with 8-10 and maxilla with 2 or 3 teeth. Lateral line incompletely pored, with 7-9 pored scales; longitudinal series with 30-32 scales; transverse series above lateral line with 3½ or 4 scale rows and below with 3 or 3½ scale rows. Dorsal fin with 10 or 11, pectoral fin with 12 or 13, pelvic fin with 8 or 9, anal fin with 19-24 and caudal fin with 19 rays. Ground color whitish; dark-brown longitudinal stripe on flank, from pseudotympanum to caudal peduncle; dark-brown rounded blotch on posterior portion of caudal peduncle and caudal-fin base, not extended to median caudal-fin rays (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 53.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná basin.
Serrapinnus sp. 2
Body elongated; greatest body depth contained 2.8 to 3.9 and caudal peduncle depth 8.0 to 10.7 times in SL; head length 4.0 to 4.6, predorsal distance 1.8 to 2.0 and caudal peduncle length 9.7 to 10.8 in SL; snout length 3.1 to 3.9, horizontal orbital diameter 2.0 to 2.6 and least interorbital width 2.5 to 3.5 in HL. Mouth terminal; premaxilla with 4 or 5, dentary with 6, and maxilla with 2 teeth. Lateral line incompletely pored, with 6 or 7 pored scales; longitudinal series with 31-34 scales; transverse series above lateral line with 3½ scale rows and below with 3 or 3½ scale rows. Dorsal fin with 11, pectoral fin with 12 or 13, pelvic fin with 8 or 9, anal fin with 20-22 and caudal fin with 19 rays. Ground color whitish; dark-brown longitudinal stripe on flank, from pseudotympanum to caudal peduncle; dark-brown rounded blotch on posterior portion of caudal peduncle and caudal-fin base, not extended to median caudal-fin rays. Hyaline fins; dorsal and anal fins with distal portion black (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 29.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná basin.
Stevardiinae
Bryconamericus
1. Greatest body depth contained 2.7 to 2.9 times in standard lenght; teeth in the outer premaxillary series forming a regular arch .................... B. aff. iheringii
1’. Greatest body depth contained 3.3 to 4.2 times in standard lenght; teeth in the outer premaxillary series irregularly arranged .................... 2
2. Distal portion of caudal-fin lobes black; dorsal and anal fins completely hyaline; mature males with bony hooks on the anal- and pelvic-fin rays; 2-3 maxillary teeth .................... B. exodon
2’. Distal portion of caudal-fin lobes hyaline; middle portion of the dorsal- and anal-fin rays dusky; bony hooks always absent on the anal- and pelvic-fin rays; 2-5 maxillary teeth .................... B. turiuba
Bryconamericus exodon Eigenmann, 1907
Body elongated; greatest body depth contained 3.8 to 4.2 and caudal peduncle depth 10.6 to 11.2 times in SL; head length 4.1 to 4.3, predorsal distance 2.1 to 2.2, and caudal peduncle length 6.7 to 7.1; snout length 3.5 to 4.0, horizontal orbital diameter 2.2 to 2.5 and interorbital distance 2.4 to 2.8 in HL. Mouth terminal; inner row of premaxilla with 4, outer row with 5; dentary with 9 or 10, and maxilla with 2 or 3 teeth. Lateral line with 36-38 pored scales; transverse series above lateral line with 4 or 5 rows and below with 3 scale rows. Dorsal fin with 11 or 12, pectoral fin with 14 or 15, pelvic fin 9, anal fin rays 22-26, and caudal with 19 rays. Ground color pale yellow; black vertically elongated humeral spot; silvery longitudinal band (brown or grey in fixed specimens), from humeral spot to caudal peduncle. Yellowish fins; distal margin of caudal-fin lobes black (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 62.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río Paraguay and upper rio Paraná basins.
Remarks. Bryconamericus exodon is a non-native species from the upper rio Paraná, and its occurrence can be associated with the functioning of the Canal da Piracema (a fish ladder that connects the region downstream from the Itaipu Dam to the region upstream from the dam).
a. Bryconamericus exodon, 55.8 mm SL, fresh specimen, uncat. b. Bryconamericus aff. iheringii, NUP 824, 50.0 mm SL, rio São Vicente, Missal, State of Paraná. c. Bryconamericus turiuba, NUP 6170, 59.3 mm SL, rio Iguatemi, tributary of the rio Paraná, Coronel Sapucaia, State of Mato Grosso do Sul. d. Diapoma guarani, NUP 17635, 31.2 mm SL, Canal da Piracema, Foz do Iguaçu, State of Paraná. e. Knodus moenkhausii, NUP 3211, 48.0 mm SL, riacho Água Nanci, Porto Rico, State of Paraná. f. Piabarchus stramineus, NUP 3298, 70.0 mm SL, rio Paraná, Porto Rico, State of Paraná. g. Piabina argentea, 68.0 mm SL, uncat. h. Planaltina britskii, NUP 11802, 27.5 mm SL, lagoa Genipapo (ilha Porto Rico, Foz do Iguaçu, State of Paraná.
Bryconamericus aff.iheringii (Boulenger, 1887)
Body deep; greatest body depth contained 2.7 to 2.9 and caudal peduncle depth 8.4 to 9.7 times in SL; head length 4.5 to 4.7, predorsal distance 1.7 to 1.9, and caudal peduncle length 7.6 to 8.1; snout length 3.7 to 3.9, horizontal orbital diameter 2.4 to 2.6 and interorbital distance 3.0 to 3.2 in HL. Mouth subterminal; inner row of premaxilla with 4, outer row with 4 or 5, dentary with 9-11, and maxilla with 3 or 4 teeth. Lateral line with 34-36 pored scales; transverse series above lateral line with 5 or 5½ rows and below with 4-5½ scale rows. Dorsal fin with 12 or 13, pectoral fin with 15, pelvic fin with 9, anal fin rays 22-24, and caudal with 19 rays. Ground color silvery to yellowish; black rounded or vertically elongated humeral spot; silvery longitudinal band (brown or grey in fixed specimens), from humeral spot to median caudal-fin rays. Yellowish or reddish fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 60.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Rio Paraná and rio Uruguay basins, and Laguna dos Patos drainage.
Bryconamericus turiuba Langeani, Lucena, Pedrini, Tarelho-Pereira, 2005Langeani F, Lucena ZMS, Pedrini JL, Tarelho Pereira FJ. Bryconamericus turiuba, a new species from the upper rio Paraná system (Ostariophysi: Characiformes). Copeia . 2005; (2):386-92.
Body elongated; greatest body depth contained 3.3 to 4.2 and caudal peduncle depth 8.6 to 11.5 times in SL; head length 3.5 to 4.7, predorsal distance 1.8 to 2.1, and caudal peduncle length 5.6 to 7.1 in SL; snout length 2.8 to 4.6, horizontal orbital diameter 2.5 to 3.3 and least interorbital width 2.7 to 4.2 in HL. Mouth terminal; inner row of premaxilla with 4 teeth, outer row with 4 or 5, rarely 3 teeth; dentary with 3 or 4 teeth, and maxilla with 2-5 teeth. Lateral line with 37-43 pored scales; transverse series above lateral line with 4 or 5 rows and below with 3 or 4 scale rows. Dorsal fin with 10, rarely 9 or 11, pectoral fin with 11-14, pelvic fin 8, rarely 7 or 9, anal fin with 17-23, and caudal with 16 or 17 rays (Langeani et al., 2005Langeani F, Lucena ZMS, Pedrini JL, Tarelho Pereira FJ. Bryconamericus turiuba, a new species from the upper rio Paraná system (Ostariophysi: Characiformes). Copeia . 2005; (2):386-92.). Ground color pale yellow; dorsal profile with dark-brown longitudinal band, from snout to caudal fin; humeral spot conspicuous and vertically elongated; silver longitudinal band (brown or grey in fixed specimens), from humeral spot to median caudal-fin rays. Fins scattered with dark-brown spots.
Maximum standard length. 61.0 mm.
Biological data. Lives in rapids, with rocky bottom, and lentic pools, with gravel bottom or sand and mud (Langeani et al., 2005Langeani F, Lucena ZMS, Pedrini JL, Tarelho Pereira FJ. Bryconamericus turiuba, a new species from the upper rio Paraná system (Ostariophysi: Characiformes). Copeia . 2005; (2):386-92.).
Distribution. Upper rio Paraná basin.
Diapoma
Diapoma guarani (Mahnert, Géry, 1987)
Body elongate; greatest body depth contained 3.2 to 3.4, caudal-peduncle depth 9.9 to 11.3 times in S L; head length 4.5 to 4.6, predorsal length 1.8 to 1.9, and caudal-peduncle length 8.3 to 10.0 in SL; snout length 3.4 to 3.8, horizontal orbital diameter 2.3 to 2.5, and least interorbital width 2.8 to 3.0 times in HL. Mouth terminal; inner and outer row of premaxilla with 3 or 4, dentary with 12-16 and maxilla with 4 or 5 teeth. Lateral line with 6 or 7 pored scales; longitudinal series with 29-31 scales; transverse series above lateral line with 4½ or 5 scales above and below with 4 scale rows. Dorsal fin with 10, pectoral fin with 12-14, pelvic fin with 7 rays, anal with 22-24 rays, and caudal fin with 19 rays. Ground color whitish to pale yellow; dark-brown longitudinal stripe, from pseudotympanum to median caudal-fin rays. Fins overall reddish; distal margin of dorsal, caudal and anal fins black.
Maximum standard length. 48.0 mm.
Distribution. Upper rio Paraná basin, in Argentina, Brazil and Paraguay.
Remarks. Diapoma guarani was identified as Hyphessobrycon sp. by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Thomaz et al. (2015Thomaz AT, Arcila D, Orti G, Malabarba LR. Molecular phylogeny of the subfamily Stevardiinae Gill, 1858 (Characiformes: Characidae): classification and the evolution of reproductive traits. BMC Evol Biol [serial on the internt]. 2015 Jan 13 [cited 2016 Dec 08]; 15:146. Available from: Available from: http://dx.doi.org/10.1186/s12862-015-0403-4
http://dx.doi.org/10.1186/s12862-015-040...
), in a molecular phylogeny of the Stevardiinae, proposed a new classification where the species of Cyanocharax Malabarba, Weitzman, 2003 and Hyphessobrycon guarani were reallocated to Diapoma Cope, 1894.
Knodus
Knodus moenkhausii (Eigenmann, Kennedy, 1903)
Body elongate; greatest body depth contained 3.5 to 4.0, caudal peduncle depth 9.7 to 12.4 times in SL; head length 3.9 to 4.5, predorsal distance 1.8 to 2.0, and caudal peduncle length 6.8 to 7.4 in SL; snout length 3.5 to 3.9, horizontal orbital diameter 1.8 to 2.3, and least interorbital width 2.5 to 3.2 times in HL. Mouth terminal; inner row of premaxilla with 4, outer row with 4-5, dentary with 8-9 and maxilla with 2-3 teeth. Lateral line with 35-39 scales; transverse series above lateral line with 4½-5½ scales above and below with 3-4½ scale rows. Dorsal fin with 10, pectoral fin with 14-15, pelvic fin with 8-9, anal with 22-24, and caudal fin with 19 rays. Ground color silvery to pale yellow; silvery longitudinal band (dark-brown in preserved specimens), from humeral spot to median caudal-fin rays; dark-brown, transversely elongate, humeral spot. Yellowish fins; reddish during reproductive period (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 54.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system, rio São Francisco and coastal drainages of Southeastern Brazil.
Piabarchus
Piabarchus stramineus (Eigenmann, 1908)
Body elongated; greatest body depth contained 4.0 to 4.2 and caudal peduncle depth 10.6 to 11.8 times in SL; head length 4.6 to 4.8, predorsal distance 2.1 to 2.2, and caudal peduncle length 6.8 to 7.4; snout length 3.6 to 4.0, horizontal orbital diameter 2.0 to 2.5 and interorbital distance 2.5 to 2.8 in HL. Mouth terminal; inner row of premaxilla with 4 teeth, outer row with 5 teeth; dentary with 9 or 10 teeth, and maxilla with 2 teeth. Lateral line with 36-38 pored scales; transverse series above lateral line with 4 rows and below with 3. Dorsal fin with 11 or 12, pectoral fin with 14 or 15, pelvic fin 9, anal fin with 20-22, and caudal with 19 rays. Ground color silvery to yellowish; silvery longitudinal band (brown or grey in preserved specimens), from humeral spot to caudal peduncle; black oval, vertically elongated, humeral spot. Caudal fin yellowish or oranged; remaining fins hyaline, with dusky border (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 84.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río de la Plata basin and rio São Francisco basin.
Remarks. Piabarchus stramineus was identified as B. stramineus by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Thomaz et al. (2015Thomaz AT, Arcila D, Orti G, Malabarba LR. Molecular phylogeny of the subfamily Stevardiinae Gill, 1858 (Characiformes: Characidae): classification and the evolution of reproductive traits. BMC Evol Biol [serial on the internt]. 2015 Jan 13 [cited 2016 Dec 08]; 15:146. Available from: Available from: http://dx.doi.org/10.1186/s12862-015-0403-4
http://dx.doi.org/10.1186/s12862-015-040...
), in a molecular phylogeny of the Stevardiinae, proposed the new combination.
Piabina
Piabina argentea Reinhardt, 1867
Body elongate; greatest body depth contained 3.8 to 4.3, caudal peduncle depth 9.2 to 11.1 times in SL; head length 3.5 to 4.1 and predorsal distance 1.9 to 2.1 in SL; snout length 3.2 to 3.7, horizontal orbital diameter 2.8 to 3.4, and least interorbital width 3.3 to 3.7 times in HL. Mouth subterminal; outer row of premaxilla with 2-3, median row with 2 and inner row with 4, dentary with 6-7 and maxilla with 2 or 3 teeth. Lateral line complete, with 37-39 pored scales; transverse series above lateral line with 5 scales above and below with 3-4 scale rows. Dorsal fin with 10, pectoral fin with 12-13, pelvic fin with 9, anal with 18-21, and caudal fin with 19 rays (Vari, Harold, 2001Vari RP, Harold AS. Phylogenetic study of the neotropical fish genera Creagrutus Günther and Piabina Reinhardt (Teleostei: Ostariophysi: Characiformes), with a revision of the Cis-Andean species. Washington (DC): Smithsonian Institution Press ; 2001. (Smithsonian Contributions to Zoology; no. 613).). Ground color silvery to pale yellow; black humeral spot, with irregular limits; dark-brown longitudinal band, from humeral spot to median caudal-fin rays. Hyaline fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 74.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río de la Plata and rio São Francisco basins.
Planaltina
Planaltina britskii Menezes, Weitzman, Burns, 2003Menezes NA, Weitzman SH, Burns JB. A systematic review of Planaltina (Teleostei: Characiformes: Characidae: Glandulocaudini: Diapomini) with a description of two new species from the upper rio Paraná, Brazil. Proc Biol Soc Wash . 2003; 116(3):557-600.
Body elongate; greatest body depth contained 3.9 to 5.4, caudal peduncle depth 7.1 to 10.0 times in SL; head length 3.9 to 4.6, predorsal distance 1.8 to 1.9, and caudal peduncle length 7.0 to 10.3 in SL; snout length 3.3 to 4.5, horizontal orbital diameter 2.4 to 2.8, and least interorbital width 2.8 to 4.3 times in HL. Mouth terminal; outer row of premaxilla with 3, inner row with 4; maxilla with 2 teeth. Lateral line complete, with 38-42 pored scales; transverse series above lateral line with 5 scales above and below with 4 scale rows. Dorsal fin with 10, pectoral fin with 10-12, pelvic fin with 7, anal with 19-24, and caudal fin with 19 rays (Menezes et al., 2003Menezes NA, Weitzman SH, Burns JB. A systematic review of Planaltina (Teleostei: Characiformes: Characidae: Glandulocaudini: Diapomini) with a description of two new species from the upper rio Paraná, Brazil. Proc Biol Soc Wash . 2003; 116(3):557-600.). Ground color silvery to pale yellow; dark-brown longitudinal stripe from humeral region to caudal fin, forming irregular spot on base of median rays (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 40.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná basin.
Crenuchidae
Characidium
1. Lateral line incompletely pored; orbit large, contained
2.3 to 3.2 times in head length .................... Characidium sp.
1’. Lateral line completely pored; orbit small, contained 3.5 to 4.4 times in head length .................... 2
2. Isthmus and region between pectoral fins without scales; fins with dark-brown stripes .................... C. gomesi
2’. Isthmus and region between pectoral fins covered by scales; fins hyaline .................... C. aff. zebra
Characidium gomesi Travassos, 1956
Body elongated; greatest body depth contained 3.9 to 4.0 and caudal peduncle depth 7.8 to 8.5 times in SL; head length 4.2, predorsal distance 2.1 to 2.4 and caudal peduncle length 4.9 to 6.0 in SL; snout length 3.8 to 3.9, horizontal orbital diameter 4.4 to 4.8 and least interorbital width 5.3 to 5.9 in HL. Mouth terminal; premaxilla with 6 or 7, dentary with 8-10 and maxilla with no teeth. Isthmus without scales. Lateral line with 34 or 35 pored scales; transverse series above lateral line with 4 scale rows and below with 2 or 2½ scale rows. Dorsal fin with 11 rays, pectoral fin with 11-13 rays, pelvic fin and anal fin with 8 or 9 rays, and caudal fin with 18 rays. Ground color brown; three dark-brown longitudinal stripes on dorsal region of body; dark-brown stripe from tip of snout to orbit; dark-brown longitudinal stripe on flank, from humeral spot caudal peduncle; dark-brown transverse bars on flank, conspicuous on caudal peduncle; dark-brown spot at base of median caudal-fin rays. Hyaline fins; dorsal fin with two dark-brown oblique stripes; adipose fin with distal portion darkened; posterior half of pectoral, pelvic and anal-fin rays darkened; caudal fin with two dark-brown transverse bars.
Maximum standard length. 56.0 mm.
Biological data. Feeding habit omnivorous with tendency to insectivory, and lives in rocks bottom with stronger current (Ferreira, 2007Ferreira KM. Biology and ecomorphology of stream fishes from the rio Mogi-Guaçu basin, Southeastern Brazil. Neotrop Ichthyol . 2007; 5(3):311-26.).
Distribution. Upper rio Paraná basin.
a. Characidium gomesi, NUP 17607, 59.3 mm SL, córrego São Lucas, tributary of the rio Amambaí, Juti, State of Mato Grosso do Sul. b. Characidium aff. zebra, 56.0 mm SL, uncat. c. Characidium sp., NUP 3450, 27.4 mm SL, lagoa da Esperança, Nova Alvorada do Sul, State of Mato Grosso do Sul. d. Cyphocharax modestus, NUP 3290, 80.0 mm SL, lagoa do Bilunga, Taquarussu, State of Mato Grosso do Sul. e. Cyphocharax nagelii, 130.0 mm SL, uncat. f. Steindachnerina brevipinna, 105.8 mm SL, fresh specimen, uncat. g. Steindachnerina insculpta, NUP 1424, 90.0 mm SL, Itaipu Reservoir, Guaíra, State of Paraná. h. Rhaphiodon vulpinus, 480.0 mm SL, fresh specimen, uncat.
Characidium aff. zebra Eigenmann, 1909
Body elongated; greatest body depth contained 3.9 to 4.8 and caudal peduncle depth 8.9 to 11.1 times in SL; head length 4.4 to 4.8, predorsal distance 2.2 to 2.4 and caudal peduncle length 8.5 to 9.7 in SL; snout length 3.6 to 3.9, horizontal orbital diameter 3.5 to 4.4 and least interorbital width 4.6 to 5.1 in HL. Mouth terminal; isthmus covered by scales; premaxilla with 9, dentary with 10-11 teeth and maxilla toothless. Lateral line with 34-37 pored scales; transverse series above lateral line with 4 scale rows and below with 3½-4 scale rows. Dorsal fin with 11, pectoral fin with 13-14, pelvic fin with 9, anal fin with 9 rays, and caudal fin with 18-19 rays. Ground color pale yellow; dark-brown longitudinal stripe from humeral spot to caudal peduncle; eight to 10 dark-brown transverse bars on flank; black spot on the base of median caudal-fin rays. Hyaline fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 70.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Amazon and río Essequibo basins and coastal drainages of Guiana Shield.
Characidium sp.
Body moderately deep; greatest body depth contained 3.4 to 4.0 and caudal peduncle depth 8.0 to 10.4 times in SL; head length 3.1 to 3.7, predorsal distance 1.9 to 2.1 and caudal peduncle length 6.3 to 7.5 in SL; snout length 3.6 to 4.5, horizontal orbital diameter 2.3 to 3.2 and least interorbital width 2.8 to 4.0 in HL. Mouth terminal; isthmus covered by scales; premaxilla with 7-9, dentary with 8-11 and maxilla toothless. Lateral line incomplete, with 9-12 pored scales; longitudinal series with 29-34 scales; transverse series above lateral line with 4-4½ scale rows and below with 3-3½ scale rows. Dorsal fin with 10-12, pectoral fin with 14, pelvic fin and anal fins with 7-9 rays, and caudal fin with 18-19 rays. Ground color pale yellow; dark-brown longitudinal stripe, occasionally inconspicuous, from humeral spot to caudal peduncle; nine to 10 dark-brown transverse bars; black spot on the base of median caudal-fin rays. Hyaline fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 32.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná floodplain and left-bank tributaries of the rio Paraná in the State of Paraná.
Curimatidae
Cyphocharax
1. Lateral line with 31 to 36 scales; transverse series above lateral line with 4½ to 7 scale rows .................... C. modestus
1’. Lateral line with 39 to 45 scales; transverse series above lateral line with 7½ to 9 scale rows .................... C. nagelii
Cyphocharax modestus (Fernández-Yépez, 1948)
Body deep; greatest body depth contained 2.4 to 2.9 and caudal peduncle depth 6.6 to 7.7 times in SL; head length 3.2 to 3.7, predorsal distance 1.9 to 2.1 and caudal peduncle length 10.7 to 10.9 in SL; snout length 3.0 to 3.7, horizontal orbital diameter 3.1 to 3.8 and least interorbital width 2.2 to 2.5 in HL. Mouth terminal; premaxilla, dentary and maxilla without teeth in adults. Lateral line with 31-36 pored scales; transverse series above lateral line with 5½-7 scale rows and below with 4½-6 scale rows. Dorsal fin with 11 or 12, pectoral fin with 14-16, pelvic fin with 9 or 10, anal fin with 8 or 9 and caudal fin with 19 rays (Vari, 1992Vari RP. Systematics of the Neotropical characiform genus Cyphocharax Fowler (Pisces, Ostariophysi). Washington (DC): Smithsonian Institution Press ; 1992. (Smithsonian Contributions to Zoology; no. 529).). Ground color silvery; dark-brown inconspicuous longitudinal band along lateral line to distal margin of median caudal-fin rays, larger on caudal peduncle. Hyaline fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 132.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system.
Cyphocharax nagelii (Steindachner, 1881)
Body elongated; greatest body depth contained 3.0 to 3.4 and caudal peduncle depth 7.7 to 8.3 times in SL; head length 3.3 to 3.7, predorsal distance 2.0 to 2.2 and caudal peduncle length 10.3 to 10.7 in SL; snout length 3.1 to 3.4, horizontal orbital diameter 3.3 to 4.0 and least interorbital width 2.6 to 2.8 in HL. Mouth terminal; premaxilla, dentary and maxilla without teeth in adults. Lateral line with 39-45 pored scales; transverse series above lateral line with 7½-9 scale rows and below with 6½-7½ scale rows. Dorsal fin with 11 or 12, pectoral fin with 13-15, pelvic fin with 9 or 10, anal fin with 8 or 9 and caudal fin with 19 rays (Vari, 1992Vari RP. Systematics of the Neotropical characiform genus Cyphocharax Fowler (Pisces, Ostariophysi). Washington (DC): Smithsonian Institution Press ; 1992. (Smithsonian Contributions to Zoology; no. 529).). Ground color silvery; dark-brown inconspicuous longitudinal band along lateral line to distal margin of median caudal-fin rays, larger on caudal peduncle. Hyaline fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 165.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná basin.
Steindachnerina
1. Dorsal fin with a black blotch on the base of median rays, sometimes little conspicuous .................... S. brevipinna
1’. Dorsal fin without black blotch on the base of median rays .................... S. insculpta
Steindachnerina brevipinna
(Eigenmann, Eigenmann, 1889)
Body elongated; greatest body depth contained 2.6 to 3.2 and caudal peduncle depth 6.6 to 8.3 times in SL; head length 3.1 to 3.7, predorsal distance 1.9 to 2.1 and caudal peduncle length 8.3 to 9.3 in SL; snout length 2.9 to 3.7, horizontal orbital diameter 2.8 to 3.6 and least interorbital width 2.2 to 2.6 in HL. Mouth terminal; premaxilla, dentary and maxilla without teeth in adults. Lateral line with 33-37 pored scales; transverse series above lateral line with 5½-6½ scale rows and below with 4½-5½ scale rows. Dorsal fin with 10-12, pectoral fin with 11-14, pelvic fin with 9, anal fin with 9 or 10 and caudal fin with 19 rays (Vari, 1991Vari RP. Systematics of the Neotropical Characiform genus Steindachnerina Fowler (Pisces: Ostariophysi). Washington (DC): Smithsonian Institution Press ; 1991. (Smithsonian Contributions to Zoology; no. 507).). Ground color silvery; black conspicuous longitudinal stripe along lateral line to distal margin of median caudal-fin rays, larger on caudal peduncle. Yellowish fins; dorsal fin black blotch on the base of median rays, sometimes little conspicuous (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 160.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río de la Plata basin.
Remarks. Steindachnerina brevipinna is a non-native species from the upper rio Paraná, and its occurrence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
Steindachnerina insculpta (Fernández-Yépez, 1948)
Body elongated; greatest body depth contained 2.9 to 3.4 and caudal peduncle depth 7.7 to 9.1 times in SL; head length 3.2 to 3.7, predorsal distance 2.0 to 2.2 and caudal peduncle length 9.1 to 9.7 in SL; snout length 3.0 to 3.4, horizontal orbital diameter 3.0 to 3.4 and least interorbital width 2.4 to 2.6 in HL. Mouth terminal; premaxilla, dentary and maxilla without teeth in adults. Lateral line with 34-40 pored scales; transverse series above lateral line with 6½-7½ scale rows and below with 4½-5½ scale rows. Dorsal fin with 11, pectoral fin with 12-15, pelvic fin with 9, anal fin with 9 or 10 and caudal fin with 19 rays (Vari, 1991Vari RP. Systematics of the Neotropical Characiform genus Steindachnerina Fowler (Pisces: Ostariophysi). Washington (DC): Smithsonian Institution Press ; 1991. (Smithsonian Contributions to Zoology; no. 507).). Ground color silvery; black conspicuous longitudinal stripe along lateral line to distal margin of median caudal-fin rays, larger on caudal peduncle. Yellowish fins; dorsal fin with few scattered black chromatophores (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 144.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná basin.
Cynodontidae
Rhaphiodon
Rhaphiodon vulpinus Spix, Agassiz, 1829
Body deep; greatest depth contained 5.2 to 6.1 and caudal peduncle depth 16.1 to 17.3 times in SL; head length 4.8 to 5.3, predorsal distance 1.3 to 1.4 and caudal peduncle length 11.9 to 14.0 in SL; snout length 3.5 to 3.7, horizontal orbital diameter 4.6 to 4.7 and least interorbital width 6.1 to 6.2 in HL. Mouth superior, its cleft oblique; premaxilla with 14-16, dentary with 21-23 and maxilla with 15-17 teeth. Lateral line with 142-145 pored scales; transverse series above lateral line with 16-21 scale rows and below with 13-16 scale rows. Dorsal fin with 14, pectoral fin with 17 or 18, pelvic fin with 9, anal fin with 40-45, and caudal fin with 19 rays (Toledo-Piza, 2000Toledo-Piza M. The neotropical fish subfamily Cynodontinae (Teleostei: Ostariophysi: Characiformes): a phylogenetic study and a revision of Cynodon and Rhaphiodon. Am Mus Novit. 2000; (3286):1-88.). Ground color silvery. Pectoral, pelvic and anal fins yellowish; dorsal and caudal fins hyaline (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 780.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río Orinoco, drainages of Guyana, Amazon and río de la Plata basins.
Erythrinidae
Erythrinus
Erythrinus erythrinus (Bloch, Schneider, 1801)
Body elongated; greatest body depth contained 3.5 to 5.0 and caudal peduncle depth 2.0 to 4.0 times in SL; head length 3.1 to 3.5, predorsal distance 1.2 to 1.7 and caudal peduncle length 10.0 to 12.1 in SL; snout length 2.6 to 3.0, horizontal orbital diameter 1.8 to 4.3 and least interorbital width 2.5 to 2.7 in HL. Mouth terminal; premaxilla with 8-10, dentary with 32-36 and maxilla with 30-33 teeth. Lateral line with 28 or 29 pored scales; transverse series above lateral line with 3-4 scale rows and below with 3 or 2½ scale rows. Dorsal fin with 12, pectoral fin with 15, pelvic fin with 8, anal fin with 10 and caudal fin with 19 rays (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Ground color pale brown, dorsal regions darker than ventral; dark-brown rounded blotch, when present, on humeral region. Fins with dark-brown spots, sometimes fused forming irregular transverse stripes, especially on dorsal and caudal fins.
Maximum standard length. 200.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Coastal rivers of Northern South America, río Orinoco and Amazon basins, Paraná-Paraguay system (Eschmeyer et al., 2017Eschmeyer WN, Fricke R, van der Laan R, editors. Catalog of fishes: genera, species, references [Internet]. San Francisco: California Academy of Science; 2017 [updated 2017 Jan 31; cited 2017 Feb 24]. Available from: Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
http://researcharchive.calacademy.org/re...
).
Remarks. Erythrinus erythrinus is a non-native species from the upper rio Paraná, and its occurrence can be associated with its introduction as a live bait by anglers.
a. Erythrinus erythrinus, NUP 16220, 77.4 mm SL, córredo da Ponte, Mundo Novo, State of Mato Grosso do Sul. b. Hoplerythrinus unitaeniatus, NUP 3437, 150.2 mm SL, lagoa Bilunguinha, Taquarussu, State of Mato Grosso do Sul. c. Hoplias intermedius, MZUSP 69370, 277.2 mm SL, rio Suaçuí Pequeno, downstream from the bridge of Procópio, Coroaci, Minas Gerais State. d. Hoplias mbigua, NUP 292, 260.0 mm SL, Itaipu Reservoir, Santa Helena, State of Paraná. e. Hoplias misionera , NUP 10408, 190.0 mm SL, lagoa Pousada das Graças, Taquarussu, State of Mato Grosso do Sul. f. Hoplias sp. 2, NUP 3457, 250.0 mm SL, ressaco do Leopoldo (ilha Mutum), Batayporã, State of Mato Grosso do Sul. g. Hoplias sp. 3, NUP 3458, 170.0 mm SL, ressaco do Leopoldo (ilha Mutum), Batayporã, State of Mato Grosso do Sul. h. Hemiodus orthonops, 137.0 mm SL, uncat.
Hoplerythrinus
Hoplerythrinus unitaeniatus (Agassiz, 1829)
Body elongated; greatest body depth contained 4.2 to 4.7 and caudal peduncle depth 6.3 to 6.6 times in SL; head length 3.1 to 3.2, predorsal distance 1.8 to 2.0 and caudal peduncle length 8.4 to 8.6 in SL; snout length 4.0 to 4.3, horizontal orbital diameter 5.2 to 5.7 and least interorbital width 2.6 to 2.7 in HL. Mouth terminal; premaxilla with 8-10, dentary with 35-38 and maxilla with 32-36 teeth. Lateral line with 35-39 pored scales; transverse series above lateral line with 3½ scale rows and below with 3 scale rows. Dorsal fin with 11, pectoral fin with 14 or 15, pelvic fin with 8, anal fin with 13 or 14 and caudal fin with 19 rays. Ground color brown, dorsal regions darker than the ventral; dark-brown rounded blotch on opercle rounded blotch; dark-brown longitudinal band, from opercle to caudal-fin base. Dark-grey fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 260.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río Orinoco, río Magdalena, Amazon and rio São Franciso basins, Paraná-Paraguay system and coastal rivers of Panamá and Northern South America (Eschmeyer et al., 2017Eschmeyer WN, Fricke R, van der Laan R, editors. Catalog of fishes: genera, species, references [Internet]. San Francisco: California Academy of Science; 2017 [updated 2017 Jan 31; cited 2017 Feb 24]. Available from: Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
http://researcharchive.calacademy.org/re...
).
Remarks. Hoplerythrinus unitaeniatus is a non-native species from the upper rio Paraná, and its occurrence can be associated with its introduction as a live bait by anglers, or with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
Hoplias
1. Inferior margins of dentaries almost parallel, in ventral view; tongue without denticles .................... H. intermedius (H. lacerdae group)
1’. Inferior margins of dentaries converging towards symphysis; tongue with denticles .................... 2 (H. malabaricus group)
2. Scales on caudal-fin base forming rounded margin .................... 3
2’. Scales on caudal-fin base forming straight margin .................... 4
3. Scales on caudal-fin base decreasing abruptely in size .................... H. misionera
3’. Scales on caudal-fin base decreasing gradually in size .................... Hoplias sp. 2
4. Lateral line, posterior to dorsal-fin origin, with 32 to 34 pored scales .................... H. mbigua
4’. Lateral line, posterior to dorsal-fin origin, with 29 to 31, rarely 32 pored scales .................... Hoplias sp. 3
Hoplias intermedius (Günther 1864)
Body elongated; greatest depth contained 3.6 to 4.2, caudal peduncle depth 6.9 to 9.3 times in SL; head length 3.2 to 3.6, predorsal distance 1.8 to 2.0 and caudal peduncle length 7.8 to 10.8; snout length 3.6 to 4.3, horizontal orbital diameter 5.5 to 12.3 and least interorbital width 3.1 to 4.6 in HL. Mouth terminal; premaxilla with 10; dentary with 35-37 and maxilla with 30-34 teeth. Lateral line with 43-45 pored scales; transverse series above lateral line with 5 or 5½ scale rows and below with 4½ or 5 scale rows. Dorsal fin with 12-15, pectoral fin with 13 or 14 rays, pelvic fin with 8, anal fin with 10-12 rays and caudal fin with 19 rays. Ground color brown; dark-brown longitudinal band over lateral line, sometimes inconspicuous; four dark-brown transverse bars. Fins with scattered dark-brown spots, sometimes forming dark-brown irregular stripes (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 320.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio São Francisco, upper rio Doce and upper rio Paraná basin.
Remarks. Hoplias intermedius was identified as Hoplias sp. (lacerdae group) by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Oyakawa, Mattox (2009Oyakawa OT, Mattox GMT. Revision of the Neotropical trahiras of the Hoplias lacerdae species-group (Ostariophysi: Characiformes: Erythrinidae) with descriptions of two new species. Neotrop Ichthyol . 2009; 7(2):117-40.), in a revisionary study of the Neotropical Hoplias lacerdae Miranda-Ribeiro, 1918 species-group, validated and designated a lectotype for H. intermedius.
Hoplias mbigua Azpelicueta, Benítez, Aichino, Mendez, 2015
Body elongated; greatest depth contained 4.5 to 5.2, caudal peduncle depth 7.2 to 8.3 times in SL; head length 2.9 to 3.6, predorsal distance 2.0 to 2.3 and caudal peduncle length 6.8 to 9.4 in SL; snout length 3.5 to 4.0, horizontal orbital diameter 5.8 to 7.5 and least interorbital width 3.6 to 4.3 in HL. Mouth terminal; premaxilla with 8, dentary with 2 rows of teeth, outer row with 13-15 and inner row with 13-16; maxilla with 30-40 teeth. Lateral line with 41-43 pored scales; series between lateral lines with 13 scale rows. Dorsal fin with 14 or 15, pectoral fin with 11-14, pelvic fin with 8 or 9, anal fin with 9-11 and caudal fin with 19 rays (Azpelicueta et al., 2015Azpelicueta MM, Benítez MF, Aichino DR, Mendez CMD. A new species of the genus Hoplias (Characiformes, Erythrinidae), a tararira from the lower Paraná River, in Misiones, Argentina. Acta Zool Lilloana. 2015; 59(1-2):71-82.; Bifi, 2013Bifi AG. Revisão taxonômica das espécies do grupo Hoplias malabaricus (Bloch, 1794) (Characiformes: Erythrinidae) da bacia do rio da Prata. [PhD Thesis on the Internet]. Maringá: Universidade Estadual de Maringá; 2013 [cited 2016 Dec 08]. Available from: Biblioteca digital Universidade Estadual de Maringá. Available from: Biblioteca digital Universidade Estadual de Maringá. http://nourau.uem.br/nou-rau/document/?code=vtls000205331
http://nourau.uem.br/nou-rau/document/?c...
). Ground color pale brown; dark-brown longitudinal band, from opercle to caudal peduncle. Some specimens with dark-brown stripes radiating ventrally and posteriorly from orbit through infraorbitals; ventral surface of dentary with light-beige stripes alternating with dark-brown stripes. Fins with dark-brown spots, sometimes forming dark-brown stripes parallel with fin base.
Maximum standard length. 293.0 mm.
Biological data. See Hoplias sp. 1 of Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system.
Remarks. Hoplias mbigua was identified as Hoplias sp. 1 by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Azpelicueta et al. (2015Azpelicueta MM, Benítez MF, Aichino DR, Mendez CMD. A new species of the genus Hoplias (Characiformes, Erythrinidae), a tararira from the lower Paraná River, in Misiones, Argentina. Acta Zool Lilloana. 2015; 59(1-2):71-82.) described the new species only from the lower río Paraná basin, in Argentina; however, the occurrence of H. mbigua in the upper rio Paraná floodplain has been reported since the submersion of the Sete Quedas Falls by the Itaipu Reservoir (e.g. Bertollo et al., 2000Bertollo LAC, Born GG, Dergam JA, Fenocchio AS, Moreira Filho O. A biodiversity approach in the Neotropical Erythrinidae fish, Hoplias malabaricus. Karyotypic survey, geographic distribution of cytotypes and cytotaxonomic considerations. Chromosome Res. 2000; 8(7):603-13.; Pazza, Júlio Júnior, 2003Pazza R, Júlio Júnior HF. Occurrence of three sympatric cytotypes of Hoplias malabaricus (Pisces, Erythrinidae) in the upper rio Paraná floodplain (Brazil). Cytologia. 2003; 68(2):159-63.; Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.; Júlio Júnior et al., 2009Júlio Júnior HF, Dei Tos C, Agostinho AA, Pavanelli CS. A massive invasion of fish species after eliminating a natural barrier in the upper rio Paraná basin. Neotrop Ichthyol . 2009; 7(4):709-18.; Bifi, 2013Bifi AG. Revisão taxonômica das espécies do grupo Hoplias malabaricus (Bloch, 1794) (Characiformes: Erythrinidae) da bacia do rio da Prata. [PhD Thesis on the Internet]. Maringá: Universidade Estadual de Maringá; 2013 [cited 2016 Dec 08]. Available from: Biblioteca digital Universidade Estadual de Maringá. Available from: Biblioteca digital Universidade Estadual de Maringá. http://nourau.uem.br/nou-rau/document/?code=vtls000205331
http://nourau.uem.br/nou-rau/document/?c...
). Additionally, Bifi (2013Bifi AG. Revisão taxonômica das espécies do grupo Hoplias malabaricus (Bloch, 1794) (Characiformes: Erythrinidae) da bacia do rio da Prata. [PhD Thesis on the Internet]. Maringá: Universidade Estadual de Maringá; 2013 [cited 2016 Dec 08]. Available from: Biblioteca digital Universidade Estadual de Maringá. Available from: Biblioteca digital Universidade Estadual de Maringá. http://nourau.uem.br/nou-rau/document/?code=vtls000205331
http://nourau.uem.br/nou-rau/document/?c...
), in a revisionary study of the Hoplias malabaricus (Bloch, 1794) species group from the río de la Plata basin, provided further useful diagnostic features for this species (identified as Hoplias sp. A). Hoplias mbigua is a non-native species from the upper rio Paraná, and its occurrence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls (Júlio Júnior et al., 2009Júlio Júnior HF, Dei Tos C, Agostinho AA, Pavanelli CS. A massive invasion of fish species after eliminating a natural barrier in the upper rio Paraná basin. Neotrop Ichthyol . 2009; 7(4):709-18.).
Hoplias misionera Rosso, Mabragaña, González-Castro, Delpiani, Avigliano, Schenone, Días de Astarloa, 2016Rosso JJ, Mabragaña E, González-Castro M, Delpiani MS, Avigliano E, Schenone N, Días de Astarloa JM. A new species of the Hoplias malabaricus species complex (Characiformes: Erythrinidae) from the La Plata River basin. Cybium . 2016: 40(3):199-208.
Body elongated; greatest depth contained 3.4 to 4.9, caudal peduncle depth 5.9 to 8.3 times in SL; head length 2.9 to 3.3, predorsal distance 1.8 to 2.1 and caudal peduncle length 7.1 to 10.0; snout length 3.4 to 4.9, horizontal orbital diameter 3.6 to 6.8 and least interorbital width 2.9 to 4.6 in HL. Mouth terminal; premaxilla with 10; dentary with 2 rows of teeth, outer row with 10 or 11 and inner row with 14-16; maxilla with 30-49 teeth. Lateral line with 39-43 pored scales; series between lateral lines with 11-13 scale rows. Dorsal fin with 14-16, pectoral fin with 11-14, pelvic fin with 8 or 9, anal fin with 9-11 and caudal fin with 19 rays (Bifi, 2013Bifi AG. Revisão taxonômica das espécies do grupo Hoplias malabaricus (Bloch, 1794) (Characiformes: Erythrinidae) da bacia do rio da Prata. [PhD Thesis on the Internet]. Maringá: Universidade Estadual de Maringá; 2013 [cited 2016 Dec 08]. Available from: Biblioteca digital Universidade Estadual de Maringá. Available from: Biblioteca digital Universidade Estadual de Maringá. http://nourau.uem.br/nou-rau/document/?code=vtls000205331
http://nourau.uem.br/nou-rau/document/?c...
; Rosso et al., 2016Rosso JJ, Mabragaña E, González-Castro M, Delpiani MS, Avigliano E, Schenone N, Días de Astarloa JM. A new species of the Hoplias malabaricus species complex (Characiformes: Erythrinidae) from the La Plata River basin. Cybium . 2016: 40(3):199-208.). Ground color pale brown; dark-brown longitudinal band, from opercle to caudal peduncle. Some specimens with dark-brown stripes radiating ventrally and posteriorly from orbit through infraorbitals; ventral surface of dentary with light-beige stripes alternating with dark-brown stripes. Fins dark-brown spots, forming dark-brown irregular stripes.
Maximum standard length. 290.5 mm.
Distribution. Río de la Plata system.
Remarks. Hoplias misionera has been captured in the upper rio Paraná floodplain since 2008 by the Nupélia staff. Additionally, Bifi (2013Bifi AG. Revisão taxonômica das espécies do grupo Hoplias malabaricus (Bloch, 1794) (Characiformes: Erythrinidae) da bacia do rio da Prata. [PhD Thesis on the Internet]. Maringá: Universidade Estadual de Maringá; 2013 [cited 2016 Dec 08]. Available from: Biblioteca digital Universidade Estadual de Maringá. Available from: Biblioteca digital Universidade Estadual de Maringá. http://nourau.uem.br/nou-rau/document/?code=vtls000205331
http://nourau.uem.br/nou-rau/document/?c...
), in a revisionary study of the H. malabaricus species group from the río de la Plata basin, provided further useful diagnostic features for this species (identified as Hoplias sp. D). Hoplias misionera is a non-native species from the upper rio Paraná, and its occurrence can be associated with the functioning of the Canal da Piracema (a fish ladder that connects the region downstream from the Itaipu Dam to the region upstream from the dam).
Hoplias sp. 2
Body elongated; greatest depth contained 4.4 to 4.8, caudal peduncle depth 7.6 to 8.0 times in SL; head length 3.2 to 3.4, predorsal distance 2.1 to 2.3 and caudal peduncle length 7.5 to 7.7 in SL; snout length 3.1 to 3.5, horizontal orbital diameter 5.6 to 6.2 and least interorbital width 3.1 to 3.5 in HL. Mouth terminal; premaxilla with 8-10; dentary with 30-33; maxilla with 31-36 teeth. Lateral line with 42-44 pored scales; series between lateral lines with 11 or 12 scale rows. Dorsal fin with 13, pectoral fin with 13 or 14, pelvic fin with 8, anal fin with 8-11 rays and caudal fin with 19 rays. Ground color pale brown; dark-brown longitudinal band, from opercle to caudal peduncle; several dark-brown irregular transverse bars. Fins with dark-brown spots, sometimes forming dark-brown stripes parallel with fin base (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 263.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná basin.
Hoplias sp. 3
Body elongated; greatest depth contained 4.7 to 4.8, caudal peduncle depth 7.8 to 9.0 times in SL; head length 3.2 to 3.3, predorsal distance 2.1 to 2.2 and caudal peduncle length 7.8 to 9.0 in SL; snout length 4.0 to 4.2, horizontal orbital diameter 5.1 to 5.4 and least interorbital width 4.0 to 4.2 in HL. Mouth terminal; premaxilla with 8-10; dentary with 30-35; maxilla with 32-36 teeth. Lateral line with 40-42 pored scales; series between lateral lines with 113 scale rows. Dorsal fin with 13, pectoral fin with 13 or 14, pelvic fin with 8, anal fin with 8-11 rays and caudal fin with 19 rays. Ground color pale brown; dark-brown longitudinal band, from opercle to caudal peduncle; several dark-brown irregular transverse bars. Fins with dark-brown spots, sometimes forming dark-brown stripes parallel with fin base (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 190.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río Paraná, rio Iguaçu and río Uruguay basins (Bifi, 2013Bifi AG. Revisão taxonômica das espécies do grupo Hoplias malabaricus (Bloch, 1794) (Characiformes: Erythrinidae) da bacia do rio da Prata. [PhD Thesis on the Internet]. Maringá: Universidade Estadual de Maringá; 2013 [cited 2016 Dec 08]. Available from: Biblioteca digital Universidade Estadual de Maringá. Available from: Biblioteca digital Universidade Estadual de Maringá. http://nourau.uem.br/nou-rau/document/?code=vtls000205331
http://nourau.uem.br/nou-rau/document/?c...
).
Hemiodontidae
Hemiodus
Hemiodus orthonops Eigenmann, Kennedy, 1903
Body elongated; greatest body depth contained 3.7 to 4.8 and caudal peduncle depth 10.0 to 12.8 times in SL; head length 3.6 to 4.6, predorsal distance 2.2to 2.4 and caudal peduncle length 6.0 to 7.2 in SL; snout length 3.0 to 3.6, horizontal orbital diameter 3.2 to 4.0 and least interorbital width 2.0 to 3.2 in HL. Mouth terminal; premaxilla with 9, dentary and maxilla with no teeth. Lateral line with 85-97 pored scales; transverse series above lateral line with 18-21 scale rows and below with 8-11 scale rows. Dorsal fin with 11 or 12, pectoral fin with 16-18, pelvic fin and anal fin with 10 or 11 and caudal fin with 19 rays. Ground color silvery; black rounded or oval, horizontally elongated, blotch approximately on vertical through end of dorsal fin. Hyaline fins, except caudal fin, with dark-brown oblique stripe on each lobe (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 230.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system.
Remarks. Hemiodus orthonops is a non-native species from the upper rio Paraná and its occurrence can be associated with the functioning of the Canal da Piracema (a fish ladder that connects the region downstream from the Itaipu Dam to the region upstream from the dam).
Lebiasinidae
Pyrrhulina
Pyrrhulina australis Eigenmann, Kennedy, 1903
Body elongated; greatest body depth contained 4.3 to 4.6 and caudal peduncle depth 7.6 to 10.0 times in SL; head length 3.7 to 4.1, predorsal distance 1.5 to 1.7 and caudal peduncle length 5.5 to 6.3 in SL; snout length 3.1 to 3.7, horizontal orbital diameter 2.6 to 2.9 and least interorbital width 2.6 to 2.7 in HL. Mouth terminal; inner row of premaxilla with 9-12, outer row with 4-6, inner row of dentary with 8-12, outer row with 4-5 and maxilla with 1 or 2 teeth. Longitudinal series with 20-25 scales; transverse series with 6-8 scale rows. Dorsal fin with 9 or 10, pectoral fin with 11 or 12, pelvic fin with 9 or 10, anal fin with 10 or 11 and caudal fin with 19 rays. Ground color pale brown; dark-brown longitunal stripe, from anterior portion of dentary, through orbit, to opercle. Yellowish fins; black irregular blotch on dorsal fin. Some specimens can present few or several black blotches on body (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 33.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río de la Plata and rio Guaporé basins.
a. Pyrrhulina australis, NUP 3345, 29.5 mm SL, lagoa do Bilunga, Taquarussu, State of Mato Grosso do Sul. b. Apareiodon affinis, 93.3 mm SL, uncat.c. Apareiodon piracicabae, 120.0 mm SL, uncat. d. Apareiodon vladii, 102.1 mm SL, uncat. e. Parodon nasus, 102.5 mm SL, fresh specimen, uncat. f. Prochilodus lineatus, 495.2 mm SL, fresh specimen, uncat.
Parodontidae
Apareiodon
1. Pre anus scales 29½ or more; premaxillary teeth with 12 to 15 cusps .................... A. affinis
1’. Pre anus scales 29 or less; premaxillary teeth with less than 12 cusps .................... 2
2. Premaxillary teeth with cusps gradually decreasing in size, giving a cutting edge almost straight, with only the corners rounded; maxilla with two (rarely one) teeth .................... A. piracicabae
2’. Premaxillary teeth pointed, with cusps abruptally decreasing in size from the central to laterals; maxilla with one tooth .................... A. vladii
Apareiodon affinis (Steindachner, 1879)
Body elongated; greatest body depth contained 3.2 to 5.4 and caudal peduncle depth 8.2 to 10.8 times in SL; head length 3.8 to 4.9, predorsal distance 1.9 to 2.2 and caudal peduncle length 7.0 to 12.1 in SL; snout length 2.6 to 3.6, horizontal orbital diameter 3.1 to 4.6, least interorbital width 2.3 to 3.4 and dentary width 3.7 to 5.9 in HL. Mouth subterminal; premaxilla with 4 or 5, maxilla with 2 or 3 and dentary with no teeth. Lateral line with 39-46 pored scales; transverse series above lateral line with 4½ or 5 scale rows and below with 3 or 4½ scale rows. Dorsal fin with 10-13, pectoral fin with 11-14, pelvic fin with 7-9, anal fin with 7 or 8 rays and caudal fin with 18 or 19 rays (Pavanelli, 1999Pavanelli CS. Revisão taxonômica da família Parodontidae (Ostariophysi: Characiformes). [PhD Thesis]. São Carlos, SP: Universidade Federal de São Carlos; 1999.). Ground color silvery to pale yellow; black longitudinal band along lateral line, from opercle to median caudal-fin rays, without adjacent brown or grey blotches downwards; six to eight dark-brown transverse bars above longitudinal band. Hyaline fins or with few scattered brown cromathophores (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 151.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system.
Apareiodon piracicabae (Eigenmann, 1907)
Body elongated; greatest body depth contained 3.4 to 5.2 and caudal peduncle depth 6.9 to 9.9 times in SL; head length 3.9 to 5.0, predorsal distance 2.0 to 2.2 and caudal peduncle length 7.3 to 13.6 in SL; snout length 2.7 to 3.4, horizontal orbital diameter 3.2 to 4.8, least interorbital width 2.3 to 3.4 and dentary width 3.7 to 5.9 in HL. Mouth subterminal; premaxilla with 4, maxilla with 1 or 2 and dentary with no teeth. Lateral line with 39-46 pored scales; transverse series above lateral line with 4½ or 5 scale rows and below with 3 or 4½ scale rows. Dorsal fin with 10-13, pectoral fin with 11-14, pelvic fin with 7-9, anal fin with 7 or 8 and caudal fin with 18 or 19 rays (Pavanelli, 1999Pavanelli CS. Revisão taxonômica da família Parodontidae (Ostariophysi: Characiformes). [PhD Thesis]. São Carlos, SP: Universidade Federal de São Carlos; 1999.). Ground color silvery to pale yellow; black longitudinal band along lateral line, from opercle to median caudal-fin rays, without adjacent brown or grey blotches downwards; six to eight dark-brown transverse bars above longitudinal band. Hyaline fins or with few scattered brown cromathophores (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 165.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná and rio São Francisco basins.
Apareiodon vladii Pavanelli, 2006
Body elongated; greatest body depth contained 3.9 to 4.7 and caudal peduncle depth 6.9 to 8.8 times in SL; head length 4.3 to 4.8, predorsal distance 2.0 to 2.2 and caudal peduncle length 6.3 to 9.0 in SL; snout length 2.7 to 3.1, horizontal orbital diameter 3.8 to 4.6 snout length 2.6 to 3.1, horizontal orbital diameter 3.8 to 4.6, least interorbital width 2.6 to 3.1 and dentary width 5.0 to 6.3 in HL. Mouth subterminal; premaxilla with 4, maxilla with 1 and dentary with no teeth. Lateral line with 37-39 pored scales; transverse series above lateral line with 4½ scale rows and below with 3 or 4½ scale rows. Dorsal fin with 11 or 12, pectoral fin with 14 or 15, pelvic fin with 8 or 9, anal fin with 7 and caudal fin with 19 rays (Pavanelli, 1999Pavanelli CS. Revisão taxonômica da família Parodontidae (Ostariophysi: Characiformes). [PhD Thesis]. São Carlos, SP: Universidade Federal de São Carlos; 1999.). Ground color silvery to pale yellow; black longitudinal band along lateral line, from opercle to median caudal-fin rays, without adjacent brown or grey blotches downwards; six to eight dark-brown transverse bars above longitudinal band. Hyaline fins or with few scattered brown cromathophores (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 113.4 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná, rio Piquiri and rio Ivaí basins.
Parodon
Parodon nasus Kner, 1859
Body elongated; greatest body depth contained 3.5 to 4.6 and caudal peduncle depth 7.0 to 8.5 times in SL; head length 4.2 to 5.0, predorsal distance 1.9 to 2.4 and caudal peduncle length 7.4 to 10.8 in SL; snout length 2.3 to 2.9, horizontal orbital diameter 3.4 to 4.9, least interorbital width 2.3 to 2.7 and dentary width 3.8 to 5.1 in HL. Mouth subterminal; premaxilla with 4, dentary with 2-4 and maxilla with 2 teeth. Lateral line with 35-39 pored scales; transverse series above lateral line with 4½ scale rows and below with 3 or 3½ scale rows. Dorsal fin with 11 or 12, pectoral fin with 13-16, pelvic fin with 8 or 9, anal fin with 8 or 9 and caudal fin with 19 rays (Pavanelli, 1999Pavanelli CS. Revisão taxonômica da família Parodontidae (Ostariophysi: Characiformes). [PhD Thesis]. São Carlos, SP: Universidade Federal de São Carlos; 1999.). Ground color pale brown dorsally and pale yellow ventrally; black longitudinal band along lateral line, from opercle to median caudal-fin rays, with projections upwards and downwards, conferring zigzag pattern. Hyaline or yellowish (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 117.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system.
Prochilodontidae
Prochilodus
Prochilodus lineatus (Valenciennes, 1836)
Body deep; greatest body depth contained 2.2 to 3.3 and caudal peduncle depth 10.7 to 14.6 times in SL; head length 2.7 to 4.3, predorsal distance 1.9 to 2.4 and caudal peduncle length 10.6 to 15.6 in SL; snout length 2.1 to 3.1, horizontal orbital diameter 3.3 to 6.6 and least interorbital width 1.7 to 2.1 in HL. Mouth terminal, with broad lips; upper lip with 95 teeth in the outer series, 13-25 in the inner series, lower lip with 75 teeth in the outer series, 9-10 in the inner series, maxilla toothless. Lateral line complete, with 44-50 pored scales; transverse series above lateral line with 7-10 scale rows and below with 6-9 scale rows. Dorsal fin with 12-13, pectoral fin with 14-19, pelvic fin with 8-9, anal fin with 10-12 and caudal fin with 19 rays (Castro, Vari, 2004bCastro RMC, Vari RP. Detritivores of South American fish family Prochilodontidae (Teleostei; Ostariophysi: Characiformes): a phylogenetic and revisionary study. Washington (DC): Smithsonian Institution Press; 2004b. (Smithsonian Contributions to Zoology; no. 622).). Ground color silvery, darker dorsally. Dorsal fin pale grey; pelvic fin reddish-yellow; remaining fins dark-gray (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 542.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system and rio Paraíba do Sul basin.
Serrasalmidae
Colossoma
Colossoma macropomum (Cuvier, 1818)
Body deep; greatest depth contained 1.8 to 1.9 and caudal peduncle depth 8.5 to 9.0 times in SL; head length 2.9 to 3.1, predorsal distance 1.5 to 1.7 and caudal peduncle length 8.5 to 9.1 in SL; snout length 3.1 to 3.4, horizontal orbital diameter 5.2 to 5.6 and least interorbital width 1.8 to 2.1 in HL. Mouth terminal; inner row of premaxilla with 2, outer row with 5; inner row of dentary with 3-4, outer with 6 teeth; maxilla toothless. Lateral line complete, with 73-88 pored scales; transverse series above lateral line with 44-50 scale rows and below with 45-50 scale rows. Ventral keel with 42-44 unpaired spines, followed by 6 pairs of spines. Dorsal fin with 14-16, pectoral fin with 12-15, pelvic fin with 8-9, anal fin with 22-25, and caudal fin with 19 rays (Parron, 2001Parron RYS. Caracterização morfológica das espécies de peixes cultivadas no Estado do Paraná, Brasil. [Monograph]. Maringá, PR: Universidade Estadual de Maringá; 2001.). Ground color brown, pale-yellowish abdominally; ventral portion of body (except abdomen) covered by large irregular patch of dark-grey pigmentation. Dark-grey fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 280.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río Orinoco and Amazon basins (introduced elsewhere).
Remarks. Colossoma macropomum is a non-native species from the upper rio Paraná and its occurrence can be associated with fish-farming.
a. Colossoma macropomum, 250.0 mm SL, uncat. b. Metynnis lippincottianus, NUP 443, 149.3 mm SL, lagoas (ilhas), Porto Rico, State of Paraná. c. Myloplus tiete, NUP 2484, 135.0 mm SL, rio Piquiri, Formosa do Oeste, State of Paraná. d. Piaractus mesopotamicus, 498.0 mm SL, fresh specimen, uncat. e. Serrasalmus maculatus, NUP 396, 157.2 mm SL, canal do Meio (ilha Porto Rico), Porto Rico, State of Paraná. f. Serrasalmus marginatus, NUP 439, 160.2 mm SL, lagoas (ilhas), Porto Rico, State of Paraná. g. Triportheus nematurus, 102.0 mm SL, fresh specimen, uncat.
Metynnis
Metynnis lippincottianus (Cope, 1870)
Body deep; greatest depth contained 1.3 to 1.5 and caudal peduncle depth 9.2 to 9.6 times in SL; head length 3.7 to 3.8, predorsal distance 1.4 to 1.6 and caudal peduncle length 17.4 to 20.0 in SL; snout length 2.6 to 3.6, horizontal orbital diameter 2.2 to 2.9 and least interorbital width 2.3 to 2.6 in HL. Mouth terminal; inner row of premaxilla with 4, outer row with 2; inner dentary row with 1, outer with 4; maxilla toothless. Lateral line complete, with 82-84 pored scales; transverse series above lateral line with 30-43 scale rows and below with 36-38 scale rows. Ventral keel with 30-33 simple spines, plus 3-5 slighlty bufurcate spines. Dorsal fin with 15-16, pectoral fin with 14-16, pelvic fin with 7, anal fin with 24-27, and caudal fin with 19 rays. Ground color silvery; black rounded humeral spot with diffuse limits; several deep-lying black spots over body; during reproductive period, several patches or stripes of red pigmentation appear mainly on ventral portion of body. Pectoral fin red, pelvic fin black in males and red in females; dorsal fin with black blotch and sparse patches of red pigmentation; anal fin hyaline; caudal fin hyaline, except for dark-grey margin (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 170.2 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Drainages of Venezuela and French Guiana, Amazon basin, drainages of Northeastern Brasil, rio São Francisco and upper rio Paraná basins (Ota, 2015Ota RR, Message HJ, Graça WJ, Pavanelli CS. Neotropical Siluriformes as a model for insights on determining biodiversity of animal groups. PLoS ONE [serial on the internet]. 2015 Jul 13 [cited 2016 Dec 08]; 10(7):e0132913. Available from: Available from: http://dx.doi.org/10.1371/journal.pone.0132913
http://dx.doi.org/10.1371/journal.pone.0...
).
Remarks. Metynnis lippincottianus is a possible non-native species from the upper rio Paraná, and its occurrence can be associated with the releasing of specimens for restocking, or with the aquarium trade (Ota, 2015Ota RR, Message HJ, Graça WJ, Pavanelli CS. Neotropical Siluriformes as a model for insights on determining biodiversity of animal groups. PLoS ONE [serial on the internet]. 2015 Jul 13 [cited 2016 Dec 08]; 10(7):e0132913. Available from: Available from: http://dx.doi.org/10.1371/journal.pone.0132913
http://dx.doi.org/10.1371/journal.pone.0...
).
Myloplus
Myloplus tiete (Eigenmann, Norris, 1900)
Body deep; greatest depth contained 1.2 to 1.6 and caudal peduncle depth 7.4 to 10.2 times in SL; head length 3.0 to 4.0, predorsal distance 1.5 to 1.7 and caudal peduncle length 10.9 to 12.0 in SL; snout length 2.6 to 3.4, horizontal orbital diameter 2.5 to 3.9 and least interorbital width 2.0 to 2.4 in HL. Mouth terminal; inner row of premaxilla with 4, outer row with 2; inner dentary row with 1, outer with 4; maxilla toothless. Lateral line complete, with 64-80 pored scales; transverse series above lateral line with 24-30 scale rows and below with 30-39 scale rows. Ventral keel with 43-45 simple spines, plus 6 pairs of spines. Dorsal fin with 24-27, pectoral fin with 14 or 15, pelvic fin with 9, anal fin with 31-38, and caudal fin with 19 rays. Ground color silvery. Dorsal, pectoral, pelvic and caudal fins hyaline; anal fin reddish with distal margin black (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 262.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system.
Piaractus
Piaractus mesopotamicus (Holmberg, 1887)
Body deep; greatest depth contained 1.5 to 2.0 and caudal peduncle depth 8.8 to 8.9 times in SL; head length 2.7 to 4.2, predorsal distance 1.6 to 1.7 and caudal peduncle length 8.8 to 9.4 in SL; snout length 2.9 to 3.3, horizontal orbital diameter 5.2 to 5.5 and least interorbital width 1.9 to 2.1 in HL. Mouth terminal; inner row of premaxilla with 2, outer row with 6-8; inner dentary row with 2, outer with 6; maxilla with 1 or 2 teeth. Lateral line complete, with 107-119 pored scales; transverse series above lateral line with 49-54 scale rows and below with 50-55 scale rows. Ventral keel with 52-54 simple spines, plus 7 pairs of spines. Dorsal fin with 15 or 16, pectoral fin with 14-17, pelvic fin with 8 or 9, anal fin with 23-25, and caudal fin with 19 rays. Ground color greyish, darker dorsally. Dorsal and pectoral fins dark-grey; pelvic anal and caudal fins yellowish-orange (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 533.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system.
Serrasalmus
1. Caudal-fin margin hyaline, preceded by a black transverse bar; dorsal profile of the head little concave .................... S. maculatus
1’. Caudal-fin margin black in variable length, sometimes extending up to the base of caudal fin; dorsal profile of the head very concave .................... S. marginatus
Serrasalmus maculatus Kner, 1858
Body deep; greatest depth contained 1.6 to 1.8 and caudal peduncle depth 8.2 to 9.7 times in SL; head length 2.8 to 3.2, predorsal distance 1.6 to 1.7 and caudal peduncle length 10.5 to 11.4 in SL; snout length 3.7 to 4.6, horizontal orbital diameter 3.5 to 4.5 and least interorbital width 2.6 to 3.0 in HL. Mouth terminal, dentary prognathous; premaxilla with 6, dentary with 7, maxilla with no teeth and palate with 4-7 teeth. Lateral line with 69-75 pored scales; transverse series above lateral line with 29-33 scale rows and below with 24-29 scale rows. Dorsal and pectoral fin with 15-17, pelvic fin with 6 or 7, anal fin with 30-33 and caudal fin with 19 rays (Jégu, Santos, 2001Jégu M, Santos GM. Mise au point à propos de Serrasalmus spilopleura Kner, 1858 et réhabilitation de S. maculatus Kner, 1858 (Characidae: Serrasalminae). Cybium. 2001; 25(2):119-43.). Ground color silvery to yellowish; several dark-brown rounded blotches on body. Yellowish fins; unpaired fins with distal margin black, except caudal fin, with hyaline margin, preceded by black transverse bar (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 268.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Amazon and Paraná-Paraguay system.
Serrasalmus marginatus Valenciennes, 1837
Body deep; greatest depth contained 1.8 to 1.9 and caudal peduncle depth 11.0 to 13.9 times in SL; head length 2.9 to 3.1, predorsal distance 1.7 to 1.8 and caudal peduncle length 13.9 to 14.6 in SL; snout length 4.2 to 4.4, horizontal orbital diameter 3.0 to 3.7 and least interorbital width 3.4 to 4.5 in HL. Mouth terminal, dentary prognathous; premaxilla with 6, dentary with 7, maxilla with no teeth and palate with 4-7 teeth. Lateral line with 74-79 pored scales; transverse series above and below lateral line with 26-30 scale rows. Dorsal and pectoral fin with 15-17, pelvic fin with 7, anal fin with 32-36 and caudal fin with 19 rays. Ground color silvery; several dark-brown rounded blotches on body. Caudal and anal fins completely or partially black, including thin hyaline distal margin; remaining fins hyaline or yellowish, with few scattered black spots (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 260.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system.
Remarks. Serrasalmus marginatus is a non-native species from the upper rio Paraná. Its occurrence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
Triportheidae
Triportheus
Triportheus nematurus (Kner, 1858)
Body deep; greatest body depth contained 2.7 to 3.2 and caudal peduncle depth 10.2 to 12.1 times in SL; head length 3.6 to 4.4, predorsal distance 1.6 to 1.8 and caudal peduncle length 9.3 to 14.7 in SL; snout length 3.9 to 5.0, horizontal orbital diameter 3.0 to 3.6 and least interorbital width 2.5 to 3.6 in HL. Mouth terminal; inner row of premaxilla with 6, outer row with 6 or 7, inner row of dentary with 1, outer row of dentary with 5 or 6 and maxilla up to 2 teeth. First gill arch with 43-48 gill rakers. Lateral line 33-37 pored scales; transverse series above lateral line with 6 scale rows and below with 2 or 3 scale rows. Dorsal fin with 11, pectoral fin with 11-13, pelvic fin with 7, anal fin with 28-34 and caudal fin with 19 rays (Malabarba, 2004Malabarba LR. Monophyly of the Cheirodontinae, characters and major clades (Ostariophysi: Characidae). In: Malabarba LR, Reis RE, Vari RP, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of Neotropical fishes. Porto Alegre: Edipucrs; 1998. p.193-233.). Ground color silvery; longitudinal series of dark-brown spots on flank scales above pectoral-fin origin; black blotch on posterior portion of caudal peduncle, extending to median caudal-fin rays (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 150.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system.
Remarks. Triportheus nematurus is a non-native species from the upper rio Paraná, and its occurrence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
GYMNOTIFORMES
Apteronotidae
Apteronotus
1. Body dark-brown, with two white transverse bars accross the tail, close to its extremity, the anterior broader, coincident with the posteriormost anal-fin rays, the posterior narrower, at the caudal-fin base; posterior margin of opercle white; and white stripe along the chin and the mid-dorsal line of head, frequently extending posterioly to about the middle of the mid-dorsal line of body .................... 2
1’. Body entirely brown, with no white marks .................... 3
2. Anterior white transverse bar of the tail immaculate .................... A. aff. albifrons
2’. Anterior white transverse bar of the tail marked with several irregular spots the same color as the body background .................... A. caudimaculosus
3. Dorsal scales large, transverse series above the lateral line with five to eight scale rows .................... A. ellisi
3’ Dorsal scales small, transverse series above the lateral line with 11 to 15 scale rows .................... A. acidops
Apteronotus acidops Triques, 2011Triques ML. Apteronotus acidops, new species of long snouted electric fish (Teleostei: Gymnotiformes: Apteronotidae) from the upper rio Paraná basin in Brazil, with a key to the apteronotid species from the area. Vertebr Zool . 2011; 61(3):299-306.
Body elongated and compressed; greatest depth contained 6.6 to 8.0 times in TL; head length 3.8 to 5.6, anal-fin base length 1.2, caudal-peduncle length 7.3 to 7.5, preanal distance 5.2 to 7.0 and prepectoral distance 4.2 to 5.2 in LEA; snout length 1.6 to 2.2, horizontal orbital diameter 12.5 to 13.2 and least interorbital width 6.2 in HL. Mouth terminal. Pectoral fin with 13-18 rays, anal fin with 162-220 rays and caudal fin with 17-20 rays; transverse series above lateral line with 11-15 scale rows (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.; Triques, 2011Triques ML. Apteronotus acidops, new species of long snouted electric fish (Teleostei: Gymnotiformes: Apteronotidae) from the upper rio Paraná basin in Brazil, with a key to the apteronotid species from the area. Vertebr Zool . 2011; 61(3):299-306.). Ground color dark-brown dorsally, and pale yellow ventrally; posterior portion of caudal peduncle and caudal-fin base with light-beige blotch. Caudal fin dark-brown.
Maximum total length. 325.0 mm.
Distribution. Upper rio Paraná basin.
Remarks. Apteronotus acidops was identified as Apteronotus sp. by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Triques (2011Triques ML. Apteronotus acidops, new species of long snouted electric fish (Teleostei: Gymnotiformes: Apteronotidae) from the upper rio Paraná basin in Brazil, with a key to the apteronotid species from the area. Vertebr Zool . 2011; 61(3):299-306.) described the new species from the upper rio Paraná basin. It is noteworthy, however, that not all specimens present the extremely elongate snout characteristic of A. acidops. R. Campos-da-Paz (in an e-mail, rcamposdapaz@gmail.com, August 2016) re-analyzed the specimen depicted by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.: 191; wrongly referenced as NUP 1750, in fact NUP 2701) and confirmed it as A. acidops. This specimen and another (NUP 2978) are typically long snouted. In contrast, some other specimens present morphometric values that are apparently intermediate between A. acidops and A. brasiliensis (see Triques, 2011Triques ML. Apteronotus acidops, new species of long snouted electric fish (Teleostei: Gymnotiformes: Apteronotidae) from the upper rio Paraná basin in Brazil, with a key to the apteronotid species from the area. Vertebr Zool . 2011; 61(3):299-306.). Rather than admitting the existence of both species in the upper rio Paraná floodplain, the range of some morphometric values in A. acidops is considered slightly wider than that reported by Triques (2011Triques ML. Apteronotus acidops, new species of long snouted electric fish (Teleostei: Gymnotiformes: Apteronotidae) from the upper rio Paraná basin in Brazil, with a key to the apteronotid species from the area. Vertebr Zool . 2011; 61(3):299-306.) and overlaps in part those of A. brasiliensis.
a. Apteronotus acidops , NUP 1750, 240.0 mm TL, Itaipu Reservoir, Guaíra, State of Paraná. b. Apteronotus aff. albifrons, 100.0 mm TL, uncat. c. Apteronotus cf. caudimaculosus, 260.0 mm TL, uncat. d. Apteronotus ellisi, 128.2 mm TL, uncat. e. Sternarchorhynchus britskii, 212.0 mm TL, uncat. f. Gymnotus inaequilabiatus, 238.0 mm TL, uncat. g. Gymnotus pantanal, NUP 13328, 138.6 mm SL, ribeirão Jacutinga, São Jorge do Ivaí, State of Paraná. h. Gymnotus paraguensis, 250.0 mm TL, uncat. i. Gymnotus sylvius, MZUSP 83538, paratype, 260.0 mm TL, rio Ribeira de Iguape, next to Miracatu, Miracatu, State of São Paulo.
Apteronotus aff. albifrons (Linnaeus, 1766)
Body elongated and compressed; greatest depth contained 5.5 to 6.2 times in TL; head length 5.5 to 6.0, anal-fin base length 1.2 to 1.5, caudal-peduncle length 5.0 to 6.6, preanal distance 6.1 to 7.5 and prepectoral distance 5.5 to 6.0 in LEA; snout length 2.7 to 4.7, horizontal orbital diameter 9.0 to 16.7 and least interorbital width 3.9 to 4.7 in HL. Mouth terminal. Pectoral fin with 15-16, anal fin with 170-192 and caudal fin with 17 to 20 rays. Transversal series above lateral line with 15-16 scale rows (Campo-da-Paz, 1997Campos-da-Paz R. Sistemática e taxonomia dos peixes elétricos das bacias dos rios Paraguai, Paraná e São Francisco, com notas sobre espécies presentes em rios costeiros do leste do Brasil (Teleostei: Ostariophysi: Gymnotiformes). [PhD Thesis]. São Paulo, SP: Universidade de São Paulo; 1997). Ground color dark-brown, with brownish-white middorsal stripe from tip of lower jaw to nape, continuing as narrower line until about middle of body (occasionally faded or discontinuous); region around branchial aperture, including isthmus, brownish-white; wide brownish-white transverse bar across tail and posterior portion of anal fin; narrower brownish-white transverse bar across posterior portion of caudal peduncle and caudal-fin base. Distal portion of caudal fin brownish white; fins otherwise dark-brown (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum total length. 150.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Drainages from Venezuela to Paraguay and río Paraná basin.
Apteronotus cf. caudimaculosus Santana, 2003
Body elongated and compressed; greatest depth contained 4.8 to 6.0 times in TL; head length 5.1 to 5.9, anal-fin base length 1.2 to 1.4, caudal-peduncle length 5.4 to 6.8, preanal distance 3.9 to 5.0 and prepectoral distance 7.0 to 7.9 in LEA; snout length 2.7 to 4.7, horizontal orbital diameter 9.0 to 16.7 and least interorbital width 3.9 to 4.7 in HL. Mouth terminal. Pectoral fin with 15-16, anal fin with 157-170 and caudal fin with 18-22 rays (Santana, 2003Santana CD. Apteronotus caudimaculosus n. sp. (Gymnotiformes: Apteronotidae), a sexually dimorphic black ghost knifefish from the Pantanal, western Brazil, with a note on the monophyly of the A. albifrons species complex. Zootaxa . 2003; 252(1):1-11.). Transversal series above lateral line with 11 to 14 scale rows. Ground color dark-brown, with brownish-white middorsal stripe from tip of lower jaw to nape, continuing as narrower line until about middle of body (occasionally faded or discontinuous); region around branchial aperture, including isthmus, brownish-white; wide brownish-white transverse bar across tail and posterior portion of anal fin, with irregular, dark-brown spots; narrower brownish-white transverse bar across posterior portion of caudal peduncle and caudal-fin base. Distal portion of caudal fin brownish white; fins otherwise dark-brown (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum total length. 280.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system.
Apteronotus ellisi (Arámburu, 1957)
Body elongated and compressed; greatest depth contained 5.8 to 6.2 times in TL; head length 5.9 to 6.5, anal-fin base length 1.1 to 1.2, caudal-peduncle length 6.2 to 7.7, preanal distance 5.8 to 7.2 and prepectoral distance 5.4 to 5.7 in LEA; snout length 2.6 to 3.0, horizontal orbital diameter 12.5 to 18.2 and least interorbital width 3.9 to 4.9 in HL. Mouth terminal. Pectoral fin with 16 or 17 rays, anal fin with 170 to 190 and caudal with 17 to 20 rays (Campos-da-Paz, 1997Campos-da-Paz R. Sistemática e taxonomia dos peixes elétricos das bacias dos rios Paraguai, Paraná e São Francisco, com notas sobre espécies presentes em rios costeiros do leste do Brasil (Teleostei: Ostariophysi: Gymnotiformes). [PhD Thesis]. São Paulo, SP: Universidade de São Paulo; 1997); transverse series above lateral linte with 5-8 scale rows. Ground color dark-brown dorsally; posterior portion of caudal peduncle and caudal-fin base with light-beige blotch. Caudal fin dark-brown.
Maximum total length. 240.0 mm.
Distribution. Rio Paraná-Paraguay basins.
Remarks. Apteronotus ellisi was identified as Porotergus ellisi by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Albert (2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Ann Arbor: University of Michigan Press; 2001. (Museum of Zoology, Miscellaneous Publications; no. 190).), in a systematic study of the American knifefishes, proposed a clade named Apteronotus sensu stricto and included A. ellisi.
Sternarchorhynchus britskii Campos-da-Paz, 2000
Body elongated and compressed; greatest depth contained 6.9 to 7.9 times in TL; head length 4.7 to 5.2, anal-fin base length 1.1, preanal distance 7.0 to 8.2 and prepectoral distance 4.6 to 5.1 in LEA; snout length (prolonged into rostrum) 1.5 to 1.6, horizontal orbital diameter 24.4 to 32.2 and least interorbital width 14.1 to 21.7 in HL. Mouth terminal. Pectoral fin with 14-15 and anal fin with 166 to 178 rays. Transversal series above lateral line with 11 to 13 scale rows (Campos-da-Paz, 2000Campos-Da-Paz R. On Sternarchorhynchus Castelnau: a South American eletric knifefish, with descriptions of two new species (Ostariophysi: Gymnotiformes: Apteronotidae). Copeia . 2000; (2):521-35.). Ground color brown, with middorsal light-brown stripe on head. Light-brown fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum total length. 212.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná basin.
Gymnotidae
Gymnotus
1. Body elongated (subcilindrical); light-beige transverse bars narrow and distant from each other along the side of the body .................... G. pantanal
1’. Body deep (knife-shaped); light-beige transverse bars narrow and close to each other along the side of the body .................... 2
2. Transversal series above lateral line with 11 or 12 scale rows (to the mid dorsum); two to seven light-beige transverse bars along the side of the body, some of them forming an inverted Y .................... G. paraguensis
2’. Transversal series above lateral line with 6 to 9 scale rows (to the mid dorsum); light-beige transverse bars along the side of the body not forming Y .................... 3
3. Head lenght contained 8.3 to 11.1 times in total length; interorbital contained 2.3 to 2.4 times in head length .................... G. inaequilabiatus
3’. Head lenght contained 7.1 to 8.2 times in total length; interorbital contained 2.6 to 2.7 times in head length .................... G. sylvius
Gymnotus inaequilabiatus (Valenciennes, 1839)
Body elongated and compressed; greatest depth contained 7.1 to 13.3 times in TL; head length 8.3 to 11.1, anal-fin base length 1.3 in LEA; snout length 2.9 to 3.3, horizontal orbital diameter 13.9 to 15.4 and least interorbital width 2.3 to 2.4 in HL. Mouth superior, dentary prognathous. Pectoral fin with 13-16 rays and anal fin with 170-260 rays; transverse series above lateral line with 6-8 scale rows. Ground color pale brown; body with light-beige transverse bars alternating with dark-brown transverse bars, visible in specimens with less than 250.0 mm TL; in larger specimens stripes can be broken or disappear, resulting in color pattern with dark-brown rounded or irregular blotches, especially on dorsal region of body; dark-brown bars wider than light-beige bars. Hyaline fins, with scattered dark-brown spots (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum total length. 600.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system.
Gymnotus pantanal Fernandes, Albert, Daniel-Silva, Lopes, Crampton, Almeida-Toledo, 2005
Body elongated and compressed; greatest depth contained 10.5 to 12.5 times in TL; head length 9.9 to 11.1, anal-fin base length 1.2 to 1.3 in LEA; snout length 2.7 to 2.9, horizontal orbital diameter 14.3 to 15.0 and least interorbital width 2.2 to 3.0 in HL. Mouth superior, dentary prognathous. Pectoral fin with 16-18 rays and anal fin with 256-270 rays; transverse series above lateral line with 7 or 8 scale rows. Ground color brown; body with seven to 25 light-beige transverse stripes, spaced apart. Hyaline fins or light-beige, with scattered dark-brown spots (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum total length. 200.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system, in Brazil and Paraguay, and río Capare-Mamoré, in Bolívia (Eschmeyer et al., 2017Eschmeyer WN, Fricke R, van der Laan R, editors. Catalog of fishes: genera, species, references [Internet]. San Francisco: California Academy of Science; 2017 [updated 2017 Jan 31; cited 2017 Feb 24]. Available from: Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
http://researcharchive.calacademy.org/re...
).
Remarks. Gymnotus pantanal is a non-native species from the upper rio Paraná, and its occurrence in the region can be associated with its introduction as a live bait by anglers, or with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
Gymnotus paraguensis Albert, Crampton, 2003
Body elongated and compressed; greatest depth contained 9.7 to 10.2 times in TL; head length 7.5 to 8.2, anal-fin base length 1.2 in LEA; snout length 2.8 to 3.0, horizontal orbital diameter 13.9 to 15.4 and least interorbital width 2.4 to 3.2 in HL. Mouth superior, dentary prognathous. Pectoral fin with 17-21 rays and anal fin with 260-270 rays; transverse series above lateral line with 11 or 12 scale rows. Ground color dark-brown; body with 23-26 light-beige oblique stripes, with well-defined margins; two to seven inverted Y-shaped stripes, or sometimes X-shaped, or sometimes interrupted on porterior half of body. Hyaline fins or light-beige, with scattered dark-brown spots (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum total length. 280.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system.
Remarks. Gymnotus paraguensis is a non-native species from the upper rio Paraná, and its occurrence in the region can be associated with its introduction as a live bait by anglers, or with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
Gymnotus sylvius Albert, Fernandes-Matioli, 1999Albert JS, Fernandes-Matioli FMC, Almeida-Toledo LF. New species of Gymnotus (Gymnotiformes, Teleostei) from southeastern Brazil: toward the deconstruction of Gymnotus carapo. Copeia. 1999; (2):410-21.
Body elongated and compressed; greatest depth contained 7.0 to 9.3 times in TL; head length 7.1 to 8.2, anal-fin base length 1.2 in LEA; snout length 3.0 to 3.3, horizontal orbital diameter 12,8 to 14.0 and least interorbital width 2.6 to 2.7 in HL. Mouth superior, dentary prognathous. Pectoral fin with 15 or 16 rays and anal fin with 220-228 rays; transverse series above lateral line with 8 or 9 scale rows (Albert et al., 1999Albert JS, Fernandes-Matioli FMC, Almeida-Toledo LF. New species of Gymnotus (Gymnotiformes, Teleostei) from southeastern Brazil: toward the deconstruction of Gymnotus carapo. Copeia. 1999; (2):410-21.). Ground color pale brown; body with light-beige transverse bars alternating with dark-brown transverse bars; light-beige bars wider than dark-brown bars. Anal fin with posterior portion light-beige (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum total length. 360.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Rio Ribeira de Iguape, rio Paraíba do Sul and rio Pardo basins.
Hypopomidae
Brachyhypopomus
Brachyhypopomus gauderio Giora, Malabarba, 2009Giora J, Malabarba LR. Brachyhypopomus gauderio, new species, a new example of underestimated species diversity of electric fishes in the southern South America (Gymnotiformes: Hypopomidae). Zootaxa . 2009; 2093:60-68.
Body elongated and compressed; greatest depth contained 5.8 to 8.1 times in TL; head length 7.6 to 8.6, anal-fin base length 1.2 to 1.4, caudal-peduncle length 2.0 to 3.1, preanal distance 3.9 to 5.0 and prepectoral distance 7.0 to 7.9 in LEA; snout length 2.4 to 4.3, horizontal orbital diameter 8.2 to 11.1 and least interorbital width 3.3 to 4.8 in HL. Mouth terminal. Pectoral fin with 14-17 rays and anal fin with 179-226 rays; transverse series above lateral line with 7-8 scale rows (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.; Giora, Malabarba, 2009Giora J, Malabarba LR. Brachyhypopomus gauderio, new species, a new example of underestimated species diversity of electric fishes in the southern South America (Gymnotiformes: Hypopomidae). Zootaxa . 2009; 2093:60-68.). Ground color yellowish to pale brown; dorsal region and lower-half of body with dark-brown transverse and irregular bars; anal fin hyaline, with dark-brown spots.
Maximum total length. 160.0 mm.
Distribution. Río de basin, Laguna dos Patos and rio Tramandaí drainages.
Remarks. Brachyhypopomus gauderio was identified as B. cf. pinnicaudatus by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Giora, Malabarba (2009Giora J, Malabarba LR. Brachyhypopomus gauderio, new species, a new example of underestimated species diversity of electric fishes in the southern South America (Gymnotiformes: Hypopomidae). Zootaxa . 2009; 2093:60-68.) described the new species from the Laguna dos Patos and rio Tramandaí drainages, río Paraguay and río Uruguay basins. Brachyhypopomus gauderio is a non-native species from the upper rio Paraná, and its occurrence in the region can be associated with its introduction as a live bait by anglers.
a.Brachyhypopomus gauderio , NUP 2510, 112.2, lagoa do Aurélio, Taquarussu, State of Mato Grosso do Sul. b. Gymnorhamphichthys britskii, NUP 3337, 115.0 mm TL, rio Baía, Taquarussu, State of Mato Grosso do Sul. c. Rhamphichthys hahni, 567.3 mm TL, uncat. d. Eigenmannia guairaca, NUP 16151, 112.3 mm TL, córrego Água Boa, tributary of the rio Iguatemi, Mundo Novo, Mato Grosso do Sul State. e. Eigenmannia trilineata, 109.2 mm TL, fresh specimen, uncat. f. Eigenmannia virescens, 205.0 mm TL, uncat. g. Sternopygus macrurus, NUP 2096, 280.0 mm TL, rio Paraná, Porto Rico, State of Paraná.
Rhamphichthyidae
Gymnorhamphichthys
Gymnorhamphichthys britskii Carvalho, Ramos,
Albert, 2011
Body elongated and compressed; greatest depth contained 16.6 to 17.9 times in TL; head length 8.5 to 8.7, anal-fin base length 1.4 to 1.6, caudal peduncle length 4.9 to 6.0, preanal distance 7.7 to 8.3 and prepectoral distance 7.8 to 8.2 in LEA; snout length 1.7 to 2.3, horizontal orbital diameter 11.5 to 17.5 and least interorbital width 8.9 to 14.9 in HL. Mouth subterminal; snout relatively short. Pectoral fin with 13-15 rays and anal fin with 141-172 rays (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.; Carvalho et al., 2011Carvalho TP, Ramos CS, Albert JS. A new species of Gymnorhamphichthys (Gymnotiformes: Rhamphichthyidae) from the Paraná-Paraguay basin. Copeia . 2011; (3):400-06.). Ground color pale yellow; three dark-brown inconspicuous longitudinal stripes on flank; dark-brown transverse bars on dorsolateral region. Hyaline fins.
Maximum total length. 180.0 mm.
Biological data. Lives in different habitats, from small streams to large size rivers (Carvalho et al., 2011Carvalho TP, Ramos CS, Albert JS. A new species of Gymnorhamphichthys (Gymnotiformes: Rhamphichthyidae) from the Paraná-Paraguay basin. Copeia . 2011; (3):400-06.).
Distribution. Río de La Plata basin.
Remarks. Gymnorhamphichthys britskii was identified as Gymnorhamphichthys sp. by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Carvalho et al. (2011Carvalho TP, Ramos CS, Albert JS. A new species of Gymnorhamphichthys (Gymnotiformes: Rhamphichthyidae) from the Paraná-Paraguay basin. Copeia . 2011; (3):400-06.) described the new species from the río de La Plata basin. Gymnorhamphichthys britskii has been sampled in the upper rio Paraná floodplain recently, with no anterior records in the basin. Therefore, this species is considered as non-native from the upper rio Paraná, and its occurrence in the region can be associated with its introduction as a live bait by anglers, or with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
Rhamphichthys
Rhamphichthys hahni (Meinken, 1937)
Body elongated and compressed; greatest depth contained 6.4 to 9.1 times in TL; head length 6.2 to 7.1, anal-fin base length 1.2, caudal-peduncle length 10.0 to 13.3 in LEA; snout length 1.9 to 2.1, horizontal orbital diameter 20.0 to 23.0 and least interorbital width 6.1 to 7.0 in HL. Mouth terminal; long snout. Pectoral fin with 17-20 rays and anal fin with 337-400 rays; transverse series above lateral line with 20-22 scale rows. Ground color pale brown; dark-brown transverse and irregular bars and blotches. Fins with dark-brown spots, sometimes forming longitudinal irregular stripes (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum total length. 800.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Rio Paraná basin.
Remarks. Rhamphichthys hahni has been recently captured in the upper rio Paraná floodplain, with no anterior records in the basin. Therefore, this species is considered as non-native from the upper rio Paraná, and its occurrence in the region can be associated with its introduction as a live bait by anglers, or with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
Sternopygidae
Eigenmannia
1. Two inconspicuous dark-brown longitudinal stripes on the flank .................... E. virescens
1’. Three or four conspicuous dark-brown longitudinal stripes on the flank .................... 2
2. Premaxilla with nine or 10 teeth distributed in two rows; pectoral fin with ii,11 or 12 rays and anal fin with 151 to 170 rays .................... E. guairaca
2’. Premaxilla with 31 to 33 teeth distributed in four rows; pectoral fin with ii,14 or 15 rays and anal fin with 176 to 217 rays .................... E. trilineata
Eigenmannia guairaca Peixoto, Dutra, Wosiacki, 2015Peixoto LAW, Dutra GM, Wosiacki WB. The electric glass knifefishes of the Eigenmannia trilineata species-group (Gymnotiformes: Sternopygidae): monophyly and description of seven new species. Zool J Linn Soc . 2015; 175(2):384-414.
Body elongated and compressed; greatest body depth contained 5.9 to 7.0 times in TL; head length 6.5 to 8.3, anal-fin base length 0.7 to 1.2, caudal filament length 2.8 to 4.5, preanal distance 5.0 to 6.0 and prepectoral distance 6.1 to 7.7 in LEA; snout length 3.8 to 4.9, horizontal orbital diameter 6.7 to 8.8 and least interorbital width 2.7 to 3.5 in HL. Mouth terminal; premaxilla with 9 or 10 teeth distributed in two rows. Pectoral fin with 12 or 13 rays and anal fin with 151-170 rays. Lateral line complete, with 110-143 pored scales; transverse series above lateral line with 9-11 scale rows. Ground color pale brown; four dark-brown longitudinal stripes on flank (one superior medial, one lateral line, one inferior medial and one at anal-fin base).
Maximum total length. 173.0 mm TL.
Distribution. Tributaries of the rio Iguatemi, and riacho Água do Ó, tributary of rio Paranapanema, upper rio Paraná basin.
Remarks. Peixoto et al. (2015Peixoto LAW, Dutra GM, Wosiacki WB. The electric glass knifefishes of the Eigenmannia trilineata species-group (Gymnotiformes: Sternopygidae): monophyly and description of seven new species. Zool J Linn Soc . 2015; 175(2):384-414.) reviewed the Eigenmannia trilineata species-group, described the new species, E. guaiaraca, from the riacho Água do Ó, tributary of the rio Paranapanema, upper rio Paraná basin, and restricted the distribution of E. trilineata to the lower rio Paraná basin. Analyzing additional material hosted at Coleção Ictiológica do Nupélia, specimens similar to E. guairaca were found in tributaries of the rio Iguatemi, right bank of the upper rio Paraná, and specimens similar to those redescribed as E. trilineata by Peixoto et al. (2015Peixoto LAW, Dutra GM, Wosiacki WB. The electric glass knifefishes of the Eigenmannia trilineata species-group (Gymnotiformes: Sternopygidae): monophyly and description of seven new species. Zool J Linn Soc . 2015; 175(2):384-414.) in the upper rio Paraná floodplain and some of its tributaries (e.g. rio Paracaí). Therefore, the occurrence of both species in the studied region has been recorded.
Eigenmannia trilineata López, Castello, 1966
Body elongated and compressed; greatest body depth contained 4.8 to 5.6 times in TL; head length 7.4 to 9.0, anal-fin base length 1.1 to 1.2, caudal filament length 2.8 to 4.0, preanal distance 5.9 to 7.8 and prepectoral distance 7.0 to 9.1 in LEA; snout length 2.5 to 3.5, horizontal orbital diameter 4.0 to 5.2 and least interorbital width 2.0 to 2.6 in HL. Mouth terminal. Pectoral fin with 16-18 and anal fin with 197-230 rays. Lateral line complete, with 110-122 pored scales. Transversal series above lateral line with 15-16 scale rows (Campos-da-paz, 1997Campos-da-Paz R. Sistemática e taxonomia dos peixes elétricos das bacias dos rios Paraguai, Paraná e São Francisco, com notas sobre espécies presentes em rios costeiros do leste do Brasil (Teleostei: Ostariophysi: Gymnotiformes). [PhD Thesis]. São Paulo, SP: Universidade de São Paulo; 1997). Ground color pale brown; four dark-brown longitudinal stripes on flank (one superior medial, one on lateral line, one inferior medial and one at anal-fin base) (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum total length. 265.0 mm TL (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río Paraná basin.
Eigenmannia virescens (Valenciennes, 1836)
Body elongated and compressed; greatest body depth contained 5.7 to 7.0 times in TL; head length 6.4 to 8.3, anal-fin base length 1.1 to 1.2, caudal filament length 2.6 to 4.9, preanal distance 5.3 to 6.4 and prepectoral distance 6.4 to 9.3 in LEA; snout length 3.1 to 4.4, horizontal orbital diameter 5.4 to 7.3 and least interorbital width 2.9 to 4.0 in HL. Mouth terminal. Pectoral fin with 14 or 17 and anal fin with 186-245 rays. Lateral line complete, with 150-162 pored scales; transverse series above lateral line with 15-16 scale rows (Campos-da-paz, 1997Campos-da-Paz R. Sistemática e taxonomia dos peixes elétricos das bacias dos rios Paraguai, Paraná e São Francisco, com notas sobre espécies presentes em rios costeiros do leste do Brasil (Teleostei: Ostariophysi: Gymnotiformes). [PhD Thesis]. São Paulo, SP: Universidade de São Paulo; 1997). Ground color pale brown; two dark-brown longitudinal stripes on flank (one on lateral line, one ventral to it) (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum total length. 320.0 mm TL (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río Orinoco and río de la Plata basins.
Sternopygus
Sternopygus macrurus (Bloch, Schneider, 1801)
Body elongated and compressed; greatest body depth contained 7.9 to 10.0 times in TL; head length 8.6 to 9.6, anal-fin base length 1.1 to 1.2, caudal filament length 2.6 to 4.9, preanal distance 6.8 to 7.6 and prepectoral distance 6.6 to 7.4 in LEA; snout length 2.6 to 3.0, horizontal orbital diameter 10.7 to 12.0 and least interorbital width 3.3 to 3.9 in HL. Mouth terminal. Pectoral fin with 15 or 17 and anal fin with 221-247 rays (Campos-da-paz, 1997Campos-da-Paz R. Sistemática e taxonomia dos peixes elétricos das bacias dos rios Paraguai, Paraná e São Francisco, com notas sobre espécies presentes em rios costeiros do leste do Brasil (Teleostei: Ostariophysi: Gymnotiformes). [PhD Thesis]. São Paulo, SP: Universidade de São Paulo; 1997). Lateral line complete, with 193-269 pored scales. Transversal series above lateral line with 20-22 scale rows. Ground color and fin rays greyish-brown, inter-radial membranes hyaline; dark-brown humeral spot, occasionally inconspicuous (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum total length. 550.0 mm TL (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Amazon and río de La Plata basin.
SILURIFORMES
Aspredinidae
Amaralia
Amaralia oviraptor Friel, Carvalho, 2016Friel JP, Carvalho TP. A new species of Amaralia Fowler (Siluriformes: Aspredinidae) from the Paraná-Paraguay River basin. Zootaxa . 2016; 4088(4):531-46.
Body depressed anteriorly, compressed posteriorly; greatest depth contained 6.5 to 10, caudal peduncle depth 9.1 to 10.5 and body width 4.0 to 4.5 times in SL; snout length 3.1 to 4.3, horizontal orbital diameter 7.8 to 14.3 and least interorbital width 2.6 to 3.9 in HL. Mouth subterminal. Dorsal fin with I,2 rays, pectoral fin with I,5 or 6 rays, pelvic fin with 6 rays and anal fin with 5 or 6 rays (Friel, Carvalho, 2016Friel JP, Carvalho TP. A new species of Amaralia Fowler (Siluriformes: Aspredinidae) from the Paraná-Paraguay River basin. Zootaxa . 2016; 4088(4):531-46.). Ground color brown; series of light-beige spots on distal portions of tubercles; fins dark-brown with light-beige distal margins.
Maximum standard length. 122.8 mm.
Biological data. Lives in the bottom of lagoons, and feeds both on eggs and developing embryos of other catfishes (Friel, 1994Friel JP. A phylogenetic study of the Neotropical banjo catfishes (Teleostei: Siluriformes: Aspredinidae). [PhD Thesis]. Durham, North Carolina: Duke University; 1994.; 2003Friel JP. Family Aspredinidae. In: Reis RE, Kullander SO, Ferraris CJ Jr., organizers. Check list of the freshwater fishes of South and Central America. Porto Alegre: Edipucs ; 2003. p.261-267.).
Distribution. Paraná-Paraguay system.
Remarks. Amaralia oviraptor was identified as Amaralia sp. by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Friel, Carvalho (2016Friel JP, Carvalho TP. A new species of Amaralia Fowler (Siluriformes: Aspredinidae) from the Paraná-Paraguay River basin. Zootaxa . 2016; 4088(4):531-46.) described the new species from the Paraná-Paraguay system.
a. Amaralia oviraptor, NUP 124, 87.1 mm SL, Itaipu Reservoir, Marechal Cândido Rondon, State of Paraná. b. Ageneiosus inermis, NUP 3161, 212.0 mm SL, male, baia Sinhá Mariana, Barão de Melgaço, State of Mato Grosso. c. Ageneiosus militaris, NUP 537, 202.0 mm SL, male, rio Ivinheima, Taquarussu, State of Mato Grosso do Sul. d. Ageneiosus ucayalensis, NUP 533, 290.0 mm SL, male, rio Iguatemi, Mundo Novo, State of Mato Grosso do Sul. e. Auchenipterus osteomystax, NUP 2627, 191.0 mm SL, male, Itaipu Reservoir, Stanta Helena, State of Paraná. f. Parauchenipterus galeatus, NUP 3302, 165.0 mm SL, ressaco do Pau Veio (ilha Porto Rico), Porto Rico, State of Paraná. g. Tatia neivai, 60.0 mm SL, male, uncat. h. Trachelyopterus sp., NUP 1885, 98.2 mm SL, rio Iguatemi, Mundo Novo, State of Mato Grosso do Sul.
Auchenipteridae
Ageneiosus
1. Caudal fin truncate; body deep, its depth contained 4.5 to 5 times in standard length .................... A. inermis
1’. Caudal fin bifurcate; body elongated, its depth contained 5.7 to 7.5 times in standard length .................... 2
2. Dorsum with a black longitudinal band, sometimes interrupted by light-beige irregular blotches; mouth cleft inverted U-shaped in ventral view .................... A. militaris
2’. Dorsum uniformly dark-grey; mouth cleft inverted V-shaped in ventral view .................... A. ucayalensis
Ageneiosus inermis (Linnaeus, 1766)
Body deep; greatest body depth contained 4.5 to 5.0 times in SL; head length 3.1 to 3.6, anal-fin base length 3.3 to 3.9 in SL; snout length 1.5 to 1.8, horizontal orbital diameter 12.5 to 15.1, least interorbital width 6.2 to 7.5 in HL; and orbital diameter 6.7 to 7.3 in interorbital width. Mouth terminal with dentigerous tooth plates in both premaxilla and dentary. Dorsal fin with I,6, pectoral fin with I,14-15, pelvic fin with I,7 and anal fin with 31-35 rays. Ground color greyish dorsally, whitish ventrally. Orange or yellowish fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 332.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Widespread in South America rivers.
Remarks. Ageneiosus inermis is a non-native species from the upper rio Paraná, and its occurrence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
Ageneiosus militaris Valenciennes, 1836
Body elongated; greatest body depth contained 6.2 to 6.6 times in SL; head length 3.2 to 4.1, anal-fin base length 3.1 to 3.3 in SL; snout length 1.8 to 2.0, horizontal orbital diameter 9.4 to 11.2, least interorbital width 4.2 to 4.7 in HL; and orbital diameter 3.7 to 4.9 in interorbital width. Mouth terminal with dentigerous tooth plates in both premaxilla and dentary. Dorsal fin with I,6, pectoral fin with I,14-15, pelvic fin with I,7 and anal fin with 32-39 rays. Ground color greyish; dorsum with black longitudinal band, sometimes interrupted by light-beige irregular blotches. Light fins; distal margin of caudal fin black (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 332.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río de la Plata basin.
Ageneiosus ucayalensis Castelnau, 1855
Body elongated; greatest body depth contained 5.7 to 7.5 times in SL; head length 3.3 to 3.9, anal-fin base length 2.9 to 3.4 in SL; snout length 1.8 to 1.9, horizontal orbital diameter 9.5 to 12.9, least interorbital width 4.9 to 6.5 in HL; and orbital diameter 3.8 to 5.5 in interorbital width. Mouth terminal with dentigerous tooth plates in both premaxilla and dentary. Dorsal fin with I,6, pectoral fin with I,14-15, pelvic fin with I,7 and anal fin with 35-39 rays. Ground color greyish dorsally. Yellowish fins; distal margin of caudal fin black (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 332.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Amazon, lower rio Tocantins and río Corantijn basins and Paraná-Paraguay system (Eschmeyer et al., 2017Eschmeyer WN, Fricke R, van der Laan R, editors. Catalog of fishes: genera, species, references [Internet]. San Francisco: California Academy of Science; 2017 [updated 2017 Jan 31; cited 2017 Feb 24]. Available from: Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
http://researcharchive.calacademy.org/re...
).
Remarks. Ageneiosus ucayalensis is a non-native species from the upper rio Paraná its occurrence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
Auchenipterus
Auchenipterus osteomystax (Miranda-Ribeiro, 1918)
Body elongated; greatest body depth contained 4.0 to 5.9 times in SL; head length 4.5 to 4.8, anal-fin base length 2.2 to 2.5 in SL; snout length 2.3 to 2.6, horizontal orbital diameter 3.2 to 3.5, least interorbital width 2.0 to 2.7 in HL; and orbital diameter 1.7 to 2.3 in interorbital width. Mouth terminal; premaxilla and dentary with several rows of thin teeth. Dorsal fin with 7, pectoral fin with 12 or 13, pelvic fin with 13 or 14 and anal fin with 42-51 rays (Ferraris, Jr., Vari, 1999Ferraris CJ Jr., Vari RP. The South American catfish genus Auchenipterus Valenciennes, 1840 (Ostariophysi: Siluriformes: Auchenipteridae): monophyly and relationships, with a revisionary study. Zool J Linn Soc. 1999; 126(4):387-450.). Ground color greyish dorsally, whitish ventrally. Dorsal, pelvic and anal fins hyaline; pectoral and caudal fins with distal margins black (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 285.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río de la Plata, rio Tocantins and lower rio Amazonas basins.
Remarks. Auchenipterus osteomystax is a non-native species from the upper rio Paraná its occurrence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
Parauchenipterus
Parauchenipterus galeatus (Linnaeus, 1766)
Body deep; greatest body depth contained 3.5 to 3.9 times in SL; head length 4.5 to 4.8, anal-fin base length 2.2 to 2.5 in SL; snout length 2.5 to 2.9, horizontal orbital diameter 4.8 to 5.2, least interorbital width 1.6 to 1.9 in HL. Mouth terminal with dentigerous tooth plates in both premaxilla and dentary. Dorsal fin with I,6, pectoral fin with I,7, pelvic fin with 7 or 8 and anal fin with 21-24 rays. Adipose fin present. Ground color pale yellow to orange with several dark-brown irregular blotches as well as fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 285.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Widespread in South America rivers.
Tatia
Tatia neivai (Ihering, 1930)
Body elongated; greatest body depth contained 4.5 to 5.2 times in SL; head length 3.6 to 4.4 in SL; snout length 2.5 to 2.9, horizontal orbital diameter 4.8 to 5.2 and least interorbital width 1.7 to 2.0 in HL. Mouth terminal with dentigerous tooth plates in both premaxilla and dentary. Dorsal and pectoral fins with I,4 or 5, pelvic fin with 7 or 8 and anal fin with 7-11 rays. Ground color dark-brown with several light-beige horizontally elongated spots, as well as fins, especially unpaired fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 68.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper río Paraguay and upper rio Paraná basins.
Trachelyopterus
Trachelyopterus sp.
Fig. 18
Body deep; greatest body depth contained 4.0 to 4.3 times in SL; head length 4.2 to 4.5, anal-fin base length 2.9 to 3.1 in SL; snout length 3.6 to 3.7, horizontal orbital diameter 6.2 to 6.4 and least interorbital width 1.6 to 1.8 in HL. Mouth terminal with dentigerous tooth plates in both premaxilla and dentary. Dorsal fin with I,6, pectoral fin with I,7, pelvic fin with 8 and anal fin with 21-25 rays. Adipose fin absent. Ground color pale yellow to orange with several dark-brown irregular blotches as well as fins. Adipose fin absent (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 128.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná floodplain.
Callichthyidae
Callichthys
Callichthys callichthys (Linnaeus, 1758)
Body elongated; greatest depth contained 4.5 to 5.0 and caudal peduncle depth 6.5 to 6.7 times in SL; head length 4.3 to 4.5, predorsal distance 2.6 to 2.7 and maxillary-barbel length 3.9 to 4.1 in SL; snout length 2.2 to 2.4, horizontal orbital diameter 9.4 to 10.1 and least interorbital width 1.4 to 1.6 in HL. Mouth terminal. Lateral line with 4 pores on upper series of plates. Dorsolateral series with 26-29 and ventrolateral series with 24-27 plates. Dorsal fin with I,7 or 8, pectoral with I,6, pelvic and anal fins with 6 rays. Ground color pale brown to dark-grey. Dark-grey fins with black spots (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 150.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Widespread in South America rivers.
a. Callichthys callichthys, MZUSP 60261, 120.0 mm SL, córrego da Onça, afluente do rio Pardo, Barra do Turvo, State of São Paulo. b. Corydoras aeneus, NUP 17578, 43.1 mm SL, córrego Guaçu, tributary of the rio Abambai, Itaquirai, State of Mato Grosso do Sul. c. Corydoras sp., NUP 16185, 33.4 mm SL, córrego Santa Maria, tributary of the rio Iguatemi, Mundo Novo, State of Mato Grosso do Sul. d. Hoplosternum littorale, NUP 161, 133.4 mm SL, lagoa Mutum, Porto Rico, State of Paraná. e. Lepthoplosternum pectorale, 100.0 mm SL, uncat. f. Cetopsis gobioides, 102.4 mm SL, uncat. g. Clarias gariepinus, NUP 11900, 329.0 mm SL, rio Lopei (Pesque-Pague Big Peixes), tributary of the rio Toledo, Cascavel, State of Paraná.
Corydoras
1. Body with a large black or dark-grey regular spot in the anterior region of the flank .................... C. aeneus
1’. Body with six dark-brown blotches on mid-line of the flank .................... Corydoras sp.
Corydoras aeneus (Gill, 1858)
Body deep and elliptical; greatest depth contained 2.0 to 2.5 and caudal peduncle depth 7.8 to 8.4 times in SL; head length 3.1 to 3.8, predorsal distance 2.1 to 2.3 and maxillary-barbel length 5.3 to 5.5 in SL; snout length 1.8 to 1.9, horizontal orbital diameter 3.6 to 4.2 and least interorbital width 1.6 to 2.3 in HL. Mouth inferior. Lateral line with 4-6 pores on upper series of plates. Dorsolateral series with 20-23 and ventrolateral series with 19-22 plates. Dorsal fin with I,7 or 8, pectoral with I,8-10, pelvic fin with 6 and anal fin with 6-8 rays. Ground color dark-grey dorsally and yellowish ventrally; large black or dargrey regular spot in anterior region of flank. Grey fins, without spots (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 52.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Drainages from Colombia to the río de la Plata basin.
Corydoras sp.
Body deep and elliptical; greatest depth contained 3.0 and caudal peduncle depth 6.8 times in SL; head length 3.1, predorsal distance 6.5 and maxillary-barbel length 6.1 in SL; snout length 1.9, horizontal orbital diameter 3.8 and least interorbital width 3.0 in HL. Mouth inferior. Lateral line with 4 pores on upper series of plates. Dorsolateral series with 24 plates and ventrolateral series with 21 plates. Dorsal fin with II,8, pectoral with I,8, pelvic and anal fins with 6 rays. Ground color yellow to pale brown; six dark-brown spots on mid-line of flank; ventrolateral plates dark-grey posteriorly to pelvic fin; middle portion of caudal-fin base with dark-brown spot.
Maximum standard length. 31.8 mm.
Distribution. Only known from the Córrego Santa Maria, tributary of the rio Iguatemi, right bank of the upper rio Paraná.
Remarks. Only two specimens of Corydoras sp. were captured in a tributary of the rio Iguatemi, in 2008 by V. F. B. Silva and collaborators. According to L. F. C. Tencatt (oral communication, 2011) this species is apparently new to science.
Hoplosternum
Hoplosternum littorale (Hancock, 1828)
Body elongated; greatest depth contained 2.8 to 3.5 and caudal peduncle depth 8.8 to 9.4 times in SL; head length 2.9 to 3.8, predorsal distance 2.0 to 2.3 and maxillary-barbel length 1.5 to 3.2 in SL; snout length 1.9 to 2.3, horizontal orbital diameter 4.7 to 7.0 and least interorbital width 1.5 to 1.8 in HL. Mouth terminal. Lateral line with 4-6 pores on upper series of plates. Dorsolateral series with 25-27 and ventrolateral series with 22-24 plates. Dorsal fin with I,7, pectoral with I,9 or 10, pelvic fin with 6 and anal fin with 6-8 rays. Ground color dark-grey, lighter ventrally. Dark-grey fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 220.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Widespread in South America rivers.
Lepthoplosternum
Lepthoplosternum pectorale (Boulenger, 1895)
Body elongated; greatest depth contained 3.1 to 3.6 and caudal peduncle depth 8.9 to 9.5 times in SL; head length 3.3 to 3.8, predorsal distance 2.0 to 2.3 and maxillary-barbel length 1.5 to 2.2 in SL; snout length 1.9 to 2.2, horizontal orbital diameter 4.7 to 5.7 and least interorbital width 1.4 to 1.7 in HL. Mouth terminal. Lateral line with 4-6 pores on upper series of plates. Dorsolateral series with 25-26 and ventrolateral series with 22-23 plates. Dorsal fin with I,7, pectoral with I,9 or 10, pelvic fin with 6 and anal fin with 8 rays. Ground color dark-grey to pale brown; several dark-brown spots on body. Dark-grey fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 140.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río Paraguay basin and upper rio Paraná floodplain.
Remarks. Lepthoplosternum pectorale is a non-native species from the upper rio Paraná basin, and its occurrence can be associated with the functioning of the Canal da Piracema, a fish ladder that connects the region downstream from the Itaipu Dam to the region upstream from the dam.
Cetopsidae
Cetopsis
Cetopsis gobioides Kner, 1858
Body elongated; greatest depth contained 3.7 to 3.9, head depth 4.9 to 5.0 and caudal peduncle depth 8.4 to 9.6 times in SL; head length 4.1 to 4.4, anal-fin base length 3.6 to 3.8, maxillary-barbel length 11.1 to 11.4 in SL; snout length 3.1 to 3.5, horizontal orbital diameter 7.8 to 8.6 and least interorbital width 2.4 to 2.8 in HL. Mouth terminal; premaxilla and dentary with viliform teeth, maxilla with no teeth and vomer with one row of conical teeth. Dorsal fin with 7, pectoral fin with 10 or 11, pelvic fin with 10 and anal fin with 21-24 rays. Ground color darker dorsally, lighter ventrally; dorsum with few scattered dark-grey chromatophores. Hyaline fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 112.4 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio São Francisco, río Paraná and río Uruguay basins.
Clariidae
Clarias
Clarias gariepinus (Burchell, 1822)
Body elongated; greatest depth contained 5.5 to 9.3 and caudal peduncle depth 9.9 to 16.1 times in SL; head length 2.9 to 3.8, anal-fin base length 2.1 to 2.7, maxillary-barbel length 1.6 to 6.5 in SL; snout length 3.7 to 5.7, horizontal orbital diameter 7.6 to 19.2, least interorbital width 2.2 to 2.7 in HL. Mouth terminal with dentigerous tooth plates in both premaxilla, vomer and dentary. Dorsal fin with 61-79, pectoral fin with I,9-12, pelvic fin with 6 and anal fin with 45-60 rays (Hanssens, 2009Hanssens M. A review of the Clarias species (Pisces; Siluriformes) from the lower Congo and the Pool Malebo. J Afrotrop Zool. 2009; 5:27-40.). Ground color grey to yellowish, abdominal region white.
Maximum standard length. 700.0 mm.
Biological data. Lives in lakes, rivers and seasonally swampy areas (Winemiller, Kelso-Winemiller, 1996Winemiller KO, Kelso-Winemiller LC. Comparative ecology of catfishes of the upper Zambezi River floodplain. J Fish Biol . 1996; 49(6):1043-61.). Feeds on plant material, plankton, arthropods, mollusks, fish, reptiles, and amphibians (Yalçin et al., 2001Yalçin S, Akyurt I, Solak K. Stomach contents of the catfish (Clarias gariepinus Burchell, 1822) in the River Asi (Turkey). Turk J Zool. 2001; 25:461-68.).
Distribution. Africa and Asia Minor; introduced elsewhere (Eschmeyer et al., 2017Eschmeyer WN, Fricke R, van der Laan R, editors. Catalog of fishes: genera, species, references [Internet]. San Francisco: California Academy of Science; 2017 [updated 2017 Jan 31; cited 2017 Feb 24]. Available from: Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
http://researcharchive.calacademy.org/re...
).
Remarks. Clarias gariepinus, native of Africa, has been captured in the upper rio Paraná floodplain since 2005 by Nupélia staff. Its occurrence in the region can be associated with fish-farming and escapes from recreational angling ponds.
Doradidae
Ossancora
Ossancora eigenmanni (Boulenger, 1895)
Body deep; greatest body depth contained 2.8 to 3.8, head length 3.4 to 4.0 times in SL; snout length contained 1.9 to 2.1, horizontal orbital diameter 2.6 to 4.9 and least interorbital width 2.6 to 3.1 times in HL; orbital diameter contained 1.3 to 1.7 times in interorbital width. Mouth terminal; with dentigerous plates in both premaxilla and dentary. Lateral line with 27-30 plates. Dorsal fin with I,6 rays, pectoral fin with I,6-7 rays, pelvic fin with 7 rays and anal fin with 12-15 rays. Ground color brownish; dark-brown spots of varied sizes irregularly distributed. Fins light-brown with dark-brown spots.
Maximum standard length. 125.0 mm.
Distribution. Upper río Paraguay and rio Paraná basins.
Remarks. Ossancora eigenmanni was identified as Oxydoras eigenmanni by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Birindelli, Sabaj-Pérez (2011Birindelli JLO, Sabaj Pérez MH. Ossancora, new genus of thorny catfish (Teleostei: Siluriformes: Doradidae) with description of one new species. Proc Acad Nat Sci Philadelphia. 2011; 161(1):117-52.), in the description of the new genus, Ossancora, proposed the new combination. Ossancora eigenmanni is a non-native species from the upper rio Paraná, and its occurrence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
a. Ossancora eigenmanni, NUP 6326, 99.1 mm SL, Rosana Reservoir, rio Paranapanema, Diamante do Norte, State of Paraná. b. Platydoras armatulus, NUP 1052, 132.9 mm SL, baia Sinhá Mariana, Barão de Melgaço, State of Mato Grosso. c. Pterodoras granulosus, 600.0 mm SL, fresh specimen, uncat. d. Rhinodoras dorbignyi, 135.5 mm SL, fresh specimen, uncat. e. Trachydoras paraguayensis, 125.0 mm SL, fresh specimen, uncat.
Platydoras
Platydoras armatulus (Valenciennes, 1840)
Body deep; greatest body depth contained 3.2 to 3.9, head length 3.8 to 4.0 times in SL; snout length contained 2.2 to 2.3, horizontal orbital diameter 4.8 to 5.5 and least interorbital width 2.5 to 2.7 times in HL; orbital diameter contained 1.8 to 2.1 times in interorbital width. Mouth terminal; with dentigerous plates in both premaxilla and dentary. Lateral line with 27-28 plates. Dorsal fin with I,6, pectoral fin with I,7, pelvic fin with 7 and anal fin with 10-12 rays (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Ground color dark-brown above pelvic-fin orign, whitish to yellowish below; yellowish longitudinal band, from dorsal region of head to distal margin of median caudal-fin rays. Dorsal, pelvic, anal and caudal fins hyaline or light-beige; dorsal fin with dark-brown blotch on distal margin; pectoral fin dark-brown, its spine light-beige; caudal fin with one dark-brown longitudinal band on each lobe.
Maximum standard length. 178.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system.
Remarks. Platydoras armatulus is a non-native species from the upper rio Paraná, and its occurrence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
Pterodoras
Pterodoras granulosus (Valenciennes, 1821)
Body deep; greatest body depth contained 2.9 to 4.4, head length 3.4 to 4.4 times in SL; snout length contained 2.2 to 2.3, horizontal orbital diameter 8.3 to 11.8 and least interorbital width 2.5 to 3.1 times in HL; orbital diameter contained 3.1 to 4.3 times in interorbital width. Mouth terminal; with dentigerous plates in both premaxilla and dentary. Lateral line with 27-28 plates. Dorsal fin with I,6, pectoral fin with I,8-10, pelvic fin with 7 and anal fin with 12 or 13 rays. Ground color pale brown to greyish, darker dorsally, lighter ventrally; dark-brown or grey chromatophores irregularly distributed on body. Dark-brown or yellowish fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 635.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Amazon, río Paraná and coastal drainages of Guyana, Surinam and French Guiana.
Remarks. Pterodoras granulosus is a non-native species from the upper rio Paraná, and its occurrence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
Rhinodoras
Rhinodoras dorbignyi (Kner, 1855)
Body deep; greatest body depth contained 3.5 to 4.5, head length 3.1 to 3.6 times in SL; snout length contained 1.9 to 2.2, horizontal orbital diameter 6.5 to 9.8 and least interorbital width 4.7 to 6.2 times in HL; orbital diameter contained 1.2 to 1.8 times in interorbital width. Mouth subterminal; with dentigerous plates in both premaxilla and dentary. Lateral line with 27-28 plates. Dorsal fin with I,6, pectoral fin with I,8-10, pelvic fin with 7 and anal fin with 8 or 9 rays. Ground color yellowish; dark-brown irregular blotches on body. Hyaline or light-beige fins with dark-brown blotches and spots (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 172.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system.
Trachydoras
Trachydoras paraguayensis (Eigenmann, Ward, 1907)
Body deep; greatest body depth contained 2.7 to 3.1, head length 3.3 to 3.8 times in SL; snout length contained 1.9 to 2.2, horizontal orbital diameter 2.5 to 3.1 and least interorbital width 2.2 to 2.8 times in HL; orbital diameter contained 1.0 to 1.3 times in interorbital width. Mouth subterminal; with dentigerous plates in both premaxilla and dentary. Lateral line with 28-30 plates. Dorsal fin with I,6, pectoral fin with I,7, pelvic fin with 7 and anal fin with 12-14 rays. Ground color greyish dorsally, whitish to yellowish ventrally. Hyaline or light-beige fins; caudal fin with dark-grey oblique stripe on each lobe (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 150.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system.
Remarks. Trachydoras paraguayensis is a non-native species from the upper rio Paraná, and its occurrence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
Heptapteridae
Cetopsorhamdia
Cetopsorhamdia iheringi Shubarti, Gomes, 1959
Body elongated; greatest body depth contained 5.7 to 7.1, head depth 6.9 to 7.5 and caudal peduncle depth 9.2 to 10.1 times in SL; head length 4.5 to 4.9, anal-fin base length 8.1 to 8.7, adipose-fin base length 8.1 to 8.4 and maxillary-barbel length 3.2 to 3.9 times in SL; snout length 2.0 to 2.3, horizontal orbital diameter 6.0 to 6.2 and least interorbital width 2.8 to 3.0 times in HL. Mouth terminal; premaxilla and dentary with several diminute and viliform teeth. Dorsal fin with 7, pectoral fin with 8-11, pelvic fin with 6 and anal fin with 11-15 rays. Ground color dark-brown. Dark-grey fins; caudal fin with dark-brown transverse bar on posterior portion of caudal pecuncle (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 65.2 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná and rio São Francisco basins.
Heptapterus
Heptapterus mustelinus (Valenciennes, 1835)
Body elongated; greatest body depth contained 10.0 to 10.6, head depth 11.9 to 12.2 and caudal peduncle depth 17.7 to 18.4 times in SL; head length 6.1 to 6.4, anal-fin base length 4.9 to 5.5, adipose-fin base length 2.0 to 2.2 and maxillary-barbel length 6.0 to 7.2 times in SL; snout length 2.9 to 3.1, horizontal orbital diameter 4.9 to 5.2 and least interorbital width 3.7 to 3.9 times in HL. Mouth terminal; premaxilla and dentary with several diminute and viliform teeth. Dorsal fin with 7, pectoral fin with 6-9, pelvic fin with 6 and anal fin with 15-17 rays. Ground color dark-brown; dark-brown transverse bars on dorsum. Hyaline or light-beige fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 130.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río de la Plata basin and costal drainages of Southern Brazil.
Remarks. Heptapterus mustelinus is a non-native species from the upper rio Paraná, and its occurrence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls (Langeani et al., 2007Langeani F, Castro RMC, Oyakawa OT, Shibatta OA, Pavanelli CS, Casatti L. Diversidade da ictiofauna do alto rio Paraná: composição atual e perspectivas futuras. Biota Neotropica , 2007; 7(3):181-97.).
All from Brazil. a. Cetopsorhamdia iheringi, NUP 14875, 51.1 mm SL, rio Curimba, Umuarama, State of Paraná. b. Heptapterus mustelinus, NUP 2500, 118.8 mm SL, Itaipu Reservoir, Santa Helena, State of Paraná. c. Imparfinis borodini, NUP 3028, 128.3 mm SL, rio Mourão, tributary of the rio Ivaí, Campo Mourão, State of Paraná. d. Imparfinis mirini, NUP 87, 64.4 mm SL, rio Alambari, Botucatu, State of São Paulo. e. Imparfinis schubarti, NUP 11369, 99.5 mm SL, rio Pitangui (cachoeira do Leajenski), Ponta Grossa, State of Paraná. f. Phenacorhamdia tenebrosa, NUP 4949, 73.4 mm SL, ribeirão Maringá, Maringá, State of Paraná.
Imparfinis
1. Body very elongate; adipose-fin base very long, extending to caudal fin, its length about half the stardard length, several times longer than anal-fin base; head depressed .................... I. borodini
1’. Body not elongate; adipose-fin base short, not extending to caudal fin, its length contained several times in stardard length, about the same as that of anal-fin base; head not depressed .................... 2
2. Dark-brown inconspicuous longitudinal band along flank; first dorsal-fin ray approximately the same size as the second .................... I. mirini
2’. Dark-brown conspicuous longitudinal band along flank; first dorsal-fin ray shorter than the second .................... I. schubarti
Imparfinis borodini Mees, Cala, 1989
Body elongated; greatest body depth contained 8.0, head depth 10.1 and caudal peduncle depth 14.4 times in SL; head length 4.0, anal-fin base length 8.8, adipose-fin base length 2.9 and maxillary-barbel length 4.8 times in SL; snout length 3.0, horizontal orbital diameter 5.7 and least interorbital width 4.5 times in HL. Mouth terminal; premaxilla and dentary with several diminute teeth. Dorsal fin with 7, pectoral fin with 8, pelvic fin with 6 and anal fin with 8 rays. Ground color pale brown; dark-brown transverse bars on dorsum; dark-brown inconspicuous longitudinal stripe along lateral line.
Maximum standard length. 105.0 mm.
Biological data. Lives in streams with sand, silt and pebbles (Sarmento-Soares et al., 2016Sarmento-Soares LM, Bristki HA, Anjos M, Zanata AM, Martins-Pinheiro RF, Barreto MG. First record of genus Imparfinis from a northeastern coastal Brazilian river basin: I. borodini Mees & Cala, 1989 in Rio de Contas, Bahia. Check List [serial on the internet]. 2016 [cited 2016 Dec 08]; 12(1):1832. Available from: Available from: http://dx.doi.org/10.15560/12.1.1832
http://dx.doi.org/10.15560/12.1.1832...
).
Distribution. Upper rio Paraná, rio São Francisco and rio Tocantins basins.
Imparfinis mirini Haseman, 1911
Body elongated; greatest body depth contained 5.1 to 5.3, head depth 7.7 to 8.0 and caudal peduncle depth 9.4 to 10.0 times in SL; head length 5.0 to 5.4, anal-fin base length 6.3 to 6.8, adipose-fin base length 4.6 to 4.8 and maxillary-barbel length 2.5 to 2.6 times in SL; snout length 1.9 to 2.4, horizontal orbital diameter 6.0 to 6.3 and least interorbital width 2.5 to 2.6 times in HL. Mouth terminal; premaxilla and dentary with several diminute and viliform teeth. Dorsal fin with 7, pectoral fin with 8-11, pelvic fin with 6 and anal fin with 11-14 rays. Ground color pale yellow; dark-brown transverse bars on dorsum; dark-brown inconspicuous longitudinal stripe along lateral line. Yellowish fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 64.4 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Araguaia and upper Paraná basins (Eschmeyer et al., 2017Eschmeyer WN, Fricke R, van der Laan R, editors. Catalog of fishes: genera, species, references [Internet]. San Francisco: California Academy of Science; 2017 [updated 2017 Jan 31; cited 2017 Feb 24]. Available from: Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
http://researcharchive.calacademy.org/re...
).
Imparfinis schubarti (Gomes, 1956)
Body elongated; greatest body depth contained 5.1 to 5.3, head depth 6.9 to 7.4 and caudal peduncle depth 8.4 to 9.0 times in SL; head length 4.8 to 5.3, anal-fin base length 6.4 to 7.0, adipose-fin base length 4.7 to 5.6 and maxillary-barbel length 2.4 to 2.7 times in SL; snout length 2.2 to 2.4, horizontal orbital diameter 3.4 to 4.5 and least interorbital width 3.6 to 3.8 times in HL. Mouth terminal; premaxilla and dentary with several diminute and viliform teeth. Dorsal fin with 7, pectoral fin with 8-11, pelvic fin with 6 and anal fin with 11-14 rays. Ground color beige; few scaterred dark-brown on body, except on ventral regdion of head and abdominal region; dark-brown transverse bars on dorsum; dark-brown conspicuous longitudinal stripe along lateral line. Yellowish or hyaline fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 70.4 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná basin.
Phenacorhamdia
Phenacorhamdia tenebrosa (Schubart, 1964)
Body elongated; greatest body depth contained 11.3 to 11.7, head depth 11.9 to 12.8 and caudal peduncle depth 15.6 to 17.1 times in SL; head length 5.0 to 5.6, anal-fin base length 6.3 to 7.3, adipose-fin base length 5.3 to 5.4 and maxillary-barbel length 6.6 to 7.3 times in SL; snout length 2.8 to 3.0, horizontal orbital diameter 9.1 to 12.2 and least interorbital width 4.4 to -6.1 times in HL. Mouth slightly prognathous; premaxilla and dentary with several diminute teeth. Dorsal fin with 7, pectoral fin with 8, pelvic fin with 6 and anal fin with 8 rays. Ground color pale yellow to pale brown; dark-brown transverse bars on dorsal region; dark-brown longitudinal, inconspicuous stripe along lateral line.
Maximum standard length. 86.4 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná and rio São Francisco basins.
Pimelodella
1. Body with two dark-brown longitudinal bands, one along the lateral line and one slightly below the dorsal fin .................... P. avanhandavae
1’. Body with only a dark-brown longitudinal band, along the lateral line .................... 2
2. First dorsal-fin ray not prolonged in a filament, in adults .................... P. gracilis
2’. First dorsal-fin ray extended in a filament, in adults .................... P. taenioptera
Pimelodella avanhandavae Eigenmann, 1917Eigenmann CH. The American Characidae. Mem Mus Comp Zool . 1917; 43:1-102. pt. 1.
Body elongated; greatest body depth contained 5.1 to 5.3, head depth 4.2 to 5.0 and caudal peduncle depth 7.4 to 8.0 times in SL; head length 4.5 to 4.7, anal-fin base length 6.6 to 7.5, adipose-fin base length 1.9 to 2.2 and maxillary-barbel length 1.1 to 1.4 times in SL; snout length 2.3 to 2.5, horizontal orbital diameter 3.1 to 3.3 and least interorbital width 3.9 to 4.1 times in HL. Mouth terminal; premaxilla and dentary with several diminute and viliform teeth. Dorsal fin with I,6, pectoral fin with I,9 or 10, pelvic fin with 6 and anal fin with 12-14 rays. Ground color beige; two dark-brown longitudinal bands, one along lateral line and one slightly below dorsal fin. Hyaline fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 236.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná basin.
a. Pimelodella avanhandavae, 125.0 mm SL, uncat. b. Pimelodella gracilis, NUP 3118, 120.0 mm SL, Itaipu Reservoir, Foz do Iguaçu, State of Paraná. c. Pimelodella taenioptera, NUP 3991, 130.0 mm SL, Itaipu Reservoir, Santa Teresinha de Itaipu, State of Paraná. d. Rhamdia quelen, 163.2 mm SL, fresh specimen, uncat. e. Ictalurus punctatus, NUP 11203, 180.5 mm SL, rio São Francisco Verdadeiro, tributary of the rio Paraná, Entre Rios do Oeste, State of Paraná.
Pimelodella gracilis (Valenciennes, 1835)
Body elongated; greatest body depth contained 5.1 to 5.9, head depth 4.2 to 4.9 and caudal peduncle depth 7.2 to 7.9 times in SL; head length 4.3 to 5.0, anal-fin base length 6.4 to 7.8, adipose-fin base length 2.3 to 2.5 and maxillary-barbel length 1.2 to 1.5 times in SL; snout length 2.3 to 2.5, horizontal orbital diameter 3.0 to 3.3 and least interorbital width 3.7 to 5.0 times in HL. Mouth terminal; premaxilla and dentary with several diminute and viliform teeth. Dorsal fin with I,6, pectoral fin with I,9 or 10, pelvic fin with 6 and anal fin with 12-14 rays. Ground color beige; dark-brown longitudinal band along lateral line. Hyaline fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 184.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río de la Plata, Amazon and río Orinoco basins.
Pimelodella taenioptera Miranda-Ribeiro, 1914
Body elongated; greatest body depth contained 4.9 to 6.2, head depth 4.3 to 5.0 and caudal peduncle depth 4.2 to 4.9 times in SL; head length 4.3 to 4.8, anal-fin base length 8.8 to 9.1, adipose-fin base length 2.3 to 2.8 and maxillary-barbel length 1.3 to 1.6 times in SL; snout length 2.0 to 2.7, horizontal orbital diameter 3.2 to 4.8 and least interorbital width 3.8 to 4.7 times in HL. Mouth terminal; premaxilla and dentary with several diminute and viliform teeth. Dorsal fin with I,6, pectoral fin with I,9 or 10, pelvic fin with 6 and anal fin with 11-14 rays. Ground color beige; dark-brown longitudinal band along lateral line. Hyaline fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 154.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná and upper río Paraguay basins.
Remarks. Pimelodella taenioptera is a non-native species from the upper rio Paraná and its occurrence can be associated with the functioning of the Canal da Piracema (a fish ladder that connects the region downstream from the Itaipu Dam to the region upstream from the dam). Despite the similarity of Pimelodella taenioptera with P. boschmai Van der Stigchel, 1964 from the rio Mogi-Guaçu, the specimens from the upper rio Paraná floodplain with the first dorsal-fin ray extended into a filament have only been recorded in the focused region after the functioning of the Canal da Piracema. Therefore, these specimens do not correspond to any other species described from the upper rio Paraná basin.
Rhamdia
Rhamdia quelen (Quoy, Gaimard, 1824)
Body elongated; greatest body depth contained 4.7 to 5.7, head depth 6.7 to 9.0 and caudal peduncle depth 5.6 to 8.4 times in SL; head length 4.1 to 4.2, anal-fin base length 7.8 to 8.8, adipose-fin base length 2.3 to 2.7 and maxillary-barbel length 2.1 to 2.7 times in SL; snout length 3.0 to 3.1, horizontal orbital diameter 5.6 to 6.1 and least interorbital width 3.0 to 3.1 times in HL. Mouth terminal; premaxilla and dentary with several diminute and viliform teeth. Dorsal fin with 7, pectoral fin with 8-10, pelvic fin with 6 and anal fin with 14-15 rays. Ground color beige to brown; some specimens can present scattered dark-brown chromatophores on body. Dark-grey or brown fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 410.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Widespread in South America rivers.
Ictaluridae
Ictalurus
Ictalurus punctatus (Rafinesque, 1818)
Body elongated; greatest body depth contained 4.4 and caudal peduncle depth 10.0 times in SL; head length 3.7, anal-fin base length 3.6, adipose-fin base length 9.9, and maxillary-barbel length 3.4 in SL; snout length 2.4, horizontal orbital diameter 5.0 and least interorbital width 2.5 in HL. Mouth terminal. Dorsal fin with I,6, pectoral fin with I,8-9, pelvic fin with I,6-7 and anal fin with 29 or 30 rays. Ground color pale brown; hyaline fins with distal margins black.
Maximum standard length. 120.0 mm.
Biological data. Lives in streams, reservoirs, ponds and lakes. Juveniles feed primarily on aquatic insects, whereas adults present feeding habit omnivorous (insects, snails, crawfish, green algae, aquatic plants, seeds and small fish) (Wellborn, 1988Wellborn TL. Channel catfish life history and biology. Stoneville: Cooperative extension work in agriculture and home economics in cooperation with the United States Department of Agriculture; 1988. (Southern Regional Aquaculture Center Publication; no. 180).). Presents parental care, which is carried out by males (Zanatta et al., 2010Zanatta AS, Ramos IP, Silva RJ, Langeani F, Carvalho ED. Pisces, Siluriformes, Ictaluridae, Ictalurus punctatus (Rafinesque, 1818): first record in middle Paranapanema River reservoir, aquaculture and exotic species dispersion. Check List . 2010; 6(4):589-91.)
Distribution. Eastern North America, from southern Canada and northern USA to eastern Mexico. Widely introduced elsewhere (Eschmeyer et al., 2017Eschmeyer WN, Fricke R, van der Laan R, editors. Catalog of fishes: genera, species, references [Internet]. San Francisco: California Academy of Science; 2017 [updated 2017 Jan 31; cited 2017 Feb 24]. Available from: Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
http://researcharchive.calacademy.org/re...
).
Remarks. Ictalurus punctatus, native of North America, has been captured in the rio São Francisco Verdadeiro, tributary of the left bank of the upper rio Paraná, since 2010 by Companhia Paranaense de Energia (Copel) staff, and in the rio Iguatemi, right bank of the upper rio Paraná, since 2007 by V. F. B. Silva and collaborators. Its occurrence in the region can be associated with fish-farming and escapes from recreational angling ponds.
Loricariidae
Hypostominae
Ancistrus
Ancistrus sp.
Body deep; greatest body depth contained 3.0 to 4.0 and caudal peduncle depth 6.0 to 7.0 times in SL; head length 2.6 to 2.8, predorsal distance 2.1 to 2.2, dorsal-fin spine length 4.1 to 4.4, pectoral-fin spine length 2.9 to 3.4 and caudal peduncle length 3.5 to 3.8 in SL; snout length 1.2 to 1.4, horizontal orbital diameter 7.9 to 8.3 and least interorbital width 2.0 to 2.4 in HL. Mouth inferior; premaxilla with 8-15 and dentary with 10-16 teeth. Mid-lateral series with 25, predorsal series with 4, and dorsal-fin base series with 12 plates. Dorsal fin with I,7-9, pectoral fin with I,6, pelvic fin with i,5 and anal fin with 6 rays. Ground color brown; light-beige rounded blotches on body, especially on head. Hyaline fins with dark-grey blotches.
Maximum standard length. 140.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Rio São Francisco Verdadeiro, upper rio Paraná basin.
a. Ancistrus sp., 110.0 mm SL, uncat. b. Hypostomus albopunctatus, NUP 1761, 210.0 mm SL, Itaipu Reservoir, Santa Helena, State of Paraná. c. Hypostomus ancistroides, 140.0 mm SL, uncat. d. Hypostomus cochliodon, NUP 2556, 230.0 mm SL, Itaipu Reservoir, Santa Helena, State of Paraná. e. Hypostomus commersoni, NUP 856, 255.0 mm SL, Mourão Reservoir (rio Mourão, Campo Mourão, State of Paraná. f. Hypostomus hermanni, NUP 4927, 142.8 mm SL, rio Piquiri, tributary of the rio Paraná, Nova Laranjeiras, State of Paraná.
Hypostomus
1. Teeth spoon-shaped .................... H. cochliodon
1’. Teeth bifid .................... 2
2. Ground color light-beige, with dark-brown spots and/or dark-brown vermiculate pattern, slightly conspicuous .................... H. ternetzi
2’.Ground color dark-brown or grey, with several conspicuous light- or dark-brown blotches .................... 3
3. Body covered by light-beige blotches .................... 4
3’. Body covered by dark-brown blotches .................... 8
4. Premaxilla with eight to 14 and dentary with 10 to 16 teeth .................... H. microstomus
4’. Premaxilla with more than 15 and dentary with more than 16 teeth .................... 5
5. Premaxilla and dentary with 19 to 32 teeth .................... H. margaritifer
5’. Premaxilla and dentary with more than 32 teeth .................... 6
6. Premaxilla with 35 to 46, and dentary with 34 to 46 teeth; pelvic fin longer or as long as pectoral fin .................... H. albopunctatus
6’. Premaxilla and dentary with more than 48 teeth; pelvic fin shorter than pectoral fin .................... 7
7. Premaxilla with 49 to 54 and dentary with 49 to 55 teeth; mid-lateral series with 25 or 26 plates; abdomen with plates concentrated on its central portion .................... H. cf. strigaticeps
7’. Premaxilla with 65 to 110 and dentary with 65 to 132 teeth; mid-lateral series with 28 or 29 plates; abdomen completely covered by plates .................... H. regani
8. Orbit large, its diameter contained 3.9 to 5.5 in head length .................... 9
8’. Orbit small, its diameter contained 6.5 a 9.5 in head length .................... 10
9. Abdomen completely covered by plates .................... H. hermanni
9’. Abdomen naked or partially covered by plates .................... H. iheringii
10. Dorsal fin long, its spine length contained 2.6 to 3.4 in standard length .................... H. commersoni
10’. Dorsal fin short, its spine length contained 3.9 to 4.3 in standard length .................... H. ancistroides
Hypostomus albopunctatus (Regan, 1908)
Body deep; greatest body depth contained 4.5 to 5.0 and caudal peduncle depth 9.5 to 10.2 times in SL; head length 2.9 to 3.4, predorsal distance 2.3 to 2.6, dorsal-fin spine length 3.3 to 5.2, pectoral-fin spine length 3.2 to 4.1, adipose-fin spine length 9.5 to 10.9 and caudal peduncle length 3.0 to 3.6 in SL; snout length 1.3 to 1.7, horizontal orbital diameter 7.0 to 9.3 and least interorbital width 2.7 to 3.3 in HL. Mouth inferior; premaxilla with 27-46 and dentary with 25-46 teeth. Mid-lateral series with 26 or 27, predorsal series with 3-5, and dorsal-fin base series with 7 or 8 plates. Dorsal fin with I,7, pectoral fin with I,6, pelvic fin with i,5 and anal fin with 6 rays. Ground color dark-brown or grey; light-beige spots on body, smaller on head. Dark-brown or grey fins with white or light-beige spots.
Maximum standard length. 315.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná basin.
Hypostomus ancistroides (Ihering, 1911)
Body deep; greatest body depth contained 4.2 to 4.7 and caudal peduncle depth 9.8 to 10.0 times in SL; head length 2.9 to 3.3, predorsal distance 2.3 to 2.6, dorsal-fin spine length 3.9 to 4.3, pectoral-fin spine length 3.7 to 3.9, adipose-fin spine length 9.9 to 9.9 to 10.9 and caudal peduncle length 2.9 to 3.3 in SL; snout length 1.5 to 1.8, horizontal orbital diameter 6.5 to 7.3 and least interorbital width 2.4 to 2.7 in HL. Mouth inferior; premaxilla with 24-33 and dentary with 23-35 teeth. Mid-lateral series with 27 or 28, predorsal series with 3, and dorsal-fin base series with 8 or 9 plates. Dorsal fin with I,7, pectoral fin with I,6, pelvic fin with i,5 and anal fin with 6 rays. Ground color brown; dark-brown blotches on body, especially on dorsal region. Dark-brown fins with black spots.
Maximum standard length. 276.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná basin.
Hypostomus cochliodon Kner, 1854
Body deep; greatest body depth contained 3.7 to 4.0 and caudal peduncle depth 9.1 to 10.4 times in SL; head length 3.7 to 4.1, predorsal distance 3.2 to 3.4, dorsal-fin spine length 3.7 to 4.1, pectoral-fin spine length 3.2 to 3.4, adipose-fin spine length 12.8 to 17.0 and caudal peduncle length 3.0 to 3.4 in SL; snout length 1.4 to 1.6, horizontal orbital diameter 6.1 to 6.3 and least interorbital width 1.8 to 2.0 in HL. Mouth inferior; premaxilla with 7 and dentary with 8 teeth. Mid-lateral series with 27 or 28, predorsal series with 3, and dorsal-fin base series with 8 plates. Dorsal fin with I,7, pectoral fin with I,6, pelvic fin with i,5 and anal fin with 6 rays. Ground color brown; dark-brown blotches on body, more conspicuous on head. Brown fins with black spots.
Maximum standard length. 270.0 mm. (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río Paraguay and middle río Paraná basins.
Remarks. Hypostomus cochliodon is a non-native species from the upper rio Paraná, and its occurrence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
Hypostomus commersoni Valenciennes, 1836
Body deep; greatest body depth contained 4.0 to 4.4 and caudal peduncle depth 9.0 to 10.5 times in SL; head length 3.0 to 3.3, predorsal distance 2.4 to 2.8, dorsal-fin spine length 2.6 to 3.4, pectoral-fin spine length 3.1 to 3.6, adipose-fin spine length 13.5 to 19.0 and caudal peduncle length 3.0 to 3.5 in SL; snout length 1.6 to 1.8, horizontal orbital diameter 6.7 to 9.5 and least interorbital width 2.4 to 2.8 in HL. Mouth inferior; premaxilla with 22-45 and dentary with 22-48 teeth. Mid-lateral series with 28-0, predorsal series with 3, and dorsal-fin base series with 8-10 plates. Dorsal fin with I,7, pectoral fin with I,6, pelvic fin with i,5 and anal fin with 6 rays. Ground color brown; black spots on body, smaller on head. Brown fins with black spots.
Maximum standard length. 270.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río Paraná basin and Laguna dos Patos drainage.
Remarks. Hypostomus commersoni is a non-native species from the upper rio Paraná, and its occurrence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
Hypostomus hermanni (Ihering, 1905)
Body deep; greatest body depth contained 4.0 to 5.3 and caudal peduncle depth 8.9 to 9.0 times in SL; head length 2.8 to 3.1, predorsal distance 2.3 to 2.5, dorsal-fin spine length 3.1 to 4.4, pectoral-fin spine length 2.9 to 3.5, adipose-fin spine length 9.3 to 16.9 and caudal peduncle length 2.9 to 3.6 in SL; snout length 1.5 to 1.8, horizontal orbital diameter 3.9 to 5.0 and least interorbital width 2.7 to 3.5 in HL. Mouth inferior; premaxilla with 29-35 and dentary with 30-47 teeth. Mid-lateral series with 25-27 plates, predorsal series with 3 plates, and dorsal-fin base series with 8 or 9 plates. Dorsal fin with I,7, pectoral fin with I,6, pelvic fin with i,5 and anal fin with 5 rays. Ground color brown; body and fins with dark-brown blotches; no blotches on ventral surface.
Maximum standard length. 170.0 mm.
Distribution. Upper rio Paraná basin.
Hypostomus iheringii (Regan, 1908)
Body deep; greatest body depth contained 4.2 to 4.7 and caudal-peduncle depth 8.3 to 8.6 times in SL; head length 2.8 to 3.0, predorsal distance 2.2 to 2.3, dorsal-fin spine length 3.4 to 3.6, pectoral-fin spine length 3.0 to 3.2, adipose-fin spine length 9.0 to 10.1, and caudal-peduncle length 3.2 to 3.4 times in SL; snout length 1.6 to 1.8, horizontal orbital diameter 4.4 to 5.5, and least interorbital width 2.7 to 2.8 in HL. Mouth inferior; pre-maxilla with 32-41 and dentary with 35-42 teeth. Mid-lateral series with 24-27 plates, predorsal series with 3 plates, and dorsal-fin base series with 8 plates. Dorsal fin with I,7, pectoral fin with I,6, pelvic fin with i,5, and anal fin with 6 rays. Ground color brownish; dark-brown blotches, occasionally inconspicuous.
Maximum standard length. 210.0 mm.
Distribution. Upper rio Paraná basin.
Remarks. Hypostomus iheringii was identified as Hypostomus sp. by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
a. Hypostomus iheringii, NUP 2577, 190.0 mm SL, rio Paraná, Porto Rico, State of Paraná. b. Hypostomus margaritifer, 235.0 mm SL, uncat. c. Hypostomus microstomus, NUP 1725, 165.0 mm SL, riacho Caracu, Porto Rico, State of Paraná. d. Hypostomus regani, NUP 2286, 170.0 mm SL, Corumbá Reservoir, Caldas Novas, State of Goiás. e. Hypostomus cf. strigaticeps, NUP 3190, 150.0 mm SL, rio Paraná, Porto Rico, State of Paraná. f. Hypostomus ternetzi, 210.0 mm SL, uncat.
Hypostomus margaritifer (Regan, 1908)
Body deep; greatest body depth contained 4.1 to 4.5 and caudal peduncle depth 9.3 to 10.0 times in SL; head length 3.0 to 3.4, predorsal distance 2.4 to 2.7, dorsal-fin spine length 2.3 to 3.1, pectoral-fin spine length 2.6 to 3.1, adipose-fin spine length 13.5 to 16.0 and caudal peduncle length 2.9 to 3.6 in SL; snout length 1.4 to 1.6, horizontal orbital diameter 5.6 to 6.5 and least interorbital width 2.4 to 3.0 in HL. Mouth inferior; premaxilla and dentary with 19-32 teeth. Mid-lateral series with 26 or 27, predorsal series with 3, and dorsal-fin base series with 8 plates. Dorsal fin with I,7, pectoral fin with I,6, pelvic fin with i,5 and anal fin with 6 rays. Ground color dark-brown or -grey; light-beige spots and blotches on body. Dark-grey fins with light-beige spots.
Maximum standard length. 312.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná basin.
Hypostomus microstomus Weber, 1987Weber C. Hypostomus microstomus sp. nov. et autres poissons-chats cuirassés du rio Paraná (Pisces, Siluriformes, Loricariidae). Arch Sci. 1987; 40(3):273-84.
Body deep; greatest body depth contained 4.0 to 4.7 and caudal peduncle depth 8.4 to 9.4 times in SL; head length 2.8 to 3.2, predorsal distance 2.4 to 2.5, dorsal-fin spine length 2.6 to 3.2, pectoral-fin spine length 2.8 to 3.1, adipose-fin spine length 13.5 to 6.0 and caudal peduncle length 3.0 to 3.5 in SL; snout length 1.4 to 1.6, horizontal orbital diameter 4.6 to 5.8 and least interorbital width 1.6 to 2.1 in HL. Mouth inferior; premaxilla with 8-14 and dentary with 10-16 teeth. Mid-lateral series with 24-27, predorsal series with 3, and dorsal-fin base series with 7 plates. Dorsal fin with I,7, pectoral fin with I,6, pelvic fin with i,5 and anal fin with 6 rays (Weber, 1987Weber C. Hypostomus microstomus sp. nov. et autres poissons-chats cuirassés du rio Paraná (Pisces, Siluriformes, Loricariidae). Arch Sci. 1987; 40(3):273-84.). Ground color brown; scattered light-beige spot on body. Brown fins with light-beige spots, sometimes inconspicuous.
Maximum standard length. 300.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río Paraná basin.
Remarks. Hypostomus microstomus is a non-native species from the upper rio Paraná, and its occurrence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
Hypostomus regani (Ihering, 1905)
Body deep; greatest body depth contained 4.3 to 4.7 and caudal peduncle depth 9.3 to 10.3 times in SL; head length 3.1 to 3.4, predorsal distance 2.5 to 2.8, dorsal-fin spine length 2.2 to 3.6, pectoral-fin spine length 3.1 to 3.7, adipose-fin spine length 10.0 to 13.3 and caudal peduncle length 2.8 to 3.3 in SL; snout length 1.6 to 1.8, horizontal orbital diameter 4.8 to 5.8 and least interorbital width 2.3 to 2.6 in HL. Mouth inferior; premaxilla with 65-110 and dentary with 65-132 teeth. Mid-lateral series with 27 or 28, predorsal series with 3-5, and dorsal-fin base series with 8 or 9 plates. Dorsal fin with I,7, pectoral fin with I,6, pelvic fin with i,5 and anal fin with 6 rays. Ground color brown; light-beige spots on body, smaller on head. Dark-brown fins with light-beige spots.
Maximum standard length. 280.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río de la Plata basin.
Hypostomus cf. strigaticeps (Regan, 1908)
Body deep; greatest body depth contained 4.2 to 4.9 and caudal peduncle depth 8.3 to 9.6 times in SL; head length 2.8 to 3.0, predorsal distance 2.2 to 2.5, dorsal-fin spine length 3.4 to 3.8, pectoral-fin spine length 3.1 to 3.3, adipose-fin spine length 8.9 to 9.7 and caudal peduncle length 3.0 to 3.4 in SL; snout length 1.5 to 1.8, horizontal orbital diameter 4.0 to 5.1 and least interorbital width 2.9 to 3.2 in HL. Mouth inferior; premaxilla with 49-54 and dentary with 49-55 teeth. Mid-lateral series with 24-26, predorsal series with 3, and dorsal-fin base series with 8 plates. Dorsal fin with I,7, pectoral fin with I,6, pelvic fin with i,5 and anal fin with 6 rays. Ground color brown; light-beige blotche on body, smaller on head. Dark-brown fins with light-beige spots.
Maximum standard length. 169.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná floodplain and rio Tietê basin.
Hypostomus ternetzi (Boulenger, 1895)
Body deep; greatest body depth contained 4.2 to 4.7 and caudal peduncle depth 7.6 to 8.2 times in SL; head length 2.8 to 3.0, predorsal distance 2.2 to 2.4, dorsal-fin spine length 2.6 to 3.5, pectoral-fin spine length 3.0 to 3.3, adipose-fin spine length 9.2 to 10.3 and caudal peduncle length 3.1 to 3.3 in SL; snout length 1.6 to 1.8, horizontal orbital diameter 6.3 to 6.5 and least interorbital width 2.6 to 3.1 in HL. Mouth inferior; premaxilla with 59-127 and dentary with 60-126 teeth. Mid-lateral series with 25-27, predorsal series with 3 or 4, and dorsal-fin base series with 8 plates. Dorsal fin with I,7, pectoral fin with I,6, pelvic fin with i,5 and anal fin with 6 rays. Ground color light-beige, with dark-brown spots and/or dark-brown vermiculate pattern, slightly conspicuous. Brown fins, sometimes with dark-brown spots.
Maximum standard length. 296.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río Paraguay, middle río Paraná and río Uruguay basins.
Remarks. Hypostomus ternetzi is a non-native species from the upper rio Paraná, and its occurrence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
Megalancistrus
Megalancistrus parananus (Peters, 1881)
Body deep; greatest body depth contained 4.0 to 4.7 and caudal peduncle depth 6.0 to 6.5 times in SL; head length 2.8 to 3.2, predorsal distance 2.2 to 2.4, dorsal-fin spine length 4.1 to 4.4, pectoral-fin spine length 3.8 to 4.3 and caudal peduncle length 3.5 to 3.8 in SL; snout length 1.2 to 1.4, horizontal orbital diameter 5.0 to 6.2 and least interorbital width 1.6 to 2.0 in HL. Mouth inferior; premaxilla with 6-8 and dentary with 8-10 teeth. Mid-lateral series with 25, predorsal series with 3, and dorsal-fin base series with 12 plates. Dorsal fin with I,10-15, pectoral fin with I,6, pelvic fin with i,5 and anal fin with 6 rays. Ground color light-beige; several dark-brown blotches on body, as well as fins.
Maximum standard length. 504.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río de la Plata basin.
a. Megalancistrus parananus, NUP 528, 205.0 mm SL, rio Baía, Taquarussu, State of Mato Grosso do Sul. b. Pterygoplichthys ambrosettii, 395.0 mm SL, fresh specimen, uncat.
Pterygoplichthys
Pterygoplichthys ambrosettii (Holmberg, 1893)
Body deep; greatest body depth contained 4.2 to 4.9 and caudal peduncle depth 8.3 to 9.6 times in SL; head length 3.4 to 3.9, predorsal distance 2.2 to 2.5, dorsal-fin spine length 2.2 to 2.7, pectoral-fin spine length 3.1 to 3.3 and adipose-fin spine length 8.9 to 9.7, and caudal-peduncle length 3.3 to 3.8 times in SL; snout length 1.5 to 1.6, horizontal orbital diameter 5.2 to 8.6 and least interorbital width 1.6 to 2.0 in HL. Mouth inferior; premaxilla with 19-36 and dentary with 17-37 teeth. Mid-lateral series with 29-31 plates, predorsal series with 3 plates, and dorsal-fin base series with 8 plates. Dorsal fin with I,11-14, pectoral fin with I,6, pelvic fin with I,5 and anal fin with 5 rays. Ground color dark-grey; body and fins with white or light-beige blotches.
Maximum standard length. 500.0 mm.
Distribution. Río de la Plata basin.
Remarks. Pterygoplichthys ambrosettii was identified as P. anisitsi by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Ferraris, Jr. (2007Ferraris CJ Jr. Checklist of catfishes, recent and fossil (Osteichthyes: Siluriformes), and catalogue of siluriform primary types. Zootaxa . 2007; 1418:1-628.), in the checklist of the catfishes, proposed the new synonym. Pterygoplichthys ambrosettii is a non-native species from the upper rio Paraná, and its occurrence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls, or with the aquarium trade (Júlio Júnior et al., 2009Júlio Júnior HF, Dei Tos C, Agostinho AA, Pavanelli CS. A massive invasion of fish species after eliminating a natural barrier in the upper rio Paraná basin. Neotrop Ichthyol . 2009; 7(4):709-18.).
Loricariinae
Farlowella
Farlowella hahni Meinken, 1937
Body depressed; greatest body depth contained 19.1 to 20.6 times in SL; head length 4.0 to 4.4, pectoral-fin length 10.2 to 11.9, and pelvic-fin length 15.1 to 17.0 in SL; snout length contained 1.6 to 2.0, horizontal orbital diameter 12.3 to 14.0, and least interorbital width 4.8 to 5.2 times in HL. Mouth inferior; pre-maxilla with 15-17 and dentary with 13-18 teeth. Mid-lateral series with 32, predorsal series with 7, series between pectoral and pelvic fins with 5 and abdomen series with 3 plates. Dorsal and pectoral fins with I,6, pelvic fin with i,4-5 and anal with 6 rays. Ground color brown; dark-brown longitudinal band from base of snout to caudal peduncle. Hyaline fins with few dark-brown spots, caudal-fin with discontinuous dark-brown longitudinal band in upper lobe.
Maximum standard length. 162.0 mm.
Distribution. Río Paraná basin.
Remarks. Farlowella hahni was identified as F. aff. amazona by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). These specimens were identified based on the assumption that they should correspond to F. paranaënse Meinken, 1937, a junior synonym of F. amazonum (correct spelling). Azpelicueta, Koerber (2014Azpelicueta MM, Koerber S. Finding of the holotype of Farlowella paranaense Meinken, 1937. Historia Natural. 2014; 4(2):95-99.) found and redescribed the holotype of F. paranaënse (which was thought to be lost since World War II), concluding that the synonymy was well justified. A comparison between the specimens from the upper rio Paraná floodplain and the revision of the genus by Retzer, Page (1996Retzer ME, Page LM. Systematics of the stick catfishes, Farlowella Eigenmann & Eigenmann (Pisces, Loricariidae). Proc Acad Nat Sci Philadelphia . 1996; 147:33-88.) showed a disagreement related to species in the F. amazonum species group in at least two important characters. First, the specimens from the upper rio Paraná floodplain present three well-developed series of abdominal scutes (vs. two in the F. amazonum group); second, their snout is relatively narrow, laterally concave in dorsal view (vs. broad, almost straight laterally in dorsal view). Therefore, the characters presented herein match the description of F. hahni. It is noteworthy that F. hahni was stated by Retzer, Page (1996Retzer ME, Page LM. Systematics of the stick catfishes, Farlowella Eigenmann & Eigenmann (Pisces, Loricariidae). Proc Acad Nat Sci Philadelphia . 1996; 147:33-88.) as indistinguishable from F. oxyrryncha (Kner, 1853) and probably its junior synonym. However, as these authors and others subsequently (e.g. Ferraris, Jr., 2003Ferraris CJ Jr. Subfamily Loricariinae. In: Reis RE, Kullander SO, Ferraris CJ Jr., organizers. Check list of the freshwater fishes of South and Central America. Porto Alegre: Edipucs; 2003. p.330-350.; 2007Ferraris CJ Jr. Checklist of catfishes, recent and fossil (Osteichthyes: Siluriformes), and catalogue of siluriform primary types. Zootaxa . 2007; 1418:1-628.; Azpelicueta, Koerber, 2014Azpelicueta MM, Koerber S. Finding of the holotype of Farlowella paranaense Meinken, 1937. Historia Natural. 2014; 4(2):95-99.) kept F. hahni as a valid species and it is native from the lower rio Paraná basin, using this name instead of the senior name was preferred. Farlowella hahni is a non-native species from the upper rio Paraná, and its occurrence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
a. Farlowella hahni, NUP 374, 130.0 mm SL, ribeirão São Pedro, São Pedro do Paraná, State of Paraná. b. Loricaria prolixa, 230.5 mm SL, uncat. c. Loricaria sp., NUP 2567, 230.5 mm SL, rio Paraná, Porto Rico, State of Paraná. d. Loricariichthys platymetopon, 210.0 mm SL, uncat. e. Loricariichthys rostratus, 250.0 mm SL, uncat. f. Rineloricaria sp., NUP 2565, 86.0 mm SL, riacho na Estrada (D. Armando), Santa Helena, State of Paraná.
Loricaria
1. Body with dark-brown rounded spots; head length contained 4.4 to 4.8 times in standard length .................... L. prolixa
1’. Body without spots; head length contained 5.8 to 6.2 times in standard length .................... Loricaria sp.
Loricaria prolixa Isbrücker, Nijssen, 1978Isbrücker IJH, Nijssen H. Two new species and a new genus of neotropical mailed catfishes of the subfamily Loricariinae Swainson, 1838 (Pisces, Siluriformes, Loricariidae). Beaufortia. 1978; 27(339):177-206.
Body depressed; greatest body depth contained 10.9 to 12.6 times in SL; head length 4.4 to 4.8, predorsal distance 3.1 to 3.3, dorsal-fin spine length 4.8 to 6.0, pectoral-fin spine length 3.5 to 4.4 and pelvic-fin spine length 3.9 to 4.7 in SL; snout length contained 1.7 to 1.8, horizontal orbital diameter 6.7 to 9.1, least interorbital width 5.2 to 5.8 and caudal peduncle depth 13.4 to 14.4 times in HL times in HL. Mouth inferior; pre-maxilla with 2-5 and dentary with 3-8 teeth. Mid-lateral series with 35-37 plates. Dorsal fin with I,7, pectoral fin with I,6, pelvic fin with i,6 or 7 and anal with 6 rays (Isbrücker, Nijssen, 1978Isbrücker IJH, Nijssen H. Two new species and a new genus of neotropical mailed catfishes of the subfamily Loricariinae Swainson, 1838 (Pisces, Siluriformes, Loricariidae). Beaufortia. 1978; 27(339):177-206.). Ground color brownish; dark-brown rounded spots. Hyaline fins with few dark-brown spots (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 450.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná basin.
Loricaria sp.
Body depressed; greatest body depth contained 10.0 to 11.9 times in SL; head length 5.8 to 6.2, predorsal distance 2.9 to 3.2, dorsal-fin spine length 4.0 to 5.1, pectoral-fin spine length 4.6 to 5.5 and pelvic-fin spine length 3.9 to 4.7 in SL; snout length contained 1.4 to 1.7, horizontal orbital diameter 6.8 to 7.1, least interorbital width 5.0 to 5.4 and caudal peduncle depth 10.4 to 12.2 times in HL. Mouth inferior; pre-maxilla with 3-5 and dentary with 5-8 teeth. Mid-lateral series with 30-35 plates. Dorsal fin with I,7, pectoral fin with I,6, pelvic fin with i,6 or 7 and anal with 6 rays. Ground color brownish; no dark-brown rounded spots; dark-brown irregular and transverse bars. Hyaline fins with few dark-brown spots (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 430.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná basin.
Remarks. According to C. H. Zawadzki (oral communication, 2018), Loricaria sp. is apparently new to science.
Loricariichthys
1. Snout short, contained 2.2 to 2.3 times in head length; orbital diameter contained 3.7 to 4.6 times in head length .................... L. platymetopon
1’. Snout elongated, contained 1.9 to 2 times in head length; orbital diameter contained 5.3 to 6.5 times in head length .................... L. rostratus
Loricariichthys platymetopon Isbrücker, Nijssen, 1979
Body depressed; greatest body depth contained 10.0 to 11.9 and caudal peduncle depth 37.0 to 58.8 times in SL; head length 4.0 to 4.5, predorsal distance 2.9 to 3.2, dorsal-fin spine length 4.0 to 5.1, pectoral-fin spine length 5.5 to 7.0 and pelvic-fin spine length 5.6 to 6.3 in SL; snout length contained 2.2 to 2.3, horizontal orbital diameter 3.7 to 4.6 and least interorbital width 3.1 to 4.5 times in HL. Mouth inferior; pre-maxilla with 7-14 and dentary with 10-20 teeth. Mid-lateral series with 31-32 plates. Dorsal fin with I,7, pectoral fin with I,6, pelvic fin with i,5 and anal with 6 rays (Reis, Pereira, 2000Reis RE, Pereira EHL. Three new species of the loricariid catfish genus Loricariichthys (Teleostei: Siluriformes) from southern South America. Copeia . 2000; (4):1029-47.). Ground color brownish; dark-brown blotches, sometimes forming irregular and transverse bars. Hyaline fins with few dark-brown spots (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 282.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río de la Plata basin.
Remarks. Loricariichthys platymetopon is a non-native species from the upper rio Paraná, and its occurrence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
Loricariichthys rostratus Reis, Pereira, 2000Reis RE, Pereira EHL. Three new species of the loricariid catfish genus Loricariichthys (Teleostei: Siluriformes) from southern South America. Copeia . 2000; (4):1029-47.
Body depressed; greatest body depth contained 7.5 to 10.3 and caudal peduncle depth 35.7 to 50.0 times in SL; head length 4.1 to 4.8, predorsal distance 2.7 to 3.1, dorsal-fin spine length 4.5 to 5.5, pectoral-fin spine length 6.2 to 7.8 and pelvic-fin spine length 6.5 to 8.0 in SL; snout length contained 1.9 to 2.0, horizontal orbital diameter 5.3 to 6.5 and least interorbital width 5.0 to 5.8 times in HL. Mouth inferior; pre-maxilla with 3-11 and dentary with 10-18 teeth. Mid-lateral series with 31-33 plates. Dorsal fin with I,7, pectoral fin with I,6, pelvic fin with i,5 and anal with 6 rays (Reis, Pereira, 2000Reis RE, Pereira EHL. Three new species of the loricariid catfish genus Loricariichthys (Teleostei: Siluriformes) from southern South America. Copeia . 2000; (4):1029-47.). Ground color brownish; dark-brown blotches on body. Hyaline fins with few dark-brown spots (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 282.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río Paraná basin, above río Paraguay mouth.
Remarks. Loricariichthys rostratus is a non-native species from the upper rio Paraná, and its occurrence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
Rineloricaria
Rineloricaria sp.
Body depressed; greatest body depth contained 9.9 and head depth 12.0 times in SL; head length 4.3, predorsal distance 2.8, dorsal-fin spine length 4.8 and pelvic-fin spine length 6.1 in SL; snout length contained 2.60, horizontal orbital diameter 8.7 and least interorbital width 4.1 times in HL. Mouth inferior; pre-maxilla and dentary with 6 teeth. Mid-lateral series with 28 plates. Dorsal fin with I,7, pectoral fin with I,6, pelvic fin with i,5 and anal with 6 rays. Ground color brownish; dark-brown transverse bars on flank. Hyaline fins with few dark-brown spots, sometimes forming irregular stripes (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 93.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná basin.
Remarks. Rineloricaria sp. is distinguished from the other two species from the upper rio Paraná, R. latirostris (Boulenger, 1900) and R. pentamaculataLangeani, Araújo, 1994Langeani F, Araújo RB. O gênero Rineloricaria Bleeker, 1862 (Ostariophysi, Siluriformes) na bacia do rio Paraná superior: Rineloricaria pentamaculata sp. n. e Rineloricaria latirostris (Boulenger, 1900) Com Mus Cien PUCRS . 1994; 7:151-66., by having conspicuous dark-brown spots and vermiculations on the head. Additionally, it is distinguished from R. pentamaculata by the absence of a naked area on the lateral corner of the snout (vs. presence; see Langeani, Araújo, 1994Langeani F, Araújo RB. O gênero Rineloricaria Bleeker, 1862 (Ostariophysi, Siluriformes) na bacia do rio Paraná superior: Rineloricaria pentamaculata sp. n. e Rineloricaria latirostris (Boulenger, 1900) Com Mus Cien PUCRS . 1994; 7:151-66., fig. 2) and by a more acute snout. From R. latirostris (sensuLangeani, Araújo, 1994Langeani F, Araújo RB. O gênero Rineloricaria Bleeker, 1862 (Ostariophysi, Siluriformes) na bacia do rio Paraná superior: Rineloricaria pentamaculata sp. n. e Rineloricaria latirostris (Boulenger, 1900) Com Mus Cien PUCRS . 1994; 7:151-66.), by having five thin, dark-brown transversal stripes on the dorsal portion of the body (vs. six, of which the third and the fourth or the fifth and the sixth may be fused to varying degrees, but in such case always forming a broad bar).
Otothyrinae
Curculionichthys
Curculionichthys insperatus ( Britski, Garavello, 2003Britski HA, Garavello JC. Hisonotus insperatus: new species, from the upper rio Paraná basin (Pisces: Ostariophysi: Loricariidae). Copeia . 2003; (3):588-93. )
Body elongated; greatest depth contained 5.2 to 6.9 times in SL; head length 3.1 to 3.9, pectoral-fin length 3.7 and pelvic-fin length 4.9 to 6.1 in SL; snout length 1.4 to 1.6, horizontal orbital diameter 4.8 to 6.7 and least interorbital width 2.0 to 2.4 in HL. Mouth inferior; premaxilla with 10-12 and dentary with 8-11 teeth. Longitudinal series with 24 plates. Dorsal fin with I,7 rays, pectoral fin with I,6, pelvic fin with 6 rays and anal fin with I,5 rays (Britski, Garavello, 2003Britski HA, Garavello JC. Hisonotus insperatus: new species, from the upper rio Paraná basin (Pisces: Ostariophysi: Loricariidae). Copeia . 2003; (3):588-93.). Ground color yellowish to pale brown; dark-brown longitudinal band, from tip of snout to median caudal-fin rays; three dark-brown transverse bars on dorsum posteriorly to dorsal fin. Hyaline fins; dorsal fin with scattered spots; caudal fin with superior and inferior margins dark-brown; dark-brown transverse stripes on distal third of both upper and lower caudal-fin lobes, their margins hyaline.
Maximum standard length. 30.2 mm.
Biological data. Lives in streams with banks covered by terrestrial vegetation partially submerged into the water; in males the urogenital papilla is conspicuous and immediately posterior to the anal opening, whereas in females the urogenital duct is opened into the cloacal cavity (Britski, Garavello, 2003Britski HA, Garavello JC. Hisonotus insperatus: new species, from the upper rio Paraná basin (Pisces: Ostariophysi: Loricariidae). Copeia . 2003; (3):588-93.).
Distribution. Upper rio Paraná basin.
a. Curculionichthys insperatus, NUP 18971, 28.9 mm SL, córrego Dourado, tributary of the rio Paraná, Japorã, State of Mato Grosso do Sul. b. Otothyropsis marapoama, NUP 9394, 28.4 mm SL, córrego Baile, tributary of the rio Ivinheima, Nova Andradina, State of Mato Grosso do Sul. c. Otothyropsis polyodon, NUP 18972, 39.1 mm SL, córrego Guaçu, tributary of the rio Iguatemi, Mundo Novo, State of Mato Grosso do Sul. d. Rhinelepis aspera, 360.0 mm SL, fresh specimen, uncat.
Otothyropsis
1. Caudal fin with dark-brown spots, sometimes forming irregular transverse stripes; ventral caudal-fin lobe without a light-beige irregular blotch .................... O. marapoama
1’. Caudal fin without dark-brown spots; ventral caudal-fin lobe dark-brown, with a light-beige irregular blotch .................... O. polyodon
Otothyropsis marapoama Ribeiro, Carvalho, Melo, 2005
Body elongated; greatest depth contained 5.0 to 5.4 times in SL; head length 2.3 to 2.4, pectoral-fin length 2.8 to 3.3 and pelvic-fin length 4.6 to 5.0 in SL; snout length 1.9 to 2.0, horizontal orbital diameter 6.9 to 7.0 and least interorbital width 2.6 to 2.7 in HL. Mouth inferior; premaxilla with 15-17 and dentary with 15-21 teeth. Longitudinal series with 22 or 23 plates. Dorsal fin with I,7, pectoral fin with I,6, pelvic fin with 6 and anal fin with 6 rays. Groundo color yellowish to brown; Hyaline fins, scattered with small dark-brown spots.
Maximum standard length. 22.6 mm.
Distribution. Middle stretch of the rio Tietê, left bank of the rio Paraná, and streams of the rio Iguatemi and rio Ivinheima, both in right bank of the upper rio Paraná.
Otothyropsis polyodon Calegari, Lehmann A., Reis, 2013Calegari BB, Lehmann-Albornoz P, Reis RE. Two new species of cascudinhos of the genus Otothyropsis (Siluriformes: Hypoptopomatinae) from the rio Paraná basin, Brazil. Zootaxa . 2013; 3619(2):130-44.
Body elongated; greatest depth contained 6.8 to 8.1 times in SL; head length 2.6 to 3.0, pectoral-fin length 3.7 to 4.7 and pelvic-fin length 4.9 to 6.9 in SL; snout length 1.9 to 2.1, horizontal orbital diameter 6.1 to 7.5 and least interorbital width 2.6 to 3.1 in HL. Mouth inferior; premaxilla and dentary with 14-21 teeth. Longitudinal series with 24 or 25 plates. Dorsal fin with I,7, rarely 8, pectoral fin with I,6, pelvic fin with 6 and anal fin with 6 rays (Calegari et al., 2013Calegari BB, Lehmann-Albornoz P, Reis RE. Two new species of cascudinhos of the genus Otothyropsis (Siluriformes: Hypoptopomatinae) from the rio Paraná basin, Brazil. Zootaxa . 2013; 3619(2):130-44.). Ground color brown; dark-brown longitudinal stripe from snout to caudal fin. Hyaline fins, with dark-brown spots; upper caudal-fin lobe with dark-brown spot close to its tip; lower caudal-fin lobe dark, with hyaline portion on its lower portion, frequently with small dark-brown spots.
Maximum standard length. 37.9 mm.
Biological data. Males of O. polyodon have a conical urogenital papilla immediately posterior to the anus opening, longer pelvic-fin spine, shorter internarial distance, and shorter prenasal length (Calegari et al., 2013Calegari BB, Lehmann-Albornoz P, Reis RE. Two new species of cascudinhos of the genus Otothyropsis (Siluriformes: Hypoptopomatinae) from the rio Paraná basin, Brazil. Zootaxa . 2013; 3619(2):130-44.).
Distribution. Rio Ocoí, tributaries of the rio Verde and the rio Iguatemi drainage, right bank of the upper rio Paraná.
Remarks. Otothyropsis polyodon was identified as Hisonotus sp. by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Besides, it has been captured in the rio Iguatemi drainage, tributary of the right bank of the upper rio Paraná, since 2008 by V. F. B. Silva and collaborators, with its first cytogenetic record provided by Fernandes et al. (2016Fernandes CA, Bailly D, Sant’Ana DRA, Alves DS. First cytogenetic record for a species of Otothyropsis Ribeiro, Carvalho & Melo, 2005 (Loricariidae, Hypoptopomatinae). Neotrop Ichthyol [serial in the internet]. 2016 Apr 14 [cited 2016 Dec 08]; 14(1):e150123. Available from: Available from: http://dx.doi.org/10.1590/1982-0224-20150123
http://dx.doi.org/10.1590/1982-0224-2015...
).
Rhinelepinae
Rhinelepis
Rhinelepis aspera Spix, Agassiz, 1829
Body elongated; greatest depth contained 3.8 to 4.3 and caudal peduncle depth 7.4 to 8.3 times in SL; head length 2.5 to 3.1, predorsal distance 2.2 to 2.5, dorsal-fin spine length 4.4 o 4.7, pectoral-fin length 4.3 to 4.7, and caudal peduncle length 3.5 to 3.9 in SL; snout length 1.5 to 1.6, horizontal orbital diameter 10.0 to 10.8 and least interorbital width 1.6 to 2.0 in HL. Mouth inferior; premaxilla with 16-57 and dentary with 19-59 teeth. Mid-lateral series with 23-25, predorsal series with 3 or 4, and dorsal-fin base series with 6-8 plates. Dorsal fin with I,7 rays, pectoral fin with I,6 rays, pelvic fin with i,6 and anal fin with 6 rays. Ground color dark-grey, without blotches, as well as fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 440.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Rio São Francisco and upper rio Paraná basins.
Pimelodidae
Hemisorubim
Hemisorubim platyrhynchos (Valenciennes, 1840)
Body elongated; greatest depth contained 6.5 to 7.6, head depth 10.6 to 10.9, caudal-peduncle depth 14.7 to 15.8 times in SL; head length 2.9 to 3.4, anal-fin base length 12.1 to 12.3, adipose-fin base length 7.0 to 7.9, maxillary-barbel length 1.8 to 2.0 in SL; snout length 2.0 to 2.6, horizontal orbital diameter 5.5 to 5.8 and least interorbital width 4.8 to 5.4 in HL. Mouth prognathous, dentary slightly prognathous; dentigerous tooth plates present in both premaxilla and dentary. Dorsal fin with 7, pectoral fin with 11-16, pelvic fin with 7 and anal fin with 10-14 rays. Ground color yellowish-green; one or two black rounded blotch on flank; sometimes one black spot on superior portion of caudal peduncle. Yellowish fins, with or without dark-grey diffuse blotches (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 495.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Amazon and río Orinoco basins and Paraná-Paraguay system.
a. Hemisorubim platyrhynchos, 470.0 mm SL, fresh specimen, uncat. b. Hypophthalmus oremaculatus, 405.0 mm SL, fresh specimen, uncat. c. Iheringichthys labrosus, 200.0 mm SL, uncat. d. Megalonema platanum, 340.0 mm SL, fresh specimen, uncat.
Hypophthalmus
Hypophthalmus oremaculatus Nani, Fuster, 1947
Body elongate; greatest body depth contained 4.3 to 4.4, head depth 7.5 to 7.9, caudal-peduncle depth 11.8 to 12.0 times in SL; head length 4.1 to 4.3, anal-fin base length 2.0 to 2.2, adipose-fin base length 31.0 to 34.2, and maxillary-barbel length 3.6 to 4.2 in SL; snout length 2.0 to 2.2, horizontal orbital diameter 7.7 to 8.6, and least interorbital width 2.2 to 2.3 in HL. Mouth terminal; toothless. Dorsal fin with 7, pectoral fin with 16-18, pelvic fin with 7 and anal fin with 55-62 rays. Ground color greyish dorsally, whitish ventrally. Whitish fins with dark-grey margins.
Maximum standard length. 440.0 mm.
Distribution. Río Paraná basin.
Remarks. Hypophthalmus oremaculatus was identified as H. edentatus by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Littmann et al. (2015Littmann MW, Azpelicueta MM, Vanegas-Rios JA, Lundberg JG. Holotype-based validation, redescription and continental-scale range extension of the South American catfish species Hypophthalmus oremaculatus Nani and Fuster, 1947, with additional information on Hypophthalmus edentatus Spix and Agassiz, 1829 (Siluriformes, Pimelodidae). Proc Acad Nat Sci Philadelphia . 2015; 164(1):159-76.) redescribed H. oremaculatus, provided additional information on H. edentatus and proposed the new identification for the specimens from the río Paraná basin. Additionally, the same authors found an exceptional specimen in a tributary of the rio Paraná (NUP 1730) that matches the description of a new species (identified as Hypophthalmus cf. n. sp. 1, Littmann, Lundberg, in preparation) by having 61 total vertebrae, seven post-Weberian vertebrae, mental barbels not reaching pectoral fins, and dorsal-fin origin posterior to anal-fin origin (vs. 55 to 59 total vertebrae, 3 to 6 post-Weberian vertebrae, long mental barbels, extending to pectoral-fin origin or beyond, and dorsal-fin origin approximately on vertical through anal-fin origin, in H. oremaculatus). However, the new species is from the rio Orinoco and Amazon basins, and, at this moment, Littmann et al. (2015Littmann MW, Azpelicueta MM, Vanegas-Rios JA, Lundberg JG. Holotype-based validation, redescription and continental-scale range extension of the South American catfish species Hypophthalmus oremaculatus Nani and Fuster, 1947, with additional information on Hypophthalmus edentatus Spix and Agassiz, 1829 (Siluriformes, Pimelodidae). Proc Acad Nat Sci Philadelphia . 2015; 164(1):159-76.) only speculated that this specimen is possible a phenotypic outlier of H. oremaculatus, or a geographic outlier of Hypophthalmus n. sp. 1. Nevertheless, H. oremaculatus is a non-native species from the upper rio Paraná, and its occurrence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
Iheringichthys
Iheringichthys labrosus (Lütken, 1874)
Body elongate; greatest body depth contained 4.1 to 4.5, head depth 5.0 to 5.3, caudal peduncle depth 12.7 to 13.8 times in SL; head length 3.4 to 3.6, anal-fin base length 10.1 to 10.8, adipose-fin base length 4.1 to 4.4, and maxillary-barbel length 0.7 to 0.8 in SL; snout length 2.0 to 3.8, horizontal orbital diameter 3.7 to 3.9, and least interorbital width 3.5 to 4.0 in HL. Mouth subterminal; dentigerous tooth plates present in both premaxilla and dentary. Dorsal fin with I,7, pectoral fin with I,13, pelvic fin with 7, and anal fin with 9-10 rays. Ground color silvery; several longitudinal series of dark-brown blotches on flank. Hyaline fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 240.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system.
Megalonema
Megalonema platanum (Günther, 1880)
Body elongate; greatest body depth contained 5.3 to 5.7, head depth 6.9 to 7.9, caudal peduncle depth 12.9 to 13.2 times in SL; head length 3.7 to 3.9, anal-fin base length 10.6 to 11.5, adipose-fin base length 4.3 to 4.5, and maxillary-barbel 1.3 to 1.8 in SL; snout length 2.0 to 2.2, horizontal orbital diameter 4.5 to 5.0 and least interorbital width 4.5 to 4.7 in HL. Mouth terminal; dentigerous tooth plates present in both premaxilla and dentary. Dorsal fin with 7, pectoral fin with 14 or 15, pelvic fin with 7, and anal fin with 9 or 10 rays. Ground color light-grey. Yellowish or hyaline fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 400.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Paraná-Paraguay system.
Pimelodus
1. A dark-brown transverse bar extending from nape almost to the pelvic fin origin; black longitudinal stripes on posterior half of flank; a dark-brown spot covering middle portion of dorsal fin rays .................... P. ornatus
1’. Body with several small to large dark-brown rounded spots, aligned to form series or randomly distributed .................... 2
2. Spots very small, randomly distributed, present only on dorsal portion of body; vomer with large patches of teeth .................... P. paranaensis
2’. Spots medium to large, aligned or not, present from dorsal region to ventral from lateral line; vomer toothless or with only a very small patch of teeth .................... 3
3. Medium-sized spots usually smaller than the pupil, not aligned, fading into a diffuse longitudinal band from below adipose fin to end of caudal peduncle; interorbital distance contained 4.0 to 6.0 times in head length .................... P. microstoma
3’. Large-sized spot usually equal to or larger than eye diameter, aligned (especially those immediately dorsal and ventral to lateral line), not fading posteriorly; interobital distance contained 2.5 to 3.8 times in head length .................... 4
4. Serration present in the basal two thirds or more of the anterior margin of pectoral spine, well developed; a small patch of teeth present on vomer; interorbital distance contained 2.5 to 3.4 times in head length .................... P. mysteriosus
4’. Serration present in the basal one half or less of the anterior margin of pectoral spine, poorly developed; vomer toothless; interorbital distance contained 3.3 to 3.8 times in head length .................... P. maculatus
Pimelodus maculatus Lacépède, 1803
Body deep; greatest depth contained 3.7 to 4.0 times in SL; head length 3.3 to 3.7, anal-fin base length 7.4 to 8.6, adipose-fin base length 4.2 to 4.4, maxillary-barbel length 0.9 to 1.4 in SL; snout length 2.0 to 2.3, horizontal orbital diameter 4.5 to 6.0, least interorbital width 3.3 to 3.8 in HL (Deprá et al., 2015Deprá GC, Ota RR, Souza F, Graça WJ, Pavanelli CS. Widening the geographical distribution of Pimelodus mysteriosus Azpelicueta 1998 (Siluriformes: Pimelodidae) to the upper Paraná River, with diagnosis for syntopic congeners. Biota Neotropica . 2015; 15(3):1-6.); orbital diameter 0.8 to 1.2 in interorbital width. Mouth terminal; dentigerous tooth plates in both premaxilla and dentary; teeth absent in vomer and metapterygoid. Dorsal fin with I,6 rays, pectoral fin with I,11-14, pelvic fin with 6 and anal fin with 11-14 rays. Ground color yellowish; longitudinal series of dark-brown blotches. Dark-grey fins with black spots, especially on caudal and adipose fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 275.0 mm (Deprá et al., 2015Deprá GC, Ota RR, Souza F, Graça WJ, Pavanelli CS. Widening the geographical distribution of Pimelodus mysteriosus Azpelicueta 1998 (Siluriformes: Pimelodidae) to the upper Paraná River, with diagnosis for syntopic congeners. Biota Neotropica . 2015; 15(3):1-6.)
Distribution. Río de la Plata basin.
a. Pimelodus maculatus, NUP 12783, 214.4 mm SL, lagoa da Onça, Batayporã, State of Mato Grosso do Sul. b. Pimelodus microstoma, NUP 18158, 117.6 mm SL, rio Azul, Palotina, State of Paraná. c. Pimelodus mysteriosus, NUP 10824, 158.0 mm SL, lagoa do Guaraná, tributary of the rio Baía, Taquarussu, State of Mato Grosso do Sul. d. Pimelodus ornatus, 208.0 mm SL, fresh specimen, uncat. e. Pimelodus paranaensis, 168.0 mm SL, uncat.
Pimelodus microstoma Steindachner, 1877
Body deep; greatest body depth contained 4.1 to 5.2 times in SL; head length 3.5 to 3.9, anal-fin base length 7.5 to 11.7, adipose-fin base length 3.5 to 3.9, maxillary-barbel length 1.0 to 1.2 in SL; snout length 2.0 to 2.2, horizontal orbital diameter 3.8 to 5.1, and least interorbital width 4.0 to 6.0 in HL; orbital diameter 0.9 to 1.5 times in interorbital width. Mouth terminal; dentigerous tooth plates in both premaxilla and dentary. Dorsal fin with I,6, pectoral fin with 12-15, pelvic fin with 6, and anal fin with 11-14 rays (Azpelicueta, 2001Azpelicueta MM. A new species of Pimelodus (Siluriformes:Pimelodidae) from the upper Paraná basin, Brazil. Ichthyol Explor Freshw. 2001; 12(3):193-200.). Ground color greyish; eight or nine dark-brown longitudinal series of spots. Light-brown fins with few dark-brown spots, occasionally inconspicuous.
Maximum standard length. 190.0 mm.
Distribution. Upper rio Paraná basin.
Remarks. Pimelodus microstoma was identified as P. heraldoi by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Ribeiro, Lucena (2010Ribeiro FRV, Lucena CAS. Pimelodus heraldoi Azpelicueta, 2001, a junior synonym of Pimelodus microstoma Steindachner, 1877 (Siluriformes: Pimelodidae). Neotrop Ichthyol . 2010; 8(2):277-81.) proposed P. heraldoi as a junior-synonym of P. microstoma.
Pimelodus mysteriosus Azpelicueta, 1998Azpelicueta MM. A new species of Pimelodus (Siluriformes: Pimelodidae) from the Paraguay and lower Paraná rivers. Neotrópica. 1998; 44(111-112):87-94.
Body deep; greatest depth contained 3.3 to 4.1 times in SL; head length 3.1 to 3.9, anal-fin base length 6.4 to 8.8, adipose-fin base length 4.2 to 5.2, maxillary-barbel length 0.7 to 1.9 in SL; snout length 2.0 to 2.3, horizontal orbital diameter 3.2 to 5.8, least interorbital width 2.5 to 3.4 in HL; orbital diameter 0.9 to 1.2 in interorbital width. Mouth subterminal; dentigerous tooth plates in both premaxilla and dentary; teeth present in vomer and metapterygoid. Dorsal fin with I,6, pectoral fin with I,9, pelvic fin with 6 rays and anal fin with 16-19 rays. Ground color silvery to pale yellow; four dark-brown longitudinal series of blotches, usually equal to or larger than eye diameter. Dark-grey fins with black spots, especially on caudal and adipose fins.
Maximum standard length. 143.2 mm.
Biological data. Lives in the mainstream, rapids and near the shore; feeds on larvae, pieces of insects, gastropods, scales and eggs; the first gonadal maturation in females occurs with 120 mm SL and in males with 100 mm SL (Azpelicueta, 1998Azpelicueta MM. A new species of Pimelodus (Siluriformes: Pimelodidae) from the Paraguay and lower Paraná rivers. Neotrópica. 1998; 44(111-112):87-94.).
Distribution. Paraná-Paraguay system.
Remarks. Some specimens of P. mysteriosus were identified as P. maculatus by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Both species occur in the upper rio Paraná floodplain, but P. mysteriosus has been captured since 2003 by Nupélia staff. Deprá et al. (2015Deprá GC, Ota RR, Souza F, Graça WJ, Pavanelli CS. Widening the geographical distribution of Pimelodus mysteriosus Azpelicueta 1998 (Siluriformes: Pimelodidae) to the upper Paraná River, with diagnosis for syntopic congeners. Biota Neotropica . 2015; 15(3):1-6.) widened the distribution of P. mysteriosus to the upper rio Paraná basin, previously known only from the río Paraguay and lower rio Paraná basins. Pimelodus mysteriosus is a non-native species from the upper rio Paraná, and its occurrence can be associated with both the filling of the Itaipu Reservoir and the functioning of the Canal da Piracema (a fish ladder that connects the region downstream from the Itaipu Dam to the region upstream from the dam).
Pimelodus ornatus Kner, 1858
Body deep; greatest depth contained 3.9 to 4.2 times in SL; head length 3.1 to 3.4, anal-fin base length 8.9 to 9.2, adipose-fin base length 4.9 to 6.0, maxillary-barbel length 1.6 to 1.8 in SL; snout length 2.1 to 2.3, horizontal orbital diameter 5.0 to 5.2, least interorbital width 3.1 to .5 in HL; orbital diameter 1.5 to 1.7 in interorbital width. Mouth terminal; dentigerous tooth plates in both premaxilla and dentary. Dorsal fin with I,6, pectoral fin with I,13-15, pelvic fin with 6 and anal fin with 11-13 rays. Ground dark-brown; light-beige longitudinal band along lateral line; light-beige oblique bar from dorsal-fin origin to pelvic-fin origin. Hyaline fins; dorsal fin with dark-brown blotch; caudal fin with dark-brown longitudinal band on each lobe (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 330.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Widespread in South America rivers.
Remarks. Pimelodus ornatus is a non-native species from the upper rio Paraná and its occurrence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
Pimelodus paranaensis Britski, Langeani, 1988Britski HA, Langeani F. Pimelodus paranensis, sp. n., um novo Pimelodidae (Pisces, Siluriformes) do alto Paraná, Brasil. Rev Bras Zool. 1988; 5(3):409-17.
Body deep; greatest depth contained 3.7 to 5.2 times in SL; head length 3.1 to 3.4, anal-fin base length 8.4 to 9.0, adipose-fin base length 4.1 to 4.6, maxillary-barbel length 1.4 to 1.8 in SL; snout length 2.1 to 2.3, horizontal orbital diameter 3.5 to 5.8, least interorbital width 4.0 to 5.8 in HL; orbital diameter 0.7 to 1.5 in interorbital width. Mouth subterminal; dentigerous tooth plates in both premaxilla and dentary. Dorsal fin with I,6, pectoral fin with I,9 or 10, pelvic fin with 6 and anal fin with 11-13 rays (Britski, Langeani, 1988Britski HA, Langeani F. Pimelodus paranensis, sp. n., um novo Pimelodidae (Pisces, Siluriformes) do alto Paraná, Brasil. Rev Bras Zool. 1988; 5(3):409-17.). Ground color light-beige; several dark-brown spots. Yellowish or brown fins, sometimes with dark-brown spots (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 190.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná basin.
Pinirampus
Pinirampus pirinampu (Agassiz, 1829)
Body deep; greatest depth contained 5.1 to 5.3 times in SL; head length 4.2 to 4.5, anal-fin base length 7.6 to 8.5, adipose-fin base length 2.3 to 2.6, maxillary-barbel length 1.1 to 1.4 in SL; snout length 2.0 to 2.2, horizontal orbital diameter 7.0 to 9.2, least interorbital width 2.6 to 2.9 in HL; orbital diameter 2.6 to 3.5 in interorbital width. Mouth terminal; dentigerous tooth plates in both premaxilla and dentary. Dorsal fin with 7, pectoral fin with 13-17, pelvic fin with 7 and anal fin with 11-15 rays. Ground color greyish to pale brown. Dorsal, pectoral and caudal fins grey, pelvic and anal fins hyaline (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 680.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Amazon and río Essequibo basins, and Paraná-Paraguay system.
a. Pinirampus pirinampu, 620.0 mm SL, fresh specimen, uncat. b. Pseudoplatystoma corruscans, 675.0 mm SL, fresh specimen, uncat. c. Pseudoplatystoma reticulatum, 580.0 mm SL, fresh specimen, uncat. d. Sorubim lima, 487.0 mm SL, fresh specimen, uncat. e. Steindachneridion scriptum, 320.0 mm SL, uncat. f. Zungaro jahu, 800.0 mm SL, fresh specimen, uncat.
Pseudoplatystoma
1. Body with several dark-brown blotches .................... P. corruscans
1’. Body with several dark-brown loop-like transverse bars .................... P. reticulatum
Pseudoplatystoma corruscans (Spix, Agassiz, 1829)
Body deep; greatest depth contained 5.8 to 6.3 times in SL; head length 2.4 to 3.2, anal-fin base length 8.4 to 8.8, adipose-fin base length 10.7 to 14.4, maxillary-barbel length 2.4 to 2.9 in SL; snout length 2.0 to 2.2, horizontal orbital diameter 9.5 to 10.8, least interorbital width 4.4 to 4.8 in HL; orbital diameter 4.4 to 4.8 in interorbital width. Mouth subterminal, premaxilla slightly longer than dentary dentigerous tooth plates in both premaxilla and dentary. Dorsal fin with 7, pectoral fin with I,9, pelvic fin with 7 and anal fin with 12-15 rays. Ground color dark-grey above lateral line, whitish below; several dark-brown blotches on body. Hyaline fins with few black spots (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 860.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. São Francisco basin and Paraná-Paraguay system.
Pseudoplatystoma reticulatum Eigenmann,
Eigenmann, 1889
Body deep; greatest depth contained 5.5 to 6.0 times in SL; head length 2.6 to 2.7, anal-fin base length 8.0 to 8.9, adipose-fin base length 11.0 to 13.0, maxillary-barbel length 2.2 to 2.5 in SL; snout length 2.0 to 2.3, horizontal orbital diameter 10.0 to 10.8, least interorbital width 4.0 to 4.9 in HL; orbital diameter 2.4 to 2.5 in interorbital width. Mouth subterminal; dentigerous tooth plates in both premaxilla and dentary. Dorsal fin with 7, pectoral fin with I,8 or 9, pelvic fin with 7 and anal fin with 14-17 rays. Ground color dark-grey above lateral line, whitish below; dark-brown loop-like transverse bars from posterior opercular flap to caudal peduncle. Dorsal and anal fins with black spots; pectoral and pelvic fins dark-grey dorsally, pale ventrally, with few black spots (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 800.0 mm.
Distribution. Central Amazon and rio Paraná basins.
Remarks. Pseudoplatystoma reticulatum was identified as P. fasciatum by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Buitrago-Suárez, Burr (2007Buitrago-Suárez UR, Burr BM. Taxonomy of the catfish genus Pseudoplatystoma Bleeker (Siluriformes: Pimelodidae) with recognition of eight species. Zootaxa. 2007; 1512:1-38.), in a taxonomic study of Pseudoplatystoma Bleeker, 1862, restricted the distribution of P. fasciatum to the Guyana region and proposed the new identification for the specimens from the rio Paraná basin. Pseudoplatystoma reticulatum is a non-native species from the upper rio Paraná, and its occurrence can be associated with the functioning of the Canal da Piracema, a fish ladder that connects the region downstream from the Itaipu Dam to the region upstream from the dam.
Sorubim
Sorubim lima (Bloch, Schneider, 1801)
Body elongated; greatest depth contained 6.2 to 7.8 times in SL; head length 2.8 to 3.0, anal-fin base length 7.0 to 7.3, adipose-fin base length 14.6 to 15.0, maxillary-barbel length 2.3 to 3.0 in SL; snout length 2.0 to 2.2, horizontal orbital diameter 9.3 to 10.5, least interorbital width 2.7 to 3.0 in HL; orbital diameter 1.9 to 2.4 in interorbital width. Mouth subterminal, premaxilla much longer than dentary; dentigerous tooth plates in both premaxilla and dentary. Dorsal fin with I,6, pectoral fin with I,8-10, pelvic fin with 7 and anal fin with 17-19 rays. Ground color dark-brown dorsally, whitish ventrally; two dark-brown longitudinal bands, with irregular borders, one along lateral line to median caudal-fin rays and another on dorsum. Hyaline fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 605.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Amazon, río Orinoco, río Paraná and rio Paranaíba basins.
Remarks. Sorubim lima is a non-native species from the upper rio Paraná, and its occurrence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
Steindachneridion
Steindachneridion scriptum (Miranda-Ribeiro, 1918)
Body deep; greatest depth contained 5.8 to 6.3 times in SL; head length 2.4 to 3.2, anal-fin base length 8.4 to 8.8, adipose-fin base length 8.0 to 10.0, maxillary-barbel length 2.4 to 2.9 in SL; snout length 2.0 to 2.2, horizontal orbital diameter 9.5 to 10.8, least interorbital width 4.4 to 4.8 in HL; orbital diameter 1.9 to 2.4 in interorbital width. Mouth terminal; dentigerous tooth plates in both premaxilla and dentary. Dorsal fin with 7, pectoral fin with I,9, pelvic fin with 7 and anal fin with 12-15 rays. Ground color greyish; dark-brown spots and irregular stripes on body. Dark-grey fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 640.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río Uruguay and upper rio Paraná basins.
Zungaro
Zungaro jahu (Ihering, 1898)
Body deep; greatest depth contained 3.5 to 3.8 times in SL; head length 2.9 to 3.1, anal-fin base length 9.0 to 9.3, adipose-fin base length 5.9 to 6.2 and maxillary-barbel length 1.9 to 2.2 in SL; snout length 2.6 to 2.8, horizontal orbital diameter 9.9 to 11.0 and least interobital width 2.7 to 3.1 in HL. Mouth terminal, tooth plates on maxilla and dentary. Dorsal fin with I,6, pectoral fin with I,11 or 12, pelvic fin with 7 rays and anal fin with 12-15 rays. Ground color pale yellow to pale green; dark-brown irregular blotches on body. All fins dark-grey.
Maximum standard length. 830.0 mm.
Distribution. Paraná-Paraguay system.
Remarks. Zungaro jahu was identified as Z. zungaro by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Lundberg, Littmann (2003Lundberg JG, Littmann MW. Family Pimelodidae. In: Reis RE, Kullander SO, Ferraris CJ Jr., organizers. Check list of the freshwater fishes of South and Central America. Porto Alegre: Edipucs ; 2003. p.432-446.) considered the specimens from the rio Paraná-Paraguay basin as Z. jahu, and the specimens from the Amazon and rio Orinoco basin as Z. zungaro.
Pseudopimelodidae
Microglanis
Microglanis garavelloi Shibatta, Benine, 2005Shibatta OA, Benine RC. A new species of Microglanis (Siluriformes: Pseudopimelodidae) from upper rio Paraná basin, Brazil. Neotrop Ichthyol . 2005; 3(4):579-85.
Body depressed anteriorly, compressed posteriorly; greatest depth contained 4.5 to 5.2 and caudal peduncle depth 6.0 to 9.3 times in SL; head length 3.3 to 4.0, adipose fin base length 3.6 to 7.0 and maxillary-barbel length 2.5 to 4.2 in SL; snout length 2.2 to 2.7, horizontal orbital diameter 6.2 to 10.9 and least interorbital width 1.8 to 2.4 in HL. Mouth terminal, teeth small and villiform; dorsal fin with I,6, pectoral fin with I,5, pelvic fin with 6 rays and anal fin with 6 rays (Shibatta, Benine, 2005Shibatta OA, Benine RC. A new species of Microglanis (Siluriformes: Pseudopimelodidae) from upper rio Paraná basin, Brazil. Neotrop Ichthyol . 2005; 3(4):579-85.). Ground color yellowish to pale brown; brown saddles on dorsal region. All fins with dark-brown spots; dorsal fin with two brown longitudinal bands, one past middle and another along base; caudal fin with two brown transverse bars, one at base and another past middle; adipose fin with brown transverse bar in middle.
Maximum standard length. 41.8 mm.
Biological data. Lives in the marginal vegetation of the streams (Shibatta, Benine, 2005Shibatta OA, Benine RC. A new species of Microglanis (Siluriformes: Pseudopimelodidae) from upper rio Paraná basin, Brazil. Neotrop Ichthyol . 2005; 3(4):579-85.).
Distribution. Rio Paranapanema, rio Tietê basins and rio Iguatemi.
a. Microglanis garavelloi, córrego Dourado, tributary of the rio Iguatemi, Japorã, State of Mato Grosso do Sul. b. Pseudopimelodus mangurus, 180.0 mm SL, uncat. c. Rhyacoglanis paranensis, NUP 14877, 42.7 mm SL, rio Curimba, tributary of the rio Ivaí, Umuarama, State of Paraná. d. Scoloplax empousa, NUP 17582, 19.6 mm SL, córrego Guaçu, tributary of the rio Abambai, Itaquirai, State of Mato Grosso do Sul. e. Paravandellia oxyptera, NUP 313, 18.0 mm SL, Itaipu Reservoir, Foz do Iguaçu, State of Paraná. f. Trichomycterus davisi, 80.9 mm SL, uncat. g. Trichomycterus diabolus, NUP 10046, 83.5 mm SL, rio Iguatemi, tributary of the rio Paraná, Sapucaia, Mato Grosso do Sul State.
Pseudopimelodus
Pseudopimelodus mangurus (Valenciennes, 1835)
Body deep; greatest depth contained 3.6 to 6.9 times in SL; head length 3.2 to 4.5, adipose fin base length 10.7 to 14.4 and maxillary-barbel length 1.1 to 1.6 in SL; snout length 2.4 to 3.0, horizontal orbital diameter 13.8 to 17.9 and least interorbital width 1.9 to 2.5 in HL. Mouth terminal, dentigerous tooth plates in both premaxilla and dentary. Dorsal fin with 7, pectoral fin with I,7, pelvic fin with 6 and anal fin with 8-13 rays (Shibatta, 1999Shibatta OA. Sistemática e evolução da família Pseudopimelodidae (Ostariophysi, Siluriformes), com a revisão taxonômica do gênero Pseudopimelodus. [PhD Thesis]. São Carlos, SP: Universidade Federal de São Carlos; 1999.). Ground color pale yellow; dorsal and lateral surfaces of head completely grey; four dark-brown transverse bars on flank, one from occipital process to lateral line, one below dorsal fin, one below adipose fin and another on posterior portion of caudal peduncle and caudal-fin base. Dorsal fin with two dark-brown longitudinal bands, one past middle and another along base; anal fin with two dark-brown stripes, one at base, other past middle; caudal fin with dark-brown transverse bar on lobes not united with dark-brown transverse bar on posterior portion of caudal peduncle.
Maximum standard length. 210.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río de la Plata basin.
Rhyacoglanis
Rhyacoglanis paranensis
Shibatta, Vari, 2017Shibatta AO, Vari RP. A new genus of Neotropical rheophilic catfishes, with four new species (Teleostei: Siluriformes: Pseudopimelodidae). Neotrop Ichthyol [serial in the internet]. 2017 Jun 12 [cited 2018 Jan 15]; 15(2):e160132. Available from: Available from: http://dx.doi.org/10.1590/1982-0224-20160132
.
http://dx.doi.org/10.1590/1982-0224-2016...
Body deep; greatest depth contained 4.2 to 5.1 and caudal peduncle 9.6 to 10.6 times in SL; head length 2.9 to 3.2, anal-fin base length 7.6 to 9.9, adipose-fin base length 4.2 to 5.2 and maxillary-barbel length 3.2 to 4.6 in SL; snout length 2.4 to 2.9, horizontal orbital diameter 6.2 to 9.4 and least interobital width 3.1 to 3.3 in HL. Mouth terminal, tooth plates on maxilla and dentary. Dorsal fin with I,6, pectoral fin with I,6, pelvic fin with 6 rays and anal fin with 8 or 9 rays. Ground color pale yellow; dorsal and lateral surfaces of head grey, with light-beige blotch on cheek; four dark-brown transverse bars on flank, one from occipital process to lateral line, one below dorsal-fin, one below adipose fin and another on posterior portion of caudal peduncle. Dorsal fin dark-brown, with distal margin hyaline (occasionally, hyaline blotch on middle of last ray); pectoral and pelvic fins with one dark-brown stripe across middle; anal fin with two dark-brown stripes, one at base, other past middle; caudal fin with dark-brown transverse bar past middle, united with dark-brown transverse bar posterior portion of caudal peduncle and caudal-fin base by thin stripe along median caudal-fin rays, demarcating one rounded light-beige blotch on each caudal-fin lobe.
Maximum standard length. 42.0 mm.
Distribution. Upper rio Paraná basin.
Remarks. Rhyacoglanis paranensis has been captured in the rio Iguatemi, right bank of the upper rio Paraná, since 2008 by V. F. B. Silva and collaborators. Shibatta, Vari (2017Shibatta AO, Vari RP. A new genus of Neotropical rheophilic catfishes, with four new species (Teleostei: Siluriformes: Pseudopimelodidae). Neotrop Ichthyol [serial in the internet]. 2017 Jun 12 [cited 2018 Jan 15]; 15(2):e160132. Available from: Available from: http://dx.doi.org/10.1590/1982-0224-20160132
.
http://dx.doi.org/10.1590/1982-0224-2016...
) described the new genus with a new species from the upper rio Paraná basin.
Scoloplacidae
Scoloplax
Scoloplax empousa Schaefer, Weitzman, Britski, 1989
Body depressed anteriorly, compressed posteriorly; greatest depth contained 5.7 to 6.8 times in SL; head length 3.7 to 4.2, pectoral fin length 4.0 to 4.2 and pelvic fin length 4.7 to 5.5 in SL; snout length 3.4 to 5.1 and least interorbital width 2.9 to 3.6 in HL. Mouth inferior, premaxilla and dentary with several small teeth. Dorsal fin with I,4, pectoral fin with I,5, pelvic fin with I,3 or 4 and anal fin with 6 rays (Britski et al., 2007Britski HA, Silimon KZS, Lopes BS. Peixes do Pantanal: manual de identificação. Brasília, DF: Embrapa Pantanal; 2007.). Ground color dark-brown, darker dorsally, lighter ventrally. Caudal-fin base with dark-brown blotch, followed by light-beige area alternating with dark-brown area; pelvic fin with dark-brown spots; dorsal, pectoral and anal fins irregularly spotted, with light-beige areas alternating with dark-brown areas (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 24.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Amazon basin and Paraná-Paraguay system.
Trichomycteridae
Paravandellia
Paravandellia oxyptera Miranda-Ribeiro, 1912
Body elongated; greatest depth contained 10.8 to 11.9 times in SL; head length 4.8 to 5.1 and predorsal distance 1.4 to 1.6 in SL; snout length 2.5 to 2.9, horizontal orbital diameter 3.6 to 3.9 and least interorbital width 7.0 to 7.2 in HL. Mouth inferior; premaxilla and dentary with several small teeth, sometimes covered by skin. Dorsal fin with 12, pectoral fin with 7, pelvic fin with 5 or 6 and anal fin with 9 or 10 rays. Ground color whitish to pale yellow; dark-brown longitudinal band, from opercle to vertical through pelvic-fin origin. Hyaline fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 27.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río de la Plata basin.
Trichomycterus
1. Caudal fin sometimes spotted and with no light-beige transverse bar .................... T. davisi
1’. Caudal fin mostly hyaline, with a light-beige transverse bar .................... T. diabolus
Trichomycterus davisi (Haseman, 1911)
Body cylindric anteriorly, compressed posteriorly; greatest depth contained 7.4 to 7.7 times in SL, head length 5.1 to 5.7 and predorsal distance 1.5 to 1.7 in SL; snout length 2.2 to 3.2, horizontal orbital diameter 7.2 to 9.3 and least interorbital width 3.7 to 3.8 in HL. Mouth terminal; premaxilla and dentary with several rows of conical teeth. Dorsal fin with 10, pectoral fin with 7, pelvic fin with 5 and anal fin with 9 or 10 rays. Ground color yellow to pale brown; dark-brown blotches over whole body. Caudal fin pale brown, mostly hyaline, sometimes with small dark-brown spots.
Maximum standard length. 100.0 mm.
Distribution. Iguaçu, Ribeira de Iguape and upper rio Paraná basin.
Remarks. Trichomycterus davisi was identified as Trichomycterus sp. by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Apparently, the specimens assigned as T. davisi belongs to a species complex.
Trichomycterus diabolus Bockmann, Casatti, de Pinna, 2004
Body cylindric anteriorly, compressed posteriorly; greatest depth contained 5.8 to 8.5 times in SL, head length 3.9 to 4.5 and predorsal distance 1.5 to 1.6 in SL; snout length 5.3 to 9.1, horizontal orbital diameter 3.2 and least interorbital width 4.0 to 5.1 in HL. Mouth terminal; premaxilla and dentary with several rows of conical teeth. Dorsal fin with 9 or 10, pectoral fin with 6, pelvic fin with 5 and anal fin with 8 rays. Ground color yellow to pale brown; dark-brown blotches over whole body. Caudal fin pale brown, mostly hyaline, with dark-brown transverse bar close to its distal margin
Maximum standard length. 54.0 mm.
Biological data. Rheophilic species, occurring in the riffles areas of the streams (Bockmann et al., 2004Bockmann FA, Casatti L, de Pinna MCC. A new species of trichomycterid catfish from the rio Paranapanema basin, Southeastern Brazil (Teleostei: Siluriformes), with comments on the phylogeny of the family. Ichthyol Explor Freshw . 2004; 15(3):225-42.). Feeds on aquatic insects in the dry season and on fish scales or fin rays in the wet season; first gonadal maturation size 37.7 mm SL in females and 31.8 mm SL in males (Casatti, 2003Casatti L. Biology of a catfish, Trichomycterus sp. (Pisces, Siluriformes), in a pristine Stream in the Morro do Diabo State Park, Southeastern Brazil. Stud Neotrop Fauna Environ. 2003; 38(2):105-10.).
Distribution. Córrego São Carlos and córrego São Pedro, tributaries of the rio Paranapanema, and rio Iguatemi, tributary of the rio Paraná.
SYNBRANCHIFORMES
Synbranchidae
Synbranchus
Synbranchus marmoratus Bloch, 1795
Body elongated, snake-shaped; greatest body depth contained 28.0 to 32.0 times in SL; head length 9.0 to 9.3 in SL; snout length 3.3 to 4.3 and least interorbital width 3.7 to 4.2 in HL. Mouth terminal; premaxilla and dentary with several small teeth. Ground color yellowish, dorsum dark-grey or -brown; several dark-grey or -brown spots on body (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 550.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Drainages from Mexico to Argentina.
a. Synbranchus marmoratus, NUP 414, 500.0 mm TL, lagoas (ilhas), Porto Rico, State of Paraná. b. Catathyridium jenynsii , NUP 1921, 107.6 mm SL, rio Paraná, Porto Rico, State of Paraná.
PLEURONECTIFORMES
Achiridae
Catathyridium
Catathyridium jenynsii (Günther, 1862)
Body deep; greatest body depth contained 1.5 to 1.8, caudal peduncle depth 4.1 to 6.0 and head depth 2.8 to 3.4 times in SL; caudal peduncle depth 2.7 to 4.0 in body depth; snout length 2.8 to 4.0, horizontal orbital diameter 8.7 to 15.9 and least interorbital width 6.1 to 11.8 in HL. Mouth terminal; premaxilla and dentary with several small conical teeth. Lateral line with 70-85 pored scales. Dorsal fin with 55-60, pelvic fin with 5 and anal fin with 40-44 rays. Ground color brownish; right side of body with dark-brown irregular spots; five or six dark-brown transverse stripes; left side of body with no pigmentation. Brown fins with dark-brown spots (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 256.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Rio Paraná and rio Uruguay basins.
Remarks. Catathyridium jenynsii is a non-native species from the upper rio Paraná and its occurrence can be associated with the filling of the Itaipu Reservoir and the consequent inundation of the Sete Quedas Falls.
CICHLIFORMES
Cichlidae
Aequidens
Aequidens plagiozonatus Kullander, 1984Kullander SO. Cichlid fishes from the La Plata basin. Part V. Description of Aequidens plagiozonatus sp.n. (Teleostei, Cichlidae) from the Paraguay River system. Zool Scr. 1984, 13(3):155-59.
Body deep; greatest body depth contained 2.0 to 2.3 and caudal peduncle depth 5.3 to 5.9 times in SL; head length 3.0 to 3.3 and caudal peduncle length 8.8 to 11.4 in SL; snout length 3.6 to 4.6, horizontal orbital diameter 2.5 to 3.0 and least interorbital width 2.4 to 2.7 in HL. Mouth terminal; premaxilla and dentary with 3 or 4 teeth rows anteriorly. Upper lateral line with 16 or 17, lower lateral line with 7 or 8 pored scales and longitudinal series with 24-26 scales. Transversal series above upper lateral line with 2½ or 3 and below lower lateral line with 4½ or 5 scale rows. Dorsal fin with XIII-XV,10-12, pectoral fin with 13 or 14, pelvic fin with I,5 and anal fin with III,8-10 rays (Kullander, 1984Kullander SO. Cichlid fishes from the La Plata basin. Part V. Description of Aequidens plagiozonatus sp.n. (Teleostei, Cichlidae) from the Paraguay River system. Zool Scr. 1984, 13(3):155-59.). Ground color yellowish-white to pale brown; dark-brown longitudinal band discontinuous; dark-brown transverse bars oblique on flank; dark-brown mid-lateral blotch; dark-brown blotch at base of upper caudal-fin lobe. Caudal fin with dark-brown spots only on its proximal portion; soft portion of dorsal anal fins with white spots.
Maximum standard length. 103.3 mm.
Biological data. Lives in shallow environments and open areas (Tondato et al., 2013Tondato KK, Fantin-Cruz I, Pedrollo OC, Súarez YR. Spatial distribution of fish assemblages along environmental gradients in the temporary ponds of Northern Pantanal, Brazil. J Limnol. 2013; 72(1):95-102.).
Distribution. Upper rio Paraná and upper rio Paraguai basins.
Remarks. Aequidens plagiozonatus has been captured in the upper rio Paraná floodplain since 2014 by the Nupélia staff. This is the first record of A. plagiozonatus to the upper rio Paraná basin, and no records of this species in the lower rio Paraná are known. Therefore, A. plagiozonatus is considered a non-native species in the region, and its recent occurrence can be associated with the aquarium trade.
a. Aequidens plagiozonatus, NUP 16948, 56.8 mm SL, lagoa sem nome, tributary of rio Baía, Taquarussu, State of Mato Grosso do Sul. b. Apistogramma commbrae, NUP 6525, 32.5 mm SL, rio Paraná, Porto Rico, State of Paraná. c. Astronotus crassipinnis, 172.3 mm SL, fresh specimen, uncat. d. Chaetobranchopsis australis, NUP 9366, 95.2 mm SL, córrego Juqueri, tributary of the rio Guiraí, Novo Horizonte do Sul, State of Mato Grosso do Sul. e. Cichla kelberi, 190.0 mm SL, fresh specimen, uncat. f. Cichla piquiti, 240.0 mm SL, fresh specimen, uncat.
Apistogramma
Apistogramma commbrae (Regan, 1906)
Body elongate; greatest depth contained 2.6 to 3.0, head depth 3.5 to 4.0 and caudal peduncle depth 6.0 to 7.0 times in SL; head length 2.8 to 3.2 and caudal peduncle length 6.4 to 8.1; snout length 5.5 to 6.2, horizontal orbital diameter 2.4 to 2.7 and least interorbital width 3.9 to 4.2 in HL. Mouth terminal; premaxilla and dentary with 3 or 4 teeth rows anteriorly. Upper lateral line with 5-15, lower lateral line with 6-9 pored scales and longitudinal series with 21 or 22 scales. Transversal series above upper lateral with 2½ or 3 and below lower lateral line with 2½ or 3 scale rows. Dorsal fin with XV-XVII,5-7, pectoral fin with 11 or 12, pelvic fin with I,5 and anal fin with III-IV,4-7 rays (Kullander, 1982aKullander SO. Cichlid fishes from the La Plata basin. Part II. Apistogramma commbrae (Regan, 1906) (Teleostei: Cichlidae). Rev Suisse Zool. 1982a; 89(1):33-48.). Ground color whitish to yellowish; dark-brown suborbital stripe; dark-brown longitudinal band, from posterior margin of orbit to caudal peduncle, and seven dark-brown transverse bars on flank; dark-brown rounded blotch on caudal-fin base; three or four dark-brown longitudinal abdominal stripes. First two or three spines of dorsal fin, outer margin of pelvic fin and distal margin of anal fin dark-brown.
Maximum standard length. 33.0 mm.
Biological data. Feeding habit iliophagous and detritivorous (Pereira et al., 2012Pereira RC, Rosa FR, Resende EK. Estrutura trófica da comunidade de peixes de riachos da porção Oeste da bacia do alto Paraná. Corumbá: Embrapa Pantanal; 2012. (Boletim de Pesquisa e Desenvolvimento; no. 117).).
Distribution. Río de la Plata basin.
Remarks. Apistogramma commbrae has been captured in the upper rio Paraná floodplain since 2007 by the Nupélia staff. Apistogramma commbrae is a possible non-native species in the upper rio Paraná basin, and its recent occurrence can be associated with the functioning of the Canal da Piracema (a fish ladder that connects the region downstream from the Itaipu Dam to the region upstream from the dam), or with the aquarium trade.
Astronotus
Astronotus crassipinnis (Heckel, 1840)
Body deep; greatest body depth contained 1.8 to 2.2 and head depth 3.0 to 3.2 times in SL; head length 2.4 to 3.2 in SL; snout length 2.9 to 3.1, horizontal orbital diameter 4.6 to 5.2 and least interorbital width 2.1 to 2.2 in HL. Mouth terminal; outer row of premaxilla with 17-20, and outer row of dentary with 15-20 teeth, followed by three rows of conical teeth. Upper lateral line with 20 or 21, lower lateral line with 22 pored scales and longitudinal series with 14-15 scales. Transversal series above upper lateral line with 5 or 5½ scale rows, and below lower lateral line with 14 or 15 scale rows. Dorsal fin with XIV,19-21, pectoral fin with 15 or 16, pelvic fin with I,6 and anal fins with III,15-17 rays. Ground color dark-brown to black; several light-beige irregular transverse bars; superior portion of caudal-fin base with black rounded ocelli, with orange margins. Dark-grey fins, sometimes dorsal, anal and caudal fins with distal margin hyaline. Males in reproductive period with yellowish-red spots on body (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 302.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Amazon basin and Paraná-Paraguay system.
Remarks. Astronotus crassipinnis is a non-native species in the upper rio Paraná basin, and its recent occurrence can be associated with the aquarium trade.
Chaetobranchopsis
Chaetobranchopsis australis Eigenmann, Ward, 1907
Body deep; greatest body depth contained 1.8, head depth 2.3 and caudal peduncle depth 5.5 times in SL; head length 2.5 and caudal peduncle length 8.3 in SL; snout length 2.7, horizontal orbital diameter 3.5 and least interorbital width 2.1 to in HL. Mouth terminal; premaxilla and dentary with many teeth rows. Upper lateral line with 20, lower lateral line with 10 pored scales and longitudinal series with 24-26 scales. Transversal series above upper lateral line with 4 scale rows, and below lower lateral line with 7½ scale rows. Dorsal fin with XV,14, pectoral fin with 14, pelvic fin with I,5 and anal fin with V,16 rays. Ground color pale brown; two dark-brown longitudinal bands on flank, one superior, from orbit to caudal fin, and another inferior, from opercle to lower caudal-fin lobe; seven brown inconspicuous bars. Dorsal fin with dark-brown spots; caudal fin with dark-brown longitudinal stripes alternating with white stripes.
Maximum standard length. 110.0 mm.
Distribution. Paraná-Paraguay system.
Remarks. Only one specimen of C. australis was captured in a tributary of the rio Ivinheima, right bank of the upper rio Paraná, in 2007 by Y. R. Súarez and collaborators. This is the first record of C. australis in the upper rio Paraná basin, and no records of this species in the lower rio Paraná are known. Therefore, it is considered a non-native species in the region, and its recent occurrence can be associated with the proximities of the headwaters of the rio Ivinheima (rio Paraná basin) with some tributaries of the río Paraguay, or with the aquarium trade. Commonly, C. australis present the two longitudinal bands much less conspicuous, and a prominent, clearly visible, black blotch coinciding with the superior band, longitudinally, and, with the encounter of the second and third bars, transversely. Nevertheless, the specimen recorded herein has an unusual color pattern, presenting the longitudinal bands very conspicuous, and no distinct black blotch.
Cichla
1. Flank with three or four dark-grey transverse bars; longitudinal series with 70 to 85 scales .................... C. kelberi
1’. Flank with six or more dark-grey transverse bars; longitudinal series with 89 to 98 scales .................... C. piquiti
Cichla kelberi Kullander, Ferreira, 2006
Body deep; greatest depth contained 2.3 to 3.7, head depth 3.0 to 3.2 and caudal peduncle depth 8.2 to 9.3 times in SL; head length 2.8 to 3.2 and caudal peduncle length 5.0 to 6.7; snout length 2.5 to 3.0, dentary length 1.9 to 2.2, horizontal orbital diameter 3.4 to 6.2 and least interorbital width 3.2 to 4.4 in HL. Mouth terminal; premaxilla with 2 or 3 and dentary with 3 or 4 teeth rows. Upper lateral line with 70-85 pored scales, lower lateral line with 52-59 pored scales and longitudinal series with 70-80 scales. Transversal series above upper lateral with 10-14 and below lower lateral line with 17-23 scale rows. Dorsal fin with XIV-XVI,12-17, pectoral fin with 14-16, pelvic fin with I,6 and anal fin with II-III,9-11 rays. Ground color yellowish-green; three or four dark-brown transverse bars; several light-beige irregular spots on body; dark-brown rounded ocelli, with yellow or white margins, on caudal-fin base. Brown or grey fins; dorsal fin with white scattered spots (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 445.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Rio Tocantins-Araguaia basin.
Remarks. Cichla kelberi is a non-native species from the upper rio Paraná and its occurence can be associated with escapes from recreational angling ponds.
Cichla piquiti Kullander, Ferreira, 2006
Body deep; greatest depth contained 3.4 to 3.5, head depth 2.8 to 3.1 and caudal peduncle depth 9.1 to 9.5 times in SL; head length 3.1 to 3.3 and caudal peduncle length 6.9 to 7.4; snout length 2.7 to 2.8, dentary length 2.0 to 2.1, horizontal orbital diameter 4.7 to 4.8 and least interorbital width 4.2 to 4.3 in HL. Mouth terminal; premaxilla with 2 or 3 and dentary with 3 or 4 teeth rows. Upper lateral line with 89-98 pored scales, lower lateral line with 58-65 pored scales and longitudinal series with 87-93 scales. Transversal series above upper lateral with 13-15 and below lower lateral line with 20-25 scale rows. Dorsal fin with XIV-XVI,16-18, pectoral fin with 14-16, pelvic fin with I,6 and anal fin with II-III,9-11 rays. Ground color greyish-blue; six or more dark-brown transverse bars; several light-beige irregular spots on body; dark-brown rounded ocelli, with yellow or white margins, on caudal-fin base. Brown or grey fins; dorsal fin with white scattered spots (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 280.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Rio Tocantins-Araguaia basin.
Remarks. Cichla piquiti is a non-native species from the upper rio Paraná and its occurence can be associated with escapes from recreational angling ponds.
Cichlasoma
Cichlasoma paranaense Kullander, 1983Kullander SO. A revision of the South American cichlid genus Cichlasoma (Teleostei: Cichlidae). Stockholm: Naturhistoriska Riksmuseet; 1983.
Body deep; greatest depth contained 1.7 to 2.5, head depth 2.5 to 3.1 and caudal peduncle depth 4.9 to 6.4 times in SL; head length 1.3 to 2.4 and caudal peduncle length 10.2 to 16.4; snout length 3.0 to 3.6, horizontal orbital diameter 3.5 to 4.4 and least interorbital width 4.2 to 5.0 in HL. Mouth terminal; premaxilla with 2 or 3 and dentary with 3 or 4 teeth rows. Upper lateral line with 14-17 pored scales, lower lateral line with 5-8 pored scales and longitudinal series with 22-23 scales. Transversal series above upper lateral with 2½-4 and below lower lateral line with 5-7 scale rows. Dorsal fin with XIII-XV,10-15, pectoral fin with 12-13, pelvic fin with I,6 and anal fin with III,8-10 rays (Kullander, 1983Kullander SO. A revision of the South American cichlid genus Cichlasoma (Teleostei: Cichlidae). Stockholm: Naturhistoriska Riksmuseet; 1983.). Ground color iridescent green; dark-brown transverse bars; dark-brown rounded blotch, slightly below upper lateral line; dark-brown blotch on superior portion of caudal peduncle (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 171.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río Paraná basin.
a. Cichlasoma paranaense, 110.0 mm SL, fresh specimen, uncat. b. Coptodon rendalli, 150.0 mm SL, uncat. c. Crenicichla britskii, 130.0 mm SL, fresh specimen, uncat. d. Crenicichla haroldoi, NUP 59, 104.3 mm SL, rio Baía, Taquarussu, State of Mato Grosso do Sul. e. Crenicichla jaguarensis, NUP 10797, 78.3 mm SL, rio Keller, tributary of the rio Ivaí, Itambé, State of Paraná. f. Crenicichla jupiaensis, NUP 5445, 75.8 mm SL, rio Piquiri, Nova Laranjeiras, State of Paraná. g. Crenicichla sp., 145.5 mm SL, uncat.
Coptodon
Coptodon rendalli (Boulenger, 1897)
Body deep; greatest body depth contained 2.4 to 2.8, head depth 3.2 to 3.6 and caudal peduncle depth 6.1 to 7.2 times in SL; head length 2.8 to 3.3 and caudal peduncle length 6.5 to 7.5 in SL; snout length 2.3 to 2.9, horizontal orbital diameter 2.0 to 4.6 and least interorbital width 2.4 to 3.8 to in HL. Mouth terminal; premaxilla and dentary with one or two teeth rows. Upper lateral line with 20 or 21, lower lateral line with 11-14 pored scales and longitudinal series with 28-33 scales. Transversal series above upper lateral line with 3½-5 scale rows and below lower lateral line with 5-7 scale row. Dorsal fin with XV-XVI,11-13 rays, pectoral fin with 11-13, pelvic fin with I,5 and anal fin with III,8-10 rays (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Ground color greyish to pale brown; black rounded blotch on posterior margin of opercle; dark-brown transverse bars on flank. Dorsal, anal and pelvic fins hyaline and scattered with dark-brown spots.
Maximum standard length. 170.0 mm.
Distribution. Africa, widely introduced everywhere (Eschmeyer et al., 2017Eschmeyer WN, Fricke R, van der Laan R, editors. Catalog of fishes: genera, species, references [Internet]. San Francisco: California Academy of Science; 2017 [updated 2017 Jan 31; cited 2017 Feb 24]. Available from: Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
http://researcharchive.calacademy.org/re...
), including Brazil.
Remarks. Coptodon rendalli was identified as Tilapia rendalli (Boulenger, 1897) by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Dunz, Schliewen (2013Dunz AR, Schliewen UK. Molecular phylogeny and revised classification of the haplotilapiine cichlid fishes formerly referred to as “Tilapia”. Mol Phylogenet Evol. 2013; 68(1):64-80.), in a molecular phylogeny of the specimens commonly known as “Tilapia”, proposed the new combination. Coptodon rendalli is an African species, widely introduced in South America by fish farming.
Crenicichla
1. Black humeral blotch present .................... C. britskii
1’. Black humeral blotch absent .................... 2
2. Flank with dark-brown transverse stripes (thin), sometimes fused to each other; suborbital stripe absent or reduced .................... C. jupiaensis
2’. Flank with dark-brown transverse bars (broad), never fused each other; suborbital stripe present .................... 3
3. Black or brown spots absent from lateral-line pores; dark-brown longitudinal band always absent on flank; 58-67 (mode 60) scales on longitudinal series (immediately above the lower lateral line); 9-12 (mode 10) gill rakers on ceratobranchial 1; snout straight, in lateral view .................... Crenicichla sp.
3’. Black or brown spots present on lateral-line pores; dark-brown longitudinal band present or absent (in some specimens of C. haroldoi, which always have very conspicuous black spots on lateral-line pores); 46-61 scales on longitudinal series; 6-9 gill rakers on ceratobranchial 1; snout slightly convex, in lateral view .................... 4
4. Inconspicuous brown spots on the lateral line pores; 46-58 scales (mode 55) on longitudinal series; 6-9 (mode 8) gill rakers on ceratobranchial 1 .................... C. jaguarensis
4’. Conspicuous black spots on the lateral line pores; 52-61 scales on longitudinal series (mode 58) on longitudinal series; 6-8 (mode 7) gill rakers on ceratobranchial 1 .................... C. haroldoi
Crenicichla britskii Kullander, 1982
Body deep; greatest depth contained 3.2 to 3.9, head depth 5.0 to 5.5 and caudal peduncle depth 7.1 to 8.1 times in SL; head length 2.9 to 3.1 and caudal peduncle length 7.8 to 8.3 in SL; snout length 1.5 to 2.3, horizontal orbital diameter 3.0 to 4.2 and least interorbital width 3.5 to 4.0 in HL. Mouth terminal; premaxilla and dentary with arranged in several teeth rows. Upper lateral line with 20-22 pored scales, lower lateral line with 7-9 pored scales and longitudinal series with 33-40 scales. Transversal series above upper lateral with 3 to 4½ and below lower lateral line with 7-9 scale rows. Dorsal fin with XVI,14-16, pectoral fin with 14 or 15, pelvic fin with I,5 and anal fin with III,9-11 rays (Kullander, 1982bKullander SO. Cichlid fishes from the La Plata basin. Part III. The Crenicichla lepidota species group (Teleostei: Cichlidae). Rev Suisse Zool . 1982b; 89(3):627-61.). Ground color greyish to greenish; dorsum with dark-brown transverse bars; dark-grey suborbital stripe; dark-brown discontinuous longitudinal band, sometimes inconspicuous, from snout to caudal peduncle; black rounded humeral blotch; black rounded ocelli on caudal-fin base. Dorsal, anal and caudal fins with white spots; pectoral and pelvic fins yellowish or hyaline (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 176.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper río Paraná basin.
Crenicichla haroldoi Luengo, Britski, 1974
Body elongate; greatest depth contained 4.1 to 5.0, head depth 4.9 to 5.4 and caudal peduncle depth 8.4 to 9.6 times in SL; head length 3.0 to 3.1 and caudal peduncle length 7.5 to 8.2 in SL; snout length 3.7 to 4.0, horizontal orbital diameter 4.0 to 4.1 and least interorbital width 4.7 to 6.5 in HL. Mouth terminal; premaxilla and dentary with several teeth rows. Upper lateral line with 22-26 pored scales, lower lateral line with 10-11 pored scales; longitudinal series with 52-61 scales. Transversal series above upper lateral with 3 or 3½ and below lower lateral line with 6-9 scale rows. Dorsal fin with XX-XXXII,9-11, pectoral fin with 15-17, pelvic fin with I,5 and anal fin with III,7-9 rays (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.; Varella, 2011Varella HR. Revisão taxonômica das espécies de Crenicichla Heckel das bacias dos rios Paraná e Paraguai (Teleostei: Cichlidae). [Master Dissertation], São Paulo, SP: Universidade de São Paulo; 2011 Oct 07 [cited 2016 Dec 08]. Available from: Biblioteca digital Universidade de São Paulo. Available from: Biblioteca digital Universidade de São Paulo. http://www.teses.usp.br/teses/disponiveis/41/41133/tde-20012012-142913/pt-br.php
http://www.teses.usp.br/teses/disponivei...
). Ground color greenish; black suborbital stripe; dark-brown longitudinal band, from opercle to caudal peduncle; dark-brown transverse bars on flank; black spots on lateral line scales. Soft portion of dorsal and anal fins with dark-brown spots; black rounded blotch on caudal-fin base (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 171.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Upper rio Paraná basin.
Crenicichla jaguarensis Haseman, 1911
Body elongated; greatest body depth contained 4.0 to 5.3, head depth 5.8 to 7.1 and caudal peduncle depth 8.2 to 10.2 times in SL; head length 2.9 to 3.4 and caudal peduncle length 5.4 to 7.6 in SL; snout length 3.1 to 4.7, horizontal orbital diameter 4.2 to 6.0 and least interorbital width 6.7 to 7.8 in HL. Mouth terminal; premaxilla and dentary with many teeth rows. Upper lateral line with 22-26 and lower lateral line with 10-13 pored scales, and longitudinal series with 46-58 scales. Transversal series above upper lateral with 3 or 3½ and below lower lateral line with 6-9 scale rows. Dorsal fin with XIX-XXI,10-12, pectoral fin with 15-17, pelvic fin with I,5 and anal fin with III,8-10 rays (Varella, 2011Varella HR. Revisão taxonômica das espécies de Crenicichla Heckel das bacias dos rios Paraná e Paraguai (Teleostei: Cichlidae). [Master Dissertation], São Paulo, SP: Universidade de São Paulo; 2011 Oct 07 [cited 2016 Dec 08]. Available from: Biblioteca digital Universidade de São Paulo. Available from: Biblioteca digital Universidade de São Paulo. http://www.teses.usp.br/teses/disponiveis/41/41133/tde-20012012-142913/pt-br.php
http://www.teses.usp.br/teses/disponivei...
). Ground color yellowish green; black suborbital stripe; dark-brown longitudinal band, from opercle to caudal peduncle; dark-brown transverse bars on flank; brown spots on lateral line scales. Soft portion of dorsal and anal fins with dark-brown spots, sometimes forming stripes; black rounded blotch on caudal-fin base.
Maximum standard length. 196.6 mm.
Distribution. Upper rio Paraná basin.
Remarks. Some specimens of C. jaguarensis were identified as C. haroldoi by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Both species occur in the upper rio Paraná floodplain, where C. jaguarensis has been captured since 2004 by the Nupélia staff. Crenicichla jaguarensis can be distinguished by presenting brown spots on the lateral line scales less conspicuous and less evident, 46-58 scales (mode 55) on longitudinal series and 6-9 (mode 8) gill rakers on ceratobranchial 1 (vs. spots on lateral line scales black and conspicuous spots on lateral line, 52-61 scales on longitudinal series (mode 58) on longitudinal series; 6-8 (mode 7) gill rakers on ceratobranchial 1 in C. haroldoi; Varella, 2011Varella HR. Revisão taxonômica das espécies de Crenicichla Heckel das bacias dos rios Paraná e Paraguai (Teleostei: Cichlidae). [Master Dissertation], São Paulo, SP: Universidade de São Paulo; 2011 Oct 07 [cited 2016 Dec 08]. Available from: Biblioteca digital Universidade de São Paulo. Available from: Biblioteca digital Universidade de São Paulo. http://www.teses.usp.br/teses/disponiveis/41/41133/tde-20012012-142913/pt-br.php
http://www.teses.usp.br/teses/disponivei...
).
Crenicichla jupiaensis Britski, Luengo, 1968
Body elongated; greatest body depth contained 2.8 to 3.0, head depth 4.9 to 5.4 and caudal peduncle depth 8.4 to 9.6 times in SL; head length 2.8 to 3.0 and caudal peduncle length 7.5 to 8.2 in SL; snout length 3.7 to 4.0, horizontal orbital diameter 4.0 to 4.1 and least interorbital width 4.7 to 6.5 in HL. Mouth terminal; premaxilla and dentary with many teeth rows. Upper lateral line with 20-22, lower lateral line with 9-11 pored scales, and longitudinal series with 488-56 scales. Transversal series above upper lateral line with 3 or 3½ scale rows, and below lower lateral line with 6-9 scale rows. Dorsal fin with XVIII-XX,9-11, pectoral fin with 16 or 17, pelvic fin with I,5 and anal fin with III,7 or 8 rays. Ground color dark-brown; black dark-brown longitudinal and discontinuous band, from opercle to caudal peduncle; 14-17 dark-brown transverse stripes, sometimes fused each other. Dark fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 150.0 mm.
Distribution. Upper rio Paraná basin.
Crenicichla sp.
Body elongated; greatest body depth contained 4.1 to 4.3, head depth 5.0 to 5.4 and caudal peduncle depth 8.4 to 9.6 times in SL; head length 2.6 to 3.0 and caudal peduncle length 7.5 to 8.5 in SL; snout length 3.1 to 4.0, horizontal orbital diameter 4.1 to 4.7 and least interorbital width 5.4 to 5.9 in HL. Mouth terminal; premaxilla and dentary teeth arranged in many rows. Upper lateral line with 26-28 and lower lateral line with 12-14 pored scales and longitudinal series with 58-67 scales. Transversal series above upper lateral line with 3 or 3½ scale rows, and below lower lateral line with 5-7 scale rows. Dorsal fin with XIX,11, pectoral fin with 12-15, pelvic fin with I,5 and anal fin with III,7 rays (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Ground color pale brown; black suborbital stripe; dark-brown longitudinal band, from opercle to caudal peduncle; dark-brown transverse bars on flank. Soft portion of dorsal and anal fins with dark-brown spots, sometimes forming stripes; pectoral and pelvic fins yellowish or hyaline; black rounded blotch on caudal-fin base. Additional blotches and ocelli may appear in reproductive period, mainly on fins.
Maximum standard length. 195.4 mm.
Distribution. Upper rio Paraná and tributaries of the stretch from Guaíra to the Itaipu Reservoir.
Remarks. Crenicichla sp. was identified as C. niederleinii by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Varella (2011Varella HR. Revisão taxonômica das espécies de Crenicichla Heckel das bacias dos rios Paraná e Paraguai (Teleostei: Cichlidae). [Master Dissertation], São Paulo, SP: Universidade de São Paulo; 2011 Oct 07 [cited 2016 Dec 08]. Available from: Biblioteca digital Universidade de São Paulo. Available from: Biblioteca digital Universidade de São Paulo. http://www.teses.usp.br/teses/disponiveis/41/41133/tde-20012012-142913/pt-br.php
http://www.teses.usp.br/teses/disponivei...
), in a revisionary study of the Crenicichla Heckel, 1840 from the Paraná-Paraguay system, considered C. niederleinii a nomen dubium from the rio Uruguay basin, and assigned the specimens from the upper rio Paraná basin to a new species, in process of formal description.
Geophagus
1. A grey to black suborbital stripe; body with light-beige longitudinal bands, sometimes bluish in live specimens; upper lateral line with 17 to 19 pored scales; longitudinal series with 24 to 27 scales .................... G. brasiliensis
1’. Suborbital stripe absent; body with several red or orange longitudinal bands in live specimens; upper lateral line with 21 to 23 pored scales; longitudinal series with 32 to 34 scales .................... G. sveni
Geophagus brasiliensis (Quoy, Gaimard, 1824)
Body deep; greatest depth contained 2.2 to 2.5 times in SL; head length 2.4 to 3.1 in SL; snout length 1.7 to 2.4, horizontal orbital diameter 2.9 to 4.7 and least interorbital width 2.7 to 3.2 in HL. Mouth terminal; premaxilla with 2 or 3 and dentary with 2 to 4 teeth rows. Upper lateral line with 17-19 pored scales, lower lateral line with 10-14 pored scales, and longitudinal series with 24-27 scales. Transversal series above upper lateral line with 4 scale rows and below lower lateral line with 5 scale rows. Dorsal fin with XVI-XVII,10-13, pectoral fin with 14 or 15, pelvic fin with I,5 and anal fin with III,7-10 rays. Ground color iridescent green; conspicuous dark-brown rounded blotch on flank; sometimes longitudinal rows of bluish spots on flank. Yellowish-red fins, with white small spots (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 175.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Coastal drainages of Eastern and Southern Brazil, río Uruguay basin and upper rio Paraná floodplain.
a. Geophagus brasiliensis, 148.0 mm SL, fresh specimen, uncat. b. Geophagus sveni, NUP 18698, 115.0 mm SL, rio Ivinheima, Naviraí, State of Mato Grosso do Sul. c. Gymnogeophagus setequedas, 81.3 mm SL, fresh specimen, uncat. d. Laetacara araguaiae, NUP 11315, 43.5 mm SL, upper rio Sucuriú, Paraíso, State of Mato Grosso do Sul. e. Oreochromis niloticus, NUP 1132, 185.0 mm SL, Corumbá Reservoir, Caldas Novas, State of Goiás. f. Satanoperca sp., 150.0 mm SL, fresh specimen, uncat.
Geophagus sveni Lucinda, Lucena, Assis, 2010Lucinda PHF, Lucena CAS, Assis NC. Two new species of cichlid fish genus Geophagus Heckel from the rio Tocantins drainage (Perciformes: Cichlidae). Zootaxa . 2010; 2429:29-42.
Body deep; greatest depth contained 2.1 to 2.5 times in SL; head length 3.0 to 3.3 in SL; snout length 1.5 to 2.4, horizontal orbital diameter 3.2 to 4.7 and least interorbital width 2.7 to 3.5 in HL. Mouth terminal; premaxilla and dentary with 2-4 teeth rows. Upper lateral line with 20-24 pored scales, lower lateral line with 17-19 pored scales, and longitudinal series with 32-34 scales. Transversal series above upper lateral line with 4 scale rows and below lower lateral line with 5 scale rows. Dorsal fin with XVI-XVIII,10-13 rays, pectoral fin with 15-16, pelvic fin with I,5 and anal fin with III,7-8 rays. Ground color greenish or silvery; iridescent marks on lachrymal, preopercle and opercle; five inconspicuous dark-brown transverse bars on flank and caudal peduncle; black rounded blotch on flank; six to 12 longitudinal series of orange spots on flank. Caudal fin reddish with four to seven iridescent blue stripes, occasionally broken into spots. Dorsal and anal fins with iridescent blue spots, sometimes forming horizontal stripes.
Maximum standard length. 200.0 mm.
Biological data. Feeds on sediment, decomposing organic matter, allochthonous plant fragments, mollusks, crustaceans, cladocerans, copepods and insects. Nests in the substrate, presents external fertilization and displays parental care. Both sexes may care for eggs and juveniles, and males usually defend the territory, while the female cares for the brood (Moretto et al., 2008Moretto EM, Marciano FT, Velludo MR, Fenerich-Verani N, Espíndola ELG, Rocha O. The recent occurrence, establishment and potential impact of Geophagus proximus (Cichlidae: Perciformes) in the Tietê River reservoirs: an Amazonian fish species introduced in the Paraná basin (Brazil). Biodivers Conserv. 2008; 17:3013-25.; Gois et al., 2015Gois KS, Pelicice FM, Gomes LC, Agostinho AA. Invasion of an Amazonian cichlid in the upper Paraná River: facilitation by dams and decline of a phylogenetically related species. Hydrobiologia. 2015; 746(1):401-13.).
Distribution. Rio Tocantins-Araguaia basin.
Remarks. Geophagus sveni was identified as G. cf. proximus by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Lucinda et al. (2010Lucinda PHF, Lucena CAS, Assis NC. Two new species of cichlid fish genus Geophagus Heckel from the rio Tocantins drainage (Perciformes: Cichlidae). Zootaxa . 2010; 2429:29-42.) described the new species from the rio Tocantins basin and distinguished it from G. proximus by not presenting a dark-grey preopercular mark. Geophaus sveni can also be distinguished by presenting four or five transverse parallel white stripes on the caudal fin, which can be broken into several spots (vs. parallel white stripes complete and horizontally directed on the caudal fin, in G. proximus) (Lucinda et al., 2010Lucinda PHF, Lucena CAS, Assis NC. Two new species of cichlid fish genus Geophagus Heckel from the rio Tocantins drainage (Perciformes: Cichlidae). Zootaxa . 2010; 2429:29-42.). A manuscript on the geographic distribution of G. sveni, with a genetic comparison between specimens from the rio Tocantins and from the upper rio Paraná floodplain and an analysis of the ontogenetic development of color patterns in this species is being prepared by GCD. Geophagus sveni is a non-native species from the upper rio Paraná basin, and its occurrence can be associated with the aquarium trade.
Gymnogeophagus
Gymnogeophagus setequedas Reis, Malabarba, Pavanelli, 1992Reis RE, Malabarba LR, Pavanelli CS. Gymnogeophagus setequedas, a new cichlid species (Teleostei: Labroidei) from middle rio Paraná system, Brazil and Paraguay. Ichthyol Explor Freshw . 1992; 3(3):265-72.
Body deep; greatest depth contained 2.1 to 2.6 and caudal peduncle depth 5.7 to 7.9 times in SL; head length 2.6 to 3.0 and caudal peduncle length 6.3 to .9 in SL; snout length 2.2 to 3.2, horizontal orbital diameter 2.7 to 3.7 and least interorbital width 3.0 to 4.0 in HL. Mouth terminal; premaxilla with 2 or 3 and dentary with 3 or 4 teeth rows. Upper lateral line with 13-18 pored scales, lower lateral line with 6-11 pored scales, and longitudinal series with 23-25 scales. Transversal series above upper lateral line with 3-5 scale rows and below lower lateral line with 7-9 scale rows. Dorsal fin with XII-XIV,9-11, pectoral fin with 11 or 13, pelvic fin with I,5 and anal fin with III-III,7-9 rays (Reis et al., 1992Reis RE, Malabarba LR, Pavanelli CS. Gymnogeophagus setequedas, a new cichlid species (Teleostei: Labroidei) from middle rio Paraná system, Brazil and Paraguay. Ichthyol Explor Freshw . 1992; 3(3):265-72.). Ground color iridescent green; dark-brown transverse bars on flank and caudal peduncle; dark-brown blotch on flank; black rounded blotch on superior portion of caudal peduncle. Hyaline fins with iridescent blue stripes or spots (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 98.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Río Paraná basin.
Laetacara
Laetacara araguaiae Ottoni, Costa, 2009Ottoni FP, Costa WJEM. Description of a new species of Laetacara Kullander, 1986 from central Brazil and re-description of Laetacara dorsigera (Heckel, 1840) (Labroidei: Cichlidae: Cichlasomatinae). Vertebr Zool. 2009; 59(1):41-48.
Body elongate; greatest depth contained 2.2 to 2.5, head depth 2.8 to 3.1 and caudal peduncle depth 5.4 to 6.1 times in SL; head length 2.6 to 3.2 and caudal peduncle length 6.8 to 8.8 in SL; snout length 3.1 to 3.7, horizontal orbital diameter 2.6 to 3.3 and least interorbital width 2.1 to 5.0 in HL. Mouth terminal; premaxilla with 2 and dentary with 2 or 3 conical teeth rows anteriorly. Upper lateral line with 12-15 pored scales, lower lateral line with 6 to 8 pored scales and longitudinal series with 22-25 scales. Transversal series above upper lateral line with 2½ to 3½ and below lower lateral line with 4 or 5 scale rows. Dorsal fin with XIV-XV,7-8, rarely 10, pectoral fin with 11-14, pelvic fin with I,5 and anal fin with III,7-9 rays (Ottoni, Costa, 2009Ottoni FP, Costa WJEM. Description of a new species of Laetacara Kullander, 1986 from central Brazil and re-description of Laetacara dorsigera (Heckel, 1840) (Labroidei: Cichlidae: Cichlasomatinae). Vertebr Zool. 2009; 59(1):41-48.). Ground color greyish; head and caudal peduncle with iridescent blue marks; seven dark-brown transverse bars on flank; one black rounded blotch on bar 5 and another at caudal-fin base. Dorsal and caudal fins yellowish with iridescent blue spots and distal margins iridescent blue; pectoral fin hyaline; pelvic and anal fins yellowish with iridescent blue stripes.
Maximum standard length. 34.0 mm.
Distribution. Rio Araguaia basin.
Remarks. Laetacara araguaiae was identified as Laetacara sp. by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Ottoni, Costa (2009Ottoni FP, Costa WJEM. Description of a new species of Laetacara Kullander, 1986 from central Brazil and re-description of Laetacara dorsigera (Heckel, 1840) (Labroidei: Cichlidae: Cichlasomatinae). Vertebr Zool. 2009; 59(1):41-48.) described the new species, L. araguaiae, from the rio Verde and rio Araguaia basins, and redescribed L. dorsigera (Heckel, 1840) from the Guaporé, Paraná-Paraguay system. It is a non-native species from the upper rio Paraná basin, and its occurrence can be associated with the aquarium trade.
Oreochromis
Oreochromis niloticus (Linnaeus, 1758)
Body deep; greatest body depth contained 2.3 to 2.9, head depth 3.0 to 4.0 and caudal peduncle depth 6.3 to 7.3 times in SL; head length 2.7 to 3.2 and caudal peduncle length 7.0 to 10.0 in SL; snout length 2.5 to 3.3, horizontal orbital diameter 3.1 to 5.0 and least interorbital width 2.2 to 3.2 to in HL. Mouth terminal; premaxilla and dentary with three or more teeth rows. Upper lateral line with 21-23, lower lateral line with 13-16 pored scales and longitudinal series with 30-35 scales. Transversal series above upper lateral line with 4-5 scale rows and below lower lateral line with 8-12 scale row. Dorsal fin with XVII-XVIII,11-15, pectoral fin with 14-16, pelvic fin with I,5 and anal fin with III,8-10 rays. Ground color greyish to pale brown; black rounded blotch on posterior margin of opercle; dark-brown transverse bars on flank. Dorsal, anal and pelvic fins hyaline and scattered with dark-brown spots; caudal fin with dark-brown spots united forming transverse stripes.
Maximum standard length. 200.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Africa, widely introduced everywhere (Eschmeyer et al., 2017Eschmeyer WN, Fricke R, van der Laan R, editors. Catalog of fishes: genera, species, references [Internet]. San Francisco: California Academy of Science; 2017 [updated 2017 Jan 31; cited 2017 Feb 24]. Available from: Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
http://researcharchive.calacademy.org/re...
), including Brazil.
Remarks. Oreochromis niloticus is an African species, widely introduced in South America by fish farming.
Satanoperca
Satanoperca sp.
Body deep; greatest body depth contained 2.0 to 2.8, head depth 2.5 to 3.3 and caudal peduncle depth 5.8 to 7.7 times in SL; head length 2.5 to 3.3 and caudal peduncle length 6.0 to 9.4 in SL; snout length 1.9 to 2.2, horizontal orbital diameter 2.8 to 3.7 and least interorbital width 3.3 to 3.6 in HL. Mouth terminal; premaxilla and dentary with one or two teeth rows. Upper lateral line with 17-21 and lower lateral line with 8-12 pored scales, and longitudinal series with 27-33 scales. Transversal series above upper lateral line with 3½-4 scale rows, and below lower lateral line with 6-9 scale rows. Dorsal fin with XIV-XVI,9-11, pectoral fin with 13-16, pelvic fin with I,5 and anal fin with III,6-8 rays (Ota, 2013Ota RR. Revisão taxonômica de Satanoperca Günther, 1862 (Perciformes, Cichlidae), com a descrição de três espécies novas. Unpublished [Master Dissertation]. Maringá, PR: Universidade Estadual de Maringá; 2013 Oct 04 [cited 2016 Dec 08]. Available from: Biblioteca digital Universidade Estadual de Maringá. Available from: Biblioteca digital Universidade Estadual de Maringá. http://nou-rau.uem.br/nou-rau/document/?code=vtls000211405
http://nou-rau.uem.br/nou-rau/document/?...
). Ground color greenish; dark-brown longitudinal band, from opercle to caudal peduncle; seven dark-brown transverse bars on flank. Soft portion of dorsal and anal fins with white spots; pelvic fin with iridescent blue stripe; black rounded blotch on base of upper caudal-fin lobe.
Maximum standard length. 173.0 mm.
Distribution. Rio Tocantins-Araguaia and upper rio Paraná basins.
Remarks. Satanoperca sp. was identified as S. pappaterra by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Ota (2013Ota RR. Revisão taxonômica de Satanoperca Günther, 1862 (Perciformes, Cichlidae), com a descrição de três espécies novas. Unpublished [Master Dissertation]. Maringá, PR: Universidade Estadual de Maringá; 2013 Oct 04 [cited 2016 Dec 08]. Available from: Biblioteca digital Universidade Estadual de Maringá. Available from: Biblioteca digital Universidade Estadual de Maringá. http://nou-rau.uem.br/nou-rau/document/?code=vtls000211405
http://nou-rau.uem.br/nou-rau/document/?...
), in a revisionary study of Satanoperca Günther, 1862, restricted the distribution of S. pappaterra to the rio Guaporé and río Paraguay basins, and proposed the new identification for the specimens from the upper rio Paraná floodplain. Satanoperca sp. is currently considered non-native from the upper rio Paraná (e.g. Langeani et al., 2007Langeani F, Castro RMC, Oyakawa OT, Shibatta OA, Pavanelli CS, Casatti L. Diversidade da ictiofauna do alto rio Paraná: composição atual e perspectivas futuras. Biota Neotropica , 2007; 7(3):181-97.; Kullander, 2012Kullander SO. A taxonomic review of Satanoperca (Teleostei: Cichlidae) from French Guiana, South America, with description of a new species. Cybium . 2012; 36(1):247-62.), and its occurence in the region has been associated with the filling of the Itaipu Reservoir (Júlio Jr. et al., 2009Júlio Júnior HF, Dei Tos C, Agostinho AA, Pavanelli CS. A massive invasion of fish species after eliminating a natural barrier in the upper rio Paraná basin. Neotrop Ichthyol . 2009; 7(4):709-18.), or with the introduction from other river basins.
CYPRINODONTIFORMES
Cynolebiidae
Melanorivulus
Melanorivulus sp.
Body elongated; greatest depth contained 4.1 to 4.6 and caudal peduncle depth 6.3 to 7.2 times in SL; head length 3.6 to 3.9, predorsal distance 1.2 to 1.3, prepelvic distance 1.7 to 1.9; dorsal-fin base length 8.6 to 10.0, anal-fin base length 4.2 to 5.1 and head width 4.6 to 5.1 times in SL; horizontal orbital diameter 2.8 to 3.3 in HL. Mouth terminal, premaxilla and dentary with several small teeth. Longitudinal series with 29-31 scales and transverse series with 8 or 9 scale rows. Dorsal fin with 8 or 9, pectoral fin with 11 or 12, pelvic fin with 6 or 7 and anal fin with 13-15 rays. Males with ground color greyish or pale brown, with dark-brown oblique and irregular stripes, V-shaped (its vertex turned forward); dark-brown transverse stripes on opercle; dorsal, anal and caudal fins with dark-brown transverse stripes. Females with similar color pattern, besides dark-brown rounded blotch on caudal-fin base.
Maximum standard length. 25.0 mm.
Distribution. Upper rio Paraná basin.
Remarks. Melanorivulus sp. was identified as Rivulus apiamici by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Costa (2011Costa WJEM. Phylogenetic position and taxonomic status of Anablepsoides, Atlantirivulus, Cynodonichthys, Laimosemion and Melanorivulus (Cyprinodontiformes: Rivulidae). Ichthyol Explor Freshw . 2011; 22(3):233-49.) included all species of the former Rivulus punctatus Boulenger, 1895 species complex in the genus Melanorivulus Costa, 2006, one of which is M. apiamici (Costa 1989). Recently, Nielsen (2016Nielsen DTB, Neves PABA, Ywamoto EV, Passos MA. Melanorivulus polychromus, a new species of killifish from the rio São José dos Dourados drainage, middle rio Paraná basin, southwestern Brazil, with a redescription of Melanorivulus apiamici Cyprinodontiformes: Rivulidae). Aqua. 2016; 22(2):79-88.) restricted the distribution of M. apiamici to the right bank of the upper rio Paraná, between Bataguassu and Três Lagoas, State of Mato Grosso do Sul. According to literature, the only two species known from the upper rio Paraná floodplain are the recently described Melanorivulus amambaiensis Volcan, Severo-Neto, Lanés 2018 and M. ivinhemensis Volcan, Severo-Neto, Lanés 2018. We analyzed preserved specimens from the rio Ivinhema (NUP 9350) and rio Amambaí (NUP 17575) basins, as well as from other localities in the upper rio Paraná floodplain (NUP 10022 and 12082). The specimens from these lots have 22-26 total caudal-fin rays, which is close to the counts recorded for M. amambaiensis in the original description, and less than the counts recorded for M. ivinhemensis. In fact, Melanorivulus specimens from other nearby localities available to us also show overlapping counts: from the rio Verde basin (NUP 6217), 24-30 rays; from the lower rio Paranapanema basin (NUP 6060, 6075, 7598), 22-28 rays. Thus, we prefer to use Melanorivulus sp. until more information, such as color pattern in life, is available.
a. Melanorivulus sp., NUP 17575, córrego Querência, Amambaí, State of Mato Grosso do Sul (top, female, 24.7 mm SL; bottom, male, 28.3 mm SL). b. Pamphorichthys hollandi, NUP 16549, rio Baia, Taquarussu, State of Mato Grosso do Sul, Brazil (top, female, 22.8 mm SL; bottom, male, 17.6 mm SL). c. Phalloceros harpagos (top, female, NUP 9360, 22.7 mm SL, female, córrego de nome desconhecido, Jatei, State of Mato Grosso do Sul; bottom, male, NUP 1938, 18.0 mm SL, riacho Caracu, Porto Rico, State of Paraná). d. Phallotorynus pankalos, NUP 5839, córrego Sossego, tributary of the rio Iguatemi, Paranhos, State of Mato Grosso do Sul (top, female, 28.4 mm SL; bottom, male, 18.6 mm SL). e. Phallotorynus victoriae, NUP 1603, lagoa do Portinho, tributary of the rio Paraná, Três Lagoas, State of Mato Grosso do Sul (top, female, 14.6 mm SL; bottom, male, 18.6 mm SL). f. Poecilia reticulata, fresh specimens, uncat (top, female, 35.2 mm SL; bottom, male, 20.3 mm SL). g. Plagioscion squamosissimus, 330.0 mm SL, fresh specimen, uncat.
Poeciliidae
Pamphorichthys
Pamphorichthys hollandi (Henn, 1916)
Body elongated; greatest depth contained 3.8 to 6.0 and caudal peduncle depth 5.1 to 6.1 times in SL; head length 3.1 to 4.2, predorsal distance 1.5 to 2.1, and gonopodium length 2.7 to 3.6 in SL; snout length 2.6 to 3.5, horizontal orbital diameter 1.8 to 2.5 and least interorbital width 1.5 to 2.1 in HL. Mouth superior, dentary prognathous; premaxilla and dentary with several small teeth. Longitudinal series with 29-31 scales and transverse series with 8 scale rows. Dorsal fin with 8 or 9 rays, pectoral fin with 9-12, pelvic fin with 6, and anal fin with 8 or 9 rays (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Ground color yellowish to pale brown; scales with dark-brown border, conferring reticulate pattern to body (except on ventral region, from one series below pectoral-fin origin). Fins yellowish; dorsal fin with dark-brown blotch on distal portion; males with dark-brown transverse bar on caudal-fin base.
Maximum standard length. 30.0 mm.
Distribution. Upper rio Paraná, rio Parnaíba and rio São Franciso basins.
Remarks. Pamphorichthys hollandi was identified as Pamphorichthys sp. by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Phalloceros
Phalloceros harpagos Lucinda, 2008Lucinda PHF. Systematics and biogeography of the genus Phalloceros Eigenmann, 1907 (Cyprinodontiformes: Poeciliidae: Poeciliinae), with the description of twenty-one new species. Neotrop Ichthyol . 2008; 6(2):113-58.
Body elongated; greatest depth contained 2.5 to 4.1 and caudal peduncle depth 5.4 to 8.1 times in SL; head length 4.0 to 5.6, predorsal distance 1.6 to 1.8, and gonopodium length 2.6 to 3.1 in SL; snout length 3.2 to 7.6, horizontal orbital diameter 1.9 to 3.0 and least interorbital width 2.3 to 2.4 in HL. Mouth superior, dentary prognathous; premaxilla and dentary with several small teeth. Longitudinal series with 26-32 scales and transverse series with 7-9 scale rows. Dorsal fin with 7-9, pectoral fin with 11-13, pelvic fin with 5 or 6 and anal fin with 8-10 (males) or 10-12 rays (females) (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.; Lucinda, 2008Lucinda PHF. Systematics and biogeography of the genus Phalloceros Eigenmann, 1907 (Cyprinodontiformes: Poeciliidae: Poeciliinae), with the description of twenty-one new species. Neotrop Ichthyol . 2008; 6(2):113-58.). Ground color yellowish brown; dark-brown lateral spot, vertically elongated; scales with dark-brown border, conferring reticulate pattern to body.
Maximum standard length. 45.2 mm.
Distribution. Paraná-Paraguay system and coastal drainages from the rio Itaboapana to the rio Araranguá.
Remarks. Phalloceros harpagos was identified as P. aff. caudimaculatus by Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.). Lucinda (2008Lucinda PHF. Systematics and biogeography of the genus Phalloceros Eigenmann, 1907 (Cyprinodontiformes: Poeciliidae: Poeciliinae), with the description of twenty-one new species. Neotrop Ichthyol . 2008; 6(2):113-58.), in a systematic study of Phalloceros, described the new species, P. harpagos, from the Paraná-Paraguay system and coastal drainages of Southern Brazil; and restricted the distribution of P. caudimaculatus to the rio Mampituba, Laguna dos Patos and rio Tramandaí drainages, lower río Uruguay and coastal drainages of Uruguay and Argentina.
Phallotorynus
1. One dark-brown spot on the lower half of the flank .................... P. victoriae
1’. Six to nine dark-brown spots on the lower half of the flank .................... P. pankalos
Phallotorynus pankalos Lucinda, Rosa, Reis, 2005Lucinda PHF, Rosa RS, Reis RE. Systematics and biogeography of the genus Phallotorynus Henn, 1916 (Cyprinodontiformes: Poeciliidae: Poeciliinae), with description of three new species. Copeia . 2005; (3):609-31.
Body elongated; greatest depth contained 4.0 to 5.0 and caudal peduncle depth 6.6 to 7.6 times in SL; head length 4.3 to 4.8, predorsal distance 1.5 to 1.6 in SL; snout length 3.3 to 3.9, horizontal orbital diameter 2.2 to 2.8 and least interorbital width 2.1 to 2.8 in HL. Mouth superior; premaxilla with 12 and dentary with 13 teeth. Longitudinal series with 28-30 scales and transverse series with 8 scale rows. Dorsal fin with 8 rays, pectoral fin with 10 or 11, pelvic fin with 5 and anal fin with 10 (males) or 11 (females) rays (Lucinda et al., 2005Lucinda PHF, Rosa RS, Reis RE. Systematics and biogeography of the genus Phallotorynus Henn, 1916 (Cyprinodontiformes: Poeciliidae: Poeciliinae), with description of three new species. Copeia . 2005; (3):609-31.; Lucinda, Graça, 2015Lucinda PHF, Graça WJ. Description of males of Phallotorynus pankalos Lucinda, Rosa & Reis, 2005 and reappraisal of Phallotorynus species relationships (Teleostei: Cyprinodontiformes: Poeciliidae). Neotrop Ichthyol . 2015; 13(1):87-92.). Ground color pale brown; scales with dark-brown border, conferring reticulate pattern to body; six to nine dark-brown rounded to irregular spots aligned on lower half of flank, alternated with dark-brown stripes.
Maximum standard length. 27.9 mm.
Biological data. First maturation size estimated at 18.2 mm SL for females, which possess small fecundity and high mortality rate after the first reproduction (Súarez et al., 2009Súarez YR, Silva JP, Vasconcelos LP, Antonialli Júnior WF. Ecology of Phallotorynus pankalos (Cyprinodontiformes: Poeciliidae) in a first-order stream of the upper Paraná basin. Neotrop Ichthyol . 2009; 7(1):49-54.).
Distribution. Only known from córrego Sossego, córrego Mirim and córrego Piraí, rio Iguatemi drainage.
Remarks. Phallotorynus pankalos has been captured in the rio Iguatemi drainage, right bank of the upper rio Paraná, since 2000 by Y. R. Súarez and collaborators. Lucinda et al. (2005Lucinda PHF, Rosa RS, Reis RE. Systematics and biogeography of the genus Phallotorynus Henn, 1916 (Cyprinodontiformes: Poeciliidae: Poeciliinae), with description of three new species. Copeia . 2005; (3):609-31.), in a systematic and biogeographic study of Phallotorynus Henn, 1916, described the new species. Another Phallotorynus species, possibly new according to P.H. Lucinda (in an e-mail, lucinda@mail.uft.edu.br, 2014), was captured in the rio Amambaí, right bank of the upper rio Paraná in 2014, also by Y. R. Súarez and collaborators. This species is similar to P. pankalos in the number of dark-brown spots on flank, but the first ones can be fused, forming a dark-brown stripe. Despite this, only two females were captured, and considering the major importance of males’ gonopodium morphology as diagnostic character, it was not included herein.
Phallotorynus victoriae Oliveros, 1983
Body elongated; greatest depth contained 3.0 to 3.5 and caudal peduncle depth 6.4 to 8.1 times in SL; head length 4.2 to 4.6, predorsal distance 1.7 to 1.8 in SL; snout length 4.0 to 5.7, and horizontal orbital diameter 2.2 to 2.6 in HL. Mouth superior; premaxilla and dentary with flattened and incisiform teeth. Longitudinal series with 26-30 scales and transverse series with 7-10 scale rows. Dorsal fin with 7-9 rays; pectoral fin with 9 to 12, pelvic fin with 5, anal fin with 10 or 11 (females) and 8 (males) (Lucinda et al., 2005Lucinda PHF, Rosa RS, Reis RE. Systematics and biogeography of the genus Phallotorynus Henn, 1916 (Cyprinodontiformes: Poeciliidae: Poeciliinae), with description of three new species. Copeia . 2005; (3):609-31.). Ground color silver-yellowish in life, and pale brown in alcohol; scales with dark-brown border, conferring reticulate pattern to body; dark-brown transverse stripes on flank; one dark-brown spot on lower half of flank, above anal fin. Dorsal-fin base hyaline, followed by dark-brown oblique stripe.
Maximum standard length. 18.0 mm.
Distribution. Río Paraná basin.
Poecilia
Poecilia reticulata Peters, 1859
Body elongated; greatest depth contained 3.4 to 4.4 and caudal peduncle depth 4.8 to 6.0 times in SL; head length 3.3 to 4.1, predorsal distance 1.5 to 1.8 and gonopodium length 3.2 to 3.6 in SL; snout length 2.1 to 3.1, horizontal orbital diameter 2.2 to 3.5 and least interorbital width 1.5 to 2.3 in HL. Mouth superior, dentary prognathous; premaxilla and dentary with several small teeth. Longitudinal series with 26-28 scales and transverse series with 8 or 9 scale rows. Dorsal fin with 8 or 9, pectoral fin with 13 or 14, pelvic fin with 6 and anal fin with 8 or 9 rays. Ground color pale yellow; scales with dark-brown border, conferring reticulate pattern to body; males with several black or colored spots and irregular stripes. Yellowish fins (males with black or colored spots); superior caudal-fin rays prolonged (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 38.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Drainages from Venezuela to río de la Plata basin.
Remarks. Poecilia reticulata is a non-native species from the upper rio Paraná and its occurrence can be associated with its introduction for insect larvae control.
INCERTAE SEDIS
Sciaenidae
Plagioscion squamosissimus (Heckel, 1840)
Body deep; greatest depth contained 2.9 to 3.1, head depth 3.4 to 3.6 and caudal peduncle depth 10.3 to 11.8 times in SL; head length 2.7 to 3.3, predorsal distance 1.5 to 1.8, prepectoral distance 1.7 to 2.2 in SL; snout length 3.7 to 4.2, horizontal orbital diameter 4.2 to 5.6 and least interorbital width 3.6 to 4.9 in HL. Mouth terminal; premaxilla and dentary with several teeth rows. Lateral line with 46-51 pored scales, transverse series above lateral with 12-15 and below lateral line with 15-20 scale rows. Dorsal fin with X,I,29-33, pectoral fin with 15-20, pelvic fin with I,6 and anal fin with II,6 rays. Ground color silvery. Light fins (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Maximum standard length. 512.0 mm (Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.).
Distribution. Amazon, río Orinoco and rio São Francisco basins, and Paraná-Paraguay system (Eschmeyer et al., 2017Eschmeyer WN, Fricke R, van der Laan R, editors. Catalog of fishes: genera, species, references [Internet]. San Francisco: California Academy of Science; 2017 [updated 2017 Jan 31; cited 2017 Feb 24]. Available from: Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
http://researcharchive.calacademy.org/re...
).
Remarks. Plagioscion squamosissimus is a non-native species from the upper rio Paraná and its occurrence can be associated with its introduction due to commercial importance.
Discussion
Our study indicated an expressive increase in the number of species in comparison with Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.), as well as an effective gain in the accuracy of geographic distribution and taxonomic information. The rio Iguatemi drainage had considerable influence on this increase, considering that 12 of the 34 new records were found in its streams, probably due to the improvement of sampling in this region (i.e., Bryconamericus turiuba, Characidium gomesi, Corydoras sp., Curculionichthys insperatus, Eigenmannia guairaca, Ictalurus punctatus, Otothyropsis marapoama, O. polyodon, Microglanis garavelloi, Phallotorhynus pankalos, Rhyacoglanis paranensis and Trichomycterus diabolus).
Therefore, the need of conservation actions directed to the tributaries of the upper rio Paraná, including the rio Iguatemi must be emphasized. This river basin, like many others in the region, is impacted by human activities (e.g. deforestation and siltation by agricultural activities) that threaten species such as P. pankalos, which is only known from the rio Iguatemi basin, and presents small fecundity and high mortality rate of females after the first reproduction (Súarez et al., 2009Súarez YR, Silva JP, Vasconcelos LP, Antonialli Júnior WF. Ecology of Phallotorynus pankalos (Cyprinodontiformes: Poeciliidae) in a first-order stream of the upper Paraná basin. Neotrop Ichthyol . 2009; 7(1):49-54.; Lucinda, Graça, 2015Lucinda PHF, Graça WJ. Description of males of Phallotorynus pankalos Lucinda, Rosa & Reis, 2005 and reappraisal of Phallotorynus species relationships (Teleostei: Cyprinodontiformes: Poeciliidae). Neotrop Ichthyol . 2015; 13(1):87-92.). Moreover, these tributaries have a major importance as migratory routes and reproductive habitats for some species, such as spawning areas (Suzuki et al., 2009Suzuki HI, Agostinho AA, Bailly D, Gimenes MF, Júlio Júnior HF, Gomes LC. Inter-annual variations in the abundance of young-of-the-year of migratory fishes in the upper Paraná River floodplain: relations with hydrographic atributes. Brazil J Biol. 2009; 69(2, Suppl.):649-60.; Reynalte-Tataje et al., 2012Reynalte-Tataje DA, Agostinho AA, Bialetzki A. Temporal and spatial distributions of the fish larval assemblages of the Ivinheima River sub-basin (Brazil). Environ Biol Fishes 2012; 96(7):811-22.); and the floodplain itself represents an important area for juveniles’ growth and for the adults to feed and recover (Nakatani et al., 1997Nakatani K, Baumgartner G, Cavicchioli M. Ecologia de ovos e larvas de peixes. In: Vazzoler AEAM, Agostinho AA, Hahn NS, editors. A planície de inundação do alto rio Paraná: aspectos físicos, biológicos e socioeconômicos. Maringá: Eduem ; 1997. p.281-306.; Vazzoler et al., 1997Vazzoler AEAM, Suzuki HI, Marques EE, Lizama MLAP. Primeira maturação gonadal, períodos e áreas de reprodução. In: Vazzoler AEAM, Agostinho AA, Hahn NS, editors. A planície de inundação do alto rio Paraná: aspectos físicos, biológicos e socioeconômicos . Maringá: Eduem ; 1997. p.249-265.).
The new records of I. punctatus and Clarias gariepinus, both non-native species from Brazil, are alarming and are associated with fish-farming (A. A. Agostinho, oral communication, 2016) and escapes from recreational angling ponds (Vitule et al., 2006Vitule JRS, Umbria SC, Aranha JMR. Introduction of the African catfish Clarias gariepinus (Burchell, 1822) into Southern Brazil. Biol Invasions. 2006; 8:677-81.; Cruz-Spindler et al., 2012Cruz-Spindler S, Leal ME, Lehmann-Albornoz PC, Schulz UH. First record of the exotic channel catfish Ictalurus punctatus (Rafinesque 1818) (Siluriformes: Ictaluridae) in the rio dos Sinos basin, RS, Brazil. Biota Neotropica. 2012; 12(3):64-67.). As the non-native species may compete for resources, prey on the native fauna, spread diseases and parasites, be physiologically tolerant and present extreme feeding plasticity, they represent one of the main threats for the aquatic biodiversity (Welcomme, 1988Welcomme RL. Fisheries ecology of floodplain rivers. London: Longman Group; 1979.; Agostinho et al., 2000Agostinho AA, Thomaz SM, Minte-Vera CV, Winemiller KO. Biodiversity in the high Paraná River floodplain. In: Gopal B, Junk WJ, Davis JA, editors. Biodiversity in wetlands: assessment, junction and conservation. Leiden: Backhuys Publishers ; 2000. p.89-118.; Vitule et al., 2009Vitule JRS, Freire CA, Simberloff D. Introduction of non-native freshwater fish can certainly be bad. Fish Fish. 2009; 10(1):98-108.). Besides, the decrease in the number of native species after the establishment of I. punctatus was reported in the Colorado river basin (USA) (e.g. Holden, Stalnaker, 1975Holden PB, Stalnaker CB. Distribution and abundance of mainstream fishes of the middle and upper Colorado River basins, 1967-1973. Trans Am Fish Soc. 1975; 104(2):217-31.; Marsh, Brooks, 1989Marsh PC, Brooks JE. Predation by ictalurid catfishes as a deterrent to re-establishment of hatchery-reared razorback suckers. Southwest Nat. 1989; 34(2):188-95.), as well as the interspecific competition for food resources (e.g. Piedras et al., 2006Piedras SRN, Pouey JLOF, Moraes PRR. Comportamento alimentar e reprodutivo de peixes exóticos e nativos cultivados na zona sul do Rio Grande do Sul. Rev Bras Agrocienc. 2006; 12(3):341-44.); and C. gariepinus is known as a large-sized top predator that can tolerate extreme environmental conditions (Vitule et al., 2006Vitule JRS, Umbria SC, Aranha JMR. Introduction of the African catfish Clarias gariepinus (Burchell, 1822) into Southern Brazil. Biol Invasions. 2006; 8:677-81.).
The geographic distribution of some species is disrupted with the inclusion of the upper rio Paraná floodplain. Previous data suggest that Aphyocheirodon hemigrammus is restricted to the rio Grande and rio Tietê basins, State of São Paulo; Apteronotus acidops, to the rio Paraná in Ilha Solteira and to a locality in the rio Mogi-Guaçu, both in State of São Paulo; Astyanax biotae, to a few localities in the lower portion of the rio Paranapanema basin, State of São Paulo; E. guairaca, to one locality in the rio Paranapanema basin, State of Paraná; M. garavelloi, to the rio Tietê and rio Paranapanema basins, in several localities in the States of São Paulo and Paraná; O. marapoama, to the rio Tietê basin, State of São Paulo; and T. diabolus, to the rio Paranapanema basin, States of Paraná and São Paulo. Serrapinnus heterodon is known from many localities in the upper rio Paraná basin, State of São Paulo (the species is also known from several other drainages, including the Paranaíba, São Francisco and minor coastal rivers), but it has never been recorded from the rio Paranapanema or any other river in the State of Paraná. Although these species are native from the upper rio Paraná basin, it is not clear whether they are native from the upper rio Paraná floodplain, because none of them has been recorded from the region before 2004.
Considering that some of the aforementioned species established themselves in the floodplain only in recent years, it must be considered that hydrological changes in the upper rio Paraná in the last few decades facilitated their dispersion downstream. Hydrological changes of this magnitude include the impoundment of several stretches of the rio Paraná and its tributaries, and the construction of navigation locks and fish passages. The impoundment of rivers enables species with lentic habits to colonize stretches of the basin from which they were previously absent, and once species have colonized the reservoir, they may disperse downstream through fish passages or through navigation locks. Although, Agostinho et al. (2007aAgostinho AA, Gomes LC, Pelicice FM, editors. Ecologia e manejo de recursos pesqueiros em reservatórios do Brasil. Maringá: Eduem; 2007a.) concluded that fish passages are basically one-way routes, permitting mostly upstream migration, but they did not evaluate their efficiency as dispersion routes for non-migratory species. In turn, little is known about navigation locks as fish dispersion facilitators, especially in a dowstream direction. However, they are not very selective, being used by fishes of different sizes and habits (Margraf, Knight, 2002Margraf FJ, Knight CT. Evaluation of fish sampling using rotenone in a navigation lock. Fish Res. 2002; 55(1-2):297-305.).
The hypothesis of dowstream dispersion through navigation locks is strengthened by the existence of the Tietê-Paraná waterway, a long navigable stretch made possible by a series of navigation locks (Júlio Júnior et al., 2009Júlio Júnior HF, Dei Tos C, Agostinho AA, Pavanelli CS. A massive invasion of fish species after eliminating a natural barrier in the upper rio Paraná basin. Neotrop Ichthyol . 2009; 7(4):709-18.). A search in the SpeciesLink database showed that several species that invaded the upper rio Paraná basin from dowstream of the former Sete Quedas falls have now established themselves throughout the rio Tietê basin. Additionally, there is evidence that Geophagus sveni (i.e.Gois et al., 2015Gois KS, Pelicice FM, Gomes LC, Agostinho AA. Invasion of an Amazonian cichlid in the upper Paraná River: facilitation by dams and decline of a phylogenetically related species. Hydrobiologia. 2015; 746(1):401-13.), Hemigrammus ora and Hyphessobrycon moniliger (all native from the rio Tocantins-Araguaia basin) were introduced somewhere upstream from the Porto Primavera Reservoir and reached the upper rio Paraná floodplain afterwards. In turn, some species, i.e. T. diabolus and M. garavelloi, are adapted to fast-flowing waters and are not likely to have dispersed through a series of reservoirs. Concurrently, only one or a few individuals of those species have been captured in the floodplain, which suggests they are rare or hardly captured. Thus, those species should probably be regarded as native from the upper rio Paraná floodplain. In any case, because good historical collections are unavailable for many tributaries of the upper rio Paraná, there will always be gap in our knowledge of the exact native range of each species.
The redirection of human and financial resources is essential to scientific studies, once it contributes to biological knowledge and allows conservations purposes (Ota, RR et al., 2015Ota RR, Message HJ, Graça WJ, Pavanelli CS. Neotropical Siluriformes as a model for insights on determining biodiversity of animal groups. PLoS ONE [serial on the internet]. 2015 Jul 13 [cited 2016 Dec 08]; 10(7):e0132913. Available from: Available from: http://dx.doi.org/10.1371/journal.pone.0132913
http://dx.doi.org/10.1371/journal.pone.0...
). In the upper rio Paraná floodplain, the Núcleo de Pesquisas em Limnologia, Ictiologia e Aquicultura (Nupélia) has been studying this area for more than 30 years through a long-term ecological research (PELD, site 6). Our results are associated with the efforts and resources from Nupélia, which enabled the monitoring and the intensive exploration of its watercourse, the training of taxonomists, and new taxonomic resolutions. Therefore, certainly this work will complement the manual of Graça, Pavanelli (2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.) and will be very useful for the scientific community, beginning students, sporting and professional fisherman, as well as for environmental inspection agencies.
Material examined. All from Brazil, upper rio Paraná basin. Acestrorhynchus lacustris, NUP 11030, 3; A. pantaneiro, NUP 7580, 1. Aequidens plagiozonatus, NUP 16948*, 2, 18.0-59.0 mm SL. Ageneiosus inermis, NUP 3161*, 2, 200.0-212.0 mm SL; A. militaris, NUP 537*, 2, 189.0-209.0 mm SL; A. ucayalensis, NUP 533*, 2, 190.0-290.0 mm SL. Amaralia oviraptor, NUP 98, 1, 50.7 mm SL; NUP 124*, 1, 87.1 mm SL. Apareiodon affinis, NUP 12646, 2; A. piracicabae, NUP 449, 2; A. vladii, NUP 3376, 9. Aphyocharax anisitsi, NUP 14259, 44; A. dentatus, NUP 13598, 3, Aphyocharax sp., NUP 3225*, 1, 32,2 mm SL. Aphyocheirodon hemigrammus, NUP 13774*, 13, 29.8-37.7 mm SL. Apistogramma commbrae, NUP 6525*, 32.5 mm SL. Apteronotus acidops, NUP 1750*, 1, 240.0 mm TL; A. aff. albifrons, NUP 4171, 4; A. cf. caudimaculosus, NUP 4674, 1; A. ellisi, NUP 14443, 1. Astyanax biotae, NUP 15137*, 18, 28.7-41.3 mm SL; A. bockmanni, NUP 765, 5, 52.9-68.4 mm SL; A. aff. fasciatus, NUP 32*, 5, 50.2-90.6 mm SL; A. lacustris, NUP 18601, 5; A. aff. paranae, NUP 133, 5, 51.4-64.8 mm SL; A. schubarti, NUP 397, 5, 40.4-52.4 mm SL. Astronotus crassipinnis, NUP 3449, 2, 208.1-210.0 mm SL. Auchenipterus osteomystax, NUP 2627*, 1, 191.0 mm SL. Brachyhypopomus gauderio, NUP 2510*, 1, 112.2 mm TL. Brycon hilarii, NUP 5956, 1; B. orbignyanus, NUP 12042, 1. Bryconamericus exodon, NUP 4911, 33, 35.0-80.5 mm SL; B. aff. iheringii, NUP 824*, 5, 56.0-58.5 mm SL; B. turiuba, NUP 6170*, 9, 46.7-55.3 mm SL. Callichthys callichthys, NUP 318, 1, 58.9 mm SL, NUP 1722, 1, 102.0 mm SL. Catathirydium jenynsii, NUP 1921*, 4, 88.2-107.5 mm SL. Cetopsis gobioides, NUP 2476*, 3, 82.4-102.4 mm SL; NUP 11673, 3. Cetopsorhamdia iheringi, NUP 3232*, 1, 65.2 mm SL; NUP 2087, 1, 42.7 mm SL. Chaetobranchopsis australis, NUP 9366, 1, 93.9 mm SL. Characidium gomesi, NUP 16179, 1, 80.3 mm SL; NUP 17607*, 1, 59,3 mm SL; C. aff. zebra, NUP 372, 5, 31.1-43.8 mm SL; Characidium sp., NUP 347, 2, 15.4-22.3 mm SL, NUP 454, 1, 116.8 mm SL, NUP 2352, 1, 21.1 mm SL, NUP 3337, 2, 23.1-24.8 mm SL, NUP 3450*, 30, 18.3-27.6 mm SL. Cichla kelberi, NUP 1746, 5, 210.-0-320.0 mm SL; C. piquiti, NUP 3379, 2, 209.0-210.5 mm SL. Cichlasoma paranaense, NUP 11382, 3. Clarias gariepinus, NUP 3246, 1, 329.0 mm SL. Coptodon rendalli, NUP 1809, 32, 102.5-122.8 mm SL. Corydoras aeneus, NUP 1526*, 5, 39.3-44.1 mm SL; Corydoras sp., NUP 16185*, 1, 31.8 mm SL. Crenicichla britskii, NUP 10586, 3; C. haroldoi, NUP 58, 106.8 mm SL; C. jaguarensis, NUP 10797*, 78.3 mm SL, State of Paraná; C. jupiaensis, NUP 19283, 1; Crenicichla sp., NUP 7330, 1, 135.7 mm SL. Curculionichthys insperatus, NUP 18971*, 2, 26.6-27.0 mm SL. Cyphocharax nagelii, NUP 1566, 1; C. modestus, NUP 3290, 2, 98.2-100.0 mm SL.. Cyprinus carpio, NUP 1414*, 6 (4, 184.0-251.2 mm SL). Eigenmannia guairaca, NUP 16151*, 1, 112.3 mm TL; E. trilineata, NUP 12241, 1; E. virescens, NUP 10766, 1. Farlowella hahni, NUP 374*, 6, 75.6-162.0 mm SL. Galeocharax gulo, NUP 263*, 125.0-187.6 mm SL. Geophagus brasiliensis, NUP 10786, 1; G. sveni, NUP 18698*, 1, 115.90 mm SL. Gymnogeophagus setequedas, NUP 18081, 3. Gymnorhamphychthys britskii, NUP 3337*, 2, 98.2-115.0 mm TL. Gymnotus inaequilabiatus, NUP 11105, 4; G. paraguensis, NUP 6289, 1; G. sylvius, MZUSP 83538, paratype, 260.0 mm TL; G. pantanal, NUP 13328, 138.6 mm TL. Hemigrammus ora, NUP 18973*, 12, 18.4-27.3 mm SL; NUP 15325, 2, 30.0-31.3 mm SL. Hemiodus orthonops, NUP 10609, 1. Hemisorubim platyrhynchos, NUP 2506, 4, 177.8-270.5 mm SL. Heptapterus mustelinus, NUP 2500*, 2, 97.9-108.6 mm SL. Hoplerythrinus unitaeniatus, NUP 3437*, 5, 143.9-176.3 mm SL. Hoplias intermedius, NUP 5810, 1; H. mbigua, NUP 292*, 7, 97.8-171.1 mm SL, NUP 3456, 5, 130.0-252.0 mm SL; H. misionera, NUP 10408*, 190.0 mm SL; Hoplias sp. 2, NUP 3457*, 5, 180.9-263.0 mm SL; Hoplias sp. 3, NUP 5458*, 3, 145.3-161.7 mm SL. Hoplosternum littorale, NUP 161*, 3, 152.0-205.0 mm SL. Hyphessobrycon eques, NUP 3282*, 5, 20.7-29.9 mm SL. H. moniliger, NUP 1248*, 1, 24.0 mm; NUP 16946*, 1, 27.0 mm SL. Hypophthalmus edentatus, NUP 1749, 2, 270.7-308.5 mm SL. Hypostomus albopunctatus, NUP 1761*, 11, 146.0-315.0 mm SL, H. ancistroides, NUP 332*, 5, 66.9-144.2 mm SL; H. cochliodon, NUP 2556*, 20, 165.7-210.0 mm SL; H. commersoni, NUP 856*, 20, 160.0-284.0 mm SL; H. hermanni, NUP 4927, 4, 142.8-183.6 mm SL; H. iheringii, NUP 2577*, 5, 123,2-186.0 mm SL; H. margaritifer, NUP1766*, 20, 227.0-312.0 mm SL; H. microstomus, NUP 1725*, 4, 194.0-260.0 mm SL; H. regani, NUP 2286*, 20, 166.0-293.0 mm SL; H. ternetzi, NUP 1765, 20, 157.0-296,0 mm SL; H. cf. strigaticeps, NUP 3190*, 4, 95.0-169.0 mm SL. Ictalurus punctatus, NUP 11203*, 173.6 mm SL. Iheringichthys labrosus, NUP 671, 2, 124.0-144.7 mm SL. Imparfinis borodini, NUP 3028*, 128.3 mm SL; NUP 17591, 1, 27.3 mm SL; I. mirini, NUP 87*, 2, 42.0-61.0 mm SL; I. schubarti, NUP 2023*, 1, 107.4 mm SL; NUP 2083, 2, 65.2-98.1 mm SL. Knodus moenkhausii, NUP 3211*, 10, 40.0-52.0 mm SL. Laetacara araguaiae, NUP 11315*, 1, 43.5 mm SL. Leporellus vittatus, NUP 1902*, 3, 79.8-103.6 mm SL. Leporinus amblyrhynchus, NUP 1898, 2; L. friderici, NUP 1180, 5, 118.2-193,4 mm SL; L. lacustris, NUP 3308*, 3, 128.0-179.8 mm SL; L. octofasciatus, NUP 281*, 3, 170.8-182.0 mm SL; L. tigrinus, NUP 17488*, 2, 174.5-174.8 mm SL; L. striatus, NUP 1905, 2. Lepthoplosternum pectorale, NUP 11107, 2. Loricaria prolixa, NUP 18690, 1. Loricaria sp., NUP 2567*, 3, 133.0-187.0 mm SL, NUP 3132, 7, 193.0-218.0 mm SL. Loricariichthys platymetopon, NUP 18613, 2; L. rostratus, NUP 18676, 4. Megalancistrus parananus, NUP 528*, 2, 230.0-280.0 mm SL. Megalonema platanum, NUP 1729, 162.7-267.0 mm SL. Megaleporinus obtusidens, NUP 763, 2, 212.0-250.3 mm SL; M. piavussu, NUP 18865, 8. Melanorivulus sp., NUP 3453, 1, 25.0 mm SL, NUP 3159, 2, 13.2-17.0 mm SL, NUP 17575*, 5, 24.7-28.3 mm SL. Microglanis garaveloi. MZUEL 15575*, 2. Metynnis lippincottianus, NUP 443*, 52.3 mm SL. Moenkhausia australe, NUP 1115*, 1, 45.0 mm SL; NUP 371, 9 (4, 26.1-31.0 mm SL); NUP 10677, 1, 41.9 mm SL; NUP 10678, 4, 25.3-36.9 mm SL; M. bonita, NUP 2384, 366, 16.8-35.8 mm SL; NUP 11700*, 9, 29.4-32.1 mm SL; M. forestii, NUP 16583*, 27.0-36.0 mm SL; M. cf. gracilima, NUP 11099*, 5, 29.8-32.6 mm SL; M. aff. intermedia, NUP 2389*, 6, 31.6-35.5 mm SL; M. sanctaefilomenae, NUP 371, 5, 31.1-50.9 mm SL. Myloplus tiete, NUP 2484*, 2, 77.9-117.0 mm SL. Odontostilbe avanhandava, NUP 1517*, 10, 23.4-26.8 mm SL. Oligorsarcus paranensis, NUP 303*, 6, 67.4-90.3 mm SL. O. pintoi, NUP 1772*, 5, 57.4-76.1 mm SL. Oreochromis niloticus, NUP 1132*, 3, 125.0-190.0 mm SL. Ossancora eigenmanni, NUP 1861*, 1, 82.9 mm SL. Otothyropsis marapoama, NUP 9394*, 4, 33.1-36.5 mm SL; NUP 9395, 3, 23.3-30.6 mm SL. O. polyodon, NUP 11646, 3, 24.1-36.9 mm SL; NUP16171, 3, 30.3-40.1 mm SL; NUP 18972*, 1, 37.7 mm SL. Pamphorichthys hollandi, NUP 16549*, 25, 17.6-22.8 mm SL. Parauchenipterus galeatus, NUP 3302*, 9, 127.5-137.4 mm SL. Paravandellia oxyptera, NUP 313*, 3, 24.0-25.9 mm SL. Parodon nasus, NUP 16169, 1. Phalloceros harpagos, NUP 9360*, 22.7 mm SL; NUP 1938*, 18.0 mm SL. Phallotorhynus pankalos, NUP 5839*, 12, 18.4-32.6 mm SL; P. victoriae, NUP 1603*, 16, 8.2-18.6 mm SL. Phenacorhamdia tenebrosa, NUP 2499, 4, 52.1-63.6 mm SL. Piabina argentea, NUP 17505, 2. Piaractus mesopotamicus, NUP 2353, 3, 119.2-131.6 mm SL. Pimelodella avanhandae, NUP 3455, 7 ,99.0-152.0 mm SL; P. gracilis, NUP 3118*, 6, 91.5-109.7 mm SL; P. taenioptera, NUP 3991*, 2, 97.9-112.3 mm SL, NUP 4404, 1, 110.2 mm SL. Pimelodus maculatus, NUP 420, 2, 76.4-100.0 mm SL, NUP 12783*, 1, 214.4 mm SL; P. microstoma, NUP 18158*, 117.6 mm SL; P. mysteriosus, NUP 10824, 2, 158.0-160.0 mm SL; P. ornatus, NUP 2492, 3, 150.0-190.0 mm SL. Pinirampus pirinampu, NUP 4487, 1. Planaltina britskii, NUP 11802*, 27.5 mm SL. Plagioscion squamosissimus, NUP 1924, 3, 212.0-215.0 mm SL. Platanichthys platana, NUP 16904*, 127, 19.5-31.3 mm SL. Platydoras armatulus, NUP 1052*, 132.9 mm SL Poecilia reticulata, NUP 3452, 10, 28.2-49.4 mm SL. Potamotrygon amandae, NUP 17036, 2; P. cf. falkneri, NUP 5708, 4. Psellogrammus kennedyi, NUP 4557, 4, 22.4-34.5 mm SL. Pseudoplatystoma corruscans, NUP 523, 2, 189.0-244.0 mm SL; P. reticulatum, NUP 4485, 1. Pseudopimelodus mangurus, NUP 539, 1. Pterodoras granulosus, NUP 4568, 12. Pterygoplichthys ambrosettii, NUP 1529, 3, 180.0-290.0 mm SL. Pyrrhulina australis, NUP 3345*, 5, 22.3-26.9 mm SL. Rhamphichthys hahni, NUP 11155, 1. Rhaphiodon vulpinus, NUP 1513, 1, 327.0 mm SL; NUP 3418, 1, 212.5 mm SL. Rhamdia quelen, NUP 2501, 3, 170.2-208.2 mm SL. Rhinelepis aspera, NUP 1726, 15, 133.0-250.0 mm SL. Rhinodoras dorbignyi, NUP 11151, 1. Rhyacoglanis paranensis, NUP 14877*, 7, 18.8-41.1 mm SL. Rineloricaria sp., NUP 2565*, 1, 86.0 mm SL. Roeboides descalvadensis, NUP 3286*, 3, 52.8-68.2 mm SL. Salminus brasiliensis, NUP 1865, 3, 212.0-282.0 mm SL; S. hilarii, NUP 1893*, 3, 160.0-214.6 mm SL. Satanoperca sp., NUP 339, 5, 150.0-200.0 mm SL. Schidozon altoparanae, NUP 434, 8; S. borellii, NUP NUP 1925, 3, 220.0-290.0 mm SL; S. nasutus, NUP 2495*, 3, 114.2-122.4 mm SL. Scoloplax empousa, NUP 4806*, 17, 18.0-21.0 mm SL. Serrapinnus calliurus, NUP 17494*, 295, 21.4-24.1 mm SL; S. heterodon, NUP 17495*, 142, 30.1-32.5 mm SL; S. notomelas, NUP 360, 10 20.2-37.9 mm SL; Serrapinnus sp. 1, NUP 3283*, 10, 28.5-39.2 mm SL; Serrapinnus sp. 2, NUP 3455*, 10, 19.0-32.0 mm SL. Serrasalmus maculatus, NUP 396*, 3, 45.7-87.3 mm SL; S. marginatus, NUP 439*, 3, 46.1-122.8 mm SL. Sorubim lima, NUP 2494, 2, 197.0-397.0 mm SL. Steindachneridium scriptum, NUP 2479, 1, 310.0 mm SL. Steindachnerina brevipinna, NUP 2372, 3, 58.4-103.6 mm SL; S. insculpta, NUP 1424, 3, 62.2-99.8 mm SL. Sternopygus macrurus, NUP 2096*, 1, 280.0 mm TL. Synbranchus marmoratus, NUP 414*, 5, 204.0-405.0 mm TL. Tatia neivai, NUP 363, 2, 30.0-110.0 mm SL. Trachelyopterus sp., NUP 1884, 2, 100.0-152.0 mm SL, NUP 1885*, 1, 180.0 mm SL. Trachydoras paraguayensis, NUP 18666, 2. Trichomycterus davisi, NUP 2325*, 15, 52.0-89.0 mm SL; T. diabolus, NUP 10046*, 1, 78.2 mm SL. Zungaro jahu, NUP 1194, 1, 394.0 mm SL.*Specimens used for photographs.
Acknowledgments
We are grateful to Alessandro G. Bifi, Carlos A. M. Oliveira, Carlos A. Figueiredo, Cibele B. Raio, Cláudio H. Zawadzki, Fernando R. Carvalho, José L. O. Birindelli, Luiz F. C. Tencatt, Manoela M. F. Marinho, Oscar A. Shibatta, Paulo H. F. Lucinda, Rafaela P. Ota, Ricardo C. Benine and Ricardo Campos-da-Paz for their expert contribution. We also thank to Oscar A. Shibatta for kindly photographing the specimen of M. garavelloi. To Elaine A. Kashiwaqui, Valéria F. B. Silva, and Yzel R. Súarez for providing their voucher material from the rio Amambaí, Iguatemi and Ivinheima drainages. To Hugo J. Message for designing the map. To Luiz C. Gomes for kindly allowing us to use his photographic equipament. To Jaime L. Pereira for kindly designing the figure plates. We also thank to the Núcleo de Pesquisas em Limnologia, Ictiologia e Aquicultura (Nupélia), Pesquisas Ecológicas de Longa Duração (PELD - site 6), and the Programa de Pós-Graduação em Ecologia de Ambientes Aquáticos Continentais (PEA) for logistic support. The Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, http://www.cnpq.br/) for granting scholarship for RRO and GCD, and providing grants to CSP; and Fundação Araucária (http://www.fappr.pr.gov.br/) for providing grants to WJG.
References
- Agostinho AA, Bonecker CC, Gomes LC. Effects of water quantity on connectivity: the case of the upper Paraná River floodplain. Ecohydrol Hidrobiol. 2009; 9(1):99-113.
- Agostinho AA, Gomes LC, Pelicice FM, editors. Ecologia e manejo de recursos pesqueiros em reservatórios do Brasil. Maringá: Eduem; 2007a.
- Agostinho AA, Gomes LC, Thomaz SM, Hahn NS. The upper Paraná River and its floodplain: main characteristics and perspectives for management and conservation. In: Thomaz SM, Agostinho AA, Hahn NS, editors. The upper Paraná River and its floodplain: physical aspects, ecology and conservation. Leiden: Backhuys Publishers; 2004a. p.381-393.
- Agostinho AA, Gomes LC, Veríssimo S, Okada EK. Flood regime, dam regulation and fish in the upper Paraná River: effects on assemblage attributes, reproduction and recruitment. Rev Fish Biol Fish. 2004b; 14(1):11-19.
- Agostinho AA, Pelicice FM, Gomes LC. Dams and the fish fauna of the Neotropical region: impacts and management related to diversity and fisheries. Braz J Biol. 2008; 68(Suppl.4):1119-32.
- Agostinho AA, Pelicice FM, Petry AC, Gomes LC, Júlio Júnior HF. Fish diversity in the upper Paraná River basin: habitats, fisheries, management and conservation. Aquat Ecosyst Health Manag. 2007b; 10(2):174-86.
- Agostinho AA, Thomaz SM, Minte-Vera CV, Winemiller KO. Biodiversity in the high Paraná River floodplain. In: Gopal B, Junk WJ, Davis JA, editors. Biodiversity in wetlands: assessment, junction and conservation. Leiden: Backhuys Publishers ; 2000. p.89-118.
- Agostinho AA, Vazzoler AEAM, Thomaz SM. The high river Paraná basin: limnological and ichthyological aspects. In: Tundisi JG, Bicudo CEM, Matsumura-Tundisi T, editors. Limnology in Brazil. Rio de Janeiro: Brazilian Academy of Science and Brazilian Limnological Society; 1995. p.59-103.
- Aguiaro T, Branco CWC, Verani JR, Caramaschi EP. Diet of the Clupeid fish Platanichthys platana (Regan, 1917) in two different Brazilian coastal lagoons. Braz Arch Biol Technol. 2003; 46(2):215-22.
- Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Ann Arbor: University of Michigan Press; 2001. (Museum of Zoology, Miscellaneous Publications; no. 190).
- Albert JS, Fernandes-Matioli FMC, Almeida-Toledo LF. New species of Gymnotus (Gymnotiformes, Teleostei) from southeastern Brazil: toward the deconstruction of Gymnotus carapo Copeia. 1999; (2):410-21.
- Azpelicueta MM. A new species of Pimelodus (Siluriformes: Pimelodidae) from the Paraguay and lower Paraná rivers. Neotrópica. 1998; 44(111-112):87-94.
- Azpelicueta MM. A new species of Pimelodus (Siluriformes:Pimelodidae) from the upper Paraná basin, Brazil. Ichthyol Explor Freshw. 2001; 12(3):193-200.
- Azpelicueta MM, Benítez MF, Aichino DR, Mendez CMD. A new species of the genus Hoplias (Characiformes, Erythrinidae), a tararira from the lower Paraná River, in Misiones, Argentina. Acta Zool Lilloana. 2015; 59(1-2):71-82.
- Azpelicueta MM, Koerber S. Finding of the holotype of Farlowella paranaense Meinken, 1937. Historia Natural. 2014; 4(2):95-99.
- Benine RC. Análise filogenética do gênero Moenkhausia Eigenmann, 1903 (Characiformes: Characidae) com uma revisão dos táxons do alto rio Paraná. [PhD Thesis]. Botucatu, SP: Universidade Estadual Paulista; 2004.
- Benine RC, Castro RMC, Sabino J. Moenkhausia bonita: a new small Characin fish from the rio Paraguay Basin, southwestern Brazil (Characiformes: Characidae). Copeia . 2004; (1):68-73.
- Benine RC, Mariguela TC, Oliveira C. New species of Moenkhausia Eigenmann, 1903 (Characiformes: Characidae) with comments on the Moenkhausia oligolepis species complex. Neotrop Ichthyol. 2009; 7(2):161-68.
- Bertollo LAC, Born GG, Dergam JA, Fenocchio AS, Moreira Filho O. A biodiversity approach in the Neotropical Erythrinidae fish, Hoplias malabaricus Karyotypic survey, geographic distribution of cytotypes and cytotaxonomic considerations. Chromosome Res. 2000; 8(7):603-13.
- Betancur-R R, Wiley EO, Arratia G, Acero A, Bailly N, Miya M, Lecointre G, Ortí G. Phylogenetic classification of bony fishes. BMC Evol Biol [serial on the Internet]. 2017 July 6. Available from: https://doi.org/10.1186/s12862-017-0958-3
» https://doi.org/10.1186/s12862-017-0958-3 - Bifi AG. Revisão taxonômica das espécies do grupo Hoplias malabaricus (Bloch, 1794) (Characiformes: Erythrinidae) da bacia do rio da Prata. [PhD Thesis on the Internet]. Maringá: Universidade Estadual de Maringá; 2013 [cited 2016 Dec 08]. Available from: Biblioteca digital Universidade Estadual de Maringá. Available from: Biblioteca digital Universidade Estadual de Maringá. http://nourau.uem.br/nou-rau/document/?code=vtls000205331
» http://nourau.uem.br/nou-rau/document/?code=vtls000205331 - Birindelli JLO, Sabaj Pérez MH. Ossancora, new genus of thorny catfish (Teleostei: Siluriformes: Doradidae) with description of one new species. Proc Acad Nat Sci Philadelphia. 2011; 161(1):117-52.
- Bockmann FA, Casatti L, de Pinna MCC. A new species of trichomycterid catfish from the rio Paranapanema basin, Southeastern Brazil (Teleostei: Siluriformes), with comments on the phylogeny of the family. Ichthyol Explor Freshw . 2004; 15(3):225-42.
- Brea M, Zucol AF. The Paraná-Paraguay basin: geology and paleoenvironments. In: Albert JS, Reis RE, editors. Historical biogeography of Neotropical freshwater fishes. Berkeley; Los Angeles; London: University of California Press; 2011. p.69-87.
- Britski HA. Sôbre uma nova espécie de Astyanax do rio Mogi-Guassu (Pisces, Characidae). Pap Avulsos Zool. 1964; 16(21):213-15.
- Britski HA, Birindelli JLO, Garavello JC. A new species of Leporinus Agassiz, 1829 from the upper rio Paraná basin (Characiformes, Anostomidae) with redescription of L. elongatus Valenciennes, 1850 and L. obtusidens (Valenciennes, 1837). Pap Avulsos Zool . 2012; 52(37):441-75.
- Britski HA, Garavello JC. Sobre Leporinus octofasciatus Steindachner da bacia do rio Paraná (Pisces, Anostomidae). Pap Avulsos Zool . 1978; 31(16):237-50.
- Britski HA, Garavello JC. Hisonotus insperatus: new species, from the upper rio Paraná basin (Pisces: Ostariophysi: Loricariidae). Copeia . 2003; (3):588-93.
- Britski HA, Langeani F. Pimelodus paranensis, sp. n., um novo Pimelodidae (Pisces, Siluriformes) do alto Paraná, Brasil. Rev Bras Zool. 1988; 5(3):409-17.
- Britski HA, Silimon KZS, Lopes BS. Peixes do Pantanal: manual de identificação. Brasília, DF: Embrapa Pantanal; 2007.
- Buitrago-Suárez UR, Burr BM. Taxonomy of the catfish genus Pseudoplatystoma Bleeker (Siluriformes: Pimelodidae) with recognition of eight species. Zootaxa. 2007; 1512:1-38.
- Calegari BB, Lehmann-Albornoz P, Reis RE. Two new species of cascudinhos of the genus Otothyropsis (Siluriformes: Hypoptopomatinae) from the rio Paraná basin, Brazil. Zootaxa . 2013; 3619(2):130-44.
- Campos AA. Contribuição ao estudo das espécies brasileiras do gênero Leporinus Pap Avulsos Zool . 1945; 5(16):141-58.
- Campos-da-Paz R. Sistemática e taxonomia dos peixes elétricos das bacias dos rios Paraguai, Paraná e São Francisco, com notas sobre espécies presentes em rios costeiros do leste do Brasil (Teleostei: Ostariophysi: Gymnotiformes). [PhD Thesis]. São Paulo, SP: Universidade de São Paulo; 1997
- Campos-Da-Paz R. On Sternarchorhynchus Castelnau: a South American eletric knifefish, with descriptions of two new species (Ostariophysi: Gymnotiformes: Apteronotidae). Copeia . 2000; (2):521-35.
- Carvalho TP, Ramos CS, Albert JS. A new species of Gymnorhamphichthys (Gymnotiformes: Rhamphichthyidae) from the Paraná-Paraguay basin. Copeia . 2011; (3):400-06.
- Casatti L. Biology of a catfish, Trichomycterus sp. (Pisces, Siluriformes), in a pristine Stream in the Morro do Diabo State Park, Southeastern Brazil. Stud Neotrop Fauna Environ. 2003; 38(2):105-10.
- Castro RMC, Vari RP. Astyanax biotae, a new species of stream fish from the rio Paranapanema basin, upper rio Parana system, southeastern Brazil (Ostariophysi: Characiformes: Characidae). Proc Biol Soc Wash. 2004a; 117(3):330-38.
- Castro RMC, Vari RP. Detritivores of South American fish family Prochilodontidae (Teleostei; Ostariophysi: Characiformes): a phylogenetic and revisionary study. Washington (DC): Smithsonian Institution Press; 2004b. (Smithsonian Contributions to Zoology; no. 622).
- Chuctaya J, Bührnheim CM, Malabarba LR. Two new species of Odontostilbe historically hidden under O. microcephala (Characiformes: Cheirodontinae). Neotrop Ichthyol [serial on the internet]. 2018 Mar 26 [cited 03 Apr 2018]; 16(1):e170047. Available from: Available from: http://dx.doi.org/10.1590/1982-0224-20170047
» http://dx.doi.org/10.1590/1982-0224-20170047 - Costa WJEM. Phylogenetic position and taxonomic status of Anablepsoides, Atlantirivulus, Cynodonichthys, Laimosemion and Melanorivulus (Cyprinodontiformes: Rivulidae). Ichthyol Explor Freshw . 2011; 22(3):233-49.
- Companhia Energética de São Paulo (CESP). Aspectos limnológicos, ictiológicos e pesqueiros de reservatórios da CESP no Período de 1986 a 1994. São Paulo: CESP; 1996.
- Cruz-Spindler S, Leal ME, Lehmann-Albornoz PC, Schulz UH. First record of the exotic channel catfish Ictalurus punctatus (Rafinesque 1818) (Siluriformes: Ictaluridae) in the rio dos Sinos basin, RS, Brazil. Biota Neotropica. 2012; 12(3):64-67.
- Deprá GC, Ota RR, Souza F, Graça WJ, Pavanelli CS. Widening the geographical distribution of Pimelodus mysteriosus Azpelicueta 1998 (Siluriformes: Pimelodidae) to the upper Paraná River, with diagnosis for syntopic congeners. Biota Neotropica . 2015; 15(3):1-6.
- Dunz AR, Schliewen UK. Molecular phylogeny and revised classification of the haplotilapiine cichlid fishes formerly referred to as “Tilapia”. Mol Phylogenet Evol. 2013; 68(1):64-80.
- Eigenmann CH. The Cheirodontinae, a subfamily of minute characid fishes of South America. Mem Mus Comp Zool. 1915; 7(1):1-100.
- Eigenmann CH. The American Characidae. Mem Mus Comp Zool . 1917; 43:1-102. pt. 1.
- Eschmeyer WN, Fricke R, van der Laan R, editors. Catalog of fishes: genera, species, references [Internet]. San Francisco: California Academy of Science; 2017 [updated 2017 Jan 31; cited 2017 Feb 24]. Available from: Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
» http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp - Fernandes CA, Bailly D, Sant’Ana DRA, Alves DS. First cytogenetic record for a species of Otothyropsis Ribeiro, Carvalho & Melo, 2005 (Loricariidae, Hypoptopomatinae). Neotrop Ichthyol [serial in the internet]. 2016 Apr 14 [cited 2016 Dec 08]; 14(1):e150123. Available from: Available from: http://dx.doi.org/10.1590/1982-0224-20150123
» http://dx.doi.org/10.1590/1982-0224-20150123 - Fernandes R, Agostinho AA, Ferreira EA, Pavanelli CS, Suzuki HI, Lima DP, Gomes LC. Effects of the hydrological regime on the ichthyofauna of riverine environments of the upper Paraná River floodplain. Braz J Biol . 2009; 69(2, Suppl.):669-80.
- Ferraris CJ Jr. Subfamily Loricariinae. In: Reis RE, Kullander SO, Ferraris CJ Jr., organizers. Check list of the freshwater fishes of South and Central America. Porto Alegre: Edipucs; 2003. p.330-350.
- Ferraris CJ Jr. Checklist of catfishes, recent and fossil (Osteichthyes: Siluriformes), and catalogue of siluriform primary types. Zootaxa . 2007; 1418:1-628.
- Ferraris CJ Jr., Vari RP. The South American catfish genus Auchenipterus Valenciennes, 1840 (Ostariophysi: Siluriformes: Auchenipteridae): monophyly and relationships, with a revisionary study. Zool J Linn Soc. 1999; 126(4):387-450.
- Ferreira KM. Biology and ecomorphology of stream fishes from the rio Mogi-Guaçu basin, Southeastern Brazil. Neotrop Ichthyol . 2007; 5(3):311-26.
- Fiori LF, Alvez GHZ, Hahn NS, Benedito E. Influence of feeding plasticity on the fitness of small Neotropical characids. Iheringia Ser Zool [serial on the internet]. 2016 May 2 [cited 2016 Dec 08]; 106:e2016006. Available from: Available from: http://dx.doi.org/10.1590/1678-4766e2016006
» http://dx.doi.org/10.1590/1678-4766e2016006 - Freitas IS, Lucinda PHF, Soares AB, Pelicice FM, Akama A. Variações espaciais na estrutura da ictiofauna entre os ambientes do reservatório de Peixe Angical. In: Agostinho CS, Pelicice FM, Marques EE, organizers. Reservatório de Peixe Angical: bases ecológicas para o manejo da ictiofauna. São Carlos: RiMa; 2009. p.41-57.
- Friel JP. A phylogenetic study of the Neotropical banjo catfishes (Teleostei: Siluriformes: Aspredinidae). [PhD Thesis]. Durham, North Carolina: Duke University; 1994.
- Friel JP. Family Aspredinidae. In: Reis RE, Kullander SO, Ferraris CJ Jr., organizers. Check list of the freshwater fishes of South and Central America. Porto Alegre: Edipucs ; 2003. p.261-267.
- Friel JP, Carvalho TP. A new species of Amaralia Fowler (Siluriformes: Aspredinidae) from the Paraná-Paraguay River basin. Zootaxa . 2016; 4088(4):531-46.
- Garavello JC. Revisão taxonômica do gênero Leporinus Spix, 1829 (Ostariophysi, Anostomidae). [PhD Thesis]. São Paulo, SP: Universidade de São Paulo; 1979.
- Garavello JC, Britski HA. Duas novas espécies do gênero Leporinus Spix, 1829, da bacia o alto rio Paraná (Teleostei, Anostomidae). Com Mus Cien PUCRS. 1987; 44:153-65.
- Garavello JC, Britski HA. Leporinus macrocephalus sp. n. da bacia do rio Paraguai (Ostariophysi, Anostomidae). Naturalia. 1988; 13:67-74.
- Garavello JC, Britski HA. Duas novas espécies do gênero Schizodon Agassiz da bacia do alto Paraná, Brasil, América do Sul (Ostariophysi, Anostomidae). Naturalia . 1990; 15:153-70.
- Garutti V, Langeani F. Redescription of Astyanax goyacensis Eigenmann, 1908 (Ostariophysi: Characiformes: Characidae). Neotrop Ichthyol . 2009; 7(3):371-76.
- Gelain D, Fialho CB, Malabarba LR. Biologia reprodutiva de Serrapinnus calliurus (Characidae, Cheirodontinae) do arroio Ribeiro, Barra do Ribeiro, Rio Grande do Sul, Brasil. Com Mus Cien PUCRS . 1999; 12:71-82.
- Giora J, Malabarba LR. Brachyhypopomus gauderio, new species, a new example of underestimated species diversity of electric fishes in the southern South America (Gymnotiformes: Hypopomidae). Zootaxa . 2009; 2093:60-68.
- Giovannetti V, Toledo-Piza M, Menezes NA. Taxonomic revision of Galeocharax Fowler, 1910 (Characiformes: Characidae: Characinae). Neotrop Ichthyol [serial in the internet]. 2017 Mar 23 [cited 2018 Jan 15]; 15(1):e160040. Available from: Available from: http://dx.doi.org/10.1590/1982-0224-20160040
» http://dx.doi.org/10.1590/1982-0224-20160040 - Gois KS, Pelicice FM, Gomes LC, Agostinho AA. Invasion of an Amazonian cichlid in the upper Paraná River: facilitation by dams and decline of a phylogenetically related species. Hydrobiologia. 2015; 746(1):401-13.
- Gonçalves CS, Souza UP, Braga FMS. Population structure, feeding and reproductive aspects of Serrapinnus heterodon (Characidae, Cheirodontinae) in a Mogi Guaçu reservoir (SP), upper Paraná River basin. Acta Limnol Bras. 2011; 23(1):13-22.
- Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem ; 2007.
- Hanssens M. A review of the Clarias species (Pisces; Siluriformes) from the lower Congo and the Pool Malebo. J Afrotrop Zool. 2009; 5:27-40.
- Holden PB, Stalnaker CB. Distribution and abundance of mainstream fishes of the middle and upper Colorado River basins, 1967-1973. Trans Am Fish Soc. 1975; 104(2):217-31.
- Isbrücker IJH, Nijssen H. Two new species and a new genus of neotropical mailed catfishes of the subfamily Loricariinae Swainson, 1838 (Pisces, Siluriformes, Loricariidae). Beaufortia. 1978; 27(339):177-206.
- Jégu M, Santos GM. Mise au point à propos de Serrasalmus spilopleura Kner, 1858 et réhabilitation de S. maculatus Kner, 1858 (Characidae: Serrasalminae). Cybium. 2001; 25(2):119-43.
- Jerep FC, Carvalho FR, Bertaco VA. Geographic distribution of Hemigrammus ora (Ostariophysi: Characiformes: Characidae) in the Amazon basin, Brazil. Zoologia. 2011; 28(4):545-50.
- Júlio Júnior HF, Dei Tos C, Agostinho AA, Pavanelli CS. A massive invasion of fish species after eliminating a natural barrier in the upper rio Paraná basin. Neotrop Ichthyol . 2009; 7(4):709-18.
- Kullander SO. Cichlid fishes from the La Plata basin. Part II. Apistogramma commbrae (Regan, 1906) (Teleostei: Cichlidae). Rev Suisse Zool. 1982a; 89(1):33-48.
- Kullander SO. Cichlid fishes from the La Plata basin. Part III. The Crenicichla lepidota species group (Teleostei: Cichlidae). Rev Suisse Zool . 1982b; 89(3):627-61.
- Kullander SO. A revision of the South American cichlid genus Cichlasoma (Teleostei: Cichlidae). Stockholm: Naturhistoriska Riksmuseet; 1983.
- Kullander SO. Cichlid fishes from the La Plata basin. Part V. Description of Aequidens plagiozonatus sp.n. (Teleostei, Cichlidae) from the Paraguay River system. Zool Scr. 1984, 13(3):155-59.
- Kullander SO. A taxonomic review of Satanoperca (Teleostei: Cichlidae) from French Guiana, South America, with description of a new species. Cybium . 2012; 36(1):247-62.
- van der Laan R, editor. Freshwater fish list: an alphabetic scientific name list of the world’s freshwater fishes and an overview of the scientific names used in the aquarium literature. 18th ed. Almere: The Netherlands; 2016.
- Langeani F, Araújo RB. O gênero Rineloricaria Bleeker, 1862 (Ostariophysi, Siluriformes) na bacia do rio Paraná superior: Rineloricaria pentamaculata sp. n. e Rineloricaria latirostris (Boulenger, 1900) Com Mus Cien PUCRS . 1994; 7:151-66.
- Langeani F, Castro RMC, Oyakawa OT, Shibatta OA, Pavanelli CS, Casatti L. Diversidade da ictiofauna do alto rio Paraná: composição atual e perspectivas futuras. Biota Neotropica , 2007; 7(3):181-97.
- Langeani F, Lucena ZMS, Pedrini JL, Tarelho Pereira FJ. Bryconamericus turiuba, a new species from the upper rio Paraná system (Ostariophysi: Characiformes). Copeia . 2005; (2):386-92.
- Lima FCT. A revision of the cis-andean species of the genus Brycon Müller & Troschel (Characiformes: Characidae). Zootaxa . 2017; 4222(1):1-189.
- Lima RS. Revisão taxonômica do gênero Aphyocharax Günther, 1868 (Aphyocharacinae, Characidae, Ostariophysi). [PhD Thesis]. São Paulo, SP: Universidade de São Paulo; 2003.
- Littmann MW, Azpelicueta MM, Vanegas-Rios JA, Lundberg JG. Holotype-based validation, redescription and continental-scale range extension of the South American catfish species Hypophthalmus oremaculatus Nani and Fuster, 1947, with additional information on Hypophthalmus edentatus Spix and Agassiz, 1829 (Siluriformes, Pimelodidae). Proc Acad Nat Sci Philadelphia . 2015; 164(1):159-76.
- Loboda TS, Carvalho MR. Systematic revision of the Potamotrygon motoro (Müller & Henle, 1841) species complex in the Paraná-Paraguay basin, with description of two new ocellated species (Chondrichthyes: Myliobatiformes: Potamotrygonidae). Neotrop Ichthyol . 2013; 11(4):693-737.
- Lucena CAS. Revisão taxonômica das espécies do gênero Roeboides grupo-affinis (Ostariophysi, Characiformes, Characidae). Iheringia Ser Zool. 2007; 97(2):117-36.
- Lucena CAS, Soares HG. Review of species of the Astyanax bimaculatus “caudal peduncle spot” subgroup sensu Garutti & Langeani (Characiformes, Characidae) from the rio La Plata and rio São Francisco drainages and coastal systems of southern Brazil and Uruguay. Zootaxa . 2016; 4072(1):101-25.
- Lucinda PHF. Systematics and biogeography of the genus Phalloceros Eigenmann, 1907 (Cyprinodontiformes: Poeciliidae: Poeciliinae), with the description of twenty-one new species. Neotrop Ichthyol . 2008; 6(2):113-58.
- Lucinda PHF, Graça WJ. Description of males of Phallotorynus pankalos Lucinda, Rosa & Reis, 2005 and reappraisal of Phallotorynus species relationships (Teleostei: Cyprinodontiformes: Poeciliidae). Neotrop Ichthyol . 2015; 13(1):87-92.
- Lucinda PHF, Lucena CAS, Assis NC. Two new species of cichlid fish genus Geophagus Heckel from the rio Tocantins drainage (Perciformes: Cichlidae). Zootaxa . 2010; 2429:29-42.
- Lucinda PHF, Rosa RS, Reis RE. Systematics and biogeography of the genus Phallotorynus Henn, 1916 (Cyprinodontiformes: Poeciliidae: Poeciliinae), with description of three new species. Copeia . 2005; (3):609-31.
- Lujan NK, Armbruster JW, Lovejoy NR, López-Fernández H. Multilocus molecular phylogeny of the suckermouth armored catfishes (Siluriformes: Loricariidae) with a focus on subfamily Hypostominae. Mol Phylogenet Evol . 2015; 82(pt. A):269-88.
- Lundberg JG, Littmann MW. Family Pimelodidae. In: Reis RE, Kullander SO, Ferraris CJ Jr., organizers. Check list of the freshwater fishes of South and Central America. Porto Alegre: Edipucs ; 2003. p.432-446.
- Makrakis S, Gomes LC, Makrakis MC, Fernandez DR, Pavanelli CS. The Canal da Piracema at Itaipu Dam as a fish pass system. Neotrop Ichthyol . 2007; 5(2):185-95.
- Malabarba LR. Monophyly of the Cheirodontinae, characters and major clades (Ostariophysi: Characidae). In: Malabarba LR, Reis RE, Vari RP, Lucena ZMS, Lucena CAS, editors. Phylogeny and classification of Neotropical fishes. Porto Alegre: Edipucrs; 1998. p.193-233.
- Margraf FJ, Knight CT. Evaluation of fish sampling using rotenone in a navigation lock. Fish Res. 2002; 55(1-2):297-305.
- Mariguela TC, Benine RC, Abe KT, Avelino GS, Oliveira C. Molecular phylogeny of Moenkhausia (Characidae) inferred from mitochondrial and nuclear DNA evidence. J Zoolog Syst Evol Res. 2013; 51(4):327-32.
- Marinho MMF. Análise filogenética e revisão taxonômica das espécies de Moenkhausia Eigenmann, 1903 do grupo M. lepidura (Ostariophysi: Characiformes: Characidae). [Master Dissertation]. São José do Rio Preto, SP: Universidade Estadual Paulista; 2009 Apr 24 [cited 2016 Dec 08]. Available from: Repositório Institucional Universidade Estadual Paulista. Available from: Repositório Institucional Universidade Estadual Paulista. http://repositorio.unesp.br/handle/11449/87587
» http://repositorio.unesp.br/handle/11449/87587 - Marinho MMF, Langeani F. Reconciling more than 150 years of taxonomic confusion: the true identity of Moenkhausia lepidura, with a key to the species of the M. lepidura group (Characiformes: Characidae). Zootaxa . 2016; 4107(3):338-52.
- Marsh PC, Brooks JE. Predation by ictalurid catfishes as a deterrent to re-establishment of hatchery-reared razorback suckers. Southwest Nat. 1989; 34(2):188-95.
- Mateussi NTB, Oliveira C, Pavanelli CS. Taxonomic revision of the Cis-Andean species of Mylossoma Eigenmann & Kennedy, 1903 (Teleostei: Characiformes: Serrasalmidae). Zootaxa . 2018; 4387(2):275-309.
- McEachran JD, Aschliman N. Phylogeny of Batoidea. In: Carrier JC, Musick JA, Heithaus MR, editors. Biology of sharks and their relatives. London: CRC Press; 2004. p.79-113. (CRC Marine Biology Series).
- Menezes NA. Três espécies novas de Oligosarcus Günther, 1864 e redefinição taxonômica das demais espécies do gênero (Osteichthyes, Teleostei, Characidae). Bolm Zool, Univ S Paulo. 1987; 11(11):1-39.
- Menezes NA. Redefinição taxonômica das espécies de Acestrorhynchus do grupo lacustris com a descrição de uma nova espécie (Osteichthyes, Characiformes, Characidae). Com Mus Cien PUCRS . 1992; 5(5):39-54.
- Menezes NA, Géry J. Seven new Acestrorhynchin characid species (Osteichthyes, Ostariophysi, Characiformes) with comments on the systematics of the group. Rev Suisse Zool . 1983; 90(3):563-92.
- Menezes NA, Weitzman SH, Burns JB. A systematic review of Planaltina (Teleostei: Characiformes: Characidae: Glandulocaudini: Diapomini) with a description of two new species from the upper rio Paraná, Brazil. Proc Biol Soc Wash . 2003; 116(3):557-600.
- Message HJ, Santos DA, Baumgartner MT. Planícies de inundação: a biodiversidade do rio Paraná ameaçada. Ciência Hoje. 2016; 56(334):36-39.
- Moreira CR, Lima FCT, Costa WJEM. Hyphessobrycon moniliger, a new characid fish from rio Tocantins basin, Central Brazil (Ostariophysi: Characiformes). Ichthyol Explor Freshw . 2002; 13(1):73-80.
- Moretto EM, Marciano FT, Velludo MR, Fenerich-Verani N, Espíndola ELG, Rocha O. The recent occurrence, establishment and potential impact of Geophagus proximus (Cichlidae: Perciformes) in the Tietê River reservoirs: an Amazonian fish species introduced in the Paraná basin (Brazil). Biodivers Conserv. 2008; 17:3013-25.
- Mota TFM, Fabrin TMC, Deprá GC, Gasques LS, Oliveira AV, Pavanelli CS, Prioli SMAP, Prioli A. Molecular characterization of Moenkhausia (Pisces: Characiformes) populations with different lateral line developmental levels. An Acad Bras Cienc. 2018, in press.
- Nakatani K, Baumgartner G, Cavicchioli M. Ecologia de ovos e larvas de peixes. In: Vazzoler AEAM, Agostinho AA, Hahn NS, editors. A planície de inundação do alto rio Paraná: aspectos físicos, biológicos e socioeconômicos. Maringá: Eduem ; 1997. p.281-306.
- Neuberger AL, Marques EE, Agostinho CS, Pelicice FM. Variações espaciais na atividade reprodutiva de peixes na área de influência do reservatório de Peixe Angical. In: Agostinho CS, Pelicice FM, Marques EE, organizers. Reservatório de Peixe Angical: bases ecológicas para o manejo da ictiofauna. São Carlos: RiMa ; 2009. p.59-68.
- Nielsen DTB, Neves PABA, Ywamoto EV, Passos MA. Melanorivulus polychromus, a new species of killifish from the rio São José dos Dourados drainage, middle rio Paraná basin, southwestern Brazil, with a redescription of Melanorivulus apiamici Cyprinodontiformes: Rivulidae). Aqua. 2016; 22(2):79-88.
- Oliveira AG, Suzuki HI, Gomes LC, Agostinho AA. Interspecific variation in migratory fish recruitment in the upper Paraná River: effects of the duration and timing of floods. Environ Biol Fish. 2014; 98(5):1327-37.
- Oliveira C, Avelino GS, Abe KT, Mariguela TC, Benine RC, Ortí G, Vari RP, Castro RMC. Phylogenetic relationships within the speciose family Characidae (Teleostei: Ostariophysi: Characiformes) based on multilocus analysis and extensive ingroup sampling. BMC Evol Biol [serial on the internet]. 2011 Sep 26 [cited 2016 Dec 08]; 11:275. Available from: Available from: http://dx.doi.org/10.1186/1471-2148-11-275
» http://dx.doi.org/10.1186/1471-2148-11-275 - Ota RP. Revisão taxonômica e análise filogenética das espécies do gênero Metynnis Cope, 1878 (Characiformes: Serrasalmidae). [PhD Thesis]. Manaus, AM: Instituto Nacional de Pesquisas da Amazônia; 2015.
- Ota RP, Lima FCT, Pavanelli CS. A new species of Hemigrammus Gill, 1858 (Characiformes: Characidae) from the central and western Amazon and rio Paraná-Paraguai basins. Zootaxa . 2015; 3948(2):218-32.
- Ota RR. Revisão taxonômica de Satanoperca Günther, 1862 (Perciformes, Cichlidae), com a descrição de três espécies novas. Unpublished [Master Dissertation]. Maringá, PR: Universidade Estadual de Maringá; 2013 Oct 04 [cited 2016 Dec 08]. Available from: Biblioteca digital Universidade Estadual de Maringá. Available from: Biblioteca digital Universidade Estadual de Maringá. http://nou-rau.uem.br/nou-rau/document/?code=vtls000211405
» http://nou-rau.uem.br/nou-rau/document/?code=vtls000211405 - Ota RR, Message HJ, Graça WJ, Pavanelli CS. Neotropical Siluriformes as a model for insights on determining biodiversity of animal groups. PLoS ONE [serial on the internet]. 2015 Jul 13 [cited 2016 Dec 08]; 10(7):e0132913. Available from: Available from: http://dx.doi.org/10.1371/journal.pone.0132913
» http://dx.doi.org/10.1371/journal.pone.0132913 - Ottoni FP, Costa WJEM. Description of a new species of Laetacara Kullander, 1986 from central Brazil and re-description of Laetacara dorsigera (Heckel, 1840) (Labroidei: Cichlidae: Cichlasomatinae). Vertebr Zool. 2009; 59(1):41-48.
- Oyakawa OT, Mattox GMT. Revision of the Neotropical trahiras of the Hoplias lacerdae species-group (Ostariophysi: Characiformes: Erythrinidae) with descriptions of two new species. Neotrop Ichthyol . 2009; 7(2):117-40.
- Parron RYS. Caracterização morfológica das espécies de peixes cultivadas no Estado do Paraná, Brasil. [Monograph]. Maringá, PR: Universidade Estadual de Maringá; 2001.
- Pavanelli CS. Revisão taxonômica da família Parodontidae (Ostariophysi: Characiformes). [PhD Thesis]. São Carlos, SP: Universidade Federal de São Carlos; 1999.
- Pavanelli CS, Graça WJ, Zawadzki CH, Britski HA, Vidotti AP, Avelino GS, Veríssimo S. Fishes from the Corumbá Reservoir, Paranaíba River drainage, upper rio Paraná basin, State of Goiás, Brazil. Check List. 2007; 3(1):58-64.
- Pazza R, Júlio Júnior HF. Occurrence of three sympatric cytotypes of Hoplias malabaricus (Pisces, Erythrinidae) in the upper rio Paraná floodplain (Brazil). Cytologia. 2003; 68(2):159-63.
- Peixoto LAW, Dutra GM, Wosiacki WB. The electric glass knifefishes of the Eigenmannia trilineata species-group (Gymnotiformes: Sternopygidae): monophyly and description of seven new species. Zool J Linn Soc . 2015; 175(2):384-414.
- Pereira RC, Rosa FR, Resende EK. Estrutura trófica da comunidade de peixes de riachos da porção Oeste da bacia do alto Paraná. Corumbá: Embrapa Pantanal; 2012. (Boletim de Pesquisa e Desenvolvimento; no. 117).
- Petry AC, Agostinho AA, Gomes LC. Fish assemblages of tropical floodplain lagoons: exploring the role of connectivity in a dry year. Neotrop Ichthyol . 2003; 1(2):111-19.
- Piedras SRN, Pouey JLOF, Moraes PRR. Comportamento alimentar e reprodutivo de peixes exóticos e nativos cultivados na zona sul do Rio Grande do Sul. Rev Bras Agrocienc. 2006; 12(3):341-44.
- Ramirez JL, Birindelli JLO, Galetti Júnior PM. A new genus of Anostomidae (Ostariophysi: Characiformes): Diversity, phylogeny and biogeography based on cytogenetic, molecular and morphological data. Mol Phylogenet Evol . 2017; 107:308-23.
- Reis RE, Malabarba LR, Pavanelli CS. Gymnogeophagus setequedas, a new cichlid species (Teleostei: Labroidei) from middle rio Paraná system, Brazil and Paraguay. Ichthyol Explor Freshw . 1992; 3(3):265-72.
- Reis RE, Pereira EHL. Three new species of the loricariid catfish genus Loricariichthys (Teleostei: Siluriformes) from southern South America. Copeia . 2000; (4):1029-47.
- Retzer ME, Page LM. Systematics of the stick catfishes, Farlowella Eigenmann & Eigenmann (Pisces, Loricariidae). Proc Acad Nat Sci Philadelphia . 1996; 147:33-88.
- Reynalte-Tataje DA, Agostinho AA, Bialetzki A. Temporal and spatial distributions of the fish larval assemblages of the Ivinheima River sub-basin (Brazil). Environ Biol Fishes 2012; 96(7):811-22.
- Ribeiro FRV, Lucena CAS. Pimelodus heraldoi Azpelicueta, 2001, a junior synonym of Pimelodus microstoma Steindachner, 1877 (Siluriformes: Pimelodidae). Neotrop Ichthyol . 2010; 8(2):277-81.
- Roberto MC, Santana NF, Thomaz SM. Limnology in the upper Paraná River floodplain: large-scale spatial and temporal patterns, and the influence of reservoirs. Braz J Biol . 2009; 69(2, Suppl.):717-25.
- Rosa RS. A systematic revision of the South American freshwater stingrays (Chondrichthyes: Potamotrygonidae). [PhD Thesis]. Virginia, The Faculty of the School of Marine Science; 1985.
- Rosso JJ, Mabragaña E, González-Castro M, Delpiani MS, Avigliano E, Schenone N, Días de Astarloa JM. A new species of the Hoplias malabaricus species complex (Characiformes: Erythrinidae) from the La Plata River basin. Cybium . 2016: 40(3):199-208.
- Roxo FF, Albert JS, Silva GSC, Zawadzki CH, Foresti F, Oliveira C. Molecular phylogeny and biogeographic history of the armored Neotropical catfish subfamilies Hypoptopomatinae, Neoplecostominae and Otothyrinae (Siluriformes: Loricariidae). PloS ONE [serial on the internet]. 2014 Aug 22 [cited 2016 Dec 08]; 9(8):e105564. Available from: Available from: http://dx.doi.org/10.1371/journal.pone.0105564
» http://dx.doi.org/10.1371/journal.pone.0105564 - Santana CD. Apteronotus caudimaculosus n. sp. (Gymnotiformes: Apteronotidae), a sexually dimorphic black ghost knifefish from the Pantanal, western Brazil, with a note on the monophyly of the A. albifrons species complex. Zootaxa . 2003; 252(1):1-11.
- Santos CJ, Tencatt LFC, Ota RR, Graça WJ. Second record of Leporinus tigrinus Borodin, 1929 (Characiformes: Anostomidae) in the upper Paraná River basin, Brasil. Check List . 2013; 9(6):1543-44.
- Sarmento-Soares LM, Bristki HA, Anjos M, Zanata AM, Martins-Pinheiro RF, Barreto MG. First record of genus Imparfinis from a northeastern coastal Brazilian river basin: I. borodini Mees & Cala, 1989 in Rio de Contas, Bahia. Check List [serial on the internet]. 2016 [cited 2016 Dec 08]; 12(1):1832. Available from: Available from: http://dx.doi.org/10.15560/12.1.1832
» http://dx.doi.org/10.15560/12.1.1832 - Shibatta OA. Sistemática e evolução da família Pseudopimelodidae (Ostariophysi, Siluriformes), com a revisão taxonômica do gênero Pseudopimelodus [PhD Thesis]. São Carlos, SP: Universidade Federal de São Carlos; 1999.
- Shibatta OA, Benine RC. A new species of Microglanis (Siluriformes: Pseudopimelodidae) from upper rio Paraná basin, Brazil. Neotrop Ichthyol . 2005; 3(4):579-85.
- Shibatta AO, Vari RP. A new genus of Neotropical rheophilic catfishes, with four new species (Teleostei: Siluriformes: Pseudopimelodidae). Neotrop Ichthyol [serial in the internet]. 2017 Jun 12 [cited 2018 Jan 15]; 15(2):e160132. Available from: Available from: http://dx.doi.org/10.1590/1982-0224-20160132
» http://dx.doi.org/10.1590/1982-0224-20160132 - Súarez YR, Silva JP, Vasconcelos LP, Antonialli Júnior WF. Ecology of Phallotorynus pankalos (Cyprinodontiformes: Poeciliidae) in a first-order stream of the upper Paraná basin. Neotrop Ichthyol . 2009; 7(1):49-54.
- Suzuki HI, Agostinho AA, Bailly D, Gimenes MF, Júlio Júnior HF, Gomes LC. Inter-annual variations in the abundance of young-of-the-year of migratory fishes in the upper Paraná River floodplain: relations with hydrographic atributes. Brazil J Biol. 2009; 69(2, Suppl.):649-60.
- Tagliacollo VA, Souza-Lima R, Benine RC, Oliveira C. Molecular phylogeny of Aphyocharacinae (Characiformes, Characidae) with morphological diagnoses for the subfamily and recognized genera. Mol Phylogenet Evol . 2012; 64(2):297-307.
- Teixeira TF, Netto-Ferreira AL, Birindelli JLO, Sousa LM. Two new species of Hyphessobrycon (Characiformes: Characidae) from the headwaters of the Tapajós and Xingu River basins, Pará, Brazil. J Fish Biol. 2015; 88(2):459-76.
- Thomaz AT, Arcila D, Orti G, Malabarba LR. Molecular phylogeny of the subfamily Stevardiinae Gill, 1858 (Characiformes: Characidae): classification and the evolution of reproductive traits. BMC Evol Biol [serial on the internt]. 2015 Jan 13 [cited 2016 Dec 08]; 15:146. Available from: Available from: http://dx.doi.org/10.1186/s12862-015-0403-4
» http://dx.doi.org/10.1186/s12862-015-0403-4 - Thomaz SM, Bini LM, Bozelli RL. Floods increase similarity among aquatic habitats in river-floodplain systems. Hydrobiologia . 2007; 579(1):1-13.
- Toledo-Piza M. The neotropical fish subfamily Cynodontinae (Teleostei: Ostariophysi: Characiformes): a phylogenetic study and a revision of Cynodon and Rhaphiodon Am Mus Novit. 2000; (3286):1-88.
- Tondato KK, Fantin-Cruz I, Pedrollo OC, Súarez YR. Spatial distribution of fish assemblages along environmental gradients in the temporary ponds of Northern Pantanal, Brazil. J Limnol. 2013; 72(1):95-102.
- Triques ML. Apteronotus acidops, new species of long snouted electric fish (Teleostei: Gymnotiformes: Apteronotidae) from the upper rio Paraná basin in Brazil, with a key to the apteronotid species from the area. Vertebr Zool . 2011; 61(3):299-306.
- Varella HR. Revisão taxonômica das espécies de Crenicichla Heckel das bacias dos rios Paraná e Paraguai (Teleostei: Cichlidae). [Master Dissertation], São Paulo, SP: Universidade de São Paulo; 2011 Oct 07 [cited 2016 Dec 08]. Available from: Biblioteca digital Universidade de São Paulo. Available from: Biblioteca digital Universidade de São Paulo. http://www.teses.usp.br/teses/disponiveis/41/41133/tde-20012012-142913/pt-br.php
» http://www.teses.usp.br/teses/disponiveis/41/41133/tde-20012012-142913/pt-br.php - Vari RP. Systematics of the Neotropical Characiform genus Steindachnerina Fowler (Pisces: Ostariophysi). Washington (DC): Smithsonian Institution Press ; 1991. (Smithsonian Contributions to Zoology; no. 507).
- Vari RP. Systematics of the Neotropical characiform genus Cyphocharax Fowler (Pisces, Ostariophysi). Washington (DC): Smithsonian Institution Press ; 1992. (Smithsonian Contributions to Zoology; no. 529).
- Vari RP, Harold AS. Phylogenetic study of the neotropical fish genera Creagrutus Günther and Piabina Reinhardt (Teleostei: Ostariophysi: Characiformes), with a revision of the Cis-Andean species. Washington (DC): Smithsonian Institution Press ; 2001. (Smithsonian Contributions to Zoology; no. 613).
- Vazzoler AEAM, Suzuki HI, Marques EE, Lizama MLAP. Primeira maturação gonadal, períodos e áreas de reprodução. In: Vazzoler AEAM, Agostinho AA, Hahn NS, editors. A planície de inundação do alto rio Paraná: aspectos físicos, biológicos e socioeconômicos . Maringá: Eduem ; 1997. p.249-265.
- Vitule JRS, Freire CA, Simberloff D. Introduction of non-native freshwater fish can certainly be bad. Fish Fish. 2009; 10(1):98-108.
- Vitule JRS, Umbria SC, Aranha JMR. Introduction of the African catfish Clarias gariepinus (Burchell, 1822) into Southern Brazil. Biol Invasions. 2006; 8:677-81.
- Weber C. Hypostomus microstomus sp. nov. et autres poissons-chats cuirassés du rio Paraná (Pisces, Siluriformes, Loricariidae). Arch Sci. 1987; 40(3):273-84.
- Welcomme RL. Fisheries ecology of floodplain rivers. London: Longman Group; 1979.
- Wellborn TL. Channel catfish life history and biology. Stoneville: Cooperative extension work in agriculture and home economics in cooperation with the United States Department of Agriculture; 1988. (Southern Regional Aquaculture Center Publication; no. 180).
- Whitehead PJP. FAO species catalogue. Rome: Food and Agriculture Organization of the United Nations; 1985. vol. 7, Clupeoid fishes of the world (suborder Clupeoidei): An annotated and illustrated catalogue of the herrings, pilchards, sprats, shads, anchovies and wolf-herrings, pt. 1, Chirocentridae, Clupeidae and Pristigasteridae. (FAO Fisheries Synopsis; no. 125).
- Winemiller KO, Kelso-Winemiller LC. Comparative ecology of catfishes of the upper Zambezi River floodplain. J Fish Biol . 1996; 49(6):1043-61.
- Wootton RJ. Ecology of teleost fishes. London; New York: Chapman and Hall; 1990. (Fish and Fisheries; 1).
- Yalçin S, Akyurt I, Solak K. Stomach contents of the catfish (Clarias gariepinus Burchell, 1822) in the River Asi (Turkey). Turk J Zool. 2001; 25:461-68.
- Zanatta AS, Ramos IP, Silva RJ, Langeani F, Carvalho ED. Pisces, Siluriformes, Ictaluridae, Ictalurus punctatus (Rafinesque, 1818): first record in middle Paranapanema River reservoir, aquaculture and exotic species dispersion. Check List . 2010; 6(4):589-91.
- Zarske A, Le Bail P-Y, Géry J. New and poorly known Characiform fishes from French Guiana. 1. Two new Tetras of the genera Hemigrammus and Hyphessobrycon (Teleostei: Characiformes: Characidae). Zool Abh. 2006; 55:17-30.
Edited by
Publication Dates
-
Publication in this collection
11 June 2018 -
Date of issue
2018
History
-
Received
02 Aug 2017 -
Accepted
12 Apr 2018