ABSTRACT
Atlantoraja cyclophora is an endemic skate to the continental shelf of the Southwestern Atlantic Ocean (22ºS-47ºS) and a by-catch species in commercial bottom trawl fisheries. The morphometric relationships, the size at maturity and the reproductive cycle of this species were analyzed, with samples collected between 34ºS and 42ºS. The size range was 190 to 674 mm total length (TL) for males and 135 to 709 mm TL for females. Sexual dimorphism between the relationships TL - disc width and TL - total weight was found, with females wider and heavier than males. The mean size at maturity for males was estimated in 530 mm TL and for females in 570 mm TL. The gonadosomatic index (GSI) in mature females varied seasonally and showed the highest value in December. The maximum follicular diameter and oviductal gland width did not show any seasonal pattern. Females with eggs in the uterus were present most of the year. The reproductive activity in males would be continuous throughout the year, evidenced by the lack of variation in the GSI between seasons. The results obtained suggest that A. cyclophora might undergo an annual reproductive cycle, in coincidence to that reported for this species in Brazilian populations.
Keywords:
Arhynchobatidae; Dimorphism; Elasmobranchs; Maturity; Reproduction
RESUMEN
Atlantoraja cyclophora es una raya endémica de las plataformas continentales del Océano Atlántico Sudoccidental (22ºS-47ºS) que se captura incidentalmente en las pesquerías comerciales de arrastre de fondo. Se estudiaron las relaciones morfométricas, el ciclo reproductivo y se estimó la longitud media de madurez sexual de esta especie con muestras colectadas entre 34ºS y 42ºS. El rango de tamaño fue 190 a 674 mm de longitud total (LT) en machos y 135 a 709 mm LT en hembras. Se observó dimorfismo sexual entre las relaciones LT - ancho de disco y LT - peso total, siendo las hembras más anchas y pesadas que los machos. La talla de madurez se estimó en 530 mm LT para machos y en 570 mm LT para hembras. El índice gonadosomático (IGS) en hembras maduras varió estacionalmente, con el valor más alto en Diciembre. No se registró variación estacional respecto al diámetro máximo folicular y al ancho de la glándula oviductal. Se observaron hembras con huevos en los úteros durante la mayor parte del año. La actividad reproductiva en los machos sería continua durante todo el año, dada la falta de variación del IGS entre estaciones. Se sugiere que A. cyclophora experimenta un ciclo reproductivo anual, similar a lo reportado para esta especie en aguas de Brasil.
Palabras Clave:
Arhynchobatidae; Dimorfismo; Elasmobranquios; Madurez; Reproducción
Introduction
The genus Atlantoraja (Menni, 1972) comprises three endemic species of the Southwestern Atlantic: A. castelnaui, (Ribeiro, 1907), A. cyclophora (Regan, 1903) and A. platana (Günther, 1880) (according to Ebert, Compagno, 2007Ebert DA, Compagno LJV. Biodiversity and systematics of skates (Chondrichthyes: Rajiformes: Rajoidei). Environ Biol Fish. 2007; 80(2-3):111-24.). Atlantoraja cyclophora is distributed from Cabo Frio, Brazil (22ºS) to San Jorge Gulf in Argentina (47ºS) and inhabits from the coast out to maximum depths of 300 m in Brazil and up to the 130 m isobath in Argentina (Oddone, Vooren, 2004Oddone MC, Vooren CM. Distribution, abundance and morphometry of Atlantoraja cyclophora (Regan, 1903) (Elasmobranchii: Rajidae) in southern Brazil, Southwestern Atlantic. Neotrop Ichthyol . 2004; 2(3):137-44.; Cousseau et al., 2007Cousseau MB, Figueroa DE, Díaz de Astarloa JM, Mabragaña E, Lucifora LO. Rayas, chuchos y otros batoideos del Atlántico sudoccidental (34ºS-55ºS). Mar del Plata: Instituto Nacional de Investigación y Desarrollo Pesquero INIDEP; 2007.). This vulnerable species (Massa et al., 2006Massa A, Hozbor N, Vooren CM. Atlantoraja cyclophora. The IUCN Red List of Threatened Species. 2006; e.T61398A12462475. http://dx.doi.org/10.2305/IUCN.UK.2006.RLTS.T61398A12462475.en
http://dx.doi.org/10.2305/IUCN.UK.2006.R...
) is taken as by-catch along all its distribution area by commercial bottom trawl fisheries (Paesch, Domingo, 2003Paesch L, Domingo A. La pesca de condrictios en el Uruguay. Frente Marítimo. 2003; 19:207-16.; Oddone, Vooren, 2005Oddone MC, Vooren CM. Reproductive biology of Atlantoraja cyclophora (Regan 1903) (Elasmobranchii: Rajidae) off southern Brazil. ICES J Mar Sci . 2005; 62(3):1095-103.; Tamini et al., 2006Tamini LL, Chiaramonte GE, Perez JE, Cappozzo HL. Batoids in a coastal trawl fishery of Argentina. Fish Res . 2006; 77(3):326-32.; Góngora et al., 2009Góngora ME, Bovcon ND, Cochia PD. Ictiofauna capturada incidentalmente en la pesquería de langostino patagónico Pleoticus muelleri Bate, 1888. Rev Biol Mar Oceanogr. 2009; 44(3):583-93.; Estalles et al., 2011Estalles M, Coller NM, Perier MR, Di Giácomo EE. Skates in the demersal trawl fishery of San Matías Gulf, Patagonia: species composition, relative abundance and maturity stages. Aquat Living Resour. 2011; 24(2):193-99.; Massa, Hozbor, 2011Massa AM, Hozbor NM. Evolución de las estimaciones de abundancia de los peces cartilaginosos demersales de mayor valor comercial del Altántico Sudoccidental capturados entre 34º y 41ºS a profundidades menores a 50m. Parte 2. Los condrictios en la actividad pesquera argentina. In: Wöhler OC, Cedrola P, Cousseau MB, editors. Contribuciones sobre la biología, pesca y comercialización de tiburones en la Argentina. Aportes para la elaboración del plan de acción nacional. Buenos Aires: Consejo Federal Pesquero; 2011. p. 193-205.; Orlando et al., 2011Orlando L, Pereyra I, Paesch L, Norbis W. Size and sex composition of two species of the genus Atlantoraja (Elasmobranchii, Rajidae) caught by the bottom trawl fisheries operating on the Uruguayan continental shelf (Southwestern Atlantic Ocean). Braz J Oceanogr. 2011; 59(4):357-64.). The coastal ecosystem of the Southwest Atlantic Ocean, between 34ºS and 42ºS and <50 m deep, constitutes the region where the greatest landings of cartilaginous fish are recorded (Massa et al., 2004Massa AM, Lucifora LO, Hozbor NM. Condrictios de la región costera bonaerense y uruguaya. In: Sánchez, RP, Bezzi, SI., editors. El Mar Argentino y sus recursos pesqueros. Los peces marinos de interés pesquero. Caracterización biológica y evaluación del estado de explotación. Mar del Plata: INIDEP; 2004. p.85-99.; Massa, Hozbor, 2011Massa AM, Hozbor NM. Evolución de las estimaciones de abundancia de los peces cartilaginosos demersales de mayor valor comercial del Altántico Sudoccidental capturados entre 34º y 41ºS a profundidades menores a 50m. Parte 2. Los condrictios en la actividad pesquera argentina. In: Wöhler OC, Cedrola P, Cousseau MB, editors. Contribuciones sobre la biología, pesca y comercialización de tiburones en la Argentina. Aportes para la elaboración del plan de acción nacional. Buenos Aires: Consejo Federal Pesquero; 2011. p. 193-205.). The multi-fleet fishery that operates in the area comprises a total of 46 species, of which 19 are cartilaginous fish (Sánchez et al., 2011Sánchez RP, Navarro G, Calvo E, Del Castillo F. La pesca y comercialización de condrictios en la Argentina. Aportes de la Dirección Nacional de Planificación Pesquera para la elaboración del plan de acción nacional. Parte 2. Los condrictios en la actividad pesquera argentina. In: Wöhler OC, Cedrola P, Cousseau MB, editors. Contribuciones sobre la biología, pesca y comercialización de tiburones en la Argentina. Aportes para la elaboración del plan de acción nacional. Buenos Aires: Consejo Federal Pesquero ; 2011. p.151-184.).
Knowledge of the reproductive biology of species is essential to understand its life history and for the development of responsible management strategies (Leonard et al., 1999Leonard BK, Summers AP, Koob TJ. Metabolic rate of embryonic little skate, Raja erinacea (Chondrichthyes: Batoidea): the cost of active pumping. J Exp Zool . 1999; 283(1):13e18.). Oddone, Vooren (2005Oddone MC, Vooren CM. Reproductive biology of Atlantoraja cyclophora (Regan 1903) (Elasmobranchii: Rajidae) off southern Brazil. ICES J Mar Sci . 2005; 62(3):1095-103.) studied the reproductive biology of A. cyclophora in southern Brazil between 100 and 300 m depths, and later, Oddone et al. (2008Oddone MC, Norbis W, Mancini PL, Amorim AF. Sexual development and reproductive cycle of the Eyespot skate Atlantoraja cyclophora (Regan, 1903) (Chondrichthyes: Rajidae: Arhynchobatinae), in southeastern Brazil. Acta Adriat. 2008; 49(1):73-87.) complemented the reproductive studies of this species in southeastern Brazil, up to 146 m depth. In Argentina, Estalles et al. (2011Estalles M, Coller NM, Perier MR, Di Giácomo EE. Skates in the demersal trawl fishery of San Matías Gulf, Patagonia: species composition, relative abundance and maturity stages. Aquat Living Resour. 2011; 24(2):193-99.) reported estimates of size at maturity for males and females of A. cyclophora in the San Matías Gulf (41°S-42°S), but there is no information on reproductive variables in the area between 34°and 42° S. Also, like other elsamobranchs (Chiaramonte, Pettovello, 2000Chiaramonte GE, Pettovello AD. The biology of Mustelus schmitti in southern Patagonia, Argentina. J Fish Biol . 2000; 57(4):930-42.; Mabragaña, Cousseau, 2004Mabragaña E, Cousseau MB. Reproductive biology of two sympatric skates in the south-west Atlantic: Psammobatis rudis and Psammobatis normani. J Fish Biol . 2004; 65(2):559-73.; Colonello et al., 2007bColonello JH, Lucifora LO, Massa AM. Reproduction of the angular angel shark (Squatina guggenheim): geographic differences, reproductive cycle, and sexual dimorphism. ICES J Mar Sci. 2007b; 64(1):131-40.), a latitudinal gradient in the maximum TL and size at maturity has been noted for A. cyclophora (Oddone, Vooren, 2005Oddone MC, Vooren CM. Reproductive biology of Atlantoraja cyclophora (Regan 1903) (Elasmobranchii: Rajidae) off southern Brazil. ICES J Mar Sci . 2005; 62(3):1095-103.; Oddone et al., 2008Oddone MC, Norbis W, Mancini PL, Amorim AF. Sexual development and reproductive cycle of the Eyespot skate Atlantoraja cyclophora (Regan, 1903) (Chondrichthyes: Rajidae: Arhynchobatinae), in southeastern Brazil. Acta Adriat. 2008; 49(1):73-87.; Estalles et al., 2011Estalles M, Coller NM, Perier MR, Di Giácomo EE. Skates in the demersal trawl fishery of San Matías Gulf, Patagonia: species composition, relative abundance and maturity stages. Aquat Living Resour. 2011; 24(2):193-99.).
The aim of this work was to increase the knowledge of the life history features of A. cyclophora in a commercially important area, where reproductive variables about the species remain unknown. In this context, we analyzed the morphometric relationships, the reproductive cycle and size at maturity of A. cyclophora in the Southwestern Atlantic Ocean, between 34°and 42°S (excluding San Matías Gulf).
Materials and Methods
Study area and sampling. A total of 974 specimens of A. cyclophora, 488 males and 486 females, were collected in the Southwestern Atlantic Ocean between 34ºS and 42ºS, from bottom trawl surveys carried out by the Instituto Nacional de Investigación y Desarrollo Pesquero (INIDEP) between 2002 and 2007 (Fig. 1). Samples were also obtained from commercial landings at Mar del Plata harbor. In the research surveys, the gear used was a standard Engel type bottom trawl of 120 mm mesh size, with a vertical height of 5 m and a horizontal opening of 20 m. The standard tow duration was 15 min at a speed for 4 knots (7.41 km h−1) and up to 50 m at depth. For the commercial hauls, a bottom trawl gear of 120 mm mesh-size was used, with variable length depending on the boat. The total length (TL, mm) from the snout tip to the tail tip, disc width (DW, mm) between lateral tips of pectoral fins, total weight (TW, g), liver weight (LW, g) and sex were recorded for each individual. Additional reproductive variables were also registered: the inner claspers length (ICL, mm) measured from the apex of the cloaca to the clasper tip, alar thorns row number (ATR) and testes weight (TTW, g) expressed as gonadosomatic index GSI for males (GSI = TTW/TW · 100); and oviductal gland width (OGW, mm), uterus width (UW, mm), ovarian weight (OW, g) expressed as gonadosomatic index (GSI = OW/TW · 100) and the largest ovarian follicles diameter (LOFD, mm) and number for females. The maximum width and length (excluding the horns) of the egg capsules obtained from the left and right uteri were also recorded. Maturity status was assessed by a macroscopic analysis of the reproductive organs, following a histologically validated maturity scale proposed by Colonello (2009Colonello JH. Ecología reproductiva de tres batoideos (Chondrichthyes): Atlantoraja castelnaui (Rajidae), Rioraja agassizi (Rajidae) y Zapteryx brevirostris (Rhinobatidae). Implicancias de distintas estrategias adaptativas en un escenario de explotación comercial intensiva. [PhD Thesis]. Argentina: Facultad de Ciencias Naturales y Museo Universidad Nacional de La Plata; 2009.), with modifications. Males were classified into 3 reproductive stages and females into 4 reproductive stages (Tab. 1).
Morphological criteria used to determine macroscopically the stage of sexual maturity of males and females Atlantoraja cyclophora. Criteria adapted from Colonello (2009Colonello JH. Ecología reproductiva de tres batoideos (Chondrichthyes): Atlantoraja castelnaui (Rajidae), Rioraja agassizi (Rajidae) y Zapteryx brevirostris (Rhinobatidae). Implicancias de distintas estrategias adaptativas en un escenario de explotación comercial intensiva. [PhD Thesis]. Argentina: Facultad de Ciencias Naturales y Museo Universidad Nacional de La Plata; 2009.).
Data analyses. Normality and homoscedasticity were tested for the whole sample by Shapiro-Wilk’s and Levene’s tests, respectively. When deviations from normality and homogeneity were detected or, there were not mean-variance relationship to apply a transformation, a nonparametric test was used (Zar, 1999Zar JH. Biostatistical analysis. 4th ed. Upper Saddle River (NJ): Prentice Hall; 1999.). The length frequency distributions between sexes were compared by the Kolmogorov-Smirnov test, using the statistic: KS=n(1)n(2)/d, where n(1) and n(2) are the sample sizes and d is the greatest common divisor of n(1) and n(2) under the normal approach based on the asymptotic distribution of KS adequately standardized (Hollander, Wolfe, 1999Hollander M, Wolfe DA. Nonparametric statistical methods. 2nd ed. New York: Wiley-Interscience; 1999.). The relationships between TL-DW, TL-TW, and TL-LW were estimated for each sex. The x and y variables for the allometric equation were log-transformed and the equation were expressed as the linear relationship between y and (Sokal, Rohlf, 1987Sokal RR, Rohlf FJ. Introduction to biostatistics. New York: WH Freeman and Company; 1987.). Parameters a and b were estimated by the least-square regression (Ordinary least square regression, Warton et al., 2006Warton DI, Wright IJ, Falster DS, Westoby M. Bivariate line-fitting methods for allometry. Biol Rev. 2006; 81(2):259-91.) and the null hypothesis of no differences between slopes was tested using ANCOVA (Zar, 1999Zar JH. Biostatistical analysis. 4th ed. Upper Saddle River (NJ): Prentice Hall; 1999.). The null hypothesis of the isometric growth (H0: b=3) (Froese, 2006Froese R. Cube law, condition factor and weight-length relationships: history, meta-analysis and recommendations. J Appl Ichthyol. 2006; 22(4):241-53.), was tested using the statistic: , where Sb is the standard error of the slope (Sokal, Rohlf, 1987Sokal RR, Rohlf FJ. Introduction to biostatistics. New York: WH Freeman and Company; 1987.). Changes in the reproductive organs as ICL, OGW, UW and GSI relative to TL, were used to further assess the onset of maturity. The symmetry and functional parity of the ovaries and the morphology of the egg capsules were tested by a paired t-test, in both cases (Zar, 1999Zar JH. Biostatistical analysis. 4th ed. Upper Saddle River (NJ): Prentice Hall; 1999.). In order to estimate the size at 50% maturity (TL50%), a logistic ogive was fitted to the data using a maximum likelihood approach and the differences in TL50% between the sexes were evaluated through a log-likelihood test (Aubone, Wöhler, 2000Aubone A, Wöhler OC. Aplicación del método de máxima verosimilitud a la estimación de parámetros y comparación de curvas de crecimiento de von Bertalanffy. INIDEP Informe Técnico Interno No 37. 2000.). Temporal changes of GSI, HSI (HSI = LW/TW·100), OGW, and LOFD of adult individuals were analyzed by ANOVA followed by post hoc comparisons with Tukey test (Zar, 1999Zar JH. Biostatistical analysis. 4th ed. Upper Saddle River (NJ): Prentice Hall; 1999.). When it was not possible to assume the normality and homogeneity of variance, the no parametric Kruskal-Wallis H-test was used (Zar, 1999Zar JH. Biostatistical analysis. 4th ed. Upper Saddle River (NJ): Prentice Hall; 1999.), followed by nonparametric multiple comparisons testing described in Conover (1999Conover WJ. Practical nonparametric statistics. 3rd ed. Toronto: John Wiley and Sons; 1999.).
Map of the study area showing trawl stations carried out in research cruises during 2002 and 2007 (dark dots) and the commercial capture (dark statistical rectangles of 1 degree by 1 degree) where Atlantoraja cyclophora were collected.
Results
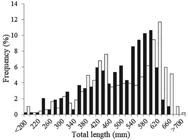
Length distributions and morphometric relationships. Males ranged from 190 mm TL to 674 mm TL, whereas females ranged from 135 mm TL to 709 mm TL. There were significant differences between sexes in the size frequency distribution (KS=0.16, n=974, p=0.01). The higher frequencies observed were in the classes between 540 - 600 mm TL for males and 600-620 mm TL for females (Fig. 2). There were significant differences between sexes in the relationships TL-DW (ANCOVA: F (1, 869)=12.74, p<0.0001) and TL-TW (ANCOVA: F(1, 877)=9.42, p<0.0001) (Fig. 3a-b). Females were found to be wider and heavier than males. This dimorphism was found to start at 290 mm TL for both cases, but it was more evident in individuals >500 mm TL. For the relationship TL-LW, the female livers were significantly heavier than those of males (ANCOVA: F(1, 721)=16, p<0.0001) (Fig. 3c).
According to the comparison of the angular coefficient “b” with the theoretical value of 3, males grew isometrically (b=3.03, d.f=444, t=1.41, p=0.15), while female growth was found to be positively allometric (b=3.16, d.f=433, t=8.26, p<0.0001) increasing their weight in greater proportion than their length (Fig. 3b).
Total length frequency distribution of males (dark bars, n= 488) and females (light bars, n = 486) of Atlantoraja cyclophora.
Relationship between total length and a. disc width (DW); b. total weight (TW) and c. liver weight (LW) of males (dark dots) and females (light dots) Atlantoraja cyclophora. The curves were fitted by: a. males DW = 33.51 + 0.71TL(r=0.96, n=438), females DW = 18.70 + 0.71TL (r=0.98, n=435); b. males TW = (5 x 10-6)TL3.031 (r=0.98, n=446), females TW = (2 x 10-6)TL3.162 (r=0.98, n=435); c. males LW = (5 x 10-7)TL2.759 (r=0.83, n=365); females LW =(9 x 10-9)TL3.459 (r=0.90, n=360).
Reproductive organs development and size at maturity. In male individuals, a gradual increase in the clasper length to TL was evident between 500 and 550 mm TL (Fig. 4a) as well as the number of alar thorn rows (Fig. 4b). The GSI does not reflect a clear trend of testicular development as a function of TL (Fig. 4c). The size of the smallest adult male was 490 mm TL and the largest juvenile was 604 mm TL. All males > 604 mm TL were adults, with inner clasper length > 178 mm, alar thorn rows > 2 and testes weight > 14 g (Tab. 2). The males TL50% was estimated in 537 mm TL (IC 95%, 425.6 ≤ TL50 ≤ 649.2), which corresponded to 79.7% of the maximum TL observed (Fig. 4d).
Relationship between total length and a. inner claspers length (ICL); b. number of alar thorns rows; c. GSI; and d. proportion by mature individuals of Atlantoraja cyclophora males according to maturity stage (stage 1, light rhombus; stage 2, dark rhombus; stage 3, light square).
Range (mean ± s.d) of the total length (TL, mm) and the reproductive variables recorded for juvenile and adults of Atlantoraja cyclophora. ICL, inner clasper length (mm); ATR, alar thorn rows (nº); TTW, testis weight (g); OGW, oviductal gland width (mm); UW, uterus width (mm); OW, ovary weight (g) and n, sample size.
In females, both ovaries were functional and similar in mass (t=-0.81, d.f=172, p=0.42). Through the macroscopic analysis it was observed that the ovaries developed simultaneously. There were no differences in the number (t=-1.43, d.f=53, p=0.16) and diameter (t=- 0.06, d.f=41, p=0.95) of largest ovarian follicles between ovaries. The relationship between TL and oviductal gland and uterus width had a phased development, with an increase between 520 and 550 mm TL (Figs. 5a-b). The GSI reflects that development of the ovaries occurs abruptly and their weight increased from 550 mm TL (Fig. 5c). The smallest adult female size was 525 mm TL and the largest juvenile was 650 mm TL. All females > 650 mm TL were adults, with oviductal gland width > 27 mm, uterus width > 10 mm and ovary weight > 23 g (Tab. 2). Total length at 50% maturity was estimated at 570 mm TL (IC 95%, 436.9 ≤ TL50 ≤ 704.8) for females, which corresponded to 80.4% of the maximum TL observed (Fig. 5d). This value was significantly greater in females than in males (t=17.56, d.f=1, p<0.0001).
Relationship between total length and a. oviductal gland width (OGW); b. uterus width (UW); c. GSI; and d. proportion by mature individuals of Atlantoraja cyclophora females according to maturity stage (stage 1, light rhombus; stage 2, dark rhombus; stage 3, light square; stage 4, dark dots).
Reproductive cycle. Males showed a significant difference in the GSI mean values between November and December (ANOVA: F(9, 129)=2.39, p=0.01) (Fig. 6a). Despite this difference, the GSI it seems to be stable throughout the year. Also, significant differences were found in male HSI (H=46.76, d.f=9, p<0.0001), which decreased in autumn and increased in spring and summer (Fig. 6b). Seasonal variations were detected in the GSI (ANOVA: F(8, 66)=2.85, p=0.008) and HSI (H=38.47, d.f=8, p<0.0001) for females (Figs. 6c-d). The highest GSI value recorded in December was significantly different from all months except November and July. The HSI was similar to male pattern with a decrease in autumn an increase in spring. The largest ovarian follicles diameter (LOFD) ranged between 13 and 40 mm and the modal value was 26 mm, for both egg-carrying and no egg-carrying females. It was possible to identify macroscopically 3 different size groups of LOFD: one between 13-25 mm, the most frequent between 26 - 33 mm, and a less frequent between 34 - 40 mm (Fig. 7a). Probably, the latter group represents the pre-ovulatory follicles. Although it was not possible to record the follicular size of all the adult females sampled, it was observed that females with follicles > 34 mm were present in March and from July to December. Females with eggs in the uterus were observed during all the sampled months excepting January, February and June (Fig. 7b). No significant differences were detected in the OGW (ANOVA: F(9,134)=1.23, p=0.28) and LOFD (ANOVA: F(7, 36)=1.24, p=0.30) throughout the year (Fig. 8a-b). However, the lowest values of the LOFD were observed in April (Fig. 8b).
Seasonal variation in gonadosomatic (GSI) and hepatosomatic (HSI) indexes for a.-b. males and c.-d. females of Atlantoraja cyclophora. The number of samples analyzed is between parentheses. The boxes represent the interquartile range between Q1 and Q3 with the 50% of data, the central line represents the median value and whiskers extend to the maximum and minimum values.
a. Frequency distribution of the largest ovarian follicles diameter (LOFD) in mature females of Atlantoraja cyclophora. b. Seasonal variation in the proportion of egg-carrying females of A. cyclophora. The number of samples analyzed is between parentheses.
Seasonal variation in a. oviductal gland width (OGW) and b. diameter of the largest ovarian follicle (LOFD) in Atlantoraja cyclophora females. The number of samples analyzed is between parentheses. The boxes represent the interquartile range between Q1 and Q3 with the 50% of data, the central line represents the median value and whiskers extend to the maximum and minimum values.
Egg capsule. The length and width of the 36 egg capsules in uteri ranged between 63 - 77 mm (71.4 ± 3.15) and 40 - 48 mm (43.9 ± 2.12) respectively. No differences were found in the mean values of the maximum length (t=-1.01, d.f=15, p=0.326) and width (t=-0.39, d.f=15, p=0.696) between the left and right egg capsules.
Discussion
The length frequency distribution significantly differed between sexes and it was more evident between adult individuals. The higher frequencies in both sexes were observed in individuals with sizes that exceeded the TL50%. However, these results are presented informatively, since part of the specimens come from commercial landings and the sample may be skewed due to the selective retention of larger specimens (Matta, Gunderson, 2007Matta ME, Gunderson DR. Age, growth, maturity, and mortality of the Alaska skate, Bathyraja parmifera, in the eastern Bering Sea. Environ Biol Fish . 2007; 80(2-3):309-23.). It has been observed that all species of the genus Atlantoraja are sexually dimorphic in TL-DW and TL-TW ratio, with females heavier and wider than males (Oddone, Vooren, 2004Oddone MC, Vooren CM. Distribution, abundance and morphometry of Atlantoraja cyclophora (Regan, 1903) (Elasmobranchii: Rajidae) in southern Brazil, Southwestern Atlantic. Neotrop Ichthyol . 2004; 2(3):137-44.; Oddone, Amorim, 2007Oddone MC, Amorim AF. Length-weight relationships, condition and population structure of the genus Atlantoraja (Elasmobranchii, Rajidae, Arhynchobatinae) in Southeastern Brazilian waters, SW Atlantic Ocean. J Northw Atl Fish Sci. 2007; 38:43-52.; Coller et al., 2011Coller NM, Perier MR, Di Giácomo EE. Dimorfismo sexual y relaciones morfométricas de Atlantoraja platana (Günther, 1880) en aguas del golfo San Matías, Patagonia. Rev Mus Argent Cienc Nat. 2011; 13(1):1-5.; Colonello et al., 2012Colonello JH, García ML, Lasta CA, Menni RC. Reproductive biology of the spotback skate Atlantoraja castelnaui in the south-west Atlantic Ocean. J Fish Biol . 2012; 80(7):2405-19.). Also, it is common in this genus that such dimorphism begins in immature individuals (Oddone, Amorim, 2007Oddone MC, Amorim AF. Length-weight relationships, condition and population structure of the genus Atlantoraja (Elasmobranchii, Rajidae, Arhynchobatinae) in Southeastern Brazilian waters, SW Atlantic Ocean. J Northw Atl Fish Sci. 2007; 38:43-52.; Coller et al., 2011Coller NM, Perier MR, Di Giácomo EE. Dimorfismo sexual y relaciones morfométricas de Atlantoraja platana (Günther, 1880) en aguas del golfo San Matías, Patagonia. Rev Mus Argent Cienc Nat. 2011; 13(1):1-5.). In this work, a change in the morphology of A. cyclophora individuals was detected around 290 mm TL. This agrees with the positive allometric growth observed in this work for females, and by Oddone, Amorim (2007Oddone MC, Amorim AF. Length-weight relationships, condition and population structure of the genus Atlantoraja (Elasmobranchii, Rajidae, Arhynchobatinae) in Southeastern Brazilian waters, SW Atlantic Ocean. J Northw Atl Fish Sci. 2007; 38:43-52.) in southeastern Brazil. However, the increment in weight of females was considerable from 500 mm TL and it was consistent with the maturity. The dimorphism in TL-LW ratio could be related to the higher reproductive energy requirements of females (Colonello et al., 2012Colonello JH, García ML, Lasta CA, Menni RC. Reproductive biology of the spotback skate Atlantoraja castelnaui in the south-west Atlantic Ocean. J Fish Biol . 2012; 80(7):2405-19.). The increase of liver weight observed in larger females (>500 mm TL), suggests higher energy storage in females during maturation, in comparison to adult males. This is consistent with the fact that the liver actively participates in the synthesis of yolk precursors (Koob, Callard, 1999Koob TJ, Callard IP. Reproductive endocrinology of female elasmobranchs: lessons from the little skate (Raja erinacea) and spiny dogfish (Squalus acanthias). J Exp Zool. 1999; 284(5):557-74.; Prisco et al., 2002Prisco M, Romano M, Ricchiari L, Limatola E, Andreuccetti P. An ultrastructural study on the vitellogenesis in the spotted ray Torpedo marmorata. Gen Comp Endocrinol. 2002; 128(3):171-79.; Díaz-Andrade et al., 2009Díaz-Andrade MC, Galíndez E, Estecondo S. The ovary of the bignose fanskate Sympterygia acuta Garman, 1877 (Chondrichthyes, Rajidae) in the Bahía Blanca estuary, Argentina: morphology and reproductive features. Braz J Biol. 2009; 69(2):405-13.).
Sexual dimorphism in maximum length and size at maturity has been observed in many chondrichthyans (Mabragaña et al., 2002Mabragaña E, Lucifora LO, Massa AM. The reproductive ecology and abundance of Sympterygia bonapartii endemic to the south-west Atlantic. J Fish Biol . 2002; 60(4):951-67.; Ungaro, 2004Ungaro N. Biological parameters of the brown ray, Raja miraletus, in the Southern Adriatic basin. 2004. Cybium, 28(2):174-76.; McFarlane, King, 2006McFarlane GA, King JR. Age and growth of big skate (Raja binoculata) and longnose skate (Raja rhina) in British Columbia waters. Fish Res . 2006; 78(2-3):169-78.; Oddone et al., 2007Oddone MC, Amorim AF, Mancini PL, Norbis W, Velasco G. The reproductive biology and cycle of Rioraja agassizi (Müller and Henle, 1841) (Chondrichthyes: Rajidae) in southeastern Brazil, SW Atlantic Ocean. Sci Mar. 2007; 71(3):593-604.; Ebert et al., 2008Ebert DA, Smith WD, Cailliet GM. Reproductive biology of two commercially exploited skates, Raja binoculata and R. rhina, in the western Gulf of Alaska. Fish Res. 2008; 94(1):48-57.; Colonello et al., 2016Colonello JH, Cortés F, Belleggia M, Massa AM. Reproductive and population parameters of spiny dogfish Squalus acanthias in the south-western Atlantic Ocean. J Fish Biol . 2016; 88(5):1758-75.; Chierichetti et al., 2017Chierichetti MA, Scenna LB, Di Giácomo EE, Ondarza PM, Figueroa DE, Miglioranza KSB. Reproductive biology of the cockfish, Callorhinchus callorynchus (Chondrichthyes: Callorhinchidae), in coastal waters of the northern Argentinean Sea. Neotrop Ichthyol . 2017; 15(2):e160137.). Viviparous elasmobranchs females usually mature and grow to large size than male, showing a positive correlation between litter and size (Colonello et al., 2011Colonello JH, García ML, Menni RC. Reproductive biology of the lesser guitarfish Zapteryx brevirostris from the south-western Atlantic Ocean. J Fish Biol . 2011; 78(1):287-302.). However, this dimorphism is quite variable among skates. In Psammobatis Günther, 1870 and Bathyraja Ishiyama, 1958, males may exceed or equal the female’s sizes and they can also reach sexual maturity at larger TL (Braccini, Chiaramonte, 2002Braccini JM, Chiaramonte GE. Reproductive biology of Psammobatis extenta. J Fish Biol. 2002; 61(1):272-88.; Mabragaña, Cousseau, 2004Mabragaña E, Cousseau MB. Reproductive biology of two sympatric skates in the south-west Atlantic: Psammobatis rudis and Psammobatis normani. J Fish Biol . 2004; 65(2):559-73.; Ebert, 2005Ebert DA. Reproductive biology of skates, Bathyraja (Ishiyama), along the eastern Bering Sea continental slope. J Fish Biol . 2005; 66(3):618-49.; San Martín et al., 2005San Martín MJ, Perez JE, Chiaramonte GE. Reproductive biology of the South West Atlantic marbled sand skate Psammobatis bergi Marini, 1932 (Elasmobranchii, Rajidae). J Appl Ichthyol . 2005; 21(6):504-10.; Perier et al., 2010Perier R, Estalles M, Coller M, Di Giacomo EE. Reproductive biology of the endemic skate Psammobatis lentiginosa in the San Matías Gulf (south-western Atlantic). J Mar Biol Assoc U.K. 2010; 91(6):1165-73.). Nonetheless, A. cyclophora females attain larger maximum size and size at maturity than males. Whatever the case, it does not seem to be a clear biological explanation for this.
In elasmobranchs with wide geographic distribution, the same species can display an increment in life history patterns with increase in latitude (Templeman, 1987Templeman W. Differences in sexual maturity and related characteristics between populations of thorny skate (Raja radiata) in the Northwest Atlantic. J Northw Atl Fish Sci . 1987; 7(2):155-67.; Chiaramonte, Pettovello, 2000Chiaramonte GE, Pettovello AD. The biology of Mustelus schmitti in southern Patagonia, Argentina. J Fish Biol . 2000; 57(4):930-42.; Yamaguchi et al., 2000Yamaguchi A, Taniuchi T, Shimizu M. Geographical variations in reproductive parameters of the starspotted dogfish, Mustelus manazo, from five localities in Japan and in Taiwan. Env Biol Fish . 2000; 57(2):221-33.; Frisk, Miller, 2006Frisk MG, Miller TJ. Age, growth, and latitudinal patterns of two Rajidae species in the northwestern Atlantic: little skate (Leucoraja erinacea) and winter skate (Leucoraja ocellata). Can J Fish Aquat Sci. 2006; 63(5):1078-91.; Colonello et al., 2007bColonello JH, Lucifora LO, Massa AM. Reproduction of the angular angel shark (Squatina guggenheim): geographic differences, reproductive cycle, and sexual dimorphism. ICES J Mar Sci. 2007b; 64(1):131-40.). There are many hypotheses to explain this fact, these variations could be a consequence of oceanographic conditions, the effect of fishing pressure (Mabragaña, Cousseau, 2004Mabragaña E, Cousseau MB. Reproductive biology of two sympatric skates in the south-west Atlantic: Psammobatis rudis and Psammobatis normani. J Fish Biol . 2004; 65(2):559-73.), or the result of phenotypic plasticity (Licandeo, Cerna, 2007Licandeo R, Cerna FT. Geographic variation in life-history traits of the endemic kite skate Dipturus chilensis (Batoidea: Rajidae), along its distribution in the fjords and channels of southern Chile. J Fish Biol . 2007; 71(2):421-40.). The maximum TL and size at maturity recorded here were similar to that previously reported in northern Patagonian waters (Estalles et al., 2011Estalles M, Coller NM, Perier MR, Di Giácomo EE. Skates in the demersal trawl fishery of San Matías Gulf, Patagonia: species composition, relative abundance and maturity stages. Aquat Living Resour. 2011; 24(2):193-99.) and greater than the registered for individuals from southern and southeastern Brazil (Oddone, Vooren, 2005Oddone MC, Vooren CM. Reproductive biology of Atlantoraja cyclophora (Regan 1903) (Elasmobranchii: Rajidae) off southern Brazil. ICES J Mar Sci . 2005; 62(3):1095-103.; Oddone et al., 2008Oddone MC, Norbis W, Mancini PL, Amorim AF. Sexual development and reproductive cycle of the Eyespot skate Atlantoraja cyclophora (Regan, 1903) (Chondrichthyes: Rajidae: Arhynchobatinae), in southeastern Brazil. Acta Adriat. 2008; 49(1):73-87.). In relation to these parameters, it is clear that A. cyclophora is a species of late maturity. Individuals analyzed in this study, mature when they reach 79.7% and 80.4% of their total growth (males and females respectively), in agreement with Brazilian populations (Tab. 3). However, the individuals of the San Matías Gulf have a later maturity despite being at the same latitude as the individuals analyzed in this work (between 41º- 42ºS and 34º- 42ºS, respectively) (Tab. 3). This could be due to the fact that these individuals are in a more protected marine environment with hydro-geographic characteristics that favor the spawning and reproduction of several species (Di Giacomo et al., 2005Di Giacomo EE, Perier MR, Pascual MS, Zampatti EA. El mar y sus recursos: golfo San Matías. In: Massera RF, Lew J, Serra Peirano G, editors. Las mesetas patagónicas que caen al mar: la costa rionegrina.Viedma: Río Negro; 2005. p.409-439.; Perier et al., 2011Perier MR, Estalles M, Coller M, Suarez MN, Mora GJ, Di Giacomo EE. Chondrichthyans of the San Matías Gulf, Patagonia, Argentina. Rev Mus Argentino Cienc Nat. 2011; 13(2):213-20.). On the other hand, although the bottom trawl fishery is developed in both regions, the fishing activity in the area between 34º- 42ºS is greater (Massa et al., 2004Massa AM, Lucifora LO, Hozbor NM. Condrictios de la región costera bonaerense y uruguaya. In: Sánchez, RP, Bezzi, SI., editors. El Mar Argentino y sus recursos pesqueros. Los peces marinos de interés pesquero. Caracterización biológica y evaluación del estado de explotación. Mar del Plata: INIDEP; 2004. p.85-99., Sánchez et al., 2011Sánchez RP, Navarro G, Calvo E, Del Castillo F. La pesca y comercialización de condrictios en la Argentina. Aportes de la Dirección Nacional de Planificación Pesquera para la elaboración del plan de acción nacional. Parte 2. Los condrictios en la actividad pesquera argentina. In: Wöhler OC, Cedrola P, Cousseau MB, editors. Contribuciones sobre la biología, pesca y comercialización de tiburones en la Argentina. Aportes para la elaboración del plan de acción nacional. Buenos Aires: Consejo Federal Pesquero ; 2011. p.151-184.).
In this work the TL-ICL relationship, together with the increase in the number of alar thorns were the best macroscopic parameters that represented the onset of sexual maturity in males, which ranged between 500 and 550 mm TL. On the other hand, size at maturity in female was established between 520 and 550 mm TL, based on a change in the OGW and in the uterus width (UW). The frequency distribution of LFOD recorded here showed that follicles with sizes bigger than 34 mm of diameter could be considered as pre-ovulatory. For this species this size had been previously estimated at 26 mm (Oddone, Vooren, 2005Oddone MC, Vooren CM. Reproductive biology of Atlantoraja cyclophora (Regan 1903) (Elasmobranchii: Rajidae) off southern Brazil. ICES J Mar Sci . 2005; 62(3):1095-103.) and 30 mm (Oddone et al., 2008Oddone MC, Norbis W, Mancini PL, Amorim AF. Sexual development and reproductive cycle of the Eyespot skate Atlantoraja cyclophora (Regan, 1903) (Chondrichthyes: Rajidae: Arhynchobatinae), in southeastern Brazil. Acta Adriat. 2008; 49(1):73-87.). However, the size of the egg cases of A. cyclophora examined in this work were similar those recorded by Oddone et al. (2004Oddone MC, Marçal AS, Vooren CM. Egg capsules of Atlantoraja cyclophora (Regan, 1903) and A. platana (Günther, 1880) (Pisces, Elasmobranchii, Rajidae). Zootaxa. 2004; 426(1):1-4.) from southern Brazil. According to Licandeo, Cerna (2007Licandeo R, Cerna FT. Geographic variation in life-history traits of the endemic kite skate Dipturus chilensis (Batoidea: Rajidae), along its distribution in the fjords and channels of southern Chile. J Fish Biol . 2007; 71(2):421-40.), the females of Zearaja chilensis (Guichenot, 1848) of the southernmost distribution from southern Chile would invest more energy in the production of larger egg cases instead of more quantity of eggs, than females of the northern region. More complementary studies of the ovarian fecundity of A. cyclophora are necessary to better understand their reproductive traits.
Size range (mm) and size at fifty percent of maturity (TL50%, mm) of Atlantoraja cyclophora, registered for different areas within its distribution range. F, females; M, males; N, number of individuals sampled; TL, total length. Other data references: (a) Oddone et al. (2008Oddone MC, Norbis W, Mancini PL, Amorim AF. Sexual development and reproductive cycle of the Eyespot skate Atlantoraja cyclophora (Regan, 1903) (Chondrichthyes: Rajidae: Arhynchobatinae), in southeastern Brazil. Acta Adriat. 2008; 49(1):73-87.), (b) Oddone, Vooren (2004Oddone MC, Vooren CM. Distribution, abundance and morphometry of Atlantoraja cyclophora (Regan, 1903) (Elasmobranchii: Rajidae) in southern Brazil, Southwestern Atlantic. Neotrop Ichthyol . 2004; 2(3):137-44.) (2005Oddone MC, Vooren CM. Reproductive biology of Atlantoraja cyclophora (Regan 1903) (Elasmobranchii: Rajidae) off southern Brazil. ICES J Mar Sci . 2005; 62(3):1095-103.), (c) Estalles et al. (2011Estalles M, Coller NM, Perier MR, Di Giácomo EE. Skates in the demersal trawl fishery of San Matías Gulf, Patagonia: species composition, relative abundance and maturity stages. Aquat Living Resour. 2011; 24(2):193-99.).
According to the results registered here, the lack of seasonal variation of LFOD and OGW and the occurrence of females with eggs in the uterus throughout the year, A. cyclophora might undergo an annual reproductive cycle. In addition, the higher values in the female GSI observed in December, might be explained by a peak of reproductive activity in spring season, as was proposed by Oddone et al. (2008Oddone MC, Norbis W, Mancini PL, Amorim AF. Sexual development and reproductive cycle of the Eyespot skate Atlantoraja cyclophora (Regan, 1903) (Chondrichthyes: Rajidae: Arhynchobatinae), in southeastern Brazil. Acta Adriat. 2008; 49(1):73-87.) in coastal waters off southeastern Brazil. Seasonal peaks of the reproductive activity are consistent with other skates that inhabit coastal (<50 m deep) and warmer waters of Southwestern Atlantic Ocean [Sympterygia bonapartii Müller & Henle, 1841 (according to Mabragaña et al., 2002Mabragaña E, Lucifora LO, Massa AM. The reproductive ecology and abundance of Sympterygia bonapartii endemic to the south-west Atlantic. J Fish Biol . 2002; 60(4):951-67.); A. castelnaui (according to Collonelo et al., 2012Colonello JH, García ML, Lasta CA, Menni RC. Reproductive biology of the spotback skate Atlantoraja castelnaui in the south-west Atlantic Ocean. J Fish Biol . 2012; 80(7):2405-19.); Rioraja agassizi (Müller & Henle, 1841) (according to Colonello et al., 2007aColonello JH, García ML, Lasta CA. Reproductive biology of Rioraja agassizi from the coastal southwestern Atlantic ecosystem between northern Uruguay (34°S) and northern Argentina (42°S). Env Biol Fish. 2007a; 80(2-3):277-84.; Oddone et al., 2007Oddone MC, Amorim AF, Mancini PL, Norbis W, Velasco G. The reproductive biology and cycle of Rioraja agassizi (Müller and Henle, 1841) (Chondrichthyes: Rajidae) in southeastern Brazil, SW Atlantic Ocean. Sci Mar. 2007; 71(3):593-604.)], where temperatures and photoperiod may affect egg laying rate (Colonello, 2009Colonello JH. Ecología reproductiva de tres batoideos (Chondrichthyes): Atlantoraja castelnaui (Rajidae), Rioraja agassizi (Rajidae) y Zapteryx brevirostris (Rhinobatidae). Implicancias de distintas estrategias adaptativas en un escenario de explotación comercial intensiva. [PhD Thesis]. Argentina: Facultad de Ciencias Naturales y Museo Universidad Nacional de La Plata; 2009.). The lack of reproductive seasonality is common in deeper waters with less environmental variability, as was observed in B. albomaculata (Norman, 1937) (according to Ruocco et al., 2006Ruocco NL, Lucifora LO, Díaz de Astarloa JM, Wöhler O. Reproductive biology and abundance of the white-dotted skate, Bathyraja albomaculata, in the Southwest Atlantic. ICES J Mar Sci . 2006; 63(1):105-16.), therefore, due to the predominant geographical location of A. cyclophora within the study area (outer coastal shelf, 28.9 - 49.6 m bottom depth, 10.6 - 14.9ºC mean bottom temperature, Jaureguizar et al., 2006Jaureguizar AJ, Menni R, Lasta C, Guerrero R. Fish assemblages of the northern Argentine coastal system: spatial patterns and their temporal variations. Fish Oceanogr. 2006; 15(4):326-44.), a mixed coastal-deep reproductive pattern might be possible. On the other hand, the reproductive activity in A. cyclophora males analyzed in this work was continuous through the year, evidenced by the lack of variation in the GSI between seasons. This agrees with results reported for southern Brazil specimens (Oddone et al., 2008Oddone MC, Norbis W, Mancini PL, Amorim AF. Sexual development and reproductive cycle of the Eyespot skate Atlantoraja cyclophora (Regan, 1903) (Chondrichthyes: Rajidae: Arhynchobatinae), in southeastern Brazil. Acta Adriat. 2008; 49(1):73-87.) and sets the possibility that males have the ability to produce sperm throughout the year. In summary, the results obtained in this work were consistent with those reported for this species in Brazilian populations (Oddone, Vooren, 2005Oddone MC, Vooren CM. Reproductive biology of Atlantoraja cyclophora (Regan 1903) (Elasmobranchii: Rajidae) off southern Brazil. ICES J Mar Sci . 2005; 62(3):1095-103.; Oddone, Amorim, 2007Oddone MC, Amorim AF. Length-weight relationships, condition and population structure of the genus Atlantoraja (Elasmobranchii, Rajidae, Arhynchobatinae) in Southeastern Brazilian waters, SW Atlantic Ocean. J Northw Atl Fish Sci. 2007; 38:43-52.; Oddone et al., 2008Oddone MC, Norbis W, Mancini PL, Amorim AF. Sexual development and reproductive cycle of the Eyespot skate Atlantoraja cyclophora (Regan, 1903) (Chondrichthyes: Rajidae: Arhynchobatinae), in southeastern Brazil. Acta Adriat. 2008; 49(1):73-87.) and suggest that the pattern of sexual and morphometric development of A. cyclophora would be similar between regions. Especially, if it is taken into account that this species maintains its feeding habits along its distribution range, using similar food resources and possibly having the same trophic role (Barbini, Lucifora, 2016Barbini SA, Lucifora LO. Diet composition and feeding habits of the eyespot skate, Atlantoraja cyclophora (Elasmobranchii: Arhynchobatidae), off Uruguay and northern Argentina. Neotrop Ichthyol. 2016; 14(3):e160032.). Ebert et al. (2008Ebert DA, Smith WD, Cailliet GM. Reproductive biology of two commercially exploited skates, Raja binoculata and R. rhina, in the western Gulf of Alaska. Fish Res. 2008; 94(1):48-57.) and Frisk, Miller (2009Frisk MG, Miller TJ. Maturation of Little Skate and Winter Skate in the Western Atlantic from Cape Hatteras to Georges Bank. Mar Coast Fish. 2009; 1(1):1-11.) exposed that for species with a large geographic range, the differences in size at maturity and other vital rates may have profound implications in the way that the species should be managed. In Argentina, A. cyclophora is one of the 5 principal species of skates landed (Massa et al., 2004Massa AM, Lucifora LO, Hozbor NM. Condrictios de la región costera bonaerense y uruguaya. In: Sánchez, RP, Bezzi, SI., editors. El Mar Argentino y sus recursos pesqueros. Los peces marinos de interés pesquero. Caracterización biológica y evaluación del estado de explotación. Mar del Plata: INIDEP; 2004. p.85-99.; Tamini et al., 2006Tamini LL, Chiaramonte GE, Perez JE, Cappozzo HL. Batoids in a coastal trawl fishery of Argentina. Fish Res . 2006; 77(3):326-32.; Perez Comesaña et al., 2011Perez Comesaña JE, Tamini LL, Chiaramonte GE. El desembarque de batoideos de interés comercial en Puerto Quequén, Provincia de Buenos Aires. Los condrictios en la actividad pesquera argentina. In: Wöhler OC, Cedrola P, Cousseau MB, editors. Contribuciones sobre la biología, pesca y comercialización de tiburones en la Argentina. Aportes para la elaboración del plan de acción nacional. Buenos Aires: Consejo Federal Pesquero ; 2011. p.207-215.) and presents latitudinal variations in maximum TL and size at maturity. This suggests the need to increase the biological studies tending to the conservation and proper management of the species.
Acknowledgments
We wish to thank crews of the INIDEP and Ana Massa. We are grateful Natalia Hozbor for the data record provided. We also thank M. Constanza Díaz Andrade and Ricardo Camina for the help provided. This work was supported by the SGC y T-UNS, PGI 24/B222 and CONICET. INIDEP contribution N° 2119.
References
- Aubone A, Wöhler OC. Aplicación del método de máxima verosimilitud a la estimación de parámetros y comparación de curvas de crecimiento de von Bertalanffy. INIDEP Informe Técnico Interno No 37. 2000.
- Barbini SA, Lucifora LO. Diet composition and feeding habits of the eyespot skate, Atlantoraja cyclophora (Elasmobranchii: Arhynchobatidae), off Uruguay and northern Argentina. Neotrop Ichthyol. 2016; 14(3):e160032.
- Braccini JM, Chiaramonte GE. Reproductive biology of Psammobatis extenta J Fish Biol. 2002; 61(1):272-88.
- Chiaramonte GE, Pettovello AD. The biology of Mustelus schmitti in southern Patagonia, Argentina. J Fish Biol . 2000; 57(4):930-42.
- Chierichetti MA, Scenna LB, Di Giácomo EE, Ondarza PM, Figueroa DE, Miglioranza KSB. Reproductive biology of the cockfish, Callorhinchus callorynchus (Chondrichthyes: Callorhinchidae), in coastal waters of the northern Argentinean Sea. Neotrop Ichthyol . 2017; 15(2):e160137.
- Coller NM, Perier MR, Di Giácomo EE. Dimorfismo sexual y relaciones morfométricas de Atlantoraja platana (Günther, 1880) en aguas del golfo San Matías, Patagonia. Rev Mus Argent Cienc Nat. 2011; 13(1):1-5.
- Colonello JH. Ecología reproductiva de tres batoideos (Chondrichthyes): Atlantoraja castelnaui (Rajidae), Rioraja agassizi (Rajidae) y Zapteryx brevirostris (Rhinobatidae). Implicancias de distintas estrategias adaptativas en un escenario de explotación comercial intensiva. [PhD Thesis]. Argentina: Facultad de Ciencias Naturales y Museo Universidad Nacional de La Plata; 2009.
- Colonello JH, Cortés F, Belleggia M, Massa AM. Reproductive and population parameters of spiny dogfish Squalus acanthias in the south-western Atlantic Ocean. J Fish Biol . 2016; 88(5):1758-75.
- Colonello JH, García ML, Lasta CA. Reproductive biology of Rioraja agassizi from the coastal southwestern Atlantic ecosystem between northern Uruguay (34°S) and northern Argentina (42°S). Env Biol Fish. 2007a; 80(2-3):277-84.
- Colonello JH, García ML, Lasta CA, Menni RC. Reproductive biology of the spotback skate Atlantoraja castelnaui in the south-west Atlantic Ocean. J Fish Biol . 2012; 80(7):2405-19.
- Colonello JH, García ML, Menni RC. Reproductive biology of the lesser guitarfish Zapteryx brevirostris from the south-western Atlantic Ocean. J Fish Biol . 2011; 78(1):287-302.
- Colonello JH, Lucifora LO, Massa AM. Reproduction of the angular angel shark (Squatina guggenheim): geographic differences, reproductive cycle, and sexual dimorphism. ICES J Mar Sci. 2007b; 64(1):131-40.
- Conover WJ. Practical nonparametric statistics. 3rd ed. Toronto: John Wiley and Sons; 1999.
- Cousseau MB, Figueroa DE, Díaz de Astarloa JM, Mabragaña E, Lucifora LO. Rayas, chuchos y otros batoideos del Atlántico sudoccidental (34ºS-55ºS). Mar del Plata: Instituto Nacional de Investigación y Desarrollo Pesquero INIDEP; 2007.
- Díaz-Andrade MC, Galíndez E, Estecondo S. The ovary of the bignose fanskate Sympterygia acuta Garman, 1877 (Chondrichthyes, Rajidae) in the Bahía Blanca estuary, Argentina: morphology and reproductive features. Braz J Biol. 2009; 69(2):405-13.
- Di Giacomo EE, Perier MR, Pascual MS, Zampatti EA. El mar y sus recursos: golfo San Matías. In: Massera RF, Lew J, Serra Peirano G, editors. Las mesetas patagónicas que caen al mar: la costa rionegrina.Viedma: Río Negro; 2005. p.409-439.
- Ebert DA. Reproductive biology of skates, Bathyraja (Ishiyama), along the eastern Bering Sea continental slope. J Fish Biol . 2005; 66(3):618-49.
- Ebert DA, Compagno LJV. Biodiversity and systematics of skates (Chondrichthyes: Rajiformes: Rajoidei). Environ Biol Fish. 2007; 80(2-3):111-24.
- Ebert DA, Smith WD, Cailliet GM. Reproductive biology of two commercially exploited skates, Raja binoculata and R. rhina, in the western Gulf of Alaska. Fish Res. 2008; 94(1):48-57.
- Estalles M, Coller NM, Perier MR, Di Giácomo EE. Skates in the demersal trawl fishery of San Matías Gulf, Patagonia: species composition, relative abundance and maturity stages. Aquat Living Resour. 2011; 24(2):193-99.
- Frisk MG, Miller TJ. Age, growth, and latitudinal patterns of two Rajidae species in the northwestern Atlantic: little skate (Leucoraja erinacea) and winter skate (Leucoraja ocellata). Can J Fish Aquat Sci. 2006; 63(5):1078-91.
- Frisk MG, Miller TJ. Maturation of Little Skate and Winter Skate in the Western Atlantic from Cape Hatteras to Georges Bank. Mar Coast Fish. 2009; 1(1):1-11.
- Froese R. Cube law, condition factor and weight-length relationships: history, meta-analysis and recommendations. J Appl Ichthyol. 2006; 22(4):241-53.
- Góngora ME, Bovcon ND, Cochia PD. Ictiofauna capturada incidentalmente en la pesquería de langostino patagónico Pleoticus muelleri Bate, 1888. Rev Biol Mar Oceanogr. 2009; 44(3):583-93.
- Hollander M, Wolfe DA. Nonparametric statistical methods. 2nd ed. New York: Wiley-Interscience; 1999.
- Jaureguizar AJ, Menni R, Lasta C, Guerrero R. Fish assemblages of the northern Argentine coastal system: spatial patterns and their temporal variations. Fish Oceanogr. 2006; 15(4):326-44.
- Koob TJ, Callard IP. Reproductive endocrinology of female elasmobranchs: lessons from the little skate (Raja erinacea) and spiny dogfish (Squalus acanthias). J Exp Zool. 1999; 284(5):557-74.
- Leonard BK, Summers AP, Koob TJ. Metabolic rate of embryonic little skate, Raja erinacea (Chondrichthyes: Batoidea): the cost of active pumping. J Exp Zool . 1999; 283(1):13e18.
- Licandeo R, Cerna FT. Geographic variation in life-history traits of the endemic kite skate Dipturus chilensis (Batoidea: Rajidae), along its distribution in the fjords and channels of southern Chile. J Fish Biol . 2007; 71(2):421-40.
- Mabragaña E, Cousseau MB. Reproductive biology of two sympatric skates in the south-west Atlantic: Psammobatis rudis and Psammobatis normani J Fish Biol . 2004; 65(2):559-73.
- Mabragaña E, Lucifora LO, Massa AM. The reproductive ecology and abundance of Sympterygia bonapartii endemic to the south-west Atlantic. J Fish Biol . 2002; 60(4):951-67.
- Massa AM, Hozbor NM. Evolución de las estimaciones de abundancia de los peces cartilaginosos demersales de mayor valor comercial del Altántico Sudoccidental capturados entre 34º y 41ºS a profundidades menores a 50m. Parte 2. Los condrictios en la actividad pesquera argentina. In: Wöhler OC, Cedrola P, Cousseau MB, editors. Contribuciones sobre la biología, pesca y comercialización de tiburones en la Argentina. Aportes para la elaboración del plan de acción nacional. Buenos Aires: Consejo Federal Pesquero; 2011. p. 193-205.
- Massa A, Hozbor N, Vooren CM. Atlantoraja cyclophora The IUCN Red List of Threatened Species. 2006; e.T61398A12462475. http://dx.doi.org/10.2305/IUCN.UK.2006.RLTS.T61398A12462475.en
» http://dx.doi.org/10.2305/IUCN.UK.2006.RLTS.T61398A12462475.en - Massa AM, Lucifora LO, Hozbor NM. Condrictios de la región costera bonaerense y uruguaya. In: Sánchez, RP, Bezzi, SI., editors. El Mar Argentino y sus recursos pesqueros. Los peces marinos de interés pesquero. Caracterización biológica y evaluación del estado de explotación. Mar del Plata: INIDEP; 2004. p.85-99.
- Matta ME, Gunderson DR. Age, growth, maturity, and mortality of the Alaska skate, Bathyraja parmifera, in the eastern Bering Sea. Environ Biol Fish . 2007; 80(2-3):309-23.
- McFarlane GA, King JR. Age and growth of big skate (Raja binoculata) and longnose skate (Raja rhina) in British Columbia waters. Fish Res . 2006; 78(2-3):169-78.
- Oddone MC, Amorim AF. Length-weight relationships, condition and population structure of the genus Atlantoraja (Elasmobranchii, Rajidae, Arhynchobatinae) in Southeastern Brazilian waters, SW Atlantic Ocean. J Northw Atl Fish Sci. 2007; 38:43-52.
- Oddone MC, Amorim AF, Mancini PL, Norbis W, Velasco G. The reproductive biology and cycle of Rioraja agassizi (Müller and Henle, 1841) (Chondrichthyes: Rajidae) in southeastern Brazil, SW Atlantic Ocean. Sci Mar. 2007; 71(3):593-604.
- Oddone MC, Marçal AS, Vooren CM. Egg capsules of Atlantoraja cyclophora (Regan, 1903) and A. platana (Günther, 1880) (Pisces, Elasmobranchii, Rajidae). Zootaxa. 2004; 426(1):1-4.
- Oddone MC, Norbis W, Mancini PL, Amorim AF. Sexual development and reproductive cycle of the Eyespot skate Atlantoraja cyclophora (Regan, 1903) (Chondrichthyes: Rajidae: Arhynchobatinae), in southeastern Brazil. Acta Adriat. 2008; 49(1):73-87.
- Oddone MC, Vooren CM. Distribution, abundance and morphometry of Atlantoraja cyclophora (Regan, 1903) (Elasmobranchii: Rajidae) in southern Brazil, Southwestern Atlantic. Neotrop Ichthyol . 2004; 2(3):137-44.
- Oddone MC, Vooren CM. Reproductive biology of Atlantoraja cyclophora (Regan 1903) (Elasmobranchii: Rajidae) off southern Brazil. ICES J Mar Sci . 2005; 62(3):1095-103.
- Orlando L, Pereyra I, Paesch L, Norbis W. Size and sex composition of two species of the genus Atlantoraja (Elasmobranchii, Rajidae) caught by the bottom trawl fisheries operating on the Uruguayan continental shelf (Southwestern Atlantic Ocean). Braz J Oceanogr. 2011; 59(4):357-64.
- Paesch L, Domingo A. La pesca de condrictios en el Uruguay. Frente Marítimo. 2003; 19:207-16.
- Perez Comesaña JE, Tamini LL, Chiaramonte GE. El desembarque de batoideos de interés comercial en Puerto Quequén, Provincia de Buenos Aires. Los condrictios en la actividad pesquera argentina. In: Wöhler OC, Cedrola P, Cousseau MB, editors. Contribuciones sobre la biología, pesca y comercialización de tiburones en la Argentina. Aportes para la elaboración del plan de acción nacional. Buenos Aires: Consejo Federal Pesquero ; 2011. p.207-215.
- Perier MR, Estalles M, Coller M, Suarez MN, Mora GJ, Di Giacomo EE. Chondrichthyans of the San Matías Gulf, Patagonia, Argentina. Rev Mus Argentino Cienc Nat. 2011; 13(2):213-20.
- Perier R, Estalles M, Coller M, Di Giacomo EE. Reproductive biology of the endemic skate Psammobatis lentiginosa in the San Matías Gulf (south-western Atlantic). J Mar Biol Assoc U.K. 2010; 91(6):1165-73.
- Prisco M, Romano M, Ricchiari L, Limatola E, Andreuccetti P. An ultrastructural study on the vitellogenesis in the spotted ray Torpedo marmorata Gen Comp Endocrinol. 2002; 128(3):171-79.
- Ruocco NL, Lucifora LO, Díaz de Astarloa JM, Wöhler O. Reproductive biology and abundance of the white-dotted skate, Bathyraja albomaculata, in the Southwest Atlantic. ICES J Mar Sci . 2006; 63(1):105-16.
- San Martín MJ, Perez JE, Chiaramonte GE. Reproductive biology of the South West Atlantic marbled sand skate Psammobatis bergi Marini, 1932 (Elasmobranchii, Rajidae). J Appl Ichthyol . 2005; 21(6):504-10.
- Sánchez RP, Navarro G, Calvo E, Del Castillo F. La pesca y comercialización de condrictios en la Argentina. Aportes de la Dirección Nacional de Planificación Pesquera para la elaboración del plan de acción nacional. Parte 2. Los condrictios en la actividad pesquera argentina. In: Wöhler OC, Cedrola P, Cousseau MB, editors. Contribuciones sobre la biología, pesca y comercialización de tiburones en la Argentina. Aportes para la elaboración del plan de acción nacional. Buenos Aires: Consejo Federal Pesquero ; 2011. p.151-184.
- Sokal RR, Rohlf FJ. Introduction to biostatistics. New York: WH Freeman and Company; 1987.
- Tamini LL, Chiaramonte GE, Perez JE, Cappozzo HL. Batoids in a coastal trawl fishery of Argentina. Fish Res . 2006; 77(3):326-32.
- Templeman W. Differences in sexual maturity and related characteristics between populations of thorny skate (Raja radiata) in the Northwest Atlantic. J Northw Atl Fish Sci . 1987; 7(2):155-67.
- Ungaro N. Biological parameters of the brown ray, Raja miraletus, in the Southern Adriatic basin. 2004. Cybium, 28(2):174-76.
- Warton DI, Wright IJ, Falster DS, Westoby M. Bivariate line-fitting methods for allometry. Biol Rev. 2006; 81(2):259-91.
- Yamaguchi A, Taniuchi T, Shimizu M. Geographical variations in reproductive parameters of the starspotted dogfish, Mustelus manazo, from five localities in Japan and in Taiwan. Env Biol Fish . 2000; 57(2):221-33.
- Zar JH. Biostatistical analysis. 4th ed. Upper Saddle River (NJ): Prentice Hall; 1999.
Edited by
Data availability
Data citations
Massa A, Hozbor N, Vooren CM. Atlantoraja cyclophora The IUCN Red List of Threatened Species. 2006; e.T61398A12462475. http://dx.doi.org/10.2305/IUCN.UK.2006.RLTS.T61398A12462475.en
Publication Dates
-
Publication in this collection
25 June 2018 -
Date of issue
2018
History
-
Received
11 Aug 2017 -
Accepted
13 Feb 2018