ABSTRACT
Gymnorhamphichthys britskii is a Neotropical electric fish of family Rhamphichthyidae described from the Paraná-Paraguay system. This study reports the first karyotypic description of G. britskii collected from the upper Paraná river basin, which presented 2n=38 chromosomes, karyotype composed of 14 metacentric, 8 submetacentric, 2 subtelocentric and 14 acrocentric chromosomes, and fundamental number as 62 for both sexes. Heteromorphic sex chromosomes were absent. A single pair of nucleolar organizing regions (NORs) was detected in the submetacentric chromosome pair number 9 by silver staining and confirmed by the 18S rDNA probe. The 5S rDNA was located in a single chromosome pair. Heterochromatic regions were clearly observed in the short arms of the NOR-bearing chromosome pair and in the telomeric positions of most acrocentric chromosomes. Besides the present data are valuable to help in understanding karyotypic evolution in Rhamphichthyidae, data from NORs confirmed the tendency of this family in presenting simple NORs sites, similar to the other Gymnotiformes clades. Yet, the presence of a large heterochromatic block in the NOR-bearing chromosome can be used as cytogenetic markers for G. britskii, and that centric fusions appear to be an important mechanism in the karyotype evolution and differentiation among Gymnotiformes species.
Keywords:
Ag-NORs; C-banding; Fish cytogenetics; Karyotype evolution; Repetitive sequences
RESUMO
Gymnorhamphichthys britskii é um peixe neotropical da família Rhamphichthyidae descrita no sistema Paraná-Paraguai. Este estudo relata a primeira descrição cariotípica de G. britskii coletado na bacia do alto rio Paraná, que apresentou 2n = 38 cromossomos, cariótipo composto por 14 metacêntricos, 8 submetacêntricos, 2 subtelocêntricos e 14 acrocêntricos, e número fundamental 62 para ambos sexos. Cromossomos sexuais heteromórficos estavam ausentes. Um único par de regiões organizadoras de nucléolos (RONs) foi detectado no par de cromossomos submetacêntricos número 9 por coloração com prata e confirmado pela sonda DNAr 18S. O DNAr 5S foi localizado em um único par cromossômico. Regiões heterocromáticas foram claramente observadas nos braços curtos do par de cromossomos que carrega a RON e nas posições teloméricas da maioria dos cromossomos acrocêntricos. Além dos dados presentes serem valiosos para auxiliar na compreensão da evolução cariotípica em Rhamphichthyidae, dados de RONs confirmaram a tendência desta família em apresentar sítios simples de RONs, semelhantes aos demais clados de Gymnotiformes. No entanto, a presença de um grande bloco heterocromático no cromossomo portador da RON, pode ser usado como marcador citogenético para G. britskii e as fusões cêntricas parecem ser um mecanismo importante na evolução e diferenciação cariotípica entre as espécies de Gymnotiformes.
Palavras-chave:
Ag-RONs; Bandeamento C; Citogenética de peixes; Evolução cariotípica; Sequências repetitivas
Introduction
The Gymnorhamphichthys Ellis, 1912 is a representant of the clade Rhamphichthyidae, a group of Neotropical electric fishes, widely distributed through the cis-Andean drainages of tropical South America, from Venezuela to northeast Argentina (Nijssen et al., 1976Nijssen H, Isbrücker IJH, Géry J. On the species of Gymnorhamphichthys Ellis, 1912, translucent sand-dwelling gymnotid fishes from South America (Pisces, Cypriniformes, Gymnotoidei). Stud Neotrop Fauna E. 1976; 11(1-2):37-63. https://doi.org/10.1080/01650527609360496
https://doi.org/10.1080/0165052760936049...
). The genus is composed of five valid species: G. rondoni (Miranda Ribeiro, 1920), G. rosamariae Schwassmann, 1989, G. bogardusae Lundberg, 2005, G. hypostomus Ellis, 1912, and G. britskiiCarvalho, Ramos & Albert, 2011Carvalho TP, Ramos CS, Albert JS. A new species of Gymnorhamphichthys (Gymnotiformes: Rhamphichthyidae) from the Paraná-Paraguay basin. Copeia. 2011; 3:400-06. https://doi.org/10.1643/CI-10-154
https://doi.org/10.1643/CI-10-154...
(Fricke et al., 2019Fricke R, Eschmeyer WN, Van Der Laan R, editors. Catalog of Fishes: Genera, Species, References [Internet]. California Academy of Sciences; 2018 [updated June, 01, 2019; cited in June, 20, 2019]. http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp.
http://researcharchive.calacademy.org/re...
).
Gymnorhamphichthys britskii is distributed from tributaries of the La Plata River system, and share with G. hypostomus a distinct color pattern of the dorsum composed of a few large, dark saddles, which is not present in any other species of Rhamphichthyidae (Carvalho et al., 2011Carvalho TP, Ramos CS, Albert JS. A new species of Gymnorhamphichthys (Gymnotiformes: Rhamphichthyidae) from the Paraná-Paraguay basin. Copeia. 2011; 3:400-06. https://doi.org/10.1643/CI-10-154
https://doi.org/10.1643/CI-10-154...
). Previously, there were no records of G. britskii from the upper Paraná River before the construction of the Itaipu Dam. The distribution of G. britskii (referred to as Gymnorhamphichthys sp. by Graça, Pavanelli, 2007Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem; 2007.) in the upper Paraná River seems to be due to the construction of Itaipu Dam, which by elevating a portion of the lower Paraná River effectively eliminated the Sete Quedas falls as a barrier for dispersal (Langeani et al., 2007Langeani F, Castro RMC, Oyakawa OT, Shibatta OA, Pavanelli CS, Casatti L. Diversidade da ictiofauna do alto rio Paraná: composição atual e perspectivas futuras. Biota Neotrop. 2007; 7(3):1-17. http://dx.doi.org/10.1590/S1676-06032007000300020
http://dx.doi.org/10.1590/S1676-06032007...
; Ota et al., 2018Ota RR, Deprá GC, Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes: revised, annotated and updated. Neotrop Ichthyol. 2018; 16(2): e170094. https://doi.org/10.1590/1982-0224-20170094
https://doi.org/10.1590/1982-0224-201700...
).
The families Hypopomidae and Rhamphichthyidae are closely related and constitute the superfamily Rhamphichthyoidea (Albert, 2001Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Publi Mus Zoo. 2001; 190:1-127). No cytogenetic information is available for species of Gymnorhamphichthys. Among the other Rhamphichthyidae, cytogenetic information is available only for Hypopygus lepturus Hoedeman, 1962 (Almeida-Toledo, 1978Almeida-Toledo LF. Contribuição a citogenética dos Gymnotoidae (Pisces, Ostariophysi). Ph.D. Thesis, Instituto de Biociências da Universidade de São Paulo, São Paulo; 1978.), Steatogenys duidae (La Monte, 1929), S. elegans (Steindachner, 1880) (Cardoso et al., 2011Cardoso AL, Pieczarka JC, Feldberg E, Milhomem SSR, Moreira-Almeida T, Silva DS, Da Silva PC, Nagamachi CY. Chromosoma l characterization of two species of genus Steatogenys (Gymnotiformes: Rhamphichthyoidea: Steatogenini) from the Amazon basin: sex chromosomes and correlations with Gymnotiformes phylogeny. Rev Fish Biol Fish. 2011; 21(3):613-21. https://doi.org/10.1007/s11160-010-9196-0
https://doi.org/10.1007/s11160-010-9196-...
), Rhamphichthys hahni (Meinken, 1937), R. pantherinus Castelnau, 1855 (cited as R. marmoratus) and R. rostratus (Linnaeus, 1766) (Mendes et al., 2012Mendes VP, Portela-Castro AL, Julio-Junior HF. First record of supernumerary (B) chromosomes in electric fish (Gymnotiformes) and the karyotype structure of three species of the same order from the upper Paraná River basin. Comp Cytogenet. 2012; 6 (1):1-16. https://doi.org/10.3897/CompCytogen.v6i1.1752
https://doi.org/10.3897/CompCytogen.v6i1...
; Silva et al., 2013Silva PC, Nagamachi CY, Silva DS, Milhomem SSR, Cardoso AL, Oliveira JA, Pieczark JC. Karyotypic similarities between two species of Rhamphichthys (Rhamphichthyidae, Gymnotiformes) from the Amazon basin. Comp Cytogenet . 2013; 7(4):279-91. https://doi.org/10.3897/CompCytogen.v7i4.4366
https://doi.org/10.3897/CompCytogen.v7i4...
). Among the Hypopomidae, it is available for Hypopomus artedi (Kaup, 1856) (Almeida-Toledo, 1978), eight Brychyhypopomus species (Almeida-Toledo, 1978Almeida-Toledo LF. Contribuição a citogenética dos Gymnotoidae (Pisces, Ostariophysi). Ph.D. Thesis, Instituto de Biociências da Universidade de São Paulo, São Paulo; 1978.; Mendes et al., 2012Mendes VP, Portela-Castro AL, Julio-Junior HF. First record of supernumerary (B) chromosomes in electric fish (Gymnotiformes) and the karyotype structure of three species of the same order from the upper Paraná River basin. Comp Cytogenet. 2012; 6 (1):1-16. https://doi.org/10.3897/CompCytogen.v6i1.1752
https://doi.org/10.3897/CompCytogen.v6i1...
; Cardoso et al., 2018Cardoso AL, Pieczarka JC, Crampton WGR, Ready JS, Figueiredo Ready WMB, Waddell JC, Oliveira JA, Nagamachi CY. Karyotypic diversity and evolution in a sympatric assemblage of neotropical electric knifefish. Front Genet. 2018; 9:81. https://doi.org/10.3389/fgene.2018.00081
https://doi.org/10.3389/fgene.2018.00081...
) and Microsternarchus bilineatus Fernández-Yépez, 1968 (de Jesus et al., 2016de Jesus IS, Ferreira M, Garcia C, Ribeiro LB, Alves-Gomes JA, Feldberg E. First cytogenetic description of Microsternarchus bilineatus (Gymnotiformes: Hypopomidae) from Negro river (Brazilian Amazon). Zebrafish . 2016; 13(6):571-77. https://doi.org/10.1089/zeb.2016.1281
https://doi.org/10.1089/zeb.2016.1281...
; Batista et al., 2017Batista JA, Cardoso AL, Milhomem-Paixão SSR, Ready JS, Pieczarka JC, Nagamachi CY. The karyotype of Microsternarchus aff. bilineatus: a first case of Y chromosome degeneration in Gymnotiformes. Zebrafish. 2017; 14(3):244-50. https://doi.org/10.1089/zeb.2016.1383
https://doi.org/10.1089/zeb.2016.1383...
).
There are still few cytogenetic studies regarding Rhamphichthyoidea, but they show that these species present great differences in relation to the number of chromosomes and karyotype macrostructure. In Rhamphichthyidae all species had 2n = 50 chromosomes but differed in their karyotypes (Almeida-Toledo, 1978Almeida-Toledo LF. Contribuição a citogenética dos Gymnotoidae (Pisces, Ostariophysi). Ph.D. Thesis, Instituto de Biociências da Universidade de São Paulo, São Paulo; 1978.; Cardoso et al., 2011Cardoso AL, Pieczarka JC, Feldberg E, Milhomem SSR, Moreira-Almeida T, Silva DS, Da Silva PC, Nagamachi CY. Chromosoma l characterization of two species of genus Steatogenys (Gymnotiformes: Rhamphichthyoidea: Steatogenini) from the Amazon basin: sex chromosomes and correlations with Gymnotiformes phylogeny. Rev Fish Biol Fish. 2011; 21(3):613-21. https://doi.org/10.1007/s11160-010-9196-0
https://doi.org/10.1007/s11160-010-9196-...
; Mendes et al., 2012Mendes VP, Portela-Castro AL, Julio-Junior HF. First record of supernumerary (B) chromosomes in electric fish (Gymnotiformes) and the karyotype structure of three species of the same order from the upper Paraná River basin. Comp Cytogenet. 2012; 6 (1):1-16. https://doi.org/10.3897/CompCytogen.v6i1.1752
https://doi.org/10.3897/CompCytogen.v6i1...
; Silva et al., 2013Silva PC, Nagamachi CY, Silva DS, Milhomem SSR, Cardoso AL, Oliveira JA, Pieczark JC. Karyotypic similarities between two species of Rhamphichthys (Rhamphichthyidae, Gymnotiformes) from the Amazon basin. Comp Cytogenet . 2013; 7(4):279-91. https://doi.org/10.3897/CompCytogen.v7i4.4366
https://doi.org/10.3897/CompCytogen.v7i4...
). Among the Hypopomidae, the diploid number ranged from 36 (Brachyhypopomus brevirostris (Steindachner, 1868)) to 48 (M. bilineatus) chromosomes (de Jesus et al., 2016de Jesus IS, Ferreira M, Garcia C, Ribeiro LB, Alves-Gomes JA, Feldberg E. First cytogenetic description of Microsternarchus bilineatus (Gymnotiformes: Hypopomidae) from Negro river (Brazilian Amazon). Zebrafish . 2016; 13(6):571-77. https://doi.org/10.1089/zeb.2016.1281
https://doi.org/10.1089/zeb.2016.1281...
; Batista et al., 2017Batista JA, Cardoso AL, Milhomem-Paixão SSR, Ready JS, Pieczarka JC, Nagamachi CY. The karyotype of Microsternarchus aff. bilineatus: a first case of Y chromosome degeneration in Gymnotiformes. Zebrafish. 2017; 14(3):244-50. https://doi.org/10.1089/zeb.2016.1383
https://doi.org/10.1089/zeb.2016.1383...
; Cardoso et al., 2018Cardoso AL, Pieczarka JC, Crampton WGR, Ready JS, Figueiredo Ready WMB, Waddell JC, Oliveira JA, Nagamachi CY. Karyotypic diversity and evolution in a sympatric assemblage of neotropical electric knifefish. Front Genet. 2018; 9:81. https://doi.org/10.3389/fgene.2018.00081
https://doi.org/10.3389/fgene.2018.00081...
).
The physical mapping of ribosomal DNAs, especially 5S and 18S rDNAs, has been frequently used by cytogenetic studies on Neotropical fish, including fishes of the order Gymnotiformes (Fernandes et al., 2017aFernandes CA, Paiz LM, Baumgärtner L, Margarido VP, Vieira MMR. Comparative cytogenetics of the black ghost knifefish (Gymnotiformes: Apteronotidae): Evidence of chromosomal fusion and pericentric inversions in karyotypes of two Apteronotus species. Zebrafish . 2017a; 14(5):471-76. https://doi.org/ 10.1089/zeb.2017.1432
https://doi.org/ 10.1089/zeb.2017.1432...
,bFernandes CA, Baumgartner L, Paiz LM, Margarido VP, Portela-Castro ALB. Chromosoma l characteristics of rDNA in a conserved karyotype of two Sternopygus macrurus (Gymnotiformes: Sternopygidae) populations from upper Paraná River basin. Biologia. 2017b; 72(6):680-85. https://doi.org/10.1515/biolog-2017-0071
https://doi.org/10.1515/biolog-2017-0071...
) to assist the clarification of taxonomic, biogeographical and phylogenetic problems. In Rhamphichthyidae, cytogenetic studies about the distribution of ribosomal genes (18S rDNA) are restricted to karyotypes of S. duidae, S. elegans (Cardoso et al., 2011Cardoso AL, Pieczarka JC, Feldberg E, Milhomem SSR, Moreira-Almeida T, Silva DS, Da Silva PC, Nagamachi CY. Chromosoma l characterization of two species of genus Steatogenys (Gymnotiformes: Rhamphichthyoidea: Steatogenini) from the Amazon basin: sex chromosomes and correlations with Gymnotiformes phylogeny. Rev Fish Biol Fish. 2011; 21(3):613-21. https://doi.org/10.1007/s11160-010-9196-0
https://doi.org/10.1007/s11160-010-9196-...
), R. hahni, R. pantherinus and R. rostratus (Mendes et al., 2012Mendes VP, Portela-Castro AL, Julio-Junior HF. First record of supernumerary (B) chromosomes in electric fish (Gymnotiformes) and the karyotype structure of three species of the same order from the upper Paraná River basin. Comp Cytogenet. 2012; 6 (1):1-16. https://doi.org/10.3897/CompCytogen.v6i1.1752
https://doi.org/10.3897/CompCytogen.v6i1...
; Silva et al., 2013Silva PC, Nagamachi CY, Silva DS, Milhomem SSR, Cardoso AL, Oliveira JA, Pieczark JC. Karyotypic similarities between two species of Rhamphichthys (Rhamphichthyidae, Gymnotiformes) from the Amazon basin. Comp Cytogenet . 2013; 7(4):279-91. https://doi.org/10.3897/CompCytogen.v7i4.4366
https://doi.org/10.3897/CompCytogen.v7i4...
), demonstrating only one chromosome pair bearing these clusters in all species.
No cytogenetic information is available for physical mapping of 5S rDNA in the Rhamphichthyidae. Therefore, in an effort to collect more information about chromosomal diversity within the family Rhamphichthyidae, this study establishes the first cytogenetic description of G. britskii by classic and molecular cytogenetics techniques.
Material and Methods
Twenty-two (8 males, 9 females and 5 sex indeterminate) individuals of G. britskii from Dourado stream, Mato Grosso do Sul State, Brazil (23°51’04.9’’S and 54°25’13.9’’W) were analysed. This stream is a tributary of the right margin of the Iguatemi River, which belongs to the upper Paraná River basin (Fig. 1).
Location of Dourado stream in the upper Paraná River basin where Gymnorhamphichthys britskii individuals were captured. The dark circle indicates the sampling spots.
Animals were captured with the permission of the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio; number 64611). Voucher specimens were deposited in the fish collection of the Núcleo de Pesquisas em Limnologia, Ictiologia e Aquicultura (NUPELIA), Universidade Estadual de Maringá, PR Brazil, as Gymnorhamphichthys britskii (NUP 16251) (Fig. 2).
This study was carried out in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals, approved by the Committee on Ethics of Animal Experiments of the Universidade Estadual do Mato Grosso do Sul (License Number: Protocol 024/2018 - CEUA/UEMS). The experiments followed the ethical conduct, and before euthanasia, the fish were anesthetized by an overdose of clove oil (Griffiths, 2000Griffiths S. The use of clove oil as an anaesthetic and method for sampling intertidal rockpool fishes. J Fish Biol. 2000; 57(6):1453-64. https://doi.org/10.1111/j.1095-8649.2000.tb02224.x
https://doi.org/10.1111/j.1095-8649.2000...
). Metaphase chromosomes were obtained from anterior kidney cells using the air-drying technique (Bertollo et al., 1978Bertollo LAC, Takahashi CS, Moreira-filho O. Cytotaxonomic considerations on Hoplias lacerdae (Pisces, Erythrinidade). Braz J Genet. 1978; 1(1):103-20.). C-positive heterochromatin (C-bands) was visualized by the procedure of Sumner (1972Sumner AT. A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res. 1972; 75(1):304-06. https://doi.org/10.1016/0014-4827(72)90558-7
https://doi.org/10.1016/0014-4827(72)905...
), with minor adaptations. NORs were detected by means of silver nitrate staining (Ag-NORs), according to Howell, Black (1980Howell WM, Black DA. Controlled silver-staining of nucleolus organizer regions with a protective coloidal developer: a 1-step method. Experientia. 1980; 36(8):1014-15. https://doi.org/10.1007/BF01953855
https://doi.org/10.1007/BF01953855...
).
At least 30 metaphases were analyzed for each individual and those with better chromosome morphology were used for the karyotype analysis. The chromosomes are classified as metacentric (m), submetacentric (sm), subtelocentric (st), and acrocentric (a) according to Levan et al. (1964Levan A, Fredga K, Sandberg AA. Nomenclature for centromeric position on chromosomes. Hereditas. 1964; 52(2):201-20. https://doi.org/10.1111/j.1601-5223.1964.tb01953.x
https://doi.org/10.1111/j.1601-5223.1964...
). The fundamental number (FN) is calculated according to the chromosomal arm numbers (the chromosomes m, sm and st are considered to contain two arms -p and q arms- and the a with one arm -only q arm).
Physical mapping of the 5S and 18S rDNA was carried out by fluorescence in situ hybridization (FISH) according to Pinkel et al. (1986Pinkel D, Straume T, Gray JW. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci. 1986; 83(9):2934-38.) and modifications suggested by Margarido, Moreira-Filho (2008Margarido VP, Moreira-Filho O. Karyotypic differentiation through chromosome fusion and number reduction in Imparfinis hollandi (Ostariophysi, Heptapteridae). Genet Mol Biol. 2008; 31(1):235-38. http://dx.doi.org/10.1590/S1415-47572008000200012
http://dx.doi.org/10.1590/S1415-47572008...
), using DNA probes obtained from the genomes of Megaleporinus elongatus (Valenciennes, 1850) (Martins, Galetti, 1999Martins C, Galetti Jr PM. Chromosome localization of 5S rDNA genes in Leporinus fish (Anostomidae, Characiformes). Chromosome Res . 1999; 7(5):363-67. https://doi.org/10.1023/A:1009216030316
https://doi.org/10.1023/A:1009216030316...
) and Prochilodus argenteus Spix & Agassiz, 1829 (Hatanaka, Galetti, 2004Hatanaka T, Galetti Jr PM. Mapping of the 18S and 5S ribosomal RNA genes in the fish Prochilodus argenteus Agassiz, 1829 (Characiformes, Prochilodontidae). Genetica. 2004; 122(3):239-44. https://doi.org/10.1007/s10709-004-2039-y
https://doi.org/10.1007/s10709-004-2039-...
), respectively. The probes were labelled through nick translation, with digoxigenin-11-dUTP (18S rDNA) and biotin-16-dUTP (5S rDNA) (Roche). Detection and amplification of the hybridization signal were carried out using avidin-FITC and anti-avidin biotin (Sigma) for probes labelled with biotin, and anti-digoxigenin rhodamine (Roche) for probes labelled with digoxigenin. Slides were counterstained with DAPI (4’6-Diamine-2’-phenylindole dihydrochloride) (50 μg ml−1 ) and analyzed in epifluorescence microscope (Olympus BX61). The images were captured using the software DP controller (Media Cybernetics) and the image composition was carried out with Adobe Photoshop CS6.
Results
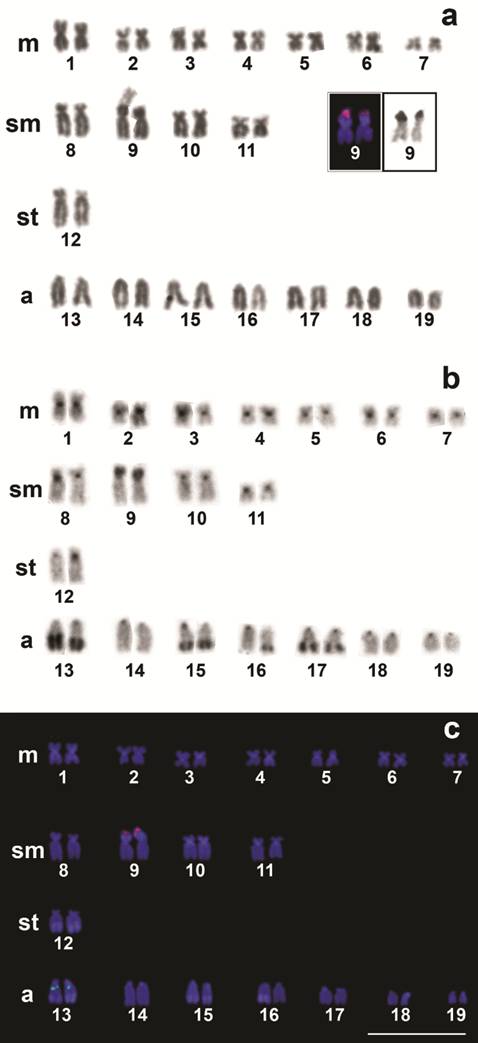
All the twenty-two individuals of G. britskii had 2n = 38 chromosomes and karyotype composed of 14 m, 8 sm, 2 st and 14 a, and FN = 62 for both sexes (Fig 3a). Heteromorphic sex chromosomes were absent. A secondary constriction was observed in the terminal region of the p arm of the sm pair number 9, which corresponds to the Ag-NORs signals (Fig. 3a, in box) and also had a clear size heteromorphism (Figs. 3a, c).
Karyotypes of Gymnorhamphichthys britskii arranged a. from Giemsa stained; b. C-banded; and c. after double-FISH with 18S rDNA (red) and 5S rDNA (green) probes.. The NOR-bearing chromosomes (pair 9) are in the box. Note the size heteromorphism involving the NORs detected by the Ag-NOR and 18S rDNA-FISH techniques. Scales bar = 10 µm.
The heterochromatins were preferentially located in the centromeric and pericentromeric regions in various chromosomes. Large terminal blocks were evidenced on the q arm of the pairs number 13, 15, 16 (one homolog) and 17, and a large heterochromatic block on the p arm of the pair number 9 flanked by NOR (Fig. 3b).
Double FISH with 18S and 5S rDNA probes confirmed the Ag-NORs sites and did not detect any further inactive major ribosomal clusters (Fig. 3c); in addition, it showed that minor rDNA clusters occur interstitially in the acrocentric pair number 13 and do not co-localize with the major rDNA clusters (Fig. 3c).
Discussion
The lack of karyotype data for several fish groups impairs comparative analyzes on their evolutionary trends and chromosomal relationships. This is the case for the family Rhamphichthyidae for which chromosomal characteristics are known only for three genera: Rhamphichthys, Hypopygus and Steatogenys, and all species presented 50 chromosomes. In this sense, this study is the first one providing classical and molecular cytogenetic data for one of its representative species, G. britskii.
Both male and female specimens of G. britskii have the same karyotype structure, with 2n = 38 chromosomes (14m + 8sm + 2st + 14a), with no evidence of differentiated sex chromosomes. Diploid number of 38 chromosomes found in G. britskii differs from the findings for every other species of the same family. Compared to other rhamphichthyids that have 2n = 50, the karyotype of G. britskii suggested a reduction in the 2n, indicating that chromosome rearrangements, such as centric fusions that can alter the chromosome number may have occurred during the diversification of this group.
Chromosome fusions were identified as important events in the diversification of Hypopomidae (the sister group of Rhamphichthyidae), in which the diploid number varies between 36 chromosomes, as in B. brevirostris, and 48 chromosomes, as in M. bilineatus (de Jesus et al., 2016de Jesus IS, Ferreira M, Garcia C, Ribeiro LB, Alves-Gomes JA, Feldberg E. First cytogenetic description of Microsternarchus bilineatus (Gymnotiformes: Hypopomidae) from Negro river (Brazilian Amazon). Zebrafish . 2016; 13(6):571-77. https://doi.org/10.1089/zeb.2016.1281
https://doi.org/10.1089/zeb.2016.1281...
; Batista et al., 2017Batista JA, Cardoso AL, Milhomem-Paixão SSR, Ready JS, Pieczarka JC, Nagamachi CY. The karyotype of Microsternarchus aff. bilineatus: a first case of Y chromosome degeneration in Gymnotiformes. Zebrafish. 2017; 14(3):244-50. https://doi.org/10.1089/zeb.2016.1383
https://doi.org/10.1089/zeb.2016.1383...
; Cardoso et al., 2018Cardoso AL, Pieczarka JC, Crampton WGR, Ready JS, Figueiredo Ready WMB, Waddell JC, Oliveira JA, Nagamachi CY. Karyotypic diversity and evolution in a sympatric assemblage of neotropical electric knifefish. Front Genet. 2018; 9:81. https://doi.org/10.3389/fgene.2018.00081
https://doi.org/10.3389/fgene.2018.00081...
). In Rhamphichthyidae, the chromosomic data of G. britskii alter the paradigm of karyotype evolution characterized by 2n conservation (50 chromosomes) and chromosome inversions (Cardoso et al., 2011Cardoso AL, Pieczarka JC, Feldberg E, Milhomem SSR, Moreira-Almeida T, Silva DS, Da Silva PC, Nagamachi CY. Chromosoma l characterization of two species of genus Steatogenys (Gymnotiformes: Rhamphichthyoidea: Steatogenini) from the Amazon basin: sex chromosomes and correlations with Gymnotiformes phylogeny. Rev Fish Biol Fish. 2011; 21(3):613-21. https://doi.org/10.1007/s11160-010-9196-0
https://doi.org/10.1007/s11160-010-9196-...
; Mendes et al., 2012Mendes VP, Portela-Castro AL, Julio-Junior HF. First record of supernumerary (B) chromosomes in electric fish (Gymnotiformes) and the karyotype structure of three species of the same order from the upper Paraná River basin. Comp Cytogenet. 2012; 6 (1):1-16. https://doi.org/10.3897/CompCytogen.v6i1.1752
https://doi.org/10.3897/CompCytogen.v6i1...
; Silva et al., 2013Silva PC, Nagamachi CY, Silva DS, Milhomem SSR, Cardoso AL, Oliveira JA, Pieczark JC. Karyotypic similarities between two species of Rhamphichthys (Rhamphichthyidae, Gymnotiformes) from the Amazon basin. Comp Cytogenet . 2013; 7(4):279-91. https://doi.org/10.3897/CompCytogen.v7i4.4366
https://doi.org/10.3897/CompCytogen.v7i4...
). Nonetheless, more rhamphichthyid species need to be cytogenetically analyzed to confirm this hypothesis (diversification by chromosome fusions). Moreover, centric fusion is the mechanism proposed in the origin of multiple sex chromosome system found in Gymnotiformes species belonging each one to different families: Eigenmannia sp. 2 (Almeida-Toledo et al., 2000aAlmeida-Toledo LF, Foresti F, Daniel MFZ, Toledo-Filho SA. Sex chromosome evolution in fish: the formation of the neo-Y chromosome in Eigenmannia (Gymnotiformes). Chromosoma. 2000a; 109(3):197-200. https://doi.org/10.1007/s004120050428
https://doi.org/10.1007/s004120050428...
), E. trilineata (Fernandes et al., 2010Fernandes CA, Bailly D, Silva VFB, Martins-Santos IC. System of multiple sex chromosomes in Eigenmannia trilineata López & Castello, 1966 (Sternopygidae, Gymnotiformes) from Iguatemi River Basin, MS, Brazil. Cytologia. 2010; 75(4):463-66. https://doi.org/10.1508/cytologia.75.463
https://doi.org/10.1508/cytologia.75.463...
), Brachyhypopomus gauderio (Mendes et al., 2012Mendes VP, Portela-Castro AL, Julio-Junior HF. First record of supernumerary (B) chromosomes in electric fish (Gymnotiformes) and the karyotype structure of three species of the same order from the upper Paraná River basin. Comp Cytogenet. 2012; 6 (1):1-16. https://doi.org/10.3897/CompCytogen.v6i1.1752
https://doi.org/10.3897/CompCytogen.v6i1...
), B. pinnicaudatus (Almeida-Toledo et al., 2000bAlmeida-Toledo LF, Daniel-Silva MFZ, Lopes CE, Toledo-Filho SA. Sex chromosome evolution in fish. II. Second occurrence of an X1X2Y sex chromosome system in Gymnotiformes. Chromosome Res. 2000b; 8(4):335-40. https://doi.org/10.1023/A:1009287630301
https://doi.org/10.1023/A:1009287630301...
), Gymnotus pantanal (cited as Gymnotus sp., Silva, Margarido, 2005), G. coropinae (da Silva et al., 2014da Silva M, Matoso DM, Artoni RF, Feldberg E. New approach data in electric fish (Teleostei: Gymnotus): Sex chromosome evolution and repetitive DNA. Zebrafish . 2014; 11(6):528-35. https://doi.org/10.1089/zeb.2013.0966
https://doi.org/10.1089/zeb.2013.0966...
). According to Silva, Margarido (2005Silva EB, Margarido VP. An X1X1X2X2/X1X2Y multiple sex chromosome system in a new species of the genus Gymnotus (Pisces, Gymnotiformes). Environ Biol Fish. 2005; 73(3):293-97. https://doi.org/10.1007/s10641-005-2144-5
https://doi.org/10.1007/s10641-005-2144-...
), the presence of a same X1X1X2X2/X1X2Y sex chromosome system in six species seems to be a homoplasic event, and it is highly probable that this apomorphic character has arisen by independent events. Thus, centric fusions appear to be an important and common mechanism acting in the karyotype evolution and differentiation among Gymnotiformes species.
NORs were located in terminal position on the p arm number 9, as revealed by the Ag-NOR and 18S rDNA-FISH techniques (Fig. 3a, box), thus characterizing a simple NORs system in G. britskii. Similar pattern was observed in karyotypes of S. duidae, S. elegans (Cardoso et al., 2011Cardoso AL, Pieczarka JC, Feldberg E, Milhomem SSR, Moreira-Almeida T, Silva DS, Da Silva PC, Nagamachi CY. Chromosoma l characterization of two species of genus Steatogenys (Gymnotiformes: Rhamphichthyoidea: Steatogenini) from the Amazon basin: sex chromosomes and correlations with Gymnotiformes phylogeny. Rev Fish Biol Fish. 2011; 21(3):613-21. https://doi.org/10.1007/s11160-010-9196-0
https://doi.org/10.1007/s11160-010-9196-...
), R. hahni, R. pantherinus and R. rostratus (Mendes et al., 2012Mendes VP, Portela-Castro AL, Julio-Junior HF. First record of supernumerary (B) chromosomes in electric fish (Gymnotiformes) and the karyotype structure of three species of the same order from the upper Paraná River basin. Comp Cytogenet. 2012; 6 (1):1-16. https://doi.org/10.3897/CompCytogen.v6i1.1752
https://doi.org/10.3897/CompCytogen.v6i1...
; Silva et al., 2013Silva PC, Nagamachi CY, Silva DS, Milhomem SSR, Cardoso AL, Oliveira JA, Pieczark JC. Karyotypic similarities between two species of Rhamphichthys (Rhamphichthyidae, Gymnotiformes) from the Amazon basin. Comp Cytogenet . 2013; 7(4):279-91. https://doi.org/10.3897/CompCytogen.v7i4.4366
https://doi.org/10.3897/CompCytogen.v7i4...
). A single chromosome pair with NORs sites is thought to be a plesiomorphic characteristic of the Rhamphichthyidae. By contrast, multiple NORs sites have characterized the karyotypes of the Hypopomidae.
In G. britskii, size heteromorphism involving the NORs was detected by the Ag-NOR and 18S rDNA-FISH techniques. This characteristic can be explained mainly by unequal recombination or random duplication (Gornung, 2013Gornung E. 2013: Twenty years of physical mapping of major ribosomal RNA genes across the teleosts: A review of research. Cytogenet Genome Res. 2013; 141(2-3):90-102. https://doi.org/10.1159/000354832
https://doi.org/10.1159/000354832...
). Events as these can be observed in chromosomes regions composed of repetitive DNA. In G. britskii, the NORs are flanked by heterochromatin. Heterochromatic regions and ribosomal genes are known to contain highly repetitive DNA. Therefore, our results reinforce the suggestion of Fernandes-Matioli et al. (1998Fernandes-Matioli FMC, Marchetto MCN, Almeida-Toledo LA, Toledo-Filho AS. High intraspecific karyological conservation in four species of Gymnotus (Pisces: Gymnotiformes) from Southeastern Brazilian basins. Caryologia. 1998; 51(3-4):221-34. https://doi.org/10.1080/00087114.1998.10797414) that heterochromatic regions bordering NORs tend to favor chromosome rearrangements in these particular chromosome areas.
The present study performed the first physical mapping of the 5S DNA in chromosomes of Rhamphichthyidae. In G. britskii, the 5S rDNA is located in a single chromosome pair and nonsyntenic with the 18S rDNA sites, a common characteristic of several fish groups (Pisano et al., 2007Pisano E, Ozouf-Costaz C, Foresti F, Kapoor BG. Fish Cytogenetics. Enfield N. H., Science Publishers Inc (USA); 2007.). Simple 5S rDNA sites were also observed in karyotypes of M. bilineatus, but in only one chromosome of the 12th pair in males showed synteny between the 18S rDNA and 5S rDNA sites (de Jesus et al., 2016de Jesus IS, Ferreira M, Garcia C, Ribeiro LB, Alves-Gomes JA, Feldberg E. First cytogenetic description of Microsternarchus bilineatus (Gymnotiformes: Hypopomidae) from Negro river (Brazilian Amazon). Zebrafish . 2016; 13(6):571-77. https://doi.org/10.1089/zeb.2016.1281
https://doi.org/10.1089/zeb.2016.1281...
). Moreover, in M. bilineatus the 5S rDNA cistrons are located in the terminal region, while in G. britskii these cistrons are located in the interstitial position.
The heterochromatin distribution follows the general pattern usually found in many other fish species, preferentially centromeric localization. In contrast, large telomeric heterochromatic blocks in acrocentric chromosomes were verified in G. britskii, but not observed in Rhamphichthys and Steatogenys (Cardoso et al., 2011Cardoso AL, Pieczarka JC, Feldberg E, Milhomem SSR, Moreira-Almeida T, Silva DS, Da Silva PC, Nagamachi CY. Chromosoma l characterization of two species of genus Steatogenys (Gymnotiformes: Rhamphichthyoidea: Steatogenini) from the Amazon basin: sex chromosomes and correlations with Gymnotiformes phylogeny. Rev Fish Biol Fish. 2011; 21(3):613-21. https://doi.org/10.1007/s11160-010-9196-0
https://doi.org/10.1007/s11160-010-9196-...
; Mendes et al., 2012Mendes VP, Portela-Castro AL, Julio-Junior HF. First record of supernumerary (B) chromosomes in electric fish (Gymnotiformes) and the karyotype structure of three species of the same order from the upper Paraná River basin. Comp Cytogenet. 2012; 6 (1):1-16. https://doi.org/10.3897/CompCytogen.v6i1.1752
https://doi.org/10.3897/CompCytogen.v6i1...
; Silva et al., 2013Silva PC, Nagamachi CY, Silva DS, Milhomem SSR, Cardoso AL, Oliveira JA, Pieczark JC. Karyotypic similarities between two species of Rhamphichthys (Rhamphichthyidae, Gymnotiformes) from the Amazon basin. Comp Cytogenet . 2013; 7(4):279-91. https://doi.org/10.3897/CompCytogen.v7i4.4366
https://doi.org/10.3897/CompCytogen.v7i4...
). Moreover, G. britskii had a conspicuous large heterochromatic block in the NOR-bearing chromosomes, adjacent to secondary constriction that can be used as cytogenetic markers for the species.
The results presented in this study allowed the first cytogenetic characterization of a species within the Gymnorhamphichthys genus, contributing to the karyotype knowledge of the family Rhamphichthyidae. Data from NORs confirmed the tendency of this family in presenting simple NORs sites, similar to the other Gymnotiformes clades. We also reported the presence of a large heterochromatic block in the NOR-bearing chromosome, adjacent to secondary constriction, which can be used as cytogenetic markers for G. britskii, and that centric fusions appear to be an important mechanism in the karyotype evolution and differentiation among Gymnotiformes species.
Acknowledgments
The authors thank Dr. Weferson Júnio da Graça for the taxonomic identification of the specimens. Besides, we are grateful to the Ministério do Meio Ambiente/ Instituto Chico Mendes de Conservação da Biodiversidade (MMA/ ICMBio - License number 64611) for authorizing the collection of the biological material.
References
- Albert JS. Species diversity and phylogenetic systematics of American knifefishes (Gymnotiformes, Teleostei). Misc Publi Mus Zoo. 2001; 190:1-127
- Almeida-Toledo LF. Contribuição a citogenética dos Gymnotoidae (Pisces, Ostariophysi). Ph.D. Thesis, Instituto de Biociências da Universidade de São Paulo, São Paulo; 1978.
- Almeida-Toledo LF, Foresti F, Daniel MFZ, Toledo-Filho SA. Sex chromosome evolution in fish: the formation of the neo-Y chromosome in Eigenmannia (Gymnotiformes). Chromosoma. 2000a; 109(3):197-200. https://doi.org/10.1007/s004120050428
» https://doi.org/10.1007/s004120050428 - Almeida-Toledo LF, Daniel-Silva MFZ, Lopes CE, Toledo-Filho SA. Sex chromosome evolution in fish. II. Second occurrence of an X1X2Y sex chromosome system in Gymnotiformes. Chromosome Res. 2000b; 8(4):335-40. https://doi.org/10.1023/A:1009287630301
» https://doi.org/10.1023/A:1009287630301 - Batista JA, Cardoso AL, Milhomem-Paixão SSR, Ready JS, Pieczarka JC, Nagamachi CY. The karyotype of Microsternarchus aff. bilineatus: a first case of Y chromosome degeneration in Gymnotiformes. Zebrafish. 2017; 14(3):244-50. https://doi.org/10.1089/zeb.2016.1383
» https://doi.org/10.1089/zeb.2016.1383 - Bertollo LAC, Takahashi CS, Moreira-filho O. Cytotaxonomic considerations on Hoplias lacerdae (Pisces, Erythrinidade). Braz J Genet. 1978; 1(1):103-20.
- Cardoso AL, Pieczarka JC, Crampton WGR, Ready JS, Figueiredo Ready WMB, Waddell JC, Oliveira JA, Nagamachi CY. Karyotypic diversity and evolution in a sympatric assemblage of neotropical electric knifefish. Front Genet. 2018; 9:81. https://doi.org/10.3389/fgene.2018.00081
» https://doi.org/10.3389/fgene.2018.00081 - Cardoso AL, Pieczarka JC, Feldberg E, Milhomem SSR, Moreira-Almeida T, Silva DS, Da Silva PC, Nagamachi CY. Chromosoma l characterization of two species of genus Steatogenys (Gymnotiformes: Rhamphichthyoidea: Steatogenini) from the Amazon basin: sex chromosomes and correlations with Gymnotiformes phylogeny. Rev Fish Biol Fish. 2011; 21(3):613-21. https://doi.org/10.1007/s11160-010-9196-0
» https://doi.org/10.1007/s11160-010-9196-0 - Carvalho TP, Ramos CS, Albert JS. A new species of Gymnorhamphichthys (Gymnotiformes: Rhamphichthyidae) from the Paraná-Paraguay basin. Copeia. 2011; 3:400-06. https://doi.org/10.1643/CI-10-154
» https://doi.org/10.1643/CI-10-154 - Fernandes CA, Bailly D, Silva VFB, Martins-Santos IC. System of multiple sex chromosomes in Eigenmannia trilineata López & Castello, 1966 (Sternopygidae, Gymnotiformes) from Iguatemi River Basin, MS, Brazil. Cytologia. 2010; 75(4):463-66. https://doi.org/10.1508/cytologia.75.463
» https://doi.org/10.1508/cytologia.75.463 - Fernandes CA, Paiz LM, Baumgärtner L, Margarido VP, Vieira MMR. Comparative cytogenetics of the black ghost knifefish (Gymnotiformes: Apteronotidae): Evidence of chromosomal fusion and pericentric inversions in karyotypes of two Apteronotus species. Zebrafish . 2017a; 14(5):471-76. https://doi.org/ 10.1089/zeb.2017.1432
» https://doi.org/ 10.1089/zeb.2017.1432 - Fernandes CA, Baumgartner L, Paiz LM, Margarido VP, Portela-Castro ALB. Chromosoma l characteristics of rDNA in a conserved karyotype of two Sternopygus macrurus (Gymnotiformes: Sternopygidae) populations from upper Paraná River basin. Biologia. 2017b; 72(6):680-85. https://doi.org/10.1515/biolog-2017-0071
» https://doi.org/10.1515/biolog-2017-0071 - Fernandes-Matioli FMC, Marchetto MCN, Almeida-Toledo LA, Toledo-Filho AS. High intraspecific karyological conservation in four species of Gymnotus (Pisces: Gymnotiformes) from Southeastern Brazilian basins. Caryologia. 1998; 51(3-4):221-34. https://doi.org/10.1080/00087114.1998.10797414
- Fricke R, Eschmeyer WN, Van Der Laan R, editors. Catalog of Fishes: Genera, Species, References [Internet]. California Academy of Sciences; 2018 [updated June, 01, 2019; cited in June, 20, 2019]. http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
» http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp - Gornung E. 2013: Twenty years of physical mapping of major ribosomal RNA genes across the teleosts: A review of research. Cytogenet Genome Res. 2013; 141(2-3):90-102. https://doi.org/10.1159/000354832
» https://doi.org/10.1159/000354832 - Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes. Maringá: Eduem; 2007.
- Griffiths S. The use of clove oil as an anaesthetic and method for sampling intertidal rockpool fishes. J Fish Biol. 2000; 57(6):1453-64. https://doi.org/10.1111/j.1095-8649.2000.tb02224.x
» https://doi.org/10.1111/j.1095-8649.2000.tb02224.x - Hatanaka T, Galetti Jr PM. Mapping of the 18S and 5S ribosomal RNA genes in the fish Prochilodus argenteus Agassiz, 1829 (Characiformes, Prochilodontidae). Genetica. 2004; 122(3):239-44. https://doi.org/10.1007/s10709-004-2039-y
» https://doi.org/10.1007/s10709-004-2039-y - Howell WM, Black DA. Controlled silver-staining of nucleolus organizer regions with a protective coloidal developer: a 1-step method. Experientia. 1980; 36(8):1014-15. https://doi.org/10.1007/BF01953855
» https://doi.org/10.1007/BF01953855 - de Jesus IS, Ferreira M, Garcia C, Ribeiro LB, Alves-Gomes JA, Feldberg E. First cytogenetic description of Microsternarchus bilineatus (Gymnotiformes: Hypopomidae) from Negro river (Brazilian Amazon). Zebrafish . 2016; 13(6):571-77. https://doi.org/10.1089/zeb.2016.1281
» https://doi.org/10.1089/zeb.2016.1281 - Langeani F, Castro RMC, Oyakawa OT, Shibatta OA, Pavanelli CS, Casatti L. Diversidade da ictiofauna do alto rio Paraná: composição atual e perspectivas futuras. Biota Neotrop. 2007; 7(3):1-17. http://dx.doi.org/10.1590/S1676-06032007000300020
» http://dx.doi.org/10.1590/S1676-06032007000300020 - Levan A, Fredga K, Sandberg AA. Nomenclature for centromeric position on chromosomes. Hereditas. 1964; 52(2):201-20. https://doi.org/10.1111/j.1601-5223.1964.tb01953.x
» https://doi.org/10.1111/j.1601-5223.1964.tb01953.x - Margarido VP, Moreira-Filho O. Karyotypic differentiation through chromosome fusion and number reduction in Imparfinis hollandi (Ostariophysi, Heptapteridae). Genet Mol Biol. 2008; 31(1):235-38. http://dx.doi.org/10.1590/S1415-47572008000200012
» http://dx.doi.org/10.1590/S1415-47572008000200012 - Martins C, Galetti Jr PM. Chromosome localization of 5S rDNA genes in Leporinus fish (Anostomidae, Characiformes). Chromosome Res . 1999; 7(5):363-67. https://doi.org/10.1023/A:1009216030316
» https://doi.org/10.1023/A:1009216030316 - Mendes VP, Portela-Castro AL, Julio-Junior HF. First record of supernumerary (B) chromosomes in electric fish (Gymnotiformes) and the karyotype structure of three species of the same order from the upper Paraná River basin. Comp Cytogenet. 2012; 6 (1):1-16. https://doi.org/10.3897/CompCytogen.v6i1.1752
» https://doi.org/10.3897/CompCytogen.v6i1.1752 - Nijssen H, Isbrücker IJH, Géry J. On the species of Gymnorhamphichthys Ellis, 1912, translucent sand-dwelling gymnotid fishes from South America (Pisces, Cypriniformes, Gymnotoidei). Stud Neotrop Fauna E. 1976; 11(1-2):37-63. https://doi.org/10.1080/01650527609360496
» https://doi.org/10.1080/01650527609360496 - Ota RR, Deprá GC, Graça WJ, Pavanelli CS. Peixes da planície de inundação do alto rio Paraná e áreas adjacentes: revised, annotated and updated. Neotrop Ichthyol. 2018; 16(2): e170094. https://doi.org/10.1590/1982-0224-20170094
» https://doi.org/10.1590/1982-0224-20170094 - Pinkel D, Straume T, Gray JW. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Natl Acad Sci. 1986; 83(9):2934-38.
- Pisano E, Ozouf-Costaz C, Foresti F, Kapoor BG. Fish Cytogenetics. Enfield N. H., Science Publishers Inc (USA); 2007.
- Silva EB, Margarido VP. An X1X1X2X2/X1X2Y multiple sex chromosome system in a new species of the genus Gymnotus (Pisces, Gymnotiformes). Environ Biol Fish. 2005; 73(3):293-97. https://doi.org/10.1007/s10641-005-2144-5
» https://doi.org/10.1007/s10641-005-2144-5 - da Silva M, Matoso DM, Artoni RF, Feldberg E. New approach data in electric fish (Teleostei: Gymnotus): Sex chromosome evolution and repetitive DNA. Zebrafish . 2014; 11(6):528-35. https://doi.org/10.1089/zeb.2013.0966
» https://doi.org/10.1089/zeb.2013.0966 - Silva PC, Nagamachi CY, Silva DS, Milhomem SSR, Cardoso AL, Oliveira JA, Pieczark JC. Karyotypic similarities between two species of Rhamphichthys (Rhamphichthyidae, Gymnotiformes) from the Amazon basin. Comp Cytogenet . 2013; 7(4):279-91. https://doi.org/10.3897/CompCytogen.v7i4.4366
» https://doi.org/10.3897/CompCytogen.v7i4.4366 - Sumner AT. A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res. 1972; 75(1):304-06. https://doi.org/10.1016/0014-4827(72)90558-7
» https://doi.org/10.1016/0014-4827(72)90558-7
Edited by
Publication Dates
-
Publication in this collection
30 Sept 2019 -
Date of issue
2019
History
-
Received
01 July 2019 -
Accepted
09 Sept 2019