ABSTRACT
A new species of Paralithoxus is described from the Ajarani River, a small tributary of the Branco River basin, Roraima State, Brazilian Amazon. The genus Paralithoxus comprises species described from the Essequibo drainage in Guyana, Approuague and Maroni in French Guiana, Suriname River in Surinam, and more recently, from Jari and Amapá rivers, in Brazil. Despite occurring in a rock-bottomed fast-flowing stream as the other species of Paralithoxus, this is the first species of the genus collected at 900 m altitude, in the Serra da Mocidade highlands, an isolated and poorly accessible small mountain chain at the southern border of the Guiana Shield. The new species is distinguished from its congeners by having truncate teeth, color pattern with green spots on dark olive-brown background, alternating dark and light blotches on fins and by the pelvic fin being as long as or longer than the pectoral fin. Sex dimorphism of the species is described. Comments on morphology and osteology are provided and compared with congeners.
Keywords:
Altitudinal fishes; Morphology; Osteology; Rio Branco basin; Taxonomy
RESUMO
Uma nova espécie de Paralithoxus é descrita do rio Ajarani, um pequeno tributário da bacia do rio Branco, Estado de Roraima, Amazônia Brasileira. O gênero Paralithoxus é composto por espécies descritas para as drenagens do Essequibo na Guiana, Approuague e Maroni na Guiana Francesa, rio Suriname no Suriname, e mais recentemente, para os rios Jari e Amapá, no Brasil. Apesar de ocorrer em pequenos riachos com fundo rochoso e correnteza forte como o restante dos representantes de Paralithoxus, esta é a primeira espécie do gênero coletada a 900 m de altitude, na Serra da Mocidade, uma pequena cadeia de montanhas localizada em uma área isolada e de difícil acesso na porção sul do Escudo das Guianas. A nova espécie distingue-se de suas congêneres pela presença de dentes truncados, pelo padrão de coloração com manchas verde claras sobre fundo oliva-marrom escuro, alternando manchas claras e escuras nas nadadeiras e pelo comprimento relativo da nadadeira pélvica igual ou maior que o comprimento da nadadeira peitoral. O dimorfismo sexual da espécie é descrito. Comentários sobre a morfologia e osteologia da espécie são feitos e comparados com suas congêneres.
Palavras-chave:
Bacia do rio Branco; Morfologia; Osteologia; Peixes de altitude; Taxonomia
Introduction
Eigenmann (1910Eigenmann CH. Catalogue of the fresh-water fishes of tropical and south temperate America. Reprints of Princeton Univ. Exp. Patagonia, 1896-1899. 1910; 3(4):375-511.) proposed Lithoxus lithoides as a new loricariid genus and species, based on specimens from Guyana. The genus was diagnosed by its reduced adult size (up to 80.0 mm SL), body extremely depressed, presence of strong spines on cheek plates, large and almost perfectly round oral disc with short fringes at the border of the lips, premaxilla extremely reduced, small mouth opening and naked abdomen. Seventy years after the original description of Ancistrus bovalliiRegan, 1906Regan CT. Notes on some loricariid fishes, with descriptions of two new species. Ann Mag Nat Hist (Ser. 7). 1906; 17(97):94-98., Isbrücker (1980Isbrücker I. Classification and catalogue of the mailed Loricariidae (Pisces, Siluriformes). Verslagen en Technische Gegevens/Instituut voor Taxonomische Zoölogie, Netherlands. 1980; 22:1-181.) assigned this species to Lithoxus.
Boeseman (1982Boeseman M. The South American mailed catfish genus Lithoxus Eigenmann, 1910, with the description of three new species from Surinam and French Guyana and records of related species (Siluriformes: Loricariidae). Proc K Ned Akad Wet Ser C: Biol Med Sci. 1982; 85(1):41-58.) proposed the new subgenus Paralithoxus and described three new species, L. (P.) planquettei, L. (P.) pallidimaculatus, and L. (P.) surinamensis, and designated L. (P.) bovallii (Regan, 1906Regan CT. Notes on some loricariid fishes, with descriptions of two new species. Ann Mag Nat Hist (Ser. 7). 1906; 17(97):94-98.) as type species of Paralithoxus. Lithoxus (Paralithoxus) was distinguished from Lithoxus (Lithoxus) based on mandibular teeth numbering 8 (vs. 12), absence of an extension of the adipose fin (vs. presence) and pectoral fin shorter than head (vs. longer than head), rendering the subgenus Lithoxus with only one species, Lithoxus (L.) lithoides.
Nijssen, Isbrücker (1990Nijssen H, Isbrücker IJH. Lithoxus stocki, a species new to science of ancistrin loricariid catfish from the Maroni River drainage, with a comparison of the primary type-specimens of the six species of Lithoxus (syn.: Paralithoxus) (Pisces, Siluriformes, Loricariidae). Bijdr Dierkd 1990; 60(3):327-33.) described Lithoxus stocki and proposed to allocate all Paralithoxus species in Lithoxus, due to the similarity between Lithoxus bovalli and L. stocki. The taxonomically simpler alternative of a single genus, Lithoxus, was judged preferable by the authors. After Nijssen, Isbrucker (1990Nijssen H, Isbrücker IJH. Lithoxus stocki, a species new to science of ancistrin loricariid catfish from the Maroni River drainage, with a comparison of the primary type-specimens of the six species of Lithoxus (syn.: Paralithoxus) (Pisces, Siluriformes, Loricariidae). Bijdr Dierkd 1990; 60(3):327-33.) redefined the genus, two new species were described, Lithoxus boujardi Muller, Isbrücker, 1993 from the Approuague River basin, French Guiana, and Lithoxus jantjaeLujan, 2008Lujan NK. Description of a new Lithoxus (Siluriformes: Loricariidae) from the Guayana Highlands with a discussion of Guiana Shield biogeography. Neotrop Ichthyol. 2008; 6(3):413-18. http://dx.doi.org/10.1590/S1679-62252008000300014
http://dx.doi.org/10.1590/S1679-62252008...
from the Ventuari River, Orinoco River basin, Venezuela.
Recently, phylogenetic studies using morphology (Armbruster et al., 2018Armbruster JW, Greene L, Lujan NK. Using morphology to test DNA-based phylogenetic relationships within the Guiana Shield catfish tribe Lithoxini (Siluriformes: Loricariidae). Copeia 2018; 106(4):671-80. https://doi.org/10.1643/CI-18-121
https://doi.org/10.1643/CI-18-121...
; de Oliveira, 2018de Oliveira RR. Sistemática de Baryancistrus Rapp Py-Daniel, 1989 e sua posição filogenética dentro da tribo Ancistrini (Loricariidae: Hypostominae). [PhD Thesis]. Manaus, Instituto Nacional de Pesquisas da Amazônia; 2018.) and molecules (Lujan et al., 2018Lujan NK, Armbruster JW, Lovejoy NR. Multilocus phylogeny, diagnosis and generic revision of the Guiana Shield endemic suckermouth armoured catfish tribe Lithoxini (loricariidae: Hypostominae). J Linn Soc London , Zool. 2018; 184(4):1169-86. https://doi.org/10.1093/zoolinnean/zly025
https://doi.org/10.1093/zoolinnean/zly02...
) recovered Lithoxus lithoides clearly separated from the remaining species of the genus, supporting the elevation of the subgenus Paralithoxus to genus and the placement of all non-L. lithoides species in it, except Lithoxus jantjae. In this same paper, Lujan et al. (2018Lujan NK, Armbruster JW, Lovejoy NR. Multilocus phylogeny, diagnosis and generic revision of the Guiana Shield endemic suckermouth armoured catfish tribe Lithoxini (loricariidae: Hypostominae). J Linn Soc London , Zool. 2018; 184(4):1169-86. https://doi.org/10.1093/zoolinnean/zly025
https://doi.org/10.1093/zoolinnean/zly02...
) created a new genus, Avalithoxus, to accommodate Lithoxus jantjae and proposed the tribe Lithoxini to contain the genera Lithoxus, Paralithoxus, Avalithoxus and Exastilithoxus.
Both Lithoxus and Paralithoxus are mainly recorded from Guianese drainages with a few records from the Trombetas River (Pará, Brazil) (Ferreira, 1993Ferreira EJG. Composição, distribuição e aspectos ecológicos da ictiofauna de um trecho do rio Trombetas, na área de influência da futura UHE Cachoeira Porteira, Estado do Pará, Brasil. Acta Amazon. 1993; 23:1-89. http://dx.doi.org/10.1590/1809-43921993235089
http://dx.doi.org/10.1590/1809-439219932...
; Lujan, 2008Lujan NK. Description of a new Lithoxus (Siluriformes: Loricariidae) from the Guayana Highlands with a discussion of Guiana Shield biogeography. Neotrop Ichthyol. 2008; 6(3):413-18. http://dx.doi.org/10.1590/S1679-62252008000300014
http://dx.doi.org/10.1590/S1679-62252008...
). Two new species were recently described, Paralithoxus jariensis from the Jari River and Paralithoxus raso from the Amapá River, both left-hand tributaries of the Amazon (Silva et al., 2017Silva GSC, Covain R, Oliveira C, Roxo FF. Description of two new species of Lithoxus (Hypostominae: Loricariidae) from rio Jari and rio Amapá basins, Brazilian Guiana Shield. Zootaxa 2017; 4347(1):151-68. http://dx.doi.org/10.11646/zootaxa.4347.1.9
http://dx.doi.org/10.11646/zootaxa.4347....
). Avalithoxus jantjae is the most westerly distributed species in the group, having been recorded only from the Ventuari River, upper Orinoco, Venezuela.
Currently, Paralithoxus comprises eigth species with a cratonic distribution restricted to basins of the Guiana Shield (Silva et al., 2017Silva GSC, Covain R, Oliveira C, Roxo FF. Description of two new species of Lithoxus (Hypostominae: Loricariidae) from rio Jari and rio Amapá basins, Brazilian Guiana Shield. Zootaxa 2017; 4347(1):151-68. http://dx.doi.org/10.11646/zootaxa.4347.1.9
http://dx.doi.org/10.11646/zootaxa.4347....
). Ferreira (1993Ferreira EJG. Composição, distribuição e aspectos ecológicos da ictiofauna de um trecho do rio Trombetas, na área de influência da futura UHE Cachoeira Porteira, Estado do Pará, Brasil. Acta Amazon. 1993; 23:1-89. http://dx.doi.org/10.1590/1809-43921993235089
http://dx.doi.org/10.1590/1809-439219932...
) presented the first record of Lithoxus lithoides and Paralithoxus bovalli for the Trombetas River, in Northern Brazil. As the Ireng River (called Maú River in Roraima), type locality of P. bovallii, is a tributary of the Takutu River in the Brazil - Guyana border, the presence of Paralithoxus representatives in other Brazilian drainages was already expected.
In 2016, a multidisciplinary expedition to remote areas of the highlands of Parque Nacional da Serra da Mocidade in Roraima State, Brazil, produced a number of specimens of an undescribed species of Paralithoxus, collected from the Ajarani River, a high-altitude tributary of Branco River basin, which is described herein. Despite other extensive collections from Branco, Mucajaí (Ferreira et al., 1988Ferreira EJG, Santos GM, Jégu M. Aspectos ecológicos da ictiofauna do Rio Mucajaí, na área da ilha Paredão, Roraima, Brasil. Amazoniana 1988; 10(3):339-52.) and Água Boa do Univini Rivers (Douglas A. Bastos, 2016, pers. obs.), Lithoxus, Paralithoxus, or Avalithoxus representatives have never been recorded from other tributaries of the Branco River basin, which strongly suggests that this new species may be restricted to high altitude streams and rivers of the southern portion of the Guiana Shield. This fieldwork resulted in the collection of several interesting new and possibly endemic species from high altitude aquatic habitats. Thus, the new Paralithoxus species from Serra da Mocidade is described herein, with comments on its morphological affinities with related species.
Material and Methods
Measurements and counts follow Boeseman (1982Boeseman M. The South American mailed catfish genus Lithoxus Eigenmann, 1910, with the description of three new species from Surinam and French Guyana and records of related species (Siluriformes: Loricariidae). Proc K Ned Akad Wet Ser C: Biol Med Sci. 1982; 85(1):41-58.), with the addition of dorsal-fin base length and interdorsal distance. Measurements were taken point-to-point with a digital caliper to the nearest 0.1 mm and expressed in percentages and ratios in the species description and Tab. 1. Standard length (SL) is expressed in millimeters; all other measurements are expressed as percentages of standard length (SL) or head length (HL). Specimens were cleared and stained (c&s) according to Taylor, Van Dyke (1985Taylor WR, Van Dyke GC. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium 1985; 9(2):107-19.). Nomenclature of body plates follows Schaefer (1997Schaefer SA. The Neotropical Cascudinhos: Systematics and Biogeography of the Otocinclus Catfishes (Siluriformes: Loricariidae). Proc Acad Nat Sci Philadelphia. 1997; 148:1-120.). Ratios in Tab. 1 were included in order to compare with the original descriptions, as type material from all Paralithoxus species was not available for examination. For P. pallidimaculatus, P. boujardi, P. jariensis and P. raso, we used only the original descriptions to compare. Photographs of c&s parts were taken with a Leica M205A stereomicroscope coupled with a Leica DMC4500 and Leica Application Suite V4.10.0 Interactive Measurements Montage. Comparative material was provided by the following institutions: AMNH, American Museum of Natural History, New York; ANSP, Academy of Natural Sciences of Drexel University, Philadelphia; CAS, California Academy of Sciences, San Francisco; INPA, Instituto Nacional de Pesquisas da Amazônia, Manaus; MNRJ, Museu Nacional, Rio de Janeiro; MPEG, Museu Paraense Emilio Goeldi, Belém; MZUSP, Museu de Zoologia da Universidade de São Paulo, São Paulo; USNM, National Museum of Natural History, Smithsonian Institution, Washington DC.
Collecting site. The Serra da Mocidade National Park (PARNA Serra da Mocidade) was officially created in 1998, comprising more than 350,000 ha, distributed across two municipalities, Caracaraí (Roraima State) and Barcelos (Amazonas State). The Serra da Mocidade is an area of extensive mountain ranges that reach up to 1920 m above sea level, and our expedition was the first to explore areas between 600 m and 1350 m altitude. The park is a federal conservation unit with highly restricted access, being limited by a Yanomami Indigenous Reserve to the west and areas of the Brazilian Army to the east and northwest bounds. The park is part of the Branco River basin, containing headwater streams of the Branco River tributaries Ajarani, Água Boa do Univini and Pacu.
Paralithoxus mocidade, new species
urn:lsid:zoobank.org:act:B5B7D3D6-F267-4ED5-A752-E5F64B461397
Holotype. INPA 54745, 50.5 mm SL, Brazil, Roraima State, Caracaraí Municipality, stream tributary to Ajarani River, Branco River basin, upstream of large waterfall, Serra da Mocidade, 01°42’52.70”N 61°48’17.64”W, 29 Jan 2016, D. A. Bastos, J. Zuanon, P. M. M. Ito and S. R. Briglia-Ferreira.
Paratypes. All from Brazil, Roraima State, Caracaraí Municipality. INPA 52422, 17, 20.6-47.2 mm SL (1 c&s, 47.2 mm SL), stream tributary to Ajarani River, Branco River basin, downstream of large waterfall, Serra da Mocidade, 01°42’50.26”N 61°48’18.65”W, 29 Jan 2016, D. A. Bastos, J. Zuanon, P. M. M. Ito and S. R. Briglia-Ferreira. INPA 52424, 17, 15.0-54.7 mm SL (1 c&s, 54.7 mm SL, 16 alc.), MNRJ 51531, 2, 23.0-26.0 mm SL, MPEG 38600, 2, 27.0-30.0 mm SL, MZUSP 125192, 2, 25.0-28.0 mm SL, ANSP 206987, 2, 26.0-33.0 mm SL, all collected with holotype.
Non-types. INPA 59028, 14, 13.8-19.0 mm SL, collected with the holotype.
Diagnosis. Paralithoxus mocidade differs from all congeners, except P. bovallii, by having truncate teeth (vs. pointed to acute teeth). It differs from P. bovallii by the truncate caudal fin (vs. caudal-fin forked). Adittionaly, P. mocidade differs from P. pallidimaculatus and P. surinamensis by the presence of adipose fin (vs. absence). It differs from P. planquettei and P. stocki by having a more depressed and elongate body: body depth 10-13.9% of SL (vs. 15.3-16.8% in P. planquettei and 15.7-16.9% in P. stocki), and body width at dorsal fin 19.3-23.0% of SL (vs. 23.3-26.5% in P. planquettei and 24.2-25.1% in P. stocki); from P. raso by the larger interorbital distance, 28.8-33 % of HL (vs. 27.1-28.6%) and larger dentaries 5.9-9.3% of HL (vs. 4.4-5.3%); from P. stocki and P. boujardi by the caudal fin conspicuously mottled, with alternating dark and light blotches (vs. caudal fin dark with clear distal band); and from P. jariensis by having seven to eight plates between dorsal and adipose fins (vs. four) and 24-26 lateral median plates (vs. 23-24).
Paralithoxus mocidade, holotype, INPA 54745, female, 50.5 mm SL, in lateral, dorsal and ventral views.
Lateral view of Paralithoxus mocidade, paratype, INPA 52424, female, 49.5 mm SL. Photographed alive by Haroldo Palo Jr.
Paralithoxus mocidade, paratype, INPA 52424, male, 50.7 mm SL in lateral, dorsal, and ventral views.
Description. Morphometrics and counts in Tab. 1. Small species of Hypostominae, largest specimen examined 54.7 mm SL. Body very depressed and short. In lateral view, dorsal profile slightly convex from snout to dorsal-fin origin, descending almost straight to dorsal procurrent rays of caudal fin. Greatest body width at pectoral-fin origin; body of similar width between posterior base of pectoral fin and origin of pelvic fin, slightly narrowing from this point towards caudal-fin base. Caudal peduncle trapezoid in cross section. Ventral profile straight from snout to caudal-fin origin.
Head short, depressed and rounded in dorsal view. Snout tip hispid, completely covered by plates. Eye moderately small, dorsally positioned; interorbital region narrow and flat. Cheek plates with up to 20 hypertrophied odontodes. Parieto-supraoccipital large, hexagonal, with process posteriorly bordered by four plates. Predorsal area with three pairs of plates, misplaced in some specimens. Nuchal plate half-moon shaped; small but conspicuous. Seven to eight plates between dorsal and adipose fins. Body almost completely plated; abdomen naked. Body plates arranged in four longitudinal series anteriorly, five series between dorsal and anal fin, and five series on caudal peduncle. All plates covered by odontodes of similar size without carinae, except for two low keels on dorsal plates between dorsal and adipose fins. Ventral series of plates largely smooth, showing large median area without odontodes, except on nuptial male. Seven plates along dorsal-fin base; low pre-adipose plate. Median series with 24-26 plates not carinate; mid-ventral series not forming prominent keel. First anal-fin pterygiophore exposed, located anterior to anal-fin origin.
Oral disc large, nearly circular. Mouth opening U-shaped, wider than long. Oral internal papillae absent. Maxillary barbel very short, projected anteriorly from oral disc. Lips well developed. Whole border of oral disc covered with large round papillae. Surface of upper and lower lips covered by round papillae not densely packed. Lip surface with shallow notches mostly without papillae distributed at top of premaxillae, around dentaries and on midline on lower lip.
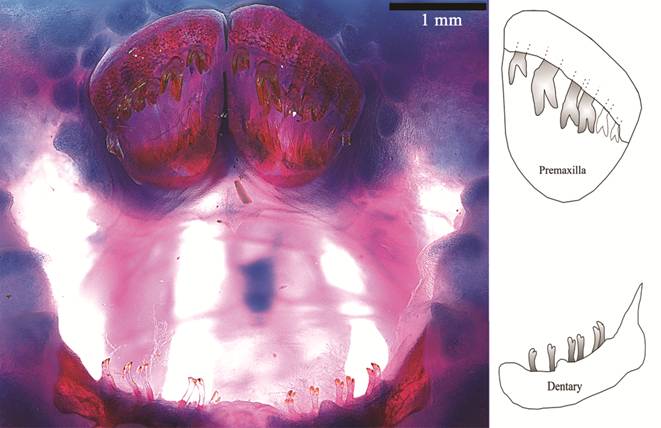
Teeth bicuspid; crown yellow and shaft translucent (Fig. 4). Premaxillary teeth much larger than mandibular teeth, arranged in crescent from lateral to mesial. Cusps of premaxillary teeth asymmetrical; larger cusp with straight border, truncate; internal cusp slightly larger. Dentary teeth extremely delicate, bifurcate; cusps small, worn to similar size. Premaxilla with 5-6 and dentary with up to 8 teeth.
Jaw teeth of Paralithoxus mocidade, INPA 52424, 55.2 mm SL. On the right, schematic representation of left mandibles.
Dorsal fin II,7, branched rays decreasing in length posteriorly; last branched ray 1/3 shorter than dorsal-fin spine. Spinelet reduced, locking mechanism not functional and with small area exposed, covered by odontodes. Pectoral fin I,6; distal tip of branched rays not surpassing insertion of pelvic fin when adpressed. Pectoral-fin spine robust, flat and slightly curved inward; spine of equal size of first branched ray. Pectoral-fin spine distal region covered with hypertrophied odontodes, mainly in mature males. Adipose fin low but conspicuous, with small membranous extension posteriorly. Pelvic fin i,5, distal tip of branched rays surpassing insertion of anal fin when adpressed. First pelvic unbranched ray leaf-like, enlarged medially; whole ray flat and covered by odontodes (Fig. 9b). Pelvic fin larger than pectoral fin in majority of specimens; ratio between pectoral/pelvic length with mean of 0.9 and standard deviation of 0.1. Anal fin ii,4, i,4 or i,5, branched rays coequal in length. Caudal fin i,14,i; seven branched rays on each lobe. Caudal fin almost truncate; principal rays mostly coequal in length, with lower rays slightly longer than upper lobe; all rays with small odontodes.
Color in alcohol. Body with dark olive-brown background with irregular light blotches; blotches slightly smaller and more densely organized on head than on trunk and fins. Four inconspicuous large transverse light bars on body: first on dorsal-fin origin, second large bar on posterior dorsal-fin base, third anterior to adipose, and fourth on base of caudal fin. Ventral surface of body uniformly cream to beige colored. All fins with checkered pattern, alternating large dark blotches with clear ones.
Color in life. Very similar to preserved coloration, but with overall body color dark olive-brown on dorsum with light green spots on snout and cheek (Fig. 2); thin creamy to light green vermiculations over interorbital area and posterior portion of head; clear marks on anterior portion of dorsum becoming larger and more irregular towards caudal peduncle. Ventral side of body uniformly beige.
Sexual dimorphism. Urogenital papilla small, but conspicuous on all specimens. The only externally mature male specimen was dissected and had a more melanic coloration, darkened on body and lips, losing bars and blotches along body and fins; dorsal, pectoral and pelvic fins with branched rays darkened showing light bar close to tip; caudal fin uniformly dark (Fig. 3). Male with odontodes on cheek plates and pectoral fin more hypertrophied than on females. Mature male with fins more developed than females: pectoral spine large, strong, bearing more developed odontodes on distal half; first pelvic-fin unbranched ray strong, rounded for entire length and enlarged medially; branched rays on pelvic fin conspicuously longer than unbranched ray. Male has caudal peduncle covered with odontodes, except around anal fin, whereas females and juveniles present caudal peduncle ventrally smooth, without aligned odontodes.
Geographic distribution and habitat. Paralithoxus mocidade has only been recorded from an unnamed tributary of the Ajarani River, in the area of Serra da Mocidade, Branco River basin, Caracaraí Municipality, Roraima State, Brazil (Fig. 5). All specimens were collected up and downstream of a 20-m high waterfall located at approximately 900 m elevation. The Ajarani River at the collection site is a small fast-flowing river (up to 15 m width and 2 m depth, as measured in dry season) with clear water, low temperature (about 21.7 ºC), acidic (pH 5.5), dissolved oxygen (DO) 20.7%, and smooth rocks on the bottom (Fig. 6). During the day, juveniles were observed grazing in the open over smooth rocks, and adults were found in narrow cracks or under rock slabs during the night. Juveniles were captured with hand-nets and adults were collected by hand while snorkeling (Fig. 7).
Distribution of all type-material of Lithoxini in northern South America (ranges not included) and detail of Serra da Mocidade, Roraima, Brazil.
Type-locality of Paralithoxus mocidade in Ajarani River, tributary of the Branco River basin, located immediately downstream of a large waterfall. Photo by Marcos Amend.
Hand collecting (a) specimens of Paralithoxus mocidade from a (b) narrow crevice in a rock slab during snorkelling.
Etymology. The specific epithet “mocidade” is in allusion to the type locality, in Serra da Mocidade National Park. A noum in apposition.
Conservation status. Paralithoxus mocidade is known only from the type locality in a geographically isolated river system, which could generate concerns about its conservation status. However, the inaccessibility of the area and the fact that nearly two-thirds of the area of Serra da Mocidade are formally protected by the Serra da Mocidade National Park and Yanomami Indigenous Territory indicate that there are no imminent threats to the species, which can be categorized as Least Concern (LC) (IUCN, 2017IUCN Standards and Petitions Subcommittee. 2017. Guidelines for Using the IUCN Red List Categories and Criteria. Version 13. Prepared by the Standards and Petitions Subcommittee. Availabe from http://cmsdocs.s3.amazonaws.com/ RedListGuidelines.pdf
http://cmsdocs.s3.amazonaws.com/ RedList...
).
Taxonomic remarks. Paralithoxus mocidade and P. bovallii were recorded from the Branco River basin: P. bovallii from the Takutu (Branco)-Essequibo drainages and P. mocidade from the Ajarani River. Recent collections in the upper Ireng River by N. K. Lujan and J. W. Armbruster produced new specimens of P. bovallii from the type-locality, as listed in the redescription of the species by Lujan et al. (2018Lujan NK, Armbruster JW, Lovejoy NR. Multilocus phylogeny, diagnosis and generic revision of the Guiana Shield endemic suckermouth armoured catfish tribe Lithoxini (loricariidae: Hypostominae). J Linn Soc London , Zool. 2018; 184(4):1169-86. https://doi.org/10.1093/zoolinnean/zly025
https://doi.org/10.1093/zoolinnean/zly02...
). Based on this and the original description (Regan, 1906Regan CT. Notes on some loricariid fishes, with descriptions of two new species. Ann Mag Nat Hist (Ser. 7). 1906; 17(97):94-98.), we conducted a morphological comparison. Both P. bovallii and P. mocidade share a mottled color pattern on body and fins, a similar number of teeth on the jaws (6 to 8), same tooth shape (truncate) (Fig. 4), and similar number of plates between dorsal and adipose fins. However, these species differ in eye size, with P. mocidade having a usually smaller eye, with orbit representing 9-12% (average 11%) vs. 11-19% (average 14.6%) in P. bovallii (Lujan et al., 2018Lujan NK, Armbruster JW, Lovejoy NR. Multilocus phylogeny, diagnosis and generic revision of the Guiana Shield endemic suckermouth armoured catfish tribe Lithoxini (loricariidae: Hypostominae). J Linn Soc London , Zool. 2018; 184(4):1169-86. https://doi.org/10.1093/zoolinnean/zly025
https://doi.org/10.1093/zoolinnean/zly02...
). Based on Regan’s original ratios, the orbital diameter in P. bovallii was contained 6-7.5 times and interorbital distance 3 times in head length, whereas in P. mocidade, the orbit is contained 8-10 times and the interorbital 3-3.5 times in head length. Besides, P. bovallii has the pectoral fin larger than the pelvic whereas in P. mocidade pectoral and pelvic are equal or the pelvic is slightly larger: pectoral length 23.8-28.7% (mean of 25.5%) in SL in P. mocidade vs. 6.9-33.0% (26.2%) in P. bovallii; pelvic fin 25.5-30.5% (27%) in SL in P. mocidade vs. 4.9-29% (22.8%) in P. bovallii (Lujan et al., 2018Lujan NK, Armbruster JW, Lovejoy NR. Multilocus phylogeny, diagnosis and generic revision of the Guiana Shield endemic suckermouth armoured catfish tribe Lithoxini (loricariidae: Hypostominae). J Linn Soc London , Zool. 2018; 184(4):1169-86. https://doi.org/10.1093/zoolinnean/zly025
https://doi.org/10.1093/zoolinnean/zly02...
).
The other species described from the Essequibo River drainage is Lithoxus lithoides (also distributed in other drainages such as the Trombetas River, following Ferreira, 1993Ferreira EJG. Composição, distribuição e aspectos ecológicos da ictiofauna de um trecho do rio Trombetas, na área de influência da futura UHE Cachoeira Porteira, Estado do Pará, Brasil. Acta Amazon. 1993; 23:1-89. http://dx.doi.org/10.1590/1809-43921993235089
http://dx.doi.org/10.1590/1809-439219932...
). Lithoxus lithoides is extremely different from Paralithoxus mocidade having larger eyes, larger pectoral fins (more developed in nuptial males), larger posterior membranous extension of the adipose fin, dorsal fin almost touching the adipose-fin plate, and three series of plates in the caudal peduncle. However, L. lithoides shares with P. mocidade the truncate teeth and membranous extension of the adipose (reduced in P. mocidade when compared to L. lithoides). Some paratypes of L. lithoides were examined and measured, and differences in orbit and caudal peduncle were highly evident between the two species: orbit diameter ranged from 19-21% of head length in L. lithoides vs. 9.2-12.2% in P. mocidade; caudal peduncle depth ranged from 18.8-23% of caudal peduncle length in L. lithoides vs. 27.6-33.5% in P. mocidade. Besides, L. lithoides has the premaxilla much smaller than the dentary, whereas in P. mocidade the toothed area in both jaws is of similar size, despite difference in the size of the teeth, reinforcing the general dissimilarity between both species.
Species of Paralithoxus recorded from French Guiana (P. planquettei, P. boujardi and P. stocki) share some features distinct from P. mocidade: teeth with acute cusps and caudal fin with distinct distal light bar vs. teeth truncate and caudal fin with alternating clear and dark blotches in P. mocidade. Besides, both P. boujardi and P. stocki have a larger pectoral fin (pectoral length 3.3-3.7 and 2.8-3.6 times in SL in P. boujardi and P. stocki respectively vs. 3.5-4.2 in P. mocidade) and shorter caudal peduncle (caudal peduncle length 3-3.25 and 3.5 times in SL in P. boujardi and P. stocki respectively vs. 3.6-4.1 in P. mocidade). Paralithoxus planquettei also has a shorter pectoral fin and a deeper caudal peduncle (caudal peduncle depth 9.8-11.75% in SL vs. 7.1-8.4% in P. mocidade) and larger mandible (dentary length 2.5-2.8 times in interorbital distance vs. 3.6-4.9 in P. mocidade).
Paralithoxus jariensis and P. raso were originally distinguished from other Paralithoxus species by a set of morphological features. Paralithoxus raso has a peculiar color pattern with light large blotches similar to P. pallidimaculatus (Silva et al., 2017Silva GSC, Covain R, Oliveira C, Roxo FF. Description of two new species of Lithoxus (Hypostominae: Loricariidae) from rio Jari and rio Amapá basins, Brazilian Guiana Shield. Zootaxa 2017; 4347(1):151-68. http://dx.doi.org/10.11646/zootaxa.4347.1.9
http://dx.doi.org/10.11646/zootaxa.4347....
). Both recently described species were recorded for the eastern part of the Amazon, in Pará and Amapá States. Paralithoxus jariensis is morphometrically very similar to P. mocidade but shows a reduction in number of plates: four plates between dorsal and adipose fin (vs. 7-8 in P. mocidade) and 23-24 lateral median plates (vs. 24-26 in P. mocidade). Paralithoxus raso differs from P. mocidade mainly by having a larger pectoral-fin spine (27-29% vs. 23-28% in SL in P. mocidade), and smaller mandibles (premaxilla 3.3-4% and dentary 4.4-5.3% in head length in P. raso vs. 6.1-9.9% and 5.9-9.3% in head length in P. mocidade). In their original description, the authors also conducted a molecular analysis using sequences of the COI mitochondrial gene for most available species of Paralithoxus (P. planquettei, P. bovallii, P. pallidimaculatus, P. boujardi and P. stocki). Paralithoxus jariensis and P. raso came out as sister group of P. planquettei, also from Eastern Guiana (Oyapock River).
Osteological remarks. Armbruster et al. (2018Armbruster JW, Greene L, Lujan NK. Using morphology to test DNA-based phylogenetic relationships within the Guiana Shield catfish tribe Lithoxini (Siluriformes: Loricariidae). Copeia 2018; 106(4):671-80. https://doi.org/10.1643/CI-18-121
https://doi.org/10.1643/CI-18-121...
) presented a morphology-based phylogeny for Lithoxini in which synapomorphies were proposed for the genera Lithoxus, Paralithoxus and Avalithoxus as a way to compare their results with the molecular phylogeny presented in Lujan et al. (2018Lujan NK, Armbruster JW, Lovejoy NR. Multilocus phylogeny, diagnosis and generic revision of the Guiana Shield endemic suckermouth armoured catfish tribe Lithoxini (loricariidae: Hypostominae). J Linn Soc London , Zool. 2018; 184(4):1169-86. https://doi.org/10.1093/zoolinnean/zly025
https://doi.org/10.1093/zoolinnean/zly02...
). Most of the synapomorphies listed for Paralithoxus were confirmed in this study with slight variation in P. mocidade, such as: first hypobranchial angled at 45° relative to the sagittal plane, maxilla distal end enlarged, and metapterygoid weakly sutured to lateral ethmoid.
Although cited as a synapomorphy for the tribe Lithoxini (Armbruster et al., 2018Armbruster JW, Greene L, Lujan NK. Using morphology to test DNA-based phylogenetic relationships within the Guiana Shield catfish tribe Lithoxini (Siluriformes: Loricariidae). Copeia 2018; 106(4):671-80. https://doi.org/10.1643/CI-18-121
https://doi.org/10.1643/CI-18-121...
), in P. mocidade the difference in size between the teeth on both mandibles and the distinct shape of the cusps are remarkable (Fig. 4): cusps truncate and subequal in size on the premaxilla, and pointed and equally forked on the dentary. As cited above, truncate teeth were also observed in L. lithoides and P. bovallii. Lithoxus lithoides, however, has the mesial cusp of premaxillary teeth almost three times the size of the lateral cusp. Also, the size and enlargement of the distal end of the maxilla deserves attention. In P. mocidade, the maxilla is well developed, its length more than 50% the length of the palatine, and its distal enlargement is rather pronounced.
The suspensorium in P. mocidade is similar to that of other Lithoxini and comparable to P. bovallii, the closest species to P. mocidade. However, the metapterygoid anterior processes, both the digitiform and the “spoon-shaped” (Armbruster, 2004Armbruster JW. Phylogenetic relationships of the suckermouth armoured catfishes (Loricariidae) with emphasis on the Hypostominae and the Ancistrinae. J Linn Soc London, Zool. 2004; 141(1):1-80. https://doi.org/10.1111/j.1096-3642.2004.00109.x
https://doi.org/10.1111/j.1096-3642.2004...
), are much more enlarged in P. mocidade than in P. bovallii and other Lithoxini. The anterior opercular process is also highly enlarged, similar to Avalithoxus jantjae (Armbruster et al., 2018Armbruster JW, Greene L, Lujan NK. Using morphology to test DNA-based phylogenetic relationships within the Guiana Shield catfish tribe Lithoxini (Siluriformes: Loricariidae). Copeia 2018; 106(4):671-80. https://doi.org/10.1643/CI-18-121
https://doi.org/10.1643/CI-18-121...
, fig. 4-A).
The branchial apparatus in P. mocidade has a strong hyoid arch, and strongly pointed, villiform teeth on both pharyngeal plates (upper and lower), a condition already registered by Armbruster (2004Armbruster JW. Phylogenetic relationships of the suckermouth armoured catfishes (Loricariidae) with emphasis on the Hypostominae and the Ancistrinae. J Linn Soc London, Zool. 2004; 141(1):1-80. https://doi.org/10.1111/j.1096-3642.2004.00109.x
https://doi.org/10.1111/j.1096-3642.2004...
) for L. lithoides and P. bovallii. Paralithoxus mocidade has only one basibranchial (BB) (Fig. 8), ossified and very elongate, almost filling the whole mesial extension of the branchial apparatus; the other basibranchial is a cartilaginous mass connected to the fourth and fifth ceratobranchials. The ordinary siluriform condition is the absence of the first basibranchial (Arratia, 2003Arratia G. Catfish head skeleton - An overview. In: Arratia G, Kapoor BG, Chardon M, Diogo R, editors. Catfishes, Volume 1. Enfield: Science Publishers, Inc.; 2003. p.3-45.), second (BB2) ossified, and third (BB3) reduced, ossified or not (Schaefer, 2003Schaefer SA. Relationships of Lithogenes villosus Eigenmann, 1909 (Siluriformes, Loricariidae): Evidence from High-Resolution Computed Microtomography. Am Mus Novit. 2003; 3401:1-54.). Most loricariids show the generalized siluriform condition. In Paralithoxus mocidade, the long ossified BB can be explained by the fusion of BB2 and BB3 (very elongate BB) or BB2 lost (Armbruster, 2004 Armbruster JW. Phylogenetic relationships of the suckermouth armoured catfishes (Loricariidae) with emphasis on the Hypostominae and the Ancistrinae. J Linn Soc London, Zool. 2004; 141(1):1-80. https://doi.org/10.1111/j.1096-3642.2004.00109.x
https://doi.org/10.1111/j.1096-3642.2004...
- for L. lithoides and P. bovallii). This condition was also observed in Exastilithoxus fimbriatus and other members of Lithoxini (Armbruster et al., 2018Armbruster JW, Greene L, Lujan NK. Using morphology to test DNA-based phylogenetic relationships within the Guiana Shield catfish tribe Lithoxini (Siluriformes: Loricariidae). Copeia 2018; 106(4):671-80. https://doi.org/10.1643/CI-18-121
https://doi.org/10.1643/CI-18-121...
).
Paralithoxus mocidade has a highly reduced accessory flange of the first ceratobranchial (CB1) (Fig. 8) (approximately 1/5 of the ceratobranchial properly). Most Hypostominae have a well-developed CB1 accessory flange (Schaefer, 1987Schaefer SA. Osteology of Hypostomus plecostomus (Linnaeus), with a phylogenetic analysis of the loricariid subfamilies (Pisces: Siluroidei). Contrib sci (Los Angel Calif). 1987; 394:1-31.; Armbruster, 2004Armbruster JW. Phylogenetic relationships of the suckermouth armoured catfishes (Loricariidae) with emphasis on the Hypostominae and the Ancistrinae. J Linn Soc London, Zool. 2004; 141(1):1-80. https://doi.org/10.1111/j.1096-3642.2004.00109.x
https://doi.org/10.1111/j.1096-3642.2004...
), an extra sheet of thin bone originating on the same point of the ceratobranchial, of similar size and, usually, bearing a row of branchial filaments. Lithogenes and astroblepids lack this accessory flange. The condition in P. mocidade has been already cited for P. bovalli, L. lithoides, and some other non-hypostomines (Armbruster, 2004Armbruster JW. Phylogenetic relationships of the suckermouth armoured catfishes (Loricariidae) with emphasis on the Hypostominae and the Ancistrinae. J Linn Soc London, Zool. 2004; 141(1):1-80. https://doi.org/10.1111/j.1096-3642.2004.00109.x
https://doi.org/10.1111/j.1096-3642.2004...
) and it was also observed in Exastilithoxus fimbriatus.
Finally, the pectoral girdle in P. mocidade has a conspicuous small hornlike process (Fig. 9a) on the anterior surface of the cleithrum. Lithoxus lithoides and P. bovallii have a short and dense bony crest at the same point, but in P. mocidade this crest comes forward as a small, blunt, cylindrical process, inclined towards the lateral sides of the body, with an extra blue-stained tissue coming off its extremity that could be a tendon or a thin cartilage. This condition is quite unusual, and it was not observed in E. fimbriatus, but it has been observed in one specimen of Hypostomus gymnorhynchus.
a. Pectoral (dorsal view), with arrows for the cleithra processes, and b. pelvic (ventral view) of Paralithoxus mocidade, INPA 52424, 55.2 mm SL.
Discussion
Paralithoxus, Lithoxus and Avalithoxus are very close to Exastilithoxus Isbrücker, Nijssen, 1979. Exastilithoxus comprises only two valid species, E. fimbriatus (Steindachner, 1915), described from the Coquenan River, Caroni basin in Venezuela, and E. hoedemani Isbrücker, Nijssen, 1985, from waterfalls in the Marauiá River, Negro River basin, Brazil. Exastilithoxus shares with Lithoxus and Paralithoxus synapomorphies related to morphology and osteology (Armbruster, 2004Armbruster JW. Phylogenetic relationships of the suckermouth armoured catfishes (Loricariidae) with emphasis on the Hypostominae and the Ancistrinae. J Linn Soc London, Zool. 2004; 141(1):1-80. https://doi.org/10.1111/j.1096-3642.2004.00109.x
https://doi.org/10.1111/j.1096-3642.2004...
; Armbruster et al., 2018Armbruster JW, Greene L, Lujan NK. Using morphology to test DNA-based phylogenetic relationships within the Guiana Shield catfish tribe Lithoxini (Siluriformes: Loricariidae). Copeia 2018; 106(4):671-80. https://doi.org/10.1643/CI-18-121
https://doi.org/10.1643/CI-18-121...
) such as: overall reduction of the branchial apparatus, loss of lateral walls of the metapterygoid channel, basipterygium with anterolateral and anteromesial processes fused, reduction in the number of plate rows on thinnest part of the caudal peduncle from five-four to three (as in Lithoxus), abdomen naked, and teeth large but not spoon-shaped.
In Lujan et al. (2018Lujan NK, Armbruster JW, Lovejoy NR. Multilocus phylogeny, diagnosis and generic revision of the Guiana Shield endemic suckermouth armoured catfish tribe Lithoxini (loricariidae: Hypostominae). J Linn Soc London , Zool. 2018; 184(4):1169-86. https://doi.org/10.1093/zoolinnean/zly025
https://doi.org/10.1093/zoolinnean/zly02...
), the Lithoxini genera Lithoxus, Paralithoxus, Exastilithoxus, and Avalithoxus were recognized by sharing a small adult size, body strongly depressed, a large oral disc with papillate lips, and small oral opening. Avalithoxus was diagnosed mainly by a reduction in the number of branched caudal-fin rays (12 vs. 14 or 13 in all other Lithoxini representatives), reduction on the number of inter dorsal plates (five vs. six to nine), and absence of digitate papillae on the lips (vs. digitate papillae present in Exastilithoxus).
The two multilocus phylogenetic analyses with Hypostominae available (Lujan et al., 2015Lujan NK, Armbruster JW, Lovejoy NR, López-Fernández H. Multilocus molecular phylogeny of the suckermouth armored catfishes (Siluriformes: Loricariidae) with a focus on subfamily Hypostominae. Mol Phylogenet Evol. 2015; 82(2015):269-88. https://doi.org/10.1016/j.ympev.2014.08.020
https://doi.org/10.1016/j.ympev.2014.08....
; 2018Lujan NK, Armbruster JW, Lovejoy NR. Multilocus phylogeny, diagnosis and generic revision of the Guiana Shield endemic suckermouth armoured catfish tribe Lithoxini (loricariidae: Hypostominae). J Linn Soc London , Zool. 2018; 184(4):1169-86. https://doi.org/10.1093/zoolinnean/zly025
https://doi.org/10.1093/zoolinnean/zly02...
) recovered two contrasting results. The first analysis, without representatives of P. bovallii, showed Lithoxus lithoides as the sister group of a large clade of undescribed species of Exastilithoxus; the second recovered L. lithoides as the sister group of the Paralithoxus species, and Avalithoxus jantjae as the sister group of a large clade comprising (Exastilithoxus (Lithoxus-Paralithoxus)).
Both Armbruster (2004Armbruster JW. Phylogenetic relationships of the suckermouth armoured catfishes (Loricariidae) with emphasis on the Hypostominae and the Ancistrinae. J Linn Soc London, Zool. 2004; 141(1):1-80. https://doi.org/10.1111/j.1096-3642.2004.00109.x
https://doi.org/10.1111/j.1096-3642.2004...
) and de Oliveira (2018de Oliveira RR. Sistemática de Baryancistrus Rapp Py-Daniel, 1989 e sua posição filogenética dentro da tribo Ancistrini (Loricariidae: Hypostominae). [PhD Thesis]. Manaus, Instituto Nacional de Pesquisas da Amazônia; 2018.), in a morphology-based phylogeny, recovered Lithoxus, Paralithoxus and Exastilithoxus nested within Ancistrini clades comprising Chaetostoma and Dekeyseria among other genera. Some of the synapomorphies shared by these taxa are corroborated here, such as the peculiar shape of the opercle in Paralithoxus, which is very similar to that of Dekeyseria.
Armbruster et al. (2018Armbruster JW, Greene L, Lujan NK. Using morphology to test DNA-based phylogenetic relationships within the Guiana Shield catfish tribe Lithoxini (Siluriformes: Loricariidae). Copeia 2018; 106(4):671-80. https://doi.org/10.1643/CI-18-121
https://doi.org/10.1643/CI-18-121...
) recovered a different ingroup arrangement from the morphological analyses cited above, with Avalithoxus jantjae as the sister-group of (Exastilithoxus (Paralithoxus, Lithoxus)), similar to the Lujan et al. (2018Lujan NK, Armbruster JW, Lovejoy NR. Multilocus phylogeny, diagnosis and generic revision of the Guiana Shield endemic suckermouth armoured catfish tribe Lithoxini (loricariidae: Hypostominae). J Linn Soc London , Zool. 2018; 184(4):1169-86. https://doi.org/10.1093/zoolinnean/zly025
https://doi.org/10.1093/zoolinnean/zly02...
) molecular analysis results. Both the 2018 morphology and molecular-based analysis were quite reductionists, dealing mainly with the ingroup terminal taxa and very few outgroups. However, those analyses comprised more ingroup taxa than the other ones, which increases confidence level in the results as well.
Paralithoxus mocidade differs from most species of Paralithoxus by the presence of a large pelvic fin, whereas in other species the pectoral is clearly larger than the pelvic fin. The new species lives in a fast-flowing river with rapids and waterfalls, similar to its congeners, and apparently represents the first record of a Paralithoxus species occurring in high-altitude water bodies. All species of Paralithoxus are adapted to high-energy waters, occupying cracks or crevices on rocks in clear water rivers with rapids and rocky/sandy substrates (Ferreira, 1993Ferreira EJG. Composição, distribuição e aspectos ecológicos da ictiofauna de um trecho do rio Trombetas, na área de influência da futura UHE Cachoeira Porteira, Estado do Pará, Brasil. Acta Amazon. 1993; 23:1-89. http://dx.doi.org/10.1590/1809-43921993235089
http://dx.doi.org/10.1590/1809-439219932...
). Other species collected with P. mocidade in the same habitat included undescribed species of Ancistrus (Loricariidae) and Trichomycterus sensu lato (Trichomycteridae).
Species of Lithoxus and Paralithoxus occur in the drainages of the Guiana Shield, as other loricariid genera such as, Corymbophanes, Exastilithoxus, Lithogenes, Metaloricaria and Neblinichthys. Distributions of loricariid taxa suggest that connections to these other areas have been important, but that within the Guiana Shield there has been little mixing of upland faunas via the Western Atlantic Coastal and Caroni-Cuyuni/Mazaruni corridors (Lujan, Armbruster, 2011Lujan NK, Armbruster JW. The Guiana Shield. In: Albert J, Reis RE, editors. Historical biogeography of neotropical freshwater fishes. Berkeley: University of California Press; 2011. p.211-24.). Most distributions within the Guiana Shield can be explained via current watershed boundaries, stream-capture events in the uplands of larger systems, and/or ancient river systems such as the proto-Berbice (Lujan, 2008Lujan NK. Description of a new Lithoxus (Siluriformes: Loricariidae) from the Guayana Highlands with a discussion of Guiana Shield biogeography. Neotrop Ichthyol. 2008; 6(3):413-18. http://dx.doi.org/10.1590/S1679-62252008000300014
http://dx.doi.org/10.1590/S1679-62252008...
; Lujan, Armbruster, 2011Lujan NK, Armbruster JW. The Guiana Shield. In: Albert J, Reis RE, editors. Historical biogeography of neotropical freshwater fishes. Berkeley: University of California Press; 2011. p.211-24.). All species of Paralithoxus occur from an eastern limit in Amapá River, Brazil (Melo et al., 2016Melo BF, Benine RC, Britzke R, Gama CS, Oliveira C. An inventory of coastal freshwater fishes from Amapá highlighting the occurrence of eight new records for Brazil. ZooKeys 2016; 606:127-40. https://doi.org/10.3897/zookeys.606.9297
https://doi.org/10.3897/zookeys.606.9297...
; Silva et al., 2017Silva GSC, Covain R, Oliveira C, Roxo FF. Description of two new species of Lithoxus (Hypostominae: Loricariidae) from rio Jari and rio Amapá basins, Brazilian Guiana Shield. Zootaxa 2017; 4347(1):151-68. http://dx.doi.org/10.11646/zootaxa.4347.1.9
http://dx.doi.org/10.11646/zootaxa.4347....
), to the west up to Cuyuni-Mazaruni River in Guyana to the North, and to the South up to Trombetas River basin, Brazil (Ferreira, 1993Ferreira EJG. Composição, distribuição e aspectos ecológicos da ictiofauna de um trecho do rio Trombetas, na área de influência da futura UHE Cachoeira Porteira, Estado do Pará, Brasil. Acta Amazon. 1993; 23:1-89. http://dx.doi.org/10.1590/1809-43921993235089
http://dx.doi.org/10.1590/1809-439219932...
). Additional unpublished records of Paralithoxus spp. from other drainages in Brazil such as Uatumã (Amazonas State), Jari, Oriximiná, Maicuru (Lúcia Rapp Py-Daniel, pers. obs.) (Pará State) and Amapá Grande (Amapá State) confirm that the current geographic distribution observed for Paralithoxus ranges from the Guiana Shield tributaries to the left hand cratonic tributaries of the Amazonas River. The recent descriptions of Paralithoxus jariensis and P. raso corroborate the putatively larger area of occurrence of the genus. Interestingly, Paralithoxus has never been recorded from the Negro River basin, where Exastilithoxus occurs, or the Orinoco River, where Avalithoxus can also be found. The Serra da Mocidade range is completely disconnected from other mountain ranges in Roraima State, Brazil. The similarity of P. mocidade with the Potaro and Takutu taxa hints at ancient and unknown connections between these areas.
Comparative material examined. Exastilithoxus fimbriatus: Guyana, AMNH 220459, 1, 48.5 mm SL, Essequibo River basin. Venezuela, ANSP 160626, 1, 43.6 mm SL, Cataniapo River, Orinoco River basin. Brazil, INPA 38957 (1 c&s 37.3 mm SL), Auaris River, shore between Sanoma and Yekuana Indigenous villages. Exastilithoxus hoedemani: Brazil, INPA 506, holotype, 50.9 mm SL, Marauiá River, Negro River basin. Exastilithoxus sp.: Brazil, INPA 049716, 1, 30 mm SL, Negro River, bedrocks close to São Sebastião village, São Gabriel da Cachoeira Municipality. Paralithoxus bovallii: Suriname, AMNH 54961, 9, 35.4-45.4 mm SL, Corintijn River basin. Lithoxus jantjae: Venezuela, ANSP 182809, 4 paratypes, 22.8-34.4 mm SL; Ventuari River, Orinoco River basin, above Salto Tencua, 58 km east-southeast of San Juan de Manapiare. Lithoxus lithoides: Guyana, AMNH 7119, 5 paratypes, 29.6-43.6 mm SL; Warraputa Falls and in rocks above falls, Essequibo River basin. ANSP 39121, 1 paratype, 38.6 mm SL, Warruputa Falls, Essequibo River basin. ANSP 185295, 1, 35.4 mm SL, Essequibo River (Atlantic Dr.) at Yukanopito Falls, 44.5 km SW of mouth of Kuyuwini River. CAS 77332, paratypes, 10, 43.1-46.8 mm SL, Amatuk cataract, Potaro River, Essequibo River basin. USNM 66223, 3 paratypes, 39.7-44.3 mm SL; Amatuk. Brazil, Amazonas State, INPA 845, 50 mm SL, 46147, 3, 24.5-41.2 mm SL, Presidente Figueiredo Municipality, Pitinga River, Uatumã River basin. Paralithoxus planquettei: Suriname, ANSP 189131, 17, 17.5-71.5 mm SL, Sipaliwini District, Litanie River at mouth and confluence with Marowini River. ANSP 189135, 1, 55.6 mm SL, Sipaliwini District, Lawa River, Marowijne River basin, Gransoela. French Guiana, INPA 3243, 2, 27.9-57.4 mm SL, Sinnamary River, igarapé Takari. Brazil, INPA 4910, 3, 27,5-30,1 mm Sl, Amapá State, Araguari River basin. INPA 7824, 2, 22.0-28.3 mm SL, Pará State, Oriximiná Municipality, Trombetas River basin. INPA 37610, 24, 32.4-58.8 mm SL, Amapá State, Macapá Municipality. MZUSP 103396, 26, 31.3-50.9 mm SL, Monte Dourado Municipality. MZUSP 101528, 17, 32.9-51.1 mm SL, Laranjal do Jari Municipality. MZUSP 101539, 6, 22.7-29.8 mm SL, Amapá State, Laranjal do Jari Municipality. Paralithoxus stocki: Suriname, ANSP 189130, 2, 67.7-68.4 mm SL, Sipaliwini District, Lawa River, Marowijne River basin. ANSP 189137, 1, 61.0 mm SL, Sipaliwini District, Lawa River, Marowijne River basin. Brazil, Pará State, Oriximiná Municipality, INPA 5537, 1 (c&s), 60 mm SL, INPA 5538, 5, 36.3-40.6 mm SL, INPA 33815, 20, 31.3-66.4 mm SL, Trombetas-Mapuera River. Paralithoxus surinamensis: Brazil, Pará State, INPA 7807, 1, 23.8 mm SL, Oriximiná Municipality, Poana River, Cachoeira Seca Superior. Pseudolithoxus sp.: Brazil, Amazonas State, INPA 16270, 7 (5 alc, 97.5-153.1 mm SL, 1 skl, 146.8 mm SL, 1 c&s, 73.6 mm SL), Presidente Figueiredo, Uatumã River, Cachoeira do Miriti. Dekeyseria picta: Brazil, Amazonas State, INPA 400, 3 (3, 42.7-74.8 mm SL, 1 c&s), Urubaxi River, near its mouth in Negro River, Barcelos Municipality.
Acknowledgments
We would like to acknowledge Renildo R. de Oliveira for a thorough review of the manuscript. Also, we thank Scott Schaefer (AMNH), Mark Sabaj (ANSP), Luiz Rocha (CAS) and Richard Vari (in memoriam) (USNM) for hosting museum visits and loaning material; Mario Cohn-Haft (INPA) for idealizing, organizing and leading a highly complex expedition to Serra da Mocidade. The expedition resulted from a collaboration between the Instituto Nacional de Pesquisas da Amazônia (INPA), the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio), the Brazilian Military Command of Amazonia (CMA), and a Brazilian film producer, Grifa Filmes. We are also in debt to Haroldo Palo Jr. (in memoriam) for providing images of the live specimen of Paralithoxus mocidade, and to Marcos Amend for the image of the collecting locality; to our colleagues from INPA, Isabel M. Soares, Shizuka Hashimoto, and Rafaela Ota for curatorial assistance; to Carlison Oliveira for editing some of the images; to Thiago Mahlmann for capturing teeth images, and the Invertebrate Collection of INPA for allowing the use of photographic equipment. Financial support to ASO was provided by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, process n. 141051/2013-2); LRP benefited from funds granted by CNPq, processes n. 474236/2004-8 and n. 309392/2011-0; and JZ received a productivity grant from CNPq (#313184/2014-7).
References
- Armbruster JW. Phylogenetic relationships of the suckermouth armoured catfishes (Loricariidae) with emphasis on the Hypostominae and the Ancistrinae. J Linn Soc London, Zool. 2004; 141(1):1-80. https://doi.org/10.1111/j.1096-3642.2004.00109.x
» https://doi.org/10.1111/j.1096-3642.2004.00109.x - Armbruster JW, Greene L, Lujan NK. Using morphology to test DNA-based phylogenetic relationships within the Guiana Shield catfish tribe Lithoxini (Siluriformes: Loricariidae). Copeia 2018; 106(4):671-80. https://doi.org/10.1643/CI-18-121
» https://doi.org/10.1643/CI-18-121 - Arratia G. Catfish head skeleton - An overview. In: Arratia G, Kapoor BG, Chardon M, Diogo R, editors. Catfishes, Volume 1. Enfield: Science Publishers, Inc.; 2003. p.3-45.
- Boeseman M. The South American mailed catfish genus Lithoxus Eigenmann, 1910, with the description of three new species from Surinam and French Guyana and records of related species (Siluriformes: Loricariidae). Proc K Ned Akad Wet Ser C: Biol Med Sci. 1982; 85(1):41-58.
- Eigenmann CH. Catalogue of the fresh-water fishes of tropical and south temperate America. Reprints of Princeton Univ. Exp. Patagonia, 1896-1899. 1910; 3(4):375-511.
- Ferreira EJG. Composição, distribuição e aspectos ecológicos da ictiofauna de um trecho do rio Trombetas, na área de influência da futura UHE Cachoeira Porteira, Estado do Pará, Brasil. Acta Amazon. 1993; 23:1-89. http://dx.doi.org/10.1590/1809-43921993235089
» http://dx.doi.org/10.1590/1809-43921993235089 - Ferreira EJG, Santos GM, Jégu M. Aspectos ecológicos da ictiofauna do Rio Mucajaí, na área da ilha Paredão, Roraima, Brasil. Amazoniana 1988; 10(3):339-52.
- IUCN Standards and Petitions Subcommittee. 2017. Guidelines for Using the IUCN Red List Categories and Criteria. Version 13. Prepared by the Standards and Petitions Subcommittee. Availabe from http://cmsdocs.s3.amazonaws.com/ RedListGuidelines.pdf
» http://cmsdocs.s3.amazonaws.com/ RedListGuidelines.pdf - Isbrücker I. Classification and catalogue of the mailed Loricariidae (Pisces, Siluriformes). Verslagen en Technische Gegevens/Instituut voor Taxonomische Zoölogie, Netherlands. 1980; 22:1-181.
- Lujan NK. Description of a new Lithoxus (Siluriformes: Loricariidae) from the Guayana Highlands with a discussion of Guiana Shield biogeography. Neotrop Ichthyol. 2008; 6(3):413-18. http://dx.doi.org/10.1590/S1679-62252008000300014
» http://dx.doi.org/10.1590/S1679-62252008000300014 - Lujan NK, Armbruster JW. The Guiana Shield. In: Albert J, Reis RE, editors. Historical biogeography of neotropical freshwater fishes. Berkeley: University of California Press; 2011. p.211-24.
- Lujan NK, Armbruster JW, Lovejoy NR, López-Fernández H. Multilocus molecular phylogeny of the suckermouth armored catfishes (Siluriformes: Loricariidae) with a focus on subfamily Hypostominae. Mol Phylogenet Evol. 2015; 82(2015):269-88. https://doi.org/10.1016/j.ympev.2014.08.020
» https://doi.org/10.1016/j.ympev.2014.08.020 - Lujan NK, Armbruster JW, Lovejoy NR. Multilocus phylogeny, diagnosis and generic revision of the Guiana Shield endemic suckermouth armoured catfish tribe Lithoxini (loricariidae: Hypostominae). J Linn Soc London , Zool. 2018; 184(4):1169-86. https://doi.org/10.1093/zoolinnean/zly025
» https://doi.org/10.1093/zoolinnean/zly025 - Melo BF, Benine RC, Britzke R, Gama CS, Oliveira C. An inventory of coastal freshwater fishes from Amapá highlighting the occurrence of eight new records for Brazil. ZooKeys 2016; 606:127-40. https://doi.org/10.3897/zookeys.606.9297
» https://doi.org/10.3897/zookeys.606.9297 - Nijssen H, Isbrücker IJH. Lithoxus stocki, a species new to science of ancistrin loricariid catfish from the Maroni River drainage, with a comparison of the primary type-specimens of the six species of Lithoxus (syn.: Paralithoxus) (Pisces, Siluriformes, Loricariidae). Bijdr Dierkd 1990; 60(3):327-33.
- de Oliveira RR. Sistemática de Baryancistrus Rapp Py-Daniel, 1989 e sua posição filogenética dentro da tribo Ancistrini (Loricariidae: Hypostominae). [PhD Thesis]. Manaus, Instituto Nacional de Pesquisas da Amazônia; 2018.
- Regan CT. Notes on some loricariid fishes, with descriptions of two new species. Ann Mag Nat Hist (Ser. 7). 1906; 17(97):94-98.
- Schaefer SA. Osteology of Hypostomus plecostomus (Linnaeus), with a phylogenetic analysis of the loricariid subfamilies (Pisces: Siluroidei). Contrib sci (Los Angel Calif). 1987; 394:1-31.
- Schaefer SA. The Neotropical Cascudinhos: Systematics and Biogeography of the Otocinclus Catfishes (Siluriformes: Loricariidae). Proc Acad Nat Sci Philadelphia. 1997; 148:1-120.
- Schaefer SA. Relationships of Lithogenes villosus Eigenmann, 1909 (Siluriformes, Loricariidae): Evidence from High-Resolution Computed Microtomography. Am Mus Novit. 2003; 3401:1-54.
- Silva GSC, Covain R, Oliveira C, Roxo FF. Description of two new species of Lithoxus (Hypostominae: Loricariidae) from rio Jari and rio Amapá basins, Brazilian Guiana Shield. Zootaxa 2017; 4347(1):151-68. http://dx.doi.org/10.11646/zootaxa.4347.1.9
» http://dx.doi.org/10.11646/zootaxa.4347.1.9 - Taylor WR, Van Dyke GC. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium 1985; 9(2):107-19.
Edited by
Publication Dates
-
Publication in this collection
02 Dec 2019 -
Date of issue
2019
History
-
Received
14 Apr 2019 -
Accepted
12 Nov 2019