Abstract
A new species of Corumbataia is described from Rio Maranhão, Rio Tocantins basin, central Brazil. The new species is distinguished from all congeners by the presence of a small, naked area on snout tip; by having the abdomen covered with small platelets forming a shield which reaches the lateral mid-ventral plates; by the anterior profile of the head rounded in dorsal view; by the lower lip not reaching the transversal line of the pectoral girdle; and by the presence of 28 or 29 vertebrae. High genetic divergence in mitochondrial cytochrome c oxidase subunit I (COI) further supports the validity of this new species. Our phylogenetic analysis shows a derived subclade in Corumbataia, herein named as the Corumbataia cuestae group, composed of the new species plus C. cuestae, C. tocantinensis, C. britskii, C. liliai, and C. lucianoi. This group is defined by having a conspicuous crest of hypertrophied odontodes on head; absence of the adipose fin or a single series of platelets at adipose-fin position; and anastomosis of the infraorbital and otic sensory canals over the pterotic-supracleithrum. Here we also restrict the distribution of C. tocantinensis to the Rio Araguaia basin.
Keywords:
Cytochrome C Oxidase; Diversity; Freshwater fishes; Hypoptopomatinae; Central Brazil
Resumo
Uma nova espécie de Corumbataiaé descrita para o Rio Maranhão, na bacia do Rio Tocantins na região central do Brasil. A nova espécie é diagnosticada dos demais congêneres pela presença de uma pequena área nua na ponta do focinho; por possuir o abdômen coberto por pequenas placas formando um escudo que alcança as placas laterais mid-ventrais; perfil anterior da cabeça arredondado em vista dorsal; lábio inferior não alcançando a linha transversal da cintura peitoral; e presença de 28 ou 29 vértebras. Altos valores de divergência genética também suportam a validade dessa nova espécie. Nossa análise filogenética encontrou um subclado derivado em Corumbataia, aqui denominado grupo Corumbataia cuestae, composto pela nova espécie mais C. cuestae, C. tocantinensis, C. britskii, C. liliai e C. lucianoi. Esse grupo é definido por possuir uma crista conspícua de odontódeos hipertrofiados na cabeça; ausência de nadadeira adiposa ou série única de placas na posição da nadadeira adiposa; anastomose dos canais sensoriais infraorbital e ótico sobre o pterótico-supracleitro. Aqui, nós também restringimos a distribuição de C. tocantinensisà bacia do Rio Araguaia.
Palabras-chave:
Brasil Central; Citocromo C Oxidase; Diversidade; Peixes de água doce; Hypoptopomatinae
INTRODUCTION
Loricariidae is the most diverse family of Siluriformes with almost 1000 valid species (Fricke et al., 2020Fricke R, Eschemeyer W, Fong JD. Species by Family/Subfamily [Internet]. San Francisco: California Academy of Science; 2020. Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/SpeciesByFamily.asp
http://researcharchive.calacademy.org/re...
) recognized mainly by having the body covered by bony plates, external tooth-like structures (odontodes), and a ventral mouth with lips forming an oral disk used to adhere to surfaces and to soft substrate for foraging (Greerinckx et al., 200Greerinckx T, Brunain M, Herrel A, Aerts P, Adrianes DA. Head with a suckermouth: A function-morphological study of the head with a suckermouth catfish Ancistrus cf. triradiatus (Loricariidae, Siluriformes). Belg J Zool. 2007; 137(1):47-66.7; Garg et al., 2010Garg KG, Domingos FXV, Almeida-Val VMF, Val AL. Histochemistry and functional organization of the dorsal skin of Ancistrus dolichopterus (Siluriformes: Loricariidae). Neotrop Ichthyol. 2010; 8(4):877-84. http://dx.doi.org/10.1590/S1679-62252010000400018
http://dx.doi.org/10.1590/S1679-62252010...
). Hypoptopomatinae is a subfamily of loricariids popularly known as cascudinhos, which contains 247 valid species (Fricke et al., 2020Fricke R, Eschemeyer W, Fong JD. Species by Family/Subfamily [Internet]. San Francisco: California Academy of Science; 2020. Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/SpeciesByFamily.asp
http://researcharchive.calacademy.org/re...
) arranged in six major clades: Hisonotini, Neoplecostomini, Otothyrini, Corumbataia clade, Otocinclus clade, and Hypoptopomatini (Roxo et al., 2019Roxo FF, Ochoa LE, Sabaj MH, Lujan NK, Covain R, Silva GSC, Melo BF, Albert JS, Chang J, Foresti F, Alfaro ME, Oliveira C. Phylogenomic reappraisal of the Neotropical catfish family Loricariidae (Teleostei: Siluriformes) using ultraconserved elements. Mol Phylogenet Evo. 2019; 135:148-65. https://doi.org/10.1016/j.ympev.2019.02.017
https://doi.org/10.1016/j.ympev.2019.02....
).
Corumbataia was proposed by Britski (1997)Britski HA. Descrição de um novo gênero de Hypoptopomatinae , com duas espécies novas (Siluriformes, Loricariidae). Pap Avulsos de Zool. 1997; 40(15):231-55. when describing Corumbataia cuestae from the Upper Paraná basin in the Rio Tietê and Corumbataia tocantinensis, from the Rio Araguaia and Rio Tocantins basins. This genus was delimited by an exposed portion in the middle of the scapular bridge formed only by the coracoid; compound hypurals 1 and 2 completely fused to the compound hypurals 3−5; atrophic maxillary barbel, and anastomosis of the infraorbital and otic sensory canals over the pterotic-supracleitrum (Britski, 1997Britski HA. Descrição de um novo gênero de Hypoptopomatinae , com duas espécies novas (Siluriformes, Loricariidae). Pap Avulsos de Zool. 1997; 40(15):231-55.). Four species were subsequently described: C. britskii Ferreira & Ribeiro, 2007, C. veadeirosCarvalho, 2008Carvalho TP. A new species of Corumbataia (Siluriformes: Loricariidae: Hypoptopomatinae) from upper Rio Tocantins Basin, Central Brazil. Copeia. 2008; 3:552-57. https://doi.org/10.1643/CI-07-064
https://doi.org/10.1643/CI-07-064...
, C. lucianoi Silva, Roxo, Souza & Oliveira, 2018 and C. liliai Silva, Roxo, Souza & Oliveira, 2018. Additionally, two species formerly described in Gymnotocinclus were transferred to Corumbataia by Roxo et al. (2019)Roxo FF, Ochoa LE, Sabaj MH, Lujan NK, Covain R, Silva GSC, Melo BF, Albert JS, Chang J, Foresti F, Alfaro ME, Oliveira C. Phylogenomic reappraisal of the Neotropical catfish family Loricariidae (Teleostei: Siluriformes) using ultraconserved elements. Mol Phylogenet Evo. 2019; 135:148-65. https://doi.org/10.1016/j.ympev.2019.02.017
https://doi.org/10.1016/j.ympev.2019.02....
: C. anosteos (Carvalho, Lehmann & Reis, 2008) and C. canoeiro (Roxo, Silva, Ochoa & Zawadzki, 2017).
Detailed examination of specimens of Corumbataia from the Rio Maranhão in the Rio Tocantins basin indicated that these represent a new species of this genus, which is formally described herein. We also provide and discuss a hypothesis for its phylogenetic position.
MATERIAL AND METHODS
Morphology. Measurements and counts were taken from the left side of 27 specimens and were made point to point to the nearest 0.1 mm with digital calipers. Nomenclature of body plates and osteology follow Schaefer (1997)Schaefer SA. The Neotropical cascudinhos: Systematic and biogeography of the Otocinclus catfishes (Siluriformes: Loricariidae). Proc Acad Nat Sci Phila. 1997; 148:1-120.. Measurements and abbreviations follow Carvalho, Reis (2009)Carvalho TP, Reis RE. Four new species of Hisonotus (Siluriformes: Loricariidae: Hypoptopomatinae) from the Upper Rio Uruguay, southerastern South America, with a review of the genus in the Rio Uruguay basin. Zootaxa. 2009; 2113(1):1-40. https://doi.org/10.11646/zootaxa.2113.1.1
https://doi.org/10.11646/zootaxa.2113.1....
, except for the measurement of body depth at dorsal-fin origin that were not included. We additionally included the following measurements: preanal length (from tip of snout until anal-fin insertion), base of dorsal-fin length (from anterior margin of dorsal-fin spinelet until insertion of last dorsal-fin ray), lower caudal-fin spine length (from posterior margin of last lateral plate of ventral plate series to the tip of the lower caudal-fin spine), body width at anal-fin insertion (from left side of the body until right side of the body at anal-fin insertion), snout-opercle length (from tip of snout until opercle opening), and head width (from opercle opening of the left side of the body until opercle opening of the right side of the body). Morphometrics are given as percentages of standard length (SL), except for subunits of head expressed as percentages of head length (HL). Four specimens were cleared and stained (c&s) according to Taylor, Van Dyke (1985)Taylor WR, Van Dyke GC. Revised procedures for staining and clearing small and other vertebrates for bones and cartilage study. Cybium. 1985; 9(2):107-19.. Vertebral counts include the five vertebrae of the Weberian apparatus and the compound caudal centrum (PU1+U1) that was counted as one element. Counts of dorsal-fin rays include the spinelet as the first unbranched ray. Institutional abbreviations follow Sabaj (2019)Sabaj MH. Standard symbolic codes for institutional resource collections in herpetology and ichthyology: an online reference. Version 7.1. Washington, DC: American Society of Ichthyologists and Herpetologists; 2019. Available from: http://www.ashi.org/
http://www.ashi.org/...
. Specimens were deposited at the Laboratório de Biologia e Genética de Peixes, Universidade Estadual Paulista, Botucatu, Brazil (LBP); Museu de Zoologia, Universidade de São Paulo, São Paulo, Brazil (MZUSP). Zoological nomenclature follows the International Code of Zoological Nomenclature (ICZN, 1999ICZN (International Commission on Zoological Nomenclature). International code of zoological nomenclature. Fourth edition. London: International Trust for Zoological Nomenclature; 1999.).
Molecular analysis. Three specimens were used for the molecular analysis: LBP 19095, 3, Rio Tocantins, tissues 77006-08. Total DNA extraction was performed using the Wizard Genomic DNA Purification Kit (Promega) from ethanol-preserved muscle, fin, or liver. Partial sequences of the cytochrome c oxidase subunit I (COI) gene were amplified in a total reaction volume of 12.5 uL. Each reaction included 1.25 uL of 10 X Buffer, 0.25 uL of MgCL2 (50 mM), 0.2 uL dNTPs (2mM), 0.5 uL of each primer (5 mM), 0.1 uL of PHT Taq DNA polymerase (Phoneutria, Belo Horizonte, Brazil), 1.0 uL of genomic DNA (20 ng) and 8.7 mL ddH2O. The conditions for the PCR reaction consisted of an initial denaturation (5 min at 94ºC), followed by 30 cycles of chain denaturation (40s at 94ºC), primer hybridization (30 s at 50-54º), nucleotide extension (1 min at 68ºC) and final extension (8 min at 68ºC). The amplified products were checked on 1% agarose gels and purified using ExoSap-IT (USB Corporation). DNA sequencing was conducted in an automatic sequencer ABI 3130 DNA Analyzer (Applied Biosystems).
All individual sequences for each species were initially assembled using the software Geneious 7.1.4 (Kearse et al., 2012Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012; 28(12):1647-49. https://doi.org/10.1093/bioinformatics/bts199
https://doi.org/10.1093/bioinformatics/b...
), and aligned by Muscle (Edgar, 2004Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucl Acids Res. 2004; 32(5):1792.) under default parameters. Alignments include the newly-generated sequences and the sequences available from the phylogenetic study of Roxo et al. (2014Roxo FF, Albert JS, Silva GSC, Zawadzki CH, Foresti F, Oliveira C. Molecular phylogeny and biogeography history of the armored Neotropical catfish subfamilies Hypoptopomatinae, Neoplecostominae and Otothyrinae (Siluriformes: Loricariidae). PLoS ONE. 2014; 9(8):e105564. https://doi.org/10.1371/journal.pone.0105564
https://doi.org/10.1371/journal.pone.010...
, 2017)Roxo FF, Silva GSC, Ochoa LE, Zawadzki CH. Description of a new species of Gymnotocinclus from the rio Tocantins basin with phylogenetic analysis of the subfamily Hypoptopomatinae (Siluriformes: Loricariidae). Zootaxa. 2017; 4268(3):337-59. http://dx.doi.org/10.11646/zootaxa.4268.3.2
http://dx.doi.org/10.11646/zootaxa.4268....
. To evaluate the occurrence of substitution saturation in our molecular data, we estimated whether the Iss (index of substitution saturation) was significantly lower than Iss.cAsym (assuming asymmetrical topology) using the method described by Xia et al. (2003)Xia X, Xie Z, Salemi M, Chen L, Wang Y. An index of substitution saturation and its application. Mol Phylogenet Evo. 2003; 26(1):1-7. https://doi.org/10.1016/S1055-7903(02)00326-3
https://doi.org/10.1016/S1055-7903(02)00...
and Xia, Lemey (2009)Xia X, Lemey P. Assessing substitution saturation with DAMBE. In: Lemey P, Salemi M, Vandamme AM, editors. The Phylogenetic Handbook: A practical approach to phylogenetic analysis and hypothesis testing. Cambridge: Cambridge University Press; 2009. p.615-30. https://doi.org/10.1017/CBO9780511819049.022
https://doi.org/10.1017/CBO9780511819049...
with the software DAMBE 5.3.38 (Xia, 2013Xia X. DAMBE5: a comprehensive software package for data analysis in molecular biology and evolution. Mol Biol and Evol. 2013; 30(7):1720-28. https://doi.org/10.1093/molbev/mst064
https://doi.org/10.1093/molbev/mst064...
). Nucleotide variation, substitution patterns, and genetic distances were examined using MEGA v.6.06 (Tamura et al., 2013Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol. 2013; 30(12):2725-29. https://doi.org/10.1093/molbev/mst197
https://doi.org/10.1093/molbev/mst197...
).
The best-fit nucleotide substitution model for the entire data set was selected under the Akaike information criterion (AICc) and Maximum Likelihood (ML) analyses were performed using the software MEGA v.6.06 (Tamura et al., 2013Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol. 2013; 30(12):2725-29. https://doi.org/10.1093/molbev/mst197
https://doi.org/10.1093/molbev/mst197...
). Bootstrap (BS) resampling (Felsenstein, 1985Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evol. 1985; 39(4):783-91. https://doi.org/10.1111/j.1558-5646.1985.tb00420.x
https://doi.org/10.1111/j.1558-5646.1985...
) was applied to assess support for individual nodes using 1,000 pseudoreplicates. Random starting trees were used for each independent ML tree search and all other parameters were set to default values. Bayesian inference (BI) (Huelsenbeck, Ronquist, 2001Huelsenbeck JP, Ronquist F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics. 2001; 17(8):754-55.) was performed in MrBayes v.3.2 (Ronquist, Huelsenbeck, 2003Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003; 19(12):1572-74.), based on the model (GTR+I). Two independent runs of 10 million steps sampling tree every 1000th generation a tree was sampled. Genetic variation within and among species groups under the best fit nucleotide model was also calculated in the MEGA v.6.06 software (Tamura et al., 2013Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol. 2013; 30(12):2725-29. https://doi.org/10.1093/molbev/mst197
https://doi.org/10.1093/molbev/mst197...
).
RESULTS
Genetic analysis. We used 18 specimens of Corumbataia representing a total of nine species and used Curculionichthys paresi(Roxo, Zawadzki & Troy, 2014), C. oliveirai (Roxo, Zawadzki & Troy, 2014), Hisonotus nigricauda (Boulenger, 1891), Rhinolekos britskii Martins, Langeani & Costa, 2011, Rhinolekos longicollum (Calegari & Reis, 2010), Pareiorhina rosai Silva, Roxo & Oyakawa, 2016, and Neoplecostomus bandeirante Roxo, Oliveira & Zawadzki, 2012 as related taxa and Hypostomus strigaticeps (Regan, 1908) to root the tree (Tab. 1). The combined sequence data resulted in a matrix with 580 bp. All sequences are deposited in GenBank (Tab. 1). The matrix does not contain any insertions, deletions or stop codons. The best nucleotide substitution model selected for the matrix was TN93+I+G (AICc = 6339.934). The nucleotide frequencies under TN93+I+G model were 0.23 (A), 0.27 (T), 0.30 (C), and 0.17 (G). Saturation was not observed, considering that the Iss < Iss.c for all NumOTU in the Xia et al. (2003)Xia X, Xie Z, Salemi M, Chen L, Wang Y. An index of substitution saturation and its application. Mol Phylogenet Evo. 2003; 26(1):1-7. https://doi.org/10.1016/S1055-7903(02)00326-3
https://doi.org/10.1016/S1055-7903(02)00...
and Xia, Lemey (2009)Xia X, Lemey P. Assessing substitution saturation with DAMBE. In: Lemey P, Salemi M, Vandamme AM, editors. The Phylogenetic Handbook: A practical approach to phylogenetic analysis and hypothesis testing. Cambridge: Cambridge University Press; 2009. p.615-30. https://doi.org/10.1017/CBO9780511819049.022
https://doi.org/10.1017/CBO9780511819049...
tests. Genetic distances in Corumbataia species and among each nominal species are shown in Tab. 2.
Specimens and species included in the molecular analysis. LBP = Laboratório de Biologia e Genética de Peixes, Universidade Estadual Paulista. The order of the species in the table follow the order of taxa in Fig. 1.
The gene tree represents a 50% majority-rule consensus obtained by maximum likelihood analysis (LogL = - 3132.01, Fig. 1). Bayesian analysis resulted in 10001 trees of which the first 2500 were discarded and the remaining 7501 were used to perform the consensus tree. In our analysis the C. canoeiro, C. veadeiros, and C. anosteos are the first groups to diverge, respectively. Corumbataia acanthodela, C. tocantinensis, C. cuestae, C. britskii, C. liliai, and C. lucianoi grouped in a clade herein named Corumbataia cuestae group. Corumbataia acanthodela is sister of the remainder members of Corumbataia-cuestae group. The phylogenetic analysis using the COI marker did not resolve the relationships among Corumbataia tocantinensis, C. cuestae, C. britskii, C. liliai, and C. lucianoi, which appeared as a polytomy.
Genetic distance and standard deviation among Corumbataia species based on the TN93+G+I model. Intraspecific genetic divergences are highlighted in diagonal bold numbers. Below the main diagonal is the value of interspecific genetic divergence. The values are shown as percentages.
A. Maximum likelihood - ML (LogL = -3132.01) tree of Corumbataia species using TN93+G+I nucleotide substitution model and based on the analysis of partial sequence of cytochrome oxidase C subunit I (COI). Numbers at nodes are bootstrap values based on 1000 pseudoreplicates. Values below 50% are not shown. B. Majority rule consensus tree obtained in Bayesian analysis using GTR+I. Numbers below branches are posterior probabilities obtained from 10000 trees.
Corumbataia acanthodela, new species
urn:lsid:zoobank.org:act:6690E445-1BCA-46AA-A212-A63D32E235E1
Corumbataia tocantinensisBristki, 1997Britski HA. Descrição de um novo gênero de Hypoptopomatinae , com duas espécies novas (Siluriformes, Loricariidae). Pap Avulsos de Zool. 1997; 40(15):231-55.:237 (original description; in part, paratypes MZUSP 51224, 51225, 50158; UFRJ 3000, 3001, 2999; Rio Maranhão, Rio Tocantins). —Carvalho, 2008Carvalho TP. A new species of Corumbataia (Siluriformes: Loricariidae: Hypoptopomatinae) from upper Rio Tocantins Basin, Central Brazil. Copeia. 2008; 3:552-57. https://doi.org/10.1643/CI-07-064
https://doi.org/10.1643/CI-07-064...
:554 (diagnosis, fig. 2A, MCN 13462).
Holotype. MZUSP 125794, 27.9 mm SL, male, Goiás, Niquelândia, Rio do Peixe, Rio Maranhão, Rio Tocantins basin, 14°30’39.3”S 48°41’10.0”W, 23 Nov 2012, B. F. Melo, J. H. Martinez, G. S. C. Silva & R. Devidé.
Paratypes. All from Brazil, Goiás: LBP 17153, 10, 28.9-16.8 mm SL, 2 c&s, 26.1-26.4 mm SL, collected with holotype. LBP 19095, 10, 27.3-16.1 mm SL, 2 c&s, 29.4-26.5 mm SL (tissues 77006-10), Goiás, Niquelândia, Rio Maranhão, Rio Tocantins basin, 14°38’16.9’’S 48°45’59.1” W, 5 Aug 2014, B. F. Melo, C. Oliveira, G. S. C. Silva & M. Taylor. LBP 25634, 1, 28.4 mm SL, Goiás, Mara Rosa, Rio Vaivém, Rio Maranhão, Rio Tocantins basin, 14º03’45.10”S 49º05’34.25”W, 25 Nov 2017, R. Devidé, B. F. Melo, C. Araya, G. S. C. Silva. NUP 22694, 3, 17.1-24.9 mm SL, Goiás, Niquelândia, Rio Maranhão, Rio Tocantins basin, 14°38’16.9’’S 48°45’59.1”W, 5 Aug 2014, B. F. Melo, C. Oliveira, G. S. C. Silva & M. Taylor.
Corumbataia acanthodela, holotype, MZUSP 125794, male 27.9 mm SL, from Rio Maranhão, Rio Tocantins basin, Niquelândia, Goiás, Brazil.
Diagnosis. The new species differs from all congeners, except for the members of Corumbataia cuestae group by having a conspicuous pair of tufts with enlarged odontodes on the tip of the supraoccipital (vs. absence of crest of enlarged odontodes in C. anosteos, C. canoeiro, and C. veadeiros); by the absence of an adipose fin or single series of platelets at adipose fin position (vs. presence of an adipose fin in C. canoeiro, or single series of platelets in C. anosteos and C. veadeiros); by anastomosis of the infraorbital and otic sensory canals over the pterotic-supracleithrum (Fig. 3A) (vs. anastomosis of infraorbital and otic sensory canals over the sphenotic in C. anosteos, C. canoeiro, and C. veadeiros) (Fig. 3B). Additionally, the new species differs from all species of Corumbataia cuestae group by having a small naked area on snout tip (vs. large naked area on snout tip, Fig. 4). Moreover, Corumbataia acanthodela differs from C. cuestae, C. liliai, C. lucianoi, and C. britskii by having abdominal platelets reaching the mid-ventral lateral plates (vs. abdominal platelets far from reaching mid-ventral lateral plates); from C. cuestae, C. liliai, and C. tocantinensis by having two rounded and more anteriorly positioned hyaline areas on the caudal-fin (Fig. 2) (vs. absence of two smaller rounded hyaline areas, see fig. 3 in Silva et al., 2018Silva GSC, Roxo FF, Souza CS, Oliveira C. Two new species of Corumbataia (Hypoptopomatinae: Loricariidae) from Rio Corrente, upper Rio Paraná basin, Brazil. Zootaxa. 2018; 4483(2):317-30. https://doi.org/10.11646/zootaxa.4483.2.5
https://doi.org/10.11646/zootaxa.4483.2....
). It differs from C. tocantinensis by having the anterior profile of the head rounded in dorsal view (vs. elliptical) and from C. lucianoi and C. liliai by the presence of plates on dorsal portion of snout (vs. presence of a broad naked area without plates or odontodes on dorsal portion of snout). It differs from C. britskii, C. lucianoi, and C. liliai by the presence of 28-29 vertebrae (vs. 27) and from C. britskii by having lower lip far from reaching pectoral girdle line (vs. reaching pectoral girdle transversal line).
Infraorbital series of Corumbataia acanthodela, LBP 19095, paratype (A), and C. anosteos, LBP 17125 (B). io1-io5 (Infraorbitals); sp (sphenotic); cpt (pterotic-supracleithrum). Scale bars = 1 mm.
Description. Morphometric and meristic data are summarized in Tab. 3. Small-sized loricariid (maximum 27.3 mm SL). Snout rounded in dorsal view. Dorsal profile of head ascending convexly approximately 45° to parieto-supraoccipital. Eyes relatively small (15.3-21.8% of HL), dorsolaterally positioned, just posterior to midpoint of head. Iris operculum present and poorly developed. Mouth moderate in size; oral disk ellipsoid with papillae randomly distributed. Lower lip larger than upper lip, not reaching cleithrum; its border fringed; lower lip inner surface covered with small papillae, similar in size. Maxillary barbel adnate to lower lip. Teeth slender with two cusps; central cusp larger than lateral cusp. Premaxillary teeth 29-42 (mode 37). Dentary teeth 22-37 (mode 32).
Frontal view of snout tip showing a naked area without odontodes. A. Corumbataia acanthodela, LBP 19095, paratype; B. Corumbataia cuestae, LBP 1309. Scale bars = 1 mm.
Morphometrics of the holotype and 27 paratypes of Corumbataia acanthodela. SD = standard deviation.
Lower surface of head naked. Head lacking ridges. Parieto-supraoccipital process elevated and with conspicuous pair of tufts of hypertrophied odontodes in specimens of all examined sizes. Predorsal region without ridges. Body elongate and compressed at caudal peduncle. Greatest body width at cleithral region, progressively narrowing anteriorly towards snout tip and posteriorly towards caudal-fin. Head and trunk covered by dermal plates, except for naked area around dorsal-fin insertion. Body dorsoventrally compressed. Dorsal profile convex from snout tip to posterior margin of parieto-supraoccipital and slightly concave from that point to dorsal-fin origin. Dorsal profile slightly concave and descending from dorsal-fin origin to first upper procurrent caudal-fin ray, rising posteriorly to insertion of caudal fin. Greatest body depth at unbranched dorsal-fin ray insertion. Cleithrum and coracoid exposed in ventral view, covered by odontodes. Arrector fossae completely enclosed by ventral lamina of coracoid.
Ventral profile straight and descending from snout tip to opercular region, slightly convex from opercular region to anal-fin origin and slightly concave from that point to lower procurrent caudal-fin ray origin. Lateral surface of body entirely covered by plates; dorsal series with 23-24 plates. Mid-dorsal plate series truncated with 18-19 plates not reaching end of caudal peduncle. Lateral plate series with 22-23 plates. Lateral line incomplete, with gaps in line of pores along mid-length of body. Mid-ventral series of lateral plates well-developed, reaching middle of caudal peduncle (17-19 plates). Ventral plates series with 20-21 plates. Body plates covered with minute odontodes.
Dorsal fin ii,7; its origin slightly posterior to vertical with pelvic-fin origin. Unbranched rays of dorsal fin slightly convex. Tip of adpressed dorsal-fin rays surpassing anal-fin origin. Dorsal-fin spinelet small and rounded (4 c&s). Pectoral-fin rays i,6; tip of adpressed pectoral fin surpassing pelvic-fin origin. Pectoral-fin axillary slit small. Pelvic-fin rays i,5. Distal margin of pelvic fin straight to slightly convex; tip of adpressed pelvic-fin ray reaching anal-fin origin in mature males, but not in females. Adipose fin absent. Anal-fin rays i,5; distal margin slightly convex. Caudal-fin rays i,7-i,7. Slightly emarginated; unbranched rays of same size. Rays of all fins covered with pointed odontodes. Lowest body depth at caudal peduncle. Caudal peduncle ellipsoid in cross section, rounded dorsally and ventrally. Hypurals elements fused in a unique hypural plate. Total vertebrae 28 or 29 (4 c&s).
Color in alcohol. Background color of dorsal region of head and trunk brown. Four dark brown saddles along dorsal portion of body: first at dorsal-fin origin, second at end of dorsal-fin base, third at middle of caudal peduncle, and fourth reaching anteriormost caudal procurrent ray. Unpigmented portion of snout appears as two parallel hyaline stripes from rostral plate to nares. Mid-lateral dark brown stripe extending from tip of snout to caudal peduncle. Ventral portion of body almost entirely yellowish, except for dark, randomly-distributed chromatophores and for a concentration of chromatophores at anal-fin origin. Dorsal, pectoral, and pelvic fins with dark, irregularly distributed chromatophores. Caudal-fin brown, with two smaller rounded hyaline areas anteriorly, and two large rounded hyaline areas posteriorly (Fig. 1).
Sexual dimorphism. Adult males possess a papilla located posterior to the urogenital opening; an unbranched pelvic-fin ray that supports dermal flap along its dorsal surface; and a wider head (84.5-88.6% vs. 80.6-85.3 of HL in female) with hypertrophied odontodes on its lateral margin (Fig. 5).
Hypertrophied odontodes on the lateral margins of head in Corumbataia acanthodela, paratypes, male (left), NUP 22694 and female (right), LBP 19095. Scale bars = 1 mm.
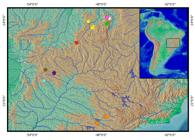
Geographical distribution.Corumbataia acanthodela is known from Rio dos Patos, Rio do Peixe, and Rio Vaivém, all tributaries of the Rio Maranhão, in the headwaters of the Rio Tocantins basin, Goiás, Central Brazil (Fig. 6). The new species was found in clear water of fast-flowing stream with rocks and marginal vegetation.
Distribution of Corumbataia species. Yellow circles: C. acanthodela, Rio Maranhão; Green circle: C. anosteos, Rio Piçarras; Blue circle: C. britskii, Rio Sucuriu; White circle: C. canoeiro, Rio Paranã; Orange circles: C. cuestae, Rio Tietê; Purple circle: C. liliai, Rio Correntes; Brown circles: C. lucianoi, Rio Correntes; Red circle: C. tocantinensis, Rio Vermelho; Pink circle: C. veadeiros, Rio Paranã.
Etymology. The specific epithet acanthodela is from Greek akantha meaning thorn, and delos meaning conspicuous, in reference to the hypertrophied odontodes in the head of mature males. An adjective.
Conservation status.Corumbataia acanthodela is known from three localities in the Rio Maranhão basin. The areas where the specimens were collected are mountainous and relatively well preserved, which do not qualify it for threatened status. Therefore,C. acanthodela is here recommended to be categorized as Least Concern (LC) under the categories and criteria of the International Union for Conservation Nature (IUCN Standards and Petitions Subcommittee, 2019IUCN Standards and Petitions Committee. Guidelines for Using the IUCN Red List Categories and Criteria. Version 14. Prepared by the Standards and Petitions Committee. 2019.Available from http://www.iucnredlist.org/documents/RedListGuidelines.pdf
http://www.iucnredlist.org/documents/Red...
).
DISCUSSION
Britski (1997)Britski HA. Descrição de um novo gênero de Hypoptopomatinae , com duas espécies novas (Siluriformes, Loricariidae). Pap Avulsos de Zool. 1997; 40(15):231-55. described Corumbataia tocantinensis based on material from Rio Vermelho, tributary of the Rio Araguaia (locality of holotype), and from the Rio Maranhão, a tributary of the Rio Tocantins. Here, we found molecular and morphological evidence that the samples from Rio Maranhão (described here as C. acanthodela) form a distinct lineage from C. tocantinensis. Our mitochondrial data analysis showed a high value of genetic divergence between these two lineages (6.0%, Tab 2). Recent DNA barcode studies in loricariids have shown lower values than 6.0%, for valid and morphologically-distinct species [i.e., 2.0% between Neoplecostomus microps (Steindachner, 1877) and N. paraty Cherobim, Lazzarotto, Langeani & 2017; 1.0% between N. botucatu Roxo, Oliveira & Zawadzki, 2012, and N. selenae Zawadzki, Pavanelli & Langeani, 2008; 1.8% between Paralithoxus jariensis (Silva, Covain, Oliveira & Roxo, 2017) and P. raso (Silva, Covain, Oliveira & Roxo, 2017) (Silva et al., 2017Silva GSC, Covain R, Oliveira C, Roxo FF. Description of two new species of Lithoxus (Hypostominae: Loricariidae) from Rio Jari and rio Amapá basin, Brazilian Guiana Shield. Zootaxa. 2017; 4347(1):151-68. http://dx.doi.org/10.11646/zootaxa.4347.1.9
http://dx.doi.org/10.11646/zootaxa.4347....
); 3.0% between Curculionichthys oliveirai and C. insperatus (Britski, Garavello, 2003Britski HA, Garavello, JC. Hisonotus insperatus: new species, from the upper rio Paraná basin (Pisces: Ostariophysi: Loricariidae). Copeia. 2003(3): 588-93. https://doi.org/10.1643/CI-02-23R
https://doi.org/10.1643/CI-02-23R...
) (Silva et al., 2014Silva GSC, Roxo FF, Oliveira C. Hisonotus acuen, a new and phenotypically variable cascudinho (Siluriformes, Loricariidae, Hypoptopomatinae) from the upper rio Xingu basin, Brazil. Zookeys. 2014; 442:105-25. https://doi.org/10.3897/zookeys.442.7870
https://doi.org/10.3897/zookeys.442.7870...
)].
On the other hand, some well-diagnosed species (Corumbataia britskii, C. cuestae, C. liliai, and C. lucianoi) showed low inter-specific pairwise divergence with their closest relatives (Tab. 1), suggesting recent divergence among these species. Curiously, C. tocantinensis showed a high intraspecific divergence (4.1%), although no morphological differences have been found between these two specimens represented in the tree (vouchers: 11827 and 11477; Tab. 1). This unexpected value of intraspecific divergence may be explained by the high number of mutations (autapomorphies) found in the sequence 11477 (Genbank: KM104484.1), probably caused by error in sequencing or manual editing.
Roxo et al. (2019)Roxo FF, Ochoa LE, Sabaj MH, Lujan NK, Covain R, Silva GSC, Melo BF, Albert JS, Chang J, Foresti F, Alfaro ME, Oliveira C. Phylogenomic reappraisal of the Neotropical catfish family Loricariidae (Teleostei: Siluriformes) using ultraconserved elements. Mol Phylogenet Evo. 2019; 135:148-65. https://doi.org/10.1016/j.ympev.2019.02.017
https://doi.org/10.1016/j.ympev.2019.02....
recovered a monophyletic Corumbataia including Gymnotocinclus (now a junior synonym of Corumbataia). In their phylogeny, the first lineage to diverge was C. canoeiro, followed by C. veadeiros and the clade formed by C. anosteos plus C. cuestae. Complementary to Roxo et al.’s study, we found a well supported clade (see Fig. 1, BS = 100 and PP = 1), named here as the “Corumbataia cuestae group”, and composed by C. acanthodela, C. britskii, C. cuestae, C. liliai, C. lucianoi, and C. tocantinensis. These six species share three putative morphological apomorphies:
(1) Conspicuous crest of hypertrophied odontodes on head (see fig. 2 in Carvalho, 2008Carvalho TP. A new species of Corumbataia (Siluriformes: Loricariidae: Hypoptopomatinae) from upper Rio Tocantins Basin, Central Brazil. Copeia. 2008; 3:552-57. https://doi.org/10.1643/CI-07-064
https://doi.org/10.1643/CI-07-064...
). The conspicuous crest on head is absent in the majority of members of Hypoptopomatinae, such as in Neoplecostomini, Otocinclus clade, Hypoptopomatini, Rhinolekos subclade, Microlepidogaster subclade and in all others species of Corumbataia clade (Microplecostomus forestii Silva, Roxo, Ochoa & Oliveira, 2016, Nannoplecostomus eleonorae Ribeiro, Lima & Pereira, 2012, Curculionichthys Roxo, Silva, Ochoa & Oliveira, 2015, species, and C. canoeiro, C. veadeiros, and C. anosteos). In contrast, we found the derived condition, a conspicuous crest of hypertrophied odontodes in the members of Corumbataia cuestae group and in some members of the Hisonotini and Otothyrini (Carvalho, Reis, 2009Carvalho TP, Reis RE. Four new species of Hisonotus (Siluriformes: Loricariidae: Hypoptopomatinae) from the Upper Rio Uruguay, southerastern South America, with a review of the genus in the Rio Uruguay basin. Zootaxa. 2009; 2113(1):1-40. https://doi.org/10.11646/zootaxa.2113.1.1
https://doi.org/10.11646/zootaxa.2113.1....
; Sarmento-Soares et al., 2009Sarmento-Soares L, Lehmann A, Martins-Pinheiro RF. Parotocinclus arandai, a new species of Hypoptopomatinae catfish (Siluriformes: Loricariidae) from the upper rios Jucuruçu and Buranhém, States of Bahia and Minas Gerais. Brazil. Neotrop Ichthyol. 2009; 7(2):191-98. https://doi.org/10.1590/S1679-62252009000200009
https://doi.org/10.1590/S1679-6225200900...
; Roxo et al., 2012Roxo FF, Oliveira C, Zawadzki CH. Three new species of Neoplecostomus (Teleostei: Siluriformes: Loricariidae) from the Upper Rio Paraná of southeastern Brazil. Zootaxa. 2012; 3233(1):1-21.).
(2) Absence of an adipose fin or platelets at the adipose-fin region. The plesiomorphic species Corumbataia canoeiro possesses a developed adipose fin, whereas in C. veadeiros and C. anosteos, the next lineages to diverge in Corumbataia, there is a single series of platelets at the adipose-fin position. This suggests that the adipose fin, present in C. canoeiro, was reduced in C. veadeiros and C. anosteos and totally lost in the species of the Corumbataia-cuestae group. The primitive condition in Corumbataia clade is the presence of adipose fin or platelets at adipose-fin region, present in the first lineages to diverge in the Corumbataia clade: Nannoplecostomus eleonorae and Microplecostomus foresti (Roxo et al., 2019Roxo FF, Ochoa LE, Sabaj MH, Lujan NK, Covain R, Silva GSC, Melo BF, Albert JS, Chang J, Foresti F, Alfaro ME, Oliveira C. Phylogenomic reappraisal of the Neotropical catfish family Loricariidae (Teleostei: Siluriformes) using ultraconserved elements. Mol Phylogenet Evo. 2019; 135:148-65. https://doi.org/10.1016/j.ympev.2019.02.017
https://doi.org/10.1016/j.ympev.2019.02....
). Curculionichthys, the sister to Corumbataia, exhibits the derived condition (absence of platelets or adipose fin), suggesting that this derived condition occurs homoplastically in Curculionichthys and in the species of Corumbataia cuestae group.
(3) Anastomosis of the Infraorbital and otic canals over the pterotic-supracleithrum (Fig. 3a). This derived condition was proposed by Britski (1997)Britski HA. Descrição de um novo gênero de Hypoptopomatinae , com duas espécies novas (Siluriformes, Loricariidae). Pap Avulsos de Zool. 1997; 40(15):231-55. to Corumbataia cuestae and C. tocantinensis, and here was also observed in all members of Corumbatia cuestae group. The plesiomorphic condition is the anostomoids of the infraorbital and otic canals over the sphenotic (See fig. 3A in Ribeiro et al., 2012Ribeiro AC, Lima FCT, Pereira EHL. A new genus and species of a minute suckermouth armored catfish (Siluriformes: Loricariidae) from the Rio Tocantins drainage, Central Brazil: The smallest known Loricariidae catfish. Copeia. 2012; 2012(4):637-47. https://doi.org/10.1643/CI-11-137
https://doi.org/10.1643/CI-11-137...
), and is found in C. canoeiro, C. veadeiros, and C. anosteos, and in the other members of Corumbataia clade.
Comparative material examined. Brazil:Corumbataia anosteos: LBP 17125, 3,18.8-33.0 mm SL, 1 c&s. Corumbataia britskii: LBP 9590, 48, 28.4-15.7 mm SL; 2 c&s, 24.5-26.8 mm SL. Corumbataia canoeiro: LBP 19469, 46, 28.0-54.3 mm SL. Corumbataia cuestae: LBP 8114, 19, 32.7-23.8 mm SL, 3 c&s, 22.7-29.4 mm SL; LBP 1309 (60, 28.9-19.4 mm SL). Corumbataia liliai: MZUSP 123826, holotype, 24.7 mm SL; LBP 9577, paratypes, 2, 18.4-23.2 mm SL; LBP 25544, paratypes, 2, 18.2-24.7 mm SL. Corumbataia lucianoi: MZUSP 123824, 24.0 mm SL; LBP 9570, paratypes 15, 12.8-25.5 mm SL. Corumbataia tocantinensis: LBP 1653, 27, 31.9-17.8 mm SL; LBP 1972, 10, 28.9-21.5, 1 c&s, 26.2 mm SL; MZUSP 50158, paratypes 2, 29.8-28.1 mm SL. Corumbataia veadeiros: LBP 19311, 2, 29.4-27.4 mm SL; LBP 19302, 3, 28.5-33.9 mm SL.
ACKNOWLEDGEMENTS
We thank Bruno F. Melo, Renato Devidé, Jefferson H. Martinez, and Martin I. Taylor for their help during field expeditions. We are also grateful to Lais Reia for helping with sequencing and figure preparation. Maxwell J. Bernt significantly contributed with comments and revision of the English on drafts of the manuscript. This research received financial support from Programa Institucional de Bolsas de Iniciação Científica e Tecnológica Unesp (MGRT), a PNPD Capes grant (GSCS), and from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq proc. 307975/2019-3; RCB). Additional support for this project came from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP grants 2018/20610-1, 2016/09204-6, 2014/26508-3) and CNPq (proc. 306054/2006-0; CO).
REFERENCES
- Britski HA. Descrição de um novo gênero de Hypoptopomatinae , com duas espécies novas (Siluriformes, Loricariidae). Pap Avulsos de Zool. 1997; 40(15):231-55.
- Britski HA, Garavello, JC. Hisonotus insperatus: new species, from the upper rio Paraná basin (Pisces: Ostariophysi: Loricariidae). Copeia. 2003(3): 588-93. https://doi.org/10.1643/CI-02-23R
» https://doi.org/10.1643/CI-02-23R - Carvalho TP. A new species of Corumbataia (Siluriformes: Loricariidae: Hypoptopomatinae) from upper Rio Tocantins Basin, Central Brazil. Copeia. 2008; 3:552-57. https://doi.org/10.1643/CI-07-064
» https://doi.org/10.1643/CI-07-064 - Carvalho TP, Reis RE. Four new species of Hisonotus (Siluriformes: Loricariidae: Hypoptopomatinae) from the Upper Rio Uruguay, southerastern South America, with a review of the genus in the Rio Uruguay basin. Zootaxa. 2009; 2113(1):1-40. https://doi.org/10.11646/zootaxa.2113.1.1
» https://doi.org/10.11646/zootaxa.2113.1.1 - Edgar RC. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucl Acids Res. 2004; 32(5):1792.
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evol. 1985; 39(4):783-91. https://doi.org/10.1111/j.1558-5646.1985.tb00420.x
» https://doi.org/10.1111/j.1558-5646.1985.tb00420.x - Fricke R, Eschemeyer W, Fong JD. Species by Family/Subfamily [Internet]. San Francisco: California Academy of Science; 2020. Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/SpeciesByFamily.asp
» http://researcharchive.calacademy.org/research/ichthyology/catalog/SpeciesByFamily.asp - ICZN (International Commission on Zoological Nomenclature). International code of zoological nomenclature. Fourth edition. London: International Trust for Zoological Nomenclature; 1999.
- Garg KG, Domingos FXV, Almeida-Val VMF, Val AL. Histochemistry and functional organization of the dorsal skin of Ancistrus dolichopterus (Siluriformes: Loricariidae). Neotrop Ichthyol. 2010; 8(4):877-84. http://dx.doi.org/10.1590/S1679-62252010000400018
» http://dx.doi.org/10.1590/S1679-62252010000400018 - IUCN Standards and Petitions Committee. Guidelines for Using the IUCN Red List Categories and Criteria. Version 14. Prepared by the Standards and Petitions Committee. 2019.Available from http://www.iucnredlist.org/documents/RedListGuidelines.pdf
» http://www.iucnredlist.org/documents/RedListGuidelines.pdf - Greerinckx T, Brunain M, Herrel A, Aerts P, Adrianes DA. Head with a suckermouth: A function-morphological study of the head with a suckermouth catfish Ancistrus cf. triradiatus (Loricariidae, Siluriformes). Belg J Zool. 2007; 137(1):47-66.
- Huelsenbeck JP, Ronquist F. MrBayes: Bayesian inference of phylogenetic trees. Bioinformatics. 2001; 17(8):754-55.
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012; 28(12):1647-49. https://doi.org/10.1093/bioinformatics/bts199
» https://doi.org/10.1093/bioinformatics/bts199 - Ribeiro AC, Lima FCT, Pereira EHL. A new genus and species of a minute suckermouth armored catfish (Siluriformes: Loricariidae) from the Rio Tocantins drainage, Central Brazil: The smallest known Loricariidae catfish. Copeia. 2012; 2012(4):637-47. https://doi.org/10.1643/CI-11-137
» https://doi.org/10.1643/CI-11-137 - Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003; 19(12):1572-74.
- Roxo FF, Albert JS, Silva GSC, Zawadzki CH, Foresti F, Oliveira C. Molecular phylogeny and biogeography history of the armored Neotropical catfish subfamilies Hypoptopomatinae, Neoplecostominae and Otothyrinae (Siluriformes: Loricariidae). PLoS ONE. 2014; 9(8):e105564. https://doi.org/10.1371/journal.pone.0105564
» https://doi.org/10.1371/journal.pone.0105564 - Roxo FF, Ochoa LE, Sabaj MH, Lujan NK, Covain R, Silva GSC, Melo BF, Albert JS, Chang J, Foresti F, Alfaro ME, Oliveira C. Phylogenomic reappraisal of the Neotropical catfish family Loricariidae (Teleostei: Siluriformes) using ultraconserved elements. Mol Phylogenet Evo. 2019; 135:148-65. https://doi.org/10.1016/j.ympev.2019.02.017
» https://doi.org/10.1016/j.ympev.2019.02.017 - Roxo FF, Oliveira C, Zawadzki CH. Three new species of Neoplecostomus (Teleostei: Siluriformes: Loricariidae) from the Upper Rio Paraná of southeastern Brazil. Zootaxa. 2012; 3233(1):1-21.
- Roxo FF, Silva GSC, Ochoa LE, Zawadzki CH. Description of a new species of Gymnotocinclus from the rio Tocantins basin with phylogenetic analysis of the subfamily Hypoptopomatinae (Siluriformes: Loricariidae). Zootaxa. 2017; 4268(3):337-59. http://dx.doi.org/10.11646/zootaxa.4268.3.2
» http://dx.doi.org/10.11646/zootaxa.4268.3.2 - Sabaj MH. Standard symbolic codes for institutional resource collections in herpetology and ichthyology: an online reference. Version 7.1. Washington, DC: American Society of Ichthyologists and Herpetologists; 2019. Available from: http://www.ashi.org/
» http://www.ashi.org/.» http://www.ashi.org/ - Sarmento-Soares L, Lehmann A, Martins-Pinheiro RF. Parotocinclus arandai, a new species of Hypoptopomatinae catfish (Siluriformes: Loricariidae) from the upper rios Jucuruçu and Buranhém, States of Bahia and Minas Gerais. Brazil. Neotrop Ichthyol. 2009; 7(2):191-98. https://doi.org/10.1590/S1679-62252009000200009
» https://doi.org/10.1590/S1679-62252009000200009 - Schaefer SA. The Neotropical cascudinhos: Systematic and biogeography of the Otocinclus catfishes (Siluriformes: Loricariidae). Proc Acad Nat Sci Phila. 1997; 148:1-120.
- Silva GSC, Covain R, Oliveira C, Roxo FF. Description of two new species of Lithoxus (Hypostominae: Loricariidae) from Rio Jari and rio Amapá basin, Brazilian Guiana Shield. Zootaxa. 2017; 4347(1):151-68. http://dx.doi.org/10.11646/zootaxa.4347.1.9
» http://dx.doi.org/10.11646/zootaxa.4347.1.9 - Silva GSC, Roxo FF, Oliveira C. Hisonotus acuen, a new and phenotypically variable cascudinho (Siluriformes, Loricariidae, Hypoptopomatinae) from the upper rio Xingu basin, Brazil. Zookeys. 2014; 442:105-25. https://doi.org/10.3897/zookeys.442.7870
» https://doi.org/10.3897/zookeys.442.7870 - Silva GSC, Roxo FF, Souza CS, Oliveira C. Two new species of Corumbataia (Hypoptopomatinae: Loricariidae) from Rio Corrente, upper Rio Paraná basin, Brazil. Zootaxa. 2018; 4483(2):317-30. https://doi.org/10.11646/zootaxa.4483.2.5
» https://doi.org/10.11646/zootaxa.4483.2.5 - Taylor WR, Van Dyke GC. Revised procedures for staining and clearing small and other vertebrates for bones and cartilage study. Cybium. 1985; 9(2):107-19.
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol Biol Evol. 2013; 30(12):2725-29. https://doi.org/10.1093/molbev/mst197
» https://doi.org/10.1093/molbev/mst197 - Xia X. DAMBE5: a comprehensive software package for data analysis in molecular biology and evolution. Mol Biol and Evol. 2013; 30(7):1720-28. https://doi.org/10.1093/molbev/mst064
» https://doi.org/10.1093/molbev/mst064 - Xia X, Lemey P. Assessing substitution saturation with DAMBE. In: Lemey P, Salemi M, Vandamme AM, editors. The Phylogenetic Handbook: A practical approach to phylogenetic analysis and hypothesis testing. Cambridge: Cambridge University Press; 2009. p.615-30. https://doi.org/10.1017/CBO9780511819049.022
» https://doi.org/10.1017/CBO9780511819049.022 - Xia X, Xie Z, Salemi M, Chen L, Wang Y. An index of substitution saturation and its application. Mol Phylogenet Evo. 2003; 26(1):1-7. https://doi.org/10.1016/S1055-7903(02)00326-3
» https://doi.org/10.1016/S1055-7903(02)00326-3
ADDITIONAL NOTES
-
HOW TO CITE THIS ARTICLE
Thimotheo MGR, Benine RC, Oliveira C, Silva GSC. New species of the Corumbataia cuestae group (Siluriformes: Loricariidae) from the Rio Tocantins basin, with comments on its phylogenetic relationships. Neotrop Ichthyol. 2020; 18(4):e200060.https://doi.org/10.1590/1982-0224-2020-0060
Edited by
Publication Dates
-
Publication in this collection
04 Dec 2020 -
Date of issue
2020
History
-
Received
8 July 2020 -
Accepted
9 Nov 2020