ABSTRACT
Parodontidae is a relatively small group of Neotropical characiform fishes consisting of three genera (Apareiodon, Parodon, and Saccodon) with 32 valid species. A vast cytogenetic literature is available on Apareiodon and Parodon, but to date, there is no cytogenetic data about Saccodon, a genus that contains only three species with a trans-Andean distribution. In the present study the karyotype of S. wagneri was described, based on both conventional (Giemsa staining, Ag-NOR, C-bands) and molecular (repetitive DNA mapping by fluorescent in situ hybridization) methods. A diploid chromosome number of 2n = 54 was observed in both sexes, and the presence of heteromorphic sex chromosomes of the ZZ/ZW type was detected. The W chromosome has a terminal heterochromatin band that occupies approximately half of the long arm, being this band approximately half the size of the Z chromosome. The FISH assay showed a synteny of the 18S-rDNA and 5S-rDNA genes in the chromosome pair 14, and the absence of interstitial telomeric sites. Our data reinforce the hypothesis of a conservative karyotype structure in Parodontidae and suggest an ancient origin of the sex chromosomes in the fishes of this family.
Keywords:
Ag-NOR; Cytogenetics; FISH; Heterochromatin; ZW sex chromosomes
RESUMO
Parodontidae é um grupo relativamente pequeno de peixes caraciformes neotropicais que consiste em três gêneros (Apareiodon, Parodon e Saccodon) com 32 espécies válidas. Uma vasta literatura citogenética está disponível sobre Apareiodon e Parodon, mas até o momento não há dados citogenéticos sobre Saccodon, um gênero que contém apenas três espécies com distribuição transandina. No presente estudo foi descrito o cariótipo de S. wagneri, baseado em métodos convencionais (coloração de Giemsa, Ag-NOR, bandas C) e moleculares (mapeamento de DNA repetitivo por hibridização fluorescente in situ). Um número cromossômico diplóide de 2n = 54 foi observado, e a presença de cromossomos sexuais heteromórficos do tipo ZZ/ZW foi revelada. O cromossomo W possui uma banda terminal heterocromática que ocupa aproximadamente metade do braço longo, sendo esta banda aproximadamente a metade do tamanho do cromossomo Z. O ensaio FISH mostrou uma sintenia dos genes 18S-rDNA e 5S-rDNA no par de cromossomos 14, e a ausência de sítios teloméricos intersticiais. Nossos dados reforçam a hipótese de uma estrutura cariotípica conservadora em Parodontidae e sugerem uma origem ancestral dos cromossomos sexuais nos peixes desta família.
Palavras-chave:
Ag-RON; Citogenética; Cromossomos sexuais ZW; FISH; Heterocromatina
INTRODUCTION
The Neotropical region has the largest repository of freshwater fish species that correspond to about 16% of the world’s fish biodiversity (Albert, Reis, 2011Albert JS, Reis RE, editors. Historical biogeography of Neotropical freshwater fishes. University of California Press; 2011.; Reis et al., 2016Reis RE, Albert JS, Dario FD, Mincarone MM, Petry P, Rocha LA. Fish biodiversity and conservation in South America. J Fish Biol . 2016; 89(1):12-47. https://doi.org/10.1111/jfb.13016
https://doi.org/10.1111/jfb.13016...
). This biodiversity has enormous ecological relevance and economic importance, as many of these species represent a fishery and aquaculture resource (Hilsdorf, Hallerman, 2017Hilsdorf AWS, Hallerman EM. Genetic resources of Neotropical fishes: Springer, Cham; 2017.). One of the most represented fish groups present in the hydrographic basins of this geographic region is Characiformes. This order includes exclusively freshwater fishes distributed in both Africa and America and shows its greatest diversity in the Neotropical Region (Malabarba, 1998Malabarba LR, Reis RE, Vari RP, Lucena ZMS, Lucena CAS. Phylogeny and Classification of Neotropical Fishes. Porto Alegre: EDIPUCRS; 1998.; Nelson et al., 2016Nelson JS, Grande TC, Wilson MVH. Fishes of the world. Hoboken, New Jersey: John Wiley & Sons; 2016.). Characiformes comprises 2,081 valid species grouped into 23 families, mostly in Characidae (1,214 species) (Fricke et al., 2020aFricke R, Eschmeyer WN, Fong JD. Eschmeyer’s catalog of fishes: species by family/subfamily. San Francisco: California Academy of Sciences; 2020a. Available from: http://researcharchive.calacademy.org/research/ ichthyology/catalog/SpeciesByFamily.asp
http://researcharchive.calacademy.org/re...
). Parodontidae is a relatively small family distributed throughout South America and part of Panama (Nelson et al., 2016), and includes 32 species (Fricke et al., 2020aFricke R, Eschmeyer WN, Fong JD. Eschmeyer’s catalog of fishes: species by family/subfamily. San Francisco: California Academy of Sciences; 2020a. Available from: http://researcharchive.calacademy.org/research/ ichthyology/catalog/SpeciesByFamily.asp
http://researcharchive.calacademy.org/re...
) organized in three genera: Apareiodon Eigenmann, 1916 (N = 15), Parodon Valenciennes, 1850 (N = 14) and Saccodon Kner, 1863 (N = 3) that differ due to some subtle morphological characters (Pavanelli, 2003Pavanelli CS. Family Parodontidae (Parodontids). In: Reis RE, Kullander SO, Ferraris JC, editors. Check List of the Freshwater Fishes of South and Central America. Porto Alegre: Edipucrs; 2003. p.46-50.).
Cytogenetic studies in Parodontidae cover about 50% of recognized valid species (Tab. 1), representing only two genera: Apareiodon and Parodon. Although the available data show that these fishes have a conserved diploid number (2n) of 54 chromosomes, differences in the number of chromosome arms (FN) and extensive variation in the position of 18S and 5S rDNA sites exist. Besides this, species with proto sex chromosomes are found together with others characterized by ZZ/ZW and ZZ/ZW1W2 multiple sex chromosome systems (Tab. 1). Sex chromosomes show different sizes among the Parodontidae species (Moreira-Filho et al., 1993Moreira-Filho O, Bertollo LAC, Galetti PM. Distribution of Sex chromosome mechanisms in Neotropical fish and description of a ZZ/ZW system in Parodon hilarii (Parodontidae). Caryologia . 1993; 46(2-3):115-25. https://doi.org/10.1080/00087114.1993.10797253
https://doi.org/10.1080/00087114.1993.10...
; Rosa et al., 2006Rosa R, Bellafronte E, Moreira Filho O, Margarido VP. Constitutive heterochromatin, 5S and 18S rDNA genes in Apareiodon sp. (Characiformes, Parodontidae) with a ZZ/ZW sex chromosome system. Genetica . 2006; 128(1-3):159-66. https://doi.org/10.1007/s10709-005-5700-1
https://doi.org/10.1007/s10709-005-5700-...
; Vicari et al., 2006Vicari MR, Moreira-Filho O, Artoni RF, Bertollo LAC. ZZ/ZW sex chromosome system in an undescribed species of the genus Apareiodon (Characiformes, Parodontidae). Cytogenet Genome Res . 2006; 114(2):163-68. https://doi.org/10.1159/000093333
https://doi.org/10.1159/000093333...
; Bellafronte et al., 2009Bellafronte E, Vicari MR, Artoni RF, Margarido VP, Moreira-Filho O. Differentiated ZZ/ZW sex chromosomes in Apareiodon ibitiensis (Teleostei, Parodontidae): cytotaxonomy and biogeography. J Fish Biol. 2009; 75(9): 2313-25. https://doi.org/10.1111/j.1095-8649.2009.02488.x
https://doi.org/10.1111/j.1095-8649.2009...
), but in all the ZW species, the W chromosome is a subtelocentric chromosome almost entirely heterochromatic, whereas the Z is smaller and usually shows heterochromatic regions only in the distal segmental portion of its short arms. The use of satellite DNA and transposable elements as probes showed that the differentiation of the sex chromosomes in the family is associated with the accumulation of these repeated sequences (Bellafronte et al., 2011Bellafronte E, Schemberger MO, Moreira-Filho O, Almeida MC et al . Chromosomal markers in Parodontidae: an analysis of new and reviewed data with phylogenetic inferences. Rev Fish Biol Fish. 2011; 21(3):559-70. https://doi.org/10.1007/s11160-010-9177-3
https://doi.org/10.1007/s11160-010-9177-...
; Schemberger et al., 2011Schemberger MO, Bellafronte E, Nogaroto V, Schühli GS, Artoni RF et al. Differentiation of repetitive DNA sites and sex chromosome systems reveal closely related group in Parodontidae (Actinopterygii: Characiformes). Genetica . 2011; 139(11-12):1499-508. https://doi.org/10.1007/s10709-012-9649-6
https://doi.org/10.1007/s10709-012-9649-...
, 2016Schemberger MO, Nogaroto V, Almeida MC, Artoni RF, Valente GT, Martins C et al . Sequence analyses and chromosomal distribution of the Tc1/Mariner element in Parodontidae Fish (Teleostei: Characiformes). Gene. 2016; 593(2):308-14. https://doi.org/10.1016/j.gene.2016.08.034
https://doi.org/10.1016/j.gene.2016.08.0...
; Nascimento et al., 2018Nascimento VD, Almeida Coelho K, Nogaroto V, Almeida RB, Ziemniczak K, Centofante L, Pavanelli CS, Torres RA, Moreira-Filho O, Vicari MR. Do multiple karyomorphs and population genetics of freshwater darter characines (Apareiodon affinis) indicate chromosomal speciation? Zoologischer Anzeiger, 2018; 272:93-103. https://doi.org/10.1016/j.jcz.2017.12.006
https://doi.org/10.1016/j.jcz.2017.12.00...
).
| Cytogenetic characteristics in Parodontidae. 1. Moreira Filho et al., 1980Moreira Filho O, Bertollo LAC, Galetti PM. Evidences for a multiple sex chromosome system with female heterogamety in Apareiodon affinis (Pisces, Parodontidae). Caryologia . 1980; 33(1):83-91. https://doi.org/10.1080/00087114.1980.10796821
https://doi.org/10.1080/00087114.1980.10... ; 2. Moreira Filho et al., 1985Moreira Filho O, Bertollo LAC, Galetti PM. Karyotypic study of some species of family Parodontidae (Pisces - Cypriniformes). Caryologia . 1985; 38(1):47-55. https://doi.org/10.1080/00087114.1985.10797729
https://doi.org/10.1080/00087114.1985.10... ; 3. Jesus et al., 1999Jesus CM, Bertollo LAC, Moreira-Filho O. Comparative cytogenetics in Apareiodon affinis (Pisces, Characiformes) and considerations regarding diversification of the group. Genetica 1999; 105:63-67. https://doi.org/10.1023/A:1003592022927
https://doi.org/10.1023/A:1003592022927... ; 4. Jorge, Moreira-Filho, 2000Jorge LC, Moreira-Filho O. Cytogenetic studies on Apareiodon affinis (Pisces, Characiformes) from Paraná river basin: sex chromosomes and polymorphism. Genetica. 2000;109(3):267-73. http://dx.doi.org/10.1023/a:1017522914023
http://dx.doi.org/10.1023/a:101752291402... ; 5. Bellafronte et al., 2009Bellafronte E, Vicari MR, Artoni RF, Margarido VP, Moreira-Filho O. Differentiated ZZ/ZW sex chromosomes in Apareiodon ibitiensis (Teleostei, Parodontidae): cytotaxonomy and biogeography. J Fish Biol. 2009; 75(9): 2313-25. https://doi.org/10.1111/j.1095-8649.2009.02488.x
https://doi.org/10.1111/j.1095-8649.2009... ; 6. Bellafronte et al., 2011Bellafronte E, Schemberger MO, Moreira-Filho O, Almeida MC et al . Chromosomal markers in Parodontidae: an analysis of new and reviewed data with phylogenetic inferences. Rev Fish Biol Fish. 2011; 21(3):559-70. https://doi.org/10.1007/s11160-010-9177-3
https://doi.org/10.1007/s11160-010-9177-... ; 7. Schemberger et al., 2011Schemberger MO, Bellafronte E, Nogaroto V, Schühli GS, Artoni RF et al. Differentiation of repetitive DNA sites and sex chromosome systems reveal closely related group in Parodontidae (Actinopterygii: Characiformes). Genetica . 2011; 139(11-12):1499-508. https://doi.org/10.1007/s10709-012-9649-6
https://doi.org/10.1007/s10709-012-9649-... ; 8. Leite, Maistro, 2004Leite MF, Maistro EL. The Karyotype of Apareiodon affinis (Pisces, Teleostei, Characiformes) from Sapucai River, Minas Gerais, Brazil. Cytologia . 2004; 69(3):319-22. https://doi.org/10.1508/cytologia.69.319
https://doi.org/10.1508/cytologia.69.319... ; 9. Calgaro et al., 2004Calgaro MR, Fenocchio AS, Pastori MC, Roncati HA. Karyology of Apareiodon affinis from Paraná River (Argentina). I. Chromosome polymorphism. Cytologia. 2004; 69(4):475-79. http://dx.doi.org/10.1508/cytologia.69.475
http://dx.doi.org/10.1508/cytologia.69.4... ; 10. Traldi et al., 2016Traldi JB, Vicari MR, Martinez JF, Blanco DR, Lui RL, Schemberger MO et al . Chromosome analyses of Apareiodon argenteus and Apareiodon davisi (Characiformes, Parodontidae): an extensive chromosomal polymorphism of 45S and 5S ribosomal DNAs. Zebrafish. 2016; 13(1):19-25. https://doi.org/10.1089/zeb.2015.1124
https://doi.org/10.1089/zeb.2015.1124... ; 11. Traldi et al., 2019Traldi JB, Ziemniczak K, de Fátima Martinez J, Blanco DR, Lui RL, Schemberger MO et al . Chromosome mapping of H1 and H4 histones in Parodontidae (Actinopterygii: Characiformes): dispersed and/ or co-opted transposable elements? Cytogenet Genome Res . 2019; 158(2):106-13. http://dx.doi.org/10.1159/000500987
http://dx.doi.org/10.1159/000500987... ; 12. Bellafronte et al., 2012Bellafronte E, Schemberger MO, Artoni RF, Filho OM, Vicari MR. Sex chromosome system ZZ/ZW in Apareiodon hasemani Eigenmann, 1916 (Characiformes, Parodontidae) and a derived chromosomal region. Genet Mol Biol . 2012; 35(4):770-76. http://dx.doi.org/10.1590/S1415-47572012005000077
http://dx.doi.org/10.1590/S1415-47572012... ; 13. Moreira-Filho et al., 1984Moreira-Filho O, Bertollo LAC, Galetti PM. Structure and variability of nucleolar organizer regions in Parodontidae Fish. Can J Genet Cytol. 1984; 26(5):564-68. https://doi.org/10.1139/g84-089
https://doi.org/10.1139/g84-089... ; 14. Vicari et al., 2006Vicari MR, Moreira-Filho O, Artoni RF, Bertollo LAC. ZZ/ZW sex chromosome system in an undescribed species of the genus Apareiodon (Characiformes, Parodontidae). Cytogenet Genome Res . 2006; 114(2):163-68. https://doi.org/10.1159/000093333
https://doi.org/10.1159/000093333... ; 15. Santos et al., 2019Santos EOD, Deon GA, Almeida RB, Oliveira EA, Nogaroto V, Silva HP et al . Cytogenetics and DNA barcode reveal an undescribed Apareiodon species (Characiformes: Parodontidae). Genet Mol Biol . 2019; 42(2):365-73. http://dx.doi.org/10.1590/1678-4685-GMB-2018-0066
http://dx.doi.org/10.1590/1678-4685-GMB-... ; 16. Rosa et al., 2006Rosa R, Bellafronte E, Moreira Filho O, Margarido VP. Constitutive heterochromatin, 5S and 18S rDNA genes in Apareiodon sp. (Characiformes, Parodontidae) with a ZZ/ZW sex chromosome system. Genetica . 2006; 128(1-3):159-66. https://doi.org/10.1007/s10709-005-5700-1
https://doi.org/10.1007/s10709-005-5700-... ; 17. Moreira-Filho et al., 1993Moreira-Filho O, Bertollo LAC, Galetti PM. Distribution of Sex chromosome mechanisms in Neotropical fish and description of a ZZ/ZW system in Parodon hilarii (Parodontidae). Caryologia . 1993; 46(2-3):115-25. https://doi.org/10.1080/00087114.1993.10797253
https://doi.org/10.1080/00087114.1993.10... ; 18. Centofante et al., 2002Centofante L, Bertollo LAC, Moreira-Filho O. A ZZ/ZW sex chromosome system in a new species of the genus Parodon (Pisces, Parodontidae). Caryologia. 2002; 55(2):139-50. https://doi.org/10.1080/00087114.2002.10589270
https://doi.org/10.1080/00087114.2002.10... ; 19. Bellafronte et al., 2005Bellafronte E, Margarido VP, Moreira-Filho O. Cytotaxonomy of Parodon nasus and Parodon tortuosus (Pisces, Characiformes): A case of synonymy confirmed by cytogenetic analyses. Genet Mol Biol. 2005; 28(4):710-16. https://doi.org/10.1590/S1415-47572005000500010
https://doi.org/10.1590/S1415-4757200500... ; 20. Paula et al., 2017Paula AA, Penha HA, Delai VA Giuliano-Caetano L, Dias AL. Occurrence of structural polymorphism and supernumerary chromosomes in a population of Parodon nasus (Parodontidae), Caryologia , 2017; 70 (3):200-05. https://doi.org/10.1080/00087114.2017.1318503
https://doi.org/10.1080/00087114.2017.13... .
The genus Saccodon is cytogenetically unexplored and contains only three valid species: S. dariensis (Meek & Hildebrand, 1913) distributed in Colombia and Panama, S. terminalis (Eigenmann & Henn, 1914) that lives in the Daule River Basin in Ecuador, and S. wagneri Kner, 1863 that inhabits the coastal basins of Ecuador and northern Peru (Pavanelli, 2003Pavanelli CS. Family Parodontidae (Parodontids). In: Reis RE, Kullander SO, Ferraris JC, editors. Check List of the Freshwater Fishes of South and Central America. Porto Alegre: Edipucrs; 2003. p.46-50.). This last one was previously known as S. cranocephalum Thominot, 1882 and Parodon ecuadoriensis Eigenmann & Henn, 1914, now considered synonym (Fricke et al., 2020bFricke R, Eschmeyer WN, Van der Laan R. Eschemeyer’s Catalog of Fishes: genera, species, references. San Francisco: California Academy of Sciences ; 2020b. Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
http://researcharchive.calacademy.org/re...
). Saccodon wagneri is adapted to live in rivers that flow rapidly with rocky bottoms near the mountains and generally above 100 m altitude (Roberts, 1974Roberts TR. Dental polymorphism and systematics in Saccodon, a Neotropical genus of freshwater fishes (Parodontidae, Characoidei). J Zool. 1974; 173(3):303-21. https://doi.org/10.1111/j.1469-7998.1974.tb04117.x
https://doi.org/10.1111/j.1469-7998.1974...
), often forming schools when swims in rapid waters (Glodek, 1978Glodek GS. The freshwater fishes of Western Ecuador [Master Dissertation]. DeKalb: Northern Illinois University, 1978.). Saccodon wagneri specimens easily adapt to confinement in aquariums where they eat algae and even balanced food, so that they could also be considered as aquarium fish, similarly to other Parodontiidae. Indeed, some species of this family as Apareiodon affinis (Steindachner, 1879), Parodon pongoensis (Allen, 1942), and P. suborbitalis Valenciennes, 1850, are included in the pet trade (Prang, 2008Prang G. An industry analysis of the freshwater ornamental fishery with particular reference to the supply of Brazilian freshwater ornamentals to the UK Market. Scientific Magazine UAKARI. 2008; 3(1):7-52. https://doi.org/10.31420/uakari.v3i1.18
https://doi.org/10.31420/uakari.v3i1.18...
) and advertised on websites dedicated to the sale of aquarium fish (https://www.aquariumglaser.de/en/fish-archives/apareiodon_affinis_en/).
In the present study, we performed a cytogenetic survey of S. wagneri based on both conventional (Giemsa staining, silver staining, C-banding) and molecular (repetitive DNA mapping methods). The study aims to verify whether morphologically differentiated sex chromosomes, that are present in some Apareiodon and Parodon species, can be identified also in the genus Saccodon and whether chromosome number and main karyotype structure are conserved in this genus. A comparative analysis of cytogenetic data on this species and the remaining Parodontidae is presented here.
MATERIAL AND METHODS
Eleven individuals (2 males and 9 females) of S. wagneri, from the Río Bonito, El Guabo, El Oro Province, 03°07’55”S 79°45’00”W, were sampled (Fig. 1). The fishes were collected with cast nets and placed in plastic bags filled up to a third of their capacity with water and oxygen the remaining two thirds, transported in cardboard boxes to the laboratory where they were confined in aquariums provided with constant aeration until they were processed.
Mitotic chromosomes were obtained from kidney cells suspension following the conventional air-drying method (Nirchio, Oliveira, 2006Nirchio M, Oliveira C. Citogenética de peces. 216 pp. Cumaná, Venezuela: Universidad de Oriente; 2006.). The animals were stimulated to increase the number of metaphases with an injection of yeast-glucose suspension (Lozano et al., 1988Lozano R, Rejon CR, Rejon MR. A method for increasing the number of mitoses available for cytogenetic analysis in rainbow trout. Stain Technol. 1988; 63(6):335-38. https://doi.org/10.3109/10520298809107608
https://doi.org/10.3109/1052029880910760...
) in the caudal peduncle 48 h before being processed. Each fish was injected with 0.0125% colchicine (1.0 ml/100 g of body weight) 50 min before being sacrificed with an overdose of benzocaine (Leary et al., 2013Leary SL, Underwood W, Anthony R, Cartner S, Grandin T, Greenacre D et al . AVMA guidelines for the euthanasia of animals: 2013 Edition. Illinois: American Veterinary Medical Association Schaumburg; 2013. https://www.spandidos-publications.com/var/AVMA%20euthanasia%20guidelines%202013.pdf
https://www.spandidos-publications.com/v...
).
Voucher specimens are preserved and deposited in the Ichthyology Collection of the Laboratório de Biologia e Genética de Peixes (LBP) of Universidade Estadual Paulista, Botucatu, São Paulo, Brazil (UNESP) (collection numbers LBP 26871-26874) and Universidad Técnica de Machala, El Oro, Ecuador (collection numbers UTMACH-0398-0399).
The metaphases were stained with 5% Giemsa solution to define the 2n and the karyotype formula. C-positive heterochromatic regions were identified by the C-banding procedure, following Sumner (1972Sumner AT. A simple technique for demonstrating centromeric heterochromatin. Exper Cell Res.1972; 75(1):304-06. https://doi.org/10.1016/0014-4827(72)90558-7
https://doi.org/10.1016/0014-4827(72)905...
), while the nucleolus organizer regions (NORs) were identified using silver nitrate impregnation (Howell, Black, 1980Howell WM, Black DA. 1980. Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method. Experientia.1980; 36(8):1014-15. https://doi.org/10.1007/BF01953855
https://doi.org/10.1007/BF01953855...
) after Giemsa staining.
The 5S rDNA and 18S rDNA (ribosomal genes), and telomeric repeats were mapped onto chromosomes by fluorescence in situ hybridization (FISH) (Pinkel et al., 1986Pinkel D, Straume T, Gray JW. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Nat Acad Sci USA. 1986; 83(9):2934-38. https://doi.org/10.1073/pnas.83.9.2934
https://doi.org/10.1073/pnas.83.9.2934...
). Probes were obtained and labeled by PCR from the genome of Hypsolebias flagellatus (Costa, 2003Costa W. The Simpsonichthys flavicaudatus species group (Cyprinodontiformes: Rivulidae: Cynolebiatinae): phylogenetic relationships, taxonomic revision and biogeography. Ichthyol Expl Freshwaters. 2003; 14(1):31-60.) using the primers described by Pendas et al. (1995Pendas AM, Moran P, Martinez JL, Garcia-Vazquez E. Applications of 5S rDNA in Atlantic salmon, brown trout, and in Atlantic salmon brown trout hybrid identification. Mol Ecol. 1995; 4(2):275-76. https://doi.org/10.1111/j.1365-294X.1995.tb00220.x
https://doi.org/10.1111/j.1365-294X.1995...
) for 5S rDNA, Utsunomia et al. (2016Utsunomia R, Silva DMZA, Ruiz-Ruano FJ, Araya-Jaime C, Pansonato-Alves JC, Scacchetti PC et al . Uncovering the ancestry of B chromosomes in Moenkhausia sanctaefilomenae (Teleostei, Characidae). PloS One. 2016; 11(3):e0150573. https://doi.org/10.1371/journal.pone.0150573
https://doi.org/10.1371/journal.pone.015...
) for 18S rDNA and Ijdo et al. (1991Ijdo JW, Wells RA, Baldini A, Reeders ST. Improved telomere detection using a telomere repeat probe (TTAGGG)n generated by PCR. Nucl Ac Res. 1991; 19(17):4780. https://doi.org/10.1093/nar/19.17.4780
https://doi.org/10.1093/nar/19.17.4780...
) for telomeric repeats. The 5S rDNA and telomeric probes were labeled with biotin-16-dUTP (2’-deoxyuridine 5’-triphosphate), and the 18S rDNA probes were labeled with digoxigenin-11-dUTP. Signals were detected with fluorescein-conjugated avidin (Sigma-Aldrich, www.sigma-aldrich.com) and antidigoxigenin-rhodamine conjugate (Roche Diagnostics, www.roche.com), respectively. Chromosomes were counterstained with 4,6-diamidino-2-phenylindole included in the Vectashield mounting medium (Vector Laboratories, Ltd., Peterborough, UK).
Images capture of chromosome spread after Giemsa, silver staining (Ag-NORs), and C-bands (constitutive heterochromatin), was performed under a CX31 Olympus microscope equipped with a Moticam 10+ digital camera coupled to a Motic Images Plus 2.0 software. FISH metaphases were analyzed under an Olympus BX53 epifluorescence microscope (Olympus Corporation, Ishikawa, Japan) with the appropriate filters; images were captured with an Olympus DP73 digital camera coupled to cellSens Dimension Software (Olympus) for image acquisition. Images were merged and edited to optimize the brightness and contrast using the Photoshop CS5 program (Adobe Systems, www.adobe.com). At least 30 metaphase spreads per individual were analyzed to confirm the diploid number, karyotype structure and FISH results. Chromosomes were classified as metacentric (m), submetacentric (sm), or subtelocentric (st) according to their arm ratios (Levan et al., 1964Levan A, Fredga K, Sandberg AA. Nomenclature for centromeric position on chromosomes. Hereditas 1964; 52:201-220.).
RESULTS
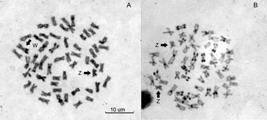
The diploid number of S. wagneri is 2n = 54 chromosomes for males and females, although differences in the FN are present between sexes. Indeed, the karyotype is composed of 31m + 16sm + 7st chromosomes in females, with FN = 101 (Fig. 2A), and of 32m + 16sm + 6st, FN = 102 (Fig. 2B) in males. This is due to the presence of morphologically differentiated sex chromosomes, i.e., to a heteromorphic ZZ/ZW sex chromosome system. The Z chromosome is submetacentric while the W is metacentric and almost twice as large as the Z (Fig. 2).
| Saccodon wagneri Giemsa karyotypes. A. Female; B. Male. Sex chromosomes are indicated. The NOR-carrying chromosomes, after silver staining, are boxed.
Sequential Giemsa and silver nitrate staining revealed a single pair of Ag-NOR positive marks located at the tip of the short arms of a small metacentric chromosome pair, probably pair 14 (Fig. 2, boxes).
C-banding revealed regions of centromeric heterochromatin in most chromosomes, as well as the presence of interstitial and terminal C-positive bands (Fig. 3). A large heterochromatic block is present on the half-distal part of the long arms of W chromosome in the female metaphases (Fig. 3B); a similar band is absent in the Z chromosome.
| Saccodon wagneri C-banded metaphases. A. Female; B. Male. The arrows indicate the sex chromosomes.
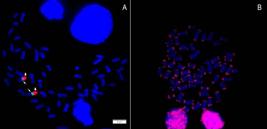
In situ hybridization using the 18S rDNA probe confirmed the presence of a single cluster of major ribosomal genes, localized on a small metacentric chromosome pair, likely coinciding with the Ag-NOR signals. Minor ribosomal genes were located on this same chromosome pair, just below the major rDNA cluster, in a syntenic condition (Fig. 4A).
| Saccodon wagneri metaphase plates after A. Double FISH with 5S rDNA (green-thin arrows) and 18S rDNA (red-thick arrows) probes; B. FISH using telomeric probes showing positive signals in the terminal positions of all chromosomes.
FISH with the telomeric repeat probe (TTAGGG)n (Fig. 4B) revealed hybridization signals only in the telomeric regions of all chromosomes, without the presence of interstitial telomeric sites (ITSs).
DISCUSSION
Recent characiform phylogenomic studies showed that Parodontidae originated about 70 million years ago (mya) and the first recognized cladogenesis occurred about 40 mya, separating Saccodon (an exclusive trans-Andean group) from Parodon and Apareiodon (wide-spread groups in the Neotropical region) (Bruno F. Melo, 2020, pers. comm.). The cytogenetics data on Parodontidae reveal a conservative 2n = 54 karyotype, that is composed predominantly of metacentric and submetacentric chromosomes (except for Apareiodon affinis, where females present 2n = 55 due to the unique ZW1W2 sex system) (Moreira Filho et al., 1980Moreira Filho O, Bertollo LAC, Galetti PM. Evidences for a multiple sex chromosome system with female heterogamety in Apareiodon affinis (Pisces, Parodontidae). Caryologia . 1980; 33(1):83-91. https://doi.org/10.1080/00087114.1980.10796821
https://doi.org/10.1080/00087114.1980.10...
) (Tab. 1). Results here obtained on Saccodon wagneri reinforce this picture, despite the ancient divergence of this genus within the family. Moreover, other Neotropical fishes closely related to Parodontidae, e.g., families Anostomidae, Prochilodontidae, Chilodontidae, and Curimatidae (Betancur et al., 2019Betancur RR, Arcila D, Vari RP, Hughes LC, Oliveira C et al . Phylogenomic incongruence, hypothesis testing, and taxonomic sampling: the monophyly of characiform fishes. Evolution. 2019; 73(2):329-45. https://doi.org/10.1111/evo.13649
https://doi.org/10.1111/evo.13649...
; Bruno F. Melo, 2020, pers. comm.), also share this feature, i.e., almost all species with 54 chromosomes, mainly metacentrics and submetacentrics, with a few exceptions (Arai, 2011Arai R. Fish karyotypes: A check list. Springer Science & Business Media; 2011.). These data indicate an ancient origin of such a karyotype, whose conservatism has been related to the population structures of these fishes, as they include many long migratory species able to form large schools (Oliveira et al., 1988Oliveira C, Almeida-Toledo LF, Foresti F, Britski HA, Toledo-Filho SD. Chromosome formulae of Neotropical freshwater fishes. Rev Brasil Genet. 1988; 11(3):577-624.).
A morphologically well-differentiated ZZ/ZW sex chromosome system is present in approximately half of all Parodon and Apareiodon species analyzed so far (Moreira-Filho et al., 1993Moreira-Filho O, Bertollo LAC, Galetti PM. Distribution of Sex chromosome mechanisms in Neotropical fish and description of a ZZ/ZW system in Parodon hilarii (Parodontidae). Caryologia . 1993; 46(2-3):115-25. https://doi.org/10.1080/00087114.1993.10797253
https://doi.org/10.1080/00087114.1993.10...
; Rosa et al., 2006Rosa R, Bellafronte E, Moreira Filho O, Margarido VP. Constitutive heterochromatin, 5S and 18S rDNA genes in Apareiodon sp. (Characiformes, Parodontidae) with a ZZ/ZW sex chromosome system. Genetica . 2006; 128(1-3):159-66. https://doi.org/10.1007/s10709-005-5700-1
https://doi.org/10.1007/s10709-005-5700-...
; Vicari et al., 2006Vicari MR, Moreira-Filho O, Artoni RF, Bertollo LAC. ZZ/ZW sex chromosome system in an undescribed species of the genus Apareiodon (Characiformes, Parodontidae). Cytogenet Genome Res . 2006; 114(2):163-68. https://doi.org/10.1159/000093333
https://doi.org/10.1159/000093333...
; Bellafronte et al., 2009Bellafronte E, Vicari MR, Artoni RF, Margarido VP, Moreira-Filho O. Differentiated ZZ/ZW sex chromosomes in Apareiodon ibitiensis (Teleostei, Parodontidae): cytotaxonomy and biogeography. J Fish Biol. 2009; 75(9): 2313-25. https://doi.org/10.1111/j.1095-8649.2009.02488.x
https://doi.org/10.1111/j.1095-8649.2009...
; Kitano, Peichel, 2012Kitano J, Peichel CL. Turnover of sex chromosomes and speciation in fishes. Environ Biol Fish. 2012; 94(3):549-58. https://doi.org/10.1007/s10641-011-9853-8
https://doi.org/10.1007/s10641-011-9853-...
). The occurrence of such sex system, characterized by an enlarged metacentric W chromosome, in S. wagneri points to its old origin inside Parodontidae. Besides this, and as frequently observed in higher vertebrates (Schartl et al., 2016Schartl M, Schmid M, Nanda I. 2016. Dynamics of vertebrate sex chromosome evolution: from equal size to giants and dwarfs. Chromosoma. 2016; 125(3):553-71. https://doi.org/10.1007/s00412-015-0569-y
https://doi.org/10.1007/s00412-015-0569-...
), rather than showing a size reduction, the sex-specific W chromosome in Parodontidae is larger than the Z, because of a huge heterochromatin amplification. Despite these common features, the W chromosomes have evolved to different shapes and sequence contents among Parodontidae species. Two main questions remain unanswered, i.e., whether (i) the Z and W chromosomes have a common origin, representing the same linkage group in all species and (ii) the absence of sex chromosomes in some species may represent a derived character, probably related to sex chromosomes turnovers, as already documented in other fishes (Kitano, Peichel, 2012Kitano J, Peichel CL. Turnover of sex chromosomes and speciation in fishes. Environ Biol Fish. 2012; 94(3):549-58. https://doi.org/10.1007/s10641-011-9853-8
https://doi.org/10.1007/s10641-011-9853-...
). Our data reinforce the hypothesis that this common ZW system has an ancient origin and it seems possible that the putative absence in some Parodontidae species would be related to subsequent specific chromosome differentiation. Further studies will make it possible to confirm the validity of this hypothesis.
In all the Parodontidae species studied so far, the presence of a single pair of NOR bearing chromosomes is the common condition, with few exceptions (Bellafronte et al., 2011Bellafronte E, Schemberger MO, Moreira-Filho O, Almeida MC et al . Chromosomal markers in Parodontidae: an analysis of new and reviewed data with phylogenetic inferences. Rev Fish Biol Fish. 2011; 21(3):559-70. https://doi.org/10.1007/s11160-010-9177-3
https://doi.org/10.1007/s11160-010-9177-...
). However, different locations of these genes have been observed among the species, probably as the result of chromosomal rearrangements (pericentric inversion), occurred along with the diversification of their karyotypes. The presence of multiple sites reported in Apareiodon davisi (Traldi et al., 2016Traldi JB, Vicari MR, Martinez JF, Blanco DR, Lui RL, Schemberger MO et al . Chromosome analyses of Apareiodon argenteus and Apareiodon davisi (Characiformes, Parodontidae): an extensive chromosomal polymorphism of 45S and 5S ribosomal DNAs. Zebrafish. 2016; 13(1):19-25. https://doi.org/10.1089/zeb.2015.1124
https://doi.org/10.1089/zeb.2015.1124...
), and A. ibitiensis (Bellafronte et al., 2009Bellafronte E, Vicari MR, Artoni RF, Margarido VP, Moreira-Filho O. Differentiated ZZ/ZW sex chromosomes in Apareiodon ibitiensis (Teleostei, Parodontidae): cytotaxonomy and biogeography. J Fish Biol. 2009; 75(9): 2313-25. https://doi.org/10.1111/j.1095-8649.2009.02488.x
https://doi.org/10.1111/j.1095-8649.2009...
; Bellafronte et al., 2011Bellafronte E, Schemberger MO, Moreira-Filho O, Almeida MC et al . Chromosomal markers in Parodontidae: an analysis of new and reviewed data with phylogenetic inferences. Rev Fish Biol Fish. 2011; 21(3):559-70. https://doi.org/10.1007/s11160-010-9177-3
https://doi.org/10.1007/s11160-010-9177-...
) represents an exception, that has been attributed to the presence of transposable elements (Bellafronte et al., 2011Bellafronte E, Schemberger MO, Moreira-Filho O, Almeida MC et al . Chromosomal markers in Parodontidae: an analysis of new and reviewed data with phylogenetic inferences. Rev Fish Biol Fish. 2011; 21(3):559-70. https://doi.org/10.1007/s11160-010-9177-3
https://doi.org/10.1007/s11160-010-9177-...
). The syntenic arrangement of the 18S and the 5S rDNA genes detected in S. wagneri, has only been reported in two other Parodontidae species, named A. davisi (Traldi et al., 2016Traldi JB, Vicari MR, Martinez JF, Blanco DR, Lui RL, Schemberger MO et al . Chromosome analyses of Apareiodon argenteus and Apareiodon davisi (Characiformes, Parodontidae): an extensive chromosomal polymorphism of 45S and 5S ribosomal DNAs. Zebrafish. 2016; 13(1):19-25. https://doi.org/10.1089/zeb.2015.1124
https://doi.org/10.1089/zeb.2015.1124...
) and P. nasus (Bellafronte et al., 2005Bellafronte E, Margarido VP, Moreira-Filho O. Cytotaxonomy of Parodon nasus and Parodon tortuosus (Pisces, Characiformes): A case of synonymy confirmed by cytogenetic analyses. Genet Mol Biol. 2005; 28(4):710-16. https://doi.org/10.1590/S1415-47572005000500010
https://doi.org/10.1590/S1415-4757200500...
), and does not represent a common condition in fishes (Sochorová et al., 2018Sochorová J, Garcia S, Gálvez F, Symonová R, Kovařík A. Evolutionary trends in animal ribosomal DNA loci: introduction to a new online database. Chromosoma. 2018; 127(1):141-50. https://doi.org/10.1007/s00412-017-0651-8
https://doi.org/10.1007/s00412-017-0651-...
). Indeed, the presence of these genes on different chromosomes/sites in fishes and in the majority of vertebrates has been interpreted in the light of their functional dynamics (Martins, Galetti, 1999Martins C, Galetti PM. Chromosomal localization of 5S rDNA genes in Leporinus fish (Anostomidae, Characiformes). 1999. Chromosome Res. 7:363-67. https://doi.org/10.1023/a:1009216030316
https://doi.org/10.1023/a:1009216030316...
) and efficiency in evolution processes associated with multiple tandem arrays (Martins, Wasko, 2004Martins C, Wasko AP. Organization and evolution of 5S ribosomal DNA in the fish genome. In: Williams CR, editor. Focus on Genome Research. Hauppauge: Nova Science Publishers; 2004.).
FISH with the telomeric probe (TTAGGG)n in S. wagneri revealed hybridization signals only in the telomeric regions of all chromosomes in females and males, without Interstitials Telomeric Sequences (ITSs) that might result from the occurrence of recent Robertsonian fusions or other chromosomal rearrangements (Ocalewicz, 2013Ocalewicz K. Telomeres in fishes. Cytogenet Genome Res. 2013; 141(2-3):114-25. https://doi.org/10.1159/000354278
https://doi.org/10.1159/000354278...
). This evidence, the common localization of constitutive heterochromatin (Moreira-Filho et al., 1984Moreira-Filho O, Bertollo LAC, Galetti PM. Structure and variability of nucleolar organizer regions in Parodontidae Fish. Can J Genet Cytol. 1984; 26(5):564-68. https://doi.org/10.1139/g84-089
https://doi.org/10.1139/g84-089...
; Jesus, 2000Jesus CM. Karyotypes of the three species of Parodon (Teleostei: Parodontidae). Ichthyol Expl Freshwaters . 2000; 11:75-80.; Jesus, Moreira-Filho, 2000Jesus CM, Moreira-Filho O. Cytogenetic studies in some Apareiodon species (Pisces, Parodontidae). Cytologia. 2000; 65:398-402. https://doi.org/10.1508/cytologia.65.398.
https://doi.org/10.1508/cytologia.65.398...
; Vicente et al., 2001Vicente VE, Jesus CM, Moreira Filho O. Chromosomal localization of 5S and 18S rRNA genes in three Parodon species (Pisces, Parodontidae). Caryologia . 2001; 54(4):365-69. https://doi.org/10.1080/00087114.2001.10589247
https://doi.org/10.1080/00087114.2001.10...
, 2003Vicente VE, Bertollo LAC, Valentini SR, Moreira Filho O. Origin and differentiation of a sex chromosome system in Parodon hilarii (Pisces, Parodontidae). Satellite DNA, G- and C-Banding. Genetica . 2003; 119(2):115-20. https://doi.org/10.1023/a:1026082904672
https://doi.org/10.1023/a:1026082904672...
; Centofante et al., 2002Centofante L, Bertollo LAC, Moreira-Filho O. A ZZ/ZW sex chromosome system in a new species of the genus Parodon (Pisces, Parodontidae). Caryologia. 2002; 55(2):139-50. https://doi.org/10.1080/00087114.2002.10589270
https://doi.org/10.1080/00087114.2002.10...
; Bellafronte et al., 2005Bellafronte E, Margarido VP, Moreira-Filho O. Cytotaxonomy of Parodon nasus and Parodon tortuosus (Pisces, Characiformes): A case of synonymy confirmed by cytogenetic analyses. Genet Mol Biol. 2005; 28(4):710-16. https://doi.org/10.1590/S1415-47572005000500010
https://doi.org/10.1590/S1415-4757200500...
; Rosa et al., 2006Rosa R, Bellafronte E, Moreira Filho O, Margarido VP. Constitutive heterochromatin, 5S and 18S rDNA genes in Apareiodon sp. (Characiformes, Parodontidae) with a ZZ/ZW sex chromosome system. Genetica . 2006; 128(1-3):159-66. https://doi.org/10.1007/s10709-005-5700-1
https://doi.org/10.1007/s10709-005-5700-...
; Vicari et al., 2006Vicari MR, Moreira-Filho O, Artoni RF, Bertollo LAC. ZZ/ZW sex chromosome system in an undescribed species of the genus Apareiodon (Characiformes, Parodontidae). Cytogenet Genome Res . 2006; 114(2):163-68. https://doi.org/10.1159/000093333
https://doi.org/10.1159/000093333...
), and the constancy of 2n suggest that diversification in Parodontidae karyotypes has not involved macro-structural reorganizations but rather microstructural ones.
In conclusion, our study, the first one to report cytogenetic data on a Saccodon species both by conventional and molecular protocols, reinforces the hypothesis of karyotype homeostasis in fishes of the family Parodontidae, by conserving the basic diploid number and chromosome formulae. The synteny of both 18S and 5S rDNA found in S. wagneri represents an uncommon trait, and its presence in species of the other two genera (A. davisi and P. nasus), suggests its ancient origin, i.e., that this is a symplesiomorphic character within the family. As an alternative hypothesis, this similarity could be due to a homoplasic condition, obtained by parallelism. Further studies with chromosomal painting, sequence analysis of microdissected sex chromosomes, and comparative mapping of transposable elements will be useful to obtain a more complete picture of the evolution of karyotype and sex chromosomes within Parodontidae.
ACKNOWLEDGMENTS
Mauro Nirchio received financial support from Centro de Investigación of Universidad Técnica de Machala, Ecuador (GPR-GEN-155); CO received financial support from Fundação de Amparo à Pesquisa do Estado de São Paulo - FAPESP grants 2018/20610-1, 2016/09204-6, 2014/26508-3 and Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq proc. 306054/2006-0; ARR received financial support from Università Sapienza (RP11816430E2E16A).
REFERENCES
- Albert JS, Reis RE, editors. Historical biogeography of Neotropical freshwater fishes. University of California Press; 2011.
- Arai R. Fish karyotypes: A check list. Springer Science & Business Media; 2011.
- Bellafronte E, Margarido VP, Moreira-Filho O. Cytotaxonomy of Parodon nasus and Parodon tortuosus (Pisces, Characiformes): A case of synonymy confirmed by cytogenetic analyses. Genet Mol Biol. 2005; 28(4):710-16. https://doi.org/10.1590/S1415-47572005000500010
» https://doi.org/10.1590/S1415-47572005000500010 - Bellafronte E, Schemberger MO, Artoni RF, Filho OM, Vicari MR. Sex chromosome system ZZ/ZW in Apareiodon hasemani Eigenmann, 1916 (Characiformes, Parodontidae) and a derived chromosomal region. Genet Mol Biol . 2012; 35(4):770-76. http://dx.doi.org/10.1590/S1415-47572012005000077
» http://dx.doi.org/10.1590/S1415-47572012005000077 - Bellafronte E, Schemberger MO, Moreira-Filho O, Almeida MC et al . Chromosomal markers in Parodontidae: an analysis of new and reviewed data with phylogenetic inferences. Rev Fish Biol Fish. 2011; 21(3):559-70. https://doi.org/10.1007/s11160-010-9177-3
» https://doi.org/10.1007/s11160-010-9177-3 - Bellafronte E, Vicari MR, Artoni RF, Margarido VP, Moreira-Filho O. Differentiated ZZ/ZW sex chromosomes in Apareiodon ibitiensis (Teleostei, Parodontidae): cytotaxonomy and biogeography. J Fish Biol. 2009; 75(9): 2313-25. https://doi.org/10.1111/j.1095-8649.2009.02488.x
» https://doi.org/10.1111/j.1095-8649.2009.02488.x - Betancur RR, Arcila D, Vari RP, Hughes LC, Oliveira C et al . Phylogenomic incongruence, hypothesis testing, and taxonomic sampling: the monophyly of characiform fishes. Evolution. 2019; 73(2):329-45. https://doi.org/10.1111/evo.13649
» https://doi.org/10.1111/evo.13649 - Calgaro MR, Fenocchio AS, Pastori MC, Roncati HA. Karyology of Apareiodon affinis from Paraná River (Argentina). I. Chromosome polymorphism. Cytologia. 2004; 69(4):475-79. http://dx.doi.org/10.1508/cytologia.69.475
» http://dx.doi.org/10.1508/cytologia.69.475 - Centofante L, Bertollo LAC, Moreira-Filho O. A ZZ/ZW sex chromosome system in a new species of the genus Parodon (Pisces, Parodontidae). Caryologia. 2002; 55(2):139-50. https://doi.org/10.1080/00087114.2002.10589270
» https://doi.org/10.1080/00087114.2002.10589270 - Costa W. The Simpsonichthys flavicaudatus species group (Cyprinodontiformes: Rivulidae: Cynolebiatinae): phylogenetic relationships, taxonomic revision and biogeography. Ichthyol Expl Freshwaters. 2003; 14(1):31-60.
- Fricke R, Eschmeyer WN, Fong JD. Eschmeyer’s catalog of fishes: species by family/subfamily. San Francisco: California Academy of Sciences; 2020a. Available from: http://researcharchive.calacademy.org/research/ ichthyology/catalog/SpeciesByFamily.asp
» http://researcharchive.calacademy.org/research/ ichthyology/catalog/SpeciesByFamily.asp - Fricke R, Eschmeyer WN, Van der Laan R. Eschemeyer’s Catalog of Fishes: genera, species, references. San Francisco: California Academy of Sciences ; 2020b. Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
» http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp - Glodek GS. The freshwater fishes of Western Ecuador [Master Dissertation]. DeKalb: Northern Illinois University, 1978.
- Hilsdorf AWS, Hallerman EM. Genetic resources of Neotropical fishes: Springer, Cham; 2017.
- Howell WM, Black DA. 1980. Controlled silver-staining of nucleolus organizer regions with a protective colloidal developer: a 1-step method. Experientia.1980; 36(8):1014-15. https://doi.org/10.1007/BF01953855
» https://doi.org/10.1007/BF01953855 - Ijdo JW, Wells RA, Baldini A, Reeders ST. Improved telomere detection using a telomere repeat probe (TTAGGG)n generated by PCR. Nucl Ac Res. 1991; 19(17):4780. https://doi.org/10.1093/nar/19.17.4780
» https://doi.org/10.1093/nar/19.17.4780 - Jesus CM, Moreira-Filho O. Cytogenetic studies in some Apareiodon species (Pisces, Parodontidae). Cytologia. 2000; 65:398-402. https://doi.org/10.1508/cytologia.65.398
» https://doi.org/10.1508/cytologia.65.398 - Jesus CM. Karyotypes of the three species of Parodon (Teleostei: Parodontidae). Ichthyol Expl Freshwaters . 2000; 11:75-80.
- Jesus CM, Bertollo LAC, Moreira-Filho O. Comparative cytogenetics in Apareiodon affinis (Pisces, Characiformes) and considerations regarding diversification of the group. Genetica 1999; 105:63-67. https://doi.org/10.1023/A:1003592022927
» https://doi.org/10.1023/A:1003592022927 - Jorge LC, Moreira-Filho O. Cytogenetic studies on Apareiodon affinis (Pisces, Characiformes) from Paraná river basin: sex chromosomes and polymorphism. Genetica. 2000;109(3):267-73. http://dx.doi.org/10.1023/a:1017522914023
» http://dx.doi.org/10.1023/a:1017522914023 - Kitano J, Peichel CL. Turnover of sex chromosomes and speciation in fishes. Environ Biol Fish. 2012; 94(3):549-58. https://doi.org/10.1007/s10641-011-9853-8
» https://doi.org/10.1007/s10641-011-9853-8 - Leary SL, Underwood W, Anthony R, Cartner S, Grandin T, Greenacre D et al . AVMA guidelines for the euthanasia of animals: 2013 Edition. Illinois: American Veterinary Medical Association Schaumburg; 2013. https://www.spandidos-publications.com/var/AVMA%20euthanasia%20guidelines%202013.pdf
» https://www.spandidos-publications.com/var/AVMA%20euthanasia%20guidelines%202013.pdf - Leite MF, Maistro EL. The Karyotype of Apareiodon affinis (Pisces, Teleostei, Characiformes) from Sapucai River, Minas Gerais, Brazil. Cytologia . 2004; 69(3):319-22. https://doi.org/10.1508/cytologia.69.319
» https://doi.org/10.1508/cytologia.69.319 - Levan A, Fredga K, Sandberg AA. Nomenclature for centromeric position on chromosomes. Hereditas 1964; 52:201-220.
- Lozano R, Rejon CR, Rejon MR. A method for increasing the number of mitoses available for cytogenetic analysis in rainbow trout. Stain Technol. 1988; 63(6):335-38. https://doi.org/10.3109/10520298809107608
» https://doi.org/10.3109/10520298809107608 - Malabarba LR, Reis RE, Vari RP, Lucena ZMS, Lucena CAS. Phylogeny and Classification of Neotropical Fishes. Porto Alegre: EDIPUCRS; 1998.
- Martins C, Galetti PM. Chromosomal localization of 5S rDNA genes in Leporinus fish (Anostomidae, Characiformes). 1999. Chromosome Res. 7:363-67. https://doi.org/10.1023/a:1009216030316
» https://doi.org/10.1023/a:1009216030316 - Martins C, Wasko AP. Organization and evolution of 5S ribosomal DNA in the fish genome. In: Williams CR, editor. Focus on Genome Research. Hauppauge: Nova Science Publishers; 2004.
- Moreira Filho O, Bertollo LAC, Galetti PM. Evidences for a multiple sex chromosome system with female heterogamety in Apareiodon affinis (Pisces, Parodontidae). Caryologia . 1980; 33(1):83-91. https://doi.org/10.1080/00087114.1980.10796821
» https://doi.org/10.1080/00087114.1980.10796821 - Moreira-Filho O, Bertollo LAC, Galetti PM. Distribution of Sex chromosome mechanisms in Neotropical fish and description of a ZZ/ZW system in Parodon hilarii (Parodontidae). Caryologia . 1993; 46(2-3):115-25. https://doi.org/10.1080/00087114.1993.10797253
» https://doi.org/10.1080/00087114.1993.10797253 - Moreira Filho O, Bertollo LAC, Galetti PM. Karyotypic study of some species of family Parodontidae (Pisces - Cypriniformes). Caryologia . 1985; 38(1):47-55. https://doi.org/10.1080/00087114.1985.10797729
» https://doi.org/10.1080/00087114.1985.10797729 - Moreira-Filho O, Bertollo LAC, Galetti PM. Structure and variability of nucleolar organizer regions in Parodontidae Fish. Can J Genet Cytol. 1984; 26(5):564-68. https://doi.org/10.1139/g84-089
» https://doi.org/10.1139/g84-089 - Nascimento VD, Almeida Coelho K, Nogaroto V, Almeida RB, Ziemniczak K, Centofante L, Pavanelli CS, Torres RA, Moreira-Filho O, Vicari MR. Do multiple karyomorphs and population genetics of freshwater darter characines (Apareiodon affinis) indicate chromosomal speciation? Zoologischer Anzeiger, 2018; 272:93-103. https://doi.org/10.1016/j.jcz.2017.12.006
» https://doi.org/10.1016/j.jcz.2017.12.006 - Nelson JS, Grande TC, Wilson MVH. Fishes of the world. Hoboken, New Jersey: John Wiley & Sons; 2016.
- Nirchio M, Oliveira C. Citogenética de peces. 216 pp. Cumaná, Venezuela: Universidad de Oriente; 2006.
- Ocalewicz K. Telomeres in fishes. Cytogenet Genome Res. 2013; 141(2-3):114-25. https://doi.org/10.1159/000354278
» https://doi.org/10.1159/000354278 - Oliveira C, Almeida-Toledo LF, Foresti F, Britski HA, Toledo-Filho SD. Chromosome formulae of Neotropical freshwater fishes. Rev Brasil Genet. 1988; 11(3):577-624.
- Paula AA, Penha HA, Delai VA Giuliano-Caetano L, Dias AL. Occurrence of structural polymorphism and supernumerary chromosomes in a population of Parodon nasus (Parodontidae), Caryologia , 2017; 70 (3):200-05. https://doi.org/10.1080/00087114.2017.1318503
» https://doi.org/10.1080/00087114.2017.1318503 - Pavanelli CS. Family Parodontidae (Parodontids). In: Reis RE, Kullander SO, Ferraris JC, editors. Check List of the Freshwater Fishes of South and Central America. Porto Alegre: Edipucrs; 2003. p.46-50.
- Pendas AM, Moran P, Martinez JL, Garcia-Vazquez E. Applications of 5S rDNA in Atlantic salmon, brown trout, and in Atlantic salmon brown trout hybrid identification. Mol Ecol. 1995; 4(2):275-76. https://doi.org/10.1111/j.1365-294X.1995.tb00220.x
» https://doi.org/10.1111/j.1365-294X.1995.tb00220.x - Pinkel D, Straume T, Gray JW. Cytogenetic analysis using quantitative, high-sensitivity, fluorescence hybridization. Proc Nat Acad Sci USA. 1986; 83(9):2934-38. https://doi.org/10.1073/pnas.83.9.2934
» https://doi.org/10.1073/pnas.83.9.2934 - Prang G. An industry analysis of the freshwater ornamental fishery with particular reference to the supply of Brazilian freshwater ornamentals to the UK Market. Scientific Magazine UAKARI 2008; 3(1):7-52. https://doi.org/10.31420/uakari.v3i1.18
» https://doi.org/10.31420/uakari.v3i1.18 - Reis RE, Albert JS, Dario FD, Mincarone MM, Petry P, Rocha LA. Fish biodiversity and conservation in South America. J Fish Biol . 2016; 89(1):12-47. https://doi.org/10.1111/jfb.13016
» https://doi.org/10.1111/jfb.13016 - Roberts TR. Dental polymorphism and systematics in Saccodon, a Neotropical genus of freshwater fishes (Parodontidae, Characoidei). J Zool. 1974; 173(3):303-21. https://doi.org/10.1111/j.1469-7998.1974.tb04117.x
» https://doi.org/10.1111/j.1469-7998.1974.tb04117.x - Rosa R, Bellafronte E, Moreira Filho O, Margarido VP. Constitutive heterochromatin, 5S and 18S rDNA genes in Apareiodon sp. (Characiformes, Parodontidae) with a ZZ/ZW sex chromosome system. Genetica . 2006; 128(1-3):159-66. https://doi.org/10.1007/s10709-005-5700-1
» https://doi.org/10.1007/s10709-005-5700-1 - Santos EOD, Deon GA, Almeida RB, Oliveira EA, Nogaroto V, Silva HP et al . Cytogenetics and DNA barcode reveal an undescribed Apareiodon species (Characiformes: Parodontidae). Genet Mol Biol . 2019; 42(2):365-73. http://dx.doi.org/10.1590/1678-4685-GMB-2018-0066
» http://dx.doi.org/10.1590/1678-4685-GMB-2018-0066 - Schartl M, Schmid M, Nanda I. 2016. Dynamics of vertebrate sex chromosome evolution: from equal size to giants and dwarfs. Chromosoma. 2016; 125(3):553-71. https://doi.org/10.1007/s00412-015-0569-y
» https://doi.org/10.1007/s00412-015-0569-y - Schemberger MO, Bellafronte E, Nogaroto V, Schühli GS, Artoni RF et al Differentiation of repetitive DNA sites and sex chromosome systems reveal closely related group in Parodontidae (Actinopterygii: Characiformes). Genetica . 2011; 139(11-12):1499-508. https://doi.org/10.1007/s10709-012-9649-6
» https://doi.org/10.1007/s10709-012-9649-6 - Schemberger MO, Nogaroto V, Almeida MC, Artoni RF, Valente GT, Martins C et al . Sequence analyses and chromosomal distribution of the Tc1/Mariner element in Parodontidae Fish (Teleostei: Characiformes). Gene. 2016; 593(2):308-14. https://doi.org/10.1016/j.gene.2016.08.034
» https://doi.org/10.1016/j.gene.2016.08.034 - Sochorová J, Garcia S, Gálvez F, Symonová R, Kovařík A. Evolutionary trends in animal ribosomal DNA loci: introduction to a new online database. Chromosoma. 2018; 127(1):141-50. https://doi.org/10.1007/s00412-017-0651-8
» https://doi.org/10.1007/s00412-017-0651-8 - Sumner AT. A simple technique for demonstrating centromeric heterochromatin. Exper Cell Res.1972; 75(1):304-06. https://doi.org/10.1016/0014-4827(72)90558-7
» https://doi.org/10.1016/0014-4827(72)90558-7 - Traldi JB, Vicari MR, Martinez JF, Blanco DR, Lui RL, Schemberger MO et al . Chromosome analyses of Apareiodon argenteus and Apareiodon davisi (Characiformes, Parodontidae): an extensive chromosomal polymorphism of 45S and 5S ribosomal DNAs. Zebrafish. 2016; 13(1):19-25. https://doi.org/10.1089/zeb.2015.1124
» https://doi.org/10.1089/zeb.2015.1124 - Traldi JB, Ziemniczak K, de Fátima Martinez J, Blanco DR, Lui RL, Schemberger MO et al . Chromosome mapping of H1 and H4 histones in Parodontidae (Actinopterygii: Characiformes): dispersed and/ or co-opted transposable elements? Cytogenet Genome Res . 2019; 158(2):106-13. http://dx.doi.org/10.1159/000500987
» http://dx.doi.org/10.1159/000500987 - Utsunomia R, Silva DMZA, Ruiz-Ruano FJ, Araya-Jaime C, Pansonato-Alves JC, Scacchetti PC et al . Uncovering the ancestry of B chromosomes in Moenkhausia sanctaefilomenae (Teleostei, Characidae). PloS One. 2016; 11(3):e0150573. https://doi.org/10.1371/journal.pone.0150573
» https://doi.org/10.1371/journal.pone.0150573 - Vicari MR, Moreira-Filho O, Artoni RF, Bertollo LAC. ZZ/ZW sex chromosome system in an undescribed species of the genus Apareiodon (Characiformes, Parodontidae). Cytogenet Genome Res . 2006; 114(2):163-68. https://doi.org/10.1159/000093333
» https://doi.org/10.1159/000093333 - Vicente VE, Bertollo LAC, Valentini SR, Moreira Filho O. Origin and differentiation of a sex chromosome system in Parodon hilarii (Pisces, Parodontidae). Satellite DNA, G- and C-Banding. Genetica . 2003; 119(2):115-20. https://doi.org/10.1023/a:1026082904672
» https://doi.org/10.1023/a:1026082904672 - Vicente VE, Jesus CM, Moreira Filho O. Chromosomal localization of 5S and 18S rRNA genes in three Parodon species (Pisces, Parodontidae). Caryologia . 2001; 54(4):365-69. https://doi.org/10.1080/00087114.2001.10589247
» https://doi.org/10.1080/00087114.2001.10589247
HOW TO CITE THIS ARTICLE
-
HOW TO CITE THIS ARTICLE
Nirchio M, Masache MC, Paim FG, Cioffi MB, Moreira Filho O, Barriga R, Oliveira C, Rossi AR. Chromosome analysis in Saccodon wagneri (Characiformes) and insights into the karyotype evolution of Parodontidae. Neotrop Ichthyol. 2021; 19(1):e200103. https://doi.org/10.1590/1982-0224-2020-0103
Edited by
Data availability
Data citations
Fricke R, Eschmeyer WN, Fong JD. Eschmeyer’s catalog of fishes: species by family/subfamily. San Francisco: California Academy of Sciences; 2020a. Available from: http://researcharchive.calacademy.org/research/ ichthyology/catalog/SpeciesByFamily.asp
Fricke R, Eschmeyer WN, Van der Laan R. Eschemeyer’s Catalog of Fishes: genera, species, references. San Francisco: California Academy of Sciences ; 2020b. Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
Publication Dates
-
Publication in this collection
22 Feb 2021 -
Date of issue
2021
History
-
Received
28 Sept 2020 -
Accepted
21 Dec 2020