Abstract
A new species of Moenkhausia is described from the rio Machado drainage, Amazon basin, Brazil. It is diagnosed from congeners by its color pattern, consisting of the concentration of chromatophores on the anterior portion of body scales, the horizontally elongate blotch on caudal peduncle, a bright golden coloration of the dorsal portion of eye when alive, and a dark line crossing the eye horizontally. The new species has variable morphology regarding trunk lateral-line canals. Most fully grown individuals do not have enclosed bony tube in many lateral line scales, resembling early developmental stages of tube formation of other species. This paedomorphic condition is interpreted as a result of developmental truncation. Such evolutionary process may have been responsible for the presence of distinct levels of trunk lateral line reductions in small characids. Variation in this feature is common, even between the sides of the same individual. We reassert that the degree of trunk lateral-line tube development must be used with care in taxonomic and phylogenetic studies, because reductions in the laterosensory system may constitute parallel loss in the Characidae. We suggest the new species to be categorized Near Threatened due to the restricted geographical distribution and continuing decline in habitat quality.
Keywords:
Developmental Truncation; Evolution; Intraspecific Variation; Paedomorphy; Scale
Resumo
Uma espécie nova de Moenkhausia é descrita da drenagem do rio Machado, bacia Amazônica, Brasil. É diagnosticada das congêneres pelo padrão de coloração, que consiste na concentração de cromatóforos na porção anterior das escamas do corpo, em uma mancha horizontalmente alongada no pedúnculo caudal, na coloração dourada brilhante da porção dorsal do olho quando vivo e na faixa escura que atravessa o olho horizontalmente. A nova espécie apresenta variação na morfologia do canal da linha lateral do corpo. A maioria dos indivíduos totalmente desenvolvidos não possuem tubo ósseo fechado em muitas escamas da linha lateral, assemelhando-se aos estágios iniciais do desenvolvimento da formação do tubo de outras espécies. Essa condição pedomórfica é interpretada como resultado do truncamento do desenvolvimento. Tal processo evolutivo pode ter sido responsável pelos diferentes níveis de redução do canal sensorial de pequenos caracídeos. A variação neste caráter é comum, até entre os lados do mesmo indivíduo. Por isso, reafirmamos que o grau de desenvolvimento do canal sensorial do corpo deve ser usado com cuidado em estudos taxonômicos e filogenéticos, porque reduções no sistema látero-sensorial podem significar perdas paralelas em Characidae. Sugerimos que a espécie nova seja categorizada como Quase Ameaçada devido à distribuição geográfica restrita e ao declínio contínuo da qualidade do habitat.
Palavras-chave:
Desenvolvimento Truncado; Escama; Evolução; Pedomorfose; Variação Intraespecífica
INTRODUCTION
Moenkhausia Eigenmann, 1903 is the third most species-rich genus among Amazonian fishes, behind only of Corydoras Lacepède, 1803 and Hyphessobrycon Durbin, 1908 (Dagosta, de Pinna, 2019Dagosta FCP, de Pinna M. The fishes of the Amazon: distribution and biogeographical patterns, with a comprehensive list of species. Bull Am Mus Nat Hist. 2019; (431):1–163. Available from: http://digitallibrary.amnh.org/handle/2246/6940
http://digitallibrary.amnh.org/handle/22...
). It is widely distributed in the Neotropical region (Fricke et al., 2020), with its greatest diversity housed within the limits of the Amazon basin, which contains more than 80% of the Moenkhausia species (Dagosta, de Pinna, 2019Dagosta FCP, de Pinna M. The fishes of the Amazon: distribution and biogeographical patterns, with a comprehensive list of species. Bull Am Mus Nat Hist. 2019; (431):1–163. Available from: http://digitallibrary.amnh.org/handle/2246/6940
http://digitallibrary.amnh.org/handle/22...
). The genus has a remarkable diversity of shapes and colors, including some of the most beautiful characids, such as Moenkhausia agnesae Géry, 1965, M. cosmopsLima, Britski & Machado, 2007Lima FCT, Britski HA, Machado FA. A new Moenkhausia (Characiformes: Characidae) from central Brazil, with comments on the area relationship between the upper rio Tapajós and upper rio Paraguai systems. Aqua. 2007; 13:45–54., and M. heikoi Géry & Zarske, 2004.
The genus was defined in a precladistic view, considering a combination of characters of common occurrence in the Characidae, which are premaxillary teeth in two rows, with at least five teeth in the inner row, caudal fin partially covered by scales, and all scales of the lateral line trunk canal pored (Eigenmann, 1917Eigenmann CH. The American Characidae (Part 1). Mem Mus Comp Zool. 1917; 43:1–102.). This classification criterion of Eigenmann (1917Eigenmann CH. The American Characidae (Part 1). Mem Mus Comp Zool. 1917; 43:1–102., 1918Eigenmann CH. The American Characidae (Part 2). Mem Mus Comp Zool. 1918; 43:103–208., 1921)Eigenmann CH. The American Characidae (Part 3). Mem Mus Comp Zool. 1921; 43:209–310. , although efficient for decades, has been subject to criticism since most of the characters used to diagnose genera are known to have independently evolved within the family (Mirande, 2010Mirande JM. Phylogeny of the family Characidae (Teleostei: Characiformes): from characters to taxonomy. Neotrop Ichthyol. 2010; 8(3):385–568. https://doi.org/10.1590/S1679-62252010000300001
https://doi.org/10.1590/S1679-6225201000...
, 2018Mirande JM. Morphology, molecules and the phylogeny of Characidae (Teleostei, Characiformes). Cladistics. 2018; 35(3):282–300. https://doi.org/10.1111/cla.12345
https://doi.org/10.1111/cla.12345...
).
Starting with Costa (1994), many authors have assigned species with an incompletely pored lateral line in Moenkhausia, arguing those were probably more closely related to species nowadays included in Moenkhausia than to species of Hemigrammus Gill, 1858, which are diagnosed from the former by having an incomplete lateral line (e.g., Lima, Toledo-Piza, 2001Lima FCT, Toledo-Piza M. New species of Moenkhausia (Characiformes: Characidae) from the rio Negro of Brazil. Copeia. 2001; (4):1058–63. https://doi.org/10.1643/0045-8511(2001)001[1058:NSOMCC]2.0.CO;2
https://doi.org/10.1643/0045-8511(2001)0...
; Lima et al., 2007Lima FCT, Britski HA, Machado FA. A new Moenkhausia (Characiformes: Characidae) from central Brazil, with comments on the area relationship between the upper rio Tapajós and upper rio Paraguai systems. Aqua. 2007; 13:45–54.; Benine et al., 2009Benine RC, Mariguela TC, Oliveira C. New species of Moenkhausia Eigenmann, 1903 (Characiformes: Characidae) with comments on the Moenkhausia oligolepis species complex. Neotrop Ichthyol. 2009; 7(2):161–68. https://doi.org/10.1590/S1679-62252009000200005
https://doi.org/10.1590/S1679-6225200900...
; Marinho, Langeani, 2010Marinho MMF, Langeani F. Moenkhausiacelibela: a new species from the Amazon basin, Brazil (Characiformes: Characidae). J Fish Biol. 2010; 77(4):879–89. http://dx.doi.org/10.1111/j.1095-8649.2010.02719.x
http://dx.doi.org/10.1111/j.1095-8649.20...
; Ohara, Lima, 2015aOhara WM, Lima FCT. Moenkhausia uirapuru, a new species from the upper Rio Guaporé, Chapada dos Parecis, Mato Grosso, Brazil (Teleostei: Characidae). Ichthyol Explor Freshw. 2015a; 26(2):159–70.). A further issue of criticism is that the trunk lateral line canal may vary within species and even at the same individual. Such observations are not recent. Lütken (1875)Lütken CF. Velhas-Flodens fiske: et bidrag til Brasiliens ichthyologi: efter Professor J. Reinhardts indsamlinger og optegnelser. Kongelige Danske videnskabernes selskab. 1875; 12(2):121–253. https://doi.org/10.5962/bhl.title.101459
https://doi.org/10.5962/bhl.title.101459...
mentioned that some specimens of Psalidodon rivularis (Lütken, 1875Lütken CF. Velhas-Flodens fiske: et bidrag til Brasiliens ichthyologi: efter Professor J. Reinhardts indsamlinger og optegnelser. Kongelige Danske videnskabernes selskab. 1875; 12(2):121–253. https://doi.org/10.5962/bhl.title.101459
https://doi.org/10.5962/bhl.title.101459...
) have complete lateral line, others incomplete. Even Eigenmann (1917:83Eigenmann CH. The American Characidae (Part 1). Mem Mus Comp Zool. 1917; 43:1–102., 1918Eigenmann CH. The American Characidae (Part 2). Mem Mus Comp Zool. 1918; 43:103–208.:110) pointed out reductions in the perforation of the lateral line scales in some populations of Moenkhausia sanctaefilomenae (Steindachner, 1907) and M. cotinhoEigenmann, 1908Eigenmann CH. Preliminary descriptions of new genera and species of tetragonopterid characins. Bull Mus Comp Zool. 1908; 52(6):91–106.. Eigenmann, Henn (1914)Eigenmann CH, Henn AW. On new species of fishes from Colombia, Ecuador, and Brazil. Indiana University Studies. 1914; 140(24):231–34. documented variation in the development of the lateral line in Hemigrammus barrigonaeEigenmann & Henn, 1914Eigenmann CH, Henn AW. On new species of fishes from Colombia, Ecuador, and Brazil. Indiana University Studies. 1914; 140(24):231–34.. For decades later, several authors have mentioned variation in this character in many species of Characidae, which we summarized in this paper.
Field expedition to upper portions of the rio Machado, rio Madeira drainage, Amazon basin, Brazil and fish collections analysis revealed a new characid with variably developed bony tube along the lateral line length, with specimens failing to develop tube in some scales of the lateral line. This paper aims to describe the new species in detail and to discuss trunk lateral-line morphology in the Characidae, considering the evolutionary development of this character and the systematic of the family.
MATERIAL AND METHODS
Counts and measurements follow Fink, Weitzman (1974)Fink WL, Weitzman SH. The so-called Cheirodontin fishes of Central America with description of two new species (Pisces, Characidae). Smithson Contrib Zool. 1974; 72:1–46. http://dx.doi.org/10.5479/si.00810282.172
http://dx.doi.org/10.5479/si.00810282.17...
and Menezes, Weitzman (1990)Menezes NA, Weitzman SH. Two new species of Mimagoniates (Teleostei: Characidae: Glandulocaudinae), their phylogeny and biogeography and a key to the glandulocaudin fishes of Brazil and Paraguay. Proc Biol Soc Wash. 1990; 103(2):380–426., except for the number of horizontal scale rows below the lateral line counted to the pelvic-fin insertion, but not including the axillary scale, and with the addition of the pelvic-fin origin to anal-fin origin distance. Standard length (SL) and notochord length (NL) is expressed in millimeters (mm) and all other measurements are expressed as percentage of SL, except for subunits of head, which are expressed as percentage of head length (HL). In the description, counts are followed by their frequency of occurrence in parentheses. Asterisk indicates the counts of the holotype. Counts of supraneurals, tooth cusps, small dentary teeth, unbranched anal-fin rays, procurrent caudal-fin rays, and the position of the pterygiophores were taken from cleared and stained (CS) specimens prepared according to Taylor, Van Dyke (1985)Taylor WR, Van Dyke GC. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium. 1985; 9(2):107–19.. Vertebrae of the Weberian apparatus were counted as four elements and the compound caudal centra (PU1+U1) as a single element. Abdominal vertebrae include the Weberian apparatus and the vertebrae associated with ribs or hemal arches without hemal spine. Caudal vertebrae are vertebra associated with hemal spine. Circuli and radii counts were taken from scale row immediately above the lateral line. Catalog numbers are followed by the number of specimens in alcohol, number of specimens measured and counted in parentheses, SL range of all specimens of the lot, and if any, the number of CS specimens and their respective SL range. Map was generated in the QGIS 3.14.16 program. Institutional abbreviations follow Sabaj (2019)Sabaj MH. Standard symbolic codes for institutional resource collections in herpetology and ichthyology. Version 7.1 [Internet]. Washington, D.C.; 2019. Available from: http://www.asih.org/standard-symbolic-codes/about-symbolic-codes
http://www.asih.org/standard-symbolic-co...
.
RESULTS
Moenkhausia cambacica, new species
urn:lsid:zoobank.org:act:329C1539-307D-4E05-B8C9-148888CEB0E3
Holotype. MZUSP 125792, 34.8 mm SL. Brazil, Rondônia State, Municipality of Vilhena, rio Madeira basin, upper rio Machado, tributary of igarapé Ávila, near BR-364 road, 12°30’36.9”S 60°28’20.29”W, 12 Nov 2014, W. M. Ohara, F. C. P. Dagosta & V. Giovannetti.
Paratypes. All from Brazil, Rondônia State, Municipality of Vilhena, upper rio Machado, rio Madeira basin. MCP 39852, 19, 16.2–28.5 mm SL, rio Ávila at BR-364 road between Vilhena and Pimenta Bueno, 12º30’18”S 60º28’15”W, 14 Sep 2004, P. Lehmann, V. A. Bertaco & F. C. T. Lima. MZUSP 125793, 12, 27.2–35.9 mm SL, 2 CS, 26.5 and 29.0 mm SL, same data of holotype. MZUSP 115277, 1, 26.1 mm SL, upper rio Machado, tributary of igarapé Piracolina, near BR-364 road, 12°48’56.5”S 60°6’37.6”W, 14 Sep 2014, W. M. Ohara, D. Hungria & B. Barros. MZUSP 118576, 5, 23.7–29.3 mm SL, BR-364 road, km 60 to Porto Velho, 12°30’37.2”S 60°28’20.9”W, 19 Nov 2013, W. M. Ohara, D. Hungria & B. Barros.
Holotype of Moenkhausia cambacica, MZUSP 125792, 34.8 mm SL, Brazil, Rondônia State, Municipality of Vilhena, rio Madeira basin, upper rio Machado drainage.
Diagnose.Moenkhausia cambacica is distinguished from all congeners, except M. chlorophthalmaSousa, Netto-Ferreira & Birindelli, 2010Sousa LM, Netto-Ferreira AL, Birindelli JLO. Two new species of Moenkhausia Eigenmann (Characiformes: Characidae) from Serra do Cachimbo, Pará, Northern Brazil. Neotrop Ichthyol. 2010; 8(2):255–64. http://dx.doi.org/10.1590/S1679-62252010000200003
http://dx.doi.org/10.1590/S1679-62252010...
, M. petymbuaba Lima & Birindelli, 2006, M. plumbea Sousa, Netto-Ferreira & Birindelli, 2010Sousa LM, Netto-Ferreira AL, Birindelli JLO. Two new species of Moenkhausia Eigenmann (Characiformes: Characidae) from Serra do Cachimbo, Pará, Northern Brazil. Neotrop Ichthyol. 2010; 8(2):255–64. http://dx.doi.org/10.1590/S1679-62252010000200003
http://dx.doi.org/10.1590/S1679-62252010...
, and M. parecisOhara & Marinho, 2016Ohara WM, Marinho MMF. A new species of Moenkhausia Eigenmann (Characiformes: Characidae) from the upper rio Machado at Chapada dos Parecis, rio Madeira basin, Brazil. Neotrop Ichthyol. 2016; 14(1):e150041. https://doi.org/10.1590/1982-0224-20150041
https://doi.org/10.1590/1982-0224-201500...
by the presence of a large dark blotch on each scale of the second to seventh longitudinal series of body which are formed by a higher concentration of cromatophores on the anterior portion of scales (vs. pigmentation absent or, when present, concentrated at the middle or posterior margin of scales, forming stripes or a reticulate pattern). Moenkhausia cambacica can be readily distinguished from all the aforementioned species by having a conspicuous, well-defined, horizontally elongate blotch on the caudal peduncle, extending to middle caudal-fin rays, not reaching the upper and lower edges of the caudal peduncle (vs. caudal peduncle blotch absent or poorly defined, continuous with the longitudinal stripe of body in M. clorophthalma, M. petymbuaba, and M. plumbea; round blotch in M. parecis). Additionally, it can be distinguished from M. petymbuaba by the absence of a conspicuous longitudinal black stripe on body (vs. black stripe present), from M. plumbea and M. clorophthalma by the absence of a dark, diffuse, slightly concave midlateral stripe on body in live specimens (vs. dark stripe present), and from M. parecis by a shorter upper jaw length (41.5–48.8% HL vs. 50.6–55.0% HL), and, in life, by having a bright golden coloration of the dorsal portion of the eye and a dark shaded line crossing the eye horizontally (vs. eye entirely bright blue, with no horizontal dark line).
Description. Morphometric data of the holotype and paratypes presented in Tab. 1. Body moderately elongate, laterally compressed. Largest specimen examined 35.9 mm SL. Greatest body depth slightly anterior to the vertical through dorsal-fin origin. Dorsal profile of head convex from anterior tip of upper jaw to vertical through anterior nostril. Straight or slightly convex from that point to tip of supraoccipital spine. Dorsal body profile straight or slightly convex from tip of supraoccipital spine to dorsal-fin origin, straight along dorsal-fin base, straight from base of last dorsal-fin ray to adipose-fin insertion and slightly concave along caudal peduncle. Ventral profile of body convex from anterior tip of dentary to anal-fin origin, straight at anal-fin base and slightly concave along caudal peduncle.
Morphometric data of Moenkhausia cambacica. Range includes the holotype. SD = Standard deviation.
Mouth terminal, jaws equal. Posterior terminus of maxilla at the vertical through middle of pupil. Maxilla approximately at 45 degrees angle relative to longitudinal axis of body. Frontals with a triangle-shaped fontanel; parietal fontanel large, extending from epiphyseal bar to supraoccipital spine. Infraorbital series with six elements. Nostrils close to each other, anterior opening circular and small, crescent-shaped posterior one, twice in size. Nostrils separated by narrow skin flap.
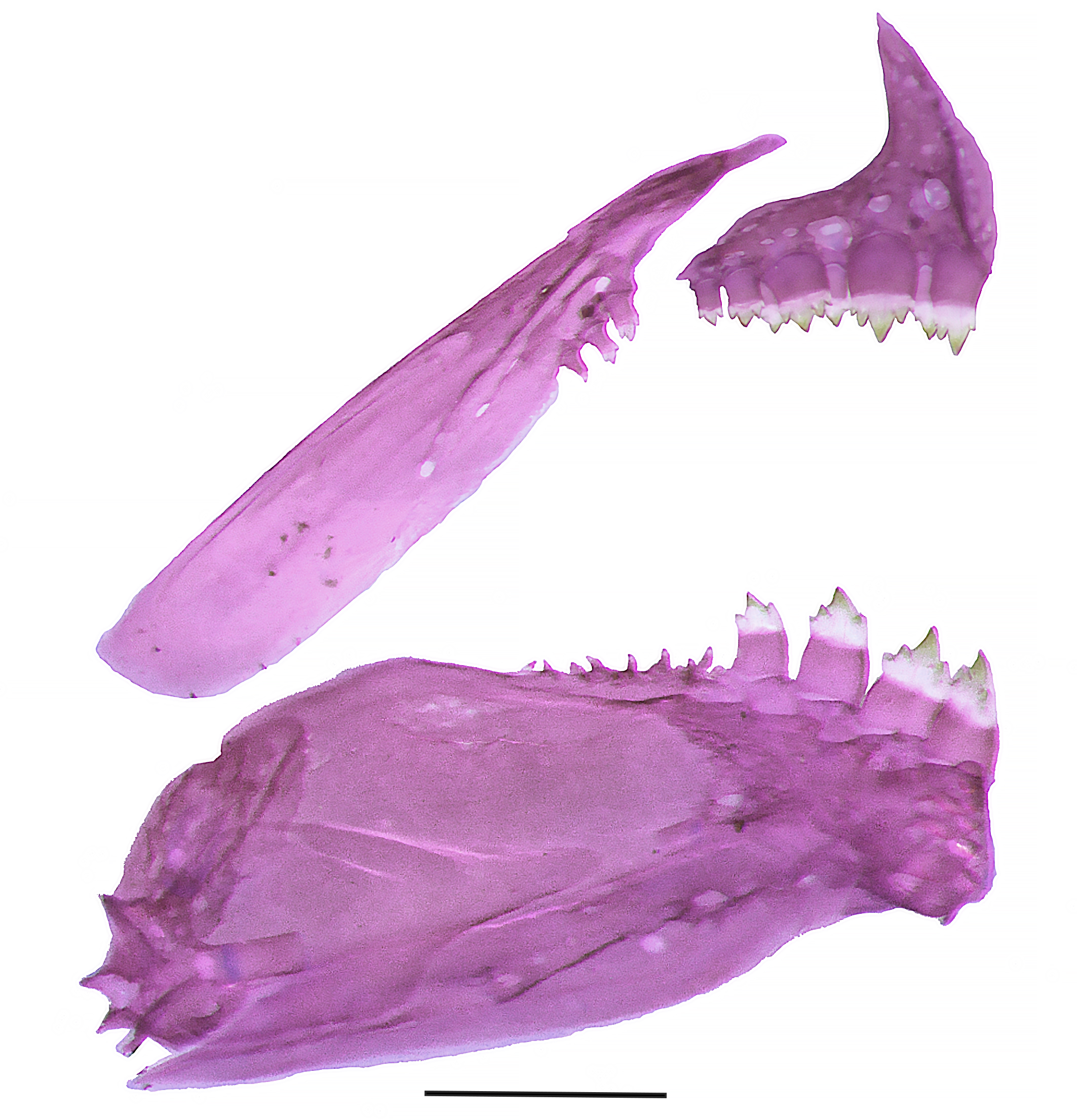
Premaxillary teeth in two rows. Outer tooth row with 3*(1), 4(25) or 5(1) tricuspid teeth; inner tooth row with 4(1) or 5*(25) teeth with three to five cusps, symphyseal tooth of inner series narrow, asymmetric, with four cuspids. Tooth cusps of inner premaxillary tooth row directed outward and arranged in an arched series. Maxilla with 2*(9), 3(17), or 4(1) teeth along its anterodorsal margin, with one to three cusps (Fig. 2). Dorsalmost tooth usually larger. Dentary with 4*(26) or 5(1) larger tri- to pentacuspid teeth, followed by a series of 9(1) or 11(1) diminute conical teeth. Tooth cusps of larger dentary teeth arranged directed inward and arranged in an arched series. Central cusp of all multicuspid teeth more developed than remaining lateral cusps.
Medial view of left side, premaxillary, maxillary, and dentary of Moenkhausia cambacica, MZUSP 125793, 27.1 mm SL, paratype. Scale bar: 1 mm.
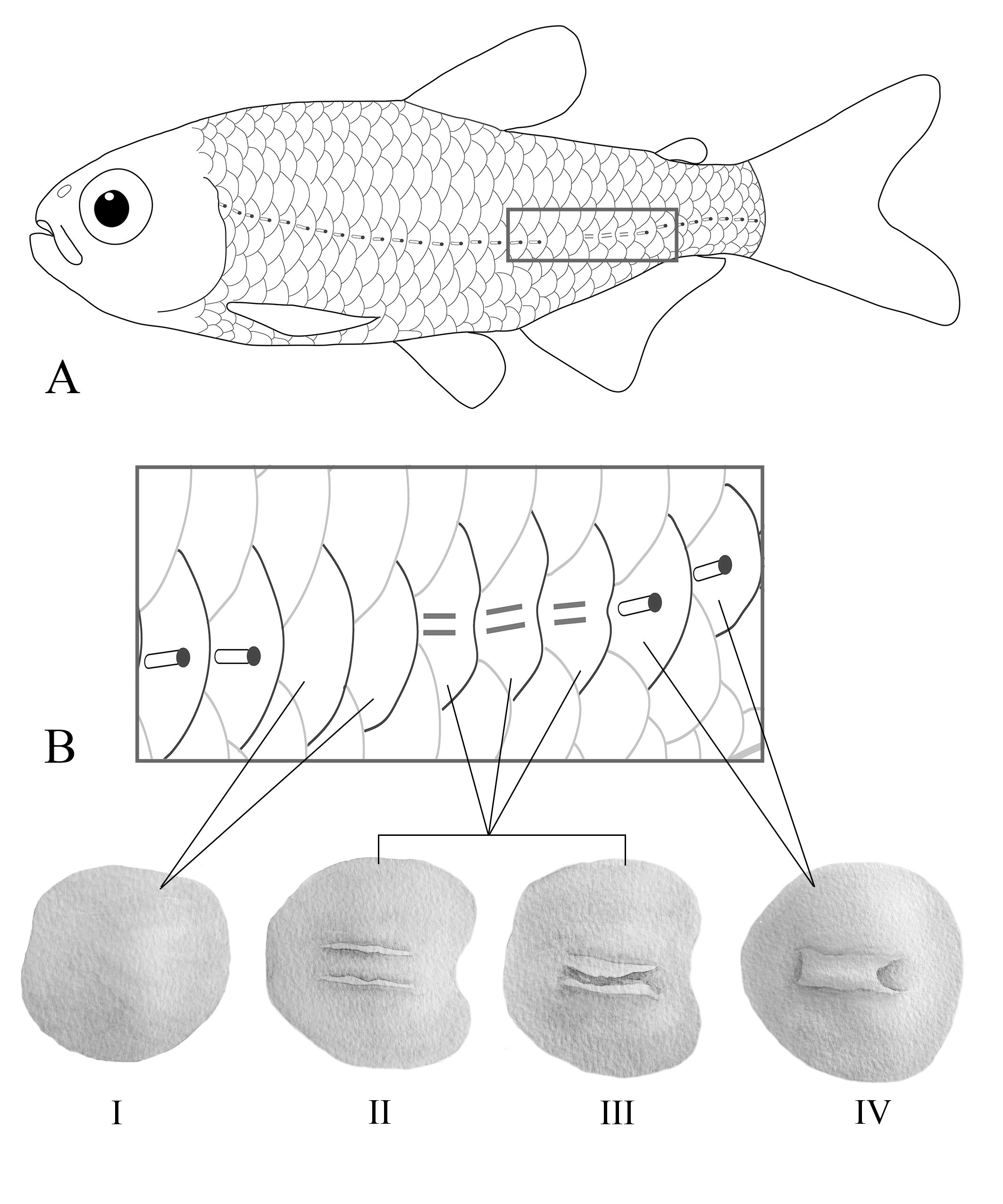
Scales cycloid, moderately large, circuli distributed over whole area of scales. Three to seven radii well defined and slightly divergent posteriorly. Lateral line slightly curved downward anteriorly, with variably developed bony tube. Four specimens (including holotype) with fully developed tube in all lateral-line scales, terminating in a pore (e.g., lateral line complete, with 31(1) and 32*(3) pored scales from supracleithrum to the end of caudal peduncle). Twenty-one specimens with fully developed tube in all lateral-line scales of the anterior and posterior portions of body, and, at the level of the anal-fin base, tubed scales interspersed by scales without bony tube and/or scales with poorly developed tube, with variable count (e.g., 22 tubed scales with pore + 2 scales without tube or pore + 3 scales with poorly developed tube and no pore + 3 tubed scales with pore) (Fig. 3), with a total of 30(2), 31(8), 32(7), or 33(1) scales in the lateral series (see details in the Discussion). Longitudinal scale rows between dorsal-fin origin and lateral line 5*(24). Longitudinal scale rows between lateral line and pelvic-fin origin 3(7) or 4*(17). Predorsal area with 9(11) or 10*(13) scales arranged in one series. Horizontal scale rows around caudal peduncle 14*(24). Single row of 4(7), 5(7), 6(3), or 7*(2) scales covering base of anteriormost anal-fin rays. Caudal fin with small scales on the basal fourth of caudal-fin lobes.
Supraneurals 4(2) with narrow bony lamellae on upper portion. Dorsal-fin rays ii*(27), 9*(27). Dorsal-fin origin at middle of standard length and slightly posterior to vertical through pelvic-fin origin. First unbranched dorsal-fin ray shorter than second unbranched ray. First dorsal-fin pterygiophore located behind neural spine of 9th(2) vertebra. Adipose fin present. Anal-fin rays v(2), 15(4), 16(15), 17*(7), or 18(1); anteriormost rays longer, subsequent rays gradually decreasing in size. Anteriormost anal-fin pterygiophore inserted posterior to haemal spine of 16th(2) vertebra. Pectoral-fin rays i*(27), 10(1), 11*(14), or 12(12). Tip of adpressed pectoral fin not reaching pelvic-fin origin in most specimens. Pelvic-fin rays i*(27), 7*(27). Tip of adpressed pelvic fin reaching the anal-fin origin. Caudal-fin with i*(26), 9*(26) rays on the upper and i*(26), 8*(26) rays on the lower lobe. Caudal-fin forked, lobes somewhat pointed and of similar size. Twelve (1) or 13(1) dorsal procurrent caudal-fin rays and 10(2) ventral procurrent caudal-fin rays. Total vertebrae 31(2): precaudal vertebrae 16(2) and caudal vertebrae 15(2).
Schematic drawing of Moenkhausia cambacica showing A. lateral-line perforation pattern observed in most specimens. B. Morphology of the lateral-line scales above anal fin: I – scale lacking tube and pore; II – scale with poorly developed tube, with small tube walls; III – scale with poorly developed tube, walls larger but not enclosed; IV– scale with fully developed bony tube, tube walls enclosed, with a posterior pore.
Color in alcohol. Overall ground color pale, with small dark chromatophores spread at the entire head and body, except the ventral portion of abdominal region, and densely concentrated in its dorsal portion, gradually fading ventrally (Fig. 1). Dorsal midline of head and body dark brown. Jaws, opercular, and infraorbital areas pigmented with dark chromatophores. Single, dark humeral blotch, vertically oriented, extending vertically two scale rows above and one scale row below the lateral line. Dorsal portion of humeral blotch wider, over three scales horizontally. Ventral portion narrow, slightly turned anteriorly, over one scale. Thin longitudinal dark stripe at horizontal septum, formed by underlying chromatophores extending from vertical through dorsal-fin origin to caudal peduncle. Conspicuous dark horizontal blotch on caudal peduncle, extending to base of midlle caudal-fin rays, never reaching the upper and lower edges of caudal peduncle. Horizontal blotch on caudal peduncle frequently extending to tip of middle caudal-fin rays. Lower portion of caudal peduncle with a clear area. Second to seventh horizontal scale rows with scales bearing dark blotches on its anterior portion. All fins with scattered dark chromatophores on interadial membranes. Distal portion of interadial membranes of dorsal fin with concentration of dark chromatophores.
Color in life. Dorsal portion of head and body light brown. Ventral half of head and body pale yellow (Fig. 4). Infraorbital and opercular areas silvery. Dorsal portion of eye bright golden, ventral portion silvery with blue hue. Dark shaded line crossing the eye horizontally (Fig. 5). Vertical arm of preopercle yellow golden. Bright yellow to orange blotch anterior and posteriorly to the humeral blotch (Fig. 5). Second to seventh horizontal scales row with scales bearing brown blotches on its anterior portion. Humeral blotch and caudal-peduncle spot conspicuous in life. All fins with orange to yellow coloration, more intense at the anterior half of caudal-fin lobes. Posterior tip of caudal and dorsal fins hyaline.
Live coloration of Moenkhausia cambacica, paratype, MZUSP 125793, Brazil, Rondônia State, Municipality of Vilhena, rio Madeira basin, upper rio Machado drainage.
Paratypes of Moenkhausia cambacica, MZUSP 125793, freshly collected, showing other aspects of its live coloration, Brazil, Rondônia State, Municipality of Vilhena, rio Madeira basin, upper rio Machado drainage.
Sexual dimorphism. Secondary dimorphic characters were not found in the examined specimens.
Geographical distribution. The new species is so far only known from two headwater tributaries of the upper rio Machado at Chapada dos Parecis, Rondônia State, Brazil (Fig. 6). Intensive ichthyological collecting efforts in the rio Madeira basin (e.g., Queiroz et al., 2013Queiroz LJ, Torrente-Vilara G, Ohara WM, Pires THS, Zuanon J, Dória CRC, organizers. Peixes do rio Madeira. São Paulo: Dialeto Latin American Documentary; 2013.), including the rio Machado drainage (e.g., Perin et al., 2007Perin L, Shibatta OA, Bernarde PS. Fish, Machado River basin, Cacoal urban area, state of Rondônia, Brazil. Check List. 2007; 3(2):94–97. https://doi.org/10.15560/3.2.94
https://doi.org/10.15560/3.2.94...
; Casatti et al., 2013Casatti L, Pérez-Mayorga MA, Carvalho FR, Brejão GL, Costa ID. The stream fish fauna from the Rio Machado basin, Rondônia State, Brazil. Check List. 2013; 9(6):1496–504. https://doi.org/10.15560/9.6.1496
https://doi.org/10.15560/9.6.1496...
; Costa et al., 2017Costa ID, Ohara WM, Almeida M. Fishes from the Jaru Biological Reserve, Machado River drainage, Madeira River basin, Rondônia State, northern Brazil. Biota Neotrop. 2017; 17(1):e20160315. http://dx.doi.org/10.1590/1676-0611-BN-2016-0315
http://dx.doi.org/10.1590/1676-0611-BN-2...
) have failed to capture M. cambacica in other streams, indicating a very restricted distribution to the tributaries draining the Chapada dos Parecis.
Distribution of Moenkhausia cambacica in the upper rio Machado, rio Madeira basin, Brazil. Black star (type-locality), blue star (other localities).
Ecological notes. The type locality of Moenkhausia cambacica is a Balneário (recreation area) upstream the Cachoeira Small Hidroeletric Dam (PCH, Pequena Central Hidrelétrica), and is located at 415 m above sea level. The stream is small, 2–4 m wide and 0.5–2 m deep, with clear waters with swift current, and bottom composed of sand and dead leaves (Fig. 7). Other species collected syntopically were: Ancistrus verecundus Fisch-Muller, Cardoso, da Silva & Bertaco, 2005, Astyanax aff. bimaculatus (Linnaeus, 1758), Bryconops piracolina Wingert & Malabarba, 2011, Erythrinus erythrinus (Bloch & Schneider, 1801), Cetopsorhamdia sp. 3 (cf.Bockmann, Slobodian, 2013Bockmann FA, Slobodian V. Heptapteridae. In: Queiroz LJ, Torrente-Vilara G, Ohara WM, Pires TS, Zuanon J, Doria CRC, editors. Peixes do rio Madeira. São Paulo: Dialeto Latim America Documentary; vol. III, 2013; p.14–77. :25), Aequidens sp., and Crenicichla sp. A single M. cambacica specimen was collected in a tributary of rio Piracolina near Vilhena at altitude 591 m a.s.l., in a small, clear water stream 1–1.5 m wide and 0.3–1.5 m deep, presenting swift water current and sandy bottom. This specimen was collected syntopically with M. parecis and other species (e.g., A. verecundus, B. piracolina, Cetopsorhamdia sp. 3, Corydoras hephaestus Ohara, Tencatt & Britto, 2016, Hyphessobrycon lucenorumOhara & Lima, 2015Ohara WM, Lima FCT. Hyphessobrycon lucenorum (Characiformes: Characidae), a new species from the rio Madeira basin, Rondônia State, Brazil. Zootaxa. 2015b; 3972(4):562–72. https://doi.org/10.11646/zootaxa.3972.4.7
https://doi.org/10.11646/zootaxa.3972.4....
, Hyphessobrycon aff. melanostichos Carvalho & Bertaco, 2006, Hyphessobrycon aff. notidanos Carvalho & Bertaco, 2006, and Pyrrhulina sp.).
Type-locality of Moenkhausia cambacica, tributary of igarapé Ávila, upper rio Machado, rio Madeira basin, Vilhena, Rondônia, Brazil.
Etymology. The specific name, cambacica, is after the one of the Brazilian popular name for Coereba flaveola (Linnaeus, 1758), a small neotropical bird whose coloration resembles that of the new species, which is bright yellow underparts, dark back coloration and a dark line crossing the region of the eye horizontally, contrasting with a light area above it. A noun in apposition.
Conservation status.Moenkhausia cambacica is another endemic species from the ‘Chapada dos Parecis’ biogeographic region, characterized by high levels of endemicity and large number of restricted-range species (Ohara, Lima, 2015aOhara WM, Lima FCT. Moenkhausia uirapuru, a new species from the upper Rio Guaporé, Chapada dos Parecis, Mato Grosso, Brazil (Teleostei: Characidae). Ichthyol Explor Freshw. 2015a; 26(2):159–70.,bOhara WM, Lima FCT. Hyphessobrycon lucenorum (Characiformes: Characidae), a new species from the rio Madeira basin, Rondônia State, Brazil. Zootaxa. 2015b; 3972(4):562–72. https://doi.org/10.11646/zootaxa.3972.4.7
https://doi.org/10.11646/zootaxa.3972.4....
; Dagosta et al., 2020Dagosta FCP, de Pinna M, Peres CA, Tagliacollo VA. Existing protected areas provide a poor safetynet for threatened Amazonian fish species. Aquatic Conserv. 2020; 1–23. https://doi.org/10.1002/aqc.3461
https://doi.org/10.1002/aqc.3461...
). This biogeographic region was considered by latter authors as one of the Endemic Amazonian Fish Areas (EAFAs), i.e., regions that should be considered as conservation priorities in the basin by presenting imminent threats and low cover of protected areas. Moenkhausia cambacica is endemic to Brazil, known by only two localities. One site is a tourist bathing resort and the other is entirely surrounded by monoculture plantation. Its area of occupancy (AOO) (B2) 8 km2 is based on these two known records. The AOO is likely underestimated, although the region has already been largely sampled. A continuing decline in habitat quality b(iii) is inferred based on the deforestation caused by still growing urbanization and agriculture activity in the region. It is not possible to meet subcriterion ‘a’ because the population is not necessarily fragmented. Therefore, we suggest this species is assessed as Near Threatened, close to meeting Critically Endangered (CR) by the following criteria B2b(iii) according to the International Union for Conservation of Nature (IUCN) categories and criteria (IUCN Standards and Petitions Subcommittee, 2019).
DISCUSSION
Moenkhausia cambacica presents a series of dark blotches on body, located at the anterior portion of scales, an unusual coloration within the Characidae. Sousa et al., (2010)Sousa LM, Netto-Ferreira AL, Birindelli JLO. Two new species of Moenkhausia Eigenmann (Characiformes: Characidae) from Serra do Cachimbo, Pará, Northern Brazil. Neotrop Ichthyol. 2010; 8(2):255–64. http://dx.doi.org/10.1590/S1679-62252010000200003
http://dx.doi.org/10.1590/S1679-62252010...
used this feature to indicate a close relationship between M. clorophthalma, M. petymbuaba, and M. plumbea. Ohara, Marinho (2016)Ohara WM, Marinho MMF. A new species of Moenkhausia Eigenmann (Characiformes: Characidae) from the upper rio Machado at Chapada dos Parecis, rio Madeira basin, Brazil. Neotrop Ichthyol. 2016; 14(1):e150041. https://doi.org/10.1590/1982-0224-20150041
https://doi.org/10.1590/1982-0224-201500...
, described the same character in M. parecis and considered it as closely related to that group of species. Additionally, Ohara, Marinho (2016)Ohara WM, Marinho MMF. A new species of Moenkhausia Eigenmann (Characiformes: Characidae) from the upper rio Machado at Chapada dos Parecis, rio Madeira basin, Brazil. Neotrop Ichthyol. 2016; 14(1):e150041. https://doi.org/10.1590/1982-0224-20150041
https://doi.org/10.1590/1982-0224-201500...
observed these species further share characters such as a relatively large head, round dorsal-fin profile, and a relatively short anal-fin base. These features are also observed in M. cambacica. These five species of Moenkhausia also have colored eyes (totally green in M. clorophthalma, partially green in M. petymbuaba, totally blue in M. parecis, yellowish with a longitudinal dark stripe in M. plumbea and M. cambacica) (Sousa et al., 2010Sousa LM, Netto-Ferreira AL, Birindelli JLO. Two new species of Moenkhausia Eigenmann (Characiformes: Characidae) from Serra do Cachimbo, Pará, Northern Brazil. Neotrop Ichthyol. 2010; 8(2):255–64. http://dx.doi.org/10.1590/S1679-62252010000200003
http://dx.doi.org/10.1590/S1679-62252010...
; Ohara, Marinho, 2016Ohara WM, Marinho MMF. A new species of Moenkhausia Eigenmann (Characiformes: Characidae) from the upper rio Machado at Chapada dos Parecis, rio Madeira basin, Brazil. Neotrop Ichthyol. 2016; 14(1):e150041. https://doi.org/10.1590/1982-0224-20150041
https://doi.org/10.1590/1982-0224-201500...
). Therefore, it is reasonable to assume M. cambacica is closely related to members of this group of species although a phylogenetic analysis is needed to corroborate this hypothesis.
Trunk lateral line and the systematics of the Characidae. The mechanoreceptive lateral-line system in fishes is typically composed of a series of neuromasts included in pored canals and of superficial neuromasts in the head and body (Webb, 1989Webb JF. Gross morphology and the evolution of the mechanoreceptive lateral-line system in teleost fishes. Brain Behav Evol. 1989; 33(1):34–53. https://doi.org/10.1159/000115896
https://doi.org/10.1159/000115896...
; Pastana et al., 2019Pastana MNL, Bockmann FA, Datovo A. The cephalic lateral-line system of Characiformes (Teleostei: Ostariophysi): anatomy and phylogenetic implications. Zool J Linn Soc. 2019; 189(1):1–46. https://doi.org/10.1093/zoolinnean/zlz105
https://doi.org/10.1093/zoolinnean/zlz10...
). Scaled fishes generally present the trunk canal contained within scales of the lateral-line series bearing tubes, which are bony canal walls and roofs that extend upward from the scale plate, surrounding the canal lumen. Adjacent lateral-line scales overlap, forming a continuous tube that is pored periodically, connecting the canal lumen to the environment (Wonsettler, Webb, 1997Wonsettler AL, Webb JF. Morphology and development of the multiple lateral line canals on the trunk in two species of Hexagrammos (Scorpaeniformes, Hexagrammidae). J Morphol. 1997; 233(3):195–214. https://doi.org/10.1002/(SICI)1097-4687(199709)233:3<195::AID-JMOR1>3.0.CO;2-3
https://doi.org/10.1002/(SICI)1097-4687(...
).
Eight trunk canal patterns are identified among teleosts (Coombs et al., 1988Coombs S, Janssen J, Webb JF. Diversity of lateral line systems: evolutionary and functional considerations. In: Atema Y, Fay RR, Popper AN, Tavolga WN, editors. Sensory biology of aquatic animals. New York: Springer-Verlag; 1988. p.553–95. Available from: https://link.springer.com/content/pdf/10.1007%2F978-1-4612-3714-3.pdf
https://link.springer.com/content/pdf/10...
; Webb, 1989Webb JF. Gross morphology and the evolution of the mechanoreceptive lateral-line system in teleost fishes. Brain Behav Evol. 1989; 33(1):34–53. https://doi.org/10.1159/000115896
https://doi.org/10.1159/000115896...
): 1) complete and straight, 2) complete and arched, 3) complete with dorsal displacement, 4) complete with ventral displacement, 5) multiple, 6) disjunct, 7) incomplete, and 8) absent. Among characids, are found (1) complete and straight, with tubed scales with a pore, extending from the supracleithrum to the caudal peduncle, (7) incomplete, with only the anteriormost scales of the lateral line tubed, from the supracleithrum to a variable extent on body, (8) absent, with scales lacking any tube or pore, and an additional condition, which is (9) discontinuous lateral line scale, with tubed scales interspersed by non-tubed scales. As discussed below, intraspecific variation can be found, i.e., species presenting complete, discontinuous, incomplete lateral line, in the same population.
Analyzed specimens of M. cambacica have variable morphology regarding trunk lateral line: four specimens (including the holotype) have a fully developed tube in all scales of the lateral-line series, which are pored posteromedially; remaining specimens (21) have scales with distinct levels of tube development along the lateral-line length (Fig. 3A). In these specimens, the anteriormost scales near supracleithrum and the posteriormost scales at the caudal peduncle bear fully developed tube with a pore (Fig. 3B, scale IV), whereas the scales located approximately at the level of the anal-fin base frequently lack tube and pore (Fig. 3B, scale I) or have poorly developed tube (i.e., tube not fully enclosed, represented by a superficial groove, frequently with a posterior slit and no apparent pore, Fig. 3B, scales II and III). In this specific area above anal-fin level, scales bearing a groove without fully developed tube are interspersed with tubed scales and/or scales lacking tube or pore, characterizing a discontinuous lateral line. Specimens with incomplete lateral line were not found.
Poorly developed bony tube of lateral line scales, i.e., those scales bearing a groove present in most specimens of M. cambacica (Fig. 3B, scales II and III), resembles early stages of formation of the bony tube of the trunk lateral line of other fish species. In both the scorpaeniform Hexagrammidae and the cypriniform zebrafish Danio rerio (Hamilton, 1822), bony tube development of lateral line scales starts as a pair of ridges that protrude outward forming a longitudinal groove which later fuses at the apical region to form the tube roof (Wonsettler, Webb, 1997Wonsettler AL, Webb JF. Morphology and development of the multiple lateral line canals on the trunk in two species of Hexagrammos (Scorpaeniformes, Hexagrammidae). J Morphol. 1997; 233(3):195–214. https://doi.org/10.1002/(SICI)1097-4687(199709)233:3<195::AID-JMOR1>3.0.CO;2-3
https://doi.org/10.1002/(SICI)1097-4687(...
; Wada et al., 2014Wada H, Iwasaki M, Kawakami K. Development of the lateral line canal system through a bone remodeling process in zebrafish. Dev Biol. 2014; 392(1):1–14. https://doi.org/10.1016/j.ydbio.2014.05.004
https://doi.org/10.1016/j.ydbio.2014.05....
). The same pattern of tube formation at scale level was observed in the development of Paracheirodon innesi (Myers, 1936) and Moenkhausia pittieri Eigenmann, 1920 (Marinho, 2017Marinho MMF. Comparative development in Moenkhausia pittieri and Paracheirodon innesi (Ostariophysi: Characiformes) with comments on heterochrony and miniaturization in the Characidae. J Fish Biol. 2017; 91(3):851–65. https://doi.org/10.1111/jfb.13384
https://doi.org/10.1111/jfb.13384...
). Also, all specimens examined are fully grown individuals, with other morphological aspects fully formed. Additionally, individuals of Moenkhausia cambacica with complete lateral line are of 28.6 to 35.9 mm SL and individuals with discontinuous lateral line are of 22.9 to 33.1 mm SL, not showing correlation between size and completeness of lateral line at this range of size.
Lack of tube formation has been repeatedly documented in Euteleostei and interpreted as a derived paedomorphic condition in many lineages (Myers, 1958Myers GS. Trends in the evolution of teleostean fishes. Stanford Ichthyol Bull. 1958; 7:27–30.; Webb, 1990Webb JF. Comparative morphology and evolution of the lateral line system in the Labridae (Perciformes: Labroidei). Copeia. 1990; (1):137–46. https://doi.org/10.2307/1445830
https://doi.org/10.2307/1445830...
; Montgomery et al., 1994Montgomery JC, Coombs S, Janssen J. Form and function relationships in lateral line systems: comparative data from six species of Antarctic notothenioid fish. Brain Behav Evol. 1994; 44(6):299–306. https://doi.org/10.1159/000113591
https://doi.org/10.1159/000113591...
; Coombs et al., 1998Coombs S, Janssen J, Webb JF. Diversity of lateral line systems: evolutionary and functional considerations. In: Atema Y, Fay RR, Popper AN, Tavolga WN, editors. Sensory biology of aquatic animals. New York: Springer-Verlag; 1988. p.553–95. Available from: https://link.springer.com/content/pdf/10.1007%2F978-1-4612-3714-3.pdf
https://link.springer.com/content/pdf/10...
; Wellenreuther et al., 2010Wellenreuther M, Brock M, Montgomery J, Clements KD. Comparative morphology of the mechanosensory lateral line system in a clade of New Zealand Triplefin fishes. Brain Behav Evol. 2010; 75:292–308. https://doi.org/10.1159/000317061
https://doi.org/10.1159/000317061...
). The same has been interpreted for characid lineages (Myers, 1958Myers GS. Trends in the evolution of teleostean fishes. Stanford Ichthyol Bull. 1958; 7:27–30.; Weitzman, 1962Weitzman SH. The osteology of Bryconmeeki, a generalized characid fish, with an osteological definition of the family. Stanford Ichthyol Bull. 1962; 8(1):1–77.; Weitzman, Fink, 1983Weitzman SH, Fink WL. Relationships of the neon tetras, a group of South American freshwater fishes (Teleostei: Characidae), with comments on the phylogeny of new world characiforms. Bull Mus Comp Zool. 1983; 150:339–95.; Weitzman, Vari, 1988Weitzman SH, Vari RP. Miniaturization in South American freshwater fishes: An overview and discussion. Proc Biol Soc Wash. 1988; 101(2):444–65. Available from: https://repository.si.edu/handle/10088/901
https://repository.si.edu/handle/10088/9...
; Mattox et al., 2016Mattox GMT, Britz R, Toledo-Piza M. Osteology of Priocharax and remarkable developmental truncation in a miniature amazonian fish (Teleostei: Characiformes: Characidae). J Morphol. 2016; 277(1):65–85. https://doi.org/10.1002/jmor.20477
https://doi.org/10.1002/jmor.20477...
; Marinho, 2017Marinho MMF. Comparative development in Moenkhausia pittieri and Paracheirodon innesi (Ostariophysi: Characiformes) with comments on heterochrony and miniaturization in the Characidae. J Fish Biol. 2017; 91(3):851–65. https://doi.org/10.1111/jfb.13384
https://doi.org/10.1111/jfb.13384...
; Pastana et al., 2017Pastana MNL, Dagosta FCP, Esguícero ALH. A new sexually dichromatic miniature Hyphessobrycon (Teleostei: Characiformes: Characidae) from the Rio Formiga, upper Rio Juruena basin, Mato Grosso, Brazil, with a review of sexual dichromatism in Characiformes. J Fish Biol. 2017; 91(5):1301–18. https://doi.org/10.1111/jfb.13449
https://doi.org/10.1111/jfb.13449...
; Camelier et al., 2018Camelier P, Dagosta FCP, Marinho MMF. New remarkable sexually dimorphic miniature species of Hyphessobrycon (Characiformes: Characidae) from the upper Rio Tapajós basin. J Fish Biol. 2018; 92(4):1149–62. https://doi.org/10.1111/jfb.13579
https://doi.org/10.1111/jfb.13579...
; Jerep et al., 2018Jerep FC, Dagosta FCP, Ohara WM. A new miniature species of Serrapinnus (Characiformes: Characidae) from the upper rio Araguaia, Brazil. Copeia. 2018; 106(1):180–87. https://doi.org/10.1643/CI-17-653
https://doi.org/10.1643/CI-17-653...
; Abrahão et al., 2019Abrahão VP, Pastana M, Marinho M. On a remarkable sexual dimorphic trait in the Characiformes related to the olfactory organ and description of a new miniature species of Tyttobrycon Géry (Characiformes: Characidae). Plos One. 2019; 14(12):e0226130. https://doi.org/10.1371/journal.pone.0226130
https://doi.org/10.1371/journal.pone.022...
). Therefore, we consider the scales with a longitudinal groove, observed in most fully grown specimens of M. cambacica, as a paedomorphic condition, a character resulted from the loss of terminal stages of development.
As widely discussed in the literature, the traditional classification of Characidae by Eigenmann (1917)Eigenmann CH. The American Characidae (Part 1). Mem Mus Comp Zool. 1917; 43:1–102. is based on features known to occur independently in numerous lineages within the family (Weitzman, Fink, 1983Weitzman SH, Fink WL. Relationships of the neon tetras, a group of South American freshwater fishes (Teleostei: Characidae), with comments on the phylogeny of new world characiforms. Bull Mus Comp Zool. 1983; 150:339–95.; Costa, 1994Costa WJEM. Description of two new species of the genus Moenkhausia (Characiformes: Characidae) from the central Brazil. Zool Anz. 1994; 232(1–2):21–29.; Mirande, 2010Mirande JM. Phylogeny of the family Characidae (Teleostei: Characiformes): from characters to taxonomy. Neotrop Ichthyol. 2010; 8(3):385–568. https://doi.org/10.1590/S1679-62252010000300001
https://doi.org/10.1590/S1679-6225201000...
; Dagosta et al., 2015Dagosta FCP, Marinho MMF, Benine RC. A new species of Moenkhausia Eigenmann (Characiformes: Characidae) from the upper rio Juruena basin, central Brazil. Zootaxa. 2015; 4032(4):417–25. https://doi.org/10.11646/zootaxa.4032.4.6
https://doi.org/10.11646/zootaxa.4032.4....
; Marinho, 2017Marinho MMF. Comparative development in Moenkhausia pittieri and Paracheirodon innesi (Ostariophysi: Characiformes) with comments on heterochrony and miniaturization in the Characidae. J Fish Biol. 2017; 91(3):851–65. https://doi.org/10.1111/jfb.13384
https://doi.org/10.1111/jfb.13384...
), resulting in non-monophyletic assemblages. One of the characters used in this classification system for establishing generic limits is the completeness of the trunk lateral line. Morphological reductions in many characids are result of loss of terminal stages in the developmental sequence that compromises late-forming structures, such as trunk lateral-line canals, resulting in incompletely pored lateral line or even absence (Weitzman, Vari, 1988Weitzman SH, Vari RP. Miniaturization in South American freshwater fishes: An overview and discussion. Proc Biol Soc Wash. 1988; 101(2):444–65. Available from: https://repository.si.edu/handle/10088/901
https://repository.si.edu/handle/10088/9...
; Marinho, 2017Marinho MMF. Comparative development in Moenkhausia pittieri and Paracheirodon innesi (Ostariophysi: Characiformes) with comments on heterochrony and miniaturization in the Characidae. J Fish Biol. 2017; 91(3):851–65. https://doi.org/10.1111/jfb.13384
https://doi.org/10.1111/jfb.13384...
). This is a common process in small characids, not exclusive to miniaturized species sensuWeitzman, Vari (1988)Weitzman SH, Vari RP. Miniaturization in South American freshwater fishes: An overview and discussion. Proc Biol Soc Wash. 1988; 101(2):444–65. Available from: https://repository.si.edu/handle/10088/901
https://repository.si.edu/handle/10088/9...
, i.e., species reaching a maximum of 26 mm SL. In view of that, distinct levels of trunk lateral line reductions observed in the family (i.e., discontinuous, incomplete or absence of lateral line) are likely associated to a distinct degree of loss of terminal stages of development. It can be observed as a process occurring at species level (e.g., Hemigrammus ataktosMarinho, Dagosta & Birindelli, 2014Marinho MMF, Dagosta FCP, Birindelli JLO. Hemigrammus ataktos: a new species from the rio Tocantins basin, central Brazil (Characiformes: Characidae). Neotrop Ichthyol. 2014; 12(2):257–64. https://doi.org/10.1590/1982-0224-20130091
https://doi.org/10.1590/1982-0224-201300...
, M. sanctaefilomenae, P. rivularis), or affecting specific populations (e.g., Astyanax aff. rupestris, Moenkhausia celibelaMarinho & Langeani, 2010Marinho MMF, Langeani F. Moenkhausiacelibela: a new species from the Amazon basin, Brazil (Characiformes: Characidae). J Fish Biol. 2010; 77(4):879–89. http://dx.doi.org/10.1111/j.1095-8649.2010.02719.x
http://dx.doi.org/10.1111/j.1095-8649.20...
, and Moenkhausia bonita Benine, Castro & Sabino, 2004) or even at individual level (see below). Absence of lateral line was only observed in miniaturized species, in which developmental truncation is extreme [e.g., individuals of Oxybrycon parvulusGéry, 1964Géry J. Poissons characoïdes de l’Amazonie péruvienne. Beiträge zur Neotropischen Fauna. 1964; 4(1):1–44. and Tyttobrycon hamatus Géry, 1973Géry J. New and little-known Aphyoditeina (Pisces, Characoidei) from the Amazon Basin. Stud Neotrop Fauna Environ. 1973; 8:81–137.; all individuals of Priocharax spp., according to Weitzman, Vari (1987)Weitzman SH, Vari RP. Two new species and a new genus of miniature characid fishes (Teleostei: Characiformes) from northern South America. Proc Biol Soc Wash. 1987; 100:640–52. and Toledo-Piza et al., (2014)Toledo-Piza M, Mattox GMT, Britz R. Priocharax nanus, a new miniature characid from the rio Negro, Amazon basin (Ostariophysi: Characiformes), with an updated list of miniature Neotropical freshwater fishes. Neotrop Ichthyol. 2014; 12(2):229–46. https://doi.org/10.1590/1982-0224-20130171
https://doi.org/10.1590/1982-0224-201301...
] (references for these observations are listed in Tab. 2).
Increased morphological variability of late-forming structures is also associated with developmental truncation (Hanken, Wake, 1993Hanken J, Wake DB. Miniaturization of body size: organismal consequences and evolutionary significance. Annu Rev Ecol Syst. 1993; 24:501–19. https://doi.org/10.1146/annurev.es.24.110193.002441
https://doi.org/10.1146/annurev.es.24.11...
). Intraspecific variations at the lateral line development have been continuously documented for small characids (Tab. 2) and distinct states can be observed even in the same individual (bilateral asymmetry) (e.g., H. barrigonae, P. rivularis). Thus, the use of such labile character in systematics, such as in species delimitation or phylogenetic analysis, needs to be made with caution. In the search for a phylogenetic classification in the family, Weitzman (1962)Weitzman SH. The osteology of Bryconmeeki, a generalized characid fish, with an osteological definition of the family. Stanford Ichthyol Bull. 1962; 8(1):1–77. stated “loss of various parts of the laterosensory system, or parts of the skeleton (…), must be used with extremely care in the studies of phyletic relationships of small fishes, since parallel loss is probably the rule rather than exception”. It is wise to look at “reductive” characters very closely (Weitzman, Fink, 1983Weitzman SH, Fink WL. Relationships of the neon tetras, a group of South American freshwater fishes (Teleostei: Characidae), with comments on the phylogeny of new world characiforms. Bull Mus Comp Zool. 1983; 150:339–95.; Mattox et al., 2016Mattox GMT, Britz R, Toledo-Piza M. Osteology of Priocharax and remarkable developmental truncation in a miniature amazonian fish (Teleostei: Characiformes: Characidae). J Morphol. 2016; 277(1):65–85. https://doi.org/10.1002/jmor.20477
https://doi.org/10.1002/jmor.20477...
). In depth investigation on the ontogeny, patterns of formation and morphology of trunk lateral line are decisive in helping to establish homology for phylogenetic analysis.
Species of Characidae with intraspecific variation in the lateral line trunk morphology. Classification of genera and subfamilies follows Mirande (2018)Mirande JM. Morphology, molecules and the phylogeny of Characidae (Teleostei, Characiformes). Cladistics. 2018; 35(3):282–300. https://doi.org/10.1111/cla.12345
https://doi.org/10.1111/cla.12345... . Burger et al., (2019)Burger R, Carvalho FR, Zanata AM. A new species of Astyanax Baird & Girard (Characiformes: Characidae) from western Chapada Diamantina, Bahia, Brazil. Zootaxa. 2019; 4604(2):369–80. https://doi.org/10.11646/zootaxa.4604.2.9
https://doi.org/10.11646/zootaxa.4604.2.... reported variation in the completeness of the lateral line in Astyanax epiagos Zanata & Camelier, 2008, Deuterodon hastatus (Myers, 1928) and D. ribeirae (Eigenmann, 1911), but specific condition were not mentioned by the authors. LL = lateral line, c = complete, d = discontinuous, i = incomplete, a = absent.
Comparative material examined. All from Brazil. Material examined are the same listed at Ohara, Marinho (2016)Ohara WM, Marinho MMF. A new species of Moenkhausia Eigenmann (Characiformes: Characidae) from the upper rio Machado at Chapada dos Parecis, rio Madeira basin, Brazil. Neotrop Ichthyol. 2016; 14(1):e150041. https://doi.org/10.1590/1982-0224-20150041
https://doi.org/10.1590/1982-0224-201500...
, with the addition of Astyanax sp.: MZUSP 110406, 11, paratypes, 23.8–30.0 mm SL; MZUSP 118302, 164, 11.9–27.0 mm SL, 3 CS. Hyphessobrycon cachimbensis: MZUSP 97586, 64, 19.9–45.0 mm SL. Hyphessobrycon sp.: MZUSP 96823, 571, 16.2–48.5 mm SL. Moenkhausia lineomaculata: MZUSP 105953, three, paratypes, 14.8–36.6 mm SL. Moenkhausia pittieri: MZUSP 120441, 129, 2.8 mm NL to 15.4 mm SL. Paracheirodon innesi: MZUSP 120442, 134, 2.4 mm NL to 13.8 mm SL.
ACKNOWLEDGEMENTS
We are grateful to Bruno Barros (UNIR), Diogo Hungria, Victor Giovanetti (USP-IB) for their help during fieldwork; Carol Doria for the donation of specimens; Michel Gianetti (MZUSP) for curatorial assistance; Rafaela Ota (UNESP) and Ricardo Benine (UNESP) for the valuable suggestions. Part of the type series was collected at field expeditions funded by the South American Characiformes Inventory (FAPESP 2011/50282–7). The authors were funded by FAPESP (grant # 2016/19075–9; MMFM: grant # 2017/09321–5; WMO: grant # 2013/22473–8) and by CNPq (FCPD: grant # 405643/2018–7).
REFERENCES
- Abrahão VP, Pastana M, Marinho M On a remarkable sexual dimorphic trait in the Characiformes related to the olfactory organ and description of a new miniature species of Tyttobrycon Géry (Characiformes: Characidae). Plos One. 2019; 14(12):e0226130. https://doi.org/10.1371/journal.pone.0226130
» https://doi.org/10.1371/journal.pone.0226130 - Almirón AE, Casciotta JR, Bechara JA, Ruíz Díaz FJ A new species of Hyphessobrycon (Characiformes, Characidae) from the Esteros del Iberá wetlands, Argentina. Rev Suisse Zool. 2004; 111(3):673–82. https://doi.org/10.5962/bhl.part.80261
» https://doi.org/10.5962/bhl.part.80261 - Benine RC, Mariguela TC, Oliveira C New species of Moenkhausia Eigenmann, 1903 (Characiformes: Characidae) with comments on the Moenkhausia oligolepis species complex. Neotrop Ichthyol. 2009; 7(2):161–68. https://doi.org/10.1590/S1679-62252009000200005
» https://doi.org/10.1590/S1679-62252009000200005 - Bockmann FA, Slobodian V Heptapteridae. In: Queiroz LJ, Torrente-Vilara G, Ohara WM, Pires TS, Zuanon J, Doria CRC, editors. Peixes do rio Madeira. São Paulo: Dialeto Latim America Documentary; vol. III, 2013; p.14–77.
- Britski HA, Silimom KZS, Lopes BS Peixes do pantanal: manual de identificacão. 2nd ed. Brasília, DF: Embrapa; 2007.
- Burger R, Carvalho FR, Zanata AM A new species of Astyanax Baird & Girard (Characiformes: Characidae) from western Chapada Diamantina, Bahia, Brazil. Zootaxa. 2019; 4604(2):369–80. https://doi.org/10.11646/zootaxa.4604.2.9
» https://doi.org/10.11646/zootaxa.4604.2.9 - Camelier P, Dagosta FCP, Marinho MMF New remarkable sexually dimorphic miniature species of Hyphessobrycon (Characiformes: Characidae) from the upper Rio Tapajós basin. J Fish Biol. 2018; 92(4):1149–62. https://doi.org/10.1111/jfb.13579
» https://doi.org/10.1111/jfb.13579 - Carvalho FR Sistemática de Hyphessobrycon Durbin, 1908 (Ostariophysi: Characidae). [PhD Thesis]. Porto Alegre: Universidade Federal do Rio Grande do Sul; 2011.
- Carvalho FR, Malabarba LR Redescription and osteology of Hyphessobrycon compressus (Meek) (Teleostei: Characidae), type species of the genus. Neotrop Ichthyol. 2015; 13(3):513–40. https://doi.org/10.1590/1982-0224-20140173
» https://doi.org/10.1590/1982-0224-20140173 - Casatti L, Pérez-Mayorga MA, Carvalho FR, Brejão GL, Costa ID The stream fish fauna from the Rio Machado basin, Rondônia State, Brazil. Check List. 2013; 9(6):1496–504. https://doi.org/10.15560/9.6.1496
» https://doi.org/10.15560/9.6.1496 - Casciotta JR, Almirón AE, Piálek L, Říčan O Cyanocharax obi, a new species (Characiformes: Characidae) and the first record of the genus from tributaries of the rio Paraná basin, Argentina. Zootaxa. 2012; 3391(1):39–51. https://doi.org/10.11646/zootaxa.3391.1.3
» https://doi.org/10.11646/zootaxa.3391.1.3 - Coombs S, Janssen J, Webb JF Diversity of lateral line systems: evolutionary and functional considerations. In: Atema Y, Fay RR, Popper AN, Tavolga WN, editors. Sensory biology of aquatic animals. New York: Springer-Verlag; 1988. p.553–95. Available from: https://link.springer.com/content/pdf/10.1007%2F978-1-4612-3714-3.pdf
» https://link.springer.com/content/pdf/10.1007%2F978-1-4612-3714-3.pdf - Costa WJEM Description of two new species of the genus Moenkhausia (Characiformes: Characidae) from the central Brazil. Zool Anz. 1994; 232(1–2):21–29.
- Costa ID, Ohara WM, Almeida M Fishes from the Jaru Biological Reserve, Machado River drainage, Madeira River basin, Rondônia State, northern Brazil. Biota Neotrop. 2017; 17(1):e20160315. http://dx.doi.org/10.1590/1676-0611-BN-2016-0315
» http://dx.doi.org/10.1590/1676-0611-BN-2016-0315 - Dagosta FCP, Marinho MMF, Benine RC A new species of Moenkhausia Eigenmann (Characiformes: Characidae) from the upper rio Juruena basin, central Brazil. Zootaxa. 2015; 4032(4):417–25. https://doi.org/10.11646/zootaxa.4032.4.6
» https://doi.org/10.11646/zootaxa.4032.4.6 - Dagosta FCP, de Pinna M The fishes of the Amazon: distribution and biogeographical patterns, with a comprehensive list of species. Bull Am Mus Nat Hist. 2019; (431):1–163. Available from: http://digitallibrary.amnh.org/handle/2246/6940
» http://digitallibrary.amnh.org/handle/2246/6940 - Dagosta FCP, de Pinna M, Peres CA, Tagliacollo VA Existing protected areas provide a poor safetynet for threatened Amazonian fish species. Aquatic Conserv. 2020; 1–23. https://doi.org/10.1002/aqc.3461
» https://doi.org/10.1002/aqc.3461 - Eigenmann CH Preliminary descriptions of new genera and species of tetragonopterid characins. Bull Mus Comp Zool. 1908; 52(6):91–106.
- Eigenmann CH The American Characidae (Part 1). Mem Mus Comp Zool. 1917; 43:1–102.
- Eigenmann CH The American Characidae (Part 2). Mem Mus Comp Zool. 1918; 43:103–208.
- Eigenmann CH The American Characidae (Part 3). Mem Mus Comp Zool. 1921; 43:209–310.
- Eigenmann CH The American Characidae (Part 4). Mem Mus Comp Zool. 1927; 43:311–428.
- Eigenmann CH, Henn AW On new species of fishes from Colombia, Ecuador, and Brazil. Indiana University Studies. 1914; 140(24):231–34.
- Eigenmann CH, Ogle F An annotated list of characin fishes in the United States National Museum and the Museum of Indiana University, with descriptions of new species. Proc U S Natl Mus. 1907; 33(1556):1–36.
- Fink WL, Weitzman SH The so-called Cheirodontin fishes of Central America with description of two new species (Pisces, Characidae). Smithson Contrib Zool. 1974; 72:1–46. http://dx.doi.org/10.5479/si.00810282.172
» http://dx.doi.org/10.5479/si.00810282.172 - Géry J Poissons characoïdes de l’Amazonie péruvienne. Beiträge zur Neotropischen Fauna. 1964; 4(1):1–44.
- Géry J New and little-known Aphyoditeina (Pisces, Characoidei) from the Amazon Basin. Stud Neotrop Fauna Environ. 1973; 8:81–137.
- Hanken J, Wake DB Miniaturization of body size: organismal consequences and evolutionary significance. Annu Rev Ecol Syst. 1993; 24:501–19. https://doi.org/10.1146/annurev.es.24.110193.002441
» https://doi.org/10.1146/annurev.es.24.110193.002441 - Jerep FC, Dagosta FCP, Ohara WM A new miniature species of Serrapinnus (Characiformes: Characidae) from the upper rio Araguaia, Brazil. Copeia. 2018; 106(1):180–87. https://doi.org/10.1643/CI-17-653
» https://doi.org/10.1643/CI-17-653 - Lima FCT, Britski HA, Machado FA A new Moenkhausia (Characiformes: Characidae) from central Brazil, with comments on the area relationship between the upper rio Tapajós and upper rio Paraguai systems. Aqua. 2007; 13:45–54.
- Lima FCT, Toledo-Piza M New species of Moenkhausia (Characiformes: Characidae) from the rio Negro of Brazil. Copeia. 2001; (4):1058–63. https://doi.org/10.1643/0045-8511(2001)001[1058:NSOMCC]2.0.CO;2
» https://doi.org/10.1643/0045-8511(2001)001[1058:NSOMCC]2.0.CO;2 - Lütken CF Velhas-Flodens fiske: et bidrag til Brasiliens ichthyologi: efter Professor J. Reinhardts indsamlinger og optegnelser. Kongelige Danske videnskabernes selskab. 1875; 12(2):121–253. https://doi.org/10.5962/bhl.title.101459
» https://doi.org/10.5962/bhl.title.101459 - Lütken CF Peixes do rio das Velhas: uma contribuição para a ictiologia do Brasil. In: Alves CBM, Pompeu PS, editors. Peixes do rio das Velhas: passado e presente. Belo Horizonte: Editora Segrac; 2001. p.23–164.
- Malabarba LR, Jerep FC Review of the species of the genus Serrapinnus Malabarba, 1998 (Teleostei: Characidae: Cheirodontinae) from the rio Tocantins-Araguaia basin, with description of three new species. Zootaxa. 2014; 3847(1):57–79. https://doi.org/10.11646/zootaxa.3847.1.3
» https://doi.org/10.11646/zootaxa.3847.1.3 - Marinho MMF Comparative development in Moenkhausia pittieri and Paracheirodon innesi (Ostariophysi: Characiformes) with comments on heterochrony and miniaturization in the Characidae. J Fish Biol. 2017; 91(3):851–65. https://doi.org/10.1111/jfb.13384
» https://doi.org/10.1111/jfb.13384 - Marinho MMF, Dagosta FCP, Birindelli JLO Hemigrammus ataktos: a new species from the rio Tocantins basin, central Brazil (Characiformes: Characidae). Neotrop Ichthyol. 2014; 12(2):257–64. https://doi.org/10.1590/1982-0224-20130091
» https://doi.org/10.1590/1982-0224-20130091 - Marinho MMF, Langeani F Moenkhausiacelibela: a new species from the Amazon basin, Brazil (Characiformes: Characidae). J Fish Biol. 2010; 77(4):879–89. http://dx.doi.org/10.1111/j.1095-8649.2010.02719.x
» http://dx.doi.org/10.1111/j.1095-8649.2010.02719.x - Mathubara K, Toledo-Piza M Taxonomic study of Moenkhausia cotinho Eigenmann, 1908 and Hemigrammus newboldi (Fernández-Yépez, 1949) with the description of two new species of Moenkhausia (Teleostei: Characiformes: Characidae). Zootaxa. 2020; 4852(1):1–40. https://doi.org/10.11646/zootaxa.4852.1.1
» https://doi.org/10.11646/zootaxa.4852.1.1 - Mattox GMT, Britz R, Toledo-Piza M Osteology of Priocharax and remarkable developmental truncation in a miniature amazonian fish (Teleostei: Characiformes: Characidae). J Morphol. 2016; 277(1):65–85. https://doi.org/10.1002/jmor.20477
» https://doi.org/10.1002/jmor.20477 - Menezes NA, Weitzman SH Two new species of Mimagoniates (Teleostei: Characidae: Glandulocaudinae), their phylogeny and biogeography and a key to the glandulocaudin fishes of Brazil and Paraguay. Proc Biol Soc Wash. 1990; 103(2):380–426.
- Mirande JM Phylogeny of the family Characidae (Teleostei: Characiformes): from characters to taxonomy. Neotrop Ichthyol. 2010; 8(3):385–568. https://doi.org/10.1590/S1679-62252010000300001
» https://doi.org/10.1590/S1679-62252010000300001 - Mirande JM Morphology, molecules and the phylogeny of Characidae (Teleostei, Characiformes). Cladistics. 2018; 35(3):282–300. https://doi.org/10.1111/cla.12345
» https://doi.org/10.1111/cla.12345 - Montgomery JC, Coombs S, Janssen J Form and function relationships in lateral line systems: comparative data from six species of Antarctic notothenioid fish. Brain Behav Evol. 1994; 44(6):299–306. https://doi.org/10.1159/000113591
» https://doi.org/10.1159/000113591 - Mota TMF, Fabrin TMC, Deprá GC, Gasques LS, Oliveira AV, Pavanelli CS, Prioli SMAP, Prioli AJ Molecular characterization of Moenkhausia (Pisces: Characiformes) populations with different lateral line developmental levels. An Acad Bras Ciênc. 2018; 90(3):2815–25. http://dx.doi.org/10.1590/0001-3765201820170493
» http://dx.doi.org/10.1590/0001-3765201820170493 - Myers GS Descriptions of new South American fresh-water fishes collected by Dr. Carl Ternetz. Bull Mus Comp Zool. 1927; 68(3):107–35.
- Myers GS Trends in the evolution of teleostean fishes. Stanford Ichthyol Bull. 1958; 7:27–30.
- Ohara WM, Lima FCT Moenkhausia uirapuru, a new species from the upper Rio Guaporé, Chapada dos Parecis, Mato Grosso, Brazil (Teleostei: Characidae). Ichthyol Explor Freshw. 2015a; 26(2):159–70.
- Ohara WM, Lima FCT Hyphessobrycon lucenorum (Characiformes: Characidae), a new species from the rio Madeira basin, Rondônia State, Brazil. Zootaxa. 2015b; 3972(4):562–72. https://doi.org/10.11646/zootaxa.3972.4.7
» https://doi.org/10.11646/zootaxa.3972.4.7 - Ohara WM, Marinho MMF A new species of Moenkhausia Eigenmann (Characiformes: Characidae) from the upper rio Machado at Chapada dos Parecis, rio Madeira basin, Brazil. Neotrop Ichthyol. 2016; 14(1):e150041. https://doi.org/10.1590/1982-0224-20150041
» https://doi.org/10.1590/1982-0224-20150041 - Oliveira CAM Revisão taxonômica do complexo de espécies Astyanax scabripinnis sensu Bertaco & Lucena (2006) (Ostariophysi: Characiformes: Characidae). [PhD Thesis]. Maringá: Universidade Estadual de Maringá; 2017. Available from: http://repositorio.uem.br:8080/jspui/handle/1/5103
» http://repositorio.uem.br:8080/jspui/handle/1/5103 - Pastana MNL, Bockmann FA, Datovo A The cephalic lateral-line system of Characiformes (Teleostei: Ostariophysi): anatomy and phylogenetic implications. Zool J Linn Soc. 2019; 189(1):1–46. https://doi.org/10.1093/zoolinnean/zlz105
» https://doi.org/10.1093/zoolinnean/zlz105 - Pastana MNL, Dagosta FCP, Esguícero ALH A new sexually dichromatic miniature Hyphessobrycon (Teleostei: Characiformes: Characidae) from the Rio Formiga, upper Rio Juruena basin, Mato Grosso, Brazil, with a review of sexual dichromatism in Characiformes. J Fish Biol. 2017; 91(5):1301–18. https://doi.org/10.1111/jfb.13449
» https://doi.org/10.1111/jfb.13449 - Perin L, Shibatta OA, Bernarde PS Fish, Machado River basin, Cacoal urban area, state of Rondônia, Brazil. Check List. 2007; 3(2):94–97. https://doi.org/10.15560/3.2.94
» https://doi.org/10.15560/3.2.94 - Queiroz LJ, Torrente-Vilara G, Ohara WM, Pires THS, Zuanon J, Dória CRC, organizers Peixes do rio Madeira. São Paulo: Dialeto Latin American Documentary; 2013.
- Sabaj MH Standard symbolic codes for institutional resource collections in herpetology and ichthyology. Version 7.1 [Internet]. Washington, D.C.; 2019. Available from: http://www.asih.org/standard-symbolic-codes/about-symbolic-codes
» http://www.asih.org/standard-symbolic-codes/about-symbolic-codes - Soares IM, Bertaco VA, Ito PMM, Zuanon J A new species of Boehlkea (Characiformes: Characidae: Stevardiinae) from the rio Japurá, Amazon basin, Brazil. Neotrop Ichthyol. 2017; 15(3):e170026. http://dx.doi.org/10.1590/1982-0224-20170026
» http://dx.doi.org/10.1590/1982-0224-20170026 - Soares IM, Bürnheim CM A new species of Moenkhausia Eigenmann, 1903 (Characiformes: Characidae) from Amazon basin, Brazil. Zootaxa. 2016; 4208(4):392–400. http://dx.doi.org/10.11646/zootaxa.4208.4.6
» http://dx.doi.org/10.11646/zootaxa.4208.4.6 - Soares IM, Ota RP, Lima FCT, Benine RC Redescription of Moenkhausia melogramma (Characiformes: Characidae), a poorly known tetra from the western Amazon basin. Neotrop Ichthyol. 2020; 18(3):e200025. http://dx.doi.org/10.1590/1982-0224-2020-0025
» http://dx.doi.org/10.1590/1982-0224-2020-0025 - Sousa LM, Netto-Ferreira AL, Birindelli JLO Two new species of Moenkhausia Eigenmann (Characiformes: Characidae) from Serra do Cachimbo, Pará, Northern Brazil. Neotrop Ichthyol. 2010; 8(2):255–64. http://dx.doi.org/10.1590/S1679-62252010000200003
» http://dx.doi.org/10.1590/S1679-62252010000200003 - Taylor WR, Van Dyke GC Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium. 1985; 9(2):107–19.
- Toledo-Piza M, Mattox GMT, Britz R Priocharax nanus, a new miniature characid from the rio Negro, Amazon basin (Ostariophysi: Characiformes), with an updated list of miniature Neotropical freshwater fishes. Neotrop Ichthyol. 2014; 12(2):229–46. https://doi.org/10.1590/1982-0224-20130171
» https://doi.org/10.1590/1982-0224-20130171 - Vanegas-Ríos JA, Azpelicueta MM, Mirande JM, García Gonzales MD Gephyrocharax torresi (Characiformes: Characidae: Stevardiinae), a new species from the río Cascajales basin, río Magdalena system, Colombia. Neotrop Ichthyol. 2013; 11(2):275–84. https://doi.org/10.1590/S1679-62252013000200005
» https://doi.org/10.1590/S1679-62252013000200005 - Wada H, Iwasaki M, Kawakami K Development of the lateral line canal system through a bone remodeling process in zebrafish. Dev Biol. 2014; 392(1):1–14. https://doi.org/10.1016/j.ydbio.2014.05.004
» https://doi.org/10.1016/j.ydbio.2014.05.004 - Webb JF Gross morphology and the evolution of the mechanoreceptive lateral-line system in teleost fishes. Brain Behav Evol. 1989; 33(1):34–53. https://doi.org/10.1159/000115896
» https://doi.org/10.1159/000115896 - Webb JF Comparative morphology and evolution of the lateral line system in the Labridae (Perciformes: Labroidei). Copeia. 1990; (1):137–46. https://doi.org/10.2307/1445830
» https://doi.org/10.2307/1445830 - Weitzman SH The osteology of Bryconmeeki, a generalized characid fish, with an osteological definition of the family. Stanford Ichthyol Bull. 1962; 8(1):1–77.
- Weitzman SH, Fink WL Relationships of the neon tetras, a group of South American freshwater fishes (Teleostei: Characidae), with comments on the phylogeny of new world characiforms. Bull Mus Comp Zool. 1983; 150:339–95.
- Weitzman SH, Vari RP Two new species and a new genus of miniature characid fishes (Teleostei: Characiformes) from northern South America. Proc Biol Soc Wash. 1987; 100:640–52.
- Weitzman SH, Vari RP Miniaturization in South American freshwater fishes: An overview and discussion. Proc Biol Soc Wash. 1988; 101(2):444–65. Available from: https://repository.si.edu/handle/10088/901
» https://repository.si.edu/handle/10088/901 - Wellenreuther M, Brock M, Montgomery J, Clements KD Comparative morphology of the mechanosensory lateral line system in a clade of New Zealand Triplefin fishes. Brain Behav Evol. 2010; 75:292–308. https://doi.org/10.1159/000317061
» https://doi.org/10.1159/000317061 - Wonsettler AL, Webb JF Morphology and development of the multiple lateral line canals on the trunk in two species of Hexagrammos (Scorpaeniformes, Hexagrammidae). J Morphol. 1997; 233(3):195–214. https://doi.org/10.1002/(SICI)1097-4687(199709)233:3<195::AID-JMOR1>3.0.CO;2-3
» https://doi.org/10.1002/(SICI)1097-4687(199709)233:3<195::AID-JMOR1>3.0.CO;2-3 - Zanata AM, Burger R, Camelier P Two new species of Astyanax Baird & Girard (Characiformes: Characidae) from the upper rio Paraguaçu basin, Chapada Diamantina, Bahia, Brazil. Zootaxa. 2018; 4438(3):471–90. https://doi.org/10.11646/zootaxa.4438.3.3
» https://doi.org/10.11646/zootaxa.4438.3.3
ADDITIONAL NOTES
-
HOW TO CITE THIS ARTICLE
Marinho MMF, Ohara WM, Dagosta FCP. A new species of Moenkhausia (Characiformes: Characidae) from the rio Madeira basin, Brazil, with comments on the evolution and development of the trunk lateral line system in characids. Neotrop Ichthyol. 2021; 19(2):e200118. https://doi.org/10.1590/1982-0224-2020-0118
Edited-by
Publication Dates
-
Publication in this collection
21 June 2021 -
Date of issue
2021
History
-
Received
20 Oct 2020 -
Accepted
11 Mar 2021