Abstract
A new species of Characidium is described from the tributaries of the rio Tocantinzinho, rio Tocantins basin, located in the southern portion of the Chapada dos Veadeiros, at about 1,200 meters of elevation, Goiás, Brazil. The new species can be diagnosed by an unusual combination of two apomorphic features present in distinct clades of Characidium, the presence of a scaleless isthmus in allied to with a single row of dentary teeth. Additionally, the new species has a unique color pattern of inconspicuous vertical bars disconnected from the dorsal midline, forming seven to nine square blotches along body sides, and the presence of a dark saddle-shaped mark at the dorsal-fin base. Osteologically, it can be diagnosed by having the first and second anal-fin proximal radials fused and contacting the third hemal spine, which is branched. The new species also has a peculiar, unusual variation of fin-ray counts among its congeners.
Keywords:
Cerrado; Characidium stigmosum; Endemism; Rio Tocantins basin; Taxonomy
Resumo
Uma nova espécie de Characidium é descrita dos riachos tributários do rio Tocantins, bacia do rio Tocantins, localizados na vertente sul da Chapada dos Veadeiros, a aproximadamente 1.200 metros de altitude, Goiás, Brasil. A nova espécie pode ser diagnosticada pela combinação não usual de dois caracteres apomórficos presentes em clados distintos de Characidium, a presença do istmo sem escama em conjunto com uma única série de dentes no dentário. Adicionalmente, a nova espécie tem um padrão de coloração único de barras verticais desconectadas na região dorsal, formando sete a nove manchas quadradas ao longo do lado do corpo, e pela presença de uma mancha em forma de sela na base da nadadeira dorsal. Osteologicamente, ela pode ser diagnosticada por possuir o primeiro e segundo radiais da nadadeira anal fusionados e em contato com o terceiro espinho hemal, que é ramificado. A espécie nova também possui uma variação peculiar e pouco usual no número de raios das nadadeiras entre os congêneres.
Palavras-chave:
Bacia do rio Tocantins; Cerrado; Characidium stigmosum; Endemismo; Taxonomia
INTRODUCTION
CharacidiumReinhardt, 1867Reinhardt JT. Om trende, formeentligt ubeskrevne fisk af characinernes eller Karpelaxenes familie. Oversigt over det Kongelige Danske Videnskabernes Selskabs Forhandlinger og dets Medlemmers Arbeider. Copenhagen. 1867; 1866:49–68. currently includes 82 valid species of small-sized fishes typical from the Neotropical region between Northern Argentina and Panama (Armbruster et al., 2021Armbruster JA, Lujan NK, Bloom DD. Redescription of the Guiana shield darter species Characidiumcrandellii and C. declivirostre (Crenuchidae) with descriptions of two new species. Ichthyol Herpetol. 2021; 109(1):102–22. https://doi.org/10.1643/i2019299
https://doi.org/10.1643/i2019299...
; Fricke et al., 2021Fricke R, Eschmeyer WN, Van der Laan R. Eschmeyer’s catalog of fishes: genera, species, references [Internet]. San Francisco: California Academy of Science; 2021. Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
http://researcharchive.calacademy.org/re...
; Melo et al., 2021Melo MRS, Ribeiro MCLB, Lima FCT. A new, narrowly distributed, and critically endangered species of Characidium (Characiformes: Crenuchidae) from the Distrito Federal, Central Brazil. Neotrop Ichthyol. 2021; 19(1):e200061. https://doi.org/10.1590/1982-0224-2020-0061
https://doi.org/10.1590/1982-0224-2020-0...
). The number of known species has rapidly increased in the past two decades, with descriptions of almost 40% of the known diversity within the group, with new findings throughout South America (e.g., Zarske, Géry, 2001Zarske A, Géry J. Beschreibung von drei neuen Arten der Gattung Characidium Reinhardt, 1866 aus Bolivien und Paraguay (Teleostei: Characiformes: Characidiidae). Zool Abh. 2001; 51(16):229–46.; Da Graça et al., 2008Da Graça WJ, Pavanelli CS, Buckup PA. Two new species of Characidium (Characiformes: Crenuchidae) from Paraguay and Xingu basins, state of Mato Grosso, Brazil. Copeia. 2008; 2008(2):326–32. https://doi.org/10.1643/CI-06-167
https://doi.org/10.1643/CI-06-167...
; Zanata, Camelier, 2015Zanata AM, Camelier P. Two new species of Characidium Reinhardt (Characiformes: Crenuchidae) from northeastern Brazilian coastal drainages. Neotrop Ichthyol. 2015; 13(3):487–98. https://doi.org/10.1590/1982-0224-20140106
https://doi.org/10.1590/1982-0224-201401...
; Agudelo-Zamora et al., 2020Agudelo-Zamora HD, Tavera J, Murillo YD, Ortega-Lara A. The unknown diversity of the genus Characidium (Characiformes: Crenuchidae) in the Chocó biogeographic region, Colombian Andes: two new species supported by morphological and molecular data. J Fish Biol. 2020; 97(6):1662–75. https://doi.org/10.1111/jfb.14527
https://doi.org/10.1111/jfb.14527...
). Until now, only three species of Characidium have been described from the rio Araguaia-Tocantins basin: C. mirimNetto-Ferreira, Birindelli & Buckup, 2013Netto-Ferreira AL, Birindelli JLO, Buckup PA. A new miniature species of Characidium Reinhardt (Ostariophysi: Characiformes: Crenuchidae) from the headwaters of the rio Araguaia, Brazil. Zootaxa. 2013; 3664(3):361–68. http://dx.doi.org/10.11646/zootaxa.3664.3.6
http://dx.doi.org/10.11646/zootaxa.3664....
, a species with narrow distribution in the upper portion of the rio das Mortes and its tributaries; C. stigmosumMelo & Buckup, 2002Melo MRS, Buckup PA. Characidiumstigmosum (Characiformes: Crenuchidae): a new species of characidiin fish from central Brazil. Copeia. 2002; 2002(4):988–93. https://doi.org/10.1643/0045-8511(2002)002[0988:CSCCAN]2.0.CO;2
https://doi.org/10.1643/0045-8511(2002)0...
, endemic to rio das Almas and rio dos Bois at the northern realm of the Chapada dos Veadeiros; and C. xanthopterumSilveira, Langeani, da Graça, Pavanelli & Buckup, 2008Silveira LGG, Langeani F, da Graça WJ, Pavanelli CS, Buckup PA. Characidium xanthopterum (Ostariophysi: Characiformes: Crenuchidae): a new species from the Central Brazilian Plateau. Neotrop ichthyol. 2008; 6(2):169–74. http://dx.doi.org/10.1590/S1679-62252008000200003
http://dx.doi.org/10.1590/S1679-62252008...
, widely distributed in the upper Paraná and upper Tocantins basins (Melo, Buckup, 2002Melo MRS, Buckup PA. Characidiumstigmosum (Characiformes: Crenuchidae): a new species of characidiin fish from central Brazil. Copeia. 2002; 2002(4):988–93. https://doi.org/10.1643/0045-8511(2002)002[0988:CSCCAN]2.0.CO;2
https://doi.org/10.1643/0045-8511(2002)0...
; Silveira et al., 2008Silveira LGG, Langeani F, da Graça WJ, Pavanelli CS, Buckup PA. Characidium xanthopterum (Ostariophysi: Characiformes: Crenuchidae): a new species from the Central Brazilian Plateau. Neotrop ichthyol. 2008; 6(2):169–74. http://dx.doi.org/10.1590/S1679-62252008000200003
http://dx.doi.org/10.1590/S1679-62252008...
; Netto-Ferreira et al., 2013Netto-Ferreira AL, Birindelli JLO, Buckup PA. A new miniature species of Characidium Reinhardt (Ostariophysi: Characiformes: Crenuchidae) from the headwaters of the rio Araguaia, Brazil. Zootaxa. 2013; 3664(3):361–68. http://dx.doi.org/10.11646/zootaxa.3664.3.6
http://dx.doi.org/10.11646/zootaxa.3664....
).
Herein, we describe a new species of Characidium from the high elevation tributaries of the upper rio Tocantins, in the southern portion of the Chapada dos Veadeiros, Goiás, Brazil. In addition, we discuss the possible relationships of the new species with its congeners based on a comparative morphological analysis across the genus, provide additional information about the distribution of the genus Characidium in the Chapada dos Veadeiros, revise the coordinates of the type locality of C. stigmosum, and give an updated list of endemic vertebrates that occur in that plateau.
MATERIAL AND METHODS
The 28 morphometric and 14 meristic characters were obtained from the left side of the individual under a stereomicroscope, according to the protocol described by Buckup, (1993a)Buckup PA. Review of the characidiin fishes (Teleostei: Characiformes), with description of four new genera and ten new species. Ichthyol Explor Freshw. 1993a; 4(2):97–154. and modified by Melo, Oyakawa, (2015)Melo MRS, Oyakawa OT. A new species of Characidium Reinhardt (Characiformes, Crenuchidae) with a distinctively dimorphic male. Copeia. 2015; 103(2):281–89. https://doi.org/10.1643/CI-14-073
https://doi.org/10.1643/CI-14-073...
, with a digital electronic caliper to 0.01 mm. All measurements are presented as proportions of standard length (SL), except for subunits of the head, which are presented as proportions of the head length (HL). Counts are given along the text with their frequency presented in parentheses and an asterisk indicating the values for the holotype. Cleared-and-stained specimens (cs) were used for counts of vertebrae, branchiostegal rays, osteological observations and to double check characteristics of dentition; the specimens were prepared according to Taylor, Van Dyke, (1985)Taylor WR, Van Dyke GC. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium. 1985; 9(2):107–19.. The anterior four vertebrae of the Weberian apparatus were counted as precaudal elements, and the fused PU1+U1 as a single caudal element. Gonads for sex identification were examined through an incision made on the left side of the specimens. Visual records were made by the senior authors with the aid of a snorkel and diving mask and photographed with an Olympus E-M5 digital camera in an underwater case. All photos and illustrations were made by MRMS otherwise stated. Institutional abbreviations follow Sabaj, (2019)Sabaj MH. Standard symbolic codes for institutional resource collections in herpetology and ichthyology: An Online Reference. Version 7.1; 2019. Available from: https://asih.org/standard-symbolic-codes
https://asih.org/standard-symbolic-codes...
.
RESULTS
Characidium kalunga, new species
urn:lsid:zoobank.org:act:1B75F475-E28A-4BD9-8571-E6FD51296B18
Holotype. MZUSP 125824, 40.5 mm SL, male, Brazil, Goiás, Alto Paraíso de Goiás, rio Almécegas at Cachoeira de Almécegas II, tributary of rio dos Couros, rio Tocantins basin, 14°11’11’’S 47°36’15’’W, 28 Nov 2012, J. L. Birindelli, F. C. Dagosta, M. V. Loeb & C. Santos.
Paratypes. All from Brazil, Goiás, Alto Paraíso de Goiás. MNRJ 52533, 4, 28.7–41.9 mm SL; MZUSP 114056, 60 (2 cs), 14.9–48.7 mm SL; ZUEC 17332, 3, 33.8–37.6 mm SL, collected with holotype. MZUSP 114058, 1, 36.6 mm SL, rio dos Couros at Cachoeira São Bento, tributary of rio Tocantins basin, 14°09’38’’S 47°35’38’’W, 28 Nov 2012, J. L. Birindelli, F. C. Dagosta, M. V. Loeb & C. Santos. UFRGS 9954, 2, 33.8–40.9 mm SL, rio das Cobras at road between Alto Paraíso de Goiás to the Chapada dos Veadeiros National Park, 14°09’39”S 47°37’55”W, 25 May 2008, T. P. Carvalho & F. C. Jerep. UFRGS 11257, 9, 22.8–39.9 mm SL, rio dos Couros, Portal da Chapada, near Fazenda São Bento, tributary of rio Tocantinzinho, 14°09’58”S 47°35’43”W, 10 Sep 2009, G. Frainer, F. R. Carvalho & V. A. Bertaco.
Characidium kalunga in lateral (top), ventral (middle) and dorsal (bottom) views, holotype, MZUSP 125824, 40.5 mm SL, rio Almécegas at Cachoeira de Almécegas, Chapada dos Veadeiros, Alto Paraíso de Goiás, Brazil.
Paratypes of Characidium kalunga, MZUSP 114054, A. 15.3 mm SL, B. 18.2 mm SL, C. 21.4 mm SL, D. 29.7 mm SP, E. 30.9 mm SL, and F. 48.7 mm SL. Scale bar = 5 mm, Chapada dos Veadeiros, Goiás, Brazil.
Diagnosis.Characidium kalunga can be distinguished from its cis-Andean congeners, except C. alipioi Travassos, 1955, C. amaila Lujan, Agudelo-Zamora, Taphorn, Booth & López-Fernández, 2013, C. boavistae Steindachner, 1915, C. bolivianum Pearson, 1924, C. crandellii Steindachner, 1915, C. duplicatum Ambruster, Lujan & Bloom, 2021, C. cricarense Malanski Sarmento-Soares, Silva-Malanski, Lopes, Ingenito & Buckup, 2019, C. declivirostre Steindachner, 1915, C. fasciatumReinhardt, 1867Reinhardt JT. Om trende, formeentligt ubeskrevne fisk af characinernes eller Karpelaxenes familie. Oversigt over det Kongelige Danske Videnskabernes Selskabs Forhandlinger og dets Medlemmers Arbeider. Copenhagen. 1867; 1866:49–68., C. gomesi Travassos, 1956, C. grajahuense Travassos, 1944, C. hasemani Steindachner, 1915, C. helmeri Zanata, Sarmento-Soares & Martins-Pinheiro, 2015, C. iaquira Zanata, Ohara, Oyakawa & Dagosta, 2020, C. japuhybensis Travassos, 1949, C. kamakan Zanata & Camelier, 2015, C. lauroi Travassos, 1949, C. macrolepidotum (Peters, 1868), C. oiticicai Travassos, 1967, C. pterostictum Gomes, 1947, C. purpuratum Steindachner, 1882, C. schubarti Travassos, 1955, C. tamata Agudelo-Zamora, Tavera, Murillo & Ortega-Lara, 2020, C. timbuiense Travassos, 1946, C. travassosi Melo, Buckup & Oyakawa, 2016, C. vidali Travassos, 1967, and C. wangyapoik Ambruster, Lujan & Bloom, 2021, by lacking scales on the isthmus (vs. isthmus completely scaled), from C. alipioi, C. amaila, C. boavistae, C. bolivianum, C. crandellii, C. cricarense, C. declivirostre, C. fasciatum, C. gomesi, C. grajahuense, C. hasemani, C. helmeri, C. iaquira, C. japuhybensis, C. kamakan, C. lauroi, C. macrolepidotum, C. oiticicai, C. pterostictum, C. purpuratum, C. schubarti, C. tamata, C. timbuiense, C. travassosi, C. vidali by having a single row of dentary teeth (vs. dentary teeth in two rows, internal row with minute conical teeth), and from C. duplicatum and C. wangyapoik by having the scalelles are extending from isthums to anterior margin of cleithra (vs. scalelles extending on isthmus, area between pectoral fins and part of belly to level of pelvic fins). It can be further distinguished from its congeners, except C. heirmostigmatada Graça & Pavanelli, 2008Da Graça WJ, Pavanelli CS. Characidiumheirmostigmata, a new characidiin fish (Characiformes: Crenuchidae) from the upper rio Paraná basin, Brazil. Neotrop ichthyol. 2008; 6(1):53–56. http://dx.doi.org/10.1590/S1679-62252008000100006
http://dx.doi.org/10.1590/S1679-62252008...
, C. papachibePeixoto & Wosiacki, 2013Peixoto LAW, Wolsiacki WB. A new species of Characidium (Characiformes: Crenuchidae) from the Lower Amazon. Copeia. 2013; 2013(1):52–57. https://doi.org/10.1643/CI-12-080
https://doi.org/10.1643/CI-12-080...
, C. satoiMelo & Oyakawa, 2015Melo MRS, Oyakawa OT. A new species of Characidium Reinhardt (Characiformes, Crenuchidae) with a distinctively dimorphic male. Copeia. 2015; 103(2):281–89. https://doi.org/10.1643/CI-14-073
https://doi.org/10.1643/CI-14-073...
, and C. serranoBuckup & Reis, 1997Buckup PA, Reis RE. Characidiin genus Characidium (Teleostei, Characiformes) in Southern Brazil, with description of three new species. Copeia. 1997; 1997(3):531–48. https://doi.org/10.2307/1447557
https://doi.org/10.2307/1447557...
, by having vertical bars on body that are disconnected dorsally, and from those species by having the bars on body as deep as wide, forming blotches two to four scales wide (vs. blotches vertically elongated, and of one scale width in C. heirmostigmata, C. papachibe, and C. serrano, or blotches forming oval dots, V-shaped, W-shaped, or diamond-shaped marks along and ventral to the lateral line in C. satoi), and bars not obliquely oriented (vs. bars oblique in C. heirmostigmata, C. papachibe, and C. serrano), and from all congeners by the presence of a saddle mark at the base of the second to eighth dorsal-fin rays. Osteologically, C. kalunga is diagnosed by having the first and second anal-fin proximal radials fused and contacting the third hemal spine (vs. separated and intercalated with the hemal spines), and by the third hemal spine branched (vs. all hemal spines unbranched).
Description. Morphometric data for holotype and paratypes summarized in Tab. 1. Largest specimen examined with 43.9 mm SL. Body fusiform. Dorsal profile moderately convex between tip of snout and dorsal-fin origin; gently arched along dorsal-fin base, nearly straight between dorsal fin and origin of first dorsal caudal-fin procurrent ray; slightly convex along anal-fin base; nearly straight between anal fin and origin of first ventral caudal-fin procurrent ray. Greatest depth of body at dorsal-fin origin.
Snout gently rounded in lateral view, its tip slightly below level of ventral margin of eye. Mouth small, subterminal. Snout-maxillary tip distance equal or slightly shorter than eye diameter; maxilla reaching level of anterior margin of orbit. Orbit circular, margin of orbit free. Nares distinctly separated by fleshy bridge; distance between nares shorter than distance between posterior naris to eye. Dermal flap along entire border of anterior naris, crescent-shaped and restricted to anterior margin of posterior naris.
Pectoral fin reaching vertical through dorsal-fin origin when adpressed to body; pectoral-fin rays iii,7 (1), iii,7,i (2), iii,8,i (6), iii,9,i* (5), iv,5,i (1), iv,6,i (1), iv,7,i (4), iv,7,ii (1), iv,8 (2), or iv,8,i (3). Pelvic fin barely reaching anal-fin origin; pelvic-fin rays i,6,i (1), i,7 (1), i,7,i (6), i,7,ii* (1), ii,5,i (2), ii,5,ii (3), ii,6,i (9), ii,7,i (1), iii,6 (1), or iii,7,i (1). Dorsal-fin rays ii,5,i (1), ii,8 (3), ii,9 (1), iii,7,i (2), iii,8 (10), iii,9 (3), or iv,8* (6), last dorsal-fin ray simple, not adnate (2 cs); dorsal-fin supranumerary element 1 (2 cs). Anal fin not reaching ventral caudal-fin procurrent rays; distal margin of anal fin rounded; anal-fin rays ii,5,i (1), ii,6 (1), ii,7 (2), iii,4 (1), iii,5 (15), iii,6 (2), iii,7* (1), iv,4 (1) or iv,5 (2); last anal-fin ray adnate (2 cs); anal-fin supranumerary element 1 (2 cs). Principal caudal-fin rays i,7,8,i (1), i,7,8,ii (1), i,8,7,ii (1), i,8,8,ii (2), i,8,9,i (3), i,9,8,i (1), ii,2,i,5-4,i,5,i (1), ii,6,8,ii (1), ii,7,8,i (1), ii,7,8,ii (10), ii,7,9,i* (3), or ii,7,9,ii (1). Adipose fin absent (1), or present* (25).
Scales cycloid; parallel radii present only on posterior field of scales. Lateral line usually complete; scales along longitudinal row 35 (2), 36 (11), or 37* (13) scales. Perforated scales in lateral line 33 (1), 35 (1), 36 (11), or 37* (13). Scales series above lateral line 4 (5) or 5* (21). Scales below lateral line 4 (1) or 5* (25). Pre-dorsal scale series 12* (5), 13 (11), or 14 (10). Circumpeduncular scales 13 (1) or 14* (25). Scales between anus and anal-fin origin 2* (8), 3 (18), or 4 (1). Isthmus lacking scales to anterior margin of cleithrum.
Premaxillary teeth 5 (3), 6* (19), or 7 (3), all conical, arranged in single row, decreasing in size posteriorly (Fig. 3A). Maxillary teeth absent. Dentary teeth 8 (6), 9 (7), 10 (6), 11* (4) or 12 (2), all conical, arranged in single row, decreasing in size posteriorly (Fig. 3B). Ectopterygoid teeth 2 (2 cs), conical. Mesopterygoid edentulous (2 cs). Branchiostegal rays 4 (2 cs); 3 attached to anterior ceratohyal (2 cs) and 1 attached to posterior ceratohyal (2 cs). Total gill rakers 10 (1 cs) or 11 (1 cs); gill rakers on basibranchial 2 (1 cs) or 3 (1 cs); gill rakers on ceratobranchial 5 (1 cs) or 6 (1 cs); and gill rakers on epibranchial 4 (1 cs) or 5 (1 cs). Parietal branch of supraorbital laterosensory canal present (2 cs), not reaching parietal bone (1 cs) or reaching parietal bone (1 cs). Fontanel triangular, limited anteriorly by frontals, laterally by frontals and parietals, and posteriorly by supraoccipital (2 cs, Fig. 3C).
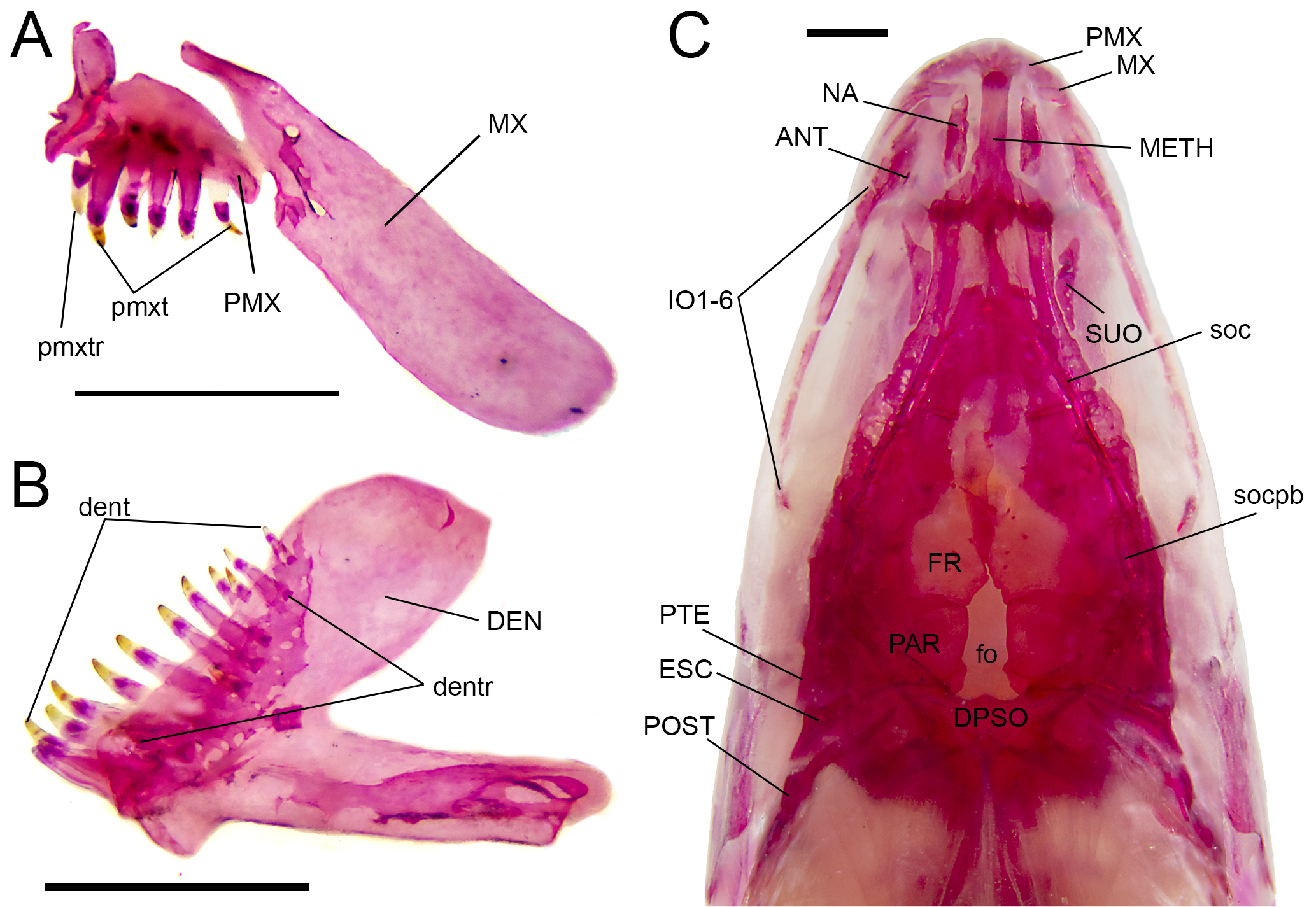
Osteological characteristics of head in Characidium kalunga, MZUSP 114054, paratype. A. Right upper jaw in medial view; B. Right dentary in medial view; C. Skull in dorsal view. Abbreviations: ANT, antorbital; DEN, dentary; dent, dentary teeth; dentr, replacement dentary teeth; DPSO, dorsal process of the supraoccipital; ESC, extrascapular; FR, frontal; fo, fontanel; IO 1–6, infraorbitals 1 to 6; METH, mesethmoid; MX, maxilla; NA, nasal; PAR, parietal; PMX, premaxilla; pmxt, premaxilarry teeth; pmxtr, replacement premaxillary teeth; PTE, pterotic; POST, posttemporal; SUO, supraorbital; soc, supraorbital canal; and socpb, parietal branch of the supraorbital canal. Scale bar = 1 mm.
Precaudal vertebrae 21 (1 cs) or 20 (1 cs); total vertebrae 37 (2 cs). Supraneurals 5 (1 cs) or 6 (1 cs). Hypurals 6 (2 cs). Epurals 2 (1 cs) or 3 (1 cs). Upper caudal-fin procurrent rays 8 (1 cs) or 9 (1 cs); lower caudal-fin procurrent rays 7 (1 cs) or 8 (1 cs). Uroneural 1 (2). Postcleithrum 1 enlarged, oval, anterodorsally elongated, and connected dorsally to ventral tip of supracleithrum; postcleithrum 2 triangular, anterodorsally elongated, connected dorsally to posterior cleithral process; postcleithrum 3 slender and elongated, rib like, connected dorsally to postcleithrum 1 (2 cs, Fig. 4A). Dorsal-fin pterygiophores 9 (1 cs) or 10 (1 cs), all disconnected, intercalating between neural spines from twelfth to eighteenth vertebrae (1 cs) or eleventh to eighteenth vertebrae (1 cs). Anal-fin pterygiophores 7 (2 cs), first and second proximal radials fused medially and contacting hemal spine of third caudal vertebra, remaining pterygiophores disconnected and inserting on musculature intercalating between hemal spines of third and fourth, fourth and fifth, and fifth and sixth caudal vertebrae (Fig. 4B).
Osteological characteristics of fins in Characidium kalunga, MZUSP 114054, paratype. A. Left pectoral girdle in lateral view; B. Caudal peduncle in lateral view – left intermuscular bones removed. Abbreviations: arrow, position of anus; asterisk, first caudal vertebra; CEN, centrum; CLE, cleithrum; COR, coracoid; ESC, extrascapular; LLS1, first lateral-line scale; HS3, third haemal spine; MCO, mesocoracoid; NS, neural spine; PCP, posterior cleithral process; PR, pleural rib; PRAF 1+2, fused anal-fin proximal radials 1 and 2; PTC1, postcleithrum 1; PTC2, postcleithrum 2; PTC3, postcleithrum 3; POST, posttemporal; SC, scapula; SCL, supracleithrum. Scale bar = 1 mm.
Humeral hiatus of obliquus superioris and obliquus inferioris muscles well developed, filled with fatty tissue and not visible externally by transparency. Humeral hiatus oval-shaped, with two chambers divided by pleural rib of fifth vertebra, anterior chamber covered by connective tissue membrane; limited dorsally by lateralis superficialis, posteroventrally by obliquuus inferioris and posterodorsally, ventrally and anteriorly by obliquus superioris. Lateral line nerve passing along dorsal margin of humeral hiatus and continuing along midlateral horizontal septum (Fig. 5).
Humeral hiatus in Characidium kalunga, MZUSP 114054, 42.0 mm SL, paratype. Abbreviations: LLN, lateral-line nerve; LS, lateralis superficialis; OI, obliquus inferioris; OS, obliquus superioris; PR5, pleural rib of fifth vertebra. Scale bar = 1 mm.
Morphometric data for Characidium kalunga. Values for the holotype (MZUSP 125824) and 25 paratypes (MZUSP 114056, 114058). Abbreviations: N, number of specimens; SD, Standard deviation.
Coloration in alcohol. General ground color tan yellow, dark brown dorsally and pale beige ventrally. Dorsal part of head snout and skull dark brown; lateral and ventral part of snout, distal portion of upper jaw, lower jaw, cheek, ventral part of opercle, and branchiostegal membranes lighter, with scarcely spaced melanophores. Scales on dorsal portion of body with melanophores more concentrated on distal margin, providing reticulated aspect on dorsal portion of body. Sides of body gradually lighter from dorsal to ventral surface, with ventral parts of belly and caudal peduncle light beige.
Preorbital stripe thin, extending obliquely between proximal third of upper jaw and anterior margin of eye. Postorbital stripe thicker than preorbital and longitudinal stripes, extending horizontally between posterior margin of eye and dorsal margin of opercle. Longitudinal stripe having less than one scale width, extending horizontally between dorsal margin of opercle to posterior portion of caudal peduncle, but not reaching base of caudal-fin rays. Humeral blotch oval located immediately posterior to upper corner of opercle, with three scales depth and two scales wide. Basicaudal spot inconspicuous and not easily discernible, positioned at middle caudal-fin rays.
Bars on body seven to nine, present in most specimens examined. Bars on dorsum irregularly distributed and disconnected from lateral portion, often fused and forming irregular longitudinal dorsal lines between head and dorsal fin. Lateral portion of bars disconnected from dorsal portion and not connected in ventral midline, forming large square blotches on sides of body but, in larger specimens, inconspicuous and not easily discernible (Fig. 2F).
Pectoral and pelvic fins mostly hyaline, with melanophores concentrated on dorsal margin of fin rays. Anal fin hyaline. Dorsal-fin mostly hyaline except for one middle, longitudinal dusk and inconspicuous stripe, and one discernible saddle-like mark on base of fourth to eight dorsal-fin rays. Caudal fin mostly hyaline, or with irregularly distributed melanophores on proximal two thirds. Adipose-fin hyaline, with scarce melanophores.
Coloration in life. General pigmentation like that of preserved specimens. Live specimens with ground color brownish, and square blotches on flanks more easily discernible (Fig. 6).
Underwater photography of Characidium kalunga in the rio Preto, at the Parque Nacional da Chapada dos Veadeiros, Goiás, Brazil (not collected).
Ontogenetic variation of pigmentation. In smaller specimens (<19 mm SL), the body is pale beige and the blotches are poorly marked and barely indistinguishable (Figs. 2A–B); in larger specimens (>20.0 mm SL), the ground color becomes yellowish and the blotches well defined (Figs. 2C–E); and the largest specimen examined (48.3 mm SL) lacks bars, has the overall body pattern dusky, including the areas on cheeks and fins and the blotches are merged into the background and indistinguishable (Fig. 2F). The humeral and basicaudal spots and the saddle mark at base of dorsal fin are visible in all specimens examined but are inconspicuous in the largest specimen. The pseudotympanum is visible by skin transparency in smaller were observed in the available specimens.
Geographical distribution.Characidiumkalunga is known only from the streams draining the southern portion of the Chapada dos Veadeiros, tributaries of the upper rio Tocantins basin. It was collected in the rio dos Couros and rio Almécegas, but field observations and photographic records also include the rio Preto, upstream from the Salto de 120 Metros waterfall (Fig. 7).
Map of the Chapada dos Veadeiros indicating the known distribution of Characidium: circles represent Characidium kalunga, triangles, C. xanthopterum, and diamonds, C. stigmosum. Black symbols indicate the type locality and white symbols other records.
Ecological notes.Characidium kalunga is a bottom dweller species, known from localities with elevation of about 1,200 meters. It inhabits rivers with fast flowing, cold, black water with rocky bottom, characterized by the presence of many rapids, canyons, and relatively large waterfalls, alternating with pools with sandy bottom. Those rivers are often impacted by sudden water flow increase caused by precipitation runoff in the watershed. The Cerrado vegetation is restricted to the margins, with no aquatic plants present in the river channel (Fig. 8).
Habitats of Characidium kalunga at the Chapada dos Veadeiros: A. Type locality, cachoeira das Almécegas II, at rio Almécegas, tributary of rio dos Couros; B. Rio Preto, at Parque Nacional da Chapada dos Veadeiros.
Etymology. The specific name honors the Comunidade Quilombola Kalunga, a resilient community of Afro-Brazilians that lives in the Chapada dos Veadeiros area, helping to protect its natural resources. Kalunga also means a sacred place in the African Bantu language. A noun in apposition.
Conservation status.Characidiumkalunga has a narrow distribution, with an area of occupancy smaller than 500 km2. It occurs within the limits of a protected area, the Parque Nacional da Chapada dos Veadeiros. Impacts such as continuing populational decline or extreme fluctuations of the extent of occurrence, area of occupancy, quality of habitat, number of locations or subpopulations, or number of mature individuals were not observed. Therefore, in accordance with the IUCN Red List Categories and Criteria (IUCN Standards and Petitions Subcommittee, 2019International Union for Conservation of Nature (IUCN). Standards and petitions subcommittee. Guidelines for Using the IUCN Red List Categories and Criteria. Version 14 [Internet]. Gland; 2019. Available from: http://www.iucnredlist.org/documents/RedListGuidelines.pdf
http://www.iucnredlist.org/documents/Red...
), C. kalunga is categorized as Least Concern (LC).
DISCUSSION
Interspecific relationships and comparisons. The genus Characidium was erected by Reinhardt, (1867)Reinhardt JT. Om trende, formeentligt ubeskrevne fisk af characinernes eller Karpelaxenes familie. Oversigt over det Kongelige Danske Videnskabernes Selskabs Forhandlinger og dets Medlemmers Arbeider. Copenhagen. 1867; 1866:49–68. to include C. fasciatum, the type species by original designation. Buckup, (1993b)Buckup PA. The monophyly of the Characidiinae, a Neotropical group of characiform fishes (Teleostei: Ostariophysi). Zool J Linn Soc. 1993b; 108(3):225–45. https://doi.org/10.1111/j.1096-3642.1993.tb00297.x
https://doi.org/10.1111/j.1096-3642.1993...
reevaluated the genus in a phylogenetic sense, proposing the only interspecific phylogenetic hypothesis to date. More recently, a few contributions to test the delimitation between species and populations of Characidium based on cytochrome oxidase I (COI) mitochondrial gene were published, but the dendrograms produced include clades with low support, indicating that the difficulties on the systematics and taxonomic practices faced by traditional morphological approaches may also apply at the molecular level in the genus (e.g., Serrano et al., 2018Serrano ÉA, Melo BF, Freitas‐Souza D, Oliveira MLM, Utsunomia R, Oliveira C, Foresti F. Species delimitation in Neotropical fishes of the genus Characidium (Teleostei, Characiformes). Zool Scr. 2018; 48(1):69–80. https://doi.org/10.1111/zsc.12318
https://doi.org/10.1111/zsc.12318...
; Malanski et al., 2019Malanski E, Sarmento-Soares LM, Silva-Malanski ACG, Lopes MM, Ingenito LFS, Buckup PA. A new species of Characidium (Characiformes: Crenuchidae) from coastal basins in the Atlantic rainforest of eastern Brazil, with phylogenetic and phylogeographic insights into the Characidium alipioi species group. Neotrop Ichthyol. 2019; 17(2):e180121. https://doi.org/10.1590/1982-0224-20180121
https://doi.org/10.1590/1982-0224-201801...
; Agudelo-Zamora et al., 2020Agudelo-Zamora HD, Tavera J, Murillo YD, Ortega-Lara A. The unknown diversity of the genus Characidium (Characiformes: Crenuchidae) in the Chocó biogeographic region, Colombian Andes: two new species supported by morphological and molecular data. J Fish Biol. 2020; 97(6):1662–75. https://doi.org/10.1111/jfb.14527
https://doi.org/10.1111/jfb.14527...
).
According to Buckup, (1993b)Buckup PA. The monophyly of the Characidiinae, a Neotropical group of characiform fishes (Teleostei: Ostariophysi). Zool J Linn Soc. 1993b; 108(3):225–45. https://doi.org/10.1111/j.1096-3642.1993.tb00297.x
https://doi.org/10.1111/j.1096-3642.1993...
, the monophyly of Characidium is supported by a single synapomorphy, the presence of a basicaudal spot near to the base of the caudal-fin rays, which is present, but inconspicuous in C. kalunga.Buckup, (1993b)Buckup PA. The monophyly of the Characidiinae, a Neotropical group of characiform fishes (Teleostei: Ostariophysi). Zool J Linn Soc. 1993b; 108(3):225–45. https://doi.org/10.1111/j.1096-3642.1993.tb00297.x
https://doi.org/10.1111/j.1096-3642.1993...
was apparently more focused on the generic relationships among the crenuchid genera and, therefore, did not investigate much further the specific interrelationships within Characidium. That analysis included relatively few representatives and characters relevant to the resolution of the internal nodes, resulting on a basal polytomy formed by C. zebra Eigenmann, 1909, C. hasemani, clade C1 (including clades C2 and C3), clade C4, and clade C5 (including clades C6 and C7).
Characidium kalunga shares a putative synapomorphy with the species placed in clade C1, the absence of scales on the isthmus. Noteworthy, Buckup, (1993b)Buckup PA. The monophyly of the Characidiinae, a Neotropical group of characiform fishes (Teleostei: Ostariophysi). Zool J Linn Soc. 1993b; 108(3):225–45. https://doi.org/10.1111/j.1096-3642.1993.tb00297.x
https://doi.org/10.1111/j.1096-3642.1993...
considered this to be a multistate character, and described three states: state 0, scaled isthmus (e.g., outgroup and C. zebra); state 1, only the isthmus lacking scales (e.g., C. lauroi); state 2, scaleless area on isthmus and area between the pectoral fins (e.g., C. gomesi); state 3, scaleless area extending along the belly to the level of the pelvic fins (e.g., C. crandellii). In C. kalunga, the scaleless area is restricted to a small, triangular area restricted to the isthmus and lined posteriorly to the level of the cleithra, therefore, as described in state 1. The two additional synapomorphies assigned for clade C1 are absent in C. kalunga, the reduction or absence of the postcleithrum 1 (vs. well developed in C. kalunga, Fig. 5A), and the fontanel reduced and limited anteriorly by the parietals (vs. fontanel extending anteriorly to the frontals, Fig. 4C). Besides, C. kalunga also lacks any of the two synapomorphies supporting clade C2 and the five synapomorphies that support clade C3. Finally, the scaleless isthmus is not a unique synapomorphy to that clade, and has at least three independent origins in the subfamily, being also present in distantly related crenuchids such as Ammocryptocharax lateralis (Eigenmann, 1909), A. vintonae (Eigenmann, 1909), Melanocharacidium depressum Buckup, 1993, and M. pectorale Buckup, 1993 Buckup, 1993aBuckup PA. Review of the characidiin fishes (Teleostei: Characiformes), with description of four new genera and ten new species. Ichthyol Explor Freshw. 1993a; 4(2):97–154.), and variable in C. lanei Travassos, 1967, which may have the isthmus fully scaled or with a small, unscaled area (MRSM pers. obs.), indicating that the condition in C. kalunga could be an additional independent origin, considering the lack of additional characters suggesting its close relationships with representatives of clade C1 of Buckup, (1993b)Buckup PA. The monophyly of the Characidiinae, a Neotropical group of characiform fishes (Teleostei: Ostariophysi). Zool J Linn Soc. 1993b; 108(3):225–45. https://doi.org/10.1111/j.1096-3642.1993.tb00297.x
https://doi.org/10.1111/j.1096-3642.1993...
.
The clade C4 was more recently revised with the inclusion of additional species and reevaluation of morphological characters (Netto-Ferreira et al., 2013Netto-Ferreira AL, Birindelli JLO, Buckup PA. A new miniature species of Characidium Reinhardt (Ostariophysi: Characiformes: Crenuchidae) from the headwaters of the rio Araguaia, Brazil. Zootaxa. 2013; 3664(3):361–68. http://dx.doi.org/10.11646/zootaxa.3664.3.6
http://dx.doi.org/10.11646/zootaxa.3664....
; Mendonça, Netto-Ferreira, 2015Mendonça MB, Netto-Ferreira AL. New species of Characidium (Characiformes: Crenuchidae) from the rio Tapajós and rio Xingu drainages, Pará, Brazil. Zootaxa. 2015; 4021(1):187–94. http://dx.doi.org/10.11646/zootaxa.4021.1.9
http://dx.doi.org/10.11646/zootaxa.4021....
). Characidium kalunga also shares one of the three synapomorphies that support the monophyly of clade C4, the absence of the dentary-teeth medial row (Fig. 5B). However, unlike the representatives of that clade, C. kalunga has the parietal branch of the supraorbital laterosensory canal, despite of being short (vs. parietal branch absent) and has seven to nine bars on body (vs. 12 or more bars). Like the characters discussed above, those three synapomorphies are not unique to that group being even variable within the species of clade C4, as discussed by Melo, Espíndola, (2016)Melo MRS, Espíndola VC. Description of a new species of Characidium Reinhardt, 1867 (Characiformes: Crenuchidae) from the Chapada Diamantina, Bahia, and redescription of Characidium bimaculatum Fowler, 1941. Zootaxa. 2016; 4196(4):552–68. http://dx.doi.org/10.11646/zootaxa.4196.4.5
http://dx.doi.org/10.11646/zootaxa.4196....
.
The overall pigmentation pattern of C. kalunga resembles that of C. heirmostigmata, C. papachibe, C. satoi, and C. serrano because the lateral portion of the vertical bars are disconnected from their dorsal portions and form a series of midlateral blotches on the flanks (Buckup, Reis, 1997Buckup PA, Reis RE. Characidiin genus Characidium (Teleostei, Characiformes) in Southern Brazil, with description of three new species. Copeia. 1997; 1997(3):531–48. https://doi.org/10.2307/1447557
https://doi.org/10.2307/1447557...
; Da Graça, Pavanelli, 2008Da Graça WJ, Pavanelli CS. Characidiumheirmostigmata, a new characidiin fish (Characiformes: Crenuchidae) from the upper rio Paraná basin, Brazil. Neotrop ichthyol. 2008; 6(1):53–56. http://dx.doi.org/10.1590/S1679-62252008000100006
http://dx.doi.org/10.1590/S1679-62252008...
; Peixoto, Wosiacki, 2013Peixoto LAW, Wolsiacki WB. A new species of Characidium (Characiformes: Crenuchidae) from the Lower Amazon. Copeia. 2013; 2013(1):52–57. https://doi.org/10.1643/CI-12-080
https://doi.org/10.1643/CI-12-080...
). Despite showing a superficial resemblance, in C. heirmostigmata, C. papachibe and C. serrano the bars form oblique bands along the body, centered at level of the lateral line and in C. satoi, oval dots, V-shaped, W-shaped, or diamond-shaped marks along and ventral to lateral line. In addition, C. serrano has more bars (10–14 vs. 8–9), which allows unambiguous distinction from C. kalunga. In addition, C. kalunga further differs from those species in the number of circumpeduncular scales (14 vs. 10 in C. papachibe and 12 in C. heirmostigmata and C. serrano).
The variation of fin-ray counts in Characidium kalunga is unusual and never recorded in any congener. In Characidium, fin-ray counts usually do not have significant interspecific variation and show a typical modal distribution. As C. kalunga has a relatively limited geographic distribution and such variations are random among the specimens, we were unable to identify any pattern related to geographical variation or sexual dimorphism, for example, and suppose that such variation may be the result of long-time inbreeding in small populations.
In summary, the phylogenetic position of C. kalunga among its congeners could not be hypothesized by us based on the available data. The discovery of a new species having synapomorphies from different well-supported clades stress the understanding that the genus Characidium urges for a phylogenetic revision, including more extensive taxa sampling, reevaluation of morphological characters and reinterpretation of homologies, in combination to molecular data.
The Chapada dos Veadeiros species richness and endemism. The Chapada dos Veadeiros is an ancient plateau located in the northern portion of the Planalto Central Goiano, formed by the collision between the Congo and São Francisco cratons during the assembly of Gondwana, which has altitudes varying from 1,000 to 1,600 (mean 1,200) meters above sea level (De Carvalho Júnior et al., 2015De Carvalho Júnior OA, Guimarães RF, Martins ÉS, Gomes RAT. Chapada dos Veadeiros: the highest landscapes in the Brazilian Central Plateau. In: Viera BC, Salgado AAR, Santos LJC, editors. Landscapes and Landforms of Brazil. Dordrecht: Springer; 2015. p.221–30. https://doi.org/10.1007/978-94-017-8023-0_20
https://doi.org/10.1007/978-94-017-8023-...
). The atmospheric temperature is mild all over the year, with means of 21º–25o C during summer (June to September) and 18º–22º C in the other months, but often reaching below 10º C in the winter. The vegetation is a typical high-altitude rocky grassland of the Cerrado (Eiten, 1972Eiten G. The Cerrado Vegetation of Brazil. Bot Rev. 1972; 38(2):201–341. https://doi.org/10.1007/BF02859158
https://doi.org/10.1007/BF02859158...
). The Chapada dos Veadeiros works as a watershed for the headwaters of rio Maranhão draining to the northwest; the rio Tocantinzinho, to the central and southern areas; and the rio Paranã to the east and northeast, all tributaries of the upper rio Tocantins (Brasil, 1982Brasil. Folha SE.21 Corumbá e parte da Folha SE.20. Geologia, geomorfologia, pedologia, vegetação e uso potencial da terra. Levantamento de recursos naturais. Volume 27. Projeto RADAMBRASIL; 1982.).
Besides C. kalunga, there are two additional congeners occurring in the Chapada dos Veadeiros, namely C. stigmosum and C. xanthopterum (Figs. 9A–B). Characidium stigmosum was described from the tributaries of rio das Almas (Melo, Buckup, 2002Melo MRS, Buckup PA. Characidiumstigmosum (Characiformes: Crenuchidae): a new species of characidiin fish from central Brazil. Copeia. 2002; 2002(4):988–93. https://doi.org/10.1643/0045-8511(2002)002[0988:CSCCAN]2.0.CO;2
https://doi.org/10.1643/0045-8511(2002)0...
). The coordinates of the type locality, a small stream tributary of córrego Ave Maria, were originally inferred as 13°47’S 47°30’W based on paper maps with 1:1.000.000 scale, but should be corrected to a more precise location: 13°45’34”S 47°27’20”W. The distribution of C. stigmosum is restricted to the northern portion of the Chapada dos Veadeiros, in the rio das Almas and rio dos Bois drainages, both tributaries of the Rio Paranã.
Additional species and habitats of Characidium in the Chapada dos Veadeiros, Goiás, Brazil: A. Characidium stigmosum, live specimen in aquarium (collected with MZUSP 113939, photo by Fernando Dagosta) collected at B. stream tributary of rio das Almas, Cavalcante (photo by Osvaldo Oyakawa); C. Characidium xanthopterum live specimen (photo by MRSM) at D. Cachoeira dos Macaquinhos, rio dos Macacos (not collected).
We also report for the first time the occurrence of C. xanthopterum in the Chapada dos Veadeiros. This species is widely distributed in the Planalto Central Goiano, occurring in both the upper rio Paraná basin and upper rio Tocantins basins, in the States of Goiás and Tocantins, and in the Distrito Federal (Silveira et al., 2008Silveira LGG, Langeani F, da Graça WJ, Pavanelli CS, Buckup PA. Characidium xanthopterum (Ostariophysi: Characiformes: Crenuchidae): a new species from the Central Brazilian Plateau. Neotrop ichthyol. 2008; 6(2):169–74. http://dx.doi.org/10.1590/S1679-62252008000200003
http://dx.doi.org/10.1590/S1679-62252008...
). In the Chapada dos Veadeiros, it was collected in sympatry with C. kalunga at Cachoeira de Almécegas (UFRGS 11257) and photographed at Cachoeira do Macaquinho, a tributary of rio Macacão in the eastern border of the Chapada dos Veadeiros, rio Paranã basin (Fig. 9B).
In addition, the Chapada dos Veadeiros hosts eight endemic species of fishes, three of which are restricted to the tributaries of the upper rio Tocantinzinho [the characids Astyanax goyanensis (Miranda-Ribeiro, 1944Miranda-Ribeiro P. Nova espécie para o gênero Astyanacinus Eigenmann, 1907 (Pisces, Characinidae). Bol Mus Nac Rio de Janeiro. 1944; 29:1–2.) and Astyanax courensisBertaco, Carvalho & Jerep, 2010Bertaco VA, Carvalho FR, Jerep FC. Astyanax goyanensis Miranda-Ribeiro, 1944), new combination and Astyanax courensis, new species (Ostariophysi: Characiformes): two Characidae from the upper rio Tocantins basin, Central Brazil. Neotrop Ichthyol. 2010; 8(2):265–75. http://dx.doi.org/10.1590/S1679-62252010000200004
http://dx.doi.org/10.1590/S1679-62252010...
, and the loricariid Corumbataia anosteos (Carvalho, Lehmann & Reis, 2008Carvalho TP, Lehmann PA, Reis RE. Gymnotocinclus anosteos, a new uniquely-plated genus and species of loricariid catfish (Teleostei: Siluriformes) from the upper rio Tocantins basin, central Brazil. Neotrop Ichthyol. 2008; 6(3):329–38. https://doi.org/10.1590/S1679-62252008000300006
https://doi.org/10.1590/S1679-6225200800...
)], and seven, to the tributaries of rio Paranã [the characids Hasemania kalungaBertaco & Carvalho, 2010Bertaco VA, Carvalho FR. New species of Hasemania (Characiformes: Characidae) from Central Brazil, with comments on the endemism of upper rio Tocantins basin, Goiás State. Neotrop Ichthyol. 2010; 8(1):27–32. http://dx.doi.org/10.1590/S1679-62252010000100004
http://dx.doi.org/10.1590/S1679-62252010...
, and Hemigrammus tocantinsiCarvalho, Bertaco & Jerep, 2010Carvalho FR, Bertaco VA, Jerep FC. Hemigrammus tocantinsi: a new species from the upper rio Tocantins basin, Central Brazil (Characiformes: Characidae). Neotrop Ichthyol. 2010; 8(2):247–54. https://doi.org/10.1590/S1679-62252010000200002
https://doi.org/10.1590/S1679-6225201000...
, Moenkhausia dasalmas Bertaco, Jerep & Carvalho, 2011; the parodontid Apareiodon cavalcante Pavanelli & Britski, 2003Pavanelli CS, Britski HA. Apareiodon (Teleostei, Characiformes), from the Tocantins-Araguaia Basin, with description of three new species. Copeia. 2003; 2003(2):337–48. https://doi.org/10.1643/0045-8511(2003)003[0337:AETCFT]2.0.CO;2
https://doi.org/10.1643/0045-8511(2003)0...
; and the loricariids Corumbataia canoeiro (Roxo, Silva, Ochoa & Zawadzki, 2017Roxo FF, Silva GSC, Ochoa LE, Zawadzki CH. Description of a new species of Gymnotocinclus from the rio Tocantins basin with phylogenetic analysis of the subfamily Hypoptopomatinae (Siluriformes: Loricariidae). Zootaxa. 2017; 4268(3):337–59. https://doi.org/10.11646/zootaxa.4268.3.2
https://doi.org/10.11646/zootaxa.4268.3....
) and Corumbataia veadeiros Carvalho, 2008Carvalho TP. A new species of Corumbataia (Siluriformes: Loricariidae: Hypoptopomatinae) from upper Rio Tocantins Basin, central Brazil. Copeia. 2008; 2008(3):552–57. https://doi.org/10.1643/CI-07-064
https://doi.org/10.1643/CI-07-064...
)] (Miranda-Ribeiro, 1944Miranda-Ribeiro P. Nova espécie para o gênero Astyanacinus Eigenmann, 1907 (Pisces, Characinidae). Bol Mus Nac Rio de Janeiro. 1944; 29:1–2.; Pavanelli, Britski, 2003Pavanelli CS, Britski HA. Apareiodon (Teleostei, Characiformes), from the Tocantins-Araguaia Basin, with description of three new species. Copeia. 2003; 2003(2):337–48. https://doi.org/10.1643/0045-8511(2003)003[0337:AETCFT]2.0.CO;2
https://doi.org/10.1643/0045-8511(2003)0...
; Carvalho, 2008Carvalho TP. A new species of Corumbataia (Siluriformes: Loricariidae: Hypoptopomatinae) from upper Rio Tocantins Basin, central Brazil. Copeia. 2008; 2008(3):552–57. https://doi.org/10.1643/CI-07-064
https://doi.org/10.1643/CI-07-064...
; Carvalho et al., 2008Carvalho TP, Lehmann PA, Reis RE. Gymnotocinclus anosteos, a new uniquely-plated genus and species of loricariid catfish (Teleostei: Siluriformes) from the upper rio Tocantins basin, central Brazil. Neotrop Ichthyol. 2008; 6(3):329–38. https://doi.org/10.1590/S1679-62252008000300006
https://doi.org/10.1590/S1679-6225200800...
, 2010Carvalho FR, Bertaco VA, Jerep FC. Hemigrammus tocantinsi: a new species from the upper rio Tocantins basin, Central Brazil (Characiformes: Characidae). Neotrop Ichthyol. 2010; 8(2):247–54. https://doi.org/10.1590/S1679-62252010000200002
https://doi.org/10.1590/S1679-6225201000...
; Bertaco, Carvalho, 2010Bertaco VA, Carvalho FR. New species of Hasemania (Characiformes: Characidae) from Central Brazil, with comments on the endemism of upper rio Tocantins basin, Goiás State. Neotrop Ichthyol. 2010; 8(1):27–32. http://dx.doi.org/10.1590/S1679-62252010000100004
http://dx.doi.org/10.1590/S1679-62252010...
; Bertaco et al., 2010Bertaco VA, Carvalho FR, Jerep FC. Astyanax goyanensis Miranda-Ribeiro, 1944), new combination and Astyanax courensis, new species (Ostariophysi: Characiformes): two Characidae from the upper rio Tocantins basin, Central Brazil. Neotrop Ichthyol. 2010; 8(2):265–75. http://dx.doi.org/10.1590/S1679-62252010000200004
http://dx.doi.org/10.1590/S1679-62252010...
; Roxo et al., 2017Roxo FF, Silva GSC, Ochoa LE, Zawadzki CH. Description of a new species of Gymnotocinclus from the rio Tocantins basin with phylogenetic analysis of the subfamily Hypoptopomatinae (Siluriformes: Loricariidae). Zootaxa. 2017; 4268(3):337–59. https://doi.org/10.11646/zootaxa.4268.3.2
https://doi.org/10.11646/zootaxa.4268.3....
; Thimotheo et al., 2020Thimotheo MGR, Benine RC, Oliveira C, Silva GSC. New species of the Corumbataia cuestae group (Siluriformes: Loricariidae) from the Rio Tocantins basin, with comments on its phylogenetic relationships. Neotrop Ichthyol. 2020; 18(4):e200060. https://doi.org/10.1590/1982-0224-2020-0060
https://doi.org/10.1590/1982-0224-2020-0...
). Additional vertebrates with narrow distribution at the Chapada dos Veadeiros and vicinity (Serra do Trombador) include four anurans [the hylids Boana phaeopleura (Caramashi & Cruz, 2000Caramaschi U, Cruz CA. Duas espécies novas de Hyla Laurenti, 1768 do estado de Goiás, Brasil (Amphibia, Anura, Hylidae). Bol Mus Nac Rio de Janeiro. 2000; 422:1–12.) and Scinax rupestrisAraujo-Vieira, Brandão & Faria, 2015Araujo-Vieira K, Brandão RA, Faria DCC. A new species of rock-dwelling Scinax Wagler (Anura: Hylidae) from Chapada dos Veadeiros, central Brazil. Zootaxa. 2015; 3915(1):52–66. http://dx.doi.org/10.11646/zootaxa.3915.1.2
http://dx.doi.org/10.11646/zootaxa.3915....
; the leptodactylid Leptodactylus tapitiSazima & Bokermann, 1978Sazima I, Bokermann WCA. Cinco novas espécies de Leptodactylus do Centro e Sudeste Brasileiro (Amphibia, Anura, Leptodactylidae). Rev. Bras. Biol. 1978; 38(4):899–912.; and the odontophrynid Proceratophrys rotundipalpebra Martins & Giaretta, 2013] and a rodent [the cricetid Oligoryzomys moojeni (Weksler & Bonvicino, 2005Weksler M, Bonvicino CR. Taxonomy of pigmy rice rats genus Oligoryzomys Bangs, 1900 (Rodentia, Sigmodontinae) of the Brazilian Cerrado, with the description of two new species. Arq Mus Nac. 2005; 63(1):113–30.)] (Sazima, Bokermann, 1978Sazima I, Bokermann WCA. Cinco novas espécies de Leptodactylus do Centro e Sudeste Brasileiro (Amphibia, Anura, Leptodactylidae). Rev. Bras. Biol. 1978; 38(4):899–912.; Caramaschi, Cruz, 2000Caramaschi U, Cruz CA. Duas espécies novas de Hyla Laurenti, 1768 do estado de Goiás, Brasil (Amphibia, Anura, Hylidae). Bol Mus Nac Rio de Janeiro. 2000; 422:1–12.; Weksler, Bonvicino, 2005Weksler M, Bonvicino CR. Taxonomy of pigmy rice rats genus Oligoryzomys Bangs, 1900 (Rodentia, Sigmodontinae) of the Brazilian Cerrado, with the description of two new species. Arq Mus Nac. 2005; 63(1):113–30.; Nogueira et al., 2011Nogueira C, Ribeiro S, Costa GC, Colli GR. Vicariance and endemism in a Neotropical savanna hotspot: distribution patterns of Cerrado squamate reptiles. J Biogeogr. 2011; 38(10):1907–22. https://doi.org/10.1111/j.1365-2699.2011.02538.x
https://doi.org/10.1111/j.1365-2699.2011...
; Santoro, Brandão, 2014Santoro GRCC, Brandão RA. Reproductive modes, habitat use, and richness of anurans from Chapada dos Veadeiros, central Brazil. North West J Zool. 2014; 10(2):365–73. ; Araujo-Vieira et al., 2015Araujo-Vieira K, Brandão RA, Faria DCC. A new species of rock-dwelling Scinax Wagler (Anura: Hylidae) from Chapada dos Veadeiros, central Brazil. Zootaxa. 2015; 3915(1):52–66. http://dx.doi.org/10.11646/zootaxa.3915.1.2
http://dx.doi.org/10.11646/zootaxa.3915....
).
In conclusion, the discovery of a new species of fish with narrow distribution emphasizes the unique species richness and endemism present on the highlands of the Chapada dos Veadeiros, which should be treated as a hotspot for vertebrates in Central Brazil and, therefore, a priority area for the conservation of the Cerrado biodiversity (Cavalcanti, Joly, 2002Cavalcanti RB, Joly CA. Biodiversity and Conservation Priorities in the Cerrado Region. In: Oliveira PS, Marquis RJ, editors. The Cerrados of Brazil: Ecology and Natural History of a Neotropical Savanna. New York: Columbia University Press; 2002. p.53–567. https://doi.org/10.7312/oliv12042-017
https://doi.org/10.7312/oliv12042-017...
; Nogueira et al., 2010Nogueira C, Buckup PA, Menezes NA, Oyakawa OT, Kasecker TP, Ramos Neto MB, da Silva JMC. Restricted-Range Fishes and the Conservation of Brazilian Freshwaters. Plos One. 2010; 5:e11390. https://doi.org/10.1371/journal.pone.0011390
https://doi.org/10.1371/journal.pone.001...
, 2011Nogueira C, Ribeiro S, Costa GC, Colli GR. Vicariance and endemism in a Neotropical savanna hotspot: distribution patterns of Cerrado squamate reptiles. J Biogeogr. 2011; 38(10):1907–22. https://doi.org/10.1111/j.1365-2699.2011.02538.x
https://doi.org/10.1111/j.1365-2699.2011...
; França, Braz, 2013França FGR, Braz VS. Diversity, activity patterns, and habitat use of the snake fauna of Chapada dos Veadeiros National Park in Central Brazil. Biota Neotrop. 2013; 13(1):74–84. https://doi.org/10.1590/S1676-06032013000100008
https://doi.org/10.1590/S1676-0603201300...
; Lima, Franco, 2014Lima PCA, Franco JLA. As RPPNs como estratégia para a conservação da Biodiversidade: O caso da Chapada dos Veadeiros. Soc Nat. 2014; 26(1):113–25. https://doi.org/10.1590/1982-451320140108
https://doi.org/10.1590/1982-45132014010...
).
Comparative material examined. Goiás, Brazil: Characidium stigmosum: UFRGS 9925, 39, 18.3–38.5 mm SL; UFRGS 11195, 26, 18.4–38.9 mm SL; MZUSP 40804, 33.7 mm SL, holotype; MNRJ 21974, 4, 27.7–38.3 mm SL, paratypes; MZUSP 70221, 15, 16.7–38.7 mm SL, paratypes; USMN 36832, 2, 31.7–31.9 mm SL, paratypes; MZUSP 40797, 2, 25.6–39.9 mm SL, paratypes; MZUSP 40810, 11, 22.0–39.5 mm SL, paratypes; MZUSP 40813, 6, 23.2–37.5 mm SL, paratypes; MCP 21715, 15, 19.6–37.5 mm SL, paratypes; MZUSP 113839, 1, 40.1 mm SL; MZUSP 113939, 61, 18.7–41.1 mm SL. Characidium xanthopterum: UFRGS 11226, 16, 26.2–36.4 mm SL; UFRGS 11299, 4, 24.6–36.6 mm SL; MZUSP 53404, 32, 30.7–40.6 mm SL, MZUSP 53422, 3, 38.8–45.2 mm SL.
ACKNOWLEDGEMENTS
For curatorial assistance, we would like to thank Alessio Datovo, Mário C. C. de Pinna, Michel D. Gianeti and Osvaldo T. Oyakawa (MZUSP), Flávio C. T. Lima (ZUEC), Cristiano R. Moreira and Emanuel B. Neuhaus (MNRJ), and Juliana M. Wingert (UFRGS). We also would like to thank Beatriz Vasconcelos and Reuber A. Brandão (Universidade de Brasília) for comments and data about the anurans, Fernando C. P. Dagosta (Universidade Federal da Grande Dourados) for pictures of the live specimen of C. stigmosum, José L. O. Birindelli (Universidade Estadual de Londrina), and Fernando Lessa for data and additional pictures of C. kalunga. Part of the type series was collected during an expedition funded by the South American Characiformes Inventory Project (FAPESP 2011/50282–7) granted to Naércio A. Menezes (MZUSP). The authors received financial support from the Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq (MRSM: 433050/2016–0), Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP (MRSM: 2017/12909–4; BBB: 2019/20913–7; FTM: 2019/20912–0), and Programa Unificado de Bolsas de Estudos da Universidade de São Paulo – PUB (RSF: 2797).
REFERENCES
- Agudelo-Zamora HD, Tavera J, Murillo YD, Ortega-Lara A The unknown diversity of the genus Characidium (Characiformes: Crenuchidae) in the Chocó biogeographic region, Colombian Andes: two new species supported by morphological and molecular data. J Fish Biol. 2020; 97(6):1662–75. https://doi.org/10.1111/jfb.14527
» https://doi.org/10.1111/jfb.14527 - Araujo-Vieira K, Brandão RA, Faria DCC A new species of rock-dwelling Scinax Wagler (Anura: Hylidae) from Chapada dos Veadeiros, central Brazil. Zootaxa. 2015; 3915(1):52–66. http://dx.doi.org/10.11646/zootaxa.3915.1.2
» http://dx.doi.org/10.11646/zootaxa.3915.1.2 - Armbruster JA, Lujan NK, Bloom DD Redescription of the Guiana shield darter species Characidiumcrandellii and C. declivirostre (Crenuchidae) with descriptions of two new species. Ichthyol Herpetol. 2021; 109(1):102–22. https://doi.org/10.1643/i2019299
» https://doi.org/10.1643/i2019299 - Bertaco VA, Carvalho FR New species of Hasemania (Characiformes: Characidae) from Central Brazil, with comments on the endemism of upper rio Tocantins basin, Goiás State. Neotrop Ichthyol. 2010; 8(1):27–32. http://dx.doi.org/10.1590/S1679-62252010000100004
» http://dx.doi.org/10.1590/S1679-62252010000100004 - Bertaco VA, Carvalho FR, Jerep FC Astyanax goyanensis Miranda-Ribeiro, 1944), new combination and Astyanax courensis, new species (Ostariophysi: Characiformes): two Characidae from the upper rio Tocantins basin, Central Brazil. Neotrop Ichthyol. 2010; 8(2):265–75. http://dx.doi.org/10.1590/S1679-62252010000200004
» http://dx.doi.org/10.1590/S1679-62252010000200004 - Brasil Folha SE.21 Corumbá e parte da Folha SE.20. Geologia, geomorfologia, pedologia, vegetação e uso potencial da terra. Levantamento de recursos naturais. Volume 27. Projeto RADAMBRASIL; 1982.
- Buckup PA Review of the characidiin fishes (Teleostei: Characiformes), with description of four new genera and ten new species. Ichthyol Explor Freshw. 1993a; 4(2):97–154.
- Buckup PA The monophyly of the Characidiinae, a Neotropical group of characiform fishes (Teleostei: Ostariophysi). Zool J Linn Soc. 1993b; 108(3):225–45. https://doi.org/10.1111/j.1096-3642.1993.tb00297.x
» https://doi.org/10.1111/j.1096-3642.1993.tb00297.x - Buckup PA, Reis RE Characidiin genus Characidium (Teleostei, Characiformes) in Southern Brazil, with description of three new species. Copeia. 1997; 1997(3):531–48. https://doi.org/10.2307/1447557
» https://doi.org/10.2307/1447557 - Caramaschi U, Cruz CA Duas espécies novas de Hyla Laurenti, 1768 do estado de Goiás, Brasil (Amphibia, Anura, Hylidae). Bol Mus Nac Rio de Janeiro. 2000; 422:1–12.
- Carvalho FR, Bertaco VA, Jerep FC Hemigrammus tocantinsi: a new species from the upper rio Tocantins basin, Central Brazil (Characiformes: Characidae). Neotrop Ichthyol. 2010; 8(2):247–54. https://doi.org/10.1590/S1679-62252010000200002
» https://doi.org/10.1590/S1679-62252010000200002 - Carvalho TP A new species of Corumbataia (Siluriformes: Loricariidae: Hypoptopomatinae) from upper Rio Tocantins Basin, central Brazil. Copeia. 2008; 2008(3):552–57. https://doi.org/10.1643/CI-07-064
» https://doi.org/10.1643/CI-07-064 - Carvalho TP, Lehmann PA, Reis RE Gymnotocinclus anosteos, a new uniquely-plated genus and species of loricariid catfish (Teleostei: Siluriformes) from the upper rio Tocantins basin, central Brazil. Neotrop Ichthyol. 2008; 6(3):329–38. https://doi.org/10.1590/S1679-62252008000300006
» https://doi.org/10.1590/S1679-62252008000300006 - De Carvalho Júnior OA, Guimarães RF, Martins ÉS, Gomes RAT Chapada dos Veadeiros: the highest landscapes in the Brazilian Central Plateau. In: Viera BC, Salgado AAR, Santos LJC, editors. Landscapes and Landforms of Brazil. Dordrecht: Springer; 2015. p.221–30. https://doi.org/10.1007/978-94-017-8023-0_20
» https://doi.org/10.1007/978-94-017-8023-0_20 - Cavalcanti RB, Joly CA Biodiversity and Conservation Priorities in the Cerrado Region. In: Oliveira PS, Marquis RJ, editors. The Cerrados of Brazil: Ecology and Natural History of a Neotropical Savanna. New York: Columbia University Press; 2002. p.53–567. https://doi.org/10.7312/oliv12042-017
» https://doi.org/10.7312/oliv12042-017 - Eiten G The Cerrado Vegetation of Brazil. Bot Rev. 1972; 38(2):201–341. https://doi.org/10.1007/BF02859158
» https://doi.org/10.1007/BF02859158 - França FGR, Braz VS Diversity, activity patterns, and habitat use of the snake fauna of Chapada dos Veadeiros National Park in Central Brazil. Biota Neotrop. 2013; 13(1):74–84. https://doi.org/10.1590/S1676-06032013000100008
» https://doi.org/10.1590/S1676-06032013000100008 - Fricke R, Eschmeyer WN, Van der Laan R Eschmeyer’s catalog of fishes: genera, species, references [Internet]. San Francisco: California Academy of Science; 2021. Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
» http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp - Da Graça WJ, Pavanelli CS Characidiumheirmostigmata, a new characidiin fish (Characiformes: Crenuchidae) from the upper rio Paraná basin, Brazil. Neotrop ichthyol. 2008; 6(1):53–56. http://dx.doi.org/10.1590/S1679-62252008000100006
» http://dx.doi.org/10.1590/S1679-62252008000100006 - Da Graça WJ, Pavanelli CS, Buckup PA Two new species of Characidium (Characiformes: Crenuchidae) from Paraguay and Xingu basins, state of Mato Grosso, Brazil. Copeia. 2008; 2008(2):326–32. https://doi.org/10.1643/CI-06-167
» https://doi.org/10.1643/CI-06-167 - International Union for Conservation of Nature (IUCN) Standards and petitions subcommittee. Guidelines for Using the IUCN Red List Categories and Criteria. Version 14 [Internet]. Gland; 2019. Available from: http://www.iucnredlist.org/documents/RedListGuidelines.pdf
» http://www.iucnredlist.org/documents/RedListGuidelines.pdf - Lima PCA, Franco JLA As RPPNs como estratégia para a conservação da Biodiversidade: O caso da Chapada dos Veadeiros. Soc Nat. 2014; 26(1):113–25. https://doi.org/10.1590/1982-451320140108
» https://doi.org/10.1590/1982-451320140108 - Malanski E, Sarmento-Soares LM, Silva-Malanski ACG, Lopes MM, Ingenito LFS, Buckup PA A new species of Characidium (Characiformes: Crenuchidae) from coastal basins in the Atlantic rainforest of eastern Brazil, with phylogenetic and phylogeographic insights into the Characidium alipioi species group. Neotrop Ichthyol. 2019; 17(2):e180121. https://doi.org/10.1590/1982-0224-20180121
» https://doi.org/10.1590/1982-0224-20180121 - Melo MRS, Buckup PA Characidiumstigmosum (Characiformes: Crenuchidae): a new species of characidiin fish from central Brazil. Copeia. 2002; 2002(4):988–93. https://doi.org/10.1643/0045-8511(2002)002[0988:CSCCAN]2.0.CO;2
» https://doi.org/10.1643/0045-8511(2002)002[0988:CSCCAN]2.0.CO;2 - Melo MRS, Espíndola VC Description of a new species of Characidium Reinhardt, 1867 (Characiformes: Crenuchidae) from the Chapada Diamantina, Bahia, and redescription of Characidium bimaculatum Fowler, 1941. Zootaxa. 2016; 4196(4):552–68. http://dx.doi.org/10.11646/zootaxa.4196.4.5
» http://dx.doi.org/10.11646/zootaxa.4196.4.5 - Melo MRS, Oyakawa OT A new species of Characidium Reinhardt (Characiformes, Crenuchidae) with a distinctively dimorphic male. Copeia. 2015; 103(2):281–89. https://doi.org/10.1643/CI-14-073
» https://doi.org/10.1643/CI-14-073 - Melo MRS, Ribeiro MCLB, Lima FCT A new, narrowly distributed, and critically endangered species of Characidium (Characiformes: Crenuchidae) from the Distrito Federal, Central Brazil. Neotrop Ichthyol. 2021; 19(1):e200061. https://doi.org/10.1590/1982-0224-2020-0061
» https://doi.org/10.1590/1982-0224-2020-0061 - Mendonça MB, Netto-Ferreira AL New species of Characidium (Characiformes: Crenuchidae) from the rio Tapajós and rio Xingu drainages, Pará, Brazil. Zootaxa. 2015; 4021(1):187–94. http://dx.doi.org/10.11646/zootaxa.4021.1.9
» http://dx.doi.org/10.11646/zootaxa.4021.1.9 - Miranda-Ribeiro P Nova espécie para o gênero Astyanacinus Eigenmann, 1907 (Pisces, Characinidae). Bol Mus Nac Rio de Janeiro. 1944; 29:1–2.
- Netto-Ferreira AL, Birindelli JLO, Buckup PA A new miniature species of Characidium Reinhardt (Ostariophysi: Characiformes: Crenuchidae) from the headwaters of the rio Araguaia, Brazil. Zootaxa. 2013; 3664(3):361–68. http://dx.doi.org/10.11646/zootaxa.3664.3.6
» http://dx.doi.org/10.11646/zootaxa.3664.3.6 - Nogueira C, Buckup PA, Menezes NA, Oyakawa OT, Kasecker TP, Ramos Neto MB, da Silva JMC Restricted-Range Fishes and the Conservation of Brazilian Freshwaters. Plos One. 2010; 5:e11390. https://doi.org/10.1371/journal.pone.0011390
» https://doi.org/10.1371/journal.pone.0011390 - Nogueira C, Ribeiro S, Costa GC, Colli GR Vicariance and endemism in a Neotropical savanna hotspot: distribution patterns of Cerrado squamate reptiles. J Biogeogr. 2011; 38(10):1907–22. https://doi.org/10.1111/j.1365-2699.2011.02538.x
» https://doi.org/10.1111/j.1365-2699.2011.02538.x - Pavanelli CS, Britski HA Apareiodon (Teleostei, Characiformes), from the Tocantins-Araguaia Basin, with description of three new species. Copeia. 2003; 2003(2):337–48. https://doi.org/10.1643/0045-8511(2003)003[0337:AETCFT]2.0.CO;2
» https://doi.org/10.1643/0045-8511(2003)003[0337:AETCFT]2.0.CO;2 - Peixoto LAW, Wolsiacki WB A new species of Characidium (Characiformes: Crenuchidae) from the Lower Amazon. Copeia. 2013; 2013(1):52–57. https://doi.org/10.1643/CI-12-080
» https://doi.org/10.1643/CI-12-080 - Reinhardt JT Om trende, formeentligt ubeskrevne fisk af characinernes eller Karpelaxenes familie. Oversigt over det Kongelige Danske Videnskabernes Selskabs Forhandlinger og dets Medlemmers Arbeider. Copenhagen. 1867; 1866:49–68.
- Roxo FF, Silva GSC, Ochoa LE, Zawadzki CH Description of a new species of Gymnotocinclus from the rio Tocantins basin with phylogenetic analysis of the subfamily Hypoptopomatinae (Siluriformes: Loricariidae). Zootaxa. 2017; 4268(3):337–59. https://doi.org/10.11646/zootaxa.4268.3.2
» https://doi.org/10.11646/zootaxa.4268.3.2 - Sabaj MH Standard symbolic codes for institutional resource collections in herpetology and ichthyology: An Online Reference. Version 7.1; 2019. Available from: https://asih.org/standard-symbolic-codes
» https://asih.org/standard-symbolic-codes - Santoro GRCC, Brandão RA Reproductive modes, habitat use, and richness of anurans from Chapada dos Veadeiros, central Brazil. North West J Zool. 2014; 10(2):365–73.
- Sazima I, Bokermann WCA Cinco novas espécies de Leptodactylus do Centro e Sudeste Brasileiro (Amphibia, Anura, Leptodactylidae). Rev. Bras. Biol. 1978; 38(4):899–912.
- Serrano ÉA, Melo BF, Freitas‐Souza D, Oliveira MLM, Utsunomia R, Oliveira C, Foresti F Species delimitation in Neotropical fishes of the genus Characidium (Teleostei, Characiformes). Zool Scr. 2018; 48(1):69–80. https://doi.org/10.1111/zsc.12318
» https://doi.org/10.1111/zsc.12318 - Silveira LGG, Langeani F, da Graça WJ, Pavanelli CS, Buckup PA Characidium xanthopterum (Ostariophysi: Characiformes: Crenuchidae): a new species from the Central Brazilian Plateau. Neotrop ichthyol. 2008; 6(2):169–74. http://dx.doi.org/10.1590/S1679-62252008000200003
» http://dx.doi.org/10.1590/S1679-62252008000200003 - Taylor WR, Van Dyke GC Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium. 1985; 9(2):107–19.
- Thimotheo MGR, Benine RC, Oliveira C, Silva GSC New species of the Corumbataia cuestae group (Siluriformes: Loricariidae) from the Rio Tocantins basin, with comments on its phylogenetic relationships. Neotrop Ichthyol. 2020; 18(4):e200060. https://doi.org/10.1590/1982-0224-2020-0060
» https://doi.org/10.1590/1982-0224-2020-0060 - Weksler M, Bonvicino CR Taxonomy of pigmy rice rats genus Oligoryzomys Bangs, 1900 (Rodentia, Sigmodontinae) of the Brazilian Cerrado, with the description of two new species. Arq Mus Nac. 2005; 63(1):113–30.
- Zanata AM, Camelier P Two new species of Characidium Reinhardt (Characiformes: Crenuchidae) from northeastern Brazilian coastal drainages. Neotrop Ichthyol. 2015; 13(3):487–98. https://doi.org/10.1590/1982-0224-20140106
» https://doi.org/10.1590/1982-0224-20140106 - Zarske A, Géry J Beschreibung von drei neuen Arten der Gattung Characidium Reinhardt, 1866 aus Bolivien und Paraguay (Teleostei: Characiformes: Characidiidae). Zool Abh. 2001; 51(16):229–46.
ADDITIONAL NOTES
-
HOW TO CITE THIS ARTICLE
Melo MRS, Bouquerel BB, Masumoto FT, França RS, Netto-Ferreira AL. A new species of Characidium (Characiformes: Crenuchidae) from the Chapada dos Veadeiros, Goiás, Brazil. Neotrop Ichthyol. 2021; 19(2):e200152. https://doi.org/10.1590/1982-0224-2020-0152
Edited-by
Publication Dates
-
Publication in this collection
25 June 2021 -
Date of issue
2021
History
-
Received
28 Dec 2020 -
Accepted
12 Apr 2021