ABSTRACT
A new species of Hyphessobrycon is described from the rio Mutum, a tributary of the rio Juruena, rio Tapajós basin, Brazil. The new taxon can be distinguished from its congeners by the presence of a well-defined and relatively narrow dark midlateral stripe on body, extending from head to the middle caudal-fin rays, presence of a humeral blotch, distal profile of the anal fin falcate in males, 13-16 branched anal-fin rays (vs. 17-26), and 11 or 12 horizontal scale rows around caudal peduncle. The new species shows polymorphism regarding the presence of the adipose fin, and a discussion on this type of polymorphism across the family and its systematic implications is presented.
Keywords:
Adipose fin; Amazon; Hyphessobrycon melanostichos; Tapajós; Taxonomy
RESUMO
Uma espécie nova de Hyphessobrycon é descrita do rio Mutum, um afluente do rio Juruena, bacia do rio Tapajós, Brasil. O táxon novo pode ser distinguido de seus congêneres pela presença de uma faixa média-lateral escura bem definida e relativamente estreita no corpo, estendendo-se da cabeça aos raios médios da nadadeira caudal, presença de uma mancha umeral, 13-16 raios da nadadeira anal ramificada (vs. 17-26) e 11-12 séries horizontais de escamas ao redor do pedúnculo caudal. A espécie nova apresenta polimorfismo em relação à presença da nadadeira adiposa. Assim, é apresentada uma discussão sobre esse tipo de polimorfismo em toda a família e suas implicações na sistemática do grupo.
Palavras-chave:
Amazonas; Hyphessobrycon melanostichos; Nadadeira adiposa; Tapajós; Taxonomia
INTRODUCTION
Hyphessobrycon Durbin, 1908 is one of the most species-rich genera of the Characidae, currently with more than 160 valid species (Marinho et al., 2016Marinho MMF, Dagosta FCP, Camelier P, Oyakawa OT. A name for the ‘blueberry tetra’, an aquarium trade popular species of Hyphessobrycon Durbin (Characiformes: Characidae), with comments on fish species descriptions lacking accurate type locality. J Fish Biol . 2016; 89(1):510-21. https://doi.org/10.1111/jfb.12991
https://doi.org/10.1111/jfb.12991...
; Carvalho et al., 2017Carvalho FR, Cabeceira FG, Carvalho LN. New species of Hyphessobrycon from the Rio Teles Pires, Rio Tapajós basin, Brazil (Ostariophysi, Characiformes). J Fish Biol. 2017; 91(3):750-63. https://doi.org/10.1111/jfb.13362
https://doi.org/10.1111/jfb.13362...
; Pastana et al., 2017Pastana MNL, Dagosta FCP, Esguícero ALH. A new sexually dichromatic miniature Hyphessobrycon (Teleostei: Characiformes: Characidae) from the Rio Formiga, upper Rio Juruena basin, Mato Grosso, Brazil, with a review of sexual dichromatism in Characiformes. J Fish Biol . 2017; 91(5):1301-18. https://doi.org/10.1111/jfb.13449
https://doi.org/10.1111/jfb.13449...
; Faria et al., 2020Faria TC, Bastos DA, Zuanon J, Lima FCT. A new Hyphessobrycon (Characiformes: Characidae) of the Hyphessobrycon heterorhabdus species-group from the Central Amazon basin, Brazil. Zootaxa. 2020; 4859(2):275-84. https://doi.org/10.11646/zootaxa.4859.2.6
https://doi.org/10.11646/zootaxa.4859.2....
; Fricke et al., 2022Fricke R, Eschmeyer WN, Van der Laan R. Eschmeyer’s catalog of fishes: genera, species, references [Internet]. San Francisco: California Academy of Science; 2022. Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
http://researcharchive.calacademy.org/re...
). It is distributed from southern Mexico to the rio de La Plata in Argentina, but most species occur in the Cis-Andean region, mainly in the Amazon basin, where it is the second most rich genus (Dagosta, de Pinna, 2019Dagosta FCP, de Pinna M. The fishes of the amazon: distribution and biogeographical patterns, with a comprehensive list of species. Bull Am Mus Nat Hist. 2019; 431:1-163. https://doi.org/10.1206/0003-0090.431.1.1
https://doi.org/10.1206/0003-0090.431.1....
). Besides being diverse in the number of species and in body shape diversity, the group is also plenty in color variation. It ranges from the flame-colored rosy-tetra clade to the elegant Hyphessobrycon loweae-group (sensuIngenito et al., 2013Ingenito LFS, Lima FCT, Buckup PA. A new species of Hyphessobrycon Durbin (Characiformes: Characidae) form the rio Juruena basin, central Brazil, with notes on H. loweae Costa & Géry. Neotrop Ichthyol . 2013; 11(1):33-44. https://doi.org/10.1590/S1679-62252013000100004
https://doi.org/10.1590/S1679-6225201300...
), with elongate fins, or to the contrasting colored, dark banded species such as the Hyphessobrycon heterorhabdus species-group (sensuFaria et al., 2021Faria TC, Guimarães KLA, Rodrigues LRR, Oliveira C, Lima FCT. A new Hyphessobrycon (Characiformes; Characidae) of the Hyphessobrycon heterorhabdus species-group from the lower Amazon basin, Brazil. Neotrop Ichthyol . 2021; 19(1):1-18. ).
A new species was collected during an expedition to the headwaters of the upper rio Juruena, rio Tapajós basin, at Chapada dos Parecis, Brazil. The new species is remarkable in coloration and has been exported by aquarists under the name Hyphessobrycon melanostichos Carvalho & Bertaco, 2006. It is popularly known as ‘emerald green tetra’. Additional specimens were recognized in the MZUSP collection and examined. The objective of the present work is to describe the new species and to evaluate its conservation status. Because the new taxon is polymorphic regarding the presence of adipose fin, we also discuss the application of this character in the systematic of characids.
MATERIAL AND METHODS
Counts and measurements follow Fink, Weitzman (1974Fink WL, Weitzman SH. The so-called Cheirodontin fishes of Central America with descriptions of two new species (Pisces: Characidae). Smithson Contrib Zool. 1974; 172:1-46.), except for not including eye-dorsal fin origin measurement and for number of horizontal scale rows below lateral line, which are counted to the pelvic-fin insertion, not including the small scale at pelvic-fin insertion, and with the addition of head depth, measured at vertical through the base of supraoccipital process. Standard length (SL) is given in millimeters (mm) and all other measurements are expressed as percentage of SL or of head length (HL) for subunits of head. In the description, counts are followed by their frequency of occurrence in parentheses, and an asterisk indicates the counts of the holotype. Number of maxillary tooth cusps, small dentary teeth, supraneurals, branchiostegal rays, gill rakers, vertebrae, unbranched anal-fin rays, and procurrent caudal-fin rays were obtained only from cleared and stained (c&s) specimens prepared according to Taylor, Van Dyke (1985Taylor WR, Van Dyke GC. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium. 1985; 9(2):107-19.). Vertebrae of the Weberian apparatus are counted as four elements and the compound caudal centra (PU1+U1) as a single element. Circuli and radii counts were taken from scale row immediately above the lateral line. The sex of specimens was confirmed by dissection and direct examination of the gonads. Diet was checked in 20 individuals from the type locality (22.3-35.0 mm SL). In the list of types and comparative material, catalog numbers are followed by the number of specimens in alcohol, their SL range, and if any, the number of c&s specimens and their respective SL range. Institutional abbreviations follow Sabaj (2020Sabaj MH. Codes for Natural History Collections in Ichthyology and Herpetology. Copeia . 2020; 108(3):593-669. https://doi.org/10.1643/ASIHCODONS2020
https://doi.org/10.1643/ASIHCODONS2020...
).
RESULTS
Hyphessobrycon comodoro, new species
urn:lsid:zoobank.org:act:2F8D142C-C2D3-4CB2-AD15-D746BB4F90E4
Holotype. MZUSP 125904, 29.6 mm SL. Brazil, Mato Grosso State, Municipality of Comodoro, lagoon at tributary of the rio Mutum, formed due to the construction of a road, tributary of rio Camararé, upper rio Juruena basin, rio Tapajós basin, 13°12’47.6”S 59°54’13.8”W, 567 m a.s.l., 19 Oct 2018, F. Dagosta, A. Ferreira & H. Lenza.
Paratypes. All from Brazil, Mato Grosso State, Municipality of Comodoro, rio Mutum drainage, upper rio Juruena basin. MZUSP 125215, 4 c&s, 211, 16.8-38.6 mm SL, UFPB 12086, 10, 24.0-31.2 mm SL, INPA 59651, 5, 26.2-31.1 mm SL, collected with holotype. MZUSP 115698, 22, 18.2-34.0 mm SL, rio Mutum at the Fazenda Mutum, at the bridge of the road between Comodoro and Vilhena, 13°05’09.2”S 59°53’33.8”W, 502 m a.s.l., 29 Aug 2013, O. Oyakawa, F. Dagosta, M. Marinho & P. Camelier. MZUSP 125217, 63, 13.9-32.1 mm SL, lagoon at tributary of the rio Mutum, formed due to the construction of a road, tributary of rio Camararé, upper rio Juruena basin, rio Tapajós basin, 13°13’23.2”S 59°54’41.8”W, 551 m a.s.l., 19 Oct 2018, F. Dagosta, A. Ferreira & H. Lenza. MZUSP 125221, 136, 14.3-29.7 mm SL, rio Mutum at the bridge of the road BR-364, 13°05’05.3”S 59°53’30.7”W, 504 m a.s.l., 20 Oct 2018, F. Dagosta, A. Ferreira & H. Lenza.
Diagnose. The new species can be distinguished from all congeners, except Hyphessobrycon. cachimbensis Travassos, 1964, H. cyanotaenia Zarske & Géry, 2006, H. fernandezi Fernández-Yépez, 1972, H. melanostichos, H. nigricinctus Zarske & Géry, 2004, H. paucilepis García-Alzate, Román-Valencia & Taphorn, 2008, H. petricolus Ohara, Lima & Barros, 2017, H. piranga Camelier, Dagosta & Marinho, 2018, H. psittacus Dagosta, Marinho, Camelier & Lima, 2016, H. scholzei Ahl, 1937, H. sovichthys Schultz, 1944, H. stegemanni Géry, 1961, H. taphorni García-Alzate, Román-Valencia & Ortega, 2013, H. tuyensis García-Alzate, Román-Valencia & Taphorn, 2008, and H. vilmae Géry, 1966 by the presence of a well-defined and relatively narrow dark midlateral stripe on body, extending from head to the middle caudal-fin rays (vs. well-defined longitudinal stripe absent, or stripe wider than the orbit, or stripe starting approximately vertically through the origin of the dorsal fin or stripe blurred posteriorly). The new species is distinguished from the aforementioned species, except H. cachimbensis, H. cyanotaenia, H. melanostichos, H. nigricinctus, and H. petricolus, by the possession of a humeral blotch (vs. humeral blotch absent). It is distinguished from H. cachimbensis and H. cyanotaenia by having the distal profile of the anal fin falcate in males (vs. approximately straight or convex) and from H. cachimbensis, H. petricolus, and H. nigricinctus by having 13-16 branched anal-fin rays (vs. 17-26). It can be further distinguished from H. cyanotaenia by lacking concentration of black pigmentation on longest rays of dorsal, pelvic, and anal fins (vs. pigmentation present). It is readily distinguished from H. melanostichos, the most similar congener, by having 11 or 12 horizontal scale rows around caudal peduncle (vs. 14), fewer branched pelvic-fin rays (6 vs. 7), humeral blotch wider than deep, with pigmentation much more intense than the dark midlateral band, with well-defined edges (vs. humeral blotch deeper than wide, with pigmentation similar to the dark midlateral band, without well-defined edges). Another useful character in distinguishing H. comodoro from H. melanostichos is the presence of 13-15, mode 14, rarely 16 (only 3 of 30 specimens), branched anal-fin rays (vs. 16-18, mode 16).
Description. Morphometric data in Tab. 1. Body compressed, moderately elongate. Greatest body depth at dorsal-fin origin. Dorsal profile of head convex from upper lip to vertical through posterior nostril; slightly convex from that point to base of supraoccipital spine Dorsal profile of body convex along predorsal region, slightly convex along dorsal-fin base, straight from terminus of dorsal-fin base to adipose-fin origin, and slightly concave to straight from that point to origin of anteriormost dorsal procurrent caudal-fin ray. Ventral profile of head and body convex from tip of lower lip to pelvic-fin origin, slightly concave between latter point to anal-fin origin, somewhat straight to convex (see Sexual Dimorphism section) along anal-fin base, and concave from the terminus of anal fin to origin of anteriormost ventral procurrent caudal-fin ray.
| Morphometric data of Hyphessobrycon comodoro. Range includes the holotype. SD = Standard deviation.
Jaws vertically aligned, mouth terminal. Premaxillary teeth in two distinct rows. Outer row with 2(1), 3*(21), or 4(9) tri- to pentacuspid teeth. Inner row with 5*(31) tri- to heptacuspid teeth. Posterior tip of maxilla at vertical through posterior half of second infraorbital. Maxilla with 1(2), 2*(28), or 3(1) conical to pentacuspid teeth. Dentary with 5*(31) larger penta- to heptacuspid teeth followed by series of 5 to 9 diminutive conical to tricuspid teeth. Central median cusp in all teeth longer than lateral cusps. Branchiostegal rays 4(4). Gill-rakers 8(2) or 9(2) in the lower and 7(1) or 8(3) in the upper branch.
Cycloid scales, with 5-7 radii from focus to posterior border, and conspicuous circulii anteriorly. Lateral line incomplete, with 6(1), 7(1), 8*(26), 9(2), or 10(1) perforated scales, and 29*(6), 30(16), or 31(7) total scales on longitudinal series. Longitudinal scale rows between dorsal-fin origin and lateral line 4(1) or 5*(30). Longitudinal scale rows between lateral line and pelvic-fin origin 3*(27) or 4(4). Scales along middorsal line between posterior tip of supraoccipital process and dorsal-fin origin 9*(13), 10(11), or 11(7). Horizontal scale rows around caudal peduncle 11(6) or 12*(25). Base of anteriormost anal-fin rays covered by series of 3 or 4 scales. Caudal fin not scaled.
Supraneurals 4(2) or 5(2). Dorsal-fin rays ii*(29), iii(2), 7(1), 8(10), or 9*(20). Base of last dorsal-fin ray at vertical anterior to anal fin. Pectoral-fin rays i*(31), 9(16), or 10*(15). Pelvic-fin rays i*(31), 6*(31). Adipose fin frequently present, of variable size, present in 27 specimens, absent in four specimens. Anal fin falcate, with iv*(4), 13(3), 14*(18), 15(7), or 16(3) branched rays. Principal caudal-fin rays i,9,8,i*(27), i,8,8,i(1), i,10,8,i(1), i,9,7,i(1); caudal fin forked, lobes somewhat pointed, of similar size. Dorsal procurrent caudal-fin rays 10(4); ventral procurrent caudal-fin rays 9(4). Total vertebrae 32(2) or 33(2): precaudal vertebrae 14(1) or 15(3) and caudal vertebrae 17(2) or 18(2).
Color in alcohol. Overall ground coloration of head and body beige (Fig. 1). Some specimens retaining guanine on opercular region. Dorsal portion of head and dorsal midline of body dark. A reticulated pattern on first three to four horizontal scale rows, formed by concentration of chromatophores on posterior margin of scales. Snout, jaws and 1st and 2nd infraorbitals with concentration of dark chromatophores, 3rd and 4th infraorbitals with scattered dark pigmentation and 5th and 6th infraorbitals densely pigmented with dark chromatophores, continuing with dark midlateral stripe. Roughly inverted teardrop-shaped humeral blotch formed by two layers of pigmentation. Superficial layer darker and conspicuous, overlapping midlateral stripe and encompassing approximately four scales horizontally and one or two vertically. Subjacent layer with scattered pigmentation encompassing approximately three scales vertically and forming a ventral projection to the humeral spot with diffuse borders. Dark midlateral stripe on body, extending from upper half of posterior portion of eye to tip of middle caudal-fin rays. Abdominal region with few scattered chromatophores. Sparse dark chromatophores above anal fin, mainly near anal-fin base. Caudal-peduncle blotch absent. Adipose fin with scattered dark chromatophores. All fins with dark chromatophores scattered along edge of lepidotrichia. Dorsal and anal fins with dark pigmentation on interadial membranes. Some specimens with sparse dark pigmentation on pelvic-fin interadial membranes.
| Hyphessobrycon comodoro, Brazil, Mato Grosso State, Municipality of Comodoro, rio Mutum, upper rio Juruena basin: A. Holotype, MZUSP 125904, 29.6 mm SL, male; B. Paratype, MZUSP 125215, 25.3 mm SL, female; C. Aquarium specimen, not measured or preserved.
| Live coloration of Hyphessobrycon comodoro, Brazil, Mato Grosso State, Municipality of Comodoro, rio Mutum, upper rio Juruena basin: A. Holotype, MZUSP 125904, 29.6 mm SL, male; B. Paratype, MZUSP 125215, 27.4 mm SL, male lacking adipose fin; C. Paratype, MZUSP 125215, 29.8 mm SL, female.
Color in life. Middorsal area olive green (Figs. 1C, 2); abdominal region silvery to yellow, with some specimens with orange pigmentation in the ventral portion. Upper portion of eye yellow to red, upper-posterior region dark pigmented. First and second infraorbitals, maxilla, lower jaw, gular area and preopercle with yellow pigmentation and scattered orange chromatophores. Remaining infraorbitals mostly silvery and with sparse orange cromatophores. Some specimens with lower portion of opercle lacking guanine, exposing red branchial filaments inside branchial chamber. Bright green midlateral stripe above and below the dark midlateral stripe, thicker at region above anal-fin base. All fins vivid orange to red coloration, more intense in caudal and anal fins. Adipose fin pale hyaline to pale yellow.
Sexual dimorphism. Males with anal-fin base slightly convex (vs. somewhat straight in females). Dark midlateral stripe in males wider and blurred (vs. midlateral stripe relatively narrow and with more defined edges in females), a type of sexual dichromatism involving the larger concentration of melanophore-based pigments in males (Pastana et al., 2017Pastana MNL, Dagosta FCP, Esguícero ALH. A new sexually dichromatic miniature Hyphessobrycon (Teleostei: Characiformes: Characidae) from the Rio Formiga, upper Rio Juruena basin, Mato Grosso, Brazil, with a review of sexual dichromatism in Characiformes. J Fish Biol . 2017; 91(5):1301-18. https://doi.org/10.1111/jfb.13449
https://doi.org/10.1111/jfb.13449...
). Bony hooks on fins not present.
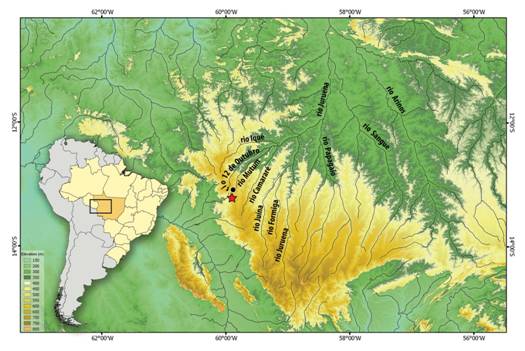
Geographical distribution. The new species is so far known from headwater of the rio Mutum, tributary of the rio Camararé, upper rio Juruena basin at Chapada dos Parecis, Mato Grosso State, Brazil (Fig. 3).
| Distribution of Hyphessobrycon comodoro in the upper rio Mutum, rio Juruena basin, Brazil. Red star (type locality), black dot (occurrence of other paratypes). Symbol can represent more than one collection event.
Ecological notes. Two collection sites of Hyphessobrycon comodoro are impoundments of tributaries of the rio Mutum formed by the road building (Fig. 4). In these habitats, the water is transparent, with maximum widths ranging 50-60 m and depth 0.3-2 m. The substrate is formed by sand, silt, and organic matter, with the presence of submerged aquatic macrophytes and large amounts of filamentous algae. The only other species collected syntopically was Hoplerythrinus unitaeniatus (Spix & Agassiz, 1829), probably a predator of the new species. The streams that form the lagoons are small, 2-4 m wide and 0.5-2 m deep, with clear rapid waters and a bottom composed of sand and leaf litter. Local vegetation is composed of secondary forest. The other known locality lies at the rio Mutum itself, downstream to the other two. At that point, the new species occurs syntopically with Hyphessobrycon hexastichos Bertaco & Carvalho, 2005 and Hasemania nambiquara Bertaco & Malabarba, 2007. The diet was mainly composed of resources autochthonous (91.6% of the volume of food items), mainly vegetable fragments (57.7%) and aquatic insects (32.7%). The vegetable fragments were composed of aquatic macrophyte structures and aquatic insects (fragments of adults, larvae, and pupae of Diptera and larvae of Trichoptera, and Odonata).
| Lagoon at the rio Mutum headwater due to the construction of a road, tributary of rio Camararé, upper rio Juruena basin, rio Tapajós basin, Comodoro, Mato Grosso, Brazil.
Specimens analyzed were sampled in a region under moderate anthropogenic pressure, which may influence the diet of fish species. Further, damming streams to road buildingchanges the taxonomic and functional of fish assemblages and limits the longitudinal dispersion (Brejão et al., 2020Brejão GL, Teresa FB, Gerhard P. When roads cross streams: fish assemblage responses to fluvial fragmentation in lowland Amazonian streams. Neotrop Ichthyol . 2020; 18(3):1-16. https://doi.org/10.1590/1982-0224-2020-0031
https://doi.org/10.1590/1982-0224-2020-0...
). The transformation from lotic to lentic environments, with an increase in the width of the canopy-opening channel, creates pelagic and benthic areas that allow the proliferation of macrophytes and algae (Brejão et al., 2020). Biological data taken from regions impacted by human action has high scientific value, but in the case of this species, it is crucial that data also be available from less impacted environments.
Etymology. The name comodoro is in reference to the Municipality of Comodoro, Mato Grosso State, where all the specimens were collected. It is also the name of a senior naval rank used in many navies, which inspired the municipality’s name. A noun in apposition.
Conservation status. Hyphessobrycon comodoro is endemic to Brazil and is a restricted-range species, a common pattern among endemic characids of the ‘Chapada dos Parecis’ biogeographic region (Dagosta et al., 2020Dagosta FCP, De Pinna M, Peres CA, Tagliacollo VAT. Existing protected areas provide a poor safety-net for threatened Amazonian fish species. Aquat Conserv. 2020; 31(5):1167-89. https://doi.org/10.1002/aqc.3461
https://doi.org/10.1002/aqc.3461...
). Despite such biogeographic region was considered by those authors as one of the Endemic Amazonian Fish Areas (EAFAs), i.e., regions that should be considered as conservation priorities in the basin by presenting imminent threats and a low cover of protected areas, the new species is endemic to one of the most preserved river basins draining the Cerrado biome - the rio Mutum drainage. Hyphessobrycon comodoro is so far known by three localities, but its EOO (Extent of occurrence) is likely underestimated since only the headwater of the rio Mutum basin was sampled. Most of the rio Mutum basin lies within the Nambikwara indigenous territory, where H. comodoro is likely to occur. Despite it has been exported in the aquarium trade it remains abundant in collection sites, which, as far as we know, are the same as those fished by the professional fishermen. Therefore, this species is assessed as Least Concern (LC) according to the International Union for Conservation of Nature (IUCN) categories and criteria (IUCN Standards and Petitions Subcommittee, 2019International Union for Conservation of Nature (IUCN). Standards and petitions committee. Guidelines for using the IUCN Red List categories and criteria. Version 14 [Internet]. Prepared by the Standards and Petitions Committee; 2019. Available from: https://www.iucnredlist.org/resources/redlistguidelines
https://www.iucnredlist.org/resources/re...
).
DISCUSSION
The adipose fin is variably developed in Hyphessobrycon comodoro, with few specimens lacking it (4 out of 27). Among Characiformes, absence of adipose fin is relatively uncommon and occurs in species of different lineages of the order (Mirande, 2019Mirande JM. Morphology, molecules and the phylogeny of Characidae (Teleostei, Characiformes). Cladistics. 2019; 35(3):282-300. https://doi.org/10.1111/cla.12345
https://doi.org/10.1111/cla.12345...
; Mattox et al., 2020Mattox GMT, Souza CS, Toledo-Piza M, Britz R, Oliveira C. A new miniature species of Priocharax (Teleostei: Characiformes: Characidae) from the Rio Madeira drainage, Brazil, with comments on the adipose fin in characiformes. Vertebr Zool. 2020; 70(3):417-33. https://doi.org/10.26049/VZ70-3-2020-11
https://doi.org/10.26049/VZ70-3-2020-11...
). Among more than 6,000 living Teleostei species bearing adipose fin (Stewart et al., 2014Stewart TA, Smith WL, Coates MI. The origins of adipose fins: an analysis of homoplasy and the serial homology of vertebrate appendages. P R Soc B. 2014; 281(1781):20133120. https://doi.org/10.1098/rspb.2013.3120
https://doi.org/10.1098/rspb.2013.3120...
, 2019Stewart TA, Bonilla MM, Ho RK, Hale ME. Adipose fin development and its relation to the evolutionary origins of median fins. Sci Rep. 2019; 9(1):1-12. https://doi.org/10.1038/s41598-018-37040-5
https://doi.org/10.1038/s41598-018-37040...
), Characiformes is the only order in which its developmental pattern differs. In the Characiformes, the adipose fin develops de novo, i.e., the fin appears after the reduction of the median larval finfold, whereas in the other orders, it develops by the retention of the larval finfold between the dorsal and caudal fin (Fuiman, 1983Fuiman RA. Ostariophysi: development and relationships. In: Moser HG, Richards WJ, Cohen DM, Fahay MP, Kendall AW Jr, Richarddson SL, editors. Ontogeny and Systematics of Fishes. California: American Society of Herpetology and Ichthyology; 1983. p. 126-37.; Bender, Moritz, 2013Bender A, Moritz T. Developmental residue and developmental novelty - different modes of adipose-fin formation during ontogeny. Zoosyst Evol. 2013; 89(2):209-14. https://doi.org/10.1002/zoos.201300007
https://doi.org/10.1002/zoos.201300007...
; Marinho, 2017Marinho MMF. Comparative development in Moenkhausia pittieri and Paracheirodon innesi (Ostariophysi: Characiformes) with comments on heterochrony and miniaturization in the Characidae. J Fish Biol . 2017; 91(3):851-65. https://doi.org/10.1111/jfb.13384
https://doi.org/10.1111/jfb.13384...
; Stewart et al., 2014Stewart TA, Smith WL, Coates MI. The origins of adipose fins: an analysis of homoplasy and the serial homology of vertebrate appendages. P R Soc B. 2014; 281(1781):20133120. https://doi.org/10.1098/rspb.2013.3120
https://doi.org/10.1098/rspb.2013.3120...
, 2019Stewart TA, Bonilla MM, Ho RK, Hale ME. Adipose fin development and its relation to the evolutionary origins of median fins. Sci Rep. 2019; 9(1):1-12. https://doi.org/10.1038/s41598-018-37040-5
https://doi.org/10.1038/s41598-018-37040...
).
Absence of adipose fin in Characiformes is more frequent among miniature to small-sized species and has long been related to miniaturization (Weitzman, Malabarba, 1999Weitzman SH, Malabarba LR. Systematics of Spintherobolus (Teleostei: Characidae: Cheirodontinae) from Eastern Brazil. Ichthyol Explor Fres . 1999; 10(1):1-43.; Bührnheim et al., 2008Bührnheim CM, Carvalho TP, Malabarba LR, Weitzman SH. A new genus and species of characid fish from the Amazon basin - the recognition of a relictual lineage of characid fishes (Ostariophysi: Cheirodontinae: Cheirodontini). Neotrop Ichthyol. 2008; 6(4):663-78. https://doi.org/10.1590/S1679-62252008000400016
https://doi.org/10.1590/S1679-6225200800...
), although large species may also lack it (e.g., Erythrinidae, Mattox et al., 2020Mattox GMT, Souza CS, Toledo-Piza M, Britz R, Oliveira C. A new miniature species of Priocharax (Teleostei: Characiformes: Characidae) from the Rio Madeira drainage, Brazil, with comments on the adipose fin in characiformes. Vertebr Zool. 2020; 70(3):417-33. https://doi.org/10.26049/VZ70-3-2020-11
https://doi.org/10.26049/VZ70-3-2020-11...
). Its absence in miniature to small species is probable a consequence of truncation in their development during the evolution of small-body size, in which late-forming structures, such as the “de novo” formation of the adipose fin, are the first to be lost (Marinho, 2017Marinho MMF. Comparative development in Moenkhausia pittieri and Paracheirodon innesi (Ostariophysi: Characiformes) with comments on heterochrony and miniaturization in the Characidae. J Fish Biol . 2017; 91(3):851-65. https://doi.org/10.1111/jfb.13384
https://doi.org/10.1111/jfb.13384...
). Besides, morphological variability of characters formed in late developmental stages is also associated with body-size reduction, resulting in intrapopulational variation of that structure (Hanken, Wake, 1993Hanken J, Wake DB. Miniaturization of Body Size: Organismal Consequences and Evolutionary Significance. Annu Rev Ecol Syst. 1993; 24:501-19. https://doi.org/10.1146/annurev.ecolsys.24.1.501
https://doi.org/10.1146/annurev.ecolsys....
; Marinho et al., 2021Marinho MMF, Ohara W, Dagosta FCP. A new species of Moenkhausia (Characiformes: Characidae) from the rio Madeira basin, Brazil, with comments on the evolution and development of the trunk lateral line system in characids. Neotrop Ichthyol . 2021; 19(2): e200118. https://doi.org/10.1590/1982-0224-2020-0118
https://doi.org/10.1590/1982-0224-2020-0...
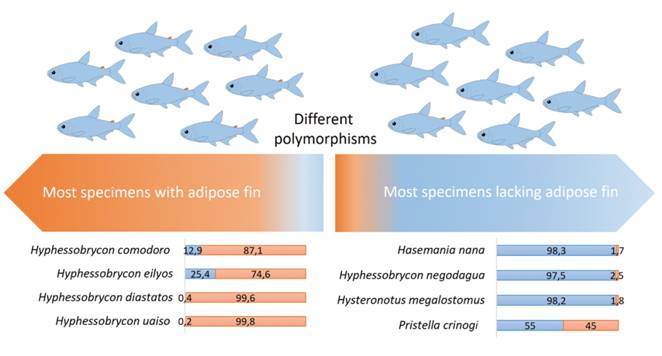
). Intraspecific variation regarding presence/absence of adipose fin has been documented for miniature to small characids (Tab. 2) and are herein interpreted as a consequence of developmental truncation. Polymorphisms are not equivalent though. The frequency of the presence of adipose fin varies among species (Fig. 5), evidencing this is a very labile character for some taxa.
| Graph showing the adipose fin variation in characids. Note that not all polymorphisms are the same condition: some species have more specimens with developed adipose whereas other species have more specimens lacking that fin. Numbers in graphic are percentages of specimens bearing adipose fin (orange) or lacking adipose fin (blue). Further details in Tab. 2.
| List of species of Characidae showing intraspecific variation in the absence/presence of the adipose fin. Counts with an asterisk include specimens with vestigial adipose fin.
Polymorphism regarding presence of adipose fin directly affect decisions on the systematic of characiforms, especially the family Characidae. This is because its absence or presence is still widely used to diagnose genera and/or species in the family. For example, HasemaniaEllis, 1911Ellis MD. On the species of Hasemania, Hyphessobrycon, and Hemigrammus collected by J. D. Haseman for the Carnegie Museum. Ann Carnegie Mus. 1911; 8(1):148-63., was originally defined as “like a Hyphessobrycon, but without an adipose” (Ellis, 1911Ellis MD. On the species of Hasemania, Hyphessobrycon, and Hemigrammus collected by J. D. Haseman for the Carnegie Museum. Ann Carnegie Mus. 1911; 8(1):148-63.), despite species of both genera present intraespecific variation in this character (Tab. 2; Fig. 5), evidencing the fragility of such definition. Therefore, the use of such labile character in systematics needs to be made with caution. Polymorphism in the presence of adipose fin in Hyphessobrycon comodoro, along with the still poorly known interspecific relationships of large polyphyletic genera within Stethaprioninae (see Mirande, 2019Mirande JM. Morphology, molecules and the phylogeny of Characidae (Teleostei, Characiformes). Cladistics. 2019; 35(3):282-300. https://doi.org/10.1111/cla.12345
https://doi.org/10.1111/cla.12345...
) raise questions on the allocation of the new species in Hyphessobrycon. The monophyletic nature of the genus has long been disputed (Weitzman, Fink, 1983Weitzman SH, Fink WL. Relationships of the neon tetras, a group of South American freshwater fishes (Teleostei, Characidae), with comments on the phylogeny of New World Characiforms. Bull Mus Comp Zool. 1983; 150(6):339-95.; Weitzman, Palmer, 1997Weitzman SH, Palmer L. A new species of Hyphessobrycon (Teleostei: Characidae) from the Neblina region of Venezuela and Brazil, with comments on the putative ‘rosy tetra clade’. Ichthyol Explor Fres . 1997; 7(3):209-42.; Mirande, 2010Mirande JM. Phylogeny of the family Characidae (Teleostei: Characiformes) from characters to taxonomy. Neotrop Ichthyol . 2010; 8(3):385-568. https://doi.org/10.1590/S1679-62252010000300001
https://doi.org/10.1590/S1679-6225201000...
, 2019Mirande JM. Morphology, molecules and the phylogeny of Characidae (Teleostei, Characiformes). Cladistics. 2019; 35(3):282-300. https://doi.org/10.1111/cla.12345
https://doi.org/10.1111/cla.12345...
) and today, Hyphessobrycon is largely accepted as polyphyletic. However, some groups are likely monophyletic, such as the rosy-tetra clade (see Mirande, 2019Mirande JM. Morphology, molecules and the phylogeny of Characidae (Teleostei, Characiformes). Cladistics. 2019; 35(3):282-300. https://doi.org/10.1111/cla.12345
https://doi.org/10.1111/cla.12345...
). The evidence of monophyly of the rosy tetras has long been proposed, based in the unique coloration pattern shared by its species (Weitzman, Palmer, 1997Weitzman SH, Palmer L. A new species of Hyphessobrycon (Teleostei: Characidae) from the Neblina region of Venezuela and Brazil, with comments on the putative ‘rosy tetra clade’. Ichthyol Explor Fres . 1997; 7(3):209-42.), and was confirmed in recent cladistic works (e.g., Mirande, 2019Mirande JM. Morphology, molecules and the phylogeny of Characidae (Teleostei, Characiformes). Cladistics. 2019; 35(3):282-300. https://doi.org/10.1111/cla.12345
https://doi.org/10.1111/cla.12345...
). Unfortunately, only a restricted sample of Hyphessobrycon could be included, not representing the whole diversity of the genus. Therefore, still needing efforts to advance the knowledge of the phylogenetic relationships of the species now attributed to Hyphessobrycon. As consequence, the composition, and limits of Hyphessobrycon remain open to question. Despite generic allocation to be tentative, the new species can be distinguished from all remaining characids by the combination of a well-defined and relatively narrow dark midlateral stripe on body extending from the upper half of the posterior margin of the eye to the middle caudal-fin ray, orange fins, a total of 33-35 scales on the longitudinal series, in which only few of them are perforated.
It is premature to infer a close evolutionary relationship between the new species with other characids, but morphological features may indicate it is more closely related to species nowadays allocated in Hyphessobrycon. The new species share with H. cachimbensis, H. cyanotaenia, H. melanostichos, H. nigricinctus, and H. petricolus a similar coloration pattern, consisting of a conspicuous, relatively narrow dark midlateral stripe from head to middle caudal-fin ray and a humeral blotch. Except for H. nigricinctus which is restricted to the rio Madre de Dios, H. cachimbensis, H. comodoro, H. cyanotaenia, H. melanostichos, H. nigricinctus, and H. petricolus are all endemic from the Brazilian Shield, occurring in tributaries of the rio Madeira and rio Tapajós basins. Among these species, H. comodoro is particularly similar to H. melanostichos, sharing other coloration details such as the bright orange caudal fin in life, the dark midlateral stripe starting in the upper half of the posterior margin of the eye, with a green to bluish stripe above it and base of anal fin sexually dimorphic, convex in males. Besides very similar morphologically, H. comodoro and H. melanostichos occur very close to each other in neighboring tributaries of the rio Camararé, Juruena river basin. They also share the fact of having a very restricted known distribution, with H. melanostichos so far known only from the rio Doze de Outubro and H. comodoro from the rio Mutum. Populations of H. melanostichos from shield tributaries of the rio Madeira basin (e.g., rio Cabixi, rio Machado) are probably closely related undescribed species that are being studied (FCPD, pers. obs.).
The description of an additional new species already known worldwide in the aquarium trade reveals how scarce is the knowledge on the diversity of Neotropical freshwater fishes (Reis et al., 2016Reis RE, Albert JS, Di Dario F, Mincarone MM, Petry P, Rocha LA. Fish biodiversity and conservation in South America. J Fish Biol . 2016, 89(1):12-47. https://doi.org/10.1111/jfb.13016
https://doi.org/10.1111/jfb.13016...
; Albert et al., 2020Albert JS, Tagliacollo VA, Dagosta FCP. Diversification of Neotropical Freshwater Fishes. Annu Rev Ecol Evol S. 2020; 51:27-53. https://doi.org/10.1146/annurev-ecolsys-011620-031032
https://doi.org/10.1146/annurev-ecolsys-...
). Despite being widely sampled in the last decade, the Chapada dos Parecis still provides new and endemic taxa that reinforces the status of being a biogeographic region distinct from the rest of the Amazon basin (Dagosta, de Pinna, 2019Dagosta FCP, de Pinna M. The fishes of the amazon: distribution and biogeographical patterns, with a comprehensive list of species. Bull Am Mus Nat Hist. 2019; 431:1-163. https://doi.org/10.1206/0003-0090.431.1.1
https://doi.org/10.1206/0003-0090.431.1....
; Dagosta et al., 2020Dagosta FCP, De Pinna M, Peres CA, Tagliacollo VAT. Existing protected areas provide a poor safety-net for threatened Amazonian fish species. Aquat Conserv. 2020; 31(5):1167-89. https://doi.org/10.1002/aqc.3461
https://doi.org/10.1002/aqc.3461...
).
Comparative material examined. Material examined is the same listed in Dagosta et al. (2016Dagosta FCP, Marinho MMF, Camelier P, Lima FCT. A new species of Hyphessobrycon (Characiformes: Characidae) from the upper Rio Juruena basin, Central Brazil, with a redescription of H. cyanotaenia. Copeia. 2016; 104(1):250-59. https://doi.org/10.1643/CI-15-243
https://doi.org/10.1643/CI-15-243...
), with the addition of Hasemania nana: Brazil, Minas Gerais, Lagoa Santa, rio São Francisco basin, MZUSP 38040, 173, 15.6-23.6 mm SL.
ACKNOWLEDGMENTS
We are grateful to Humberto Lenza for his help during fieldwork and to Michel Gianetti (MZUSP) for curatorial assistance. The authors were indebted to Karolina Reis for her support in examining ichthyological material in MZUSP. We thank Fabiano Antunes, Márcia R. Russo, and Adnara R. Gomide for administrative support at Universidade Federal da Grande Dourados (UFGD). Type series was collected at field expeditions partially funded by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP #2017/09321-5) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq # 405643/2018-7). Authors were supported by CNPq # 405643/2018-7, FCPD and MMFM by FAPESP # 2016/19075-9, and MMFM by FAPESP # 2018/11415-0.
REFERENCES
- Albert JS, Tagliacollo VA, Dagosta FCP. Diversification of Neotropical Freshwater Fishes. Annu Rev Ecol Evol S. 2020; 51:27-53. https://doi.org/10.1146/annurev-ecolsys-011620-031032
» https://doi.org/10.1146/annurev-ecolsys-011620-031032 - Bender A, Moritz T. Developmental residue and developmental novelty - different modes of adipose-fin formation during ontogeny. Zoosyst Evol. 2013; 89(2):209-14. https://doi.org/10.1002/zoos.201300007
» https://doi.org/10.1002/zoos.201300007 - Bührnheim CM, Carvalho TP, Malabarba LR, Weitzman SH. A new genus and species of characid fish from the Amazon basin - the recognition of a relictual lineage of characid fishes (Ostariophysi: Cheirodontinae: Cheirodontini). Neotrop Ichthyol. 2008; 6(4):663-78. https://doi.org/10.1590/S1679-62252008000400016
» https://doi.org/10.1590/S1679-62252008000400016 - Brejão GL, Teresa FB, Gerhard P. When roads cross streams: fish assemblage responses to fluvial fragmentation in lowland Amazonian streams. Neotrop Ichthyol . 2020; 18(3):1-16. https://doi.org/10.1590/1982-0224-2020-0031
» https://doi.org/10.1590/1982-0224-2020-0031 - Carvalho FR, Langeani F. Hyphessobrycon uaiso: new characid fish from the rio Grande, upper rio Paraná basin, Minas Gerais State (Ostariophysi: Characidae), with a brief comment about some types of Hyphessobrycon Neotrop Ichthyol . 2013; 11(3):525-36. https://doi.org/10.1590/S1679-62252013000300006
» https://doi.org/10.1590/S1679-62252013000300006 - Carvalho FR, Cabeceira FG, Carvalho LN. New species of Hyphessobrycon from the Rio Teles Pires, Rio Tapajós basin, Brazil (Ostariophysi, Characiformes). J Fish Biol. 2017; 91(3):750-63. https://doi.org/10.1111/jfb.13362
» https://doi.org/10.1111/jfb.13362 - Dagosta FCP, Marinho MMF, Camelier P. A new species of Hyphessobrycon Durbin (Characiformes: Characidae) from the middle rio São Francisco and upper and middle rio Tocantins basins, Brazil, with comments on its biogeographic history. Neotrop Ichthyol . 2014; 12(2):365-75. http://dx.doi.org/10.1590/1982-0224-20130179
» http://dx.doi.org/10.1590/1982-0224-20130179 - Dagosta FCP, Marinho MMF, Camelier P, Lima FCT. A new species of Hyphessobrycon (Characiformes: Characidae) from the upper Rio Juruena basin, Central Brazil, with a redescription of H. cyanotaenia Copeia. 2016; 104(1):250-59. https://doi.org/10.1643/CI-15-243
» https://doi.org/10.1643/CI-15-243 - Dagosta FCP, de Pinna M. The fishes of the amazon: distribution and biogeographical patterns, with a comprehensive list of species. Bull Am Mus Nat Hist. 2019; 431:1-163. https://doi.org/10.1206/0003-0090.431.1.1
» https://doi.org/10.1206/0003-0090.431.1.1 - Dagosta FCP, De Pinna M, Peres CA, Tagliacollo VAT. Existing protected areas provide a poor safety-net for threatened Amazonian fish species. Aquat Conserv. 2020; 31(5):1167-89. https://doi.org/10.1002/aqc.3461
» https://doi.org/10.1002/aqc.3461 - Ellis MD. On the species of Hasemania, Hyphessobrycon, and Hemigrammus collected by J. D. Haseman for the Carnegie Museum. Ann Carnegie Mus. 1911; 8(1):148-63.
- Faria TC, Bastos DA, Zuanon J, Lima FCT. A new Hyphessobrycon (Characiformes: Characidae) of the Hyphessobrycon heterorhabdus species-group from the Central Amazon basin, Brazil. Zootaxa. 2020; 4859(2):275-84. https://doi.org/10.11646/zootaxa.4859.2.6
» https://doi.org/10.11646/zootaxa.4859.2.6 - Faria TC, Guimarães KLA, Rodrigues LRR, Oliveira C, Lima FCT. A new Hyphessobrycon (Characiformes; Characidae) of the Hyphessobrycon heterorhabdus species-group from the lower Amazon basin, Brazil. Neotrop Ichthyol . 2021; 19(1):1-18.
- Fink WL, Weitzman SH. The so-called Cheirodontin fishes of Central America with descriptions of two new species (Pisces: Characidae). Smithson Contrib Zool. 1974; 172:1-46.
- Fricke R, Eschmeyer WN, Van der Laan R. Eschmeyer’s catalog of fishes: genera, species, references [Internet]. San Francisco: California Academy of Science; 2022. Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
» http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp - Fuiman RA. Ostariophysi: development and relationships. In: Moser HG, Richards WJ, Cohen DM, Fahay MP, Kendall AW Jr, Richarddson SL, editors. Ontogeny and Systematics of Fishes. California: American Society of Herpetology and Ichthyology; 1983. p. 126-37.
- Géry J. Characoids of the World. New Jersey: T. F. H. Publications; 1977.
- Hanken J, Wake DB. Miniaturization of Body Size: Organismal Consequences and Evolutionary Significance. Annu Rev Ecol Syst. 1993; 24:501-19. https://doi.org/10.1146/annurev.ecolsys.24.1.501
» https://doi.org/10.1146/annurev.ecolsys.24.1.501 - Ingenito LFS, Lima FCT, Buckup PA. A new species of Hyphessobrycon Durbin (Characiformes: Characidae) form the rio Juruena basin, central Brazil, with notes on H. loweae Costa & Géry. Neotrop Ichthyol . 2013; 11(1):33-44. https://doi.org/10.1590/S1679-62252013000100004
» https://doi.org/10.1590/S1679-62252013000100004 - International Union for Conservation of Nature (IUCN). Standards and petitions committee. Guidelines for using the IUCN Red List categories and criteria. Version 14 [Internet]. Prepared by the Standards and Petitions Committee; 2019. Available from: https://www.iucnredlist.org/resources/redlistguidelines
» https://www.iucnredlist.org/resources/redlistguidelines - Lima FCT, Gerhard P. A new Hyphessobrycon (Characiformes: Characidae) from Chapada Diamantina, Bahia, Brazil, with notes on its natural history. Ichthyol Explor Fres. 2001; 12(2):105-14.
- Lima FCT, Moreira CR. Three new species of Hyphessobrycon (Characiformes: Characidae) from the upper rio Araguaia basin in Brazil. Neotrop Ichthyol . 2003; 1(1):21-33. https://doi.org/10.1590/S1679-62252003000100003
» https://doi.org/10.1590/S1679-62252003000100003 - Lima FCT, Caires RA, Conde-Saldaña CC, Mirande JM, Carvalho FR. A new miniature Pristella (Actinopterygii: Characiformes: Characidae) with reversed sexual dimorphism from the rio Tocantins and rio São Francisco basins, Brazil. Can J Zool. 2021; 99:339-48. https://doi.org/10.1139/cjz-2020-0241
» https://doi.org/10.1139/cjz-2020-0241 - Marinho MMF, Dagosta FCP, Camelier P, Oyakawa OT. A name for the ‘blueberry tetra’, an aquarium trade popular species of Hyphessobrycon Durbin (Characiformes: Characidae), with comments on fish species descriptions lacking accurate type locality. J Fish Biol . 2016; 89(1):510-21. https://doi.org/10.1111/jfb.12991
» https://doi.org/10.1111/jfb.12991 - Marinho MMF, Ohara W, Dagosta FCP. A new species of Moenkhausia (Characiformes: Characidae) from the rio Madeira basin, Brazil, with comments on the evolution and development of the trunk lateral line system in characids. Neotrop Ichthyol . 2021; 19(2): e200118. https://doi.org/10.1590/1982-0224-2020-0118
» https://doi.org/10.1590/1982-0224-2020-0118 - Marinho MMF. Comparative development in Moenkhausia pittieri and Paracheirodon innesi (Ostariophysi: Characiformes) with comments on heterochrony and miniaturization in the Characidae. J Fish Biol . 2017; 91(3):851-65. https://doi.org/10.1111/jfb.13384
» https://doi.org/10.1111/jfb.13384 - Mattox GMT, Souza CS, Toledo-Piza M, Britz R, Oliveira C. A new miniature species of Priocharax (Teleostei: Characiformes: Characidae) from the Rio Madeira drainage, Brazil, with comments on the adipose fin in characiformes. Vertebr Zool. 2020; 70(3):417-33. https://doi.org/10.26049/VZ70-3-2020-11
» https://doi.org/10.26049/VZ70-3-2020-11 - Menezes NA, Weitzman SH, Teixeira TF. Redescription of Hysteronotus megalostomus (Characiformes: Characidae: Stevardiinae), a poorly known characid from tributaries of the Rio São Fancisco, Brazil with comments on the conservation of the species. J Fish Biol . 2016; 89(1):495-509. https://doi.org/10.1111/jfb.13000
» https://doi.org/10.1111/jfb.13000 - Mirande JM. Phylogeny of the family Characidae (Teleostei: Characiformes) from characters to taxonomy. Neotrop Ichthyol . 2010; 8(3):385-568. https://doi.org/10.1590/S1679-62252010000300001
» https://doi.org/10.1590/S1679-62252010000300001 - Mirande JM. Morphology, molecules and the phylogeny of Characidae (Teleostei, Characiformes). Cladistics. 2019; 35(3):282-300. https://doi.org/10.1111/cla.12345
» https://doi.org/10.1111/cla.12345 - Pastana MNL, Dagosta FCP, Esguícero ALH. A new sexually dichromatic miniature Hyphessobrycon (Teleostei: Characiformes: Characidae) from the Rio Formiga, upper Rio Juruena basin, Mato Grosso, Brazil, with a review of sexual dichromatism in Characiformes. J Fish Biol . 2017; 91(5):1301-18. https://doi.org/10.1111/jfb.13449
» https://doi.org/10.1111/jfb.13449 - Reis RE, Albert JS, Di Dario F, Mincarone MM, Petry P, Rocha LA. Fish biodiversity and conservation in South America. J Fish Biol . 2016, 89(1):12-47. https://doi.org/10.1111/jfb.13016
» https://doi.org/10.1111/jfb.13016 - Sabaj MH. Codes for Natural History Collections in Ichthyology and Herpetology. Copeia . 2020; 108(3):593-669. https://doi.org/10.1643/ASIHCODONS2020
» https://doi.org/10.1643/ASIHCODONS2020 - Stewart TA, Smith WL, Coates MI. The origins of adipose fins: an analysis of homoplasy and the serial homology of vertebrate appendages. P R Soc B. 2014; 281(1781):20133120. https://doi.org/10.1098/rspb.2013.3120
» https://doi.org/10.1098/rspb.2013.3120 - Stewart TA, Bonilla MM, Ho RK, Hale ME. Adipose fin development and its relation to the evolutionary origins of median fins. Sci Rep. 2019; 9(1):1-12. https://doi.org/10.1038/s41598-018-37040-5
» https://doi.org/10.1038/s41598-018-37040-5 - Taylor WR, Van Dyke GC. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium. 1985; 9(2):107-19.
- Weitzman SH, Palmer L. A new species of Hyphessobrycon (Teleostei: Characidae) from the Neblina region of Venezuela and Brazil, with comments on the putative ‘rosy tetra clade’. Ichthyol Explor Fres . 1997; 7(3):209-42.
- Weitzman SH, Malabarba LR. Systematics of Spintherobolus (Teleostei: Characidae: Cheirodontinae) from Eastern Brazil. Ichthyol Explor Fres . 1999; 10(1):1-43.
- Weitzman SH, Fink WL. Relationships of the neon tetras, a group of South American freshwater fishes (Teleostei, Characidae), with comments on the phylogeny of New World Characiforms. Bull Mus Comp Zool. 1983; 150(6):339-95.
ADDITIONAL NOTES
-
HOW TO CITE THIS ARTICLE
Dagosta FCP, Seren TJ, Ferreira A, Marinho MMF. The emerald green tetra: a new restricted-range Hyphessobrycon (Characiformes: Characidae) from the upper rio Juruena, Chapada dos Parecis, Brazil. Neotrop Ichthyol. 2022; 20(1):e210119. https://doi.org/10.1590/1982-0224-2021-0119 -
ZOOBANK REGISTER
urn:lsid:zoobank.org:pub:75800B1A-D828-4801-8AC5-C5CA22484B2D
Edited by
Data availability
Data citations
Fricke R, Eschmeyer WN, Van der Laan R. Eschmeyer’s catalog of fishes: genera, species, references [Internet]. San Francisco: California Academy of Science; 2022. Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
Publication Dates
-
Publication in this collection
28 Mar 2022 -
Date of issue
2022
History
-
Received
24 July 2021 -
Accepted
03 Dec 2021