Abstract
South America is home to more miniature fishes (<26 mm in standard length) than any other continent. Despite this diversity, the ecology of miniature fishes is poorly studied. To promote the study of miniature fish ecology, we investigated patterns in total richness, assemblage structure and environmental correlates for miniature fishes in the rio Jacundá drainage of the Lower Amazon River basin, Pará State. Based on multi-pass dip-netting of leaf litter at 20 locations distributed across two sites, we collected miniature species and used rarefaction to estimate 9 to 14 species might be present. The miniature fish assemblage at the upstream site was a nested subset of the downstream site, and water pH and canopy cover, two features known to be altered by deforestation, correlated most strongly with assemblage variation. Our work represents one of the first quantitative assessments of environmental correlates with miniature fish assemblages and highlights research topics that should be investigated further to promote conservation and preservation of the overlooked and understudied Amazonian diminutive freshwater fish fauna.
Keywords:
Community; Conservation; Miniaturization; Ordination; Rarefaction
Resumo
A América do Sul abriga o maior número de peixes miniaturas (<26 mm de comprimento padrão) do que qualquer outro continente. Apesar dessa diversidade, a ecologia dos peixes miniaturas é pouco estudada. Visando promover estudos de ecologia de peixes miniaturas, investigamos padrões de riqueza total, estrutura da assembleia e fatores ambientais correlacionados para peixes miniaturas no rio Jacundá, drenagem do baixo rio Amazonas, Pará. Com base em múltiplas passagens de redes no sedimento em 20 pontos distribuídos em dois locais, coletamos espécies miniaturas e usamos rarefação para estimar que 9 a 14 espécies podem estar presentes. A assembleia de peixes miniaturas no local à montante foi um subgrupo aninhado na assembleia no local à jusante, e pH da água e cobertura de copas, dois fatores sabidamente alterados por desmatamento, foram os mais correlacionados com a variação na assembleia. Nosso trabalho representa um dos primeiros estudos quantitativos de fatores ambientais correlacionados às assembleias de peixes miniaturas e ressalta um tópico de pesquisa que deveria ser melhor investigado para promover a conservação da pouco conhecida fauna de peixes diminutos da Amazônia.
Palavras-chave:
Comunidade; Conservação; Miniaturização; Ordenação; Rarefação
INTRODUCTION
The evolution of extremely small adult body size is known as miniaturization and is a common theme in animal evolution with examples from all major metazoan taxa (Hanken, Wake, 1993Hanken J, Wake BD. Miniaturization of body size: organismal consequences and evolutionary significance. Annu Rev Ecol Syst. 1993; 24(1993):501–19. https://doi.org/10.1146/annurev.es.24.110193.002441
https://doi.org/10.1146/annurev.es.24.11...
; Rundell, Leander, 2010Rundell RJ, Leander BS. Masters of miniaturization: convergent evolution among interstitial eukaryotes. BioEssays. 2010; 32(5):430–37. https://doi.org/10.1002/bies.200900116
https://doi.org/10.1002/bies.200900116...
). The process of miniaturization is particularly notable among freshwater fishes in South America, including species ≤26 mm standard length (SL) at maximum size (reviewed by Weitzman, Vari, 1988Weitzman SH, Vari RP. Miniaturization in South American freshwater fishes: An overview and discussion. Proc Biol Soc Wash. 1988; 101(2):444–65. https://repository.si.edu/handle/10088/901?show=full
https://repository.si.edu/handle/10088/9...
; Toledo-Piza et al., 2014Toledo-Piza M, Mattox GMT, Britz R. Priocharax nanus, a new miniature characid from the rio Negro, Amazon basin (Ostariophysi: Characiformes), with an updated list of miniature Neotropical freshwater fishes. Neotrop Ichthyol. 2014; 12(2):229–46. https://doi.org/10.1590/1982-0224-20130171
https://doi.org/10.1590/1982-0224-201301...
). More than 200 species of miniature freshwater fishes are known in South America, a number that exceeds that of any other continent (Toledo-Piza et al., 2014Toledo-Piza M, Mattox GMT, Britz R. Priocharax nanus, a new miniature characid from the rio Negro, Amazon basin (Ostariophysi: Characiformes), with an updated list of miniature Neotropical freshwater fishes. Neotrop Ichthyol. 2014; 12(2):229–46. https://doi.org/10.1590/1982-0224-20130171
https://doi.org/10.1590/1982-0224-201301...
) and continues to grow through the description of new species (e.g., Abrahão et al., 2019Abrahão VP, Pastana M, Marinho M. On a remarkable sexual dimorphic trait in the Characiformes related to the olfactory organ and description of a new miniature species of Tyttobrycon Géry (Characiformes: Characidae). PLoS ONE. 2019; 14(12):e0226130. https://doi.org/10.1371/journal.pone.0226130
https://doi.org/10.1371/journal.pone.022...
; Carvalho, Reis, 2020Carvalho TP, Reis RE. A new miniature species of Acanthobunocephalus (Silurifomes: Aspredinidae) from the Lower Purus River basin, Amazon basin, Brazil. Copeia. 2020; 108(2):347–57. https://doi.org/10.1643/CI-19-309
https://doi.org/10.1643/CI-19-309...
; Mattox et al., 2020Mattox GMT, Souza CS, Toledo-Piza M, Britz R, Oliveira C. A new miniature species of Priocharax (Teleostei: Characiformes: Characidae) from the rio Madeira drainage, Brazil, with comments on the adipose fin in characiforms. Verteb Zool. 2020; 70(3):417–33. https://doi.org/10.26049/VZ70-3-2020-11
https://doi.org/10.26049/VZ70-3-2020-11...
, 2021Mattox GMT, Souza CS, Toledo-Piza M, Oliveira C. A new miniature species of Priocharax (Characiformes: Characidae) from the upper rio Ipixuna, Purus drainage, Brazil. Neotrop Ichthyol. 2021; 19(2):e210048. https://doi.org/10.1590/1982-0224-2021-0048
https://doi.org/10.1590/1982-0224-2021-0...
; Rodrigues, Netto-Ferreira, 2020Rodrigues EKDQ, Netto-Ferreira AL. A new miniature species of Odontocharacidium (Characiformes: Crenuchidae) from the Río Orinoco basin, Venezuela. Neotrop Ichthyol. 2020; 18(2):e190008. https://doi.org/10.1590/1982-0224-2019-0008
https://doi.org/10.1590/1982-0224-2019-0...
). In their description of “the almost invisible league”, Carvalho et al., (2006)Carvalho LN, Zuanon J, Sazima I. The almost invisible league: crypsis and association between minute fishes and shrimps as a possible defense against visually hunting predators. Neotrop Ichthyol. 2006; 4(2):219–24. https://doi.org/10.1590/S1679-62252006000200008
https://doi.org/10.1590/S1679-6225200600...
described communities of miniature fishes that occupy the benthos of leaf-littered, black-water streams in the Amazon. These miniature fishes tend to occupy benthic (Henderson, Walker, 1990Henderson PA, Walker I. Spatial organization and population density of the fish community of the litter banks within a central Amazonian blackwater stream. J Fish Biol. 1990; 37(3):401–11. https://doi.org/10.1111/j.1095-8649.1990.tb05871.x
https://doi.org/10.1111/j.1095-8649.1990...
) and floating (Carvalho et al., 2013Carvalho LN, Fidelis L, Arruda R, Galuch A, Zuanon J. Second floor, please: the fish fauna of floating litter banks in Amazonian streams and rivers. Neotrop Ichthyol. 2013; 11(1):85–94. http://dx.doi.org/10.1590/S1679-62252013000100010
http://dx.doi.org/10.1590/S1679-62252013...
) litter banks, suggesting some level of specialized habitat requirements. However, the ecology of miniature fishes is not well studied, having remained underexplored in the same way as most of the freshwater fish diversity in South America (Reis et al., 2016Reis RE, Albert JS, Di Dario F, Mincarone MM, Petry P, Rocha LA. Fish biodiversity and conservation in South America. J Fish Biol. 2016; 89(1):12–47. https://doi.org/10.1111/jfb.13016
https://doi.org/10.1111/jfb.13016...
).
Miniature fishes are overlooked and understudied for a number of reasons. The capture of miniature species requires specialized sampling gear with mesh sizes that are finer than what is typically used, and gears must be deployed in a manner that involves digging into the substrate or benthic debris (e.g., Carvalho et al., 2014Carvalho MS, Zuanon J, Ferreira EJG. Diving in the sand: the natural history of Pygidianops amphioxus (Siluriformes: Trichomycteridae), a miniature catfish of Central Amazonian streams in Brazil. Environ Biol Fishes. 2014; 97(1):59–68. https://doi.org/10.1007/s10641-013-0123-9
https://doi.org/10.1007/s10641-013-0123-...
). When they are captured, miniature fishes are sometimes dismissed as juvenile forms of other, larger species or are disregarded altogether in favor of focus on larger species (Weitzman, Vari, 1988Weitzman SH, Vari RP. Miniaturization in South American freshwater fishes: An overview and discussion. Proc Biol Soc Wash. 1988; 101(2):444–65. https://repository.si.edu/handle/10088/901?show=full
https://repository.si.edu/handle/10088/9...
). Confident identification requires voucher specimens be returned to the laboratory because identification in the field can be extremely challenging (Van der Sleen, Albert, 2018Van der Sleen P, Albert JS. Field guide to the fishes of the Amazon, Orinoco, and Guianas (Vol. 115). Princeton University Press; 2018.). Moreover, an overall similarity in general appearance between congeners and uncertainty regarding the taxonomy of miniature fishes can complicate identification and phylogenetic placement (e.g., Britz et al., 2014Britz R, Conway KW, Rüber L. Miniatures, morphology and molecules: Paedocypris and its phylogenetic position (Teleostei, Cypriniformes). Zool J Linn Soc. 2014; 172(3):556–615. https://doi.org/10.1111/zoj.12184
https://doi.org/10.1111/zoj.12184...
). Despite these challenges, miniature fishes represent a significant and growing component of global biodiversity with new species descriptions outpacing our understanding of their ecology (Costa, Le Bail, 1999Costa WJEM, Le Bail P. Fluviphylaxpalikur: a new poeciliid from the rio Oiapoque basin, Northern Brazil (Cyprinodontiformes: Cyprinodontoidei), with comments on miniaturization in Fluviphylax and other Neotropical freshwater fishes. Copeia. 1999; 1999(4):1027–34. https://doi.org/10.2307/1447977
https://doi.org/10.2307/1447977...
; Toledo-Piza et al., 2014Toledo-Piza M, Mattox GMT, Britz R. Priocharax nanus, a new miniature characid from the rio Negro, Amazon basin (Ostariophysi: Characiformes), with an updated list of miniature Neotropical freshwater fishes. Neotrop Ichthyol. 2014; 12(2):229–46. https://doi.org/10.1590/1982-0224-20130171
https://doi.org/10.1590/1982-0224-201301...
).
In their review of miniaturization in South American freshwater fishes, Weitzman, Vari, (1988)Weitzman SH, Vari RP. Miniaturization in South American freshwater fishes: An overview and discussion. Proc Biol Soc Wash. 1988; 101(2):444–65. https://repository.si.edu/handle/10088/901?show=full
https://repository.si.edu/handle/10088/9...
noted that the majority of miniature species inhabit lentic or slow flowing waters. Although the authors noted many streams inhabited by miniature fishes were characterized by low pH, Weitzman, Vari, (1988)Weitzman SH, Vari RP. Miniaturization in South American freshwater fishes: An overview and discussion. Proc Biol Soc Wash. 1988; 101(2):444–65. https://repository.si.edu/handle/10088/901?show=full
https://repository.si.edu/handle/10088/9...
were careful not to identify specific environmental parameters as potential selective agents for miniaturization and called for further investigations on miniature fishes, especially studies that would shed light on potential physical environmental parameters shaping the ecology and evolution of miniaturization. Subsequent reviews of miniature fishes in other regions (Kottelat, Vidthayanon, 1993Kottelat M, Vidthayanon C. Boraras micros, a new genus and species of minute freshwater fish from Thailand (Teleostei: Cyprinidae). Ichthyol Expl Freshw. 1993; 4(2):161–76.; Conway, Moritz, 2006Conway KW, Moritz T. Barboidesbritzi, a new species of miniature cyprinid from Benin (Ostariophysi: Cyprinidae), with a neotype designation for B. gracilis. Ichthyol Explor Freshw. 2006; 17(1):73–84.; Bennett, Conway, 2010Bennett MG, Conway KW. An overview of North America’s diminutive freshwater fish fauna. Ichthyol Explor Freshw. 2010; 21(1):63–72.) also highlight the link between small body size and lentic or slow flowing habitats, especially swamps that may experience annual periods of drought in which they are reduced to a small series of pools (Kottelat, Vidthayanon, 1993Kottelat M, Vidthayanon C. Boraras micros, a new genus and species of minute freshwater fish from Thailand (Teleostei: Cyprinidae). Ichthyol Expl Freshw. 1993; 4(2):161–76.; Kottelat et al., 2006Kottelat M, Britz R, Hui TH, Witte KE. Paedocypris, a new genus of Southeast Asian cyprinid fish with a remarkable sexual dimorphism, comprises the world’s smallest vertebrate. Proc R Soc Lond [Biol]. 2006; 273(1589):895–99. https://doi.org/10.1098/rspb.2005.3419
https://doi.org/10.1098/rspb.2005.3419...
). Additional research on the ecology of miniature fishes is needed to advance our understanding of the mechanisms that generate and maintain miniature fish diversity. This is especially true in the Amazon, where anthropogenic alterations to natural rainforest land cover may be altering the structure and function of the riverscapes that contributed to the impressive diversity of miniature fishes in this region of the Neotropics. For example, Ríos-Villamizar et al., (2017)Ríos-Villamizar EA, Piedade MT, Junk WJ, Waichman AV. Surface water quality and deforestation of the Purus river basin, Brazilian Amazon. Int Aquat Res. 2017; 9(1):81–88. https://doi.org/10.1007/s40071-016-0150-1
https://doi.org/10.1007/s40071-016-0150-...
found that accumulated total deforestation in the rio Purus correlated with a decrease in water pH, and Carvalho, Uieda, (2010)Carvalho EM, Uieda VS. Input of litter in deforested and forested areas of a tropical headstream. Braz J Biol. 2010; 70(2):283–88. https://doi.org/10.1590/S1519-69842010005000015
https://doi.org/10.1590/S1519-6984201000...
found deforestation contributed to open canopies over streams and ultimately reduced the leaf-litter habitats commonly occupied by miniature fishes (Henderson, Walker, 1990Henderson PA, Walker I. Spatial organization and population density of the fish community of the litter banks within a central Amazonian blackwater stream. J Fish Biol. 1990; 37(3):401–11. https://doi.org/10.1111/j.1095-8649.1990.tb05871.x
https://doi.org/10.1111/j.1095-8649.1990...
; Carvalho et al., 2006Carvalho LN, Zuanon J, Sazima I. The almost invisible league: crypsis and association between minute fishes and shrimps as a possible defense against visually hunting predators. Neotrop Ichthyol. 2006; 4(2):219–24. https://doi.org/10.1590/S1679-62252006000200008
https://doi.org/10.1590/S1679-6225200600...
). Deforestation is also linked to changes in stream fish body size at both the individual and assemblage levels (Ilha et al., 2018Ilha P, Schiesari L, Yanagawa FI, Jankowski K, Navas CA. Deforestation and stream warming affect body size of Amazonian fishes. PloS ONE. 2018; 13(5):e0196560. https://doi.org/10.1371/journal.pone.0196560
https://doi.org/10.1371/journal.pone.019...
), meaning natural fish body size gradients are affected by anthropogenic activities. The effects of deforestation on larger-bodied Amazonian stream fishes point to high importance of local habitat factors (Montag et al., 2019Montag LF, Winemiller KO, Keppeler FW, Leão H, Benone NL, Torres NR et al. Land cover, riparian zones and instream habitat influence stream fish assemblages in the eastern Amazon. Ecol Freshw Fish. 2019; 28(2):317–29. https://doi.org/10.1111/eff.12455
https://doi.org/10.1111/eff.12455...
), but local habitat correlates of miniature fish assemblages have not been the focus of quantitative studies to our knowledge. Consequently, research pertaining to relationships between local habitat variables and miniature fish assemblages is necessary to improve our understanding of how future changes to Neotropical river systems might influence these remarkable diminutive fishes (Albert et al., 2020Albert JS, Tagliacollo VA, Dagosta F. Diversification of Neotropical freshwater fishes. Annu Rev Ecol Evol Syst. 2020; 51:27–53. https://doi.org/10.1146/annurev-ecolsys-011620-031032
https://doi.org/10.1146/annurev-ecolsys-...
).
This study aimed to investigate patterns in total richness, assemblage structure and environmental correlates for miniature fishes in the rio Jacundá, a tributary of the Lower Amazon River Basin. We chose this tributary because it houses a large number of endemic and miniature fishes and because of ongoing and rapid habitat loss in the region (Claro-García, Shibatta, 2013Claro-García A, Shibatta OA. The fish fauna of streams from the upper rio Tocantins basin, Goiás State, Brazil. Check List. 2013; 9(1):28–33. https://doi.org/10.15560/9.1.28
https://doi.org/10.15560/9.1.28...
). First, we used rarefaction and species accumulation curves to estimate miniature and non-miniature fish richness sampled with specialized gear targeting miniature species. We expected that the number of miniature fish species would plateau with less effort compared to non-miniature fishes given that sampling methods favored collection of smaller fishes. Second, we assessed differences in assemblage composition at two sites located within the same tributary stream. We expected that either nesting or turnover in the identity of miniature fishes would contribute to differences in assemblage composition between the two sites and that species would partition habitats such that they rarely occurred together (Henderson, Walker, 1990Henderson PA, Walker I. Spatial organization and population density of the fish community of the litter banks within a central Amazonian blackwater stream. J Fish Biol. 1990; 37(3):401–11. https://doi.org/10.1111/j.1095-8649.1990.tb05871.x
https://doi.org/10.1111/j.1095-8649.1990...
). Collectively, our analyses fill an important knowledge gap concerning the ecology of miniature freshwater fishes and provide insight into habitat features that might be important for conservation.
MATERIAL AND METHODS
We collected fishes from the rio Jacundá, a stream in the lower Amazon (Pará State) that flows into the Furo de Santa Maria, which empties into the Atlantic Ocean with the rio Tocantins. The rio Jacundá is an oligotrophic, black-water, floodplain river characterized by low pH and high density of leaf litter. During the dry season in August 2019, we visited two sites 20 km apart along the rio Jacundá on the western edge of Reserva Extrativista Ipaú-Anilzinho, Pará, Brazil and sampled 20 locations (11 at the upstream site and nine at the downstream site; Fig. 1). We distributed sampling locations along gradients of habitat alteration associated with road crossing construction, particularly clear-cutting at the road edge transitioning to natural forest cover with distance from road crossings. At the center of each sampling location, we measured water temperature (°C), pH, conductivity (µs/cm), total suspended solids (parts per million), dissolved oxygen (mg/L), and water depth (cm). We visually estimated percent of benthos covered by leaf litter, emergent vegetation, detritus, and open water, and we used a concave densitometer to measure percent canopy cover above each sampling location. We collected the global position system (GPS) coordinates for each sampling location to develop spatial variables that could be used to assess spatial relationships among the sampling locations, specifically asymmetric eigenvector maps (Blanchet et al., 2008Blanchet FG, Legendre P, Borcard D. Modelling directional spatial processes in ecological data. Ecol Modell. 2008; 215(4):325–36. https://doi.org/10.1016/j.ecolmodel.2008.04.001
https://doi.org/10.1016/j.ecolmodel.2008...
). We first measured the hydrographic distance (m) between each point along the river course and created a graph theoretic representation of the locations denoted as nodes with edges (lines) connecting these nodes. We used the inverse distance between nodes to weight the edges (Dray et al., 2006Dray S, Legendre P, Peres-Neto PR. Spatial modelling: a comprehensive framework for principal coordinate analysis of neighbour matrices (PCNM). Ecol Modell. 2006; 196(3–4):483–93. https://doi.org/10.1016/j.ecolmodel.2006.02.015
https://doi.org/10.1016/j.ecolmodel.2006...
) and directed edges according to the flow of water. We then used the ‘aem’ function from the ‘adespatial’ package in R to create multivariate vectors representing spatial relationships among fish sampling locations (Dray et al., 2020Dray S, Blanchet G, Borcard D, Guenard G, Jombart T, Larocque G et al. adespatial: Multivariate multiscale spatial analysis. 2020. Available from: https://CRAN.R-project.org/package=adespatial
https://CRAN.R-project.org/package=adesp...
).
Study area map illustrating A. The location of the rio Jacundá in the lower Amazon Basin, B. The two areas sampled on the western edge of the Reserva Extrativista Ipaú-Anilzinho, Pará, Brazil, C. The distribution of 11 sampling locations and a site photograph at the upstream area, and D. The distribution of nine sampling locations and a site photograph at the downstream area.
We used a combination of fine-mesh (0.8 mm) dip-netting and seining to collect fishes from a 2 m by 2 m area within wadeable (i.e., <1 m deep) portions along the stream bank at each sampling location. Our standardized sampling protocol at each location included an initial seine haul through the 2 m x 2 m area, followed by three rounds of dip netting (by two netters) to scoop leaf litter from the benthos, followed by a second seine haul. Dip netters covered the entire benthos of the sampling area by digging handheld nets into the leaf litter and retrieving both litter and fishes. Leaf litter collected via dip netting was inspected for fishes prior to being discarded back into the stream. Fishes collected via seine and dip nets at each location were euthanized immediately in a solution of river water and clove oil (eugenol). Euthanized fishes were fixed in solution of a 4% buffered formalin and transferred to a solution of 70% ethanol for preservation. In the laboratory, specimens were identified to the lowest possible taxonomic level (i.e., species) using appropriate taxonomic keys (e.g., Van der Sleen, Albert, 2018Van der Sleen P, Albert JS. Field guide to the fishes of the Amazon, Orinoco, and Guianas (Vol. 115). Princeton University Press; 2018.) and measured for SL (mm). Specimens were classified as miniature if the known adult size was ≤26 mm SL (sensuWeitzman, Vari, 1988Weitzman SH, Vari RP. Miniaturization in South American freshwater fishes: An overview and discussion. Proc Biol Soc Wash. 1988; 101(2):444–65. https://repository.si.edu/handle/10088/901?show=full
https://repository.si.edu/handle/10088/9...
) based on Toledo-Piza et al., (2014)Toledo-Piza M, Mattox GMT, Britz R. Priocharax nanus, a new miniature characid from the rio Negro, Amazon basin (Ostariophysi: Characiformes), with an updated list of miniature Neotropical freshwater fishes. Neotrop Ichthyol. 2014; 12(2):229–46. https://doi.org/10.1590/1982-0224-20130171
https://doi.org/10.1590/1982-0224-201301...
. Voucher specimens are deposited at Laboratório de Ictiologia de Sorocaba (LISO), UFSCar, campus Sorocaba.
We used a subsampling-based statistical analysis to assess the richness of the miniature fish assemblage in the sampled area of the rio Jacundá. For our first objective, we used the rarefaction and extrapolation techniques of Chao et al., (2014)Chao A, Gotelli NJ, Hsieh TC, Sander EL, Ma KH, Colwell RK et al. Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol Mono. 2014; 84(1):45–67. https://doi.org/10.1890/13-0133.1
https://doi.org/10.1890/13-0133.1...
to assess accumulation of species (separated by miniature vs. non-miniature) across our samples and projected richness to two points, including twice our sampling effort (i.e., n = 40 samples) and asymptotic richness. We used the ‘iNEXT’ function from the ‘iNEXT’ package in R (Hsieh et al., 2020Hsieh TC, Ma KH, Chao A. iNEXT: iNterpolation and EXTrapolation for species diversity. R package version 2.0.20. 2020. Available from: http://chao.stat.nthu.edu.tw/wordpress/software-download/
http://chao.stat.nthu.edu.tw/wordpress/s...
) to generate curves for miniature and non-miniature fishes independently. Preliminary results from this analysis illustrated complete sampling of miniature fishes but incomplete sampling of non-miniature fishes, so we proceeded with analysis of only miniature fish assemblages. We reviewed the conservation status listed by the International Union for the Conservation of Nature (IUCN; https://www.iucnredlist.org/) for each of the miniature species identified.
To examine our second objective, we used non-metric multidimensional scaling (NMDS) only on miniature fish assemblages to visualize differences in assemblage structure between the two sampling sites and among sampling locations (n = 20). The NMDS was implemented with the ‘vegan’ package in R (Oksanen et al., 2019Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P et al. vegan: Community Ecology Package. R package version 2.5-6. 2019. Available form: https://CRAN.R-project.org/package=vegan
https://CRAN.R-project.org/package=vegan...
) and based on the Bray-Curtis distance metric (Bray, Curtis, 1957Bray JR, Curtis JT. An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr. 1957; 27(4):326–49. https://doi.org/10.2307/1942268
https://doi.org/10.2307/1942268...
) calculated from Hellinger-transformed abundance data (López-Delgado et al., 2019López-Delgado EO, Winemiller KO, Villa-Navarro FA. Do metacommunity theories explain spatial variation in fish assemblage structure in a pristine tropical river? Freshw Biol. 2019; 64(2):367–79. https://doi.org/10.1111/fwb.13229
https://doi.org/10.1111/fwb.13229...
). We used the ‘envfit’ function from the ‘vegan’ package to identify environmental and spatial variables that significantly correlated with assemblage structure. We quantified species co-occurrence by calculating all pairwise Pearson correlation coefficient between species using the ‘corrplot’ function from the ‘corrplot’ package in R (Taiyun, Viliam, 2017Taiyun W, Viliam S. R package “corrplot”: Visualization of a correlation matrix (Version 0.84). 2017. Available from: https://github.com/taiyun/corrplot
https://github.com/taiyun/corrplot...
). Prior to analyses, we log-transformed all continuous environmental variables that were measured on a non-bound scale, logit-transformed all continuous environmental variables measured on a percentage scale, and then standardized all variables to a mean of zero and standard deviation of one (i.e., z-score transformation) to better approximate normal distributions. Following the NMDS, we tested for a significant difference in assemblage structure between the two sampling locations using permuted multivariate analysis of variance (pmanova) implemented with the ‘adonis2’ function from the ‘vegan’ package using the transformed miniature fish assemblage matrix as the response variable and a two-level factor representing the two sites. All analyses were conducted in R version 3.6.2 (R Development Core Team, 2019R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2019. Available from: http://www.R-project.org/
http://www.R-project.org/...
).
RESULTS
We collected nine miniature and 44 non-miniature species across the 20 sampling locations, yielding a total of 717 miniature and 1,199 non-miniature specimens. One of the miniature and one of the non-miniature species we collected are not formally described (GMTM, pers. obs.), and two specimens were represented only by juveniles that could not be identified below the level of genus (Tab. 1). None of the miniature fishes collected were evaluated by the IUCN (Tab. 2). Accumulation of miniature fish richness across sampling effort leveled out between 10 and 20 samples, while accumulation of non-miniature fishes continued past our 20 samples and was still increasing at twice our sample size (Fig. 2). The asymptotic richness estimation (95% confidence range) was 9 (9–14) species for miniatures and 59 (49–96) species for non-miniatures. Average SL was 12.1 mm (range = 8.3–20.1) for miniature fishes and 24.1 mm (range = 4.5–151.1) for non-miniature fishes.
Fishes collected from the rio Jacundá in the Amazon basin, Brazil, during August 2019, including the number of specimens collected and mean standard length of specimens. Miniature fishes are shown in bold text. Lengths were not recorded for two juvenile non-miniature specimens (“–“). Vouchers are deposited at Laboratório de Ictiologia de Sorocaba (LISO).
Abundances of miniature fish species collected at the upstream and downstream sampling sites (see Fig. 1 for locations) and conservation status according to the International Union for the Conservation of Nature (IUCN). NE = Not Evaluated.
Accumulation (solid lines), extrapolation (dashed lines), and 95% confidence intervals (shaded areas) for diversity of miniature (red) and non-miniature (blue) freshwater fishes collected from the rio Jacundá, rio Amazonas basin, Brazil.
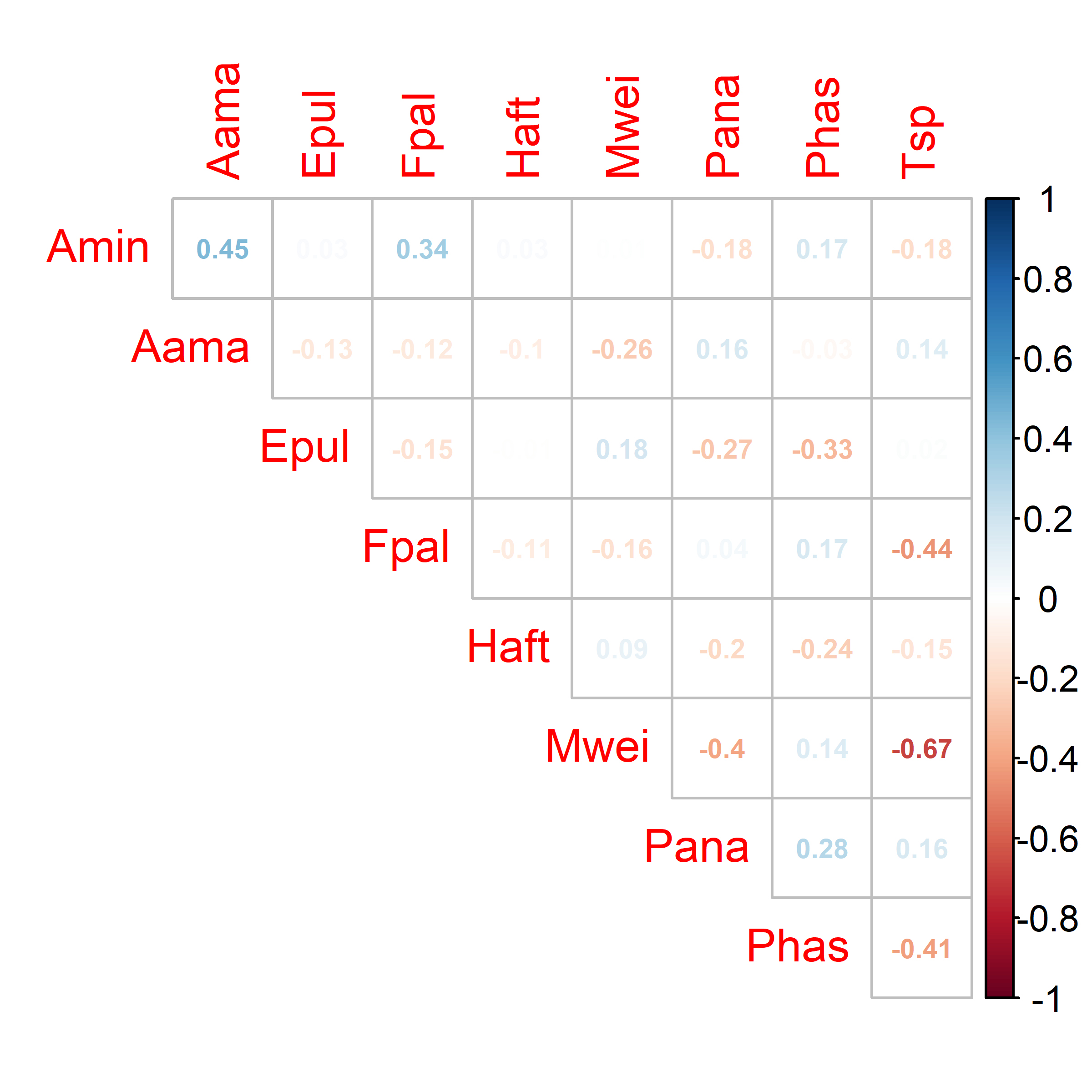
The NMDS analysis showed clear separation of fish assemblages within and among the two sites, with a two-dimensional stress value of 0.13 (Fig. 3). The upstream site (Fig. 1C) was a nested subset of the downstream site (Fig. 1D) with four of the nine miniature species occurring at only the downstream site, including Ammocryptocharax minutus Buckup, 1993, Elachocharax pulcher Myers, 1927, Hemigrammus aff. tridens, and Tyttobrycon sp. (Tab. 2). With the exception of Ammoglanis amapaensis Mattos, Costa & Gama, 2008, all species that occurred upstream had higher abundances upstream relative to downstream. These patterns contributed to a significant difference between the upstream and downstream sites based on the pmanova test (F1,18 = 9.7, P < 0.01, R2 = 0.35). The environmental variables that correlated with assemblage structure based on the ‘envfit’ analysis (Tab. 3) were pH (R2 = 0.33, P = 0.03), water temperature (R2 = 0.32, P = 0.04), conductivity (R2 = 0.31, P = 0.04), percent canopy cover (R2 = 0.30, P = 0.04), and total suspended solids (R2 = 0.29, P = 0.04). The only spatial variable that correlated with assemblage structure was the first axis of the asymmetric eigenvector map (i.e., AEM1, R2 = 0.50, P < 0.01). Pairwise correlations of species abundance at the 20 sampling locations showed low correlations in species occurrence, with only Microcharacidium weitzmani Buckup, 1993 and Tyttobrycon sp. having an absolute correlation coefficient >0.50 (Fig. 4).
Non-metric multidimensional scaling (NMDS) plot illustrating 20 sampling locations (points) at upstream (blue boxes) and downstream (red circles) sampling sites. Miniature fish scores are shown as green text, including Aama (Ammoglanis amapaensis), Amin (Ammocryptocharax minutus), Epul (Elachocharax pulcher), Fpal (Fluviphylax palikur), Haft (Hemigrammus aff. tridens), Mwei (Microcharacidium weitzmani), Pana (Physopyxis ananas), Phas (Potamoglanis hasemani), and Tsp (Tyttobrycon sp.). Environmental and spatial correlates with miniature fish assemblage structure are shown as gray vectors and text, including water overhead canopy cover (%), water temperature (C), pH, total suspended solids (TSS, ppm), conductivity (µs/cm), and the spatial relationship among sites from the first axis of an asymmetric eigenvector map (AEM1).
Environmental variables that showed significant correlation with miniature fish assemblages in the non-metric multidimensional scaling plot.
Pairwise Pearson correlations in species abundance across the 20 sampling locations showing positive correlations in blue and negative correlations in red. Species codes are Aama (Ammoglanis amapaensis), Amin (Ammocryptocharax minutus), Epul (Elachocharax pulcher), Fpal (Fluviphylax palikur), Haft (Hemigrammus aff. tridens), Mwei (Microcharacidium weitzmani), Pana (Physopyxis ananas), Phas (Potamoglanis hasemani) and Tsp (Tyttobrycon sp.).
DISCUSSION
Few studies have quantitatively assessed miniature fish local richness or relationships with environmental variables. Henderson, Walker (1986Henderson PA, Walker I. On the leaf litter community of the Amazonian blackwater stream Tarumazinho. J Trop Ecol. 1986; 2(1):1–16. https://doi.org/10.1017/s0266467400000547
https://doi.org/10.1017/s026646740000054...
, 1990Henderson PA, Walker I. Spatial organization and population density of the fish community of the litter banks within a central Amazonian blackwater stream. J Fish Biol. 1990; 37(3):401–11. https://doi.org/10.1111/j.1095-8649.1990.tb05871.x
https://doi.org/10.1111/j.1095-8649.1990...
) used hand nets to study the fish assemblages in litter banks of the small rio Tarumã-Mirim. Henderson, Walker, (1990)Henderson PA, Walker I. Spatial organization and population density of the fish community of the litter banks within a central Amazonian blackwater stream. J Fish Biol. 1990; 37(3):401–11. https://doi.org/10.1111/j.1095-8649.1990.tb05871.x
https://doi.org/10.1111/j.1095-8649.1990...
used an early form of rarefaction to illustrate species accumulation across sampling efforts and challenged earlier notions that leaf litter banks were depauperate by collecting 20 species that apparently spatially segregated their habitats. However, no quantitative assessment of habitat was presented despite Henderson, Walker, (1986)Henderson PA, Walker I. On the leaf litter community of the Amazonian blackwater stream Tarumazinho. J Trop Ecol. 1986; 2(1):1–16. https://doi.org/10.1017/s0266467400000547
https://doi.org/10.1017/s026646740000054...
reporting water quality parameters. Carvalho et al., (2006)Carvalho LN, Zuanon J, Sazima I. The almost invisible league: crypsis and association between minute fishes and shrimps as a possible defense against visually hunting predators. Neotrop Ichthyol. 2006; 4(2):219–24. https://doi.org/10.1590/S1679-62252006000200008
https://doi.org/10.1590/S1679-6225200600...
conducted behavioral observations of miniature fishes and noted the apparent use of transparency and camouflage to avoid predation, but the authors provided no quantitative assessment of habitats used by fishes. Carvalho et al., (2013)Carvalho LN, Fidelis L, Arruda R, Galuch A, Zuanon J. Second floor, please: the fish fauna of floating litter banks in Amazonian streams and rivers. Neotrop Ichthyol. 2013; 11(1):85–94. http://dx.doi.org/10.1590/S1679-62252013000100010
http://dx.doi.org/10.1590/S1679-62252013...
used fine-mesh hand nets to collect fishes from floating litter banks in four rivers of the Amazon basin. The authors used rarefaction analysis to compare richness across four river systems, but did not differentiate miniature fishes from juvenile forms of non-miniature fishes. Our work adds to this body of literature by revealing richness specific to miniature fishes as well as local habitat variables that correlate with benthic assemblage structure.
We found that assemblage composition was not uniform between the two sampling sites. The upstream site was a nested subset of the downstream site, yet abundances were greater upstream among the majority of miniature species present at both sites. Although our inference is limited because of only two sampling sites, this pattern should be explored in future research targeting miniature fishes across gradients of stream size, habitat types, and anthropogenic alterations. Our fine scale study uncovered two environmental variables that are likely important at broader scales. First, water pH strongly correlated with differences in assemblage structure between the two sites. Ríos-Villamizar et al., (2017)Ríos-Villamizar EA, Piedade MT, Junk WJ, Waichman AV. Surface water quality and deforestation of the Purus river basin, Brazilian Amazon. Int Aquat Res. 2017; 9(1):81–88. https://doi.org/10.1007/s40071-016-0150-1
https://doi.org/10.1007/s40071-016-0150-...
noted that pH increased in a downstream direction in the rio Purus, but pH decreased with greater accumulated upstream total deforestation. The environmental or ecological factors driving the evolution of miniaturization in Neotropical freshwater fishes have yet to be identified (Weitzman, Vari, 1988Weitzman SH, Vari RP. Miniaturization in South American freshwater fishes: An overview and discussion. Proc Biol Soc Wash. 1988; 101(2):444–65. https://repository.si.edu/handle/10088/901?show=full
https://repository.si.edu/handle/10088/9...
). Because miniature fishes are frequently encountered in still or slow-flowing waters with low pH, anthropogenic alterations to water pH caused by deforestation could alter miniature fish assemblages over ecological and evolutionary time scales. A second environmental variable that strongly correlated with miniature fish assemblage structure was percent canopy cover. Previous work established the link between deforestation and reductions in leaf litter density within streams (Carvalho, Uieda, 2010Carvalho EM, Uieda VS. Input of litter in deforested and forested areas of a tropical headstream. Braz J Biol. 2010; 70(2):283–88. https://doi.org/10.1590/S1519-69842010005000015
https://doi.org/10.1590/S1519-6984201000...
). The NMDS analysis showed a trajectory towards convergence in assemblage structure at sampling locations with greater canopy cover and lower pH (negative values along NMDS 1 and 2) regardless of upstream versus downstream sampling site identity. These locations were dominated by Hemigrammus aff. tridens and Elachocharax pulcher at the downstream site and Microcharacidium weitzmani at both upstream and downstream sites. Henderson, Walker, (1990)Henderson PA, Walker I. Spatial organization and population density of the fish community of the litter banks within a central Amazonian blackwater stream. J Fish Biol. 1990; 37(3):401–11. https://doi.org/10.1111/j.1095-8649.1990.tb05871.x
https://doi.org/10.1111/j.1095-8649.1990...
noted that E. pulcher was most abundant at locations with newly fallen leaves that had not yet began to decompose, suggesting a link between this species and high canopy cover. Our fine-scale results combined with patterns from previous studies suggest future research on miniature fishes across broader spatial scales and abiotic gradients should consider pH and canopy cover during hypothesis development.
Our results support previous suggestions that miniature fishes spatially partition habitats, a finding that highlights future research avenues. Henderson, Walker, (1990)Henderson PA, Walker I. Spatial organization and population density of the fish community of the litter banks within a central Amazonian blackwater stream. J Fish Biol. 1990; 37(3):401–11. https://doi.org/10.1111/j.1095-8649.1990.tb05871.x
https://doi.org/10.1111/j.1095-8649.1990...
suggested miniature fishes in the litter banks of the rio Tarumã-Mirim spatially partitioned their habitats, perhaps as a means of reducing competition for limited resources. Our analyses of miniature fish assemblages advance this assertion in two ways. First, miniature fish species showed clear separation in multivariate space in our NMDS analysis, highlighting that different species dominated different sampling locations distributed across gradients of primarily pH and canopy cover. Olden et al., (2006)Olden JD, Poff NL, Bestgen KR. Life-history strategies predict fish invasions and extirpations in the Colorado River basin. Ecol Mono. 2006; 76(1):25–40. https://doi.org/10.1890/05-0330
https://doi.org/10.1890/05-0330...
used functional traits of fishes to suggest that the filling of “vacant” multivariate space by non-native species was evidence of niche segregation that ultimately allowed for co-occurrence of native and non-native species in the Colorado River basin in North America. Hypotheses regarding niche segregation by miniature fishes could be tested as functional trait information becomes available in the future. Second, results from pairwise correlations quantifying co-occurrence showed generally low or negative similarities in species abundances among sampling locations. Strong negative relationships might be expected if local assemblages were structured by competition (e.g., Peres-Neto, 2004Peres-Neto PR. Patterns in the co-occurrence of fish species in streams: the role of site suitability, morphology and phylogeny versus species interactions. Oecologia. 2004; 140(2):352–60. https://doi.org/10.1007/s00442-004-1578-3
https://doi.org/10.1007/s00442-004-1578-...
), whereas strong positive relationships might be expected if facilitation structured assemblages (e.g., Peoples, Frimpong, 2016Peoples BK, Frimpong EA. Biotic interactions and habitat drive positive co-occurrence between facilitating and beneficiary stream fishes. J Biogeogr. 2016; 43(5):923–31. https://doi.org/10.1111/jbi.12699
https://doi.org/10.1111/jbi.12699...
). This is a promising avenue for future research as hypotheses could easily be tested in small mesocosms given the minute adult size of the species involved.
Recent evidence from fishes suggests that miniaturization may arise from an acceleration of development rate (Marinho, 2017Marinho MMF. Comparative development in Moenkhausia pittieri and Paracheirodon innesi (Ostariophysi: Characiformes) with comments on heterochrony and miniaturization in the Characidae. J Fish Biol. 2017; 91(3):851–65. https://doi.org/10.1111/jfb.13384
https://doi.org/10.1111/jfb.13384...
) and/or truncated ontogeny (Britz, Conway, 2009Britz R, Conway KW. Osteology of Paedocypris, a miniature and highly developmentally truncated fish (Teleostei: Ostariophysi: Cyprinidae). J Morphol. 2009; 270(4):389–412. https://doi.org/10.1002/jmor.10698
https://doi.org/10.1002/jmor.10698...
; Mattox et al., 2016Mattox GMT, Britz R, Toledo-Piza M. Osteology of Priocharax and remarkable developmental truncation in a miniature Amazonian fish (Teleostei: Characiformes: Characidae). J Morphol. 2016; 277(1):65–85. https://doi.org/10.1002/jmor.20477
https://doi.org/10.1002/jmor.20477...
). Miniaturization of South American freshwater fishes is also hypothesized to arise in response to biotic factors such as enhanced survival (as species) associated with minute body size and year-round reproduction (Roberts, 1984Roberts TR. Amazonsprattus scintilla, new genus and species from the rio Negro, Brazil, the smallest known clupeomorph fish. Proc Calif Acad Sci. 1984; 43(20):317–21.). Thus, body size likely interacts with life history traits to track species-specific optima in tradeoffs among growth, mortality, and reproduction (Kozlowski, Gawelczyk, 2002Kozlowski J, Gawelczyk AT. Why are animal body size distributions usually skewed to the rigth? Funct Ecol. 2002; 16(4):419–32. http://dx.doi.org/10.1046/j.1365-2435.2002.00646.x
http://dx.doi.org/10.1046/j.1365-2435.20...
). We suggest priority research areas pertaining to miniature fish ecology include life history traits, experiments involving competition, and tracking movements. Enhancing our understanding of the ecology of these understudied and overlooked fishes will benefit both basic research on their evolutionary history and applied research pertaining to conservation. The former is necessary to provide context to the repeated and widespread evolution of miniature body size in freshwater fishes (Rüber et al., 2007Rüber L, Kottelat M, Tan HH, Ng PKL, Britz R. Evolution of miniaturization and the phylogenetic position of Paedocypris, comprising the world’s smallest vertebrate. BMC Evol Biol. 2007; 7(1):1–10. https://doi.org/10.1186/1471-2148-7-38
https://doi.org/10.1186/1471-2148-7-38...
), while the latter is important given impending and on-going changes to Amazonian streams inhabited by miniature fishes (e.g., Winemiller et al., 2016Winemiller KO, McIntyre PB, Castello L, Fluet-Chouinard E, Giarrizzo T, Nam S et al. Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science. 2016; 351(6269):128–29. https://doi.org/10.1126/science.aac7082
https://doi.org/10.1126/science.aac7082...
; Albert et al., 2020Albert JS, Tagliacollo VA, Dagosta F. Diversification of Neotropical freshwater fishes. Annu Rev Ecol Evol Syst. 2020; 51:27–53. https://doi.org/10.1146/annurev-ecolsys-011620-031032
https://doi.org/10.1146/annurev-ecolsys-...
).
ACKNOWLEDGEMENTS
This research resulted from an international collaboration between Texas A&M University (TAMU) and FAPESP SPRINT/UFSCar (FAPESP Proc. 2018/22592-0). The fieldtrip was partially funded by FAPESP (Proc. 2017/01970-4) and TAMU (TAMU/FAPESP #2018-3-16). Alberto Akama and Wolmar Wosiacki (MPEG) provided crucial supplies and information that ensured the success of our fieldtrip. Manoela Marinho (UFPB) and Vinícius Reis (MZUSP) confirmed the identification of Tyttobrycon sp. and the trichomycterids Ammoglanis amapaensis and Potamoglanis hasemani, respectively. Flávio Lima (ZUEC) aided in Hemigrammus and Hyphessobrycon identifications.
REFERENCES
- Abrahão VP, Pastana M, Marinho M. On a remarkable sexual dimorphic trait in the Characiformes related to the olfactory organ and description of a new miniature species of Tyttobrycon Géry (Characiformes: Characidae). PLoS ONE. 2019; 14(12):e0226130. https://doi.org/10.1371/journal.pone.0226130
» https://doi.org/10.1371/journal.pone.0226130 - Albert JS, Tagliacollo VA, Dagosta F. Diversification of Neotropical freshwater fishes. Annu Rev Ecol Evol Syst. 2020; 51:27–53. https://doi.org/10.1146/annurev-ecolsys-011620-031032
» https://doi.org/10.1146/annurev-ecolsys-011620-031032 - Bennett MG, Conway KW. An overview of North America’s diminutive freshwater fish fauna. Ichthyol Explor Freshw. 2010; 21(1):63–72.
- Blanchet FG, Legendre P, Borcard D. Modelling directional spatial processes in ecological data. Ecol Modell. 2008; 215(4):325–36. https://doi.org/10.1016/j.ecolmodel.2008.04.001
» https://doi.org/10.1016/j.ecolmodel.2008.04.001 - Bray JR, Curtis JT. An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr. 1957; 27(4):326–49. https://doi.org/10.2307/1942268
» https://doi.org/10.2307/1942268 - Britz R, Conway KW. Osteology of Paedocypris, a miniature and highly developmentally truncated fish (Teleostei: Ostariophysi: Cyprinidae). J Morphol. 2009; 270(4):389–412. https://doi.org/10.1002/jmor.10698
» https://doi.org/10.1002/jmor.10698 - Britz R, Conway KW, Rüber L. Miniatures, morphology and molecules: Paedocypris and its phylogenetic position (Teleostei, Cypriniformes). Zool J Linn Soc. 2014; 172(3):556–615. https://doi.org/10.1111/zoj.12184
» https://doi.org/10.1111/zoj.12184 - Carvalho EM, Uieda VS. Input of litter in deforested and forested areas of a tropical headstream. Braz J Biol. 2010; 70(2):283–88. https://doi.org/10.1590/S1519-69842010005000015
» https://doi.org/10.1590/S1519-69842010005000015 - Carvalho LN, Fidelis L, Arruda R, Galuch A, Zuanon J. Second floor, please: the fish fauna of floating litter banks in Amazonian streams and rivers. Neotrop Ichthyol. 2013; 11(1):85–94. http://dx.doi.org/10.1590/S1679-62252013000100010
» http://dx.doi.org/10.1590/S1679-62252013000100010 - Carvalho LN, Zuanon J, Sazima I. The almost invisible league: crypsis and association between minute fishes and shrimps as a possible defense against visually hunting predators. Neotrop Ichthyol. 2006; 4(2):219–24. https://doi.org/10.1590/S1679-62252006000200008
» https://doi.org/10.1590/S1679-62252006000200008 - Carvalho MS, Zuanon J, Ferreira EJG. Diving in the sand: the natural history of Pygidianops amphioxus (Siluriformes: Trichomycteridae), a miniature catfish of Central Amazonian streams in Brazil. Environ Biol Fishes. 2014; 97(1):59–68. https://doi.org/10.1007/s10641-013-0123-9
» https://doi.org/10.1007/s10641-013-0123-9 - Carvalho TP, Reis RE. A new miniature species of Acanthobunocephalus (Silurifomes: Aspredinidae) from the Lower Purus River basin, Amazon basin, Brazil. Copeia. 2020; 108(2):347–57. https://doi.org/10.1643/CI-19-309
» https://doi.org/10.1643/CI-19-309 - Chao A, Gotelli NJ, Hsieh TC, Sander EL, Ma KH, Colwell RK et al Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecol Mono. 2014; 84(1):45–67. https://doi.org/10.1890/13-0133.1
» https://doi.org/10.1890/13-0133.1 - Claro-García A, Shibatta OA. The fish fauna of streams from the upper rio Tocantins basin, Goiás State, Brazil. Check List. 2013; 9(1):28–33. https://doi.org/10.15560/9.1.28
» https://doi.org/10.15560/9.1.28 - Conway KW, Moritz T Barboidesbritzi, a new species of miniature cyprinid from Benin (Ostariophysi: Cyprinidae), with a neotype designation for B. gracilis Ichthyol Explor Freshw. 2006; 17(1):73–84.
- Costa WJEM, Le Bail P Fluviphylaxpalikur: a new poeciliid from the rio Oiapoque basin, Northern Brazil (Cyprinodontiformes: Cyprinodontoidei), with comments on miniaturization in Fluviphylax and other Neotropical freshwater fishes. Copeia. 1999; 1999(4):1027–34. https://doi.org/10.2307/1447977
» https://doi.org/10.2307/1447977 - Dray S, Blanchet G, Borcard D, Guenard G, Jombart T, Larocque G et al adespatial: Multivariate multiscale spatial analysis. 2020. Available from: https://CRAN.R-project.org/package=adespatial
» https://CRAN.R-project.org/package=adespatial - Dray S, Legendre P, Peres-Neto PR. Spatial modelling: a comprehensive framework for principal coordinate analysis of neighbour matrices (PCNM). Ecol Modell. 2006; 196(3–4):483–93. https://doi.org/10.1016/j.ecolmodel.2006.02.015
» https://doi.org/10.1016/j.ecolmodel.2006.02.015 - Hanken J, Wake BD. Miniaturization of body size: organismal consequences and evolutionary significance. Annu Rev Ecol Syst. 1993; 24(1993):501–19. https://doi.org/10.1146/annurev.es.24.110193.002441
» https://doi.org/10.1146/annurev.es.24.110193.002441 - Henderson PA, Walker I. On the leaf litter community of the Amazonian blackwater stream Tarumazinho. J Trop Ecol. 1986; 2(1):1–16. https://doi.org/10.1017/s0266467400000547
» https://doi.org/10.1017/s0266467400000547 - Henderson PA, Walker I. Spatial organization and population density of the fish community of the litter banks within a central Amazonian blackwater stream. J Fish Biol. 1990; 37(3):401–11. https://doi.org/10.1111/j.1095-8649.1990.tb05871.x
» https://doi.org/10.1111/j.1095-8649.1990.tb05871.x - Hsieh TC, Ma KH, Chao A. iNEXT: iNterpolation and EXTrapolation for species diversity. R package version 2.0.20. 2020. Available from: http://chao.stat.nthu.edu.tw/wordpress/software-download/
» http://chao.stat.nthu.edu.tw/wordpress/software-download/ - Ilha P, Schiesari L, Yanagawa FI, Jankowski K, Navas CA. Deforestation and stream warming affect body size of Amazonian fishes. PloS ONE. 2018; 13(5):e0196560. https://doi.org/10.1371/journal.pone.0196560
» https://doi.org/10.1371/journal.pone.0196560 - Kottelat M, Britz R, Hui TH, Witte KE Paedocypris, a new genus of Southeast Asian cyprinid fish with a remarkable sexual dimorphism, comprises the world’s smallest vertebrate. Proc R Soc Lond [Biol]. 2006; 273(1589):895–99. https://doi.org/10.1098/rspb.2005.3419
» https://doi.org/10.1098/rspb.2005.3419 - Kottelat M, Vidthayanon C Boraras micros, a new genus and species of minute freshwater fish from Thailand (Teleostei: Cyprinidae). Ichthyol Expl Freshw. 1993; 4(2):161–76.
- Kozlowski J, Gawelczyk AT. Why are animal body size distributions usually skewed to the rigth? Funct Ecol. 2002; 16(4):419–32. http://dx.doi.org/10.1046/j.1365-2435.2002.00646.x
» http://dx.doi.org/10.1046/j.1365-2435.2002.00646.x - López-Delgado EO, Winemiller KO, Villa-Navarro FA. Do metacommunity theories explain spatial variation in fish assemblage structure in a pristine tropical river? Freshw Biol. 2019; 64(2):367–79. https://doi.org/10.1111/fwb.13229
» https://doi.org/10.1111/fwb.13229 - Marinho MMF. Comparative development in Moenkhausia pittieri and Paracheirodon innesi (Ostariophysi: Characiformes) with comments on heterochrony and miniaturization in the Characidae. J Fish Biol. 2017; 91(3):851–65. https://doi.org/10.1111/jfb.13384
» https://doi.org/10.1111/jfb.13384 - Mattox GMT, Britz R, Toledo-Piza M. Osteology of Priocharax and remarkable developmental truncation in a miniature Amazonian fish (Teleostei: Characiformes: Characidae). J Morphol. 2016; 277(1):65–85. https://doi.org/10.1002/jmor.20477
» https://doi.org/10.1002/jmor.20477 - Mattox GMT, Souza CS, Toledo-Piza M, Britz R, Oliveira C. A new miniature species of Priocharax (Teleostei: Characiformes: Characidae) from the rio Madeira drainage, Brazil, with comments on the adipose fin in characiforms. Verteb Zool. 2020; 70(3):417–33. https://doi.org/10.26049/VZ70-3-2020-11
» https://doi.org/10.26049/VZ70-3-2020-11 - Mattox GMT, Souza CS, Toledo-Piza M, Oliveira C. A new miniature species of Priocharax (Characiformes: Characidae) from the upper rio Ipixuna, Purus drainage, Brazil. Neotrop Ichthyol. 2021; 19(2):e210048. https://doi.org/10.1590/1982-0224-2021-0048
» https://doi.org/10.1590/1982-0224-2021-0048 - Montag LF, Winemiller KO, Keppeler FW, Leão H, Benone NL, Torres NR et al Land cover, riparian zones and instream habitat influence stream fish assemblages in the eastern Amazon. Ecol Freshw Fish. 2019; 28(2):317–29. https://doi.org/10.1111/eff.12455
» https://doi.org/10.1111/eff.12455 - Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P et al vegan: Community Ecology Package. R package version 2.5-6. 2019. Available form: https://CRAN.R-project.org/package=vegan
» https://CRAN.R-project.org/package=vegan - Olden JD, Poff NL, Bestgen KR. Life-history strategies predict fish invasions and extirpations in the Colorado River basin. Ecol Mono. 2006; 76(1):25–40. https://doi.org/10.1890/05-0330
» https://doi.org/10.1890/05-0330 - Peoples BK, Frimpong EA. Biotic interactions and habitat drive positive co-occurrence between facilitating and beneficiary stream fishes. J Biogeogr. 2016; 43(5):923–31. https://doi.org/10.1111/jbi.12699
» https://doi.org/10.1111/jbi.12699 - Peres-Neto PR. Patterns in the co-occurrence of fish species in streams: the role of site suitability, morphology and phylogeny versus species interactions. Oecologia. 2004; 140(2):352–60. https://doi.org/10.1007/s00442-004-1578-3
» https://doi.org/10.1007/s00442-004-1578-3 - R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria; 2019. Available from: http://www.R-project.org/
» http://www.R-project.org/ - Reis RE, Albert JS, Di Dario F, Mincarone MM, Petry P, Rocha LA. Fish biodiversity and conservation in South America. J Fish Biol. 2016; 89(1):12–47. https://doi.org/10.1111/jfb.13016
» https://doi.org/10.1111/jfb.13016 - Ríos-Villamizar EA, Piedade MT, Junk WJ, Waichman AV. Surface water quality and deforestation of the Purus river basin, Brazilian Amazon. Int Aquat Res. 2017; 9(1):81–88. https://doi.org/10.1007/s40071-016-0150-1
» https://doi.org/10.1007/s40071-016-0150-1 - Roberts TR Amazonsprattus scintilla, new genus and species from the rio Negro, Brazil, the smallest known clupeomorph fish. Proc Calif Acad Sci. 1984; 43(20):317–21.
- Rodrigues EKDQ, Netto-Ferreira AL. A new miniature species of Odontocharacidium (Characiformes: Crenuchidae) from the Río Orinoco basin, Venezuela. Neotrop Ichthyol. 2020; 18(2):e190008. https://doi.org/10.1590/1982-0224-2019-0008
» https://doi.org/10.1590/1982-0224-2019-0008 - Rüber L, Kottelat M, Tan HH, Ng PKL, Britz R. Evolution of miniaturization and the phylogenetic position of Paedocypris, comprising the world’s smallest vertebrate. BMC Evol Biol. 2007; 7(1):1–10. https://doi.org/10.1186/1471-2148-7-38
» https://doi.org/10.1186/1471-2148-7-38 - Rundell RJ, Leander BS. Masters of miniaturization: convergent evolution among interstitial eukaryotes. BioEssays. 2010; 32(5):430–37. https://doi.org/10.1002/bies.200900116
» https://doi.org/10.1002/bies.200900116 - Taiyun W, Viliam S. R package “corrplot”: Visualization of a correlation matrix (Version 0.84). 2017. Available from: https://github.com/taiyun/corrplot
» https://github.com/taiyun/corrplot - Toledo-Piza M, Mattox GMT, Britz R Priocharax nanus, a new miniature characid from the rio Negro, Amazon basin (Ostariophysi: Characiformes), with an updated list of miniature Neotropical freshwater fishes. Neotrop Ichthyol. 2014; 12(2):229–46. https://doi.org/10.1590/1982-0224-20130171
» https://doi.org/10.1590/1982-0224-20130171 - Van der Sleen P, Albert JS. Field guide to the fishes of the Amazon, Orinoco, and Guianas (Vol. 115). Princeton University Press; 2018.
- Weitzman SH, Vari RP. Miniaturization in South American freshwater fishes: An overview and discussion. Proc Biol Soc Wash. 1988; 101(2):444–65. https://repository.si.edu/handle/10088/901?show=full
» https://repository.si.edu/handle/10088/901?show=full - Winemiller KO, McIntyre PB, Castello L, Fluet-Chouinard E, Giarrizzo T, Nam S et al Balancing hydropower and biodiversity in the Amazon, Congo, and Mekong. Science. 2016; 351(6269):128–29. https://doi.org/10.1126/science.aac7082
» https://doi.org/10.1126/science.aac7082
ADDITIONAL NOTES
-
HOW TO CITE THIS ARTICLE
Perkin JS, Montaña CG, Nogueira EJ, Brandão BB, Mattox GMT, Conway KW. Estimated richness and environmental correlates of miniature fish assemblages in the rio Jacundá, Brazil. Neotrop Ichthyol. 2022; 20(2):e210051. https://doi.org/10.1590/1982-0224-2021-0051
Edited-by
Publication Dates
-
Publication in this collection
11 May 2022 -
Date of issue
2022
History
-
Received
17 Feb 2021 -
Accepted
31 Mar 2022