Abstract
The Bacanga River Estuary has a hydrodynamic behavior and its tidal flow is limited by a dam. It is considered as a hypertrophic environment that receives daily high loads of domestic sewage without treatment. This study aimed to evaluate the spatial and temporal variation of phytoplankton community and its relationship with environmental parameters. Bi-monthly sampling campaigns were carried out at six fixed sites between 2012 and 2013. Physical-chemical and biological parameters were collected (chlorophyll a, phytoplankton composition and abundance) to perform the statistical correlations. The results indicate that phytoplankton community is mostly represented by diatoms, with Skeletonema costatum being the dominant species responsible for bloom in April and June of 2012. The dominance of this species is related to the high silicate concentrations, pH and turbidity. Other blooms events as well as the Euglena gracilis and Chlamydomonas sp. were recorded in February 2013, when the total phosphorus concentrations were high and the dissolved oxygen concentrations were higher. Dinoflagellates, cyanobacteria and diatom Thallassiosira sp. were widely distributed in the dry period and highly correlated with salinity, water transparency and nutrients. Hence, the distribution of phytoplankton community is more defined seasonally, rather than spatially.
Descriptors:
Algal bloom; Microalgae; Nutrients
O estuário do rio Bacanga apresenta um comportamento hidrodinâmico com fluxo de marés limitado por uma barragem. Ele é considerado como um ambiente hipereutrófico que recebe diariamente altas cargas de esgoto doméstico sem tratamento. Este trabalho teve como objetivo avaliar a variação espaço-sazonal da comunidade fitoplanctônica e suas relações com parâmetros ambientais. Amostragens bimestrais foram realizadas em seis pontos fixos entre 2012 e 2013, obtendo valores dos parâmetros físico-químicos e biológicos (clorofila a, composição e abundância do fitoplâncton) para realização das análises estatísticas. Os resultados indicam que a comunidade fitoplanctônica é representada por diatomáceas, sendo Skeletonema costatum a espécie dominante responsável por pulsos de florações em abril e junho de 2012. O predomínio dessa espécie está relacionado aos elevados teores de silicato, pH e turbidez da água. Outros eventos de florações como da Euglena gracilis e Chlamydomonas sp. foram registrados em fevereiro de 2013, quando os teores de fósforo total estiveram elevados e as taxas de oxigênio dissolvido foram superiores. Os dinoflagelados, cianobactérias e a diatomácea Thallassiosira sp. apresentaram ampla distribuição no período de estiagem e estão altamente correlacionados com a salinidade, transparência da água e nutrientes. Desta forma, a distribuição da comunidade fitoplanctônica é mais definida sazonalmente que espacialmente
Descritores:
Florações algas; Microalgas; Nutrientes
INTRODUCTION
Estuaries are hydrodynamic coastal environments, where the freshwater flow of rivers is mixed with the salt water from the oceans causing extensive environmental fluctuations (NASCIMENTO et al., 2003NASCIMENTO, F. C. R.; MUNIZ, K.; FEITOSA, F. A. N.; ARAÚJO, J. P.; SILVA, R. M. S.; SILVA, G. S.; FLORES-MONTES, M. J. Disponibilidade nutricional da Bacia do Pina e rio Tejipió (Recife-PE-Brasil) em relação aos nutrientes e biomassa primária (setembro/2000). Trop. Oceanogr., v. 31, n. 2, p. 149-169, 2003.; MASUDA et al., 2011MASUDA, L. S. M.; MOSER, G. A. O.; BARRERA-ALBA, J. J. Variação temporal do fitoplâncton no canal estuarino de Santos (sp). Braz. J. Aquat. Sci. Technol., v. 15, n. 1, p. 79-93, 2011.). Those conditions create a biologically productive system, considered “hotspots” in the cycling and export of nutrients, increasing the rates of biological metabolism and the local primary production (GREGO et al., 2009GREGO, C. K. S.; FEITOSA, F. A. N.; SILVA, M. H.; SILVA-CUNHA, M. G. G.; NASCIMENTO FILHO, G. A. Fitoplâncton do ecossistema estuarino do rio Ariquindá (Tamandaré, Pernambuco, Brasil): variáveis ambientais, biomassa e produtividade primária. Atlântica, v. 31, n. 2, p. 183-198, 2009.; CALLAWAY et al., 2014CALLAWAY, R.; GRENFELL, S.; LØNBORG, C. Small estuaries: Ecology, Environmental drivers and management challenges. Estuar. Coast. Shelf Sci. , v. 150, p. 193-195, 2014.; CLOERN et al., 2014CLOERN, J. E.; FOSTER, S. Q.; KLECKNER, A. E. Phytoplankton primary production in the world’s estuarine-coastal ecosystems. Biogeosciences, v. 11, p. 2477-2501, 2014.).
Those environments are such good places for ecological studies, not only because of the spatial and temporal variations of biotic and abiotic processes, but also because they are considered as the most degraded environments due the coastal urban development, which leads to aquatic eutrophication (MATOS et al., 2011MATOS, J. B., SODRÉ, D. K. L., COSTA, K. G.; PEREIRA, L. C. C.; COSTA, R. M. Spatial and temporal variation in the composition and biomass of phytoplankton in an Amazonian estuary. J. Coast. Res. SI 64, p. 1525-1529, 2011.; CAREY et al., 2013CAREY, R. O.; HOCHMUTH, G. J.; CHRISTOPHER, J.; MARTINEZ, C. J. M.; BOYER, T. H.; DUKES, M. D.; TOOR, G. S.; CISAR, J. L. Evaluating nutrient impacts in urban watersheds: challenges and research opportunities. Environ. Pollu., v. 173, p. 138-149, 2013.; WILD-ALLEN et al., 2013WILD-ALLEN, K.; SKERRATT, J.; WHITEHEAD, J.; RIZWI, F.; PARSLOW, J. Mechanisms driving estuarine water quality: A 3D biogeochemical model for informed management. Estuar. Coast. Shelf Sci., v. 135, p. 33-45, 2013.).
The high load of nutrients in coastal habitats promotes an increase in the magnitude and frequency of phytoplankton blooms, affecting the biogeochemistry and structure of the trophic web being a challenge for the continued activity of these systems (PAERL et al., 2014PAERL, H. W.; HALL, N. S.; PEIERLS, B. L.; ROSSIGNOL, K. L. Evolving Paradigms and Challenges in Estuarine and Coastal Eutrophication Dynamics in a Culturally and Climatically Stressed World. Estuar. Coast., v. 37, n. 2, p. 243-258, 2014.; LU; GAN, 2015LU, Z.; GAN, J. Controls of seasonal variability of phytoplankton blooms in the Pearl River Estuary. Deep Sea Res Part II: Top Stud Oceanogr., v.117, p. 86-96, 2015.; SIN et al., 2015SIN, Y.; LEE, E.; LEE, Y.; SHIN, K. H. The river-estuarine continuum of nutrients and phytoplankton communities in an estuary physically divided by a sea dike. Estuar. Coast. Shelf Sci., v. 163, Pt. B, p. 279-289, 2015.). Thus, the phytoplankton acts as an outstanding environmental indicator (GUENTHER et al., 2015GUENTHER, M.; ARAÚJO, M.; FLORES-MONTES, M. J.; GONZALEZ-RODRIGUEZ, E.; NEUMANN-LEITÃO, S. Eutrophication effects on phytoplankton size-fractioned biomass and production at a tropical estuary. Mar. Pollut. Bull, v. 91, p. 537-547, 2015.), once it is the transfer link and energy in estuarine trophodynamics. The phytoplankton community is capable of changing its composition and structure in response to different modifications of the environmental conditions caused by humans (REYNOLDS, 2006REYNOLDS, C. S. The Ecology of phytoplanktonCambridge: Cambridge University Press, 2006. 550 p.; GREGO et al., 2009GREGO, C. K. S.; FEITOSA, F. A. N.; SILVA, M. H.; SILVA-CUNHA, M. G. G.; NASCIMENTO FILHO, G. A. Fitoplâncton do ecossistema estuarino do rio Ariquindá (Tamandaré, Pernambuco, Brasil): variáveis ambientais, biomassa e produtividade primária. Atlântica, v. 31, n. 2, p. 183-198, 2009.; SEGURO et al., 2015SEGURO, I.; GARCÍA, C. M.; PAPASPYROU, S.; GÁLVEZ, J. A.; GARCÍA-ROBLEDO, E.; NAVARRO, G.; SORIA-PÍRIZ, S.; AGUILAR, V.; LIZANO, O. G.; MORALES-RAMÍREZ, A.; CORZO, A. Seasonal changes of the microplankton community along a tropical estuary. Reg. Stud. Mar. Sci., v. 2, p. 189-202, 2015.).
This study was conducted in a hypereutrophic tropical estuary (SILVA et al., 2014SILVA, G. S.; SANTOS, E. A.; CORRÊA, L. B.; MARQUES, A. L. B.; MARQUES, E. P.; SOUSA, E. R.; SILVA, G. S. Avaliação integrada da qualidade de águas superficiais: grau de trofia e proteção da vida aquática nos rios Anil e Bacanga, São Luís (MA). Eng. Sanit. Ambient., v. 19, p. 245-250, 2014.), located in a densely populated area of the São Luís Island. The area receives daily high loads of domestic sewage without treatment (Instituto da Cidade, Pesquisa e Planejamento Urbano e Rural - INCID). The estuary of the Bacanga River has a hydrodynamic behavior of raising and lowering of tides limited by a transverse dam installed in its mouth. The opening and closing of the dam gates is irregular, which hinders the water level control up to 4 meters (PITOMBEIRA; MORAIS, 1977PITOMBEIRA, E. S.; MORAIS, J. O. Comportamento hidrodinâmico e sedimentológico do estuário do Rio Bacanga (São Luis, Estado do Maranhão, Brasil). Arq. Ciênc. Mar. Fortaleza. v. 17, n. 2, p. 165-174, 1977.). Studies about the microphytoplankton community are scarce, unlike the ones about the use and occupation of soil, degradation and availability of water resources, geomorphology and rehabilitation of degraded areas (CARVALHO et al., 2000CARVALHO, G. P.; CAVALCANTE, P. R. S.; CASTRO, A. C. L.; ROJAS, M. O. A. I. Preliminary assessment of heavy metal levels in Mytella falcata (Bivalvia, Mytilidae) from Bacanga River estuary, São Luis, State of Maranhão, Northeastern Brazil. Rev. Bras. Biol., v. 60, n. 1, p. 11-16, 2000.; POTAZIO et al., 2004POTAZIO, L.; TANAKA, S. M. C. N.; CAVALCANTE, P. R. S. Avaliação de procedimentos de extração sequencial de fósforo em sedimento. Rev. Analyt., n. 8, p. 35-41, 2004.; RIBEIRO et al., 2005RIBEIRO, F. V.; FURTADO, M. S.; LIMA, N. F. C.; BRITO, L. C.; FEITOSA, A. C. Erosive Processes at Bacanga State Park Area. Rev. Soc. Nat., v. 1. n. 1, p. 142-147, 2005.; COELHO; DAMÁZIO, 2006COELHO, C. J. C.; DAMÁZIO, E. Aspectos da Disponibilidade e dos Usos da Água na Bacia do Rio Bacanga/Ilha do Maranhão (I. DE SÃO LUÍS) - MA. Bol. Lab. Hidrobiol., v. 19, n. 1, p. 73-84, 2006.; ANDRADE; CASTRO, 2007ANDRADE, A. A. S.; CASTRO, A. C. L. Evaluation of the management plan of the Bacanga State Park, island of Maranhão, northeast Brazil. Bol. Lab. Hidrobiol., v. 20, n. 1, p. 61-68, 2007.; ROJAS et al., 2007ROJAS, M. O. A. I.; CAVALCANTE, P. R. S.; SOUZA, R. C.; DOURADO, E. C. S. Teores de Zinco e Cobre em ostra (Crassotrea rhizophorae) e sururu (Mytella falcata) do estuário do rio Bacanga em São Luís (MA). Bol. Lab. Hidrobiol., v. 20, n. 1, 2007.; BEZERRA et al., 2009BEZERRA, P. S. S.; TAKIYAMA, L. R.; BEZERRA, C. W. B. Complexação de íons de metais por matéria orgânica dissolvida: modelagem e aplicação em sistemas reais. Acta Amaz., v. 39, n. 3, p. 639-648, 2009.; SILVA et al., 2014SILVA, G. S.; SANTOS, E. A.; CORRÊA, L. B.; MARQUES, A. L. B.; MARQUES, E. P.; SOUSA, E. R.; SILVA, G. S. Avaliação integrada da qualidade de águas superficiais: grau de trofia e proteção da vida aquática nos rios Anil e Bacanga, São Luís (MA). Eng. Sanit. Ambient., v. 19, p. 245-250, 2014.). In this context, this study was established as the first record of the phytoplankton in that area, in order to evaluate the spatial and temporal variation of that community and its relationship with environmental parameters over an annual cycle.
MATERIAL AND METHODS
Study area
The Bacanga River Estuary is located in the northwest portion of the São Luís Island, Maranhão (2°32’26’ 2°38’07”S and 44°16’00” and 44°19’16”W) and from its source to its mouth it is about 22 Km covering an area of 11,030 hectares and then it flows into São Marcos Bay. It is featured as a shallow system formed by two tributaries (Bicas River and Gapara River), which together are part of an Environmental Protection Area called Bacanga State Park (Figure 1). The area has a tropical humid climate with high temperatures throughout the year, represented by two well-defined seasonal periods: rainy season (January to July) and dry season (August to December) (AZEVEDO et al., 2008AZEVEDO, A. C. G.; FEITOSA, F. A. N.; KOENING, M. L. Distribuição espacial e temporal da biomassa fitoplanctônica e variáveis ambientais no Golfão Maranhense, Brasil. Acta Bot. Bras., v. 22, n. 3, p. 870-877, 2008.).
Samples were collected at six sites (Figure 1) along the estuary, following the annual cycle between 2012 and 2013. Six bimonthly samples were taken, three of which occurred in the rainy season (April/12, June/12 and February/13) and three in the dry season (August/12, September/12 and December/12). The sampling followed the normal inflow of seawater (downstream-upstream) during the ebb tide in compliance with the lunar cycle.
Quality water and meteorological analysis
The evaluation of water quality was based on physical and chemical parameters, such as water temperature, pH, salinity and total dissolved solids (TDS) were obtained with the aid a multi-parameter probe (Hanna-9878). The water transparence was obtained using the Secchi disk and turbidity was measured using a turbidimeter (Lamotte 2020), these parameters were analyzed in situ. For nutrient analysis 2 liters of water were collected at the surface layer (50 cm depth) using a Von Dorn bottle. Quantification of DIN (NH4 + + NO2 - + NO3 -), DIP (PO4-3), total phosphorus (TP) and inorganic silicate (SiO2-3) followed the methodology described in “Standard Methods for Water and Wastewater” (APHA, 2012AMERICAN PUBLIC HEALTH ASSOCIATION (APHA). Standard Methods For The Examination Of Water And Wastewater. 22nd ed. Washington: American Public Health Association, 2012.). Total phosphorus and nitrate were determined using the methodology described by KOROLEFF (1983)KOROLEFF, K. Determination of phosphorus. In: GRASSHOFF, K.; EHRHARDT, M.; KREMLING, K. (Eds.). Methods of sea water analysis2nd ed. Weinhein: Verlag Chemie, 1983. p. 125-139.. Nitrite determination was based on the method of STRICKLAND and PARSONS (1972)STRICKLAND, J. D. H.; PARSONS, T. R. A Practical Handbook of Seawater AnalysisOttawa: Fisheries Research Board of Canada, 1972., silicate and orthophosphate (GRASSHOFF et al., 1983GRASSHOFF, K.; EHRHARDT, M.; KREMLING, K. Methods of seawater analisis. 2nd ed. New York: Verlag Chemie, 1983. 419 p.). Dissolved oxygen was determined by the chemical method of Winkler modified by GOLTERMAN et al. (1978)GOLTERMAN, H. L.; CLYMO, R. S.; OHNSTAD, M. A. M. Methods for physical and chemical analysis of freshwater2nd ed. Oxford: Blackwell Scientific Publications,1978. 213 p.. The historical mean (2003-2013) and total monthly rainfall were obtained by the Laboratory of Meteorology of the Maranhão State University (LabMet/UEMA).
Phytoplankton composition
For qualitative analysis of microphytoplankton, horizontal hauls were taken in the euphotic zone (20 cm depth). The samples were stored in plastic bottles and kept with buffered formalin 4%. In the laboratory, the identification was made through mounting of temporary and permanent slides (MÜLLER-MELCHERS; FERRANDO, 1956MÜLLER-MELCHERS, F. C.; FERRANDO, H. J. Técnicas para el estudio de las diatomáceas. Bol. Inst. Oceanogr., 1956. v. 7, n. 1/2, p. 151-160.), using the recommended bibliography. For classification and updating of the taxa the website AlgaeBase was used (http://www.algaebase.org).
The frequency of occurrence of taxa was calculated according to MATEUCCI and COLMA (1982)MATEUCCI, S. D.; COLMA, A. La metodologia para el estudo de la vegetaciónWashington: OEA, 1982. 168 p., using the ratio between the numbers of samples in which each taxon occurred, and the total number of samples tested. The following categories were obtained: very frequent (≥75%), frequent (<75% and ≥50%) occasional (<50% and ≥25%) and rare (<25%).
Chlorophyll A and microphytoplankton density
To obtain the chlorophyll data, two liters of water were collected in the surface and placed in matte vials and then they were vacuum filtered through glass-fiber filters Whatman GF/C (0.48µm pore size and 24 mm diameter). Two total filtrations and two fractionated filtrations were performed resulting in the largest and in the smallest fraction of the phytoplankton, microphytoplankton (>20µm) and nano/pico phytoplankton (<20µm), respectively. For the samples fractioning, it was used a screen with a mesh aperture of 20µm. The filtrate volume ranged from 0.10 to 0.25 liters. After drying, the filters were kept in a freezer (-18°C) until the extraction of the chlorophyll pigments using the method of spectrophotometry (UNESCO, 1966UNESCO. Determination of photosynthetic pigments in sea waterParis: UNESCO, 1966. 66 p.) and it was used the calculation suggested by STRICKLAND and PARSONS (1972)STRICKLAND, J. D. H.; PARSONS, T. R. A Practical Handbook of Seawater AnalysisOttawa: Fisheries Research Board of Canada, 1972..
The sampling for phytoplankton density followed the same methodology used to obtain chlorophyll. However, 250 mL aliquots in Lugol’s solution were kept until cell counting through the Utermöhl technique (FERRARIO et al., 1995FERRARIO, M.; SARS, E.; SALA, S. Metodologia básica para el estúdio de fitolancton com especial referencia a las diatomáceas. In: ALVEAR, K.; FERARIO, M. E.; OLIVEIRA FILHO, E. C.; SARS, E. (Eds.). Manual de métodos ficológicosConcepción: Universidad de Concepción, 1995. p. 1-24.). Based on the cell counts per liter, ecological diversity indexes were calculated (SHANNON; WIENNER, 1963SHANNON, C. E.; WEAVER, W. The Mathematical Theory of CommunicationUrbana: University Illinois Press, 1963. 117 p.) according to the classification to VALENTIN (2000)VALENTIN, J. L. Ecologia numérica: Uma introdução à análise multivariada de dados ecológicosRio de Janeiro: Interciência, 2000. 117 p., species richness (MARGALEF, 1958MARGALEF, R. Temporal succession and spatial heterogeneity in phytoplankton. In: BUZZATI-TRAVERSO, A. A. (Ed.). Perspectives in Marine BiologyBerkeley: University of California Press, 1958. p. 323-349.) and species evenness (PIELOU, 1966PIELOU, E. C. Species-diversity and pattern-diversity in the study of ecological succession. J. Theor. Biol., v. 10, n. 2, p. 370-383, 1966.).
Statistical analysis
PERMANOVA and Two-way ANOVA were performed in order to determine the significant differences (p<0.05) of the physical-chemical factors among seasonal periods and sampled sites. Principal Component Analysis was applied to standardized data in order to obtain the correlation among the environmental variables. To see the correlation of environmental variables among dominant species groups in the counting of cell per liter, it was used the Canonical Correspondence Analysis not rectified (CCA). Pearson’s Correlation Analysis was performed to correlate the phytoplankton density in relation to different physical-chemical factors analyzed. SIMPER analysis was used to determine the contribution of taxa between seasonal periods. All statistical analyzes were performed with the aid of the software STATISTIC 10.0 and Past 2.16.
RESULTS
Physical and chemical water parameters and meteorological characteristics
The characteristics of the estuarine waters of the Bacanga River are summarized in Table 1, with significant spatial and temporal differences of environmental variables according to the PERMANOVA analysis (F=6.51 and p=0.0001).
Statistic summary of environmental parameters collected from surface water in the estuary of the Bacanga River.
The pH (overall average 8.35±0.56; F=1.59, p>0.05) and the dissolved oxygen (6.06±0.56 mg L-1; F=0.65, p>0.05) showed no significant temporal and spatial differences. However, the salinity values (20.43±09.04; F=34.79, p<0.001) were significant regarding the seasonal and spatial variations, showing lower values in the rainy period and increasing in the downstream direction (Table 1).
Turbidity (10.99±4.79 NTU F=5.77, p<0.05) and TDS (19.00±6.26 mg L-1; F=5.51, p<0.05) were significant in the rainy season, whereas the water transparency (0.76±0.26 m; F=0.34; p>0.05) and depth (2.39±2.34 m; F=0.99; p>0.05) were significant only spatially. The water temperature (29.99±1.03ºC; F=0.13; p>0.05) was significant in regard to the spatial variation with higher values in section 5 (Table 1).
The concentrations of NO3- (0.13±0.81 µmol L-1; F=0.65, p>0.05) showed no significant spatio-temporal differences. However, the concentrations of NO2 - (0.47±0.36 µmol L-1; F=5.03, p<0.05) were significant considering the seasonal periods and the spatial variation, showing higher values in the dry season at site 3 (Table 1). The NH4+ ion (84.92±100.44 µmol L-1; F=5.56, p<0.05) was significant considering both seasonal and spatial variations, showing higher values in the dry season, specifically at the sites 2 and 4. The DIN showed an overall average of 100.44±85.58 µmol L-1 (F=5.66, p<0.05) and had the NH4 + as its main component, indicating a recent pollution of the environment.
TP rates (6.08±5.56 µmol L-1; F=1.08, p>0.05) were high all the year, and the concentrations of DIP (PO4-3) had an average of 26.53±46.73 µmol L-1(F=3.19, p>0.05). The SIO2- had an average of 66.24±86.37 µmol L-1 (F=3.70, p>0.05) showing no significant difference with DIN: DIS ratio (02.04±1.29 µmol L-1; F=2.88, p<0.05). The DIN: DIP molar ratio was lower than 16:1 in both seasonal periods, showing nitrogen limitation. However, the months of February (at the sites 5 and 6) showed high Redfield ratio, having the phosphate as the limiting factor.
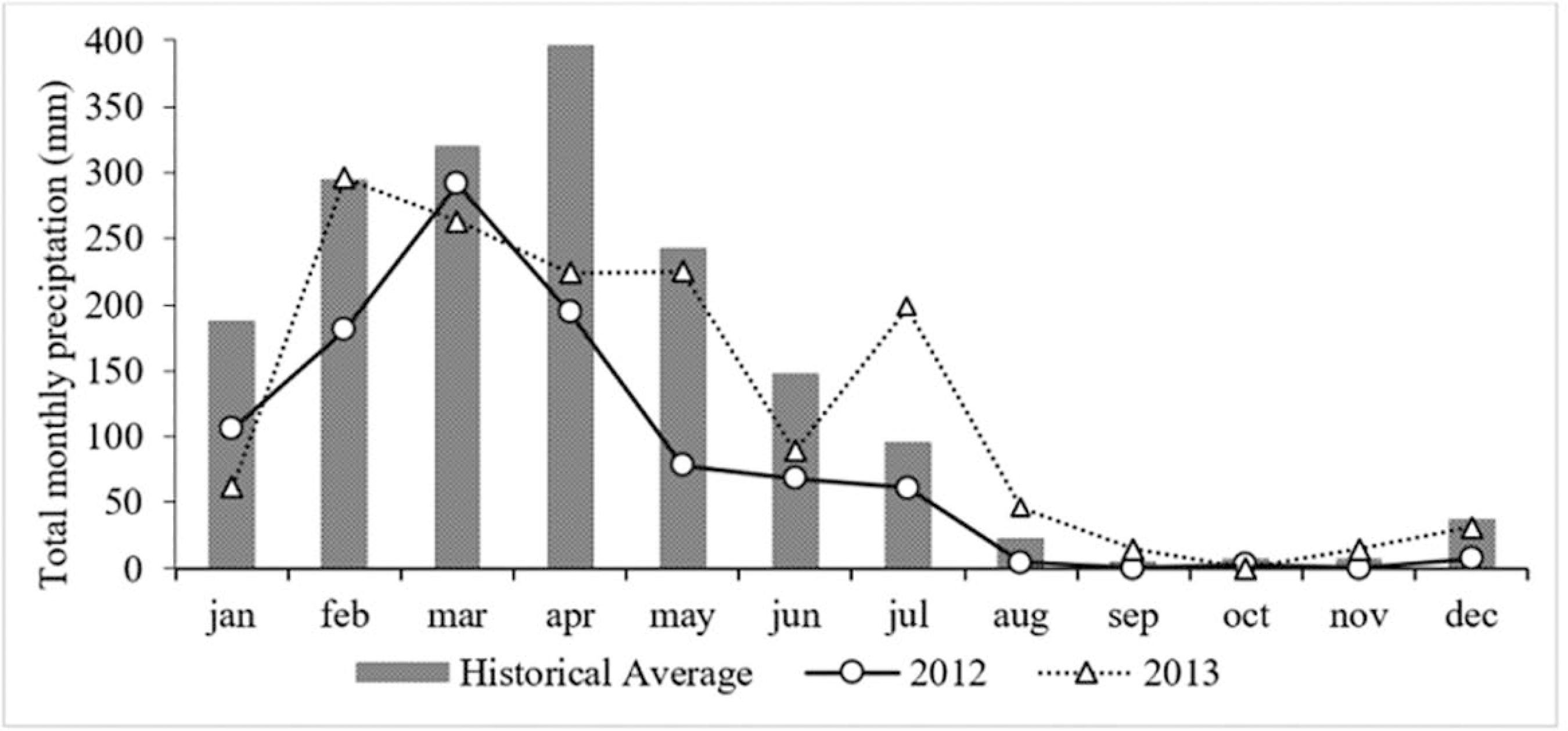
The historical mean rainfall (2003 - 2013) showed an average of 1790.43 mm, ranging from 995 mm in 2012 to 2536.10 mm in 2011. It was observed a seasonal cycle well defined with an intensification of the rainy season from January to July and April as the month that presents the highest rainfall events, and a dry period from August to December, in which September is the driest month. The heaviest rainfall occurred in February/2013 (296 mm) and the lowest in October/2012 (2.8) (Figure 2).
Historical mean (2003-2013) and total monthly rainfall during the study period (April 2012 to February 2013).
Phytoplankton composition
With the qualitative analysis of phytoplankton composition at the investigated area of the estuary of the Bacanga River, 133 phytoplankton species have been distributed in five divisions: Bacillariophyta (64.66%), Dinophyta (12.03%), Cyanobacteria (28.11%), Euglenophyta (27.8%) and Chlorophyta (3.76%) (Figure 3).
Seasonal variation of phytoplankton distribution by representative group in the estuary of the River Bacanga.
Regard the seasonal distribution, diatoms predominated in the rainy season with a percentage of 67.96% and in the dry season with 61.61%. Dinoflagellates were more representative in the dry season with 14.29% and cyanobacteria in the rainy season with 12.62% (Figure 3). The most representative class was Mediophyceae with 41 species, especially the Chaetocerotaceae family, in which Chaetoceros was the most expressive genus with 23 species. Within the dinoflagellate group, eight species of the genus Protoperidinium were identified. For the spatial distribution, the diatoms (site 1, 78.31%), dinoflagellates (site 2, 3.93%), and cyanobacteria (site 2, 16.79%) showed higher contribution at the sites closer to the dam; however, euglenophytes (site 4, 13.74%) and chlorophytes (site 5, 2.27%) at the sites further from the dam (Table 2).
Composition, taxonomic classification and occurrence of microphytoplankton identified in the estuary of the Bacanga River (1, 2, 3, 4, 5 and 6 sites).
In relation to the occurrence frequency, 81.20% of the species were classified as rare, 10.53% as occasional, 2.06% as frequent and only 2.26% as very frequent. Within this group, Gyrosigma balticum, Navicula sp. and Nitzschia sigma occur in 100% of the samples. As for seasonal variation, Oscillatoria principes occurred in 100% of the samples collected in the rainy season, whereas Coscinodiscus rothii and Protoperidinium sp1 occurred in 100% of the dry season samples.
Chlorophyll aand microphytoplankton density
Chlorophylla
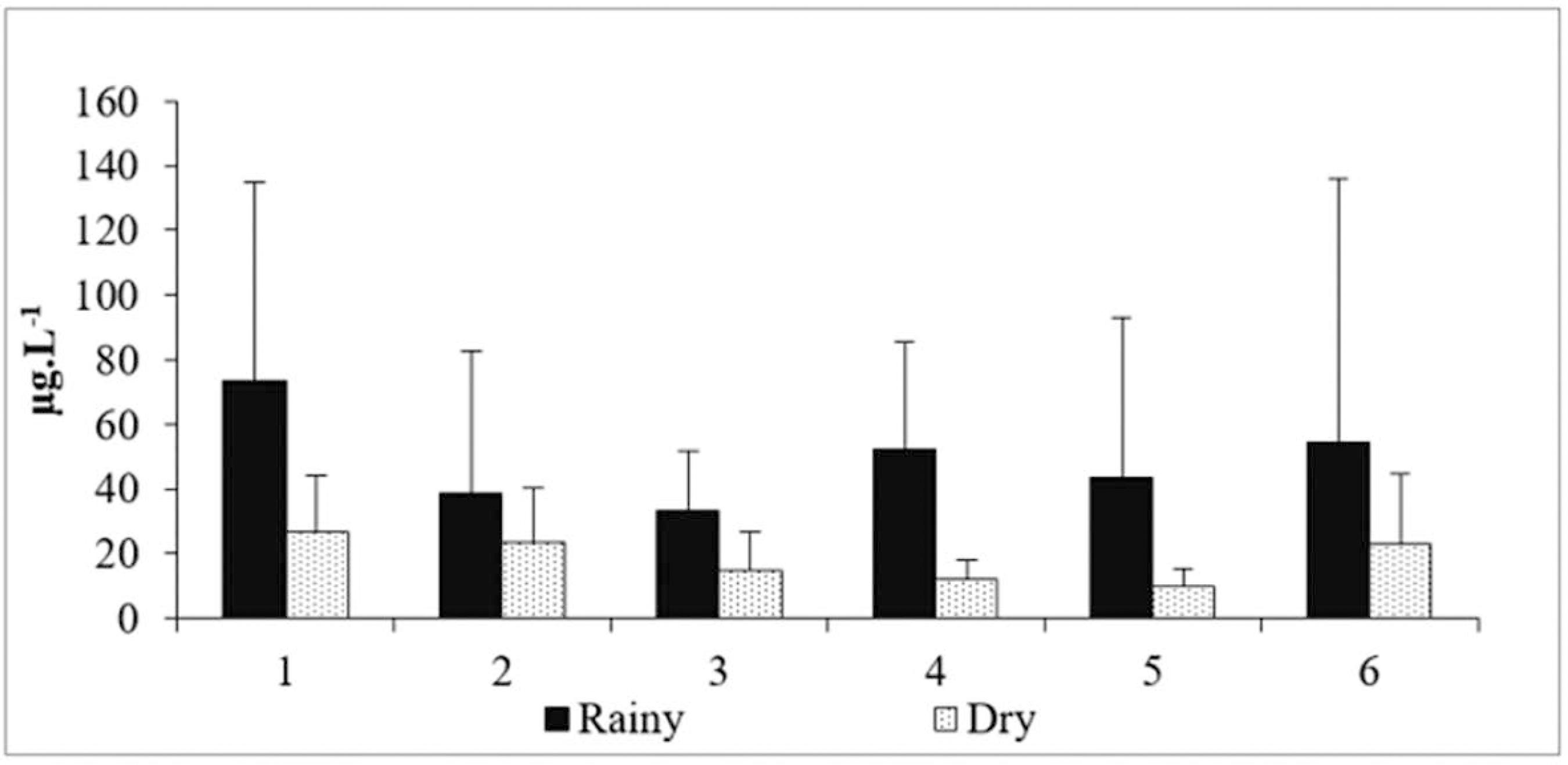
Chlorophyll a concentrations ranged from 10.06±5.44 µg L-1 (dry season - site 5) to 73.29±61.42 µg L-1 (rainy season - site 1) and it was more significant in the rainy season (F=5.99 and p<0.05) (Figure 4).
Spatial-temporal variation of the total chlorophyll concentrations in the estuary of the River Bacanga.
The microphytoplankton was the main contributor to the photosynthetic process in 47.22% of the samples, ranging from 1.69±0.16 at site 3 during the dry season, to 54.97±54.71 µg L-1 at site 1 during the rainy season with a higher level of significance (F=4.35 and p<0.05). However, the nano/pico phytoplankton (< 20µm) was not significant between seasonal periods and the sampled sites. It was considered the most representative portion of the sample 52.78%, with the lowest value of 7.67±5.40 µg L-1 in the dry season and the higher value of 41.60±51.18 µg L-1 (F=3.54 and p>0.05) in the rainy season, both at site 5 (Figure 5).
Spatial-temporal variation of chlorophyll concentrations (µg L-1) of microphytoplankton and nanopikophytoplankton in the estuary of the River Bacanga.
Density of microphytoplankton
The phytoplankton density was lower during the dry season (369.024±203.786 cell L-1) at site 3 and higher in the rainy season (15,671,680±27,039,949 cell L-1) at site 6 (F=1.47; p>0.05) (Figure 6), and in the rainy season occurred the major algal blooms.
Variation spatial-temporal density of phytoplankton and ecological index in the estuary of the River Bacanga.
Some algal bloom events were recorded over the study period. The increase in algae recorded at sites 1 and 2 was due to the specie Skeletonema costatum (814,775±1,968,950 cells L-1) in April and June 2012. While the proliferation of Euglena gracilis (19,144,334±7,523,378 cells L-1) and Chlamydomonas sp. (731,154±310,224 cells L-1) occurred at site 6 in February 2013. In the rainy season, the number of Thalassiosira sp. (F=5.12 and p=0.03) and dinoflagellates (F=4.10 and p=0.005) decreased significantly (Table 3).
Mean values of phytoplankton density (103 cells L- 1) in the estuary of the Bacanga River. (-) absent.
Regarding ecological indexes, the Bacanga River estuary showed a low species diversity (H’) with values ranging from 0.90±0.58 bits. cell-1 at site 6 during the dry season to 1.87±0.56 bits. cell-1 at site 5 in the rainy season. The richness was very low throughout the year, with the lowest value of 1.33±0.58 in the rainy season at site 6 and the highest value of 2.60±0.35 in the dry season at site 3. The evenness (J’) ranged from 0.37±0.31 (site 2) to 0.78±0.16 (site 5), both in the dry season (Figure 6). The moderate evenness is common to both seasonal periods, with no significant difference between rain and drought.
Statistical analysis
The PCA explained 55.46% of the total variance (Factor 1=21.15%, Factor 2=18.99% and Factor 3=15.31%) (Table 4) showing a seasonal scenario well defined in relation to environmental variables in factor 1, where the phytoplankton density (-0.68) and chlorophyll a (-0.69) were directly correlated with turbidity (-0.76) and dissolved oxygen (-0.60). Rainfall (0.67) was directly correlated with SiO2 - (0.81) and inversely correlated with salinity (-0.62) in the factor 2. The NH4+ (-0.92) and NID (-0.92) showed strong correlation in factor 3 (Figure 7).
Principal taxon contributing to the mean dissimilarities between rainy and dry seasons, as determined by the SIMPER analysis.
Principal components analysis performed on environmental parameters, where: Secchi - water transparence; TDS - total dissolved solids; Pluv - pluviosity; DO - dissolved oxygen concentrations; Den -density; TºC - water temperature; NO-3 - nitrate concentrations; NO-2 - nitrite concentrations; NH4 + - amnion concentrations; DIN - dissolved inorganic nitrogen concentrations; DIP - dissolved inorganic phosphorus concentrations; SiO2 - silicate concentrations; TP - total phosphorus concentrations; Chl-a - chlorophyll a concentrations.
The Canonical Correspondence Analysis (CCA) explained 80.72% of the relationships between species and environmental variables. The first axis (54.96%) showed that rain favors blooms of Skeletonema costatum at sites 1 and 2 and it was directly related to silicate concentrations, pH, and turbidity, causing an increase of chlorophyll concentrations. Also in the first axis, the proliferation of Euglena gracilis and Chlamydomonas sp. in February/2013 at sites 5 and 6, was strongly influenced by the high levels of total phosphorus that contributed to an increase in dissolved oxygen rates. The second axis 2 (26.45%) showed that the dinoflagellates, cyanobacteria and Thallassiosira sp. have a wide distribution in the dry season and are highly correlated with salinity, transparency of water and nutrients (DIP and DIN) (Figure 8).
Canonical correspondence analysis performed with environmental parameters and phytoplankton groups, where: Secchi - water transparence; Turb - Turbidity, Pluv - pluviosity, DO - Dissolved oxygen concentrations, DIN - dissolved inorganic nitrogen concentrations, DIP - dissolved inorganic phosphorus concentrations, SiO2 - silicate concentrations, TP - total phosphorus concentrations, Chl-a - chlorophyll a concentration, Sal - salinity, Dino - Dinoflagellates, Ciano - Cyanobacteria, Euglena - Euglena gracilis, Chlam - Chlamydomonas sp., S.costatum - Skeletonema costatum, Chaetoceros - Chaetoceros group and Thallsp - Thallassiosira sp.
The SIMPER analysis showed dissimilarity of 82.74% in the distribution of dominant groups of phytoplankton between rainy season and dry season. The species Skeletonema costatum (25.96%), Euglena gracilis (19.31%), Chaetoceros sp. (10.54%) and Chlamydomonas sp. (1.88%) were dominant in the rainy season. Whereas the dry season had Thalassiosira sp. (23.10%), the dinoflagellates group (15.90%) and cyanobacteria (3.30%) as the most representative (Table 5).
Eigenvalues, proportion of variation explained by each factor and variable loads of Principal Component Analysis (PCA).
Regarding the linear correlations, the phytoplankton density showed a strong positive correlation with NH4+ (0.80), rain (0.80), DIN (0.81) and NO3- (0.86), and the DIP showed strong correlation with the rain (0.98). The blooms of Skeletonema costatum (0.86), Euglena gracilis (0.83) and Chlamydomonas sp. (0.86) showed high correlation with NO3- concentrations. Thalassiosira sp. showed a strong relationship with water transparency (0.98), dinoflagellates (0.97) and cyanobacteria (0.82) groups were correlated with pH (Table 6).
DISCUSSION
Matrix of the Pearson correlation between environmental parameters and phytoplankton groups, where: Sec - water transparence; NTU - Turbidity, Pluv - pluviosity, DO - Dissolved oxygen concentrations, NO3 - nitrate concentrations, NO2 - nitrite concentrations, NH4 - amnion concentrations, DIN - dissolved inorganic nitrogen concentrations, DIP - dissolved inorganic phosphorus concentrations, Si - silicate concentrations, TP - total phosphorus concentrations, pH - pH, Chla - chlorophyll a concentration, Sal - salinity, Dino - Dinoflagellates, Cyan - Cyanobacteria, Eug- Euglena gracilis, Chlam - Chlamydomonas sp., S.cost - Skeletonema costatum, and Thall - Thallassiosira sp. Significant correlations are indicated in bold text.
These results show that environmental variations in the Bacanga River estuary are strongly affected by rainfall, which during the study showed a seasonal cycle well defined. However, it was atypical, according to the historical series (AZEVEDO et al., 2008AZEVEDO, A. C. G.; FEITOSA, F. A. N.; KOENING, M. L. Distribuição espacial e temporal da biomassa fitoplanctônica e variáveis ambientais no Golfão Maranhense, Brasil. Acta Bot. Bras., v. 22, n. 3, p. 870-877, 2008.). That is a typical scenario for the Amazon regimes (MONTEIRO et al., 2016MONTEIRO, M. C.; JIMÉNEZ, J. A.; PEREIRA, L. C. C. Natural and human controls of water quality of an Amazon estuary (Caeté-PA, Brazil). Ocean Coast. Manag., v. 124, p. 42-52, 2016.). The following diagram shows the interrelationships among those variables involved and the phytoplankton community, in which the rain was the enhancing agent of nutrient concentrations and the increase in local blooms.
These rainfall variations cause changes in the dynamics of the estuary such as reduced water transparency and salinity (GREGO et al., 2004GREGO, C. K. S.; FEITOSA, F.A. N.; HONORATO DA SILVA, M.; FLORES-MONTES, M. J. Distribuição Espacial e Sazonal da clorofila a fitoplanctônica e hidrologia do estuário do rio Timbó (Paulista - Pe). Trop. Oceanogr. Recife, v. 32, n. 2, p. 181-199, 2004.; MOSER et al., 2005MOSER, G. A. O.; GIANESELLA, S. M. F.; ALBA, J. J. B.; BÉRGAMO, A. L.; SALDANHA-CORRÊA, F. M. P.; MIRANDA, L. B.; HARARI, J. Instantaneous transport of salt, nutrients, suspended matter and chlorophyll-a in the tropical estuarine system of Santos. Rev. Bras. Oceanogr., v. 53, n. 3-4, p. 115-127, 2005.; LEÃO et al., 2008LEÃO, B. M.; PASSAVANTE, J. Z. O.; SILVA-CUNHA, M. G. G.; SANTIAGO, M. F. Ecologia do microfitoplâncton do estuário do rio Igarassu, PE, Brasil. Acta Bot. Bras., v. 22, n. 3, p. 711-722, 2008.; SANTIAGO et al., 2010SANTIAGO, M. F.; SILVA-CUNHA, M. G. G.; NEUMANN-LEITÃO, S.; COSTA, K. M. P.; BORGES, G. C. P.; PORTO NETO, F. F.; NUNES, F. S. Phytoplankton Dynamics in a Highly Eutrophic Estuary in Tropical Brazil. Braz. J. Oceanogr., v. 58, n. 3, p. 189-205, 2010.). The Bacanga River Estuary was characterized as a shallow system, low water transparency and high turbidity. According to CONLEY et al. (1993)CONLEY D, J.; SCHELSKE C, L.; STOERMER, E. F. Modification of the biogeochemical cycle of sílica with eutrophication. Mar. Ecol. Prog. Ser., v. 101, p. 179-192, 1993. and CLOERN (2001)CLOERN, J. E. Our evolving conceptual model of the coastal eutrophication problem. Mar. Ecol. Prog. Ser., v. 210, p. 223-253, 2001., the increasing of phytoplankton biomass contributes to reduce water transparency and decreased availability of light energy.
The average concentrations of dissolved oxygen, classifies the estuary area as a low saturation zone, according to MACÊDO and COSTA (1978)MACÊDO, S. J.; COSTA, K. M. P. Estudos ecológicos da região de Itamaracá. Pernambuco - Brasil. Condições Hidrológicas do Estuário do Rio Botafogo. Ciênc. Cult., v. 30, p. 346- 368, 1978.. However, in February/2013 oxygen super saturation zones were recorded at sites 5 and 6. These high concentrations of dissolved oxygen are related to the high chlorophyll rates (LU; GAN, 2015LU, Z.; GAN, J. Controls of seasonal variability of phytoplankton blooms in the Pearl River Estuary. Deep Sea Res Part II: Top Stud Oceanogr., v.117, p. 86-96, 2015.). According to GUENTHER et al. (2015)GUENTHER, M.; ARAÚJO, M.; FLORES-MONTES, M. J.; GONZALEZ-RODRIGUEZ, E.; NEUMANN-LEITÃO, S. Eutrophication effects on phytoplankton size-fractioned biomass and production at a tropical estuary. Mar. Pollut. Bull, v. 91, p. 537-547, 2015., this scenario is common to restricted circulation environments and to the high primary production rates, exceeding the depletion of oxygen by heterotrophic organisms.
The pH is alkaline, showing a reduction in the water quality. However, it is within the limits for marine life (NASCIMENTO et al., 2003NASCIMENTO, F. C. R.; MUNIZ, K.; FEITOSA, F. A. N.; ARAÚJO, J. P.; SILVA, R. M. S.; SILVA, G. S.; FLORES-MONTES, M. J. Disponibilidade nutricional da Bacia do Pina e rio Tejipió (Recife-PE-Brasil) em relação aos nutrientes e biomassa primária (setembro/2000). Trop. Oceanogr., v. 31, n. 2, p. 149-169, 2003.). The renewal time of Bacanga River Estuary waters depends on the seasonality. During the dry season, the gates remain closed most of the time. This residence time of freshwater in estuaries generates stabilization of the water column, and it is essential to add nutrients and allow faster growth of phytoplankton (ODEBRECHT et al., 2015ODEBRECH, C.; ABREU, P. C.; CARSTENSEN, J. Retention time generates short-term phytoplankton blooms in a shallow microtidal subtropical estuary. Estuar. Coast. Shelf Sci., v. 162, p. 35-44, 2015.).
The water temperature is stable in both seasons, which is common in tropical estuaries (MOSER et al., 2005MOSER, G. A. O.; GIANESELLA, S. M. F.; ALBA, J. J. B.; BÉRGAMO, A. L.; SALDANHA-CORRÊA, F. M. P.; MIRANDA, L. B.; HARARI, J. Instantaneous transport of salt, nutrients, suspended matter and chlorophyll-a in the tropical estuarine system of Santos. Rev. Bras. Oceanogr., v. 53, n. 3-4, p. 115-127, 2005.; AZEVEDO et al., 2008AZEVEDO, A. C. G.; FEITOSA, F. A. N.; KOENING, M. L. Distribuição espacial e temporal da biomassa fitoplanctônica e variáveis ambientais no Golfão Maranhense, Brasil. Acta Bot. Bras., v. 22, n. 3, p. 870-877, 2008.; RODRIGUES; CUTRIM, 2010RODRIGUES, E. I.; CUTRIM, M. V. J. Relações entre as variáveis físicas, químicas e fitoplanctônicas de três áreas estuarinas da costa Norte do Brasil - São José de Ribamar, Cedral e Cajapió, MA. Arq. Ciênc. Mar. Fortaleza, v. 43, n. 2, p. 45-54, 2010.). The rain influences directly the salinity, which showed values significantly lower during the months of April and June, and it causes an increased salinity gradient toward the downstream, similar to that registered by PAMPLONA et al. (2013)PAMPLONA, F. C.; PAES, E. T.; NEPOMUCENO A. Nutrient fluctuations in the Quatipuru river: A macrotidal estuarine mangrove system in the Brazilian Amazonian basin. Estuar. Coast. Shelf Sci., v. 133, p. 273-284, 2013. in the Amazon estuary. The blooms of Skeletonema costatum, Euglena gracilis and Chlamydomonas sp occur when there is an intensification of the rainfall (LEÃO et al., 2008LEÃO, B. M.; PASSAVANTE, J. Z. O.; SILVA-CUNHA, M. G. G.; SANTIAGO, M. F. Ecologia do microfitoplâncton do estuário do rio Igarassu, PE, Brasil. Acta Bot. Bras., v. 22, n. 3, p. 711-722, 2008.; BAZIN et al., 2014BAZIN, P.; JOUENNE, F.; FRIEDL, T.; DETON-CABANILLAS, A. F.; LE ROY, B.; VÉRON, B. Phytoplankton Diversity and Community Composition along the Estuarine Gradient of a Temperate Macrotidal Ecosystem: Combined Morphological and Molecular Approaches. PloS ONE, v. 9, n. 4, p. e94110, 2014.; JENDYK et al., 2014JENDYK, J.; HEMRAJ, D. A.; BROWN, M. H.; ELLIS, A. V.; LETERME, S. C. Environmental variability and phytoplankton dynamics in a South Australian inverse estuary. Cont. Shelf Res., v. 91, p. 134-144, 2014.).
The combination of high nutrient concentrations may limit the growth of phytoplankton on a short time scale (WU; CHU, 2003WU, J. T.; CHOU, T. L. Silicate as the limiting nutrient for phytoplankton in a subtropical eutrophic estuary of Taiwan. Estuar. Coast. Shelf Sci., v. 58, n. 1, p. 155-162, 2003.) and the N: P ratio has been related to the occurrence of algal blooms in coastal waters (SOUZA et al., 2014SOUZA, M. M.; GASTALDINI, M. C. C. Avaliação da qualidade da água em bacias hidrográficas com diferentes impactos antrópicos. Eng. Sanit. Ambient., v. 19, n. 3, p. 263-274, 2014.). Based on that, the nitrogen was shown to be a limiting factor for the diatoms blooms and the phosphorus was a limiting factor for the proliferation of green algae and euglenoids. According to SIN et al. (2015)SIN, Y.; LEE, E.; LEE, Y.; SHIN, K. H. The river-estuarine continuum of nutrients and phytoplankton communities in an estuary physically divided by a sea dike. Estuar. Coast. Shelf Sci., v. 163, Pt. B, p. 279-289, 2015. and SEGURO et al. (2015)SEGURO, I.; GARCÍA, C. M.; PAPASPYROU, S.; GÁLVEZ, J. A.; GARCÍA-ROBLEDO, E.; NAVARRO, G.; SORIA-PÍRIZ, S.; AGUILAR, V.; LIZANO, O. G.; MORALES-RAMÍREZ, A.; CORZO, A. Seasonal changes of the microplankton community along a tropical estuary. Reg. Stud. Mar. Sci., v. 2, p. 189-202, 2015. it is common the nitrogen being the main limiting factor for marine phytoplankton and the phosphorus for continental phytoplankton. According to RODRIGUES and CUTRIM (2010)RODRIGUES, E. I.; CUTRIM, M. V. J. Relações entre as variáveis físicas, químicas e fitoplanctônicas de três áreas estuarinas da costa Norte do Brasil - São José de Ribamar, Cedral e Cajapió, MA. Arq. Ciênc. Mar. Fortaleza, v. 43, n. 2, p. 45-54, 2010., phosphorus is usually the main nutrient for microphytoplankton in the estuaries of Maranhão, suggesting that this community is physiologically well adapted to the conditions of nutrients mixing.
This scenario shows a distribution of the phytoplankton community well-defined seasonally. However, it does not happen spatially, once the algal bloom was potentiated by high concentrations of nutrients. The entry of nutrients can occur naturally through runoff, or through the discharge of domestic sewage (THOMAS et al., 2005THOMAS, H.; BOZEC, Y.; ELKALAY, K.; BAAR, H. J. W.; BORGES, A. V.; SCHIETTECATTE, L. S. Controls of the surface water partial pressure of CO2 in the North Sea. Biogeosciences, v. 2, n. 4, p. 323-334, 2005.; PAERL et al., 2010PAERL, H. W.; ROSSIGNOL, K. L.; HALL, N. S.; PEIERLS, B. L.; WETZ, M. S. Phytoplankton Community Indicators of Short- and Long-term Ecological Change in the Anthropogenically and Climatically Impacted Neuse River Estuary, North Carolina, USA. Estuar. Coast., v. 33, n. 2, p. 485-497, 2010.; SOUZA et al., 2014SOUZA, M. M.; GASTALDINI, M. C. C. Avaliação da qualidade da água em bacias hidrográficas com diferentes impactos antrópicos. Eng. Sanit. Ambient., v. 19, n. 3, p. 263-274, 2014.), and when dominants (ammonium and phosphorus) the N:P ratio is less than the Redfield ratio 16:1 (COELHO et al., 2015COELHO, S.; PÉREZ-RUZAFA, A.; GAMITO S. Phytoplankton community dynamics in an intermittently open hypereutrophic coastal lagoon in southern Portugal. Estuar. Coast. Shelf Sci., v. 167, Pt. A, p. 102-112, 2015.; SILVA et al., 2015SILVA, M. A. M.; SOUZA, M. F. L.; ABREU, P. C. Spatial and temporal variation of dissolved inorganic nutrients, and chlorophyll-a in a tropical estuary in northeastern Brazil: Dynamics of nutrient removal. Braz. J. Oceanogr., v. 63, n. 1, p. 1-15, 2015.).
Based on that, the seasonal rainfall in tropical areas produce fluctuations in salinity, nutrient concentrations, turbidity and biological productivity (SANTIAGO et al., 2010SANTIAGO, M. F.; SILVA-CUNHA, M. G. G.; NEUMANN-LEITÃO, S.; COSTA, K. M. P.; BORGES, G. C. P.; PORTO NETO, F. F.; NUNES, F. S. Phytoplankton Dynamics in a Highly Eutrophic Estuary in Tropical Brazil. Braz. J. Oceanogr., v. 58, n. 3, p. 189-205, 2010.) and the ammonium was the main inorganic form of DIN in the Bacanga River Estuary, corroborating with the data of MOSER et al. (2005)MOSER, G. A. O.; GIANESELLA, S. M. F.; ALBA, J. J. B.; BÉRGAMO, A. L.; SALDANHA-CORRÊA, F. M. P.; MIRANDA, L. B.; HARARI, J. Instantaneous transport of salt, nutrients, suspended matter and chlorophyll-a in the tropical estuarine system of Santos. Rev. Bras. Oceanogr., v. 53, n. 3-4, p. 115-127, 2005.; PAMPLONA et al. (2013)PAMPLONA, F. C.; PAES, E. T.; NEPOMUCENO A. Nutrient fluctuations in the Quatipuru river: A macrotidal estuarine mangrove system in the Brazilian Amazonian basin. Estuar. Coast. Shelf Sci., v. 133, p. 273-284, 2013. and SILVA et al. (2015)SILVA, M. A. M.; SOUZA, M. F. L.; ABREU, P. C. Spatial and temporal variation of dissolved inorganic nutrients, and chlorophyll-a in a tropical estuary in northeastern Brazil: Dynamics of nutrient removal. Braz. J. Oceanogr., v. 63, n. 1, p. 1-15, 2015..
According to WU and CHOU (2003)WU, J. T.; CHOU, T. L. Silicate as the limiting nutrient for phytoplankton in a subtropical eutrophic estuary of Taiwan. Estuar. Coast. Shelf Sci., v. 58, n. 1, p. 155-162, 2003. and SIN et al. (2015)SIN, Y.; LEE, E.; LEE, Y.; SHIN, K. H. The river-estuarine continuum of nutrients and phytoplankton communities in an estuary physically divided by a sea dike. Estuar. Coast. Shelf Sci., v. 163, Pt. B, p. 279-289, 2015., high concentrations of nitrogen in the environment may reduce productivity in eutrophic estuaries due to the increase of urban pollution.
Regarding the microphytoplankton composition, diatoms were the most representative group, followed by dinoflagellates (SEGURO et al., 2015SEGURO, I.; GARCÍA, C. M.; PAPASPYROU, S.; GÁLVEZ, J. A.; GARCÍA-ROBLEDO, E.; NAVARRO, G.; SORIA-PÍRIZ, S.; AGUILAR, V.; LIZANO, O. G.; MORALES-RAMÍREZ, A.; CORZO, A. Seasonal changes of the microplankton community along a tropical estuary. Reg. Stud. Mar. Sci., v. 2, p. 189-202, 2015.), which are transported by tidal currents, and are more frequent in the dry season (PAIVA et al., 2006PAIVA, R. S.; ESKINAZI-LEÇA, E.; PASSAVANTE, J. Z. O.; SILVA-CUNHA, M. G. G.; MELO, N.F.A.C. Considerações ecológicas sobre o fitoplâncton da baía do Guajará e foz do rio Guamá, Pará, Brasil. Bol. Mus. Para. Emílio Goeldi. Ciênc. Natu., v. 1, n. 2, p. 133-146, 2006.). The dominance of diatoms is common in estuarine systems due to their euryhaline nature (SENA et al., 2015SENA, B. A.; COSTA, V. B.; NAKAYAMA, L.; ROCHA, R. M. Composition of microphytoplankton of an estuarine Amazon River, Pará, Brazil. Biot. Amaz., v. 5, n. 2, p. 1-9, 2015.) and it is correlated directly with the silicate content, since the major source of silica is from the continent and the diatoms frustules have silica in their composition. In estuaries of Maranhão, the predominance of that group occurs in over 80% of the phytoplankton composition (FERREIRA-CORREIA et al., 2004FERREIRA-CORREIA, M. M.; ALMEIDA, I. C. S.; DOURADO, E. C. S. Microalgas da Baía de Turiaçu, APA das Reentrâncias Maranhenses-Uma abordagem qualitativa. Bol. Lab. Hidrobiol., v.17, n. 1, 2004.; RODRIGUES; CUTRIM, 2010RODRIGUES, E. I.; CUTRIM, M. V. J. Relações entre as variáveis físicas, químicas e fitoplanctônicas de três áreas estuarinas da costa Norte do Brasil - São José de Ribamar, Cedral e Cajapió, MA. Arq. Ciênc. Mar. Fortaleza, v. 43, n. 2, p. 45-54, 2010.) and when eutrophication happens, blooms of diatoms become more frequent due to the wide availability of nutrients and high salinity (MATOS et al., 2011MATOS, J. B., SODRÉ, D. K. L., COSTA, K. G.; PEREIRA, L. C. C.; COSTA, R. M. Spatial and temporal variation in the composition and biomass of phytoplankton in an Amazonian estuary. J. Coast. Res. SI 64, p. 1525-1529, 2011.). Under these conditions, the local phytoplankton community consists mostly of centric taxa classified as rare and occasional. That is due to the high hydrodynamic environment that can reach areas far from the mouth, that are considered lentic zones (PAIVA et al., 2006PAIVA, R. S.; ESKINAZI-LEÇA, E.; PASSAVANTE, J. Z. O.; SILVA-CUNHA, M. G. G.; MELO, N.F.A.C. Considerações ecológicas sobre o fitoplâncton da baía do Guajará e foz do rio Guamá, Pará, Brasil. Bol. Mus. Para. Emílio Goeldi. Ciênc. Natu., v. 1, n. 2, p. 133-146, 2006.; SOUSA et al., 2008SOUSA, E. B.; COSTA, V. B.; PEREIRA, L. C. C.; COSTA, R. M. Microfitoplâncton de águas costeiras amazônicas: Ilha Canela (Bragança, PA, Brasil). Acta Bot. Bras., v. 22, n. 3, p. 626-636, 2008.; REZENDE et al., 2015REZENDE, K. R.V.; HATHERLY, M. M. F.; PIMENTA, C. M. M.; EDUARDO, J.; VIANNA, S. C.; MANGIAVACCHI, N. Phytoplankton community structure in one sector of Guanabara Bay (RJ, Brazil) during 2011 and 2012. Braz. J. Oceanogr., v. 63, n. 3, p. 239-254, 2015.; SENA et al., 2015SENA, B. A.; COSTA, V. B.; NAKAYAMA, L.; ROCHA, R. M. Composition of microphytoplankton of an estuarine Amazon River, Pará, Brazil. Biot. Amaz., v. 5, n. 2, p. 1-9, 2015.).
Thalassiosira sp. was the most representative species in the dry season, and it is related to the marine influence. In estuarine ecosystems it is common the events of biomass production by the microphytoplankton, specifically diatoms, which are highly adapted to rapid population growth opportunities in shallow, turbulent, turbid waters and in waters enriched by nutrients (CARSTENSEN et al., 2015CARSTENSEN, J.; KLAIS R.; CLOERN, J. E. Phytoplankton blooms in estuarine and coastal waters: Seasonal patterns and key species. Estuar. Coast. Shelf Sci., v. 162, p. 98-109, 2015.).
Among the main representatives of phytoplankton blooms, the Skeletonema costatum is evident. It is a cosmopolitan and euryhaline species, one of the most abundant in estuaries, considered opportunistic in eutrophic environments (VILLAC; TENEBAUM, 2010VILLAC, M. C.; TENENBAUM, D. R. The phytoplankton of Guanabara Bay, Brazil. I. Historical account of its biodiversity. Biota Neotrop, v. 10, n. 2, p. 271-293, 2010.; MASUDA et al., 2011MASUDA, L. S. M.; MOSER, G. A. O.; BARRERA-ALBA, J. J. Variação temporal do fitoplâncton no canal estuarino de Santos (sp). Braz. J. Aquat. Sci. Technol., v. 15, n. 1, p. 79-93, 2011.; REZENDE et al., 2015REZENDE, K. R.V.; HATHERLY, M. M. F.; PIMENTA, C. M. M.; EDUARDO, J.; VIANNA, S. C.; MANGIAVACCHI, N. Phytoplankton community structure in one sector of Guanabara Bay (RJ, Brazil) during 2011 and 2012. Braz. J. Oceanogr., v. 63, n. 3, p. 239-254, 2015.; BARRERA-ALBA et al., 2016BARRERA-ALBA, J. J.; MOSER G. A. O. Short-term response of phytoplankton community to over-enrichment of nutrients in a well-preserved sub-tropical estuary. Braz. J. Oceanogr, v. 64, n. 2, p. 191-196, 2016.). Skeletonema costatum is classified as a harmful species and it is associated with toxic microorganisms in some cases, causing economic losses for aquaculture (KHAN et al., 1998KHAN, S.; HAQUE, M. M.; ARAKAWA, O.; ONOUE, Y. Physiological observations on a diatom Skeletonema costatum (Greville) Cleve. Bangladesh J. Fish. Res., v. 2, n. 2, p. 109-118, 1998.; UNESCO, 2004UNESCO; HALLEGRAEFF, G. M.; ANDERSON, D. M.; CEMBELLA, A. D. (eds). Manual on Harmful Marine MicroalgaeParis: UNESCO , 2004. 793 p.).
The euglenoids occurred in low salinity and high total phosphorus concentrations during the rainy season (LEÃO et al., 2008LEÃO, B. M.; PASSAVANTE, J. Z. O.; SILVA-CUNHA, M. G. G.; SANTIAGO, M. F. Ecologia do microfitoplâncton do estuário do rio Igarassu, PE, Brasil. Acta Bot. Bras., v. 22, n. 3, p. 711-722, 2008.; HONORATO DA SILVA et al., 2009HONORATO DA SILVA, M.; SILVA-CUNHA, M. G. G.; PASSAVANTE, J. Z. O.; GREGO, C. K. S.; MUNIZ, K. Estrutura sazonal e espacial do microfitoplâncton no estuário tropical do rio Formoso, PE, Brasil. Acta Bot. Bras., v. 23, n. 2, p. 355-368, 2009.). Chlamydomonas sp. became dominant also on these same conditions (BAZIN et al., 2014BAZIN, P.; JOUENNE, F.; FRIEDL, T.; DETON-CABANILLAS, A. F.; LE ROY, B.; VÉRON, B. Phytoplankton Diversity and Community Composition along the Estuarine Gradient of a Temperate Macrotidal Ecosystem: Combined Morphological and Molecular Approaches. PloS ONE, v. 9, n. 4, p. e94110, 2014.; JENDYK et al., 2014JENDYK, J.; HEMRAJ, D. A.; BROWN, M. H.; ELLIS, A. V.; LETERME, S. C. Environmental variability and phytoplankton dynamics in a South Australian inverse estuary. Cont. Shelf Res., v. 91, p. 134-144, 2014..). This species tolerates a wide range of salinity. However, the highest numbers occur in low salinities, showing that there are other factors influencing the presence of this genus (NCHE-FAMBO et al., 2015NCHE-FAMBO, F. A.; SCHARLER, U. M.; TIROK, K. Resilience of estuarine phytoplankton and their temporal variability along salinity gradients during drought and hypersalinity. Estuar. Coast. Shelf Sci., v. 158, p. 40-52, 2015.).
Coastal estuarine waters are mainly composed of high density of diatoms and dinoflagellates, and green algae and cyanobacteria blooms are restricted to low salinity habitats. These blooms generate important ecological and biochemical implications for the food web and nutrient cycling (CARSTENSEN et al., 2015CARSTENSEN, J.; KLAIS R.; CLOERN, J. E. Phytoplankton blooms in estuarine and coastal waters: Seasonal patterns and key species. Estuar. Coast. Shelf Sci., v. 162, p. 98-109, 2015.).
Based on the chlorophyll concentrations the estuary was classified as hypereutrophic, corroborating with SANTOS et al. (2009)SANTOS, T. G.; BEZERRA-JUNIOR, J. L.; COSTA, K. M. P.; FEITOSA, F. A. N. Dinâmica da Biomassa Fitoplanctônica e variáveis ambientais em um estuário tropical (Bacia do Pina, Recife, Pe). Rev. Bras. Enga. Pesca, v. 4, n. 1, p. 95-109, 2009., SILVA et al. (2014)SILVA, G. S.; SANTOS, E. A.; CORRÊA, L. B.; MARQUES, A. L. B.; MARQUES, E. P.; SOUSA, E. R.; SILVA, G. S. Avaliação integrada da qualidade de águas superficiais: grau de trofia e proteção da vida aquática nos rios Anil e Bacanga, São Luís (MA). Eng. Sanit. Ambient., v. 19, p. 245-250, 2014., COTOVICZ JR. et al. (2012)COTOVICZ JR., L. C.; BRANDINI, N.; KNOPPERS, B. A.; SOUZA, W. F. L.; MEDEIROS, P. R. P. Comparação de Modelos e Índices para Avaliação do Estado Trófico do Complexo Estuarino-Lagunar Mundaú-Manguaba, (AL). Geochim Brasiliensis, v. 26, n. 1, p. 7-18, 2012. in which the higher values of the pigment were associated with the period of highest rainfall events, unlike TEIXEIRA et al. (1988)TEIXEIRA, C.; ARANHA, F. J.; BARBIERI, R.; MELO, O. T. Produção primária e clorofila a do fitoplâncton e parâmetros físicos e químicos do estreito dos Coqueiros, Maranhão. Brasil. Rev. Bras. Biol., v. 48, n. 1, p. 29-39, 1988. and AZEVEDO et al. (2008)AZEVEDO, A. C. G.; FEITOSA, F. A. N.; KOENING, M. L. Distribuição espacial e temporal da biomassa fitoplanctônica e variáveis ambientais no Golfão Maranhense, Brasil. Acta Bot. Bras., v. 22, n. 3, p. 870-877, 2008. in their studies in other estuaries.
Thus, the Bacanga River estuary showed a rapid growth of microphytoplankton due to the discharge of domestic sewage and consequently affected the environmental changes, which conducted to ecological instability scenarios especially in the rainy season, defined by low diversity and species richness. According to VALENTIN et al. (1999)VALENTIN, J. L.; TENENBAUM, D. R.; BONECKER, A. C. T.; BONECKER, S. L. C.; NOGUEIRA, C. R.; VILLAC, M. C. O. Sistema planctônico da Baía de Guanabara: síntese do conhecimento. In: SILVA, S. H. G.; LAVRADO, H. P. (Eds.). Ecologia de Ambientes Costeiros do Estado do Rio de JaneiroSérie Oecologia Brasilienses. Rio de Janeiro: PPGE-UFRJ, 1999. p. 35-59., that is a response to the intense eutrophication process in large and sporadic nutrients pulsing favoring the dominance of larger cells. However, when the photosynthetic efficiencies are compared, the nano/pico phytoplankton are superior and the synthesis is always of large scale (RODRIGUES; CUTRIM, 2010RODRIGUES, E. I.; CUTRIM, M. V. J. Relações entre as variáveis físicas, químicas e fitoplanctônicas de três áreas estuarinas da costa Norte do Brasil - São José de Ribamar, Cedral e Cajapió, MA. Arq. Ciênc. Mar. Fortaleza, v. 43, n. 2, p. 45-54, 2010.; GUENTHER et al., 2012GUENTHER, M.; LIMA, I.; MUGRABE, G.; TENENBAUM, D. R.; GONZALEZ-RODRIGUEZ, E.; VALENTIN, J. L. Small time scale plankton structure variations at the entrance of a tropical eutrophic bay (Guanabara Bay, Brazil). Braz. J. Oceanogr., v. 60, n. 4, p. 405-414, 2012.).
CONCLUSION
The results confirm that the distribution of phytoplankton community is more defined seasonally, rather than spatially. Therefore, the rain is the factor that intensifies the concentrations of nitrogen compounds and phosphate, which favors blooms at different periods. During the rainy season, the Skeletonema costatum proliferates using nitrogen, whereas Euglena gracilis and Chlamydomonas sp. proliferates using phosphate. The Thalassiosira sp. is the dominant species in the dry season, which is the period when the residence time in the estuary is higher and domestic effluents are concentrated in the form of ammonium. The environmental impact on the community is evidenced by the low diversity, species evenness and species richness with high chlorophyll concentrations determined by the microphytoplankton. Thus, the eutrophication process promotes algal blooms events that affect the quality of the water and show ecological instability scenarios. This study contributes to a better understanding of the microphytoplankton and suggests the control of the local eutrophication as a mitigation measure for these blooms pulsing. In addition, this study can be used as a model for the characterization and monitoring of the Bacanga River Estuary.
ACKNOWLEDGEMENTS
We thank the grant given by FINEP (01.10.0714-00 CT-HIDRO) and the Master’s scholarship granted to the first author by CAPES (Coordination for the Improvement of Higher Education Personnel).
REFERENCES
- ANDRADE, A. A. S.; CASTRO, A. C. L. Evaluation of the management plan of the Bacanga State Park, island of Maranhão, northeast Brazil. Bol. Lab. Hidrobiol., v. 20, n. 1, p. 61-68, 2007.
- AMERICAN PUBLIC HEALTH ASSOCIATION (APHA). Standard Methods For The Examination Of Water And Wastewater 22nd ed. Washington: American Public Health Association, 2012.
- AZEVEDO, A. C. G.; FEITOSA, F. A. N.; KOENING, M. L. Distribuição espacial e temporal da biomassa fitoplanctônica e variáveis ambientais no Golfão Maranhense, Brasil. Acta Bot. Bras., v. 22, n. 3, p. 870-877, 2008.
- BARRERA-ALBA, J. J.; MOSER G. A. O. Short-term response of phytoplankton community to over-enrichment of nutrients in a well-preserved sub-tropical estuary. Braz. J. Oceanogr, v. 64, n. 2, p. 191-196, 2016.
- BAZIN, P.; JOUENNE, F.; FRIEDL, T.; DETON-CABANILLAS, A. F.; LE ROY, B.; VÉRON, B. Phytoplankton Diversity and Community Composition along the Estuarine Gradient of a Temperate Macrotidal Ecosystem: Combined Morphological and Molecular Approaches. PloS ONE, v. 9, n. 4, p. e94110, 2014.
- BEZERRA, P. S. S.; TAKIYAMA, L. R.; BEZERRA, C. W. B. Complexação de íons de metais por matéria orgânica dissolvida: modelagem e aplicação em sistemas reais. Acta Amaz., v. 39, n. 3, p. 639-648, 2009.
- CALLAWAY, R.; GRENFELL, S.; LØNBORG, C. Small estuaries: Ecology, Environmental drivers and management challenges. Estuar. Coast. Shelf Sci. , v. 150, p. 193-195, 2014.
- CAREY, R. O.; HOCHMUTH, G. J.; CHRISTOPHER, J.; MARTINEZ, C. J. M.; BOYER, T. H.; DUKES, M. D.; TOOR, G. S.; CISAR, J. L. Evaluating nutrient impacts in urban watersheds: challenges and research opportunities. Environ. Pollu., v. 173, p. 138-149, 2013.
- CARSTENSEN, J.; KLAIS R.; CLOERN, J. E. Phytoplankton blooms in estuarine and coastal waters: Seasonal patterns and key species. Estuar. Coast. Shelf Sci., v. 162, p. 98-109, 2015.
- CARVALHO, G. P.; CAVALCANTE, P. R. S.; CASTRO, A. C. L.; ROJAS, M. O. A. I. Preliminary assessment of heavy metal levels in Mytella falcata (Bivalvia, Mytilidae) from Bacanga River estuary, São Luis, State of Maranhão, Northeastern Brazil. Rev. Bras. Biol., v. 60, n. 1, p. 11-16, 2000.
- CLOERN, J. E. Our evolving conceptual model of the coastal eutrophication problem. Mar. Ecol. Prog. Ser., v. 210, p. 223-253, 2001.
- CLOERN, J. E.; FOSTER, S. Q.; KLECKNER, A. E. Phytoplankton primary production in the world’s estuarine-coastal ecosystems. Biogeosciences, v. 11, p. 2477-2501, 2014.
- COELHO, C. J. C.; DAMÁZIO, E. Aspectos da Disponibilidade e dos Usos da Água na Bacia do Rio Bacanga/Ilha do Maranhão (I. DE SÃO LUÍS) - MA. Bol. Lab. Hidrobiol., v. 19, n. 1, p. 73-84, 2006.
- COELHO, S.; PÉREZ-RUZAFA, A.; GAMITO S. Phytoplankton community dynamics in an intermittently open hypereutrophic coastal lagoon in southern Portugal. Estuar. Coast. Shelf Sci., v. 167, Pt. A, p. 102-112, 2015.
- CONLEY D, J.; SCHELSKE C, L.; STOERMER, E. F. Modification of the biogeochemical cycle of sílica with eutrophication. Mar. Ecol. Prog. Ser., v. 101, p. 179-192, 1993.
- COTOVICZ JR., L. C.; BRANDINI, N.; KNOPPERS, B. A.; SOUZA, W. F. L.; MEDEIROS, P. R. P. Comparação de Modelos e Índices para Avaliação do Estado Trófico do Complexo Estuarino-Lagunar Mundaú-Manguaba, (AL). Geochim Brasiliensis, v. 26, n. 1, p. 7-18, 2012.
- FERRARIO, M.; SARS, E.; SALA, S. Metodologia básica para el estúdio de fitolancton com especial referencia a las diatomáceas. In: ALVEAR, K.; FERARIO, M. E.; OLIVEIRA FILHO, E. C.; SARS, E. (Eds.). Manual de métodos ficológicosConcepción: Universidad de Concepción, 1995. p. 1-24.
- FERREIRA-CORREIA, M. M.; ALMEIDA, I. C. S.; DOURADO, E. C. S. Microalgas da Baía de Turiaçu, APA das Reentrâncias Maranhenses-Uma abordagem qualitativa. Bol. Lab. Hidrobiol., v.17, n. 1, 2004.
- GOLTERMAN, H. L.; CLYMO, R. S.; OHNSTAD, M. A. M. Methods for physical and chemical analysis of freshwater2nd ed. Oxford: Blackwell Scientific Publications,1978. 213 p.
- GRASSHOFF, K.; EHRHARDT, M.; KREMLING, K. Methods of seawater analisis. 2nd ed. New York: Verlag Chemie, 1983. 419 p.
- GREGO, C. K. S.; FEITOSA, F. A. N.; SILVA, M. H.; SILVA-CUNHA, M. G. G.; NASCIMENTO FILHO, G. A. Fitoplâncton do ecossistema estuarino do rio Ariquindá (Tamandaré, Pernambuco, Brasil): variáveis ambientais, biomassa e produtividade primária. Atlântica, v. 31, n. 2, p. 183-198, 2009.
- GREGO, C. K. S.; FEITOSA, F.A. N.; HONORATO DA SILVA, M.; FLORES-MONTES, M. J. Distribuição Espacial e Sazonal da clorofila a fitoplanctônica e hidrologia do estuário do rio Timbó (Paulista - Pe). Trop. Oceanogr. Recife, v. 32, n. 2, p. 181-199, 2004.
- GUENTHER, M.; ARAÚJO, M.; FLORES-MONTES, M. J.; GONZALEZ-RODRIGUEZ, E.; NEUMANN-LEITÃO, S. Eutrophication effects on phytoplankton size-fractioned biomass and production at a tropical estuary. Mar. Pollut. Bull, v. 91, p. 537-547, 2015.
- GUENTHER, M.; LIMA, I.; MUGRABE, G.; TENENBAUM, D. R.; GONZALEZ-RODRIGUEZ, E.; VALENTIN, J. L. Small time scale plankton structure variations at the entrance of a tropical eutrophic bay (Guanabara Bay, Brazil). Braz. J. Oceanogr., v. 60, n. 4, p. 405-414, 2012.
- HONORATO DA SILVA, M.; SILVA-CUNHA, M. G. G.; PASSAVANTE, J. Z. O.; GREGO, C. K. S.; MUNIZ, K. Estrutura sazonal e espacial do microfitoplâncton no estuário tropical do rio Formoso, PE, Brasil. Acta Bot. Bras., v. 23, n. 2, p. 355-368, 2009.
- JENDYK, J.; HEMRAJ, D. A.; BROWN, M. H.; ELLIS, A. V.; LETERME, S. C. Environmental variability and phytoplankton dynamics in a South Australian inverse estuary. Cont. Shelf Res., v. 91, p. 134-144, 2014.
- KHAN, S.; HAQUE, M. M.; ARAKAWA, O.; ONOUE, Y. Physiological observations on a diatom Skeletonema costatum (Greville) Cleve. Bangladesh J. Fish. Res., v. 2, n. 2, p. 109-118, 1998.
- KOROLEFF, K. Determination of phosphorus. In: GRASSHOFF, K.; EHRHARDT, M.; KREMLING, K. (Eds.). Methods of sea water analysis2nd ed. Weinhein: Verlag Chemie, 1983. p. 125-139.
- LEÃO, B. M.; PASSAVANTE, J. Z. O.; SILVA-CUNHA, M. G. G.; SANTIAGO, M. F. Ecologia do microfitoplâncton do estuário do rio Igarassu, PE, Brasil. Acta Bot. Bras., v. 22, n. 3, p. 711-722, 2008.
- LU, Z.; GAN, J. Controls of seasonal variability of phytoplankton blooms in the Pearl River Estuary. Deep Sea Res Part II: Top Stud Oceanogr., v.117, p. 86-96, 2015.
- MACÊDO, S. J.; COSTA, K. M. P. Estudos ecológicos da região de Itamaracá. Pernambuco - Brasil. Condições Hidrológicas do Estuário do Rio Botafogo. Ciênc. Cult., v. 30, p. 346- 368, 1978.
- MARGALEF, R. Temporal succession and spatial heterogeneity in phytoplankton. In: BUZZATI-TRAVERSO, A. A. (Ed.). Perspectives in Marine BiologyBerkeley: University of California Press, 1958. p. 323-349.
- MASUDA, L. S. M.; MOSER, G. A. O.; BARRERA-ALBA, J. J. Variação temporal do fitoplâncton no canal estuarino de Santos (sp). Braz. J. Aquat. Sci. Technol., v. 15, n. 1, p. 79-93, 2011.
- MATEUCCI, S. D.; COLMA, A. La metodologia para el estudo de la vegetaciónWashington: OEA, 1982. 168 p.
- MATOS, J. B., SODRÉ, D. K. L., COSTA, K. G.; PEREIRA, L. C. C.; COSTA, R. M. Spatial and temporal variation in the composition and biomass of phytoplankton in an Amazonian estuary. J. Coast. Res. SI 64, p. 1525-1529, 2011.
- MONTEIRO, M. C.; JIMÉNEZ, J. A.; PEREIRA, L. C. C. Natural and human controls of water quality of an Amazon estuary (Caeté-PA, Brazil). Ocean Coast. Manag., v. 124, p. 42-52, 2016.
- MOSER, G. A. O.; GIANESELLA, S. M. F.; ALBA, J. J. B.; BÉRGAMO, A. L.; SALDANHA-CORRÊA, F. M. P.; MIRANDA, L. B.; HARARI, J. Instantaneous transport of salt, nutrients, suspended matter and chlorophyll-a in the tropical estuarine system of Santos. Rev. Bras. Oceanogr., v. 53, n. 3-4, p. 115-127, 2005.
- MÜLLER-MELCHERS, F. C.; FERRANDO, H. J. Técnicas para el estudio de las diatomáceas. Bol. Inst. Oceanogr., 1956. v. 7, n. 1/2, p. 151-160.
- NASCIMENTO, F. C. R.; MUNIZ, K.; FEITOSA, F. A. N.; ARAÚJO, J. P.; SILVA, R. M. S.; SILVA, G. S.; FLORES-MONTES, M. J. Disponibilidade nutricional da Bacia do Pina e rio Tejipió (Recife-PE-Brasil) em relação aos nutrientes e biomassa primária (setembro/2000). Trop. Oceanogr., v. 31, n. 2, p. 149-169, 2003.
- NCHE-FAMBO, F. A.; SCHARLER, U. M.; TIROK, K. Resilience of estuarine phytoplankton and their temporal variability along salinity gradients during drought and hypersalinity. Estuar. Coast. Shelf Sci., v. 158, p. 40-52, 2015.
- ODEBRECH, C.; ABREU, P. C.; CARSTENSEN, J. Retention time generates short-term phytoplankton blooms in a shallow microtidal subtropical estuary. Estuar. Coast. Shelf Sci., v. 162, p. 35-44, 2015.
- PAERL, H. W.; ROSSIGNOL, K. L.; HALL, N. S.; PEIERLS, B. L.; WETZ, M. S. Phytoplankton Community Indicators of Short- and Long-term Ecological Change in the Anthropogenically and Climatically Impacted Neuse River Estuary, North Carolina, USA. Estuar. Coast., v. 33, n. 2, p. 485-497, 2010.
- PAERL, H. W.; HALL, N. S.; PEIERLS, B. L.; ROSSIGNOL, K. L. Evolving Paradigms and Challenges in Estuarine and Coastal Eutrophication Dynamics in a Culturally and Climatically Stressed World. Estuar. Coast., v. 37, n. 2, p. 243-258, 2014.
- PAIVA, R. S.; ESKINAZI-LEÇA, E.; PASSAVANTE, J. Z. O.; SILVA-CUNHA, M. G. G.; MELO, N.F.A.C. Considerações ecológicas sobre o fitoplâncton da baía do Guajará e foz do rio Guamá, Pará, Brasil. Bol. Mus. Para. Emílio Goeldi. Ciênc. Natu., v. 1, n. 2, p. 133-146, 2006.
- PAMPLONA, F. C.; PAES, E. T.; NEPOMUCENO A. Nutrient fluctuations in the Quatipuru river: A macrotidal estuarine mangrove system in the Brazilian Amazonian basin. Estuar. Coast. Shelf Sci., v. 133, p. 273-284, 2013.
- PIELOU, E. C. Species-diversity and pattern-diversity in the study of ecological succession. J. Theor. Biol., v. 10, n. 2, p. 370-383, 1966.
- PITOMBEIRA, E. S.; MORAIS, J. O. Comportamento hidrodinâmico e sedimentológico do estuário do Rio Bacanga (São Luis, Estado do Maranhão, Brasil). Arq. Ciênc. Mar. Fortaleza. v. 17, n. 2, p. 165-174, 1977.
- POTAZIO, L.; TANAKA, S. M. C. N.; CAVALCANTE, P. R. S. Avaliação de procedimentos de extração sequencial de fósforo em sedimento. Rev. Analyt., n. 8, p. 35-41, 2004.
- REYNOLDS, C. S. The Ecology of phytoplanktonCambridge: Cambridge University Press, 2006. 550 p.
- REZENDE, K. R.V.; HATHERLY, M. M. F.; PIMENTA, C. M. M.; EDUARDO, J.; VIANNA, S. C.; MANGIAVACCHI, N. Phytoplankton community structure in one sector of Guanabara Bay (RJ, Brazil) during 2011 and 2012. Braz. J. Oceanogr., v. 63, n. 3, p. 239-254, 2015.
- RIBEIRO, F. V.; FURTADO, M. S.; LIMA, N. F. C.; BRITO, L. C.; FEITOSA, A. C. Erosive Processes at Bacanga State Park Area. Rev. Soc. Nat., v. 1. n. 1, p. 142-147, 2005.
- RODRIGUES, E. I.; CUTRIM, M. V. J. Relações entre as variáveis físicas, químicas e fitoplanctônicas de três áreas estuarinas da costa Norte do Brasil - São José de Ribamar, Cedral e Cajapió, MA. Arq. Ciênc. Mar. Fortaleza, v. 43, n. 2, p. 45-54, 2010.
- ROJAS, M. O. A. I.; CAVALCANTE, P. R. S.; SOUZA, R. C.; DOURADO, E. C. S. Teores de Zinco e Cobre em ostra (Crassotrea rhizophorae) e sururu (Mytella falcata) do estuário do rio Bacanga em São Luís (MA). Bol. Lab. Hidrobiol., v. 20, n. 1, 2007.
- SANTIAGO, M. F.; SILVA-CUNHA, M. G. G.; NEUMANN-LEITÃO, S.; COSTA, K. M. P.; BORGES, G. C. P.; PORTO NETO, F. F.; NUNES, F. S. Phytoplankton Dynamics in a Highly Eutrophic Estuary in Tropical Brazil. Braz. J. Oceanogr., v. 58, n. 3, p. 189-205, 2010.
- SANTOS, T. G.; BEZERRA-JUNIOR, J. L.; COSTA, K. M. P.; FEITOSA, F. A. N. Dinâmica da Biomassa Fitoplanctônica e variáveis ambientais em um estuário tropical (Bacia do Pina, Recife, Pe). Rev. Bras. Enga. Pesca, v. 4, n. 1, p. 95-109, 2009.
- SEGURO, I.; GARCÍA, C. M.; PAPASPYROU, S.; GÁLVEZ, J. A.; GARCÍA-ROBLEDO, E.; NAVARRO, G.; SORIA-PÍRIZ, S.; AGUILAR, V.; LIZANO, O. G.; MORALES-RAMÍREZ, A.; CORZO, A. Seasonal changes of the microplankton community along a tropical estuary. Reg. Stud. Mar. Sci., v. 2, p. 189-202, 2015.
- SENA, B. A.; COSTA, V. B.; NAKAYAMA, L.; ROCHA, R. M. Composition of microphytoplankton of an estuarine Amazon River, Pará, Brazil. Biot. Amaz., v. 5, n. 2, p. 1-9, 2015.
- SHANNON, C. E.; WEAVER, W. The Mathematical Theory of CommunicationUrbana: University Illinois Press, 1963. 117 p.
- SILVA, G. S.; SANTOS, E. A.; CORRÊA, L. B.; MARQUES, A. L. B.; MARQUES, E. P.; SOUSA, E. R.; SILVA, G. S. Avaliação integrada da qualidade de águas superficiais: grau de trofia e proteção da vida aquática nos rios Anil e Bacanga, São Luís (MA). Eng. Sanit. Ambient., v. 19, p. 245-250, 2014.
- SILVA, M. A. M.; SOUZA, M. F. L.; ABREU, P. C. Spatial and temporal variation of dissolved inorganic nutrients, and chlorophyll-a in a tropical estuary in northeastern Brazil: Dynamics of nutrient removal. Braz. J. Oceanogr., v. 63, n. 1, p. 1-15, 2015.
- SIN, Y.; LEE, E.; LEE, Y.; SHIN, K. H. The river-estuarine continuum of nutrients and phytoplankton communities in an estuary physically divided by a sea dike. Estuar. Coast. Shelf Sci., v. 163, Pt. B, p. 279-289, 2015.
- SOUSA, E. B.; COSTA, V. B.; PEREIRA, L. C. C.; COSTA, R. M. Microfitoplâncton de águas costeiras amazônicas: Ilha Canela (Bragança, PA, Brasil). Acta Bot. Bras., v. 22, n. 3, p. 626-636, 2008.
- SOUZA, M. M.; GASTALDINI, M. C. C. Avaliação da qualidade da água em bacias hidrográficas com diferentes impactos antrópicos. Eng. Sanit. Ambient., v. 19, n. 3, p. 263-274, 2014.
- STRICKLAND, J. D. H.; PARSONS, T. R. A Practical Handbook of Seawater AnalysisOttawa: Fisheries Research Board of Canada, 1972.
- TEIXEIRA, C.; ARANHA, F. J.; BARBIERI, R.; MELO, O. T. Produção primária e clorofila a do fitoplâncton e parâmetros físicos e químicos do estreito dos Coqueiros, Maranhão. Brasil. Rev. Bras. Biol., v. 48, n. 1, p. 29-39, 1988.
- THOMAS, H.; BOZEC, Y.; ELKALAY, K.; BAAR, H. J. W.; BORGES, A. V.; SCHIETTECATTE, L. S. Controls of the surface water partial pressure of CO2 in the North Sea. Biogeosciences, v. 2, n. 4, p. 323-334, 2005.
- UNESCO. Determination of photosynthetic pigments in sea waterParis: UNESCO, 1966. 66 p.
- UNESCO; HALLEGRAEFF, G. M.; ANDERSON, D. M.; CEMBELLA, A. D. (eds). Manual on Harmful Marine MicroalgaeParis: UNESCO , 2004. 793 p.
- VALENTIN, J. L. Ecologia numérica: Uma introdução à análise multivariada de dados ecológicosRio de Janeiro: Interciência, 2000. 117 p.
- VALENTIN, J. L.; TENENBAUM, D. R.; BONECKER, A. C. T.; BONECKER, S. L. C.; NOGUEIRA, C. R.; VILLAC, M. C. O. Sistema planctônico da Baía de Guanabara: síntese do conhecimento. In: SILVA, S. H. G.; LAVRADO, H. P. (Eds.). Ecologia de Ambientes Costeiros do Estado do Rio de JaneiroSérie Oecologia Brasilienses. Rio de Janeiro: PPGE-UFRJ, 1999. p. 35-59.
- VILLAC, M. C.; TENENBAUM, D. R. The phytoplankton of Guanabara Bay, Brazil. I. Historical account of its biodiversity. Biota Neotrop, v. 10, n. 2, p. 271-293, 2010.
- WILD-ALLEN, K.; SKERRATT, J.; WHITEHEAD, J.; RIZWI, F.; PARSLOW, J. Mechanisms driving estuarine water quality: A 3D biogeochemical model for informed management. Estuar. Coast. Shelf Sci., v. 135, p. 33-45, 2013.
- WU, J. T.; CHOU, T. L. Silicate as the limiting nutrient for phytoplankton in a subtropical eutrophic estuary of Taiwan. Estuar. Coast. Shelf Sci., v. 58, n. 1, p. 155-162, 2003.
Publication Dates
-
Publication in this collection
Jul-Sep 2017
History
-
Received
17 Aug 2016 -
Accepted
28 June 2017