Abstract
A comparative study on size and shape of Leptuca uruguayensis was carried out between populations from Garças River, Brazil (BP), and Solís Grande River, Uruguay (UP). The size of the onset of sexual maturity was also estimated for UP. A total of 36 crabs BP and 387 crabs UP were analyzed. In the relative growth analysis, carapace width (CW) for both sexes, major cheliped length (LMC) for males and abdomen width (AW) for females were measured. The centroid size of carapace (1.40±0.19 cm BP and 1.88±0.30 cm UP) and cheliped (1.16±0.22 cm BP and 1.58±0.45 cm UP) differed significantly (p<0.001). The shape also differed significantly (p<0.001), having UP wider carapace than BP, rostrum projected forward and posterior margin positioned more anteriorly; the cheliped of UP was also wider than BP. In UP, males' CW ranged 4.28-19.5 mm and females' 2.53-16.3 mm CW; males' LMC ranged 1.79-31.60 mm and females' AW, 0.80-8.53 mm. The onset of sexual maturity of UP was estimated in 12.20 mm CW for males and 7.81 mm for females. These differences are likely related to abiotic variables acting distinctly in the two localities.
Descriptors:
Body shape; Body dimension; Geometric morphometry; Guaratuba Bay; Solís Grande River
Resumo
Um estudo comparativo sobre o tamanho e a forma de Leptuca uruguayensis foi realizado entre as populações do Rio das Garças, Brasil (BP) e do Rio Solís Grande, Uruguai (UP). O tamanho do início da maturidade sexual, também foi estimado para UP. Foi analisado um total de 36 indivíduos para BP e 387 para UP. Para a análise do crescimento relativo, a largura da carapaça (LC) para ambos os sexos, comprimento do maior quelípodo (CMQ) dos machos e a largura do abdome (LA) das fêmeas foram mensurados. O tamanho da carapaça e do quelípodo foi maior em UP. A forma também diferiu, sendo a carapaça de UP mais ampla do que BP; rostro projetado para a frente e margem posterior posicionada mais anteriormente; o quelípodo de UP também foi mais amplo do que BP. Em UP, a LC variou de 4,28-19,5 mm (machos) e 2,53-16,3 mm (fêmeas); o CMQ variou de 1,79-31,60 mm (machos) e a LA de 0,80-8,53 mm (fêmeas). O início da maturidade sexual para UP foi estimado em 12,20 mm LC (machos) e 7,81 mm (fêmeas). Essas diferenças podem indicar que variáveis abióticas estão atuando distintamente nas duas localidades.
Descritores:
Forma do corpo; Dimensão do corpo; Morfometria geométrica; Baía de Guaratuba; Rio Solís Grande
INTRODUCTION
The comprehension of morphological variations have been crucial to answer fundamental questions in evolutionary biology (HOPKINS; THURMAN, 2010HOPKINS, M. J.; THURMAN, C. L. The geographic structure of morphological variation in eight species of fiddler crabs (Ocypodidae: genus Uca) from the eastern United States and Mexico. Biol. J. Linn. Soc., v. 100, n. 1, p. 248-270, 2010.; LEZCANO et al., 2012LEZCANO, A. H.; GONZÁLEZ-JOSÉ, R.; SPIVAK, E. D.; DELLATORRE, F. G. Geographic differences in the carapace shape of the crab Cyrtograpsus affinis (Decapoda: Varunidae) and its taxonomic implications. Sci. Mar., v. 76, n. 2, p. 329-337, 2012.). Along its geographical distribution, a species may face distinct environmental and selective pressures that act upon morphology, reproductive patterns, growth rates and mortality (HOFFMANN; SHIRRIFFS, 2002HOFFMANN, A. A.; SHIRRIFFS, J. Geographic variation for wing shape in Drosophila serrata. Evolution, v. 56, n. 5, p. 1068-1073, 2002.). In some situations, this variation could be related to phenotypic plasticity of the species that react to different environmental conditions in a particular way. Therefore, morphological analyses are useful to recognize those adaptive differences (ORENSANZ et al., 1991ORENSANZ, J.; PARMA, A.; IRIBARNE, O. Population dynamics and management of natural stocks. In: SHUMWAY, S. E. (Ed.). Scallops: Biology, Ecology and Aquaculture. Elsevier, 1991. p. 625-714.; CADRIN, 2000CADRIN, S. X. Advances in morphometric identification of fishery stocks. Rev. Fish Biol. Fish., v. 10, n. 1, p. 91-112, 2000.).
Geometric morphometry is a technique focused on the geometric shape of animals, revealing information about the spatial covariance between the landmarks of an organism (ROHLF; MARCUS, 1993ROHLF, F. J.; MARCUS, L. F. A revolution in morphometrics. Trends Ecol. Evol., v. 8, n. 4, p. 129-132, 1993.). These landmarks are effective to demonstrate the shape of an organism in an accurate and detailed way, allowing population, ontogenetic, and sexual comparisons (CAVALCANTI et al., 1999CAVALCANTI, M. J.; MONTEIRO, R. L.; LOPES, P. R. D. Landmark based morphometric analysis in selected species of serranid fishes (Perciformes: Teleostei). Zool. Stud., v. 38, n. 3, p. 287-294, 1999.; CLABAUT et al., 2007CLABAUT, C.; BUNJE, P. M.; SALZBURGER, W.; MEYER, A. Geometric morphometric analyses provide evidence for the adaptive character of the Tanganyikan cichlid radiations. Evolution, v. 61, n. 3, p. 560-578, 2007.; SILVA; PAULA, 2008SILVA, I. C.; PAULA, J. Is there a better cheliped to use for geometric morphometric differentiation in brachyuran crabs? A case study using Pachygrapsus marmoratus and Carcinus maenas. J. Mar. Biol. Assoc. U.K., v. 88, n. 5, p. 941-953, 2008.). The hard exoskeleton of most decapod crustaceans facilitates the use of geometric morphometry in the studies of body parts such carapace and cheliped propodus (ROSENBERG, 1997ROSENBERG, M. S. Evolution of shape differences between the major and minor chelipeds of Uca pugnax (Decapoda: Ocypodidae). J. Crust. Biol., v. 17, n. 1, p. 52-59, 1997.; CLARK et al., 2001CLARK, P. F.; NEALE, M.; RAINBOW, P. S. A morphometric analysis of regional variation in Carcinus Leach, 1814 (Brachyura: Portunidae: Carcininae) with particular reference to the status of the two species C. maenas (Linnaeus, 1758) and C. aestuarii Nardo, 1847. J. Crust. Biol., v. 21, n. 1, p. 288-303, 2001.; RUFINO et al., 2006RUFINO, M. M.; ABELLÓ, P.; YULE, A. B. Geographic and gender shape differences in the carapace of Liocarcinus depurator (Brachyura: Portunidae) using geometric morphometrics and the influence of a digitizing method. J. Zool., v. 269, n. 4, p. 458-465, 2006.).
Some somatic dimensions of the animals can grow at higher rates than others, resulting in changes in the proportion of the body parts during the ontogenetic development; this phenomenon is called relative growth (HARTNOLL, 1978HARTNOLL, R. G. The determination of relative growth in Crustacea. Crustaceana, v. 34, n. 3, p. 281-293, 1978.). In decapod crustaceans, this technique the relative growth is widely used to estimate the onset of morphological sexual maturity. Generally, the dimensions most analyzed are the major cheliped length for males and the width of abdomen for females, because they constitute parameters of sexual dimorphism closely associated with reproductive activities (HARTNOLL, 1982HARTNOLL, R. G. Growth. In: Bliss, D. E. (Eds.). The biology of Crustacea: embryology, morphology and ecology. Vol. 2. New York: Academic Press, 1982. p. 111-196.).
Ten species of fiddler crab are known from Brazil: Leptuca cumulanta (CRANE, 1943), Leptuca leptodactyla (RATHUM, 1898), Leptuca uruguayensis (NOBILI, 1901), Minuca burgersi (HOLTHUIS, 1967), Minuca mordax (SMITH, 1870), Minuca rapax (SMITH, 1870), Leptuca thayeri (RATHBUN, 1900), Minuca victoriana (VON HAGEN, 1987), Minuca vocator (HERBST, 1804) and Uca maracoani (LATREILLE, 1802-1803) (MELO, 1996MELO, G. A. S. Manual de Identificação dos Brachyura (Caranguejos e Siris) do Litoral Brasileiro. São Paulo: Plêiade/FAPESP, 1996. 604 p.). On the other hand, L. uruguayensis constitutes the only fiddler crabs species recorded from Uruguayan coast, occurring in sandy or muddy substrates of the estuarine intertidal region (SCARABINO, 2006SCARABINO, F. Faunística y taxonomía de invertebrados bentônicos marinos y estuarinos de La costa uruguaya. In: MENAFRA, R.; RODRÍGUEZ-GALLEGO, L.; SCARABINO, F.; CONDE, D. (Eds.). Bases para la conservación y el manejo de La costa uruguaya. Montevideo: Vida Silvestre, 2006. p. 113-142.). The population of L. uruguayensis living in the banks of Solís Grande River was studied by GONZÁLEZ (1980)GONZÁLEZ, L. A. Primeira comunicación a um studio morfológico y bioecológico de Uca uruguayensis Nobili, 1901. Rev. Fac. Human. Cienc. Uruguay, v. 1, n. 1, p. 153-200, 1980..
Leptuca uruguayensis is widely distributed along 2,400 Km of the Western Atlantic coast, from Rio de Janeiro State (Brazil) to Quequén River in the Province of Buenos Aires (Argentina) (BOSCHI, 2000BOSCHI, E. E. Species of decapod crustaceans and their distribution in the American marine zoogeographic provinces. Rev. Invest. Desarr. Pesq., v. 13, p. 7-136, 2000.). Generally, this species is associated with estuarine marshes in the Uruguayan and Argentinean coast (SPIVAK, et al., 1991SPIVAK, E. D.; GAVIO, M. A.; NAVARRO, C. E. Life history and structure of the world’s southernmost Uca population: Uca uruguayensis (Crustacea, Brachyura) in Mar Chiquita Lagoon (Argentina). Bull. Mar. Sci., v. 48, n. 3, p. 679-688, 1991.). In Guaratuba Bay, Paraná State, Brazil, the species occurs along the sandy margins of streams that cross mangrove areas (MARTINS; MASUNARI, 2013aMARTINS, S. B.; MASUNARI, S. Relative growth in the fiddler crab Uca uruguayensis Nobili, 1901 (Brachyura, Ocypodidae) from Garças River mangrove, Guaratuba Bay, southern Brazil. Nauplius, v. 21, n. 1, p. 35-41, 2013a.).
Based on cytochrome c oxidase I gene data, LAURENZANO et al. (2012)LAURENZANO, C.; FARÍAS, N. E.; SCHUBART, C. D. Mitochondrial genetic structure of two populations of Uca uruguayensis fails to reveal an impact of the Rio de la Plata on gene flow. Nauplius, v. 20, n. 1, p. 15-25, 2012. did not find any significant restriction in the gene flux between the Brazilian (São Paulo State) and Argentinean (Mar Chiquita) populations of L. uruguayensis, that are living as far as 2,000 Km, and separated by a barrier consisting of Río de La Plata.
On the other hand, due to the wideness of the L. uruguayensis distribution, it is supposed that populations living in quite diverse climates would present intraspecific variations in the size and morphology. Actually, there is a contrasting size difference between the population from Guaratuba Bay, Brazil (MARTINS; MASUNARI, 2013aMARTINS, S. B.; MASUNARI, S. Relative growth in the fiddler crab Uca uruguayensis Nobili, 1901 (Brachyura, Ocypodidae) from Garças River mangrove, Guaratuba Bay, southern Brazil. Nauplius, v. 21, n. 1, p. 35-41, 2013a.) and that from Province of Maldonado, Uruguay (type locality). In Guaratuba Bay, the largest male showed 8.33 mm of carapace width (CW) and the largest female 7.79 mm, and the onset of morphological sexual maturity is 4.14 mm CW for males and 3.52 mm CW for females (MARTINS; MASUNARI, 2013aMARTINS, S. B.; MASUNARI, S. Relative growth in the fiddler crab Uca uruguayensis Nobili, 1901 (Brachyura, Ocypodidae) from Garças River mangrove, Guaratuba Bay, southern Brazil. Nauplius, v. 21, n. 1, p. 35-41, 2013a.). In contrast, maximum CW value is 16.5 mm for both sexes in the population from Province of Maldonado, Uruguay (CRANE, 1975CRANE, J. Fiddler crabs of the world (Ocypodidae, Genus Uca). Princeton: Princeton University, 1975. 736 p.), a value about twice the largest males from Guaratuba Bay.
The objectives of this work were to identify morphological differences of the crab L. uruguayensis in two distinct regions and to analyze the morphological sexual maturity for the Uruguayan population.
MATERIAL AND METHODS
Description of the study areas
The Brazilian population of Leptuca uruguayensis was obtained from margins of Garças River, Guaratuba Bay, Paraná State, southern Brazil (25°53’S and 48°38’W) and the Uruguayan one, from margins of Solís Grande River, Province of Maldonado, southeast littoral of Uruguay (34°46’S and 55°23’W).
Garças River is a narrow and short watercourse (2.4 km long and 45.6 m wide). The sampling site is located in a mangrove forest area and riverbanks with muddy substrate. In this area also occur the crabs Leptuca thayeri (RATHBUN, 1900) and Ucides cordatus (LINNAEUS, 1763) (personal observation). This coastal area is under semi-diurnal tide, with tidal mean high of 0.79 m and range of -0.2 to 1.6 m (MARINHA DO BRASIL, 2013MARINHA DO BRASIL. 2013. Available: <http://www.mar.mil.br/dhn/chm/box-previsao-mare/tabuas/> Accessed: 2017 May 27.
http://www.mar.mil.br/dhn/chm/box-previs...
). The salinity oscillated from 12 to 23, but it can drop up to 7 after sudden strong rainfall; the monthly precipitation oscillated from 103.0 mm to 428.6 mm. Furthermore, the monthly average air temperature oscillated from 16.6°C to 25.7°C and the monthly water temperature from 17.0°C (winter month) to 27.0°C (summer month) during the period from April 2011 to March 2012 (MARTINS; MASUNARI, 2013bMARTINS, S. B.; MASUNARI, S. Temporal distribution in the abundance of the fiddler crab Uca (Leptuca) uruguayensis Nobili, 1901 (Decapoda: Ocypodidae) from Garças River mangrove, Guaratuba Bay, southern Brazil. Nauplius, v. 21, n. 2, p. 151-159, 2013b.).
The Solís Grande River is a small watercourse that crosses perpendicularly to the coastline with 90 Km extension (with a average of 2 m depth). The collection site is located in sandy/muddy riverbank with the presence of the smooth cordgrass Spartina alterniflora LOISEL (Poaceae), the spiny rush Juncus acutus LINNAEUS (Juncaceae) and the crab Neohelice granulata (DANA, 1851). The organic matter content in the sediments is high, in comparison with other studies for estuarine systems of similar latitudes (SEYS et al., 1994SEYS, J. J.; MEIRE, P. M.; COOSEN, J.; GRAEYMEERSCH, J. A. Long-term changes (1979-89) in the intertidal macrozoobenthos of the Oosterschelde estuary: are patterns in total density, biomass and diversity induced by the construction of the strom-surge barrier? Hydrobiologia, v. 282, n. 1, p. 251-264, 1994.; IENO; BASTIDA, 1998IENO, E. N.; BASTIDA, R. O. Spatial and temporal patterns in coastal macrobenthos of Samborombon Bay, Argentina: A case of study of very low diversity. Estuaries, v. 21, n. 4, p. 690-699, 1998.; MUNIZ; VENTURINI, 2001MUNIZ, P.; VENTURINI, N. Spatial distribution of the macrozoobenthos in the Solís Grande Stream estuary (Canelones-Maldonado, Uruguay). Braz. J. Biol., v. 61, n. 3, p. 409-420, 2001.).
Sampling of crabs
The crabs were sampled manually from Garças River in March 2012, and from Solís Grande River in April, 29th, 2013 and September, 14th, 2013. Beyond the sampled crabs, 50 males deposited in Museo Nacional de História Natural (MNAHNM 255 and MNAHNM 1461), Montevideo, Uruguay, coming from Solís Grande River were added for sexual maturity analysis. All the crabs were preserved in 75% ethanol.
Geometric morphometric analysis
Photographs in dorsal view of 30 carapaces and 36 major cheliped propodus were taken from Guaratuba Bay, and 32 carapaces and 47 major cheliped propodus from Solís Grande River, with a digital camera with a resolution of 10 megapixels. Only intact males were used. Twelve anatomical two-dimensional landmarks were established on the carapace and 10 on the major cheliped propodus (Figure 1), using the TPS Dig2 software, version 2.16 (ROHLF, 2010ROHLF, F. J. 2010. TpsDig, Digitize Landmarks and Outlines, version 2.16. Department of Ecology and Evolution, State University of New York at Stony Brook, Stony Brook. 2011. Available: http://life.bio.sunysb.edu/ee/rohlf/software.html> Acessed: 2017 May 27.
http://life.bio.sunysb.edu/ee/rohlf/soft...
).
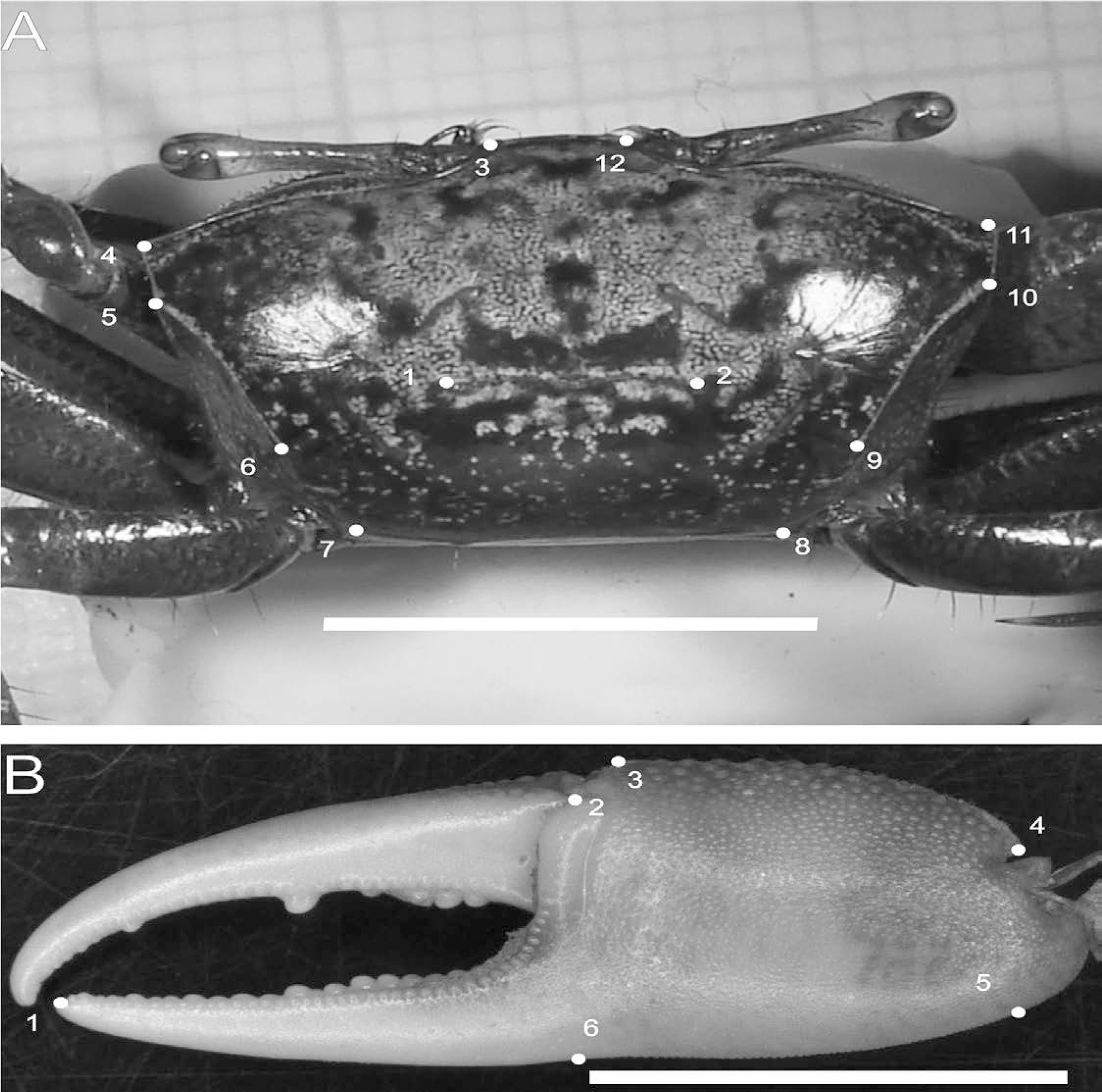
Leptuca uruguayensis. Position of the anatomical landmarks on dorsal view of carapace (A) and on outer view of major cheliped propodus of male (B). Scale: 10 mm. (A) 1 and 2: Ends of posterior groove of cardiac region, 3 and 12: Angles between the rostrum and the frontal margin, 4 and 11: Anterolateral corners, 5 and 10: Beginnings of laterovertical margin, 6 and 9: Posterior ends of lateral margin, 7 and 8: Posterolateral corners. (B) 1: Tip of fixed finger, 2: Suture between “pré-dactilar” lobe and palm base, 3: Distal corner of palm, 4: Proximal end of carpopropodus articulation, 5: Vertical line traced from landmark 4, 6: Vertical line traced from landmark 2.
A Generalized Procrustes Analysis (GPA) was performed for each body part to remove the effect of position, orientation and size of the configurations of anatomical landmarks, and aligned configurations now corresponding only to the shape of the structures (MONTEIRO; REIS, 1999MONTEIRO, L. R.; REIS, S. F. Princípios de Morfometria Geométrica. Ribeirão Preto: Holos, 1999. 189 p.). GPA consists in overlapping the configuration through the centroid (the center of mass of the configuration), escaling the centroid size of each configuration to the value of “one” and rotating the configurations, and by then obtain a least squares fit to the corresponding anatomical landmarks (ADAMS et al., 2004ADAMS, D. C; ROHLF, F. J.; SLICE, D. E. Geometric morphometrics: ten years of progress following the ‘revolution’. Ital. J. Zool., v. 71, n. 1, p. 5-16, 2004.).
Considering that the carapace is a symmetrical object, the shape components can be separated into symmetrical and asymmetrical components (KLINGENBERG et al., 2002KLINGENBERG, C. P.; BARLUENGA, M.; MEYER, A. Shape analysis of symmetric structures: quantifying variation among individuals and asymmetry. Evolution, v. 56, n. 10, p. 1909-1920, 2002.). However, only symmetrical components of the carapace were used in the present analysis of the shape variation. The size of the carapace and of the major chelip propodus was estimated as the size of the centroid, defined as the square root of the sum of the squared distances of a group of points to that centroid (MONTEIRO; REIS, 1999MONTEIRO, L. R.; REIS, S. F. Princípios de Morfometria Geométrica. Ribeirão Preto: Holos, 1999. 189 p.).
To evaluate the variation on the size of the carapace and of the major cheliped propodus between the populations, a Student t-test was used. The analyses were performed with R software (R DEVELOPMENT CORE TEAM, 2011R DEVELOPMENT CORE TEAM. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. 2011. ISBN 3-900051-07-0. Available: <http://www.R-project.org/> Accessed: 2016 Jan 19.
http://www.R-project.org/...
). The shape variation between the populations were analysed using a principal component analysis (PCA) that was performed on the variance-covariance matrix of the residuals from the GPA. The principal component scores were used as new variables to characterize the shape. This approach allowed the scores to be used as independent variables and served to reduce the dimensionality of the data (KLINGENBERG; MONTEIRO, 2005KLINGENBERG, C. P.; MONTEIRO, L. R. Distances and directions in multidimensional shape spaces: implications for morphometric applications. Syst. Biol., v. 54, n. 4, p. 678-688, 2005.; FORNEL et al., 2010FORNEL, R.; CORDEIRO-ESTRELA, P.; DE FREITAS, T. R. O. Skull shape and size variation in Ctenomys minutes (Rodentia: Ctenomyidae) in geographical, chromosomal polymorphism, and environmental contexts. Biol. J. Linn. Soc., v. 101, n. 3, p. 705-720, 2010.). The shape variance was tested with a Discriminant function analysis (DFA) in with a permutation test. This analysis computed the classification percentages and performed a cross-validation between the groups (VISCOSI; CARDINI, 2011VISCOSI, V.; CARDINI, A. Leaf Morphology, Taxonomy and Geometric Morphometrics: A Simplified Protocol for Beginners. Plos ONE, v. 6, n. 10, p. e25630, 2011.). Although all of the specimens used in the study are from the same ontogeny class (adults), they vary in size and exhibit some allometric growth. In order to generate a shape variable without influence of size (allometry-free shape variables) a multivariate regression of symmetric components of shape variables (carapace) or Procrustes coordinates (cheliped propodus) on Log centroid size was done (MONTEIRO; REIS, 1999MONTEIRO, L. R.; REIS, S. F. Princípios de Morfometria Geométrica. Ribeirão Preto: Holos, 1999. 189 p.; KLINGENBERG, 2016KLINGENBERG, C. P. Size, shape, and form: concepts of allometry in geometric morphometrics. Dev. Genes Evol., v. 226, n. 3, p. 113-137, 2016.). These analyses were performed with the MorphoJ program (KLINGENBERG, 2011KLINGENBERG, C. P. MorphoJ: an integrated software package for geometric morphometrics. Mol. Ecol. Resour., v. 11, n. 2, p. 353-357, 2011.).
Allometric analysis
The crabs were sexed and the morphometric measurements were taken with a digital caliper (precision 0.01 mm). The carapace width (CW) and length (CL) were measured for both sexes. The length of the major chela (LMC), from the proximal border of the propodus to the distal end of the fixed finger was measured. On the other hand, the maximum width of the abdomen (AW) at the fourth abdominal segment was measured. These dimensions (LMC and AW) were chosen because they are related to the reproductive activities of respective sex, and constitute elements of sexual dimorphism in crab adulthood (HARTNOLL, 1982HARTNOLL, R. G. Growth. In: Bliss, D. E. (Eds.). The biology of Crustacea: embryology, morphology and ecology. Vol. 2. New York: Academic Press, 1982. p. 111-196.).
The power equation Y=aXb was used, where ‘a’ is the intersection of ‘Y’, and ‘b’ is the constant allometric growth. The equation was linearized (log y=log a + b. logx), where the CW was considered the independent variable (x), since it is the most representative of overall size of the animal (HARTNOLL, 1982HARTNOLL, R. G. Growth. In: Bliss, D. E. (Eds.). The biology of Crustacea: embryology, morphology and ecology. Vol. 2. New York: Academic Press, 1982. p. 111-196.). The remaining dimensions (LMC and AW) were considered as dependent variables (y). The similarity between the slopes and intercepts of straight lines representing each phase of development (juveniles and adults) in the dispersion graphs was tested through covariance analysis (ANCOVA) with 95% confidence (ZAR, 1999ZAR, J. H. Bioestatistical analysis. 4th ed. New Jersey: Prentice Hall, 1999. 663 p.). After the groups of juveniles and adults were recognized by the analysis described above and by evaluate the adhesion of the abdomen to the external, the average size at morphological maturity was determined by interpolation of the equation obtained from a logistic regression analysis of the maturation condition of each individual (0=immature and 1=mature), as a function of the CW dispersion points according to the methodological procedures proposed by PAGANO and GAUVREAU (2006)PAGANO, M.; GAUVREAU, K. Princípios de Bioestatística. 2ª ed. São Paulo: Thompson, 2006. 506 p.. This average size indicates that 50% of the individuals in the population showed evidence of morphometric maturation. The logistic regression was calculated in Statistica 7.1.
RESULTS
A total of 36 males of Leptuca uruguayensis from Garças River and 387 individuals (173 males and 214 females) from Solís Grande River were analysed. The larger number of individuals used in Uruguayan population is due the sexual maturity analysis in which juveniles males and adults and juveniles females were also included. The onset of the morphological sexual maturity in the BP population was already estimated by MARTINS; MASUNARI (2013a)MARTINS, S. B.; MASUNARI, S. Relative growth in the fiddler crab Uca uruguayensis Nobili, 1901 (Brachyura, Ocypodidae) from Garças River mangrove, Guaratuba Bay, southern Brazil. Nauplius, v. 21, n. 1, p. 35-41, 2013a..
In the Garças River population the maximum CW for males was 8.30 mm and 7.79 mm for females, while for Solís Grande River these values were 19.5 mm CW and 16.3 mm CW, respectively.
The depth of the crab burrow was quite different in the two sampling sites. While at Garças River the burrows were at most 10 cm deep, at Solís Grande River, they attained up to 80 cm. As the riverbank of Garças River was narrow, a dense population (up to 71 ind.m-2) of L. uruguayensis was living in apparent competition for space (MARTINS; MASUNARI, 2013bMARTINS, S. B.; MASUNARI, S. Temporal distribution in the abundance of the fiddler crab Uca (Leptuca) uruguayensis Nobili, 1901 (Decapoda: Ocypodidae) from Garças River mangrove, Guaratuba Bay, southern Brazil. Nauplius, v. 21, n. 2, p. 151-159, 2013b.). In contrast, at Solís Grande River the sandy margins were spacious, and the burrows were located about as far as 0.5-2.0 m each other. The salinity range from 7-23 PSU at Garças River and 10-23 PSU at Solís Grande River.
Geometric morphometry: size variation in the carapace and major cheliped propodus
The carapace centroid size (t=-12.82, p<0.001, mean±SD: Garças River=1.40±0.19 cm, Solís Grande River=1.88±0.30 cm) and cheliped propodus centroid size (t= -9.01, p<0.001, mean±SD: Garças River=1.16±0.22 cm, Solís Grande River=1.58±0.45 cm) differed significantly between the two populations of Leptuca uruguayensis.
Geometric morphometry: shape variation in the carapace and cheliped propodus
The shape of the carapace and cheliped propodus also differed significantly between the two populations of L. uruguayensis. For carapace (Procrustes distance=0.026, p<0.001; Mahalanobis distance=1.42, p<0.001), the percentage of correct classification was 65% and for cheliped propodus (Procrustes distance=0.039, p<0.001; Mahalanobis distance=1.99, p<0.001) was 75%.
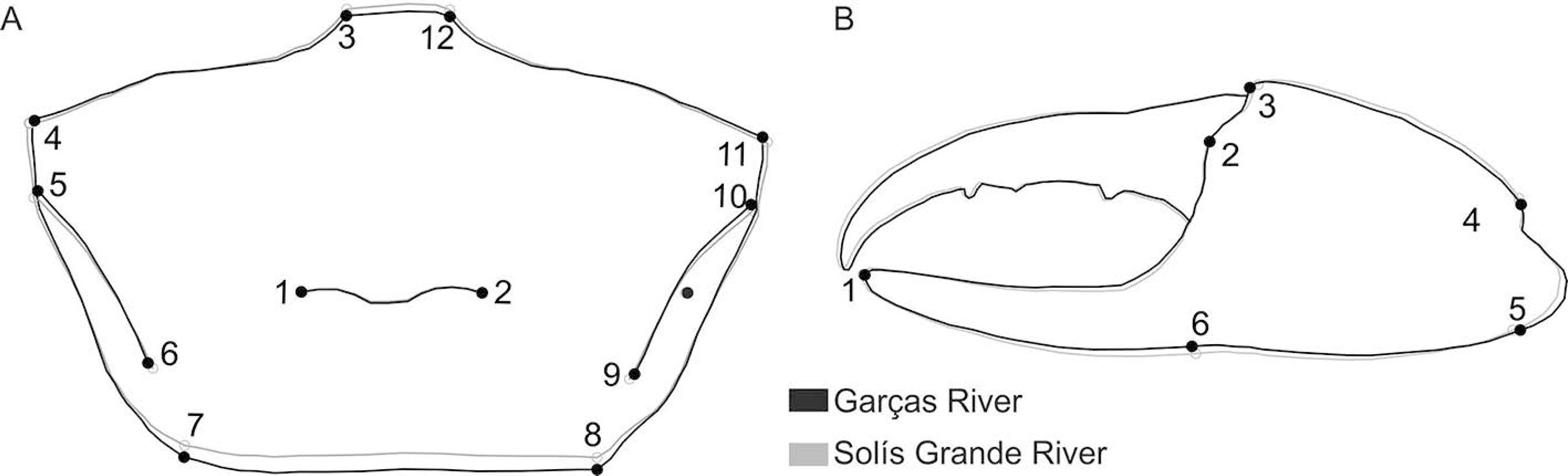
The main differences in the carapace shape between these populations were observed in the orbital region (landmarks 3 and 12; landmarks 4 and 11) and in the posterior margin (landmarks 7 and 8) (Figure 2A). Solís Grande River population showed a wider and projected forward carapace than that from Garças River; furthermore, the posterior margin is slightly retracted in the first population. For the major cheliped propodus the differences were observed in the landmarks 6 and 5 (Figure 2B), showing a slightly wider propodus in the population from Solís Grande River than that from Garças River.
Leptuca uruguayensis. Variation in the carapace shape (A) and in the major cheliped propodus (B) in the populations from Garças River and Solís Grande River. Deformations magnified only once.
Allometric analyses
In Solís Grande River a total of 387 sexually differentiated crabs were measured: 173 males and 214 females. Male CW ranged from 4.28 to 19.5 mm, while females’ from 2.53 to 16.3 mm. Male LMC ranged from 1.79 to 31.60 mm and female AW from 0.80 to 8.53 mm.
In the dispersion graph of the empirical points between LMC and CW of males, it was possible to discriminate the groups of juvenile (4.28-12.10 mm CW, n=83) and adult (12.35-19.50 mm CW, n=90). In the same way, the dispersion graph between AW and CW of females, juveniles measured from 2.53 to 6.48 mm CW (n=107) while adults, from 7.75 to16.3 mm CW (n=107). Both dimensions (LMC and AW) showed a positive allometric growth during the juvenile and adult phases (Table 1).
Leptuca uruguayensis from Solís Grande River, Maldonado, Uruguay. Statistics of linear relationships between the length of the major chela propodus (LMC) and the carapace width (CW) of males, and between the abdomen width (AW) and the carapace width (CW) of females. (N) sample size, (r) correlation coefficient, (r2) coefficient of determination, (a) intersection and (b) declivity.
The average size attaining morphometric maturity of males was calculated as 12.26 mm CW and that of females 7.81 mm CW (Figure 3).
Leptuca uruguayensis from Solís Grande River, Maldonado, Uruguay. Logistic regressions (dotted lines) fitted to the percentage of the presence (value of 1) and the absence (value of 0) of mature individuals, at a given carapace width to establish the average CW at which the species attains morphological maturity for males (A) and females (B).
DISCUSSION
Considering all populations of Leptuca uruguayensis occurring along its distribution without barriers for gene flux as suggested by LAURENZANO et al. (2012)LAURENZANO, C.; FARÍAS, N. E.; SCHUBART, C. D. Mitochondrial genetic structure of two populations of Uca uruguayensis fails to reveal an impact of the Rio de la Plata on gene flow. Nauplius, v. 20, n. 1, p. 15-25, 2012., the intraspecific difference in the carapace width between the two populations found in the present work is remarkable. Actually, the largest male found in the present study (=19.5 mm CW) was bigger than that recorded by CRANE (1975)CRANE, J. Fiddler crabs of the world (Ocypodidae, Genus Uca). Princeton: Princeton University, 1975. 736 p. in the same Province of Maldonado, Uruguay. Similarly, the major cheliped propodus has proportionally followed the carapace width.
In a general way, L. uruguayensis from temperate climate are bigger than those from subtropical or tropical one (Table 2). This inverse correlation between size and temperature was also observed for Carcinus maenas (LINNAEUS, 1758) (KELLEY et al., 2015KELLEY, A. L.; DE RIVERA, C. E.; GROSHOLZ, E. D.; RUIZ, G. M.; YAMADA, S. B.; GILLESPIE, G. Thermogeographic variation in body size of Carcinus maenas, the European green crab. Mar. Biol., v. 162, n. 8, p. 1625-1635, 2015.). The temperature can act regulating the growth rate through two distinct hypothesis: 1) the temperature can act as a selective agent, producing evolutionary changes (through genetic mutations) in the genes controlling growth rates and the size of adults (PARTRIDGE et al., 1994PARTRIDGE, L.; BARRIE, B.; FOWLER, K.; VERNON, F. Evolution and Development of Body Size and Cell Size in Drosophila melanogaster in Response to Temperature. Evolution, v. 48, n. 4, p. 1269-1276, 1994.); 2) the variation in temperature during development can change the expression of genes that regulate growth, resulting in a phenotypically plastic response (NUNNEY; CHEUNG, 1997NUNNEY, L.; CHEUNG, W. The Effect of Temperature on Body Size and Fecundity in Female Drosophila melanogaster: Evidence for Adaptive Plasticity. Evolution, v. 51, n. 5, p. 1529-1535, 1997.).
Leptuca uruguayensis. Maximum carapace width (CW) of the populations from various localities that are ordered in growing latitudinal degrees.
However, abiotic variables other than temperature are certainly influencing the size of these crabs. Among probable variables, the content of organic matter in the substrate may be the most important, as consequence of the abundance of the cordgrass Spartina in Solís Grande River but absent in Garças River. This vegetation is known to be the principal producer of organic matter in shallow coastal areas (NETTO; LANA, 1997NETTO, S. A.; LANA, P. C. Influence of Spartina alterniflora on superficial sediment characteristics of tidal flats in Paranaguá Bay (South-eastern Brazil). Estuar. Coast. Shelf Sci, v. 44, n. 55, p. 641-648, 1997.) and, therefore, it would favoring the bigness of the Uruguayan population. Although mangrove forest is also known as strong organic matter producer in coastal areas (SCHAEFFER-NOVELLI, 1995SCHAEFFER-NOVELLI, Y. Manguezal: ecossistema entre a terra e o mar. São Paulo: Caribbean Ecological Research, 1995. 64 p.), apparently, L. uruguayensis at Garças River do not share this resource.
Another abiotic variable that may influence the size difference between the two studied populations seems the tidal regime: in Maldonado coast, the low tide (exposed condition) stands for longer time period (at least eight hours in the collection day) than in Paraná State coast (at most four hours), allowing Uruguayan crabs to enhance their feeding activity and, consequently, to attain larger size than Brazilian ones. Furthermore, the sympatry and syntopy of L. uruguayensis with the grapsid crab Neohelice granulata (DANA, 1851) in Solís Grande River suggests absence of competition for resources such as space and food. On the other hand, the population of L. uruguayensis is the only species living in the limited riverbank of Garças River; in the contiguous mangrove forest the sympatric but not syntopic fiddler crab Leptuca thayeri and the ocypodid crab Ucides cordatus (LINNAEUS, 1763) occur in the muddy and shaded substrate inside the mangrove. The salinity do not seems to influence the size of L. uruguayensis, as its range and values were quite similar in both study sites (MARTINS; MASUNARI, 2013bMARTINS, S. B.; MASUNARI, S. Temporal distribution in the abundance of the fiddler crab Uca (Leptuca) uruguayensis Nobili, 1901 (Decapoda: Ocypodidae) from Garças River mangrove, Guaratuba Bay, southern Brazil. Nauplius, v. 21, n. 2, p. 151-159, 2013b.).
The phenotypic plasticity in the carapace shape of L. uruguayensis found in the present study seems to be a common feature among fiddler crab populations. WIEMAN et al. (2014)WIEMAN, A. C.; BERENDZEN, P. B.; HAMPTON, K. R.; JANG, J.; HOPKINS, M. J.; JURGENSON, J.; MCNAMARA, J. C.; THURMAN, C. L. A panmictic fiddler crab from the coast of Brazil? Impact of divergent ocean currents and larval dispersal potential on genetic and morphological variation in Uca maracoani. Mar. Biol., v. 161, n. 1, p. 173-185, 2014. also recorded an incongruity between genetic and morphological assessments among populations of Uca maracoani (LATREILLE, 1802-1803) living in eight different geographical locations along Brazilian coast (Amapá, Maranhão, Ceará, Pernambuco, Bahia, Espírito Santo, Rio de Janeiro and Paraná States).
Another example of phenotypic plasticity in the carapace shape of fiddler crabs was reported by HAMPTON et al. (2014)HAMPTON, K. R.; HOPKINS, M. J.; MCNAMARA, J. C.; THURMAN, C. L. Intraspecific variation in carapace morphology among fiddler crabs (Genus Uca) from the Atlantic coast of Brazil. Aquat. Biol., v. 20, p. 53-67, 2014., who found a significant morphological divergence in the carapace (variations in the hepatic or branchial regions) among the northern and southern populations of eight species living along Brazilian coast (between Amapá and Santa Catarina States). These authors attributed this divergence to the existence of a geographic barrier (Central South Equatorial Current that splits into two circulation patterns, North Brazil and South Brazil Current) for the larval planktonic stage of these species. However, the shape difference in carapace observed in the present study would not explained by such barrier. Future detailed studies using geometric morphometric techniques for all populations of L. uruguayensis perhaps may clarify the meaning of this carapace shape divergence. On the other hand, the significant but weak shape difference in the major cheliped propodus between the two populations reinforce the propodus as a good systematic character for the species.
The CW in which the Uruguayan population attain onset of morphological sexual maturity was the largest for both sexes among studied populations by other authors (Table 3). Distinct ecological and physical factors can be responsible for this populational difference in the onset of maturity like: predator pressure, temperature and photoperiod, food availibity, kind of substrate and population density, as observed for other brachyurans (WENNER et al., 1974WENNER, A. M.; FUSARO, C. OATEN, A. Size at onset of sexual maturity and growth rate in crustacean populations. Can. J. Zool., v. 52, n. 9, p. 1095-1106, 1974.; HINES, 1989HINES, A. H. Geographic variation in size at maturity in brachyuran crabs. Bull. Mar. Sci., v. 45, n. 2, p. 356-368, 1989.). All those factors would act on the size of the onset of morphological sexual maturity through the modulation of growth rates and longevity (HINES, 1989HINES, A. H. Geographic variation in size at maturity in brachyuran crabs. Bull. Mar. Sci., v. 45, n. 2, p. 356-368, 1989.).
Leptuca uruguayensis from various localities. Level of allometry (b) in the relative growth and the carapace width (CW) value in which the populations attain their onset of morphological sexual maturity. CW was considered as independent variable. LMC=length of the major cheliped propodus, AW= abdomen width, (0) isometry, (+) positive allometry, (-) negative allometry.
Beyond the possible ecological and physical factors mentioned, the sampled methods and the number of juveniles and adults sampled from each sex can also influence the onset of sexual maturity.
Both the variation in shape and size may be related to the phenotypic plasticity of these two populations of L. uruguayensis or to the genetic divergence in course. The last hypothesis can only be confirmed with future molecular studies using more accurate genetic markers (such as microsatellites) to try to better understand the recent historical processes.
ACKNOWLEDGEMENTS
We are grateful to Dr. Mariana Baptista Lacerda from Aquaplan Consultoria e Tecnologia Ambiental Ltda for helping us during the collection of fiddler crabs at Solís Grande River in September, 14th, 2013. All biological samplings of the present study complies with the current laws of Paraná State and Brazilian Federal Government, and they were conducted with the permission of SISBIO (Authorization system and information on biodiversity license No. 34856-1). This is Contribution No. 1936 of Department of Zoology, Federal University of Paraná.
REFERENCES
- ADAMS, D. C; ROHLF, F. J.; SLICE, D. E. Geometric morphometrics: ten years of progress following the ‘revolution’. Ital. J. Zool., v. 71, n. 1, p. 5-16, 2004.
- BOSCHI, E. E. Species of decapod crustaceans and their distribution in the American marine zoogeographic provinces. Rev. Invest. Desarr. Pesq., v. 13, p. 7-136, 2000.
- CADRIN, S. X. Advances in morphometric identification of fishery stocks. Rev. Fish Biol. Fish., v. 10, n. 1, p. 91-112, 2000.
- CAVALCANTI, M. J.; MONTEIRO, R. L.; LOPES, P. R. D. Landmark based morphometric analysis in selected species of serranid fishes (Perciformes: Teleostei). Zool. Stud., v. 38, n. 3, p. 287-294, 1999.
- CLABAUT, C.; BUNJE, P. M.; SALZBURGER, W.; MEYER, A. Geometric morphometric analyses provide evidence for the adaptive character of the Tanganyikan cichlid radiations. Evolution, v. 61, n. 3, p. 560-578, 2007.
- CLARK, P. F.; NEALE, M.; RAINBOW, P. S. A morphometric analysis of regional variation in Carcinus Leach, 1814 (Brachyura: Portunidae: Carcininae) with particular reference to the status of the two species C. maenas (Linnaeus, 1758) and C. aestuarii Nardo, 1847. J. Crust. Biol., v. 21, n. 1, p. 288-303, 2001.
- CRANE, J. Fiddler crabs of the world (Ocypodidae, Genus Uca) Princeton: Princeton University, 1975. 736 p.
- FORNEL, R.; CORDEIRO-ESTRELA, P.; DE FREITAS, T. R. O. Skull shape and size variation in Ctenomys minutes (Rodentia: Ctenomyidae) in geographical, chromosomal polymorphism, and environmental contexts. Biol. J. Linn. Soc., v. 101, n. 3, p. 705-720, 2010.
- GONZÁLEZ, L. A. Primeira comunicación a um studio morfológico y bioecológico de Uca uruguayensis Nobili, 1901. Rev. Fac. Human. Cienc. Uruguay, v. 1, n. 1, p. 153-200, 1980.
- HAMPTON, K. R.; HOPKINS, M. J.; MCNAMARA, J. C.; THURMAN, C. L. Intraspecific variation in carapace morphology among fiddler crabs (Genus Uca) from the Atlantic coast of Brazil. Aquat. Biol., v. 20, p. 53-67, 2014.
- HARTNOLL, R. G. The determination of relative growth in Crustacea. Crustaceana, v. 34, n. 3, p. 281-293, 1978.
- HARTNOLL, R. G. Growth. In: Bliss, D. E. (Eds.). The biology of Crustacea: embryology, morphology and ecology Vol. 2. New York: Academic Press, 1982. p. 111-196.
- HINES, A. H. Geographic variation in size at maturity in brachyuran crabs. Bull. Mar. Sci., v. 45, n. 2, p. 356-368, 1989.
- HIROSE, G. L.; FRANSOZO, V.; TROPEA, C.; LÓPEZ-GRECO, L. S.; NEGREIROS-FRANSOZO, M. L. Comparison of body size, relative growth and size at onset sexual maturity of Uca uruguayensis (Crustacea: Decapoda: Ocypodidae) from different latitudes in the southwestern Atlantic. J. Mar. Biol. Assoc. U.K., v. 93, n. 3, p. 781-788, 2013.
- HOFFMANN, A. A.; SHIRRIFFS, J. Geographic variation for wing shape in Drosophila serrata. Evolution, v. 56, n. 5, p. 1068-1073, 2002.
- HOPKINS, M. J.; THURMAN, C. L. The geographic structure of morphological variation in eight species of fiddler crabs (Ocypodidae: genus Uca) from the eastern United States and Mexico. Biol. J. Linn. Soc., v. 100, n. 1, p. 248-270, 2010.
- IENO, E. N.; BASTIDA, R. O. Spatial and temporal patterns in coastal macrobenthos of Samborombon Bay, Argentina: A case of study of very low diversity. Estuaries, v. 21, n. 4, p. 690-699, 1998.
- KELLEY, A. L.; DE RIVERA, C. E.; GROSHOLZ, E. D.; RUIZ, G. M.; YAMADA, S. B.; GILLESPIE, G. Thermogeographic variation in body size of Carcinus maenas, the European green crab. Mar. Biol., v. 162, n. 8, p. 1625-1635, 2015.
- KLINGENBERG, C. P.; BARLUENGA, M.; MEYER, A. Shape analysis of symmetric structures: quantifying variation among individuals and asymmetry. Evolution, v. 56, n. 10, p. 1909-1920, 2002.
- KLINGENBERG, C. P.; MONTEIRO, L. R. Distances and directions in multidimensional shape spaces: implications for morphometric applications. Syst. Biol., v. 54, n. 4, p. 678-688, 2005.
- KLINGENBERG, C. P. MorphoJ: an integrated software package for geometric morphometrics. Mol. Ecol. Resour., v. 11, n. 2, p. 353-357, 2011.
- KLINGENBERG, C. P. Size, shape, and form: concepts of allometry in geometric morphometrics. Dev. Genes Evol., v. 226, n. 3, p. 113-137, 2016.
- LAURENZANO, C.; FARÍAS, N. E.; SCHUBART, C. D. Mitochondrial genetic structure of two populations of Uca uruguayensis fails to reveal an impact of the Rio de la Plata on gene flow. Nauplius, v. 20, n. 1, p. 15-25, 2012.
- LEZCANO, A. H.; GONZÁLEZ-JOSÉ, R.; SPIVAK, E. D.; DELLATORRE, F. G. Geographic differences in the carapace shape of the crab Cyrtograpsus affinis (Decapoda: Varunidae) and its taxonomic implications. Sci. Mar., v. 76, n. 2, p. 329-337, 2012.
- MARINHA DO BRASIL. 2013. Available: <http://www.mar.mil.br/dhn/chm/box-previsao-mare/tabuas/> Accessed: 2017 May 27.
» http://www.mar.mil.br/dhn/chm/box-previsao-mare/tabuas/ - MARTINS, S. B.; MASUNARI, S. Relative growth in the fiddler crab Uca uruguayensis Nobili, 1901 (Brachyura, Ocypodidae) from Garças River mangrove, Guaratuba Bay, southern Brazil. Nauplius, v. 21, n. 1, p. 35-41, 2013a.
- MARTINS, S. B.; MASUNARI, S. Temporal distribution in the abundance of the fiddler crab Uca (Leptuca) uruguayensis Nobili, 1901 (Decapoda: Ocypodidae) from Garças River mangrove, Guaratuba Bay, southern Brazil. Nauplius, v. 21, n. 2, p. 151-159, 2013b.
- MELO, G. A. S. Manual de Identificação dos Brachyura (Caranguejos e Siris) do Litoral Brasileiro São Paulo: Plêiade/FAPESP, 1996. 604 p.
- MONTEIRO, L. R.; REIS, S. F. Princípios de Morfometria Geométrica Ribeirão Preto: Holos, 1999. 189 p.
- MUNIZ, P.; VENTURINI, N. Spatial distribution of the macrozoobenthos in the Solís Grande Stream estuary (Canelones-Maldonado, Uruguay). Braz. J. Biol., v. 61, n. 3, p. 409-420, 2001.
- NETTO, S. A.; LANA, P. C. Influence of Spartina alterniflora on superficial sediment characteristics of tidal flats in Paranaguá Bay (South-eastern Brazil). Estuar. Coast. Shelf Sci, v. 44, n. 55, p. 641-648, 1997.
- NUNNEY, L.; CHEUNG, W. The Effect of Temperature on Body Size and Fecundity in Female Drosophila melanogaster: Evidence for Adaptive Plasticity. Evolution, v. 51, n. 5, p. 1529-1535, 1997.
- ORENSANZ, J.; PARMA, A.; IRIBARNE, O. Population dynamics and management of natural stocks. In: SHUMWAY, S. E. (Ed.). Scallops: Biology, Ecology and Aquaculture Elsevier, 1991. p. 625-714.
- PAGANO, M.; GAUVREAU, K. Princípios de Bioestatística 2ª ed. São Paulo: Thompson, 2006. 506 p.
- PARTRIDGE, L.; BARRIE, B.; FOWLER, K.; VERNON, F. Evolution and Development of Body Size and Cell Size in Drosophila melanogaster in Response to Temperature. Evolution, v. 48, n. 4, p. 1269-1276, 1994.
- R DEVELOPMENT CORE TEAM. R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria. 2011. ISBN 3-900051-07-0. Available: <http://www.R-project.org/> Accessed: 2016 Jan 19.
» http://www.R-project.org/ - ROHLF, F. J. 2010. TpsDig, Digitize Landmarks and Outlines, version 2.16. Department of Ecology and Evolution, State University of New York at Stony Brook, Stony Brook 2011. Available: http://life.bio.sunysb.edu/ee/rohlf/software.html> Acessed: 2017 May 27.
» http://life.bio.sunysb.edu/ee/rohlf/software.html - ROHLF, F. J.; MARCUS, L. F. A revolution in morphometrics. Trends Ecol. Evol., v. 8, n. 4, p. 129-132, 1993.
- ROSENBERG, M. S. Evolution of shape differences between the major and minor chelipeds of Uca pugnax (Decapoda: Ocypodidae). J. Crust. Biol., v. 17, n. 1, p. 52-59, 1997.
- RUFINO, M. M.; ABELLÓ, P.; YULE, A. B. Geographic and gender shape differences in the carapace of Liocarcinus depurator (Brachyura: Portunidae) using geometric morphometrics and the influence of a digitizing method. J. Zool., v. 269, n. 4, p. 458-465, 2006.
- SCARABINO, F. Faunística y taxonomía de invertebrados bentônicos marinos y estuarinos de La costa uruguaya. In: MENAFRA, R.; RODRÍGUEZ-GALLEGO, L.; SCARABINO, F.; CONDE, D. (Eds.). Bases para la conservación y el manejo de La costa uruguaya Montevideo: Vida Silvestre, 2006. p. 113-142.
- SCHAEFFER-NOVELLI, Y. Manguezal: ecossistema entre a terra e o mar São Paulo: Caribbean Ecological Research, 1995. 64 p.
- SEYS, J. J.; MEIRE, P. M.; COOSEN, J.; GRAEYMEERSCH, J. A. Long-term changes (1979-89) in the intertidal macrozoobenthos of the Oosterschelde estuary: are patterns in total density, biomass and diversity induced by the construction of the strom-surge barrier? Hydrobiologia, v. 282, n. 1, p. 251-264, 1994.
- SILVA, I. C.; PAULA, J. Is there a better cheliped to use for geometric morphometric differentiation in brachyuran crabs? A case study using Pachygrapsus marmoratus and Carcinus maenas. J. Mar. Biol. Assoc. U.K., v. 88, n. 5, p. 941-953, 2008.
- SPIVAK, E. D.; GAVIO, M. A.; NAVARRO, C. E. Life history and structure of the world’s southernmost Uca population: Uca uruguayensis (Crustacea, Brachyura) in Mar Chiquita Lagoon (Argentina). Bull. Mar. Sci., v. 48, n. 3, p. 679-688, 1991.
- VISCOSI, V.; CARDINI, A. Leaf Morphology, Taxonomy and Geometric Morphometrics: A Simplified Protocol for Beginners. Plos ONE, v. 6, n. 10, p. e25630, 2011.
- WENNER, A. M.; FUSARO, C. OATEN, A. Size at onset of sexual maturity and growth rate in crustacean populations. Can. J. Zool., v. 52, n. 9, p. 1095-1106, 1974.
- WIEMAN, A. C.; BERENDZEN, P. B.; HAMPTON, K. R.; JANG, J.; HOPKINS, M. J.; JURGENSON, J.; MCNAMARA, J. C.; THURMAN, C. L. A panmictic fiddler crab from the coast of Brazil? Impact of divergent ocean currents and larval dispersal potential on genetic and morphological variation in Uca maracoani. Mar. Biol., v. 161, n. 1, p. 173-185, 2014.
- ZAR, J. H. Bioestatistical analysis 4th ed. New Jersey: Prentice Hall, 1999. 663 p.
Publication Dates
-
Publication in this collection
Jul-Sep 2017
History
-
Received
15 Sept 2016 -
Accepted
11 May 2017