Abstract
The susceptibility of coastal environments to shifts in the biogeochemical cycles of carbon and nutrients driven by anthropogenic pressure and climate change is a real challenge for the scientific community. This paper evaluated the effects of an extreme rainfall event over the nutrients and carbonate parameters in two polluted tropical estuaries. Surface water samples were taken seasonally along a salinity gradient in the Capibaribe and Barra de Jangadas estuaries in order to investigate the spatial and seasonal variability of dissolved nutrients, chlorophyll-a, dissolved oxygen, total alkalinity, inorganic carbon, partial pressure of CO2 (pCO2) and CO2 fluxes. The increased riverine influence caused by the fluvial flooding during the extremely rainy season augmented the nitrogen concentrations in the plumes, which also presented reduced salinity, alkalinity and dissolved oxygen values. In the Capibaribe plume it has also shifted the mean CO2 flux value of - 4.01 mmolC m-2 d-1 during the dry season, to a positive mean flux of + 5.7 mmolC m-2 d-1 during the rainy season. Within the estuaries the BOD5,20 and dissolved phosphorus values were higher during the dry season (p<0.0001), they showed positive correlation with the phytoplanktonic blooms that reached a chl-a value of 85 mg m-3 in the Capibaribe. The high alkalinity found in both estuaries, with mean values between dry and wet seasons respectively from 1808 to 1373 µmol kg-1 in the Capibaribe estuary and 1616 to 1058 µmol kg-1 in Barra de Jangadas estuary, may act as a buffer to the process of coastal acidification due to eutrophication. The increased rivers discharge lead to a greater transport of organic matter and nutrients to the coast, decreasing the oxygen availability and shifting the metabolic status of the estuarine plume to heterotrophic, whereas increased the water quality within the estuaries due the flushing promoted by the extreme rainfall event.
Descriptors:
Anthropogenic pollution; Ocean acidification; Eutrophication; Extreme event
Resumo
A suscetibilidade dos ambientes costeiros às mudanças nos ciclos biogeoquímicos do carbono e nutrientes impulsionados pela pressão antrópica e mudanças climáticas é um verdadeiro desafio para a comunidade científica. Este artigo avaliou os efeitos de um evento de precipitação extrema sobre os parâmetros de oxigênio, nutrientes e do sistema carbonato em dois estuários tropicais poluídos. As amostras de água superficial foram retiradas sazonalmente ao longo de um gradiente de salinidade nos estuários do Capibaribe e Barra de Jangadas, a fim de investigar a variabilidade espacial e sazonal dos nutrientes dissolvidos, clorofila-a, oxigênio dissolvido, alcalinidade total, carbono inorgânico, pressão parcial de CO2 (pCO2) e fluxos de CO2. O aumento da influência ribeirinha causada pelas inundações fluviais durante a estação de precipitação extrema aumentou as concentrações de nitrogênio nas plumas dos estuários, que também apresentaram valores reduzidos de salinidade, alcalinidade e oxigênio dissolvido. Na pluma do Capibaribe o valor médio de fluxo de CO2 também mudou, passou de - 4,01 mmolC m-2 d-1 durante a estação seca, para um fluxo médio positivo de + 5,7 mmolC m-2 d-1 durante a estação chuvosa. Dentro dos estuários, os valores de BOD5,20 e fosfato dissolvido foram maiores durante a estação seca (p<0,0001) e apresentaram correlação positiva com os blooms fitoplanctônicos que atingiram um valor de chl-a de 85 mg m-3 no Capibaribe. A alta alcalinidade encontrada em ambos os estuários, com valores médios entre estações seca e chuvosa, respectivamente, de 1808 a 1373 µmol kg-1 no estuário Capibaribe e 1616 a 1058 µmol kg-1 no estuário Barra de Jangadas, pode atuar como um amortecedor para o processo de acidificação costeira devido à eutrofização. O aumento da descarga dos rios levou a um maior transporte de matéria orgânica e nutrientes para o litoral, diminuindo a disponibilidade de oxigênio e deslocando o estado metabólico da pluma estuarina para heterotrófico, enquanto aumentou a qualidade da água dentro dos estuários devido ao aumento de descarga promovido pela precipitação extrema.
Descritores:
Poluição antrópica; Acidificação marinha; Eutrofização; Evento extremo
INTRODUCTION
Estuaries play an essential role in the transportation to and transformation of carbon of continental and atmospheric origin in the ocean (Sabine et al., 2004SABINE, C. L., FEELY, R. A., FEELY, N., KEY, R. M., LEE, K., BULLISTER, J. L., WANNINKHOF, R., WONG, C. S., WALLACE, D. W. R., TILBROOK, B., MILLERO, F. J., PENG, T. H., KOZYR, A., ONO, T. & RIOS, A. F. 2004. The oceanic sink for anthropogenic CO2. Science, 305, 367-71. DOI: 10.1126/science.1097403
https://doi.org/10.1126/science.1097403...
; Chen, 2004CHEN, C. T. A. 2004. Exchanges of carbon in the coastal seas. In: Field C. B. & RAUPACH M. R. (eds) The global carbon cycle: Integrating human, climate and the natural world. Washungton: Island Press.; Flores Montes et al., 2011FLORES MONTES, M. DE J., PAULO, J. G., NASCIMENTO-FILHO, G. A., GASPAR, F. L., FEITOSA, F. A., SANTOS-JÚNIOR, A. C., BATISTA, T. N. F., TRAVASSOS, R. K. & PITANGA, M. E. 2011. The trophic status of an urban estuarine complex in Northeast Brazil. Journal of Coastal Research, 64, 408-411.; Travassos et al., 2016TRAVASSOS, R. K., FLORES MONTES, M. J., COSTA, B. V, M. & SILVA JÚNIOR, J, M. 2016. The Influence of Urban Effluents on the Elemental C/N Ratio in a Tropical Coastal Area of Northeastern Brazil. Journal of Coastal Research, 75, 168-172.; Guenther et al., 2015GUENTHER, M., ARAÚJO, M., FLORES-MONTES, M., GONZALEZ-RODRIGUEZ, E. & NEUMANN-LEITÃO, S. 2015. Eutrophication effects on phytoplankton size-fractioned biomass and production at a tropical estuary. Marine pollution bulletin, 91, 537-547.). Some studies have shown that changes in land use and land cover in river basins can alter the natural balance of bicarbonate ions and contribute to alkalinity inputs into estuaries (Cai and Wang, 1998CAI, W. & WANG, Y. 1998. The chemistry, fluxes, and sources of carbon dioxide in the estuarine waters of the Satilla and Altamaha Rivers, Georgia. Limnology and Oceanography, 43, 657-668.; Wang and Cai, 2004WANG, Z. A. & CAI, W. J. 2004. Carbon dioxide degassing and inorganic carbon export from a marsh-dominated estuary (the Duplin River): A marsh CO2 pump. Limnology and Oceanography, 49, 341-354.). However, the effect of the allochtonous and autochtonous inputs on the balance of alkalinity and nutrients in estuaries remains uncertain (Flores Montes et al, 2011FLORES MONTES, M. DE J., PAULO, J. G., NASCIMENTO-FILHO, G. A., GASPAR, F. L., FEITOSA, F. A., SANTOS-JÚNIOR, A. C., BATISTA, T. N. F., TRAVASSOS, R. K. & PITANGA, M. E. 2011. The trophic status of an urban estuarine complex in Northeast Brazil. Journal of Coastal Research, 64, 408-411.). This may affect the magnitude of the capture/emission of CO2 and lead to changes in the natural balance of production and mineralization of organic matte remains uncertain (Borges, 2011BORGES, A.V. 2011. Present day carbon dioxide fluxes in the coastal ocean and possible feedbacks under global change. In: DUARTE, P. & SANTANA-CASIANO, J. M. (eds.) Oceans and the atmospheric carbon content. Dordrecht: Springer Netherlands. 47-77.).
The focus of recent studies of inorganic carbon dynamics are the large rivers and estuaries, especially in mid and high latitudes, despite the significant contribution of small tropical rivers and estuaries, which together cover an area larger than the temperate estuaries (orges). As a result of limited data from tropical regions, current estimates of the global average flux of CO2 are biased (Cai, 2011CAI, W. 2011. Estuarine and coastal ocean carbon paradox: CO2 sinks or sites of terrestrial carbon incineration? Annual Review of Marine Science, 3, 123-145.). Moreover, the CO2 flux to the atmosphere depends on the calculation of the exchange rate k, which shows great variability related to local environmental features such as tide, depth, direction and wind intensity (Raymond and Cole, 2001RAYMOND, P. A. & COLE, J. J. 2001. Gas exchange in rivers and estuaries: Choosing a gas transfer velocity. Estuaries and Coasts, 24, 312-317.; Abril and Borges, 2005ABRIL, G. & BORGES, A. 2005. Carbon dioxide and methane emissions from estuaries. In: TREMBLAY, A., VARFALVY, l., ROEHM, C. & GAMEAU, M. (eds) Greenhouse gas emissions-fluxes and processes, hydroelectric reservoirs and Natural Environments. Berlim Heildelberg New York: Springer.; Borges et al., 2004BORGES, V., DELILLE, B., SCHIETTECATTE, L., GAZEAU, F., GAZEAU, F., ABRIL, G. & FRANKIGNOULLE, M. 2004. Gas transfer velocities of CO2 in three European estuaries (Randers Fjord, Scheldt, and Thames). Limnology and Oceanography, 49, 1630-1641.).
Anthropogenic pressure adds various stressors such as pollution, overfishing, changes in the land use and cover, amongst others to the coastal environments. These impacts are expected to increase in the future with continued changes in the global climate system and increases in human population levels (Howard et al., 2013HOWARD J., BABIJ, E., GRIFFIS, R., HELMUTH, B., HIMES-CORNELL, A., NIEMIER, P., ORBACH, M., PETES, L., ALLEN, S., AUAD, G., AUER, C., BEARD, R., BOATMAN, M., BOND, N., BOYER, T., BROWN, D., CLAY, P., CRANE, K., CROSS, S., DALTON, M., DIAMOND, J., DIAZ, R., DORTCH, Q., DUFFY, E., FAUQUIER, D., FISHER, W., GRAHAM, M., HALPERN, B., HANSEN, L., HAYUM, B., HERRICK, S., HOLLOWED, A., HUTCHINS, D., JEWETT, E., JIN, D., KNOWLTON, N., KOTOWICZ, D., KRISTIANSEN, T., MORRISON, J. R., MURRAY, J., NORMAN, K., O'DONNELL, J., OVERLAND, J., PARSONS, R., PETTIGREW, N., PFEIFFER, L., PIDGEON, E., PLUMMER, M., POLOVINA, J., QUINTRELL, J., ROWLES, T., RUNGE, J., RUST, M., SANFORD, E., SEND, U., SINGER, M., SPEIR, C., STANITSKI, D., THORNBER, C., WILSON, C. & XUE, Y. 2013. Oceans and marine resources in a changing climate. In: HUGHES, R.N., HUGHES, D. J. & SMITH, I. P. (eds) Oceanography and Marine Biology: An Annual Review. Boca Raton: CRC Press. 51, 71-192.). According to Trenberth (2011)TRENBERTH, K. E. 2011. Changes in precipitation with climate change. Climate Research, 47, 123-138., it is expected that the effect of climate change on winds and precipitation may be moderate, but will vary regionally. Consequently, these climate change effects may alter the magnitude of the CO2 fluxes at the air/water interface and the water residence time in estuaries, which is an important factor in the regulation of alkalinity in estuarine environments (Cai, 2011CAI, W. 2011. Estuarine and coastal ocean carbon paradox: CO2 sinks or sites of terrestrial carbon incineration? Annual Review of Marine Science, 3, 123-145.).
The Brazilian coast extends for over 8000 km and is home to 50.7 million people, equivalent to 26.6% of the country's population (IBGE, 2010IBGE (Instituto Brasileiro de Geografia Estatística). Censo 2010. [cited 2013 Oct]. Available from: http://www.censo2010.ibge.gov.br/
http://www.censo2010.ibge.gov.br/...
). Population growth in the coastal region has led to an increase in nutrient export to the coast, due to the release of untreated sewage into rivers and estuaries (Noriega and Araujo, 2009NORIEGA, C. & ARAUJO, M. 2009. Nitrogen and phosphorus loading in coastal watersheds in northeastern Brazil. Journal of Coastal Research, 56, 871-875.). According to Noriega et al. (2013)NORIEGA, C. E. D., ARAUJO, M. & LEFÈVRE, N. 2013. Spatial and temporal variability of the CO2 fluxes in a tropical, highly urbanized estuary. Estuaries and Coasts, 36, 1054-1072. the estimated average flux for the Capibaribe estuary ranges from 10.95 to 17.52 molCO2 m-2 y-1 during high tide in a regular rainfall year. Those authors also reported the average emissions of ~ 0.35 Tg C y-1 for estuaries in the Brazilian Northeast.
In the present study, we analyzed the seasonal and spatial distribution of nutrients, alkalinity, dissolved inorganic carbon, and pCO2 and CO2 fluxes in two estuaries located in areas of high population density during a seasonal cycle of extremely heavy rainfall using measurements of total alkalinity, pH, temperature, salinity, dissolved inorganic nitrogen, total phosphorus (TP) and silicate.
METHODS
Study site
There are three major rivers in the Recife Metropolitan Region (RMR), which are Capibaribe, Jaboatão and Pirapama, whose waters are used for public water supply and the disposal of industrial effluents and domestic sewage without appropriate treatment. The land use activity in these watersheds is primarily urban and industrial, including areas of sugarcane monoculture, and some vestigial areas of Atlantic Forest and mangrove forest (CPRH, 2011CPRH (Agencia estadual de meio ambiente e recursos hídricos). 2006. Relatório de monitoramento de bacias hidrográficas do estado de Pernambuco 2011, Recife. [cited 2013 Oct 15]. Available from: http://www.cprh.pe.gov.br
http://www.cprh.pe.gov.br...
). Several studies have identified an increase in nutrient concentrations, human eutrophication and algal blooms in this region in recent years (Travassos et al., 1993TRAVASSOS, P. E. P. F., MACÊDO, S. J. & KOENING, M. L., 1993. Aspectos hidrológicos do estuário do rio Capibaribe (Recife, PE). Trabalhos do Instituto de Oceanografia da Universidade Federal de Pernambuco, 22, 9-38.; Koening et al., 1995KOENING, M. L., MACÊDO, S. J., TRAVASSOS, P. E. P. F. & PASSAVANTE, J. Z. O. 1995. Biomassa fitoplanctônica do estuário do rio Capibaribe (Recife - PE - Brasil). Arquivos de Biologia e Tecnologia, 38, 1071-1083.; Feitosa et al., 1999FEITOSA, F. A. N.; NASCIMENTO, F. C. R; COSTA, K. M. P. Distribuição espacial e temporal da biomassa fitoplanctônica relacionada com parâmetros hidrológicos na Bacia do Pina (Recife-PE). Trabalhos Oceanográficos da Universidade Federal de Pernambuco, v. 27, n. 2, p. 1-13, 1999.; Noriega and Araujo, 2009NORIEGA, C. & ARAUJO, M. 2009. Nitrogen and phosphorus loading in coastal watersheds in northeastern Brazil. Journal of Coastal Research, 56, 871-875.; Noriega and Araujo, 2011NORIEGA, C. E. D., ARAUJO, M. 2011. Nutrient budgets (C, N and P) and trophic dynamicsof a Brazilian tropical estuary: Barra das Jangadas. Anais da Academia Brasileira de Ciências, 83, 441-456.; Flores Montes et al., 2011FLORES MONTES, M. DE J., PAULO, J. G., NASCIMENTO-FILHO, G. A., GASPAR, F. L., FEITOSA, F. A., SANTOS-JÚNIOR, A. C., BATISTA, T. N. F., TRAVASSOS, R. K. & PITANGA, M. E. 2011. The trophic status of an urban estuarine complex in Northeast Brazil. Journal of Coastal Research, 64, 408-411.; Guenther et al., 2015GUENTHER, M., ARAÚJO, M., FLORES-MONTES, M., GONZALEZ-RODRIGUEZ, E. & NEUMANN-LEITÃO, S. 2015. Eutrophication effects on phytoplankton size-fractioned biomass and production at a tropical estuary. Marine pollution bulletin, 91, 537-547.) and organic pollution (Paulo et al., 2011PAULO, J. G., FLORES MONTES, M. J., SANTOS JUNIOR, A. C., BATISTA, T. N. F., TRAVASSOS, R. K., NASCIMENTO FILHO, G. A., FEITOSA, F. A., GASPAR, F. L. & PITANGA, M. E. 2011. Allochthonous and autochthonous organic matter in an urban tropical estuarine area of northeastern Brazil. Journal of Coastal Research, 64, 1798-1801.; Travassos et al., 2016TRAVASSOS, R. K., FLORES MONTES, M. J., COSTA, B. V, M. & SILVA JÚNIOR, J, M. 2016. The Influence of Urban Effluents on the Elemental C/N Ratio in a Tropical Coastal Area of Northeastern Brazil. Journal of Coastal Research, 75, 168-172.).
The Capibaribe River watershed covers a drainage area of ≈7,557 km2 and is used by a population of approximately 1,328,361 inhabitants (CPRH, 2011CPRH (Agencia estadual de meio ambiente e recursos hídricos). 2006. Relatório de monitoramento de bacias hidrográficas do estado de Pernambuco 2011, Recife. [cited 2013 Oct 15]. Available from: http://www.cprh.pe.gov.br
http://www.cprh.pe.gov.br...
). The Capibaribe estuary has a mean depth of 3m and an approximate area of ≈19 km2 located entirely within the city of Recife.
The estuarine area of the Barra de Jangadas (BJ) is formed by the confluence of the Jaboatão and Pirapama rivers, which together drain an area of approximately 1,022 km2 and whose waters are used by a population of approximately 1,347,053 inhabitants (CPRH, 2011CPRH (Agencia estadual de meio ambiente e recursos hídricos). 2006. Relatório de monitoramento de bacias hidrográficas do estado de Pernambuco 2011, Recife. [cited 2013 Oct 15]. Available from: http://www.cprh.pe.gov.br
http://www.cprh.pe.gov.br...
). This estuary, covering approximately 14 km2, and has an average depth of 2.6 m and a variable width of approximately 150 m.
Both river basins are located within the geological Formation called "Tabuleiros Costeiros do Nordeste", characterized by Tertiary and Quaternary sediments which forms coastal plains constituted by sandy soils closer to the coast and inland there are the occurrence of yellow-red latosols and podsols, moreover small stretches of alluvial eutrophic soils along river valleys (Silva, 1995SILVA, L F. 1995. Solos tropicais: aspectos pedológicos, ecológicos e de manejo, São Paulo, Terra Brasilis.).
The river flows are intermittent in the inner semi-arid portions of the Pernambuco state depending on the amount of rainfall, but are permanent where they flow into the Atlantic Ocean. The estuaries located in the RMR come under a semidiurnal tidal regime with present mean ranges of 1.3 m during the neap tide and 1.8 m during spring tide (Araujo et al., 1999ARAUJO, M., MEDEIROS, C. & RIBEIRO, C 1999. Energy balance and time-scales of mixing and stratification in the Jaboatão estuary, NE-Brazil. Revista Brasileira de Oceanografia, 47, 145-154.).
Sampling
The estuaries were divided into different segments according to the longitudinal saline gradient classification proposed by McLusky (1992)MCLUSKY, D. S. 1992. Marine and estuarine gradientes, Marine Pollution Bulletin, 24, 55-56.. The upper estuary was defined as the area where salinities are <2.5, the medium estuary 2.5<S≤30 and the upper estuary S>30 . Six sampling campaigns were performed bimonthly between November 2010 and September 2011 at 12 stations distributed across the Capibaribe estuary and 11 stations in the Barra de Jangadas estuary (Fig. 1). There were also four sampling stations located within each estuarine plume.
Map of the study area with the respective sampling stations. The numbers indicate the river plume stations. The M indicates the meteorological stations at which the precipitation, evaporation and wind speed data were recorded.
The samples were taken from the surface layer during the low-water spring tide to assess the influence of the riverine and urban inputs to the coastal areas. The water samples were collected with Niskin oceanographic bottles and sent to the Laboratory of Chemical Oceanography and the Primary Productivity Lab, at the Federal University of Pernambuco (UFPE) where they were processed and analyzed.
Temperature and salinity were determined in situ with a CTD SBE19. pH was measured on board with a Ross combination electrode, measured on the total scale with a precision of ±0.01 units and accuracy of 0.1%.
The monthly meteorological data of precipitation, air temperature, atmospheric pressure and wind speed at 10m above sea surface were obtained from the Pernambuco Agency of Water and Climate (APAC) and the National Institute of Meteorology (INMET). River discharge data were obtained from the National Water Agency (ANA).
Analyses
Dissolved oxygen (DO) was determined using the modified Winkler method (Strickland and Parsons, 1972STRICKLAND, J. D. H & PARSONS, T. R. 1972. A Practical Handbook of Seawater Analysis, Ottawa, Fisheries Research Board of Canada.) with an accuracy of ± 1.3 µmol.L-1. Oxygen saturation was calculated using temperature and salinity data, in accordance with Garcia and Gordon (1992)GARCIA, H. E. & GORDON, L. I. 1992. Oxygen solubility in seawater: Better fitting equations. Limnology and Oceanography, 37, 1307-1312..
Samples for the analysis of the biological oxygen demand (BOD) were collected in accordance with the recommendations described in Standard Methods - APHA (1995)APHA - AMERICAN PUBLIC HEALTH ASSOCIATION. 1995. Standard methods for the examination of water and wastewater, Washington, APHA., and incubated for five days at 20°C (BOD5,20).
The alkalinity was determined in unfiltered water samples by potentiometric titration with H2SO4 0.016 mol L-1 as described in Radtke et al. (2012)RADTKE, D. B., WILDE, F. D., DAVIS, J. V., & POPOWSKI, T. J. 2012. Alkalinity and acid neutralizing capacity (version 4.0). National field manual for the collection of water-quality data: US Geological Survey techniques of water-resources investigations, Reston, US Geological Survey. with a precision of 15 µmol kg-1 and an accuracy of 2%.
The samples for determination of total dissolved phosphorus (TDP) precision 0.1 µmol kg-1 , and accuracy of 1%, dissolved reactive silicate Si(OH)4 - precision 1 µmol kg-1 and accuracy 2%, dissolved inorganic nitrogen (DIN), which is the sum of ammonia-N - (NH4+ + NH3) precision 0.1 µmol kg-1 and accuracy 2%, nitrite (NO2-) precision 0.1 µmol kg-1 and accuracy 1%; and nitrate (NO3-) precision 0.5 µmol kg-1 and accuracy 1% , were analyzed by the methods described in Grasshoff et al. (1983)GRASSHOFF, K., EHRHARDT, M. & KREMLING, K. 1983. Methods of Seawater Analysis, Weinheim, Wiley-Verlag. and are expressed in µmol kg-1. The method for determining phytoplankton biomass (Chl-a) was the spectrophotometric analysis described in UNESCO (1966)UNESCO. 1966. Determination of Photosynthetic Pigments in Sea-water, Paris: UNESCO..
Calculations
Carbon dioxide solubility in the water was calculated in accordance with Weiss (1974)WEISS, R. F. 1974. Carbon dioxide in water and seawater: the solubility of a non-ideal gas. Marine chemistry, 2, 203-215.. Total inorganic carbon TCO2 = [CO2*] + [HCO3-] + [CO32-] and the partial pressure of CO2 (pCO2) were calculated with the CO2calc software, developed by Robbins et al. (2010)ROBBINS, L. L., HANSEN, M. E., KLEYPAS, J. A. & MEYLAN, S. C. 2010. CO2calc: A user-friendly seawater carbon calculator for Windows, Mac OS X, and iOS (iPhone), Washington, US Geological Survey., using alkalinity, pH, temperature, salinity, TP and reactive silica data. We used the dissociation constants of carbonic acid in accordance with Millero et al. (2006)MILLERO, F, J., GRAHAM, T. B., HUANG, F., BUSTOS-SERRANO, H. & PIERROT, D. 2006. Dissociation constants of carbonic acid in seawater as a function of salinity and temperature. Marine Chemistry, 100, 80-94., and the dissociation constants of borate and sulfate given, respectively, by Dickson (1990a)DICKSON, A. G. 1990a. Standard potential of the reaction: AgCl (s)+ 12H2 (g)= Ag (s)+ HCl (aq), and and the standard acidity constant of the ion HSO4− in synthetic sea water from 273.15 to 318.15 K. The Journal of Chemical Thermodynamics, 22, 113-127. and Dickson (1990b)DICKSON, A. G. 1990b. Thermodynamics of the dissociation of boric acid in synthetic seawater from 273.15 to 318.15 K. Deep Sea Research Part A. Oceanographic Research Papers, 37, 755-766..
CO2 flux was calculated as follows:
Where F(CO2) is the air/water flux of CO2 (mmol m-2 d-1), k(CO2) is the transfer velocity of CO2, KHCO2 is the solubility of CO2 (Weiss, 1974WEISS, R. F. 1974. Carbon dioxide in water and seawater: the solubility of a non-ideal gas. Marine chemistry, 2, 203-215.) and ΔpCO2 is the difference of pCO2 between surface water and the atmosphere. Positive values indicate outgassing towards the atmosphere.
The pCO2 in the atmosphere was calculated as follows:
Where Patm is the atmospheric pressure and xCO2atm is the molar concentration of CO2 in dry air in parts per million, obtained from the NOAA station located in Maxaranguape, 330 km north of Recife. Finally pH2O is the vapor pressure of water at the sea surface.
The gas transfer velocity k(CO2) was calculated using the formula of Raymond and Cole (2001)RAYMOND, P. A. & COLE, J. J. 2001. Gas exchange in rivers and estuaries: Choosing a gas transfer velocity. Estuaries and Coasts, 24, 312-317.:
Where u (m.s-1) is the mean wind speed 10 m above the sea surface on the sampling campaign day, and Sc is the Schmidt number in salt water for the CO2, calculated as a function of temperature.
An empirical model developed by Cole and Cloern (1987)COLE, B. E. & CLOERN, J. E. 1987. An empirical model for estimating phytoplankton productivity in estuaries. Marine Ecology Progress Series, 36, 299-305. for estimating phytoplankton productivity in the coastal areas was used. The above authors found a mean coefficient of determination r2=0.82 using nine data sets and a highly significant relationship (p<0.001) between production and B, Zp, and I for the pooled data (n=211) given by:
where B represents the phytoplankton biomass (mg m-3 of chlorophyll-a), Zp is the photic depth in meters (measured by Secchi disk), and I is the surface irradiance.
The depth limit of the photic layer (Zp), which receives 1% of the radiation that reaches the surface, was calculated using the equation Zp=4.61/k proposed by Cole and Cloern (1987)COLE, B. E. & CLOERN, J. E. 1987. An empirical model for estimating phytoplankton productivity in estuaries. Marine Ecology Progress Series, 36, 299-305., where k is the coefficient of light attenuation calculated using the depth of disappearance of the Secchi disk (Zds), (k=1,7/Zds) as described by Poole and Atkins (1929)POOLE, H. H. & ATKINS, W. R. G. 1929. Photo-electric measurements of submarine illumination throughout the year. Journal of the Marine Biological Association of the United Kingdom, 16, 297-324..
The radiometric irradiance data used to calculate productivity were obtained from the Center for Weather Forecasting and Climate Studies/National Institute for Space Research - CPTEC/INPE, available at www.cptec.inpe.br. These data were converted to photosynthetically active radiation (PAR - cal.g. cm-2.d-1) by applying the correction factor of 0.47, as described by Vollenweider (1974)VOLLENWEIDER, R, A., TALLING, J. F. & WESTLAKE, D. F. 1974. A manual on methods for measuring primary production in aquatic environments, Oxford, Blackwell Scientific Pub.. Values of PAR were converted into values of quantum E (m2 d-1) using a correction factor of 0.192, as described by Colijn (1982)COLIJN, F. 1982. Light absorption in the waters of the Ems-Dollard estuary and its consequences for the growth of phytoplankton and microphytobenthos. Netherlands Journal of Sea Research, 15, 196-216..
The seasonal water and salt flux average budgets for each estuary were calculated according to Gordon et al. (1996)GORDON, D. C.; BOUDREAU, P. R.; MANN, K. H.; ONG, J.-E., SILVERT, W. L.; SMITH, S. V.; WATTAYAKORN, G.; WULLF, F. & YANAGI, T. 1996. LOICZ biogeochemical modelling guidelines, Yerseke, Netherlands Institute for Sea Research. and the residence time we calculated by equation 5.
Statistics
The data normality was tested using the Shapiro-Wilks test. We used the t-test to compare the historical means with the 2010/2011 monthly means of precipitation and wind velocities, with a significance level α=0.05. Since some of the variables didn't showed a normal distribution due to a reduced data pool, we used the Kruskal-Wallis test, with a level of significance α=0.05 to identify whether there were seasonal and/or spatial differences in the pooled data.
In order to examine the links between all the variables measured, a principal component analysis (PCA) was used. Linear regressions and Pearson's correlations were calculated to assess variables distributions along the salinity gradient and under different rivers discharge conditions.
RESULTS
Climatology and river discharge
The average air temperature ranged between 23.71°C and 27.91°C. The mean wind speed for the area was 1.82 m s-1, varying between 0.6 and 2.86 m s-1. These values are close to the average wind speed for 1990-2010 of 2.2 m s-1.
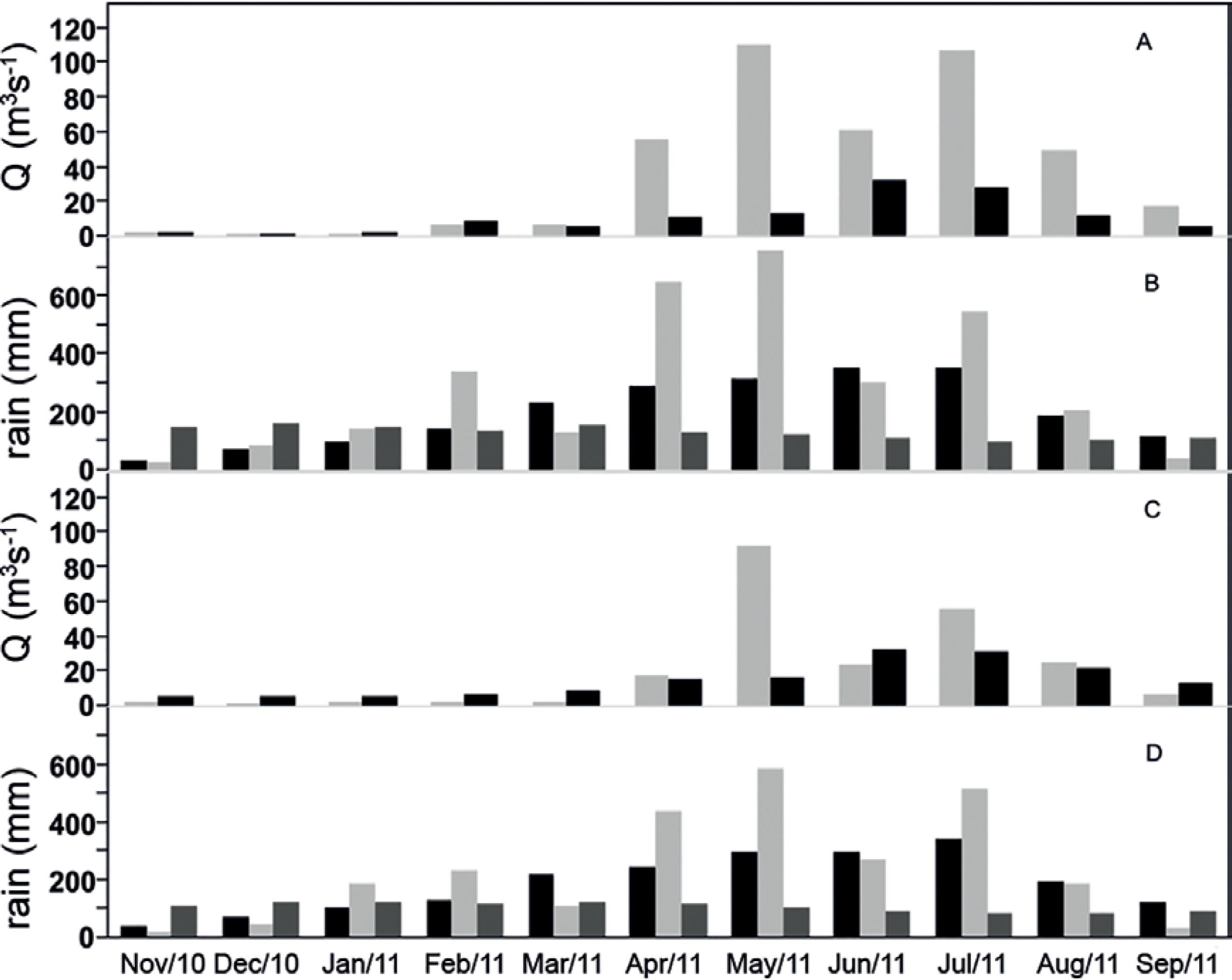
During the period from 2010 to 2011, the rainfall was above the expected values for some months of the rainy season (April to July), relative to the climatological average registered for 1990 to 2010 (Fig. 2 B, D). There was no significant difference in annual precipitation (t-test, p=0.3149), only in the monthly distribution of the rainfall as shown by the t test (t-test, p=0.017). The river discharge values for the Capibaribe (37.7 m3 s-1) and Pirapama (20.7 m3 s-1) found during this study were higher than the averages calculated from data provided by the Brazilian National Water Agency (Fig. 2 A, C).
River discharge (gray) and mean discharge (black) from 1986 to 2009 in the Capibaribe (A) and from 1990 to 2009 in Barra de Jangadas (C). Monthly precipitation in the cities of Recife (B) and Jaboatão (D),monthly precipitation (gray), Thornthwaite's potential evapotranspiration (dark gray), average precipitation from 1990 to 2010 (black). There was no difference in annual rainfall (t-test p >0.05) only in the monthly precipitation distribution (t-test p=0.017).
The mixing flow (Vx) in the Capibaribe estuary was always below the residual flow (Vr), while the opposite dynamic was observed at the Barra de Jangadas estuary (Table 1). This latter act as an importer of salt, particulate and dissolved material while the Capibaribe estuary act as an exporter to the coastal zone. The residence time during the extreme rainfall event decreased ≈3.5 times and ≈10 times in the Capibaribe and Barra de Jangadas estuaries respectively.
Seasonal water and salt flux average budgets for each estuary. Vq river discharge; Vp precipitation; Ve evaporation; Vr residual flow; Soc ocean salinity; Ssys river salinity; Sr residual salinity; Vx mixing flux; t residence time do bod x sal
Dissolved oxygen
Seasonally, the maximum dissolved oxygen values registered in the Capibaribe estuary were found during the rainy season (Kruskal-Wallis, p<0.0001 between seasons). During the dry season, in November 2010 and September 2011, the oxygen values found in this estuary increased during phytoplanktonic blooms. No seasonal variability was identified for this parameter in the Barra de Jangadas estuary with low values throughout the study (Figure 3).
Overall distribution of the BOD and OD for the Capibaribe (A, B) and Barra de Jangadas (C,D) (black=dry season; gray = rainy season).*estuarine plume; circles - lower estuary; triangles - middle estuary; rectangles -upper estuary; z Pirapama.
The highest dissolved oxygen saturations (%DO) were found in the estuarine plumes, with annual mean values ± sd of 98±10% and 86±14% for Barra de Jangadas and Capibaribe, respectively. A seasonal difference was identified only in the BJ plume, where the mean %DO decreased from 104% during the dry season to 88% (Kruskal-Wallis, p=0.0024) during the rainy season (Table 2).
The most extreme variability of the %DO was found in the Capibaribe estuary. The spatial distribution pattern of the %DO tended to decrease toward the lower estuary, with an annual mean value ± standard deviation (sd) of 74±33% in the upper estuary and 29±34% in the lower estuary, where anoxic conditions were not rare. In Barra de Jangadas, the maximum %DO annual average was found at the low estuary 50±20%, decreasing towards the upper estuary (14.8±8.7%), and resulting in an average overall oxygen saturation below 30% throughout the study period.
The Capibaribe estuary showed an annual average BOD5,20 of 5.6 mg l-1, three times higher than the mean found for the BJ estuary (1.82 mg l-1). There was no clear variability linked to seasonal cycles. Spatially, most of the high values were found within the estuaries (Table 2) but these values showed no correlation with salinity (Figure 3).
DIN, TP, Silicate and Chl-a
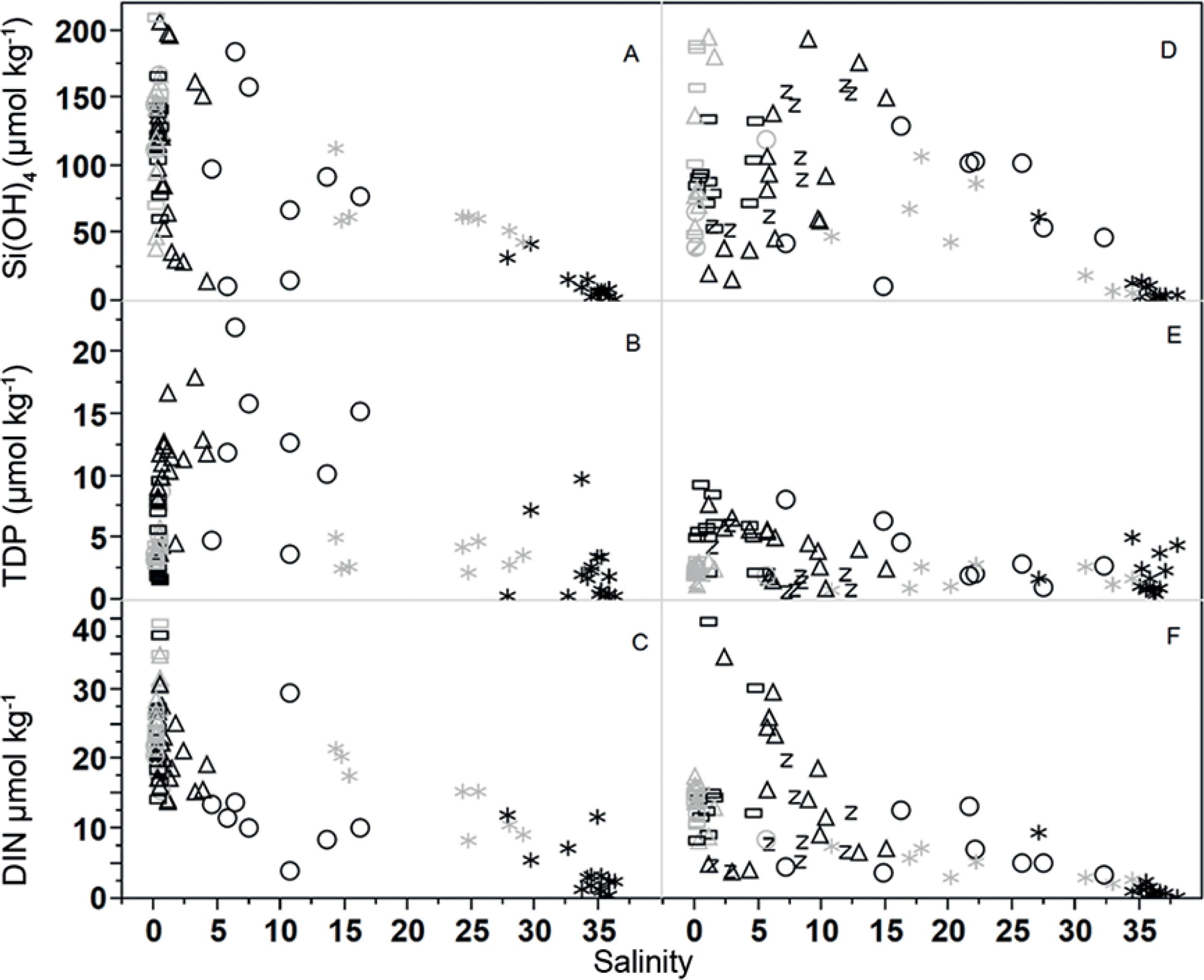
The results of dissolved inorganic nutrients are presented in Table 2 for estuaries and Table 3 for the estuarine plumes. The Capibaribe estuary and its plume showed the highest average concentrations of all nutrients. In this estuary, dissolved inorganic nitrogen (DIN) was negatively correlated with salinity, decreasing seaward (r2=0.69). No correlation between these parameters was identified in the Barra de Jangadas estuary (Figure 4). The mean values of DIN showed a significant difference between rainy and dry seasons only within the Capibaribe estuary (p<0.0001). The annual overall average DIN concentrations were 22.5±7.34 µmol kg-1 for the Capibaribe and 12.9±7.65 µmol kg-1 for the Barra de Jangadas estuary.
Seasonal mean, minimum and maximum values of the parameters measured in the Capibaribe and Barra de Jangadas estuaries
Seasonal mean, minimum and maximum values of the parameters measured in the Capibaribe and Barra de Jangadas estuarine plumes
Overall distribution of the dissolved nutrients along the salinity gradient in the estuaries of the Capibaribe (A,B,C) and Barra de Jangadas (D,E,F), (black = dry season; gray = rainy season). *river plume; circle - lower estuary; triangle - middle estuary; rectangle - upper estuary; z - Pirapama.
The estuarine plumes showed a clear correlation between DIN and rainfall r2=0.60 and 0.78 for the Capibaribe and Barra de Jangadas plumes, respectively. Through a seasonal comparison of DIN, we found p values of 0.0011 and 0.0006 for the BJ and Capibaribe plumes respectively. The increased riverine influence caused by the fluvial flooding during the extremely rainy season augmented the DIN concentrations in the plumes (Table 3), as was also seen by the salinity decrease.
The total dissolved phosphorus (TDP) maximum values were found during the dry season in both estuaries (p=0.0191) for Barra de Jangadas and (p<0.0001) for the Capibaribe estuary. The mean overall TDP concentrations found within the BJ and Capibaribe estuaries were 3.53±2.11 µmol kg-1 and 7.18±4.51 µmol kg-1, respectively.
Seasonally, there were significant variations in the silicate concentrations (Table 2) in the Capibaribe estuary (p=0.0289) and in the plumes of the Capibaribe (p=0.0001) and BJ (p=0.0041). The mean ± sd concentrations increased from 5.98±4.74 µmol kg-1 in the dry season to 48.11±37.31 µmol kg-1 in the rainy season in the BJ plume. In the Capibaribe plume the mean values ranged from 11.8±11.15 µmol kg-1 in the dry season to 64.44±21.05 µmol kg-1 during the rainy season (p=0.0001).
The phytoplanktonic biomass within the estuaries is shown in Figures 5 A and 5 B. There was no correlation between rainfall and chl-a in the Barra de Jangadas estuary (r2=0.01). In the Capibaribe estuary the average chl-a concentration was higher during the dry season, influenced by the recurrent algal blooms, and resulted in a negative correlation between rainfall and chl-a r2=0.50 with a significant seasonal difference (p=0.0002). During the campaigns, it was possible to identify an algal bloom event in November 2010 in both estuaries, when the chl-a values reached the maximum values of 85.11 mg m-3 in the Capibaribe, and 34.71 mg m-3 in the BJ estuary. A second bloom event was registered in September 2011, though less intense and only in the Capibaribe estuary, where the chl-a values ranged from a minimum of 22.94 mg m-3 to a maximum of 50.34 mg m-3. In the estuarine plumes (Figures 5 C, D) the chlorophyll a was lower than in the estuaries throughout the year and showed only a significant seasonal variation in the Capibaribe plume due to the algal blooms (p=0.0011).
Monthly variability of the CO2 fluxes (gray) and primary productivity (black) within the estuaries of the Capibaribe (A) and Barra de Jangadas (B), and in the river plumes (C), (D). Error bars represents standard deviation.
Total Alkalinity, TCO2, pH
The spatial variability of total alkalinity (TA) and pH are shown in Figure 6. Within the estuaries the alkalinity increases towards the lower estuary as a result of greater seawater influence. A positive correlation between TA and salinity was weak in the Capibaribe estuary r2=0.42, but the correlation between these parameters was more evident in the BJ estuary r2=0.62 (Figure 6 A, C).
Overall distribution along a salinity gradient of total alkalinity (TA) and pH in the estuaries of the Capibaribe (A,B) and Barra de Jangadas (C,D) (black=dry season; gray=rainy season).*river plume; circle - lower estuary; triangle - middle estuary; rectangle - upper estuary; z Pirapama).
It was found a significant seasonal and spatial differences in the TA values (p<0.0001 and p=0.0278, respectively), total alkalinity was higher in the Capibaribe estuary, with an annual average of 1649±390 µmol kg-1, ranging from 1557±315 µmol kg-1 in the upper estuary to 1860±49 µmol kg-1 in the lower estuary. For the Barra de Jangadas estuary, the annual average alkalinity was 1430±375 µmol kg-1 and ranged from 1138±288 µmol kg-1 in the upper estuary to 1668±361 µmol kg-1 in the lower estuary.
The lowest average alkalinities were recorded during the rainy season in both estuaries (Table 2), with means± sd for this season of 1078±244 and 1372±375 µmol kg-1 in the BJ and Capibaribe estuaries, respectively. There was no significant difference in TA between river plumes, with an annual average of 2142±308 µmol kg-1 in the BJ and 2196±198 µmol kg-1 in the Capibaribe.
With regard to pH, both estuaries showed a positive correlation with salinity, r2=0.62 and 0.79 for the Capibaribe and Barra de Jangadas, respectively (Figure 6 B, D). Average pH values within the BJ estuary were lower during the rainy months (6.70±0.21) and higher in the dry season (7.21±0.32). For the Capibaribe estuary, the pH values varied seasonally with mean ± sd for the rainy and dry seasons of 7.42±0.11 and 7.33 ± 0.23 (Table 2).
The pH values also varied seasonally in both river plumes, with the lowest values recorded during the rainy season for the Capibaribe (p=0.0372) and Barra de Jangadas (p=0.0301) (Table 3).
The PCA was used here to determine the influence of the pluviometry on the chemical parameters measured over time and space scales. There is a clear difference between dry and rainy period (Figure 7) where was found more elevated values of DIN, silicate in the estuaries and in the river plumes. The phytolpanktonic blooms events which occurred during the dry period were linked to the elevated TDP and BOD5,20 values, thus the %DO increased in the rainy season in response to the decrease in the organic load in the estuaries (Figure 7).
Principal component analysis obtained from the observed data in the Capibaribe (open markers) and Barra de Jangadas (solid markers). The numbers in F1 and F2 axis labels are the explained variance in each factor. (black=dry season; gray=rainy season).*river plume Capibaribe; Y river plume BJ; circle - lower estuary; triangle - middle estuary; rectangle - upper estuary; z Pirapama).
During the dry season the lower estuary station in BJ is grouped with the estuarine plume (Figure 7A) it changes in the rainy season due the increased influence of freshwater promoted by the extreme rainfall. Such event leaded to a decrease in the salinity of the river plumes and decreased the differences among river plume and lower estuary, especially in the Capibaribe (Figure 7).
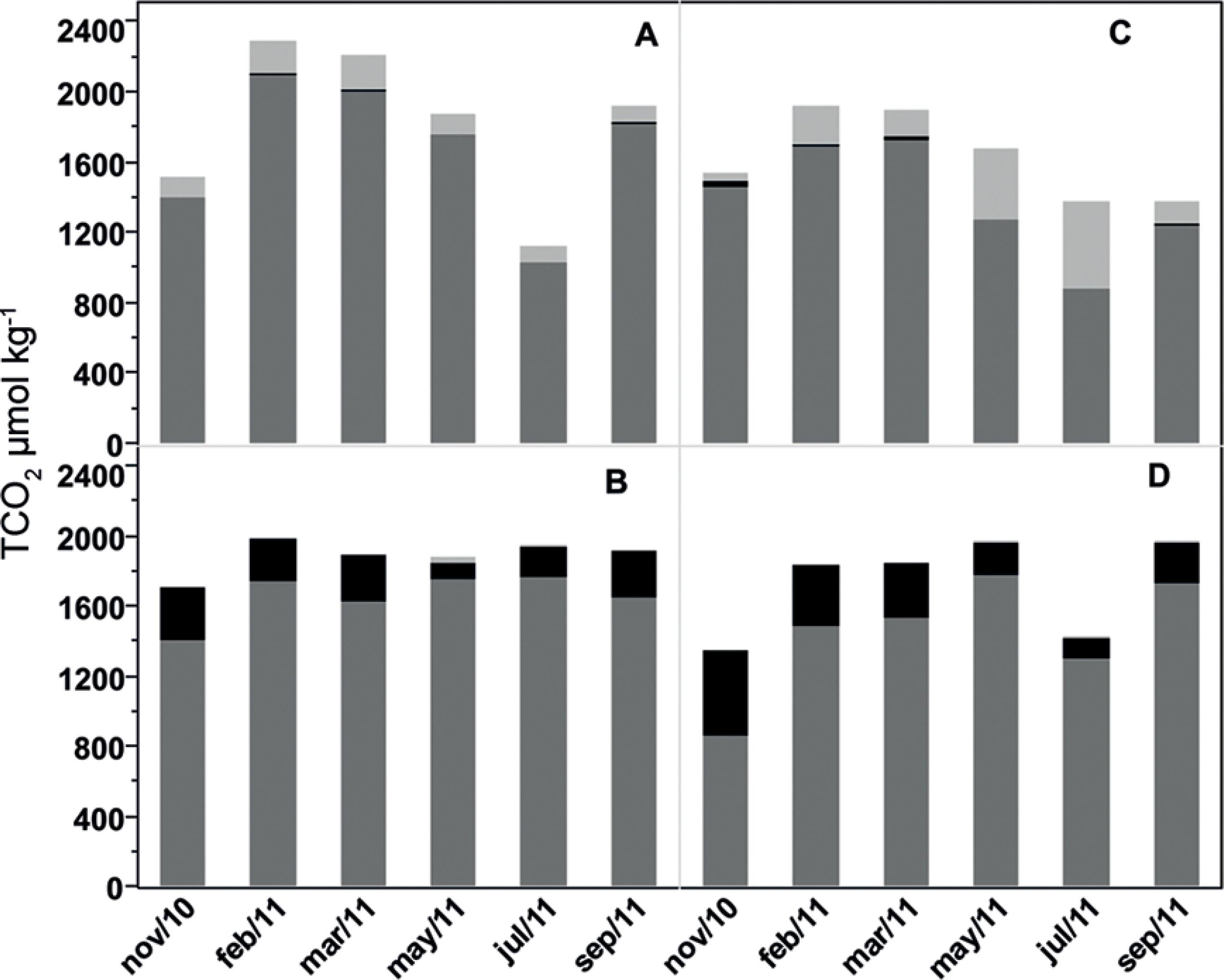
The TCO2 was also higher in the Capibaribe than in BJ (p=0.0027), with annual averages of 1764±407 µmol kg-1 and 1638±306 µmol kg-1, respectively. Variations in TCO2 values accompanied the variation in alkalinity and were lower during the rainy season in both estuaries. The maximum relative HCO3- contributions to TCO2 were registered in the Capibaribe estuary with a mean±sd of 91±3.68% of the TCO2 composed by HCO3- (Figure 8).
Monthly variability of the mean inorganic carbon species to TCO2 within the estuaries of the Capibaribe (A), Barra de Jangadas (C), and in the river plumes of the (B) Capibaribe, and (D) Barra de Jangadas. (dark gray - [HCO3-], Black - [CO3 2-], light gray - [CO2]).
Although the TCO2 did not show any seasonal variation in the river plumes, the relative contribution of inorganic carbon species varied, with a decrease in the percentage of CO32- ions and a relative increase of HCO3- ions and CO2(aq) (Figure 8), accompanied by a pH decrease during the high discharge months of April and May 2011.
Water pCO2 and CO2 fluxes
The estuaries showed a different seasonal pattern in the partial pressure of CO2 (pCO2) and CO2 fluxes (FCO2), for each estuary. The pCO2 and the FCO2 were higher during the rainy season in the Barra de Jangadas estuary and during the dry season in the Capibaribe estuary (Figures 5 and 8).
In the estuarine plumes, values of TA, pH and salinity were lower during the rainy season, increasing the pCO2, with mean values ± sd of 667±537 µatm in the Capibaribe and 332±189 µatm in the BJ (Figure 8 B). During the dry season the mean pCO2 values found were 237±86 µatm and 166±112 µatm for the estuarine plumes of the Capibaribe (p=0.0110) and Barra de Jangadas (p=0.0131), respectively.
The maximum pCO2 values were registered in the upper estuary, with low salinity, pH and alkalinity, mainly in the Barra de Jangadas estuary and decreased seawards r2=0.53 (Figure 9 D). A significant correlation was not found in the Capibaribe estuary r2=0.36 (Figure 9 B). The annual overall mean pCO2 in the Capibaribe estuary was 3317±2034 µatm, ranging from 3142±2282 µatm in the upper estuary to 2316±710 µatm in the lower estuary. In Barra de Jangadas the annual overall pCO2 mean was 6018±4589 µatm, with average values ± sd ranging from 7852±4308 µatm in the upper estuary to 3533±4615 µatm in the lower estuary, where CO2 subsaturation states were frequent during the dry season.
Correlation between river discharge (Q) and pCO2 within the Capibaribe (A); and Barra de Jangadas (B) estuaries. Overall distribution of pCO2 in the Capibaribe (C), and Barra de Jangadas (D), along a salinity gradient (black = dry season; gray = rainy season). *river plume; circle - lower estuary; triangle - middle estuary; rectangle - upper estuary; z Pirapama).
Monthly variability of FCO2 in the estuaries is presented in Figure 5 A and B. In the Capibaribe estuary the values ranged from 106.6 mmolC m-2 d-1 to 61.62 mmolC m-2 d-1 between dry and rainy periods, resulting in an annual average of 90 mmolC m-2 d-1. In the BJ estuary, the average FCO2 ranged from 117 mmolC m-2 d-1 to 348 mmolC m-2 d-1 in the dry and rainy seasons, respectively, resulting in an annual average of 186 mmolC m-2 d-1. This annual FCO2 value found for the BJ estuary showed a considerable influence of the freshwater input, when the maximum values ranged from 180 mmolC m-2 d-1 in the lower estuary to 653 mmolC m-2 d-1, in the upper estuary. During the dry season, the average FCO2 (117 mmolC m2 d-1) was close to that found for the Capibaribe (106 mmolC m-2 d-1).
DISCUSSION
The elevated organic load has driven the nutrient availability in the studied areas especially in the Capibaribe estuary where recurrent phytolanktonic blooms were registered during the dry period, which was correlated with the elevated concentrations of phosphorus and DBO5,20 also influenced by the high residence time of 25 days in the Capibaribe. The rivers flooding increased the TDP, DIN and silicate concentrations and reduce the residence time in the estuaries to values below the previously reported for these estuarine systems in the wet season. Noriega et al. (2013NORIEGA, C. E. D., ARAUJO, M. & LEFÈVRE, N. 2013. Spatial and temporal variability of the CO2 fluxes in a tropical, highly urbanized estuary. Estuaries and Coasts, 36, 1054-1072.) calculated an average residence time varying from 9-15 days for the Capibaribe estuary and Noriega and Araujo (2011)NORIEGA, C. E. D., ARAUJO, M. 2011. Nutrient budgets (C, N and P) and trophic dynamicsof a Brazilian tropical estuary: Barra das Jangadas. Anais da Academia Brasileira de Ciências, 83, 441-456. calculated a residence time of 8-16 days for the BJ estuary. The extreme precipitation event decreased these residence times to ≈7 and ≈4 days for the Capibaribe and BJ estuaries respectively, thus increasing the transport to the coastal area and such a process brings more organic matter to be mineralized over the continental shelf (Martins et al., 2016MARTINS, S. E. M., BARCELLOS, R. L., FLORES-MONTES, M. J. & FRANÇA, E. J. 2016. Depositional evolution in a estuarine lagoonal system under a port influence in Northeastern Brazil. Journal of Coastal Research, 75, 84-88.).
Different studies discussed about sources of organic matter and dissolved inorganic nutrients to the coast in the Pernambuco state (Noriega and Araujo, 2009NORIEGA, C. & ARAUJO, M. 2009. Nitrogen and phosphorus loading in coastal watersheds in northeastern Brazil. Journal of Coastal Research, 56, 871-875.; Paulo et al., 2011PAULO, J. G., FLORES MONTES, M. J., SANTOS JUNIOR, A. C., BATISTA, T. N. F., TRAVASSOS, R. K., NASCIMENTO FILHO, G. A., FEITOSA, F. A., GASPAR, F. L. & PITANGA, M. E. 2011. Allochthonous and autochthonous organic matter in an urban tropical estuarine area of northeastern Brazil. Journal of Coastal Research, 64, 1798-1801.; Travassos et al., 2016TRAVASSOS, R. K., FLORES MONTES, M. J., COSTA, B. V, M. & SILVA JÚNIOR, J, M. 2016. The Influence of Urban Effluents on the Elemental C/N Ratio in a Tropical Coastal Area of Northeastern Brazil. Journal of Coastal Research, 75, 168-172.) and trophic state (Flores Montes et al., 2011FLORES MONTES, M. DE J., PAULO, J. G., NASCIMENTO-FILHO, G. A., GASPAR, F. L., FEITOSA, F. A., SANTOS-JÚNIOR, A. C., BATISTA, T. N. F., TRAVASSOS, R. K. & PITANGA, M. E. 2011. The trophic status of an urban estuarine complex in Northeast Brazil. Journal of Coastal Research, 64, 408-411.; Guenther et al., 2015GUENTHER, M., ARAÚJO, M., FLORES-MONTES, M., GONZALEZ-RODRIGUEZ, E. & NEUMANN-LEITÃO, S. 2015. Eutrophication effects on phytoplankton size-fractioned biomass and production at a tropical estuary. Marine pollution bulletin, 91, 537-547.), and they indicated the wastewaters the major source in this interface land-water. Noriega and Araujo (2009)NORIEGA, C. & ARAUJO, M. 2009. Nitrogen and phosphorus loading in coastal watersheds in northeastern Brazil. Journal of Coastal Research, 56, 871-875. estimated a phosphorus input to the ocean of 37,048 t P y-1, where anthropogenic sources accounted for 99.7% of the emissions.
The link between nutrient dynamics and the ecosystem response under different hydrological regimes on various spatio-temporal scales is still a challenge for the scientific community (Chen and Hong, 2012CHEN, N. & HONG, H. 2012. Integrated management of nutrients from the watershed to coast in the subtropical region. Current Opinion in Environmental Sustainability, 4, 233-242.; Nixon et al., 2015NIXON, S. W., OCZKOWSKI, A. J., PILSON, M. E. Q., FIELDS, M., OVIATT, C. A. & HUNT, C. W. 2015. On the response of pH to inorganic nutrient enrichment in well-mixed coastal marine waters. Estuaries and Coasts, 38, 232-241.). There are some opposing views, with reports of nutrient enrichment exacerbating the larger scale decline in oceanic pH (Cai et al., 2011CAI, W., HU, X., HUANG, W., MURRELL, M. C., LEHRTER, J. C., LOHRENZ, S. E., CHOU, W., ZHAI, W., HOLLIBAUGH, J. T., WANG, Y., ZHAO, P., GUO, X., GUNDERSEN, K., DAI, M. & GONG, G. 2011. Acidification of subsurface coastal waters enhanced by eutrophication. Nature Geoscience, 4, 766-770.; Duarte et al., 2013DUARTE, C. M., HENDRIKS, I. E., MOORE, T. S., OLSEN, Y. S., STECKBAUER, A., RAMAJO, L., CARSTENSEN, J., TROTTER, J. A. & MCCULLOCH, M. 2013. Is ocean acidification an open-ocean syndrome? Understanding anthropogenic impacts on seawater pH. Estuaries and Coasts, 36, 221-236.). While other authors reported that the increase in the primary productivity due to eutrophication may counter the effects of ocean acidification due to the increased productivity leading to CO2 absorption. (Borges and Gypens, 2010BORGES, A. V. & GYPENS, N. 2010. Carbonate chemistry in the coastal zone responds more strongly to eutrophication than to ocean acidification. Limnology Oceanography, 55, 346-353.; Guenther et al., 2015GUENTHER, M., ARAÚJO, M., FLORES-MONTES, M., GONZALEZ-RODRIGUEZ, E. & NEUMANN-LEITÃO, S. 2015. Eutrophication effects on phytoplankton size-fractioned biomass and production at a tropical estuary. Marine pollution bulletin, 91, 537-547.).
Despite the increased nutrient concentrations in the river plumes during the wet season, the Chl-a and productivity values were higher during the dry season, because of the algal blooms which. Such seasonal variation was also previously reported by Koening et al. (1995)KOENING, M. L., MACÊDO, S. J., TRAVASSOS, P. E. P. F. & PASSAVANTE, J. Z. O. 1995. Biomassa fitoplanctônica do estuário do rio Capibaribe (Recife - PE - Brasil). Arquivos de Biologia e Tecnologia, 38, 1071-1083. and Feitosa et al. (1999)FEITOSA, F. A. N.; NASCIMENTO, F. C. R; COSTA, K. M. P. Distribuição espacial e temporal da biomassa fitoplanctônica relacionada com parâmetros hidrológicos na Bacia do Pina (Recife-PE). Trabalhos Oceanográficos da Universidade Federal de Pernambuco, v. 27, n. 2, p. 1-13, 1999.. Those authors related the decrease in productivity to the increased turbidity in the study area. According to Resurreição et al. (1996)RESURREIÇÃO, M. G., PASSAVANTE, J. Z. O. & MACEDO, S. J. 1996. Estudo da Plataforma Continental na área do Recife (Brasil): variação sazonal da biomassa fitoplanctónica. Trabalhos Oceanográficos da Universidade Federal de Pernambuco, 39-59., who studied productivity during a seasonal cycle on a transect of the continental shelf in front of the Capibaribe, there is no significant seasonal variation in the chl-a concentration at a distance of approximately 4 nautical miles (nm) from the coast.
During the extreme rainfall events of April and May 2011, it was registered the lowest concentrations of CO32- with a relative increase in the CO2 at the same months Such a variability in the inorganic carbon species must be influenced by the decrease in the primary productivity in the study area during the extreme rainfall months, due the reduced uptake of CO2 by the phytoplanktonic communities. Several metabolic and diagenetic processes are responsible for the TCO2 and total alkalinity consumption/production in estuaries. Different authors related that the photosynthesis consumes, and both aerobic and anaerobic organic matter oxidation produce CO2. These processes decrease/increase the TCO2 in seawater, but not the total alkalinity since there is an adjustment of carbonate species and pH according to the equilibrium conditions (Zeebe and Wolf-Gladrow, 2001ZEEBE, R. E. & WOLF-GLADROW, D. 2001. CO2 in Seawater: Equilibrium, Kinetics, Isotopes, New York, Elsevier.; Wolf-Gladrow et al., 2007WOLF-GLADROW, D. A., ZEEBE, R. E., KLAAS, C., KÖRTZINGER, A. & DICKSON, A. G. 2007. Total alkalinity: The explicit conservative expression and its application to biogeochemical processes. Marine Chemistry, 106, 287-300.). Sulfate reduction and methane production release HCO3-, but also CO2 and lowers pH, and the final result depends on the total sediment chemistry (Van der Weijden, 1992VAN DER WEIJDEN, C. H. 1992. Early Diagenesis and Marine Pore Water. Developments in Sedimentology, 47, 13-134.). Additionally, the anaerobic oxidation of organic matter, which favors HCO3- production and increases alkalinity is an important aspect in the alkalinity regulation in polluted estuaries (Frankignoulle et al., 1996FRANKIGNOULLE, M., BOURGE, I. & WOLLAST, R. 1996. Atmospheric CO2 fluxes in a highly polluted estuary (the Scheldt). Limnology and Oceanography, 41, 365-369.; Abril and Frankignoulle, 2001ABRIL, G. & FRANKIGNOULLE, M. 2001. Nitrogen-alkalinity interactions in the highly polluted scheldt basin (belgium). Water Research, 35, 844-850.; Abril; Borges, 2004BORGES, V., DELILLE, B., SCHIETTECATTE, L., GAZEAU, F., GAZEAU, F., ABRIL, G. & FRANKIGNOULLE, M. 2004. Gas transfer velocities of CO2 in three European estuaries (Randers Fjord, Scheldt, and Thames). Limnology and Oceanography, 49, 1630-1641.; Thomas et al., 2009THOMAS, H., SCHIETTECATTE, L. S., SUYKENS, KONÉ, Y. J. M., SHADWICK, E. H., PROWE, A. E. F., BOZEC, Y., BAAR, H. J. W. & BORGES, A. V. 2009. Enhanced ocean carbon storage from anaerobic alkalinity generation in coastal sediments. Biogeosciences, 6, 267-274.).
The input of freshwater with high alkalinity to the Capibaribe and BJ estuaries may counterbalance the free hydrogen ions, except from estuarine organic matter respiration, from natural and anthropogenic sources, which decreases pH. Araujo et al. (2013)ARAUJO, M., NORIEGA, C., VELEDA, D. & LEFÈVRE, N. 2013. Nutrient input and CO2 flux of a tropical coastal fluvial system with high population density in the Northeast region of Brazil. Journal of Water Resource and Protection, 5, 362-375. calculated the inorganic carbon species for the 2002-2010 period, in a fluvial section of the Capibaribe river, and found values of TCO2 ranging from 596 to 2235 µmol kg-1, increasing over time, and attributed the pattern to an enhanced organic load associated with population growth over the period.
During the study period, it was found a residual organic load of 18.24 t BOD5, 20 d-1 in the Capibaribe, calculated through the integration of the average annual river's discharge (37.7 m3 s-1) and annual mean BOD5, 20 (5.6 mg.l-1) (Qmean x BOD5,20mean). The BJ received a residual organic load of 3.25 t BOD5, 20 d-1. It is 5.6 times less residual organic load than the found in the Capibaribe estuary and may explain the difference in alkalinity found between these estuaries. Since the Capibaribe estuary has a residual volume more elevated than the exchange volume and the BJ estuary has the opposite dynamic, this latter is more susceptible to a CO2 enrichment and consequently pH decrease, since the organic matter keeps retained inside the estuary where is mineralized.
The prevalence of anaerobic reactions in anoxic estuarine waters and sediments is a drawback to carbonate system calculations using pH and total alkalinity since the real amount of carbonate alkalinity is unknown. The contribution of the organic alkalinity to the TA may be relevant, thus leading to an underestimation of the calculated pCO2 values using pH and TA (Ko et al., 2016KO, Y. H., LEE, K., EOM, K. H. & HAN, I. S. 2016. Organic alkalinity produced by phytoplankton and its effect on the computation of ocean carbon parameters. Limnology and Oceanography, 61, 1462-1471.). Such underestimation in the pCO2 may vary around 30% to 50% (Kuliński et al., 2014KULIŃSKI, K., SCHNEIDER, B., HAMMER, K., MACHULIK, U. & SCHULZ-BULL, D. 2014. The influence of dissolved organic matter on the acid-base system of the Baltic Sea. Journal of Marine Systems, 132, 106-115.). From the low oxygen saturation found in the Barra de Jangadas estuary, <30% throughout the year, and in the Capibaribe estuary during the dry season, as well as the high DBO5,20 values and total phosphorus it is possible to assume that the alkalinity from dissolved organic matter probably influence the alkalinity regulation in these estuaries as has recently been discussed by Kullinsky et al. (2014), Abril et al. (2014)ABRIL, G., MARTINEZ, J., ARTIGAS, L. F., MOREIRA-TURCQ, P., BENEDETTI, M. F., VIDAL, L., MEZIANE, T., KIM, J., BERNARDES, M. C., SAVOYE, N., DEBORDE, J., SOUZA, E. L., ALBÉRIC, P., SOUZA, M. F. L &. ROLAND, F. 2014. Amazon River carbon dioxide outgassing fuelled by wetlands. Nature, 505, 395-398. and Ko et al., (2016)KO, Y. H., LEE, K., EOM, K. H. & HAN, I. S. 2016. Organic alkalinity produced by phytoplankton and its effect on the computation of ocean carbon parameters. Limnology and Oceanography, 61, 1462-1471.. So further studies are necessary to understand which processes control the alkalinity and the inorganic and organic carbon variability in the Capibaribe and BJ estuaries, including possible submarine groundwater discharge and organic alkalinity contributions.
However, the elevated values found in the present study, with an average pCO2 in the BJ estuary of 6081 µatm is within the range of 400 to 10000 µatm proposed by Chen et al. (2012)CHEN, C. T. A., HUANG, T. H., FU, Y., HE, X. & BAI, Y. 2012. Strong sources of CO2 in upper estuaries become sinks of CO2 in large river plumes. Current Opinion in Environmental Sustainability, 4, 179-185. in a review of several studies in estuarine environments. Recently, Noriega et al. (2015)NORIEGA, C. E. D., ARAUJO, M., LEFÈVRE, N., FLORES MONTES, M., GASPAR, F. & VELEDA, D. 2015. Spatial and temporal variability of CO2 fluxes in tropical estuarine systems near areas of high population density in Brazil. Regional Environmental Change, 15, 619-630. has reported values of pCO2 in 12 estuaries of northeastern Brazil ranging from 987 to 8970 µatm. Lower pCO2 values have been reported by Cotovicz et al. (2016)COTOVICZ JR, L. C., LIBARDONI, B. G., BRANDINI, N., KNOPPERS, B, A. & ABRIL, G. 2016. Comparações entre medições em tempo real da pco2 aquática com estimativas indiretas em dois estuários tropicais contrastantes: o estuário eutrofizado da baía de guanabara (RJ) e o estuário oligotrófico do Rio São Francisco (al). Química Nova, 39, 1206-1214. for the Guanabara Bay (418±431 µatm) - an eutrophic ecosystem where the primary productivity significantly lowers the pCO2 - and in the São Francisco estuary (1469±2948 µatm) which is oligotrophic and dominated by heterotrophy and thus presents higher pCO2 values.
The rivers' discharge is usually correlated with pCO2 when there is a significant contribution to the estuary from the upper river (Jiang et al., 2008JIANG, L. Q., CAI, W. J. & WANG, Y. 2008. A comparative study of carbon dioxide degassing in river-and marine-dominated estuaries. Limnology and Oceanography, 53, 2603-2615.). The absence of correlation between these parameters in the Capibaribe and BJ estuaries (Figure 8A, B), may be related to the land use in the watershed. The Capibaribe estuary, which is located entirely within an urban area, showed a decrease in pCO2 during the rainy season, in accordance with previous studies of Noriega et al. (2013)NORIEGA, C. E. D., ARAUJO, M. & LEFÈVRE, N. 2013. Spatial and temporal variability of the CO2 fluxes in a tropical, highly urbanized estuary. Estuaries and Coasts, 36, 1054-1072.. This kind of decrease has also been reported by Abril et al. (2000)ABRIL, G., ETCHEBER, H., BORGES, A. V. & FRANKIGNOULLE, M. 2000. Excess atmospheric carbon dioxide transported by rivers into the Scheldt estuary. Sciences - Series IIA - Earth and Planetary Science, 330, 761-768. in the Scheldt estuary. In such environments, the processes of organic matter mineralization and the microbial respiration rates are the main drivers of CO2 variability inside the estuary (Zhai et al., 2005ZHAI, W., DAI, M., CAI, W. J., WANG, Y. & WANG, Z. 2005. High partial pressure of CO2 and its maintaining mechanism in a subtropical estuary: the Pearl River estuary, China. Marine Chemistry, 93, 21-32.; Borges and Guenther et al., 2015).
The variability of CO2 (aq) and pH in rivers and estuaries are influenced by the soil CO2 runoff from wetlands and mangrove areas, which have a potential effect on the process of pCO2 increase in water bodies, as reported by Abril and Borges (2005)ABRIL, G. & BORGES, A. 2005. Carbon dioxide and methane emissions from estuaries. In: TREMBLAY, A., VARFALVY, l., ROEHM, C. & GAMEAU, M. (eds) Greenhouse gas emissions-fluxes and processes, hydroelectric reservoirs and Natural Environments. Berlim Heildelberg New York: Springer., Cai et al. (2011)CAI, W., HU, X., HUANG, W., MURRELL, M. C., LEHRTER, J. C., LOHRENZ, S. E., CHOU, W., ZHAI, W., HOLLIBAUGH, J. T., WANG, Y., ZHAO, P., GUO, X., GUNDERSEN, K., DAI, M. & GONG, G. 2011. Acidification of subsurface coastal waters enhanced by eutrophication. Nature Geoscience, 4, 766-770., Sarma et al. (2012)SARMA, V. V. S. S., VISWANADHAM, R., RAO, G. D., PRASAD, R, V., KUMAR, B. S. K., NAIDU, S. A., KUMAR, N. A., RAO, D. B., SRIDEVI, T., KRISHNA, M. S., REDDY, N. P. C., SADHURAM, Y. & MURTY, T. V. R. 2012. Carbon dioxide emissions from Indian monsoonal estuaries. Geophysical Research Letters, 39. and Abril et al. (2014)ABRIL, G., MARTINEZ, J., ARTIGAS, L. F., MOREIRA-TURCQ, P., BENEDETTI, M. F., VIDAL, L., MEZIANE, T., KIM, J., BERNARDES, M. C., SAVOYE, N., DEBORDE, J., SOUZA, E. L., ALBÉRIC, P., SOUZA, M. F. L &. ROLAND, F. 2014. Amazon River carbon dioxide outgassing fuelled by wetlands. Nature, 505, 395-398.. The BJ estuary appears to be more susceptible to this type of input because its banks still have dense mangrove areas and extensive wetlands which are exposed during the low tide period. The weak correlation with river discharge (r2=0.49) suggests that it has also been influenced by the high alkalinity freshwater input and the predominance of estuarine biological processes (production/remineralization of organic matter), as compared with advective fluxes. Once estuaries are predominantly heterotrophic ecosystems with high pCO2 values in the freshwater endmember, decreasing seaward (Chen et al., 2012CHEN, C. T. A., HUANG, T. H., FU, Y., HE, X. & BAI, Y. 2012. Strong sources of CO2 in upper estuaries become sinks of CO2 in large river plumes. Current Opinion in Environmental Sustainability, 4, 179-185.; 2013CHEN, C. T. A., HUANG, T. H., CHEN, Y. C., HE, X. & KANG, Y. 2013. Air-sea exchanges of CO2 in the world's coastal seas. Biogeosciences, 10, 6509-6544.) similar to the pattern found in the present study.
The river plumes of the BJ and Capibaribe estuaries, except during the extreme rainy months, acted like autotrophic areas, although their low productivity they were predominantly in a state of CO2 undersaturation (Figure 5 C, D). During the rainy season in the Capibaribe river plume, especially in May, a CO2 supersaturation was observed, which has driven the mean flux value of - 4.01 mmolC m-2 d-1 during the dry season, to a positive mean of + 5.7 mmolC m-2 d-1 during the rainy season, driven by the influence of the CO2 rich freshwater.
The average annual CO2 fluxes found for the urban tropical estuaries evaluated here, of +32,8±20.5 molCm-2 y-1 and+68.2±58.8 molC m-2 y-1 for the Capibaribe and BJ estuaries, respectively, are higher than those reported for another tropical Brazilian estuary by Souza et al. (2009)SOUZA, M. F. L., GOMES, V. R., FREITAS, ANDRADE, R. C. B. & KNOPPERS, B. 2009. Net ecosystem metabolism and nonconservative fluxes of organic matter in a tropical mangrove estuary, Piauí River (NE of Brazil). Estuaries and Coasts, 32, 111-122. of 13 molCm-2 y-1 and are above the average for tropical estuaries proposed by Borges et al. (2005)BORGES, A.V., DELILLE, B. & FRANKIGNOULLE, M. 2005. Budgeting sinks and sources of CO2 in the coastal ocean: Diversity of ecosystems counts. Geophysical Research Letters, 32. of 17 molC m-2 y-1.
The FCO2 average values found in the present study are comparable to those recently reported for 28 estuaries of the northern and northeastern regions of Brazil by Noriega et al. (2014)NORIEGA, C. E. D. & ARAUJO, M. 2014. Carbon dioxide emissions from estuaries of northern and northeastern Brazil. Scientific Reports, 4, 6164.. They proposed a mean value of 20±16 molC m-2 y-1during a normal rainfall season. The FCO2 averages found in the present study are also comparable to those values proposed by Borges (2005)BORGES, A. V. 2005. Do we have enough pieces of the jigsaw to integrate CO2 fluxes in the coastal ocean? Estuaries, 28, 3-27., for polluted environments at mid and high latitudes (46 molC m-2 y-1) and the average flux of 62 molC m-2 y-1 proposed by Frankignoulle et al. (1998)FRANKIGNOULLE, M., ABRIL, G., BORGES, A., BOURGE, I., CANON, C., LIBERT, E. & THÉATE, J. M. 1998. Carbon dioxide emission from European estuaries. Science, 282, 434-436. and 68 molC m-2 y-1 by Chen et al. (2012)CHEN, N. & HONG, H. 2012. Integrated management of nutrients from the watershed to coast in the subtropical region. Current Opinion in Environmental Sustainability, 4, 233-242. for the inner portion of estuaries.
CONCLUSION
The low correlations of pCO2 with river discharges, which we found in both estuaries, is due to the high alkalinity freshwater input and the predominance of biogeochemical processes of production/remineralization of organic matter, from natural and anthropogenic sources. The high residence time of both estuaries and especially in Barra de Jangadas estuary leads to a CO2 enrichment of its waters and consequently a pH decrease despite the reduced organic load when compared to the Capibaribe estuary. Such intense organic load is also increasing the nutrients concentrations and lowering the %DO in the study area and also promoting phytoplanktonic blooms during the dry season when the conditions of transparency and high residence time of the waters are favorable to its development. The extreme precipitation event in the Barra de Jangadas estuary led to an increase of nearly 300% in the average pCO2 values during the months of extreme rainfall. The high TA values found in the freshwater of both estuaries may have a major component of organic alkalinity as seen by the high residual organic loads which these environments are subjected. The final alkalinity balance in these estuaries may constitute an additional buffer to estuarine proton inputs from organic matter respiration. However, further studies are needed to investigate the causes of the alkalinity variability in the area such as submarine groundwater discharges and organic alkalinity contribution. The CO2 fluxes found for both estuaries are considered high and are comparable to the proposed averages for polluted estuaries in high and intermediate latitudes, illustrating the effects of anthropogenic pressure on rivers and estuaries in densely populated tropical areas.
ACKNOWLEDGMENTS
We thank the National Counsel of Technological and Scientific Development (CNPq) (MCT/CNPq/CT-Hidro/CT-Infra nº. 38/2009), the Foundation for Science and Technology Support of Pernambuco (FACEPE/FAPESP- APQ-0074-1.08/11) for their financial support for this project and the Research Support Foundation of the State of São Paulo (FAPESP/FACEPE/ANR 2011/50582-0) for the cooperation activities developed in scope of the thematic project. We also thank the Coordination for the Improvement of Higher Education Personnel (CAPES) for scholarship support.
REFERENCES
- ABRIL, G., ETCHEBER, H., BORGES, A. V. & FRANKIGNOULLE, M. 2000. Excess atmospheric carbon dioxide transported by rivers into the Scheldt estuary. Sciences - Series IIA - Earth and Planetary Science, 330, 761-768.
- ABRIL, G. & FRANKIGNOULLE, M. 2001. Nitrogen-alkalinity interactions in the highly polluted scheldt basin (belgium). Water Research, 35, 844-850.
- ABRIL, G. & BORGES, A. 2005. Carbon dioxide and methane emissions from estuaries. In: TREMBLAY, A., VARFALVY, l., ROEHM, C. & GAMEAU, M. (eds) Greenhouse gas emissions-fluxes and processes, hydroelectric reservoirs and Natural Environments. Berlim Heildelberg New York: Springer.
- ABRIL, G., MARTINEZ, J., ARTIGAS, L. F., MOREIRA-TURCQ, P., BENEDETTI, M. F., VIDAL, L., MEZIANE, T., KIM, J., BERNARDES, M. C., SAVOYE, N., DEBORDE, J., SOUZA, E. L., ALBÉRIC, P., SOUZA, M. F. L &. ROLAND, F. 2014. Amazon River carbon dioxide outgassing fuelled by wetlands. Nature, 505, 395-398.
- APHA - AMERICAN PUBLIC HEALTH ASSOCIATION. 1995. Standard methods for the examination of water and wastewater, Washington, APHA.
- ARAUJO, M., MEDEIROS, C. & RIBEIRO, C 1999. Energy balance and time-scales of mixing and stratification in the Jaboatão estuary, NE-Brazil. Revista Brasileira de Oceanografia, 47, 145-154.
- ARAUJO, M., NORIEGA, C., VELEDA, D. & LEFÈVRE, N. 2013. Nutrient input and CO2 flux of a tropical coastal fluvial system with high population density in the Northeast region of Brazil. Journal of Water Resource and Protection, 5, 362-375.
- BORGES, V., DELILLE, B., SCHIETTECATTE, L., GAZEAU, F., GAZEAU, F., ABRIL, G. & FRANKIGNOULLE, M. 2004. Gas transfer velocities of CO2 in three European estuaries (Randers Fjord, Scheldt, and Thames). Limnology and Oceanography, 49, 1630-1641.
- BORGES, A.V., DELILLE, B. & FRANKIGNOULLE, M. 2005. Budgeting sinks and sources of CO2 in the coastal ocean: Diversity of ecosystems counts. Geophysical Research Letters, 32.
- BORGES, A. V. 2005. Do we have enough pieces of the jigsaw to integrate CO2 fluxes in the coastal ocean? Estuaries, 28, 3-27.
- BORGES, A. V. & GYPENS, N. 2010. Carbonate chemistry in the coastal zone responds more strongly to eutrophication than to ocean acidification. Limnology Oceanography, 55, 346-353.
- BORGES, A.V. 2011. Present day carbon dioxide fluxes in the coastal ocean and possible feedbacks under global change. In: DUARTE, P. & SANTANA-CASIANO, J. M. (eds.) Oceans and the atmospheric carbon content Dordrecht: Springer Netherlands. 47-77.
- CAI, W. & WANG, Y. 1998. The chemistry, fluxes, and sources of carbon dioxide in the estuarine waters of the Satilla and Altamaha Rivers, Georgia. Limnology and Oceanography, 43, 657-668.
- CAI, W. 2011. Estuarine and coastal ocean carbon paradox: CO2 sinks or sites of terrestrial carbon incineration? Annual Review of Marine Science, 3, 123-145.
- CAI, W., HU, X., HUANG, W., MURRELL, M. C., LEHRTER, J. C., LOHRENZ, S. E., CHOU, W., ZHAI, W., HOLLIBAUGH, J. T., WANG, Y., ZHAO, P., GUO, X., GUNDERSEN, K., DAI, M. & GONG, G. 2011. Acidification of subsurface coastal waters enhanced by eutrophication. Nature Geoscience, 4, 766-770.
- CHEN, C. T. A. 2004. Exchanges of carbon in the coastal seas. In: Field C. B. & RAUPACH M. R. (eds) The global carbon cycle: Integrating human, climate and the natural world Washungton: Island Press
- CHEN, C. T. A., HUANG, T. H., FU, Y., HE, X. & BAI, Y. 2012. Strong sources of CO2 in upper estuaries become sinks of CO2 in large river plumes. Current Opinion in Environmental Sustainability, 4, 179-185.
- CHEN, C. T. A., HUANG, T. H., CHEN, Y. C., HE, X. & KANG, Y. 2013. Air-sea exchanges of CO2 in the world's coastal seas. Biogeosciences, 10, 6509-6544.
- CHEN, N. & HONG, H. 2012. Integrated management of nutrients from the watershed to coast in the subtropical region. Current Opinion in Environmental Sustainability, 4, 233-242.
- COLE, B. E. & CLOERN, J. E. 1987. An empirical model for estimating phytoplankton productivity in estuaries. Marine Ecology Progress Series, 36, 299-305.
- COLIJN, F. 1982. Light absorption in the waters of the Ems-Dollard estuary and its consequences for the growth of phytoplankton and microphytobenthos. Netherlands Journal of Sea Research, 15, 196-216.
- COTOVICZ JR, L. C., LIBARDONI, B. G., BRANDINI, N., KNOPPERS, B, A. & ABRIL, G. 2016. Comparações entre medições em tempo real da pco2 aquática com estimativas indiretas em dois estuários tropicais contrastantes: o estuário eutrofizado da baía de guanabara (RJ) e o estuário oligotrófico do Rio São Francisco (al). Química Nova, 39, 1206-1214.
- CPRH (Agencia estadual de meio ambiente e recursos hídricos). 2006. Relatório de monitoramento de bacias hidrográficas do estado de Pernambuco 2011, Recife [cited 2013 Oct 15]. Available from: http://www.cprh.pe.gov.br
» http://www.cprh.pe.gov.br - DUARTE, C. M., HENDRIKS, I. E., MOORE, T. S., OLSEN, Y. S., STECKBAUER, A., RAMAJO, L., CARSTENSEN, J., TROTTER, J. A. & MCCULLOCH, M. 2013. Is ocean acidification an open-ocean syndrome? Understanding anthropogenic impacts on seawater pH. Estuaries and Coasts, 36, 221-236.
- DICKSON, A. G. 1990a. Standard potential of the reaction: AgCl (s)+ 12H2 (g)= Ag (s)+ HCl (aq), and and the standard acidity constant of the ion HSO4− in synthetic sea water from 273.15 to 318.15 K. The Journal of Chemical Thermodynamics, 22, 113-127.
- DICKSON, A. G. 1990b. Thermodynamics of the dissociation of boric acid in synthetic seawater from 273.15 to 318.15 K. Deep Sea Research Part A. Oceanographic Research Papers, 37, 755-766.
- FEITOSA, F. A. N.; NASCIMENTO, F. C. R; COSTA, K. M. P. Distribuição espacial e temporal da biomassa fitoplanctônica relacionada com parâmetros hidrológicos na Bacia do Pina (Recife-PE). Trabalhos Oceanográficos da Universidade Federal de Pernambuco, v. 27, n. 2, p. 1-13, 1999.
- FRANKIGNOULLE, M., BOURGE, I. & WOLLAST, R. 1996. Atmospheric CO2 fluxes in a highly polluted estuary (the Scheldt). Limnology and Oceanography, 41, 365-369.
- FRANKIGNOULLE, M., ABRIL, G., BORGES, A., BOURGE, I., CANON, C., LIBERT, E. & THÉATE, J. M. 1998. Carbon dioxide emission from European estuaries. Science, 282, 434-436.
- FLORES MONTES, M. DE J., PAULO, J. G., NASCIMENTO-FILHO, G. A., GASPAR, F. L., FEITOSA, F. A., SANTOS-JÚNIOR, A. C., BATISTA, T. N. F., TRAVASSOS, R. K. & PITANGA, M. E. 2011. The trophic status of an urban estuarine complex in Northeast Brazil. Journal of Coastal Research, 64, 408-411.
- GARCIA, H. E. & GORDON, L. I. 1992. Oxygen solubility in seawater: Better fitting equations. Limnology and Oceanography, 37, 1307-1312.
- GORDON, D. C.; BOUDREAU, P. R.; MANN, K. H.; ONG, J.-E., SILVERT, W. L.; SMITH, S. V.; WATTAYAKORN, G.; WULLF, F. & YANAGI, T. 1996. LOICZ biogeochemical modelling guidelines, Yerseke, Netherlands Institute for Sea Research.
- GRASSHOFF, K., EHRHARDT, M. & KREMLING, K. 1983. Methods of Seawater Analysis, Weinheim, Wiley-Verlag.
- GUENTHER, M., ARAÚJO, M., FLORES-MONTES, M., GONZALEZ-RODRIGUEZ, E. & NEUMANN-LEITÃO, S. 2015. Eutrophication effects on phytoplankton size-fractioned biomass and production at a tropical estuary. Marine pollution bulletin, 91, 537-547.
- HOWARD J., BABIJ, E., GRIFFIS, R., HELMUTH, B., HIMES-CORNELL, A., NIEMIER, P., ORBACH, M., PETES, L., ALLEN, S., AUAD, G., AUER, C., BEARD, R., BOATMAN, M., BOND, N., BOYER, T., BROWN, D., CLAY, P., CRANE, K., CROSS, S., DALTON, M., DIAMOND, J., DIAZ, R., DORTCH, Q., DUFFY, E., FAUQUIER, D., FISHER, W., GRAHAM, M., HALPERN, B., HANSEN, L., HAYUM, B., HERRICK, S., HOLLOWED, A., HUTCHINS, D., JEWETT, E., JIN, D., KNOWLTON, N., KOTOWICZ, D., KRISTIANSEN, T., MORRISON, J. R., MURRAY, J., NORMAN, K., O'DONNELL, J., OVERLAND, J., PARSONS, R., PETTIGREW, N., PFEIFFER, L., PIDGEON, E., PLUMMER, M., POLOVINA, J., QUINTRELL, J., ROWLES, T., RUNGE, J., RUST, M., SANFORD, E., SEND, U., SINGER, M., SPEIR, C., STANITSKI, D., THORNBER, C., WILSON, C. & XUE, Y. 2013. Oceans and marine resources in a changing climate. In: HUGHES, R.N., HUGHES, D. J. & SMITH, I. P. (eds) Oceanography and Marine Biology: An Annual Review Boca Raton: CRC Press. 51, 71-192.
- IBGE (Instituto Brasileiro de Geografia Estatística). Censo 2010 [cited 2013 Oct]. Available from: http://www.censo2010.ibge.gov.br/
» http://www.censo2010.ibge.gov.br/ - JIANG, L. Q., CAI, W. J. & WANG, Y. 2008. A comparative study of carbon dioxide degassing in river-and marine-dominated estuaries. Limnology and Oceanography, 53, 2603-2615.
- KO, Y. H., LEE, K., EOM, K. H. & HAN, I. S. 2016. Organic alkalinity produced by phytoplankton and its effect on the computation of ocean carbon parameters. Limnology and Oceanography, 61, 1462-1471.
- KOENING, M. L., MACÊDO, S. J., TRAVASSOS, P. E. P. F. & PASSAVANTE, J. Z. O. 1995. Biomassa fitoplanctônica do estuário do rio Capibaribe (Recife - PE - Brasil). Arquivos de Biologia e Tecnologia, 38, 1071-1083.
- KULIŃSKI, K., SCHNEIDER, B., HAMMER, K., MACHULIK, U. & SCHULZ-BULL, D. 2014. The influence of dissolved organic matter on the acid-base system of the Baltic Sea. Journal of Marine Systems, 132, 106-115.
- MARTINS, S. E. M., BARCELLOS, R. L., FLORES-MONTES, M. J. & FRANÇA, E. J. 2016. Depositional evolution in a estuarine lagoonal system under a port influence in Northeastern Brazil. Journal of Coastal Research, 75, 84-88.
- MCLUSKY, D. S. 1992. Marine and estuarine gradientes, Marine Pollution Bulletin, 24, 55-56.
- MILLERO, F, J., GRAHAM, T. B., HUANG, F., BUSTOS-SERRANO, H. & PIERROT, D. 2006. Dissociation constants of carbonic acid in seawater as a function of salinity and temperature. Marine Chemistry, 100, 80-94.
- NIXON, S. W., OCZKOWSKI, A. J., PILSON, M. E. Q., FIELDS, M., OVIATT, C. A. & HUNT, C. W. 2015. On the response of pH to inorganic nutrient enrichment in well-mixed coastal marine waters. Estuaries and Coasts, 38, 232-241.
- NORIEGA, C. & ARAUJO, M. 2009. Nitrogen and phosphorus loading in coastal watersheds in northeastern Brazil. Journal of Coastal Research, 56, 871-875.
- NORIEGA, C. E. D., ARAUJO, M. 2011. Nutrient budgets (C, N and P) and trophic dynamicsof a Brazilian tropical estuary: Barra das Jangadas. Anais da Academia Brasileira de Ciências, 83, 441-456.
- NORIEGA, C. E. D., ARAUJO, M. & LEFÈVRE, N. 2013. Spatial and temporal variability of the CO2 fluxes in a tropical, highly urbanized estuary. Estuaries and Coasts, 36, 1054-1072.
- NORIEGA, C. E. D., ARAUJO, M., LEFÈVRE, N., FLORES MONTES, M., GASPAR, F. & VELEDA, D. 2015. Spatial and temporal variability of CO2 fluxes in tropical estuarine systems near areas of high population density in Brazil. Regional Environmental Change, 15, 619-630.
- NORIEGA, C. E. D. & ARAUJO, M. 2014. Carbon dioxide emissions from estuaries of northern and northeastern Brazil. Scientific Reports, 4, 6164.
- OTSUKA, A. Y., FEITOSA, F. A. N., FLORES-MONTES, M. J. & SILVA, A. 2016. Dynamics of Chlorophyll a and Oceanographic Parameters in the Coastal Zone: Barra das Jangadas-Pernambuco, Brazil. Journal of Coastal Research, 32, 490-499.
- PAULO, J. G., FLORES MONTES, M. J., SANTOS JUNIOR, A. C., BATISTA, T. N. F., TRAVASSOS, R. K., NASCIMENTO FILHO, G. A., FEITOSA, F. A., GASPAR, F. L. & PITANGA, M. E. 2011. Allochthonous and autochthonous organic matter in an urban tropical estuarine area of northeastern Brazil. Journal of Coastal Research, 64, 1798-1801.
- PEEL, M. C., FINLAYSON, B. L. & MCMAHON, T. A. 2007. Updated world map of the Köppen-Geiger climate classification. Hydrology and Earth System Sciences, 11, 1633-1644.
- POOLE, H. H. & ATKINS, W. R. G. 1929. Photo-electric measurements of submarine illumination throughout the year. Journal of the Marine Biological Association of the United Kingdom, 16, 297-324.
- RAYMOND, P. A. & COLE, J. J. 2001. Gas exchange in rivers and estuaries: Choosing a gas transfer velocity. Estuaries and Coasts, 24, 312-317.
- ROBBINS, L. L., HANSEN, M. E., KLEYPAS, J. A. & MEYLAN, S. C. 2010. CO2calc: A user-friendly seawater carbon calculator for Windows, Mac OS X, and iOS (iPhone), Washington, US Geological Survey.
- RESURREIÇÃO, M. G., PASSAVANTE, J. Z. O. & MACEDO, S. J. 1996. Estudo da Plataforma Continental na área do Recife (Brasil): variação sazonal da biomassa fitoplanctónica. Trabalhos Oceanográficos da Universidade Federal de Pernambuco, 39-59.
- RADTKE, D. B., WILDE, F. D., DAVIS, J. V., & POPOWSKI, T. J. 2012. Alkalinity and acid neutralizing capacity (version 4.0). National field manual for the collection of water-quality data: US Geological Survey techniques of water-resources investigations, Reston, US Geological Survey.
- SABINE, C. L., FEELY, R. A., FEELY, N., KEY, R. M., LEE, K., BULLISTER, J. L., WANNINKHOF, R., WONG, C. S., WALLACE, D. W. R., TILBROOK, B., MILLERO, F. J., PENG, T. H., KOZYR, A., ONO, T. & RIOS, A. F. 2004. The oceanic sink for anthropogenic CO2 Science, 305, 367-71. DOI: 10.1126/science.1097403
» https://doi.org/10.1126/science.1097403 - SARMA, V. V. S. S., VISWANADHAM, R., RAO, G. D., PRASAD, R, V., KUMAR, B. S. K., NAIDU, S. A., KUMAR, N. A., RAO, D. B., SRIDEVI, T., KRISHNA, M. S., REDDY, N. P. C., SADHURAM, Y. & MURTY, T. V. R. 2012. Carbon dioxide emissions from Indian monsoonal estuaries. Geophysical Research Letters, 39.
- SILVA, L F. 1995. Solos tropicais: aspectos pedológicos, ecológicos e de manejo, São Paulo, Terra Brasilis.
- SOUZA, M. F. L., GOMES, V. R., FREITAS, ANDRADE, R. C. B. & KNOPPERS, B. 2009. Net ecosystem metabolism and nonconservative fluxes of organic matter in a tropical mangrove estuary, Piauí River (NE of Brazil). Estuaries and Coasts, 32, 111-122.
- STRICKLAND, J. D. H & PARSONS, T. R. 1972. A Practical Handbook of Seawater Analysis, Ottawa, Fisheries Research Board of Canada.
- THOMAS, H., SCHIETTECATTE, L. S., SUYKENS, KONÉ, Y. J. M., SHADWICK, E. H., PROWE, A. E. F., BOZEC, Y., BAAR, H. J. W. & BORGES, A. V. 2009. Enhanced ocean carbon storage from anaerobic alkalinity generation in coastal sediments. Biogeosciences, 6, 267-274.
- TRAVASSOS, P. E. P. F., MACÊDO, S. J. & KOENING, M. L., 1993. Aspectos hidrológicos do estuário do rio Capibaribe (Recife, PE). Trabalhos do Instituto de Oceanografia da Universidade Federal de Pernambuco, 22, 9-38.
- TRAVASSOS, R. K., FLORES MONTES, M. J., COSTA, B. V, M. & SILVA JÚNIOR, J, M. 2016. The Influence of Urban Effluents on the Elemental C/N Ratio in a Tropical Coastal Area of Northeastern Brazil. Journal of Coastal Research, 75, 168-172.
- TRENBERTH, K. E. 2011. Changes in precipitation with climate change. Climate Research, 47, 123-138.
- UNESCO. 1966. Determination of Photosynthetic Pigments in Sea-water, Paris: UNESCO.
- UNESCO. 1973. International oceanographic tables National Institute of Oceanography of Great Britain, Wormley, and United Nations Educational, Scientific and Cultural Organization (UNESCO), Paris, France.
- VOLLENWEIDER, R, A., TALLING, J. F. & WESTLAKE, D. F. 1974. A manual on methods for measuring primary production in aquatic environments, Oxford, Blackwell Scientific Pub.
- WANG, Z. A. & CAI, W. J. 2004. Carbon dioxide degassing and inorganic carbon export from a marsh-dominated estuary (the Duplin River): A marsh CO2 pump. Limnology and Oceanography, 49, 341-354.
- WEISS, R. F. 1974. Carbon dioxide in water and seawater: the solubility of a non-ideal gas. Marine chemistry, 2, 203-215.
- VAN DER WEIJDEN, C. H. 1992. Early Diagenesis and Marine Pore Water. Developments in Sedimentology, 47, 13-134.
- WOLF-GLADROW, D. A., ZEEBE, R. E., KLAAS, C., KÖRTZINGER, A. & DICKSON, A. G. 2007. Total alkalinity: The explicit conservative expression and its application to biogeochemical processes. Marine Chemistry, 106, 287-300.
- ZAPPA, C. J., RAYMOND, P. A., TERRAY, E. A. & MCGILLES, W. R. 2003. Variation in surface turbulence and the gas transfer velocity over a tidal cycle in a macro-tidal estuary. Estuaries and Coasts, 26, 1401-1415.
- ZEEBE, R. E. & WOLF-GLADROW, D. 2001. CO2 in Seawater: Equilibrium, Kinetics, Isotopes, New York, Elsevier.
- ZHAI, W., DAI, M., CAI, W. J., WANG, Y. & WANG, Z. 2005. High partial pressure of CO2 and its maintaining mechanism in a subtropical estuary: the Pearl River estuary, China. Marine Chemistry, 93, 21-32.
Publication Dates
-
Publication in this collection
Jan-Mar 2018
History
-
Received
30 June 2017 -
Accepted
21 Feb 2018