Abstracts
Tuberculosis remains a serious social and public health problem, affecting millions of people annually. The bacille Calmette-Guerin (BCG) vaccine, used prophylactically, does not impede the progression of the disease, which usually manifests as decreased cellular immunity. Early diagnosis, together with polychemotherapy, can control the dissemination of the tuberculosis infection. The current diagnostic methods present certain problems. Such problems include the low sensitivity of sputum smear microscopy, the fact that performing microbiological cultures is quite time-consuming, and the low specificity of the skin test with the purified protein derivative of M. tuberculosis. New diagnostic methods, which use specific antigens such as the early secreted antigenic target 6-kDa and culture filtrate protein 10kDa, are being evaluated. The genes that encode these antigens are located in the DNA region of difference 1 of M. tuberculosis, M. africanum and M. bovis. However, they are absent from the M. bovis (BCG) and from most environmental mycobacteria. Diagnostic methods such as QuantiFERON-TB® and T SPOT.TB®, which are based on the production of interferon-gamma by T lymphocytes, in response to those antigens, are being tested and have been found to outstrip the purified protein derivative skin test in the following characteristics: greater sensitivity; lower cross-reactivity due to BCG vaccination or infection with environmental mycobacteria; and execution time. The introduction of diagnostic methods that are more specific and sensitive, together with gaining a better understanding of the molecular and cellular mechanisms that regulate the parasite-host interaction, can increase the efficiency of strategies devised to combat tuberculosis.
Tuberculosis; Mycobacterium tuberculosis; Diagnosis; Antigens; bacterial/ESAT-6 protein; Immunity
A tuberculose continua sendo um grave problema social e de saúde, afetando milhões de pessoas anualmente. A vacina Bacille Calmette-Guerin (BCG), usada no controle profilático, é incapaz de conter a progressão da doença, que usualmente se manifesta através da queda da imunidade celular do indivíduo. O diagnóstico da tuberculose em seus estágios iniciais, aliado à poliquimioterapia, pode contribuir para o controle da disseminação da infecção. Os atuais métodos de diagnóstico apresentam problemas, como: baixa sensibilidade da baciloscopia; longo tempo de realização das culturas microbiológicas; e baixa especificidade do teste cutâneo com o derivado protéico purificado do M. tuberculosis. Novos métodos de diagnóstico que utilizam antígenos específicos (por exemplo, os conhecidos em inglês como o early secreted antigenic target 6-kDa e o culture filtrate protein 10-kDa), estão sendo testados. Os genes que codificam esses antígenos estão localizados na região de diferença 1 do M. tuberculosis, M. africanum e M. bovis, mas estão ausentes no M. bovis (BCG) e na maioria das micobactérias do meio ambiente. Métodos de diagnóstico baseados na produção de interferon-gama por linfócitos T, em resposta a esses antígenos, como o QuantiFERON-TB® e o T SPOT.TB®, estão sendo testados, e superam o teste cutâneo com o derivado protéico purificado nas seguintes características: maior sensibilidade; menor reatividade cruzada devido à vacinação com o BCG ou infecção por micobactérias do meio ambiente; e tempo de execução. A introdução de métodos de diagnóstico mais específicos e sensíveis, assim como um maior entendimento dos mecanismos moleculares e celulares que regulam a interação parasito-hospedeiro, pode contribuir para um eficiente combate à tuberculose.
Tuberculose; Mycobacterium tuberculosis; Diagnóstico; Antígenos de bactérias; Proteínas de bactérias; Imunidade
REVIEW ARTICLE
Immunological diagnosis of tuberculosis: problems and strategies for success* Correspondence to: Dr. Martin Emilio Munk Genmab A/S, Toldbodgade 59B, 1253 Copenhagen, Denmark Phone 00 45 2540-3016 E-mail: mmu@genmab.com
Henrique Couto TeixeiraI; Clarice AbramoII; Martin Emilio MunkIII
IAssociate Professor of Immunology at the Universidade Federal de Juiz de Fora UFJF, Federal University of Juiz de Fora Juiz de Fora (MG) Brazil
IIAdjunct Professor of Parasitology at the Universidade Federal de Juiz de Fora UFJF, Federal University of Juiz de Fora Juiz de Fora (MG) Brazil
IIIPhD in Medicine from the University of Ulm Ulm, Germany
Correspondence to Correspondence to: Dr. Martin Emilio Munk Genmab A/S, Toldbodgade 59B, 1253 Copenhagen, Denmark Phone 00 45 2540-3016 E-mail: mmu@genmab.com
ABSTRACT
Tuberculosis remains a serious social and public health problem, affecting millions of people annually. The bacille Calmette-Guerin (BCG) vaccine, used prophylactically, does not impede the progression of the disease, which usually manifests as decreased cellular immunity. Early diagnosis, together with polychemotherapy, can control the dissemination of the tuberculosis infection. The current diagnostic methods present certain problems. Such problems include the low sensitivity of sputum smear microscopy, the fact that performing microbiological cultures is quite time-consuming, and the low specificity of the skin test with the purified protein derivative of Mycobacterium tuberculosis. New diagnostic methods, which use specific antigens such as the early secreted antigenic target 6-kDa and culture filtrate protein 10 kDa, are being evaluated. The genes that encode these antigens are located in the DNA region of difference 1 of M. tuberculosis, M. africanum and M. bovis. However, they are absent from the M. bovis (BCG) and from most environmental mycobacteria. Diagnostic methods such as QuantiFERON-TB® and T SPOT.TB®, which are based on the production of interferon-gamma by T lymphocytes, in response to those antigens, are being tested and have been found to outstrip the purified protein derivative skin test in the following characteristics: greater sensitivity; lower cross-reactivity due to BCG vaccination or infection with environmental mycobacteria; and execution time. The introduction of diagnostic methods that are more specific and sensitive, together with gaining a better understanding of the molecular and cellular mechanisms that regulate the parasite-host interaction, can increase the efficiency of strategies devised to combat tuberculosis.
Keywords: Tuberculosis; Mycobacterium tuberculosis; Diagnosis; Antigens, bacterial/ESAT-6 protein; Immunity.
Tuberculosis as a global problem
Mycobacterium tuberculosis is responsible for tuberculosis (TB), a disease that annually affects 8 to 9 million people worldwide, accounting for approximately 2 to 3 million deaths each year. India, China, Indonesia, Bangladesh, and Pakistan, the most heavily populated countries in Asia, present the highest incidence of the disease and, together, have over half the cases in the world.,In Brazil, the number of new cases is nearly 100,000/year.(1,2) It is estimated that the latent form of TB affects approximately one-third of mankind. Individuals with latent TB constitute a large reservoir of M. tuberculosis, although, in this phase of infection, the microorganisms are metabolically inactive, which has been associated with the absence of clinical manifestations in these infected individuals.(3,4) The increase in the incidence of TB, as of 1990, has been related to the onset of the AIDS epidemic, as well as to the emergence of multidrug-resistant strains.(13) The principal characteristic of HIV infection is the gradual destruction of CD4+ T lymphocytes, which play a key role in the immune response to M. tuberculosis and in the immunological diagnosis of TB.
The natural history of TB shows that most individuals- are resistant to the infection, probably due to their capacity to generate an efficient immune response to M. tuberculosis. However, such individuals are incapable of completely ridding themselves of the bacteria. Of the individuals exposed to M. tuberculosis, 10 to 30% become infected. Of those, only 5 to 10% develop active TB. Consequently, the TB can be disseminated or localized (in the pulmonary, lymph node, renal, or bone forms) and can also affect any other organ.(3)
Among susceptible immunocompetent individuals with active TB, 85% present the exclusively pulmonary form. In such individuals, the infection with M. tuberculosis is not associated with greater susceptibility to other infectious agents. However, many patients can present a state of immunosuppression against M. tuberculosis-specific antigens, which can favor the accelerated growth of the bacilli and the development of the disease. In immunocompromised individuals, such as those infected with HIV, TB can frequently become a disseminated disease, most commonly extrapulmonary TB.
Immune response to M. tuberculosis
Since TB is basically a pulmonary disease, the lung is the point of entry for the microorganism and the principal manifestation site of the infection. Immediately after a primary infection, air particles, alveolar macrophages, and dendritic cells, which phagocytosed the M. tuberculosis, migrate through the lymphatic system toward the regional lymph node, forming the Ghon complex. Simultaneously, phagocytic cells can penetrate the pulmonary parenchyma, initiating an inflammatory focus to which other macrophages will be attracted. In this case, the accumulation of inflammatory cells around the microorganism initiates the formation of a granuloma, coordinated by T lymphocytes. The T cells become indispensable to the formation of stable granulomas, contacting mononuclear phagocytes and influencing their differentiation and activation status. The M. tuberculosis is contained in the granuloma, and can persist in the lesions for decades, in latent form, without triggering the disease.
Immunosuppression, either due to the poor health status of the individual, HIV infection, or use of immunosuppressants, is the most frequent cause of the multiplication of bacilli enclosed in the granuloma and of the reactivation of TB (endogenous reactivation), as compared to the reinfection (exogenous) with M. tuberculosis.(2,4)
Macrophages in the tissue constitute one of the first lines of defense against mycobacteria. After being phagocytosed, the bacilli remain within the phagosome. After the phagosome-lysosome fusion, antigens can be processed and subsequently presented to T- helper (Th) lymphocytes (CD4+), through the major histocompatibility complex class II (MHC II) molecules (also known as antigen-presenting cells), which are found only in macrophages, dendritic cells, and B lymphocytes. It is known that T-helper type 1 (Th1) CD4+ cells play the principal role in the immune response to mycobacteria.(3) However, cytotoxic T cells (CD8+), which recognize antigens from the cytoplasm (tumor or viral), also participate in the immune response to M. tuberculosis.(3) The CD8+ T cells can recognize peptide fragments bound to MHC class I cells, which are expressed in practically all differentiated or mature cells of the organism. In the case of mycobacteria, it has been demonstrated that apoptotic vesicles from infected cells containing antigens of the bacillus associated with MHC class I can specifically stimulate CD8+ T cells.(5) Alternatively, in a phenomenon known as cross-presentation, antigens of intracellular pathogens can directly access the presentation via MHC class I cells, owing to the capacity of the phagosomes to fuse with the endoplasmic reticulum, and to the protein recruitment from the endoplasmic reticulum to the phagosome. Consequently, phagocytosed antigens can access the cytoplasm, suffer degradation by proteases, known as proteasomes, return to the phagosome through transporters associated with antigen processing (TAPs), and bind to MHC class I molecules located in the phagosome, leading to the subsequent expression on the cell surface and to the recognition by CD8+ cells.(6,7)
Atypical lymphocytes (CD4- and CD8-) have receptors containing gamma/delta polypeptide chains and recognize phosphoric components of M. tuberculosis,(8) regardless of MHC class I or II, whereas T lymphocyte receptors restricted only to CD1 can be stimulated by glycolipids derived from the cell wall of the mycobacteria.(9) Therefore, the immune system can recognize and effectively respond to a broad spectrum of antigenic determinants of different biochemical characteristics. In this recognition, there is a hierarchy among the T cell subpopulations that contribute to the immune response to mycobacteria, and the CD4+ and CD8+ T lymphocytes are the most important in this hierarchy.(10,11)
Regarding the innate immune response, neutrophils are the first inflammatory cells to arrive at the bacillus multiplication site, followed by natural killer (NK) cells and macrophages. The NK cells can destroy pathogens directly or by killing the infected monocytes, as well as being able to activate phagocytic cells at the site of the infection.(3) However, it has been shown that mice depleted of NK1.1 cells do not present greater susceptibility to mycobacterial infection.(12) The recognition and phagocytosis of bacteria by innate immunity cells (neutrophils, macrophages, and dendritic cells) occur via recognition receptors, such as the mannose receptor, receptors for the Fc portion of antibodies (FcRs), and receptors for activation products of the complement system, such as C3b and C4b (CR1), among others.(3) The activation of standard recognition receptors, such as Toll-like receptors (TLRs), leads to an important connection between innate and acquired immune response. The expression of co-stimulating molecules such as CD80 and CD86, on the surface of macrophages and dendritic cells, is induced after TLRs recognize specific molecules of the pathogens, such as lipoarabinomannans, lipoproteins and other lipid derivatives of M. tuberculosis.(13,14) The activation of CD4+ lymphocytes involves the recognition of the peptide bound to MHC class II and the interaction between co-stimulating molecules, such as the CD80/CD86-CD28 interaction. In addition, cytokines produced by antigen-presenting cells, such as interleukin (IL)-12, and cytokines produced by activated T lymphocytes, such as IL-2, are involved in the activation and proliferation of T lymphocytes. Consequently, M. tuberculosis-specific antigens interact with TLRs and other receptors present on the surface of macrophages and dendritic cells, thereby inducing a predominantly pro-inflammatory cellular immune response (Figure 1).
Cytokines, molecules produced and secreted by different immunocompetent cells after some stimulus, are a central component in the defense against mycobacteria. At all stages of the immune response, the cytokines produced participate in the regulatory processes, as well as in effector functions.(3) The recognition of the mycobacteria and posterior secretion of IL-12 by macrophages are processes initiated before the M. tuberculosis antigens are presented to T lymphocytes (Figure 1). The production of interferon-gamma (IFN-g) in NK cells is induced by IL-12 in the initial phase of the immune response. In addition, IL-12 induces the activation, differentiation, and production of IFNg, as well as the expansion of antigen-specific Th1 cells. Recently, other cytokines have been described, produced by macrophages and dendritic cells, which present similar activity to that of IL12.(15) The production of IFN-g is also induced by IL-23, IL18, and IL27, a process that is accelerated when IL18 and IL27 act in synergy with IL-12 (Figure 1). It is believed that IL-27 acts in an early phase of the immune response, preceding IL-12 in the inducement of the production of IFN-g, whereas IL-12 presents strong activity in the amplification of IFN-g production and Th1 lymphocyte expansion at a subsequent stage(15) (Figure 1). Constituting the principal source of IL2 and IFN-g during the acquired immune response, Th1 cells are necessary for the control of the chronic phase of the infection, due to the effect that IL2 and IFN-g have on T cells and macrophages. Produced by dendritic cells and macrophages, IL-12 is active in T cells, forming a link between the innate and acquired responses. Individuals with mutations in genes IL-12p40 and IL-12R present reduced T-cell production of IFN-g and are more susceptible to infections disseminated by the bacille Calmette-Guerin (BCG) vaccine and M. avium.(16)
The bactericidal capacity of the macrophage against M. tuberculosis needs to be previously activated, and IFN-g is the principal and most potent mediator of this process.(16,17) Increased production of IFN-g can have a variety of effects: increasing the expression of various genes in the macrophage; increasing the expression of the MHC (greater presentation of antigens) and of immunoglobulin receptors (FcRs; greater capacity for phagocytosis); recruiting T lymphocytes that participate in the destruction of bacteria; and promoting the production of nitric oxide. Although IFN-g production alone cannot control the bacillus, IFN-g is one of the crucial components of the protective response against the pathogen.(3,16-17) In synergy with tumor necrosis factor alpha (TNF-a), IFNg activates infected macrophages, initiating an important effector mechanism of the cell-mediated immunity. Due to its importance, defects in IFN-g genes or IFN-g receptors predispose individuals to serious mycobacterial infections.(18) Although the production capacity of IFN-g can vary among individuals, some studies suggest that IFN-g levels are decreased in patients with active TB.(19) These levels are even lower in patients with advanced pulmonary disease.(20) In addition, it has been demonstrated that M. tuberculosis can prevent macrophages from adequately responding to IFN-g.(21)
However, the importance of IFN-g in the protection against various pathogens, including parasites, bacteria, and viruses, has been well established.(22) Therefore, in various biological systems, the presence of IFN-g or IFN-g-producing cells after antigen exposure is frequently used as a marker of effector cell activity. Cytokines such as IL-4, IL-5, and IL10, which are involved in the activation of B cells and the production of antibodies, are produced by Th2 cells. However, immunity against TB is mediated by Th1 cells. Nevertheless, it has been recently reported that, in human TB, in addition to the Th1-produced cytokines, IL-4 is produced.(23-24) It has been demonstrated that, due to the strong antagonist effect that IL-4 has on the Th1 response, that response can be jeopardized even when the Th2 response is weak.(25) The TLR2 expression and the activation of macrophages can be negatively regulated by IL4.(14) Recently, CD4+ and CD25+ regulatory T cells have been identified. These cells produce IL10 and transforming growth factor-beta, as well as expressing TLRs (which can react with mycobacteria) and participating in the suppression of protective immunity. Therefore, they constitute a potentially important factor at the onset of the infection, since they can influence the latency or progression of TB.(4)
In addition, the immune system contains molecules known as chemokines, which induce chemotaxis or signaling. Chemokines can potentially intensify the immune response through their capacity to recruit and focus distinct populations of leukocytes. In in vivo and in vitro murine models, M. tuberculosis induces the production of a variety of chemokines, including macrophage inflammatory protein 1-alpha (MIP-1a), MIP-2, monocyte chemoattractant protein 1 (MCP-1), MCP-3, MCP-5, and IFN-g-inducible protein 10.(26) The production of IFN-g can regulate that of various chemokines. The monokine induced by IFN-g (MIG, or CXCL9) can accomplish this task and be used as a sensitive and specific measure of IFN-g production. One of the primary effects of IFN-g release is macrophage production of MIG.(27) It is believed that MIG is an important mediator of the protective immune response. In fact, peripheral blood mononuclear cells of patients with TB produce MIG in response to M. tuberculosis-specific antigens, and this production is significantly lower in control individuals residing in an endemic area and vaccinated with BCG.(28)
Successes and problems in the prevention and diagnosis of TB
The principal measures to prevent the progression of TB worldwide include early diagnosis, effective treatment for resistant forms of TB, and the search for a vaccine that is more advanced and protective than the current BCG vaccine.(2) The low efficiency of the BCG vaccine has been demonstrated in epidemiologic studies carried out worldwide, and its efficacy against pulmonary TB ranges from 0 to 80%.(29) The principal causes of the low efficiency of the BCG vaccine can be related to the following factors: 1) exposure to environmental mycobacteria;(30) 2) genetic variations in the target population or in the vaccine strains; (4) 3) nutritional differences among vaccinated individuals;(29) and 4) co-infections.(31) Nevertheless, in areas of low TB prevalence, vaccination is recommended for children (except for children with AIDS), immediately after birth or at the first contact with public health facilities, in order to prevent meningitis.(32) Despite continuous efforts to develop more efficacious vaccines against TB, no new vaccines have as yet been approved. Even if a vaccine is developed, it will not prevent the progression of the active disease among the more than 2 billion people already infected with M. tuberculosis. Therefore, even if a new vaccine is implemented worldwide, more effective diagnostic systems and treatment will be necessary, in the decades to come, in order to contain TB.(1-2)
The diagnostic methods currently used, such as sputum smear microscopy, microbiological culture, chest X-rays, and the intradermal purified protein derivative (PPD) test, or tuberculin test, have not been as successful as expected, failing to significantly reduce the incidence of TB.(1-2) In individuals with pulmonary TB, the most common symptom at the onset of the disease is nonproductive cough. As the infection progresses, sputum production is induced by the increased inflammation and necrosis of the lung tissue. Consequently, sputum smear microscopy is the method of choice for diagnosis and control in the treatment of TB. The principal method for testing bacilli in sputum is the Ziehl-Neelsen (ZN) staining technique, which is an affordable method involving hot carbol fuchsin staining, followed by decolorization with acid-alcohol. Since only the mycobacteria are acid-fast, only they retain the red color. Fluorescence microscopy of auramine-stained smears presents accuracy equal to that of ZN staining, and requires less time to read. However, it is rarely used, since it demands trained personnel and is quite costly. In addition, slides testing positive in the fluorescence microscopy technique need to be confirmed through ZN staining. The principal disadvantage of sputum smear microscopy, despite its simplicity and low cost, is the fact that it provides false-negative results in 30 to 50% of individuals infected with M. tuberculosis, partially due to the need for at least 5000 bacilli/mL of sputum.(33)
The microbiological culture, generally used in suspected pulmonary cases and sputum smear microscopy negative cases, has the advantage of allowing the detection and isolation of the mycobacteria, the identification of the species and/or of the isolated complex, and the determination of the sensitivity of the microorganism to chemotherapeutic agents for TB. The principal culture media used are Löwenstein-Jensen (egg-based solid medium) and Middlebrook (solid or liquid, in agar medium). Despite its importance, the culture of M. tuberculosis is time-consuming, due to the slow growth of the bacillus (15-20 h), and the test does not always present 100% positivity.(1) Automated systems for the detection of mycobacteria, such as the BACTEC 460 TB®, BACTEC 9000® and the MGIT®, which use enriched media that promote the acceleration of bacterial growth, are promising, although they can also produce false-positive results due to contamination by other bacteria.(33) In addition to sputum, gastric aspirate, bronchoalveolar lavage fluid, transbronchial biopsy, urine, blood, and liquor, as well as pleural and peritoneal fluid, can be submitted to smear microscopy and culture for mycobacteria. The sputum induction technique, with ultrasonic nebulization of 3% hypertonic saline solution, has proven to be an easily performed alternative, presenting the best cost-benefit ratio for the diagnosis of pulmonary TB with nonproductive cough. This technique precedes invasive studies such as fiberoptic bronchoscopy and is always used in conjunction with sputum smear microscopy and mycobacteria culture.(33-34)
Chest X-rays are indicated as an auxiliary method in the diagnosis of TB in symptomatic and smear-negative patients, family of patients with active tuberculosis, and even in those suspected of having extrapulmonary TB. The method is based on the presence of characteristic radiographic opacities and is useful in the diagnosis of primary pulmonary TB (more homogeneous opacity and increase in the volume of regional lymph nodes) and secondary pulmonary TB (heterogeneous opacity, cavities, and nodules).(33) The radiologic analysis, however, is not a specific test to detect patients with TB, since pulmonary lesions similar to those caused by M. tuberculosis can occur in other diseases. In practice, chest X-rays and sputum tests are applicable in patients suspected of having pulmonary TB.(34) Although they are quite costly and are only available at referral centers, computed tomography scans of the chest are a high-resolution radiologic tool, more sensitive than chest X-rays.(33)
Since immunosuppression favors uncontrolled growth of M. tuberculosis in the lungs, as well as hematogenous dissemination and subsequent involvement of one or more extrapulmonary sites, computed tomography is more frequently used in immunocompromised patients, principally those infected with HIV. Extrapulmonary forms of TB are more difficult to diagnose, in part because they are less well known by physicians. Extrapulmonary TB can involve difficult-to-access sites; in addition, due to the nature of these sites, some bacilli can cause great damage. The combination of bacilli and difficult-to-access sites makes bacteriological confirmation difficult, and invasive processes are frequently needed in order to make a diagnosis. Due to the variety of organic systems involved in extrapulmonary and disseminated (miliary) TB, clinical manifestations vary greatly. The signs and symptoms presented are generally nonspecific and systemic, such as fever, weight loss, night sweats, anorexia, and weakness. Other symptoms are related to the severity of the disease in the organ involved.(34)
Due to its high positivity, the PPD test has been long used as an auxiliary method in the diagnosis of TB. It is also used as a screening method for the diagnosis of TB. Although the PPD test can detect infection with M. tuberculosis, including latent infection, it is insufficient for the diagnosis of TB as a disease. Originally developed by Robert Koch, in 1890, and formerly known as tuberculin, this method is based on the cellular reaction (accumulation of inflammatory cells) developed in the skin, 24 to 72 h after intradermal inoculation with PPD, a mixture of proteins of low molecular weight. The tuberculin used is PPD-RT23 (prepared by Statens Serum Institute, Copenhagen, Denmark) delivered intradermally, in the forearm, in a dose of 0.1 mL. An induration that is larger in diameter than a certain size (ranging from 5 mm to 15 mm, depending on the risk factors presented by the individual) is considered a positive result and indicates infection with M. tuberculosis. Despite its importance, the PPD test does not present 100% sensitivity (percentage of sick individuals who test positive) or specificity (percentage of healthy individuals who test negative). On average, 10 to 25% patients with active TB present no reaction to PPD, and specificity varies. Population surveys in areas that present different risk of infection with M. tuberculosis have shown a wide range of induration diameters, with significant differences among different geographical areas.
The PPD test has lower sensitivity in populations of immunocompromised patients, recently infected individuals, and very young children. Specificity is low, since the PPD contains various antigens widely shared among different species of mycobacteria, such as environmental mycobacteria, M. tuberculosis, M. bovis, and M. bovis (BCG).(35) Various studies have demonstrated that the PPD does not safely distinguish individuals vaccinated with BCG from those exposed to environmental mycobacteria or infected with M. tuberculosis.(36) The fact that the PPD test is still used, despite these limitations, speaks to the urgent need for TB diagnostic tests that are more specific.
Initiatives for a precise diagnosis of TB
An immunological diagnostic test is directly related to the immune response of the patient. Therefore, the advantage of an immunological test lies in its capacity to demonstrate whether the patient has been previously sensitized to the mycobacterium and confirm an infection, without the need to detect the bacillus in sputum or any other biological sample of the patient. An in vitro test, using a small peripheral blood sample of the patient, can be sufficient to perform a rapid immunological investigation.(37) The inclusion of positive controls (mitogens) in an immunological test allows us to distinguish immunocompetent individuals (who do not present a specific cellular response, but respond to mitogens) from immunocompromised individuals (HIV+ individuals who do not respond, or respond poorly, to specific antigenic stimuli and mitogens).
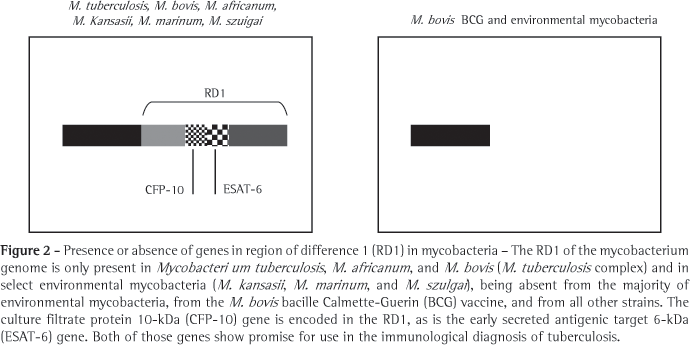
In the search for new antigens that would replace PPD, various regions of the genome of the M. tuberculosis have been defined as being expressed only by strains of M. tuberculosis; and are therefore not found in the strain used in the M. bovis (BCG) vaccine or in other species of mycobacteria.(38) Therefore, these genomic regions that encode M. tuberculosis-specific antigens are the principal instruments for the development of new methods for the diagnosis of TB, since they represent expressed molecules with great potential for the development of specific immune response. These regions, present in the genome of M. tuberculosis and absent from that of M. bovis (BCG), are known as regions of difference (RDs), 16 of which have been characterized.(39) In RD1 (Figure 2), at least two promising antigens for the detection of TB are encoded: early secreted antigenic target 6-kDa (ESAT-6),(38) and culture filtrate protein 10-kDa (CFP-10).(40) These two proteins are essentially present in pathogenic mycobacteria, such as M. tuberculosis, M. bovis and M. africanum.(41) The two, both of which are strongly immunodominant, are secreted in great quantity when these mycobacteria are cultured or infect the host.(35,42) Since molecules such as ESAT-6 and CFP10 are incapable of activating T cells at the onset of TB, molecules such as ESAT-6 and CFP-10 play an important role in certain stages of mycobacterial growth and intracellular survival. Some studies involving mutations in the genes that codify ESAT-6 and CFP-10 have shown lack of induction of T cells specific response and that the mycobacteria were rendered avirulent.(43) The practical consequence of this immunodominance is that these two molecules are ideal partners for a diagnostic method.(44-47) In addition, the two antigens can be used in isolation or together in the form of a recombinant hybrid molecule.(28,44)
Immunological tests related to IFN-g production by T cells, in response to antigens present in M. tuberculosis and absent in M. bovis (BCG), such as ESAT-6 and CFP-10, have been developed in an attempt to replace the PPD skin test.(35) Immunological tests are based on the concept that T cells of individuals previously sensitized by antigens of M. tuberculosis (memory T cells) release IFN-g when restimulated with M. tuberculosisspecific antigens. This test, in contrast to the PPD skin test, is performed ex vivo, that is, through the culture of a sample of cells from the peripheral blood of the patient, for 24 h, in the presence of M. tuberculosis antigens.(44) Consequently, the sensitized and specific cells produce and secrete IFN-g in the culture supernatant, which can be subsequently detected through an enzyme-linked immunosorbent assay (ELISA). High IFN-g production in response to M. tuberculosis-specific antigens indicates previous sensitization, although not necessarily in active disease. In this aspect, the IFN-g analysis derived from an immunological test is similar to that of the PPD test, that is, it is not easy to distinguish latent infection from active disease.(33,45-46)
Two tests based on the IFN-g production by T lymphocytes in culture, using antigens expressed by genes present in RD1, are commercially available. The first to be introduced was the QuantiFERON-TB® test (Cellestis Limited, Carnegie, Australia), approved by the United States Food and Drug Administration (FDA) in 2001, in which a sample of the peripheral blood is cultured in previous prepared plates, and the supernatant is analyzed using ELISA.(47-48) This first generation of immunological assays used the PPD as principal antigen and presented the same specificity problems as did the PPD skin test: high sensitivity but low specificity.(35) The initial test was replaced by the QuantiFERON-TB-Gold®, using ESAT-6 and CFP-10 rather than PPD. This new immunological assay was approved by the FDA in December of 2004.(47-48) The test offers some advantages, such as being performed in a single patient visit, providing results within 24 h, and not requiring a second challenge to the immune system of the individual, and is not affected by previous vaccination with BCG. However, the test requires processing the blood sample within 12 h after collection, and there are still few data related to its use in determining the risk of contracting TB.(49)
Another tool developed for IFN-g detection is the enzyme-linked immunospot (ELISPOT), in which the number of IFN-g production cells can be quantified.(35,50-52) In the ELISPOT, IFN-g molecules secreted by cells of the peripheral blood bind specifically to anti-IFN-g monoclonal antibodies previously immobilized on a plate, avoiding the problem of cytokine consumption during culture, thus increasing the sensitivity of the ELISPOT in comparison with the ELISA.The T SPOT.TB® assay (Oxford Immunotec, Oxon, UK) uses ESAT-6 and CFP-10 as specific antigens for the stimulation of blood T lymphocytes. This assay is awaiting FDA approval.(47)
Some studies have been developed in order to study the concordance between the PPD skin test and IFN-g detection tests, considering the PPD test as the standard determinant. Most studies demonstrate a concordance ranging from modest to high (60-80%) between the two tests.(5253) Various studies suggest that the tests directed at the detection of RD1 antigen-induced IFN-g production surpass the PPD test in terms of the following characteristics: specificity; correlation with indirect measurements of exposure to M. tuberculosis; lower cross reactivity with BCG vaccination or with infection caused by environmental mycobacteria; and faster laboratory test results.(35,46-47) Due to the lack of a standard determinant in the diagnosis of TB, it is impossible to define with precision the sensitivity and specificity of the tests that can quantify IFN-g production for the diagnosis of latent infection.(35)
Serologic testing can detect specific antibodies to mycobacteria in serum and is an attractive diagnostic method due to its ease of application, capacity to determine events related to humoral response after infection, and possible application in the diagnosis of the initial phase of the disease. Therefore, serology testing can be rapid and strong enough to be implemented in adverse conditions, such as those encountered in developing countries.(54) It is noteworthy that, in patients suffering from AIDS, in whom the number of T cells is decreased or even null, determining humoral response can be an invaluable tool in making an early diagnosis and gaining epidemiological control over TB.
Serologic tests present low sensitivity and specificity. This is due to the great heterogeneity of the humoral response in patients with TB and to the cross reactivity with other antigens, such as those in environmental mycobacteria, hindering their application.(55) Various trials have been conducted in attempts to improve serologic testing in the diagnosis of TB. For this reason, various antigenic preparations, such as bacterial suspensions, bacterial culture filtrates, bacterial extracts, and even PPD, have been studied. Obtaining purified and specific mycobacterial proteins has increased the potential of serologic testing in the diagnosis of TB. Various antigens of considerable serologic value, such as antigen 85A, 38-kDa protein, alpha-crystallin (16 kDa), MTB48, and PGL-Tb1, have been identified.(35) Some of these antigens are secreted or are present in the cell wall of the bacillus. Although the mycobacterium is an intracellular microorganism, thereby protected from the biological effect of antibodies, the fact that some secreted antigens are simultaneously immunodominant justifies the evaluation of the humoral response and its application in the diagnosis of TB. It is believed that, in the future, efficacious serologic assays will contain various specific antigens (an antigen cocktail) in order to evaluate the humoral immune response.(56-57)
Recently, a new diagnostic test for TB, known as the e-nose and capable of detecting volatile components in the serum, was introduced.(58) The volatile components are likely released from the lung into the circulation by the mycobacteria present in an active infection. This new method can distinguish changes in the physical properties of the serum (conductivity, resistance, and frequency) in response to certain chemical groups originating in the mycobacteria and has therefore been nominated as a potential diagnostic test for bovine TB. The test is easy to perform and affordable, which makes it possible to apply it on a large scale. However, it is unclear whether this method will be able to discriminate between infections caused by pathogenic mycobacteria and those caused by nonpathogenic mycobacteria. The effect of BCG vaccination on the sensitivity of this diagnostic method is equally uncertain.
The in vitro amplification of the DNA of the mycobacteria through polymerase chain reaction can in turn provide a rapid diagnostic response, although this method requires a specialized laboratory and trained personnel.(59,60) The method is not very practical in cases of extrapulmonary TB or in pediatric patients, in whom invasive procedures are required in order to obtain samples for analysis. Nevertheless, the comparison of results obtained in different laboratories has shown that there is great variety in the reproduction of this highly sensitive technique, resulting in a high index of false-positive results. In addition, methods of this kind that are commercially available do not reach the level of sensitivity achieved using the traditional culture method,(35) principally in the cases of negative sputum smear microscopy, commonly found in patients with HIV.
Final considerations
The rapid and specific diagnosis of TB is vital to the control of infection with the bacillus. Despite the enormous public health problem caused by TB worldwide, the medical resources available for treatment and prevention of the disease are still limited. It is worthy of mention that no new chemotherapeutic or biological agent against TB have been introduced in the past 40 years, and a uniformly effective vaccine has yet to be developed. The low specificity of the diagnostic methods still in use precludes any change in the degree to which M. tuberculosis infection is controlled. Due to these limitations, we need to gain a better understanding the pathologic bases of TB. It is especially important to elucidate the molecular and cellular mechanisms that regulate parasite-host interaction, in order to urgently develop more efficacious weapons against TB.
Acknowledgments
The authors are grateful to the Conselho Nacional de Pesquisa e Desenvolvimento (CNPq, National Council on Research and Development) for the research productivity grant awarded to Henrique Couto Teixeira, as well as to the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG, Foundation for the Support of Research in the state of Minas Gerais) for providing general financial support.
References
1. Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. Lancet. 2003;362(9387):887-99.
2. World Health Organization. Global tuberculosis control. WHO/CDS/TB/2001.287.
3. North RJ, Jung YJ. Immunity to Tuberculosis. Annu Rev Immunol. 2004;(22):599-623.
4. Kaufmann SH. Recent findings in immunology give tuberculosis vaccines a new boost. Trends Immunol. 2005;26(12):660-7.
5. Winau F, Weber S, Sad S, de Diego J, Hoops SL, Breiden B, et al. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity. 2006;24(1):105-17.
6. Guermonprez P, Saveanu L, Kleijmeer M, Davoust J, Van Endert P, Amigorena S. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425(6956):397-402.
7. Houde M, Bertholet S, Gagnon E, Brunet S, Goyette G, Laplante A, et al. Phagosomes are competent organelles for antigen cross-presentation. Nature. 2003;425(6956):402-6.
8. Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995;375(6527): 155-8.
9. Grant EP, Degano M, Rosat JP, Stenger S, Modlin RL, Wilson IA, et al. Molecular recognition of lipid antigens by T cell receptors. J Exp Med. 1999;189(1):195-205.
10. Kaufmann SH, Schaible UE. Antigen presentation and recognition in bacterial infections. Curr Opin Immunol. 2005;17(1):79-87.
11. Orme IM, Andersen P, Boom WH. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993; 167(6):1481-97.
12. Teixeira HC, Munk ME, Kaufmann SH. Frequencies of IFN gamma- and IL-4-producing cells during Mycobacterium bovis (BCG) infection in two genetically susceptible mouse strains: role of alpha/beta T cells and NK1.1 cells. Immunol Lett. 1995;46(1-2):15-9.
13. Medzhitov R, Janeway C Jr. Innate Immunity. N Engl J Med. 2000;343(5):338-44.
14. Krutzik SR, Modlin RL. The role of Toll-like receptors in combating mycobacteria. Semin Immunol. 2004; 16(1):35-41.
15. Ottenhoff TH, Verreck FA, Hoeve MA, van de Vosse E. Control of human host immunity to mycobacteria. Tuberculosis (Edinb). 2005;85(1-2):53-64.
16. Ottenhoff TH, Kumararatne D, Casanova JL. Novel human immunodeficiencies reveal the essential role of type-I cytokines in immunity to intracellular bacteria. Immunol Today. 1998;19(11):491-4.
17. Salgame P. Host innate and th1 responses and the bacterial factors that control Mycobacterium tuberculosis infection. Curr Opin Immunol. 2005;17(4):374-80.
18. Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile JF, Newport M, et al. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N Engl J Med. 1996;335(26):1956-61.
19. Lin Y, Zhang M, Hofman FM, Gong J, Barnes PF. Absence of a prominent Th2 cytokine response in human tuberculosis. Infect Immun. 1996;64(4):1351-6.
20. Swaminathan S, Gong J, Zhang M, Samten B, Hanna LE, Narayanan PR, et al. Cytokine production in children with tuberculous infection and disease. Clin Infect Dis. 1999;28(6):1290-3.
21. Ting LM, Kim AC, Cattamanchi A, Ernst JD. Mycobacterium tuberculosis inhibits IFN-gamma transcriptional responses without inhibiting activation of STAT1. J Immunol. 1999;163(7):3898-906.
22. Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749-95.
23. Seah GT, Scott GM, Rook GA. Type 2 cytokine gene activation and its relationship to extent of disease in patients with tuberculosis. J Infect Dis. 2000;181(1):385-9.
24. van Crevel R, Karyadi E, Preyers F, Leenders M, Kullberg BJ, Nelwan RH, et al. Increased production of interleukin 4 by CD4+ and CD8+ T cells from patients with tuberculosis is related to the presence of pulmonary cavities. J. Infect Dis. 2000;181(3):1194-7.
25. Rook GA, Hernandez-Pando R, Dheda K, Teng Seah G. IL-4 in tuberculosis: implications for vaccine design. Trends Immunol. 2004;25(9): 483-8.
26. Rhoades ER, Cooper AM, Orme IM. Chemokine response in mice infected with Mycobacterium tuberculosis. Infect Immun. 1995;63(10):3871-7.
27. Brice GT, Graber NL, Hoffman SL, Doolan DL. Expression of the chemokine MIG is a sensitive and predictive marker for antigen-specific, genetically restricted IFN-gamma production and IFN-gamma-secreting cells. J Immunol Methods. 2001;257(1-2):55-69.
28. Abramo C, Meijgaarden KE, Garcia D, Franken KL, Klein MR, Kolk AJ, et al. Monokine induced by interferon gamma and IFN-gamma response to a fusion protein of Mycobacterium tuberculosis ESAT-6 and CFP-10 in Brazilian tuberculosis patients. Microbes Infect. 2006;8(1):45-51.
29. Bloom BR, Fine PEM. The BCG experience: implications for future vaccine against tuberculosis. In: Bloom BR, editor. Tuberculosis: pathogenesis, protection and control. Washington: American Society of Microbiology; 1994. p. 531-57.
30. Black GF, Weir RE, Floyd S, Bliss L, Warndorff DK, Crampin AC, et al. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet. 2002;359(9315):1393-401.
31. Ferreira AP, Aguiar AS, Fava MW, Corrêa JO, Teixeira FM, Teixeira HC. Can the efficacy of bacille calmette-guerin tuberculosis vaccine be affected by intestinal parasitic infections? J Infect Dis. 2002;186(3):441-2. 32. World Health Organization. Core information for the development of immunization policy: 2002 update. WHO/V&B/02.28.
33. Sociedade Brasileira de Pneumologia e Tisiologia. II Consenso Brasileiro de tuberculose. Diretrizes brasileiras para tuberculose 2004. J Bras Pneumol. 2004;30(Supl 1):S1-S55.
34. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This Official Statement of the American Thoracic Society and the Centers for Disease Control and Prevention was Adopted by the ATS Board of Directors, July 1999. This Statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000;161(4 Pt 1):1376-95.
35. Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet. 2000; 356(9235):1099-104.
36. Fine PE, Sterne JA, Ponnighaus JM, Rees RJ. Delayed type hypersensitivity, mycobacterial vaccines and protective immunity. Lancet. 1994;344(8932):1245-9.
37. Ravn P, Munk ME, Andersen AB, Lundgren B, Lundgren JD, Nielsen LN, et al. Prospective evaluation of a whole-blood test using Mycobacterium tuberculosis-specific antigens ESAT-6 and CFP-10 for diagnosis of active tuberculosis. Clin Diagn Lab Immunol. 2005;12(4):491-6.
38. Sorensen AL, Nagai S, Houen G, Andersen P, Andersen AB. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1995;63(5):1710-7.
39. Brosch R, Gordon SV, Pym A, Eiglmeier K, Garnier T, Cole ST. Comparative genomics of the mycobacteria. Int J Med Microb. 2000;290(2):143-52.
40. Berthet FX, Rasmussen PB, Rosenkrands I, Andersen P, Gicquel B. Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10). Microbiology. 1998;144(Pt 11):3195-203.
41. Dillon DC, Alderson MR, Day CH, Bement T, Campos-Neto A, Skeiky YA, et al. Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis (BCG). J Clin Microbiol. 2000;38(9):3285-90.
42. Ulrichs T, Munk ME, Mollenkopf H, Behr-Perst S, Colangeli R, Gennaro ML, et al. Differential T cell responses to Mycobacterium tuberculosis ESAT6 in tuberculosis patients and healthy donors. Eur J Immunol. 1998;28(12):3949-58.
43. Brodin P, Jonge MI, Majlessi L, Leclerc C, Nilges M, Cole ST, et al. Functional analysis of early secreted antigenic target-6, the dominant T-cell antigen of Mycobacterium tuberculosis, reveals key residues involved in secretion, complex formation, virulence, and immunogenicity. J Biol Chem. 2005;280(40):33953-9.
44. Munk ME, Arend SM, Brock I, Ottenhoff TH, Andersen P. Use of ESAT-6 and CFP-10 antigens for diagnosis of extrapulmonary tuberculosis. J Infect Dis. 2001;183(1):175-6.
45. Cardoso FL, Antas PR, Milagres AS, Geluk A, Franken KL, Oliveira EB, et al. T-cell responses to the Mycobacterium tuberculosis-specific antigen ESAT-6 in Brazilian tuberculosis patients. Infect Immun. 2002;70(12):6707-14.
46. Brock I, Munk ME, Kok-Jensen A, Andersen P. Performance of whole blood IFN-gamma test for tuberculosis diagnosis based on PPD or the specific antigens ESAT-6 and CFP-10. Int J Tuberc Lung Dis. 2001;5(5):462-7.
47. Pai M, Riley LW, Colford JM Jr. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis. 2004;4(12):761-76.
48. Mazurek GH. Guidelines for using the QuantiFERON®-TB test for diagnosing latent Mycobacterium tuberculosis infection. Morbidity and Mortality Weekly Report [serial on the Internet]. 2003 [cited 2005 Jan 31]; 52 (RR02). Available from: http://www.cdc.gov/mmwr/preview/ mmwrhtml/rr5202a2.htm.
49. Guidelines for the investigation of contacts of persons with infectious tuberculosis: recommendations from the National Tuberculosis Controllers Association and CDC. Morbidity and Mortality Weekly Report [serial on the Internet]. 2005 [cited 2005 Dec 16]; 54(RR15). Available from: http://www.cdc.gov/mmwr/pdf/rr/rr5415.pdf
50. Ulrichs T, Anding R, Kaufmann SH, Munk ME. Numbers of IFN-gamma-producing cells against ESAT-6 increase in tuberculosis patients during chemotherapy. Int J Tuberc Lung Dis. 2000;4(12):1181-13.
51. Lalvani A. Spotting latent infection: the path to better tuberculosis control. Thorax. 2003;58(11):916-8.
52. Hill PC, Brookes RH, Fox A, Fielding K, Jeffries DJ, Jackson-Sillah D, et al. Large-scale evaluation of enzyme-linked immunospot assay and skin test for diagnosis of Mycobacterium tuberculosis infection against a gradient of exposure in The Gambia. Clin Infect Dis. 2004;38(7):966-73.
53. Richeldi L, Ewer K, Losi M, Bergamini BM, Roversi P, Deeks J, et al. T cell-based tracking of multidrug resistant tuberculosis infection after brief exposure. Am J Respir Crit Care Med. 2004;170(3):288-95.
54. Mukhopadhyay A, Guan M, Chen HY, Lu Y, Lim TK. Prospective study of a new serological test (ASSURE TB Rapid Test) for the diagnosis of pulmonary tuberculosis. Int J Tuberc Lung Dis. 2006;10(6):620-4.
55. Lyashchenko K, Colangeli R, Houde M, Al Jahdali H, Menzies D, Gennaro ML. Heterogeneous antibody responses in tuberculosis. Infect Immun. 1998;66(8):3936-40.
56. Gennaro ML. Immunologic diagnosis of tuberculosis. Clin Infect Dis. 2000;30 (Suppl 1):S243-S6.
57. Houghton RL, Lodes MJ, Dillon DC, Reynolds LD, Day CH, McNeill PD, et al. Use of multiepitope polyproteins in serodiagnosis of active tuberculosis. Clin Diagn Lab. Immunol. 2002;9(4):883-91.
58. Fend R, Geddes R, Lesellier S, Vordermeier HM, Corner LA, Gormley E, et al. Use of an electronic nose to diagnose Mycobacterium bovis infection in badgers and cattle. J Clin Microbiol. 2005;43(4):1745-51.
59. Mello FCQ, Fonseca-Costa J, Fávero Al, Oliveira MM, Baptista RLR, Kritski AL, et al. Evaluation of an amplification test - AMPLICOR Mycobacterium tuberculosis (MTB test - Roche Molecular Systems - for the diagnosis of smear negative pulmonary tuberculosis (SNPT) at a teaching hospital, in Rio de Janeiro, Brasil [abstract]. Am J Respir Crit Care Med. 2002;165:A628.
60. Sperhacke RD, Mello FC, Zaha A, Kritski Al, Rosseti ML. Detection of Mycobacterium tuberculosis by a polymerase chain reaction colorimetric dot-blot assay. Int J Tuberc Lung Dis. 2004;8(3):312-7.
Submitted: 1 September 2006. Accepted, after review: 25 October 2006.
* Study carried out in the Immunology Laboratory of the Department of Parasitology, Microbiology and Immunology of the Institute of Biological Sciences of the Universidade Federal de Juiz de Fora UFJF, Federal University of Juiz de Fora Juiz de Fora (MG) Brazil, and at the Department of Preclinical investigations, Genmab A/S Copenhagen, Denmark.
- 1. Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C. Tuberculosis. Lancet. 2003;362(9387):887-99.
- 2. World Health Organization. Global tuberculosis control. WHO/CDS/TB/2001.287.
- 3. North RJ, Jung YJ. Immunity to Tuberculosis. Annu Rev Immunol. 2004;(22):599-623.
- 4. Kaufmann SH. Recent findings in immunology give tuberculosis vaccines a new boost. Trends Immunol. 2005;26(12):660-7.
- 5. Winau F, Weber S, Sad S, de Diego J, Hoops SL, Breiden B, et al. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity. 2006;24(1):105-17.
- 6. Guermonprez P, Saveanu L, Kleijmeer M, Davoust J, Van Endert P, Amigorena S. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature. 2003;425(6956):397-402.
- 7. Houde M, Bertholet S, Gagnon E, Brunet S, Goyette G, Laplante A, et al. Phagosomes are competent organelles for antigen cross-presentation. Nature. 2003;425(6956):402-6.
- 8. Tanaka Y, Morita CT, Tanaka Y, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995;375(6527): 155-8.
- 9. Grant EP, Degano M, Rosat JP, Stenger S, Modlin RL, Wilson IA, et al. Molecular recognition of lipid antigens by T cell receptors. J Exp Med. 1999;189(1):195-205.
- 10. Kaufmann SH, Schaible UE. Antigen presentation and recognition in bacterial infections. Curr Opin Immunol. 2005;17(1):79-87.
- 11. Orme IM, Andersen P, Boom WH. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993; 167(6):1481-97.
- 12. Teixeira HC, Munk ME, Kaufmann SH. Frequencies of IFN gamma- and IL-4-producing cells during Mycobacterium bovis (BCG) infection in two genetically susceptible mouse strains: role of alpha/beta T cells and NK1.1 cells. Immunol Lett. 1995;46(1-2):15-9.
- 13. Medzhitov R, Janeway C Jr. Innate Immunity. N Engl J Med. 2000;343(5):338-44.
- 14. Krutzik SR, Modlin RL. The role of Toll-like receptors in combating mycobacteria. Semin Immunol. 2004; 16(1):35-41.
- 15. Ottenhoff TH, Verreck FA, Hoeve MA, van de Vosse E. Control of human host immunity to mycobacteria. Tuberculosis (Edinb). 2005;85(1-2):53-64.
- 16. Ottenhoff TH, Kumararatne D, Casanova JL. Novel human immunodeficiencies reveal the essential role of type-I cytokines in immunity to intracellular bacteria. Immunol Today. 1998;19(11):491-4.
- 17. Salgame P. Host innate and th1 responses and the bacterial factors that control Mycobacterium tuberculosis infection. Curr Opin Immunol. 2005;17(4):374-80.
- 18. Jouanguy E, Altare F, Lamhamedi S, Revy P, Emile JF, Newport M, et al. Interferon-gamma-receptor deficiency in an infant with fatal bacille Calmette-Guerin infection. N Engl J Med. 1996;335(26):1956-61.
- 19. Lin Y, Zhang M, Hofman FM, Gong J, Barnes PF. Absence of a prominent Th2 cytokine response in human tuberculosis. Infect Immun. 1996;64(4):1351-6.
- 20. Swaminathan S, Gong J, Zhang M, Samten B, Hanna LE, Narayanan PR, et al. Cytokine production in children with tuberculous infection and disease. Clin Infect Dis. 1999;28(6):1290-3.
- 21. Ting LM, Kim AC, Cattamanchi A, Ernst JD. Mycobacterium tuberculosis inhibits IFN-gamma transcriptional responses without inhibiting activation of STAT1. J Immunol. 1999;163(7):3898-906.
- 22. Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749-95.
- 23. Seah GT, Scott GM, Rook GA. Type 2 cytokine gene activation and its relationship to extent of disease in patients with tuberculosis. J Infect Dis. 2000;181(1):385-9.
- 24. van Crevel R, Karyadi E, Preyers F, Leenders M, Kullberg BJ, Nelwan RH, et al. Increased production of interleukin 4 by CD4+ and CD8+ T cells from patients with tuberculosis is related to the presence of pulmonary cavities. J. Infect Dis. 2000;181(3):1194-7.
- 25. Rook GA, Hernandez-Pando R, Dheda K, Teng Seah G. IL-4 in tuberculosis: implications for vaccine design. Trends Immunol. 2004;25(9): 483-8.
- 26. Rhoades ER, Cooper AM, Orme IM. Chemokine response in mice infected with Mycobacterium tuberculosis. Infect Immun. 1995;63(10):3871-7.
- 27. Brice GT, Graber NL, Hoffman SL, Doolan DL. Expression of the chemokine MIG is a sensitive and predictive marker for antigen-specific, genetically restricted IFN-gamma production and IFN-gamma-secreting cells. J Immunol Methods. 2001;257(1-2):55-69.
- 28. Abramo C, Meijgaarden KE, Garcia D, Franken KL, Klein MR, Kolk AJ, et al. Monokine induced by interferon gamma and IFN-gamma response to a fusion protein of Mycobacterium tuberculosis ESAT-6 and CFP-10 in Brazilian tuberculosis patients. Microbes Infect. 2006;8(1):45-51.
- 29. Bloom BR, Fine PEM. The BCG experience: implications for future vaccine against tuberculosis. In: Bloom BR, editor. Tuberculosis: pathogenesis, protection and control. Washington: American Society of Microbiology; 1994. p. 531-57.
- 30. Black GF, Weir RE, Floyd S, Bliss L, Warndorff DK, Crampin AC, et al. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet. 2002;359(9315):1393-401.
- 31. Ferreira AP, Aguiar AS, Fava MW, Corrêa JO, Teixeira FM, Teixeira HC. Can the efficacy of bacille calmette-guerin tuberculosis vaccine be affected by intestinal parasitic infections? J Infect Dis. 2002;186(3):441-2.
- 32. World Health Organization. Core information for the development of immunization policy: 2002 update. WHO/V&B/02.28.
- 33. Sociedade Brasileira de Pneumologia e Tisiologia. II Consenso Brasileiro de tuberculose. Diretrizes brasileiras para tuberculose 2004. J Bras Pneumol. 2004;30(Supl 1):S1-S55.
- 34. Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This Official Statement of the American Thoracic Society and the Centers for Disease Control and Prevention was Adopted by the ATS Board of Directors, July 1999. This Statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000;161(4 Pt 1):1376-95.
- 35. Andersen P, Munk ME, Pollock JM, Doherty TM. Specific immune-based diagnosis of tuberculosis. Lancet. 2000; 356(9235):1099-104.
- 36. Fine PE, Sterne JA, Ponnighaus JM, Rees RJ. Delayed type hypersensitivity, mycobacterial vaccines and protective immunity. Lancet. 1994;344(8932):1245-9.
- 37. Ravn P, Munk ME, Andersen AB, Lundgren B, Lundgren JD, Nielsen LN, et al. Prospective evaluation of a whole-blood test using Mycobacterium tuberculosis-specific antigens ESAT-6 and CFP-10 for diagnosis of active tuberculosis. Clin Diagn Lab Immunol. 2005;12(4):491-6.
- 38. Sorensen AL, Nagai S, Houen G, Andersen P, Andersen AB. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1995;63(5):1710-7.
- 39. Brosch R, Gordon SV, Pym A, Eiglmeier K, Garnier T, Cole ST. Comparative genomics of the mycobacteria. Int J Med Microb. 2000;290(2):143-52.
- 40. Berthet FX, Rasmussen PB, Rosenkrands I, Andersen P, Gicquel B. Mycobacterium tuberculosis operon encoding ESAT-6 and a novel low-molecular-mass culture filtrate protein (CFP-10). Microbiology. 1998;144(Pt 11):3195-203.
- 41. Dillon DC, Alderson MR, Day CH, Bement T, Campos-Neto A, Skeiky YA, et al. Molecular and immunological characterization of Mycobacterium tuberculosis CFP-10, an immunodiagnostic antigen missing in Mycobacterium bovis (BCG). J Clin Microbiol. 2000;38(9):3285-90.
- 42. Ulrichs T, Munk ME, Mollenkopf H, Behr-Perst S, Colangeli R, Gennaro ML, et al. Differential T cell responses to Mycobacterium tuberculosis ESAT6 in tuberculosis patients and healthy donors. Eur J Immunol. 1998;28(12):3949-58.
- 43. Brodin P, Jonge MI, Majlessi L, Leclerc C, Nilges M, Cole ST, et al. Functional analysis of early secreted antigenic target-6, the dominant T-cell antigen of Mycobacterium tuberculosis, reveals key residues involved in secretion, complex formation, virulence, and immunogenicity. J Biol Chem. 2005;280(40):33953-9.
- 44. Munk ME, Arend SM, Brock I, Ottenhoff TH, Andersen P. Use of ESAT-6 and CFP-10 antigens for diagnosis of extrapulmonary tuberculosis. J Infect Dis. 2001;183(1):175-6.
- 45. Cardoso FL, Antas PR, Milagres AS, Geluk A, Franken KL, Oliveira EB, et al. T-cell responses to the Mycobacterium tuberculosis-specific antigen ESAT-6 in Brazilian tuberculosis patients. Infect Immun. 2002;70(12):6707-14.
- 46. Brock I, Munk ME, Kok-Jensen A, Andersen P. Performance of whole blood IFN-gamma test for tuberculosis diagnosis based on PPD or the specific antigens ESAT-6 and CFP-10. Int J Tuberc Lung Dis. 2001;5(5):462-7.
- 47. Pai M, Riley LW, Colford JM Jr. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect Dis. 2004;4(12):761-76.
- 48. Mazurek GH. Guidelines for using the QuantiFERON®-TB test for diagnosing latent Mycobacterium tuberculosis infection. Morbidity and Mortality Weekly Report [serial on the Internet]. 2003 [cited 2005 Jan 31]; 52 (RR02). Available from: http://www.cdc.gov/mmwr/preview/ mmwrhtml/rr5202a2.htm
-
49Guidelines for the investigation of contacts of persons with infectious tuberculosis: recommendations from the National Tuberculosis Controllers Association and CDC. Morbidity and Mortality Weekly Report [serial on the Internet]. 2005 [cited 2005 Dec 16]; 54(RR15). Available from: http://www.cdc.gov/mmwr/pdf/rr/rr5415.pdf
- 50. Ulrichs T, Anding R, Kaufmann SH, Munk ME. Numbers of IFN-gamma-producing cells against ESAT-6 increase in tuberculosis patients during chemotherapy. Int J Tuberc Lung Dis. 2000;4(12):1181-13.
- 51. Lalvani A. Spotting latent infection: the path to better tuberculosis control. Thorax. 2003;58(11):916-8.
- 52. Hill PC, Brookes RH, Fox A, Fielding K, Jeffries DJ, Jackson-Sillah D, et al. Large-scale evaluation of enzyme-linked immunospot assay and skin test for diagnosis of Mycobacterium tuberculosis infection against a gradient of exposure in The Gambia. Clin Infect Dis. 2004;38(7):966-73.
- 53. Richeldi L, Ewer K, Losi M, Bergamini BM, Roversi P, Deeks J, et al. T cell-based tracking of multidrug resistant tuberculosis infection after brief exposure. Am J Respir Crit Care Med. 2004;170(3):288-95.
- 54. Mukhopadhyay A, Guan M, Chen HY, Lu Y, Lim TK. Prospective study of a new serological test (ASSURE TB Rapid Test) for the diagnosis of pulmonary tuberculosis. Int J Tuberc Lung Dis. 2006;10(6):620-4.
- 55. Lyashchenko K, Colangeli R, Houde M, Al Jahdali H, Menzies D, Gennaro ML. Heterogeneous antibody responses in tuberculosis. Infect Immun. 1998;66(8):3936-40.
- 56. Gennaro ML. Immunologic diagnosis of tuberculosis. Clin Infect Dis. 2000;30 (Suppl 1):S243-S6.
- 57. Houghton RL, Lodes MJ, Dillon DC, Reynolds LD, Day CH, McNeill PD, et al. Use of multiepitope polyproteins in serodiagnosis of active tuberculosis. Clin Diagn Lab. Immunol. 2002;9(4):883-91.
- 58. Fend R, Geddes R, Lesellier S, Vordermeier HM, Corner LA, Gormley E, et al. Use of an electronic nose to diagnose Mycobacterium bovis infection in badgers and cattle. J Clin Microbiol. 2005;43(4):1745-51.
- 59. Mello FCQ, Fonseca-Costa J, Fávero Al, Oliveira MM, Baptista RLR, Kritski AL, et al. Evaluation of an amplification test - AMPLICOR Mycobacterium tuberculosis (MTB test - Roche Molecular Systems - for the diagnosis of smear negative pulmonary tuberculosis (SNPT) at a teaching hospital, in Rio de Janeiro, Brasil [abstract]. Am J Respir Crit Care Med. 2002;165:A628.
- 60. Sperhacke RD, Mello FC, Zaha A, Kritski Al, Rosseti ML. Detection of Mycobacterium tuberculosis by a polymerase chain reaction colorimetric dot-blot assay. Int J Tuberc Lung Dis. 2004;8(3):312-7.
Publication Dates
-
Publication in this collection
25 Sept 2007 -
Date of issue
June 2007
History
-
Accepted
25 Oct 2006 -
Received
01 Sept 2006