ABSTRACT
In patients with severe respiratory failure, either hypoxemic or hypercapnic, life support with mechanical ventilation alone can be insufficient to meet their needs, especially if one tries to avoid ventilator settings that can cause injury to the lungs. In those patients, extracorporeal membrane oxygenation (ECMO), which is also very effective in removing carbon dioxide from the blood, can provide life support, allowing the application of protective lung ventilation. In this review article, we aim to explore some of the most relevant aspects of using ECMO for respiratory support. We discuss the history of respiratory support using ECMO in adults, as well as the clinical evidence; costs; indications; installation of the equipment; ventilator settings; daily care of the patient and the system; common troubleshooting; weaning; and discontinuation.
Keywords:
Extracorporeal membrane oxygenation; Respiratory distress syndrome, adult; Hypoxia; Hypercapnia
RESUMO
Em pacientes com insuficiência respiratória grave (hipoxêmica ou hipercápnica), o suporte somente com ventilação mecânica pode ser insuficiente para suas necessidades, especialmente quando se tenta evitar o uso de parâmetros ventilatórios que possam causar danos aos pulmões. Nesses pacientes, extracorporeal membrane oxygenation (ECMO, oxigenação extracorpórea por membrana), que também é muito eficaz na remoção de dióxido de carbono do sangue, pode manter a vida, permitindo o uso de ventilação pulmonar protetora. No presente artigo de revisão, objetivamos explorar alguns dos aspectos mais relevantes do suporte respiratório por ECMO. Discutimos a história do suporte respiratório por ECMO em adultos; evidências clínicas; custos; indicações; instalação do equipamento; parâmetros ventilatórios; cuidado diário do paciente e do sistema; solução de problemas comuns; desmame e descontinuação.
Descritores:
Oxigenação por membrana extracorpórea; Síndrome do desconforto respiratório do adulto; Anóxia; Hipercapnia
INTRODUCTION
Patients with severe ARDS presenting with refractory hypoxemia or hypercapnia have mortality rates that vary from 45% to 90% depending on the definition of refractoriness.11. Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):637-45. https://doi.org/10.1001/jama.299.6.637
https://doi.org/10.1001/jama.299.6.637...
,22. Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):646-55. https://doi.org/10.1001/jama.299.6.646
https://doi.org/10.1001/jama.299.6.646...
Extracorporeal membrane oxygenation (ECMO) has been used worldwide with encouraging results as rescue therapy for severe ARDS in adult patients.33. Lindén V, Palmér K, Reinhard J, Westman R, Ehrén H, Granholm T, et al. High survival in adult patients with acute respiratory distress syndrome treated by extracorporeal membrane oxygenation, minimal sedation, and pressure supported ventilation. Intensive Care Med. 2000;26(11):1630-7. https://doi.org/10.1007/s001340000697
https://doi.org/10.1007/s001340000697...
4. Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351-63. https://doi.org/10.1016/S0140-6736(09)61069-2
https://doi.org/10.1016/S0140-6736(09)61...
5. Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA. 2011;306(15):1659-68. https://doi.org/10.1001/jama.2011.1471
https://doi.org/10.1001/jama.2011.1471...
-66. Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators, Davies A, Jones D, Bailey M, Beca J, Bellomo R, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA. 2009;302(17):1888-95. https://doi.org/10.1001/jama.2009.1535
https://doi.org/10.1001/jama.2009.1535...
ECMO is able to provide blood oxygenation, carbon dioxide removal, and circulatory support when suitable, allowing protective/ultraprotective mechanical ventilation.77. Peek GJ, Moore HM, Moore N, Sosnowski AW, Firmin RK. Extracorporeal membrane oxygenation for adult respiratory failure. Chest. 1997;112(3):759-64. https://doi.org/10.1378/chest.112.3.759
https://doi.org/10.1378/chest.112.3.759...
In the present review, we aimed to explore some of the most relevant aspects involved in respiratory support using ECMO.
ECMO FOR RESPIRATORY SUPPORT IN ADULTS: HISTORY AND CLINICAL EVIDENCE
After an initial sequence of positive results with the use of ECMO in severely hypoxemic patients with ARDS,88. Hill JD, O'Brien TG, Murray JJ, Dontigny L, Bramson ML, Osborn JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med. 1972;286(12):629-34. https://doi.org/10.1056/NEJM197203232861204
https://doi.org/10.1056/NEJM197203232861...
,99. Zapol WM, Snider MT, Schneider RC. Extracorporeal membrane oxygenation for acute respiratory failure. Anesthesiology. 1977;46(4):272-85. https://doi.org/10.1097/00000542-197704000-00008
https://doi.org/10.1097/00000542-1977040...
the growing interest in ECMO support led to the first randomized clinical trial funded by the National Institutes of Health.1010. Zapol WM, Snider MT, Hill JD, Fallat RJ, Bartlett RH, Edmunds LH, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA. 1979;242(20):2193-6. https://doi.org/10.1001/jama.1979.03300200023016
https://doi.org/10.1001/jama.1979.033002...
That trial was based on the rationale that hypoxemia and hypercapnia are the determinants of death in ARDS patients. Therefore, patients in the intervention group were supported with ECMO in order to improve their blood gases, and the ventilator settings were kept similar to those in the control group. The trial was interrupted early due to the equally high mortality rates in both groups.
After that trial, a group in Italy1111. Gattinoni L, Pesenti A, Mascheroni D, Marcolin R, Fumagalli R, Rossi F, et al. Low-frequency positive-pressure ventilation with extracorporeal CO2 removal in severe acute respiratory failure. JAMA. 1986;256(7):881-6. https://doi.org/10.1001/jama.1986.03380070087025
https://doi.org/10.1001/jama.1986.033800...
published their experience using ECMO support in 43 patients, in whom high positive end-expiratory pressure (PEEP) values (15-25 cmH2O), together with what they considered low peak pressures (35-45 cmH2O) and low RR (3-5 breaths/min), were applied with the rationale of reducing the lung injury caused by the ventilator. In that case series, with an expected mortality rate of over 90%, survival was considered good in the 21 patients (49%) who had been discharged home. The hypothesis that the quasi-apneic ventilation (made possible because of ECMO) promoted lung protection generated enthusiasm again, leading to the second trial of ECMO for respiratory support in patients with severe ARDS in the United States, also funded by the National Institutes of Health.1212. Morris AH, Wallace CJ, Menlove RL, Clemmer TP, Orme JF Jr, Weaver LK, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149(2 Pt 1):295-305. https://doi.org/10.1164/ajrccm.149.2.8306022
https://doi.org/10.1164/ajrccm.149.2.830...
Again, the trial results were disappointing, showing similar rates of survival in both groups (38%).
Despite that second setback, respiratory ECMO remained in use as a rescue therapy for severe hypoxemia or hypercapnia. With the growing knowledge of the substantial contribution of ventilator-induced lung injury as a cause of death in ARDS patients,1313. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med . 2000;342(18):1301-8. https://doi.org/10.1056/NEJM200005043421801
https://doi.org/10.1056/NEJM200005043421...
various groups published their experiences with the novel rationale of using respiratory ECMO to promote protective or ultraprotective ventilation, using peak airway pressures < 20-25 cmH2O and very low tidal volumes (0.5-1.0 mL/kg). Some authors also showed that it was possible to use less sedation in order to allow more efficient interaction with the patient.33. Lindén V, Palmér K, Reinhard J, Westman R, Ehrén H, Granholm T, et al. High survival in adult patients with acute respiratory distress syndrome treated by extracorporeal membrane oxygenation, minimal sedation, and pressure supported ventilation. Intensive Care Med. 2000;26(11):1630-7. https://doi.org/10.1007/s001340000697
https://doi.org/10.1007/s001340000697...
,77. Peek GJ, Moore HM, Moore N, Sosnowski AW, Firmin RK. Extracorporeal membrane oxygenation for adult respiratory failure. Chest. 1997;112(3):759-64. https://doi.org/10.1378/chest.112.3.759
https://doi.org/10.1378/chest.112.3.759...
In those studies, survival rates among those severely ill patients with ARDS were as high as 88%.
Based on those favorable experiences, a multicenter randomized controlled trial was performed in the United Kingdom (UK) by the National Health System.44. Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351-63. https://doi.org/10.1016/S0140-6736(09)61069-2
https://doi.org/10.1016/S0140-6736(09)61...
A total of 180 patients with severe ARDS were randomized to undergo conventional low airway pressure ventilation (in a local hospital) or ultraprotective ventilation using ECMO for respiratory support. Patients randomized to the ECMO group were transferred by the British Royal Air Force to Glenfield Hospital in Leicester, UK, without ECMO support. At that hospital, after approximately 12 h of protective ventilation, ECMO support was started if severe respiratory failure persisted. In that trial, ECMO was considered a cost-effective strategy for the UK. However, only 68 of the 90 patients randomized to the ECMO group actually received ECMO. The remaining 22 patients (24%) improved during the 12-h period of observation on protective mechanical ventilation. In a sense, the real hypothesis tested was whether the referral strategy, rather than the ECMO support, was effective.
The big ECMO boom occurred in 2009 with the publication of the abovementioned trial,44. Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351-63. https://doi.org/10.1016/S0140-6736(09)61069-2
https://doi.org/10.1016/S0140-6736(09)61...
as well as with the outbreak of pandemic influenza A (H1N1). A great number of patients developed severe hypoxemic respiratory failure and received ECMO support. Survival rates in those patients were surprisingly high (above 70%)55. Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA. 2011;306(15):1659-68. https://doi.org/10.1001/jama.2011.1471
https://doi.org/10.1001/jama.2011.1471...
,66. Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators, Davies A, Jones D, Bailey M, Beca J, Bellomo R, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA. 2009;302(17):1888-95. https://doi.org/10.1001/jama.2009.1535
https://doi.org/10.1001/jama.2009.1535...
,1414. Pham T, Combes A, Rozé H, Chevret S, Mercat A, Roch A, et al. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome. A cohort study and propensity-matched analysis. Am J Respir Crit Care Med . 2013;187(3):276-85. https://doi.org/10.1164/rccm.201205-0815OC
https://doi.org/10.1164/rccm.201205-0815...
,1515. Extracorporeal Life Support Organization [homepage on the Internet]. Ann Arbor (MI): the Organization [cited 2016 Sep 1]. H1N1 Registry; [about 4 screens]. Available from: Available from: https://www.elso.org/Registry/H1N1Registry.aspx
https://www.elso.org/Registry/H1N1Regist...
despite the severity of their respiratory failure.
In conclusion, the current evidence favors the use of ECMO for respiratory support in adult patients with severe ARDS. However, the evidence is still scant and weak while we await the results of the ongoing Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome (EOLIA) trial, which is estimated to be completed in February of 2018.1616. ClinicalTrials.gov [homepage on the Internet]. Bethesda: U.S. National Institutes of Health [cited 2016 Sep 1]. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome (EOLIA) [about 7 screens]. Available from: Available from: https://clinicaltrials.gov/ct2/show/NCT01470703
https://clinicaltrials.gov/ct2/show/NCT0...
ECMO CONFIGURATION
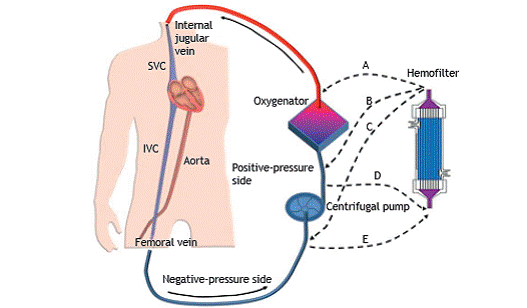
The venovenous (vv) and venoarterial (va) ECMO configurations are shown in Figure 1. For respiratory purposes, we favor the use of the vv-ECMO configuration, which can adequately oxygenate blood and remove CO2, allowing the use of gentle, protective mechanical ventilation. In the vv configuration, the oxygenator is in series with the native lungs; therefore, the residual function of the lungs remains important for systemic oxygenation.
Basic extracorporeal membrane oxygenation (ECMO) configurations. Panel A shows the venovenous ECMO configuration, in which the extracorporeal system is in series with the lungs, providing only respiratory support. Panel B shows the venoarterial ECMO configuration, in which the extracorporeal system is in parallel with the heart and lungs, providing respiratory and cardiovascular support.
The vv configuration does not provide circulatory support. For patients with respiratory failure but without severe circulatory dysfunction, vv-ECMO is associated with outcomes similar to those of va-ECMO, but with fewer complications.1717. Oshima K, Kunimoto F, Hinohara H, Ohkawa M, Mita N, Tajima Y, et al. Extracorporeal membrane oxygenation for respiratory failure: comparison of venovenous versus venoarterial bypass. Surg Today. 2010;40(3):216-22. https://doi.org/10.1007/s00595-008-4040-z
https://doi.org/10.1007/s00595-008-4040-...
,1818. Zahraa JN, Moler FW, Annich GM, Maxvold NJ, Bartlett RH, Custer JR. Venovenous versus venoarterial extracorporeal life support for pediatric respiratory failure: are there differences in survival and acute complications? Crit Care Med. 2000;28(2):521-5. https://doi.org/10.1097/00003246-200002000-00039
https://doi.org/10.1097/00003246-2000020...
In patients with severe ARDS and shock, hemodynamic instability can be due to an associated medical condition (e.g., sepsis and severe left ventricular dysfunction). In that scenario, va-ECMO is indicated in order to provide respiratory and circulatory support. Alternatively, shock can be secondary to acute cor pulmonale, a condition found in more than 22% of the patients on protective ventilation, with severe hypoxemia, or with acidemia. 1919. Mekontso Dessap A, Charron C, Devaquet J, Aboab J, Jardin F, Brochard L, et al. Impact of acute hypercapnia and augmented positive end-expiratory pressure on right ventricle function in severe acute respiratory distress syndrome. Intensive Care Med . 2009;35(11):1850-8. https://doi.org/10.1007/s00134-009-1569-2
https://doi.org/10.1007/s00134-009-1569-...
Such conditions justify an attempt to use vv-ECMO even in patients with shock. Restoration of blood gases towards normal obtained with vv-ECMO can reduce pulmonary artery pressure and improve right ventricular function.2020. Reis Miranda D, van Thiel R, Brodie D, Bakker J. Right ventricular unloading after initiation of venovenous extracorporeal membrane oxygenation. Am J Respir Crit Care Med . 2015;191(3):346-8. https://doi.org/10.1164/rccm.201408-1404LE
https://doi.org/10.1164/rccm.201408-1404...
If that attempt with vv-ECMO fails, the patient can be switched to a hybrid configuration or to a va configuration.
Depending on the clinical indication, different hybrid cannulations can be used.2121. Napp LC, Kühn C, Hoeper MM, Vogel-Claussen J, Haverich A, Schäfer A, et al. Cannulation strategies for percutaneous extracorporeal membrane oxygenation in adults. Clin Res Cardiol. 2016;105(4):283-96. https://doi.org/10.1007/s00392-015-0941-1
https://doi.org/10.1007/s00392-015-0941-...
The two classical reasons to use a hybrid configuration are to improve blood drainage (to provide circulatory support) and to avoid the Harlequin syndrome (hypoxemia restricted to the upper part of the body). Sometimes the blood flow through the vein does not meet the demand generated by the pump. As a result, the negative pressure can suck the venous wall into the access ports of the cannula, a situation referred to as the "sucking-up" phenomenon, leading to transient obstruction of the inlets and to a decrease in ECMO blood flow. The obstruction is usually brief (sometimes fractions of a second) until the vein is again filled with blood originating from the venous return. The process repeats itself indefinitely and usually leads to the chattering of the ECMO circuit. Prompt corrections involve either reducing ECMO blood flow (which might worsen hypoxemia) or administering fluid challenges (which can worsen lung function). An alternative solution is the insertion of an additional venous cannula using a "Y" piece in order to improve venous drainage. This hybrid configuration is known as venoarterial-venous (vav)-ECMO.
To avoid the Harlequin syndrome, a single-lumen (venous) cannula can be inserted, and blood return can occur through arterial and venous cannulas connected to a "Y" piece (vav-ECMO configuration). This configuration can be derived from an ongoing va- or vv-ECMO support session.
ECMO PHYSIOLOGY AND CIRCUIT
To reach adequate arterial oxygenation, extracorporeal blood flows higher than 60% of the cardiac output (> 3 L/min) are frequently necessary. Such flows are obtained by using peristaltic or centrifugal pumps, large-bore cannulas, and a circuit. The large diameter of the cannulas is important to avoid significant hemolysis. Although peristaltic and centrifugal pumps induce comparable amounts of hemolysis, we favor the use of the latter in the ICU, because they are able to avoid circuit rupture in the rare instance that blood flow obstruction occurs. Blood drains through a venous circuit, in which negative pressures as low as between −30 and −104 mmHg can be reached, passes through the pump, is propelled to the oxygenator with pressures up to the 350-400 mmHg range, and is then returned to the patient (Figure 2). In modern oxygenators, resistance to the passage of blood is low, although it still increases at higher blood flows and at higher temperatures.2222. Park M, Costa EL, Maciel AT, Barbosa EV, Hirota AS, Schettino Gde P, et al. Effect of flow rate and temperature on transmembrane blood pressure drop in an extracorporeal artificial lung. Perfusion. 2014;29(6):517-25. https://doi.org/10.1177/0267659114525986
https://doi.org/10.1177/0267659114525986...
Venovenous extracorporeal membrane oxygenation (ECMO) configuration and possible connections with a hemofilter, with or without a dialysis machine. The drainage cannula is inserted into the femoral vein and advanced up to the inferior vena cava (IVC), whereas the return cannula is inserted into the internal jugular vein and advanced up to the superior vena cava (SVC). In this example, blood is drained by the suctioning action of a centrifugal pump into the negative-pressure side of the extracorporeal circuit. Downstream of the pump, blood is propelled into the positive-pressure side of the circuit, crosses the oxygenator, and is returned to the SVC. The dashed lines indicate different possibilities of connection to a hemofilter-downstream of the oxygenator (path A); downstream of the pump and upstream of the oxygenator (path B); upstream of the ECMO pump (path C); downstream of the ECMO pump (positive-pressure side, path D); and upstream of the ECMO pump (negative-pressure side, path E)-in patients requiring simultaneous renal replacement therapy.
The oxygenator promotes gas exchange based on the diffusion principle. Fresh gas (or sweep gas), rich in oxygen and devoid of CO2, crosses a respiratory membrane (polymethylpentene hollow fibers) in countercurrent flow with venous blood. The CO2 removal from the blood is highly effective, depending on the blood flow, sweep gas flow, and venous CO2 concentration.2323. Kolobow T, Gattinoni L, Tomlinson T, White D, Pierce J, Iapichino G. The carbon dioxide membrane lung (CDML): a new concept. Trans Am Soc Artif Intern Organs. 1977;23:17-21. https://doi.org/10.1097/00002480-197700230-00005
https://doi.org/10.1097/00002480-1977002...
Oxygen transfer can be more complicated, because it depends on blood flow, total oxygen-carrying capacity of the blood (which is mainly determined by hemoglobin concentration), and the effectiveness of oxygen diffusion through the respiratory membrane, which is 16-20 times lower than that of CO2 diffusion. At low blood flows, the partial pressure of oxygen in the return circuit is very high, and oxygen transfer is mainly perfusion-limited. At higher blood flows, the partial oxygen pressure in the return circuit decreases, even though hemoglobin saturation remains high and oxygen transfer is both diffusion- and perfusion-limited. As a result, arterial blood oxygenation improves with increases in blood flow through the ECMO system despite the diffusion-determined loss of efficiency. 2424. Park M, Mendes PV, Costa EL, Barbosa EV, Hirota AS, Azevedo LC. Factors associated with blood oxygen partial pressure and carbon dioxide partial pressure regulation during respiratory extracorporeal membrane oxygenation support: data from a swine model. Rev Bras Ter Intensiva. 2016;28(1):11-8. https://doi.org/10.5935/0103-507X.20160006
https://doi.org/10.5935/0103-507X.201600...
The sweep gas flow has little impact on oxygen transfer.2525. Park M, Costa EL, Maciel AT, Silva DP, Friedrich N, Barbosa EV, et al. Determinants of oxygen and carbon dioxide transfer during extracorporeal membrane oxygenation in an experimental model of multiple organ dysfunction syndrome. PLoS One. 2013;8(1):e54954. https://doi.org/10.1371/journal.pone.0054954
https://doi.org/10.1371/journal.pone.005...
In the vv-ECMO support configuration, systemic oxygenation depends on cardiac output, residual function of the native lungs, and vv-ECMO blood flow, whereas arterial CO2 elimination mainly depends on the ECMO sweep gas flow, cardiac output, CO2 production, and residual function of the native lungs. In the peripheral va-ECMO configuration (the most common venoarterial insertion used in the ICU at the bedside), blood is returned to the aorta against the flow of blood ejected by the left ventricle during systole. At low cardiac outputs, the coronary arteries, brachiocephalic trunk, left carotid artery, and left subclavian artery receive oxygenated blood returning from the ECMO system. If cardiac output improves and the lungs continue to show poor residual function, the upper half of the body can receive deoxygenated blood coming from the pulmonary veins, whereas the lower half of the body receives oxygenated blood coming from the ECMO circuit, a situation known as the Harlequin syndrome.
ECMO COSTS AND COST-EFFECTIVENESS
The cost of ECMO support is high. At current prices in Brazil, the equipment costs from US$ 8,000 to US$ 36,000 per patient. The gain in cost-effectiveness per quality-adjusted life year is reasonable in developed countries,44. Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351-63. https://doi.org/10.1016/S0140-6736(09)61069-2
https://doi.org/10.1016/S0140-6736(09)61...
and it is possibly acceptable in Brazil.2626. Park M, Mendes PV, Zampieri FG, Azevedo LC, Costa EL, Antoniali F, et al. The economic effect of extracorporeal membrane oxygenation to support adults with severe respiratory failure in Brazil: a hypothetical analysis. Rev Bras Ter Intensiva . 2014;26(3):253-62. https://doi.org/10.5935/0103-507X.20140036
https://doi.org/10.5935/0103-507X.201400...
SUITABLE CANDIDATES FOR ECMO FOR RESPIRATORY SUPPORT
ECMO for respiratory support is potentially useful in patients with severe hypoxemia or severe hypercapnia, resulting in low pH (usually < 7.20) despite lung-protective mechanical ventilation. ECMO support is often tried after failed attempts at multiple rescue therapies, such as prone positioning, alveolar recruitment maneuvers, and use of nitric oxide, alone or in combination.
Our group uses the following inclusion criteria2727. Park M, Azevedo LC, Mendes PV, Carvalho CR, Amato MB, Schettino GP, et al. First-year experience of a Brazilian tertiary medical center in supporting severely ill patients using extracorporeal membrane oxygenation. Clinics (Sao Paulo). 2012;67(10):1157-63. https://doi.org/10.6061/clinics/2012(10)07
https://doi.org/10.6061/clinics/2012(10)...
:
Major criteria (both required)
-
Acute pulmonary disease
-
Possibility of recovery from disease
-
Complementary criteria (at least one required)
-
PaO2/FiO2 ratio < 50 mmHg with an FiO2 = 1 for at least 1 h, with or without rescue maneuvers
-
PaO2/FiO2 ratio < 50 mmHg with an FiO2 > 0.8 for at least 3 h, despite rescue maneuvers
-
Hypercapnia with a pH < 7.2 despite an RR > 35 breaths/min, requiring tidal volumes ≥ 6 mL/kg of ideal body weight and plateau pressures > 30 cmH2O
-
Murray's score (lung injury score) > 3.0 in the presence of clinical deterioration
ECMO support is contraindicated in patients with chronic illnesses that impair their quality of life and in those with severe acute multiorgan dysfunction. Some scores can be used in order to predict mortality in patients on ECMO for respiratory support.2828. Schmidt M, Zogheib E, Rozé H, Repesse X, Lebreton G, Luyt CE, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med . 2013;39(10):1704-13. https://doi.org/10.1007/s00134-013-3037-2
https://doi.org/10.1007/s00134-013-3037-...
,2929. Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med . 2014;189(11):1374-82. https://doi.org/10.1164/rccm.201311-2023OC
https://doi.org/10.1164/rccm.201311-2023...
One of those (Respiratory Extracorporeal Membrane Oxygenation Survival Prediction)2929. Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med . 2014;189(11):1374-82. https://doi.org/10.1164/rccm.201311-2023OC
https://doi.org/10.1164/rccm.201311-2023...
is easily accessible from the Internet (www.respscore.com) and provides prognostic information that can be used in order to help select the patients. Being > 75 years of age is considered by some a relative contraindication. Long duration of mechanical ventilation prior to ECMO support initiation (generally > 7 days) is strongly associated with a poor prognosis and is also a relative contraindication to ECMO support.2828. Schmidt M, Zogheib E, Rozé H, Repesse X, Lebreton G, Luyt CE, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med . 2013;39(10):1704-13. https://doi.org/10.1007/s00134-013-3037-2
https://doi.org/10.1007/s00134-013-3037-...
,2929. Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med . 2014;189(11):1374-82. https://doi.org/10.1164/rccm.201311-2023OC
https://doi.org/10.1164/rccm.201311-2023...
VASCULAR CANNULATION
Only peripheral venous cannulations, which are usually done at the bedside using the Seldinger technique, will be discussed here. An ultrasound vascular examination helps to select the cannulas, generally with a gauge 2 mm smaller than the vessel diameter.2727. Park M, Azevedo LC, Mendes PV, Carvalho CR, Amato MB, Schettino GP, et al. First-year experience of a Brazilian tertiary medical center in supporting severely ill patients using extracorporeal membrane oxygenation. Clinics (Sao Paulo). 2012;67(10):1157-63. https://doi.org/10.6061/clinics/2012(10)07
https://doi.org/10.6061/clinics/2012(10)...
The ultrasound also allows safe vascular punctures and the correct positioning of the cannulas.3030. Sidebotham D, McGeorge A, McGuinness S, Edwards M, Willcox T, Beca J. Extracorporeal membrane oxygenation for treating severe cardiac and respiratory failure in adults: part 2-technical considerations. J Cardiothorac Vasc Anesth. 2010;24(1):164-72. https://doi.org/10.1053/j.jvca.2009.08.002
https://doi.org/10.1053/j.jvca.2009.08.0...
Using the vv-ECMO configuration, the drainage cannula (19-25 Fr/55-70 cm of length) is usually inserted into the femoral vein. The oxygenated venous blood returns to the patient using the reinfusion cannula, also known as arterial cannula (17-19 Fr/30-40 cm in length). The femoral-jugular configuration (with venous and arterial cannulas, respectively) is the most widely studied configuration and can provide blood flows within the 6-7 L/min range with little recirculation.3131. Rich PB, Awad SS, Crotti S, Hirschl RB, Bartlett RH, Schreiner RJ. A prospective comparison of atrio-femoral and femoro-atrial flow in adult venovenous extracorporeal life support. J Thorac Cardiovasc Surg. 1998;116(4):628-32. https://doi.org/10.1016/S0022-5223(98)70170-9
https://doi.org/10.1016/S0022-5223(98)70...
To optimize drainage, we favor placing the venous cannula at the junction of the inferior vena cava with the right atrium. The jugular-femoral configuration (with venous and arterial cannulas, respectively)33. Lindén V, Palmér K, Reinhard J, Westman R, Ehrén H, Granholm T, et al. High survival in adult patients with acute respiratory distress syndrome treated by extracorporeal membrane oxygenation, minimal sedation, and pressure supported ventilation. Intensive Care Med. 2000;26(11):1630-7. https://doi.org/10.1007/s001340000697
https://doi.org/10.1007/s001340000697...
and the femoral-femoral configurations have also been used by experienced ECMO groups,66. Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators, Davies A, Jones D, Bailey M, Beca J, Bellomo R, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA. 2009;302(17):1888-95. https://doi.org/10.1001/jama.2009.1535
https://doi.org/10.1001/jama.2009.1535...
although higher recirculation has been reported when those configurations are compared with the femoral-jugular configuration.3131. Rich PB, Awad SS, Crotti S, Hirschl RB, Bartlett RH, Schreiner RJ. A prospective comparison of atrio-femoral and femoro-atrial flow in adult venovenous extracorporeal life support. J Thorac Cardiovasc Surg. 1998;116(4):628-32. https://doi.org/10.1016/S0022-5223(98)70170-9
https://doi.org/10.1016/S0022-5223(98)70...
,32) The double-lumen (bicaval) cannula is another option to provide vv-ECMO support. The cannula is inserted through the jugular vein and must be positioned using transesophageal echocardiography or fluoroscopy. These double-lumen cannulas, which are still unavailable for clinical use in Brazil, have been associated with lower blood flows, high recirculation rate,3333. van Heijst AF, van der Staak FH, de Haan AF, Liem KD, Festen C, Geven WB, et al. Recirculation in double lumen catheter veno-venous extracorporeal membrane oxygenation measured by an ultrasound dilution technique. ASAIO J. 2001;47(4):372-6. https://doi.org/10.1097/00002480-200107000-00015
https://doi.org/10.1097/00002480-2001070...
hemolysis,3434. Chimot L, Marqué S, Gros A, Gacouin A, Lavoué S, Camus C, et al. Avalon(c) bicaval dual-lumen cannula for venovenous extracorporeal membrane oxygenation: survey of cannula use in France. ASAIO J . 2013;59(2):157-61. https://doi.org/10.1097/MAT.0b013e31827db6f3
https://doi.org/10.1097/MAT.0b013e31827d...
and venous thrombosis.3535. Kalem V, Buchwald D, Strauch J, Sidiropoulos A, Meindl R, Schildhauer TA, et al. Surgical extraction after thrombosis around the Avalon dual lumen cannula. Ann R Coll Surg Engl. 2014;96(1):106E-108E. https://doi.org/10.1308/003588414X13824511649814
https://doi.org/10.1308/003588414X138245...
Conversely, in patients requiring long-term ECMO support, such as those awaiting lung transplantation, the double-lumen cannula facilitates mobilization and can be a good choice.
ECMO SUPPORT SETTINGS
After the circuit has been primed (typically with crystalloid solutions warmed to 36°C), the ECMO blood flow is initiated at 500 mL/min, and the sweep gas flow is maintained at 1-2 L/min (FiO2 = 1) until the extracorporeal system is filled with blood. Blood flow and the sweep gas flow are subsequently increased to 2,000 mL/min when using the vv-ECMO configuration. Blood flow and the sweep gas flow are then elevated to a 1:1 ratio, with a target SpO2 of at least 85%. In extracorporeal support due to hypercapnic respiratory failure, it is important to avoid overly rapid corrections of hypercapnia when initiating ECMO support. Failing to do so might promote alkalemia and a right shift of the oxygen-hemoglobin dissociation curve, as well as cerebral vasoconstriction. In those patients, we usually start the sweep gas flow at half of blood flow. For all patients, subsequent fine tuning of ECMO settings should be based on arterial blood gases: ECMO blood flow should be adjusted based on oxygen levels, and the sweep gas flow should be managed for adequate CO2 and pH. The interval between blood sample collections varies according to ECMO blood flow; using low blood flows (~2,000 mL/min), intervals as long as 1.5 h are usually necessary to reach PaCO2 equilibrium. With high blood flows (~3,500 mL/min), a shorter time (30 min) is usually sufficient.3636. Mendes PV, Park M, Maciel AT, E Silva DP, Friedrich N, Barbosa EV, et al. Kinetics of arterial carbon dioxide during veno-venous extracorporeal membrane oxygenation support in an apnoeic porcine model. Intensive Care Med Exp. 2016;4(1):1. https://doi.org/10.1186/s40635-015-0074-x
https://doi.org/10.1186/s40635-015-0074-...
The oxygenation target varies among ECMO centers. Our group sought to reach a PaO2 within the 55-60 mmHg range3737. Nunes LB, Mendes PV, Hirota AS, Barbosa EV, Maciel AT, Schettino GP, et al. Severe hypoxemia during veno-venous extracorporeal membrane oxygenation: exploring the limits of extracorporeal respiratory support. Clinics (Sao Paulo) . 2014;69(3):173-8. https://doi.org/10.6061/clinics/2014(03)05
https://doi.org/10.6061/clinics/2014(03)...
with a normal pH (7.35-7.40).2727. Park M, Azevedo LC, Mendes PV, Carvalho CR, Amato MB, Schettino GP, et al. First-year experience of a Brazilian tertiary medical center in supporting severely ill patients using extracorporeal membrane oxygenation. Clinics (Sao Paulo). 2012;67(10):1157-63. https://doi.org/10.6061/clinics/2012(10)07
https://doi.org/10.6061/clinics/2012(10)...
In patients with high cardiac outputs and very low residual lung function, such targets can be impossible to reach, despite the use of ECMO. In such patients, instead of further damaging the lungs with injurious ventilator settings, it might be preferable to tolerate lower levels of oxygenation (permissive hypoxemia) in the absence of hypercapnic acidemia.3838. Mendes PV, Moura E, Barbosa EV, Hirota AS, Scordamaglio PR, Ajjar FM, et al. Challenges in patients supported with extracorporeal membrane oxygenation in Brazil. Clinics (Sao Paulo) . 2012;67(12):1511-5. https://doi.org/10.6061/clinics/2012(12)27
https://doi.org/10.6061/clinics/2012(12)...
This strategy of permissive hypoxemia has also been proposed as a means of promoting ultraprotective or protective mechanical ventilation. An experienced ECMO group in Sweden, for example, allows systemic arterial saturation as low as 70% during the vv-ECMO initial run, when the residual function of the native lungs can be close to zero.33. Lindén V, Palmér K, Reinhard J, Westman R, Ehrén H, Granholm T, et al. High survival in adult patients with acute respiratory distress syndrome treated by extracorporeal membrane oxygenation, minimal sedation, and pressure supported ventilation. Intensive Care Med. 2000;26(11):1630-7. https://doi.org/10.1007/s001340000697
https://doi.org/10.1007/s001340000697...
That group identified no significant neurocognitive or physical deficits in a cohort of patients with severe hypoxemia supported with vv-ECMO.3939. Lindén VB, Lidegran MK, Frisén G, Dahlgren P, Frenckner BP, Larsen F. ECMO in ARDS: a long-term follow-up study regarding pulmonary morphology and function and health-related quality of life. Acta Anaesthesiol Scand. 2009;53(4):489-95. https://doi.org/10.1111/j.1399-6576.2008.01808.x
https://doi.org/10.1111/j.1399-6576.2008...
It is important to note, however, that other authors found an association between severe hypoxemia and long-term neurocognitive dysfunction in ARDS patients not supported by ECMO.4040. Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson BT, Bellamy SL, et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med . 2012;185(12):1307-15. https://doi.org/10.1164/rccm.201111-2025OC
https://doi.org/10.1164/rccm.201111-2025...
The protective or ultraprotective mechanical ventilation during ECMO support is commonly achieved by using a PEEP of 10-15 cmH2O, a plateau pressure of 20-25 cmH2O, an RR of 10-15 breaths/min, an inspiratory time of 0.8-1.0 s, and an FiO2 of 0.3 (or the minimum to achieve the desired PaO2).44. Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351-63. https://doi.org/10.1016/S0140-6736(09)61069-2
https://doi.org/10.1016/S0140-6736(09)61...
,77. Peek GJ, Moore HM, Moore N, Sosnowski AW, Firmin RK. Extracorporeal membrane oxygenation for adult respiratory failure. Chest. 1997;112(3):759-64. https://doi.org/10.1378/chest.112.3.759
https://doi.org/10.1378/chest.112.3.759...
,4141. Schmidt M, Pellegrino V, Combes A, Scheinkestel C, Cooper DJ, Hodgson C. Mechanical ventilation during extracorporeal membrane oxygenation. Crit Care. 2014;18(1):203. https://doi.org/10.1186/cc13702
https://doi.org/10.1186/cc13702...
In patients with more severe lung injury, the use of higher PEEP values (13-15 cmH2O) is associated with improved outcomes.4242. Schmidt M, Stewart C, Bailey M, Nieszkowska A, Kelly J, Murphy L, et al. Mechanical ventilation management during extracorporeal membrane oxygenation for acute respiratory distress syndrome: a retrospective international multicenter study. Crit Care Med . 2015;43(3):654-64. https://doi.org/10.1097/CCM.0000000000000753
https://doi.org/10.1097/CCM.000000000000...
,4343. Marhong JD, Telesnicki T, Munshi L, Del Sorbo L, Detsky M, Fan E. Mechanical ventilation during extracorporeal membrane oxygenation. An international survey. Ann Am Thorac Soc. 2014;11(6):956-61. https://doi.org/10.1513/AnnalsATS.201403-100BC
https://doi.org/10.1513/AnnalsATS.201403...
The approach of our group consists in first reducing the plateau pressure to < 25 cmH2O after ECMO initiation, then setting the PEEP to 10-15 cmH2O and the RR to 10 breaths/min, thereafter reducing the FiO2.2727. Park M, Azevedo LC, Mendes PV, Carvalho CR, Amato MB, Schettino GP, et al. First-year experience of a Brazilian tertiary medical center in supporting severely ill patients using extracorporeal membrane oxygenation. Clinics (Sao Paulo). 2012;67(10):1157-63. https://doi.org/10.6061/clinics/2012(10)07
https://doi.org/10.6061/clinics/2012(10)...
Sometimes, at very low tidal volumes (e.g., < 0.5 mL/kg), chest expansion can be difficult to notice. That finding should not raise concern unless it is associated with an elevation of the HR, hypotension, or any other sign of worsening pulmonary hypertension.44. Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351-63. https://doi.org/10.1016/S0140-6736(09)61069-2
https://doi.org/10.1016/S0140-6736(09)61...
,2727. Park M, Azevedo LC, Mendes PV, Carvalho CR, Amato MB, Schettino GP, et al. First-year experience of a Brazilian tertiary medical center in supporting severely ill patients using extracorporeal membrane oxygenation. Clinics (Sao Paulo). 2012;67(10):1157-63. https://doi.org/10.6061/clinics/2012(10)07
https://doi.org/10.6061/clinics/2012(10)...
When tidal volume is reduced to such extreme levels, the inhaled air should be warmed and humidified using an active (heated) humidifier.4444. Campbell RS, Davis K Jr, Johannigman JA, Branson RD. The effects of passive humidifier dead space on respiratory variables in paralyzed and spontaneously breathing patients. Respir Care. 2000;45(3):306-12. A closed tracheal suctioning system can also be used in order to avoid depressurization during airway suctioning. Whenever possible, suctioning should be performed using assisted pressure-controlled mode or pressure-support mode, because the ventilator can compensate for the loss of flow to the suctioning system, thus minimizing alveolar derecruitment.4545. Maggiore SM, Lellouche F, Pigeot J, Taille S, Deye N, Durrmeyer X, et al. Prevention of endotracheal suctioning-induced alveolar derecruitment in acute lung injury. Am J Respir Crit Care Med . 2003;167(9):1215-24. https://doi.org/10.1164/rccm.200203-195OC
https://doi.org/10.1164/rccm.200203-195O...
After clinical stabilization, when the acute phase of lung inflammation subsides, spontaneous ventilation using pressure support can be allowed. The sweep gas flow can be adjusted aiming at optimal patient comfort. Higher sweep gas flows are associated with lower PaCO2 levels and less respiratory effort.4646. Mauri T, Grasselli G, Suriano G, Eronia N, Spadaro S, Turrini C, et al. Control of Respiratory Drive and Effort in Extracorporeal Membrane Oxygenation Patients Recovering from Severe Acute Respiratory Distress Syndrome. Anesthesiology . 2016;125(1):159-67. https://doi.org/10.1097/ALN.0000000000001103
https://doi.org/10.1097/ALN.000000000000...
PATIENT MANAGEMENT
The ECMO-supported patient should be treated as a regular critically ill patient. Analgesia can be administered preemptively in all cannulated patients, at least until an objective evaluation of the pain is possible. Sedation should be titrated to promote or facilitate the protective or ultraprotective ventilation during the first hours of ECMO support, always avoiding life-threatening agitation. Some patients will require neuromuscular blocking agents to guarantee adequate mechanical ventilation in the first hours of ECMO support. Later in the course of the underlying disease, when the acute inflammatory phase has waned, titrating the sweep gas flow to a normal or slightly high pH can allow protective mechanical ventilation even in patients who are awake and interacting with family members and health care providers.(3) Although feeding should be initiated as soon as possible, its impact on fluid balance during the first days of ECMO support should be taken into account.4747. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network., Rice TW, Wheeler AP, Thompson BT, Steingrub J, Hite RD, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307(8):795-803. https://doi.org/10.1001/jama.2012.137
https://doi.org/10.1001/jama.2012.137...
Antimicrobials must not be used prophylactically. Infectious complications during ECMO support, especially ventilator-associated pneumonia, are common and should be treated as usual.4848. Schmidt M, Bréchot N, Hariri S, Guiguet M, Luyt CE, Makri R, et al. Nosocomial infections in adult cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation. Clin Infect Dis. 2012;55(12):1633-41. https://doi.org/10.1093/cid/cis783
https://doi.org/10.1093/cid/cis783...
The adequate level of hemoglobin is still a matter of debate. Oftentimes, patients remain hypoxemic despite the use of ECMO. To optimize systemic oxygen delivery, some centers use transfusion of blood components to maintain hemoglobin levels > 14 g/dL44. Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351-63. https://doi.org/10.1016/S0140-6736(09)61069-2
https://doi.org/10.1016/S0140-6736(09)61...
or > 10 g/dL and platelet counts > 100,000/mm3.4949. Brogan TV, Thiagarajan RR, Rycus PT, Bartlett RH, Bratton SL. Extracorporeal membrane oxygenation in adults with severe respiratory failure: a multi-center database. Intensive Care Med . 2009;35(12):2105-14. https://doi.org/10.1007/s00134-009-1661-7
https://doi.org/10.1007/s00134-009-1661-...
In the absence of hypoxemia, we adopt a more conservative approach: at an SaO2 > 88%, our hemoglobin threshold for transfusion of red blood cells is 7.0 g/dL and our platelet count threshold for platelet transfusion is 50,000/mm3.5050. Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med . 2011;365(20):1905-14. https://doi.org/10.1056/NEJMct1103720
https://doi.org/10.1056/NEJMct1103720...
We use those thresholds as general guidelines, and the decision is always individualized after thorough evaluation of each patient. Early mobilization and early ambulation of patients on ECMO support improve motor outcomes and rehabilitation.5151. Abrams D, Javidfar J, Farrand E, Mongero LB, Agerstrand CL, Ryan P, et al. Early mobilization of patients receiving extracorporeal membrane oxygenation: a retrospective cohort study. Crit Care . 2014;18(1):R38. https://doi.org/10.1186/cc13746
https://doi.org/10.1186/cc13746...
RENAL REPLACEMENT THERAPY
The renal replacement therapy (RRT) circuit can be connected to a separate vascular access or directly to the ECMO circuit (Figure 2). Keeping dialysis independent from ECMO makes all the procedures related to the RRT occur exactly as they would in patients without ECMO support. However, that decision involves the risk of obtaining a new central venous access in patients who have often been fully anticoagulated.
Alternatively, the RRT circuit can be connected in series with the ECMO circuit (Figure 2). The best configuration of the inlet and outlet lines of the dialysis machine is a matter of controversy. We do not recommend connecting the dialysis system downstream of the oxygenator, because of the risk of air embolism. The choice between making the connection upstream of the ECMO pump (negative-pressure side) and making it downstream of the ECMO pump (positive-pressure side) will depend on the dialysis machine. In some RRT machines, negative pressure will trigger low-pressure alarms if the outlet lines are connected upstream of the ECMO pump. Connection on the positive-pressure side (i.e., downstream of the pump and upstream of the oxygenator), where pressures can reach up to 350 mmHg, is a safe and effective option.5252. Santiago MJ, Sánchez A, López-Herce J, Pérez R, del Castillo J, Urbano J, et al. The use of continuous renal replacement therapy in series with extracorporeal membrane oxygenation. Kidney Int. 2009;76(12):1289-92. https://doi.org/10.1038/ki.2009.383
https://doi.org/10.1038/ki.2009.383...
Finally, it is possible to take advantage of the pressure differences within the ECMO circuit to drive blood across the hemofilter without a dialysis machine. To that end, the inlet line of the hemofilter drains blood from the positive-pressure side and returns blood to the negative-pressure side. With that configuration, it is possible to deliver slow continuous ultrafiltration therapy, with an infusion pump controlling the amount of fluid removal or the amount of dialysate in countercurrent with blood (which would require two pumps). This option can be hazardous, because the amount of flow through the hemofilter will vary depending on the ECMO-circuit pressures, as well as because infusion pumps can underestimate fluid loss.5252. Santiago MJ, Sánchez A, López-Herce J, Pérez R, del Castillo J, Urbano J, et al. The use of continuous renal replacement therapy in series with extracorporeal membrane oxygenation. Kidney Int. 2009;76(12):1289-92. https://doi.org/10.1038/ki.2009.383
https://doi.org/10.1038/ki.2009.383...
The risks and benefits of each of the options should be considered. We believe that, at centers where the staff have sufficient experience, connecting the dialysis machine directly to the ECMO circuit might be advantageous because it avoids the risk of central venous access and has been associated with a lower risk of infection.5252. Santiago MJ, Sánchez A, López-Herce J, Pérez R, del Castillo J, Urbano J, et al. The use of continuous renal replacement therapy in series with extracorporeal membrane oxygenation. Kidney Int. 2009;76(12):1289-92. https://doi.org/10.1038/ki.2009.383
https://doi.org/10.1038/ki.2009.383...
PHARMACOKINETICS
Modern ECMO circuits produce significant pharmacokinetic changes. First, hemodilution due to the priming volume of approximately 500-600 mL can dilute drugs soon after the initiation of ECMO support. Second and most importantly, the ECMO circuit is coated with biopassive and bioactive products to enhance biocompatibility and to reduce thrombogenesis, respectively.5353. Zimmermann AK, Weber N, Aebert H, Ziemer G, Wendel HP. Effect of biopassive and bioactive surface-coatings on the hemocompatibility of membrane oxygenators. J Biomed Mater Res B Appl Biomater. 2007;80(2):433-9. https://doi.org/10.1002/jbm.b.30614
https://doi.org/10.1002/jbm.b.30614...
The circuit coating is able to adsorb lipophilic5454. Shekar K, Roberts JA, Barnett AG, Diab S, Wallis SC, Fung YL, et al. Can physicochemical properties of antimicrobials be used to predict their pharmacokinetics during extracorporeal membrane oxygenation? Illustrative data from ovine models. Crit Care . 2015;19:437. https://doi.org/10.1186/s13054-015-1151-y
https://doi.org/10.1186/s13054-015-1151-...
and protein-bound drugs. There is information on the pharmacokinetics of some drugs (Table 1).5555. Shekar K, Fraser JF, Smith MT, Roberts JA. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J Crit Care . 2012;27(6):741.e9-18. https://doi.org/10.1016/j.jcrc.2012.02.013
https://doi.org/10.1016/j.jcrc.2012.02.0...
,5656. Shekar K, Fraser JF, Taccone FS, Welch S, Wallis SC, Mullany DV, et al. The combined effects of extracorporeal membrane oxygenation and renal replacement therapy on meropenem pharmacokinetics: a matched cohort study. Crit Care . 2014;18(6):565. https://doi.org/10.1186/s13054-014-0565-2
https://doi.org/10.1186/s13054-014-0565-...
Whenever possible (e.g., in the case of analgesics and vasopressors), close clinical surveillance of the pharmacokinetic effects of ECMO support is indicated.
Pharmacokinetics of some medications when using extracorporeal membrane oxygenation support.
Anticoagulation
The coating of the ECMO circuit contains heparin, which provides partial protection against clotting. Further anticoagulation is necessary to avoid clot formation in the ECMO system and to prevent vascular thrombosis in the insertion sites of the cannulas, thus avoiding dysfunction of the oxygenator, hemolysis, post-phlebitis syndrome, arterial ischemia, and pulmonary embolism. Between 10% and 33% of patients on ECMO support experience thrombotic or bleeding events.5757. Bembea MM, Annich G, Rycus P, Oldenburg G, Berkowitz I, Pronovost P. Variability in anticoagulation management of patients on extracorporeal membrane oxygenation: an international survey. Pediatr Crit Care Med . 2013;14(2):e77-84. https://doi.org/10.1097/PCC.0b013e31827127e4
https://doi.org/10.1097/PCC.0b013e318271...
Monitoring of anticoagulation varies according to the experience of each center.5757. Bembea MM, Annich G, Rycus P, Oldenburg G, Berkowitz I, Pronovost P. Variability in anticoagulation management of patients on extracorporeal membrane oxygenation: an international survey. Pediatr Crit Care Med . 2013;14(2):e77-84. https://doi.org/10.1097/PCC.0b013e31827127e4
https://doi.org/10.1097/PCC.0b013e318271...
Table 2 shows the anticoagulation techniques used at 187 ECMO centers worldwide.5757. Bembea MM, Annich G, Rycus P, Oldenburg G, Berkowitz I, Pronovost P. Variability in anticoagulation management of patients on extracorporeal membrane oxygenation: an international survey. Pediatr Crit Care Med . 2013;14(2):e77-84. https://doi.org/10.1097/PCC.0b013e31827127e4
https://doi.org/10.1097/PCC.0b013e318271...
Measuring the activity of anti-factor Xa is considered the gold standard, being the only monitoring tool that correlates well with serum levels of heparin. However, it is unavailable at many centers and is associated with high costs.5757. Bembea MM, Annich G, Rycus P, Oldenburg G, Berkowitz I, Pronovost P. Variability in anticoagulation management of patients on extracorporeal membrane oxygenation: an international survey. Pediatr Crit Care Med . 2013;14(2):e77-84. https://doi.org/10.1097/PCC.0b013e31827127e4
https://doi.org/10.1097/PCC.0b013e318271...
Continuous infusions of unfractionated heparin with usual control of activated partial thromboplastin time (aPTT) are associated with a long system lifespan.5858. Nishinaka T, Tatsumi E, Katagiri N, Ohnishi H, Mizuno T, Shioya K, et al. Up to 151 days of continuous animal perfusion with trivial heparin infusion by the application of a long-term durable antithrombogenic coating to a combination of a seal-less centrifugal pump and a diffusion membrane oxygenator. J Artif Organs. 2007;10(4):240-4. https://doi.org/10.1007/s10047-007-0390-3
https://doi.org/10.1007/s10047-007-0390-...
Our group uses unfractionated heparin and aPTT obtained at intervals from 6 h to 24 h, depending on the moment of ECMO support, always maintaining the aPTT ratio between 2.0 and 3.0. When the heparin infusion needs to be discontinued, high ECMO blood flows, usually above 3,500 mL/min, help avoid clot formation. During vv-ECMO support, some periods without anticoagulation can be allowed.
ECMO MONITORING
The ECMO system must be checked at least once a day. The presence of clots in the circuit, oxygenator, or pump head indicates insufficient anticoagulation. The auscultation of a friction noise in the pump head indicates fibrin deposition on the impeller blades. We daily perform brief (less than a second) elevations of the sweep gas flow to the maximum in order to remove water condensation from the oxygenator ("the oxygenator cough maneuver"), improving the exchange surface. In patients at risk of cerebral hypoperfusion, such as those with increased intracranial hypertension, we avoid the routine use of the cough maneuver, because it can lead to transient hypocapnia and consequent cerebral vasoconstriction. In those patients, we perform the maneuver in selected circumstances (when there is worsening of oxygenation due to ineffective gas exchange of the membrane). The battery can be checked by turning off the external power source. We then provide adequate gel lubrication of the ultrasonic blood flow meter. It is always important to have emergency equipment on the pump console cart: a hand crank, an empty pack to use in cases of massive air embolism, three clamps (at least), and gel for the flow meter. Avoid the use of alcohol on the polycarbonate material to prevent cracks.
Some ECMO groups routinely monitor blood gases and pressures upstream and downstream of the oxygenator. Increased transmembrane pressure is an early warning sign of oxygenator failure due to thrombus formation. Conversely, a concomitant increase in upstream and downstream oxygenator pressures without a change in pump rotations may indicate kinking of the arterial cannula. Our group restricts that monitoring to situations in which hypoxemia or hypercapnia occurs. We also monitor the blood flow/pump rotation ratio, which depends on the preload and afterload of the pump, to identify circuit or oxygenator obstructions to blood flow.5959. Park M, Mendes PV, Hirota AS, dos Santos EV, Costa EL, Azevedo LC. Blood flow/pump rotation ratio as an artificial lung performance monitoring tool during extracorporeal respiratory support using centrifugal pumps. Rev Bras Ter Intensiva . 2015;27(2):178-84. https://doi.org/10.5935/0103-507X.20150030
https://doi.org/10.5935/0103-507X.201500...
The fixation and insertion of the cannulas must also be checked daily. The determination of SpO2 can be used in order to monitor arterial oxygenation. In patients on va-ECMO, it is important to determine the SpO2 on the right arm, which more closely represents the oxygen availability to the brain and myocardium. Once-daily determination of arterial blood gases is usually enough for patients on vv-ECMO. In patients on va-ECMO, cardiac activity can occasionally be extremely depressed, and-in the absence of pulsatility-pulse oximetry will not work. In those patients, blood samples for arterial blood gas analysis should be obtained more frequently, preferably from the right arm.
Twice a week, we screen for hemolysis by measuring lactate dehydrogenase, bilirubins, haptoglobin, aspartate aminotransferase, alanine aminotransferase, and, if possible, free hemoglobin. Measuring D-dimer levels can be also useful for predicting the progression of clotting within the membrane.
Transesophageal echocardiography can be an important tool to monitor patients on vv- or va-ECMO. In vv-ECMO, transesophageal echocardiography can monitor for incident right heart failure, whereas in va-ECMO it can be used in order to diagnose thrombus formation in the aortic root and in the left ventricle, as well as to monitor the function of native cardiac output.
REFRACTORY HYPOXEMIA AND HYPERCAPNIA DURING ECMO SUPPORT
Hypercapnia during ECMO support is very rare, considering that relatively low ECMO blood flows are usually enough to wash out CO2. Sometimes, at low ECMO blood flows, CO2 removal has a "ceiling effect", and further increases in the sweep gas flow have little impact on PaCO2. Under those circumstances, the ECMO blood flow and the sweep gas flow should both be increased in order to treat hypercapnia.
Conversely, hypoxemia during ECMO support is a more common and difficult-to-manage situation, and knowledge of the interaction between the patient and ECMO might help at the bedside. We favor a stepwise approach for such situations, as described below:
-
Check ECMO blood flow: initial settings include keeping ECMO blood flow at a minimum of 60% of the cardiac output in order to reduce the fraction of non-oxygenated venous blood flow that will mix with oxygenated blood.
-
Check for recirculation: the oxygenated blood from the arterial cannula might be once again drained by the ECMO system, causing recirculation, which can reduce ECMO efficiency. The relative position of the cannulas can be assessed with chest X-ray and ultrasound at the bedside. When the tips of arterial and venous cannulas are in close proximity (Figure 3), recirculation is likely to occur. It is recommended that the tip of the femoral venous cannula be placed at the level of the suprahepatic inferior vena cava. If oxygen saturation in the drainage cannula is > 70% or if its difference in relation to the SaO2 is < 20%, recirculation can be a contributing factor to persistent hypoxemia. Consider repositioning the drainage cannula or changing to a hybrid configuration.
An X-ray of the chest (taken with a portable X-ray machine at the bedside) of a patient with hypoxemic respiratory failure submitted to extracorporeal membrane oxygenation support. Note the positioning of the cannulas (dashed lines) with their tips (arrows) in close proximity, which may favor the occurrence of recirculation.
-
Check for excessive oxygen consumption: aggressively treat fever and agitation. Consider neuromuscular blockers if the patient exhibits increased respiratory effort.
-
Check for oxygenator failure: thrombus formation due to inappropriate anticoagulation or to prolonged ECMO support might impair gas exchange. Consider replacing the oxygenator if there are signs of hemolysis or inappropriate fibrinolysis.
-
Optimize ventilator settings if possible: increase the FiO2 if it is < 60%, and increase the PEEP if appropriate. Also consider using alveolar recruitment maneuvers and the prone position, as well as neuromuscular blockers or inhaled nitric oxide.
-
Consider reducing the cardiac output of the patient, if appropriate, with beta-blockers. Although this option is associated with improvement in oxygenation, it has not been tested formally in a randomized clinical trial and therefore remains experimental.
The decision of whether and how far hypoxemia or hypercapnia can be tolerated should be individualized, considering the clinical condition of the patient.
TROUBLESHOOTING
Communication with the hospital blood bank is important to make sure there is enough blood in stock in case of emergency.
If for any reason the ECMO device stops, prompt restoration of mechanical ventilation is mandatory with predefined "emergency settings." Those settings must be written down, visible, and placed near the ventilator (for instance, FiO2 = 1, PEEP = 10 cmH2O, peak pressure = 30 cmH2O, and RR = 35 breaths/min).
In cases of interruption of power supply or any other pump console failure, the hand crank must be used in order to pump the blood. The previously mentioned "sucking-up" phenomenon is common. It can cause sudden falls in the measured ECMO blood flow with preserved pump rotations, which is commonly associated with the chattering of the ECMO circuit. Additionally, the pump head, normally dark red because it is filled with venous blood, can become light red or even white (the color of the impeller blades) when the flow from the drainage cannula is insufficient (a phenomenon referred to as cavitation). Some maneuvers can be of help: 1) increasing the bed angle; 2) placing the patient in the lateral decubitus position; 3) decreasing the ECMO blood flow and then increasing it slowly; 4) increasing the PEEP; 5) transfusing packed red blood cells if appropriate; 6) attempting fluid challenges; and 7) starting or increasing epinephrine infusion when appropriate.2121. Napp LC, Kühn C, Hoeper MM, Vogel-Claussen J, Haverich A, Schäfer A, et al. Cannulation strategies for percutaneous extracorporeal membrane oxygenation in adults. Clin Res Cardiol. 2016;105(4):283-96. https://doi.org/10.1007/s00392-015-0941-1
https://doi.org/10.1007/s00392-015-0941-...
Air embolism can occur when the venous pre-pump system, where the pressure is negative, is being handled. If air embolism occurs, immediately interrupt the ECMO blood flow (the outlet of the pump head must be turned down in order to avoid pushing the air forward) and change to emergency mechanical ventilation settings (see above). Then proceed to remove the air with syringes or emergency bags. If a circuit rupture is identified, the ECMO flow must be interrupted and the damaged segment of the circuit must be replaced. Hemolysis is relatively common and can be avoided by reducing the ECMO blood flow, correcting cannula malpositioning, and enhancing anticoagulation, especially if there are clots in the circuit. If the RRT circuit is connected to the ECMO circuit, the use of a separate dialysis catheter should be considered.6060. Betrus C, Remenapp R, Charpie J, Kudelka T, Brophy P, Smoyer WE, et al. Enhanced hemolysis in pediatric patients requiring extracorporeal membrane oxygenation and continuous renal replacement therapy. Ann Thorac Cardiovasc Surg. 2007;13(6):378-83.) During vv-ECMO support, cardiac arrest should be treated as usual, except that ventilation is unnecessary.
ECMO DISCONTINUATION
Every day, we perform an autonomy test by reducing the sweep gas flow to zero. To guarantee the absence of sweep gas flow, we usually clamp the air line that is connected to the oxygenator. Immediately before the autonomy test, acceptable mechanical ventilation parameters must be set. In patients on controlled mechanical ventilation, we favor the use of a PEEP of 10-15 cmH2O, a tidal volume of 6 mL/kg of ideal body weight, a RR of 35 breaths/min, and an FiO2 of 0.6.44. Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351-63. https://doi.org/10.1016/S0140-6736(09)61069-2
https://doi.org/10.1016/S0140-6736(09)61...
If the patient is on pressure support ventilation, the pressure support can be adjusted to between 6 cmH2O and 20 cmH2O depending on the clinical and physiological condition of the patient. When the sweep gas flow is turned off, patient comfort and SpO2 must be observed closely. If the patient is stable for 1-6 h, arterial blood gas analysis is performed. If the PaO2 is > 55 mmHg and the pH is within the normal range (7.35-7.45), we consider (depending on the clinical status) the withdrawal of ECMO support.2323. Kolobow T, Gattinoni L, Tomlinson T, White D, Pierce J, Iapichino G. The carbon dioxide membrane lung (CDML): a new concept. Trans Am Soc Artif Intern Organs. 1977;23:17-21. https://doi.org/10.1097/00002480-197700230-00005
https://doi.org/10.1097/00002480-1977002...
Patients with borderline oxygenation and right ventricular dysfunction might not tolerate sudden changes in oxygenation, because hypoxia can worsen or the patient can develop cor pulmonale . In those patients, we gradually change the FiO2 of the sweep gas flow from 1.0 to 0.21, after which we slowly reduce it to zero.
We remove the venous cannulas at the bedside and apply pressure to the insertion sites for at least 30 min. We later place suture reinforcements around the orifices and leave the sutures in place for at least 24 h. Arterial cannulas are withdrawn in the operating room.
FINAL CONSIDERATIONS
ECMO for respiratory support is a suitable rescue therapy for patients with severe ARDS or other causes of respiratory failure, assuming that the patients are not beyond hope. Costs do not seem prohibitive. A trained multidisciplinary ICU team is fully capable of initiating and conducting ECMO support. Although emergencies are infrequent among patients on vv-ECMO support, a training program is necessary to keeping the team competent and confident.
Our group has created a YouTube channel, designated "Grupo de ECMO HCFMUSP ", with the purpose of training our educational team. The channel is open access.
REFERENCES
-
1Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):637-45. https://doi.org/10.1001/jama.299.6.637
» https://doi.org/10.1001/jama.299.6.637 -
2Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):646-55. https://doi.org/10.1001/jama.299.6.646
» https://doi.org/10.1001/jama.299.6.646 -
3Lindén V, Palmér K, Reinhard J, Westman R, Ehrén H, Granholm T, et al. High survival in adult patients with acute respiratory distress syndrome treated by extracorporeal membrane oxygenation, minimal sedation, and pressure supported ventilation. Intensive Care Med. 2000;26(11):1630-7. https://doi.org/10.1007/s001340000697
» https://doi.org/10.1007/s001340000697 -
4Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374(9698):1351-63. https://doi.org/10.1016/S0140-6736(09)61069-2
» https://doi.org/10.1016/S0140-6736(09)61069-2 -
5Noah MA, Peek GJ, Finney SJ, Griffiths MJ, Harrison DA, Grieve R, et al. Referral to an extracorporeal membrane oxygenation center and mortality among patients with severe 2009 influenza A(H1N1). JAMA. 2011;306(15):1659-68. https://doi.org/10.1001/jama.2011.1471
» https://doi.org/10.1001/jama.2011.1471 -
6Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators, Davies A, Jones D, Bailey M, Beca J, Bellomo R, et al. Extracorporeal Membrane Oxygenation for 2009 Influenza A(H1N1) Acute Respiratory Distress Syndrome. JAMA. 2009;302(17):1888-95. https://doi.org/10.1001/jama.2009.1535
» https://doi.org/10.1001/jama.2009.1535 -
7Peek GJ, Moore HM, Moore N, Sosnowski AW, Firmin RK. Extracorporeal membrane oxygenation for adult respiratory failure. Chest. 1997;112(3):759-64. https://doi.org/10.1378/chest.112.3.759
» https://doi.org/10.1378/chest.112.3.759 -
8Hill JD, O'Brien TG, Murray JJ, Dontigny L, Bramson ML, Osborn JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med. 1972;286(12):629-34. https://doi.org/10.1056/NEJM197203232861204
» https://doi.org/10.1056/NEJM197203232861204 -
9Zapol WM, Snider MT, Schneider RC. Extracorporeal membrane oxygenation for acute respiratory failure. Anesthesiology. 1977;46(4):272-85. https://doi.org/10.1097/00000542-197704000-00008
» https://doi.org/10.1097/00000542-197704000-00008 -
10Zapol WM, Snider MT, Hill JD, Fallat RJ, Bartlett RH, Edmunds LH, et al. Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA. 1979;242(20):2193-6. https://doi.org/10.1001/jama.1979.03300200023016
» https://doi.org/10.1001/jama.1979.03300200023016 -
11Gattinoni L, Pesenti A, Mascheroni D, Marcolin R, Fumagalli R, Rossi F, et al. Low-frequency positive-pressure ventilation with extracorporeal CO2 removal in severe acute respiratory failure. JAMA. 1986;256(7):881-6. https://doi.org/10.1001/jama.1986.03380070087025
» https://doi.org/10.1001/jama.1986.03380070087025 -
12Morris AH, Wallace CJ, Menlove RL, Clemmer TP, Orme JF Jr, Weaver LK, et al. Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med. 1994;149(2 Pt 1):295-305. https://doi.org/10.1164/ajrccm.149.2.8306022
» https://doi.org/10.1164/ajrccm.149.2.8306022 -
13Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med . 2000;342(18):1301-8. https://doi.org/10.1056/NEJM200005043421801
» https://doi.org/10.1056/NEJM200005043421801 -
14Pham T, Combes A, Rozé H, Chevret S, Mercat A, Roch A, et al. Extracorporeal membrane oxygenation for pandemic influenza A(H1N1)-induced acute respiratory distress syndrome. A cohort study and propensity-matched analysis. Am J Respir Crit Care Med . 2013;187(3):276-85. https://doi.org/10.1164/rccm.201205-0815OC
» https://doi.org/10.1164/rccm.201205-0815OC -
15Extracorporeal Life Support Organization [homepage on the Internet]. Ann Arbor (MI): the Organization [cited 2016 Sep 1]. H1N1 Registry; [about 4 screens]. Available from: Available from: https://www.elso.org/Registry/H1N1Registry.aspx
» https://www.elso.org/Registry/H1N1Registry.aspx -
16ClinicalTrials.gov [homepage on the Internet]. Bethesda: U.S. National Institutes of Health [cited 2016 Sep 1]. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome (EOLIA) [about 7 screens]. Available from: Available from: https://clinicaltrials.gov/ct2/show/NCT01470703
» https://clinicaltrials.gov/ct2/show/NCT01470703 -
17Oshima K, Kunimoto F, Hinohara H, Ohkawa M, Mita N, Tajima Y, et al. Extracorporeal membrane oxygenation for respiratory failure: comparison of venovenous versus venoarterial bypass. Surg Today. 2010;40(3):216-22. https://doi.org/10.1007/s00595-008-4040-z
» https://doi.org/10.1007/s00595-008-4040-z -
18Zahraa JN, Moler FW, Annich GM, Maxvold NJ, Bartlett RH, Custer JR. Venovenous versus venoarterial extracorporeal life support for pediatric respiratory failure: are there differences in survival and acute complications? Crit Care Med. 2000;28(2):521-5. https://doi.org/10.1097/00003246-200002000-00039
» https://doi.org/10.1097/00003246-200002000-00039 -
19Mekontso Dessap A, Charron C, Devaquet J, Aboab J, Jardin F, Brochard L, et al. Impact of acute hypercapnia and augmented positive end-expiratory pressure on right ventricle function in severe acute respiratory distress syndrome. Intensive Care Med . 2009;35(11):1850-8. https://doi.org/10.1007/s00134-009-1569-2
» https://doi.org/10.1007/s00134-009-1569-2 -
20Reis Miranda D, van Thiel R, Brodie D, Bakker J. Right ventricular unloading after initiation of venovenous extracorporeal membrane oxygenation. Am J Respir Crit Care Med . 2015;191(3):346-8. https://doi.org/10.1164/rccm.201408-1404LE
» https://doi.org/10.1164/rccm.201408-1404LE -
21Napp LC, Kühn C, Hoeper MM, Vogel-Claussen J, Haverich A, Schäfer A, et al. Cannulation strategies for percutaneous extracorporeal membrane oxygenation in adults. Clin Res Cardiol. 2016;105(4):283-96. https://doi.org/10.1007/s00392-015-0941-1
» https://doi.org/10.1007/s00392-015-0941-1 -
22Park M, Costa EL, Maciel AT, Barbosa EV, Hirota AS, Schettino Gde P, et al. Effect of flow rate and temperature on transmembrane blood pressure drop in an extracorporeal artificial lung. Perfusion. 2014;29(6):517-25. https://doi.org/10.1177/0267659114525986
» https://doi.org/10.1177/0267659114525986 -
23Kolobow T, Gattinoni L, Tomlinson T, White D, Pierce J, Iapichino G. The carbon dioxide membrane lung (CDML): a new concept. Trans Am Soc Artif Intern Organs. 1977;23:17-21. https://doi.org/10.1097/00002480-197700230-00005
» https://doi.org/10.1097/00002480-197700230-00005 -
24Park M, Mendes PV, Costa EL, Barbosa EV, Hirota AS, Azevedo LC. Factors associated with blood oxygen partial pressure and carbon dioxide partial pressure regulation during respiratory extracorporeal membrane oxygenation support: data from a swine model. Rev Bras Ter Intensiva. 2016;28(1):11-8. https://doi.org/10.5935/0103-507X.20160006
» https://doi.org/10.5935/0103-507X.20160006 -
25Park M, Costa EL, Maciel AT, Silva DP, Friedrich N, Barbosa EV, et al. Determinants of oxygen and carbon dioxide transfer during extracorporeal membrane oxygenation in an experimental model of multiple organ dysfunction syndrome. PLoS One. 2013;8(1):e54954. https://doi.org/10.1371/journal.pone.0054954
» https://doi.org/10.1371/journal.pone.0054954 -
26Park M, Mendes PV, Zampieri FG, Azevedo LC, Costa EL, Antoniali F, et al. The economic effect of extracorporeal membrane oxygenation to support adults with severe respiratory failure in Brazil: a hypothetical analysis. Rev Bras Ter Intensiva . 2014;26(3):253-62. https://doi.org/10.5935/0103-507X.20140036
» https://doi.org/10.5935/0103-507X.20140036 -
27Park M, Azevedo LC, Mendes PV, Carvalho CR, Amato MB, Schettino GP, et al. First-year experience of a Brazilian tertiary medical center in supporting severely ill patients using extracorporeal membrane oxygenation. Clinics (Sao Paulo). 2012;67(10):1157-63. https://doi.org/10.6061/clinics/2012(10)07
» https://doi.org/10.6061/clinics/2012(10)07 -
28Schmidt M, Zogheib E, Rozé H, Repesse X, Lebreton G, Luyt CE, et al. The PRESERVE mortality risk score and analysis of long-term outcomes after extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. Intensive Care Med . 2013;39(10):1704-13. https://doi.org/10.1007/s00134-013-3037-2
» https://doi.org/10.1007/s00134-013-3037-2 -
29Schmidt M, Bailey M, Sheldrake J, Hodgson C, Aubron C, Rycus PT, et al. Predicting survival after extracorporeal membrane oxygenation for severe acute respiratory failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) score. Am J Respir Crit Care Med . 2014;189(11):1374-82. https://doi.org/10.1164/rccm.201311-2023OC
» https://doi.org/10.1164/rccm.201311-2023OC -
30Sidebotham D, McGeorge A, McGuinness S, Edwards M, Willcox T, Beca J. Extracorporeal membrane oxygenation for treating severe cardiac and respiratory failure in adults: part 2-technical considerations. J Cardiothorac Vasc Anesth. 2010;24(1):164-72. https://doi.org/10.1053/j.jvca.2009.08.002
» https://doi.org/10.1053/j.jvca.2009.08.002 -
31Rich PB, Awad SS, Crotti S, Hirschl RB, Bartlett RH, Schreiner RJ. A prospective comparison of atrio-femoral and femoro-atrial flow in adult venovenous extracorporeal life support. J Thorac Cardiovasc Surg. 1998;116(4):628-32. https://doi.org/10.1016/S0022-5223(98)70170-9
» https://doi.org/10.1016/S0022-5223(98)70170-9 -
32Guervilly C, Dizier S, Thomas G, Jaussaud N, Morera P, Hraiech S, et al. Comparison of femorofemoral and femorojugular configurations during venovenous extracorporeal membrane oxygenation for severe ARDS. Intensive Care Med . 2014;40(10):1598-9. https://doi.org/10.1007/s00134-014-3427-0
» https://doi.org/10.1007/s00134-014-3427-0 -
33van Heijst AF, van der Staak FH, de Haan AF, Liem KD, Festen C, Geven WB, et al. Recirculation in double lumen catheter veno-venous extracorporeal membrane oxygenation measured by an ultrasound dilution technique. ASAIO J. 2001;47(4):372-6. https://doi.org/10.1097/00002480-200107000-00015
» https://doi.org/10.1097/00002480-200107000-00015 -
34Chimot L, Marqué S, Gros A, Gacouin A, Lavoué S, Camus C, et al. Avalon(c) bicaval dual-lumen cannula for venovenous extracorporeal membrane oxygenation: survey of cannula use in France. ASAIO J . 2013;59(2):157-61. https://doi.org/10.1097/MAT.0b013e31827db6f3
» https://doi.org/10.1097/MAT.0b013e31827db6f3 -
35Kalem V, Buchwald D, Strauch J, Sidiropoulos A, Meindl R, Schildhauer TA, et al. Surgical extraction after thrombosis around the Avalon dual lumen cannula. Ann R Coll Surg Engl. 2014;96(1):106E-108E. https://doi.org/10.1308/003588414X13824511649814
» https://doi.org/10.1308/003588414X13824511649814 -
36Mendes PV, Park M, Maciel AT, E Silva DP, Friedrich N, Barbosa EV, et al. Kinetics of arterial carbon dioxide during veno-venous extracorporeal membrane oxygenation support in an apnoeic porcine model. Intensive Care Med Exp. 2016;4(1):1. https://doi.org/10.1186/s40635-015-0074-x
» https://doi.org/10.1186/s40635-015-0074-x -
37Nunes LB, Mendes PV, Hirota AS, Barbosa EV, Maciel AT, Schettino GP, et al. Severe hypoxemia during veno-venous extracorporeal membrane oxygenation: exploring the limits of extracorporeal respiratory support. Clinics (Sao Paulo) . 2014;69(3):173-8. https://doi.org/10.6061/clinics/2014(03)05
» https://doi.org/10.6061/clinics/2014(03)05 -
38Mendes PV, Moura E, Barbosa EV, Hirota AS, Scordamaglio PR, Ajjar FM, et al. Challenges in patients supported with extracorporeal membrane oxygenation in Brazil. Clinics (Sao Paulo) . 2012;67(12):1511-5. https://doi.org/10.6061/clinics/2012(12)27
» https://doi.org/10.6061/clinics/2012(12)27 -
39Lindén VB, Lidegran MK, Frisén G, Dahlgren P, Frenckner BP, Larsen F. ECMO in ARDS: a long-term follow-up study regarding pulmonary morphology and function and health-related quality of life. Acta Anaesthesiol Scand. 2009;53(4):489-95. https://doi.org/10.1111/j.1399-6576.2008.01808.x
» https://doi.org/10.1111/j.1399-6576.2008.01808.x -
40Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson BT, Bellamy SL, et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med . 2012;185(12):1307-15. https://doi.org/10.1164/rccm.201111-2025OC
» https://doi.org/10.1164/rccm.201111-2025OC -
41Schmidt M, Pellegrino V, Combes A, Scheinkestel C, Cooper DJ, Hodgson C. Mechanical ventilation during extracorporeal membrane oxygenation. Crit Care. 2014;18(1):203. https://doi.org/10.1186/cc13702
» https://doi.org/10.1186/cc13702 -
42Schmidt M, Stewart C, Bailey M, Nieszkowska A, Kelly J, Murphy L, et al. Mechanical ventilation management during extracorporeal membrane oxygenation for acute respiratory distress syndrome: a retrospective international multicenter study. Crit Care Med . 2015;43(3):654-64. https://doi.org/10.1097/CCM.0000000000000753
» https://doi.org/10.1097/CCM.0000000000000753 -
43Marhong JD, Telesnicki T, Munshi L, Del Sorbo L, Detsky M, Fan E. Mechanical ventilation during extracorporeal membrane oxygenation. An international survey. Ann Am Thorac Soc. 2014;11(6):956-61. https://doi.org/10.1513/AnnalsATS.201403-100BC
» https://doi.org/10.1513/AnnalsATS.201403-100BC -
44Campbell RS, Davis K Jr, Johannigman JA, Branson RD. The effects of passive humidifier dead space on respiratory variables in paralyzed and spontaneously breathing patients. Respir Care. 2000;45(3):306-12.
-
45Maggiore SM, Lellouche F, Pigeot J, Taille S, Deye N, Durrmeyer X, et al. Prevention of endotracheal suctioning-induced alveolar derecruitment in acute lung injury. Am J Respir Crit Care Med . 2003;167(9):1215-24. https://doi.org/10.1164/rccm.200203-195OC
» https://doi.org/10.1164/rccm.200203-195OC -
46Mauri T, Grasselli G, Suriano G, Eronia N, Spadaro S, Turrini C, et al. Control of Respiratory Drive and Effort in Extracorporeal Membrane Oxygenation Patients Recovering from Severe Acute Respiratory Distress Syndrome. Anesthesiology . 2016;125(1):159-67. https://doi.org/10.1097/ALN.0000000000001103
» https://doi.org/10.1097/ALN.0000000000001103 -
47National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network., Rice TW, Wheeler AP, Thompson BT, Steingrub J, Hite RD, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307(8):795-803. https://doi.org/10.1001/jama.2012.137
» https://doi.org/10.1001/jama.2012.137 -
48Schmidt M, Bréchot N, Hariri S, Guiguet M, Luyt CE, Makri R, et al. Nosocomial infections in adult cardiogenic shock patients supported by venoarterial extracorporeal membrane oxygenation. Clin Infect Dis. 2012;55(12):1633-41. https://doi.org/10.1093/cid/cis783
» https://doi.org/10.1093/cid/cis783 -
49Brogan TV, Thiagarajan RR, Rycus PT, Bartlett RH, Bratton SL. Extracorporeal membrane oxygenation in adults with severe respiratory failure: a multi-center database. Intensive Care Med . 2009;35(12):2105-14. https://doi.org/10.1007/s00134-009-1661-7
» https://doi.org/10.1007/s00134-009-1661-7 -
50Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med . 2011;365(20):1905-14. https://doi.org/10.1056/NEJMct1103720
» https://doi.org/10.1056/NEJMct1103720 -
51Abrams D, Javidfar J, Farrand E, Mongero LB, Agerstrand CL, Ryan P, et al. Early mobilization of patients receiving extracorporeal membrane oxygenation: a retrospective cohort study. Crit Care . 2014;18(1):R38. https://doi.org/10.1186/cc13746
» https://doi.org/10.1186/cc13746 -
52Santiago MJ, Sánchez A, López-Herce J, Pérez R, del Castillo J, Urbano J, et al. The use of continuous renal replacement therapy in series with extracorporeal membrane oxygenation. Kidney Int. 2009;76(12):1289-92. https://doi.org/10.1038/ki.2009.383
» https://doi.org/10.1038/ki.2009.383 -
53Zimmermann AK, Weber N, Aebert H, Ziemer G, Wendel HP. Effect of biopassive and bioactive surface-coatings on the hemocompatibility of membrane oxygenators. J Biomed Mater Res B Appl Biomater. 2007;80(2):433-9. https://doi.org/10.1002/jbm.b.30614
» https://doi.org/10.1002/jbm.b.30614 -
54Shekar K, Roberts JA, Barnett AG, Diab S, Wallis SC, Fung YL, et al. Can physicochemical properties of antimicrobials be used to predict their pharmacokinetics during extracorporeal membrane oxygenation? Illustrative data from ovine models. Crit Care . 2015;19:437. https://doi.org/10.1186/s13054-015-1151-y
» https://doi.org/10.1186/s13054-015-1151-y -
55Shekar K, Fraser JF, Smith MT, Roberts JA. Pharmacokinetic changes in patients receiving extracorporeal membrane oxygenation. J Crit Care . 2012;27(6):741.e9-18. https://doi.org/10.1016/j.jcrc.2012.02.013
» https://doi.org/10.1016/j.jcrc.2012.02.013 -
56Shekar K, Fraser JF, Taccone FS, Welch S, Wallis SC, Mullany DV, et al. The combined effects of extracorporeal membrane oxygenation and renal replacement therapy on meropenem pharmacokinetics: a matched cohort study. Crit Care . 2014;18(6):565. https://doi.org/10.1186/s13054-014-0565-2
» https://doi.org/10.1186/s13054-014-0565-2 -
57Bembea MM, Annich G, Rycus P, Oldenburg G, Berkowitz I, Pronovost P. Variability in anticoagulation management of patients on extracorporeal membrane oxygenation: an international survey. Pediatr Crit Care Med . 2013;14(2):e77-84. https://doi.org/10.1097/PCC.0b013e31827127e4
» https://doi.org/10.1097/PCC.0b013e31827127e4 -
58Nishinaka T, Tatsumi E, Katagiri N, Ohnishi H, Mizuno T, Shioya K, et al. Up to 151 days of continuous animal perfusion with trivial heparin infusion by the application of a long-term durable antithrombogenic coating to a combination of a seal-less centrifugal pump and a diffusion membrane oxygenator. J Artif Organs. 2007;10(4):240-4. https://doi.org/10.1007/s10047-007-0390-3
» https://doi.org/10.1007/s10047-007-0390-3 -
59Park M, Mendes PV, Hirota AS, dos Santos EV, Costa EL, Azevedo LC. Blood flow/pump rotation ratio as an artificial lung performance monitoring tool during extracorporeal respiratory support using centrifugal pumps. Rev Bras Ter Intensiva . 2015;27(2):178-84. https://doi.org/10.5935/0103-507X.20150030
» https://doi.org/10.5935/0103-507X.20150030 -
60Betrus C, Remenapp R, Charpie J, Kudelka T, Brophy P, Smoyer WE, et al. Enhanced hemolysis in pediatric patients requiring extracorporeal membrane oxygenation and continuous renal replacement therapy. Ann Thorac Cardiovasc Surg. 2007;13(6):378-83.
-
Financial support: None
-
2
Study carried out at the Faculdade de Medicina do ABC, Santo André (SP), at Hospital Sírio-Libanês, and at the Instituto do Coração, Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo, São Paulo (SP) Brasil
Publication Dates
-
Publication in this collection
Jan-Feb 2017
History
-
Received
22 Sept 2016 -
Accepted
05 Jan 2017