Abstract
The aim of this study was to evaluate the effect of industrialised foods and drinks on primary tooth enamel previously eroded with hydrochloric acid (HCl). The crowns of one hundred two specimens were subjected to an erosive challenge with HCl and randomly divided into six groups (n = 17): Chocolate Milk (Toddynho® - Pepsico) - negative control; Petit Suisse Yogurt (Danoninho® - Danone); Strawberry Yogurt (Vigor); Apple puree (Nestlé); Fermented Milk (Yakult® - Yakult); and Home Squeezed Style Orange Juice (del Valle) - positive control. The 28-day immersion cycles for the test products were performed twice daily and were interspersed with exposure of the test substrate to artificial saliva. Measurements of enamel surface microhardness (SMH) were performed initially, after immersion in HCl and at 7, 14, 21 and 28 days of experimentation. A two-way ANOVA, according to a split-plot design, followed by the sum of squares decomposition and Tukey’s test, revealed a significant effect for the interaction between Foods and Drinks and Length of Exposure (p < 0.00001). Orange juice resulted in greater mineral loss of enamel after 28 days. None of the test products was associated with recovery of tooth enamel microhardness.
Tooth Erosion; Tooth, Deciduous; Hydrochloric Acid; Food; Beverages
Introduction
Dental erosion is the chronic loss of tooth substance in the presence of acids, without the involvement of bacteria,11 Lussi A, Megert B, Shellis RP, Wang X. Analysis of the erosive effect of different dietary substances and medications. Br J Nutr. 2012 Jan;107(2):252-62. characterised clinically as corrosive-abrasive wear.22 Serra MC, Messias DCF, Turssi CP. Control of erosive tooth wear: possibilities and rationale. Braz Oral Res. 2009 Jun;23(Suppl 1):49-55. It is a multifactorial condition with a complex aetiology and is common in children and adolescents.33 Lussi A, Schaffner M, Jaeggi T. Dental erosion – diagnosis and prevention in children and adults. Int Dent J. 2007 Dec;57(6):385-98. It involves contact between acids and tooth structure, which may be due to intrinsic factors such as vomiting, regurgitation, and gastro-oesophageal reflux disease (GORD), and/or extrinsic factors such as industrial sources,44 Addy M, Shellis RP. Interaction between attrition, abrasion and erosion in tooth wear. In: Lussi A. Dental erosion. Monogr Oral Sci. Bern: Basel, Karger; 2006. p.17-21. acid medicines, and diet.22 Serra MC, Messias DCF, Turssi CP. Control of erosive tooth wear: possibilities and rationale. Braz Oral Res. 2009 Jun;23(Suppl 1):49-55. Gastro-oesophageal reflux is a physiological process that commonly affects paediatric patients and is characterised by involuntary passage of gastric contents from the oesophagus55 Davies AEM, Sandhu BK. Diagnosis and treatment of gastro-oesophageal reflux. Arch Dis Child. 1995 Jul;73(1):82-6. into the oral cavity. This is considered pathological when both intensity and frequency increase, causing GORD.55 Davies AEM, Sandhu BK. Diagnosis and treatment of gastro-oesophageal reflux. Arch Dis Child. 1995 Jul;73(1):82-6. Although the hydrochloric acid (HCl) present in gastric juice induces the demineralisation and loss of dental hard tissues,66 Bartlett D. Intrinsic causes of erosion. In: Lussi A. Dental erosion. Monogr Oral Sci. Bern: Basel, Karger 2006. p.119-39. diet is the most important cause of dental erosion77 Lussi A, Schlueter N, Rakhmatullina E, Ganss C. Dental erosion – an overview with emphasis on chemical and histopathological aspects. Caries Res. 2011 May;45(1 Suppl):2-12. due to individuals’ high consumption of acidic foods and drinks.88 Lussi A, Kohler N, Zero D, Schaffner M, Megert B. A comparison of the erosive potential of different beverages in primary and permanent teeth using an in vitro model. Eur J Oral Sci. 2000 Apr;108(2):110-14. The erosive potential of acidic drinks or foods depends on the pH, acid concentration, length of exposure,99 Larsen MJ, Nyvad B. Enamel erosion by soft drinks and orange juices relative to their pH, buffering effect and contents of calcium phosphate. Caries Res. 1999 May;33(1):81-7. titratable acidity, mineral content, clearance on tooth surface, and its calcium-chelating properties.1010 Lussi A, Jaeggi T. Erosion—diagnosis and risk factors. Clin Oral Investig. 2008 Mar;12(Suppl 1):S5–S13.
In seeking to provide practical snacks to children, caregivers often choose manufactured products, including yogurt, fermented milk, petit suisse, fruit puree, juices, and chocolate milk because they are easily transported, well accepted and routinely ingested by children due to their good taste. The literature shows conflicting results regarding the erosive effect of yogurt. Whereas some authors state that yogurt, despite its low pH, does not have an erosive effect,77 Lussi A, Schlueter N, Rakhmatullina E, Ganss C. Dental erosion – an overview with emphasis on chemical and histopathological aspects. Caries Res. 2011 May;45(1 Suppl):2-12. , 88 Lussi A, Kohler N, Zero D, Schaffner M, Megert B. A comparison of the erosive potential of different beverages in primary and permanent teeth using an in vitro model. Eur J Oral Sci. 2000 Apr;108(2):110-14. , 1111 Rytömaa I, Meurman JH, Koskinen J, Laakso T, Gharazi L, Turunen R. In vitro erosion of bovine enamel caused by acids drinks and other foodstuffs. Scand J Dent Res. 1988 Aug;96(4):324-33. a report has shown yogurt significantly increases wear and reduces the hardness of dental enamel.1212 Jitpukdeebodintra S, Chuenarrom C, Muttarak C, Khonsuphap P, Prasattakarn S. Effects of 1.23% acidulated phosphate fluoride gel and drinkable yogurt on human enamel erosion, in vitro. Quintessence Int. 2010 Jul-Aug;41(7):595–604. In a recent study, fermented milk drinks, which are rich in lactobacilli and bifidobacteria and are used in the prevention and treatment of gastrointestinal disorders,1313 Haukioja A. Probiotics and Oral Health. Eur J Dent. 2010 Jul;4(3):348-55. produced enamel mineral loss after four cycles of demineralisation and remineralisation.1414 Lodi CS, Sassaki KT, Fraiz FC, Delbem AC, Martinhon CC. Evaluation of some properties of fermented milk beverages that affect the demineralization of dental enamel. Braz Oral Res. 2010 Jan-Mar;24(1):95-101. Even though there are some studies on food items widely consumed by children, there is a paucity of studies on the effect of petit suisse yogurt, which is fresh cheese derived from milk fermentation and pasteurisation, rich in minerals and vitamins, with the addition of fruit pulp. Pureed Fruit are practical, doughy foods offered to children by the spoonful; however, no studies have evaluated their potential and/or erosive effect. Studies have shown that orange juice has a high erosive potential1515 Hunter L, Patel S, Rees J. The in vitro erosive potential of a range of baby drinks. Int J Paediatr Dent. 2009 Sep;19(5):325-29. and an erosive effect,88 Lussi A, Kohler N, Zero D, Schaffner M, Megert B. A comparison of the erosive potential of different beverages in primary and permanent teeth using an in vitro model. Eur J Oral Sci. 2000 Apr;108(2):110-14. , 99 Larsen MJ, Nyvad B. Enamel erosion by soft drinks and orange juices relative to their pH, buffering effect and contents of calcium phosphate. Caries Res. 1999 May;33(1):81-7. , 1111 Rytömaa I, Meurman JH, Koskinen J, Laakso T, Gharazi L, Turunen R. In vitro erosion of bovine enamel caused by acids drinks and other foodstuffs. Scand J Dent Res. 1988 Aug;96(4):324-33. , 1212 Jitpukdeebodintra S, Chuenarrom C, Muttarak C, Khonsuphap P, Prasattakarn S. Effects of 1.23% acidulated phosphate fluoride gel and drinkable yogurt on human enamel erosion, in vitro. Quintessence Int. 2010 Jul-Aug;41(7):595–604. , 1616 Torres CP, Chinellati MA, Gomes-Silva JM, Rizóli FA, Oliveira MA, Palma-Dibb RG, et al. Surface and subsurface erosion of primary enamel by acid beverages over time. Braz Dent J. 2010 Sep;21(4):337-45. due to its acidic nature, although it is considered a healthy drink. However, foods and drinks containing milk and, hence, high levels of calcium and phosphate (e.g., chocolate milk) are more likely to have a remineralising effect1111 Rytömaa I, Meurman JH, Koskinen J, Laakso T, Gharazi L, Turunen R. In vitro erosion of bovine enamel caused by acids drinks and other foodstuffs. Scand J Dent Res. 1988 Aug;96(4):324-33. on dental enamel. Due to the constant consumption of some industrialised foods and drinks by children and the dearth of literature on this issue, the aim of this paper was to evaluate the erosive effect of yogurt, fermented milk, petit suisse, fruit puree, and orange juice, as well as the remineralising effect of chocolate milk on primary tooth enamel previously eroded by HCl, simulating what occurs in patients with gastro-oesophageal reflux.
Methodology
This study was independently reviewed and approved by the Research Ethics Committee of the School of Dentistry of Ribeirão Preto, Universidade de São Paulo – USP (Process #2011.1.1012.58.7).
Composition of foods and beverages and measurement of initial pH
The information about the compositions of the tested products was obtained from the products’ packaging. The pH of each product was measured with a previously calibrated digital pH meter (Analion AN2000, Ribeirão Preto, Brazil) (Table 1).
Experimental design
Arrangements were made to perform a randomised complete block experiment according to a split-plot design in order to compare the response variable (microhardness; SMH) and the factors under study (two factors: experimental groups and time effect), as follows:
-
Experimental groups – six different types of snacks, foods and drinks for children, including (1) Negative Control [Milk Chocolate (Toddynho® - Pepsico do Brasil Ltda., São Paulo, Brazil)], (2) Positive Control [Orange Juice Home Squeezed Style Orange Juice (del Valle, Sucos del Valle do Brasil Ltda., Santa Bárbara D’Oeste, Brazil)], (3) Apple Puree (Nestlé - Nestlé Brasil Ltda., São Paulo, Brazil), (4) Strawberry Yogurt (Vigor - Vigor Alimentos S.A., São Paulo, Brazil), (5) Fermented Milk (Yakult® - Yakult S.A. Indústria e Comércio, São Bernardo do Campo, Brazil), and (6) Petit Suisse Yogurt (Danoninho® - Danone®Ltda., Poços de Caldas, Brazil);
-
Time effect – repeated measurements were taken from the same experimental group over time after immersion in HCl to obtain responses at baseline (before immersion) and at 7, 14, 21, and 28 days after immersion.
Enamel surface loss measured by surface microhardness (SMH) was used as the response variable.
Selection of teeth and preparation of specimens
Two hundred human primary central incisors were obtained from the Human Tooth Bank of the School of Dentistry of Ribeirão Preto, Universidade de São Paulo. They were stored in 0.1% thymol at 4°C for 48 h, cleaned with a pumice-water slurry using Robinson bristle brushes, and later examined under a stereomicroscope (Carl Zeiss, Jena, EUA) at 20x magnification, and eventually 78 teeth with cracks or structural anomalies were excluded. Using a low-speed water-cooled diamond saw (Isomet 1000, Buehler, Lake Bluff, Illinois, USA), the teeth were sectioned at the cementoenamel junctions. The crowns were fixed with sticky wax (Kota Ind. Com. Ltda., São Paulo, Brazil) in acrylic resin cylinders (1.5 x 1.5 x 1.0 cm) with the labial surfaces facing up. Grinding and polishing were performed with a water-cooled mechanical grinder (Buehler Beta Grinder Polisher, Lake Bluff, USA) using aluminium oxide abrasive papers (600- and 1200-grit) (Norton Saint-Gobain, São Paulo, Brazil) and a 0.3-μm alumina suspension (Buheler, Lake Bluff,, USA). Subsequently, the specimens were ultrasonically cleaned (T1440D, Odontobrás, Ribeirão Preto, Brazil) in deionised water for 10 minutes. The specimens were insulated with a piece of insulating tape, leaving exposed circular areas that were 2 mm in diameter on each surface, and were subsequently randomly assigned to each experimental group and stored at 37°C in 100% relative humidity.
Prior to the initial microhardness test, the specimens were immersed in artificial saliva as described by McKnight-Hanes and Whitford1717 McKnight-Hanes C, Whitford GM. Fluoride release from three glass ionomer materials and the effects of varnishing with or without finishing. Caries Res. 1992; (5):345-50. doi: 10.1159/000261466. and modified according to Amaechi et al. 1818 Amaechi BT, Higham SM, Edgar WM. Factors influencing the development of dental erosion in vitro: enamel type, temperature and exposure time. J Oral Rehabil. 1999 Aug;26(8):624-30. for 24 h at 37ºC.
Erosive challenge and immersion cycles
In an attempt to simulate intrinsic erosive challenge in patients with gastro-oesophageal reflux, each specimen was individually immersed in 10 mL of 0.01 M HCl solution (pH 2) for 2 minutes.1919 Hove LH, Holme B, Øgaard B, Willumsen T, Tveit AB. The protective effect of TiF4, SnF2 and NaF on erosion of enamel by hydrochloric acid in vitro measured by white light interferometry. Caries Res. 2006 Sep;40(5):440-43. doi: 10.1159/000094291. They were then washed with deionised water for approximately 10 seconds.
After the erosive challenge with HCl, each specimen was individually immersed in 10 mL of the test products for 1 min at room temperature twice daily (at 9 a.m. and 3 p.m.) for 28 days, without agitation. Immediately afterwards, each specimen was washed with deionised water and stored in 10 mL of saliva for 6 h (between cycles) and 18 h (between the first and last cycles on the next day) at 37°C. The saliva was changed on a daily basis before the first cycle.
Surface Microhardness Tests
The samples were placed on glass slides in a parallelometer, and three Knoop microhardness indentations (at baseline, after hydrochloric acid (HCl) challenge and at 7, 14, 21 and 28 days of immersion cycles), totalling 18 indentations, were carried out in an HMV-2 microhardness tester (Shimadzu, Kyoto, Japan) (25 g, for 30 s).
The indentations were separated by a distance of 100 µm from the central region of each fragment, allowing 500 µm from the top and bottom edges with the aid of Newage Computer Assisted Microhardness System (C.A.M.S.) dedicated software (Newage testing instruments, Inc., Southampton, USA). After the measurements, the specimens whose microhardness values corresponded to ± 10% of the average value were excluded, and 102 specimens were selected and randomly assigned to six groups (n = 17).
Statistical Analysis
The data were analysed by Stata 9.0 software (StataCorp, College Station, USA), and the significance level was set as α = 0.05. Split-plot ANOVA was used to evaluate the entire dataset at once, considering variance components from repeated measures. Any significant interaction between experimental groups and the time effect was assessed using the sum of squares decomposition followed by Tukey’s test.
Results
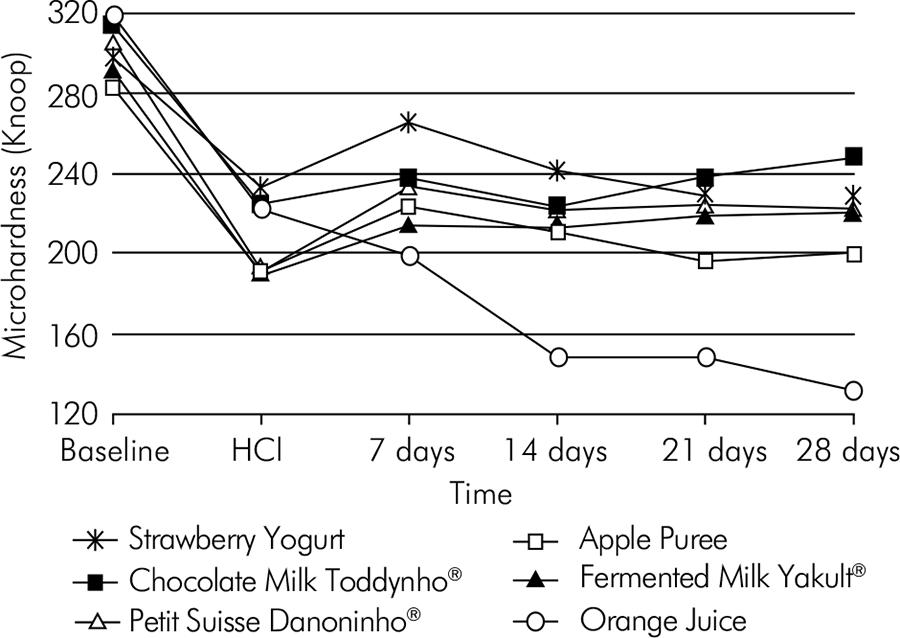
ANOVA, with normal distribution, yielded a well-fitted model of data (R2 = 0.7046) and revealed a significant effect concerning the interaction between time and snacks (p < 0.00001), as shown in Table 2. Orange juice resulted in greater enamel mineral loss after 28 days (Figure).
Discussion
Despite the convenience, durability, and acceptance of processed food and beverages, the acid content in the diet often result in wear, such as dental erosion.1010 Lussi A, Jaeggi T. Erosion—diagnosis and risk factors. Clin Oral Investig. 2008 Mar;12(Suppl 1):S5–S13. Apart from an acidic diet, erosion is also caused by frequent contact of the oral cavity66 Bartlett D. Intrinsic causes of erosion. In: Lussi A. Dental erosion. Monogr Oral Sci. Bern: Basel, Karger 2006. p.119-39. , 1919 Hove LH, Holme B, Øgaard B, Willumsen T, Tveit AB. The protective effect of TiF4, SnF2 and NaF on erosion of enamel by hydrochloric acid in vitro measured by white light interferometry. Caries Res. 2006 Sep;40(5):440-43. doi: 10.1159/000094291. with HCl, being commonly observed in paediatric patients with gastro-oesophageal reflux.55 Davies AEM, Sandhu BK. Diagnosis and treatment of gastro-oesophageal reflux. Arch Dis Child. 1995 Jul;73(1):82-6.
The proposed protocol to simulate an episode of gastro-oesophageal reflux was adapted from the in vitro model of Hove et al. 1919 Hove LH, Holme B, Øgaard B, Willumsen T, Tveit AB. The protective effect of TiF4, SnF2 and NaF on erosion of enamel by hydrochloric acid in vitro measured by white light interferometry. Caries Res. 2006 Sep;40(5):440-43. doi: 10.1159/000094291. HCl, used in intrinsic erosion models,2020 Young A, Tenuta LMA. Initial erosion models. Caries Res. 2011 May;45(1 Suppl):33-42. is a strong acid,2121 West NX, Hughes JA, Addy M. The effect of pH on the erosion of dentine and enamel by dietary acids in vitro. J Oral Rehabil. 2001 Sep;28(9):860-64. which, in the presence of water, dissociates completely into hydrogen ions and chloride ions, removing the mineral surface.2222 Featherstone JD, Lussi A. Understanding the chemistry of dental erosion. In: Lussi A. Dental erosion. Monogr Oral Sci. Bern: Basel, Karger 2006; p.66-76. Short-term acid exposures cause surface changes observed before the loss of tooth structure.2323 Ferreira FV, Pozzobon RT. Processed dairy beverages pH evaluation: consequences of temperature variation. J Clin Pediatr Dent. 2009 Summer;33(4):319–24.Although there has been a standardisation of the methodology applied in the choice of substrate, size of specimens, polishing procedure, pre-treatment with artificial saliva, baseline microhardness measurements, and volume and duration of acid exposure, this study showed a significant difference across samples exposed to HCl, limiting the comparison of snacks within each time period. If any errors occurred during or after the HCl challenge, this error was random and it could not be controlled.
Processed foods and beverages, commonly given to infants as snacks, were used, assuming that the diet could interfere in the process of enamel demineralisation and remineralisation already caused by the intrinsic erosive challenge. All the selected products, except for chocolate milk (pH 6.41), had pH levels below the critical pH for dissolution of hydroxyapatite.77 Lussi A, Schlueter N, Rakhmatullina E, Ganss C. Dental erosion – an overview with emphasis on chemical and histopathological aspects. Caries Res. 2011 May;45(1 Suppl):2-12. Although Ferreira and Pozzobon2323 Ferreira FV, Pozzobon RT. Processed dairy beverages pH evaluation: consequences of temperature variation. J Clin Pediatr Dent. 2009 Summer;33(4):319–24. claimed that the pH of chocolate-based products would not have the potential to cause dental erosion, no studies to date have specifically tested their remineralising effect. Milk-based products may have a protective effect and may not cause enamel demineralisation, depending on their amount of calcium and phosphate.1111 Rytömaa I, Meurman JH, Koskinen J, Laakso T, Gharazi L, Turunen R. In vitro erosion of bovine enamel caused by acids drinks and other foodstuffs. Scand J Dent Res. 1988 Aug;96(4):324-33. Cow’s milk was found to cause remineralisation of the bovine enamel surface after an erosive challenge with chlorinated water.2424 Vongsawan K, Surarit R, Rirattanapong P. The effect of high calcium milk and casein phosphopeptide-amorphous calcium phosphate on enamel erosion caused by chlorinated water. Southeast Asian J Trop Med Public Health. 2010 Nov;41(6):1494-99. Chocolate milk contains calcium and was chosen as a negative control, in the expectation that it could remineralise the tooth surface previously exposed to HCl. The results of this experiment showed that chocolate milk (Toddynho®) did not remineralise the enamel surface during the study period.
Orange juice was chosen as a positive control due to its erosive potential1515 Hunter L, Patel S, Rees J. The in vitro erosive potential of a range of baby drinks. Int J Paediatr Dent. 2009 Sep;19(5):325-29. and to the presence of citric acid. However, the erosive potential of an acidic drink is not influenced only by the type of acid, but also by its buffering property, chelating property, and frequency and duration of intake.1010 Lussi A, Jaeggi T. Erosion—diagnosis and risk factors. Clin Oral Investig. 2008 Mar;12(Suppl 1):S5–S13. The erosive effect of orange juice was observed in previous studies88 Lussi A, Kohler N, Zero D, Schaffner M, Megert B. A comparison of the erosive potential of different beverages in primary and permanent teeth using an in vitro model. Eur J Oral Sci. 2000 Apr;108(2):110-14. , 99 Larsen MJ, Nyvad B. Enamel erosion by soft drinks and orange juices relative to their pH, buffering effect and contents of calcium phosphate. Caries Res. 1999 May;33(1):81-7. , 1111 Rytömaa I, Meurman JH, Koskinen J, Laakso T, Gharazi L, Turunen R. In vitro erosion of bovine enamel caused by acids drinks and other foodstuffs. Scand J Dent Res. 1988 Aug;96(4):324-33. , 1414 Lodi CS, Sassaki KT, Fraiz FC, Delbem AC, Martinhon CC. Evaluation of some properties of fermented milk beverages that affect the demineralization of dental enamel. Braz Oral Res. 2010 Jan-Mar;24(1):95-101. , 1616 Torres CP, Chinellati MA, Gomes-Silva JM, Rizóli FA, Oliveira MA, Palma-Dibb RG, et al. Surface and subsurface erosion of primary enamel by acid beverages over time. Braz Dent J. 2010 Sep;21(4):337-45. by different protocols in relation to volume of liquid, length of exposure, duration of the erosive challenge, and salivary exposure. No study has evaluated the effect of orange juice or other foods and drinks after simulation of an endogenous erosive challenge in primary teeth, as was conducted in this study. Although Torres et al. 1616 Torres CP, Chinellati MA, Gomes-Silva JM, Rizóli FA, Oliveira MA, Palma-Dibb RG, et al. Surface and subsurface erosion of primary enamel by acid beverages over time. Braz Dent J. 2010 Sep;21(4):337-45. evaluated the erosive effect of orange juice on primary enamel and observed surface mineral loss after 45 days, the present study showed that enamel microhardness decreased after only 14 days and remained constant at 21 and 28 days of experimentation. Perhaps, the decrease in enamel microhardness with a shorter length of exposure is justified because the orange juice (pH 3.56) used in this study was home-squeezed, while the orange juice used by Torres et al. 1616 Torres CP, Chinellati MA, Gomes-Silva JM, Rizóli FA, Oliveira MA, Palma-Dibb RG, et al. Surface and subsurface erosion of primary enamel by acid beverages over time. Braz Dent J. 2010 Sep;21(4):337-45. was soy-based (pH 3.86). Another possible explanation is the cumulative effect of erosion caused by HCl challenge, followed by exposure to orange juice containing citric acid. Although it is a weak acid, citric acid chelates calcium from hydroxyapatite1818 Amaechi BT, Higham SM, Edgar WM. Factors influencing the development of dental erosion in vitro: enamel type, temperature and exposure time. J Oral Rehabil. 1999 Aug;26(8):624-30. and promotes the dissolution of crystals by binding the hydrogen ion to the carbonate and/or phosphate ion.2222 Featherstone JD, Lussi A. Understanding the chemistry of dental erosion. In: Lussi A. Dental erosion. Monogr Oral Sci. Bern: Basel, Karger 2006; p.66-76.
Although the manufacturer does not mention the presence of acidulants (e.g., citric acid) or any other acids, fermented milk (Yakult®) has a pH of 3.67 and may cause superficial mineral loss in bovine surface enamel after 20 min of exposure, interspersed with exposure to artificial saliva.1414 Lodi CS, Sassaki KT, Fraiz FC, Delbem AC, Martinhon CC. Evaluation of some properties of fermented milk beverages that affect the demineralization of dental enamel. Braz Oral Res. 2010 Jan-Mar;24(1):95-101. In this study, fermented milk did not exacerbate the mineral loss caused by endogenous simulation challenge at 28 days and by a total exposure of 56 minutes. One possible explanation for this difference would be the greater erosive challenge in Lodi et al.,
1414 Lodi CS, Sassaki KT, Fraiz FC, Delbem AC, Martinhon CC. Evaluation of some properties of fermented milk beverages that affect the demineralization of dental enamel. Braz Oral Res. 2010 Jan-Mar;24(1):95-101. where dental enamel erosion was assessed using surface microhardness and surface profilometry. Regarding the apple puree, it was expected to decrease enamel microhardness because of its acidic pH (3.58) and its erosive effect on permanent teeth.2525 Lussi A, Jaeggi T. The potential of various oral care products compared to foodstuffs and beverages. Schweiz Monatsschr Zahnmed [Internet]. 2001[cited 2012 Feb 17];111(3):274-81. Available from: http://www.gaba-bv.nl/data/docs/nl_NL/1186/Studie-Lussi-Jaeggi-2001.pdf
http://www.gaba-bv.nl/data/docs/nl_NL/11...
In this study, even after 56 min of total exposure, the apple puree did not aggravate the mineral loss caused by the HCl challenge. However, it should be considered that, in this study, the specimens were exposed to apple puree twice daily for 1 min, interspersed with exposure to saliva for about 24 hours. This design was different from the study of Lussi and Jaeggi,2525 Lussi A, Jaeggi T. The potential of various oral care products compared to foodstuffs and beverages. Schweiz Monatsschr Zahnmed [Internet]. 2001[cited 2012 Feb 17];111(3):274-81. Available from: http://www.gaba-bv.nl/data/docs/nl_NL/1186/Studie-Lussi-Jaeggi-2001.pdf
http://www.gaba-bv.nl/data/docs/nl_NL/11...
which used one single exposure to apple puree, for 10 or 20 min, not interspersing it with saliva exposure. There are no studies evaluating petit suisse strawberry yogurt, and therefore comparisons are not possible. Although petit suisse yogurt increased enamel microhardness on the seventh day of experimentation, its value was not different from that of HCl after 14 days of exposure. Although it contains two acids (citric and tartaric acid) and its pH is below 7, petit suisse yogurt contains calcium and phosphorus in its composition. Some studies have shown that yogurt does not have an erosive effect77 Lussi A, Schlueter N, Rakhmatullina E, Ganss C. Dental erosion – an overview with emphasis on chemical and histopathological aspects. Caries Res. 2011 May;45(1 Suppl):2-12.
,
88 Lussi A, Kohler N, Zero D, Schaffner M, Megert B. A comparison of the erosive potential of different beverages in primary and permanent teeth using an in vitro model. Eur J Oral Sci. 2000 Apr;108(2):110-14.
,
1111 Rytömaa I, Meurman JH, Koskinen J, Laakso T, Gharazi L, Turunen R. In vitro erosion of bovine enamel caused by acids drinks and other foodstuffs. Scand J Dent Res. 1988 Aug;96(4):324-33.
,
2525 Lussi A, Jaeggi T. The potential of various oral care products compared to foodstuffs and beverages. Schweiz Monatsschr Zahnmed [Internet]. 2001[cited 2012 Feb 17];111(3):274-81. Available from: http://www.gaba-bv.nl/data/docs/nl_NL/1186/Studie-Lussi-Jaeggi-2001.pdf
http://www.gaba-bv.nl/data/docs/nl_NL/11...
on dental enamel. However, there are studies in which yogurt reduced enamel microhardness.1212 Jitpukdeebodintra S, Chuenarrom C, Muttarak C, Khonsuphap P, Prasattakarn S. Effects of 1.23% acidulated phosphate fluoride gel and drinkable yogurt on human enamel erosion, in vitro. Quintessence Int. 2010 Jul-Aug;41(7):595–604.
,
2525 Lussi A, Jaeggi T. The potential of various oral care products compared to foodstuffs and beverages. Schweiz Monatsschr Zahnmed [Internet]. 2001[cited 2012 Feb 17];111(3):274-81. Available from: http://www.gaba-bv.nl/data/docs/nl_NL/1186/Studie-Lussi-Jaeggi-2001.pdf
http://www.gaba-bv.nl/data/docs/nl_NL/11...
Lussi et al.
2626 Lussi A, Merget B, Shellis RP, Wang X. Analysis of the erosive effect of different dietary substances and medications. Br J Nutr. 2012 Jan;107(2):252-62. described that plain yogurt did not cause dental erosion, but the presence of acidic additives in flavoured yogurt resulted in a clinically negligible reduction in after 2 min of exposure. In this experimental model, strawberry yogurt (pH 4.19) did not reduce the enamel microhardness of primary teeth. In addition to the differences between the types of acids used in the previous challenge, there was also a difference in the type of challenge and in the type of yogurt.
In an attempt to simulate snack time at school, the specimens were submitted to one-minute tests twice a day, interspersed with exposure to artificial saliva, which contains calcium, phosphate and magnesium in its composition and has a similar remineralising effect to that of fresh human saliva.1616 Torres CP, Chinellati MA, Gomes-Silva JM, Rizóli FA, Oliveira MA, Palma-Dibb RG, et al. Surface and subsurface erosion of primary enamel by acid beverages over time. Braz Dent J. 2010 Sep;21(4):337-45. Considering the lengths of exposure, the option for 2 min in HCl and 1 min in the test products was based on the simulation of in vivo conditions, which should not exceed 2 minutes.2020 Young A, Tenuta LMA. Initial erosion models. Caries Res. 2011 May;45(1 Suppl):33-42. The initial erosive lesions may be caused by acid exposure and short cycles of exposure to acid, followed by exposure to saliva.2020 Young A, Tenuta LMA. Initial erosion models. Caries Res. 2011 May;45(1 Suppl):33-42. In this test, the initial erosive lesions were produced by simulating the endogenous challenge (i.e., short-term acid exposure) and immersions in test products for 1 min (i.e., short-exposure cycles). The duration of an in vitro test may range between 15 s to 40 min per cycle,2727 Wiegand A, Attin T. Design of erosion/abrasion studies – insights and rational concepts. Caries Res. 2011 May;45(1 Suppl):53-9. depending on the degree of injury desired. Enamel erosion initially manifests itself by partial surface demineralisation due to high mineral content.2828 Schlueter N, Hara A, Shellis RP, Ganss C. Methods for the measurement and characterization of erosion in enamel and dentine. Caries Res. 2011 May;45(1 Suppl):13-23. In this study, we decided to perform enamel microhardness measurements because they are the most useful method to assess enamel softening.2929 Barbour ME, Rees JS. The laboratory assessment of enamel erosion: a review. J Dent. 2004 Nov;32(8):591–602. In addition, this quantitative method is simple, inexpensive, and easily applied,3030 Shellis RP, Ganss C, Ren Y, Zero DT, Lussi A. Methodology and models in erosion research: discussion and conclusions. Caries Res. 2011 May;45(1 Suppl):69-77.and consists in measuring the resistance of a substrate to indentation, which can involve Vickers (tetra-pyramidal) or Knoop (rhomboid) measures.2828 Schlueter N, Hara A, Shellis RP, Ganss C. Methods for the measurement and characterization of erosion in enamel and dentine. Caries Res. 2011 May;45(1 Suppl):13-23. Knoop microhardness was chosen in this experiment because it is considered more sensitive to changes in the surface layer of an erosive lesion2727 Wiegand A, Attin T. Design of erosion/abrasion studies – insights and rational concepts. Caries Res. 2011 May;45(1 Suppl):53-9. than other microhardness tests.
Conclusion
Laboratory studies are important for future scientific contributions, despite their limitations and the fact that results may not be conclusive in some clinical situations. Further studies are needed to support definitive conclusions about the erosive potential and effect of chocolate milk, petit suisse strawberry yogurt, strawberry yogurt, apple puree, and fermented milk. However, orange juice should be avoided as a snack option, especially in children exposed to intrinsic acids.
Acknowledgements
The authors are grateful to Patricia Marchi for technical assistance and to Oficina do Texto for revising the English in the manuscript. This study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - CAPES and Conselho Nacional de Desenvolvimento Científico e Tecnológico - CNPq (309219/2009-4).
References
-
1Lussi A, Megert B, Shellis RP, Wang X. Analysis of the erosive effect of different dietary substances and medications. Br J Nutr. 2012 Jan;107(2):252-62.
-
2Serra MC, Messias DCF, Turssi CP. Control of erosive tooth wear: possibilities and rationale. Braz Oral Res. 2009 Jun;23(Suppl 1):49-55.
-
3Lussi A, Schaffner M, Jaeggi T. Dental erosion – diagnosis and prevention in children and adults. Int Dent J. 2007 Dec;57(6):385-98.
-
4Addy M, Shellis RP. Interaction between attrition, abrasion and erosion in tooth wear. In: Lussi A. Dental erosion. Monogr Oral Sci. Bern: Basel, Karger; 2006. p.17-21.
-
5Davies AEM, Sandhu BK. Diagnosis and treatment of gastro-oesophageal reflux. Arch Dis Child. 1995 Jul;73(1):82-6.
-
6Bartlett D. Intrinsic causes of erosion. In: Lussi A. Dental erosion. Monogr Oral Sci. Bern: Basel, Karger 2006. p.119-39.
-
7Lussi A, Schlueter N, Rakhmatullina E, Ganss C. Dental erosion – an overview with emphasis on chemical and histopathological aspects. Caries Res. 2011 May;45(1 Suppl):2-12.
-
8Lussi A, Kohler N, Zero D, Schaffner M, Megert B. A comparison of the erosive potential of different beverages in primary and permanent teeth using an in vitro model. Eur J Oral Sci. 2000 Apr;108(2):110-14.
-
9Larsen MJ, Nyvad B. Enamel erosion by soft drinks and orange juices relative to their pH, buffering effect and contents of calcium phosphate. Caries Res. 1999 May;33(1):81-7.
-
10Lussi A, Jaeggi T. Erosion—diagnosis and risk factors. Clin Oral Investig. 2008 Mar;12(Suppl 1):S5–S13.
-
11Rytömaa I, Meurman JH, Koskinen J, Laakso T, Gharazi L, Turunen R. In vitro erosion of bovine enamel caused by acids drinks and other foodstuffs. Scand J Dent Res. 1988 Aug;96(4):324-33.
-
12Jitpukdeebodintra S, Chuenarrom C, Muttarak C, Khonsuphap P, Prasattakarn S. Effects of 1.23% acidulated phosphate fluoride gel and drinkable yogurt on human enamel erosion, in vitro. Quintessence Int. 2010 Jul-Aug;41(7):595–604.
-
13Haukioja A. Probiotics and Oral Health. Eur J Dent. 2010 Jul;4(3):348-55.
-
14Lodi CS, Sassaki KT, Fraiz FC, Delbem AC, Martinhon CC. Evaluation of some properties of fermented milk beverages that affect the demineralization of dental enamel. Braz Oral Res. 2010 Jan-Mar;24(1):95-101.
-
15Hunter L, Patel S, Rees J. The in vitro erosive potential of a range of baby drinks. Int J Paediatr Dent. 2009 Sep;19(5):325-29.
-
16Torres CP, Chinellati MA, Gomes-Silva JM, Rizóli FA, Oliveira MA, Palma-Dibb RG, et al. Surface and subsurface erosion of primary enamel by acid beverages over time. Braz Dent J. 2010 Sep;21(4):337-45.
-
17McKnight-Hanes C, Whitford GM. Fluoride release from three glass ionomer materials and the effects of varnishing with or without finishing. Caries Res. 1992; (5):345-50. doi: 10.1159/000261466.
-
18Amaechi BT, Higham SM, Edgar WM. Factors influencing the development of dental erosion in vitro: enamel type, temperature and exposure time. J Oral Rehabil. 1999 Aug;26(8):624-30.
-
19Hove LH, Holme B, Øgaard B, Willumsen T, Tveit AB. The protective effect of TiF4, SnF2 and NaF on erosion of enamel by hydrochloric acid in vitro measured by white light interferometry. Caries Res. 2006 Sep;40(5):440-43. doi: 10.1159/000094291.
-
20Young A, Tenuta LMA. Initial erosion models. Caries Res. 2011 May;45(1 Suppl):33-42.
-
21West NX, Hughes JA, Addy M. The effect of pH on the erosion of dentine and enamel by dietary acids in vitro. J Oral Rehabil. 2001 Sep;28(9):860-64.
-
22Featherstone JD, Lussi A. Understanding the chemistry of dental erosion. In: Lussi A. Dental erosion. Monogr Oral Sci. Bern: Basel, Karger 2006; p.66-76.
-
23Ferreira FV, Pozzobon RT. Processed dairy beverages pH evaluation: consequences of temperature variation. J Clin Pediatr Dent. 2009 Summer;33(4):319–24.
-
24Vongsawan K, Surarit R, Rirattanapong P. The effect of high calcium milk and casein phosphopeptide-amorphous calcium phosphate on enamel erosion caused by chlorinated water. Southeast Asian J Trop Med Public Health. 2010 Nov;41(6):1494-99.
-
25Lussi A, Jaeggi T. The potential of various oral care products compared to foodstuffs and beverages. Schweiz Monatsschr Zahnmed [Internet]. 2001[cited 2012 Feb 17];111(3):274-81. Available from: http://www.gaba-bv.nl/data/docs/nl_NL/1186/Studie-Lussi-Jaeggi-2001.pdf
» http://www.gaba-bv.nl/data/docs/nl_NL/1186/Studie-Lussi-Jaeggi-2001.pdf -
26Lussi A, Merget B, Shellis RP, Wang X. Analysis of the erosive effect of different dietary substances and medications. Br J Nutr. 2012 Jan;107(2):252-62.
-
27Wiegand A, Attin T. Design of erosion/abrasion studies – insights and rational concepts. Caries Res. 2011 May;45(1 Suppl):53-9.
-
28Schlueter N, Hara A, Shellis RP, Ganss C. Methods for the measurement and characterization of erosion in enamel and dentine. Caries Res. 2011 May;45(1 Suppl):13-23.
-
29Barbour ME, Rees JS. The laboratory assessment of enamel erosion: a review. J Dent. 2004 Nov;32(8):591–602.
-
30Shellis RP, Ganss C, Ren Y, Zero DT, Lussi A. Methodology and models in erosion research: discussion and conclusions. Caries Res. 2011 May;45(1 Suppl):69-77.
Publication Dates
-
Publication in this collection
2015
History
-
Accepted
23 Oct 2014 -
Accepted
13 Apr 2015 -
Reviewed
29 June 2015