Abstract
The present study compared IgA specificity against oral streptococci in colostrum and saliva samples. Sixty-two mother-and-child pairs were included; samples of colostrum (C) and saliva (MS) were collected from the mothers and saliva samples were collected from babies (BS). The specificity of IgA against Streptococcus mutans and S. mitis were analyzed by western blot. Only 30% of babies’ samples presented IgA reactivity to S. mutans, while 74 and 80% of MS and C, respectively, presented this response. IgA reactivity to S. mutans virulence antigens (Ag I/II, Gtf and GbpB) in positive samples showed differences between samples for Gtf and especially for GbpB (p < 0.05), but responses to Ag I/II were similar (p > 0.05). The positive response of Gtf-reactive IgA was different between C (90%) and MS (58%) samples (p < 0.05), but did not differ from BS (p > 0.05). GbpB was the least detected, with 48 and 26% of C and MS, and only 5% of BS samples presenting reactivity (p > 0.05). Eight percent of MS and C samples presented identical bands to SM in the same time-point. In conclusion, the differences of IgA response found between C and MS can be due to the different ways of stimulation, proliferation and transportation of IgA in those secretions. The colostrum has high levels of IgA against S. mutans virulence antigens, which could affect the installation and accumulation process of S. mutans, mainly by supplying anti-GbpB IgA to the neonate.
Streptococcus mutans; Colostrum; Saliva; Immunoglobulin A
Introduction
The oral cavity is an important access route into the human body for several microorganisms, some of which become residents that facilitate the adhesion and accumulation of other species, increasing the complexity of the oral microbial communities. Streptococcus mitis is the main bacteria that initially colonizes the oral cavity.11. Hohwy J, Reinholdt J, Kilian M. Population dynamics of Streptococcus mitis in its natural habitat. Infect Immun. 2001;69(10):6055-63. https://doi.org/10.1128/IAI.69.10.6055-6063.2001
https://doi.org/10.1128/IAI.69.10.6055-6...
,22. Seow WK, Lam JH, Tsang AK, Holcombe T, Bird PS. Oral Streptococcus species in pre-term and full-term children - a longitudinal study. Int J Paediatr Dent. 2009;19(6):406-11. https://doi.org/10.1111/j.1365-263X.2009.01003.x
https://doi.org/10.1111/j.1365-263X.2009...
After tooth eruption new species are more commonly found, such as Streptococcus mutans.33. Caufield PW, Cutter GR, Dasanayake AP. Initial acquisition of mutans streptococci by infants: evidence for a discrete window of infectivity. J Dent Res. 1993;72(1):37-45. https://doi.org/10.1177/00220345930720010501
https://doi.org/10.1177/0022034593072001...
However, these species may also be detected in predentate children highly exposed to S. mutans.44. Alves AC, Nogueira RD, Stipp RN, Pampolini F, Moraes AB, Gonçalves RB et al. Prospective study of potential sources of Streptococcus mutans transmission in nursery school children. J Med Microbiol. 2009;8(4):476-8. https://doi.org/10.1099/jmm.0.005777-0
https://doi.org/10.1099/jmm.0.005777-0...
Some major antigens expressed in the S. mutans cell surface, such as antigen I/II (Ag I/II), glucosyltransferase (Gtf) and glucan-binding protein B (GbpB), are involved in the ability of these microorganisms to adhere and accumulate in the oral biofilm, promoting the development of dental caries.55. Lee SF, Progulske-Fox A, Bleiweis AS. Molecular cloning and expression of a Streptococcus mutans major surface protein antigen. P1 (I/II), in Escherichia coli. Infect Immun. 1998;56(8):2114–9.,66. Smith DJ, Taubman MA. Experimental immunization of rats with a Streptococcus mutans 59-kilodalton glucan-binding protein protects against dental caries. Infect Immun. 1996;64(8):3069-73.,77. Yamashita Y, Bowen WH, Burne RA, Kuramitsu HK. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect Immun. 1993;61(9):7-11. Several studies have shown that the induction of specific antibodies against these antigens can confer protection against the development of dental caries in animal models.88. Koga T, Oho T, Shimazaki Y, Nakano Y. Immunization against dental caries. Vaccine. 2002;20(16):2027-44. https://doi.org/10.1016/S0264-410X(02)00047-6
https://doi.org/10.1016/S0264-410X(02)00...
Some studies support the use of these antigens in clinical trials on vaccines against caries in older children and adults.99. Childers NK, Tong G, Mitchell S, Kirk K, Russell MW, Michalek SM. A controlled clinical study of the effect of nasal immunization with a Streptococcus mutans antigen alone or incorporated into lipossomes on induction of immune responses. Infect Immun. 1999;67(2):618-23.
The main immunoglobulin present in mucosal secretions is the secretory IgA (SIgA), representing the first line of defense of the oral cavity. SIgA is associated to the oral microbiota control by reducing the adherence of bacteria on the mucosa and teeth.1010. Clapp DW. Developmental regulation of the immune system. Semin Perinatol. 2006;30(2):69-72. https://doi.org/10.1053/j.semperi.2006.02.004
https://doi.org/10.1053/j.semperi.2006.0...
Previous prospective studies with 5- to 24-month-old children heavily exposed to S. mutans showed a complex pattern of salivary IgA reactivity to S. mutans antigens,1111. Nogueira RD, Alves AC, Napimoga MH, Smith DJ, Mattos-Graner RO. Characterization of salivary immunoglobulin A responses in children heavily exposed to the oral bacterium Streptococcus mutans: influence of specific antigen recognition in infection. Infect Immun. 2005;73(9):5675-84. https://doi.org/10.1128/IAI.73.9.5675-5684.2005
https://doi.org/10.1128/IAI.73.9.5675-56...
,1212. Nogueira RD, Alves AC, King WF, Gonçalves RB, Höfling JF, Smith DJ. Age-specific salivary immunoglobulin A response to Streptococcus mutans GbpB. Clin. Vaccine Immunol. 2007;14(6):804-7. https://doi.org/10.1128/CVI.00098-07
https://doi.org/10.1128/CVI.00098-07...
suggesting that responses to virulence-associated antigens, especially against GbpB, may influence the ability of S. mutans to colonize the oral cavity. Thus, the salivary IgA response to GbpB can modulate the infection by S. mutans. Saliva from newborns with undetectable levels of S. mutans and detectable IgA levels, presented IgA reactivity to Ag I/II and Gtf in 16% of samples, but no reactivity to GbpB was detected.1313. Nogueira RD, Sesso ML, Borges MC, Mattos-Graner RO, Smith DJ, Ferriani VP. Salivary IgA antibody responses to Streptococcus mitis and Streptococcus mutans in preterm and fullterm newborn children. Arch Oral Biol. 2012;57(6):647-53. https://doi.org/10.1016/j.archoralbio.2011.11.011
https://doi.org/10.1016/j.archoralbio.20...
After 3 months, IgA reactivity to Gtf and Ag I/II was unchanged, but 7% of the children presented salivary IgA response to GbpB.1414. Borges MC, Sesso ML, Roberti LR, Oliveira MA, Nogueira RD, Geraldo-Martins VR et al. Salivary antibody response to streptococci in preterm and fullterm children: a prospective study. Arch Oral Biol. 2015;60(1):116-25. https://doi.org/10.1016/j.archoralbio.2014.08.003
https://doi.org/10.1016/j.archoralbio.20...
Breastfeeding represents an important source of immune components for the mucosal membranes of neonates, especially by the mother-to-child transfer of SIgA, the main mediator of anti-bacterial activity in human colostrums.1515. Pribylova J, Krausova K, Kocourkova I, Rossmann P, Klimesova K, Kverka M et al. Colostrum of healthy mothers contains broad spectrum of secretory IgA autoantibodies. J Clin Immunol. 2012;32(6):1372-80. https://doi.org/10.1007/s10875-012-9733-9
https://doi.org/10.1007/s10875-012-9733-...
,1616. Ovono Abessolo F, Essomo Owono Megne-Mbo M, Ategbo S, N’negue MA, Lendoye E, Mvé Abaga R et al. [Profile of immunoglobulins A, G, and M during breast milk maturation in a tropical area (Gabon)]. Sante. 2011;21(1):15-9. French. https://doi.org/10.1684/san.2011.0230
https://doi.org/10.1684/san.2011.0230...
Several studies showed the passive protection conferred by breast milk against bacterial infections through binding mechanisms and also by covering the mucosal surfaces, preventing the adhesion and invasion of a large variety of pathogenic microorganisms.1717. Bertoldo BB, Corrêa NF, Nogueira RD. Influência do aleitamento materno no estabelecimento de microrganismos cariogênico e desenvolvimento de cárie. Unopar Cient Cienc Biol Saúde. 2012;15(4):1-8. https://doi.org/10.17921/2447-8938.2013v15n4p%25p
https://doi.org/10.17921/2447-8938.2013v...
,1818. Van de Perre P. Transfer of antibody via mother’s milk. Vaccine. 2003;21(24):3374-6.https://doi.org/10.1016/S0264-410X(03)00336-0
https://doi.org/10.1016/S0264-410X(03)00...
The IgA antibodies present in breast milk are directed against microbes and food proteins from the mother that the breastfeeding child may also have contact.1919. Tomicić S, Johansson G, Voor T, Björkstén B, Böttcher MF, Jenmalm MC. Breast milk cytokine and IgA composition differ in Estonian and Swedish mothers-relationship to microbial pressure and infant allergy. Pediatr Res. 2010;68(4):330-4. https://doi.org/10.1203/PDR.0b013e3181ee049d
https://doi.org/10.1203/PDR.0b013e3181ee...
This antigenic exposure of the gut associated lymphoid tissues (GALT) and upper respiratory tract tissues results in sensitized B-lymphocytes in those tissues via the circulation to mesenteric lymph nodes.
Previous studies showed that colostrum has detectable levels of IgA against all S. mutans antigens, although GbpB has been significantly less detected than other antigens.2020. Petrechen LN, Zago FH, Sesso ML, Bertoldo BB, Silva CB, Azevedo KP. Levels and complexity of IgA antibody against oral bacteria in samples of human colostrums. Immunobiol. 2014;220(1):142-6. https://doi.org/10.1016/j.imbio.2014.08.009
https://doi.org/10.1016/j.imbio.2014.08....
Therefore, colostrum provides to the neonate significant levels of specific IgA against S. mutans virulence, which can disrupt the installation and accumulation process of these microorganisms in the oral cavity2020. Petrechen LN, Zago FH, Sesso ML, Bertoldo BB, Silva CB, Azevedo KP. Levels and complexity of IgA antibody against oral bacteria in samples of human colostrums. Immunobiol. 2014;220(1):142-6. https://doi.org/10.1016/j.imbio.2014.08.009
https://doi.org/10.1016/j.imbio.2014.08....
and supply the immature mucosal immune system in early life, especially against GbpB, as anti-GpbB IgA is absent at birth.1313. Nogueira RD, Sesso ML, Borges MC, Mattos-Graner RO, Smith DJ, Ferriani VP. Salivary IgA antibody responses to Streptococcus mitis and Streptococcus mutans in preterm and fullterm newborn children. Arch Oral Biol. 2012;57(6):647-53. https://doi.org/10.1016/j.archoralbio.2011.11.011
https://doi.org/10.1016/j.archoralbio.20...
,1414. Borges MC, Sesso ML, Roberti LR, Oliveira MA, Nogueira RD, Geraldo-Martins VR et al. Salivary antibody response to streptococci in preterm and fullterm children: a prospective study. Arch Oral Biol. 2015;60(1):116-25. https://doi.org/10.1016/j.archoralbio.2014.08.003
https://doi.org/10.1016/j.archoralbio.20...
There is little information about the immune response during the early stages of S. mutans bacterial challenge and the role of IgA provided by breastfeeding against the installation of these bacteria. It is possible that some factors can affect the initial response of pioneer commensal species of the oral cavity, and influence the patterns of immune response and the susceptibility to colonization. In the present study, the levels of anti-S. mutans IgA and anti-S. mitis IgA were analyzed on saliva (MS) and colostrum (C) samples from mothers and in saliva samples from newborn babies (BS).
Methodology
Study design
A total of 62 pairs of mothers and newborns were enrolled in this study, after the mother’s written consent for participation. The Ethical Committee of the Medical School of Ribeirao Preto, SP, Brazil, 13290/2012, approved this study. Only healthy mothers submitted to a cesarean section within the previous 12 hours were included. Information about maternal and gestational background was obtained by interviewing the expectant mother at arrival in the maternity hospital. After birth, newborn’s saliva samples were obtained. Collection of the mothers’ samples (C and MS) occurred 12 hours after giving birth. Detectable levels of S. mutans were found in all MS samples, which were processed for immunoassay analysis.
Collection of samples
Samples of colostrum were collected manually and stored into sterile polypropylene Falcon tubes. After collection, the samples were transported to the laboratory, and centrifuged at 1,300 g to remove lipid components. Samples of whole non-stimulated saliva were collected using sterile polypropylene transfer pipettes. In newborns, collection saliva samples was performed at approximately 5 minutes after birth. A solution of 250 mM EDTA, pH 5.2 (Sigma, St Louis, USA) was added to each sample prior to transporting it on ice to the laboratory for storage at -80ºC until analysis.
Colostrum and saliva IgA reactivity against streptococcal antigens
Total levels of IgA, IgM and IgG were determined in capture ELISA assays as previously described.1111. Nogueira RD, Alves AC, Napimoga MH, Smith DJ, Mattos-Graner RO. Characterization of salivary immunoglobulin A responses in children heavily exposed to the oral bacterium Streptococcus mutans: influence of specific antigen recognition in infection. Infect Immun. 2005;73(9):5675-84. https://doi.org/10.1128/IAI.73.9.5675-5684.2005
https://doi.org/10.1128/IAI.73.9.5675-56...
,2020. Petrechen LN, Zago FH, Sesso ML, Bertoldo BB, Silva CB, Azevedo KP. Levels and complexity of IgA antibody against oral bacteria in samples of human colostrums. Immunobiol. 2014;220(1):142-6. https://doi.org/10.1016/j.imbio.2014.08.009
https://doi.org/10.1016/j.imbio.2014.08....
Patterns of reactivity of salivary IgA antibody against S. mutans (UA159) and S. mitis (ATCC506) antigens were determined in western blot assays as previously described.2020. Petrechen LN, Zago FH, Sesso ML, Bertoldo BB, Silva CB, Azevedo KP. Levels and complexity of IgA antibody against oral bacteria in samples of human colostrums. Immunobiol. 2014;220(1):142-6. https://doi.org/10.1016/j.imbio.2014.08.009
https://doi.org/10.1016/j.imbio.2014.08....
Sixteen micrograms of antigen extracts were loaded per lane, separated by 6% sodium dodecyl sulfate polyacrylamide gel electrophoresis, and transferred to nitrocellulose membranes. Membranes were washed and blocked overnight at 4°C (in Tris-buffered saline–Tween, pH 7.5, 5% nonfat milk, Sigma). Incubations with diluted samples (normalized by the IgA concentration) were performed at room temperature for 2 h. Membranes incubated with blocking buffer were used as negative controls, and membranes incubated with a standard saliva sample obtained from an adult whose pattern of reaction with antigen extracts had been previously measured were used as positive controls.
The secondary antibody was goat IgG anti-human IgA conjugated with horseradish peroxidase (Sigma, 1:4,000 dilutions). Antibody reactions were developed using an ECL system (GE Healthcare UK, Little Chalfont, United Kingdom). Immunoblots were incubated with ECL detection solution and then exposed to the same X-ray film for 5 min. The developed X-ray films were scanned in a scanning densitometer (GE Healthcare) to analyze patterns of antigen recognition, including the number of reactive bands. A blank value was subtracted from the value of the reactive band.
Statistical analysis
Frequencies of children with IgA antibody specificities were compared by chi-square test. The mean number of IgA-reactive bands in antigen extracts was also determined and compared between samples with ANOVA. Associations between concentrations of specific IgA and patterns of antibody reactions between samples were tested by Pearson correlation analysis. A p-value of <0.05 was considered statistically significant.
Results
The mean concentrations of IgA in C, MS and BS were 2850.2 (± 2567.2); 435.2 (± 86.7) and 2.4 (± 7.6) µg/mL, respectively. There were no correlations in the levels of IgA between the samples (p > 0.05, r < 0.23). The concentration of IgA in C and MS was significantly higher than in BS (p < 0.05). No associations were found between immunoglobulin levels based on race, maternal age, oral health, type of birth and socioeconomic data (p > 0.05).
The percentage of samples (C, MS and BS) with positive and negative IgA response to S. mutans and S. mitis is shown in Figure 1. The mean number of reactive bands to S. mutans and the frequency of positive response to the virulence antigens (Ag I/II, Gtf and GbpB), in C (n = 50), MS (n = 4 0) and BS (n = 1 6) samples presenting positive IgA response to S. mutans is shown in Table 1. The analysis in each group showed that the frequency of positive response to Ag I/II and Gtf were statistically higher than the negative (Table 1, p < 0.05). Concerning GbpB, there was no difference between positive and negative detection in C, but the number of negative IgA response was statistically higher than the positive in MS and BS (p < 0.05). The mean number of S. mitis reactive bands and the frequency of positive responses to 56kDa-Ag of S. mitis in the samples is shown in Table 2. The number of IgA reactive bands to S. mitis was higher in C and MS than in BS (Table 2).
Number of samples in C (colostrum), MS (maternal saliva) and BS (newborn saliva) with IgA reactive or not to SMI (Streptococcus mitis) and SM (S. mutans).
The number of samples with IgA positive to S. mutans and S. mitis in BS was significantly lower than in C and MS (p < 0.001, Figure 1). Contrarily, the majority of C and MS samples were IgA-reactive to S. mutans and S. mitis, and did not differ statistically (p < 0.05, Figure 1). The mean number of IgA-reactive bands to S. mutans was significantly different between samples (p < 0.021), being higher in C, followed by MS and BS (Table 1). The mean number of IgA-reactive bands to S. mitis was similar between MS and C samples (p = 0.08) and lower in comparison with BS ( p< 0.006, Table 2).
The positivity of samples with IgA reactive to Gtf was different between C and MS (p < 0.001, q = 18.8) and between C and BS (p < 0.001, q = 28.9), with colostrum showing the greatest number of samples with Gtf-reactive IgA. GpbB-positive IgA was less frequent than other antigens in the samples (p < 0.05), especially in BS, in which only 5.6% of samples were GbpB-positive, while 36.9 and 50.0% of MS and C, respectively, showed such response (p<0.05 for between-group differences).
Comparative analysis of IgA response patterns between groups
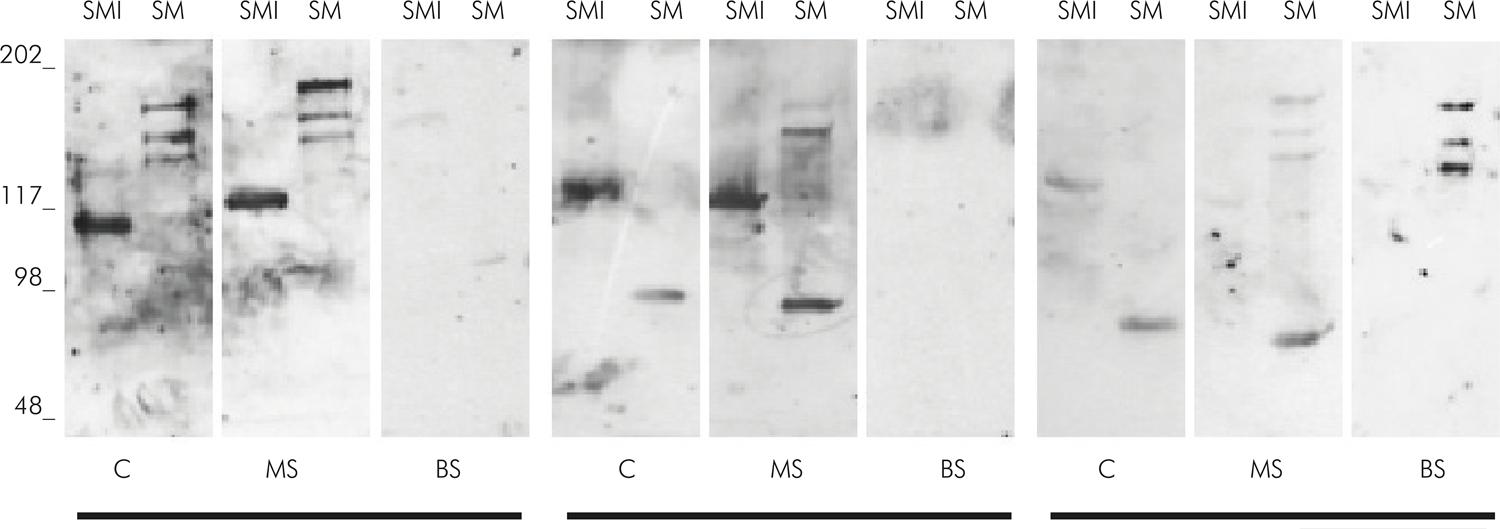
Figure 2 shows a diagram of IgA responses to S. mutans and S. mitis and their virulence antigens in C, MS and BS. Examples of western blot from 3 samples of mothers (MS and C) and their respective babies’ saliva (BS) are illustrated in Figure 3. Pattern similarity analysis of IgA response to S. mutans between samples shows a positive correlation between positive response to S. mutans and Ag I/II (p < 0.05, r > 0.79) and between S. mutans and Gtf (p < 0.05, r > 0.69) in all samples. A positive correlation occurred between IgA response to S. mutans and GbpB in MS and C (p < 0.05, r > 0.36), but not in BS (p > 0.05). There was a positive correlation between C and MS in the IgA response to S. mutans (p = 0.03, r = 0.27) and MS and BS in the IgA response to Gtf (p = 0.003, r = 0.36).
Schematic representation of positive (gray) and negative (white) IgA reactive to SMI (Streptococcus mitis) and SM (S. mutans) and their virulence antigens (Ag I/II, Gtf, GbpB) in C (colostrum), MS (maternal saliva) and BS (newborn saliva) in the 62 pairs of samples
Patterns of IgA specificities against antigens from SMI (Streptococcus mitis) and SM (S. mutans) in C (colostrum), MS (maternal saliva) and BS (newborn saliva) in the 3 groups of samples. Standard molecular sizes (kDa) are indicated in left the immunoblots.
The comparative analysis of IgA response patterns to S. mutans, in MS and C showed that only 8% of those samples presented identical bands in the same time-point, and 9.6% of samples did not present any band. C and MS samples presented some similarities in the response to S. mutans; for example, 48 pairs of C and MS (77,4%) presented the same response to S. mutans, which was not found when the comparisons were done between MS and BS (43,5%), C and BS (38,7%), and C, BS and MS (29,0%).
The number of C and MS that presented a similar positive response to Ag I/II and Gtf was higher than other antigens and samples (p < 0.05), because 29 pairs of those samples presented IgA response to Gtf and Ag I/II in the same time-point (p < 0.05). In general, GbpB was different and less recognized by the samples, which was confirmed by the elevated number of sample pairs with a negative response to GbpB (p < 0.05) and a decreased number of pairs with similar IgA response to GbpB and other antigens in the same time-point (p < 0.05).
Discussion
IgA concentration was significantly higher in colostrum than in maternal and newborn saliva, which is compatible with the type of secretion and maturation of the mucosal system. IgA antibodies reactive to S. mutans and S. mitis were found in most of the MS and C samples (Figure 1). The analysis of reactive bands showed that colostrum has a more complex IgA response than MS or BS.
Although having an immature mucosal immune system, the newborns presented antibody production against oral streptococcal colonizers, as found by Borges et al.1414. Borges MC, Sesso ML, Roberti LR, Oliveira MA, Nogueira RD, Geraldo-Martins VR et al. Salivary antibody response to streptococci in preterm and fullterm children: a prospective study. Arch Oral Biol. 2015;60(1):116-25. https://doi.org/10.1016/j.archoralbio.2014.08.003
https://doi.org/10.1016/j.archoralbio.20...
Since saliva collection was performed before starting breastfeeding, the presence of IgA in babies’ saliva supports the hypothesis that oral bacterial antigens can be transferred from mother to child via the umbilical cord blood and/or through the amniotic fluid. Past studies suggest that during the intrauterine life there is an efflux of commensal bacteria from the mother to the child.2323. Martín R, Langa S, Reviriego C, Jimínez E, Marín ML, Xaus J. Human milk is a source of lactic acid bacteria for the infant gut. J Pediatr. 2003;143(6):754-8. https://doi.org/10.1016/j.jpeds.2003.09.028
https://doi.org/10.1016/j.jpeds.2003.09....
The presence of Enterococcus, Staphylococcus epidermidis and Streptococcus sanguinis was identified in cord blood samples2424. Jiménez E, Fernández L, Marín ML, Martín R, Odriozola JM, Nueno-Palop C et al. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr Microbiol. 2005;51(4):270-4. https://doi.org/10.1007/s00284-005-0020-3
https://doi.org/10.1007/s00284-005-0020-...
and these species are frequently detected during the first days of life, generally regarded as commensal organisms in healthy children.2323. Martín R, Langa S, Reviriego C, Jimínez E, Marín ML, Xaus J. Human milk is a source of lactic acid bacteria for the infant gut. J Pediatr. 2003;143(6):754-8. https://doi.org/10.1016/j.jpeds.2003.09.028
https://doi.org/10.1016/j.jpeds.2003.09....
,2424. Jiménez E, Fernández L, Marín ML, Martín R, Odriozola JM, Nueno-Palop C et al. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr Microbiol. 2005;51(4):270-4. https://doi.org/10.1007/s00284-005-0020-3
https://doi.org/10.1007/s00284-005-0020-...
Thus, the bacteria detected in the babies’ saliva could have been delivered to the fetus in utero and thereby stimulating the development of the mucosal immune system.
The analysis of S. mutans-specific IgA antigens showed that the majority of samples with positive response to S. mutans presented IgA response against Ag I/II and Gtf, which was found in colostrum samples2020. Petrechen LN, Zago FH, Sesso ML, Bertoldo BB, Silva CB, Azevedo KP. Levels and complexity of IgA antibody against oral bacteria in samples of human colostrums. Immunobiol. 2014;220(1):142-6. https://doi.org/10.1016/j.imbio.2014.08.009
https://doi.org/10.1016/j.imbio.2014.08....
and saliva from children at birth,1414. Borges MC, Sesso ML, Roberti LR, Oliveira MA, Nogueira RD, Geraldo-Martins VR et al. Salivary antibody response to streptococci in preterm and fullterm children: a prospective study. Arch Oral Biol. 2015;60(1):116-25. https://doi.org/10.1016/j.archoralbio.2014.08.003
https://doi.org/10.1016/j.archoralbio.20...
at three months1414. Borges MC, Sesso ML, Roberti LR, Oliveira MA, Nogueira RD, Geraldo-Martins VR et al. Salivary antibody response to streptococci in preterm and fullterm children: a prospective study. Arch Oral Biol. 2015;60(1):116-25. https://doi.org/10.1016/j.archoralbio.2014.08.003
https://doi.org/10.1016/j.archoralbio.20...
and 5–11 months of age,1212. Nogueira RD, Alves AC, King WF, Gonçalves RB, Höfling JF, Smith DJ. Age-specific salivary immunoglobulin A response to Streptococcus mutans GbpB. Clin. Vaccine Immunol. 2007;14(6):804-7. https://doi.org/10.1128/CVI.00098-07
https://doi.org/10.1128/CVI.00098-07...
regardless of S. mutans colonization. On the other hand, many samples had a negative IgA response to GbpB, especially in the salivary samples. The S. mutans virulence is related, among other mechanisms, to its ability to synthesize GbpB, a heterogeneous group of proteins that promote cell adhesion to the tooth surface.2525. Hoshino T, Fujiwara T, Kawabata S. Evolution of cariogenic character in Streptococcus mutans: horizontal transmission of glycosyl hydrolase family 70 genes. Sci Rep. 2012;2:518. https://doi.org/10.1038/srep00518
https://doi.org/10.1038/srep00518...
Thus, studies on the natural immune response against this protein can provide evidence of its importance and immunogenic behavior, especially considering that the positive response of IgA to GbpB in saliva samples was more commonly found in children not colonized by S. mutans.1212. Nogueira RD, Alves AC, King WF, Gonçalves RB, Höfling JF, Smith DJ. Age-specific salivary immunoglobulin A response to Streptococcus mutans GbpB. Clin. Vaccine Immunol. 2007;14(6):804-7. https://doi.org/10.1128/CVI.00098-07
https://doi.org/10.1128/CVI.00098-07...
It is critical to investigate the transfer of antibodies from the mother to the newborn by breastfeeding as an important way of fighting several infectious challenges. Recent studies show that colostrum may supplement the lack of antibodies in the newborn, since colostrum have high levels of IgA against S. mutans antigens.2020. Petrechen LN, Zago FH, Sesso ML, Bertoldo BB, Silva CB, Azevedo KP. Levels and complexity of IgA antibody against oral bacteria in samples of human colostrums. Immunobiol. 2014;220(1):142-6. https://doi.org/10.1016/j.imbio.2014.08.009
https://doi.org/10.1016/j.imbio.2014.08....
Here, the differences found in the frequency and specificity of IgA between C and BS support the importance of breastfeeding against oral colonization early in life. Clearly, the high levels of IgA specific to S. mutans antigens in colostrum can address the lack of antibodies in the newborn’s saliva, especially against GbpB, a protein which is absent in saliva of the absolute majority of babies’ saliva samples. However, to attest the function and protection of colostrum in the prevention of caries disease, it is necessary to study the influence of colostrum in the S. mutans biofilm formation.
Little is known about the role of S. mutans antigens in the immune system of mucosal membranes, and the results of this study highlight some important points. First, the presence of antibodies against S. mutans in colostrum and saliva of mothers is related to the presence of the S. mutans colonizing the oral cavity. In both samples, there was a positive correlation between the IgA responses to S. mutans, showing that the stimulation pathways are the same (by oral colonization).
Assuming that the stimulation is by intra-uterine route, GbpB protein is not transferred and does not stimulate the immune system of the fetus, which justifies a smaller amount of salivary antibodies against GbpB. With time, the baby is exposed to S. mutans, and IgA reactive to GbpB antibodies become detectable at 3 months, as described by Borges et al.1414. Borges MC, Sesso ML, Roberti LR, Oliveira MA, Nogueira RD, Geraldo-Martins VR et al. Salivary antibody response to streptococci in preterm and fullterm children: a prospective study. Arch Oral Biol. 2015;60(1):116-25. https://doi.org/10.1016/j.archoralbio.2014.08.003
https://doi.org/10.1016/j.archoralbio.20...
At 6 months of age, this reactivity has an important modulatory action on infection, as reported by Nogueira et al.1212. Nogueira RD, Alves AC, King WF, Gonçalves RB, Höfling JF, Smith DJ. Age-specific salivary immunoglobulin A response to Streptococcus mutans GbpB. Clin. Vaccine Immunol. 2007;14(6):804-7. https://doi.org/10.1128/CVI.00098-07
https://doi.org/10.1128/CVI.00098-07...
In summary, the complexity of IgA in colostrum and saliva of mothers is similar and suggest a common pathway, although the concentration of Igs in colostrum is significantly higher. In newborn’s saliva, the levels of IgA are low especially against GbpB, which strengthens the importance of breastfeeding in controlling early infections by S. mutans.
Acknowledgments
This study was supported by Conselho Nacional de Pesquisa (CNPq), proc. 472928/2007-4.
References
-
1Hohwy J, Reinholdt J, Kilian M. Population dynamics of Streptococcus mitis in its natural habitat. Infect Immun. 2001;69(10):6055-63. https://doi.org/10.1128/IAI.69.10.6055-6063.2001
» https://doi.org/10.1128/IAI.69.10.6055-6063.2001 -
2Seow WK, Lam JH, Tsang AK, Holcombe T, Bird PS. Oral Streptococcus species in pre-term and full-term children - a longitudinal study. Int J Paediatr Dent. 2009;19(6):406-11. https://doi.org/10.1111/j.1365-263X.2009.01003.x
» https://doi.org/10.1111/j.1365-263X.2009.01003.x -
3Caufield PW, Cutter GR, Dasanayake AP. Initial acquisition of mutans streptococci by infants: evidence for a discrete window of infectivity. J Dent Res. 1993;72(1):37-45. https://doi.org/10.1177/00220345930720010501
» https://doi.org/10.1177/00220345930720010501 -
4Alves AC, Nogueira RD, Stipp RN, Pampolini F, Moraes AB, Gonçalves RB et al. Prospective study of potential sources of Streptococcus mutans transmission in nursery school children. J Med Microbiol. 2009;8(4):476-8. https://doi.org/10.1099/jmm.0.005777-0
» https://doi.org/10.1099/jmm.0.005777-0 -
5Lee SF, Progulske-Fox A, Bleiweis AS. Molecular cloning and expression of a Streptococcus mutans major surface protein antigen. P1 (I/II), in Escherichia coli Infect Immun. 1998;56(8):2114–9.
-
6Smith DJ, Taubman MA. Experimental immunization of rats with a Streptococcus mutans 59-kilodalton glucan-binding protein protects against dental caries. Infect Immun. 1996;64(8):3069-73.
-
7Yamashita Y, Bowen WH, Burne RA, Kuramitsu HK. Role of the Streptococcus mutans gtf genes in caries induction in the specific-pathogen-free rat model. Infect Immun. 1993;61(9):7-11.
-
8Koga T, Oho T, Shimazaki Y, Nakano Y. Immunization against dental caries. Vaccine. 2002;20(16):2027-44. https://doi.org/10.1016/S0264-410X(02)00047-6
» https://doi.org/10.1016/S0264-410X(02)00047-6 -
9Childers NK, Tong G, Mitchell S, Kirk K, Russell MW, Michalek SM. A controlled clinical study of the effect of nasal immunization with a Streptococcus mutans antigen alone or incorporated into lipossomes on induction of immune responses. Infect Immun. 1999;67(2):618-23.
-
10Clapp DW. Developmental regulation of the immune system. Semin Perinatol. 2006;30(2):69-72. https://doi.org/10.1053/j.semperi.2006.02.004
» https://doi.org/10.1053/j.semperi.2006.02.004 -
11Nogueira RD, Alves AC, Napimoga MH, Smith DJ, Mattos-Graner RO. Characterization of salivary immunoglobulin A responses in children heavily exposed to the oral bacterium Streptococcus mutans: influence of specific antigen recognition in infection. Infect Immun. 2005;73(9):5675-84. https://doi.org/10.1128/IAI.73.9.5675-5684.2005
» https://doi.org/10.1128/IAI.73.9.5675-5684.2005 -
12Nogueira RD, Alves AC, King WF, Gonçalves RB, Höfling JF, Smith DJ. Age-specific salivary immunoglobulin A response to Streptococcus mutans GbpB. Clin. Vaccine Immunol. 2007;14(6):804-7. https://doi.org/10.1128/CVI.00098-07
» https://doi.org/10.1128/CVI.00098-07 -
13Nogueira RD, Sesso ML, Borges MC, Mattos-Graner RO, Smith DJ, Ferriani VP. Salivary IgA antibody responses to Streptococcus mitis and Streptococcus mutans in preterm and fullterm newborn children. Arch Oral Biol. 2012;57(6):647-53. https://doi.org/10.1016/j.archoralbio.2011.11.011
» https://doi.org/10.1016/j.archoralbio.2011.11.011 -
14Borges MC, Sesso ML, Roberti LR, Oliveira MA, Nogueira RD, Geraldo-Martins VR et al. Salivary antibody response to streptococci in preterm and fullterm children: a prospective study. Arch Oral Biol. 2015;60(1):116-25. https://doi.org/10.1016/j.archoralbio.2014.08.003
» https://doi.org/10.1016/j.archoralbio.2014.08.003 -
15Pribylova J, Krausova K, Kocourkova I, Rossmann P, Klimesova K, Kverka M et al. Colostrum of healthy mothers contains broad spectrum of secretory IgA autoantibodies. J Clin Immunol. 2012;32(6):1372-80. https://doi.org/10.1007/s10875-012-9733-9
» https://doi.org/10.1007/s10875-012-9733-9 -
16Ovono Abessolo F, Essomo Owono Megne-Mbo M, Ategbo S, N’negue MA, Lendoye E, Mvé Abaga R et al. [Profile of immunoglobulins A, G, and M during breast milk maturation in a tropical area (Gabon)]. Sante. 2011;21(1):15-9. French. https://doi.org/10.1684/san.2011.0230
» https://doi.org/10.1684/san.2011.0230 -
17Bertoldo BB, Corrêa NF, Nogueira RD. Influência do aleitamento materno no estabelecimento de microrganismos cariogênico e desenvolvimento de cárie. Unopar Cient Cienc Biol Saúde. 2012;15(4):1-8. https://doi.org/10.17921/2447-8938.2013v15n4p%25p
» https://doi.org/10.17921/2447-8938.2013v15n4p%25p -
18Van de Perre P. Transfer of antibody via mother’s milk. Vaccine. 2003;21(24):3374-6.https://doi.org/10.1016/S0264-410X(03)00336-0
» https://doi.org/10.1016/S0264-410X(03)00336-0 -
19Tomicić S, Johansson G, Voor T, Björkstén B, Böttcher MF, Jenmalm MC. Breast milk cytokine and IgA composition differ in Estonian and Swedish mothers-relationship to microbial pressure and infant allergy. Pediatr Res. 2010;68(4):330-4. https://doi.org/10.1203/PDR.0b013e3181ee049d
» https://doi.org/10.1203/PDR.0b013e3181ee049d -
20Petrechen LN, Zago FH, Sesso ML, Bertoldo BB, Silva CB, Azevedo KP. Levels and complexity of IgA antibody against oral bacteria in samples of human colostrums. Immunobiol. 2014;220(1):142-6. https://doi.org/10.1016/j.imbio.2014.08.009
» https://doi.org/10.1016/j.imbio.2014.08.009 -
21Mickleson KN, Moriarty KM. Immunoglobulin levels in human colostrum and milk. J Pediatr Gastroenterol Nutr. 1982;1(3):381-4. https://doi.org/10.1097/00005176-198201030-00018
» https://doi.org/10.1097/00005176-198201030-00018 -
22Wan AK, Seow WK, Purdie DM, Bird PS, Walsh LJ, Tudehope DI. Immunoglobulins in saliva of preterm and full-term infants: a longitudinal study from 0-18 months of age. Oral Microbiol Immunol, 2003;18(2):72-8. https://doi.org/10.1034/j.1399-302X.2003.00044.x
» https://doi.org/10.1034/j.1399-302X.2003.00044.x -
23Martín R, Langa S, Reviriego C, Jimínez E, Marín ML, Xaus J. Human milk is a source of lactic acid bacteria for the infant gut. J Pediatr. 2003;143(6):754-8. https://doi.org/10.1016/j.jpeds.2003.09.028
» https://doi.org/10.1016/j.jpeds.2003.09.028 -
24Jiménez E, Fernández L, Marín ML, Martín R, Odriozola JM, Nueno-Palop C et al. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr Microbiol. 2005;51(4):270-4. https://doi.org/10.1007/s00284-005-0020-3
» https://doi.org/10.1007/s00284-005-0020-3 -
25Hoshino T, Fujiwara T, Kawabata S. Evolution of cariogenic character in Streptococcus mutans: horizontal transmission of glycosyl hydrolase family 70 genes. Sci Rep. 2012;2:518. https://doi.org/10.1038/srep00518
» https://doi.org/10.1038/srep00518
Publication Dates
-
Publication in this collection
2017
History
-
Received
16 Sept 2015 -
Reviewed
27 Mar 2017 -
Accepted
10 Apr 2017


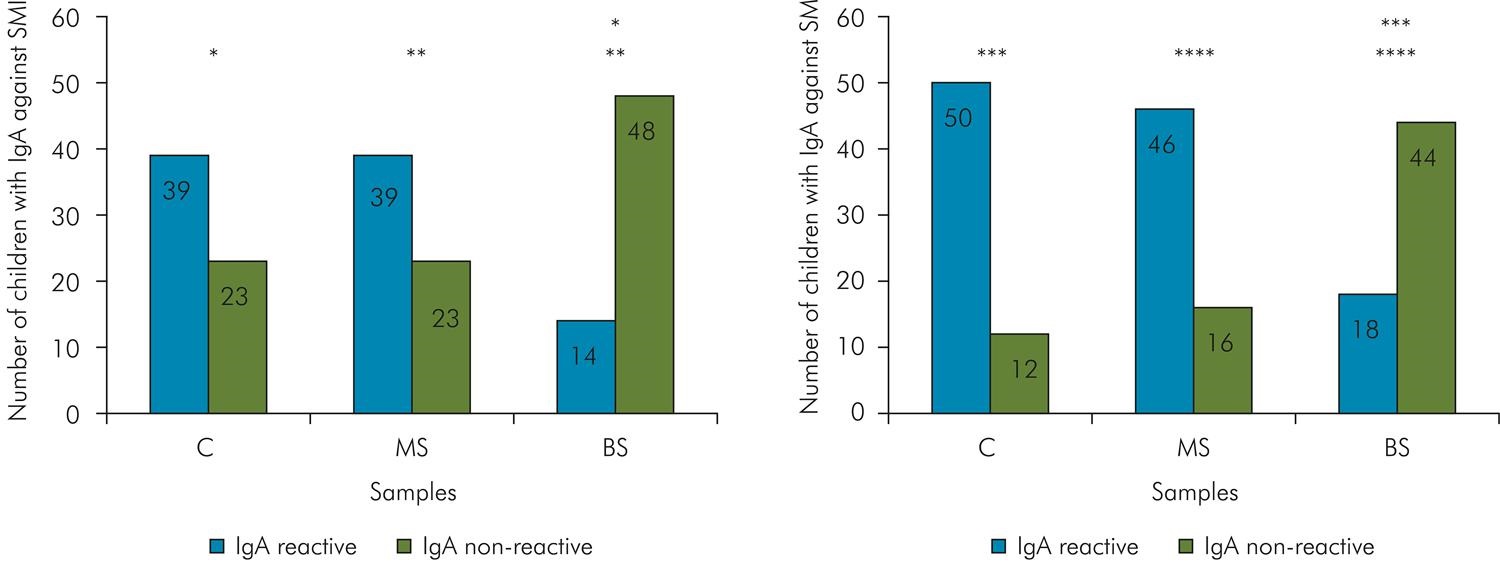 * Chi-square, p<0.01, q=23.2** Chi-square, p<0.01, q=23.2*** Chi-Square, p<0.01, q=33.3**** Chi-square, p<0.01, q=25.3
* Chi-square, p<0.01, q=23.2** Chi-square, p<0.01, q=23.2*** Chi-Square, p<0.01, q=33.3**** Chi-square, p<0.01, q=25.3