Abstract
OBJECTIVE: The aim of this study was to perform a detailed tomographic analysis of the skull base, craniocervical junction, and the entire spine in seven patients with spondylocostal dysostosis syndrome. METHOD: Detailed scanning images have been organized in accordance with the most prominent clinical pathology. The reasons behind plagiocephaly, torticollis, short immobile neck, scoliosis and rigid back have been detected. Radiographic documentation was insufficient modality. RESULTS: Detailed computed tomography scans provided excellent delineation of the osseous abnormality pattern in our patients. CONCLUSION: This article throws light on the most serious osseous manifestations of spondylocostal dysostosissyndrome.
Spondylocostal dysostosis syndrome (SCD); Plagiocephaly; Torticollis; Scoliosis; CT scan
CLINICAL SCIENCE
Tomographic assessment of the spine in children with spondylocostal dysotosis syndrome
Ali Al KaissiI,II; Klaus KlaushoferI; Franz GrillII
ILudwig Boltzmann Institute of Osteology, Hanusch Hospital of WGKK and the AUVA Trauma Centre Meidling, 4th Medical Department, Hanusch Hospital, Vienna, Austria
IIOrthopaedic Hospital of Speising, Paediatric Department, Vienna, Austria
ABSTRACT
OBJECTIVE: The aim of this study was to perform a detailed tomographic analysis of the skull base, craniocervical junction, and the entire spine in seven patients with spondylocostal dysostosis syndrome.
METHOD: Detailed scanning images have been organized in accordance with the most prominent clinical pathology. The reasons behind plagiocephaly, torticollis, short immobile neck, scoliosis and rigid back have been detected. Radiographic documentation was insufficient modality.
RESULTS: Detailed computed tomography scans provided excellent delineation of the osseous abnormality pattern in our patients.
CONCLUSION: This article throws light on the most serious osseous manifestations of spondylocostal dysostosissyndrome.
Keywords: Spondylocostal dysostosis syndrome (SCD); Plagiocephaly; Torticollis; Scoliosis; CT scan.
INTRODUCTION
Spondylocostal dysostosis (SCD) is a heterogeneous group of disorders of axial skeletal malformation, characterized by multiple vertebral segmentation defects and rib anomalies.
Autosomal dominant and recessive forms have been described, with the latter being on average more severe. The main clinical features are plagiocephaly, facial asymmetry, a short rigid neck and scoliosis. Radiographically, hemi-and fused vertebrae give rise to a short trunk, in severe cases. The spinal defects can be associated with absent or fused ribs. 1-3
Since the description of the disorder in 1938 by Jarcho-Levin3 , numerous studies have described the different aspects of the disorder.1,2,4-7 Morbidity and mortality among patients with spondylocostal dysostosis syndrome/ spondylothoracic dysplasia syndrome and Jarcho-Levine syndrome almost always have been attributed to congenital thoracic cage abnormalities and eventually the prognosis has been considered directly related to respiratory complications.3,4,8-9
CT scans have been applied to evaluate the entire spine in some patients with spondylothoracic dysplasia.4 Specific and or detailed anatomical abnormalities were not included.
This article defies previous reports concerning the underlying pathological reasons behind the development of the aforementioned sequelae in patients with spondylocostal dysostosis syndrome. Moreover, it highlighted for the first time the diverse features of the anatomical abnormalities of the spine via detailed CT scanning analysis.
Cervical radiographs were made, but the superimposition of the shoulders and the mandible combined with the extremely short neck made conventional radiographic evaluation inadequate.
METHODS
The study protocol was approved by the Medical University of Vienna (Ethics committee, EK Nr: 921/2009) and informed consent was obtained from the patient's guardians. Seven patients of different ethnic origins were ascertained through the osteogenetic department (Orthopaedic Hospital of Speising, Vienna). The mean age of patients in our series was 6 years and the mean age of diagnosis was 1 year. Plagiocephaly and torticollis were present in all patients; a short immobile neck was present in five patients. Short trunk dwarfism occurred in all patients and a rigid lumbar spine was present in three patients. Radiographs were of limited value in understanding the different symptomatology. CT scanning was the modality of choice
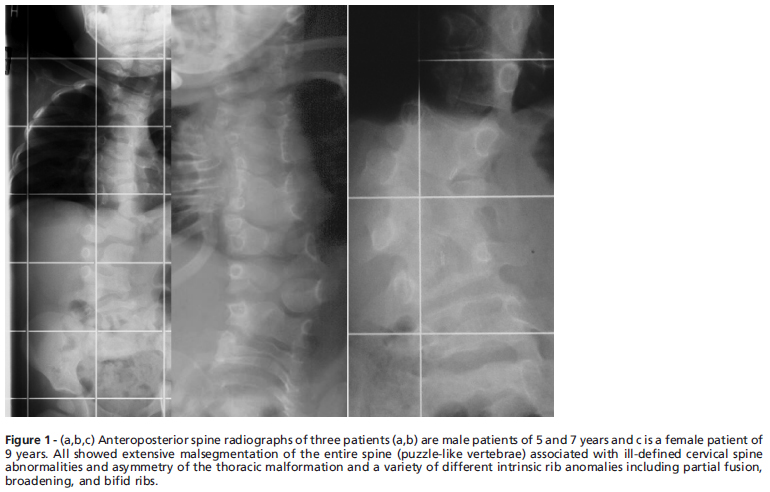
The diagnosis of SCD was based on details of the phenotype and the spine radiographs as well. All patients manifested the classic radiographic features of SCD (consisting of progressive or constant scoliosis and severe defects of the entire spine segmentation associated with rib maldevelopment). Radiographs taken for the evaluation of the cervical/thoracic spine abnormalities were fraught with difficulty. Anteroposterior radiographs for all patients were the first modality, nonetheless; these showed ill-defined cervical spine maldevelopment ( a puzzle-like formation) associated with asymmetry of the thorax and a variety of intrinsic rib anomalies including partial fusion, broadening, and bifid ribs (Fig 1 a,b,c). Patients were then divided principally into two groups with subsequent subgroup divisions. The grouping of patients was organized in view of the most prominent clinical pathology. Group 1 included patients with remarkable torticollis, plagiocephaly, short neck and restricted neck movements. Group 2 included patients with significant thoracic and lumbar abnormalities.
Group I
A. Skull base abnormalities. Plagiocephaly is a pathologic craniofacial asymmetry that involves every part of the skull skeleton,10-12 particularly in the calvaria, which represents seven -eighths of the cranial volume in the infant.12 It involves the face too and can result in an occlusion disorder of the permanent teeth in affected children. 13 To study the reason behind the deformational plagiocephaly and the facial asymmetry, 3D reconstruction CT scan was applied in two patients.
B. Atlas anomalies. The atlas serves as a washer between the occipital condyles and the axis vertebra. The atlantooccipital articulations allows for some flexion/extension, minimal lateral bending, and little axial rotation. The neurocentral synchondroses, formed at the juncture of the anterior arch and lateral mass ossification centers, typically fuse by 7 years of age. Two or even 3 ossification centers may contribute to the development of the anterior arch, yielding 3 or 4 synchondroses. Rarely there is another anterior ossification center, and the anterior arch forms from the posterior ossification centers with a midline synchondrosis. There are large variances in ages of completion of fusion of ossification centers, but failure of a synchondrosis to fuse by adulthood results in an arch defect or clefting. Synchondrosis fractures or dislocations are difficult to appreciate radiographically and contribute to the high diagnostic error rate for cervical spine injury (24%) in young children. However, synchondroses can also be the site of injury. The majority of atlas anomalies are posterior arch anomalies, probably due to developmental failure of chondrogenesis. These include complete aplasia, Keller-type aplasia with persistence of the posterior tubercle, aplasia with a unilateral or bilateral remnant and midline rachischisis, and hemiaplasia or partial hemiaplasia of the posterior arch. Partial hemiaplasia/ neurocentral synchondrosis and or lateral mass synchondrosis may simulate Jefferson fractures.14-17 Our patients in this group manifested torticollis, marked restriction of neck mobility accompanied with mild pain.
C. Odontoid abnormalities. At birth the odontoid is separated from the body of C2 by a cartilaginous band called the neurocentral synchondrosis. The neurocentral synchondrosis actually is an embryonic ''Joint'' between the vertebral arches and the centrum (embryonic vertebral bodies). The synchondrosis sits well within the body of the axis. It is seen in virtually all children of age 3 and absent by the age of 6 years. The apical portion of the dens is derived from the fourth occipital sclerotome and ossifies by the age of 3-5 years, with the fusion with the rest of the odontoid taking place around age 12. Two main pathologic categories of odontoid abnormalities are included in this study. Odontoid aplasia and hypoplasia have been encountered in patients with SCD. In instances of both hypoplasia and aplasia, the attachments for the apical and alar ligaments are absent, predisposing patients to atlantoaxial instability and resultant cord compression at the level of C1 arch. 18,19 An open mouth radiograph to a 13-year-old patient showed no trace of odontoid.
Group II
A. Thoracic vertebrae. Neural tube defects are considered to result from the failure of normal neural tube closure between the third and fourth week of embryonic development .In general, they vary in severity, the mildest form being spina bifida occulta, in which the osseous fusion of one or more vertebral arches is lacking, without involvement of the underlying meninges or neural tissue.20-21 To investigate the reason for the entire thoracic vertebral maldevelopment (a puzzle-like vertebrae, the short trunk and the chest asymmetry) we further referred to a reconstruction CT scan.
B. Lumbar spine abnormalities. Defects of vertebral segmentation may be either unilateral or bilateral. They occur most frequently in the thoracic or thoracolumbar regions. A unilateral failure of the segmentation of two or more vertebrae results in a unilateral unsegmented bar, which is one of the most common causes of congenital scoliosis.22-23 This type of anomaly consists of a bar of bone fusing the disc spaces and facet joints on one side of the spine while leaving the other side relatively unaffected. A bilateral failure of the segmentation of a number of adjacent vertebrae results in a block vertebrae. The disc spaces between the affected vertebrae are very narrow or fused. As a result, a longitudinal growth that is impaired on both sides of the spine, producing a shortened segment, canoccur. Two patients of 9 and 15 years respectively have been investigated by means of 3 DCT scan because of significant lumbar scoliosis.
RESULTS
The anatomical changes along the entire spine (skull base, craniocervical, thoracic and lumbar) changes were analyzed via CT scanning. Plagiocephaly and torticollis were correlated to prenatal endocranial skull base asymmetry in connection with endocranial synchondrosis. Short immobile neck was appeared to be connected with atlas and odontoid abnormalities and extensive neurocentral synchondrosis of the cervical spine. Short and fixed lumbar scoliosis was found to be due to the persistence of posterior mid-line unsegmented bar and associated with extensive spina bifida occulta.
Group I (A)
All showed endocranial base asymmetric 3D position of the external acoustic meatus. The asymmetry was secondary to extensive skull base synchondrosis. More marked in the middle and the posterior cranial fossa while the anterior cranial fossa was minimally involved (Figs. 2,3).
Group I (B)
Coronal reformatted CT scan showed neurocentral synchondrosis and a pseudo-Jefferson fracture of the lateral mass (Fig 4). 3D reconstruction CT scans showed posterior arch rachischisis making a split atlas (Fig 5).
Group I (C)
Axial reformatted CT scans showed assimilation of the posterior arch of the atlas, a splitting deformity of the anterior arch and agenesis of the odontoid process (Fig. 6).
Group II (A)
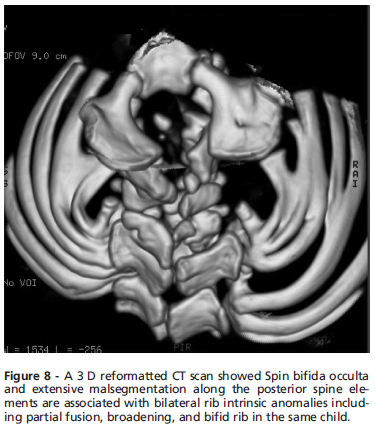
Massive malsegmentation throughout the entire cervical spine was noted anteriorly, with smooth outlines to the vertebral bodies, the so-called ''pebble beach sign'' in a-3year-old boy with spondylocostal dysostosis syndrome (Fig. 7). Spina bifida occulta in the same patient was associated with bilateral rib intrinsic anomalies including partial fusion, broadening, and bifid rib (Fig. 8)
Group II (B)
In one of our patients, lumbar scoliosis was investigated further via 3 D reconstruction CT scan. A posterior unsegmented bar including the upper three lumbar verteb rae was evident (Fig. 9). In other patient, a 3D reformatted CT scan showed extensive failure of fusion of the spinous processes of the lumbar vertebrae (Fig. 10).
DISCUSSION
Developmentally, the vertebral column is derived from mesodermal cells that are generated initially as epithelial spheres from the segmental plate on both sides of the neural tube. Influenced by inductive signals, the cells of the ventral half of somites undergo an epithelial-mesenchymal transition and form the sclerotome, the progenitor cell population of the axial skeleton. The Notch signalling pathway is crucial in three types of processes: lateral inhibition, lineage decisions, and boundary formation. Axial skeletal defects have been reported when abnormalities of this pathway are present, due in part to the fact that somite segmentation relies on boundary formation. Both core Notch signalling components and modifiers are required for normal somite formation and anteroposterior somite polarity.6,7,14
The skull base asymmetry in plagiocephaly has been known since the craniometric study of Manouvrier,24 which showed the asymmetric position of the external acoustic meatus in the frontal and transverse planes. This asymmetry is secondary to the deviation of the petrous part of the temporal bone. In patients with unilateral suture synostosis, the petrous part of the temporal bone is deviated forward and upward and the hemicranium is smaller on the synostotic side. Our patients manifested skull base asymmetry secondary to extensive endocranial synchondrosis. The asymmetry is more marked in the middle and the posterior cranial fossa while the anterior cranial fossae were slightly asymmetric.
The neurocentral synchondroses of the atlas, formed at the juncture of the anterior arch and lateral mass ossification centers, typically fuse by 7 years of age. Two or even 3 ossification centers may contribute to the development of the anterior arch, yielding 3 or 4 synchondroses. Rarely, there is another anterior ossification center, and the anterior arch forms from the posterior ossification centers with a midline synchondrosis. There are large variances in ages of completion of fusion of the ossification centers, but the failure of a synchondrosis to fuse by adulthood results in an arch defect or clefting. Synchondrosis fractures or dislocations are difficult to appreciate radiographically and contribute to the high diagnostic error rate for cervical spine injury (24%) in young children. However, synchondroses can also be the site of injury. 14-17 The majority of atlas anomalies are posterior arch anomalies, probably due to the developmental failure of chondrogenesis. These include complete aplasia, Keller type aplasia, with persistence of the posterior tubercle, aplasia, with a unilateral or bilateral remnant and midline rachischisis, and hemiaplasia or partial hemiaplasia of the posterior arch.14 Jefferson fractures15 have been described infrequently in the immature spine, but not in association with skeletally dysplastic patients. The term ''Jefferson fracture'' is commonly associated with a 4-part burst pattern. Fractures may be confined to a single arch (type I), include both arches (type II), or involve the lateral masses (Type III). Two-part and 3-part burst variants have been shown to be more common than the 4-part burst pattern. Gehweiler et al.25 described congenital malfor mations of the atlas simulating Jefferson fracture in a nonsyndromic patient.
Odontoid hypoplasia has been reported in association with a variety of skeletal dysplasias such as spondyloepiphyseal dysplasia and mucopolysaccharoidoses, and in metatropic dwarfism. In instances of both hypoplasia and aplasia, the attachments for the apical and alar ligaments are absent, predisposing to atlanto-axial instability and resultant cord compression at the level of the C1 arch.5,14,18
Defects of vertebral segmentation may be either unilateral or bilateral. They occur most frequently in the thoracic or thoracolumbar regions. A unilateral failure of segmentation of two or more vertebrae results in a unilateral unsegmented bar, which is one of the most common causes of congenital scoliosis. This type of anomaly consists of a bar of bone fusing the disc spaces and facet joints on one side of the spine while leaving the other side relatively unaf fected. 6,7,22,23
The association of segmental costo-vertebral malformations and neural tube defects has been reported, and it has been proposed that this association is not coincidental.20,21 Giacoia and say 9 reported SCD associated with neural tube defects. They concluded that SCD and neural tube defects are aetiologically related. Radiographs were the only applied modality.
Mortier et al.,26 proposed dividing Jarcho-Levin syndrome into three clinical presentations: Jarcho-Levin for autosomal recessive usually a lethal condition, spondy lothoracic for autosomal recessive usually a less severe phenotype; and spondylocostal dysplasia for the autosomal and or recessive patterns of inheritance. Conventional radiography was the prime adopted modality. Specific and or detailed anatomical abnormalities have not been clarified.
Patients with SCD have vertebral abnormalities and numeric or structural rib anomalies that lead to thoracic asymmetry. Rib anomalies and dysmorphism are the typical features that differentiate this syndrome from spondylothoracic dysplasia (STD). Spondylothoracic dysplasia is characterized by segmentation and formation defects of the cervical, thoracic, and lumbar spine, such as hemivertebrae, block vertebrae, and unsegmented bars, with fusion of all of the ribs at the costovertebral junction. Lavy et al.27 described a crablike configuration of the thorax in patients with STD. They explained the poor prognosis of STD due to thoracic cage abnormalities. Neither craniocervical nor upper cervical spine abnormalities have been elucidated.
A literature review does not show patients with SCD syndrome matching the tomographic changes as seen in our patients. Kusumi and Turnpenny 6 described the various syndromes that occur due to formation errors in the spinal column. They emphasized the significant role of the radiographic interpretation in classifying the various forms of SCD. 3D scanning studies were not included.
Turnpenny et al.7 reported the clinical, radiographic and molecular findings in 10 families with autosomal recessive SCD, abnormal vertebral segmentation and the notch signalling pathway. Abnormal segmentation throughout the entire spine has been described as a predominating and a sole pathology.
Computed tomography scans provide excellent delineation of osseous deformity patterns in children with syndromic association. Three-dimensional studies and sagittal and coronal reconstructions provide the orthopaedic surgeon with a detailed understanding of the spinal osseous deformity and the development of subsequent traumatic atrophy of the spinal cord. 28 These investigations have important implications in understanding the mechanism of injury, judging potential instability, evaluating deformity, and planning surgical procedures.
CONCLUSION
The reason for presenting this study is four-fold. One is that radiographic documentation is an insufficient modality in assessing the spine malformation complex in patients with SCD. Second, previous reports used tomographic analysis to detect thoracic cage abnormalities in SCD patients. Third, this is the first study to analyze the spine in a remarkable number of patients. Fourth, using 3DCT, we were able to visualize with greater clarity the spine malformation complex along several anatomical segments. Finally we wish to stress that, the optimal imaging strategy for a particular patient will depend on accurate clinical and radiographic assessment of the individual's underlying pathology.
Received for publication on June 24, 2010; First review completed on July 8, 2010; Accepted for publication on July 9, 2010
E-mail: ali.alkaissi@osteologie.at
Tel.: +43 (0) 1 91021 86724
- 1. Bonafe L, Giunta C, Gassner M, Steinmann B, Superti Furga A. A cluster of autosomal recessive spondylocostal dysostosis caused by three newly identified DLL3 mutations segregating in a small village. Clin Genet. 2003;63:28-35.
- 2. Gellis SS, Feingold M. Spondylothoracic dysplasia (costovertebral dysplasia, Jarcho-Levin syndrome). Am J Dis Child. 1976;130:513-4.
- 3. Jarcho S, Levin PM. Hereditary malformations of the vertebral bodies. Johns Hopkins Med. 1938;62:216-26.
- 4. Ramírez N, Cornier AS, Campbell RM, Carlo S, Arroyo S, Romeu J. Natural history of thoracic insufficiency syndrome: a spondylothoracic dysplasia perspective. J Bone Joint Surg Am. 2007;89:2663-75.
- 5. Maroteuax P, Le Merrer M. Maladies osseuses de l'enfant, 4th edn. Paris. 2002; Medeicine-Sciences Flammarion.
- 6. Kusumi K, Turnpenny P. Formation errors of the vertebral column. J Bone and Joint Surg (Am). 2007;89:64-71.
- 7. Turnpenny PD, Whittock N, Duncan J, Dunwoodie S, Kusumi K, Ellard S. Novel mutations in DLL3, a somitogenesis gene encoding a ligand for the Notch signalling pathway, cause a consistent pattern of abnormal vertebral segmentation in spondylocostal dysostosis. J Med Genet. 2003;40:333-9.
- 8. Takikawa K, Haga N, Maruyama T, Nakatomi A, Kondoh T, Makita Y, Hata A, Kawabata H, Ikegawa S. Spine and rib abnormalities and stature in spondylocostal dysostosis. Spine. 2006;31:E192-7.
- 9. Giacoia GP, Say B. Spondylocostal dysplasia and neural tube defects. J Med Genet. 1991;18:51-3.
- 10. Sperber GH. Craniofacial embryology. Boston: Wright. 1981;87-101.
- 11. Kreiborg S, Bjork A. Craniofacial asymmetry of a dry skull with plagiocephaly. Eur J Orthod. 1981;3:195-203.
- 12. Bories J. Le crâne du nouveau-né. Springer, Berlin, Heidelberg, New York, 1996.
- 13. Delaire J, Billet J, Ferré J, Faucher O, Julia P. Malformations faciales et asymétrie de la base du crane (un nouveau syndrome malformatif intéressant l'orthodontiste). Rev Stomatol. 1965;66:379-96.
- 14. Bailey DK. The normal cervical spine in infants and children. Radiology. 1952;59:712-9.
- 15. Landells CD, Van Peteghem PK. Fractures of the atlas: classification, treatment and morbidity. Spine. 1988;13:450-2.
- 16. Judd DB, Liem LK, Petermann G. Pediatric atlas fracture: a case of fracture through a synchondrosis and review of the literature. Neurosurgery. 2000;46:991-4.
- 17. Galindo MJ Jr, Francis WR. Atlantal fracture in a child through congenital anterior and posterior arch defects. A case report. Clin Orthop. 1983;178:220-2.
- 18. McManner T. Odontoid hypoplasia. Br J Radiol. 1983;56:907-10.
- 19. Menzes AH. Craniocervical developmental anatomy and its implications. Clin Neurosurg. 2005;52:5364.
- 20. Botto LD, Moore CA, Khoury MJ, Erickson JD. Neural-tube defects. New Eng. J. Med. 1999;341:1509-19.
- 21. Duru S, Ceylan S, Güvenc BH. Segmental costovertebral malformations: association with neural tube defects. Report of 3 cases and review of the literature. Pediatr Neurosurg. 1999;30:272-7.
- 22. Al Kaissi A, Ghachem MB, Nassib N, Ben Chehida F, Kozlowski K. Spondylocarpotarsal synostosis syndrome (with a posterior midline unsegmented bar). Skeletal Radiol. 2005;34(6):364-6.
- 23. Akbarnia BA, Moe JH. Familial congenital scoliosis with unilateral unsegmented bar. J Bone Joint Surg Am. 1978;60:259-61.
- 24. Manouvrier L. Etude craniométrique sur la plagiocephalie. Bull Soc Anthrop Paris. 1883;526-53.
- 25. Gehweiler J, Daffner R, Roberts LJ. Malformations of the atlas vertebra simulating the Jefferson fracture. AJR Am J Roentgenol. 1983;149:1083-6.
- 26. Mortier GR, Lachman RS, Bocian M, Rimoin D. Multiple vertebral segmentation defects. Am J Med Genet. 1996;61:310-9.
- 27. Lavy NW, Palmer CG, Merritt AD. A syndrome of bizarre vertebral anomalies. J Pediatr 1966;69:1121-5.
- 28. Al Kaissi A, Ben Chehida F, Ben Ghachem M, Klaushofer K, Grill F. Diffuse skull base/cervical fusion syndromes in two siblings with spondylocostal dysostosis syndrome: analysis via three dimensional computed tomography scanning. Spine. 2008;33:E425-8.
Publication Dates
-
Publication in this collection
22 Nov 2010 -
Date of issue
2010
History
-
Received
24 June 2010 -
Accepted
09 July 2010 -
Reviewed
08 July 2010