Abstract
BACKGROUND: In Brazil, despite the recommendations of the Brazilian Society of Hemodynamics and Interventional Cardiology, the National Health System has not yet approved the use of drug-eluting stents. In percutaneous coronary interventions performed in the public and part of the private health care system, bare metal stents are used as the only option. Therefore, new information on bare metal stents is of great importance. The primary endpoint was to evaluate the influence of the alloy and the profile of coronary stents on late loss and restenosis rates 6 months after implantation in patients with multivessel coronary disease. METHODS: Single center, randomized and prospective study comparison of cobalt-chromium versus stainless steel stent implantation in 187 patients with multivessel coronary disease. At least one cobalt-chromium and one stainless steel stent were implanted per patient. RESULTS: Mean age of patients was 59.5 + 10.1 years with a prevalence of males (66.3%) and patients with acute coronary syndrome (56%). Baseline clinical characteristics were similar with hypertension in 146 (78%), dyslipidemia in 85 (45.5%) and diabetes in 68 (36.4%). Two hundred and twenty-nine cobalt-chromium and 284 stainless steel stents were implanted. Angiographic variables showed no statistically significant difference. Angiographic follow-up to 6 months after implantation showed similar late loss and restenosis rates. CONCLUSION: The use of two different alloys, stainless steel and cobalt-chrome stents, in the same patient and in the same vessel produced similar 6-month restenosis and late loss rates.
Coronary artery disease; Coronary restenosis; Percutaneous transluminal coronary angioplasty; Coronary stents; Chromium alloy
CLINICAL SCIENCE
IHospital Stella Maris, Guarulhos, SP, Brazil
IIInstituto do Coração da Universidade de São Paulo, São Paulo, SP, Brazil
IIIInstituto de Assistência Médica ao Servidor Público Estadual-IAMSPE Postgraduate Program, São Paulo, SP, Brazil
ABSTRACT
BACKGROUND: In Brazil, despite the recommendations of the Brazilian Society of Hemodynamics and Interventional Cardiology, the National Health System has not yet approved the use of drug-eluting stents. In percutaneous coronary interventions performed in the public and part of the private health care system, bare metal stents are used as the only option. Therefore, new information on bare metal stents is of great importance. The primary endpoint was to evaluate the influence of the alloy and the profile of coronary stents on late loss and restenosis rates 6 months after implantation in patients with multivessel coronary disease.
METHODS: Single center, randomized and prospective study comparison of cobalt-chromium versus stainless steel stent implantation in 187 patients with multivessel coronary disease. At least one cobalt-chromium and one stainless steel stent were implanted per patient.
RESULTS: Mean age of patients was 59.5 + 10.1 years with a prevalence of males (66.3%) and patients with acute coronary syndrome (56%). Baseline clinical characteristics were similar with hypertension in 146 (78%), dyslipidemia in 85 (45.5%) and diabetes in 68 (36.4%). Two hundred and twenty-nine cobalt-chromium and 284 stainless steel stents were implanted. Angiographic variables showed no statistically significant difference. Angiographic follow-up to 6 months after implantation showed similar late loss and restenosis rates.
CONCLUSION: The use of two different alloys, stainless steel and cobalt-chrome stents, in the same patient and in the same vessel produced similar 6-month restenosis and late loss rates.
Keywords: Coronary artery disease; Coronary restenosis; Percutaneous transluminal coronary angioplasty; Coronary stents; Chromium alloy.
INTRODUCTION
In Brazil, coronary heart disease is an important cause of death and hospitalization.1,2 Despite the recommendations of the Brazilian Society of Hemodynamics and Interventional Cardiology, a significant percentage of patients are still treated with bare-metal stents. The National Health System has not yet incorporated the use of drug-eluting stents (DES).3 Thus, baremetal stents are the only option for percutaneous coronary interventions performed in our public health care system. The National Agency for Sanitary Surveillance has registered about 108 bare-metal stents for clinical use; none has fully met the "optimal stent" criteria.4,5
There is renewed interest in the development of baremetal stents because, as these stents improve, so does the platform for DES. Coronary stents for clinical use are made from metal alloys, and stainless steel alloy is the most frequently used. These stents may release heavy metal ions (nickel, chromium and molybdenum), which can cause allergic and hypersensitivity reactions resulting in a stimulus for the proliferation and migration of smooth muscle cells and consequently restenosis.6
Lately, alloys of nickel-chrome and cobalt-chromium have been used to improving certain characteristics of stainless steel stents. Owing to its greater density, this alloy enables the construction of stents with thinner struts and the same radiopacity and radial strength as stainless steel with thicker struts. In turn, the reduced thickness of the struts provides more flexibility and lower crossing profiles to the stents, thus reducing the inflammatory response at the implant site and neointimal thickening, with potentially lower restenosis and target vessel revascularization rates.7-11
The evidence in experimental studies in animal models shows that the cobalt-chromium stents with thinner struts cause less neointimal thickening and percentage of stenosis compared with the thicker strut stents.8
Clinical evidence has suggested that patients treated with thin metal strut stents (<100 μm thick)12 have less neointimal proliferation and better outcomes than patients treated with thicker stents.13-15 The first post-implant study of a cobalt-chromium stent (Multilink Vision registry), with thick 81-μm struts, showed late loss of 0.83 mm, which is comparable to the thinnest stainless steel Guidant Multilink stent with thinner struts (50 μm) and late loss of 0.78 mm. Restenosis and target lesion revascularization rates were 15.7% and 4.3% respectivelly.16
Unlike stainless steel stents, which have been widely studied, there are few studies on the efficacy and safety of cobalt-chromium stents, as the clinical use of these stents took place in the era of DES.7,8,16-18
The primary endpoint was to evaluate the influence of the alloy and the profile of coronary stents on late loss and restenosis rates at 6 months after implantation in patients with multivessel coronary disease.
METHODS
This is a single center, prospective and randomized trial, comparing cobalt-chromium stent versus stainless steel stent implantation in patients with multivessel coronary disease to evaluate the influence of metal alloys and their profile on the late loss and restenosis rates 6 months after implantation in patients with multivessel coronary disease. The study protocol was approved by the ethics committee of Instituto de Assistencia Medica ao Servidor Publico Estadual (IAMSPE) and was conducted according to the principles of the Declaration of Helsinki. All patients gave written informed consent.
The study included patients with stable angina or acute coronary syndrome (unstable angina or myocardial infarction) with de novo multivessel coronary artery lesions of >70% by quantitative coronary angiographic analysis who were suitable for stent implantation.
Patients were excluded if there was failure to provide written informed consent, contraindication to any emergency myocardial revascularization surgery, patients with single-vessel disease, restenotic lesions, chronic total occluded lesions, significant left main disease, patients undergoing primary angioplasty, contraindications to the use of acetylsalicylic acid or clopidogrel and a left ventricular ejection fraction of <30%. Patients with cardio-genic shock, malignancies or other comorbidities with life expectancy < 12 months or that may result in non-compliance with the protocol, or pregnancy were considered ineligible for the study.
Procedural success was defined as a final reduction in lumen diameter stenosis of < 20% after stent deployment, and obtaining TIMI3 flow in the absence of major in-laboratory and in-hospital complications (death, emergency bypass surgery, development of a new non-ST elevation myocardial infarction or ST-elevation myocardial infarction and target vessel revascularization). ST-elevation myocar-dial infarction was diagnosed as the development of ST-elevations on the electrocardiogram and creatine kinase-MB increase above normal laboratory values. Non-ST-elevation myocardial infarction was defined as creatine kinase-MB values three times greater than the normal range with or without persistent ST-segment or T-wave changes on the post-procedure electrocardiogram. Major adverse cardiac events included death, Q-wave myocardial infarction and any target lesion revascularization. Angiographic restenosis was defined as stenosis diameter >50% in stent segment or up to 5 mm proximal and distal to the edge of the stent at the 6-month angiography. All deaths were considered cardiac deaths unless clearly documented as non-cardiac deaths. Angioplasty for subacute stent thrombosis is considered as target lesion revascularization. Late loss was defined as a difference between the minimal luminal diameter immediately after stent implantation and the minimal luminal diameter at angiographic follow-up. A control coronary angiography was performed 6 months after last stent implantation.
Coronary angiography was performed routinely. Measurements of the interpolated reference diameter, lesion length and diameter, diameter stenosis and minimal luminal diameter were obtained before and after the intervention and at angiography follow-up, from an angiogram performed after an intracoronary bolus injection of 20 mg of isosorbide mononitrate in all patients for whom follow-up angiogram was available. Quantitative coronary arteriography was performed by an independent radiology technician at the core laboratories of the Stella Maris Hospital using the validated automatic edge detection of Philips System (CAAS II, Pie Medical Imaging, Maastricht, Netherlands).
Randomization was performed by obtaining a random sequence of numbers in two columns at www.random.org. Following the randomization sequence, patients were divided into two groups (stainless steel stents and cobalt-chromium stents). After assessing the eligibility of patients and obtaining informed consent, patients were randomized according to the sequence for stent implantation (cobalt-chromium or stainless steel). Implants of at least one stainless steel stent and a cobalt-chromium stent were performed per patient in different vessels or in the same vessel provided there was a distance >10 mm between the stents. Implantation of both stents was performed in the same procedure or in another procedure on different dates.
The lesions were treated by standard stenting techniques, with access through the femoral artery, at the investigator's criteria to pre- or post-dilatation. In the case of dissection or obstructions in proximal or distal areas of the stent, balloon dilation was carried out. The cobalt-chromium PRO-Kinetic (Biotronik), the only cobalt-chromium stent available at our service, and stainless steel stents available at our service were used. Left ventricular function was expressed as the global ejection fraction as a percentage, as measured by angiography before the procedure.
Patients were treated with 200 mg of aspirin daily and 300 mg of clopidogrel on the day before the procedure. Isosorbide-5 mononitrate (20 mg) was administered intra-coronarily before the analysis of quantitative coronary angiography (before and immediately after), and heparin at a dose of 100 IU/kg was administered intravenously before implantation. After hospital discharge, 75 mg of clopidogrel was administered for 30 days and aspirin indefinitely.
Hospital discharge was schedule for 24-48 hours after stent implantation if there were no complications.
Patients were evaluated at 6 months post-implant. If the patient did not come for the scheduled visit, contact was attempted by various means (telephone, letter, contact your doctor) to obtain information. In the case of death, telephone contact was made with the family and a death certificate was requested.
During hospitalization and at the 6-month follow-up visit, the following clinical events were assessed: cardiac death, myocardial infarction, coronary artery bypass graft (CABG), any new procedure for percutaneous revascularization (site of the target lesion or another arterial segment), subacute coronary occlusion. Creatine kinase and its CK-MB fraction activity were measured 18 hours after the procedure.
All statistical analyses were performed with Prime. Results are presented as means + SDs for continuous variables and as percentages for categorized variables. Sample size calculation was performed by McNemar's test, because these are not independent populations and based on the assumption of an error of 0.05, with b test power of 0.8 and a rate of target lesion revascularization of 15% for stainless steel stents and 8% for cobalt-chrome stents. Differences in continuous variables between groups were compared by the Student t-test and one-way ANOVA for comparing more than two groups. Comparison of restenosis rate by risk factors was performed by Chi-square test, and late loss was performed by the Kruskal-Wallis test.
RESULTS
Between July 2006 and November 2008,190 patients with multivessel coronary disease were selected to receive at least one cobalt-chromium stent and one stainless steel stent. There were two deaths from cardiac causes (1.05%) and one subacute thrombosis (0.5%) successfully treated with angioplasty in the post-hospitalization follow-up. These three patients were excluded from analysis, and the remaining 187 patients were implanted with 229 cobalt-chromium and 284 stainless steel stents.
The clinical characteristics of the patients are listed in Table 1. Baseline clinical characteristics were the same as comparisons were made for the same patient. Mean age was 59.5 + 10.1 years with a prevalence of males (66.3%) and patients with acute coronary syndrome (56%).
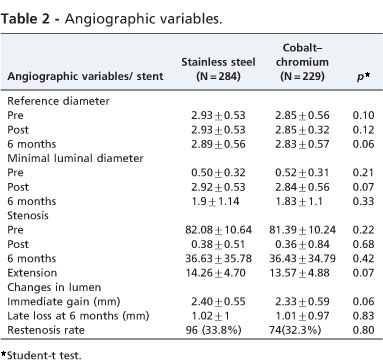
Table 2 lists the angiographic variables. No statistically significant differences were observed between the vessel diameter, diameter stenosis and lesion length. No statistically significant differences occurred between late loss and restenosis 6 months after the implant.
The restenosis rate and late loss were not associated with gender, hypertension and hypercholesterolemia. However, they were associated with diabetes (Table 3).
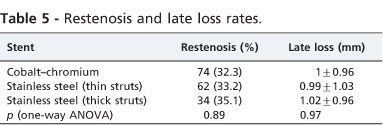
The Pro-Kinetic cobalt-chromium stent and stainless steel stents available at our service were used in this study (Table 4). Among the stainless steel stents, there was a prevalence of thin strut stents (58.1%). When comparing the rate of restenosis and late loss at 6 months after implantation between the stainless steel stents with thin or thick struts and the cobalt chrome stents, there were no statistically significant differences (Table 5).
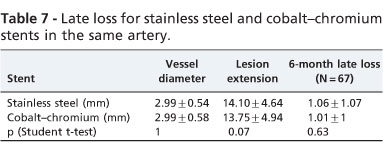
In a subanalysis of the sample, 56 patients had two lesions in one artery (112 lesions), four patients had two lesions in two arteries (16 lesions) and one patient had two lesions in three arteries (six lesions), totaling 61 patients (67 arteries, 134 lesions). In these 67 arteries, 67 cobalt-chromium stents and 67 stainless steel stents were implanted. In 15 arteries (22.5%), there was restenosis agreement; in 40 arteries (59.7%), there was agreement on the lack of restenosis and disagreement in 12 arteries (17.8%), p = 0.8, McNemar's test (Table 6). There was no statistically significant difference between the late loss 6 months after implantation when the stents were implanted in the same artery (Table 7).
DISCUSSION
This prospective study with a randomized sequence of stent implantation, comparing the Pro-Kinetic cobalt-chromium stent and stainless steel stents, showed no statistically significant difference in restenosis and late loss rates at 6 months post-implantation between the two groups.
The strength of this study is that the comparison between the two stents was performed in the same patient and, in about one third of patients, the implant was performed in the same artery, matching not only the biological factors but, in most patients, the angiographic variables as well. Therefore, the result was influenced solely by the type of stent. Another advantage in comparing the implantation of two types of stents in the same patient is the smaller sample required to achieve significance.19
In-stent late loss assessed by quantitative coronary angiography is the measure that best reflects the real and pure biological effect of the performance of coronary stents.20 In the current study, which used the same clinical variables and where the angiographic variables showed no statistically significant difference, it was observed that late loss was similar for both stents, whether implanted in different vessels or in the same vessel.
The cobalt-chromium stent and most stainless steel stents (58.1%) used in this study are considered to be thin strut stents (100 μm). Clinical evidence has suggested that patients treated with thin strut stents (< 100 μm thick) show less neointimal proliferation and better outcomes than patients treated with stents with thicker struts. In the national registry, Salles et al. (2008)21 assessed the rate of target vessel revascularization, hospital stay and mean 18-month patient follow-up with the contemporary thin strut cobalt-chromium stents (Driver, Medtronic, United States -World Headquaters, 710 Medtronic Parkway, Minneapolis, MN 55432-5604) and stainless steel (Liberte, Boston Scientific) stents and observed no significant statistically differences between them.
Randomized quantitative coronary angiographic studies comparing stents with the same configuration and different thicknesses (Multi-Link versus Multi-Link Duet with struts of 50 μm and 140 μm respectively)13 and stents with different configurations and different strut thicknesses (Multi-Link versus BX Velocity and Multi-Link versus GFX)14,15 showed a lower restenosis rate when the thinnest stent struts were used. In this study, stainless steel and cobalt-chromium stents showed similar restenosis rates, although not all stainless steel stents had thin struts (41.9%), which should have a negative impact.
A recent randomized trial comparing the rate of target lesion revascularization and percentage diameter stenosis at 9 months after implantation of stainless steel stents (Taxus Express, struts 96 μm thick) and platinum chromium (Taxus Element, struts 81 μm thick), both paclitaxel-eluting stents, showed no differences in the results, which reinforces the outcomes of the present study.22
In this study, there was restenosis agreement in 15 arteries (22.5%), agreement of no restenosis in 40 arteries (59.7%) and disagreement in 12 arteries (17.8%). These findings indicate that there are populations of patients that are likely to develop coronary restenosis and that the rates of angio-graphic restenosis (including late luminal loss) showed a bimodal, non-Gaussian distribution.23-25 Diabetes was the only clinical variable correlated with higher probability of development of restenosis and higher late loss.
The clinical implications of this study are the compatibility of the use of stainless steel and cobalt-chromium stents in the same patient and in the same vessel, and possibly that restenosis depends largely on the patient's predisposition to develop it.
One limitation of this study was the use of different types of stainless steel stents of different sizes, which may have influenced the results. Another limitation was the non-performance of intracoronary ultrasound to assess atherosclerotic plaques, as it included patients with stable angina and patients with acute coronary syndrome, which could influence the results.
CONCLUSION
Comparison of stents made from two different alloys, stainless steel and cobalt-chrome stents, in the same patient and in the same vessel showed similar 6-month restenosis and late loss rates.
Received for publication on November 25, 2010; First review completed on February 28, 2011; Accepted for publication on March 2, 2011
E-mail: george.ximenes@terra.com.br Tel.: 55 11 91574544
- 1. Mansur A de P, Favarato D, Avakian SD, Ramires JA. Trends in ischemic heart disease and stroke death ratios in Brazilian women and men. Clinics. 2010;65:1143-7, doi: 10.1590/S1807-59322010001100016.
- 2. Bampi AB, Rochitte CE, Favarato D, Lemos PA, da Luz PL. Comparison of non-invasive methods for the detection of coronary atherosclerosis. Clinics. 2009;64:675-82, doi: 10.1590/S1807-59322009000700012.
- 3. Lima VC, Matos LAP, Caramori PRA, Perin MA, Mangione JM, Machado BM, et al. Consenso de Especialistas (SBC/SBHCI) Sobre o Uso de Stents Farmacológicos. Recomendações da Sociedade Brasileira Cardiologia/Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista ao Sistema Único de Saúde. Arq Bras Cardiol. 2006;87:e162-7, doi: 10.1590/S0066-782X2006001700037.
-
4Stents farmacológicos e stents metálicos no tratamento da doenca arterial coronariana. Boletim Brasileiro de Avaliação de Tecnologia em Saúde (BRATS), June 2009. Available at www.anvisa.gov.br/divulga/newsletter/brats/2009/BRATS8.pdf (accessed 22 February 2009).
- 5. Chamie D, Abizaid A. Stent cronus: chegou o momento de adotarmos um stent nacional. Rev Bras Cardiol Invas. 2009;17:300-4.
- 6. Koster R, Vieluf D, Kiehn M, Sommerauer M, Kahler J, Baldus S, et al. Nickel and molybdenum contact allergies in patients with coronary in-stent restenosis. Lancet. 2000;356:1895-7, doi: 10.1016/S0140-6736(00)03262-1.
- 7. Lemos PA, Laurindo FRM, Morato SP, Takimura C, Camps CA, Gutierrez PS, et al. Stent Coronaario de Liga Cobalto-Cromo Concebido no Brasil: Achados Histológicos Preliminares em Modelo Experimental Porcino. Rev Bras Cardiol Invas. 2007;15:378-85.
- 8. Jabara R, Geva S, Riberio HB, Chen JP, Hou D, Li J, king SB, et al. A third generation ultra-thin strut cobalt chromium stent: histopathological evaluation in porcine coronary arteries. EuroIntervention. 2009;5:619-26, doi: 10.4244/EIJV5I5A99.
- 9. Donachie M. Biomedical alloys. Adv Materials Processes. 1998;7:63-5.
- 10. Hagemeister J, Baer FM, Schwinger RHG, Hopp HW. Compliance of a cobalt chromium coronary stent alloy. The CIVIS trial. Cardiovasc Med. 2005;6:17-20.
- 11. Gotman I. Characteristics of metals used in implants. J Endourol. 1997;11:383-9, doi: 10.1089/end.1997.11.383.
- 12. Briguori C, Sarais C, Pagnotta P, Liistro F, Montorfano M, Chieffo A, et al. In-stent restenosis in small coronary arteries: impact of strut thickness. J Am Coll Cardiol. 2002;40:403-9, doi: 10.1016/S0735-1097(02)01989-7.
- 13. Kastrati A, Mehili J, Dirschinger J, Dotzer F, Schülen H, Neuman FJ, et al. Intracoronary stenting and angiographic results - strut thickness effect on restenosis outcome (ISAR-STERO) trial. Circulation. 2001;103:2816-21.
- 14. PacheJ,KastratiA,Mehilli J, Schühlen H, Dotzer F, Hausleiter J, et al. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2). J Am Coll Cardiol. 2003;41(8):1283-8, doi: 10.1016/S0735-1097(03)00119-0.
- 15. Yoshitomi Y, Kojima S, Yano M, Sugi T, Matsumoto Y, Saotome M, et al. Does stent design affect probability of restenosis? A randomized trial comparing MULT-LINK stents with GFX stents. Am Heart J. 2001;142:445-51, doi: 10.1067/mhj.2001.117321.
- 16. Kereiakes DJ, Cox DA, Hermiller JB, Midei MG, Bachinsky WB, Nukta ED. Usefulness of a cobalt chromium coronary stent alloy. Am J Cardiol. 2003;92:463-6.
- 17. Kaiser C, Rocca HPBL, Buser PT, Bonetti PO, Osswald S, Linka A, et al. Increment cost-effectiveness of drug-eluting stents compared with a third-generation bare-metal stent in a real-world setting: randomized Basel Stent Kosten Effektivitats trial (BASKET). Lancet. 2005;366:921-9, doi: 10.1016/S0140-6736(05)67221-2.
- 18. Campos CAHM, Ribeiro EE, Lemos PA, Obregon A, Ribeiro H, Spadaro AG, et al. Resultados clínicos do primeiro stent de cromo-cobalto concebido no Brasil. Rev Bras Cardiol Invas. 2009;17:314-19.
- 19. Luiz RR, Magnanini MMF. A Lógica da Determinação do Tamanho da Amostra em Investigates Epidemiológicas. Cadernos Saúde Coletiva, Rio de Janeiro, 2000;8:9-28.
- 20. Sousa AGMR, Sousa JEMR. CYPHER ou TAXUS: diferentes ou semelhantes? Rev Bras de Cardiol Inv. 2004;12:1-3.
- 21. Sales JAB, Andreía JCM, Cortes LA, Camilis F, Carestiato L, Figueira HR. Implante contemporâneo de stents convencionais: comparação dos stents de hastes finas de acço inoxidável versus cromo-cobalto. Rev Bras Cardiol Invas. 2008;16:59-63.
- 22. Kereiakes DJ, Cannon LA, Feldman RL, Popma JJ, Magorien R, Whitbourn R, et al. Clinical and angiographic outcomes after treatment of de novo coronary stenosis with a novel platinum chromium thin-strut stent: primary results of the PERSEUS (Prospective Evaluation in a Randomized Trial of the Safety and Efficacy of the Use of the TAXUS Element Paclitaxel-Eluting Coronary Stent System) trial. J Am Coll Cardiol 2010;56:264-71, doi: 10.1016/j.jacc.2010.04.011.
- 23. Resing BJ, Hermans WR, Deckers JW, de Feyter PJ, Tijssen JGP, Serruys PW. Lumen narrowing after percutaneous transluminal coronary balloon angioplasty study in 1,445 successfully dilated lesions. J Am Coll Cardiol 1992;19:939-45, doi: 10.1016/0735-1097(92)90274-Q.
- 24. Kuntz RE, Safian RD, Levine MJ, Reis GJ, Diver DJ, Baim DS. Novel approach to the analysis of restenosis after the use of three coronary devices. J Am Coll Cardiol 1992;19:1493-9, doi: 10.1016/0735-1097(92)90609-Q.
- 25. Lehmann KG, Melkert R, Serruys PW. Contributions of frequency distribution analysis to the understanding of coronary restenosis: a reappraisal of the Gaussian curve. Circulation 1996;93:1123-32.
Influence of metal alloy and the profile of coronary stents in patients with multivessel coronary disease
Publication Dates
-
Publication in this collection
21 July 2011 -
Date of issue
2011
History
-
Accepted
02 Mar 2011 -
Reviewed
28 Feb 2011 -
Received
25 Nov 2010