Abstract
OBJECTIVE:
Intestinal ischemia-reperfusion injury occurs in several clinical conditions and after intestinal transplantation. The aim of the present study was to investigate the phenomena of apoptosis and cell proliferation in a previously described intestinal ischemia-reperfusion injury autograft model using immunohistochemical markers. The molecular mechanisms involved in ischemia-reperfusion injury repair were also investigated by measuring the expression of the early activation genes c-fos and c-jun, which induce apoptosis and cell proliferation.
MATERIALS AND METHODS:
Thirty adult male Wistar rats were subjected to surgery for a previously described ischemia-reperfusion model that preserved the small intestine, the cecum and the ascending colon. Following reperfusion, the cecum was harvested at different time points as a representative segment of the intestine. The rats were allocated to the following four subgroups according to the reperfusion time: subgroup 1: 5 min; subgroup 2: 15 min; subgroup 3: 30 min; and subgroup 4: 60 min. A control group of cecum samples was also collected. The expression of c-fos, c-jun and immunohistochemical markers of cell proliferation and apoptosis (Ki67 and TUNEL, respectively) was studied.
RESULTS:
The expression of both c-fos and c-jun in the cecum was increased beginning at 5 min after ischemia-reperfusion compared with the control. The expression of c-fos began to increase at 5 min, peaked at 30 min, and exhibited a declining tendency at 60 min after reperfusion. A progressive increase in c-jun expression was observed. Immunohistochemical analyses confirmed these observations.
CONCLUSION:
The early activation of the c-fos and c-jun genes occurred after intestinal ischemia-reperfusion injury, and these genes can act together to trigger cell proliferation and apoptosis.
Ischemia-reperfusion injury; Immediate early genes; Activator protein 1; Small intestine; C-fos; C-jun gene
INTRODUCTION
Intestinal ischemia-reperfusion (I/R) injury occurs in several clinical conditions
and after intestinal transplantation. The hypoxia and tissue damage caused by I/R
injury can, in turn, result in severe complications, and following intestinal
transplantation, this type of injury plays a determining role in early graft
dysfunction and long-term graft survival 1. HaglundU. Gut ischaemia. Gut. 1994;35(1
Suppl):S73-6.2. SantosMM, TannuriU, TannuriAC, CoelhoMC. Description of a new
model of intestinal denervation and in?situ ischemia-reperfusion injury using
the cecal artery for perfusion.Transplant Proc. 2009;41(9):3617-21,
http://dx.doi.org/10.1016/j.transproceed.2009.05.037.
http://dx.doi.org/10.1016/j.transproceed...
3. IkedaH, SuzukiY, SuzukiM, KoikeM, TamuraJ, TongJ, et al.
Apoptosis is a major mode of cell death caused by ischaemia and
ischaemia/reperfusion injury to the rat intestinal epithelium.Gut.
1998;42(4):530-7, http://dx.doi.org/10.1136/gut.42.4.530.
http://dx.doi.org/10.1136/gut.42.4.530...
1-44. ShimaY, TajiriT, TaguchiT, SuitaS. Increased expression of c-fos
and c-jun in the rat small intestinal epithelium after ischemia-reperfusion
injury: a possible correlation with the proliferation or apoptosis of intestinal
epithelial cells.J Pediatr Surg. 2006;41(4):830-6,
http://dx.doi.org/10.1016/j.jpedsurg.2005.12.025.
http://dx.doi.org/10.1016/j.jpedsurg.200...
. The
number of cells present in adult tissue is determined based on the balance between
destroyed and regenerated cells, and apoptosis plays an important role in this
process of homeostasis under both physiological and pathological conditions 55. HuppertzB, FrankHG, KaufmannP. The apoptosis
cascade--morphological and immunohistochemical methods for its
visualization.Anat Embryol (Berl). 1999;200(1):1-18.. As a function of apoptosis, some
gastrointestinal mucosal cells are periodically lost, leaving room for their
physiological renewal, which occurs in association with the proliferation of local
totipotent cells. Apoptosis and regeneration can also be triggered by I/R injury at
the molecular level 66. ShahKA, ShureyS, GreenCJ. Characterization of apoptosis in
intestinal ischaemia-reperfusion injury--a light and electron microscopic
study.Int J Exp Pathol. 1997;78(5):355-63,
http://dx.doi.org/10.1046/j.1365-2613.1997.420369.x.
http://dx.doi.org/10.1046/j.1365-2613.19...
. In
addition, apoptosis has been described as a marker of intestinal graft rejection
77. PasternakBA, CollinsMH, TiaoGM, RudolphJA, AlonsoMH, RyckmanFC,
et al. Anatomic and histologic variability of epithelial apoptosis in small
bowel transplants.Pediatr Transplant2010;14(1):72-6,
http://dx.doi.org/10.1111/ptr.2009.14.issue-1.
http://dx.doi.org/10.1111/ptr.2009.14.is...
. The process of intestinal
regeneration after I/R injury involves increased DNA synthesis as well as the rapid
and brief expression of immediate early response genes (IEGs) 44. ShimaY, TajiriT, TaguchiT, SuitaS. Increased expression of c-fos
and c-jun in the rat small intestinal epithelium after ischemia-reperfusion
injury: a possible correlation with the proliferation or apoptosis of intestinal
epithelial cells.J Pediatr Surg. 2006;41(4):830-6,
http://dx.doi.org/10.1016/j.jpedsurg.2005.12.025.
http://dx.doi.org/10.1016/j.jpedsurg.200...
, including those which encode the transcription
factors c-fos and c-jun. IEGs, which are expressed in a rapid and transient manner,
can be induced by several factors, including I/R injury.
The cellular genes c-fos and c-jun dimerize to form
the activator protein-1 (AP-1) transcription factor complex. In addition, each forms
several other stable homo- or heterodimeric complexes that bind to DNA regulatory,
promoter, and potentiation regions thus controlling the transcription of numerous
target genes. Both c-fos and c-jun contain leucine zippers that are responsible for
their dimerization and for the regulation of cellular response genes following
injury. Those genes can be expressed within minutes after the exposure of cells to a
given stimulus, and the resulting products participate in several metabolic
processes, such as the regulation of cell proliferation, differentiation, and
apoptosis 88. WielandE, OellerichM, BraunF, SchtuzE. c-fos and c-jun mRNA
expression in a pig liver model of ischemia/reperfusion: effect of extended cold
storage and the antioxidant idebenone.Clin Biochem2000;33(4):285-90,
http://dx.doi.org/10.1016/S0009-9120(00)00070-9.
http://dx.doi.org/10.1016/S0009-9120(00)...
. The aim of the
present study was to investigate the phenomena of apoptosis and cell proliferation
in a previously described intestinal I/R injury autograft model involving cold
preservation of the intestine. This model allows for the study of the physiological
changes that occur following intestinal transplantation without the interference of
immune aggression induced by the graft 22. SantosMM, TannuriU, TannuriAC, CoelhoMC. Description of a new
model of intestinal denervation and in?situ ischemia-reperfusion injury using
the cecal artery for perfusion.Transplant Proc. 2009;41(9):3617-21,
http://dx.doi.org/10.1016/j.transproceed.2009.05.037.
http://dx.doi.org/10.1016/j.transproceed...
.
In cold ischemic preservation of the intestine, hypothermia protects against I/R
injury 9. HassounHT, FischerUM, AttuwaybiBO, MooreFA, SafiHJ, AllenSJ, et
al. Regional hypothermia reduces mucosal NFkappaB and PMN priming via gut lymph
during canine mesenteric ischemia/reperfusion.J Surg Res. 2003;115(1):121-6,
http://dx.doi.org/10.1016/S0022-4804(03)00298-1.
http://dx.doi.org/10.1016/S0022-4804(03)...
9,1010. AttuwaybiBO, HassounHT, ZouL, KozarRA, KoneBC, WeisbrodtNW, et
al. Hypothermia protects against gut ischemia/reperfusion-induced impaired
intestinal transit by inducing heme oxygenase-1.J Surg Res. 2003;115(1):48-55,
http://dx.doi.org/10.1016/S0022-4804(03)00313-5.
http://dx.doi.org/10.1016/S0022-4804(03)...
. We quantified apoptosis and the cell
proliferation index via histological examination and immunohistochemical analysis.
We also investigated the molecular mechanisms involved in I/R injury repair by
measuring the expression of the early activation genes c-fos and
c-jun, which induce apoptosis and cell proliferation. A better
understanding of the phenomena underlying I/R injury may allow for the
identification of interventions to reduce its occurrence and, thus, to achieve
better preservation of the intestinal graft.
MATERIALS AND METHODS
Thirty adult male Wistar rats were subjected to surgery using the previously
described I/R model involving perfusion through the cecal artery 22. SantosMM, TannuriU, TannuriAC, CoelhoMC. Description of a new
model of intestinal denervation and in?situ ischemia-reperfusion injury using
the cecal artery for perfusion.Transplant Proc. 2009;41(9):3617-21,
http://dx.doi.org/10.1016/j.transproceed.2009.05.037.
http://dx.doi.org/10.1016/j.transproceed...
. Briefly, under an operating
microscope (10X magnification), the mesocolon was sectioned from the cecum to the
level of the middle colic artery. In the root of the mesentery, the mesenteric
artery and vein were completely dissected and isolated by sectioning all of the
nervous and lymphatic tissues to an extension of approximately 1 cm.
All of the mesentery between the superior mesenteric artery and the jejunum (6-8 cm below the ligament of Treitz) was also sectioned. After these procedures, the distal branch of the superior mesenteric artery corresponding to the cecum was dissected (under a microscope at 40X magnification) in an extension that was sufficient for cannulation with a 1.9 French silicone catheter (Figure 1A). Close to the cecal valve, there was a consistently located lymph node that served as a reference point for the localization of the cecal artery. The jejunum and the ascending colon were clamped, and the superior mesenteric artery and vein were occluded using non-traumatic vascular clamps. The superior mesenteric artery was irrigated with lactated Ringer'´s solution at 4°C through a previously inserted cannula in the cecal artery, and perfusion was performed using an infusion pump at a rate of 40 ml of solution per hour, accompanied by the additional cooling of the peritoneal cavity with ice (Figure 1B). The effluent flowed out through a small leak in the superior mesenteric vein. The perfusion was considered adequate when the intestine and the mesentery were completely pale and the effluent was clear. Then, perfusion was interrupted (the total volume necessary for perfusion was 2.5 to 5.0 ml), the opening of the mesenteric vein was sutured with 10-0 prolene, and the catheter was removed. Next, the clamps were immediately removed from the mesenteric artery and vein, and reperfusion of the intestine (graft) was confirmed based on the return of pulsatile mesenteric blood flow. The average duration of ischemia was 18.37±5.06 min. Following reperfusion, the cecum was harvested at different time points as representative intestinal samples. The rats were allocated to the following four subgroups according to the reperfusion time: subgroup 1: 5 min (n=9); subgroup 2: 15 min (n=7); subgroup 3: 30 min (n=8); and subgroup 4: 60 min (n=6). Additionally, a control group (n=7) was subjected to the same procedures except for I/R injury.
A) Cannulation of the distal branch of the superior mesenteric artery using a silicone catheter. B) Aspect of the intestine after irrigation with lactated Ringer’s solution at 4°C.
The harvested material was divided into two fragments One fragment was immersed in 10% formaldehyde for histological processing and immunohistochemical analysis, and the other fragment was immediately frozen in liquid nitrogen at -170°C for RNA extraction.
Immunohistochemical methods
TUNEL assay
The TUNEL assay is a method used to detect DNA fragmentation that results
from the activation of apoptotic cascades. In the present study, intestinal
histological sections were fixed in 10% formalin, embedded in paraffin,
sliced into 5-μm-thick sections, and then deparaffinized. Following
hydration, the sections were subjected to TUNEL staining and enzymatic
digestion with proteinase K, followed by the blocking of endogenous
peroxidase activity. Finally, the sections were immersed in sodium citrate
and Triton X-100 solution to induce cell permeabilization, incubated in TdT
enzyme solution and a mixture of nucleotides, washed with phosphate-buffered
saline (PBS), and incubated in Converter-POD. The sections were processed to
detect apoptotic cells using the chromogenic substrate
3,3’-diaminobenzidine (DAB) (Sigma¯), followed by
counterstaining with Harris hematoxylin, dehydration, and mounting. This
assay was performed using intestinal samples from the control group, and the
samples were harvested at 60 min after I/R to quantify apoptosis. This time
point was selected because it exhibited the greatest expression of the
c-fos and c-jun genes 22. SantosMM, TannuriU, TannuriAC, CoelhoMC. Description of a new
model of intestinal denervation and in?situ ischemia-reperfusion injury using
the cecal artery for perfusion.Transplant Proc. 2009;41(9):3617-21,
http://dx.doi.org/10.1016/j.transproceed.2009.05.037.
http://dx.doi.org/10.1016/j.transproceed...
.
Ki-67 staining
To quantify intestinal cell proliferation, immunohistochemical staining for Ki-67 was performed on intestinal samples from the control group and the group of samples harvested at 60 min after I/R.
The monoclonal anti-Ki-67 antibody used was a mouse IgG1 antibody against a nuclear fraction of the L428 Hodgkin’s lymphoma cell line. The anti-Ki-67 antibody recognizes and reacts with Ki-67, which is a nuclear protein detected at all active stages of the cell cycle except for G0; therefore, it serves as a marker of cell proliferation.
After fixation with 10% formaldehyde, the tissues were embedded in paraffin, sliced into 5-μm-thick sections, deparaffinized, and hydrated. The sections were then processed for antigen recovery, blocking of endogenous peroxidase activity, and the immunohistochemical detection of Ki-67. For this purpose, the sections were incubated in the mouse monoclonal anti-rat Ki-67 antibody (clone MIB-5; DAKO) diluted 1:400. Staining was performed using the chromogenic substrate DAB (Sigma¯), followed by counterstaining with Harris hematoxylin, dehydration, and mounting.
Histomorphometric analysis
Histological analysis was performed using a Nikon¯ eclipse 50i microscope (Nikon Corporation Tokyo, Japan). Images were acquired using 10x ocular and 40x objective lenses. The full surface of the intestinal mucosa of each section was photographed for all of the slides. NIS-Elements BR¯ software controlled by an IBM computer was used to acquire digital color microscopic images. Histomorphometric analyses were performed using slides from the control group and the group of samples harvested at 60 min after reperfusion. To quantify the cells labeled in the TUNEL assay and by Ki-67 immunostaining, the digital images were analyzed using Image-Pro Plus 4.5 software (Media Cybernetics, Silver Spring, USA). TUNEL-positive cells were quantified by counting individual apoptotic cells in a defined area of interest, the size of which was automatically calculated by a previously calibrated computer system. Thus, the number of apoptotic cells per mm3 was obtained. A total of 47 mucosal fragments from six animals in the control group and 80 mucosal fragments from six animals in the 60-min after reperfusion group were measured.
A macro (computer routine) was developed for use with Image-Pro Plus software, for which the interval of positive labeling was predefined by an experienced pathologist to perform the automatic counting of Ki-67-positive areas that were dark brown in color, as well as to calculate the total villus/delimited crypt areas. These calculations allowed us to determine the proportions of Ki-67-positive areas in the villi and the crypts. A total of 390 Ki-67-positive villus areas were measured in six animals from the control group, and 652 were measured from six animals in the group of cecum samples harvested at 60 min after I/R.
Molecular methods
Extraction of total RNA and reverse transcription
The intestinal fragments that were freshly collected and immediately frozen in liquid nitrogen were stored at -170°C until processing to study the expression of the c-fos and c-jun genes in association with cell proliferation and apoptosis. First, total RNA was extracted from intestinal samples using TRIZOL™ reagent (Invitrogen, Carlsbad, CA, USA). Approximately 100 mg of intestine was fragmented in a tissue mill (Mikro Dismembrator U, Sartorius AG, Goettingen, Germany) after the addition of liquid nitrogen and was then homogenized in 700 μl of TRIZOL solution according to the manufacturer’s protocol.
The total RNA concentration was measured using a spectrophotometer (BioPhotometer, Eppendorf AG, Germany) at 260 nm, and RNA quality was assessed by calculating the 260 nm/280 nm absorbance ratio. One microgram of RNA was subjected to agarose gel electrophoresis to assess its integrity by visualizing the fragments corresponding to 18S and 28S ribosomal RNAs.
Complementary DNA (cDNA) was prepared from 2 μg of total RNA via reverse transcription using 200 U of SuperScript III RNase H-RT (Invitrogen) and oligo(dT)s as primers. The resulting cDNA solution was stored at -20°C.
Gene expression was evaluated by two methods, conventional semiquantitative RT-polymerase chain reaction (PCR) and real-time RT-PCR analyses, and the results of these two methods were compared.
PCR and semiquantitative analysis of PCR products
The specific primers for c-fos and c-jun used in the present study were
previously described by Shima et al. 44. ShimaY, TajiriT, TaguchiT, SuitaS. Increased expression of c-fos
and c-jun in the rat small intestinal epithelium after ischemia-reperfusion
injury: a possible correlation with the proliferation or apoptosis of intestinal
epithelial cells.J Pediatr Surg. 2006;41(4):830-6,
http://dx.doi.org/10.1016/j.jpedsurg.2005.12.025.
http://dx.doi.org/10.1016/j.jpedsurg.200...
. The β-actin gene
was amplified as an internal control of RNA transcription. The primer
sequences and the amplification conditions are described in Table 1. Double-distilled
water was used in place of cDNA as a negative control. The total PCR volume
of 25 μl included the following reagents: 3 μl of cDNA, 2 pmol
of each forward and reverse primer, 200 μM of each deoxynucleotide
(dATP, dCTP, dGTP, and dTTP), 2.5 μl of enzyme buffer, and 2 U of
Taq DNA polymerase. A separate reaction was prepared
for each investigated gene and was amplified according to the optimal
parameters for the given primers using the same thermocycler (MJ Research,
CA, USA). After an initial denaturation step at 95°C for 5 min, cycles
of denaturation at 95°C for 60 sec, annealing for 60 sec (Table 1), and extension at
72°C were performed, followed by a final extension step at 72°C
for 10 min. Each PCR reaction was repeated three times to ensure data
consistency. The amplified product (10 μl volume) was subjected to
electrophoresis in a 2% agarose gel.
Semiquantitative analysis of PCR products
The gel was viewed using a Kodak Gel Logic 100 Imaging System (Kodak, Rochester, NY, USA). The magnitude of the expression of each gene was assessed by band densitometry using Kodak Molecular Imaging Software. The amounts of the c-fos and c-jun PCR products were normalized to those of β-actin. The expression levels of the c-jun and c-fos genes were presented as the means ± standard deviation of the average relative band densities.
Real-time PCR and quantitative analysis of PCR products
The primers used to amplify the c-fos and β-actin genes were the same as those used in semiquantitative RT-PCR, but other primers were synthesized to amplify the c-jun gene because in real-time PCR, the reactions for the target gene and the housekeeping gene are performed together, and the same annealing temperature should be used for both reactions. In addition, a smaller amplicon is required. The primer sequences for the c-jun gene were as follows: 5’ caggtggcacagcttaaaca 3’ (forward); 5’ cgcaaccagtcaagttctca 3’ (reverse). The amplicon size was 162 base pairs.
Quantitative real-time PCR was performed in a 15.0 µL reaction mixture consisting of 7.5 µL of Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen, Carlsbad, CA), 0.3 µL each of gene-specific forward and reverse primers (10 µM), 1.0 µL of cDNA, and 5.9 µL of nuclease-free water using a Rotor-Gene Q 5plex HRM thermal cycler (Qiagen, Germany).
The cycling conditions were as follows: initial template denaturation at 95 °C for 1 min, followed by 40 cycles of denaturation at 95 °C for 20 seconds, annealing at 60 °C for 30 seconds, and extension at 72 °C for 30 seconds. Fluorescence detection was performed during each cycle at 72°C to identify the positive samples. Each sample was assessed in triplicate, and controls lacking the template were included in parallel for each reaction.
Amplification was followed by melting curve analysis to assess PCR product
specificity. The data were analyzed using the 2-ΔΔCT
method of relative quantification 1111. LivakKJ, SchmittgenTD. Analysis of relative gene expression data
using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method.Methods.
2001;25(4):402-8, http://dx.doi.org/10.1006/meth.2001.1262.
http://dx.doi.org/10.1006/meth.2001.1262...
.
Statistical analysis
To compare the gene expression data obtained via semiquantitative RT-PCR, one-way analysis of variance (ANOVA) was performed, and the Bonferroni test was applied for comparisons between the groups. The gene expression data obtained via real-time PCR were analyzed using the Kruskal-Wallis test and the post hoc Tukey test because they had a non-parametric distribution. The TUNEL and Ki-67 immunofluorescence data were analyzed using PASW Statistics Base v.15.0 for Windows (SPSS Inc.©). The Mann-Whitney U test was used to compare pairs of groups. The data were expressed as the medians (interquartile ranges). Significance was established at p<0.05.
RESULTS
Molecular studies
Semiquantitative and quantitative analysis of PCR products
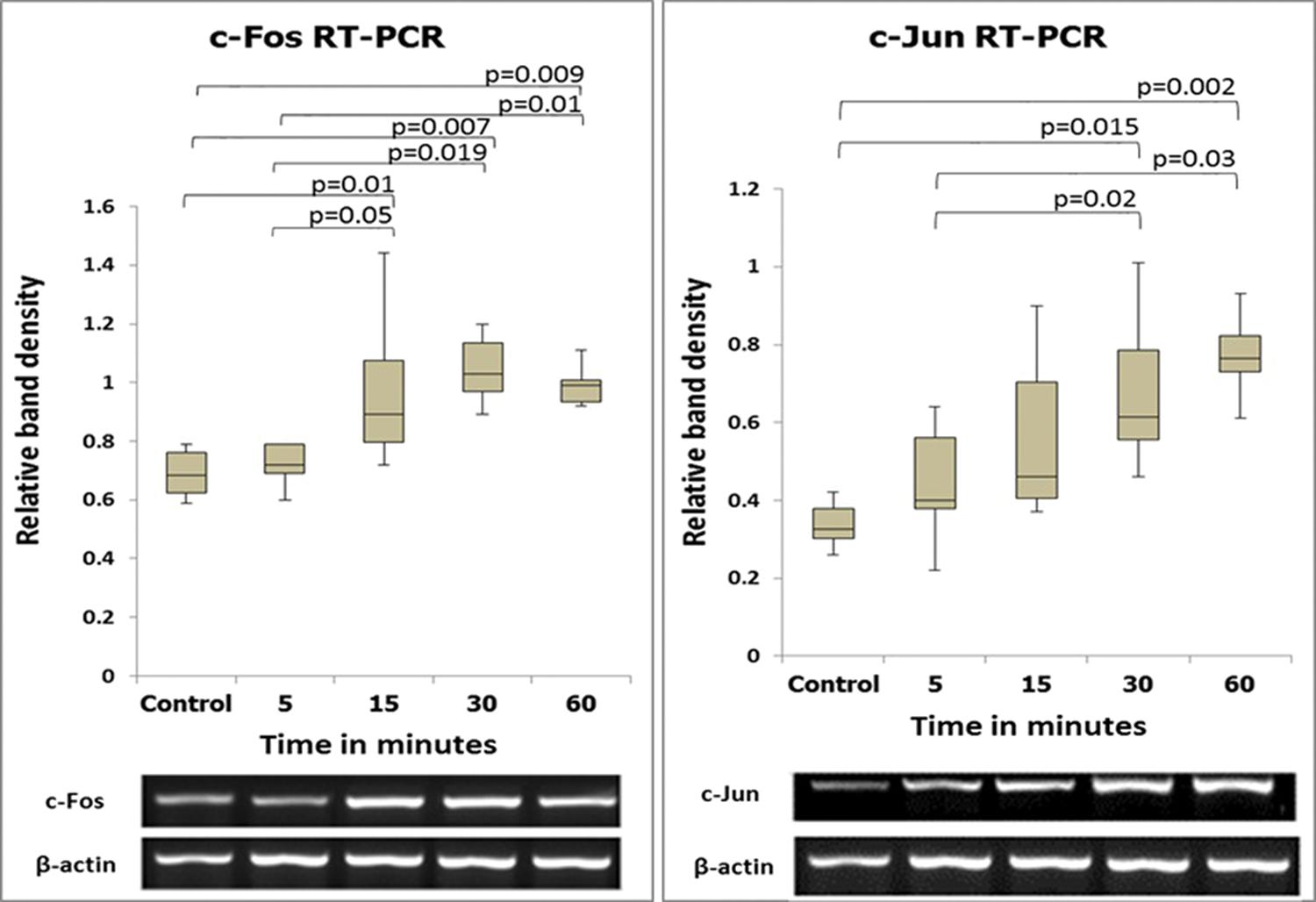
The expression levels of the c-fos and c-jun genes as determined by semiquantitative and quantitative analyses are shown in Figures 2 and 3. The expression levels of these two genes coincided, with increases beginning at 5 min after reperfusion.
The sequential expression patterns of c-fos and c-jun mRNA (semiquantitative method) and images of the respective bands. The numbers in the vertical axis correspond to the relative expression levels normalized to the internal control gene (β-actin).
The sequential expression patterns of c-fos and c-jun mRNA (quantitative method). Note that these results are similar to those obtained using the semiquantitative method.
Immunohistochemical analysis
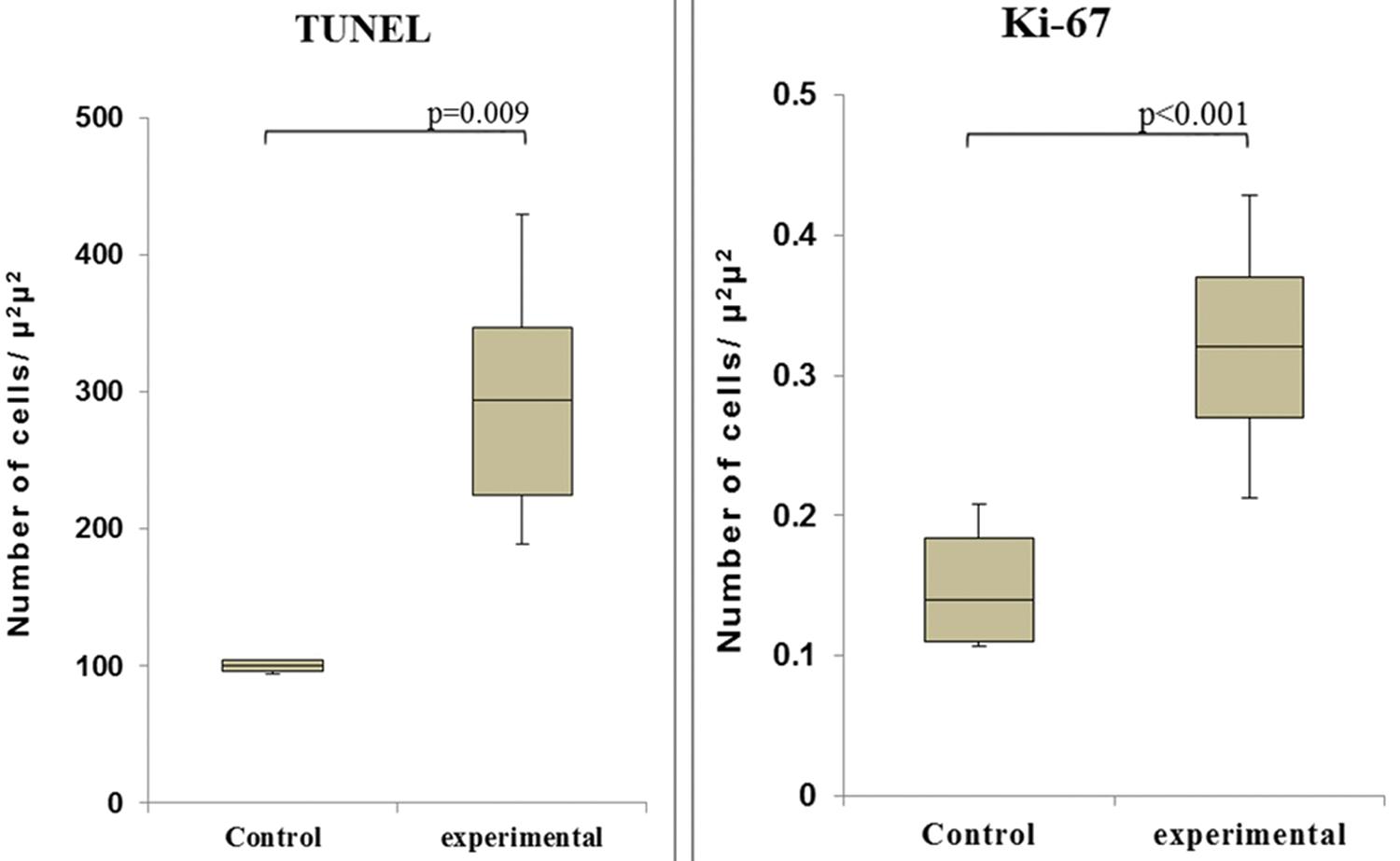
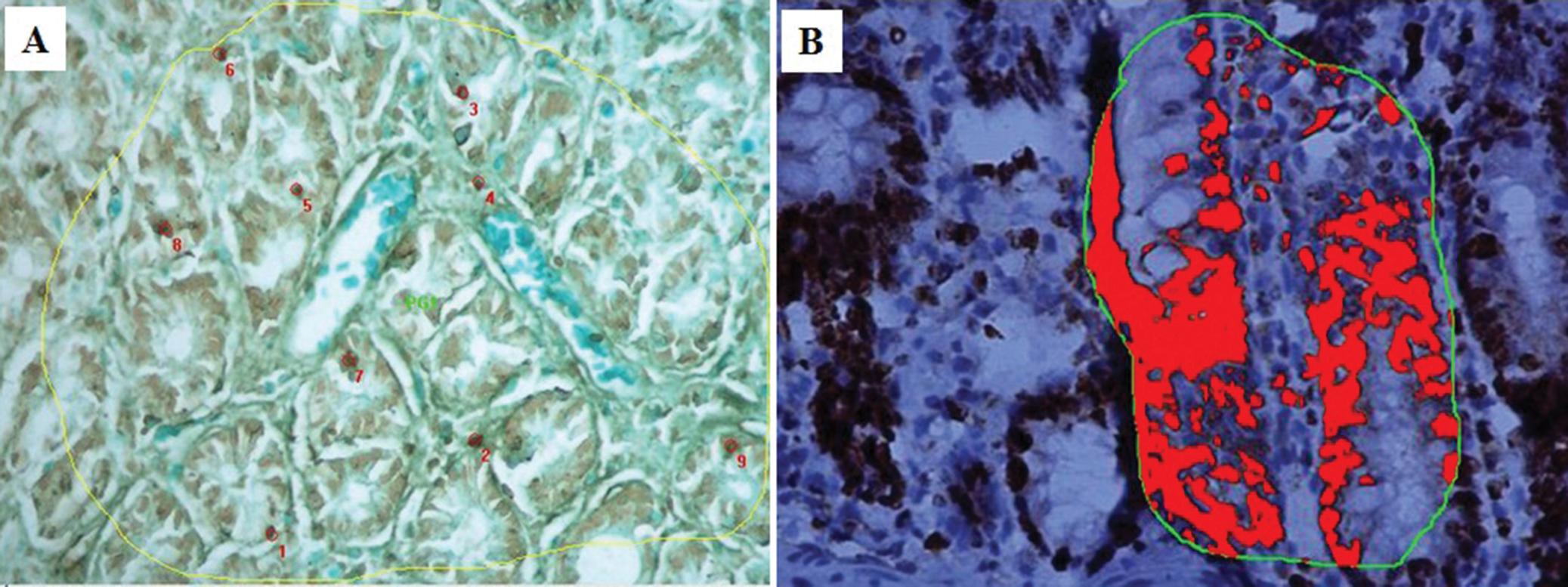
Observation of the TUNEL-stained sections under a microscope revealed the presence of dark brown apoptotic bodies, predominantly in the mucosa. Counting of the apoptotic bodies revealed that the number of apoptotic cells in the group of cecum samples harvested at 60 min after I/R was greater than that in the control group (p=0.009). In addition, the proportion of Ki-67-positive area was significantly higher in this experimental group than in the control group (p<0.001) (Figures 4 and 5).
The numbers of apoptotic cells (left - TUNEL assay) and proliferating cells (right - Ki-67 staining) in the control and I/R-injured groups (60 min after reperfusion).
Immunohistochemical analyses of TUNEL (A) and Ki-67 (B) staining at 60 min after reperfusion. Note the increased numbers of positively stained cells at this time point compared to the control.
DISCUSSION
In the present study, we investigated the expression of the IEGs c-fos and c-jun in a rat I/R model in which the intestine was perfused with lactated Ringer’s solution at 4°C. The intestine was kept cool in situ during ischemia. In addition, markers of cell proliferation and apoptosis were investigated in intestinal samples harvested at the time point of maximal IEG expression. Two analysis methods, semiquantitative and quantitative PCR, were utilized in the current investigation to provide a better estimation of the results. The expression levels of both genes coincided, in agreement with previous results of investigations performed in our laboratory 1212. GonçalvesJO, TannuriAC, CoelhoMC, BenditI, TannuriU. Dynamic expression of desmin, α-SMA and TGF-β1 during hepatic fibrogenesis induced by selective bile duct ligation in young rats. Braz J Med Biol Res.2014;47(10):850-7..
A previous study of IEGs in a pig liver I/R model during the acute stage, i.e., 1-3 h
after reperfusion, suggested that c-fos and c-jun
participate in both tissue regeneration and apoptosis 88. WielandE, OellerichM, BraunF, SchtuzE. c-fos and c-jun mRNA
expression in a pig liver model of ischemia/reperfusion: effect of extended cold
storage and the antioxidant idebenone.Clin Biochem2000;33(4):285-90,
http://dx.doi.org/10.1016/S0009-9120(00)00070-9.
http://dx.doi.org/10.1016/S0009-9120(00)...
. The same study showed that the duration of cold
ischemia for liver preservation and the levels of oxygen free radicals are
associated with the expression of IEGs 88. WielandE, OellerichM, BraunF, SchtuzE. c-fos and c-jun mRNA
expression in a pig liver model of ischemia/reperfusion: effect of extended cold
storage and the antioxidant idebenone.Clin Biochem2000;33(4):285-90,
http://dx.doi.org/10.1016/S0009-9120(00)00070-9.
http://dx.doi.org/10.1016/S0009-9120(00)...
. Taguchi et al. investigated the expression of c-fos
and c-jun in a rat model of intestinal transplantation. These
authors reported a lack of gene activity during the first 30 min after
transplantation. They found that at 4 h after transplantation,
c-fos only began to be expressed and c-jun
exhibited little activity. However, at 72 h after transplantation, the expression of
both genes was not detectable. These authors concluded that c-fos
and c-jun are associated with the adaptive process of intestinal
transplantation 1313. TaguchiT, FujiiY, NakaoM, OgitaK, ShimaY, SuitaS. Expression of
immediate-early genes c-fos and c-jun in small intestinal
transplantation.Transplant Proc. 2000;32(6):1279,
http://dx.doi.org/10.1016/S0041-1345(00)01224-0.
http://dx.doi.org/10.1016/S0041-1345(00)...
. The results of
our study are similar, showing that these two IEGs are expressed early. However, our
measurements were performed earlier and at shorter intervals within the first hour
after reperfusion. The c-fos and c-jun expression
levels in our samples began to increase at 5 min, and this alteration was maintained
at 60 min, in contrast to the observations of the aforementioned previous study.
Shima et al. studied the expression of c-fos and
c-jun in rat intestines after I/R 44. ShimaY, TajiriT, TaguchiT, SuitaS. Increased expression of c-fos
and c-jun in the rat small intestinal epithelium after ischemia-reperfusion
injury: a possible correlation with the proliferation or apoptosis of intestinal
epithelial cells.J Pediatr Surg. 2006;41(4):830-6,
http://dx.doi.org/10.1016/j.jpedsurg.2005.12.025.
http://dx.doi.org/10.1016/j.jpedsurg.200...
and found that apoptosis and cell proliferation
depend on their expression, similar to the conclusions of other studies 8. WielandE, OellerichM, BraunF, SchtuzE. c-fos and c-jun mRNA
expression in a pig liver model of ischemia/reperfusion: effect of extended cold
storage and the antioxidant idebenone.Clin Biochem2000;33(4):285-90,
http://dx.doi.org/10.1016/S0009-9120(00)00070-9.
http://dx.doi.org/10.1016/S0009-9120(00)...
8,1414. OnishiI, ShimizuK, TaniT, HashimotoT, MiwaK. JNK activation and
apoptosis during ischemia-reperfusion.Transplant Proc. 1999;31(1-2):1077-9,
http://dx.doi.org/10.1016/S0041-1345(98)01912-5.
http://dx.doi.org/10.1016/S0041-1345(98)...
. The authors further suggested that the joint expression of these
IEGs results in the induction of cell proliferation and apoptosis after I/R. Shima
et al. proposed a more specific correlation between the roles of
c-fos and c-jun after I/R in which
c-fos induces cell proliferation and c-jun is
a key factor involved in apoptosis 44. ShimaY, TajiriT, TaguchiT, SuitaS. Increased expression of c-fos
and c-jun in the rat small intestinal epithelium after ischemia-reperfusion
injury: a possible correlation with the proliferation or apoptosis of intestinal
epithelial cells.J Pediatr Surg. 2006;41(4):830-6,
http://dx.doi.org/10.1016/j.jpedsurg.2005.12.025.
http://dx.doi.org/10.1016/j.jpedsurg.200...
. In the present study, the early expression of c-fos
and c-jun indicated that these inducers of apoptosis and cell
proliferation had been activated by I/R. We sought to understand and to establish
the correlation between these genetic phenomena and the immunohistochemical markers
of apoptosis (by TUNEL staining) and cell proliferation (by Ki-67 staining).
Immunohistochemical analysis via the TUNEL assay revealed a statistically significant
increase in the number of apoptotic cells at 60 min after I/R in the I/R-injured
cecum compared with the control cecum, indicating that the induction of apoptosis,
but not necrosis, occurred early 1515. LiY, AritaY, KooHC, DavisJM, KazzazJA. Inhibition of c-jun
N-terminal kinase pathway improves cell viability in response to oxidant
injury.Am J Respir Cell Mol Biol2003;29(6):779-83,
http://dx.doi.org/10.1165/rcmb.2003-0087RC.
http://dx.doi.org/10.1165/rcmb.2003-0087...
. This finding is in agreement with reports in the literature showing
that apoptotic cells appear several minutes after stimulation and that the full
sequence of apoptosis is completed within 1-4 h 1616. NodaT, IwakiriR, FujimotoK, MatsuoS, AwTY. Programmed cell death
induced by ischemia-reperfusion in rat intestinal mucosa.Am J Physiol.
1998;274(2 Pt 1):G270-6.. We also found a statistically significant
difference in the number of proliferating cells at 60 min after reperfusion, as
demonstrated by the increased expression of Ki-67. Thus, the IEGs
c-fos and c-jun together may be responsible
for cell proliferation and apoptosis. The major questions that remain to be answered
concern the exact time at which these phenomena occur and whether it is possible to
control apoptosis to improve the preservation of intestinal grafts. Our results
showed that cold graft preservation was not sufficiently protective because the IEGs
were induced quite early after reperfusion. Oxygen free radicals are likely an
important trigger of the expression of IEGs, and these species may also play
definitive roles in cold ischemic graft preservation; however, these possibilities
require further study. In conclusion, the early activation of the
c-fos and c-jun genes occurred after
intestinal I/R injury, and these genes together can trigger cell proliferation and
apoptosis.
REFERENCES
-
1HaglundU. Gut ischaemia. Gut. 1994;35(1 Suppl):S73-6.
-
2SantosMM, TannuriU, TannuriAC, CoelhoMC. Description of a new model of intestinal denervation and in?situ ischemia-reperfusion injury using the cecal artery for perfusion.Transplant Proc. 2009;41(9):3617-21, http://dx.doi.org/10.1016/j.transproceed.2009.05.037.
» http://dx.doi.org/10.1016/j.transproceed.2009.05.037 -
3IkedaH, SuzukiY, SuzukiM, KoikeM, TamuraJ, TongJ, et al. Apoptosis is a major mode of cell death caused by ischaemia and ischaemia/reperfusion injury to the rat intestinal epithelium.Gut. 1998;42(4):530-7, http://dx.doi.org/10.1136/gut.42.4.530.
» http://dx.doi.org/10.1136/gut.42.4.530 -
4ShimaY, TajiriT, TaguchiT, SuitaS. Increased expression of c-fos and c-jun in the rat small intestinal epithelium after ischemia-reperfusion injury: a possible correlation with the proliferation or apoptosis of intestinal epithelial cells.J Pediatr Surg. 2006;41(4):830-6, http://dx.doi.org/10.1016/j.jpedsurg.2005.12.025.
» http://dx.doi.org/10.1016/j.jpedsurg.2005.12.025 -
5HuppertzB, FrankHG, KaufmannP. The apoptosis cascade--morphological and immunohistochemical methods for its visualization.Anat Embryol (Berl). 1999;200(1):1-18.
-
6ShahKA, ShureyS, GreenCJ. Characterization of apoptosis in intestinal ischaemia-reperfusion injury--a light and electron microscopic study.Int J Exp Pathol. 1997;78(5):355-63, http://dx.doi.org/10.1046/j.1365-2613.1997.420369.x.
» http://dx.doi.org/10.1046/j.1365-2613.1997.420369.x -
7PasternakBA, CollinsMH, TiaoGM, RudolphJA, AlonsoMH, RyckmanFC, et al. Anatomic and histologic variability of epithelial apoptosis in small bowel transplants.Pediatr Transplant2010;14(1):72-6, http://dx.doi.org/10.1111/ptr.2009.14.issue-1.
» http://dx.doi.org/10.1111/ptr.2009.14.issue-1 -
8WielandE, OellerichM, BraunF, SchtuzE. c-fos and c-jun mRNA expression in a pig liver model of ischemia/reperfusion: effect of extended cold storage and the antioxidant idebenone.Clin Biochem2000;33(4):285-90, http://dx.doi.org/10.1016/S0009-9120(00)00070-9.
» http://dx.doi.org/10.1016/S0009-9120(00)00070-9 -
9HassounHT, FischerUM, AttuwaybiBO, MooreFA, SafiHJ, AllenSJ, et al. Regional hypothermia reduces mucosal NFkappaB and PMN priming via gut lymph during canine mesenteric ischemia/reperfusion.J Surg Res. 2003;115(1):121-6, http://dx.doi.org/10.1016/S0022-4804(03)00298-1.
» http://dx.doi.org/10.1016/S0022-4804(03)00298-1 -
10AttuwaybiBO, HassounHT, ZouL, KozarRA, KoneBC, WeisbrodtNW, et al. Hypothermia protects against gut ischemia/reperfusion-induced impaired intestinal transit by inducing heme oxygenase-1.J Surg Res. 2003;115(1):48-55, http://dx.doi.org/10.1016/S0022-4804(03)00313-5.
» http://dx.doi.org/10.1016/S0022-4804(03)00313-5 -
11LivakKJ, SchmittgenTD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method.Methods. 2001;25(4):402-8, http://dx.doi.org/10.1006/meth.2001.1262.
» http://dx.doi.org/10.1006/meth.2001.1262 -
12GonçalvesJO, TannuriAC, CoelhoMC, BenditI, TannuriU. Dynamic expression of desmin, α-SMA and TGF-β1 during hepatic fibrogenesis induced by selective bile duct ligation in young rats. Braz J Med Biol Res.2014;47(10):850-7.
-
13TaguchiT, FujiiY, NakaoM, OgitaK, ShimaY, SuitaS. Expression of immediate-early genes c-fos and c-jun in small intestinal transplantation.Transplant Proc. 2000;32(6):1279, http://dx.doi.org/10.1016/S0041-1345(00)01224-0.
» http://dx.doi.org/10.1016/S0041-1345(00)01224-0 -
14OnishiI, ShimizuK, TaniT, HashimotoT, MiwaK. JNK activation and apoptosis during ischemia-reperfusion.Transplant Proc. 1999;31(1-2):1077-9, http://dx.doi.org/10.1016/S0041-1345(98)01912-5.
» http://dx.doi.org/10.1016/S0041-1345(98)01912-5 -
15LiY, AritaY, KooHC, DavisJM, KazzazJA. Inhibition of c-jun N-terminal kinase pathway improves cell viability in response to oxidant injury.Am J Respir Cell Mol Biol2003;29(6):779-83, http://dx.doi.org/10.1165/rcmb.2003-0087RC.
» http://dx.doi.org/10.1165/rcmb.2003-0087RC -
16NodaT, IwakiriR, FujimotoK, MatsuoS, AwTY. Programmed cell death induced by ischemia-reperfusion in rat intestinal mucosa.Am J Physiol. 1998;274(2 Pt 1):G270-6.
Publication Dates
-
Publication in this collection
May 2015
History
-
Received
3 Dec 2014 -
Reviewed
28 Jan 2015 -
Accepted
6 Feb 2015