Abstract
OBJECTIVES:
We aimed to evaluate the incidence of pancreatic alterations in Crohn's disease using endoscopic ultrasound (EUS) and to correlate the number of alterations with current clinical data.
METHODS:
Patients diagnosed with Crohn's disease (n=51) were examined using EUS, and 11 variables were analyzed. A control group consisted of patients with no history of pancreatic disease or Crohn's disease. Patients presenting with three or more alterations underwent magnetic resonance imaging (MRI). Pancreatic function was determined using a fecal elastase assay.
RESULTS:
Two of the 51 patients (3.9%) presented with four EUS alterations, 3 (5.9%) presented with three, 11 (21.5%) presented with two, and 13 (25.5%) presented with one; in the control group, only 16% presented with one EUS alteration (p<0.001). Parenchymal abnormalities accounted for 39 of the EUS findings, and ductal abnormalities accounted for 11. Pancreatic lesions were not detected by MRI. Low fecal elastase levels were observed in 4 patients, none of whom presented with significant pancreatic alterations after undergoing EUS. Ileal involvement was predictive of the number of EUS alterations.
CONCLUSION:
A higher incidence of pancreatic abnormalities was found in patients with Crohn's disease than in individuals in the control group. The majority of these abnormalities are related to parenchymal alterations. In this group of patients, future studies should be conducted to determine whether such morphological abnormalities could evolve to induce exocrine or endocrine pancreatic insufficiency and, if so, identify the risk factors and determine which patients should undergo EUS.
KEYWORDS:
Crohn's Disease; Pancreatitis; Pancreatic Exocrine Insufficiency; Endosonography; Cholangiopancreatography
INTRODUCTION
Chronic pancreatic involvement in patients diagnosed with Crohn's disease (CD) has been the focus of studies in recent years (11. Dobrilla G. Regional enteritis and pancreatitis. Gastroenterology. 1972;63(4):713-4.
2. Axon AT, Ashton MG, Lintott DJ. Chronic pancreatitis and inflammatory bowel disease. Clin Radiol. 1979;30(2):179-82. https://doi.org/10.1016/S0009-9260(79)80146-4
https://doi.org/10.1016/S0009-9260(79)80...
3. Seyrig JA, Jian R, Modigliani R, Golfain D, Florent C, Messing B, et al. Idiopathic pancreatitis associated with inflammatory bowel disease. Dig Dis Sci. 1985;30(12):1121-6. https://doi.org/10.1007/BF01314044
https://doi.org/10.1007/BF01314044...
4. Eisner TD, Goldman IS, McKinley MJ. Crohn's disease and pancreatitis. Am J Gastroenterol. 1993;88(4):583-6.
5. Angelini G, Cavallini G, Bovo P, Brocco G, Castagnini A, Lavarini E, et al. Pancreatic function in chronic inflammatory bowel disease. Int J Pancreatol. 1988;3(2-3):185-93. https://doi.org/10.1007/BF02798930
https://doi.org/10.1007/BF02798930...
6. Hegnhøj J, Hansen CP, Rannem T, Søbirk H, Andersen LB, Andersen JR. Pancreatic function in Crohn's disease. Gut. 1990 Sep;31(9):1076-9.-77. Barthet M, Lesavre N, Desplats S, Panuel M, Gasmi M, Bernard JP, et al. Frequency and characteristics of pancreatitis in patients with inflammatory bowel disease. Pancreatology. 2006;6(5):464-71. https://doi.org/10.1159/000094564
https://doi.org/10.1159/000094564...
). Medications, primary sclerosing cholangitis, inflammatory involvement of the duodenum and autoimmune pancreatitis (AIP) are all well-established causes of pancreatitis in this population (88. Piontek M, Hengels KJ, Strohmeyer G. Crohn's disease: what about the pancreas? J Clin Gastroenterol. 1990;12(5):491-3.
9. Rasmussen HH, Fonager K, Sørensen HT, Pedersen L, Dahlerup JF, Steffensen FH. Risk of acute pancreatitis in patients with chronic inflammatory bowel disease. A Danish 16-year nationwide follow-up study. Scand J Gastroenterol. 1999;34(2):199-201. https://doi.org/10.1080/00365529950173096
https://doi.org/10.1080/0036552995017309...
10. Pitchumoni CS, Rubin A, Das K. Pancreatitis in inflammatory bowel diseases. J Clin Gastroenterol. 2010;44(4):246-53. https://doi.org/10.1097/MCG.0b013e3181cadbe1
https://doi.org/10.1097/MCG.0b013e3181ca...
11. Pitchumoni CS, Chari S. Ulcerative colitis and autoimmune pancreatitis. J Clin Gastroenterol. 2013;47(6):469. https://doi.org/10.1097/MCG.0b013e31828a7099
https://doi.org/10.1097/MCG.0b013e31828a...
-1212. Ravi K, Chari ST, Vege SS, Sandborn WJ, Smyrk TC, Loftus EV Jr. Inflammatory bowel disease in the setting of autoimmune pancreatitis. Inflamm Bowel Dis. 2009;15(9):1326-30. https://doi.org/10.1002/ibd.20898
https://doi.org/10.1002/ibd.20898...
). Studies on this topic are limited and extremely heterogeneous, mainly because there is no established consensus method to study the pancreas. The prevalence of this combination (CD and pancreatitis) ranges from 1.2% to 58% depending on the population studied and the method of pancreatic evaluation employed (55. Angelini G, Cavallini G, Bovo P, Brocco G, Castagnini A, Lavarini E, et al. Pancreatic function in chronic inflammatory bowel disease. Int J Pancreatol. 1988;3(2-3):185-93. https://doi.org/10.1007/BF02798930
https://doi.org/10.1007/BF02798930...
,66. Hegnhøj J, Hansen CP, Rannem T, Søbirk H, Andersen LB, Andersen JR. Pancreatic function in Crohn's disease. Gut. 1990 Sep;31(9):1076-9.,1313. Niemelä S, Lehtola J, Karttunen T, Lähde S. Pancreatitis in patients with chronic inflammatory bowel disease. Hepatogastroenterology. 1989;36(3):175-7.
14. Barthet M, Hastier P, Bernard JP, Bordes G, Frederick J, Allio S, et al. Chronic pancreatitis and inflammatory bowel disease: true or coincidental association? Am J Gastroenterol. 1999;94(8):2141-8. https://doi.org/10.1111/j.1572-0241.1999.01287.x
https://doi.org/10.1111/j.1572-0241.1999...
15. Chapin LE, Scudamore HH, Baggenstoss AH, Bargen JA. Regional enteritis: associated visceral changes. Gastroenterology. 1956;30(3):404-15. https://doi.org/10.1016/S0016-5085(56)80104-2
https://doi.org/10.1016/S0016-5085(56)80...
16. Heikius B, Niemelä S, Lehtola J, Karttunen T, Lähde S. Pancreatic duct abnormalities and pancreatic function in patients with chronic inflammatory bowel disease. Scand J Gastroenterol. 1996;31(5):517-23. https://doi.org/10.3109/00365529609006775
https://doi.org/10.3109/0036552960900677...
17. Seibold F, Scheurlen M, Müller A, Jenss H, Weber P. Impaired pancreatic function in patients with Crohn's disease with and without pancreatic autoantibodies. J Clin Gastroenterol. 1996;22(3):202-6. https://doi.org/10.1097/00004836-199604000-00010
https://doi.org/10.1097/00004836-1996040...
-1818. Oishi Y, Yao T, Matsui T, Ueki T, Sakurai T, Sakaguchi S. Abnormal pancreatic imaging in Crohn's disease: prevalence and clinical features. J Gastroenterol. 2004;39(1):26-33. https://doi.org/10.1007/s00535-003-1241-5
https://doi.org/10.1007/s00535-003-1241-...
).
Postmortem studies of patients diagnosed with CD that did not present with pancreatic symptoms have found pancreatic fibrosis (38% of cases) distributed in the interlobular and periductal areas, as well as acinar dilation (31% of cases) (1515. Chapin LE, Scudamore HH, Baggenstoss AH, Bargen JA. Regional enteritis: associated visceral changes. Gastroenterology. 1956;30(3):404-15. https://doi.org/10.1016/S0016-5085(56)80104-2
https://doi.org/10.1016/S0016-5085(56)80...
,1919. Ball WP, Baggenstoss AH, Bargen JA. Pancreatic lesions associated with chronic ulcerative colitis. Arch Pathol (Chic). 1950;50(3):347-58.). Pancreatic lesions are believed to result from the formation of immunocomplexes and autoantibodies against the pancreas, which are known as glycoprotein 2 (GP2) –specific pancreatic autoantibodies (PAB) (11. Dobrilla G. Regional enteritis and pancreatitis. Gastroenterology. 1972;63(4):713-4.,99. Rasmussen HH, Fonager K, Sørensen HT, Pedersen L, Dahlerup JF, Steffensen FH. Risk of acute pancreatitis in patients with chronic inflammatory bowel disease. A Danish 16-year nationwide follow-up study. Scand J Gastroenterol. 1999;34(2):199-201. https://doi.org/10.1080/00365529950173096
https://doi.org/10.1080/0036552995017309...
,2020. Ectors N, Maillet B, Aerts R, Geboes K, Donner A, Borchard F, et al. Non-alcoholic duct destructive chronic pancreatitis. Gut. 1997;41(2):263-8. https://doi.org/10.1136/gut.44.4.579
https://doi.org/10.1136/gut.44.4.579...
,2121. Pavlidis P, Romanidou O, Roggenbuck D, Mytilinaiou MG, Al-Sulttan F, Liaskos C, et al. Ileal inflammation may trigger the development of GP2-specific pancreatic autoantibodies in patients with Crohn's disease. Clin Dev Immunol. 2012;2012:640835. https://doi.org/10.1155/2012/640835
https://doi.org/10.1155/2012/640835...
). Furthermore, the formation of epithelioid granulomas has been described in patients with CD (1414. Barthet M, Hastier P, Bernard JP, Bordes G, Frederick J, Allio S, et al. Chronic pancreatitis and inflammatory bowel disease: true or coincidental association? Am J Gastroenterol. 1999;94(8):2141-8. https://doi.org/10.1111/j.1572-0241.1999.01287.x
https://doi.org/10.1111/j.1572-0241.1999...
,2222. Gschwantler M, Kogelbauer G, Klose W, Bibus B, Tscholakoff D, Weiss W. The pancreas as a site of granulomatous inflammation in Crohn's disease. Gastroenterology. 1995;108(4):1246-9. https://doi.org/10.1016/0016-5085(95)90226-0
https://doi.org/10.1016/0016-5085(95)902...
). Epithelial cells of the gastrointestinal tract and pancreatic tissue may share similar target molecular or cellular structures vulnerable to injuries (2323. Martinelli M, Strisciuglio C, Illiceto MT, Cardile S, Guariso G, Vignola S, et al. Natural history of pancreatic involvement in paediatric inflammatory bowel disease. Dig Liver Dis. 2015;47(5):384-9. https://doi.org/10.1016/j.dld.2015.01.155
https://doi.org/10.1016/j.dld.2015.01.15...
), as observed in animal studies where there was overexpression of abnormal proinflammatory hypoglycosylated mucinin, both in the colonic epithelium of patients with inflammatory bowel disease (IBD) and in the pancreatic ductal epithelium (2424. Trikudanathan G, Navaneethan U. Association of pancreatitis with inflammatory bowel disease. J Clin Gastroenterol. 2011;45(1):83. https//doi.org/10.1097/MCG.0b013e3181d9f935
https//doi.org/10.1097/MCG.0b013e3181d9f...
).
Endoscopic retrograde cholangiopancreatography (ERCP) is considered the gold standard for the study of pancreatic morphology. However, in addition to morbidity risks, this method evaluates only the pancreatic ducts, providing no information on the conditions of the pancreatic parenchyma (2525. Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120(3):682-707. https://doi.org/10.1053/gast.2001.22586
https://doi.org/10.1053/gast.2001.22586...
,2626. Owiang C. Chronic pancreatitis. In: Yamada T. Textbook of gastroenterology. Philadelphia: Lippincott Willians & Wilkins; 1999. Vol 2, p. 2151-77.). Endoscopic ultrasound (EUS) is more sensitive, is safer, evaluates both the parenchyma and the pancreatic ducts and correlates strongly with histological findings (2727. Miyakawa H, Suga T, Okamura K. Usefulness of endoscopic ultrasonography for the diagnosis of chronic pancreatitis. J Gastroenterol. 2007;42 Suppl 17:85-9. https://doi.org/10.1007/s00535-006-1935-6
https://doi.org/10.1007/s00535-006-1935-...
28. Catalano MF. Diagnosing early-stage chronic pancreatitis: is endoscopic ultrasound a reliable modality? J Gastroenterol. 2007;42 Suppl 17:78-84. https://doi.org/10.1007/s00535-006-1915-x
https://doi.org/10.1007/s00535-006-1915-...
29. Raimondo M, Wallace MB. Diagnosis of early chronic pancreatitis by endoscopic ultrasound. Are we there yet? JOP. 2004;5(1):1-7.
30. Wiersema MJ, Hawes RH, Lehman GA, Kochman ML, Sherman S, Kopecky KK. Prospective evaluation of endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in patients with chronic abdominal pain of suspected pancreatic origin. Endoscopy. 1993;25(9):555-64. https://doi.org/10.1055/s-2007-1010405
https://doi.org/10.1055/s-2007-1010405...
31. Irisawa A, Katakura K, Ohira H, Sato A, Bhutani MS, Hernandez LV, et al. Usefulness of endoscopic ultrasound to diagnose the severity of chronic pancreatitis. J Gastroenterol. 2007;42 Suppl 17:90-4. https://doi.org/10.1007/s00535-006-1916-9
https://doi.org/10.1007/s00535-006-1916-...
32. Hollerbach S, Klamann A, Topalidis T, Schmiegel WH. Endoscopic ultrasonography (EUS) and fine-needle aspiration (FNA) cytology for diagnosis of chronic pancreatitis. Endoscopy. 2001;33(10):824-31. https://doi.org/10.1055/s-2001-17337
https://doi.org/10.1055/s-2001-17337...
-3333. Jung JG, Lee JK, Lee KH, Lee KT, Woo YS, Paik WH, et al. Comparison of endoscopic retrograde cholangiopancreatography with papillary biopsy and endoscopic ultrasound-guided pancreatic biopsy in the diagnosis of autoimmune pancreatitis. Pancreatology. 2015;15(3):259-64. https://doi.org/10.1016/j.pan.2015.03.011
https://doi.org/10.1016/j.pan.2015.03.01...
).
Although there is considerable agreement between EUS and ERCP when the EUS results are either normal or when they reveal moderate to severe pancreatitis, many patients have presented with mild EUS alterations but have normal ERCP results (3434. Sahai AV, Zimmerman M, Aabakken L, Tarnasky PR, Cunningham JT, van Velse A, et al. Prospective assessment of the ability of endoscopic ultrasound to diagnose, exclude, or establish the severity of chronic pancreatitis found by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc. 1998;48(1):18-25. https://doi.org/10.1016/S0016-5107(98)70123-3
https://doi.org/10.1016/S0016-5107(98)70...
35. Catalano MF, Lahoti S, Geenen JE, Hogan WJ. Prospective evaluation of endoscopic ultrasonography, endoscopic retrograde pancreatography, and secretin test in the diagnosis of chronic pancreatitis. Gastrointest Endosc. 1998;48(1):11-7. https://doi.org/10.1016/S0016-5107(98)70122-1 · Source: PubMed
https://doi.org/10.1016/S0016-5107(98)70...
36. Buscail L, Escourrou J, Moreau J, Delvaux M, Louvel D, Lapeyre F, et al. Endoscopic ultrasonography in chronic pancreatitis: a comparative prospective study with conventional ultrasonography, computed tomography, and ERCP. Pancreas. 1995;10(3):251-7.-3737. Nattermann C, Goldschmidt AJ, Dancygier H. Endosonography in chronic pancreatitis - A comparison between endoscopic retrograde pancreatography and endoscopic ultrasonography. Endoscopy. 1993;25(9):565-70. https://doi.org/10.1055/s-2007-1010406
https://doi.org/10.1055/s-2007-1010406...
). Elderly patients, obese patients, and patients with diabetes mellitus, as well as patients who consume excessive amounts of alcohol, present with EUS alterations in the pancreas that are unrelated to disease. With the exception of these patients, studies have hypothesized that patients presenting with altered EUS but normal ERCP results are suffering from pancreatitis in its initial stages because, pathophysiologically, parenchymatous alterations precede ductal alterations in the necrosis-fibrosis sequence in the development of chronic pancreatitis (3838. Glasbrenner B, Kahl S, Malfertheiner P. Modern diagnostics of chronic pancreatitis. Eur J Gastroenterol Hepatol. 2002;14(9):935-41. https://doi.org/10.1097/00042737-200209000-00003
https://doi.org/10.1097/00042737-2002090...
). To increase the specificity of EUS, some authors have suggested that the pancreas should be considered normal if only one or two endoscopic criteria are met, whereas a pancreas displaying three or more of these criteria should be considered abnormal (3030. Wiersema MJ, Hawes RH, Lehman GA, Kochman ML, Sherman S, Kopecky KK. Prospective evaluation of endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in patients with chronic abdominal pain of suspected pancreatic origin. Endoscopy. 1993;25(9):555-64. https://doi.org/10.1055/s-2007-1010405
https://doi.org/10.1055/s-2007-1010405...
,3131. Irisawa A, Katakura K, Ohira H, Sato A, Bhutani MS, Hernandez LV, et al. Usefulness of endoscopic ultrasound to diagnose the severity of chronic pancreatitis. J Gastroenterol. 2007;42 Suppl 17:90-4. https://doi.org/10.1007/s00535-006-1916-9
https://doi.org/10.1007/s00535-006-1916-...
,3434. Sahai AV, Zimmerman M, Aabakken L, Tarnasky PR, Cunningham JT, van Velse A, et al. Prospective assessment of the ability of endoscopic ultrasound to diagnose, exclude, or establish the severity of chronic pancreatitis found by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc. 1998;48(1):18-25. https://doi.org/10.1016/S0016-5107(98)70123-3
https://doi.org/10.1016/S0016-5107(98)70...
,3939. Hawes RH. Comparison of diagnostic modalities: EUS, ERCP, and fluid analysis. Gastrointest Endosc. 1999;49(3 Suppl):S74-6. https://doi.org/10.1016/S0016-5107(99)70531-6
https://doi.org/10.1016/S0016-5107(99)70...
).
Exocrine pancreatic insufficiency has been reported in IBD in 18 to 80% of cases, as measured by the fecal elastase, paraminobenzoic acid (PABA), amylase, lipase meal and secretin-cerulein tests. (77. Barthet M, Lesavre N, Desplats S, Panuel M, Gasmi M, Bernard JP, et al. Frequency and characteristics of pancreatitis in patients with inflammatory bowel disease. Pancreatology. 2006;6(5):464-71. https://doi.org/10.1159/000094564
https://doi.org/10.1159/000094564...
,1414. Barthet M, Hastier P, Bernard JP, Bordes G, Frederick J, Allio S, et al. Chronic pancreatitis and inflammatory bowel disease: true or coincidental association? Am J Gastroenterol. 1999;94(8):2141-8. https://doi.org/10.1111/j.1572-0241.1999.01287.x
https://doi.org/10.1111/j.1572-0241.1999...
,4040. Bokemeyer B. Asymptomatic elevation of serum lipase and amylase in conjunction with Crohn's disease and ulcerative colitis. Z Gastroenterol. 2002;40(1):5-10. https://doi.org/10.1055/s-2002-19636
https://doi.org/10.1055/s-2002-19636...
). Fecal elastase is considered the gold standard among noninvasive tests of pancreatic function (4141. Lankisch PG. The problem of diagnosing chronic pancreatitis. Dig Liver Dis. 2003;35(3):131-4. https://doi.org/10.1016/S1590-8658(03)00019-7
https://doi.org/10.1016/S1590-8658(03)00...
). Although the results obtained by this method are in agreement with the tests of pancreatic function that use duodenal intubation (4242. Chowdhury RS, Forsmark CE. Review article: Pancreatic function testing. Aliment Pharmacol Ther. 2003;17(6):733-50.), it can yield false positives for intestinal diseases (because of dilution and consequent reduction in enzymatic concentration) and displays low sensitivity to mild or moderate pancreatitis (3838. Glasbrenner B, Kahl S, Malfertheiner P. Modern diagnostics of chronic pancreatitis. Eur J Gastroenterol Hepatol. 2002;14(9):935-41. https://doi.org/10.1097/00042737-200209000-00003
https://doi.org/10.1097/00042737-2002090...
,4141. Lankisch PG. The problem of diagnosing chronic pancreatitis. Dig Liver Dis. 2003;35(3):131-4. https://doi.org/10.1016/S1590-8658(03)00019-7
https://doi.org/10.1016/S1590-8658(03)00...
,4343. Löser C, Möllgaard A, Fölsch UR. Faecal elastase 1: a novel, highly sensitive, and specific tubeless pancreatic function test. Gut. 1996;39(4):580-6.
44. Lankisch PG, Schmidt I, König H, Lehnick D, Knollmann R, Löhr M, et al. Faecal elastase 1: not helpful in diagnosing chronic pancreatitis associated with mild to moderate exocrine pancreatic insufficiency. Gut. 1998;42(4):551-4. https://doi.org/10.1136/gut.42.4.551
https://doi.org/10.1136/gut.42.4.551...
45. Gullo L, Ventrucci M, Tomassetti P, Migliori M, Pezzilli R. Fecal elastase 1 determination in chronic pancreatitis. Dig Dis Sci. 1999;44(1):210-3. https://doi.org/10.1023/A:1026691209094
https://doi.org/10.1023/A:1026691209094...
-4646. Hardt PD, Marzeion AM, Schnell-Kretschmer H, Wüsten O, Nalop J, Zekorn T, et al. Fecal elastase 1 measurement compared with endoscopic retrograde cholangiopancreatography for the diagnosis of chronic pancreatitis. Pancreas. 2002;25(1):e6-9. https://doi.org/10.1097/00006676-200207000-00004
https://doi.org/10.1097/00006676-2002070...
).
In the present study, we sought to evaluate the incidence of pancreatic alterations identified using EUS in patients with established CD and correlate these results with factors such as duration of the disease, disease site, medication use, and level of disease activity. To correlate these morphological alterations with pancreatic exocrine function, we decided to measure fecal elastase levels. We also compared the EUS findings with magnetic resonance cholangiography (MRCP) findings. To the best of our knowledge, this study is the first to analyze pancreatic EUS abnormalities in CD patients, which is an important tool to detect preliminary alterations of the pancreas.
MATERIALS AND METHODS
Fifty-one patients between 18 and 60 years of age who were diagnosed with CD on the basis of clinical, radiological, endoscopic, or anatomopathological criteria were selected to participate in this prospective study.
Patients previously diagnosed with pancreatitis, sclerosing cholangitis, or diabetes mellitus; patients who were obese; or patients who had previous gastrectomy or gastro-jejunal bypass were excluded, as were those who consumed more than 30 g of alcohol/day or who were active smokers (less than 5 years after quitting).
Data regarding age, gender, elapsed time since disease onset, CD activity index (4747. Sandborn WJ, Feagan BG, Hanauer SB, Lochs H, Löfberg R, Modigliani R, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn's disease. Gastroenterology. 2002;122(2):512-30. https://doi.org/10.1053/gast.2002.31072
https://doi.org/10.1053/gast.2002.31072...
), anatomical location of the disease, extraintestinal manifestations, and surgical history, as well as prior and current use of medications, were collected. Serum levels of amylase and lipase were also determined for all of the patients studied.
Endoscopic ultrasound
EUS was conducted by the endoscopy team at the Hospital das Clínicas using a 7.5 MHz linear transducer (GFUCT 140; Olympus, Hamburg, Germany) under propofol sedation. The pancreas was evaluated by 2placing the transducer in the duodenal bulb (for visualizing head of the pancreas), second and third part of the duodenum (uncinated process) and over the stomach (body and pancreatic tail). The diameter of the pancreatic duct was measured at the confluence of the splenic vein and the upper mesenteric vein.
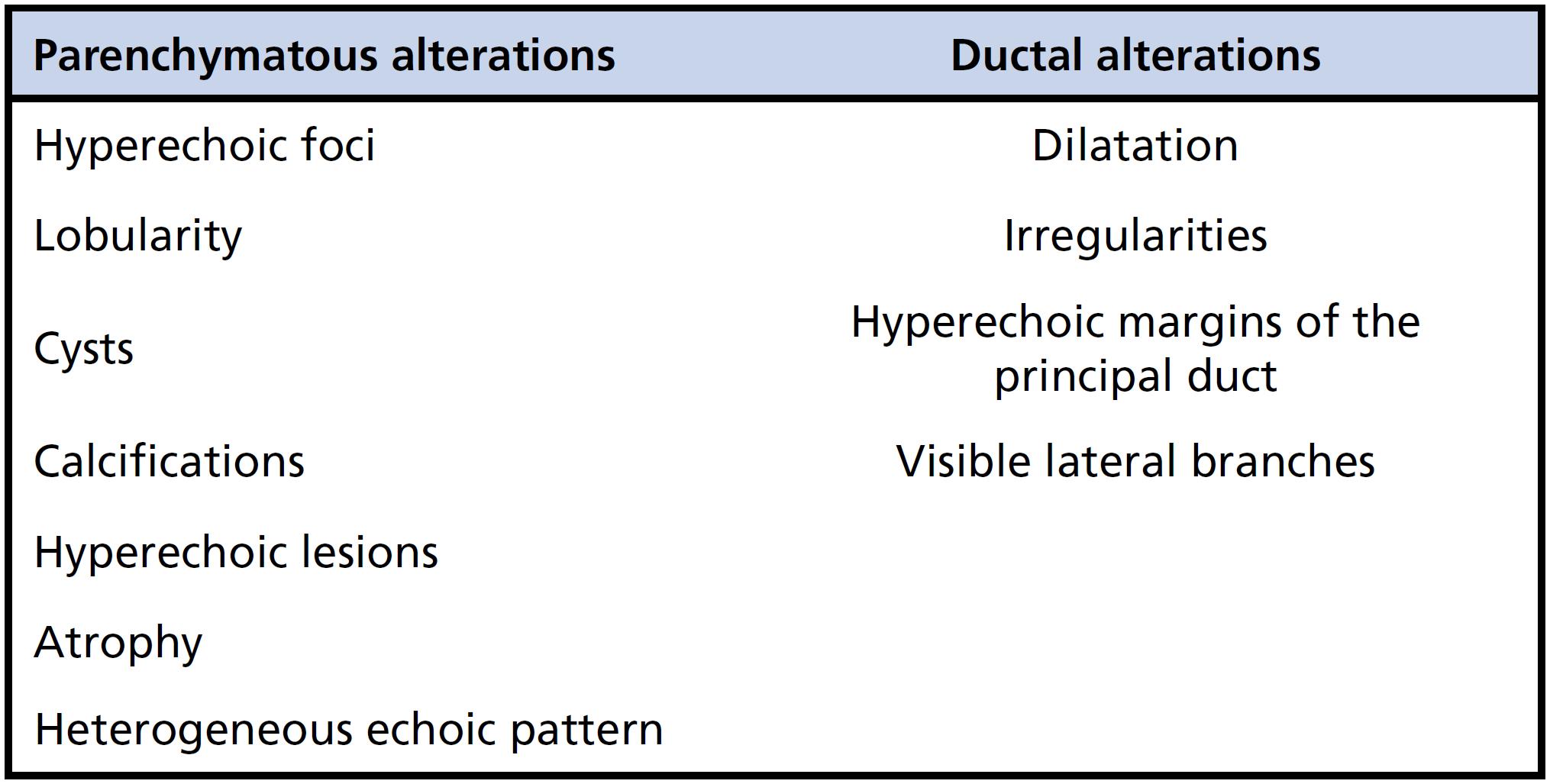
As shown in Figure 1, pancreatic alterations identified through EUS were classified as either parenchymatous or ductal (2828. Catalano MF. Diagnosing early-stage chronic pancreatitis: is endoscopic ultrasound a reliable modality? J Gastroenterol. 2007;42 Suppl 17:78-84. https://doi.org/10.1007/s00535-006-1915-x
https://doi.org/10.1007/s00535-006-1915-...
,2929. Raimondo M, Wallace MB. Diagnosis of early chronic pancreatitis by endoscopic ultrasound. Are we there yet? JOP. 2004;5(1):1-7.,4747. Sandborn WJ, Feagan BG, Hanauer SB, Lochs H, Löfberg R, Modigliani R, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn's disease. Gastroenterology. 2002;122(2):512-30. https://doi.org/10.1053/gast.2002.31072
https://doi.org/10.1053/gast.2002.31072...
). Three or more of these criteria were considered abnormal. Twenty patients in the control group who did not have pancreatic disease, biliary disease, or a history of inflammatory intestinal disease also underwent EUS (EUS control group).
Magnetic resonance cholangiography
Patients who presented with three or more EUS alterations suggestive of chronic pancreatic also underwent MRCP to determine whether there was a correlation between these two methods.
A 1.5 T magnetic resonance imaging (MRI) scanner was used (Sigma; GE Medical Systems, Milwaukee, Wisconsin, USA). The sequence carried out for each pass was as follows: axial T2-weighted fast spin-echo; axial spoiled gradient-recalled dual-echo (in-phase and out-phase); coronal single-shot fast spin-echo with fat saturation; axial spoiled gradient-recalled echo with fat saturation; and axial dynamic spoiled gradient-recalled echo (arterial and portal).
Sequences were obtained during apnea with the cooperation of the patients who had fasted for 8 h prior. No type of contrast agent or sedation was administered. The sequences were performed in three spatial planes and, occasionally, in oblique planes. Images were projected at maximum intensity and analyzed using multiplane reconstruction at a workstation.
Fecal elastase
Pancreatic fecal elastase levels were determined using an enzyme-linked immunosorbent assay (ELISA) that used two polyclonal antibodies that recognize different epitopes at defined sequences of human pancreatic elastase according to the manufacturer´s instructions (BIOSERV Diagnostics, Rostok, Germany).
Samples were collected from 39 patients in the CD group. Samples were collected from 10 patients with chronic alcoholic pancreatitis (chronic pancreatitis group) and from 10 individuals without any clinical symptoms, radiological findings, or laboratory test results indicative of pancreatic disease (fecal elastase control group) to serve as positive and negative controls. All tests were carried out in duplicate.
Ethical aspects
The present study was approved by the Ethics in Research committee of the University of Sao Paulo Medicine School - Hospital das Clínicas. All patients gave written informed consent.
Statistical analysis
Statistical analysis was carried out using the Statistical Package for the Social Sciences (SPSS) program for Windows, version 10.0. Comparisons between patients with CD and the EUS control group were made using Student's t-test. One-way ANOVA was used for comparisons among the CD group, chronic pancreatitis group and fecal elastase control group. For analysis of the correlation between different variables in the CD group, Spearman's correlation coefficient was used. Values of p<0.050 were considered statistically significant.
RESULTS
Of the 51 patients examined by EUS, 28 (56%) were female. The mean age was 38 years (range, 18–59 years). The mean elapsed time since diagnosis of the disease was 7 years (range, 1–25 years), and the mean CD activity index was 102 (range, 20-419). The majority of patients presented with ileal involvement, either isolated (47% of cases) or in combination with colonic involvement (approximately 37% of cases). In 8% of the cases, only the upper gastrointestinal tract was affected.
Approximately 40% of the patients currently presented with or had a history of enteral fistulas, and 20% presented with stenosing CD. Extraintestinal manifestations of the disease (mostly osteoarticular) were observed in 58% of the patients. With respect to current and previous medication use, 56% reported having used azathioprine (Table 1).
Although none of the patients presented with an elevated level of serum lipase, 9% presented with an increase in serum amylase (less than 1.5 times higher than the upper limit of normal).
Endoscopic ultrasound
All 51 patients underwent EUS examination. According to the EUS results, 2 patients (3.9%) presented with four of the abnormalities suggestive of chronic pancreatic disease (as described in the Methods section), and three patients (5.9%) presented with three abnormalities (Table 2).
The prevalence of parenchymatous abnormalities (39 out of 51) was significantly higher than that of ductal abnormalities (11 out of 51) among patients with CD (Table 3).
Of the control group, 3 patients (16%) presented with one alteration by EUS examination. The remaining control group patients presented with no abnormalities on the EUS (Table 2). The difference between the two groups was statistically significant (p<0.001). The only variable that correlated with the EUS data was the ileal location of the disease (p=0.040).
Magnetic resonance cholangiography
The patients presenting with three or more abnormalities by EUS were examined by MCRP. One of these patients died prior to MCRP. The cause of death was unrelated to CD or to the tests that were carried out in this study.
None of the patients who were examined by MCRP presented with any abnormalities.
Fecal elastase
The mean levels of fecal elastase (μg of elastase/g of feces) for each group were as follows: 450 μg/g for the CD group, 487 μg/g for the control group, and 125 μg/g for the chronic pancreatitis group. No significant difference was observed between the CD group and the fecal elastase control group. There was no correlation between the CD activity index and the level of fecal elastase.
Four patients (10%) presented with results suggestive of exocrine pancreatic insufficiency (a fecal elastase value <200 μg/g of feces), and 2 of these patients were classified as having severe exocrine insufficiency. For EUS, none of these 4 patients presented with any abnormalities in pancreatic morphology. In contrast, patients with three or more EUS abnormalities presented fecal elastase levels that were within the normal range. In addition, no correlation was found between the levels of fecal elastase and the epidemiological characteristics of the population.
DISCUSSION
There is a growing number of studies linking IBD and pancreatitis, as shown in a recent review by Srinath et al. (4848. Srinath AI, Gupta N, Husain SZ. Probing the Association of Pancreatitis in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22(2):465-75. https://doi.org/10.1097/MIB.0000000000000611
https://doi.org/10.1097/MIB.000000000000...
). The main subtypes are acute pancreatitis, chronic pancreatitis, AIP and asymptomatic abnormalities. The present study shows that 9.8% of the patients with CD presented with pancreatic involvement (three or more abnormalities) in morphological evaluations using EUS, predominantly in patients with ileal involvement. In other words, ileal involvement was predictive of the number of EUS alterations.
This finding is in line with those published in 2012 by Pavlidis et al. (2121. Pavlidis P, Romanidou O, Roggenbuck D, Mytilinaiou MG, Al-Sulttan F, Liaskos C, et al. Ileal inflammation may trigger the development of GP2-specific pancreatic autoantibodies in patients with Crohn's disease. Clin Dev Immunol. 2012;2012:640835. https://doi.org/10.1155/2012/640835
https://doi.org/10.1155/2012/640835...
), showing that ileal inflammation may trigger the development of GP2-specific PAB in patients with CD. This protein is present on the apical surface of microfold (M) intestinal cells located in the ileum but not in colonic cells, which explains the close relation between higher positive rates in CD (almost 40%) versus fewer than 8% in UC.
Our findings are also in agreement with other studies on the morphological evaluation of the pancreas in patients with CD. In a retrospective study involving 255 patients with CD, the prevalence of pancreatic abnormalities in individuals presenting with no signs or symptoms of chronic pancreatitis on abdominal ultrasound, computed tomography, or ERCP was 6.3% (1818. Oishi Y, Yao T, Matsui T, Ueki T, Sakurai T, Sakaguchi S. Abnormal pancreatic imaging in Crohn's disease: prevalence and clinical features. J Gastroenterol. 2004;39(1):26-33. https://doi.org/10.1007/s00535-003-1241-5
https://doi.org/10.1007/s00535-003-1241-...
). In a prospective study evaluating pancreatic morphology in patients with CD, Heikius et al. (1616. Heikius B, Niemelä S, Lehtola J, Karttunen T, Lähde S. Pancreatic duct abnormalities and pancreatic function in patients with chronic inflammatory bowel disease. Scand J Gastroenterol. 1996;31(5):517-23. https://doi.org/10.3109/00365529609006775
https://doi.org/10.3109/0036552960900677...
) used ERCP as a diagnostic tool. The authors observed abnormalities suggestive of chronic pancreatitis in 4 (8.7%) out of 46 ERCP procedures performed. In a prospective study, Barthet et al. (77. Barthet M, Lesavre N, Desplats S, Panuel M, Gasmi M, Bernard JP, et al. Frequency and characteristics of pancreatitis in patients with inflammatory bowel disease. Pancreatology. 2006;6(5):464-71. https://doi.org/10.1159/000094564
https://doi.org/10.1159/000094564...
) found a 30% prevalence of pancreatic insufficiency among IBD patients with no previous history of pancreatopathy and a 50% prevalence among patients with previous symptoms. In that study, patients were evaluated by pancreato-MRI and exocrine function by fecal elastase test, amylase, lipase, C-reactive protein, pancreatitis associated protein (PAP), IgG4 and PAB.
In our study, the higher prevalence of pancreatic abnormalities (9.8% of the patients presented with three or more EUS abnormalities) can be explained by differences in the morphological evaluation method. Importantly, three or more positive EUS signs are suggestive of chronic pancreatopathy instead of chronic pancreatitis. The sensitivity of EUS in evaluating the pancreatic duct and the parenchyma, which is crucial to the early detection of chronic pancreatitis, appears to be greater than that of ERCP (3838. Glasbrenner B, Kahl S, Malfertheiner P. Modern diagnostics of chronic pancreatitis. Eur J Gastroenterol Hepatol. 2002;14(9):935-41. https://doi.org/10.1097/00042737-200209000-00003
https://doi.org/10.1097/00042737-2002090...
,4949. Kahl S, Glasbrenner B, Leodolter A, Pross M, Schulz HU, Malfertheiner P. EUS in the diagnosis of early chronic pancreatitis: a prospective follow-up study. Gastrointest Endosc. 2002;55(4):507-11. https://doi.org/10.1067/mge.2002.122610
https://doi.org/10.1067/mge.2002.122610...
). For the evaluation of pancreatic diseases in patients with CD, EUS is the most rational choice because pancreatic abnormalities in this situation are predominantly parenchymatous, as has been shown in anatomopathological studies (1515. Chapin LE, Scudamore HH, Baggenstoss AH, Bargen JA. Regional enteritis: associated visceral changes. Gastroenterology. 1956;30(3):404-15. https://doi.org/10.1016/S0016-5085(56)80104-2
https://doi.org/10.1016/S0016-5085(56)80...
,5050. Moon SH, Kim MH. Autoimmune pancreatitis: role of endoscopy in diagnosis and treatment. Gastrointest Endosc Clin N Am. 2013;23(4):893-915. https://doi.org/10.1016/j.giec.2013.06.005
https://doi.org/10.1016/j.giec.2013.06.0...
). By evaluating our patients endoscopically, we found more parenchymatous abnormalities predominated than ductal abnormalities, accounting for 39 and 11 findings, respectively. Based on these results, we may presume that CD patients have pancreatic abnormalities similar to those of patients with obesity, diabetes and alcohol intake, and the vast majority are asymptomatic. However, our results are from only one observer. We could not integrate interobserver agreement into the study, which would have led to more reliable results. Additionally, the patients who had EUS alterations underwent MRCP without any contrast agent. The use of gadolinium could provide more information on minimal parenchymal changes. All patients in the study who submitted to this procedure had no abnormalities.
We identified pancreatic insufficiency in 10% of patients with CD by fecal elastase level determination, although all 4 patients who had values below the threshold of 200 μg/g did not have any ultrasonographic changes, suggesting that some patients with CD might have a functional pancreatic insufficiency not related to morphologic changes in the gland. This finding is in agreement with previous results obtained for patients with idiopathic pancreatitis (5151. Maartense S, Ledeboer M, Masclee AA. Chronic pancreatitis: relation between function and morphology. Dig Liver Dis. 2004;36(1):61-7. https://doi.org/10.1016/j.dld.2003.06.004
https://doi.org/10.1016/j.dld.2003.06.00...
), as well as for patients with mild or moderate pancreatitis (3535. Catalano MF, Lahoti S, Geenen JE, Hogan WJ. Prospective evaluation of endoscopic ultrasonography, endoscopic retrograde pancreatography, and secretin test in the diagnosis of chronic pancreatitis. Gastrointest Endosc. 1998;48(1):11-7. https://doi.org/10.1016/S0016-5107(98)70122-1 · Source: PubMed
https://doi.org/10.1016/S0016-5107(98)70...
,5252. Mee AS, Girdwood AH, Walker E, Gilinsky NH, Kottler RE, Marks IN. Comparison of the oral (PABA) pancreatic function test, the secretin-pancreozymin test and endoscopic retrograde pancreatography in chronic alcohol induced pancreatitis. Gut. 1985;26(11):1257-62.
53. Malfertheiner P, Büchler M, Stanescu A, Ditschuneit H. Exocrine pancreatic function in correlation to ductal and parenchymal morphology in chronic pancreatitis. Hepatogastroenterology. 1986;33(3):110-4.-5454. Lankisch PG, Seidensticker F, Otto J, Lubbers H, Mahlke R, Stockmann F, et al. Secretin-pancreozymin test (SPT) and endoscopic retrograde cholangiopancreatography (ERCP): both are necessary for diagnosing or excluding chronic pancreatitis. Pancreas. 1996;12(2):149-52.). Lindstrom et al. analyzed patients with inflammatory intestinal disease and primary sclerosing cholangitis (5555. Lindström E, Bodemar G, Rydén BO, Ihse I. Pancreatic ductal morphology and exocrine function in primary sclerosing cholangitis. Acta Chir Scand. 1990;156(6-7):451-6.). The authors observed primary abnormalities in morphology rather than in pancreatic function. The fecal elastase test has an insufficient sensitivity (68–77%) and is inappropriate to demonstrate early chronic pancreatitis with a cut-off value of 200 g/g stool (4545. Gullo L, Ventrucci M, Tomassetti P, Migliori M, Pezzilli R. Fecal elastase 1 determination in chronic pancreatitis. Dig Dis Sci. 1999;44(1):210-3. https://doi.org/10.1023/A:1026691209094
https://doi.org/10.1023/A:1026691209094...
,5656. Amann ST, Bishop M, Curington C, Toskes PP. Fecal pancreatic elastase 1 is inaccurate in the diagnosis of chronic pancreatitis. Pancreas. 1996;13(3):226–30.
57. Lankisch PG. Now that fecal elastase is available in the United States, should clinicians start using it? Curr Gastroenterol Rep. 2004;6(2):126-31.https://doi.org/10.1007/s11894-004-0039-9
https://doi.org/10.1007/s11894-004-0039-...
-5858. Keim V, Teich N, Moessner J. Clinical value of a new fecal elastase test for detection of chronic pancreatitis. Clin Lab. 2003;49(5-6):209-15.). The sensitivity of the fecal elastase test is closely related to the severity of pancreatic insufficiency (4545. Gullo L, Ventrucci M, Tomassetti P, Migliori M, Pezzilli R. Fecal elastase 1 determination in chronic pancreatitis. Dig Dis Sci. 1999;44(1):210-3. https://doi.org/10.1023/A:1026691209094
https://doi.org/10.1023/A:1026691209094...
,5757. Lankisch PG. Now that fecal elastase is available in the United States, should clinicians start using it? Curr Gastroenterol Rep. 2004;6(2):126-31.https://doi.org/10.1007/s11894-004-0039-9
https://doi.org/10.1007/s11894-004-0039-...
).
Only four previous studies have analyzed exocrine pancreatic function in patients with CD (55. Angelini G, Cavallini G, Bovo P, Brocco G, Castagnini A, Lavarini E, et al. Pancreatic function in chronic inflammatory bowel disease. Int J Pancreatol. 1988;3(2-3):185-93. https://doi.org/10.1007/BF02798930
https://doi.org/10.1007/BF02798930...
,66. Hegnhøj J, Hansen CP, Rannem T, Søbirk H, Andersen LB, Andersen JR. Pancreatic function in Crohn's disease. Gut. 1990 Sep;31(9):1076-9.,1616. Heikius B, Niemelä S, Lehtola J, Karttunen T, Lähde S. Pancreatic duct abnormalities and pancreatic function in patients with chronic inflammatory bowel disease. Scand J Gastroenterol. 1996;31(5):517-23. https://doi.org/10.3109/00365529609006775
https://doi.org/10.3109/0036552960900677...
,1717. Seibold F, Scheurlen M, Müller A, Jenss H, Weber P. Impaired pancreatic function in patients with Crohn's disease with and without pancreatic autoantibodies. J Clin Gastroenterol. 1996;22(3):202-6. https://doi.org/10.1097/00004836-199604000-00010
https://doi.org/10.1097/00004836-1996040...
). Among the patients studied, the proportion presenting with a decrease in pancreatic function ranged from 4.2% to 58%. Such variation occurred due to the differences among the methods used to evaluate pancreatic function. Among the studies employing invasive tests (e.g., those involving duodenal intubation), one used a secretin test to evaluate pancreatic function in 54 patients, 2 (4.2%) of which presented with abnormalities (1616. Heikius B, Niemelä S, Lehtola J, Karttunen T, Lähde S. Pancreatic duct abnormalities and pancreatic function in patients with chronic inflammatory bowel disease. Scand J Gastroenterol. 1996;31(5):517-23. https://doi.org/10.3109/00365529609006775
https://doi.org/10.3109/0036552960900677...
). Angelini et al. used the secretin-cerulein test in 17 patients with CD (55. Angelini G, Cavallini G, Bovo P, Brocco G, Castagnini A, Lavarini E, et al. Pancreatic function in chronic inflammatory bowel disease. Int J Pancreatol. 1988;3(2-3):185-93. https://doi.org/10.1007/BF02798930
https://doi.org/10.1007/BF02798930...
). The authors observed that in the duodenal aspirate, 35% of the patients presented with a decrease in the secretion of enzymes and bicarbonate, whereas 58% presented with a decrease only in the concentration of lipase. Using the Lundh meal test, Hegnohj et al. noted a significant decrease in the concentration of amylase and lipase in the duodenal aspirate of 143 patients with CD and that the decrease was significantly greater in patients presenting with extensive ileal involvement (66. Hegnhøj J, Hansen CP, Rannem T, Søbirk H, Andersen LB, Andersen JR. Pancreatic function in Crohn's disease. Gut. 1990 Sep;31(9):1076-9.). Seibold et al. used noninvasive methods (fluorescein dilaurate and fecal chymotrypsin) to evaluate pancreatic function in patients with CD (1717. Seibold F, Scheurlen M, Müller A, Jenss H, Weber P. Impaired pancreatic function in patients with Crohn's disease with and without pancreatic autoantibodies. J Clin Gastroenterol. 1996;22(3):202-6. https://doi.org/10.1097/00004836-199604000-00010
https://doi.org/10.1097/00004836-1996040...
). The authors found that the prevalence of impaired pancreatic function was approximately 15%, regardless of the method employed and that such impairment was more common in patients with autoantibodies against the exocrine pancreas.
AIP was recently described (5959. Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40(7):1561-8. https://doi.org/10.1007/BF02285209
https://doi.org/10.1007/BF02285209...
). AIP is characterized by serum IgG4 elevation associated with predominantly lymphoplasmocytic inflammatory infiltrate and is accompanied by pancreatic fibrosis, thereby provoking pancreatic insufficiency (6060. Finkelberg DL, Sahani D, Deshpande V, Brugge WR. Autoimmune pancreatitis. N Engl J Med. 2006;355(25):2670-6. https://doi.org/10.1056/NEJMra061200
https://doi.org/10.1056/NEJMra061200...
61. Kim KP, Kim MH, Kim JC, Lee SS, Seo DW, Lee SK. Diagnostic criteria for autoimmune chronic pancreatitis revisited. World J Gastroenterol. 2006;12(16):2487-96. https://doi.org/10.3748/wjg.v12.i16.2487
https://doi.org/10.3748/wjg.v12.i16.2487...
-6262. Morselli-Labate AM, Pezzilli R. Usefulness of serum IgG4 in the diagnosis and follow up of autoimmune pancreatitis: A systematic literature review and meta-analysis. J Gastroenterol Hepatol. 2009;24(1):15-36. https://doi.org/10.1111/j.1440-1746.2008.05676.x
https://doi.org/10.1111/j.1440-1746.2008...
). AIP can be associated with inflammatory intestinal disease in 5% to 22% of cases (1212. Ravi K, Chari ST, Vege SS, Sandborn WJ, Smyrk TC, Loftus EV Jr. Inflammatory bowel disease in the setting of autoimmune pancreatitis. Inflamm Bowel Dis. 2009;15(9):1326-30. https://doi.org/10.1002/ibd.20898
https://doi.org/10.1002/ibd.20898...
,2020. Ectors N, Maillet B, Aerts R, Geboes K, Donner A, Borchard F, et al. Non-alcoholic duct destructive chronic pancreatitis. Gut. 1997;41(2):263-8. https://doi.org/10.1136/gut.44.4.579
https://doi.org/10.1136/gut.44.4.579...
,6060. Finkelberg DL, Sahani D, Deshpande V, Brugge WR. Autoimmune pancreatitis. N Engl J Med. 2006;355(25):2670-6. https://doi.org/10.1056/NEJMra061200
https://doi.org/10.1056/NEJMra061200...
,6363. Bor R, Farkas K, Bálint A, Wittmann T, Nagy F, Tiszlavicz L, et al. [Autoimmune pancreatitis in a patient with ulcerative colitis simulating a pancreatic tumor]. Orv Hetil. 2014;155(25):1000-4. https://doi.org/10.1556/OH.2014.29936
https://doi.org/10.1556/OH.2014.29936...
), and an elevation of IgG4 was observed in colonic biopsies from IBD patients even without pancreatic disease (6464. Virk R, Shinagare S, Lauwers GY, Yajnik V, Stone JH, Deshpande V. Tissue IgG4-positive plasma cells in inflammatory bowel disease: a study of 88 treatment-naive biopsies of inflammatory bowel disease. Mod Pathol. 2014;27(3):454-9. https://doi.org/10.1038/modpathol.2013.12
https://doi.org/10.1038/modpathol.2013.1...
). The most common EUS findings are a focal or diffuse increase in the size of the pancreas, together with diffusely or focally hypoechoic pancreatic parenchyma (6565. Farrell JJ, Garber J, Sahani D, Brugge WR. EUS findings in patients with autoimmune pancreatitis. Gastrointest Endosc. 2004;60(6):927-36. https://doi.org/10.1016/S0016-5107(04)02230-8
https://doi.org/10.1016/S0016-5107(04)02...
). In a recent study, Hoki et al. concluded that the Sahai criteria (3434. Sahai AV, Zimmerman M, Aabakken L, Tarnasky PR, Cunningham JT, van Velse A, et al. Prospective assessment of the ability of endoscopic ultrasound to diagnose, exclude, or establish the severity of chronic pancreatitis found by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc. 1998;48(1):18-25. https://doi.org/10.1016/S0016-5107(98)70123-3
https://doi.org/10.1016/S0016-5107(98)70...
) for chronic pancreatitis are inadequate for the evaluation of AIP, where the average score was only 2 (6666. Hoki N, Mizuno N, Sawaki A, Tajika M, Takayama R, Shimizu Y, et al. Diagnosis of autoimmune pancreatitis using endoscopic ultrasonography. J Gastroenterol. 2009;44(2):154-9. https://doi.org/10.1007/s00535-008-2294-2
https://doi.org/10.1007/s00535-008-2294-...
). A periductal infiltrate composed of lymphocytes and plasmocytes accompanied by inter and intralobular fibrosis is histologically observed. Atrophy of the parenchyma can also occur (6060. Finkelberg DL, Sahani D, Deshpande V, Brugge WR. Autoimmune pancreatitis. N Engl J Med. 2006;355(25):2670-6. https://doi.org/10.1056/NEJMra061200
https://doi.org/10.1056/NEJMra061200...
). It is possible that some of our patients had IgG4-related disease, which might alter the sonographic appearance of the organ.
In a study of the pancreas, EUS findings were correlated with the histopathological findings (2929. Raimondo M, Wallace MB. Diagnosis of early chronic pancreatitis by endoscopic ultrasound. Are we there yet? JOP. 2004;5(1):1-7.). In our study, the principal findings were hyperechoic foci (35%), hyperechoic ductal margins (21%), hyperechoic lesions (21%) and a heterogeneous echoic pattern (11%). These alterations correspond, respectively, to focal fibrosis, periductal fibrosis, bridge fibrosis and edema, characteristics that are quite prevalent in AIP.
The pancreatic alterations observed in CD are specifically due to the activation of immunocomplexes (44. Eisner TD, Goldman IS, McKinley MJ. Crohn's disease and pancreatitis. Am J Gastroenterol. 1993;88(4):583-6.,88. Piontek M, Hengels KJ, Strohmeyer G. Crohn's disease: what about the pancreas? J Clin Gastroenterol. 1990;12(5):491-3.,2020. Ectors N, Maillet B, Aerts R, Geboes K, Donner A, Borchard F, et al. Non-alcoholic duct destructive chronic pancreatitis. Gut. 1997;41(2):263-8. https://doi.org/10.1136/gut.44.4.579
https://doi.org/10.1136/gut.44.4.579...
) since some of these patients present with high titers of circulating autoantibodies against the exocrine pancreas (1717. Seibold F, Scheurlen M, Müller A, Jenss H, Weber P. Impaired pancreatic function in patients with Crohn's disease with and without pancreatic autoantibodies. J Clin Gastroenterol. 1996;22(3):202-6. https://doi.org/10.1097/00004836-199604000-00010
https://doi.org/10.1097/00004836-1996040...
,6767. Stöcker W, Otte M, Ulrich S, Normann D, Finkbeiner H, Stöcker K, et al. Autoimmunity to pancreatic juice in Crohn's disease. Results of an autoantibody screening in patients with chronic inflammatory bowel disease. Scand J Gastroenterol Suppl. 1987;139:41-52. https://doi.org/10.3109/00365528709089774
https://doi.org/10.3109/0036552870908977...
68. Koutroubakis IE, Drygiannakis D, Karmiris K, Drygiannakis I, Makreas S, Kouroumalis EA. Pancreatic autoantibodies in Greek patients with inflammatory bowel disease. Dig Dis Sci. 2005;50(12):2330-4. https://doi.org/10.1007/s10620-005-3056-0
https://doi.org/10.1007/s10620-005-3056-...
-6969. Papp M, Sipeki N, Tornai T, Altorjay I, Norman GL, Shums Z, et al. Rediscovery of the Anti-Pancreatic Antibodies and Evaluation of their Prognostic Value in a Prospective Clinical Cohort of Crohn's Patients: The Importance of Specific Target Antigens [GP2 and CUZD1]. J Crohns Colitis. 2015;9(8):659-68. https://doi.org/10.1093/ecco-jcc/jjv087
https://doi.org/10.1093/ecco-jcc/jjv087...
), which have been directly related to the presence of pancreatic insufficiency (1717. Seibold F, Scheurlen M, Müller A, Jenss H, Weber P. Impaired pancreatic function in patients with Crohn's disease with and without pancreatic autoantibodies. J Clin Gastroenterol. 1996;22(3):202-6. https://doi.org/10.1097/00004836-199604000-00010
https://doi.org/10.1097/00004836-1996040...
). These autoantibodies are believed to induce tropism in exocrine pancreatic acinar cells (1414. Barthet M, Hastier P, Bernard JP, Bordes G, Frederick J, Allio S, et al. Chronic pancreatitis and inflammatory bowel disease: true or coincidental association? Am J Gastroenterol. 1999;94(8):2141-8. https://doi.org/10.1111/j.1572-0241.1999.01287.x
https://doi.org/10.1111/j.1572-0241.1999...
). Histologically, B lymphocyte aggregates are observed in the pancreatic tissue (2020. Ectors N, Maillet B, Aerts R, Geboes K, Donner A, Borchard F, et al. Non-alcoholic duct destructive chronic pancreatitis. Gut. 1997;41(2):263-8. https://doi.org/10.1136/gut.44.4.579
https://doi.org/10.1136/gut.44.4.579...
). Pancreatic alterations are believed to be less common and less prominent than other extraintestinal manifestations of CD because autoantigens come into contact with the immune system only outside of the pancreas (6767. Stöcker W, Otte M, Ulrich S, Normann D, Finkbeiner H, Stöcker K, et al. Autoimmunity to pancreatic juice in Crohn's disease. Results of an autoantibody screening in patients with chronic inflammatory bowel disease. Scand J Gastroenterol Suppl. 1987;139:41-52. https://doi.org/10.3109/00365528709089774
https://doi.org/10.3109/0036552870908977...
).
CONCLUSION
In conclusion, the present study showed that in a sample of 51 patients, 5 (9.8%) presented with three or more EUS abnormalities suggestive of chronic pancreatitis, which is significantly different than the number observed for the control group. However, none of these 5 patients presented with any clinical signs or laboratory test results (e.g., fecal elastase levels) indicative of pancreatic exocrine insufficiency.
In this group of patients, future studies should be conducted to determine whether such morphological abnormalities could evolve to induce exocrine or endocrine pancreatic insufficiency and, if so, identify the risk factors and determine which patients should undergo EUS (88. Piontek M, Hengels KJ, Strohmeyer G. Crohn's disease: what about the pancreas? J Clin Gastroenterol. 1990;12(5):491-3.,1010. Pitchumoni CS, Rubin A, Das K. Pancreatitis in inflammatory bowel diseases. J Clin Gastroenterol. 2010;44(4):246-53. https://doi.org/10.1097/MCG.0b013e3181cadbe1
https://doi.org/10.1097/MCG.0b013e3181ca...
,2323. Martinelli M, Strisciuglio C, Illiceto MT, Cardile S, Guariso G, Vignola S, et al. Natural history of pancreatic involvement in paediatric inflammatory bowel disease. Dig Liver Dis. 2015;47(5):384-9. https://doi.org/10.1016/j.dld.2015.01.155
https://doi.org/10.1016/j.dld.2015.01.15...
).
REFERENCES
-
1Dobrilla G. Regional enteritis and pancreatitis. Gastroenterology. 1972;63(4):713-4.
-
2Axon AT, Ashton MG, Lintott DJ. Chronic pancreatitis and inflammatory bowel disease. Clin Radiol. 1979;30(2):179-82. https://doi.org/10.1016/S0009-9260(79)80146-4
» https://doi.org/10.1016/S0009-9260(79)80146-4 -
3Seyrig JA, Jian R, Modigliani R, Golfain D, Florent C, Messing B, et al. Idiopathic pancreatitis associated with inflammatory bowel disease. Dig Dis Sci. 1985;30(12):1121-6. https://doi.org/10.1007/BF01314044
» https://doi.org/10.1007/BF01314044 -
4Eisner TD, Goldman IS, McKinley MJ. Crohn's disease and pancreatitis. Am J Gastroenterol. 1993;88(4):583-6.
-
5Angelini G, Cavallini G, Bovo P, Brocco G, Castagnini A, Lavarini E, et al. Pancreatic function in chronic inflammatory bowel disease. Int J Pancreatol. 1988;3(2-3):185-93. https://doi.org/10.1007/BF02798930
» https://doi.org/10.1007/BF02798930 -
6Hegnhøj J, Hansen CP, Rannem T, Søbirk H, Andersen LB, Andersen JR. Pancreatic function in Crohn's disease. Gut. 1990 Sep;31(9):1076-9.
-
7Barthet M, Lesavre N, Desplats S, Panuel M, Gasmi M, Bernard JP, et al. Frequency and characteristics of pancreatitis in patients with inflammatory bowel disease. Pancreatology. 2006;6(5):464-71. https://doi.org/10.1159/000094564
» https://doi.org/10.1159/000094564 -
8Piontek M, Hengels KJ, Strohmeyer G. Crohn's disease: what about the pancreas? J Clin Gastroenterol. 1990;12(5):491-3.
-
9Rasmussen HH, Fonager K, Sørensen HT, Pedersen L, Dahlerup JF, Steffensen FH. Risk of acute pancreatitis in patients with chronic inflammatory bowel disease. A Danish 16-year nationwide follow-up study. Scand J Gastroenterol. 1999;34(2):199-201. https://doi.org/10.1080/00365529950173096
» https://doi.org/10.1080/00365529950173096 -
10Pitchumoni CS, Rubin A, Das K. Pancreatitis in inflammatory bowel diseases. J Clin Gastroenterol. 2010;44(4):246-53. https://doi.org/10.1097/MCG.0b013e3181cadbe1
» https://doi.org/10.1097/MCG.0b013e3181cadbe1 -
11Pitchumoni CS, Chari S. Ulcerative colitis and autoimmune pancreatitis. J Clin Gastroenterol. 2013;47(6):469. https://doi.org/10.1097/MCG.0b013e31828a7099
» https://doi.org/10.1097/MCG.0b013e31828a7099 -
12Ravi K, Chari ST, Vege SS, Sandborn WJ, Smyrk TC, Loftus EV Jr. Inflammatory bowel disease in the setting of autoimmune pancreatitis. Inflamm Bowel Dis. 2009;15(9):1326-30. https://doi.org/10.1002/ibd.20898
» https://doi.org/10.1002/ibd.20898 -
13Niemelä S, Lehtola J, Karttunen T, Lähde S. Pancreatitis in patients with chronic inflammatory bowel disease. Hepatogastroenterology. 1989;36(3):175-7.
-
14Barthet M, Hastier P, Bernard JP, Bordes G, Frederick J, Allio S, et al. Chronic pancreatitis and inflammatory bowel disease: true or coincidental association? Am J Gastroenterol. 1999;94(8):2141-8. https://doi.org/10.1111/j.1572-0241.1999.01287.x
» https://doi.org/10.1111/j.1572-0241.1999.01287.x -
15Chapin LE, Scudamore HH, Baggenstoss AH, Bargen JA. Regional enteritis: associated visceral changes. Gastroenterology. 1956;30(3):404-15. https://doi.org/10.1016/S0016-5085(56)80104-2
» https://doi.org/10.1016/S0016-5085(56)80104-2 -
16Heikius B, Niemelä S, Lehtola J, Karttunen T, Lähde S. Pancreatic duct abnormalities and pancreatic function in patients with chronic inflammatory bowel disease. Scand J Gastroenterol. 1996;31(5):517-23. https://doi.org/10.3109/00365529609006775
» https://doi.org/10.3109/00365529609006775 -
17Seibold F, Scheurlen M, Müller A, Jenss H, Weber P. Impaired pancreatic function in patients with Crohn's disease with and without pancreatic autoantibodies. J Clin Gastroenterol. 1996;22(3):202-6. https://doi.org/10.1097/00004836-199604000-00010
» https://doi.org/10.1097/00004836-199604000-00010 -
18Oishi Y, Yao T, Matsui T, Ueki T, Sakurai T, Sakaguchi S. Abnormal pancreatic imaging in Crohn's disease: prevalence and clinical features. J Gastroenterol. 2004;39(1):26-33. https://doi.org/10.1007/s00535-003-1241-5
» https://doi.org/10.1007/s00535-003-1241-5 -
19Ball WP, Baggenstoss AH, Bargen JA. Pancreatic lesions associated with chronic ulcerative colitis. Arch Pathol (Chic). 1950;50(3):347-58.
-
20Ectors N, Maillet B, Aerts R, Geboes K, Donner A, Borchard F, et al. Non-alcoholic duct destructive chronic pancreatitis. Gut. 1997;41(2):263-8. https://doi.org/10.1136/gut.44.4.579
» https://doi.org/10.1136/gut.44.4.579 -
21Pavlidis P, Romanidou O, Roggenbuck D, Mytilinaiou MG, Al-Sulttan F, Liaskos C, et al. Ileal inflammation may trigger the development of GP2-specific pancreatic autoantibodies in patients with Crohn's disease. Clin Dev Immunol. 2012;2012:640835. https://doi.org/10.1155/2012/640835
» https://doi.org/10.1155/2012/640835 -
22Gschwantler M, Kogelbauer G, Klose W, Bibus B, Tscholakoff D, Weiss W. The pancreas as a site of granulomatous inflammation in Crohn's disease. Gastroenterology. 1995;108(4):1246-9. https://doi.org/10.1016/0016-5085(95)90226-0
» https://doi.org/10.1016/0016-5085(95)90226-0 -
23Martinelli M, Strisciuglio C, Illiceto MT, Cardile S, Guariso G, Vignola S, et al. Natural history of pancreatic involvement in paediatric inflammatory bowel disease. Dig Liver Dis. 2015;47(5):384-9. https://doi.org/10.1016/j.dld.2015.01.155
» https://doi.org/10.1016/j.dld.2015.01.155 -
24Trikudanathan G, Navaneethan U. Association of pancreatitis with inflammatory bowel disease. J Clin Gastroenterol. 2011;45(1):83. https//doi.org/10.1097/MCG.0b013e3181d9f935
» https//doi.org/10.1097/MCG.0b013e3181d9f935 -
25Etemad B, Whitcomb DC. Chronic pancreatitis: diagnosis, classification, and new genetic developments. Gastroenterology. 2001;120(3):682-707. https://doi.org/10.1053/gast.2001.22586
» https://doi.org/10.1053/gast.2001.22586 -
26Owiang C. Chronic pancreatitis. In: Yamada T. Textbook of gastroenterology. Philadelphia: Lippincott Willians & Wilkins; 1999. Vol 2, p. 2151-77.
-
27Miyakawa H, Suga T, Okamura K. Usefulness of endoscopic ultrasonography for the diagnosis of chronic pancreatitis. J Gastroenterol. 2007;42 Suppl 17:85-9. https://doi.org/10.1007/s00535-006-1935-6
» https://doi.org/10.1007/s00535-006-1935-6 -
28Catalano MF. Diagnosing early-stage chronic pancreatitis: is endoscopic ultrasound a reliable modality? J Gastroenterol. 2007;42 Suppl 17:78-84. https://doi.org/10.1007/s00535-006-1915-x
» https://doi.org/10.1007/s00535-006-1915-x -
29Raimondo M, Wallace MB. Diagnosis of early chronic pancreatitis by endoscopic ultrasound. Are we there yet? JOP. 2004;5(1):1-7.
-
30Wiersema MJ, Hawes RH, Lehman GA, Kochman ML, Sherman S, Kopecky KK. Prospective evaluation of endoscopic ultrasonography and endoscopic retrograde cholangiopancreatography in patients with chronic abdominal pain of suspected pancreatic origin. Endoscopy. 1993;25(9):555-64. https://doi.org/10.1055/s-2007-1010405
» https://doi.org/10.1055/s-2007-1010405 -
31Irisawa A, Katakura K, Ohira H, Sato A, Bhutani MS, Hernandez LV, et al. Usefulness of endoscopic ultrasound to diagnose the severity of chronic pancreatitis. J Gastroenterol. 2007;42 Suppl 17:90-4. https://doi.org/10.1007/s00535-006-1916-9
» https://doi.org/10.1007/s00535-006-1916-9 -
32Hollerbach S, Klamann A, Topalidis T, Schmiegel WH. Endoscopic ultrasonography (EUS) and fine-needle aspiration (FNA) cytology for diagnosis of chronic pancreatitis. Endoscopy. 2001;33(10):824-31. https://doi.org/10.1055/s-2001-17337
» https://doi.org/10.1055/s-2001-17337 -
33Jung JG, Lee JK, Lee KH, Lee KT, Woo YS, Paik WH, et al. Comparison of endoscopic retrograde cholangiopancreatography with papillary biopsy and endoscopic ultrasound-guided pancreatic biopsy in the diagnosis of autoimmune pancreatitis. Pancreatology. 2015;15(3):259-64. https://doi.org/10.1016/j.pan.2015.03.011
» https://doi.org/10.1016/j.pan.2015.03.011 -
34Sahai AV, Zimmerman M, Aabakken L, Tarnasky PR, Cunningham JT, van Velse A, et al. Prospective assessment of the ability of endoscopic ultrasound to diagnose, exclude, or establish the severity of chronic pancreatitis found by endoscopic retrograde cholangiopancreatography. Gastrointest Endosc. 1998;48(1):18-25. https://doi.org/10.1016/S0016-5107(98)70123-3
» https://doi.org/10.1016/S0016-5107(98)70123-3 -
35Catalano MF, Lahoti S, Geenen JE, Hogan WJ. Prospective evaluation of endoscopic ultrasonography, endoscopic retrograde pancreatography, and secretin test in the diagnosis of chronic pancreatitis. Gastrointest Endosc. 1998;48(1):11-7. https://doi.org/10.1016/S0016-5107(98)70122-1 · Source: PubMed
» https://doi.org/10.1016/S0016-5107(98)70122-1 -
36Buscail L, Escourrou J, Moreau J, Delvaux M, Louvel D, Lapeyre F, et al. Endoscopic ultrasonography in chronic pancreatitis: a comparative prospective study with conventional ultrasonography, computed tomography, and ERCP. Pancreas. 1995;10(3):251-7.
-
37Nattermann C, Goldschmidt AJ, Dancygier H. Endosonography in chronic pancreatitis - A comparison between endoscopic retrograde pancreatography and endoscopic ultrasonography. Endoscopy. 1993;25(9):565-70. https://doi.org/10.1055/s-2007-1010406
» https://doi.org/10.1055/s-2007-1010406 -
38Glasbrenner B, Kahl S, Malfertheiner P. Modern diagnostics of chronic pancreatitis. Eur J Gastroenterol Hepatol. 2002;14(9):935-41. https://doi.org/10.1097/00042737-200209000-00003
» https://doi.org/10.1097/00042737-200209000-00003 -
39Hawes RH. Comparison of diagnostic modalities: EUS, ERCP, and fluid analysis. Gastrointest Endosc. 1999;49(3 Suppl):S74-6. https://doi.org/10.1016/S0016-5107(99)70531-6
» https://doi.org/10.1016/S0016-5107(99)70531-6 -
40Bokemeyer B. Asymptomatic elevation of serum lipase and amylase in conjunction with Crohn's disease and ulcerative colitis. Z Gastroenterol. 2002;40(1):5-10. https://doi.org/10.1055/s-2002-19636
» https://doi.org/10.1055/s-2002-19636 -
41Lankisch PG. The problem of diagnosing chronic pancreatitis. Dig Liver Dis. 2003;35(3):131-4. https://doi.org/10.1016/S1590-8658(03)00019-7
» https://doi.org/10.1016/S1590-8658(03)00019-7 -
42Chowdhury RS, Forsmark CE. Review article: Pancreatic function testing. Aliment Pharmacol Ther. 2003;17(6):733-50.
-
43Löser C, Möllgaard A, Fölsch UR. Faecal elastase 1: a novel, highly sensitive, and specific tubeless pancreatic function test. Gut. 1996;39(4):580-6.
-
44Lankisch PG, Schmidt I, König H, Lehnick D, Knollmann R, Löhr M, et al. Faecal elastase 1: not helpful in diagnosing chronic pancreatitis associated with mild to moderate exocrine pancreatic insufficiency. Gut. 1998;42(4):551-4. https://doi.org/10.1136/gut.42.4.551
» https://doi.org/10.1136/gut.42.4.551 -
45Gullo L, Ventrucci M, Tomassetti P, Migliori M, Pezzilli R. Fecal elastase 1 determination in chronic pancreatitis. Dig Dis Sci. 1999;44(1):210-3. https://doi.org/10.1023/A:1026691209094
» https://doi.org/10.1023/A:1026691209094 -
46Hardt PD, Marzeion AM, Schnell-Kretschmer H, Wüsten O, Nalop J, Zekorn T, et al. Fecal elastase 1 measurement compared with endoscopic retrograde cholangiopancreatography for the diagnosis of chronic pancreatitis. Pancreas. 2002;25(1):e6-9. https://doi.org/10.1097/00006676-200207000-00004
» https://doi.org/10.1097/00006676-200207000-00004 -
47Sandborn WJ, Feagan BG, Hanauer SB, Lochs H, Löfberg R, Modigliani R, et al. A review of activity indices and efficacy endpoints for clinical trials of medical therapy in adults with Crohn's disease. Gastroenterology. 2002;122(2):512-30. https://doi.org/10.1053/gast.2002.31072
» https://doi.org/10.1053/gast.2002.31072 -
48Srinath AI, Gupta N, Husain SZ. Probing the Association of Pancreatitis in Inflammatory Bowel Disease. Inflamm Bowel Dis. 2016;22(2):465-75. https://doi.org/10.1097/MIB.0000000000000611
» https://doi.org/10.1097/MIB.0000000000000611 -
49Kahl S, Glasbrenner B, Leodolter A, Pross M, Schulz HU, Malfertheiner P. EUS in the diagnosis of early chronic pancreatitis: a prospective follow-up study. Gastrointest Endosc. 2002;55(4):507-11. https://doi.org/10.1067/mge.2002.122610
» https://doi.org/10.1067/mge.2002.122610 -
50Moon SH, Kim MH. Autoimmune pancreatitis: role of endoscopy in diagnosis and treatment. Gastrointest Endosc Clin N Am. 2013;23(4):893-915. https://doi.org/10.1016/j.giec.2013.06.005
» https://doi.org/10.1016/j.giec.2013.06.005 -
51Maartense S, Ledeboer M, Masclee AA. Chronic pancreatitis: relation between function and morphology. Dig Liver Dis. 2004;36(1):61-7. https://doi.org/10.1016/j.dld.2003.06.004
» https://doi.org/10.1016/j.dld.2003.06.004 -
52Mee AS, Girdwood AH, Walker E, Gilinsky NH, Kottler RE, Marks IN. Comparison of the oral (PABA) pancreatic function test, the secretin-pancreozymin test and endoscopic retrograde pancreatography in chronic alcohol induced pancreatitis. Gut. 1985;26(11):1257-62.
-
53Malfertheiner P, Büchler M, Stanescu A, Ditschuneit H. Exocrine pancreatic function in correlation to ductal and parenchymal morphology in chronic pancreatitis. Hepatogastroenterology. 1986;33(3):110-4.
-
54Lankisch PG, Seidensticker F, Otto J, Lubbers H, Mahlke R, Stockmann F, et al. Secretin-pancreozymin test (SPT) and endoscopic retrograde cholangiopancreatography (ERCP): both are necessary for diagnosing or excluding chronic pancreatitis. Pancreas. 1996;12(2):149-52.
-
55Lindström E, Bodemar G, Rydén BO, Ihse I. Pancreatic ductal morphology and exocrine function in primary sclerosing cholangitis. Acta Chir Scand. 1990;156(6-7):451-6.
-
56Amann ST, Bishop M, Curington C, Toskes PP. Fecal pancreatic elastase 1 is inaccurate in the diagnosis of chronic pancreatitis. Pancreas. 1996;13(3):226–30.
-
57Lankisch PG. Now that fecal elastase is available in the United States, should clinicians start using it? Curr Gastroenterol Rep. 2004;6(2):126-31.https://doi.org/10.1007/s11894-004-0039-9
» https://doi.org/10.1007/s11894-004-0039-9 -
58Keim V, Teich N, Moessner J. Clinical value of a new fecal elastase test for detection of chronic pancreatitis. Clin Lab. 2003;49(5-6):209-15.
-
59Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40(7):1561-8. https://doi.org/10.1007/BF02285209
» https://doi.org/10.1007/BF02285209 -
60Finkelberg DL, Sahani D, Deshpande V, Brugge WR. Autoimmune pancreatitis. N Engl J Med. 2006;355(25):2670-6. https://doi.org/10.1056/NEJMra061200
» https://doi.org/10.1056/NEJMra061200 -
61Kim KP, Kim MH, Kim JC, Lee SS, Seo DW, Lee SK. Diagnostic criteria for autoimmune chronic pancreatitis revisited. World J Gastroenterol. 2006;12(16):2487-96. https://doi.org/10.3748/wjg.v12.i16.2487
» https://doi.org/10.3748/wjg.v12.i16.2487 -
62Morselli-Labate AM, Pezzilli R. Usefulness of serum IgG4 in the diagnosis and follow up of autoimmune pancreatitis: A systematic literature review and meta-analysis. J Gastroenterol Hepatol. 2009;24(1):15-36. https://doi.org/10.1111/j.1440-1746.2008.05676.x
» https://doi.org/10.1111/j.1440-1746.2008.05676.x -
63Bor R, Farkas K, Bálint A, Wittmann T, Nagy F, Tiszlavicz L, et al. [Autoimmune pancreatitis in a patient with ulcerative colitis simulating a pancreatic tumor]. Orv Hetil. 2014;155(25):1000-4. https://doi.org/10.1556/OH.2014.29936
» https://doi.org/10.1556/OH.2014.29936 -
64Virk R, Shinagare S, Lauwers GY, Yajnik V, Stone JH, Deshpande V. Tissue IgG4-positive plasma cells in inflammatory bowel disease: a study of 88 treatment-naive biopsies of inflammatory bowel disease. Mod Pathol. 2014;27(3):454-9. https://doi.org/10.1038/modpathol.2013.12
» https://doi.org/10.1038/modpathol.2013.12 -
65Farrell JJ, Garber J, Sahani D, Brugge WR. EUS findings in patients with autoimmune pancreatitis. Gastrointest Endosc. 2004;60(6):927-36. https://doi.org/10.1016/S0016-5107(04)02230-8
» https://doi.org/10.1016/S0016-5107(04)02230-8 -
66Hoki N, Mizuno N, Sawaki A, Tajika M, Takayama R, Shimizu Y, et al. Diagnosis of autoimmune pancreatitis using endoscopic ultrasonography. J Gastroenterol. 2009;44(2):154-9. https://doi.org/10.1007/s00535-008-2294-2
» https://doi.org/10.1007/s00535-008-2294-2 -
67Stöcker W, Otte M, Ulrich S, Normann D, Finkbeiner H, Stöcker K, et al. Autoimmunity to pancreatic juice in Crohn's disease. Results of an autoantibody screening in patients with chronic inflammatory bowel disease. Scand J Gastroenterol Suppl. 1987;139:41-52. https://doi.org/10.3109/00365528709089774
» https://doi.org/10.3109/00365528709089774 -
68Koutroubakis IE, Drygiannakis D, Karmiris K, Drygiannakis I, Makreas S, Kouroumalis EA. Pancreatic autoantibodies in Greek patients with inflammatory bowel disease. Dig Dis Sci. 2005;50(12):2330-4. https://doi.org/10.1007/s10620-005-3056-0
» https://doi.org/10.1007/s10620-005-3056-0 -
69Papp M, Sipeki N, Tornai T, Altorjay I, Norman GL, Shums Z, et al. Rediscovery of the Anti-Pancreatic Antibodies and Evaluation of their Prognostic Value in a Prospective Clinical Cohort of Crohn's Patients: The Importance of Specific Target Antigens [GP2 and CUZD1]. J Crohns Colitis. 2015;9(8):659-68. https://doi.org/10.1093/ecco-jcc/jjv087
» https://doi.org/10.1093/ecco-jcc/jjv087
Publication Dates
-
Publication in this collection
30 May 2019 -
Date of issue
2019
History
-
Received
01 July 2018 -
Accepted
17 Jan 2019