Abstract
OBJECTIVES:
Although the practice of physical exercise in patients with intermittent claudication (IC) is often encouraged, adherence is low. The difficulty in performing physical training may be related to the psychological characteristics of patients with claudication. To verify the association between anxiety and depression symptoms and barriers to physical exercise and walking capacity in patients with IC.
METHODS:
One-hundred and thirteen patients with a clinical diagnosis of IC were included in the study. Patients underwent clinical evaluation by a vascular surgeon, answered the Beck Depression Inventory, and Beck Anxiety Inventory tests were applied by the psychologist. The patients performed the 6-minute test and reported their barriers to physical activity practice in a questionnaire.
RESULTS:
Patients with signs of depression had a shorter pain-free walking distance (p=0.015) and total walking distance (p=0.035) compared to patients with no signs of depression. Pain-free walking distance (p=0.29) and total walking distance (p=0.07) were similar between patients with and without signs of anxiety. Patients with symptoms of moderate to severe depression reported more barriers to physical activity practice compared to patients without signs of depression.
CONCLUSION:
Symptoms of anxiety and depression are prevalent among patients with peripheral arterial occlusive disease (PAD). Depression symptoms are associated with personal barriers to exercise, while anxiety symptoms are not. The main barriers to physical activity among patients with IC are exercise-induced pain and the presence of other diseases.
Intermittent Claudication; Depression; Anxiety; Physical Activity; Barriers; Peripheral Arterial Obstructive Disease
INTRODUCTION
Depression and anxiety are related to several chronic diseases, including coronary disease (11. Smolderen KG, Hoeks SE, Pedersen SS, van Domburg RT, de Liefde II, Poldermans D. Lower-leg symptoms in peripheral arterial disease are associated with anxiety, depression, and anhedonia. Vasc Med. 2009;14(4):297-304. https://doi.org/10.1177/1358863X09104658.
https://doi.org/10.1177/1358863X09104658...
). Depression is more frequent in the cardiac patient population and is an independent risk factor for worse coronary disease prognosis (22. Columbo JA, Stone DH, Goodney PP, Nolan BW, Stableford JA, Brooke BS, et al. The Prevalence and Regional Variation of Major Depressive Disorder Among Patients With Peripheral Arterial Disease in the Medicare Population. Vasc Endovascular Surg. 2016;50(4):235-40. https://doi.org/10.1177/1538574416644529.
https://doi.org/10.1177/1538574416644529...
). In addition, the combination of anxiety and depression further increases the risk of cardiovascular events (33. Tully PJ, Cosh SM, Baumeister H. The anxious heart in whose mind? A systematic review and meta-regression of factors associated with anxiety disorder diagnosis, treatment and morbidity risk in coronary heart disease. J Psychosom Res. 2014;77(6):439-48. https://doi.org/10.1016/j.jpsychores.2014.10.001.
https://doi.org/10.1016/j.jpsychores.201...
). Similar to coronary disease, the major etiology of peripheral arterial occlusive disease (PAD) is atherosclerosis. Thus, the relationship between depression/anxiety and PAD has also aroused interest.
Smolderen et al. (11. Smolderen KG, Hoeks SE, Pedersen SS, van Domburg RT, de Liefde II, Poldermans D. Lower-leg symptoms in peripheral arterial disease are associated with anxiety, depression, and anhedonia. Vasc Med. 2009;14(4):297-304. https://doi.org/10.1177/1358863X09104658.
https://doi.org/10.1177/1358863X09104658...
) studied 628 patients with PAD and found anxiety symptoms in 29%, depression in 30%, and anhedonia in 28% of these individuals. These psychiatric disorders were more prevalent in patients with more severe PAD (11. Smolderen KG, Hoeks SE, Pedersen SS, van Domburg RT, de Liefde II, Poldermans D. Lower-leg symptoms in peripheral arterial disease are associated with anxiety, depression, and anhedonia. Vasc Med. 2009;14(4):297-304. https://doi.org/10.1177/1358863X09104658.
https://doi.org/10.1177/1358863X09104658...
). In addition to controlling the risk factors for atherosclerosis, PAD treatment involves routine physical activity, especially walking (44. Grizzo Cucato G, de Moraes Forjaz CL, Kanegusuku H, da Rocha Chehuen M, Riani Costa LA, Wolosker N, et al. Effects of walking and strength training on resting and exercise cardiovascular responses in patients with intermittent claudication. Vasa. 2011;40(5):390-7. https://doi.org/10.1024/0301-1526/a000136.
https://doi.org/10.1024/0301-1526/a00013...
,55. Ritti-Dias RM, Correia MA, Andrade-Lima A, Cucato GG. Exercise as a therapeutic approach to improve blood pressure in patients with peripheral arterial disease: current literature and future directions. Expert Rev Cardiovasc Ther. 2019;17(1):65-73. https://doi.org/10.1080/14779072.2019.1553676.
https://doi.org/10.1080/14779072.2019.15...
). Arterial obstruction causes pain due to muscle ischemia triggered by exercise, a condition that defines intermittent claudication (IC) and makes walking a painful activity (66. Farah BQ, Ritti-Dias RM, Cucato GG, Chehuen Mda R, Barbosa JP, Zeratti AE, et al. Effects of clustered comorbid conditions on walking capacity in patients with peripheral artery disease. Ann Vasc Surg. 2014;28(2):279-83. https://doi.org/10.1016/j.avsg.2013.01.020.
https://doi.org/10.1016/j.avsg.2013.01.0...
,77. Chehuen M, Cucato GG, Carvalho CRF, Ritti-Dias RM, Wolosker N, Leicht AS, et al. Walking training at the heart rate of pain threshold improves cardiovascular function and autonomic regulation in intermittent claudication: A randomized controlled trial. J Sci Med Sport. 2017;20(10):886-92. https://doi.org/10.1016/j.jsams.2017.02.011.
https://doi.org/10.1016/j.jsams.2017.02....
).
Thus, the initial clinical treatment of PAD depends on an unpleasant practice that is painful to most patients. Barbosa et al. (88. Barbosa JP, Farah BQ, Chehuen M, Cucato GG, Farias Júnior JC, Wolosker N, et al. Barriers to physical activity in patients with intermittent claudication. Int J Behav Med. 2015;22(1):70-6. https://doi.org/10.1007/s12529-014-9408-4.
https://doi.org/10.1007/s12529-014-9408-...
) studied 150 patients with IC and observed that the most prevalent barriers to physical activity were personal and environmental. The emotional state might play a role in enhancing these barriers, thereby decreasing the willingness of patients with IC to perform physical activity and consequently aggravate their PAD. However, no study has analyzed the relationship between the barriers to the practice of exercise and the presence of anxiety/depression.
This study evaluated the relationship between anxiety/depression symptoms and the presence of barriers to exercise with regard to the physical activity levels of patients with IC.
MATERIAL AND METHODS
Study design and patients
This prospective, cross-sectional study examined 113 patients with IC of atherosclerotic etiology in one or both legs who were treated at the Vascular Surgery Outpatient Clinic between 2016 and 2018. The institutional ethics committee approved this study, and all patients signed an informed consent document prior to inclusion. The inclusion criteria were: decreased intensity or absence of arterial pulse in at least one of the extremities, ankle-brachial index less than 0.90, ≥20% reduction in systolic blood pressure in the ankle immediately after walking the maximum claudication distance in a standardized treadmill test with progressive load (Gardner protocol), and patients without limiting claudication (walking distance >50 m). The following patients were excluded: association of other limiting factors such as osteoarticular disease in the lower limbs, major lower limb amputation, severe chronic obstructive pulmonary disease, decompensated coronary disease, previous diagnosis of depression or anxiety, use of antidepressants and/or anxiolytics.
Intermittent claudication assessment
At the initial consultation, the participants’ medical histories were taken, and a general and vascular physical examination was performed to obtain anthropometric data, including a measurement of the ankle brachial index. The patients also reported their physical activity routines. After this consultation, the patients performed the 6-minute walk test (6MWT) to measure their pain-free walking distance and maximum walking distance. Participants also filled a questionnaire to assess the barriers to physical activity, and the Beck Depression Inventory (BDI) to assess their depression and anxiety symptoms. Based on these symptoms, the patients were classified into two groups: asymptomatic or symptomatic.
The ankle brachial index was calculated by dividing the ankle systolic blood pressure by the brachial systolic blood pressure, which permits an assessment of the level of hemodynamic changes of the lower limbs (99. Wolosker N, Rosoky RA, Nakano L, Basyches M, Puech-Leão P. Predictive value of the ankle-brachial index in the evaluation of intermittent claudication. Rev Hosp Clin Fac Med Sao Paulo. 2000;55(2):61-4. https://doi.org/10.1590/S0041-87812000000200005.
https://doi.org/10.1590/S0041-8781200000...
). This measure was taken in one or both legs with PAD (1010. Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126(24):2890-909. https://doi.org/Erratum in: Circulation. 2013;127(1):e264..
https://doi.org/Erratum in: Circulation....
). Systolic ankle and brachial pressures were obtained using a mercury sphygmomanometer and a doppler ultrasound (Martec DV 6000, Ribeirão Preto, Brazil). In the 6MWT, patients walked down a flat 30-meter hallway for 6 minutes. Patients were encouraged to complete as many laps as possible, even if the patient needed to rest. It was important that the patient maintained his or her typical walking rhythm. The distance to the initial pain and the total distance over 6 minutes were recorded (1111. Farah BQ, Ritti-Dias RM, Montgomery P, Cucato GG, Gardner A. Exercise Intensity during 6-Minute Walk Test in Patients with Peripheral Artery Disease. Arq Bras Cardiol. 2020;114(3):486-92.).
The barriers analyzed refer to the difficulties encountered by patients that prevent them from practicing physical activity. To evaluate the perceived difficulty, an adapted version of the Neighborhood Environmental Walkability Scale (NEWS) (1212. Booth ML, Bauman A, Owen N, Gore CJ. Physical activity preferences, preferred sources of assistance, and perceived barriers to increased activity among physically inactive Australians. Prev Med. 1997;26(1):131-7. https://doi.org/10.1006/pmed.1996.9982.
https://doi.org/10.1006/pmed.1996.9982...
) was used. This was previously validated by Malavasi et al. (1313. Saelens BE, Sallis JF, Black JB, Chen D. Neighborhood-based differences in physical activity: an environment scale evaluation. Am J Public Health. 2003;93(9):1552-8. https://doi.org/10.2105/AJPH.93.9.1552.
https://doi.org/10.2105/AJPH.93.9.1552...
) and later used in IC patients (77. Chehuen M, Cucato GG, Carvalho CRF, Ritti-Dias RM, Wolosker N, Leicht AS, et al. Walking training at the heart rate of pain threshold improves cardiovascular function and autonomic regulation in intermittent claudication: A randomized controlled trial. J Sci Med Sport. 2017;20(10):886-92. https://doi.org/10.1016/j.jsams.2017.02.011.
https://doi.org/10.1016/j.jsams.2017.02....
).
Barriers to physical activity assessment
Personal barriers to physical activity were assessed using questions that assessed the following items: fatigue, lack of time, no one to accompany patients while walking, lack of money to practice physical activity, lack of knowledge, and uncertainty regarding the benefits of exercise, lack of energy, pain induced by walking, and the need to rest due to pain. This questionnaire was validated for use among the elderly and was administered by the same physician (88. Barbosa JP, Farah BQ, Chehuen M, Cucato GG, Farias Júnior JC, Wolosker N, et al. Barriers to physical activity in patients with intermittent claudication. Int J Behav Med. 2015;22(1):70-6. https://doi.org/10.1007/s12529-014-9408-4.
https://doi.org/10.1007/s12529-014-9408-...
). Patients answered yes or no to all questions regarding barriers that might affect their ability to engage in physical activity.
Depression/anxiety questionnaires
For a better understanding, symptoms of depression and anxiety were investigated with an interview with a psychologist who applied two tests to assess the symptoms of depression and anxiety. The BDI and the Beck Anxiety Inventory (BAI) were chosen for this analysis because of their ease of application, validation in other diseases, and other questionnaires (1414. Aben I, Verhey F, Lousberg R, Lodder J, Honig A. Validity of the beck depression inventory, hospital anxiety and depression scale, SCL-90, and hamilton depression rating scale as screening instruments for depression in stroke patients. Psychosomatics. 2002;43(5):386-93. https://doi.org/10.1176/appi.psy.43.5.386.
https://doi.org/10.1176/appi.psy.43.5.38...
). The BDI is a self-reported questionnaire consisting of 21 items scored on a 4-point scale ranging from 0 to 3, which measures the severity of depressive symptoms. The total score ranges from 0 to 63, with 0 to 13 indicating minimal depression, 14 to 19 indicating mild depression, 20 to 28 indicating moderate depression, and 29 to 63 indicating severe depression.
Anxiety symptoms were investigated using the BAI. The BAI is a list that describes 21 anxiety symptoms. Patients assessed the symptoms that they experienced during the past week and reported how much these symptoms bothered them. The items were scored on a 3-point scale ranging from 0 to 3, and the total score ranges from 0 to 63, where 0-9 indicate normal or no anxiety, 10-18 indicate mild-to-moderate anxiety, 19-29 indicate moderate-to-severe anxiety, and 30-63 indicates severe anxiety.
Statistics
SPSS version 17.0 (Chicago, USA) was used to analyze the data. The results are presented as means, standard deviations, and frequencies. Data normality was confirmed using the Shapiro-Wilk test and visual inspection of the histograms. Categorical variables were tested using the Pearson's chi-square test, and Student's t-test was used for independent samples. A p-value of ≤0.05 was adopted as the significance level.
RESULTS
The characteristics of the sample, which was predominantly male (67.8%) with a mean age of 66.7 years, depressive symptoms were more frequent than anxious symptoms, as detailed in Table 1.
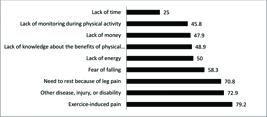
Fig. 1 shows the prevalence of personal barriers to walking. The two most prevalent barriers were pain induced by exercise and the presence of other diseases, injuries, or disabilities that impeded physical activity.
Patients with symptoms of depression had a shorter pain-free walking distance (p=0.015) and total walking distance (p=0.035) than those without symptoms of depression. The pain-free walking distance (p=0.29) and the total walking distance (p=0.07) of patients with and without symptoms of anxiety were similar (Table 2).
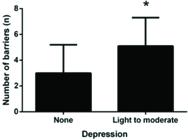
A comparison of the number of barriers between the presence of anxiety/depression symptoms is shown in Figs. 2 and 3. We observed that patients with symptoms of depression had significantly more barriers (p<0.005). This pattern was not observed among patients with symptoms of anxiety. Most patients reported more than one barrier, so the data overlap.
Table 3 shows the relationship between barriers to physical activity and depressive symptoms. Only three barriers were more significant among patients with depression than among those without depression.
Table 4 presents the association between barriers to physical activity and anxiety symptoms. There was no difference in the barriers present between patients with and without anxiety.
DISCUSSION
IC is the clinical manifestation of PAD that typically hinders patients’ usual activities by decreasing their ability to walk daily (1515. Gerage AM, Correia MA, Oliveira PML, Palmeira AC, Domingues WJR, Zeratti AE, et al. Physical Activity Levels in Peripheral Artery Disease Patients. Arq Bras Cardiol. 2019;113(3):410-6.,1616. Gardner AW, Ritti-Dias RM, Stoner JA, Montgomery PS, Scott KJ, Blevins SM. Walking economy before and after the onset of claudication pain in patients with peripheral arterial disease. J Vasc Surg. 2010;51(3):628-33. https://doi.org/10.1016/j.jvs.2009.09.053.
https://doi.org/10.1016/j.jvs.2009.09.05...
) and consequently reduce their quality of life (1717. McDermott MM, Greenland P, Guralnik JM, Liu K, Criqui MH, Pearce WH, et al. Depressive symptoms and lower extremity functioning in men and women with peripheral arterial disease. J Gen Intern Med. 2003;18(6):461-7. https://doi.org/10.1046/j.1525-1497.2003.20527.x.
https://doi.org/10.1046/j.1525-1497.2003...
). The initial treatment of IC is often clinical because, with conservative treatment, most patients experience improvement or stability in their clinical manifestations combined with a low risk of amputation. This treatment consists of the use of antiplatelet therapy and statins, the modification of aggressive risk factors (i.e., smoking, hyperlipidemia, and hypertension), and physical activity (1818. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33Suppl 1:S1-75. https://doi.org/10.1016/j.ejvs.2006.09.024.
https://doi.org/10.1016/j.ejvs.2006.09.0...
). When clinical treatment does not produce results, conventional or endovascular revascularization becomes an alternative (1818. Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33Suppl 1:S1-75. https://doi.org/10.1016/j.ejvs.2006.09.024.
https://doi.org/10.1016/j.ejvs.2006.09.0...
).
The sex ratio of our sample (67.9% male, 31.1% female) was comparable to that of other studies that have evaluated patients with IC (1919. Grenon SM, Cohen BE, Smolderen K, Vittinghoff E, Whooley MA, Hiramoto J. Peripheral arterial disease, gender, and depression in the Heart and Soul Study. J Vasc Surg. 2014;60(2):396-403. https://doi.org/10.1016/j.jvs.2014.02.013.
https://doi.org/10.1016/j.jvs.2014.02.01...
). The high rates of hypertension, smoking, diabetes, and acute myocardial infarction in our sample were similar to those expected for patients with PAD (2020. Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295(2):180-9. https://doi.org/10.1001/jama.295.2.180.
https://doi.org/10.1001/jama.295.2.180...
).
Psychological disorders such as depression and anxiety are significantly correlated with smoking. Several hypotheses have been proposed to explain the high rates of smoking among people with depression and anxiety (2121. Thomas AJ, Kalaria RN, O'Brien JT. Depression and vascular disease: what is the relationship? J Affect Disord. 2004;79(1-3):81-95. https://doi.org/10.1016/S0165-0327(02)00349-X.
https://doi.org/10.1016/S0165-0327(02)00...
). These hypotheses include the following: the individual smokes to alleviate their symptoms and therefore, the symptoms of depression and anxiety stimulate smoking; alternatively, smoking might lead to depression or anxiety through their effects on an individual's neurocircuits, which increases susceptibility to environmental stressors (2121. Thomas AJ, Kalaria RN, O'Brien JT. Depression and vascular disease: what is the relationship? J Affect Disord. 2004;79(1-3):81-95. https://doi.org/10.1016/S0165-0327(02)00349-X.
https://doi.org/10.1016/S0165-0327(02)00...
). In our sample, the incidence of active smoking was 19.5%, and this behavior was more frequent among patients with depression; however, no significant difference was observed with regard to patients without depression. The same result was observed with regard to patients with anxiety. It was not possible to establish a causal relationship between psychological symptoms and active smoking.
The daily physical activity of patients with IC decreases as the severity of PAD increases due to functional status deterioration (1717. McDermott MM, Greenland P, Guralnik JM, Liu K, Criqui MH, Pearce WH, et al. Depressive symptoms and lower extremity functioning in men and women with peripheral arterial disease. J Gen Intern Med. 2003;18(6):461-7. https://doi.org/10.1046/j.1525-1497.2003.20527.x.
https://doi.org/10.1046/j.1525-1497.2003...
). As a result of this process, patients tend to avoid physical activities because they lead to pain. Once patients stop adhering to regular exercise, they enter a vicious circle that can lead to a progressive worsening of functional status and quality of life (2222. Tew GA, Allen L, Askew CD, Chetter I, Cucato G, Doherty P, et al. Infographic. Exercise for intermittent claudication. Br J Sports Med. 2020:bjsports-2019-101930. https://doi.org/10.1136/bjsports-2019-101930.
https://doi.org/10.1136/bjsports-2019-10...
).
Although walking is one of the most accessible forms of physical activity and can easily be incorporated into patients' routines, especially those with IC, these patients do not easily adhere to treatment (2323. Harwood AE, Smith GE, Cayton T, Broadbent E, Chetter IC. A Systematic Review of the Uptake and Adherence Rates to Supervised Exercise Programs in Patients with Intermittent Claudication. Ann Vasc Surg. 2016;34:280-9. https://doi.org/10.1016/j.avsg.2016.02.009.
https://doi.org/10.1016/j.avsg.2016.02.0...
). Understanding the barriers associated with this low tendency to adhere to treatment can help solve a major problem with treatment.
The barriers analyzed here refer to the difficulties encountered by patients which prevent them from practicing physical activity. These barriers were studied through a previously validated questionnaire (88. Barbosa JP, Farah BQ, Chehuen M, Cucato GG, Farias Júnior JC, Wolosker N, et al. Barriers to physical activity in patients with intermittent claudication. Int J Behav Med. 2015;22(1):70-6. https://doi.org/10.1007/s12529-014-9408-4.
https://doi.org/10.1007/s12529-014-9408-...
). The analysis of the barriers to the practice of physical activity in our patients, regardless of mood, revealed that the most prevalent barrier was related to claudication symptoms (difficulty in walking with pain), which is consistent with previous studies (88. Barbosa JP, Farah BQ, Chehuen M, Cucato GG, Farias Júnior JC, Wolosker N, et al. Barriers to physical activity in patients with intermittent claudication. Int J Behav Med. 2015;22(1):70-6. https://doi.org/10.1007/s12529-014-9408-4.
https://doi.org/10.1007/s12529-014-9408-...
,2424. Cavalcante BR, Farah BQ, dos A Barbosa JP, Cucato GG, da Rocha Chehuen M, et al. Are the barriers for physical activity practice equal for all peripheral artery disease patients? Arch Phys Med Rehabil. 2015;96(2):248-52. https://doi.org/10.1016/j.apmr.2014.09.009.
https://doi.org/10.1016/j.apmr.2014.09.0...
,2525. de Sousa ASA, Correia MA, Farah BQ, Saes G, Zerati AE, Puech-Leao P, et al. Barriers and Levels of Physical Activity in Patients With Symptomatic Peripheral Artery Disease: Comparison Between Women and Men. J Aging Phys Act. 2019;27(5):719-24. https://doi.org/10.1123/japa.2018-0206.
https://doi.org/10.1123/japa.2018-0206...
). Patients with PAD are prone to lack of exercising and a sedentary lifestyle, which is the reverse of the therapeutic proposal. Other associated diseases such as arthrosis and cataracts as well as pulmonary and cardiac symptoms, are important barriers that hamper an exercise program. Several studies have demonstrated an association between anxiety/depression and cardiovascular disease (1717. McDermott MM, Greenland P, Guralnik JM, Liu K, Criqui MH, Pearce WH, et al. Depressive symptoms and lower extremity functioning in men and women with peripheral arterial disease. J Gen Intern Med. 2003;18(6):461-7. https://doi.org/10.1046/j.1525-1497.2003.20527.x.
https://doi.org/10.1046/j.1525-1497.2003...
,1919. Grenon SM, Cohen BE, Smolderen K, Vittinghoff E, Whooley MA, Hiramoto J. Peripheral arterial disease, gender, and depression in the Heart and Soul Study. J Vasc Surg. 2014;60(2):396-403. https://doi.org/10.1016/j.jvs.2014.02.013.
https://doi.org/10.1016/j.jvs.2014.02.01...
,2121. Thomas AJ, Kalaria RN, O'Brien JT. Depression and vascular disease: what is the relationship? J Affect Disord. 2004;79(1-3):81-95. https://doi.org/10.1016/S0165-0327(02)00349-X.
https://doi.org/10.1016/S0165-0327(02)00...
). The structural brain abnormalities resulting from PAD might predispose patients to geriatric depressive symptoms known as “vascular depression” (2626. Chun HY, Whiteley WN, Dennis MS, Mead GE, Carson AJ. Anxiety After Stroke: The Importance of Subtyping. Stroke. 2018;49(3):556-64. https://doi.org/10.1161/STROKEAHA.117.020078.
https://doi.org/10.1161/STROKEAHA.117.02...
). In addition, the number of studies that have assessed anxiety and depression symptoms in people with PAD has been increasing.
Several tests can be applied to identify patients’ moods (1414. Aben I, Verhey F, Lousberg R, Lodder J, Honig A. Validity of the beck depression inventory, hospital anxiety and depression scale, SCL-90, and hamilton depression rating scale as screening instruments for depression in stroke patients. Psychosomatics. 2002;43(5):386-93. https://doi.org/10.1176/appi.psy.43.5.386.
https://doi.org/10.1176/appi.psy.43.5.38...
). The tests are mostly self-assessments in which the patient reads multiple-choice questions and selects the best answer. The BDI was chosen by our group as it is among the most used and validated scales for various purposes such as the analysis of depressive symptoms after stroke, and it is equivalent to other tests (1414. Aben I, Verhey F, Lousberg R, Lodder J, Honig A. Validity of the beck depression inventory, hospital anxiety and depression scale, SCL-90, and hamilton depression rating scale as screening instruments for depression in stroke patients. Psychosomatics. 2002;43(5):386-93. https://doi.org/10.1176/appi.psy.43.5.386.
https://doi.org/10.1176/appi.psy.43.5.38...
,2727. Leyfer OT, Ruberg JL, Woodruff-Borden J. Examination of the utility of the Beck Anxiety Inventory and its factors as a screener for anxiety disorders. J Anxiety Disord. 2006;20(4):444-58. https://doi.org/10.1016/j.janxdis.2005.05.004.
https://doi.org/10.1016/j.janxdis.2005.0...
). Definitive cut-offs to classify anxiety or depression as “minimal”, “mild”, “moderate”, and “severe” are not defined in the literature. In our study, we decided to use lower cutoff values to increase the sensitivity to detect anxiety and depressive symptoms.
Studies have shown a depressive symptom prevalence of approximately 16% among patients with IC (2828. Torrent DJ, Maness MR, Capps TC, Sears SF, Whited AL, Yamaguchi DJ, et al. Impact of psychological factors on objective ambulatory measures in patients with intermittent claudication. J Vasc Surg. 2014;60(3):708-14. https://doi.org/10.1016/j.jvs.2014.03.281.
https://doi.org/10.1016/j.jvs.2014.03.28...
). We observed a higher prevalence in our sample (40%). This difference is most likely due to the exclusion of patients previously treated with antidepressants. When patients are treated, they might not report mood swings.
Anxiety has recently received increased attention, especially with regard to heart patients because both anxiety and depression have a strong relationship with the increased risk of adverse outcomes during surgery (33. Tully PJ, Cosh SM, Baumeister H. The anxious heart in whose mind? A systematic review and meta-regression of factors associated with anxiety disorder diagnosis, treatment and morbidity risk in coronary heart disease. J Psychosom Res. 2014;77(6):439-48. https://doi.org/10.1016/j.jpsychores.2014.10.001.
https://doi.org/10.1016/j.jpsychores.201...
). Anxiety is more prevalent among patients with severe PAD (critical ischemia). Smolderen et al. (11. Smolderen KG, Hoeks SE, Pedersen SS, van Domburg RT, de Liefde II, Poldermans D. Lower-leg symptoms in peripheral arterial disease are associated with anxiety, depression, and anhedonia. Vasc Med. 2009;14(4):297-304. https://doi.org/10.1177/1358863X09104658.
https://doi.org/10.1177/1358863X09104658...
) demonstrated that anxiety was less prevalent in asymptomatic patients and increased progressively with worsening symptoms. Thus, patients with pain at rest had a higher degree of anxiety than those who were asymptomatic. Therefore, we expected that anxious patients would have shorter (pain-free and total) walking distances, and the reporting of pain symptoms would occur in advance because anxiety about having pain would reduce the pain-free distance, but this result was not evident in our series. Patients with and without anxiety had similar walking limitations, which is consistent with what we expected, the total distance would not change because it is more related to physical disability resulting from arterial obstruction.
Patients with depression walk less than non-depressed patients, most likely because they exercise less. This finding was noted when we observed that patients with depression reported more barriers than non-depressed patients. Non-depressed patients are likely able to overcome these barriers and exercise regularly, thereby generating more expressive results with regard to walking distances. Non-depressed patients walk more than depressed patients, unlike anxious patients. Anxious patients do not report more barriers than non-anxious patients; therefore, they likely exercise regularly, regardless of their mood.
Interestingly, the lack of money, fear of falling, and worsening of PAD were the most important barriers among patients with depression. The walking instructions provided by the doctors included walking around the house, on flat streets, or in parks without slopes or hills, and avoiding rough terrain and bumpy sidewalks (which is not always feasible). An earlier study found that individuals who did not live near parks were 35% more likely to be inactive (88. Barbosa JP, Farah BQ, Chehuen M, Cucato GG, Farias Júnior JC, Wolosker N, et al. Barriers to physical activity in patients with intermittent claudication. Int J Behav Med. 2015;22(1):70-6. https://doi.org/10.1007/s12529-014-9408-4.
https://doi.org/10.1007/s12529-014-9408-...
). Patients who need to leave their environment and go to other places to exercise, which is associated with a lack of money, might have personal reasons to engage in physical activity.
Depressive symptoms alone can inhibit healthy behaviors and lead to functional declines through psychological and/or biological pathways. In general, the depressive symptoms of patients with IC can generate greater daily limitations than claudication alone.
Considering the high prevalence of depressive symptoms and its relationship to the barriers that hamper clinical treatment (i.e., daily physical activity), the treatment of depression should most likely result in improved walking distances and quality of life.
Our study had certain limitations, including the questionnaires used to investigate the barriers to physical activity. These questionnaires were adapted for patients with PAD; however, only minor changes were made (88. Barbosa JP, Farah BQ, Chehuen M, Cucato GG, Farias Júnior JC, Wolosker N, et al. Barriers to physical activity in patients with intermittent claudication. Int J Behav Med. 2015;22(1):70-6. https://doi.org/10.1007/s12529-014-9408-4.
https://doi.org/10.1007/s12529-014-9408-...
), and these changes might not have altered the results regarding both the prevalence of barriers and their association with physical activity level. Another limitation was the number of patients recruited, which was small primarily because most eligible patients received treatment from other medical specialties or were taking psychotropic medications.
CONCLUSIONS
Symptoms of anxiety and depression are prevalent among patients with PAD. Depression symptoms are associated with personal barriers to exercise, while anxiety symptoms are not.
The main barriers to physical activity among patients with IC are exercise-induced pain and the presence of other diseases.
REFERENCES
-
1Smolderen KG, Hoeks SE, Pedersen SS, van Domburg RT, de Liefde II, Poldermans D. Lower-leg symptoms in peripheral arterial disease are associated with anxiety, depression, and anhedonia. Vasc Med. 2009;14(4):297-304. https://doi.org/10.1177/1358863X09104658
» https://doi.org/10.1177/1358863X09104658 -
2Columbo JA, Stone DH, Goodney PP, Nolan BW, Stableford JA, Brooke BS, et al. The Prevalence and Regional Variation of Major Depressive Disorder Among Patients With Peripheral Arterial Disease in the Medicare Population. Vasc Endovascular Surg. 2016;50(4):235-40. https://doi.org/10.1177/1538574416644529
» https://doi.org/10.1177/1538574416644529 -
3Tully PJ, Cosh SM, Baumeister H. The anxious heart in whose mind? A systematic review and meta-regression of factors associated with anxiety disorder diagnosis, treatment and morbidity risk in coronary heart disease. J Psychosom Res. 2014;77(6):439-48. https://doi.org/10.1016/j.jpsychores.2014.10.001
» https://doi.org/10.1016/j.jpsychores.2014.10.001 -
4Grizzo Cucato G, de Moraes Forjaz CL, Kanegusuku H, da Rocha Chehuen M, Riani Costa LA, Wolosker N, et al. Effects of walking and strength training on resting and exercise cardiovascular responses in patients with intermittent claudication. Vasa. 2011;40(5):390-7. https://doi.org/10.1024/0301-1526/a000136
» https://doi.org/10.1024/0301-1526/a000136 -
5Ritti-Dias RM, Correia MA, Andrade-Lima A, Cucato GG. Exercise as a therapeutic approach to improve blood pressure in patients with peripheral arterial disease: current literature and future directions. Expert Rev Cardiovasc Ther. 2019;17(1):65-73. https://doi.org/10.1080/14779072.2019.1553676
» https://doi.org/10.1080/14779072.2019.1553676 -
6Farah BQ, Ritti-Dias RM, Cucato GG, Chehuen Mda R, Barbosa JP, Zeratti AE, et al. Effects of clustered comorbid conditions on walking capacity in patients with peripheral artery disease. Ann Vasc Surg. 2014;28(2):279-83. https://doi.org/10.1016/j.avsg.2013.01.020
» https://doi.org/10.1016/j.avsg.2013.01.020 -
7Chehuen M, Cucato GG, Carvalho CRF, Ritti-Dias RM, Wolosker N, Leicht AS, et al. Walking training at the heart rate of pain threshold improves cardiovascular function and autonomic regulation in intermittent claudication: A randomized controlled trial. J Sci Med Sport. 2017;20(10):886-92. https://doi.org/10.1016/j.jsams.2017.02.011
» https://doi.org/10.1016/j.jsams.2017.02.011 -
8Barbosa JP, Farah BQ, Chehuen M, Cucato GG, Farias Júnior JC, Wolosker N, et al. Barriers to physical activity in patients with intermittent claudication. Int J Behav Med. 2015;22(1):70-6. https://doi.org/10.1007/s12529-014-9408-4
» https://doi.org/10.1007/s12529-014-9408-4 -
9Wolosker N, Rosoky RA, Nakano L, Basyches M, Puech-Leão P. Predictive value of the ankle-brachial index in the evaluation of intermittent claudication. Rev Hosp Clin Fac Med Sao Paulo. 2000;55(2):61-4. https://doi.org/10.1590/S0041-87812000000200005
» https://doi.org/10.1590/S0041-87812000000200005 -
10Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126(24):2890-909. https://doi.org/Erratum in: Circulation. 2013;127(1):e264.
-
11Farah BQ, Ritti-Dias RM, Montgomery P, Cucato GG, Gardner A. Exercise Intensity during 6-Minute Walk Test in Patients with Peripheral Artery Disease. Arq Bras Cardiol. 2020;114(3):486-92.
-
12Booth ML, Bauman A, Owen N, Gore CJ. Physical activity preferences, preferred sources of assistance, and perceived barriers to increased activity among physically inactive Australians. Prev Med. 1997;26(1):131-7. https://doi.org/10.1006/pmed.1996.9982
» https://doi.org/10.1006/pmed.1996.9982 -
13Saelens BE, Sallis JF, Black JB, Chen D. Neighborhood-based differences in physical activity: an environment scale evaluation. Am J Public Health. 2003;93(9):1552-8. https://doi.org/10.2105/AJPH.93.9.1552
» https://doi.org/10.2105/AJPH.93.9.1552 -
14Aben I, Verhey F, Lousberg R, Lodder J, Honig A. Validity of the beck depression inventory, hospital anxiety and depression scale, SCL-90, and hamilton depression rating scale as screening instruments for depression in stroke patients. Psychosomatics. 2002;43(5):386-93. https://doi.org/10.1176/appi.psy.43.5.386
» https://doi.org/10.1176/appi.psy.43.5.386 -
15Gerage AM, Correia MA, Oliveira PML, Palmeira AC, Domingues WJR, Zeratti AE, et al. Physical Activity Levels in Peripheral Artery Disease Patients. Arq Bras Cardiol. 2019;113(3):410-6.
-
16Gardner AW, Ritti-Dias RM, Stoner JA, Montgomery PS, Scott KJ, Blevins SM. Walking economy before and after the onset of claudication pain in patients with peripheral arterial disease. J Vasc Surg. 2010;51(3):628-33. https://doi.org/10.1016/j.jvs.2009.09.053
» https://doi.org/10.1016/j.jvs.2009.09.053 -
17McDermott MM, Greenland P, Guralnik JM, Liu K, Criqui MH, Pearce WH, et al. Depressive symptoms and lower extremity functioning in men and women with peripheral arterial disease. J Gen Intern Med. 2003;18(6):461-7. https://doi.org/10.1046/j.1525-1497.2003.20527.x
» https://doi.org/10.1046/j.1525-1497.2003.20527.x -
18Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg. 2007;33Suppl 1:S1-75. https://doi.org/10.1016/j.ejvs.2006.09.024
» https://doi.org/10.1016/j.ejvs.2006.09.024 -
19Grenon SM, Cohen BE, Smolderen K, Vittinghoff E, Whooley MA, Hiramoto J. Peripheral arterial disease, gender, and depression in the Heart and Soul Study. J Vasc Surg. 2014;60(2):396-403. https://doi.org/10.1016/j.jvs.2014.02.013
» https://doi.org/10.1016/j.jvs.2014.02.013 -
20Bhatt DL, Steg PG, Ohman EM, Hirsch AT, Ikeda Y, Mas JL, et al. International prevalence, recognition, and treatment of cardiovascular risk factors in outpatients with atherothrombosis. JAMA. 2006;295(2):180-9. https://doi.org/10.1001/jama.295.2.180
» https://doi.org/10.1001/jama.295.2.180 -
21Thomas AJ, Kalaria RN, O'Brien JT. Depression and vascular disease: what is the relationship? J Affect Disord. 2004;79(1-3):81-95. https://doi.org/10.1016/S0165-0327(02)00349-X
» https://doi.org/10.1016/S0165-0327(02)00349-X -
22Tew GA, Allen L, Askew CD, Chetter I, Cucato G, Doherty P, et al. Infographic. Exercise for intermittent claudication. Br J Sports Med. 2020:bjsports-2019-101930. https://doi.org/10.1136/bjsports-2019-101930
» https://doi.org/10.1136/bjsports-2019-101930 -
23Harwood AE, Smith GE, Cayton T, Broadbent E, Chetter IC. A Systematic Review of the Uptake and Adherence Rates to Supervised Exercise Programs in Patients with Intermittent Claudication. Ann Vasc Surg. 2016;34:280-9. https://doi.org/10.1016/j.avsg.2016.02.009
» https://doi.org/10.1016/j.avsg.2016.02.009 -
24Cavalcante BR, Farah BQ, dos A Barbosa JP, Cucato GG, da Rocha Chehuen M, et al. Are the barriers for physical activity practice equal for all peripheral artery disease patients? Arch Phys Med Rehabil. 2015;96(2):248-52. https://doi.org/10.1016/j.apmr.2014.09.009
» https://doi.org/10.1016/j.apmr.2014.09.009 -
25de Sousa ASA, Correia MA, Farah BQ, Saes G, Zerati AE, Puech-Leao P, et al. Barriers and Levels of Physical Activity in Patients With Symptomatic Peripheral Artery Disease: Comparison Between Women and Men. J Aging Phys Act. 2019;27(5):719-24. https://doi.org/10.1123/japa.2018-0206
» https://doi.org/10.1123/japa.2018-0206 -
26Chun HY, Whiteley WN, Dennis MS, Mead GE, Carson AJ. Anxiety After Stroke: The Importance of Subtyping. Stroke. 2018;49(3):556-64. https://doi.org/10.1161/STROKEAHA.117.020078
» https://doi.org/10.1161/STROKEAHA.117.020078 -
27Leyfer OT, Ruberg JL, Woodruff-Borden J. Examination of the utility of the Beck Anxiety Inventory and its factors as a screener for anxiety disorders. J Anxiety Disord. 2006;20(4):444-58. https://doi.org/10.1016/j.janxdis.2005.05.004
» https://doi.org/10.1016/j.janxdis.2005.05.004 -
28Torrent DJ, Maness MR, Capps TC, Sears SF, Whited AL, Yamaguchi DJ, et al. Impact of psychological factors on objective ambulatory measures in patients with intermittent claudication. J Vasc Surg. 2014;60(3):708-14. https://doi.org/10.1016/j.jvs.2014.03.281
» https://doi.org/10.1016/j.jvs.2014.03.281
Publication Dates
-
Publication in this collection
20 Jan 2021 -
Date of issue
2021
History
-
Received
14 Sept 2020 -
Accepted
13 Oct 2020