Abstracts
The effect of organic manure on the community of mycorrhizal fungi (AMF) was evaluated in maize, cowpea and cotton intercropping systems in a semi-arid region of Brazil over two consecutive years. The experiment was conducted using a randomized block design with four replicates and six soil treatments: (M) goat manure incorporated in the soil before planting; (G) gliricidia prunings incorporated before planting; (M+G) manure and gliricidia incorporated before planting; (GS) gliricidia applied on the soil surface 45 days after planting; (M+GS) manure incorporated before planting and gliricidia applied to the surface 45 days after planting; and (C) control treatment without incorporation. It was not possible to identify the best treatment for both years in terms of spore density, mycorrhizal colonization and glomalin content. However, there was a significant effect from the fertilization treatments when compared to the control in most treatments. In both years, M+GS favored glomalin and AMF sporulation in the cotton plots. In the second year, which had low rainfall, there was an increase in production of spores, glomalin, colonization and AMF species richness irrespective of soil incorporation and culture. Plots that were fertilized with manure presented greater AMF species richness regardless of the year and crop type.
mycorrhizal association; Gliricidia sepium; goat manure; green fertilizer
O efeito da adubação orgânica sobre a comunidade de fungos micorrízicos arbusculares (FMA) em sistemas consorciados com milho, feijão e algodão foi avaliado no semi-árido paraibano, ao longo de dois anos. O experimento foi conduzido em delineamento de blocos casualizados com quatro repetições e seis tratamentos: (M) esterco incorporado no solo antes do plantio; (G) ramas de gliricídia incorporadas antes do plantio; (M+G) esterco e gliricídia incorporados no solo antes do plantio; (GS) gliricídia aplicada em cobertura 45 dias após o plantio; (M+GS) esterco incorporado antes do plantio e gliricídia aplicada em cobertura 45 dias após o plantio; (C) tratamento controle. Não foi possível identificar um melhor tratamento, em ambos os anos, em termos de densidade de esporos, colonização micorrízica e teor de glomalina. Entretanto, houve efeito significativo das incorporações na maioria dos tratamentos quando comparados ao controle. Em ambos os anos, M+GS favoreceu a produção de glomalina e a esporulação de FMA no sistema com algodoeiro. No segundo ano, de poucas chuvas, houve aumentos na produção de esporos, glomalina, colonização e riqueza de espécies de FMA, independentemente do efeito das incorporações e da cultura. Os tratamentos com esterco apresentaram maior riqueza de espécies, independentemente do ano e da cultura.
associação micorrízica; Gliricidia sepium; esterco caprino; adubos verdes
AGRICULTURE MICROBIOLOGY
Occurrence of arbuscular mycorrhizal fungi after organic fertilization in maize, cowpea and cotton intercropping systems
Ocorrência de fungos micorrízicos arbusculares após adubação orgânica em sistemas consorciados de milho, feijão e algodão
Carla da Silva SousaI, * * Author for correspondence. E-mail: cssagro@yahoo.com.br ; Rômulo Simões Cezar MenezesII; Everardo Valadares de Sá Barreto SampaioII; Fritz OehlIII; Leonor Costa MaiaIV; Marlon da Silva GarridoV; Francisco de Sousa LimaI
ICentro de Ciências Agrárias, Ambientais e Biológicas, Universidade Federal do Recôncavo da Bahia, R. Rui Barbosa, s/n, Campus Universitário, 44380-000, Cruz das Almas, Bahia, Brazil
IIDepartamento de Energia Nuclear, Universidade Federal de Pernambuco,Recife, Pernambuco, Brazil
IIIAgroscope Reckenholz-Tänikon Research Station, Reckenholzstrasse, Zürich, Switzerland
IVDepartamento de Micologia, Universidade Federal de Pernambuco, Recife, Pernambuco, Brazil
VUniversidade Federal do Vale do São Francisco, Petrolina, Pernambuco, Brazil
ABSTRACT
The effect of organic manure on the community of mycorrhizal fungi (AMF) was evaluated in maize, cowpea and cotton intercropping systems in a semi-arid region of Brazil over two consecutive years. The experiment was conducted using a randomized block design with four replicates and six soil treatments: (M) goat manure incorporated in the soil before planting; (G) gliricidia prunings incorporated before planting; (M+G) manure and gliricidia incorporated before planting; (GS) gliricidia applied on the soil surface 45 days after planting; (M+GS) manure incorporated before planting and gliricidia applied to the surface 45 days after planting; and (C) control treatment without incorporation. It was not possible to identify the best treatment for both years in terms of spore density, mycorrhizal colonization and glomalin content. However, there was a significant effect from the fertilization treatments when compared to the control in most treatments. In both years, M+GS favored glomalin and AMF sporulation in the cotton plots. In the second year, which had low rainfall, there was an increase in production of spores, glomalin, colonization and AMF species richness irrespective of soil incorporation and culture. Plots that were fertilized with manure presented greater AMF species richness regardless of the year and crop type.
Keywords: mycorrhizal association, Gliricidia sepium, goat manure, green fertilizer.
RESUMO
O efeito da adubação orgânica sobre a comunidade de fungos micorrízicos arbusculares (FMA) em sistemas consorciados com milho, feijão e algodão foi avaliado no semi-árido paraibano, ao longo de dois anos. O experimento foi conduzido em delineamento de blocos casualizados com quatro repetições e seis tratamentos: (M) esterco incorporado no solo antes do plantio; (G) ramas de gliricídia incorporadas antes do plantio; (M+G) esterco e gliricídia incorporados no solo antes do plantio; (GS) gliricídia aplicada em cobertura 45 dias após o plantio; (M+GS) esterco incorporado antes do plantio e gliricídia aplicada em cobertura 45 dias após o plantio; (C) tratamento controle. Não foi possível identificar um melhor tratamento, em ambos os anos, em termos de densidade de esporos, colonização micorrízica e teor de glomalina. Entretanto, houve efeito significativo das incorporações na maioria dos tratamentos quando comparados ao controle. Em ambos os anos, M+GS favoreceu a produção de glomalina e a esporulação de FMA no sistema com algodoeiro. No segundo ano, de poucas chuvas, houve aumentos na produção de esporos, glomalina, colonização e riqueza de espécies de FMA, independentemente do efeito das incorporações e da cultura. Os tratamentos com esterco apresentaram maior riqueza de espécies, independentemente do ano e da cultura.
Palavras-chave: associação micorrízica, Gliricidia sepium, esterco caprino, adubos verdes.
Introduction
In the semi-arid region of northeastern Brazil, high rainfall variability, recurrent droughts and low soil fertility limit the agriculture and cattle productivity of the land (SILVA; MENEZES, 2007). The use of chemical fertilizers to improve soil fertility is not viable for most producers in this region due to irregular rainfall and low agriculture profitability. Therefore, organic fertilization is the most viable alternative to replenish the soil nutrients (TIESSEN et al., 1994).
Livestock manure is the main organic fertilizer used in the region; however, the amount produced is insufficient to fertilize all of the agricultural land (GARRIDO et al., 2009). Furthermore, the available manure is usually low quality and contains low nitrogen and high lignin content, which can lead to N immobilization and hinder crop growth (MENEZES; SALCEDO, 2007; SILVA; MENEZES, 2007).
In addition to livestock manure, the use of green manure, which consists of biomass from plant species, is a low-cost practice that may increase crop yield in low-input agricultural systems such as those in the semi-arid region of NE Brazil (TIESSEN
et al., 1994). When used in combination with livestock manure, green manure can supply the necessary N to enable faster decomposition of the low quality livestock manure and supply other nutrients such as P (SILVA; MENEZES, 2007).
An example of green manure with a potential use in semi-arid conditions is gliricidia, which provides high quality forage to feed livestock (BARRETO; FERNANDES, 2001) and is able to fix significant amounts of atmospheric N in association with diazotrophic bacteria (BALA et al., 2003). In addition, gliricidia produces biomass under conditions of low water availability (MARIN et al., 2007). Pruned gliricidia leaves and thin twigs (< 1 cm in diameter) have a low secondary metabolite content and high N mineralization rate (MAFONGOYA et al., 2000).
Arbuscular mycorrhizal fungi (AMF) are important organisms in plant nutrition because they contribute to increased root absorption of low mobility nutrients in the soil, such as P, Zn and Cu (MIRANDA et al., 2008). The beneficial effects of organic fertilizers were also observed for infectivity (PALENZUELA et al., 2002), mycorrhizal colonization (GRYNDLER et al., 2005) and production of propagules in the field (GAUR; ADHOLEYA, 2005).
The organic fertilizers benefit AMF by improving the soil properties and production of certain substances during their decomposition (GRYNDLER et al., 2005). Although the use of organic sources favors the intra-root phase, factors such as dose, type of residue, plant species and AMF should be considered for the success of symbiosis in organic cultivation (SILVA et al., 2007).
Negative responses of AMF to the incorporation of organic residues have been observed and attributed to the high nutrient content of these materials, presence of phytotoxic substances (MARTIN et al., 2002), the specific composition of the residue (BORIE et al., 2002) and/or presence of pathogens (ELORRIETA et al., 2003).
The effects of organic fertilization on AMF are variable, and more studies are needed to clarify their relationship. Therefore, the objective of the present work was to evaluate the effect of organic fertilization with gliricidia and/or goat manure on AMF in an intercropped system of maize, cowpea and cotton in the semi-arid region of Brazil.
Material and methods
Description of the experimental area
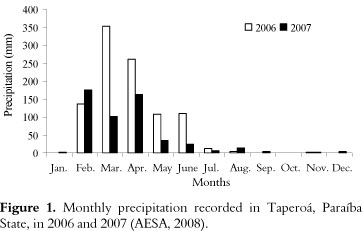
The experiment was performed in 2006 and 2007 at the Agroecological Station of Vila Maria Rita, in Taperoá municipality, Paraíba State, Brazil, which is located at 7º 12"23" South and 36º 49"25" West and has anaverage altitude of 520 m. The soil from the experimental area is classified as a Fluvent. The average annual precipitation is 558 mm, and the average annual temperature is 26ºC. The total monthly rainfall was recorded at the study site during the two years of the experiment (Figure 1).
Experiment
The experiment was established in random blocks with four replicates. The intercropping systems included maize, Zea mays L. (Sergipano variety); cowpea, Vigna unguiculata (L.) Walp (Moitinha variety); herbaceous cotton, Gossipium hirsutum L. (H8 variety, Embrapa CNPA) and the following organic fertilization systems: M - goat manure incorporated in the soil before planting; G - gliricidia prunings incorporated before planting; M+G - gliricidia and manure incorporated before planting; GS - gliricidia applied on the soil surface 45 days after planting; M+GS - manure incorporated before planting and gliricidia applied 45 days after planting; and C - control treatment without incorporation. The experimental plots were 5 x 7 m with a harvest area of 4 x 5 m. The intercropping system consisted of alternating rows with holes planted with cotton, cowpea and maize. Four seeds of each crop species were placed in each hole, and the seedlings were thinned to maintain only two plants per hole approximately two weeks after emergence.
Goat manure and gliricidia were applied in doses equivalent to 20 ton. ha-1 of dry matter for manure and 20 ton. ha-1 of fresh matter for gliricidia in treatments with a single fertilizer source. For treatments with both fertilizers, 10 ton. ha-1 of dry matter of manure and 10 ton. ha-1 of fresh matter of gliricidia were applied. The average dry matter content of the gliricidia fresh prunings was 250 g kg-1. The fertilizers were incorporated to a depth of 15 cm using a manual hoe when applied to the soil before planting or were spread evenly on the soil throughout the entire experimental plot when surface applied.
Fertilizer incorporation and crop planting were performed immediately after the first rains in 2006 and 2007, which was between January and March. Weeding was manually performed using a hoe three times throughout the crop cycle, and insect pests were controlled with neem oil. The nutrient content of the gliricidia and manure used, on a dry matter basis, was 34 and 12 g N kg-1; 6.1 and 6.5 g P kg-1; and 21 and 27 g K kg-1, respectively, as determined by Embrapa (1999).
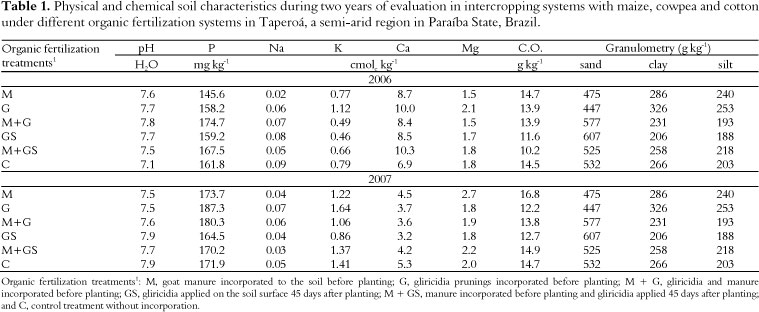
Soil samples were collected from the 0-15 cm layer from each experimental plot for chemical and physical analysis (EMBRAPA, 1999) in 2006 and 2007 (Table 1). Rhizospheric soil and root samples were collected in 2006 and 2007. The samples were air-dried, separated, homogenized and sieved with 2-mm mesh. Fine roots (< 2 mm) were separated from the soil, washed in water and stored in plastic containers with 50% alcohol for conservation until analysis.
AMF spore density and species identification
AMF spores were extracted from 50-g subsamples using the humid sieving technique (GERDEMANN; NICOLSON, 1963), which employs superposed sieves of 50-µm, 100-µm and 250-µm sieves followed by centrifugation in water (3000 g) and 45% sucrose (2000 g) for 3 and 1 min., respectively. After, the spores were counted in channeled plates using a stereomicroscope (40x).
For the identification of the AMF species, trap cultures were prepared using soil samples diluted in autoclaved sand (1:1), which were, transferred to 500 mL plastic pots planted with Italian millet (Panicum miliaceum L.). After three millet multiplication cycles, the spores were extracted from the soil, separated according to their morphological characteristics and mounted on glass slides with PVLG (polyvinyl-lactoglycerol alcohol) and Melzer + PVLG (1:1; v v-1). Species richness was considered as the number of species that occurred in the plot. The frequency of occurrence was estimated according to Equation 1: Fi = Ji/k*100, where: Ji was the number of samples in which the species was observed, and k was the number of total soil samples.
Quantification of glomalin-related soil proteins
The content of glomalin-related soil proteins was quantified by the Wright and Upadhyaya (1998) method, in which 0,25 g of soil were autoclaved with 2 mL of sodium citrate (20 mM; pH 7.0) for 30 min. at 121ºC, and afterwards centrifuged at 10000 g for 5 min. An aliquot of 50 µL of the supernatant together with 2,5 mL of the shiny comassie brilliant blue dye G-250 was used for the quantification of glomalin content. Bovine serum albumin was used as standard.
Mycorrhizal colonization
The percentage of mycorrhizal colonization was determined using the split-plate intersect method (GIOVANNETTI; MOSSE, 1980). The preparation of the samples began with the clarification of the roots with KOH (10%) for 24 hours, at room temperature.
After this, the roots were treated with H2O2 for 45 minutes, with HCl (1%) for 3 minutes and colored with Trypan blue (0.05%). One-hundred colored root segments were separated for visualization of fungal structures (arbuscules, vesicles and hyphae) using a stereomicroscope (40x).
Statistical analysis
Results were submitted to analysis of variance, and the averages were compared by the Scott and Knott test at 5% probability using the SISVAR software package. Data of spore density and percentage of mycorrhizal colonization were transformed by (x + 0.5)½ and arc sen  , respectively.
, respectively.
Results and discussion
In 2006, treatment with G, M+G and GS increased AMF spore production (81, 73 and 75 spores in 50 g soil, respectively) in the rhizosphere of maize (Table 2). In the following year, the M+G treatment continued favoring sporulation (278 spores in 50 g soil); however, the spore numbers did not significantly differ from the M system (258 spores in 50 g soil). No significant differences were observed among the fertilization treatments regarding mycorrhizal colonization; however, in 2006, they were superior to the control treatment. The contents of glomalin-related soil proteins (GRSP) in all treatments, except for the M+G treatment, were superior to the control treatment in 2006 but not in 2007.
M and GS favored spore production in the rhizosphere of cowpea in 2006 (53 and 61 spores in 50 g soil, respectively). In the following year, greater values were observed in the G system (292 spores in 50 g soil). There was no significant effect on the fertilization treatments in terms of mycorrhizal colonization regardless of the year. In both years, the GRSP contents increased in all fertilization systems, except G, when compared to the control treatment.
Spore density, mycorrhizal colonization and GRSP content varied among the fertilization treatments; therefore, it was not possible to identify the consistently best system for maize and cowpea for both years. However, in most situations, there was a significant effect of the fertilization systems when compared to the control.
There are many explanations for the beneficial effects of organic fertilization on AMF. According to Gryndler et al. (2005), humic substances, such as fulvic acids that result from the decomposition of organic fertilizers, adsorb free cations from the soil solution and may favor the physiological functions of the fungal mycelia (absorption and transport). The improvement in soil structure also contributes to AMF mycelia development because it reduces the mechanical resistance to hyphal growth. Furthermore, bacterial communities that promote spore germination and increase the rate of AMF colonization (JOHANSSON et al., 2004) are favored by the addition of organic fertilizers (CRECCHIO et al., 2001). According to Muthukumar and Udaiyan (2002), conditions that favor plant growth, especially growth of the root systems, also promote the establishment of the mycorrhizal association because they increase the chance for contact between the surface of the roots and AMF propagules in the soil.
The M+GS system favored spore and glomalin production in the rhizosphere of cotton in both years. The cotton plants had the greatest mycorrhizal colonization; however, in 2006, it did not significantly differ from the GS system (60 and 56%, respectively). In 2007, except for the M+G fertilization system, mycorrhizal colonization of cotton did not significantly differ among the fertilization systems; however, they were superior to the control.
Garrido et al. (2009) reported that the M+GS treatment had the highest cotton biomass production, which was linked to slow crop growth in phase with the gradual nutrient release by the manure. The large biomass production likely resulted in an increase of photosynthesis and photosynthate allocation to the roots, which stimulated sporulation, mycorrhizal colonization and glomalin production by the AMF associated with these plants.
It has been shown that moderate quantities of organic fertilizers have small less adverse effects on AMF than the equivalent quantities of mineral fertilizers, which is likely due to a temporal difference in P availability that results from its gradual release that is concomitant with demands by the plants (BODDINGTON; DODD, 2000).
Significant increases in spore density (215-1270%) and mycorrhizal colonization (95-257%) occurred in the 2007 crop cycle compared to the 2006 cycle regardless of the fertilization system. These increases may represent a resistance mechanism of AMF to adverse conditions because the rainfall was much lower in 2007 (Figure 1). Root colonization and sporulation are crucial strategies for survival of AMF under adverse conditions (HART; READER, 2002).
An increase in the GRSP content was observed in the rhizosphere of maize and cowpea in all fertilization systems in 2007 when compared to 2006, which varied from 107 to 576%. This increase was not observed in the rhizosphere of cotton. It is possible that the AMF increased the number of propagules, which is used as a survival strategy, and favored the increase of glomalin production. According to Driver et al. (2005), glomalin deposition in the soil is a result of the decomposition of spores and hyphae (> 80%), and to a lesser degree, is due to the passive release or secretion of the hyphae.
A total of 21 AMF species that belong to the genera Glomus (12), Acaulospora (6), Ambispora (1), Entrophosphora (1) and Kuklospora (1) were registered during both crop years (Table 3). With the highest number of known species, the genus Glomus had the greatest presence, which may indicate a possible relationship with the soil pH in the region (above 7.5) because species belonging to this genus generally predominate in a pH from 6.0 to 8.0 (MOREIRA; SIQUEIRA, 2006). According to Carrenho et al. (2001), species of Glomus have the capacity to adapt to different soil organic matter contents, such as liming and texture. In other studies with organic fertilization (FOCCHI et al., 2004; OEHL et al., 2004; PURIN et al., 2006), the predominance of Glomus species have also been reported.
Species of Acaulospora tend to be found in soils with a pH lower than 6.5 (GAI et al., 2006). However, in our work, species of this genus were observed even when the pH was greater than 7.5. Similarly, Silva et al. (2005) reported the occurrence of Acaulospora species in regions with a pH above 7.0, which indicates that this species is likely tolerant to a broad pH spectrum.
Genera such as Glomus and Acaulospora, which produce small spores, are more able to survive by adapting their sporulation patterns under unfavorable conditions, such as in semi-arid environments (GAI et al., 2006; LI et al., 2007; SHI et al., 2007; TAO; ZHIWEI, 2005). This dominance may also indicate a selective adaptation to water stress in hot and arid regions.
In general, there were more species (64%) in 2007 regardless of the fertilization system, and the presence of A. elegans, A. spinosa, E. infrequens, G. aggregatum, G. constrictum, G. sp resembling, G. eburneum, G. glomerulatum, G. sinuosum, G. tortuosum and Kuklospora colombiana were all detected. It is possible that the increase in sporulation in this year enabled the recovery of a greater number of AMF species. The M+GS system had an increased species richness (12 species) compared to the other fertilization systems regardless of the crop and year. Oehl et al. (2004) observed greater species diversity of AMF in organic systems than in other cultivation systems; among these species were A. longula, G. etunicatum, G. constrictum and G. mosseae, which were also observed in the present study. Purin et al. (2006) reported the presence of 20 AMF species in conventional orchards and 30 AMF species in organic apple orchards in the southern region of Brazil and found eight species (A. morrowiae, A. spinosa, A. scrobiculata, E. infrequens, G. claroideum, G. etunicatum, G. mosseae and G. sinuosum) that were also detected in the present study. In organic systems in western Pennsylvania, USA, Franke-Snyder et al. (2001) detected the presence of G. mosseae, G. etunicatum and G. claroideum.
Usually, the actual AMF species richness may be greater than what is reported because the size and morphological resemblance among Glomus spores may hinder species identification (MAIA et al., 2006). The eventual spore production by some species and the presence of non-viable spores may also hinder species identification (SOUZA et al., 2010). Furthermore, spore multiplication in pot cultures likely did not enable the recovery and identification of all species present in the plots.
Entrophosphora infrequens was exclusively registered in the M+G system in maize rhizospheric soil while A. scrobiculata, G. sinuosum and G. aggregatum were identified only in the M+GS system associated with cowpea. In addition, A. elegans and G. eburneum-like were only detected in the M system in maize and cowpea soil, respectively. Glomus glomerulatum was only observed in the GS system with cowpea. Kuklospora colombiana was identified only incotton plots without organic fertilizer application. Oehl et al. (2010) stated that abiotic factors were more important than biotic factors to the establishment of AMF populations. Furthermore, local conditions determine the selection of AMF species, which are more demanding for environmental adaptation and produce favorable responses to plant development.
Thirteen, fifteen and eleven AMF species were observed in the maize, cowpea and cotton rhizosphere when considering all organic fertilization systems and both years. Glomus claroideum and G. etunicatum had the greatest presence in 2006 and 2007, respectively. However, it is important to highlight that species with a low frequency of occurrence may be more efficient than species that were more frequent despite the fact that the latter may be more adapted to the soil and climate conditions (PURIN et al., 2006).
Conclusion
The application of organic fertilizers favored AMF activity and diversity in the rhizosphere of maize and cowpea when compared to the control treatment.
The system M+GS favored spore and glomalin production in the rhizosphere of cotton.
Plots that were fertilized with systems M or M+GS had greater species richness than the other fertilization systems, regardless of the crop and year.
In the second crop year there was a significant increase in sporulation, mycorrhizal colonization, glomalin production (except for cotton) and AMF species richness, regardless of the fertilization system and the crop.
Acknowledgements
The authors thank the financial support provided by CNPq - Edital MCT/CNPq 15/2007 (Process 478138/2007-5), Edital MCT/CNPq 01/2005 (Project Imsear- Process n. 420294/2005-8) and Edital MCT/CNPq/CT-Agro 43/2008 (Process 574893/2008-3). The authors also thank the support of the Inter American Institute for Global Change Research (IAI) - Project Amfoods (CRN2-014) and from Facepe - Edital PPP 2006 (Processo APQ-0633-5.01/06).
Received on April 13, 2011.
Accepted on July 2, 2011.
License information: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- AESA-Agência Executiva de Gestão das Águas do Estado da Paraíba. Avaliable from: <http://www.site2.aesa.pb.gov.br/aesa/monitoramentoPluviometria.do?metodo=listarMesesChuvasMensais>. Access on: Nov. 25, 2008.
» link - BALA, A.; MURPHY, P.; GILLER, K. E. Distribution and diversity of rhizobia nodulating agroflorestry legumes in soil from three continents in the tropics. Molecular Ecology, v. 12, n. 4, p. 917-929, 2003.
- BARRETO, A. C.; FERNANDES, F. M. Cultivo de Gliricidia sepium e Leucaena leucocephala em alamedas visando a melhoria dos solos dos tabuleiros costeiros. Pesquisa Agropecuária Brasileira, v. 36, n. 10, p. 1287-1293, 2001.
- BODDINGTON, C. L.; DODD, J. C. The effect of agricultural practices on the development of indigenous arbuscular mycorrhizal fungi. I. Field studies in an Indonesian ultisol. Plant and Soil, v. 218, n. 1-2, p. 137-144, 2000.
- BORIE, F.; REDEL, Y.; RUBIO, R.; ROUANET, J. L.; BAREA, J. M. Interations between crop residues application and mycorrhizal developments and some root interface properties and mineral acquisition by plants in an acidic soil. Biology and Fertility of Soils, v. 36, n. 2, p. 151-160, 2002.
- CARRENHO, R.; TRUFEM, S. F. B.; BONONI, V. L. R. Fungos micorrízicos arbusculares em rizosferas de três espécies de fitobiontes instaladas em área de mata ciliar revegetada. Acta Botânica Brasílica, v. 15, n. 1, p. 115-124, 2001.
- CRECCHIO, C.; CURCI, M.; MININNI, R.; RICCIUTI, P.; RUGGIERO, P. Short-term effects of municipal solid waste compost amendments on soil carbon and nitrogen content, some enzyme activities and genetic diversity. Biology and Fertility of Soils, v. 34, n. 5, p. 311-318, 2001.
- DRIVER, J. D.; HOLBEN, W. E.; RILLIG, M. C. Characterization of glomalin as a hyphal wall component of arbuscular mycorrhizal fungi. Soil Biology and Biochemistry, v. 37, n. 1, p. 101-106, 2005.
- ELORRIETA, M. A.; SUÁREZ-ESTRELA, F.; LÓPEZ, M. J.; VARGAS-GÁRCIA, M. C.; MORENO, J. Survival of phytopathogenic bacteria during waste composting. Agriculture, Ecosystems and Environment, v. 96, n. 1-3, p. 141-146, 2003.
- EMBRAPA-Empresa Brasileira de Pesquisa Agropecuária. Manual de análises químicas de solos, plantas e fertilizantes / Embrapa Solos Embrapa Informática agropecuária. Organizador Fábio César da Silva. Brasília: Embrapa Comunicação para Transferência de Tecnologia, 1999.
- FOCCHI, S. S.; SOGLIO, F. K. D.; CARRENHO, R.; SOUZA, P. V. D.; LOVATO, P. E. Fungos micorrízicos arbusculares em cultivos de citros sob manejo convencional e orgânico. Pesquisa Agropecuária Brasileira, v. 39, n. 5, p. 469-476, 2004.
- FRANKE-SNYDER, M.; DOUDS JR., D. D.; GALVEZ, L.; PHILLIPS, J. G.; WAGONER, P.; DRINKWATER, L.; MORTON, J. B. Diversity of communities of arbuscular mycorrhizal (AM) fungi present in conventional versus low-input agricultural sites in Eastern Pennsylvania, USA. Applied Soil Ecology, v. 16, n. 1, p. 35-48, 2001.
- GAI, J. P.; FENG, G.; CAI, X. B.; CHRISTIE, P.; LI, X. L. A preliminary survey of the arbuscular mycorrhizal status of grassland plants in southern Tibet. Mycorrhiza, v. 16, n. 3, p. 191-196, 2006.
- GARRIDO, M. S.; MENEZES, R. S. C.; SAMPAIO, E. V. S. B.; MARQUES, T. R. R. Crescimento e absorção de nutrientes pelo algodoeiro e pela mamoneira adubados com gliricídia e esterco. Revista Brasileira de Engenharia Agrícola e Ambiental, v. 13, n. 5, p. 531-536, 2009.
- GAUR, A.; ADHOLEYA, A. Diverse response of five ornamental plant species to mixed indigenous and single isolate arbuscular mycorrhizal inocula in marginal soil amended with organic matter. Journal of Plant Nutrition, v. 28, n. 4, p. 707-723, 2005.
- GERDEMANN, J. W.; NICOLSON, T. H. Spores of mycorrhizal Endogonespecies extracted from soil by wet sieving and decanting. Transactions of the British Mycological Society, v. 46, n. 2, p. 235-244, 1963.
- GIOVANNETTI, M.; MOSSE, B. An evaluation of techniques to measure vesicular-arbuscular mycorrhizal infection in roots. New Phytologist, v. 84, n. 3, p. 484-500, 1980.
- GRYNDLER, M.; HRSELOVÁ, H.; SUDOVÁ, R.; GRYNDLEROVÁ, H.; REZÁCOVÁ, V.; MERHAUTOVÁ, V. Hyphal growth and mycorrhiza formation by the arbuscular fungus Glomus calaroideum BEG23 is stimulated by humic substances. Mycorrhiza, v. 15, n. 7, p. 483-488, 2005.
- HART, M. H.; READER, R. J. Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytologist, v. 153, n. 2, p. 335-344, 2002.
- JOHANSSON, J.; PAUL, L. R.; FINLAY, R. D. Microbial interactions in the mycorrhizosphere and their significance for sustainable agriculture. FEMS Microbiology Ecology, v. 48, n. 1, p. 1-13, 2004.
- LI, L.-F.; LI, T.; ZHAO, Z.-W. Differences of arbuscular mycorrhizal fungal diversity and community between a cultivated land, and old field, and a never-cultivated field in a hot and arid ecosystem of southwest China. Mycorrhiza, v. 17, n. 8, p. 655-665, 2007.
- MAFONGOYA, P. L.; BARAK, P.; REED, J. D. Carbon, nitrogen and phosphorus mineralization of tree leaves and manure. Biology and Fertility of Soils, v. 30, n. 4, p. 298-305, 2000.
- MAIA, L. C.; YANO-MELO, A. M.; GOTO, B. T. Filo Glomeromycota. In: PORTO, K. C.; CORTEZ, A.; TABARELLI, M. (Ed.). Diversidade biológica e conservação da floresta atlântica ao Norte do Rio São Francisco Brasília: MMA, 2006. p. 109-126.
- MARIN, A. M. P.; MENEZES, R. S. C.; SALCEDO, I. H. Produtividade de milho solteiro ou em aléias de gliricídia adubadas com duas fontes orgânicas. Pesquisa Agropecuária Brasileira, v. 42, n. 5, p. 669-677, 2007.
- MARTIN, T.; SAMPEDRO, I.; GARCIA-ROMERA, I.; GARCÍA-GARRIDO, J. M.; OCAMPO, J. A. Arbuscular mycorrhizal colonization and growth of soybean (Glycine max) and lettuce (Lactuca sativa) and phytotoxic effect of olive mill residues. Soil Biology and Biochemistry, v. 34, n. 11, p. 1769-1775, 2002.
- MENEZES, R. S. C.; SALCEDO, I. H. Mineralização de N após incorporação de adubos orgânicos em um Neossolo Regolítico cultivado com milho. Revista Brasileira de Engenharia Agrícola e Ambiental, v. 11, n. 4, p. 361-367, 2007.
- MIRANDA, E. M.; SAGGIN JUNIOR, O. J.; SILVA, E. M. R. Seleção de fungos micorrízicos arbusculares para o amendoim forrageiro consorciado com braquiária. Pesquisa Agropecuária Brasileira, v. 43, n. 9, p. 1185-1191, 2008.
- MOREIRA, F. M. S.; SIQUEIRA, J. O. Microbiologia e bioquímica de solo Lavras: UFLA, 2006.
- MUTHUKUMAR, T.; UDAIYAN, K. Growth and yield of cowpea as influenced by changes in arbuscular mycorrhiza in responde to organic manuring. Journal of Agronomy and Crop Science, v. 188, n.2, p. 123-132, 2002.
- OEHL, F.; LACZKO, E.; BOGENRIEDER, A.; STAHR, K.; BÖSCH, R.; VAN DER HEIJDEN, M. G. A.; SIEVERDING, E. Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities. Soil Biology and Biochemistry, v. 42, n. 5, p. 724-732, 2010.
- OEHL, F.; SIEVERDING, E.; MÄDER, P.; DUBOIS, D.; INEICHEN, K.; BOLLER, T.; WIEMKEN, A. Impact of long-term conventional and organic farming on the diversity of arbuscular mycorrhizal fungi. Oecologia, v. 138, n. 4, p. 574-583, 2004.
- PALENZUELA, J.; AZCÓN-AGUIAR, C.; FIGUEROA, D.; CARAVANA, F.; ROLDÁN, A.; BAREA, J. M. Effects of mycorrhizal inoculation of shrubs from Mediterranean ecosystems and composed residue application on transplant performance and mycorrhizal developments in a desertified soil. Biology and Fertility of Soils, v. 36, n. 2, p. 170-175, 2002.
- PURIN, S.; KLAUBERG FILHO, O.; STÜRMER, S. L. Mycorrhizae activity and diversity in conventional and organic apple orchards from Brazil. Soil Biology and Biochemistry, v. 38, n. 7, p. 1831-1839, 2006.
- SHI, Z. Y.; ZHANG, L. Y.; LI, X. L.; FENG, G.; TIAN, C. Y.; CHRISTIE, P. Diversity of arbuscular mycorrhizal fungi associated with desert ephemerals in plant communities of Junggar Basin, northwest China. Applied Soil Ecology, v. 35, n. 1, p. 10-20, 2007.
- SILVA, F. S. B.; MELO, A. M. Y.; MAIA, L. C. Production and infectivity of inoculum of arbuscular mycorrhizal fungi multiplied in a substrate supplemented with tris-HCl buffer. Brazilian Journal of Microbiology, v. 38, n. 4, p. 752-755, 2007.
- SILVA, G. A.; TRUFEM, S. F. B.; SAGGIN JÚNIOR, O. J.; MAIA, L. C. Arbuscular mycorrhizal fungi in a semiarid copper mining area in Brazil. Mycorrhiza, v. 15, n. 1, p. 47-53, 2005.
- SILVA, T. O.; MENEZES, R. S. C. Adubação orgânica da batata com esterco e, ou, Crotalaria juncea II - Disponibilidade de N, P e K no solo ao longo do ciclo de cultivo. Revista Brasileira de Ciência do Solo, v. 31, n. 1, p. 51-61, 2007.
- SOUZA, R. G.; GOTO, B. T.; SILVA, D. K. A.; SILVA, F. S. B.; SAMPAIO, E. V. S. B.; MAIA, L. C. The role of arbuscular mycorrhizal fungi and cattle manure in the establishment of Tocoyena selloana Schum. in mined dune areas. European Journal of Soil Biology, v.46, n. 3-4, p. 237-242, 2010.
- TAO, L.; ZHIWEI, Z. Arbuscular mycorrhizas in hot and arid ecosystem in southwest China. Applied Soil Ecology, v. 29, n. 2, p. 135-141, 2005.
- TIESSEN, H.; CUEVAS, E.; CHACON, P. The role of soil organic matter in sustaining soil fertility. Nature, v. 371, n. 6500, p. 783-785, 1994.
- WRIGHT, S. F.; UPADHYAYA, A. A. A survey of soils for aggregate stability and glomalina, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant and Soil, v. 198, n. 1, p. 97-107, 1998.
Publication Dates
-
Publication in this collection
05 June 2012 -
Date of issue
June 2012
History
-
Received
13 Apr 2011 -
Accepted
02 July 2011