Abstracts
It is possible to determine the optimum time for permanence of vegetative propagules (mini-cuttings) inside a greenhouse for rooting, and this value can be used to optimize the structure of the nursery. The aim of this study was to determine the dynamics of adventitious rooting in mini-cuttings of three clones of Eucalyptus benthamii x Eucalyptus dunnii. Sprouts of H12, H19 and H20 clones were collected from mini-stumps that were planted in gutters containing sand and grown in a semi-hydroponic system. The basal region of the mini-cuttings was immersed in 2,000 mg L-1 indole-3-butyric acid (IBA) solution for 10 seconds. The rooting percentage of the mini-cuttings, the total length of the root system and the rooting rate per mini-cutting were also evaluated at 0 (time of planting), 7, 14, 21, 28, 35, 42, 49 and 56 days. We used logistic and exponential regression to mathematically model the speed of rhizogenesis. The rooting percentage was best represented as a logistic model, and the total length of the root system was best represented as an exponential model. The clones had different speeds of adventitious rooting. The optimum time for permanence of the mini-cuttings inside the greenhouse for rooting was between 35 and 42 days, and varied depending on the genetic material.
vegetative propagation; clonal forestry; organogenesis; mathematical modeling; rhizogenesis; clonal mini-garden
O tempo ideal de permanência de propágulos vegetativos (miniestacas) no interior da casa de vegetação para a rizogênese é possível de ser determinado matematicamente, o que pode otimizar as instalações do viveiro. O objetivo deste estudo foi determinar a dinâmica de enraizamento de miniestacas de três clones de Eucalyptus benthamii x Eucalyptus dunnii. Brotações dos clones H19, H12 e H20 foram coletadas de minicepas plantadas em canaletão com areia e cultivadas sob sistema semi-hidropônico. A região basal da miniestaca foi imersa em solução de 2.000 mg L-1 de ácido indolbutírico (AIB) por 10 segundos. A porcentagem de enraizamento de miniestacas, o comprimento total de raízes e a taxa de enraizamento por miniestaca foram avaliados a cada sete dias (0 - instante da estaquia, 14, 21, 28, 35, 42, 49 e 56 dias). Foram utilizadas as regressões logística e exponencial para a modelagem matemática da velocidade dos processos rizogênicos. O modelo logístico teve o melhor ajuste para o percentual de enraizamento, e o modelo exponencial para o comprimento total do sistema radicular. Os clones apresentaram diferentes velocidades de enraizamento. O tempo ideal de permanência das miniestacas dentro da casa de vegetação para o enraizamento está entre 35 a 42 dias, podendo variar em função do material genético.
propagação vegetativa; silvicultural clonal; organogênese; modelagem matemática; rizogênese; minijardim clonal
CROP PRODUCTION
Dynamics of adventitious rooting in mini-cuttings of Eucalyptus benthamii x Eucalyptus dunnii
Dinamica de enraizamento adventício em miniestacas de Eucalyptus benthamii x Eucalyptus dunnii
Gilvano Ebling BrondaniI, * * Authors for correspondence. E-mail: gebrondani@yahoo.com.br; ivar@cnpf.embrapa.br ; Ivar WendlingII, * * Authors for correspondence. E-mail: gebrondani@yahoo.com.br; ivar@cnpf.embrapa.br ; André Ebling BrondaniIII; Marla Alessandra AraujoIV; André Luís Lopes da SilvaV; Antônio Natal GonçalvesI
ILaboratório de Fisiologia das Árvores, Programa de Pós-graduação em Recursos Florestais, Escola Superior de Agricultura "Luiz de Queiroz", Universidade de São Paulo, Av. Pádua Dias, 11, Cx. Postal 9, 13418-900, Piracicaba, São Paulo, Brazil
IILaboratório de Propagação de Plantas, Empresa Brasileira de Pesquisa Agropecuária, Embrapa Florestas, Colombo, Paraná, Brazil
IIIDepartamento de Matemática, Instituto de Ciências Exatas, Polo Universitário de Volta Redonda, Universidade Federal Fluminense, Volta Redonda, Rio de Janeiro, Brazil
IVLaboratório de Análise Foliar, Universidade Federal de Lavras, Lavras, Minas Gerais, Brazil
VLaboratório de Bioprocessos e Biotecnologia, Universidade Federal do Paraná, Curitiba, Paraná, Brazil
ABSTRACT
It is possible to determine the optimum time for permanence of vegetative propagules (mini-cuttings) inside a greenhouse for rooting, and this value can be used to optimize the structure of the nursery. The aim of this study was to determine the dynamics of adventitious rooting in mini-cuttings of three clones of Eucalyptus benthamii x Eucalyptus dunnii. Sprouts of H12, H19 and H20 clones were collected from mini-stumps that were planted in gutters containing sand and grown in a semi-hydroponic system. The basal region of the mini-cuttings was immersed in 2,000 mg L-1 indole-3-butyric acid (IBA) solution for 10 seconds. The rooting percentage of the mini-cuttings, the total length of the root system and the rooting rate per mini-cutting were also evaluated at 0 (time of planting), 7, 14, 21, 28, 35, 42, 49 and 56 days. We used logistic and exponential regression to mathematically model the speed of rhizogenesis. The rooting percentage was best represented as a logistic model, and the total length of the root system was best represented as an exponential model. The clones had different speeds of adventitious rooting. The optimum time for permanence of the mini-cuttings inside the greenhouse for rooting was between 35 and 42 days, and varied depending on the genetic material.
Keywords: vegetative propagation, clonal forestry, organogenesis, mathematical modeling, rhizogenesis, clonal mini-garden.
RESUMO
O tempo ideal de permanência de propágulos vegetativos (miniestacas) no interior da casa de vegetação para a rizogênese é possível de ser determinado matematicamente, o que pode otimizar as instalações do viveiro. O objetivo deste estudo foi determinar a dinâmica de enraizamento de miniestacas de três clones de Eucalyptus benthamii x Eucalyptus dunnii. Brotações dos clones H19, H12 e H20 foram coletadas de minicepas plantadas em canaletão com areia e cultivadas sob sistema semi-hidropônico. A região basal da miniestaca foi imersa em solução de 2.000 mg L-1 de ácido indolbutírico (AIB) por 10 segundos. A porcentagem de enraizamento de miniestacas, o comprimento total de raízes e a taxa de enraizamento por miniestaca foram avaliados a cada sete dias (0 - instante da estaquia, 14, 21, 28, 35, 42, 49 e 56 dias). Foram utilizadas as regressões logística e exponencial para a modelagem matemática da velocidade dos processos rizogênicos. O modelo logístico teve o melhor ajuste para o percentual de enraizamento, e o modelo exponencial para o comprimento total do sistema radicular. Os clones apresentaram diferentes velocidades de enraizamento. O tempo ideal de permanência das miniestacas dentro da casa de vegetação para o enraizamento está entre 35 a 42 dias, podendo variar em função do material genético.
Palavras-chave: propagação vegetativa, silvicultural clonal, organogênese, modelagem matemática, rizogênese, minijardim clonal.
Introduction
The Eucalyptus genus was originally limited to Australia and the regions around it, and comprises about 600 species, ranging from small shrubs to large trees that occur in a vast range of habitats (TURNBULL, 1999). Eucalyptus are the most widely planted hardwood trees all over the world (CABRAL et al., 2011; TURNBULL, 1999) due to their large number of species, wide adaptability to soils and climates, fast growth rates, the wide knowledge and technology for their culturing and the variety of wood and non-wood products that come from them. The commercial cloning of Eucalyptus began in 1975 in the Republic of Congo (DELWAULLE et al., 1983) and was introduced in Brazil in 1979 (CAMPINHOS; IKEMORI, 1983). The development of clonal propagation techniques has allowed for the full maintenance of genetic characteristics of a selected plant (donor of mini-cuttings), which has provided uniform forests with rapid growth and the production of homogeneous raw materials (FERREIRA et al., 2004; SCHWAMBACH et al., 2008). In general, the advantages of vegetative propagation are the uniformity of forests, the adaptation of specific clones for production areas and the maximization of timber production in quantity and quality, as compared to forest plantations produced by seeds (seedlings) (GOULART; XAVIER, 2008; HARTMANN et al., 2002; SCHWAMBACH et al., 2008). Moreover, clonal forests can allow for the accurate prediction of yields and, therefore, the profits.
Rooting processes for the production of vegetative propagules of Eucalyptus such as micro-cutting and mini-cutting techniques have been used for the propagation of selected clones (superior trees), which allows considerable gains for commercial production due to higher rooting rates and a reduced time for mini-cutting formation (STAPE et al., 2001; TITON et al., 2002). Mini-cutting is a propagation technique that has largely been used in recent years for the commercial cloning of Eucalyptus in Brazil. This mass propagation technique originated as a response to the limitations of the micro-cutting technique used to obtain rejuvenated material in micropropagation laboratories, including technical, structural and operational limitations as well as the cost. The mini-cutting technique can be divided into the following stages: (i) production of mini-cuttings in clonal mini-garden, (ii) induction of adventitious rooting in the greenhouse under intermittent mist and with a controlled temperature, (iii) acclimation in a shade house and (iv) growth and hardening in outdoor conditions (BRONDANI et al., 2010a).
Plant growth regulators have had positive effects on rooting in Eucalyptus species that have poor rooting capacities. Indole-3-butyric acid (IBA) is a commonly used growth regulator that has been applied in different forms, including powder, liquid (ALMEIDA et al., 2007; BRONDANI et al., 2010a and b; DAY et al., 1998; WENDLING; XAVIER, 2005) and, more recently, gel (BRONDANI et al., 2008). However, considering the conditions favorable to disease incidence in the greenhouse during root formation and the need to optimize the installations in the nursery, a technical criterion can be used to determine the optimum time for permanence of mini-cuttings in the greenhouse. This value can be calculated by taking the intercept between the curves of the daily current increment (DCI) and the daily medium increment (DMI) for the rooted mini-cuttings, similar to what has been done in the field of forestry biometrics (FERREIRA et al., 2004). Using this criterion, Ferreira et al. (2004) determined the optimum time for rooting of mini-cuttings of two clones of Eucalyptus spp., obtaining the optimal time for permanence of the mini-cuttings inside the greenhouse and demonstrating the potential of mathematical modeling of rhizogenic processes for the management of selected genotypes. Despite recent advances in forest biotechnology, current scientific reports are insufficient in terms of the rooting efficiency of the mini-cutting technique for the optimization of installations in the nursery.
In regions where the incidence of severe frosts affects the establishment of Eucalyptus forests, the adaptation to low temperatures is one of the most important characteristics that can be introduced via genetic transformation or hybridization. The hybrids Eucalyptus benthamii and Eucalyptus dunnii were of economic importance to cold regions in Brazil. Nevertheless, subtropical species of Eucalyptus generally have poor adventitious rooting abilities (ASSIS; MAFIA, 2007; BRONDANI et al., 2010b). Additionally, the production of interspecific hybrids of elite genotypes may provide additional benefits (TRUEMAN; RICHARDSON, 2007), considering the adaptive advantages of the parental species.
We aimed to determine the dynamics of rooting through mathematical modeling of the emissions and root growth of three clones of Eucalyptus benthamii Maiden & Cambage x Eucalyptus dunnii Maiden propagated by the mini-cutting technique.
Material and methods
General characterization of the experiment
The experiment was conducted from April to June of 2006 in a greenhouse belonging to the National Research Center for Forestry Research - Embrapa Florestas, Colombo, Paraná State, Brazil (25°20' S and 49°14' W; 950 m). The climate of the region is Cfb, according to the Köppen classification.
Source of clones and formation of the clonal mini-garden
The sprouts used to obtain the mini-cuttings originated from Eucalyptus benthamii x Eucalyptus dunnii mini-stumps (clones H12, H19 and H20) (Figure 1A), grown in a semi-hydroponic system of the 'canaletão' (gutters) type, containing medium sand. The mini-stumps were produced by conventional cuttings from trees selected on the field at one year of age (BRONDANI et al., 2010b), which were planted in gutters with a spacing of 10.0 x 15.0 cm. At 21 days after planting, pruning of the mini-stumps was conducted in the apical sprouts at 7 cm above the root collar, keeping a pair of leaves per mini-stump to induce new axilar sprouts (Figure 1B). The first collection of sprouts was conducted 45 days after the mini-stumps were pruned (Figure 1C and D), forming a clonal mini-garden (Figure 1E).
Management and nutrition of mini-stumps
The clonal mini-garden was kept in a greenhouse covered with polyethylene. The mini-stumps received nutrient solution three times per day (daily total of 5 L m-2), prepared from a commercial fertilizer mixture.
The nutrient solution was composed of: monoammonium phosphate (Krista®, 0.04 g L-1), magnesium sulfate (Citral®, 0.40 g L-1), potassium nitrate (Krista®, 0.44 g L-1), ammonium sulfate (Boutin®, 0.31 g L-1), calcium chloride (Hydro®, 0.79 g L-1), boric acid (Nutriplant®, 2.88 mg L-1), manganese sulfate (Multitécnica®, 3.70 mg L-1), sodium molybdate (IPC®, 0.18 mg L-1), zinc sulfate (Nutriplant®, 0.74 mg L-1) and wetted iron dust (LibFer®, 81.80 mg L-1). The pH of the nutrient solution was adjusted to 5.5 (± 0.1) with NaOH or HCl, both at 1.0 M. The nutrient solution was replaced every three weeks or if the electrical conductivity of the water drained from the canal was higher than 4.0 mS m-2 at 25°C. During each replacement of nutrient solution, the semi-hydroponic system was washed with 11 L m-2 tap water to remove excess salt. The chemical analysis of the water showed the following characteristics: 1.4 mg L-1 N-NO3-; 0.4 mg L-1 N-NH4+; 0.93 mg L-1 P; 1.44 mg L-1 K; 26.08 mg L-1 Ca; 5.07 mg L-1 Mg; 0.4 mg L-1 Cu; 0.09 mg L-1 Fe; 0.04 mg L-1 Mn and 0.04 mg L-1 Zn.
Collection of sprouts and preparation of the mini-cuttings
Before the sprouts were pruned, plant materials were sterilized with a 70% water-ethanol solution (v v-1), and the sprouts of three hybrid clones of Eucalyptus benthamii x Eucalyptus dunnii (H12, H19 and H20) were collected. The sprouts were stored in styrofoam boxes containing water to prevent the loss of cellular turgor in the tissues and reduce oxidation. After the sprouts were collected, shoot tips from the mini-cuttings were eliminated, and, for each mini-cutting, a pair of leaves was maintained with a reduction of 50% of the total leaf area. Each mini-cutting was 6 cm (± 1 cm) in total length.
The basal region of each mini-cutting was immersed in a solution containing 2,000 mg L-1 IBA (water:ethanol, 1:1, v v-1, Merck®) for 10 seconds. The mini-cuttings were planted in conical plastic tubes (55 cm3) with 2 cm of the basal region inserted in the substrate. The mixture substrate was prepared with carbonized rice husk and medium vermiculite (1:1, v v-1). The conical plastic tubes were immersed in 0.25% active chlorine solution (NaOCl) for 48 hours for disinfection before the mini-cuttings were inserted.
Rooting of the mini-cuttings
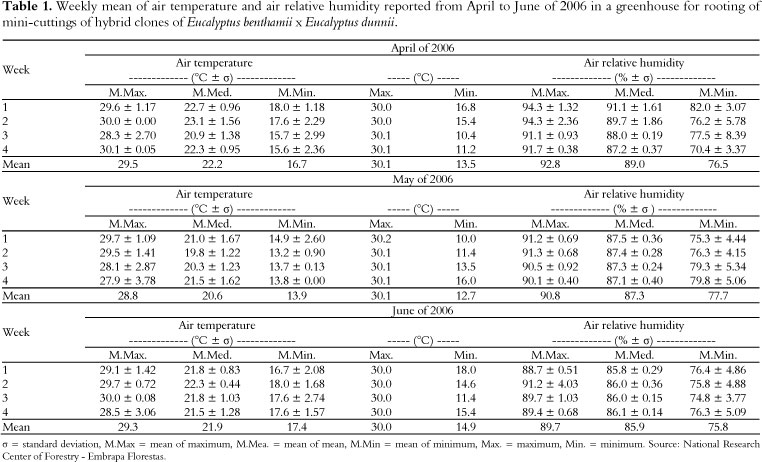
The mini-cuttings were kept in a greenhouse with an intermittent mist system set at a misting flow rate of 7 L h-1. The relative humidity and the temperature of the air were automatically controlled by a humidistat and timer, respectively. Air temperatures and relative humidities in the greenhouse were reported weekly during the experimental period (April to June of 2006) (Table 1).
Response variables and experimental design
Rooting was determined every seven days: at 0 (time of planting), 7, 14, 21, 28, 35, 42, 49 and 56 days after the mini-cuttings were brought into the greenhouse. Each evaluation used 10 randomly sampled mini-cuttings per replicate, and the number of mini-cuttings rooted, the total length of the root system (TENNANT, 1975) and the rooting rate were evaluated. The sampling was destructive. Through weekly evaluations, the daily current increment (DCI) and the daily medium increment (DMI) were determined. The optimum time for permanence of the mini-cuttings in the greenhouse was calculated as the intercept between the DCI and DMI curves (FERREIRA et al., 2004). The DCI and DMI curves were determined using the following formula:
where:
i = time of evaluation; X(i+1) = total length of root system in the time i+1; X(i) = root total length in the time i; T(i) = days in the time i; DCI = daily current increment and DMI = daily medium increment.
The experiment was conducted using a completely randomized design with a factorial arrangement. The experiments were performed using three clones (H12, H19 and H20) with eight weekly evaluations (7, 14, 21, 28, 35, 42, 49 and 56 days) of five replicates of 70 mini-cuttings.
Statistical analysis
The data were analyzed using the Bartlett test (p < 0.05) to test for homogeneity of variance between treatments. Analysis of variance (ANOVA) was used to test for significant effects of the treatments (p < 0.01 and p < 0.05). According to significance, the data were subjected to logistic and exponential regression (p < 0.05).
Results and discussion
Rooting percentage
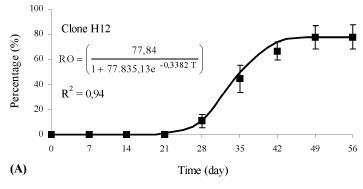
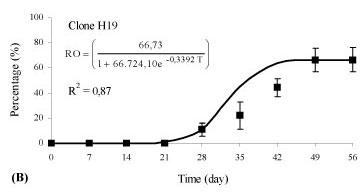
There was an interaction between the rooting percentage (p < 0.01) and the weekly evaluations (time) for clones of Eucalyptus benthamii x Eucalyptus dunnii propagated by the mini-cutting technique. Different clones showed different behaviors in relation to rhizogenesis, and, therefore, different equations were adjusted (p < 0.05). The variation of the rooting percentage of the mini-cuttings in relation to time (days) was adequately represented by an adjusted logistic equation and was statistically significant (p < 0.05).
Rhizogenesis of the mini-cuttings started at day 21 (Figure 2), independent of the clone evaluated. For the H12 clone, 66.7% of the mini-cuttings had rooted at 42 days (Figure 2A), and rooting stabilization occurred during this evaluation period. For the H19 and H20 clones, 44.4% of the mini-cuttings had rooted at 42 days (Figures 2B and C ), with a similar growth rate between the two genotypes. Thus, the time for permanence of mini-cuttings (clones H12, H19 and H20) in the greenhouse for an adequate rooting percentage can vary, and the permanence time can be longer or shorter, depending on the genetic material.
Total length of the root system
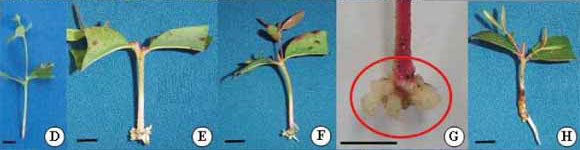
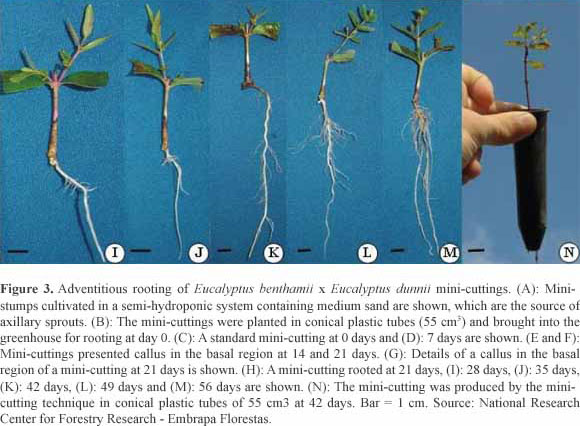
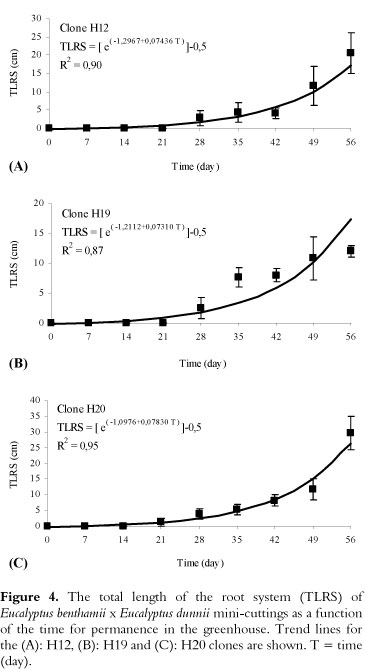
The semi-hydroponic system, containing mini-stumps of hybrids (Figure 3A), was efficient for the propagation process, from sprout production to the preparation of mini-cuttings (Figure 3B and C). During the sampling period of the mini-cuttings, 7 days after entry into the greenhouse, morphological changes in the basal region were not observed (Figure 3D). However, at 14 days, callus formation in the basal region of the mini-cuttings was verified, without the presence of adventitious roots (Figure 3E-G). The first adventitious roots were detected at 21 days (Figure 3H), regardless of the clone evaluated, and the roots grew exponentially up to 56 days (Figures 3I-M and 4). Again, there was a growth rate differential between the clones (Figure 4).
The total length of the root system varied between the clones (p < 0.05) and increased exponentially until 56 days (Figure 4). At 35 days (Figure 3J), the total length of the root system of the mini-cuttings for the recipients (conical plastic tubes of 55 cm3) was 5.0 cm, 7.7 cm and 5.4 cm for the H12, H19 and H20 clones, respectively (Figure 4A-C).
During this time, the roots showed good formation and development but were very fragile. At 42 days, the roots were better formed, with a greater number of secondary and tertiary roots (Figure 3K) for all clones (Figure 3N).
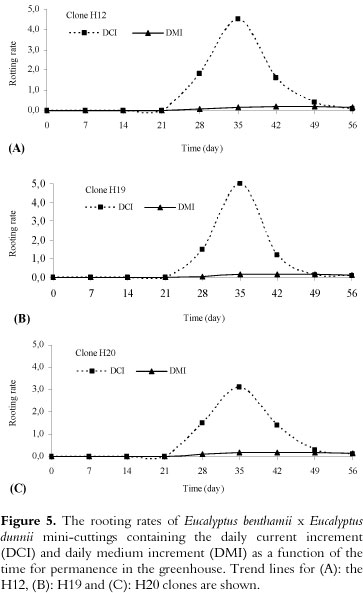
Curves of the DCI (daily current increment) and the DMI (daily medium increment)
Regardless of the clone evaluated, the maximum growth of the roots was reached at 35 days, and then a growth reduction occurred until day 56 (Figure 5A-C). This behavior is associated with the restriction of root growth by the conical plastic tubes, which have a specific volume limiting the growth and development of roots (55 cm3), until the mini-cutting is formed (Figure 3N). The intercept between the DCI and DMI curves occurred at approximately 49 days, indicating that the mini-cutting should be transferred for acclimation before that stage (49 days).
Mini-cutting is an important cloning technique in the forestry sector, mainly used for the production of selected tree mini-cuttings, allowing genetic characteristics to be maintained (MAJADA et al., 2011; ROMERO, 2004; SCHWAMBACH et al., 2008; STAPE et al., 2001; WENDLING; XAVIER, 2005; WENDLING et al., 2010). Despite advances in plant biotechnology, rhizogenesis in mini-cuttings (adventitious root formation) is poorly understood, including the different factors (chemical and physical) that influence their induction and formation (HARTMANN et al., 2002; HUNT et al., 2011; HUSEN; PAL, 2007; RASMUSSEN et al., 2009; RISOPATRON et al., 2010), as well as the speed of root growth. The root growth can be evaluated and used as a parameter to mathematically model the time of permanence of mini-cuttings in the greenhouse (FERREIRA et al., 2004; GOULART; XAVIER, 2008; TITON et al., 2002), optimizing the installations and mini-cuttings production in a nursery.
Thus, in accordance with the practices adopted in each nursery, numerous factors may influence the rooting rate, which can cause variations within the management system based on the genetic material, increasing or decreasing the time for permanence of mini-cuttings in the greenhouse for rooting. Considering the mass production of mini-cuttings, it is desirable to relate the appropriate management of structures in the nursery, to optimize all the installations, such as greenhouses and environments, for acclimation and hardening and to use the shortest time, ensuring the quantity and quality of the mini-cutting produced.
Among the limiting factors for the cloning of plants via the mini-cutting technique, the minimum permanence time of vegetative propagules in the greenhouse for rooting has been highlighted (GOULART; XAVIER, 2008). The greenhouse has a high relative humidity and air temperature, which, in certain conditions, are favorable to the spread of disease. Unlike the growth speed of the adventitious root, several mathematical models (e.g., exponential and logistic models) can be used to represent rhizogenesis (FERREIRA et al., 2004), and moreover, can provide a basis for determining the dynamics of rooting for different genetic materials. The optimization of the rooting time in the greenhouse can reduce the negative effects of the high humidity on the adventitious root system, avoiding mortality after rooting.
Based on the data analyzed, our results revealed that the rooting percentages varied between the clones (H12, H19 and H20), and it was possible to use different mathematical equations to model their behavior. Regardless of the clone studied, rhizogenesis began 21 days after the mini-cuttings were brought into the greenhouse and reached their maximum growth at 35 days. According to Ferreira et al. (2004), it is common to overestimate the permanence time of vegetative propagules in greenhouse conditions for the induction of adventitious roots. However, with the use of mathematical models, we were able to determine a better time to transfer the mini-cuttings to acclimation conditions in a shade-house, for each genetic material.
The formation of adventitious roots in mini-cuttings involves the formation of meristematic cells niches (i.e., initial cells and/or target cells), which are dependent on external and internal factors (DETTMER et al., 2009; PAPP; PLATH, 2011; RISOPATRON et al., 2010; SMET; BEECKMAN, 2011; SMET et al., 2009). The differentiation of these groups of cells in the root primordia and the emergence of new roots involve the rupture of other tissues of the stem and the formation of vascular connections with the conductive tissues (HARTMANN et al., 2002; LI et al., 2009). There are two routes that can originate or induce the formation of adventitious roots in vegetative propagules: (i) direct organogenesis from different cellular tissues such as the vascular cambium and (ii) from callus tissue followed by mechanical damage, which occurs when vegetative propagules are cut (LI et al., 2009). Generally, the presence of callus at a region of vascular connection is considered a problem, because the connection region is very fragile, and callus can compromise the functionality of the adventitius root (LI et al., 2009). Nevertheless, it is desirable that the adventitius root has a direct connection with the vascular cambium, which influences the functioning of the root.
The rooting rates evaluated in our study are suitable for this type of hybrid (subtropical Eucalyptus) and are in accordance with studies performed by Brondani et al. (2010a and b). More broadly, the data are also similar to those recorded for the mini-cutting technique for other eucalypt species (FERREIRA et al., 2004; GOULART; XAVIER, 2008; SCHWAMBACH et al., 2008; STAPE et al., 2001; TITON et al., 2002).
The H12 clone showed a higher rooting rate than the other clones (H19 and H20). This difference in the rooting speed between genotypes has been previously reported (FERREIRA et al., 2004; HARTMANN et al., 2002). Many factors may be related to differences in the behavior of rooting, including enzymes, genes, proteins, the temperature of the rooting substrate, the ontogenetic age of the plant tissue and the application of growth regulators (ANDRÉS et al., 2002; CORRÊA; FETT-NETO, 2004; DAI et al., 2004; FOGAÇA; FETT-NETO, 2005; HUSEN; PAL, 2007; LI et al., 2009; PAPP; PLATH, 2011; RASMUSSEN et al., 2009; RISOPATRON et al., 2010; THORPE, 2004; TRUEMAN; RICHARDSON, 2008).
The total root length also varied significantly between the clones and was an important variable to consider for modeling the rooting behavior of different clones. This information is important for the management of each clone (e.g., permanence time in the greenhouse for rooting) and the optimization of the installations in the nursery.
The DCI and DMI curves allowed us to determine the maximum rooting time (i.e., establish the time of the greatest growth), which was followed by a reduction in root growth, similar to what was reported by Ferreira et al. (2004). The reduction in root growth is related to the type of recipient used for mini-cutting production (HARTMANN et al., 2002). In this study, we use conical plastic tubes with a capacity of 55 cm3, which are commonly used in Brazil, and the roots of the mini-cuttings were limited by this volume until day 56. Due to this factor, the roots grew until a maximum was reached (35 days) and then tended to reduce their growth (Figure 5).
The optimal time for the transfer of mini-cuttings to the greenhouse must properly match the stage of root development, as mortality of the mini-cutting can occur during the process of acclimation in a shade house if the roots are not developed. Based on our observations, the suitable period for the formation and growth of the adventitious root of the Eucalyptus hybrid is between 35 and 42 days, which is the ideal time interval for the transfer of mini-cuttings to acclimate in a shade house.
In summary, it was possible to model mathematically the growth and speed of adventitious rooting in mini-cuttings of Eucalyptus benthamii x Eucalyptus dunnii. The data obtained in this study provide a basis for the management of mass mini-cutting production of specific genetic materials and can be adopted at commercial nurseries to optimize their installations.
Conclusion
The rooting percentage and the total length of the root system of Eucalyptus benthamii x Eucalyptus dunnii mini-cuttings are possible to model mathematically.
The genotypes show different speeds of rooting.
Considering the experimental conditions, the rooting percentage is best represented by a logistic model and the total length of the root system is best represented by an exponential model.
The optimum time for permanence of mini-cuttings in a greenhouse is between 35 and 42 days, which varies depending on the genotype.
Acknowledgements
The authors thank to EMBRAPA and the Ministry of Agriculture of Brazil for support.
Received on April 3, 2011.
Accepted on June 1, 2011.
License information: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
- ALMEIDA, F. D.; XAVIER, A.; DIAS, J. M. M.; PAIVA, H. N. Auxin (IBA and NAA) effects on minicutings rooting of Eucalyptus cloeziana F. Muell. clones. Revista Árvore, v. 31, n. 3, p. 455-463, 2007.
- ANDRÉS, H.; FERNÁNDEZ, B.; RODRÍGUEZ, R.; RODRÍGUEZ, A. Phytohormone contents in Corylus avellana and their relationship to age and other developmental processes. Plant Cell, Tissue and Organ Culture, v. 70, n. 2, p. 173-180, 2002.
- ASSIS, T. F.; MAFIA, R. G. Hibridação e clonagem. In: BORÉM, A. (Ed.). Biotecnologia florestal Viçosa: Suprema, 2007. p. 93-121.
- BRONDANI, G. E.; WENDLING, I.; ARAUJO, M. A.; PIRES, P. P. Indolbutyric acid in gel for minicuttings rooting of Eucalyptus benthamii Maiden & Cambage x Eucalyptus dunnii Maiden. Scientia Agraria, v. 9, n. 2, p. 153-158, 2008.
- BRONDANI, G. E.; GROSSI, F.; WENDLING, I.; DUTRA, L. F.; ARAUJO, M. A. IBA application for rooting of Eucalyptus benthamii Maiden & Cambage x Eucalyptus dunnii Maiden minicuttings. Acta Scientiarum. Agronomy, v. 32, n. 4, p. 667-674, 2010a.
- BRONDANI, G. E.; WENDLING, I.; GROSSI, F.; DUTRA, L. F.; ARAUJO, M. A. Eucalyptus benthamii x Eucalyptus dunnii minicutting technique: (ii) minicutting survival and rooting in relation to collection and seasons. Ciência Florestal, v. 20, n. 3, p. 453-465, 2010b.
- CABRAL, O. M. R.; GASH, J. H. C.; ROCHA, H. R.; MARSDEN, C.; LIGO, M. A. V.; FREITAS, H. C.; TATSCH, J. D.; GOMES, E. Fluxes of CO2 above a plantation of Eucalyptus in southeast Brazil. Agricultural and Forest Meteorology, v. 151, n. 1, p. 49-59, 2011.
- CAMPINHOS, E.; IKEMORI, Y. K. Nova técnica para a produção de mudas de essências florestais. Revista-IPEF, n. 23, p. 43-46, 1983.
- CORRÊA, L. R.; FETT-NETO, A. G. Effects of temperature on adventitious root development in microcuttings of Eucalyptus saligna Smith and Eucalyptus globulus Labill. Journal of Thermal Biology, v. 29, n. 6, p. 315-324, 2004.
- DAI, W.; CHENG, Z. M.; SARGENT, W. A. Expression of the rolB gene enhances adventitious root formation in hardwood cuttings of aspen. In Vitro Cellular and Developmental Biology - Plant, v. 40, n. 4, p. 366-370, 2004.
- DAY, J. S.; GOULD, K. S.; JAMESON, P. E. Adventitious root initiation, plasticity, and response to plant growth regulator treatments of seedling, juvenile, and adult Elaeocarpus hookerianus plants. New Zealand Journal of Botany, v. 36, n. 3, p. 477-484, 1998.
- DELWAULLE, J. C.; LAPLACE, Y.; QUILLET, G. Production massive de boutures d' Eucalyptus en République Populaire du Congo. Silvivultura, v. 8, n. 2, p. 779-781, 1983.
- DETTMER, J.; ELO, A.; HELARIUTTA, Y. Hormone interactions during vascular development. Plant Molecular Biology, v. 69, n. 4, p. 347-360, 2009.
- FERREIRA, E. M.; ALFENAS, A. C.; MAFIA, R. G.; LEITE, H. G.; SARTORIO, R. C.; PENCHEL FILHO, R. M. Determination of the optimum time for rooting of mini-cuttings of Eucalyptus spp. clones. Revista Árvore, v. 28, n. 2, p. 183-187, 2004.
- FOGAÇA, C. M.; FETT-NETO, A. G. Role of auxin and its modulators in the adventitious rooting of Eucalyptus species differing in recalcitrance. Plant Growth Regulation, v. 45, n. 1, p. 1-10, 2005.
- GOULART, P. B.; XAVIER, A. Efeito do tempo de armazenamento de miniestacas no enraizamento de clones de Eucalyptus grandis x E urophylla Revista Árvore, v. 32, n. 4, p. 671-677, 2008.
- HARTMANN, H. T.; KESTER, D. E.; DAVIES JÚNIOR, F. T.; GENEVE, R. L. Plant propagation: principles and practices. 7th ed. New Jersey: Prentice-Hall, 2002.
- HUNT, M. A.; TRUEMAN, S. J.; RASMUSSEN, A. Indole-3-butyric acid accelerates adventitious root formation and impedes shoot growth of Pinus elliottii var. elliottii x P caribaea var. hondurensis cuttings. New Forests, v. 41, n. 3, p. 349-360, 2011.
- HUSEN, A.; PAL, M. Metabolic changes during adventitious root primordium development in Tectona grandis Linn. f. (teak) cuttings as affected by age of donor plants and auxin (IBA and NAA) treatment. New Forests, v. 33, n. 3, p. 309-323, 2007.
- LI, S. W.; XUE, L.; XU, S.; FENG, H.; AN, L. Mediators, genes and signaling in adventitious rooting. The Botanical Review, v. 75, n. 2, p. 230-247, 2009.
- MAJADA, J.; MARTÍNEZ-ALONSO, C.; FEITO, I.; KIDELMAN, A.; ARANDA, I.; ALÍA, R. Mini-cuttings: an effective technique for the propagation of Pinus pinaster Ait. New Forests, v. 41, n. 3, p. 399-412, 2011.
- PAPP, B.; PLATH, K. Reprogramming to pluripotency: stepwise resetting of the epigenetic landscape. Cell Research, v. 21, n. 3, p. 486-501, 2011.
- RASMUSSEN, A.; SMITH, T. E.; HUNT, M. A. Cellular stages of root formation, root system quality and survival of Pinus elliottii var. elliottii x P caribaea var. hondurensis cuttings in different temperature environments. New Forests, v. 38, n. 3, p. 285-294, 2009.
- RISOPATRON, J. P. M.; SUN, Y.; JONES, B. J. The vascular cambium: molecular control of cellular structure. Protoplasma, v. 247, n. 3-4, p. 145-161, 2010.
- ROMERO, J. L. A review of propagation programs for Gmelina arborea New Forests, v. 28, n. 2-3, p. 245-254, 2004.
- SCHWAMBACH, J.; RUEDELL, C. M.; ALMEIDA, M. R.; PENCHEL, R. M.; ARAÚJO, E. F.; FETT-NETO, A. Adventitious rooting of Eucalyptus globulus x maidennii mini-cuttings derived from mini-stumps grown in sand bed and intermittent flooding trays: a comparative study. New Forests, v. 36, n. 3, p. 261-271, 2008.
- SMET, I.; BEECKMAN, T. Asymmetric cell division in land plants and algae: the driving force for differentiation. Nature Reviews Molecular Cell Biology, v. 12, n. 3, p. 177-188, 2011.
- SMET, I.; VOß, U.; JÜRGENS, G.; BEECKMAN, T. Receptor-like kinases shape the plant. Nature Cell Biology, v. 11, n. 10, p. 1166-1173, 2009.
- STAPE, J. L.; GONÇALVES, J. L. M.; GONÇALVES, A. N. Relationships between nursery practices and field performance for Eucalyptus plantations in Brazil. New Forests, v. 22, n. 1-2, p. 19-41, 2001.
- TENNANT, D. A test of a modified line intersect method of estimating root length. Journal of Ecology, v. 63, n. 3, p. 995-1001, 1975.
- THORPE, T. A. Turning point article to root or not to root, that is the question: reflections of a developmental plant physiologist. In Vitro Cellular and Developmental Biology - Plant, v. 40, n. 2, p. 128-142, 2004.
- TITON, M.; XAVIER, A.; OTONI, W. C. Rooting dynamics of microcuttings and minicuttings of Eucalyptus grandis clones. Revista Árvore, v. 26, n. 6, p. 665-673, 2002.
- TRUEMAN, S. J.; RICHARDSON, D. M. In vitro propagation of Corymbia torelliana x C citriodora (Myrtaceae) via cytokinin-free node culture. Australian Journal of Botany, v. 55, n. 4, p. 471-481, 2007.
- TRUEMAN, S. J.; RICHARDSON, D. M. Relationships between indole-3-butyric acid, photoinhibition and adventitious rooting of Corymbia torelliana, C citriodora and F1 hybrid cuttings. Tree and Forestry Science and Biotechnology, v. 2, n. 1, p. 26-33, 2008.
- TURNBULL, J. W. Eucalypt plantations. New Forests, v. 17, n. 1-3, p. 37-52, 1999.
- WENDLING, I.; XAVIER, A. Indolbutiric acid and serial minicutting technique on rooting and vigor of Eucalyptus grandis clone minicuttings. Revista Árvore, v. 29, n. 6, p. 921-930, 2005.
- WENDLING, I.; BRONDANI, G. E.; DUTRA, L. F.; HANSEL, F. A. Mini-cuttings technique: a new ex vitro method for clonal propagation of sweetgum. New Forests, v. 39, n. 3, p. 343-353, 2010.
Publication Dates
-
Publication in this collection
05 June 2012 -
Date of issue
June 2012
History
-
Received
03 Apr 2011 -
Accepted
01 June 2011