Abstracts
The aim of this study was to evaluate the storage potential of the pollen grains of Brazil Green Dwarf (BGD), Brazilian Tall (BRA) and Cameroon Red Dwarf (CRD) coconut accessions under different storage conditions. Representative plants of BGD, BRA and CRD accessions were selected from the Coconut Active Germplasm Bank of Embrapa Coastal Tablelands. The pollen grains were collected, placed in cryotubes and maintained under the following storage conditions: T1, refrigerator (-4ºC); T2, freezer (-20ºC); T3, ultra-freezer (- 80ºC) and T4, liquid nitrogen (-196ºC). The pollen grain viability was determined by staining with 1% acetic carmine and in vitro germination in a Lora culture medium at 30 and 60 days after the storage. Storage under refrigerator (-4ºC), freezer (-80ºC) and liquid nitrogen (-196ºC) conditions promoted a better pollen grain viability of the Brazilian Tall coconut accession. Storage under freezer (-20ºC) and liquid nitrogen (-196ºC) conditions promoted a better pollen grain viability of the Cameroon Red Dwarf coconut accession. Storage under freezer (-20ºC and -80ºC) and liquid nitrogen (-196ºC) conditions promoted a better pollen grain viability of the Brazil Green Dwarf coconut accession. The pollen grain viability of the Brazilian Tall coconut accession is stable after 30 and 60 days of storage.
Cocos nucifera L.; genetic resources; conservation; germinação in vitro; culture media
O objetivo deste estudo foi avaliar o potencial de armazenamento de grãos de pólen de acessos de coqueiro Anão Verde do Brasil de Jiqui (AVeBrJ), coqueiro Gigante do Brasil Praia do Forte (GBrPF) e coqueiro Anão Vermelho dos Camarões (AVC) em diferentes condições de armazenamento. Plantas matrizes dos acessos foram selecionados no Banco Ativo de Germoplasma de Coco da Embrapa Tabuleiros Costeiros. Os grãos de pólen foram coletados, armazenados em criotubos e mantidos nas seguintes condições de armazenamento: T1: Congelador (-4ºC) T2: Freezer (-20ºC), T3: Freezer (-80ºC) e T4: Nitrogênio líquido (-196ºC). A viabilidade grãos de pólen foi determinada por coloração com carmim acético a 1% e por germinação in vitro em meio de cultura Lora aos 30 e 60 dias após o armazenamento. O armazenamento em refrigeração (-4ºC), freezer (-80ºC) e nitrogênio líquido (-196ºC) promoveu uma melhor viabilidade de grãos de pólen para o acesso GBrPF . O armazenamento em freezer (-20ºC) e nitrogênio líquido (-196ºC) proporcionou melhor viabilidade de grãos de pólen para o acesso AVC. O armazenamento em freezer (-20ºC e -80ºC) e nitrogênio líquido (-196ºC) promoveu uma melhor viabilidade de grãos de pólen do acesso AVeBrJ. A viabilidade de grãos de pólen do acesso GBrPF é estável após 30 e 60 dias de armazenamento.
meio de cultura; Cocos nucifera L.; recursos genéticos; conservação; in vitro germination
CROP PRODUCTION
Pollen grain viability of coconut accessions at low temperatures
Viabilidade de grãos de pólen de acessos de coqueiro em baixas temperaturas
Caroline de Araújo MachadoI; Catrine Regina Feitosa MouraI; Eurico Eduardo Pinto de LemosII; Semíramis Rabelo Ramalho RamosI; Francisco Elias RibeiroI; Ana da Silva LédoIII,* * Author for correspondence. E-mail: ana.ledo@embrapa.br
IUniversidade Federal de Sergipe, São Cristovão, Sergipe, Brazil
IICentro de Ciências Agrárias, Universidade Federal de Alagoas, São Cristovão, Sergipe, Brazil
IIIEmbrapa Tabuleiros Costeiros, Av. Beira Mar, 3250, 49025-040, Aracajú, Sergipe, Brazil
ABSTRACT
The aim of this study was to evaluate the storage potential of the pollen grains of Brazil Green Dwarf (BGD), Brazilian Tall (BRA) and Cameroon Red Dwarf (CRD) coconut accessions under different storage conditions. Representative plants of BGD, BRA and CRD accessions were selected from the Coconut Active Germplasm Bank of Embrapa Coastal Tablelands. The pollen grains were collected, placed in cryotubes and maintained under the following storage conditions: T1, refrigerator (-4ºC); T2, freezer (-20ºC); T3, ultra-freezer (- 80ºC) and T4, liquid nitrogen (-196ºC). The pollen grain viability was determined by staining with 1% acetic carmine and in vitro germination in a Lora culture medium at 30 and 60 days after the storage. Storage under refrigerator (-4ºC), freezer (-80ºC) and liquid nitrogen (-196ºC) conditions promoted a better pollen grain viability of the Brazilian Tall coconut accession. Storage under freezer (-20ºC) and liquid nitrogen (-196ºC) conditions promoted a better pollen grain viability of the Cameroon Red Dwarf coconut accession. Storage under freezer (-20ºC and -80ºC) and liquid nitrogen (-196ºC) conditions promoted a better pollen grain viability of the Brazil Green Dwarf coconut accession. The pollen grain viability of the Brazilian Tall coconut accession is stable after 30 and 60 days of storage.
Keywords: Cocos nucifera L., genetic resources, conservation, meio de cultura, germinação in vitro.
RESUMO
O objetivo deste estudo foi avaliar o potencial de armazenamento de grãos de pólen de acessos de coqueiro Anão Verde do Brasil de Jiqui (AVeBrJ), coqueiro Gigante do Brasil Praia do Forte (GBrPF) e coqueiro Anão Vermelho dos Camarões (AVC) em diferentes condições de armazenamento. Plantas matrizes dos acessos foram selecionados no Banco Ativo de Germoplasma de Coco da Embrapa Tabuleiros Costeiros. Os grãos de pólen foram coletados, armazenados em criotubos e mantidos nas seguintes condições de armazenamento: T1: Congelador (-4ºC) T2: Freezer (-20ºC), T3: Freezer (-80ºC) e T4: Nitrogênio líquido (-196ºC). A viabilidade grãos de pólen foi determinada por coloração com carmim acético a 1% e por germinação in vitro em meio de cultura Lora aos 30 e 60 dias após o armazenamento. O armazenamento em refrigeração (-4ºC), freezer (-80ºC) e nitrogênio líquido (-196ºC) promoveu uma melhor viabilidade de grãos de pólen para o acesso GBrPF . O armazenamento em freezer (-20ºC) e nitrogênio líquido (-196ºC) proporcionou melhor viabilidade de grãos de pólen para o acesso AVC. O armazenamento em freezer (-20ºC e -80ºC) e nitrogênio líquido (-196ºC) promoveu uma melhor viabilidade de grãos de pólen do acesso AVeBrJ. A viabilidade de grãos de pólen do acesso GBrPF é estável após 30 e 60 dias de armazenamento.
Palavras chave: Cocos nucifera L., recursos genéticos, conservação, culture media, in vitro germination.
Introduction
Coconut palm (Cocos nucifera L.) originates from tropical and subtropical Pacific Ocean islands. Southeast Asia is its main reference point as the center of origin and diversity, and its cultivation has spread to Latin America, Caribbean and Tropical Africa. Currently, coconut palm is grown in over 200 countries and is found in large crop areas between 23ºN and 23ºS (FOALE; HARRIES, 2009).
The conservation of this germplasm is considered of worldwide importance and has resulted in the International Treaty on Genetic Resources for Food and Agriculture, organized by FAO, with 146 signatory countries, including Brazil (FAO, 2009).
Several biotechnological techniques have been applied in the conservation of genetic resources, with plant tissue culture and both short- and longterm in vitro conservation being emphasized. In general, short-term storage is performed in studies focused on genetics and breeding programs, whereas long-term storage is aimed at genetic conservation (ENGELMANN, 2004). Most of the methods used involve reducing the moisture content of pollen and maintaining the grains at low temperatures to avoid fluctuations (GANESHAN et al., 2008).
Studies on stored pollen viability are more numerous in species of temperate and subtropical climates, such as pepper, onion, elephant grass, pearl millet, peach, citrus, papaya, banana, grape, pistachio, garlic, rose, wheat and castor bean (SHAKED et al., 2004; EINHARDT et al., 2006; GOMES et al., 2003; TECHIO et al., 2006; PIO et al., 2007; DAMASCENO JUNIOR et al., 2008; GANESHAN et al., 2008; SOARES et al., 2008; ACAR; KAKANI, 2010; CUCHIARA et al., 2012).
In such studies, coconut palm has received special attention due to the production of hybrids, which is dependent on the use of pollen grains, both fresh and stored at low temperatures (OLIVEIRA et al., 2001; SOUSA et al., 2010). However, few studies have been published that examine coconut genotypes (ARMENDARIZ et al., 2006; KARUN et al., 2006; KARUN; SAJINI, 2010).
In addition to physiological and genetic factors, the main factors that could affect the viability of stored pollen are moisture and the storage temperature (GANESHAN et al., 2008). Therefore, studies have been aimed at developing storage methods that interfere as little as possible with the purposes of conservation.
Accordingly, the aim of this study was to evaluate the storage potential of pollen grains of Brazil Green Dwarf (BGD), Brazilian Tall (BRA) and Cameroon Red Dwarf (CRD) coconut accessions at low temperatures.
Material and methods
Representative plants of Brazil Green Dwarf (BGD), Brazilian Tall (BRA) and Cameroon Red Dwarf (CRD) coconut accessions were selected from the Coconut Active Germplasm Bank of Embrapa Coastal Tablelands, Sergipe State, located at the experimental fields of Itaporanga, SE. For each plant, a spathe close to maturation was marked before opening, removed from the plants and maintained at room temperature in the laboratory (28 ± 1ºC) until anthesis. The pollen grains were collected, placed in cryotubes and maintained under the following storage conditions: T1, refrigerator (-4ºC); T2, freezer (-20ºC); T3, ultra-freezer (-80ºC) and T4, liquid nitrogen (-196ºC). These storage conditions were selected based on their availability in biotechnology laboratories and may be applied to other species to optimize storage costs.
To assess the viability of the pollen grains, the samples were removed from the storage conditions and subjected to rehydration for two hours in a moist chamber. To determine the viability of the pollen grains by a staining method, a pollen sample of approximately 0.02 grams was placed on a slide, and a drop of 1% acetic carmine was added, followed by homogenization. The slide was then placed in a Petri dish (80 mm, Labomax Inc.) and incubated in a biological incubator at 37 ± 1ºC for 25-30 minutes. The slides were analyzed for the number of viable and nonviable pollen grains per quadrant using a microscope (model DMSL, Leica, Bernsheim, Germany) at 10x magnification and a digital camera (model Moticam C2300, Motic Instruments, Hong Kong, China). The pollen grains that stained red with intact walls were considered viable (by the reaction of the presence of enzymatic activity), and those that were colorless or stained red with ruptured walls were considered nonviable.
For viability studies using in vitro germination, the pollen samples were inoculated onto Petri dishes containing 2 mL of the culture medium of Lora et al. (2006), which is composed of 200 mg L-1 MgSO4.7H2O; 300 mg L-1 Ca(NO3)O2.4H2O; 100 mg L-1 KNO3; 100 mg L-1 H3BO3; and 40 g L-1 of sucrose. The Petri dishes (80 mm, Labomax Inc.) were kept in an incubator for 24 hours at a temperature of 24 ± 1ºC. The Petri dishes were analyzed for the number of germinated pollen grains using a microscope (model DMSL, Leica, Bernsheim, Germany) at 10x magnification with a digital camera (model Moticam C2300, Motic Instruments, Hong Kong, China). The pollen grains were considered germinated when exhibiting a pollen tube length greater than the diameter.
The evaluations were performed at 30 and 60 days, and a completely randomized design in a [(4 x 2) + 1] factorial scheme was used (four storage conditions combined with two different storage periods plus a control treatment at ambient temperature 28 ± 1ºC) with three replications.
The mean viability percentage of the pollen grains by staining with acetic carmine and in vitro germination under different storage conditions and the control were subjected to an analysis of variance. The mean viability percentage of the pollen grains by both techniques after 30 and 60 days under different storage conditions were compared using the Tukey test at a 5% probability with SISVAR software (FERREIRA, 2011). For the viability percentage by in vitro germination, the control was compared to all of the treatments using Dunnett's t-test at a 5% probability with SAS software.
Results and discussion
Pollen grain viability of the Brazilian Tall accession under different storage conditions
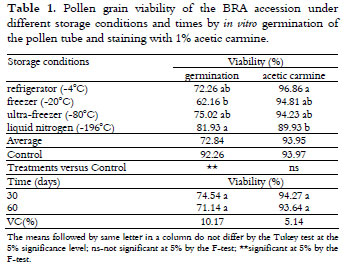
There was a significant effect of the storage condition and storage time on the viability of the pollen grains of the Brazilian Tall accession according to the in vitro germination of the pollen tube. As shown in Table 1, there was a higher viability for pollen germination (81.93%) after storage in liquid nitrogen (-196ºC).
Karun and Sajini (2010) obtained lower values for the in vitro germination of the West Coast Tall (WCT) coconut accession after 0, 1, 2, and 3 years of storage in liquid nitrogen (32.07, 32.16, 40.05 and 34.32%, respectively). Oliveira et al. (2001) studied the preservation of assai plant (Euterpe oleracea) pollen using Baker staining and observed an average germination percentage at 30 days and 79.6% and 77.4% at three months.
Regarding the storage time, no significant difference was observed between 30 and 60 days, which is in contrast to the results of Pereira et al. (2002) who observed a loss of viability of eucalyptus (Eucalyptus) pollen grains and in several species of this genus during storage time in a desiccator at 10ºC and 4ºC.
Higher viability percentages were observed in the grains stained with 1% acetic carmine (94.27 and 93.64%, respectively) when compared to viability determined by in vitro germination (74.54 and 71.14%, respectively).
Using in vitro germination, intermediate viability (62.16%) was only observed under the freezer condition (-20ºC). In contrast, both viability assessment methods after the other storage conditions resulted in high viability values, according to Souza et al. (2002).
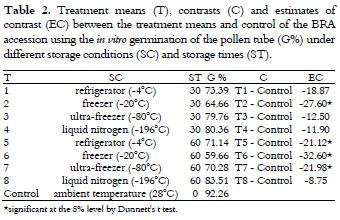
When comparing the storage conditions with the control, there were no differences with regard to the viability staining, whereas the control was higher than the average of treatments for the germination of the pollen grains (Table 1). However, there was a drastic decrease in the viability of the BRA pollen grains at ambient temperature, thus justifying an appropriate storage condition.
Comparing all of the treatments with control by Dunnett's t-test (Table 2), the control was greater than the freezing treatment (-20ºC) for 30 days and greater than all treatments, except liquid nitrogen, for 60 days.
The storage in liquid nitrogen, with an average of 81.93% viability in the germination tests, can be considered a good result when compared to the 92.26% obtained at the time of pollen collection. In addition, the storage stability over time may suggest that this strategy may be suitable for germplasm storage for longer periods, though this requires further study.
With a reduction in metabolism to levels so low that all biochemical processes are significantly reduced and even biological deterioration is virtually halted, the cryopreservation technique has the potential to preserve germplasm for an unlimited time (ALMEIDA et al., 2002).
Pollen grain viability of dwarf coconut accessions under different storage conditions
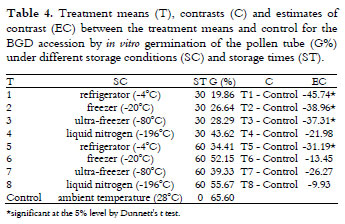
There was a significant effect of the storage condition and time on the viability of the Brazil Green Dwarf (BGD) accession pollen grains using the in vitro germination of the pollen tube. According to Table 3, storage in liquid nitrogen resulted in a higher viability (49.64%) when compared to refrigeration (27.13%). Karun and Sajini (2010) obtained an in vitro pollen tube germination viability for Chowghat Orange Dwarf (COD) that was similar to that of the BGD accession after 0, 1, 2, and 3 years of storage in liquid nitrogen (46. 34, 32.69, 44.14 and 32.40%, respectively).
Cuchiara et al. (2012) and Bettiol Neto et al. (2009) also obtained higher pollen viability for castor bean (Ricinus communis) and Annonaceae, respectively, stored in liquid nitrogen.
According to Souza et al. (2002), values above 70% are considered to indicate highly viable pollen, from 31 to 69% indicates intermediate viability, and up to 30% indicates low viability. Using the staining method, all of the storage conditions showed a high viability; however, the in vitro germination of the pollen tub indicated that the viability was intermediate under freezer and liquid nitrogen storage conditions (-20ºC and -80ºC) and low at refrigerator (-4ºC) and ambient temperatures. With regard to the storage time, there was a significant increase in viability by in vitro germination from 30 to 60 days.
According to Stanley and Linskens (1974), staining reactions may not correlate well with in vitro germination or the ability to fertilize, particularly for stored pollen and may explain the discrepant results shown in Tables 1 and 2. Einhardt et al. (2006) studied peach (Prunus persica) pollen reported that the staining of pollen grains overestimated the percentage of viable pollen. Techio et al. (2006) reported similar observations for pollen grains of elephant grass (Pennisetum purpureum) and pearl millet (P. glaucum). According to Stanley and Linskens (1974), no test is quite satisfactory, particularly if the pollen has been stored, for the following reasons: chemical tests use dyes that react with the chemical constituents or structures whose presence may not reflect the capacity of the pollen grain to germinate; and pollen grain samples that germinate well in vitro may not produce enough elongation of the pollen tube, thus affecting fertilization. Conversely, pollen samples that seem nonviable through in vitro tests can produce a high percentage of in vivo seeds, and stored pollen can germinate differently in repeated samples or different media.
The control (40.62%) was higher than the average of the treatments (37.49%) for the germination of the pollen grains, and no difference was observed for the viability using the staining technique (Table 3).
According to Dunnett's t-test (Table 4), the control was greater than all of the treatments, except liquid nitrogen, for 30 days and refrigeration (-4ºC) for 60 days.
Storage in liquid nitrogen can be considered a promising strategy for the conservation of BGD pollen grains.
The Cameroon Red Dwarf (CRD) accession results are presented in Table 5.
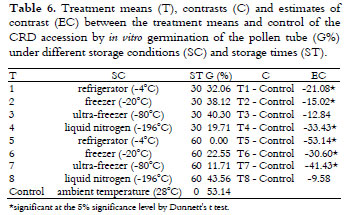
There was a significant effect of the storage condition and time on the viability of the Cameroon Red Dwarf (CRD) accession pollen grains by both of the techniques. As shown in Table 5, freezer (-20ºC) and liquid nitrogen storage did not differ and promoted a greater viability using the in vitro germination of the pollen tube than the other treatments.
The control (53.14%) was higher than the average of the treatments (23.77%) for the germination of the pollen grains and, no difference was observed for the viability staining results.
Determining the viability using acetic carmine staining revealed that storage in liquid nitrogen (-196ºC) resulted in a lower viability than the other treatments, with the storage time showing a decrease in viability up to 60 days (Figure 1).
According to Table 6, the control was greater than all of the treatments, except for the ultra-freezer (-80ºC), at 30 days and for all of the treatments, except liquid nitrogen, at 60 days.
In studies with onion (Allium cepa), Gomes et al. (2003) observed that the best condition for the storage of pollen grains during a period of two years is in liquid nitrogen. This storage condition also promoted a higher viability of the BGD accession pollen.
A comparison between Tables 4 and 6 shows that the average viability of the CRD accession under all of the storage conditions was less than that of BGD. Regardless of the conservation period, successful pollen preservation depends mainly on such factors as the temperature and relative humidity of the storage environment and on the pollen moisture content (GOMES et al., 2003) and genotype. In Pistacia genotypes, Acar and Kakani (2010) observed the interference of cardinal temperatures (minimum, optimum and maximum) associated with the genotypes on the pollen germination percentage and pollen tube growth.
According to the results found by Armendariz et al. (2006) and Song (2001), the mean viability determined by the staining method showed lower variation coefficients and higher values when compared with those obtained by the in vitro germination of the pollen tube. This is reinforced by the non-significance of the viability by staining when compared to the control under all of the storage conditions for the studied accessions (Tables 1, 3 and 5).
However, a comparison between the accessions showed that accession BRA achieved the highest viability; this variation is probably linked to a greater sensitivity of dwarf accession pollen.
Further studies should be conducted to improve the performance of the storage conditions on the pollen viability and longevity to obtain efficient protocols for the storage of pollen grains of the BGD and CRD accessions.
Conclusion
Storage under refrigerator (-4ºC), freezer (-80ºC) and liquid nitrogen (-196ºC) conditions up to 60 days promoted a better pollen grain viability using the in vitro germination of the Brazilian Tall coconut accession.
Storage under freezer (-20ºC) and liquid nitrogen (-196ºC) conditions up to 60 days promoted a better pollen grain viability using the in vitro germination of the Cameroon Red Dwarf coconut accession.
Storage under freezer (-20ºC and -80ºC) and liquid nitrogen (-196ºC) conditions up to 60 days promoted a better pollen grain viability using the in vitro germination of the Brazil Green Dwarf coconut accession.
The pollen grain viability of the Brazilian Tall coconut accession is stable after 30 and 60 days of storage.
Acknowledgements
Thanks are extended to Brazilian Agricultural Research Corporation (EMBRAPA), International Coconut Genetic Resources Network (COGENT) and National Biodiversity Mainstreaming and Institutional Consolidation Project (PROBIO II) for financial support, CAPES/FAPITEC-SE for a scholarship and Ph.D. Carlos Alberto da Silva Lédo (EMBRAPA) for statistical analysis.
Received on May 24, 2012.
Accepted on September 9, 2012.
- ACAR, I.; KAKANI, V. G. The effects of temperature on in vitro pollen germination and pollen tube growth of Pistacia spp. Scientia Horticulturae, v. 125, n. 4, p. 569-572, 2010.
- ALMEIDA, F. A. C.; MORAIS, A. M.; CARVALHO, J. M. F. C.; GOUVEIA, J. P. G. Crioconservação de sementes de mamona das variedades nordestina e pernambucana. Revista Brasileira de Engenheira Agrícola e Ambiental, v. 6, n. 2, p. 295-302, 2002.
- ARMENDARIZ, B. H. C.; OROPEZA, C.; CHAN, J. L.; MAUST, B.; AGUILAR, C. C. C.; SÁENZ, L. Pollen fertility and female flower anatomy of micropropagated coconut palms. Revista Fitotécnica Mexicana, v. 29, n. 4, p. 373-378, 2006.
- BETTIOL NETO, J. E.; DEL NERO, M.; KAVATI, R.; PINTO-MAGLIO, C. A. F. Viabilidade e conservação de pólen de três anonas comerciais. Bragantia, v. 68, n. 4, p. 825-837, 2009.
- CUCHIARA, C. C.; SILVA, S. D. A.; BROBOWSKI, V. L. Conservação de grãos de pólen de mamoneira a baixas temperaturas. Revista Ceres, v. 59, n. 1, p. 82-87, 2012.
- DAMASCENO JUNIOR, P. C.; PEREIRA, T. N. S.; PEREIRA, M. G.; SILVA, F. F. Conservação de grão de pólen de mamoeiro. Revista Ceres, v. 55, n. 5, p. 433-438, 2008.
- EINHARDT, P. M.; CORREA, E. R.; RASEIRA, M. C. B. Comparação entre métodos para testar a viabilidade de pólen de pessegueiro. Revista Brasileira Fruticultura,v. 28, n. 1, p. 5-7, 2006.
- ENGELMANN, F. Plant cryopreservation: progress and prospects. In Vitro Cellular and Developmental Biology - Plant, v. 40, n. 5, p. 427-433. 2004.
- FAO-Food and Agriculture Organization of the United Nations. International treaty on plant genetic resources for food and agriculture Rome: FAO, 2009.
- FERREIRA, D. F. SISVAR: a computer statistical analysis system. Ciência e Agrotecnologia, v. 35, n. 6, p. 1039-1042, 2011.
- FOALE, M.; HARRIES, H. Farm and forestry production and marketing profile for coconut (Cocos nucifera). In: ELEVITCH, C. R. (Ed.). Specialty crops for pacific island agroforestry Holualoa: Permanent Agricultur e Resources (PAR), 2009.
- GANESHAN, S.; RAJASEKHARAN, P. E.; SHASHIKUMAR, S.; DECRUZE, W. Cryopreservation of pollen. In: REED, B. M. (Ed.). Plant cryopreservation: a practical guide. New York: Springer, 2008. p. 443-447.
- GOMES, P. R.; RASEIRA, M. C. B.; BAUDET, L. L.; PESKE, S. T. Armazenamento do grão de pólen de cebola (Allium cepa L.). Revista Brasileira de Sementes, v. 25, n. 1, p. 14-17, 2003.
- KARUN, A.; SAJINI, K. K. S.; NAIR, M.; KUMARAN, P. M.; SAMSUDHEEN, K. Cryopreservation of coconut (Cocos nucifera L.) pollen. Journal of Plantation Crops, v. 3, n. 3, p. 568-571, 2006.
- KARUN, A.; SAJINI, K. K. Cryopreservation of coconut zygotic embryos and pollen Kasaragod: Central Plantation Crops Research Institute, 2010.
- LORA, M. A. J.; OTEYZA, P.; FUENTETAJA, P.; HORMAZA, J. I. Low temperature storage and in vitro germination of cherimoya (Annona cherimola Mill) pollen. Scientia Horticulturae, v. 108, n. 1, p. 91-94, 2006.
- OLIVEIRA, M. S.; PADILHA, M. M. M.; KALUME, M. A. A. Viabilidade de pólen in vivo e in vitro em genótipos de açaizeiro. Acta Botanica Brasílica, v. 15, n. 1, p. 27-33, 2001.
- PEREIRA, R. C.; DAVIDE, L. C.; RAMALHO, M. A. P.; ANDRADE, H. B. Alternativas para aumentar a eficiência dos cruzamentos em programas de melhoramento de Eucalyptus Cerne, v. 8, n. 2, p. 60-69, 2002.
- PIO, L. A. S.; RAMOS, J. D.; PASQUAL, M.; JUNQUEIRA, K. P.; SANTOS, F. C.; RUFINI, J. C. M. Viabilidade do pólen de laranjas doces em diferentes condições de armazenamento. Ciência e Agrotecnologia, v. 31, n. 1, p. 147-153, 2007.
- SONG, Z. P. A study of pollen viability and longevity in Orysa rufipogan, O. sativa, and their hybrids. International Rice Research Notes, v. 26, n. 2, p. 31-32, 2001.
- SOARES, T. L.; SILVA, S. O.; COSTA, M. A. P. C.; SANTOS-SEREJO, J. A.; SOUZA, A. S.; LINO, L. S. M.; SOUZA, E. H.; JESUS, O. N. In vitro germination and viability of pollen grains of banana diploids. Crop Breeding and Applied Biotechnology, v. 8, n. 2, p. 111-118, 2008.
- SOUSA, V. A.; SCHEMBERG, E. A.; AGUIAR, A. V. Germinação in vitro do pólen de jerivá (Syagrus romanzoffiana (S.) Cham). Scientia Forestalis, v. 38, n. 86, p. 147-151, 2010.
- SOUZA, M. M.; PEREIRA, T. N. S.; MARTINS, E. R. Microsporogênese e microgametogênese associadas ao tamanho do botão floral e da antera e viabilidade polínica em maracujazeiro amarelo (Passiflora edulis Sims f. flavicarpa Degener). Ciência e Agrotecnologia, v. 26, n. 6, p. 1209-1217, 2002.
- SHAKED, R.; ROSENFELD, K.; PRESSMA, E. The effect of low night temperatures on carbohydrates metabolism in developing pollen grains of pepper in relation to their number and functioning. Scientia Horticulturae, v. 102, n. 1, p. 29-36, 2004.
- STANLEY, R. G.; LINSKENS, H. F. Pollen: biology, biochemistry and management. New York: Springer, 1974.
- TECHIO, V. H.; CHAMMA, D. L.; PEDROZO, C. A.; VANDER, P. A. Viabilidade do grão de pólen de acessos de capim-elefante, milheto e híbridos interespecíficos (capimelefante x milheto) Acta Scientiarum. Biological Sciences, v. 28, n. 1, p. 7-12, 2006.
Publication Dates
-
Publication in this collection
03 June 2014 -
Date of issue
June 2014
History
-
Received
24 May 2012 -
Accepted
09 Sept 2012