ABSTRACT.
This study was carried out with the objective of evaluating the effect of fungicide application on grain rot in commercial maize hybrids and the relation between grain rot and the expression of lipoxygenase enzyme in grain in conventional row spacing of 0.70 m and reduced row spacing of 0.45 m. Treatments were made in a 3 x 8 factorial scheme, using three forms of management with fungicide (Trifloxystrobin + Prothioconazole) and eight maize hybrids divided into two groups (tolerant and susceptible) with three repetitions, totaling 72 plots in each environment (conventional and reduced spacing) in the 2013/2014 crop. The following characteristics were evaluated: grain rot percentage and lipoxygenase enzyme expression (LOX) in the grain. The hybrid and the fungicide utilized influenced the grain rot percentage. Grain rot percentage was reduced by the use of the fungicide, and the highest reduction was in susceptible hybrids with two applications, V8 and V8+VT. There was higher expression of LOX enzyme in maize hybrids that belong to the group tolerant of fungi that cause grain rot .The use of the fungicide in two applications, V8 (eight leaves) and VT (tasseling), increased the intensity of the LOX enzyme, which was more evident for the reduced spacing.
Keywords:
LOX; spindle rot; chemical control; reduced spacing; Zea mays
RESUMO.
O objetivo deste trabalho foi avaliar o efeito da aplicação de fungicida sobre a podridão de grãos em híbridos comerciais de milho e a relação entre os grãos ardidos e a expressão da enzima lipoxigenase no grão, em espaçamento convencional de 0,70 m e reduzido de 0,45 m. Os tratamentos foram no esquema fatorial 3 x 8, com três formas de manejo com fungicida (Trifloxistrobina + Protioconazol) e oito híbridos de milho, sendo divididos em dois grupos (tolerantes e suscetíveis), com três repetições, totalizando 72 parcelas em cada ambiente (espaçamento convencional e reduzido), na safra agrícola 2013/2014. As seguintes caractrísticas foram avaliadas: porcentagem de grãos ardidos e expressão da enzima lipoxigenase (LOX) nos grãos. O fungicida reduziu a incidência de grãos ardidos, e a maior redução ocorreu nos híbridos suscetíveis, com duas aplicações, V8 e V8+VT. Houve maior expressão da enzima LOX em híbridos de milho pertencentes ao grupo tolerante aos fungos causadores de grãos ardidos. A aplicação do fungicida em duas aplicações, V8 (oito folhas) e VT (pendoamento), aumentou a intensidade da enzima LOX, mais evidente no espaçamento reduzido.
Palavras-chave:
LOX; podridão de espigas; controle químico; espaçamento reduzido; Zea mays
Introduction
Maize (Zea mays L.) is one of the most important and ancient crops of the world (Werle, Nicolay, Santos, Borsoi, & Secco, 2011Werle, A. J. K., Nicolay, R. J., Santos, R. F., Borsoi, A., & Secco, D. (2011). Avaliação de híbridos de milho convencional e transgênico (Bt), com diferentes aplicações de inseticida em cultivo safrinha. Revista Brasileira de Tecnologia Aplicada nas Ciências Agrárias, 4(1), 150-159.). This species is cultivated in many different environments and climates at different latitudes, from Russia to Argentina. The culture has a wide geographic range of cultivation and can be developed in many different soil and climate conditions; consequently, it is possible to use various
commercial hybrids that have sundry tolerance levels to leaf and grain pathogens (Pozar, Butruille, Diniz, & Viglioni, 2009Pozar, G., Butruille, D., Diniz, H. S., & Viglioni, J. P. (2009). Mapping and validation of quantitative trait loci for resistance to Cercospora infection in tropical maize (Zea mays L.). Theoretical and Applied Genetics, 118(3), 553-564.). The occurrence of these pathogens causes reductions in grain yield and grain health quality because infection by these fungi results in paralysis of the normal process of grain filling and reduces maize cob weight.
Northern leaf blight (caused by Exserohilum turcicum), common rust (caused by Puccinia sorghi) and gray leaf spot (caused by Cercospora zeae-maydis) are important foliar diseases of maize (Juliati & Souza, 2005Juliatti, F. C., & Souza, R. M. (2005). Efeitos de Épocas de Plantio na severidade de doenças foliares e produtividade de híbridos de milho. Bioscience Journal, 21(1), 103-112. ). Mendes, Von Pinho, Machado, Albuquerque, and Falquete (2011Mendes, M. C., Von Pinho, R. G., Machado, J. C., Albuquerque, C. J. B., & Falquete, J. C. F. (2011). Qualidade sanitária de grãos de milho com e sem inoculação a campo dos fungos causadores de podridões de espiga. Ciência e Agrotecnologia, 35(5), 931-939.) highlights the rot of the cob caused mainly by fungi present in the field such as Fusarium verticilioides, Stenocarpella maydis, and Stenocarpella macrospora, which cause grain rot in maize. The indiscriminate use of susceptible hybrids, intensive planting systems and the improper use of high technology, associated with the occurrence of favorable climate for epidemic development, have contributed to an increase of the diseases of importance in maize and the use of fungicides (Brito, Von Pinho, Pozza, Pereira, & Faria Filho, 2007Brito, A. H., Von Pinho, R. G., Pozza, E. A., Pereira, J. L. A. R., & Faria Filho, E. M. (2007). Efeito da Cercosporiose no rendimento de híbridos comerciais de milho. Fitopatologia Brasileira, 32(6), 472-479.). Thus, it becomes increasingly important to select the correct genetic material to be used (Mendes, Pereira, Von Pinho, & Balestre, 2012Mendes, M. C., Von Pinho, R. G., Von Pinho, E. V. R., & Faria, M. V. (2012). Comportamento de híbridos de milho inoculados com os fungos causadores do complexo grãos ardidos e associação com parâmetros químicos e bioquímicos. Ambiência, 8(2), 277-279.), and in recent years, the discussion of management strategies to reduce the disease in a sustainable manner has increased, using crop rotation, genotype and especially the adoption of chemical control.
Sequential degradation of lipids, which are primary products of the lipoxygenase reaction, occurs when plant tissues are damaged by pathogens or mechanically. These enzymes are activated and oxidize fatty acids, producing a certain concentration of aldehydes and volatile compounds that inhibit the formation and development of fungus in grain (Mendes et al., 2012Mendes, M. C., Von Pinho, R. G., Von Pinho, E. V. R., & Faria, M. V. (2012). Comportamento de híbridos de milho inoculados com os fungos causadores do complexo grãos ardidos e associação com parâmetros químicos e bioquímicos. Ambiência, 8(2), 277-279.).
Currently, the adoption of reduced spacing associated with the use of modern and not modern maize hybrids changes the spatial arrangement of maize plants in the field. This can change the microclimate in a positive way, increasing the photosynthetically active radiation interception by the canopy; it can also be observed that there is an increase in the absorption efficiency of nutrients and water, exerting influence on maize grain yield, possibly negatively influencing the hybrids' tolerance to grain diseases and the efficiency of control by the active component of fungicides.
The efficiency of chemical control in the management of grain rot in maize is still a matter of doubt in relation to the efficiency of fungicides and number of applications, and research that can clarify the effects of fungicides on pathogens associated with cob diseases and the relation to the susceptibility of the hybrid used is lacking.
Thus, due to the new maize hybrids that have been launched in the market every year with high yield potential, it is evident the importance of this research to elucidate the results obtained from the use of fungicide in maize, its association with the activity of the specific enzymes, the presence of pathogen agents that cause cob rot and different plant spacing. Therefore, the objective of this work was to evaluate the effect of fungicide application on grain rot in commercial maize hybrids, and the relation between grain rot and lipoxygenase enzyme expression in the grain, in conventional and reduced spacing.
Material and methods
Two experiments were conducted in the crop season of 2013/2014, in Guarapuava, State Paraná, Brazil. The first experiment (environment 1) was conducted at Cedeteg with conventional row spacing of 0.70 m and at a latitude of 25º 23' 04.83'' south, a longitude of 51º 29' 44.32'' west, and an altitude of 1,028 m. The second experiment (environment 2) was installed at Três Capões Farm with reduced row spacing of 0.45 m and at a latitude of 25º 26' 57.79'' south, a longitude of 51º 38' 29.18'' west, and an altitude of 948 m. In both experiments, a population of 75,000 plants per hectare was used. This experiment was conducted on the no-tillage system, in areas where there was white oat (Avena sativa) as ground cover. The topography is considered plane. The region has a humid mesothermal subtropical climate. The climate classification proposed by Köppen is Cfb type without a defined dry season, cool summers and winters with severe and frequent frosts. The annual average temperature is 16.8ºC, ranging from 6.8oC (minimum average) to 36°C (maximum average), and the annual average total rainfall is 1,500 mm with an annual average relative humidity of 77.9%. The soil is classified as Dystrophic Haplohumox Oxisol, clayey texture (Empresa Brasileira de Pesquisa Agropecuária [Embrapa], 2006).
The experimental design was in random blocks, with three replications, in a 3 x 8 factorial scheme, totaling 24 treatments and 72 plots in each environment (conventional and reduced spacing) in the crop season of 2013/2014. The first factor consisted of three levels of foliar fungicide application (Trifloxystrobin 150.0 g L-1 (15.0% m/v) + Prothioconazole 175.0 g L-1 (17.5% m/v): at stage V8 (eight expanded leaves) with 0.4 L ha-1, at stage VT (tasseling) with 0.5 L ha-1 and control (without fungicide). The second factor had eight maize hybrids divided into two groups according to their reaction to the causative fungus of the grain rot complex: tolerant (AG 9045PRO, AG 8041PRO, DKB 245PRO2, and 2B707PW) and susceptible (P 32R48H, DKB 390PRO, P 30F53H, and P 30R50H).
The experiments were installed in the first fortnight of October 2013, and the harvest occurred in the second fortnight of March 2014, after physiological maturity. The first experiment had a row spacing of 0.70 m and a length of 5 m, four rows and a total area of 14 m² per plot. The second experiment had a row spacing of 0.45 m and a length of 5 m, four rows and a total area of 9 m². In both experiments, the two central rows of each plot were used.
The fungicide applications were carried out with the aid of a CO2 pressurized costal sprayer, equipped with four nozzles, hollow cone spray nozzles (0.3) spaced at 0.5 m, consumption of 200 L ha-1 and 3.6 km h-1 of speed displacement. Climatic variables at the time of application (start and end) were monitored by a digital anemometer. Meteorological data were collected every 10 days at the IAPAR/UNICENTRO/ CEDETEG experimental station located approximately 500 meters from the area of the first experiment.
The percentage of grain rot and the evaluation of the lipoxygenase enzyme were performed. The percentage of grain rot was determined according to the procedure proposed in ordinance No. 11 of April 12, 1996 (Brasil, 1996). The electrophoretic enzyme analyses, in both environments, were performed in the central laboratory of seeds belonging to the Department of Agriculture of the Universidade Federal de Lavras, Lavras, Minas Gerais State, Brazil. To perform the analysis, composite grain samples derived from three replicates of each treatment were used, and the gel analysis was made as in Alfenas (2006Alfenas, A. C. (2006). Eletroforese e marcadores bioquímicos em plantas e microrganismos. Viçosa, MG: UFV .). To carry out the electrophoretic run, 50 mL of each supernatant was applied in the gel groove, and the running was at 4°C at 120 V for approximately 8 hours. After that, the gels were revealed in the presence of a substrate specific for the particular enzyme (Alfenas, 1998).
Percentage of grain rot was submitted to homogeneity of variances testing by Harley’s test (Ramalho, Ferreira, & Oliveira, 2000Ramalho, M. A. P., Ferreira, D. F., & Oliveira, A. C. (2000). Experimentação em genética e melhoramento de plantas. Lavras, MG: UFLA.). After that, individual and joint variance analyses involving the cultivation environments were performed, and the averages were grouped by the Scott-Knott test at the 5% probability level using SISVAR® software as the statistical program (Ferreira, 2011).
Results and discussion
There was an accumulated rainfall level of 1,008 mm in environment 1 (CEDETEG) throughout the culture cycle. During the initial phase of the experiment implementation, shortly after the sowing in October and November, 230 mm of accumulated rainfall volume was verified, ensuring good initial development for the culture. Cumulatively, 778 mm of rainfall was observed during the fungicide application and in the subsequent months until the harvest. In environment 2 (Três Capões Farm) with reduced spacing, there was an accumulated rainfall of 896 mm during the culture cycle. During the initial phase of implementation, shortly after the sowing in October and November, there was good rainfall volume; 215 mm of accumulated rainfall volume was observed, ensuring good initial development for the culture. During the fungicide application and in the subsequent months until the harvest, rainfall was observed for the study area with accumulation of 681 mm (Figure 1).
Pluviometry and temperature for ten days in Guarapuava, Paraná State, Brazil, CEDETEG (environment 1) and Três Capões Farm (environment 2) from October to April in the 2013/2014 crop season.
¶Regarding the results obtained in relation to the minimum and maximum temperatures, it is important to note that the two experiments are in the same municipality and that the season is close in the two experiments, so it was decided to use the average temperature. According to the results of the joint variance analyses, for the percentage of grain rot, a significant effect was observed (p < 0.01) in the interaction hybrid x environment (Table 1).
In environment 1, a significant difference was verified between the hybrids within each treatment with fungicide; in the check treatment, for grain rot, there was a higher percentage in DKB 390PRO, P 32R48H and P 30F53H (susceptible hybrids), all belonging to group 2 (Table 1). In Brito, Pereira, Von Pinho, and Balestre (2012Brito, A. H., Pereira, J. L. A. R., Von Pinho, R. G., & Balestre, M. (2012). Controle químico de doenças foliares e grãos ardidos em milho (Zea mays L.). Revista Brasileira de Milho e Sorgo, 11(1), 49-59.), the use of fungicides (Azoxystrobin + Cyproconazole) in maize possibly caused a reduction in the grain rot percentage.
In treatment V8, there was a higher percentage for hybrid AG 9045PRO (group 1), but it did not differ statistically from hybrids DKB 390PRO and P 30F53H (belonging to group 2). It is possible to note that hybrids AG 8041PRO and 2B707PW (belonging to group 1) had the lowest percentage of grain rot. In relation to V8 + VT, taking into consideration the grain rot characteristic, there was a higher percentage for hybrids P 32R48H and DKB 390PRO (belonging to group 2) (Table 1). According to Casa, Reis, and Zambolim (2006Casa, R. T., Reis, E. M., & Zambolim, L. (2006). Doenças do milho causadas por fungos do gênero Stenocarpella. Fitopatologia Brasileira, 31(5), 427-439.), the fungus Stenocarpella spp. is mainly associated with grain rot, and it may be the principal agent of grain rot in maize, which may have occurred in this research. The use of fungicides is recommended for maize in hybrids that present susceptibility to the disease (Costa Cota, Silva, Lanza, & Figueiredo, 2012Costa, R. V., Cota, L. V., Silva, D. D., Lanza, F. E. & Figueiredo, J. E. F. (2012). Eficiência de fungicidas para o controle de mancha branca no milho. Revista Brasileira de Milho e Sorgo, 11(3), 291-301.).
Evaluating the utilized treatments in the hybrid groups, in groups 1 and 2, there was no significant difference between treatments (Table 1). However, when the hybrid groups are compared for each treatment, there was a significant difference in all treatments with evaluated fungicide (check, V8 and V8 + VT); group 2 (susceptible) presented higher percentage of grain rot in environment 1 (conventional spacing), emphasizing the importance of the genotype evaluated (Table 1). Juliatti, Zuza, Souza, and Polizel (2007Juliatti, F. C., Zuza, J. L. M. F., Souza, P. P., & Polizel, A. C. (2007). Efeito do genótipo de milho e da aplicação foliar de fungicidas na incidência de grãos ardidos. Bioscience Journal, 23(2), 34-41. ), which used the fungicides Pyraclostrobin + Epoxiconazole in two applications, reported the efficiency of the products at reducing the percentage of grain rot. The performance of two foliar applications (V8 + pre-tasseling) or one application at pre-tasseling of the fungicide azoxystrobin + cyproconazole resulted in a lower percentage of the fungus Fusarium sp. in the harvested grain in the two sowing periods (Stefanello, Bachi, Gavassoni, Hirata, & Pontim, 2012Stefanello, J., Bachi, L. M. A., Gavassoni, W. L., Hirata, L. M., & Pontim, B. C. A. (2012). Incidência de fungos em grãos de milho em função de diferentes épocas de aplicação foliar de fungicida. Pesquisa Agropecuária Tropical, 42(4), 476-481.).
In environment 2, it was verified that in the check treatment, there was no significant difference when analyzing the hybrids (Table 1). One of the factors that should be further studied is the maize culture response to the arrangement of plants in the area. It is possible to distribute the plants in many ways in an area, variation of the spacing between rows and between plants being responsible for the different plant arrangements. This practice has been intensified in producer regions, yet the different hybrids respond differently to variations in the arrangement and density of plants (Demetrio, Filho, Cazetta, & Cazetta, 2008Demetrio, C. S., Filho, D. F., Cazetta, J. O., & Cazetta, D. A. (2008). Desempenho de híbridos de milho submetidos a diferentes espaçamentos e densidades populacionais. Pesquisa Agropecuária Brasileira, 43(12), 1691-1697.) and also in relation to the disease severity.
In the V8 treatment, there was a higher percentage for hybrid AG 9045PRO (group 1), but it did not differ statistically from hybrids P 32R48H, DKB 390PRO, P 30F53H, and P 30R50H hybrids (belonging to group 2). It can be noted that in hybrid AG 9045PRO of group 1 (tolerant), even the foliar fungicide application did not show effectiveness at reducing the grain rot percentage (Table 1). Studies by Duarte, Juliatti, Lucas, and Freitas (2009Juliatti, F. C., Pedrosa, M. G., Silva, H. D., & Silva, J. V. C. (2009). Genetic mapping for resistance to Gray leaf spot in maize. Euphytica, 169(2), 227-238.) corroborate the results obtained in this study, and the foliar application of fungicides in some maize genotypes did not result in fungus percentage control.
In the V8 + VT treatment, there was higher percentage for the P 32R48H and DKB 390PRO hybrids (belonging to the group 2) (Table 1). These data corroborate Brito et al. (2012Brito, A. H., Pereira, J. L. A. R., Von Pinho, R. G., & Balestre, M. (2012). Controle químico de doenças foliares e grãos ardidos em milho (Zea mays L.). Revista Brasileira de Milho e Sorgo, 11(1), 49-59.), who noted that the use of foliar fungicide application makes a reduction of the grain rot percentage possible.
Comparing the groups of hybrids with fungicide treatments, in group 1 (tolerant), there was a significant difference between treatments for grain rot, where the higher percentage occurred in the check treatment compared to the V8 and V8 + VT treatments; therefore, the fungicide application reduced the grain rot percentage (Table 1). It is possible to favor a microclimate, using reduced spacing, with the increase or decrease in plant population, and this favors the percentage of disease. There was no significant difference between treatments from group 2 (susceptible) evaluated for grain rot (Table 1). Silva, Cunha Junior, Assis, and Imolesi (2008Silva, A. G., Cunha Junior, C. R., Assis, R. L., & Imolesi, A. S. (2008). Influência da população de plantas e do espaçamento entre linhas nos caracteres agronômicos do híbrido de milho P30K75 em Rio Verde, Goiás. Bioscience Journal, 24(2) 89-96.) found that hybrid P30K75, when it is cultivated with reduced spacing (50 cm of row spacing), showed higher severity index of foliar diseases. For group 2 (susceptible), there was no significant difference between the evaluated treatments for grain rot (Table 1).
However, when the groups of hybrids in each treatment were compared, there was a significant difference for grain rot, and in the V8 and V8 + VT treatments, group 1 showed a lower grain rot percentage compared to group 2; in the check treatment, there was no significant difference between the groups of evaluated hybrids (Table 1). Fungicide application is effective in the control of foliar diseases, provides higher grain yield and reduces the percentage of grain rot. An important factor in foliar disease development is the effect of climate, and the environment is an important component of host-pathogen-environment interactions. Cunha and Pereira (2009Cunha, J. P. A. R., & Pereira, R. G. (2009). Efeito de pontas e volumes de pulverização no controle químico de doenças do milho. Revista Ciência Agronômica, 40(4), 533-538. ) used the fungicide Pyraclostrobin + Epoxiconazole, which provided disease control, as reflected in the yield, which was on average 16.3% higher than the evaluated check.
When the P 30R50H hybrid was evaluated for grain rot (environment 2), a similar behavior to the previous was observed a lower percentage of grain rot only in treatment V8 + VT; there was a significant difference when compared with the check, showing that the response to the fungicide application (Trifloxystrobin + Prothioconazole) may be higher susceptibility due to the reduced spacing, presenting a lower response to fungal treatments in a preventive way. In environment 1, this hybrid showed a lower percentage of grain rot in all treatments, even in the check treatment, possibly with interference from the space, which is higher in environment 1, with less percentage of grain rot (Table 1).
The high percentage of grain rot from group 2 (susceptible) is closely related to the susceptibility of these hybrids to the diseases of the grain rot complex. Duarte et al. (2009Duarte, R. P., Juliatti, F. C., Lucas, B. V., & Freitas, P. T. (2009). Comportamento de diferentes genótipos de milho com aplicação foliar de fungicida quanto à incidência de fungos causadores de grãos ardidos. Bioscience Journal, 25(4), 112-122.) tested fungicides on maize and reported that there was a reduction in the percentage of grain rot due to the foliar application and fungicide association (Azoxystrobin + Cyproconazole).
Another factor that may have contributed to this result of higher percentage of grain rot in the hybrids of group 2 is the high volume of rainfall at the flowering stage of the culture, corresponding to the period of December and January, which are months considered critical for the occurrence of grain rot. There is evidence that the use of fungicide in maize culture enables the reduction in the percentage of grain rot; according to Juliatti et al. (2007Juliatti, F. C., Zuza, J. L. M. F., Souza, P. P., & Polizel, A. C. (2007). Efeito do genótipo de milho e da aplicação foliar de fungicidas na incidência de grãos ardidos. Bioscience Journal, 23(2), 34-41. ), the foliar application of triazole and strobilurinfungicides (Pyraclostrobin + Epoxiconazole, Azoxystrobin + Cyproconazole and Azoxystrobin) resulted in a lower percentage of grain rot. In recent years, research has shown the effectiveness of fungicide application in the management of foliar diseases and in reducing the damage caused by them to productivity (Cunha, Silva, Boller, & Rodrigues, 2010Cunha, J. P. A. R., Silva, L. L., Boller, W., & Rodrigues, J. F. (2010). Aplicação aérea e terrestre de fungicida para o controle de doenças do milho. Revista Ciência Agronômica, 41(3), 366-372.).
It was also verified that for grain rot, there was a higher percentage in almost all groups of maize hybrids from group 2, in both environments evaluated, with the exception of the P 30F53H hybrid; regarding environment 1, in the treatments V8 and V8 + VT, there was a significant difference, and a lower percentage of grain rot occurred compared with the check treatment, showing a response to the Trifloxystrobin + Prothioconazole fungicide application. In many cases, the damage caused by foliar diseases in maize is considered indirect, leaving the plant weakened by reducing the leaf area and thus vulnerable to the entry of pathogens that cause rot of the stalk, grains and roots (Jardine and Laca-Buendía, 2009Jardine, D. F., & Laca-Buendía, J. P. (2009). Eficiência de fungicidas no controle de doenças foliares na cultura do milho. Fazu em Revista, 26(6), 11-52.).
There was no significant difference for grain rot in environment 2; the hybrid P 30F53H showed a lower percentage only for the V8 + VT treatment. There may be higher susceptibility due to the reduced spacing, having a lower response to the fungicide treatments in a preventive way (Table 1). The use of Azoxystrobin + Cyproconazole in foliar application at pre-tasseling reduced the percentage of grain rot 5.12%, beyond an increase in productivity by 12.4% of different hybrids cultivated under high disease severity with and without fungicide application (Brito, Von Pinho, Souza Filho, & Altoé, 2008Brito, A. H., Von Pinho, R. G., Souza Filho, A. X., & Altoé, T. F. (2008). Avaliação da severidade da cercosporiose e rendimento de grãos em híbridos comerciais de milho. Revista Brasileira de Milho e Sorgo, 7(1), 19-31. ). Juliatti, Nascimento, and Rezende (2010Juliatti, F. C., Nascimento, C., & Rezende, A. A. (2010). Avaliação de diferentes pontas e volumes de pulverização na aplicação de fungicida na cultura do milho. Summa Phytopathological, 36(3), 216-221.) observed that all treatments with fungicide (strobilurin + triazole) provided an increase in thousand grain weight compared to the check treatment, showing a direct relation of the control of diseases with grain filling.
In relation to the averages of grain rot in the treatments used in environments 1 and 2, it was observed that there was a significant difference between the environments; analyzing the hybrids, a higher percentage of grain rot occurred in the P 32R48H and DKB 390PRO hybrids (both belonging to group 2) in environment 1 (Table 1). Additionally, as shown in Table 1, the percentage was high and similar in environments 1 and 2. Compared with the data obtained for precipitation (Figure 1), it can be observed that there was no interference of precipitation and temperature.
However, it was found that there was a higher percentage of grain rot in maize hybrids belonging to group 2 in both evaluated environments, but in environment 2, a positive response was noted only with two fungicide applications of Trifloxystrobin + Prothioconazole (V8 and V8 + VT); therefore, there was environmental interference in the percentage of grain rot, which possibly the reduced the microclimate conditions caused by spacing, causing an increase of causative agent attack. When the plant population is larger, there is higher demand for nutrients and water, resulting in plants more sensitive to pathogen infection. Therefore, hybrid yield potential must be evaluated using the recommended population. Increases in population, thousand grain weight and plant weight favor higher grain yields, which are decreased by the increased occurrence of foliar diseases (Silva, Teixeira, Martins, Simon, & Francischini, 2014Silva, A. G., Teixeira, I. R., Martins, P. D. S., Simon, G. A., & Francischini, R. (2014). Desempenho agronômico e econômico de híbridos de milho na safrinha. Revista Agroambiente On-line, 8(2), 261-271.).
Comparing the data obtained in environments 1 and 2 and the grain rot shown in Table 1, it can be verified that in the check, V8 and V8 + VT treatments, the hybrids belonging to group 1 (tolerant to rot grains) showed a lower percentage of grain rot, except th e check treatment in environment 2, where there was no significant difference between the groups of hybrids.
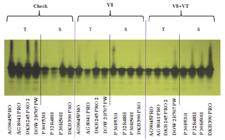
LOX expression was determined for all hybrids studied and all the treatments evaluated in environment 1 (Figure 2). In the check treatment, it was possible to note higher expression of LOX enzyme for hybrids belonging to the group considered tolerant of grain rot.
Lipoxygenase electrophoretic pattern in maize hybrids tolerant(T) and susceptible (S) to fungi that cause grain rot; the use of fungicide in stages V8, V8 + VT and the control produced in environment 1 in Guarapuava, Paraná State, Brazil, crop season of 2013/14
The hybrids belonging to group 1 have a lower percentage of grain rot compared with group 2 (Table 1), proving the results obtained by Zeringue, Brown, Neucere, and Cleveland (1996Zeringue, H. J., Brown, R. L., Neucere, J. N., & Cleveland, T. E. (1996). Relationships between C6-C12 alkanal and alkenal volatile contents and resistance of maize genotypes to Aspergillus flavus and aflatoxin prodution. Journal of Agricultural and Food Chemistry, 44(2),403-407.), which included higher expression of LOX and a consequently lower percentage of grain rot. The same authors suggest that the LOX enzyme, by lipoxygenation of linoleic acid, would be able to produce volatile aldehydes with chains of 6 to 12 carbons, inhibiting or preventing fungus development and even mycotoxin formation. According to Mendes et al. (2012Mendes, M. C., Von Pinho, R. G., Von Pinho, E. V. R., & Faria, M. V. (2012). Comportamento de híbridos de milho inoculados com os fungos causadores do complexo grãos ardidos e associação com parâmetros químicos e bioquímicos. Ambiência, 8(2), 277-279.), genotypes of maize with a high activity of lipoxygenase enzyme have greater ability to resist a fungal attack, resulting in lower rates of grain rot in the harvest. In addition, the fungus infection affects the quality of maize grain by the production of mycotoxins that cause damage to health (human and animal) due to the toxic activity that can be exerted on the organism (Kumar, Basu, & Rajendran, 2008Kumar, V., Basu, M. S., & Rajendran, T. P. (2008). Mycotoxin research and mycoflora in some commercially important agricultural commodities. Crop Protection, 27(6), 891-905.).
Studies have shown that the resistance of maize genotypes to grain rot is also correlated with the content of polyunsaturated fatty acids, specifically with linoleic acid, and the resistance mechanism is through the production of LOX. Lipoxygenase is related to the oxylipin formation through the oxygenation of fatty acids. The oxylipins are a group of signaling molecules such as jasmonate, mitel-jasmonate (mitel-jasmonato), etc., compounds that act in plant defense (Kazan & Manners, 2008Kazan, K., & Manners, J. M. (2008). Jasmonate Signaling: toward na integrated view. Plant Physiology, 146(4), 1459-1468.). Other studies have reported that other derivatives of lipoxygenase act in plant defense through the regulation of gene expression, cell death or antimicrobial products (Kishimoto, Matsui, Ozawa, & Takabayashi, 2008Kishimoto, K., Matsui, K., Ozawa, R., & Takabayashi, J. (2008). Direct fungicidal activities of C6-aldehydes are important constituents for defense responses in Arabidopsis against Botrytis cinerea. Phytochemistry, 69(11), 2127-2132.).
In the V8 treatment, as shown in Figure 2, the same enzyme expression was noted. For the two groups of hybrids, the fungicide application of Trifloxystrobin + Prothioconazole did not change the expression of LOX.
However, for the V8 + VT treatment, the same did not occur; therefore, it was possible to notice a higher expression of LOX enzyme for hybrids belonging to the group considered susceptible to grain rot, hybrids P 30F53H, P 32R48H, P 30R50H, and DKB 390PRO (Figure 2).
Based on the data presented in Table 1, the P32R48H and DKB 390PRO hybrids are in the group with the highest percentage of grain rot, but for the P30F53H and P30R50H hybrids, the percentage of grain rot was comparable to the hybrids considered tolerant. In this sense, it can be inferred that one of the possible contributions of the fungicide used, based on Trifloxystrobin + Prothioconazole when applied in V8 + VT, is to the route of the expression of the LOX enzyme. Studies related to the influence of chemical compounds and the activity of specific enzymes in maize grain on the resistance of genotypes associated with the pathogens that cause grain rot are being carried out in Brazil.
Several controlling loci of quantitative traits (QTLs) with the additive action of genes are associated with the resistance, which shows that this resistance is a horizontal or polygenic type. These QTLs may be determined by the use of microsatellite markers (Juliatti et al., 2009Juliatti, F. C., Pedrosa, M. G., Silva, H. D., & Silva, J. V. C. (2009). Genetic mapping for resistance to Gray leaf spot in maize. Euphytica, 169(2), 227-238.) and SNPs (Pozar et al., 2009Pozar, G., Butruille, D., Diniz, H. S., & Viglioni, J. P. (2009). Mapping and validation of quantitative trait loci for resistance to Cercospora infection in tropical maize (Zea mays L.). Theoretical and Applied Genetics, 118(3), 553-564.).
In research conducted by Pozar et al. (2009Pozar, G., Butruille, D., Diniz, H. S., & Viglioni, J. P. (2009). Mapping and validation of quantitative trait loci for resistance to Cercospora infection in tropical maize (Zea mays L.). Theoretical and Applied Genetics, 118(3), 553-564.) in Brazil, QTLs related to gray leaf spot resistance have been mapped and characterized through SNP and SSR markers.
The US Department of Agriculture conducted several experiments to prove the resistance of some varieties of maize due to different levels of fatty acids, mainly linoleic acid associated with lipoxygenase enzyme presence (Zeringue et. al., 1996Zeringue, H. J., Brown, R. L., Neucere, J. N., & Cleveland, T. E. (1996). Relationships between C6-C12 alkanal and alkenal volatile contents and resistance of maize genotypes to Aspergillus flavus and aflatoxin prodution. Journal of Agricultural and Food Chemistry, 44(2),403-407.).
The expression of LOX was possible to observe for all hybrids studied and all treatments evaluated in environment 2. In the check treatment, it was possible to notice higher expression of the LOX enzyme for the hybrids belonging to the group considered tolerant of grain rot, represented in figure 3 by the letter T, when compared with the group considered susceptible to the fungus, represented by S, in this same treatment. These results corroborate Mendes et al. (2012Mendes, M. C., Von Pinho, R. G., Von Pinho, E. V. R., & Faria, M. V. (2012). Comportamento de híbridos de milho inoculados com os fungos causadores do complexo grãos ardidos e associação com parâmetros químicos e bioquímicos. Ambiência, 8(2), 277-279.), which evaluated groups of hybrids considered resistant and hybrids considered susceptible to the grain rot complex for the LOX enzyme; in this study, the electrophoretic profiles for lipoxygenase revealed a higher intensity of bands for the hybrids resistant to fungus that causes grain rot in maize.
In the V8 treatment (Figure 3), it was possible to note higher expression of LOX for the hybrids belonging to the group considered susceptible to grain rot, showing that the fungicide application at V8 interfered in the expression of LOX.
Electrophoretic pattern of lipoxygenase in maize hybrids considered tolerant and susceptible to fungi that cause grain rot; the use of fungicide in stages V8, V8 + VT and the control produced in environment 2 in Guarapuava, Paraná State, Brazil, crop season of 2013/14
In the V8 + VT treatment, it was possible to note higher expression of the LOX enzyme in the hybrids belonging to the group considered susceptible to grain rot, hybrids P 30F53H, P 32R48H, P 30R50H, and DKB 390PRO, represented in Figure 3 by the letter S, when compared to the group considered tolerant to fungus, represented by the letter T; however, even in the group considered susceptible to grain rot, strong expression of LOX was noted.
When the results in Table 1 are compared, hybrids P 30F53H and P 30R50H reduced the percentage of grain rot; therefore, it is economically viable, it affects the quality and price obtained for the grain, and this occurred in the reduced spacing and in the conventional spacing. Therefore, with two fungicide applications, it is possible to infer that there is a relation of the active principle's action and LOX expression. The effects of LOX action can contribute to defense reactions, inhibiting pathogen development and inducing the phytoalexins (Latunde-Dada & Lucas, 2001Latunde-Dada, A. O., & Lucas, J. A. (2001). The plant defense activador acibenzolar-S-Methyl primes cowpea [(Vigna unguiculata (L.) Walp.)] seedlings for rapid induction of resistance. Physiological and Molecular Plant Pathology, 58(5), 199-208.).
Thus, for the maize hybrids with high activity of the enzyme lipoxygenase, these genotypes have a greater ability to resist fungal attack at the end of the cycle; with that, lower production of grain rot occurs in the harvest. The lipoxygenase product can act either as an intermediate or as an end product of a metabolic route. In plants, lipoxygenases are involved in the biosynthesis of jasmonic acid and aldehyde that are responsible for defense mechanism signaling (Quaglia, Fabrizi, Zazzerini, & Zadra, 2012Quaglia, M., Fabrizi, M., Zazzerini, A., & Zadra, C. (2012). Role of pathogen-induced volatiles in the Nicotiana tabacum and Golovinomyces cichoracearum interaction. Plant Physiology and Biochemistry, 52(3), 9-20.; Senthilraja, Anand, Kennedy, Raguchander, & Samiyappan, 2013Senthilraja, G., Anand, T., Kennedy, J. S., Raguchander, T., & Samiyappan, R. (2013). Plant growth promoting rhizobacteria (PGPR) and entomopathogenic fungus bioformulation enhance the expression of defense enzymes and pathogenesis-related proteins in groundnut plants against leafminer insect and collar rot pathogen. Physiological and Molecular Plant Pathology, 82(4), 10-19.).
Analyzing the data in environments 1 and 2 associated with grain rot (Table 1), it is possible to note that in the check, V8 and V8 + VT treatments, the hybrids belonging to group 1 (considered tolerant of grain rot) present a lower percentage of grain rot, except the check treatment in environment 2, where there was no significant difference between the groups of hybrids; the reduced spacing had a possible influence in this environment. However, in the check treatment, an increase of LOX enzyme expression occurred (Figures 2 and 3) for the hybrids belonging to the group considered tolerant of grain rot, as shown in Table 1. The hybrids belonging to group 1 have a lower percentage of grain rot when compared to group 2, and there was a direct relation between the lower percentage of grain rot and the higher expression of the lipoxygenase enzyme, proving the results obtained by Zeringue et al. (1996Zeringue, H. J., Brown, R. L., Neucere, J. N., & Cleveland, T. E. (1996). Relationships between C6-C12 alkanal and alkenal volatile contents and resistance of maize genotypes to Aspergillus flavus and aflatoxin prodution. Journal of Agricultural and Food Chemistry, 44(2),403-407.), which reported that higher expression of the LOX enzyme indicates a lower percentage of grain rot. The same authors suggest that the LOX enzyme, via lipoxygenation of linoleic acid, would be able to produce volatile aldehyde chains with 6 to 12 carbons, inhibiting or preventing development and even mycotoxin formation. Thus, the increase in enzyme expression may be related to the application of the fungicide Trifloxystrobin + Prothioconazole at V8 + VT reducing the cob rot.
Therefore, a positive response to the fungicide application (Trifloxystrobin + Prothioconazole) was observed, mainly in the percentage of grain rot in these hybrids; the reduction of grain rot was 2.3% and 3.4% in V8 + VT in the group of hybrids (tolerant and susceptible) in the 1st experiment, and in the 2nd experiment, the reduction was 3.4% and 0.7%, respectively, and in relation to lipoxygenase enzyme activity, evidencing the importance to continue this line of research and providing better understanding of active ingredient application and the action of the enzymes in the grain.
Conclusion
The fungicide (Trifloxystrobin + Prothioconazole) reduced the incidence of grain rot in two applications, V8 and V8 + VT, and these results were more evident in the hybrids considered susceptible.
There was higher expression of the enzyme LOX in maize hybrids belonging to the group considered tolerant of fungi that cause grain rot.
The use of the fungicide (Trifloxystrobin + Prothioconazole) in two applications, V8 (eight leaves) and VT (tasseling), increased the intensity of the LOX enzyme, which was more evident in the reduced spacing.
Acknowledgements
CAPES is acknowledged for providing a master's scholarship to the first author. CNPq, FINEP and Fundação Araucária are thanked for financial support.
References
- Alfenas, A. C. (1998). Eletroforese de isoenzimas e proteínas afins: fundamentos e aplicações em plantas e microrganismos Viçosa, MG: UFV.
- Alfenas, A. C. (2006). Eletroforese e marcadores bioquímicos em plantas e microrganismos Viçosa, MG: UFV .
- Brasil (1996). Portaria n° 11, de 12 de abril de 1996 estabelece critérios complementares para classificação do milho. Diário Oficial da União, Brasília, n. 72.
- Brito, A. H., Von Pinho, R. G., Pozza, E. A., Pereira, J. L. A. R., & Faria Filho, E. M. (2007). Efeito da Cercosporiose no rendimento de híbridos comerciais de milho. Fitopatologia Brasileira, 32(6), 472-479.
- Brito, A. H., Von Pinho, R. G., Souza Filho, A. X., & Altoé, T. F. (2008). Avaliação da severidade da cercosporiose e rendimento de grãos em híbridos comerciais de milho. Revista Brasileira de Milho e Sorgo, 7(1), 19-31.
- Brito, A. H., Pereira, J. L. A. R., Von Pinho, R. G., & Balestre, M. (2012). Controle químico de doenças foliares e grãos ardidos em milho (Zea mays L.). Revista Brasileira de Milho e Sorgo, 11(1), 49-59.
- Casa, R. T., Reis, E. M., & Zambolim, L. (2006). Doenças do milho causadas por fungos do gênero Stenocarpella Fitopatologia Brasileira, 31(5), 427-439.
- Costa, R. V., Cota, L. V., Silva, D. D., Lanza, F. E. & Figueiredo, J. E. F. (2012). Eficiência de fungicidas para o controle de mancha branca no milho. Revista Brasileira de Milho e Sorgo, 11(3), 291-301.
- Cunha, J. P. A. R., Silva, L. L., Boller, W., & Rodrigues, J. F. (2010). Aplicação aérea e terrestre de fungicida para o controle de doenças do milho. Revista Ciência Agronômica, 41(3), 366-372.
- Cunha, J. P. A. R., & Pereira, R. G. (2009). Efeito de pontas e volumes de pulverização no controle químico de doenças do milho. Revista Ciência Agronômica, 40(4), 533-538.
- Demetrio, C. S., Filho, D. F., Cazetta, J. O., & Cazetta, D. A. (2008). Desempenho de híbridos de milho submetidos a diferentes espaçamentos e densidades populacionais. Pesquisa Agropecuária Brasileira, 43(12), 1691-1697.
- Duarte, R. P., Juliatti, F. C., Lucas, B. V., & Freitas, P. T. (2009). Comportamento de diferentes genótipos de milho com aplicação foliar de fungicida quanto à incidência de fungos causadores de grãos ardidos. Bioscience Journal, 25(4), 112-122.
- Empresa Brasileira de Pesquisa Agropecuária [Embrapa]. (2006). Sistema Brasileiro de Classificação de Solos (2a ed.). Rio de Janeiro, RJ: Embrapa Solos.
- Ferreira, D. F. (2011). Sisvar: a computer statistical analysis system. Ciência e Agrotecnologia, 35(6), 1039-1042.
- Jardine, D. F., & Laca-Buendía, J. P. (2009). Eficiência de fungicidas no controle de doenças foliares na cultura do milho. Fazu em Revista, 26(6), 11-52.
- Juliatti, F. C., & Souza, R. M. (2005). Efeitos de Épocas de Plantio na severidade de doenças foliares e produtividade de híbridos de milho. Bioscience Journal, 21(1), 103-112.
- Juliatti, F. C., Zuza, J. L. M. F., Souza, P. P., & Polizel, A. C. (2007). Efeito do genótipo de milho e da aplicação foliar de fungicidas na incidência de grãos ardidos. Bioscience Journal, 23(2), 34-41.
- Juliatti, F. C., Pedrosa, M. G., Silva, H. D., & Silva, J. V. C. (2009). Genetic mapping for resistance to Gray leaf spot in maize. Euphytica, 169(2), 227-238.
- Juliatti, F. C., Nascimento, C., & Rezende, A. A. (2010). Avaliação de diferentes pontas e volumes de pulverização na aplicação de fungicida na cultura do milho. Summa Phytopathological, 36(3), 216-221.
- Kazan, K., & Manners, J. M. (2008). Jasmonate Signaling: toward na integrated view. Plant Physiology, 146(4), 1459-1468.
- Kishimoto, K., Matsui, K., Ozawa, R., & Takabayashi, J. (2008). Direct fungicidal activities of C6-aldehydes are important constituents for defense responses in Arabidopsis against Botrytis cinerea Phytochemistry, 69(11), 2127-2132.
- Kumar, V., Basu, M. S., & Rajendran, T. P. (2008). Mycotoxin research and mycoflora in some commercially important agricultural commodities. Crop Protection, 27(6), 891-905.
- Latunde-Dada, A. O., & Lucas, J. A. (2001). The plant defense activador acibenzolar-S-Methyl primes cowpea [(Vigna unguiculata (L.) Walp.)] seedlings for rapid induction of resistance. Physiological and Molecular Plant Pathology, 58(5), 199-208.
- Mendes, M. C., Von Pinho, R. G., Machado, J. C., Albuquerque, C. J. B., & Falquete, J. C. F. (2011). Qualidade sanitária de grãos de milho com e sem inoculação a campo dos fungos causadores de podridões de espiga. Ciência e Agrotecnologia, 35(5), 931-939.
- Mendes, M. C., Von Pinho, R. G., Von Pinho, E. V. R., & Faria, M. V. (2012). Comportamento de híbridos de milho inoculados com os fungos causadores do complexo grãos ardidos e associação com parâmetros químicos e bioquímicos. Ambiência, 8(2), 277-279.
- Pozar, G., Butruille, D., Diniz, H. S., & Viglioni, J. P. (2009). Mapping and validation of quantitative trait loci for resistance to Cercospora infection in tropical maize (Zea mays L.). Theoretical and Applied Genetics, 118(3), 553-564.
- Quaglia, M., Fabrizi, M., Zazzerini, A., & Zadra, C. (2012). Role of pathogen-induced volatiles in the Nicotiana tabacum and Golovinomyces cichoracearum interaction. Plant Physiology and Biochemistry, 52(3), 9-20.
- Ramalho, M. A. P., Ferreira, D. F., & Oliveira, A. C. (2000). Experimentação em genética e melhoramento de plantas Lavras, MG: UFLA.
- Senthilraja, G., Anand, T., Kennedy, J. S., Raguchander, T., & Samiyappan, R. (2013). Plant growth promoting rhizobacteria (PGPR) and entomopathogenic fungus bioformulation enhance the expression of defense enzymes and pathogenesis-related proteins in groundnut plants against leafminer insect and collar rot pathogen. Physiological and Molecular Plant Pathology, 82(4), 10-19.
- Silva, A. G., Cunha Junior, C. R., Assis, R. L., & Imolesi, A. S. (2008). Influência da população de plantas e do espaçamento entre linhas nos caracteres agronômicos do híbrido de milho P30K75 em Rio Verde, Goiás. Bioscience Journal, 24(2) 89-96.
- Silva, A. G., Teixeira, I. R., Martins, P. D. S., Simon, G. A., & Francischini, R. (2014). Desempenho agronômico e econômico de híbridos de milho na safrinha. Revista Agroambiente On-line, 8(2), 261-271.
- Stefanello, J., Bachi, L. M. A., Gavassoni, W. L., Hirata, L. M., & Pontim, B. C. A. (2012). Incidência de fungos em grãos de milho em função de diferentes épocas de aplicação foliar de fungicida. Pesquisa Agropecuária Tropical, 42(4), 476-481.
- Werle, A. J. K., Nicolay, R. J., Santos, R. F., Borsoi, A., & Secco, D. (2011). Avaliação de híbridos de milho convencional e transgênico (Bt), com diferentes aplicações de inseticida em cultivo safrinha. Revista Brasileira de Tecnologia Aplicada nas Ciências Agrárias, 4(1), 150-159.
- Zeringue, H. J., Brown, R. L., Neucere, J. N., & Cleveland, T. E. (1996). Relationships between C6-C12 alkanal and alkenal volatile contents and resistance of maize genotypes to Aspergillus flavus and aflatoxin prodution. Journal of Agricultural and Food Chemistry, 44(2),403-407.
Publication Dates
-
Publication in this collection
Oct 2017
History
-
Received
05 Aug 2016 -
Accepted
07 Feb 2017