Abstracts
The objective was to evaluate the effect of propolis-based products (PBP) on performance, digestibility, microbial production and carcass characteristics of feedlot young bulls. Twenty-seven crossbred young bulls were used, with 353 ± 28 kg of body weight in a completely randomized experimental design, divided in three treatments: two diets with PBP with different dosages (PBP1= 0.018 mg g-1 and PBP2= 0.036 mg g-1 of total flavonoids in chrysin) and control diet (CON). To determine total digestibility, the indigestible dry matter was used as an internal marker, while microbial production was estimated from purine derivatives in urine, collected by the spot method. The evaluated carcass characteristics were: hot carcass weight, dressing percentage, conformation, Longissimus muscle area, fat thickness, colour, texture, marbling, pH, cushion thickness and percentages of muscle, bone and fat. The studied variables were subjected to analysis of variance with 5% probability. The addition of propolis had no effect on DM and nutrients digestibility (except the ADF, which was higher) or efficiency of microbial synthesis. Carcass characteristics were not affected by the experimental treatments. The PBP in the used dosages should be reviewed and higher dosages should be tested.
additive; flavonoids; meat quality; microbial efficiency; ruminant
Objetivou-se avaliar o efeito de produtos à base de própolis (PBP) sobre o desempenho, digestibilidade, produção microbiana e características de carcaça de bovinos confinados. Foram utilizados 27 bovinos com 353 ± 28 kg de peso corporal em um delineamento inteiramente casualizado dividido em três tratamentos: duas dietas contendo PBP em diferentes dosagens (PBP1 = 0.018 mg g-1 e PBP2 = 0.036 mg g-1 de flavonoides totais em crisina) e dieta controle (CON) sem adição de própolis. Para a determinação da digestibilidade total, a matéria seca indigestível foi usada como marcador interno, enquanto a produção microbiana foi estimada pelos derivados de purina na urina, coletadas pelo método spot. As características de carcaça avaliadas foram: peso de carcaça quente, rendimento de carcaça quente, conformação, área de olho de lombo, espessura de gordura, coloração, textura, marmoreio, pH, espessura de coxão e percentagens de músculo, osso e gordura. As variáveis estudadas foram submetidas à análise de variância com 5% de probabilidade. A adição da própolis não teve efeito sobre a digestibilidade da MS e nutrientes (exceto para FDA, que foi maior) e eficiência de síntese microbiana. As características de carcaça não foram afetadas pelos tratamentos experimentais. As dosagens utilizadas nos PBP devem ser revistas e dosagens mais elevadas devem ser testadas.
aditivo; flavonoides; qualidade de carne; eficiência de síntese microbiana; ruminante
RUMINANT NUTRITION NUTRIÇÃO DE RUMINANTES
Performance, digestibility, microbial production and carcass characteristics of feedlot young bulls fed diets containing propolis
Desempenho, digestibilidade, produção microbiana e características de carcaça de bovinos confinados que receberam dietas contendo própolis
Sílvia Cristina de Aguiar; Lúcia Maria Zeoula* * Author for correspondence. E-mail: lmzeoula@uem.br License information: This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. ; Lucimar Pontara Peres de Moura; Ivanor Nunes do Prado; Eduardo Marostegan de Paula; Rafael Barreiros Samensari
Departamento de Zootecnia, Universidade Estadual de Maringá, Av. Colombo 5790, 87020-900, Maringá, Paraná, Brazil
ABSTRACT
The objective was to evaluate the effect of propolis-based products (PBP) on performance, digestibility, microbial production and carcass characteristics of feedlot young bulls. Twenty-seven crossbred young bulls were used, with 353 ± 28 kg of body weight in a completely randomized experimental design, divided in three treatments: two diets with PBP with different dosages (PBP1= 0.018 mg g-1 and PBP2= 0.036 mg g-1 of total flavonoids in chrysin) and control diet (CON). To determine total digestibility, the indigestible dry matter was used as an internal marker, while microbial production was estimated from purine derivatives in urine, collected by the spot method. The evaluated carcass characteristics were: hot carcass weight, dressing percentage, conformation, Longissimus muscle area, fat thickness, colour, texture, marbling, pH, cushion thickness and percentages of muscle, bone and fat. The studied variables were subjected to analysis of variance with 5% probability. The addition of propolis had no effect on DM and nutrients digestibility (except the ADF, which was higher) or efficiency of microbial synthesis. Carcass characteristics were not affected by the experimental treatments. The PBP in the used dosages should be reviewed and higher dosages should be tested.
Keywords: additive, flavonoids, meat quality, microbial efficiency, ruminant.
RESUMO
Objetivou-se avaliar o efeito de produtos à base de própolis (PBP) sobre o desempenho, digestibilidade, produção microbiana e características de carcaça de bovinos confinados. Foram utilizados 27 bovinos com 353 ± 28 kg de peso corporal em um delineamento inteiramente casualizado dividido em três tratamentos: duas dietas contendo PBP em diferentes dosagens (PBP1 = 0.018 mg g-1 e PBP2 = 0.036 mg g-1 de flavonoides totais em crisina) e dieta controle (CON) sem adição de própolis. Para a determinação da digestibilidade total, a matéria seca indigestível foi usada como marcador interno, enquanto a produção microbiana foi estimada pelos derivados de purina na urina, coletadas pelo método spot. As características de carcaça avaliadas foram: peso de carcaça quente, rendimento de carcaça quente, conformação, área de olho de lombo, espessura de gordura, coloração, textura, marmoreio, pH, espessura de coxão e percentagens de músculo, osso e gordura. As variáveis estudadas foram submetidas à análise de variância com 5% de probabilidade. A adição da própolis não teve efeito sobre a digestibilidade da MS e nutrientes (exceto para FDA, que foi maior) e eficiência de síntese microbiana. As características de carcaça não foram afetadas pelos tratamentos experimentais. As dosagens utilizadas nos PBP devem ser revistas e dosagens mais elevadas devem ser testadas.
Palavras-chave: aditivo, flavonoides, qualidade de carne, eficiência de síntese microbiana, ruminante.
Introduction
Ionophore additives are used in ruminant nutrition; however, no animal-based food products containing these substances can be produced in or enter in Europe since January 2006, according to European Union legislation, as published in the Official Journal of the European Union (2003). With these facts, alternatives have been sought to replace these additives with natural ones. The propolis may serve this promising purpose,
as it is a product with numerous pharmacological properties, including antimicrobial activity (PARK et al., 2002). Propolis appears to provide an action similar to ionophore when administered to animals, as observed in in vivo and in vitro experiments (ÍTAVO et al., 2011; OLIVEIRA et al., 2004, 2006; STRADIOTTI JÚNIOR et al., 2004).
The ability of propolis to inhibit the growth of microorganisms is its most popularly known and scientifically proven pharmacological activity. Its antimicrobial property is mainly attributed to the flavanone pinocembrin, to the flavonol galangin and to the caffeic acid phenethyl ester, with a mechanism of action probably based on the inhibition of bacterial RNA polymerase (TAKAISI-KIKUNI; SCHILCHER, 1994). Other compo-nents, such as flavonoids, caffeic acid, benzoic acid and cinnamic acid, probably act on the membrane or cell wall of the microbe and cause structural and functional damage (SCAZZOCCHIO et al., 2005). Propolis has greater antimicrobial activity against Gram-positive bacteria, with limited effectiveness against Gram-negative ones (FERNANDES JÚNIOR et al., 2006; LU et al., 2005; MARCUCCI et al., 2001).
The propolis-based product (PBP), developed by Franco and Bueno (1999), has shown positive results in studies involving ruminant nutrition, such as increase in the in vitro dry matter digestibility, increase in the flow and higher digestibility of crude protein (CP) in the intestines and better feed conversion ratio (PRADO et al, 2010a and b; ZAWADZKI et al., 2011).
However, more research on the propolis use in animal production is needed to determine whether its antimicrobial action has any effect on energy efficiency in diets for ruminants, as well as on food efficiency, nutrient digestibility, rumen microbial protein and carcass characteristics. The aim of the present research was to evaluate the effects of propolis-based products in diets containing the same forage:concentrate ratio (50:50) on performance, digestibility, microbial production and carcass characteristics of feedlot young bulls.
Material and methods
Source of propolis-based product (PBP)
Propolis-based product, a powder, contains dried propolis extract and is registered in the National Institute of Industrial Property - Brazil, under no. 0605768-3. The preparation of PBP consists of the hydroalcoholic extraction of raw propolis to release its active substances - flavonoids, mainly. Subsequently, the alcohol is evaporated with the aid of a rotary evaporator and the extract is dried.
The levels of total flavonoids, quantified in chrysin by Prado et al. (2010b) through high performance liquid chromatography (HPLC), with 0.018 mg g-1 for the PBP1 and 0.036 mg g-1 for the PBP2. Doses of PBP supplied daily to the animals were added in 75 g of excipient (soya bean meal and corn meal based) and included in the experimental diet.
Location, animals, management and experimental diets
The experiment was conducted in the city of Maringá, Paraná State, Brazil. Twenty-seven crossbred young bulls (½ Nelore x ½ Angus) were used, with 353 ± 28 kg of average body weight (ABW) and twenty-four months old, housed in individual stalls (10 m2) surrounded with steel rebar and concrete floor, half of the bay covered with sheets of zinc. The diet of the animals consisted of 50% of corn silage and 50% of commercial concentrate (Table 1), containing two treatments with propolis-based products at different dosages (PBP1 and PBP2) and a control treatment without addition of additives (CON).
The experimental diet was formulated according to the recommendations proposed by the NRC (1996), containing 70.2% of total digestible nutrients (TDN) and 13.5% of CP. The animals were fed twice daily, at 8 and 16h, with forage and concentrate mixed on the trough. All animals received the same experimental diet, differing only in the addition of propolis or not (control). The PBP1 and PBP2 products were added to the feed at the time of feeding.
Performance, digestibility and microbial production
The ration, weighed daily, was provided ad libitum, so that the refusals represented 10% of the total. The animals were weighed at the beginning of the experiment and then every twenty-eight days after a solids fasting period of eight hours, until the end of the experiment (84 days), in order to determine performance.
Fecal collection was performed for a period of five days, on the thirty-seventh day of confinement, to obtain the total digestibility coefficient of dry matter and nutrients. Fecal samples were stored in labeled plastic bags and stored in a freezer at -20oC, for later laboratory analysis. Daily feed intake was estimated by the difference between the supplied feed and refusals in the trough. During the experimental period, samples of the supplied feed and refusals were collected and a representative composite sample was drafted per animal in each treatment.
To estimate the flux of fecal dry matter, indigestible DM (iDM) was used as an internal marker. Feeds, remains and feces composite samples were milled through a 2 mm sieve, packed (6 g of sample) in 10 x 20 cm ANKON® nylon bags (Ankon Technology Co. Fairport, NY, USA), previously weighed, and incubated for 6 days in the rumen of a Holstein cow (545 kg BW), fed on a mixed diet of equal parts of forage (corn silage) and concentrate (the same one used in this study), based on DM. After incubation, the bags were removed, washed with water until total clearance, dried in a ventilated oven (55°C for 72 hours), once again removed and dried in an oven at 105oC. The iDM was estimated by weight difference obtained before and after ruminal incubation of the samples. The fecal excretion was calculated by the following equation: FE = iDMI/ iDMCF; where: FE = fecal excretion (kg day-1); iDMI = iDM intake (kg day-1); iDMCF = iDM concentration in feces (kg kg-1).
The total digestibility coefficients (TDC) for DM and nutrients were estimated according to the equations described by Coelho da Silva and Leão (1979). The analysis to determine dry matter (DM, method no. 934.01), organic matter determined by ash (OM, method no. 924.05), crude protein (CP, method no. 920.87) and ether extract (EE, method no. 920.85) in the samples milled to 1 mm, were conducted in accordance to the AOAC (1990). Neutral detergent fiber (NDF) was determined according to Van Soest et al. (1991) and acid detergent fiber (ADF) determined according to method no. 973.18 (AOAC, 1990). The total carbohydrates (TC) were obtained by using the following equation: TC = 100 - (% CP +% EE +% Ash) (SNIFFEN et al., 1992). Non-fiber carbohydrates (NFC) were determined by the difference between TC and NDF (without correction for protein). TDN content of diets was obtained by the CNCPS (Cornell Net Carbohydrate and Protein System): TDN (%) = DCP (%) + 2.25*DEE (%) + DTC; where: DCP = digestible crude protein, DEE = digestible ether extract, and DTC = digestible total carbohydrates.
In order to determine microbial production, spot urine samples were collected approximately 4 hours after feeding, during voluntary urination. The analyses of allantoin were performed using methods described by Chen and Gomes (1992). To determine creatinine and uric acid, urine samples were sent to the Laboratory Diagnosis Center (CEDLAB), located in the city of Maringá, Paraná State. Urine volume (expressed in L) was estimated from the concentration of creatinine in each spot urine sample, dividing the daily excretion of creatinine (mg kg-1 of BW) by creatinine concentration (mg L-1). Microbial nitrogen production was calculated using the equation described by Chen and Gomes (1992).
Slaughter and carcass characteristics
At the end of the experiment, the animals were slaughtered at a slaughterhouse located near the experimental farm, where the physical characteristics of carcass and meat were determined. The dressing percentage (DP) was obtained from the fasting BW of the animal prior to going to slaughter, and hot carcass weight (HCW) was determined at slaughter.
Carcass conformation was assessed according to a point scale suggested by Müller (1980), and was scored with grade values from 18 (superior conformation) to 1 (inferior conformation). Cushion thickness (CUT) was determined using a compass, finding the distance between the lateral and medial upper portion of the cushion measured with a millimeter tape.
The Longissimus muscle area (LMA) was determined in the right half of the carcass, where a cross section was taken between the 12th and 13th ribs, exposing the surface of the muscle, on which the outline of the muscle was traced on paper. Later, the area was calculated with the aid of a planimeter and expressed as total area in cm2 and per 100 kg of carcass (LMA, cm2 100 kg-1).
The determination of fat thickness (FT) was performed in the cut between the 12th and 13th ribs, above the Longissimus dorsi muscle, with the aid of a caliper, by calculating the average of three measurements per carcass. The percentages of bone (BP), muscle (MP) and fat (FP) in the carcass were determined using the section of the L. dorsi obtained through the methodology described by Hankins and Howe (1946). Marbling was determined in the exposed side of the L. dorsi between the 12th and 13th ribs, and was evaluated visually according to the methodology described by Müller (1980), where the marbling was scored with grade values from 18 (abundant marbling) to 1 (traces of marbling).
The texture and colour of the muscle were evaluated subjectively using a point scale proposed by Müller (1987) in the same sample used to rate marbling. Texture was scored with grade values from 5 (very thin texture) to 1 (very coarse texture), while colour was scored with grade values from 5 (bright red) to 1 (dark).
Statistical analysis
The data were analyzed using the GENMOD procedure of the SAS statistical software package (SAS, 2000). The experimental design was completely randomized. For performance, digestibility, microbial synthesis and carcass characteristics, nine replications were used per treatment. Differences between treatments means were determined by Tukey test. Tests that had p-values < 0.05 were considered statistically significant; those that had values < 0.10 suggested trends.
Results and discussion
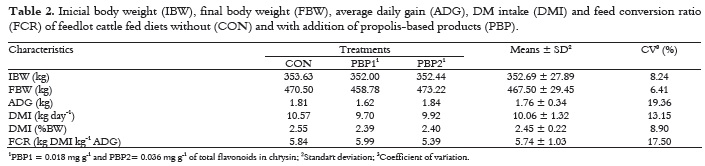
The experimental diets did not influence (p > 0.05) DM intake (mean = 2.45, %BW), average daily gain (1.76 kg) and feed conversion ratio (FCR) with mean of 5.74 for crossbred young bulls (Table 2).
These data disagree with those observed by Bonomi and Bonomi (2002), who observed a better performance (p < 0.05) in feedlot young bulls receiving propolis in the diets. Due to higher levels of propolis extracts (20, 40 and 60 ppm) in the diet of Limousin young bulls, the authors found improved weight gain by 4.5, 9.0 and 12.0%, respectively and improvement in FCR of 5.0, 10.0 and 15.0%, respectively. The absence of propolis effects in this study may be related to lower levels of flavonoids present in the propolis-based products used. Zawadzki et al. (2011) tested the same PBP used in this experiment, but at higher dosage (0.0054 mg g-1), in feedlot finished bulls and found greater weight gain and better FCR (p < 0.05) for animals that received propolis in the diet. The propolis-based product increased ADG at 25.6 and 19.6% when compared to control and sodium monensin treatments, respectively, and reduced FCR at 26.2 and 17.3% for the same treatments, respectively. Probably the PBP dosage given to animals in this work did not contain a sufficient amount of phenolic compounds to act on the rumen microflora and, consequently, improve animal performance.
An effect of propolis addition was observed in the second and third feedlot period (28 days period-1), as shown in Table 3.
There was significant difference (p < 0.05) for ADG in the second period of experiment. The CON treatment had greater weight gain (1.98 kg day-1), but did not differ from treatment with the highest dosage of propolis (PBP2), with an average of 1.85 kg day-1; for the PBP1 treatment, it was observed the lowest weight gain during this period.
However, between the second and last period, there was a trend for reduced feed intake (p = 0.08) in both PBP. This fact seems to show that propolis-based products would be exerting antimicrobial action throughout the feedlot period, which cannot be characterized as microbe resistance to propolis. The action of flavonoids on the microbial and animal metabolism appears to be related to the amount and availability of flavonoids and to the diet composition. These effects were demonstrated by Prado et al. (2010b) for diets containing the same forage:concentrate ratio (50:50) and 100% forage. The addition of PBP1 reduced the fermentation of cellulose when expressed as a percentage of tolerant bacterial strains, but this effect was lower in diets based on forage.
The use of PBP did not affect (p > 0.05) dry matter and nutrient digestibility (Table 4).
The TDN values obtained, with an average of 69.6%, are close to pre-established values, 70.2%. The results observed for DM digestibility differ from those reported by Prado et al. (2010b) for diets containing the same forage:concentrate ratio (50:50), who observed an increase from 8.3% and 6.2% in vitro DM digestibility with the addition of PBP1 (p < 0.05) compared to the control and to monensin, respectively. Probably the differences of previous results in vitro are related to ruminal volume, the dry matter intake, passage rate in the rumen and basal diet. For the ADF digestibility, the PBP2 did not differ from control treatment, however, there was a trend (p = 0.08) for higher digestibility of ADF, when compared to the lower dosage treatment (PBP1). It is probably necessary adjust the dosage of PBP to feedlot young bulls, to provide more energy for animal metabolism.
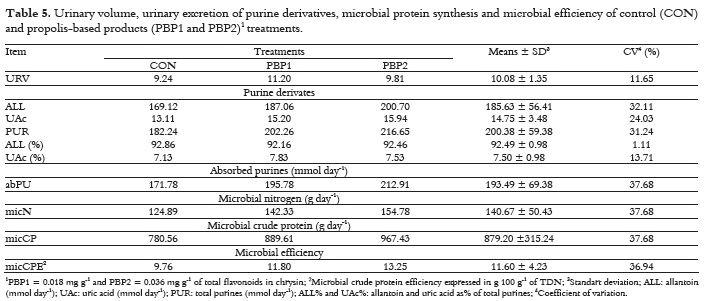
The propolis-based products did not affect (p > 0.05) microbial protein synthesis (g day-1) and microbial efficiency (g 100 g-1 of TDN) (Table 5).
The PBP2 provided a value of 13.3 g 100 g-1 of TDN for microbial efficiency and, according to NRC (1996), the value of 13.0 g of CP 100 g-1 of TDN for micCPE is a good estimate. A significant increase in protein flow to the intestine was observed by Prado et al. (2010a) for cattle fed on forage with the addition of PBP1 compared to control treatment (705.0 vs. 788.0 g day-1 of microbial CP). The antimicrobial activity of propolis has also been related to rumen protozoa, as observed by Broudiscou et al. (2000), who reported a decrease in these, for the treatment with propolis extract in continuous culture.
The excretion of allantoin and uric acid did not differ (p > 0.05) for the treatments. In relation to total purine, there was an average of allantoin excretion of 92.49%. This value is similar to that observed by Rennó et al. (2008).
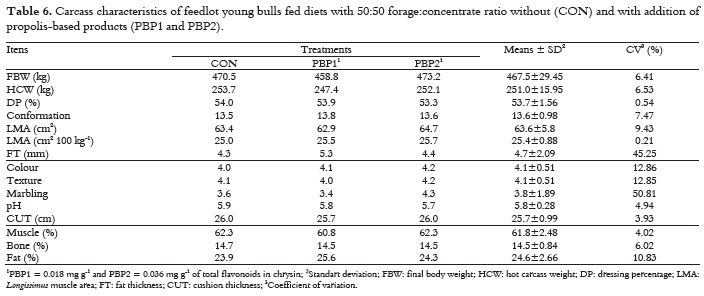
The treatments used in this experiment did not influence (p > 0.05) carcass characteristics in cattle (Table 6). Zawadzki et al. (2011), evaluated the carcass characteristics of young bulls that received the same PBP, but at higher dosage, and also found no differences between the control, sodium monensin and propolis treatments. Though, opposite to the observed data, Bonomi and Bonomi (2002) found higher carcass weight in Limousin young bulls that received higher dosage of propolis extract compared to control diet. Some studies that assessed the effect of ionophore food additives on carcass characteristics found no influence, regardless of sex, breed, age and housing system (MENEZES et al., 2006; OSMARI et al., 2008). Although there is no difference among treatments, dressing percentage (DP, %) is within expectations (53.7%), as same as carcass conformation, with an average score of 13.6. Thus, conformation was described as very good, and this in an important data, since conformation indicates the carcass meat:bone ratio. The average value obtained for the Longissimus muscle area (LMA) is also within the desired range (25.4 cm2 100 kg-1), as well as fat thickness (FT).
The marbling in this work was very low, and is characterized as very light; however, authors (RESTLE et al., 2000; RODRIGUES; ANDRADE, 2004) reported that higher levels of marbling are found in castrated than in non-castrated animals. Moreover, it is important to underline that the animals were also very young, which may have influenced the observed marbling.
Conclusion
The addition of propolis-based product (PBP) in the diet of crossbred young bulls did not affect productive performance, carcass characteristics, total digestibility of DM and nutrients and the efficiency of microbial synthesis. Therefore, it is necessary to review the dosages of propolis extracts used for crossbred feedlot young bulls.
Acknowledgements
The authors would like to acknowledge the financial assistance provided by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
References
AOAC-Association of Official Analytical Chemists. Official methods of analysis. 15th ed. Arlington: AOAC International, 1990.
BONOMI, A.; BONOMI, B. M. L'impiego della propoli nell'alimentazione dei vitelloni. La Rivista di Scienza dell'alimentazione, v. 31, n. 1, p. 91-103, 2002.
BROUDISCOU, L. P.; PAPON, Y.; BROUDISCOU, A. F. Effects of dry plant extracts on fermentation and methanogenesis in continuous culture of rumen microbes. Animal Feed Science and Technology, v. 87, n. 3-4, p. 263-277, 2000.
CHEN, X. B.; GOMES, M. J. Estimation of microbial protein supply to sheep and cattle based on urinary excretion of purine derivatives: An overview of technical details. International Feed Resources Unit, p. 1-21, 1992. (Occasional Publication. Rowett Research Institute, Aberdeen, United Kingdom).
COELHO DA SILVA, J. F.; LEÃO, M. I. Fundamentos de nutrição de ruminantes. Piracicaba: Livroceres, 1979.
FERNANDES JÚNIOR, A.; LOPES, M. M. R.; COLOMBARI, V.; MONTEIRO, A. C. M.; VIEIRA, E. P. Atividade antimicrobiana de própolis de Apis mellifera obtidas em três regiões do Brasil. Ciência Rural, v. 36, n. 1, p. 294-297, 2006.
FRANCO, S. L.; BUENO, J. H. F. Otimização do processo extrativo de própolis. Infarma, v. 11, n. 11-12, p. 48-51, 1999.
HANKINS, O. G.; HOWE, P. E. Estimation of the composition of beef carcasses and cuts. Washington, D.C.: United States Department of Agriculture, 1946.
ÍTAVO, C. C. B. F.; MORAIS, M. G.; COSTA, C.; ÍTAVO, L. C. V.; FRANCO, G. L.; SILVA, J. A.; REIS, F. A. Addition of propolis or monensin in the diet: behavior and productivity of lambs in feedlot. Animal Feed Science and Technology, v. 165, n. 3-4, p. 161-166, 2011.
LU, L.; CHEN, Y.; CHOU, C. Antibacterial activity of propolis against Staphylococcus aureus. International Journal of Food Microbiology, v. 102, n. 2, p. 213-220, 2005.
MARCUCCI, M. C.; FERRERES, F.; GARCÍA-VIGUERA, C.; BANKOVA, V. S.; DE CASTRO, S. L.; DANTAS, A. P.; VALENTE, P. H. M.; PAULINO, N. Phenolic compounds from Brazilian propolis with pharmacological activities. Journal of Ethnopharmacology, v. 4, n. 2, p. 105-112, 2001.
MENEZES, F. L. G.; KOZLOSKI, G. V.; RESTLE, J.; DESCHAMPS, F. C.; BRONDANI, I. L.; SANTOS, A. P.; FIAMONCINI, J. Perfil de ácidos graxos de cadeia longa e qualidade da carne de novilhos terminados em confinamento com diferentes níveis de monensina sódica na dieta. Ciência Rural, v. 36, n. 1, p. 186-190, 2006.
MÜLLER, L. Normas para avaliação de carcaças e concurso de carcaça de novilhos. 1. ed. Santa Maria: UFSM, 1980.
MÜLLER, L. Normas para avaliação de carcaças e concurso de carcaça de novilhos. 2. ed. Santa Maria: UFSM, 1987.
NRC-National Research Concil. Nutrient requirements of beef cattle. 7th ed. Washington, D.C.: National Academy, 1996.
OFFICIAL JOURNAL OF THE EUROPEAN UNION. Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. L 268, p. 29-43, Oct.18, 2003.
OLIVEIRA, J. S.; LANA, R. P.; BORGES, A. C.; QUEIROZ, A. C.; ALMEIDA, I. C. C. Efeito da monensina e extrato de própolis sobre a produção de amônia e degradabilidade in vitro da proteína bruta de diferentes fontes de nitrogênio. Revista Brasileira de Zootecnia, v. 33, n. 2, p. 504-510, 2004.
OLIVEIRA, J. S.; QUEIROZ, A. C.; LANA, R. P.; MANTOVANI, H. C.; GENEROSO, R. A. R. Efeito da monensina e da própolis sobre a atividade de fermentação de aminoácidos in vitro pelos microrganismos ruminais. Revista Brasileira de Zootecnia, v. 35, n. 1, p. 275-281, 2006.
OSMARI, M. P.; ARBOITTE, M. Z.; BRONDANI, I. L.; KUSS, F.; ALVES FILHO, D. C.; RESTLE, J. Vacas terminadas em campo nativo suplementadas com farelo de trigo ou farelo de arroz integral contendo ou não monensina sódica. Ciência e Agrotecnologia, v. 32, n. 6, p. 1974-1980, 2008.
PARK, Y. K.; ALENCAR, S. M.; SCAMPARINI; R. P.; AGUIAR, C. L. Própolis produzida no sul do Brasil, Argentina e Uruguai: evidências fitoquímicas de sua origem vegetal. Ciência Rural, v. 32, n. 6, p. 997-1003, 2002.
PRADO, O. P. P.; ZEOULA, L. M.; MOURA, L. P. P.; FRANCO, S. L.; PRADO, I. N.; GOMES, H. C. C. Digestibilidade e parâmetros ruminais de dietas à base de forragem com adição de própolis e monensina sódica para bovinos. Revista Brasileira de Zootecnia, v. 39, n. 6, p. 1336-1345, 2010a.
PRADO, O. P. P.; ZEOULA, L. M.; PONTARA, L. P. M.; FRANCO, S. L.; NOVELLO, C. R.; GERON, L. J. V. Adição de própolis ou monensina sódica sobre digestibilidade in vitro da matéria seca em dietas 50:50% volumoso:concentrado e 100% volumoso. Revista Brasileira de Saúde e Produção Animal, v. 11, n. 4, p. 1023-1032, 2010b.
RENNÓ, L. N.; VALADARES FILHO, S. C.; VALADARES, R. F. D.; PAULINO, M. F.; RENNÓ, F. P.; SILVA, P. A. Níveis de uréia na ração de novilhos de quatro grupos genéticos: estimativa da produção de proteína microbiana por meio dos derivados de purinas na urina utilizando duas metodologias de coleta. Revista Brasileira de Zootecnia, v. 37, n. 3, p. 546-555, 2008.
RESTLE, J.; VAZ, F. N.; FEIJÓ, G. L. D.; BRONDAN, I. L.; ALVES FILHO, D. C.; BERNARDES, R. A. C.; FATURI, C.; PACHECO, P. S. Características de carcaça de bovinos de corte inteiros ou castrados de diferentes composições raciais Charolês x Nelore. Revista Brasileira de Zootecnia, v. 29, n. 5, p. 1371-1379, 2000.
RODRIGUES, V. C.; ANDRADE, I. F. Características físico-químicas da carne de bubalinos e de bovinos castrados e inteiros. Revista Brasileira de Zootecnia, v. 33, n. 6, p. 1839-1849, 2004.
SAS-Statistical Analysis System. SAS user's guide: statistics, version 8.1. 4th ed. Cary: Statistical Analysis System Institute, 2000.
SCAZZOCCHIO, F.; D'AURIA, F. D.; ALESSANDRINI, D.; PANTANELLA, F. Multifactorial aspects of antimicrobial activity of propolis. Microbiological Research, v. 161, n. 4, p. 327-333, 2005.
SNIFFEN, C. J.; O'CONNOR, J. D.; VAN SOEST, P. J.; FOX, D. G.; RUSSELL, J. B. A net carbohydrate and protein system for evaluating cattle diets: II. Carbohydrate and protein availability. Journal of Dairy Science, v. 70, n. 11, p. 3562-3577, 1992.
STRADIOTTI JÚNIOR, D.; QUEIROZ, A. C.; LANA, R. P.; PACHECO, C. G.; EIFERT, E. C.; NUNES, P. M. M. Ação da própolis sobre a desaminação de aminoácidos e a fermentação ruminal. Revista Brasileira de Zootecnia, v. 33, n. 4, p. 1086-1092, 2004.
TAKAISI-KIKUNI, N. B.; SCHILCHER, H. Electron microscopy and microcalorimetric investigations of the possible mechanism of the antibacterial action of a defined propolis provenance. Planta Medica, v. 60, n. 3, p. 222-227, 1994.
VAN SOEST, P. J.; ROBERTSON, J. B.; LEWIS, B. A. Symposim: carbohydrate methodology, metabolism, and nutritional implications in dairy cattle. Journal of Dairy Science, v. 74, n. 10, p. 3583-3597, 1991.
ZAWADZKI, F.; PRADO, I. N.; MARQUES, J. A.; ZEOULA, L. M.; ROTTA, P. P.; SESTARI, B. B.; VALERO, M. V.; RIVAROLLI, D. C. Sodium monensin or propolis extract in the diets of feedlot-finished bulls: effects on animal performance and carcass characteristics. Journal of Animal and Feed Sciences, v. 20, n. 1, p. 16-25, 2011.
Received on October 31, 2011.
Accepted on February 15, 2012.
- AOAC-Association of Official Analytical Chemists. Official methods of analysis 15th ed. Arlington: AOAC International, 1990.
- BONOMI, A.; BONOMI, B. M. L'impiego della propoli nell'alimentazione dei vitelloni. La Rivista di Scienza dell'alimentazione, v. 31, n. 1, p. 91-103, 2002.
- BROUDISCOU, L. P.; PAPON, Y.; BROUDISCOU, A. F. Effects of dry plant extracts on fermentation and methanogenesis in continuous culture of rumen microbes. Animal Feed Science and Technology, v. 87, n. 3-4, p. 263-277, 2000.
- CHEN, X. B.; GOMES, M. J. Estimation of microbial protein supply to sheep and cattle based on urinary excretion of purine derivatives: An overview of technical details. International Feed Resources Unit, p. 1-21, 1992. (Occasional Publication. Rowett Research Institute, Aberdeen, United Kingdom).
- COELHO DA SILVA, J. F.; LEÃO, M. I. Fundamentos de nutrição de ruminantes Piracicaba: Livroceres, 1979.
- FERNANDES JÚNIOR, A.; LOPES, M. M. R.; COLOMBARI, V.; MONTEIRO, A. C. M.; VIEIRA, E. P. Atividade antimicrobiana de própolis de Apis mellifera obtidas em três regiões do Brasil. Ciência Rural, v. 36, n. 1, p. 294-297, 2006.
- FRANCO, S. L.; BUENO, J. H. F. Otimização do processo extrativo de própolis. Infarma, v. 11, n. 11-12, p. 48-51, 1999.
- HANKINS, O. G.; HOWE, P. E. Estimation of the composition of beef carcasses and cuts Washington, D.C.: United States Department of Agriculture, 1946.
- ÍTAVO, C. C. B. F.; MORAIS, M. G.; COSTA, C.; ÍTAVO, L. C. V.; FRANCO, G. L.; SILVA, J. A.; REIS, F. A. Addition of propolis or monensin in the diet: behavior and productivity of lambs in feedlot. Animal Feed Science and Technology, v. 165, n. 3-4, p. 161-166, 2011.
- LU, L.; CHEN, Y.; CHOU, C. Antibacterial activity of propolis against Staphylococcus aureus International Journal of Food Microbiology, v. 102, n. 2, p. 213-220, 2005.
- MARCUCCI, M. C.; FERRERES, F.; GARCÍA-VIGUERA, C.; BANKOVA, V. S.; DE CASTRO, S. L.; DANTAS, A. P.; VALENTE, P. H. M.; PAULINO, N. Phenolic compounds from Brazilian propolis with pharmacological activities. Journal of Ethnopharmacology, v. 4, n. 2, p. 105-112, 2001.
- MENEZES, F. L. G.; KOZLOSKI, G. V.; RESTLE, J.; DESCHAMPS, F. C.; BRONDANI, I. L.; SANTOS, A. P.; FIAMONCINI, J. Perfil de ácidos graxos de cadeia longa e qualidade da carne de novilhos terminados em confinamento com diferentes níveis de monensina sódica na dieta. Ciência Rural, v. 36, n. 1, p. 186-190, 2006.
- MÜLLER, L. Normas para avaliação de carcaças e concurso de carcaça de novilhos 1. ed. Santa Maria: UFSM, 1980.
- MÜLLER, L. Normas para avaliação de carcaças e concurso de carcaça de novilhos 2. ed. Santa Maria: UFSM, 1987.
- NRC-National Research Concil. Nutrient requirements of beef cattle 7th ed. Washington, D.C.: National Academy, 1996.
- OFFICIAL JOURNAL OF THE EUROPEAN UNION. Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition L 268, p. 29-43, Oct.18, 2003.
- OLIVEIRA, J. S.; LANA, R. P.; BORGES, A. C.; QUEIROZ, A. C.; ALMEIDA, I. C. C. Efeito da monensina e extrato de própolis sobre a produção de amônia e degradabilidade in vitro da proteína bruta de diferentes fontes de nitrogênio. Revista Brasileira de Zootecnia, v. 33, n. 2, p. 504-510, 2004.
- OLIVEIRA, J. S.; QUEIROZ, A. C.; LANA, R. P.; MANTOVANI, H. C.; GENEROSO, R. A. R. Efeito da monensina e da própolis sobre a atividade de fermentação de aminoácidos in vitro pelos microrganismos ruminais. Revista Brasileira de Zootecnia, v. 35, n. 1, p. 275-281, 2006.
- OSMARI, M. P.; ARBOITTE, M. Z.; BRONDANI, I. L.; KUSS, F.; ALVES FILHO, D. C.; RESTLE, J. Vacas terminadas em campo nativo suplementadas com farelo de trigo ou farelo de arroz integral contendo ou não monensina sódica. Ciência e Agrotecnologia, v. 32, n. 6, p. 1974-1980, 2008.
- PARK, Y. K.; ALENCAR, S. M.; SCAMPARINI; R. P.; AGUIAR, C. L. Própolis produzida no sul do Brasil, Argentina e Uruguai: evidências fitoquímicas de sua origem vegetal. Ciência Rural, v. 32, n. 6, p. 997-1003, 2002.
- PRADO, O. P. P.; ZEOULA, L. M.; MOURA, L. P. P.; FRANCO, S. L.; PRADO, I. N.; GOMES, H. C. C. Digestibilidade e parâmetros ruminais de dietas à base de forragem com adição de própolis e monensina sódica para bovinos. Revista Brasileira de Zootecnia, v. 39, n. 6, p. 1336-1345, 2010a.
- PRADO, O. P. P.; ZEOULA, L. M.; PONTARA, L. P. M.; FRANCO, S. L.; NOVELLO, C. R.; GERON, L. J. V. Adição de própolis ou monensina sódica sobre digestibilidade in vitro da matéria seca em dietas 50:50% volumoso:concentrado e 100% volumoso. Revista Brasileira de Saúde e Produção Animal, v. 11, n. 4, p. 1023-1032, 2010b.
- RENNÓ, L. N.; VALADARES FILHO, S. C.; VALADARES, R. F. D.; PAULINO, M. F.; RENNÓ, F. P.; SILVA, P. A. Níveis de uréia na ração de novilhos de quatro grupos genéticos: estimativa da produção de proteína microbiana por meio dos derivados de purinas na urina utilizando duas metodologias de coleta. Revista Brasileira de Zootecnia, v. 37, n. 3, p. 546-555, 2008.
- RESTLE, J.; VAZ, F. N.; FEIJÓ, G. L. D.; BRONDAN, I. L.; ALVES FILHO, D. C.; BERNARDES, R. A. C.; FATURI, C.; PACHECO, P. S. Características de carcaça de bovinos de corte inteiros ou castrados de diferentes composições raciais Charolês x Nelore. Revista Brasileira de Zootecnia, v. 29, n. 5, p. 1371-1379, 2000.
- RODRIGUES, V. C.; ANDRADE, I. F. Características físico-químicas da carne de bubalinos e de bovinos castrados e inteiros. Revista Brasileira de Zootecnia, v. 33, n. 6, p. 1839-1849, 2004.
- SAS-Statistical Analysis System. SAS user's guide: statistics, version 8.1. 4th ed. Cary: Statistical Analysis System Institute, 2000.
- SCAZZOCCHIO, F.; D'AURIA, F. D.; ALESSANDRINI, D.; PANTANELLA, F. Multifactorial aspects of antimicrobial activity of propolis. Microbiological Research, v. 161, n. 4, p. 327-333, 2005.
- SNIFFEN, C. J.; O'CONNOR, J. D.; VAN SOEST, P. J.; FOX, D. G.; RUSSELL, J. B. A net carbohydrate and protein system for evaluating cattle diets: II. Carbohydrate and protein availability. Journal of Dairy Science, v. 70, n. 11, p. 3562-3577, 1992.
- STRADIOTTI JÚNIOR, D.; QUEIROZ, A. C.; LANA, R. P.; PACHECO, C. G.; EIFERT, E. C.; NUNES, P. M. M. Ação da própolis sobre a desaminação de aminoácidos e a fermentação ruminal. Revista Brasileira de Zootecnia, v. 33, n. 4, p. 1086-1092, 2004.
- TAKAISI-KIKUNI, N. B.; SCHILCHER, H. Electron microscopy and microcalorimetric investigations of the possible mechanism of the antibacterial action of a defined propolis provenance. Planta Medica, v. 60, n. 3, p. 222-227, 1994.
- VAN SOEST, P. J.; ROBERTSON, J. B.; LEWIS, B. A. Symposim: carbohydrate methodology, metabolism, and nutritional implications in dairy cattle. Journal of Dairy Science, v. 74, n. 10, p. 3583-3597, 1991.
- ZAWADZKI, F.; PRADO, I. N.; MARQUES, J. A.; ZEOULA, L. M.; ROTTA, P. P.; SESTARI, B. B.; VALERO, M. V.; RIVAROLLI, D. C. Sodium monensin or propolis extract in the diets of feedlot-finished bulls: effects on animal performance and carcass characteristics. Journal of Animal and Feed Sciences, v. 20, n. 1, p. 16-25, 2011.
Publication Dates
-
Publication in this collection
22 Oct 2012 -
Date of issue
Dec 2012
History
-
Received
31 Oct 2011 -
Accepted
15 Feb 2012