ABSTRACT.
Experiments were conducted to improve the fertilization rate of cryopreserved semen of dourado, Salminus brasiliensis. The experiment tested two cryoprotectant solutions at different semen: cryoprotectant ratios (1:5, 1:15, 1:25 and 1:50). The standard solution for the species (mixture of dimethyl sulfoxide, glucose, egg yolk and distilled water) was compared to a 350 mOsm ACP(r)-104 solution, which is composed of powdered coconut water diluted in distilled water and methylglycol. Differences between the dilutions tested were significant only for ACP(r). The fertilization potential by using the standard solution at the lowest dilution (1:5) is equated when the sperm is diluted in ACP(r) at 1:25 or 1:50. These results show that the standard solution is the most suitable for the cryopreservation of dourado sperm, since the dilution did not alter the fertilization rate, requiring smaller storage space.
Keywords:
cryopreservation; sperm; fertilization; cryoprotectant; coconut water
RESUMO.
Experimentos foram realizados, visando melhorar a taxa de fertilização com a utilização de sêmen criopreservado do dourado, Salminus brasiliensis. O experimento testou duas soluções crioprotetoras em diferentes diluições de sêmen:solução crioprotetora (1:5, 1:15, 1:25 e 1:50). A solução padrão para a espécie (mistura de glicose, água destilada, gema de ovo e dimetilsulfóxido) foi comparada com a solução ACP(r)-104 350 mOsm, composta por água de coco em pó diluída em água destilada e metilglicol. Foi observada diferença entre as distintas diluições testadas apenas para a ACP(r). O potencial de fertilização com o uso da solução padrão na menor diluição testada (1:5) é igualado apenas quando o sêmen em ACP(r) é diluído em 1:25 ou 1:50. Os resultados apontam que a solução padrão é mais vantajosa para a criopreservação de sêmen do dourado, uma vez que a variação da diluição do sêmen não altera a taxa de fertilização, requerendo, assim, menor espaço para armazenamento das amostras.
Palavras-chave:
criopreservação; esperma; fertilização; crioprotetor; água de coco
Introduction
The storage of cryopreserved fish sperm has different applications in both the conservation of genetic resources of species and in aquaculture. Gamete banks have been created to protect endangered species and assist in programs related to biodiversity conservation and breeding (Kopeika, Kopeika, & Zhang, 2007Kopeika, E., Kopeika, J., & Zhang, T. (2007). Cryopreservation of Fish Sperm. Series: Methods in Molecular Biology. In J. G. Day, & G. N. Stacey, (Eds.), Cryopreservation and Freeze-Drying Protocols (2a. ed., Vol. 368, p. 203-217). Totowa, NJ: Humana Press Inc.; Paniagua-Chávez, Ortiz-Gallarza, & Aguilar-Juárez, 2011Paniagua-Chávez, C. G., Ortiz-Gallarza, S. M., & Aguilar-Juárez, M. (2011). Subsistema Nacional de Recursos Genéticos Acuáticos: uso de la criopreservación para la conservación de los recursos genéticos acuáticos en México. Hidrobiológica21(3), 415-429. ). In fish farming, maintenance of representative samples of a given population in a cryopreserved sperm bank allow to reduce the number of male individuals in the breeding stock and facilitate routine work in induced reproduction, given the ready availability of semen from several males for reproduction, favoring the concentration of efforts only in the management of female specimens (Carneiro, 2007Carneiro, P. C. F. (2007). Tecnologias de produção e armazenamento de sêmen de peixes. Revista Brasileira de Reprodução Animal31 (3), 361-366. ).
S. brasiliensis (Cuvier, 1816, Characidae), known as dourado, is a fish with high quality meat appreciated by sport fishermen. The species is with reduced stocks in several South American river basins, mainly because of human action. Within the genus Salminus, this species reaches the largest size, has the greatest number of studies related to aquaculture and is considered the one with the highest potential for fish farming (Weingartner & Zaniboni-Filho, 2010Weingartner, M., & Zaniboni-Filho, E. (2010). Biologia e cultivo do Dourado. In B. Baldisserotto, & L. C. Gomes, (Orgs.), Espécies Nativas para Piscicultura no Brasil (245-281). Santa Maria, RS: Editora UFSM. ).
The use of cryopreserved dourado sperm has begun with Coser, Godinho and Ribeiro (1984Coser, A. M., Godinho, H., & Ribeiro, D. (1984). Cryogenic preservation of spermatozoa from Prochilodus scrofa and Salminus maxillosus. Aquaculture37(4), 387-390. ), and resumed years later by Carolsfeld, Godinho, Zaniboni-Filho and Harvey (2003Carolsfeld, J., Godinho, H. P., Zaniboni-Filho, E., & Harvey, B. J. (2003). Cryopreservation of sperm in Brazilian migratory fish conservation. Journal of Fish Biology63(2), 472-489.), Viveiros, Oliveira, Maria, Orfão, and Souza (2009bViveiros, A. T. M., Oliveira, A. V., Maria, A. N., Orfão, L. H., & Souza, J. C. (2009b). Sensibilidade dos espermatozóides de dourado (Salminus brasiliensis) a diferentes soluções crioprotetora. Arquivo Brasileiro de Medicina Veterinária e Zootecnia61(4), 883-889. ), Zanandrea, Weingartner and Zaniboni-Filho (2014Zanandrea, A. C.V., Weingartner, M., & Zaniboni-Filho, E. (2014). Use of ACP-104 at different dilutions for sperm cryopreservation of dourado, Salminus brasiliensis. Journal of the World AquacultureSociety45(1), 82-87. ) and Weingartner, Zanandrea and Zaniboni-Filho (2015Weingartner, M., Zanandrea, A. C. V., & Zaniboni-Filho, E. (2015). Cryopreserved sperm for oocyte fertilization of dourado Salminus brasiliensis. Ciência Rural45(5), 892-897. ). Most studies use sperm motility as a parameter for sperm quality, but, according to Bobe & Labbé (2010Bobe, J., & Labbé, C. (2010). Egg and sperm quality in fish. General and Comparative Endocrinology165(3), 535-548.), only the success of fertilization allows to prove accurately the quality of sperm and oocyte.
Over the last decade, several studies have been conducted to test different combinations of cryoprotectants and protocols for semen cryopreservation of South American Characiformes, seeking the best combinations to increase fertilization rate with the use of cryopreserved semen (Maria, Viveiros, Freitas, & Oliveira, 2006Maria, A. N., Viveiros, A. T. M., Freitas, R. T. F., & Oliveira, A.V. (2006). Extenders and cryoprotectants for cooling and freezing of piracanjuba (Brycon orbignyanus) semen, an endangered Brazilian teleost fish. Aquaculture, 260(1-4), 298-306. ; Ninhaus-Silveira, Foresti, Veríssimo-Silveira & Senhorini, 2006Ninhaus-Silveira, A., Foresti, F., Veríssimo-Silveira, R., & Senhorini, J. A. (2006). Seminal analysis, cryogenic preservation, and fertility in matrinxã fish, Brycon cephalus (Günther, 1869). Brazilian Archives of Biology and Technology49(4), 651-659. ; Taitson, Chami & Godinho, 2008Taitson, P. F., Chami, E., & Godinho, H. P. (2008). Gene banking of the neotropical fish Leporinus obtusidens (Valenciennes, 1836): A protocol to freeze its sperm in the field. Animal Reproduction Science105(3), 283-291. ; Viveiros, Orfão, Maria & Allaman, 2009aViveiros, A. T. M., Orfão, L. H., Maria, A. N., & Allaman, I. B. (2009a). A simple, inexpensive and successful freezing method for curimba Prochilodus lineatus (Characiformes) semen. Animal Reproduction Science, 112(3-4), 293-300. ; Viveiros, Orfão, Nascimento, Corrêa & Caneppele, 2012Viveiros, A. T. M., Orfão, L. H., Nascimento, A. F., Corrêa, F. M., & Caneppele, D. (2012). Effects of extenders, cryoprotectants and freezing methods on sperm quality of the threatened Brazilian freshwater fish pirapitinga-do-sul Brycon opalinus (Characiformes). Theriogenology, 78(2), 361-368. ).
Among these studies, a product specifically designed for cryopreservation of freshwater teleost semen was developed and registered as ACP(r)-104 (Viveiros, Maria, Orfão, Carvalho & Nunes, 2008Viveiros, A. T. M, Maria, A. N., Orfão, L. H., Carvalho, M. A., & Nunes, J. F. (2008). Powder coconut water (ACP-104) as extender for semen cryopreservation of Brazilian migratory fish species. Cybium32(2), suppl. 215. ). This product consists of a stable and standardized powder coconut water, which has suitable characteristics for use as an extracellular cryoprotectant (Silva et al., 2012Silva, M. A., Peixoto, G. C. X., Lima, G. L., Bezerra, J. A. B., Campos, L. B., Paiva, A. L. C., ... Silva, A. R. (2012). Cryopreservation of collared peccaries (Tayassu tajacu) semen using a powdered coconut water (ACP-116c) based extender plus various concentrations of egg yolk and glycerol. Theriogenology, 78(3), 605-611. ).
This study aimed at evaluating the effect of ACP(r)-104 as a cryoprotectant solution for cryopreservation of dourado sperm on the fertilization rate, comparing these results with those obtained when using the cryoprotectant solution that has been recommended for the species (Carolsfeld et al., 2003Carolsfeld, J., Godinho, H. P., Zaniboni-Filho, E., & Harvey, B. J. (2003). Cryopreservation of sperm in Brazilian migratory fish conservation. Journal of Fish Biology63(2), 472-489.; Zanandrea et al, 2014Zanandrea, A. C.V., Weingartner, M., & Zaniboni-Filho, E. (2014). Use of ACP-104 at different dilutions for sperm cryopreservation of dourado, Salminus brasiliensis. Journal of the World AquacultureSociety45(1), 82-87. ), testing four different semen: cryoprotectant ratios.
Material and methods
Selection of breeders and gamete collection
This study used specimens of S. brasiliensis of the first generation born in captivity from wild fish descendants caught in the upper Uruguay river (Brazil). Animals were kept in earth ponds and fed daily with commercial feed containing 40% crude protein. For the experiments, males that released semen upon slight abdominal pressure and females with distended abdomen were taken to the laboratory and kept in circular tanks (1,000 L) supplied with running water and recirculation rate of 17 times a day, 26ºC average temperature and average concentration of dissolved oxygen at 7.0 mg L-1.
Gametes were obtained through hormonal induction for final maturation with two intramuscular injections of crude extract of carp pituitary (CPE; Danúbio Aquacultura, Blumenau, Santa Catarina, Brazil), containing the equivalent to 0.4 and 4 mg CPE kg-1 for males and 0.5 and 5 mg CPE kg-1 for females, keeping an interval of 12 hours between applications (Weingartner & Zaniboni-Filho, 2010Weingartner, M., & Zaniboni-Filho, E. (2010). Biologia e cultivo do Dourado. In B. Baldisserotto, & L. C. Gomes, (Orgs.), Espécies Nativas para Piscicultura no Brasil (245-281). Santa Maria, RS: Editora UFSM. ).
For the collection of semen, males were taken from the tanks and the urogenital region was dried with paper towels. Semen was collected by gentle abdominal pressure with movements in the cranio-caudal direction, avoiding contamination of the semen by feces and urine, and collected in Falcon tubes for immediate evaluation of semen quality. Semen from only one male was used in the experiment, which showed the following characteristics: milky consistency, light yellow color, motility time longer than 40 seconds and motility intensity greater than 80%.
Cryopreservation
The cryoprotectant solution commonly used in dourado is the generic formula designed for South American migratory fish (Carolsfeld et al., 2003Carolsfeld, J., Godinho, H. P., Zaniboni-Filho, E., & Harvey, B. J. (2003). Cryopreservation of sperm in Brazilian migratory fish conservation. Journal of Fish Biology63(2), 472-489.), consisting in mixing 10% (15 mL) DMSO (dimethylsulfoxide), 5% (7.5 g) glucose, a chicken egg yolk and 135 mL distilled water, and defined in this study as the standard cryoprotectant solution - CS. This standard solution was compared to the ACP(r)-104 350 mOsm solution, made up of powdered coconut water (3.74 g) mixed with methylglycol (10% v v-1) diluted in distilled water to a final volume of 50 mL, and defined in this study as - CA.
For the experiments, semen was collected two hours before fertilization and the fresh semen samples were kept refrigerated for use as a control treatment. For cryopreservation, fresh semen was mixed with the cryoprotectant solutions at appropriate proportions, packaged in 0.5 mL straws and frozen in nitrogen vapor (Taylor-Wharton, CP model 300, Harsco Corp., Theodore, AL, U.S.A.).
Fertilization
Female gametes were obtained by extrusion of oocytes from only one female through ventral massage immediately before fertilization. The use of oocytes from a single female aimed at eliminating interference other than the treatments tested, once oocytes from different females may present distinct quality and thus affect the results.
Fertilization took place through the method known as 'dry fertilization', using the same water of the recirculation system that supplies breeding tanks and incubators to promote the activation of gametes, using the 1:10 oocyte:water ratio. In this experiment, for each incubator of 10 L, 10 grams of oocytes were placed.
Thawing was done by immersing the straws in water at 60°C for eight seconds. Then, the thawed content of each straw (semen + cryoprotectant solution) was mixed with 10 g oocyte samples, respecting the proportion of 5 mL semen per kilogram of oocyte. For each dilution tested due correction was made to maintain the same ratio between sperm and oocyte. A sample with the same amount of semen used to fertilize the same amount of oocytes was kept cold, consisting in the control treatment.
Eggs were kept in cylindrical-conical incubators in a recirculating water system at 25°C with a flow rate of 2 L per minute for each incubator. About seven hours after fertilization, at the blastopore closure stage, the fertilization rate was estimated. To this, at least 260 eggs of each incubator were sampled, analyzed under a stereomicroscope (40x) to calculate the fertilization rate by the formula: Fertilization rate (%) = number of normal eggs at the blastopore closure stage x 100 / total number of eggs observed.
Experiment - Comparison of cryoprotectant solutions at different dilutions
The two cryoprotectant solutions - CS and CA - were tested in a factorial arrangement with four different dilutions of semen:cryoprotectant solution. Fresh semen was mixed to different dilutions of cryoprotectant solutions at the proportions: 1:5, 1:15, 1:25 and 1:50 semen:cryoprotectant solution. Fresh semen samples were kept refrigerated for use in the control treatment, each test was run with three replicates.
Statistical analysis
To eliminate the effect of oocyte quality on the results, we adjusted the fertilization rates according to Carolsfeld et al. (2003Carolsfeld, J., Godinho, H. P., Zaniboni-Filho, E., & Harvey, B. J. (2003). Cryopreservation of sperm in Brazilian migratory fish conservation. Journal of Fish Biology63(2), 472-489.). This consists of estimating the relative fertility rate of each treatment compared to the control treatment, which is considered 100% (maximum fertilization rate of oocytes of every female obtained by mixing with fresh semen). Values of fertilization rates of treatments were corrected considering the fertilization rate obtained by the treatment control and are expressed as mean ± standard deviation.
Data are expressed as percentage and were subjected to angular transformation (arc sine √y/100). Factorial ANOVA was applied, obeying the assumptions of normality and homogeneous variance, followed by Tukey's test to determine significant differences between the means (p < 0.05) (Zar, 1996Zar, J. H. (1996). Biostatistical analysis. New Jersey: Prentice Hall.). The effect of different dilutions of cryoprotectant solutions was analyzed by linear regression.
Results
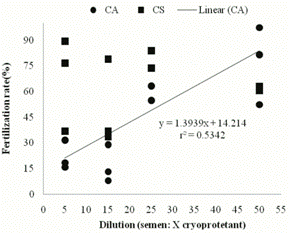
Table 1 lists the results of fertilization obtained when using two cryoprotectant solutions at different dilutions. Despite the statistical difference between the standard solution and ACP(r)-104 at lower dilutions (1:5 and 1:15), the standard solution showed no statistical difference between the different dilutions.
Linear regression partly explained the increase in fertilization rate with increasing dilutions for the cryoprotectant solution composed of powdered coconut water (Figure 1), but this correlation was not found for the standard solution (B = 0), indicating that fertilization rate was not affected by variation in semen dilution in standard cryoprotectant solution.
Fertilization rate (%) of dourado (S. brasiliensis) fertilized with cryopreserved sperm at different dilutions of two cryoprotectant solutions: ACP(r)-104 350 mOsm (CA) and the standard cryoprotectant solution for the species (CS).
Discussion
Increasing the dilution had a different effect on the fertilization rate obtained for the two cryoprotectant solutions tested: while the powdered coconut water-based solution reached the highest fertilization rates using more diluted semen (≥ 1:25), the standard solution provided similar fertilization rate independent of the dilution tested. A possible explanation for the better results of fertilization with higher dilution of semen in coconut water-based solution is the potassium concentration in solution. Yasui, Fujimoto, Arias-Rodriguez, Takagi, and Arai (2012Yasui, G. S., Fujimoto, T., Arias-Rodriguez, L., Takagi, Y., & Arai, K. (2012). The effect of ions and cryoprotectants upon sperm motility and fertilization success in the loach Misgurnus anguillicaudatus. Aquaculture, 344-349(1), 147-152.), in a study with loach, M. anguillicaudatus, found that the fertilization rates were reduced with the addition of certain substances to cryoprotectant solutions, and concluded that high potassium concentrations should be avoided in the preparation of cryoprotectants. The ACP(r) is derived from coconut water, which is composed of many minerals as potassium (175 mg 100 mL-1) and calcium (17.5 mg 100 mL-1) at higher concentrations, besides other minerals in smaller quantities (Kwiatkowski, Clemente, Scarcelli, & Vida, 2008Kwiatkowski, A., Clemente, E., Scarcelli, A., & Vida, J. B. (2008). Quality of coconut water "in natura" belonging to Green Dwarf fruit variety in different stages of development, in plantation on the northwest area of Paraná, Brazil. Journal of Food, Agriculture and Environment, 6(1), 102-105. ; Viveiros, Nascimento, Orfão, & Isaú, 2010Viveiros, A. T. M., Nascimento, A. F., Orfão, L. H., & Isaú, Z. A. (2010). Motility and fertility of the subtropical freshwater fish streaked prochilod (Prochilodus lineatus) sperm cryopreserved in powdered coconut water. Theriogenology, 74, 551-556.).
In freshwater fish species, mature spermatozoa require a hypo-osmotic shock to trigger sperm motility. Other factors also affect motility, such as pH, temperature, ion concentration and osmolarity (Islam & Akhter, 2011Islam, M. S., & Akhter, T. (2011). Tale of Fish Sperm and Factors Affecting Sperm Motility: A Review. International Journal of Advanced Life Sciences1(1), 11-19.). Among these factors, the potassium ion concentration is essential, which, combined with the osmotic pressure, controls sperm motility in several species. This control takes place by means of influx of Ca2+ and K+ or by Na+ flux through specific ion channels, changing the membrane potential and signaling the onset of motility (Alavi & Cosson, 2006Alavi, S. M. H., & Cosson, J. (2006). Sperm motility in fishes. (II) Effects of ions and osmolality: A review. Cell Biology International30(1), 1-14. ). Studies on different fish groups indicate differences in this interaction, suggesting that the concentration of potassium ion can also inhibit sperm motility as seen for salmonids (Morisawa, Suzuki, & Morisawa, 1983Morisawa, M., Suzuki, K., & Morisawa, S. (1983). Effects of potassium and osmolality on spermatozoan motility of salmonid fishes. The Journal of Experimental Biology 107(1), 105-113.), cyprinids (Krasznai, Márián, Balkay, Gáspár & Trón, 1995Krasznai, Z., Márián, T., Balkay, L., Gáspár, J. R., & Trón, L. (1995). Potassium channels regulate hypo-osmotic shock-induced motility of common carp (Cyprinus carpio) sperm. Aquaculture, 129(1-4), 123-128. ) and sturgeon (Li, Li, Hulak, Rodina & Linhart, 2012Li, P., Li, Z., Hulak, M., Rodina, M., & Linhart, O. (2012). Regulation of spermatozoa motility in response to cations in Russian sturgeon Acipenser gueldenstaedtii. Theriogenology78(1), 102-109.). A study with Brycon henna, belonging to a close family of dourado, showed that sperm motility was dramatically reduced during activation induced with a potassium solution at a concentration above 140 mM (Tabares, Rufz, Arboleda, & Olivera, 2007Tabares, J., Rufz, T., Arboleda, L., & Olivera, M. (2007). Effect of some ions on sperm activation in Brycon henni (Eigenmann 1913). Acta Biológica Colombiana12(1), 87-98.).
Cryoprotectants are used to reduce the damage caused to the cells by freezing and thawing, and are divided into two categories: intracellular and extracellular (Silva & Guerra, 2011Silva, S. V., & Guerra, M. M. P. (2011). Efeitos da criopreservação sobre as células espermáticas e alternativas para redução das crioinjúrias. Revista Brasileira de Reprodução Animal, 35(4), 370-384. ). The combination of these cryoprotectants to prepare the cryoprotectant solution, and the dilution of such cryoprotectant solution in relation to semen may influence fertilization rates, suggesting that the preparation of cryoprotectant solution is probably species-specific (Yasui et al., 2012Yasui, G. S., Fujimoto, T., Arias-Rodriguez, L., Takagi, Y., & Arai, K. (2012). The effect of ions and cryoprotectants upon sperm motility and fertilization success in the loach Misgurnus anguillicaudatus. Aquaculture, 344-349(1), 147-152.). The high concentration of minerals in ACP(r) compared to the standard solution may have influenced the efficacy of ACP(r) as cryoprotectant for dourado, thus affecting the fertilization rate. In this way, the increase in dilution of semen in the cryoprotectant solution based on coconut water may have led the concentration of salts to a level that favored the sperm performance, positively influencing the fertilization rate. For the standard solution, this better performance apparently has been attained at lower dilutions.
Studies with ACP(r)-104 at 1:9 dilution (semen:cryoprotectant solution) in curimbatá, P. lineatus, reported that semen cryopreserved with this product exhibited greater motility than when cryopreserved in a mixture with glucose, however, the velocity of sperm and fertilization rate were similar between the two solutions, concluding that both substances are suitable for cryopreservation of P. lineatus semen (Viveiros et al., 2010Viveiros, A. T. M., Nascimento, A. F., Orfão, L. H., & Isaú, Z. A. (2010). Motility and fertility of the subtropical freshwater fish streaked prochilod (Prochilodus lineatus) sperm cryopreserved in powdered coconut water. Theriogenology, 74, 551-556.). Evaluations of sperm quality of S. brasiliensis semen cryopreserved in standard solution showed that the highest values of motility were obtained in samples diluted in cryoprotectant solution at a ratio of 1:5, compared to samples diluted at 1:10 (Viveiros et al., 2009b). Carolsfeld et al. (2003Carolsfeld, J., Godinho, H. P., Zaniboni-Filho, E., & Harvey, B. J. (2003). Cryopreservation of sperm in Brazilian migratory fish conservation. Journal of Fish Biology63(2), 472-489.) recommend a dilution between 1:3 and 1:5 for the sperm of South American fish. In the present study, the fertilization rate of dourado oocytes using semen cryopreserved with the standard solution (based on glucose, egg yolk and DMSO) was not affected by the different dilutions tested, indicating that the lowest dilution (1:5) is enough to achieve the best results of fertilization after cryopreservation of sperm.
Our findings demonstrated high fertilization rates with sperm cryopreserved in glucose-based standard solution, reaching values ranging between 49 and 88% of the values obtained with fresh sperm. Dourado sperm cryopreserved in standard solution presented fertilization rate similar or higher than observed for other South American Characiformes, as P. lineatus, between 68 and 77% (Carolsfeld et al., 2003Carolsfeld, J., Godinho, H. P., Zaniboni-Filho, E., & Harvey, B. J. (2003). Cryopreservation of sperm in Brazilian migratory fish conservation. Journal of Fish Biology63(2), 472-489.) and B. opalinus, 69.4% (Viveiros et al., 2012Viveiros, A. T. M., Orfão, L. H., Nascimento, A. F., Corrêa, F. M., & Caneppele, D. (2012). Effects of extenders, cryoprotectants and freezing methods on sperm quality of the threatened Brazilian freshwater fish pirapitinga-do-sul Brycon opalinus (Characiformes). Theriogenology, 78(2), 361-368. ).
Conclusion
Regarding the conditions of this study, the cryoprotectant solution ACP(r)-104 demonstrated similar fertilization rates compared to the standard solution only when used a dilution equal to or greater than 1:25 (semen:cryoprotectant solution). Therefore, the standard solution proved to be more advantageous for the cryopreservation of the dourado sperm, because it requires smaller amounts of cryoprotectant solution to attain the maximum fertilization rate. Thereby, it requires smaller space to store the same volume of sperm, which reduces costs of cryopreservation.
Acknowledgements
We thank the funding agencies that support research, Capes and CNPq, for scholarships.
References
- Alavi, S. M. H., & Cosson, J. (2006). Sperm motility in fishes. (II) Effects of ions and osmolality: A review. Cell Biology International30(1), 1-14.
- Bobe, J., & Labbé, C. (2010). Egg and sperm quality in fish. General and Comparative Endocrinology165(3), 535-548.
- Carneiro, P. C. F. (2007). Tecnologias de produção e armazenamento de sêmen de peixes. Revista Brasileira de Reprodução Animal31 (3), 361-366.
- Carolsfeld, J., Godinho, H. P., Zaniboni-Filho, E., & Harvey, B. J. (2003). Cryopreservation of sperm in Brazilian migratory fish conservation. Journal of Fish Biology63(2), 472-489.
- Coser, A. M., Godinho, H., & Ribeiro, D. (1984). Cryogenic preservation of spermatozoa from Prochilodus scrofa and Salminus maxillosus Aquaculture37(4), 387-390.
- Islam, M. S., & Akhter, T. (2011). Tale of Fish Sperm and Factors Affecting Sperm Motility: A Review. International Journal of Advanced Life Sciences1(1), 11-19.
- Kopeika, E., Kopeika, J., & Zhang, T. (2007). Cryopreservation of Fish Sperm. Series: Methods in Molecular Biology. In J. G. Day, & G. N. Stacey, (Eds.), Cryopreservation and Freeze-Drying Protocols (2a. ed., Vol. 368, p. 203-217). Totowa, NJ: Humana Press Inc.
- Krasznai, Z., Márián, T., Balkay, L., Gáspár, J. R., & Trón, L. (1995). Potassium channels regulate hypo-osmotic shock-induced motility of common carp (Cyprinus carpio) sperm. Aquaculture, 129(1-4), 123-128.
- Kwiatkowski, A., Clemente, E., Scarcelli, A., & Vida, J. B. (2008). Quality of coconut water "in natura" belonging to Green Dwarf fruit variety in different stages of development, in plantation on the northwest area of Paraná, Brazil. Journal of Food, Agriculture and Environment, 6(1), 102-105.
- Li, P., Li, Z., Hulak, M., Rodina, M., & Linhart, O. (2012). Regulation of spermatozoa motility in response to cations in Russian sturgeon Acipenser gueldenstaedtii Theriogenology78(1), 102-109.
- Maria, A. N., Viveiros, A. T. M., Freitas, R. T. F., & Oliveira, A.V. (2006). Extenders and cryoprotectants for cooling and freezing of piracanjuba (Brycon orbignyanus) semen, an endangered Brazilian teleost fish. Aquaculture, 260(1-4), 298-306.
- Morisawa, M., Suzuki, K., & Morisawa, S. (1983). Effects of potassium and osmolality on spermatozoan motility of salmonid fishes. The Journal of Experimental Biology 107(1), 105-113.
- Ninhaus-Silveira, A., Foresti, F., Veríssimo-Silveira, R., & Senhorini, J. A. (2006). Seminal analysis, cryogenic preservation, and fertility in matrinxã fish, Brycon cephalus (Günther, 1869). Brazilian Archives of Biology and Technology49(4), 651-659.
- Paniagua-Chávez, C. G., Ortiz-Gallarza, S. M., & Aguilar-Juárez, M. (2011). Subsistema Nacional de Recursos Genéticos Acuáticos: uso de la criopreservación para la conservación de los recursos genéticos acuáticos en México. Hidrobiológica21(3), 415-429.
- Silva, S. V., & Guerra, M. M. P. (2011). Efeitos da criopreservação sobre as células espermáticas e alternativas para redução das crioinjúrias. Revista Brasileira de Reprodução Animal, 35(4), 370-384.
- Silva, M. A., Peixoto, G. C. X., Lima, G. L., Bezerra, J. A. B., Campos, L. B., Paiva, A. L. C., ... Silva, A. R. (2012). Cryopreservation of collared peccaries (Tayassu tajacu) semen using a powdered coconut water (ACP-116c) based extender plus various concentrations of egg yolk and glycerol. Theriogenology, 78(3), 605-611.
- Taitson, P. F., Chami, E., & Godinho, H. P. (2008). Gene banking of the neotropical fish Leporinus obtusidens (Valenciennes, 1836): A protocol to freeze its sperm in the field. Animal Reproduction Science105(3), 283-291.
- Tabares, J., Rufz, T., Arboleda, L., & Olivera, M. (2007). Effect of some ions on sperm activation in Brycon henni (Eigenmann 1913). Acta Biológica Colombiana12(1), 87-98.
- Viveiros, A. T. M, Maria, A. N., Orfão, L. H., Carvalho, M. A., & Nunes, J. F. (2008). Powder coconut water (ACP-104) as extender for semen cryopreservation of Brazilian migratory fish species. Cybium32(2), suppl. 215.
- Viveiros, A. T. M., Nascimento, A. F., Orfão, L. H., & Isaú, Z. A. (2010). Motility and fertility of the subtropical freshwater fish streaked prochilod (Prochilodus lineatus) sperm cryopreserved in powdered coconut water. Theriogenology, 74, 551-556.
- Viveiros, A. T. M., Oliveira, A. V., Maria, A. N., Orfão, L. H., & Souza, J. C. (2009b). Sensibilidade dos espermatozóides de dourado (Salminus brasiliensis) a diferentes soluções crioprotetora. Arquivo Brasileiro de Medicina Veterinária e Zootecnia61(4), 883-889.
- Viveiros, A. T. M., Orfão, L. H., Maria, A. N., & Allaman, I. B. (2009a). A simple, inexpensive and successful freezing method for curimba Prochilodus lineatus (Characiformes) semen. Animal Reproduction Science, 112(3-4), 293-300.
- Viveiros, A. T. M., Orfão, L. H., Nascimento, A. F., Corrêa, F. M., & Caneppele, D. (2012). Effects of extenders, cryoprotectants and freezing methods on sperm quality of the threatened Brazilian freshwater fish pirapitinga-do-sul Brycon opalinus (Characiformes). Theriogenology, 78(2), 361-368.
- Weingartner, M., Zanandrea, A. C. V., & Zaniboni-Filho, E. (2015). Cryopreserved sperm for oocyte fertilization of dourado Salminus brasiliensis Ciência Rural45(5), 892-897.
- Weingartner, M., & Zaniboni-Filho, E. (2010). Biologia e cultivo do Dourado. In B. Baldisserotto, & L. C. Gomes, (Orgs.), Espécies Nativas para Piscicultura no Brasil (245-281). Santa Maria, RS: Editora UFSM.
- Yasui, G. S., Fujimoto, T., Arias-Rodriguez, L., Takagi, Y., & Arai, K. (2012). The effect of ions and cryoprotectants upon sperm motility and fertilization success in the loach Misgurnus anguillicaudatus Aquaculture, 344-349(1), 147-152.
- Zanandrea, A. C.V., Weingartner, M., & Zaniboni-Filho, E. (2014). Use of ACP-104 at different dilutions for sperm cryopreservation of dourado, Salminus brasiliensis Journal of the World AquacultureSociety45(1), 82-87.
- Zar, J. H. (1996). Biostatistical analysis. New Jersey: Prentice Hall.
Publication Dates
-
Publication in this collection
Mar 2016
History
-
Received
14 Aug 2015 -
Accepted
27 Oct 2015