ABSTRACT.
An experiment was carried out to evaluate the requirement of digestible methionine for growing pullets at growth phase (7 to 12 weeks of age). A completely randomized design was distributed in five treatments, six replicates, and 15 pullets per experimental unit. 450 Dekalb White pullets from the 7th weeks of age, with an average initial weight of 313.14 ± 12.49 g were used. Dietary treatments consisted in five diets supplemented with DL-Methionine which resulted in five levels of digestible methionine (0.266, 0.294, 0.322, 0.350, and 0.378 %). Performance, serological blood, histopathology and histomorphometry data were evaluated. Quadratic responses were observed for final live weight (p < 0.0143), weight gain (p < 0.0073), feed conversion ratio (p < 0.0058), glycogen deposition in the liver (p < 0.0001), gamma-glutamyl transferase enzyme activity (p < 0.0008), and villus height (p < 0.0024) with digestible dMet levels. In conclusion, the use of 0.343 % dMet, corresponding to a dMet:dLys ratio 55, is recommended for white-egg pullets from 7 to 12 weeks of age.
Keywords:
enzymatic profile; essential amino acid; histology; morphometry
Introduction
Methionine is the first limiting amino acid for laying hens, and can be effective for the protein synthesis, for the development of muscles, organs, feather, beaks, and for the production and quality of eggs.
The update of dietary requirements for layer pullets was justified by improvement the lineages, with superior productive characteristics as well as unprecedented knowledge of the nutrition, management, and ambience fields (D’Agostini et al., 2012D’Agostini, P., Gomes, P. C., Calderano, A. A., Melo, H. H. C., Sá, L. M., Rostagno, H. S., & Albino, L. F. T. (2012). Requirement of methionine+cysteine for pullets in the growing phase from 7 to 12 weeks old. Brazilian Journal Veterinarian Animal Science, 64(6), 1699-1706. doi: 10.1590/S0102-09352012000600040
https://doi.org/10.1590/S0102-0935201200...
).
Methionine is amino acid involved in the control of some functions in the bird’s body that potentiate weight gain and growth rates (Del Vesco et al., 2013Del Vesco, A. P., Gasparino, E., Oliveira Neto, A. R., Guimarães, S. E. F., Marcato, S. M. M., & Voltoline, D. M. (2013). Dietary methionine effects on IGF-I and GHR mRNA expression in broilers. Genetics Molecular Research, 12(4), 6414-6423. doi: 10.4238/2013.December.10.2
https://doi.org/10.4238/2013.December.10...
), protection against liver damage (Wu et al., 2012Wu, B., Chui, H., Peng, X., Fang, J., Cui, W., & Liu, X. (2012). Pathology of spleen in chickens fed on a diet deficient in methionine. Health, 4(1), 32-38. doi: 10.4236/health.2012.41007
https://doi.org/10.4236/health.2012.4100...
), intestinal development and morphology (Shen, Ferket, Park, Malheiros & Kim, 2015Shen, Y. B., Ferket, P., Park, I., Malheiros, R. D., & Kim, S. W. (2015). Effects of feed grade L- methionine on intestinal redox status, intestinal development, and growth performance of young chickens compared with conventional DL-methionine. Journal of Animal Science, 93(6), 2977-2986. doi: 10.2527/jas.2015-8898
https://doi.org/10.2527/jas.2015-8898...
), and formation and functioning of the immune system (Jankowski, Kubinska & Zdunczyk, 2014Jankowski, J., Kubinska, M., & Zdunczyk, Z. (2014). Nutritional and immunomodulatory function of methionine in poultry diets - a review. Annals of Animal Science, 14(1), 17-31. doi: 10.2478/aoas-2013-0081
https://doi.org/10.2478/aoas-2013-0081...
).
The proper methionine nutritional level not only boosts productive performance rates, tissue formation, and the functioning of physiological systems, but also maximizes the preparation of pullets for the subsequent developer and egg-laying phases.
Thus, the Granja Planalto (2009Granja Planalto (2009). Manual de manejo das poedeiras Dekalb White. Uberlândia, MG: Granja Planalto. ) manual and Rostagno et al. (2017Rostagno, H. S., Albino, L. F. T., Hannas, M. I., Donzele, J. L., Sakomura, N. K., Perazzo, F. G., ... Brito, C.O. (2017). Tabelas brasileiras para aves e suínos: composição de alimentos e exigências nutricionais . Viçosa, MG: Universidade Federal de Viçosa .) recommended dMet levels of 0.52g 100g-1 for white-egg pullets from 6 to 10 weeks of age, and 0.44g 100g-1 digestible methionine for white-egg pullets from 5 to 10 weeks of age, respectively.
This study aimed to determine the digestible methionine requirement for white-egg pullets from 7 to 12 weeks of age.
Material and methods
The experiment was conducted in Poultry Sector, Department of Animal Science at Federal University of Paraíba. The project had ethical approval from the Animal Use and Care Committee of the Federal University of Paraíba, Brazil, under protocol number 149/2015. The total experimental period was 42 days in the growth phase for pullets from 7 to 12 weeks of age.
A number of 450 Dekalb White pullets, 7 weeks old, with an average initial weight of 313.14 ± 12.49 g, were housed in experimental pens, measuring 1.0 × 1.5 each. The litter was sugarcane bagasse bedding and each box contained an incandescent 100 W light bulb for heating the pullets in the seventh weeks of life, a tubular feeder, and a pendulum chick drinker. Water and feed were available ad libitum, and the vaccination and lighting programs adopted were those suggested for the sanitation challenge in the region and by guide of the lineage, respectively.
The experiment comprised a completely randomized design, with five treatments and six replicates with 15 pullets per experimental unit. Treatments consisted of five experimental diets, a basal diet was formulated to meet the requirements of all nutrients, according to recommendations of Rostagno et al. (2011Rostagno, H. S., Albino, L. F. T., Donzele, J. L., Gomes, P. C., Oliveira, R. F., Lopes, D. C., ... Euclides, R. F. (2011). Tabelas brasileiras para aves e suínos: composição de alimentos e exigências nutricionais. Viçosa, MG: Universidade Federal de Viçosa.), except dMet, which was supplemented by DL-Methionine (0.0, 0.05, 0.10, 0.15, and 0.20 %), resulting in five dMet levels (0.266, 0.294, 0.322, 0.350 and 0.378 %), shown in Table 1.
The variables were analyzed feed intake (FI, g pullet-1); final live weight (FLW, g pullet-1); weight gain (WG, g pullet-1); feed conversion ratio (FCR, g g-1); the growth curve; empty body weight (EBW, g pullet-1); weights of liver (g pullet-1), spleen (g pullet-1), and abdominal fat (AF, g pullet-1); glycogen deposition in the liver (GDL, Ishak Score), serum analysis of alanine aminotransferase (ALT, U L-1), aspartate aminotransferase (AST, U L-1), gamma-glutamyl transferase (GGT, U L-1), creatinine (CRE, mg dL-1), albumin (ALB, g dL-1), and protein (PTN, g dL-1); villus height (VH, µm) villus width (VW, µm), crypt depth (CD, µm), villus: crypt ratio (VH:CD, µm/µm); and goblet cell count (GCC, cells/villus). Additionally, a histopathology analysis of liver, kidneys, intestine, magnum, and uterus was performed.
The diets samples were analyzed for DM by placing duplicate samples in a drying oven at 105°C for 24h (Association of Official Analytical Chemists [AOAC], 1990Association of Official Analytical Chemists [AOAC]. (1990). Official methods of analysis. Washington, DC: AOAC International.). Nitrogen content of feed samples were determined on a 0.25 g sample with a combustion analyzer (Leco model FP-2000 N analyzer, St. Joseph, MI) using EDTA as a calibration standard, with CP being calculated by multiplying percentage N by 6.25. For AA analysis, samples were prepared by 6 N HCL hydrolysis for 24h at 110°C followed by neutralization with 4 mL 25% (wt/vol) NaOH, and then cooled to room temperature. Afterward, sodium citrate buffer was added and the mixture was equalized to a 50 mL volume (AOAC, 1990Association of Official Analytical Chemists [AOAC]. (1990). Official methods of analysis. Washington, DC: AOAC International.; method 982.30). Methionine and cystine (sulfur containing amino acids) were analyzed by performic acid oxidation at 0°C, followed by acid hydrolysis. The amino acids (AOAC, 1990Association of Official Analytical Chemists [AOAC]. (1990). Official methods of analysis. Washington, DC: AOAC International.; method 982.30) in the hydrolysate were determined by an AA analyzer (Biochrom 30. 30 plus, Biochrom Ltd, Cambridge, United Kingdom). Calcium (Ca) and phosphorus (P) were determined according methodologies describe by Silva and Queiroz (2002Silva, D. J., & Queiroz, A. C. (2002). Análise de alimentos: métodos químicos e biológicos. Viçosa, MG: UFV.). The Ca content was determined in a PerlinElmer atomic absorption spectrophotometer (AAnalyst 200) at 422.7 nm wavelength, and P content was determined by Kasuaki digital colorimetric spectrophotometer at 725 nm wavelength.
Concerning the growth curve, the pullets live weight was measured weekly in the period of seven to twelve weeks of age, totaling five curves, and the obtained data were submitted to the equation models proposed by Von Bertalanffy (1957Bertalanffy, L. V. (1957). Quantitative laws in metabolism and growth. The Quarterly Review of Biology, 32(3), 217-231. Doi: 10.1086/401873
https://doi.org/10.1086/401873...
), Brody (1945Brody, S. (1945). Bioenergetics and growth. New York, NY: Reinhold Publishing. ), Gompertz (1825Gompertz, G. (1825). On the nature of the function expressive of the law of human mortality, and on the new mode of determining the value of life contingencies. Philosophical Transactions of the Royal Society, 115, 513-585. doi: 10.1098/rstl.1825.0026
https://doi.org/10.1098/rstl.1825.0026...
), Logistic (Verhulst, 1845Verhulst, P. F. (1845). Recherches mathématiques sur la loi d’accroissement de la population. Nouveaux Mémoires de L’Académie Royale des Sciences et Belles-Lettres de Bruxelles, 18, 1-41. ), and Richards (1959Richards, F. J. A. (1959). A flexible growth function for empirical use. Journal of Experimental Botany, 10(2), 290-301. doi: 10.1093/jxb/10.2.290
https://doi.org/10.1093/jxb/10.2.290...
), using the GOSA Statistical Software, which gave the best adjustment for mathematical equations. Akaike’s Information Criterio (AIC) (Akaike, 1987Akaike, H. (1987). Factor analysis and AIC. Psychometrika, 52(3), 317-332. doi: 10.0007/978-1-4612-1694-0_29
https://doi.org/10.0007/978-1-4612-1694-...
) was used to choose the mathematical model that best fit the pullets age-weight data on the growth curves.
On the last day of the experimental period, ten birds per treatment were slaughtered for serum and histopathological analyses and measurements of organs. Serum analyses were performed using a VetTest Blood Chemistry Analyzer. For the histopathological analyses, fragments of liver, kidneys, intestine, magnum, and uterus were immersed in a methacarn fixing solution (60% methanol, 30% chloroform, and 10% acetic acid) for 12 h, and the standard histopathological procedure was performed.
For the optical microscopy, fragments were included in paraplast and subsequently sectioned into series of 5 µm depth. The histological staining methods were performed: hematoxylin and eosin, Periodic Acid Schiff (PAS) and Masson’s trichrome. Photomicrographs were captured with a micro-camera coupled to an Olympus BX-51 microscope (Olympus, Tokyo, Japan), and images were digitalized on the KS 400.3 software (Carl Zeiss Vision GmbH, Germany).
In the evaluation of glycogen deposition in the liver, a score was given to each evaluated animal (ten birds per treatment), considering the degree of positivity to PAS stain, as follows: 0 (no glycogen deposition), 1 (little glycogen deposition), 2 (medium glycogen deposition), 3 (elevated glycogen deposition), and 4 (large glycogen deposition), according to Ishak’s (modified) semi-quantitative scoring (Ishak et al., 1995Ishak, K., Baptista, A., Bianchi, L., Callea, F., Groote, J. D., Gudat, F., ... Thaler, H. (1995). Histological grading and staging of chronic hepatitis. Journal Hepatology, 22(6), 696-699. doi: 10.1016/0168-8278(95)80226-6
https://doi.org/10.1016/0168-8278(95)802...
). Assessments were undertaken by the same histologist.
The morphometric study of villus height, crypt depth, and duodenal villus width was undertaken through microscope visualizations and scanning of at least two images per bird, each treatment consisting of 10 animals. For each image, at least three morphometric measurements were taken for villus height, villus width, and respective crypt depth, with 50x magnification, making 60 “n”-shaped curves per treatment.
For the morphometry of goblet cells from the duodenal villi, samples of 10 animals per treatment were also used. Several images were digitalized, with all samples magnified 200x. At least two images of each animal were chosen at random, and the intestinal epithelium was measured linearly until 10,000 µm were covered. In these measured linear-epithelium areas, the number of goblet cells was counted using PAS stain, being goblet cells magenta. Based on the results, the number of goblet cells in 1,000 µm per treatment was defined.
Statistical analyses of the evaluated traits were run on SAS software (Statistical Analysis System [SAS], 2011Statistical Analysis System [SAS]. (2011). SAS/STAT User’s guide, Version 9.4. Cary, NC: SAS Institute Inc. ). The estimate of digestible methionine requirement was determined by regression analyses.
Variables FLW, WG, and FCR were the ones that best adjusted to the quadratic regression model, with maximum and minimum points of 0.351, 0.353, and 0.343%, respectively. On the other hand, the feed intake variable, showed best adjustment in the linear regression model (Table 2).
Results and discussion
Based on the results obtained in this study, it can be inferred that the best level found for feed conversion is related to the better use of the dietary nutrients for weight gain. This improvement in the efficiency of use of the feed for weight gain with the increase in the dietary levels of digestible methionine + cystine levels is probably a result of the better amino acid balance and consequently improvement on protein synthesis (Shen, Ferket, Park, Malheiros, & Kim, 2015Shen, Y. B., Ferket, P., Park, I., Malheiros, R. D., & Kim, S. W. (2015). Effects of feed grade L- methionine on intestinal redox status, intestinal development, and growth performance of young chickens compared with conventional DL-methionine. Journal of Animal Science, 93(6), 2977-2986. doi: 10.2527/jas.2015-8898
https://doi.org/10.2527/jas.2015-8898...
).
The dMet levels found in this study for white-egg pullets from 7 to 12 weeks of age are lower than 0.52 % and 0.40 %, respectively recommended by the Dekalb White (Granja Planalto, 2009Granja Planalto (2009). Manual de manejo das poedeiras Dekalb White. Uberlândia, MG: Granja Planalto. ) from 6 to 10 weeks, and W-36 strain from 6 to 12 weeks of age (Hy-Line International, 2016Hy-Line International. (2016). Management Guide. Hy-Line international. Des Moines, IA: Hy-Line International.). What is recommended on the Dekalb White manual (Granja Planalto, 2009Granja Planalto (2009). Manual de manejo das poedeiras Dekalb White. Uberlândia, MG: Granja Planalto. ) and W-36 (Hy-Line International, 2016), is 0.82 %and 0.88 %dLys respectively, and the dMet:dLys ratio 63 and 45. In the present study, the optimal ratio obtained was 55, due to the best level of dMet 0.343 % by FCR and the use of dLys 0.621 %.
Feed intake increased as the dMet levels were increased, to meet the pullet’s nutritional requirements. This increased nutrient balance resulted in better weight gain rates, as they are part of the body composition or participate in its formation process (Islam & Dutta, 2014Islam, M. S., & Dutta, R. K. (2014). Impacts of vitamin A and essential amino acids on growth performance, survivability, carcass characteristics and profitability of a crossbred chicken (Gallus domesticus L.) in Rajshahi, Bangladesh. International Journal Science Research Environment Science, 2(5), 174-183. doi: 10.12983/ijsres-2014-p0174-0183
https://doi.org/10.12983/ijsres-2014-p01...
).
Some studies have shown that this increased protein synthesis is related to the induction of higher amounts of IGF-1 and GH in the body of birds. IGF-I and GH genes are known to be relevant in the production of functional substances, acting directly on the metabolism and growth of birds. However, dietary levels of excess methionine cause a decrease in growth, as it results in an amino acid imbalance, in addition to its excess it suffers an oxidation that promotes the production of sulfate, leading to a metabolic acidosis (Flora et al., 2017Flora, R. P. D., Dionello, N. J. L., Benitez, L., Germano, J. M., Gotuzzo, A. G., & Freitas, S. (2017). Expressão gênica de IGF-1 e GHR no fígado e no músculo do peito de codornas de corte suplementadas com diferentes níveis de metionina em duas gerações sucessivas. Arquivos Brasileiro de Medicina Veterinária e Zootecnia, 69(1), 205-213. doi: 10.1590/1678-4162-9143
https://doi.org/10.1590/1678-4162-9143...
).
On the growth curve data on the pullets from 7 to 12 weeks of age, the best adjustment of the mathematical model proposed by Gompertz, with the parameters estimated by the function showing the lowest AIC (1442.120) (Table 3).
Accordingly, this model best described the average growth curve of the pullets from 7 to 12 weeks of age (Figure 1). In the growth curve, the better performance of the pullets that received the diet containing the levels of 0.350 and 0.378 % dMet was visible. The worst growth performances represented mathematically were obtained by the pullets that were fed diets with lowest dMet levels (0.294 and 0.266 %).
The results obtained in the graphic analysis for growth pullets from 7 to 12 weeks of age is a consequence of the better productive efficiency, serological, and histomorphometric performance data observed in the pullets that received slightly higher levels of dMet than those recommended by the Rostagno et al. (2011Rostagno, H. S., Albino, L. F. T., Donzele, J. L., Gomes, P. C., Oliveira, R. F., Lopes, D. C., ... Euclides, R. F. (2011). Tabelas brasileiras para aves e suínos: composição de alimentos e exigências nutricionais. Viçosa, MG: Universidade Federal de Viçosa.), 0.273 % dMet and dMet:dLys 44, which resulted in better use of nutrients and amino acids, greater protein synthesis, and better tissue formation in their body.
No statistical effect was detected on the relative final body weight or weights of liver, spleen, and abdominal fat of the birds from 7 to 12 weeks of age (Table 4).
The lack of significant differences between the dMet levels on the relative weights of the empty body, liver, spleen, and abdominal fat was probably because the most part of the development and formation of organs occurred in the starter phase (from 1 to 6 weeks of age), and thus they were not much affected by the dMet levels in the subsequent stage.
Effects of treatments on the relative final weights of empty body (EBW), liver (LIV), spleen (SPL), and abdominal fat (AF) of pullets from 7 to 12 weeks of age.
According to Hy-Line International (2016), the development of pullets is a multi-stage process, in which organs grow from 1 to 5 weeks of age; bones, from 6 to 12 weeks; and reproductive tract, from 13 to 18 weeks, and the best nutritional adequacy should follow these physiological development stages.
However, in the pre-laying phase, the higher relative liver weight may be associated with higher laying precocity, since the onset of female reproductive activity implies greater activity of this organ for synthesis of the yolk material and to be decoded in the ovary (Braz et al., 2011Braz, N. M., Freitas, E. R., Bezerra, R. M., Cruz, C. E. B., Farias, N. A. P., Silva, N. M., ... Xavier, R. P. S. (2011). Fibra na ração de crescimento e seus efeitos no desempenho de poedeiras nas fases de crescimento e postura. Revista Brasileira de Zootecnia, 40(12), 2744-2753. doi: 10.1590/S1516-35982011001200019
https://doi.org/10.1590/S1516-3598201100...
), as well as an interaction between body fat and laying reproductive system development (Börnelov et al., 2018Bornelöv, S., Seroussi, E., Yosefi, S., Benjamini, S., Miyara, S., Ruzal, M., & Friedman-Einat, M. (2018). Comparative omics and feeding manipulations in chicken indicate a shift of the endocrine role of visceral fat towards reproduction. BMC Genomics, 19(1), 295. doi: 10.1186/s12864-018-4675-0
https://doi.org/10.1186/s12864-018-4675-...
).
No signs of liver steatosis were detected in the analyzed treatments, as demonstrated in the histopathological studies. The minimum point found for glycogen deposition in the liver was 0.348g/100g dMet, estimated by the derivative of the regression equation (Table 5).
Methionine plays a role in liver fat metabolism because it is necessary for fat methylation processes, and as consequence of amino acid deficiency, there is accumulation in the liver of the birds, and a relationship between methylation and gene of lipoprotein lipase in birds in nutrient deficiency situations of the sulfur amino acids (Aggrey, González-Cerón, Rekaya, & Mercier, 2018Aggrey, S. E., González-Cerón, F., Rekaya, R., & Mercier, Y. (2018). Gene expression differences in the methionine demethylation and transulphuration pathways under methionine restriction and recovery with D,L-methionine or D,L-HMTBA in meat-type chickens. Journal of Animal Physiology and Animal Nutrition, 102(1), 468-475. doi: 10.1111/jpn.12779
https://doi.org/10.1111/jpn.12779...
).
Nevertheless, the methionine levels used in the study at this rearing stage were not sufficient to cause clinical liver damage as found in the analyzes. The amino acid imbalance caused by the excess or deficiency of methionine in the diets was limited to changes in the pullet’s productive performance, where the treatments containing the methionine levels closer to the amino acid balance showed a better productive performance and metabolic activity. According to Flora et al. (2017Flora, R. P. D., Dionello, N. J. L., Benitez, L., Germano, J. M., Gotuzzo, A. G., & Freitas, S. (2017). Expressão gênica de IGF-1 e GHR no fígado e no músculo do peito de codornas de corte suplementadas com diferentes níveis de metionina em duas gerações sucessivas. Arquivos Brasileiro de Medicina Veterinária e Zootecnia, 69(1), 205-213. doi: 10.1590/1678-4162-9143
https://doi.org/10.1590/1678-4162-9143...
) high concentrations of methionine in the diet causes toxicity in birds, initially reducing feed intake and growth retardation.
The lack of significant effects on the relative weights of the spleen may have stemmed from factors associated with the lower dietary requirement of dMet for the normal formation and operation of this component of the immune system of growing pullets, from 7 to 12 weeks of age. In this regard, Kidd (2004Kidd, M. T. (2004). Nutritional modulation of immune function in broilers. Poultry Science, 83(4), 650-657. doi: 10.1093/ps/83.4.650
https://doi.org/10.1093/ps/83.4.650...
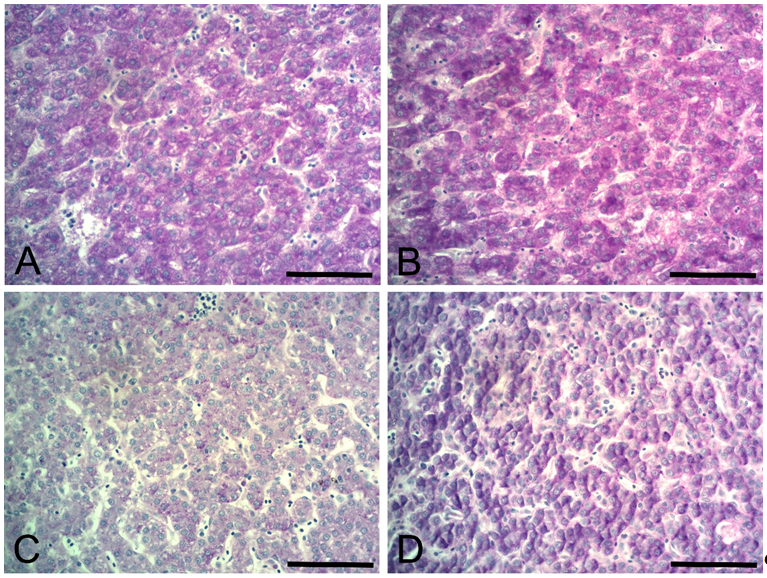
) observed in nutrition-immunology studies, that the nutritional requirements for immune responses do not coincide with those for growth or tissue deposition. Diets whose treatments contained the lowest dMet (0.266 e 0.294%) levels presented the lowest efficiency in the aminoacidic use, being its majority carried to formation of energetic sources, such as glycogen deposition in the liver. It can be seen in the histologic studies on Figure 2. The deposition of glycogen in the liver was verified by the higher positivity to periodic acid Schiff stain (Figure 2).
The increase dMet levels had a quadratic adjustment on the variable gamma-glutamyl transferase, whose minimum obtained value was at 0.321 % (Table 6). Serum levels of alanine aminotransferase, albumin, and protein responded linearly (p < 0.05) to the digestible methionine + cystine levels tested.
Photomicrographs of the liver from white-egg pullets at 12 weeks of age supplemented with dMet levels. A) Liver from white-egg pullets representing the treatment supplemented with 0.266 % dMet. B) Liver from white-egg pullets representing the treatment supplemented with 0.294 % dMet. C) Liver from white-egg pullets representing the treatment supplemented with 0.350 % dMet. D) Liver from white-egg pullets representing the treatment supplemented with 0.378 % dMet. Periodic acid Schiff staining. Bar: 200 µm.
The diets in the treatments containing higher levels of dMet (0.350 and 0.378 %) provided a greater efficiency in the use of amino acids.
Although there were no signs of liver injury on the treatments studied, the lower serum gamma-glutamyl transferase values found in treatments that were supplemented with 0.322 and 0.350% dMet is indicative of lower fat deposition, as found in histological analyzes (Figure 2). Montonen et al. (2012Montonen, J., Boeing, H., Fritsche, A., Schleicher, E., Joost, H. G., Schulze, B., ... Pischon, T. (2012). Consumption of red meat and whole-grain bread in relation to biomarkers of obesity, inflammation, glucose metabolism and oxidative stress. European Journal of Nutrition, 52(1), 337-345. doi: 10.1007/s00394-012-0340-6
https://doi.org/10.1007/s00394-012-0340-...
) correlated the elevation in serum levels of gamma-glutamyl transferase and alanine aminotransferase with an increase in hepatic fat deposition. Similarly, Samuel et al. (2004Samuel, V. T., Liu, Z. X., Qu, X., Elder, B. D., Bilz, S., Befroy, D., ... Shulman, G. I. (2004). Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. Journal of Biological Chemistry, 279(31), 32345-32353. doi: 10.1074/jbc.M313478200
https://doi.org/10.1074/jbc.M313478200...
) proposed that liver enzymes, especially gamma-glutamyl transferase, may serve as indicators of hepatic fat, which is related to increased glycogenesis. The enzymatic activity values found in this study are within the normal range for species (Kaneko, Harvey & Bruss, 2008Kaneko, J. J., Harvey, J. W., & Bruss, M. L. (2008). Clinical biochemistry of domestic animals. New York, NY: Academic Press.).
The dMet levels stimulated the formation of albumin and protein. The higher availability of these compounds may promote better development of the body and reproductive system in the pullets. Emadi et al. (2010Emadi, M., Kaveh, K., Bejo, M. H., Ideris, A., Jahanshiri, F., Ivan, M., & Alimon, R. (2010). Growth performance and blood parameters as influenced by different levels of dietary arginine in broiler chickens. Journal of Animal and Veterinary Advances, 9(1), 70-74.) reported that decreased total serum protein and albumin levels are directly associated with low levels of dietary protein and amino acids for poultry, thus evidencing the importance of donors of the methyl group in protein metabolism and maximization of the metabolic functioning of birds.
Handique, Saikia, Dowarah, Saikia, and Tamuly (2019Handique, B., Saikia, G., Dowarah, R., Saikia, B. N., & Tamuly, S. (2019). Effect of supplementation of synthetic lysine and methionine on serum biochemical profile, carcass characteristics and meat composition in broiler chicken. Indian Journal of Animal Nutrition, 36(1), 40-46. doi: 10.5958/2231-6744.2019.00007.0
https://doi.org/10.5958/2231-6744.2019.0...
) observed that higher serum protein values may be attributed to higher crude protein metabolism with higher nitrogen retention due to adequate dietary amino acid balance, resulting in increased absorption and circulation of amino acids in the blood. With the increased availability of amino acids in the poultry organism, through the optimal level of methionine in the diet, it is expected that the anabolism process will be maximized, while a reduction in the catabolic process, favoring protein synthesis in different tissues body (Zeitz et al., 2019Zeitz, J. O., Mohrmann, S., Käding, S. C., Devlikamov, M., Niewalda, I., Whelan, R., ... Eder, K. (2019). Effects of methionine on muscle protein synthesis and degradation pathways in broilers. Journal of Animal Physiology and Animal Nutrition , 103(1), 191-203. doi: 10.1111/jpn.13026
https://doi.org/10.1111/jpn.13026...
).
As regards creatinine, the lack of statistical effects on this variable (p > 0.05) indicates that it does not affect the urinary system, which corroborated the histopathological findings. In other words, the increased amounts of dMet did not cause kidney damage.
In the analysis of the histomorphometric variables, no statistical effect (p > 0.05) was found between the treatments, except for villus height, which showed a quadratic response in the regression analysis; the maximum point for this variable was found at 0.324 % (Table 7).
The increase of dMet levels provided an increase in villus height, reflected mainly at 0.322 % and 0.350 %, respectively (Figure 3).
Photomicrographs of the intestine of white-egg pullets at 12 weeks of age supplemented with dMet levels. A) Intestine of white-egg pullets representing the treatment supplemented with 0.266, 0.294, and 0.378 % dMet. B) Intestine of white-egg pullets representing the treatment supplemented with 0.322 and 0.350 % dMet. Hematoxylin and eosin staining. Bar: 1 mm.
Methionine is required for enterocyte and intestinal development. It decreases crypt proliferation, limits the number of enterocytes available and the villus growth. Therefore, the villus increases are linked to a higher digestive activity and intestinal absorption, resulting from an increase in the area of absorption, enzymatic secretion in the membrane brush border, nutrient transport system, and consequently potentiation of the productive performance of the bird (Norouzian, Alirezaei, Dezfoulian & Taati, 2018Norouzian, H., Alirezaei, M., Dezfoulian, O., & Taati, M. (2018). The effects of post-hatch feeding with betaine on the intestinal development of broiler chickens. Poultry Science , 20(3), 403-412. doi: 10.1590/1806-9061-2017-0468
https://doi.org/10.1590/1806-9061-2017-0...
).
In addition, methionine is a precursor of cystine, which plays a key role in maintaining the protein function and activity of glutathione peroxidase (GSH), which is an important cellular antioxidant (Ruan et al., 2018Ruan, D., Fouad, A. M., Fan, Q., Xia, W., Wang, S., Chen, W., ... Zheng, C. (2018). Effects of dietary methionine on productivity, reproductive performance, antioxidant capacity, ovalbumin and antioxidant-related gene expression in laying duck breeders. British Journal of Nutrition, 199(2), 121-130. doi: 10.1017/S0007114517003397
https://doi.org/10.1017/S000711451700339...
). Therefore, Shen, Ferket, Park, Malheiros, and Kim (2015Shen, Y. B., Ferket, P., Park, I., Malheiros, R. D., & Kim, S. W. (2015). Effects of feed grade L- methionine on intestinal redox status, intestinal development, and growth performance of young chickens compared with conventional DL-methionine. Journal of Animal Science, 93(6), 2977-2986. doi: 10.2527/jas.2015-8898
https://doi.org/10.2527/jas.2015-8898...
) was found that methionine supplementation increases GSH levels and total antioxidant capacity in the duodenum mucosa, improving the development of intestinal villi, indicating that these beneficial effects are due to their antioxidant function.
Conclusion
Based on feed conversion results, a level of 0.343% dMet with dMet:Lys ratio 55, is recommended for white-egg pullets from 7 to 12 weeks of age.
Acknowledgements
We are grateful to Granja Planalto (Uberlândia, Minas Gerais, Brazil) and Adisseo Animal Nutrition (São Paulo, Brazil) for their support throughout the research
References
- Aggrey, S. E., González-Cerón, F., Rekaya, R., & Mercier, Y. (2018). Gene expression differences in the methionine demethylation and transulphuration pathways under methionine restriction and recovery with D,L-methionine or D,L-HMTBA in meat-type chickens. Journal of Animal Physiology and Animal Nutrition, 102(1), 468-475. doi: 10.1111/jpn.12779
» https://doi.org/10.1111/jpn.12779 - Akaike, H. (1987). Factor analysis and AIC. Psychometrika, 52(3), 317-332. doi: 10.0007/978-1-4612-1694-0_29
» https://doi.org/10.0007/978-1-4612-1694-0_29 - Association of Official Analytical Chemists [AOAC]. (1990). Official methods of analysis Washington, DC: AOAC International.
- Bertalanffy, L. V. (1957). Quantitative laws in metabolism and growth. The Quarterly Review of Biology, 32(3), 217-231. Doi: 10.1086/401873
» https://doi.org/10.1086/401873 - Bornelöv, S., Seroussi, E., Yosefi, S., Benjamini, S., Miyara, S., Ruzal, M., & Friedman-Einat, M. (2018). Comparative omics and feeding manipulations in chicken indicate a shift of the endocrine role of visceral fat towards reproduction. BMC Genomics, 19(1), 295. doi: 10.1186/s12864-018-4675-0
» https://doi.org/10.1186/s12864-018-4675-0 - Braz, N. M., Freitas, E. R., Bezerra, R. M., Cruz, C. E. B., Farias, N. A. P., Silva, N. M., ... Xavier, R. P. S. (2011). Fibra na ração de crescimento e seus efeitos no desempenho de poedeiras nas fases de crescimento e postura. Revista Brasileira de Zootecnia, 40(12), 2744-2753. doi: 10.1590/S1516-35982011001200019
» https://doi.org/10.1590/S1516-35982011001200019 - Brody, S. (1945). Bioenergetics and growth New York, NY: Reinhold Publishing.
- D’Agostini, P., Gomes, P. C., Calderano, A. A., Melo, H. H. C., Sá, L. M., Rostagno, H. S., & Albino, L. F. T. (2012). Requirement of methionine+cysteine for pullets in the growing phase from 7 to 12 weeks old. Brazilian Journal Veterinarian Animal Science, 64(6), 1699-1706. doi: 10.1590/S0102-09352012000600040
» https://doi.org/10.1590/S0102-09352012000600040 - Del Vesco, A. P., Gasparino, E., Oliveira Neto, A. R., Guimarães, S. E. F., Marcato, S. M. M., & Voltoline, D. M. (2013). Dietary methionine effects on IGF-I and GHR mRNA expression in broilers. Genetics Molecular Research, 12(4), 6414-6423. doi: 10.4238/2013.December.10.2
» https://doi.org/10.4238/2013.December.10.2 - Granja Planalto (2009). Manual de manejo das poedeiras Dekalb White Uberlândia, MG: Granja Planalto.
- Emadi, M., Kaveh, K., Bejo, M. H., Ideris, A., Jahanshiri, F., Ivan, M., & Alimon, R. (2010). Growth performance and blood parameters as influenced by different levels of dietary arginine in broiler chickens. Journal of Animal and Veterinary Advances, 9(1), 70-74.
- Flora, R. P. D., Dionello, N. J. L., Benitez, L., Germano, J. M., Gotuzzo, A. G., & Freitas, S. (2017). Expressão gênica de IGF-1 e GHR no fígado e no músculo do peito de codornas de corte suplementadas com diferentes níveis de metionina em duas gerações sucessivas. Arquivos Brasileiro de Medicina Veterinária e Zootecnia, 69(1), 205-213. doi: 10.1590/1678-4162-9143
» https://doi.org/10.1590/1678-4162-9143 - Gompertz, G. (1825). On the nature of the function expressive of the law of human mortality, and on the new mode of determining the value of life contingencies. Philosophical Transactions of the Royal Society, 115, 513-585. doi: 10.1098/rstl.1825.0026
» https://doi.org/10.1098/rstl.1825.0026 - Handique, B., Saikia, G., Dowarah, R., Saikia, B. N., & Tamuly, S. (2019). Effect of supplementation of synthetic lysine and methionine on serum biochemical profile, carcass characteristics and meat composition in broiler chicken. Indian Journal of Animal Nutrition, 36(1), 40-46. doi: 10.5958/2231-6744.2019.00007.0
» https://doi.org/10.5958/2231-6744.2019.00007.0 - Hy-Line International. (2016). Management Guide. Hy-Line international Des Moines, IA: Hy-Line International.
- Ishak, K., Baptista, A., Bianchi, L., Callea, F., Groote, J. D., Gudat, F., ... Thaler, H. (1995). Histological grading and staging of chronic hepatitis. Journal Hepatology, 22(6), 696-699. doi: 10.1016/0168-8278(95)80226-6
» https://doi.org/10.1016/0168-8278(95)80226-6 - Islam, M. S., & Dutta, R. K. (2014). Impacts of vitamin A and essential amino acids on growth performance, survivability, carcass characteristics and profitability of a crossbred chicken (Gallus domesticus L.) in Rajshahi, Bangladesh. International Journal Science Research Environment Science, 2(5), 174-183. doi: 10.12983/ijsres-2014-p0174-0183
» https://doi.org/10.12983/ijsres-2014-p0174-0183 - Jankowski, J., Kubinska, M., & Zdunczyk, Z. (2014). Nutritional and immunomodulatory function of methionine in poultry diets - a review. Annals of Animal Science, 14(1), 17-31. doi: 10.2478/aoas-2013-0081
» https://doi.org/10.2478/aoas-2013-0081 - Kaneko, J. J., Harvey, J. W., & Bruss, M. L. (2008). Clinical biochemistry of domestic animals New York, NY: Academic Press.
- Kidd, M. T. (2004). Nutritional modulation of immune function in broilers. Poultry Science, 83(4), 650-657. doi: 10.1093/ps/83.4.650
» https://doi.org/10.1093/ps/83.4.650 - Montonen, J., Boeing, H., Fritsche, A., Schleicher, E., Joost, H. G., Schulze, B., ... Pischon, T. (2012). Consumption of red meat and whole-grain bread in relation to biomarkers of obesity, inflammation, glucose metabolism and oxidative stress. European Journal of Nutrition, 52(1), 337-345. doi: 10.1007/s00394-012-0340-6
» https://doi.org/10.1007/s00394-012-0340-6 - Norouzian, H., Alirezaei, M., Dezfoulian, O., & Taati, M. (2018). The effects of post-hatch feeding with betaine on the intestinal development of broiler chickens. Poultry Science , 20(3), 403-412. doi: 10.1590/1806-9061-2017-0468
» https://doi.org/10.1590/1806-9061-2017-0468 - Richards, F. J. A. (1959). A flexible growth function for empirical use. Journal of Experimental Botany, 10(2), 290-301. doi: 10.1093/jxb/10.2.290
» https://doi.org/10.1093/jxb/10.2.290 - Rostagno, H. S., Albino, L. F. T., Donzele, J. L., Gomes, P. C., Oliveira, R. F., Lopes, D. C., ... Euclides, R. F. (2011). Tabelas brasileiras para aves e suínos: composição de alimentos e exigências nutricionais Viçosa, MG: Universidade Federal de Viçosa.
- Rostagno, H. S., Albino, L. F. T., Hannas, M. I., Donzele, J. L., Sakomura, N. K., Perazzo, F. G., ... Brito, C.O. (2017). Tabelas brasileiras para aves e suínos: composição de alimentos e exigências nutricionais . Viçosa, MG: Universidade Federal de Viçosa .
- Ruan, D., Fouad, A. M., Fan, Q., Xia, W., Wang, S., Chen, W., ... Zheng, C. (2018). Effects of dietary methionine on productivity, reproductive performance, antioxidant capacity, ovalbumin and antioxidant-related gene expression in laying duck breeders. British Journal of Nutrition, 199(2), 121-130. doi: 10.1017/S0007114517003397
» https://doi.org/10.1017/S0007114517003397 - Samuel, V. T., Liu, Z. X., Qu, X., Elder, B. D., Bilz, S., Befroy, D., ... Shulman, G. I. (2004). Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. Journal of Biological Chemistry, 279(31), 32345-32353. doi: 10.1074/jbc.M313478200
» https://doi.org/10.1074/jbc.M313478200 - Statistical Analysis System [SAS]. (2011). SAS/STAT User’s guide, Version 9.4 Cary, NC: SAS Institute Inc.
- Shen, Y. B., Ferket, P., Park, I., Malheiros, R. D., & Kim, S. W. (2015). Effects of feed grade L- methionine on intestinal redox status, intestinal development, and growth performance of young chickens compared with conventional DL-methionine. Journal of Animal Science, 93(6), 2977-2986. doi: 10.2527/jas.2015-8898
» https://doi.org/10.2527/jas.2015-8898 - Silva, D. J., & Queiroz, A. C. (2002). Análise de alimentos: métodos químicos e biológicos Viçosa, MG: UFV.
- Verhulst, P. F. (1845). Recherches mathématiques sur la loi d’accroissement de la population. Nouveaux Mémoires de L’Académie Royale des Sciences et Belles-Lettres de Bruxelles, 18, 1-41.
- Wu, B., Chui, H., Peng, X., Fang, J., Cui, W., & Liu, X. (2012). Pathology of spleen in chickens fed on a diet deficient in methionine. Health, 4(1), 32-38. doi: 10.4236/health.2012.41007
» https://doi.org/10.4236/health.2012.41007 - Zeitz, J. O., Mohrmann, S., Käding, S. C., Devlikamov, M., Niewalda, I., Whelan, R., ... Eder, K. (2019). Effects of methionine on muscle protein synthesis and degradation pathways in broilers. Journal of Animal Physiology and Animal Nutrition , 103(1), 191-203. doi: 10.1111/jpn.13026
» https://doi.org/10.1111/jpn.13026
Publication Dates
-
Publication in this collection
18 May 2020 -
Date of issue
2020
History
-
Received
29 Mar 2019 -
Accepted
21 Oct 2019