ABSTRACT.
This study was designed to examine the protective effects of nano-selenium and nano-zinc oxide on queen and workers performance under heat stress condition and gene expression of heat shock protein 70 (hsp70) as an index of heat tolerance. Sixty colonies were randomly assigned to five treatments with 12 replicates from June until early September. Sugar syrup (50%) containing no supplement or nano-selenium at levels of 50 and 100 µg L-1 or nano-zinc at levels of 100 and 200 µg L-1 was fed to colonies. Nano-selenium supplementations had no effect, but nano-zinc at level of 100 µg L-1 significantly decreased body malondialdehyde concentration. The highest bee population was seen in nano-zinc at level of 100 µg L-1 and the lowest one in control group. The lowest and the highest body weight, fat and protein deposition was found in group received nano-zinc at level of 100 µg L-1 and control, respectively. The highest gene expression was for group received nano-zinc at level of 100 µg L-1 In group received nano-zinc at level of 100 µg L-1, an increase in hsp70 gene expression was found. In conclusion, nano-zinc oxide at level of 100 µg L-1 could increase queen and worker performance and heat resistance of bees in the hot climate condition.
Keywords:
nano-particle; antioxidant; honey bee; hot climate; heat tolerance
Introduction
In the tropical and subtropical areas coupled with high humidity, honey bees will congregate on the bottom of frames or outside of the hive on very hot days and prefer not to work (Blazyte-Cereskiene et al., 2010Blazyte-Cereskiene, L., Vaitkevičienė, G., Venskutonytė, S., & Būda, V. (2010). Honey bee foraging in spring oilseed rape crops under high ambient temperature conditions. Zemdirbyste-Agriculture, 97, 61-70. ). In these conditions, water and pollen deficiency and thermal stress affect the survival rate and performance of colonies (Atmowidjojo et al., 1997Atmowidjojo, A. H., Wheeler, D. E., Erickson, E. H., & Cohen, A. C. (1997). Temperature tolerance and water balance in feral and domestic honey bees, Apis mellifera L. Comparative Biochemistry and Physiology Part A: Physiology, 118(4), 1399-1403.; Joshi & Joshi, 2010Joshi, N. C., & Joshi, P. C. (2010). Foraging behaviour of Apis spp. on apple flowers in a subtropical environment. New York Science Journal, 3(3), 71-76. ). In thermal stress, some possible toxic materials may be generated in the body of queen, drones and workers, of which reactive oxygen species (Collins, Williams, & Evans, 2004Collins, A. M., Williams, V., & Evans, J. D. (2004). Sperm storage and antioxidative enzyme expression in the honey bee, Apis mellifera. Insect Molecular Biology, 13(2), 141-146. doi: 10.3896/IBRA.1.53.4.02
https://doi.org/10.3896/IBRA.1.53.4.02...
; Stabentheiner, Kovac, & Brodschneider, 2010Stabentheiner, A., Kovac, H., & Brodschneider, R. (2010). Honeybee colony thermoregulation-regulatory mechanisms and contribution of individuals in dependence on age, location and thermal stress. PLoS One, 5(1), e8967. ; Kovac, Käfer, Stabentheiner, & Costa, 2014Kovac, H., Käfer, H., Stabentheiner, A., & Costa, C. (2014). Metabolism and upper thermal limits of Apis mellifera carnica and A. m. ligustica. Apidologie, 45(6), 664-677. doi: 10.1007/s13592-014-0284-3
https://doi.org/10.1007/s13592-014-0284-...
; Agarwal, Virk, Ong, & Du Plessis, 2014Agarwal, A., Virk, G., Ong, C., & Du Plessis, S. S. (2014). Effect of oxidative stress on male reproduction. The World Journal of Men's Health, 32(1), 1-17. doi: 10.5534/wjmh.2014.32.1.1
https://doi.org/10.5534/wjmh.2014.32.1.1...
). High levels of reactive oxygen species induce oxidative stress (Burton & Jauniaux, 2010Burton, G. J., & Jauniaux, E. (2011). Oxidative stress. Best Practice & Research: Clinical Obstetrics & Gynaecology, 25(3), 287-299. doi: 10.1016/j.bpobgyn.2010.10.016
https://doi.org/10.1016/j.bpobgyn.2010.1...
) which can damage the cells integrity, and macromolecules such as DNA, lipids, and proteins. Oxidative stress is one of the factors that cause disturbance of reproductive organs in drones and queen bees and finally swarming or queen replacement (Tarpy & Oliverez Junior, 2014Tarpy, D. R., & Olivarez Junior, R. (2014). Measuring sperm viability over time in honey bee queens to determine patterns in stored-sperm and queen longevity. Journal of Apicultural Research, 53(4), 493-495. ). During heat stress period, activation of some genes like heat shock protein 70 is important for heat resistance (Paynter et al., 2017Paynter, E., Millar, A. H., Welch, M., Baer-Imhoof, B., Cao, D., & Baer, B. (2017). Insights into the molecular basis of long-term storage and survival of sperm in the honeybee (Apis mellifera). Scientific Reports, 7(1), 1-9. doi: 10.1038/srep40236
https://doi.org/10.1038/srep40236...
), because this protein protect the cells from heat and stress damages.
Selenium is known to be an antioxidant as it is able to decrease the Malondialdehyde concentrations near normal concentration (Boostani et al., 2015Boostani, A., Sadeghi, A. A., Mousavi, S. N., Chamani, M., & Kashan, N. (2015). The effects of organic, inorganic, and nano-selenium on blood attributes in broiler chickens exposed to oxidative stress. Acta Scientiae Veterinariae, 43, 1-6. ). Zinc is an essential element for stability of antioxidant enzymes (Lee, 2018Lee, S. R. (2018). Critical role of zinc as either an antioxidant or a prooxidant in cellular systems. Oxidative Medicine and Cellular Longevity, 2018, e9156285. doi: 10.1155/2018/9156285
https://doi.org/10.1155/2018/9156285...
). Pollen with a Se content of 10 mg kg-1 and Zn content of 60 mg kg−1 is sufficient to satisfy the nutritional requirements for royal jelly production and to improve the health of larvae (Zhang, Zhang, Cui, & Xu, 2015Zhang, G., Zhang, W., Cui, X., & Xu, B. (2015). Zinc nutrition increases the antioxidant defenses of honey bees. Entomologia Experimentalis et Applicata, 156(3), 201-210. doi: 10.1111/eea.12342
https://doi.org/10.1111/eea.12342...
). The pollen entrance in hot climate is poor, because its availability is low and collection is very hard for workers. Therefore, in these conditions Se and Zn supplementation could satisfy the needs. Nano particle of Selenium and Zinc oxide has recently attracted in nutrition attentions, because of high absorbability, great specific surface area, high surface activity, a lot of surface active centers and high catalytic efficiency, high absorbed ability and low toxicity than micron-sized particles (Skalickova et al., 2016Skalickova, S., Milosavljevic, V., Cihalova, K., Horky, P., Richtera, L., & Adam, V. (2016). Perspective of selenium nanoparticles as a nutrition supplement. Nutrition, 33(1), 83-90. doi: 10.1016/j. nut
https://doi.org/10.1016/j. nut...
).
In the literature, limited study was found concerning the Se and Zn supplementation in honey bee, especially their nano particles form on queen and worker population and gene expression. Therefore, the present study was designed to examine the protective effect of nano-selenium and nano-zinc oxide on queen and workers performance under heat stress condition and gene expression heat shock protein as an index of heat tolerance.
Material and methods
Nano-particle of selenium (10 to 30 nm with purity ≥ 99 %) was prepared from US Research Nanomaterials, Inc. (Houston, USA) and nano-zinc oxide (50 nm and purity ≥ 97%) was prepared from Sigma Co. (St. Louis, MO, USA). The particle size of nano-materials was determined by the particle size Mastersizer and zeta potential analyzer (Malvern Instruments, Malvern, UK). The nano-particles of Se and Zn were suspended in normal saline with 1% stabilizer (sodium carboxymethyl cellulose), stirred for 15 minutes and then dispersed by ultrasonic vibration bath for 20 min as described previously (Rastgoo & Sadeghi, 2015 Rastgoo, S., & Sadeghi, A. (2015). Effect of Nano-selenium on Plasma Antioxidant status and Reproductive system function of Female Rats exposed to Oxidative Stress Induced by Doxorubicin. Biological Forum, 7(1), 187-191. ). Fresh suspension was prepared before every use to avoid the aggregation of the particles.
For selecting the experimental levels of nano-particles, groups of newly emerged Carnica honey bees (n=100) were collected from five colonies in a research apiary in Karaj (Alborz province, Iran), then mixed and placed into cages with ventilation holes. The cages were kept in an incubator (temp 30ºC and relative humidity 65%). Different levels of nano-particles were supplemented in syrup and dead bees were counted and finally the dose-response was assessed. For farm experiment, two safe doses of nano-selenium and two doses of nano-zinc oxide was selected based on dose-response bioassays (data not shown).
European honeybee (Apis mellifera Carnica) colonies were used in the farm trial. The colonies were kept in one Langstroth hive body. Measurements of bee population size and brood area were done at the start of experiment, and colonies were equalized to have the same numbers of bees and brood. Honeybee colonies (n = 60) with two month sister queens were randomly assigned to one of five treatments with 12 replicates. Treatments were performed from the months of June until early September (hot climate season, 42-44°C). One liter of sugar syrup with a concentration of 1:1 (one parts of sugar and one part of water) containing no supplement or nano-selenium at levels of 50 and 100 µg L-1 or nano-zinc oxide at the levels of 100 and 200 µg L-1 was fed to all the colonies. The rate of the eggs laid by the queen was measured by measuring the surface of the comb containing broods using a grid squares. Measurements of all frames with brood were summed for each colony. In the final of study, bee population size of each colony was measured by estimating the area of each comb covered with bees and the number of combs.
For body weight and deposit measurements, in the final of study a cup of adult bees from the frames in sides of hive was taken, weighted and counted. Then 50 bees were placed in freezer at -20°C. After drying and grinding of bees to 1 mm particle size, protein and fat contents were measured using Kjeldahl method and Soxlhet method (Association of Official Analytical Chemists [AOAC], 2016Association of Official Analytical Chemists [AOAC]. (2016). Official Methods of Analysis (20th ed.). Arlington, VA: AOAC International.). Malondialdehyde (MDA) production was determined using the thiobarbituric acid assay described by Esterbauer and Cheeseman (1990Esterbauer, H., & Cheeseman, K. (1990). Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynoneal. Methods in Enzymology, 186(1), 407-421.).
Bees sample (n = 50) was placed in cry-protectant tube, then frozen in liquid nitrogen and stored in freezer (at -70°C) until analysis for hsp70 gene expression. The relative abundance of hsp70 mRNA was determined by quantitative PCR as described by Sahebzadeh and Lau (2017Sahebzadeh, N., & Lau, W. H. (2017). Expression of heat-shock protein genes in Apis mellifera meda (Hymenoptera: Apidae) after exposure to monoterpenoids and infestation by Varroa destructor mites (Acari: Varroidae). European Journal of Entomology, 114, 195.). The frozen liver was crushed in a sterile mortar, and the powder was applied for total RNA extraction using Accuzol reagent (Cat. No. K-2102, Bioneer Co., Seoul, South Korea). A quantity of 1 ug of each RNA sample was reverse transcribed to cDNA using commercial kit (Bioneer Co., Seoul, South Korea). The resulting cDNA was stored at -20°C prior to use. Quantitative PCR was performed with a specific primer pairs (Koo, Son, Kim, & Lee, 2015Koo, J., Son, T. G., Kim, S. Y., & Lee, K. Y. (2015). Differential responses of Apis mellifera heat shock protein genes to heat shock, flower-thinning formulations, and imidacloprid. Journal of Asia-Pacific Entomology, 18(3), 583-589. doi: 10.1016/j.aspen.2015.06.011
https://doi.org/10.1016/j.aspen.2015.06....
) using Quanti Fast SYBER Green PCR kit (Cat. No: 204052, QIAGEN, Tehran, Iran). Β-actin was applied as a reference gene (Lourenço, Mackert, Santos Cristino, & Simões, 2008Lourenço, A. P., Mackert, A., Santos Cristino, A., & Simões, Z. L. P. (2008). Validation of reference genes for gene expression studies in the honey bee, Apis mellifera, by quantitative real-time RT-PCR. Apidologie, 39(3), 372-385. doi: 10.1051/apido:2008015
https://doi.org/10.1051/apido:2008015...
). The relative expression ratio of hsp70 as a target gene was normalized to β-actin gene based on method of Livak and Schmittgen (2001Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods, 25(4), 402-408.). Quantification was performed in triplicates for each treatment group.
Statistical Analysis System [SAS] (2010Statistical Analysis System [SAS]. (2010). SAS/Stat User Guide: Version 9.1. Cary, NC: SAS Institute Inc.) was used for statistical analysis. Analysis of variance was performed and differences between treatment means were separated using Tukey tests. The level of significance was p < 0.05.
Results and discussion
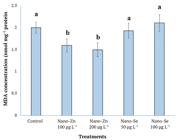
The effect of nano-particles supplementation on malondialdehyde concentration in the body of worker bees are presented in Figure 1. Nano-selenium supplementation at the levels of 50 and 100 µg L-1 had no effect compared to the control group, but nano-zinc oxide supplementation at the level of 100 100 µg L-1 decreased significantly body malondialdehyde concentration.
The end product of lipid peroxidation is malondialdehyde and this compound is an index of lipid peroxidation degree. In the spring, malodialdehyde concentration was assayed and its level was below 1.10 nmol mg-1 protein. In the control group, thermal stress increased free radicals and these oxidants accumulated in the body which finally results in increases in lipid peroxidation and malondialdehyde concentration (Suji & Sivakami 2008Suji, G., & Sivakami, S. (2008). Malondialdehyde, a lipid-derived aldehyde alters the reactivity of Cys34 and the esterase activity of serum albumin. Toxicology in Vitro, 22(3), 618-624. ; Bitzer-Quintero et al., 2010Bitzer-Quintero, O. K., Dávalos-Marín, A. J., Ortiz, G. G., del Angel Meza, A. R., Torres-Mendoza, B. M., Robles, R. G. & Beas-Zárate, C. (2010). Antioxidant activity of tryptophan in rats under experimental endotoxic shock. Biomedicine & Pharmacotherapy, 64(1), 77-81. doi: 10.1016/j.biopha.2009.07.002
https://doi.org/10.1016/j.biopha.2009.07...
). Total antioxidant capacity and MDA content correlated negatively.
The lower MDA concentration means the higher total antioxidant capacity and activity (Tamer et al., 2002Tamer, L., Sucu, N., Polat, G., Ercan, B., Aytacoglu, B., Yücebilgiç, G. & Atik, U. (2002). Decreased serum total antioxidant status and erythrocyte-reduced glutathione levels are associated with increased serum malondialdehyde in atherosclerotic patients. Archives of Medical Research, 33(3), 257-260. ). The low concentration of MDA in group received nano-zinc at two levels is beneficial for worker bees, because in this group, the total antioxidant capacity is high. Nano- -selenium at two levels could not significantly influence on malondialdehyde concentration and numerically increased body MDA level. Zinc is involved in defense against reactive oxygen species, because it acts in the stabilization of membranes, inhibits a pro-oxidant enzyme (NADPH-Oxidase), and induces metallothionein synthesis (Marreiro et al., 2017Marreiro, D. D. N., Cruz, K. J. C., Morais, J. B. S., Beserra, J. B., Severo, J. S., & De Oliveira, A. R. S. (2017). Zinc and oxidative stress: current mechanisms. Antioxidants, 6(2), 24. doi: 10.3390/antiox6020024).
The effects of nano-selenium and nano-zinc oxid supplementation on egg laying area, bee population and body composition were presented in Table 1. There was significant difference for egg laying area in colonies received nano-zinc at the two levels compared to the other treatments and the control group. The positive effect of nano-zinc on laying area and worker population may be related to overcome the thermal stress and defense against free radicals. Based on the study of Sahu et al. (2015Sahu, S., Dandapat, J., & Mohanty, N. (2015). Foliar supplementation of zinc modulates growth and antioxidant defense system of tasar silkworm Antheraea mylitta. Journal of Entomology and Zoology Studies, 3, 25-35. ), zinc plays a vital role in the synthesis of proteins, lipids and carbohydrates and also reduces the duration of larval and pupal stages. In addition zinc is needed as a cofactor for activity and structure stability of antioxidant enzymes. The antioxidant enzymes of insects are generally composed of catalase and Zinc is function as cofactor for antioxidant enzymes (Tasaki, Kobayashi, Matsuura, & Iuchi, 2017Tasaki, E., Kobayashi, K., Matsuura, K., & Iuchi, Y. (2017). An efficient antioxidant system in a long-lived termite queen. PLoS One, 12(1), e0167412. doi: 10.1371/journal.pone.0167412
https://doi.org/10.1371/journal.pone.016...
). Mariani et al. (2008Mariani, E., Mangialasche, F., Feliziani, F. T., Cecchetti, R., Malavolta, M., Bastiani, P., & Jajte, J. (2008). Effects of zinc supplementation on antioxidant enzyme activities in healthy old subjects. Experimental Gerontology, 43(5), 445-451. doi: 10.1016/j.exger.2007.10.012
https://doi.org/10.1016/j.exger.2007.10....
) reported that the potential beneficial effects of Zn supplementation on Zn-dependent antioxidant enzymes. Sahu et al. (2015Sahu, S., Dandapat, J., & Mohanty, N. (2015). Foliar supplementation of zinc modulates growth and antioxidant defense system of tasar silkworm Antheraea mylitta. Journal of Entomology and Zoology Studies, 3, 25-35. ) reported that foliar supplementations of Zinc increased the activity of catalase and Glutathione S-transferase significantly in the whole body homogenate of insects. Also, Zhang et al. (2015Zhang, G., Zhang, W., Cui, X., & Xu, B. (2015). Zinc nutrition increases the antioxidant defenses of honey bees. Entomologia Experimentalis et Applicata, 156(3), 201-210. doi: 10.1111/eea.12342
https://doi.org/10.1111/eea.12342...
) reported that Zinc nutrition increases the antioxidant defenses of honey bees.
The highest bee population was seen in nano-zinc at level of 100 and 200 µg L-1 and the lowest one in those received nano-Se. Nano-selenium maybe had toxic effect on larvae as previousely investigated (Hladun,.Kaftanoglu, Parker, Tran, & Trumble, 2013Hladun, K. R., Kaftanoglu, O., Parker, D. R., Tran, K. D., & Trumble, J. T. (2013). Effects of selenium on development, survival, and accumulation in the honeybee (Apis mellifera L.). Environmental Toxicology and Chemistry, 32(11), 2584-2592. doi: 10.1002/etc.2357
https://doi.org/10.1002/etc.2357...
). Vitellogenin is a Zinc binding glycolipoprotein that has been implicated in the regulation of honey bee immune integrity and the longevity. Lack or deficiency of Zinc in honey bees induces pycnosis, which may explain the loss of functional hemocytes in foragers and results in increase of mortality and decrease of population.
The queen laying area, population and body composition of worker bees received nano-particles
There was no significant difference among treatments for body weight. The lowest fat and protein deposition was found in group received nano-Zinc at two levels and the highest fat and protein deposition was for the control group. Body fat and protein deposition of workers in group received nano-zinc mobilized for supporting the queen and larva feeding. In the control group thermal stress resulted in lower bee’s activity and working and lower laying and larval feeding, therefore the deposition remained higher than the other groups.
Figure 2 presents the effect of nano-particles supplementation on the relative hsp gene expression. The highest gene expression was for group received nano-zinc at the level of 200 µg L-1. In group received nano-zinc at level of 100 µg L-1, an increase in the hsp 70 gene expression was found. Supplementation of nano-Se had no effect on the relative hsp gene expression compare to the control group. Enhancement in the expression of hsp70 protect cells from damages as it help them to recover after and during stressors (Paynter et al., 2017Paynter, E., Millar, A. H., Welch, M., Baer-Imhoof, B., Cao, D., & Baer, B. (2017). Insights into the molecular basis of long-term storage and survival of sperm in the honeybee (Apis mellifera). Scientific Reports, 7(1), 1-9. doi: 10.1038/srep40236
https://doi.org/10.1038/srep40236...
). When organisms are exposed to heat stress, the synthesis of many proteins retarded, but the synthesis of heat shock proteins rapidly increased. In this condition, the heat shock proteins bind to important proteins for body that are heat sensitive. Heat shock proteins protect these important sensitive proteins from degradation (Paynter et al., 2017Paynter, E., Millar, A. H., Welch, M., Baer-Imhoof, B., Cao, D., & Baer, B. (2017). Insights into the molecular basis of long-term storage and survival of sperm in the honeybee (Apis mellifera). Scientific Reports, 7(1), 1-9. doi: 10.1038/srep40236
https://doi.org/10.1038/srep40236...
).
Conclusion
Nano-zinc supplementation at the level of 200 µg L-1 could increase queen and worker performance and could increase heat resistance of bees under thermal stress conditions. Nano-Selenium supplementation had no significant effect on colony performance and gene expression. Further studies are needed to find the mechanism of the nano-material defensive effects in honey bee workers and queen.
Acknowledgements
The authors would like to thanks Dr. S. Rastgo and her Company for providing nano-particles samples.
References
- Agarwal, A., Virk, G., Ong, C., & Du Plessis, S. S. (2014). Effect of oxidative stress on male reproduction. The World Journal of Men's Health, 32(1), 1-17. doi: 10.5534/wjmh.2014.32.1.1
» https://doi.org/10.5534/wjmh.2014.32.1.1 - Association of Official Analytical Chemists [AOAC]. (2016). Official Methods of Analysis (20th ed.). Arlington, VA: AOAC International.
- Atmowidjojo, A. H., Wheeler, D. E., Erickson, E. H., & Cohen, A. C. (1997). Temperature tolerance and water balance in feral and domestic honey bees, Apis mellifera L. Comparative Biochemistry and Physiology Part A: Physiology, 118(4), 1399-1403.
- Bitzer-Quintero, O. K., Dávalos-Marín, A. J., Ortiz, G. G., del Angel Meza, A. R., Torres-Mendoza, B. M., Robles, R. G. & Beas-Zárate, C. (2010). Antioxidant activity of tryptophan in rats under experimental endotoxic shock. Biomedicine & Pharmacotherapy, 64(1), 77-81. doi: 10.1016/j.biopha.2009.07.002
» https://doi.org/10.1016/j.biopha.2009.07.002 - Blazyte-Cereskiene, L., Vaitkevičienė, G., Venskutonytė, S., & Būda, V. (2010). Honey bee foraging in spring oilseed rape crops under high ambient temperature conditions. Zemdirbyste-Agriculture, 97, 61-70.
- Boostani, A., Sadeghi, A. A., Mousavi, S. N., Chamani, M., & Kashan, N. (2015). The effects of organic, inorganic, and nano-selenium on blood attributes in broiler chickens exposed to oxidative stress. Acta Scientiae Veterinariae, 43, 1-6.
- Burton, G. J., & Jauniaux, E. (2011). Oxidative stress. Best Practice & Research: Clinical Obstetrics & Gynaecology, 25(3), 287-299. doi: 10.1016/j.bpobgyn.2010.10.016
» https://doi.org/10.1016/j.bpobgyn.2010.10.016 - Collins, A. M., Williams, V., & Evans, J. D. (2004). Sperm storage and antioxidative enzyme expression in the honey bee, Apis mellifera Insect Molecular Biology, 13(2), 141-146. doi: 10.3896/IBRA.1.53.4.02
» https://doi.org/10.3896/IBRA.1.53.4.02 - Esterbauer, H., & Cheeseman, K. (1990). Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynoneal. Methods in Enzymology, 186(1), 407-421.
- Hladun, K. R., Kaftanoglu, O., Parker, D. R., Tran, K. D., & Trumble, J. T. (2013). Effects of selenium on development, survival, and accumulation in the honeybee (Apis mellifera L.). Environmental Toxicology and Chemistry, 32(11), 2584-2592. doi: 10.1002/etc.2357
» https://doi.org/10.1002/etc.2357 - Joshi, N. C., & Joshi, P. C. (2010). Foraging behaviour of Apis spp. on apple flowers in a subtropical environment. New York Science Journal, 3(3), 71-76.
- Koo, J., Son, T. G., Kim, S. Y., & Lee, K. Y. (2015). Differential responses of Apis mellifera heat shock protein genes to heat shock, flower-thinning formulations, and imidacloprid. Journal of Asia-Pacific Entomology, 18(3), 583-589. doi: 10.1016/j.aspen.2015.06.011
» https://doi.org/10.1016/j.aspen.2015.06.011 - Kovac, H., Käfer, H., Stabentheiner, A., & Costa, C. (2014). Metabolism and upper thermal limits of Apis mellifera carnica and A. m. ligustica. Apidologie, 45(6), 664-677. doi: 10.1007/s13592-014-0284-3
» https://doi.org/10.1007/s13592-014-0284-3 - Lee, S. R. (2018). Critical role of zinc as either an antioxidant or a prooxidant in cellular systems. Oxidative Medicine and Cellular Longevity, 2018, e9156285. doi: 10.1155/2018/9156285
» https://doi.org/10.1155/2018/9156285 - Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods, 25(4), 402-408.
- Lourenço, A. P., Mackert, A., Santos Cristino, A., & Simões, Z. L. P. (2008). Validation of reference genes for gene expression studies in the honey bee, Apis mellifera, by quantitative real-time RT-PCR. Apidologie, 39(3), 372-385. doi: 10.1051/apido:2008015
» https://doi.org/10.1051/apido:2008015 - Mariani, E., Mangialasche, F., Feliziani, F. T., Cecchetti, R., Malavolta, M., Bastiani, P., & Jajte, J. (2008). Effects of zinc supplementation on antioxidant enzyme activities in healthy old subjects. Experimental Gerontology, 43(5), 445-451. doi: 10.1016/j.exger.2007.10.012
» https://doi.org/10.1016/j.exger.2007.10.012 - Marreiro, D. D. N., Cruz, K. J. C., Morais, J. B. S., Beserra, J. B., Severo, J. S., & De Oliveira, A. R. S. (2017). Zinc and oxidative stress: current mechanisms. Antioxidants, 6(2), 24. doi: 10.3390/antiox6020024
- Paynter, E., Millar, A. H., Welch, M., Baer-Imhoof, B., Cao, D., & Baer, B. (2017). Insights into the molecular basis of long-term storage and survival of sperm in the honeybee (Apis mellifera). Scientific Reports, 7(1), 1-9. doi: 10.1038/srep40236
» https://doi.org/10.1038/srep40236 - Rastgoo, S., & Sadeghi, A. (2015). Effect of Nano-selenium on Plasma Antioxidant status and Reproductive system function of Female Rats exposed to Oxidative Stress Induced by Doxorubicin. Biological Forum, 7(1), 187-191.
- Sahebzadeh, N., & Lau, W. H. (2017). Expression of heat-shock protein genes in Apis mellifera meda (Hymenoptera: Apidae) after exposure to monoterpenoids and infestation by Varroa destructor mites (Acari: Varroidae). European Journal of Entomology, 114, 195.
- Sahu, S., Dandapat, J., & Mohanty, N. (2015). Foliar supplementation of zinc modulates growth and antioxidant defense system of tasar silkworm Antheraea mylitta. Journal of Entomology and Zoology Studies, 3, 25-35.
- Skalickova, S., Milosavljevic, V., Cihalova, K., Horky, P., Richtera, L., & Adam, V. (2016). Perspective of selenium nanoparticles as a nutrition supplement. Nutrition, 33(1), 83-90. doi: 10.1016/j. nut
» https://doi.org/10.1016/j. nut - Stabentheiner, A., Kovac, H., & Brodschneider, R. (2010). Honeybee colony thermoregulation-regulatory mechanisms and contribution of individuals in dependence on age, location and thermal stress. PLoS One, 5(1), e8967.
- Statistical Analysis System [SAS]. (2010). SAS/Stat User Guide: Version 9.1 Cary, NC: SAS Institute Inc.
- Suji, G., & Sivakami, S. (2008). Malondialdehyde, a lipid-derived aldehyde alters the reactivity of Cys34 and the esterase activity of serum albumin. Toxicology in Vitro, 22(3), 618-624.
- Tamer, L., Sucu, N., Polat, G., Ercan, B., Aytacoglu, B., Yücebilgiç, G. & Atik, U. (2002). Decreased serum total antioxidant status and erythrocyte-reduced glutathione levels are associated with increased serum malondialdehyde in atherosclerotic patients. Archives of Medical Research, 33(3), 257-260.
- Tarpy, D. R., & Olivarez Junior, R. (2014). Measuring sperm viability over time in honey bee queens to determine patterns in stored-sperm and queen longevity. Journal of Apicultural Research, 53(4), 493-495.
- Tasaki, E., Kobayashi, K., Matsuura, K., & Iuchi, Y. (2017). An efficient antioxidant system in a long-lived termite queen. PLoS One, 12(1), e0167412. doi: 10.1371/journal.pone.0167412
» https://doi.org/10.1371/journal.pone.0167412 - Zhang, G., Zhang, W., Cui, X., & Xu, B. (2015). Zinc nutrition increases the antioxidant defenses of honey bees. Entomologia Experimentalis et Applicata, 156(3), 201-210. doi: 10.1111/eea.12342
» https://doi.org/10.1111/eea.12342
Publication Dates
-
Publication in this collection
25 Sept 2020 -
Date of issue
2021
History
-
Received
12 July 2019 -
Accepted
15 Oct 2019