Abstracts
The aim of this study was to investigate the interaction of human FSH (10ng/ml) with T4 (20ng/mL) on survival, activation and growth of preantral follicles cultured in vitro for 28 days. Fragments of non-cultured and cultured ovarian tissue were processed for classic histology and transmission electron microscopy. The results showed a reduction in the survival rate in all the media tested (one to 28 days) when compared to the fresh control. However the treatment with T4/hFSH for seven days of culture maintained the rate similar to the control. The media tested by one and 28 days reduced the percentage of primordial follicles in all periods of culture. However, T4/hFSH on day one of culture remained similar to the fresh control. None of the media were able to keep the percentage of the developing follicles. It was observed that the follicular diameter in the medium with T4/hFSH remained similar to the fresh control. The ultrastructural analysis confirmed the integrity of follicles cultured for seven days in a medium supplemented with T4/hFSH. In conclusion, the medium with T4/hFSH is able to maintain the survival, promote the activation, and the ultrastructural integrity of caprine preantral follicles for until seven days.
Activation; caprine; hFSH; preantral follicles; T4
O objetivo deste estudo foi investigar a interação do FSH humano (10 ng/mL) com T4 (20 ng/mL) na sobrevivência, ativação e crescimento de folículos pré-antrais cultivados in vitro, por um período de longa duração (28 dias). Fragmentos de tecido ovariano não cultivado e cultivados foram processados para histologia clássica e microscopia eletrônica de transmissão. Os resultados mostraram uma redução na taxa de sobrevivência em todos os meios testados (um a 28 dias) quando comparado ao controle fresco. Entretanto, o tratamento com T4/hFSH por sete dias de cultivo resultou em taxa semelhante ao controle. Os meios testados por 28 dias reduziram o percentual de folículos primordiais em todos os períodos de cultivo. Contudo, T4/hFSH no dia um de cultivo manteve-se semelhante ao controle fresco. Nenhum dos meios foi capaz de manter o percentual dos folículos em desenvolvimento. Observou-se que o diâmetro folicular cultivado no meio com T4/hFSH manteve-se semelhante ao controle fresco. As análises ultraestruturais confirmaram a integridade de folículos cultivados por sete dias em meio suplementado com T4/hFSH. Em conclusão, o meio com T4/hFSH é capaz de manter a sobrevivência, promover a ativação e a integridade ultraestrutural de folículos pré-antrais caprinos, por até sete dias.
Ativação; caprino; folículos pré-antrais; hFSH; T4
Introduction
The limitation in the supply of fertilizable mature oocytes represents an important obstacle to increase the success of assisted reproduction techniques. Accordingly, the use of in vitro oocytes of cultured and matured follicles is an alternative to the improvement of these techniques(11 Telfer E.E., Mclaughlin M., Ding C., Thong K.J. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Human Reproduction, 2008; 23(5):1151-1158.). Over the last decades, some advances have been achieved during in vitro culture of preantral follicles; however the rate of embryo production is still low in domestic animals(22 Arunakumari G., Shanmugasundaram N., Rao V.H. Development of morulae from the oocytes of cultured sheep preantral follicles. Theriogenology, 2010; 74:884-894.,33 Saraiva M.V.A., Rossetto R., Brito I.R., Celestino J.J.H., Silva C.M.G., Faustino L.R., Almeida A.P., Bruno J.B., Magalhães D.M., Matos M.H.T., Campello C.C., Figueiredo J.R. Dynamic medium produces caprine embryo from preantral follicles grown in vitro. Reproductive Sciences, 2010; 17:1135-1143.). This low efficiency can be caused by the media composition used during the culture period. These are unable to withstand the constant evolution of the developing follicle needs(44 Peng X., Yang M., Wang L., Tong C., Guo Z. In vitro culture of sheep lamb ovarian cortical tissue in a sequential culture medium. The Journal of Assisted Reproduction and Genetics, 2010; 27(5):247-257.).
During the reproductive life of the female, a large number of primordial follicles are stimulated to grow, in a process known as follicular activation, and the vast majority (99.9%) can become atretic before ovulation(55 Skinner M.K. Regulation of primordial follicle assembly and development. Human Reproduction Update, 2005; 11:461-471.). Despite the high rate of atresia that occurs in primordial follicles during in vivo growth and maturation, these can be a valuable source for studies about the in vitro development of oocytes, and the production of embryos(66 Carroll J.D.G., Whittingham M.J., Wood E., Telfer R.G. Gosden. Extraovarian production of mature viable mouse oocytes from frozen primary follicles. Journal Reproduction and Fertility, 1990; 90:321-327.,77 O'Brien M.J., Pendola J.K., Eppig J.J. A revised protocol for in vitro development of mouse oocyte from primordial follicles dramatically improves their development competence. Biology of Reproduction, 2003; 68:1682-1686.). Until recently, little was known about how the primordial follicles are activated and stimulated to grow and develop. In domestic mammals, early follicular development follows a very rigorous and complex process(88 Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocrine Reviews, 1996; 17:121-154.); therefore, a long period of culture may be required for the development of preantral follicles. However, few studies of long-term in vitro culture have obtained follicular activation rates (sheep(99 Muruvi W., Picton H.M., Rodway R.G., Joyce I.M. In vitro growth of oocytes from primordial follicles isolated from frozen-thawed lamb ovaries. Theriogenology, 2005; 64:1357-1370.), humans(1010 Sadeu J.C., Cortvrindt R., Ron-El R., Kasterstein E., Smitz J. Morphological and ultrastructural evaluation of cultured frozen-thawed human fetal ovarian tissue. Fertility and Sterility, 2006; 85:1130-1141.), goat(1111 Rossetto R., Lima-Verd, I.B., Matos M.H.T., Saraiva M.V.A., Martins F.S., Faustino L.R., Araújo V.R., Silva C.M.G., Name K.P.O., Báo S.N., Campello C.C., Figueiredo J.R., Blume H. Interaction between ascorbic acid and follicle-stimulating hormone maintains follicular viability after long-term in vitro culture of caprine preantral follicles. Domestic Animal Endocrinology, 2009; 37:112-123.)). This may be related to the use of simple culture medium, without the addition of a series of growth factors or hormones that can satisfy the demands required by the follicles during the different stages of growth.
Among the most commonly used hormones in the culture medium, the follicle stimulating Hormone (FSH) outstands for its role in early folliculogenesis in mammals. FSH is a gonadotropin known for regulating the proliferation and differentiation of granulosa cells during the beginning (antral) of in vivo follicle development(1212 Gutierrez C.G., Campbell B.K., Webb R. Development of a long-term bovine granulosa cell culture system: induction and maintenance of estradiol production, response to follicle stimulating hormone and morphological characteristics. Biology of Reproduction, 1997; 56:608-616.,1313 Hillier S.G. Gonadotropic control of ovarian follicular growth and development. Molecular and Cellular Endocrinology, 2001; 179:39-46.). In goats, the addition of 50 ng/mL of FSH to the culture medium of preantral follicles maintained the survival, and ultrastructural integrity of the follicles(1414 Matos M.H.T., Lima-Verde I.B., Luque M.C.A., Maia Jr J.E., Silva J.R.V., Celestino J.J.H., Martins F.S., Báo S.N., Lucci C.M., Figueiredo J.R. Essential role of follicle stimulating hormone in the maintenance of caprine preantral follicle viability in vitro. Zygote, 2007; 15:173-182.).
The thyroxine hormone (T4) has also been implicated for its participation in the fertility of mammals(1515 Cecconi S., Rossi G., Coticchio G., Maccharelli G., Borini A., Canipari R. Influence of thyroid hormone on mouse preantral follicle development in vitro. Fertility and Sterility, 2004; 81:919-924.). The effects of these hormones on ovarian folliculogenesis and steroidogenesis are related to the differentiation of granulosa cells(1616 Silva J.R.V., Van Den Hurk R., Matos M.H.T., Santos R.R., Pessoa C., Moraes M.O., Figueiredo J.R. Influences of FSH and EGF on primordial follicles during in vitro culture of caprine ovarian cortical tissue. Theriogenology, 2004; 61:1691-1704.), and stimulation of ovulation(1717 Serakides R., Nunes V.A., Nascimento E., Ribeiro A.F.C., Silva C.M. Foliculogênese e esteroidogênese ovarianas em ratas adultas hipertireóideas. Arquivo Brasileiro de Endocrinologia & Metabologia, 2001; 45:258-264.). Research has confirmed the addition of FSH and T3 to the in vitro culture medium promotes the growth of preantral follicles, and that this interaction is mediated through the increase in the expression of mRNA to FSH receptors (rFSH) through a GDF-9 dependent mechanism. It is believed that these two hormones (FSH and T3) interact synergistically inhibiting the apoptosis of granulosa cells in small preantral follicles(1818 Asahara S., Sato A., Ali Aljonaid A., Maruo T. Thyroid Hormone Synergizes with Follicle Stimulating Hormone to Inhibit Apoptosis in Porcine Granulosa Cells Selectively from Small Follicles. Kobe Journal of Medical Sciences, 2003; 49:107-116.).
The present study was designed with the aim to investigate the influence of human FSH (10 ng/mL) and T4 (20 ng/mL) on the survival, activation, and growth of preantral follicles cultured in vitro for a long duration period (28 days). These concentrations were selected considering previous studies conducted by our team, which showed promising results with the use of these hormones.
Material and Methods
This study was approved by the Ethics Committee on Animal Use, CEUA-UFV under case No 45/2012 on August 06, 2012. Ovaries (n = 10) from five adult non-pregnant mixed-breed goats with 1 to 3 y.o. were collected from a local slaughterhouse. Immediately postmortem, the ovaries were washed in 70% alcohol for 10 seconds and then washed again in MEM supplemented with 100 μg/mL penicillin and 100 μg/mL streptomycin. The pairs of ovaries were transported to the laboratory in MEM at 4 °C within 1 h postmortem. Unless otherwise mentioned, human FSH (hFSH), thyroxine and other chemicals used in the present study were purchased from Sigma Chemical Co. (St Louis, USA).
The organ culture system utilized herein was previously described(1414 Matos M.H.T., Lima-Verde I.B., Luque M.C.A., Maia Jr J.E., Silva J.R.V., Celestino J.J.H., Martins F.S., Báo S.N., Lucci C.M., Figueiredo J.R. Essential role of follicle stimulating hormone in the maintenance of caprine preantral follicle viability in vitro. Zygote, 2007; 15:173-182.,1919 Celestino J.J.H., Bruno J.B., Lima-Verde I.B., Matos M.H., Saraiva M.V., Chaves R.N., Martins F.S., Almeida A.P., Cunha R.M., Lima L.F., Name K.P., Campello C.C., Silva J.R., Báo S.N., Figueiredo J.R. Steadystate level of kit ligand mRNA in goat ovaries and the role of kit ligand in preantral follicle survival and growth in vitro. Molecular Reproduction and Development, 2010; 77(3):231-40.). In the laboratory, the ovaries from each animal were stripped of surrounding fat tissue and ligaments. Subsequently, ovarian cortex tissue samples from each ovarian pair were cut into nine slices (approximate size: 3 x 3 mm, with 1 mm thickness) using a scalpel under sterile conditions. The tissue pieces were then either fixed for histological and ultrastructural analysis (fresh control) or placed in culture for 1 (d1), 7 (d7), 14 (d14), 21 (d21) and 28 (d28) days. Caprine tissues were transferred to 24-well culture dishes containing 1 mL of culture media. Culture was performed at 39 °C in 5% CO2 in a humidified incubator and all the media were incubated for 1 h prior to use. The basic culture medium consisted of α-MEM (pH 7.2 to 7.4) supplemented with 10 μg/mL insulin, 5.5 μg/mL transferrin and 5 ng/mL selenium (ITS), 2 mM glutamine, 2 mM hypoxanthine, 1.25 mg/mL bovine serum albumin (BSA), which was called α-MEM+ (culture control).
The ovarian cortex fragments were cultured in α-MEM+ alone (culture control) and α-MEM+ supplemented with hFSH (10 ng/mL) in association with T4 (20 ng/mL) as showed in the Table 1. Each treatment was repeated five times and the culture medium was changed every 2 days.
Before (fresh control) and after 1, 7, 14, 21 or 28 days in culture, all tissue pieces were fixed in Carnoy's solution for four hours, and then dehydrated in increasing concentrations of ethanol. After paraffin embedding (Vetec, Rio de Janeiro, Brazil), caprine ovarian cortex tissues were cut into 7-µm sections, which were mounted on glass slides and stained by Periodic Acid Schiff (PAS) - hematoxylin. Follicle stage and morphology were assessed microscopically on serial sections. Coded anonymized slides were examined by a microscope (Olympus, Japan) under 400x magnification.
The developmental stages of follicles have been defined previously(1616 Silva J.R.V., Van Den Hurk R., Matos M.H.T., Santos R.R., Pessoa C., Moraes M.O., Figueiredo J.R. Influences of FSH and EGF on primordial follicles during in vitro culture of caprine ovarian cortical tissue. Theriogenology, 2004; 61:1691-1704.)as primordial (one layer of flattened granulosa cells around the oocyte) or growing follicles (intermediate: one layer of flattened and cuboidal granulosa cells; primary: one layer of cuboidal granulosa cells; and secondary: two or more layers of cuboidal granulosa cells around the oocyte). Only the preantral follicles (primordial, intermediate, primary and secondary) were considered in the analysis, and the antral stage was not included because of the very small number of antral follicles present in the ovarian fragments. The preantral follicles were also classified individually as histologically normal when an intact oocyte was present, surrounded by granulosa cells that were well organized in one or more layers and lacked a pyknotic nucleus. Degenerated follicles were defined as those with a retracted oocyte, pyknotic nucleus, and/or disorganized granulosa cells detached from the basement membrane. Overall, 150 follicles were evaluated for each treatment (30 follicles per treatment in one repetition x five repetitions = 150 follicles).
To evaluate follicular activation, the percentages of healthy primordial and growing follicles were calculated before (fresh control) and after culture in each medium. In addition, follicle and oocyte diameters were measured in healthy follicles only. Follicle diameter was recorded from edge to edge of the outer layer of granulosa cells or from the outside edge of the theca cell layer when present. Oocyte diameter was recorded from edge to edge of the oocyte membrane. Two perpendicular diameters were recorded in each measurement and the average of these two values was calculated. Each follicle was examined in every section in which it appeared and matched with the same follicle on adjacent sections to avoid double counting, thus ensuring that each follicle was only counted once.
To better evaluate follicular morphology the ultrastructural analysis was performed on preantral follicles from non-cultured tissue (fresh control), follicles cultured in the treatments that had the best results for morphology, activation and growth. Briefly, small pieces (1 mm3) of caprine ovarian tissues were fixed for 4 h at room temperature in karnovsky fixative (2.5% glutaraldehyde and 4% formaldehyde in 0.1 M cacodylate buffer, pH 7.2). After fixation, the samples were washed twice in 0.1 M cacodylate buffer, post-fixed in 1% osmium tetroxide, dehydrated in acetone, and embedded in Epon 812 resin. Afterwards, semi-thin sections (3 μm) were cut using an ultramicrotome (MT2-B, Sorvall, USA) stained with toluidine blue, and analyzed by light microscopy under 400x magnification. Ultra-thin sections (60 to 70 nm) were obtained from preantral follicles classified as morphologically normal in semi-thin sections, according to the criteria adopted for histological analysis. Subsequently, ultra-thin sections were contrasted with uranyl acetate, and lead citrate. A transmission electron microscope EM 109 Zeiss (EM, Berlin, Germany) operating at 80 Kv20 was used to examine each sample. Parameters such as density and integrity of ooplasmic and granulosa cell organelles, vacuolization and integrity of the basement membrane were evaluated.
The variables were submitted to Normality (Lilliefors) and Homoscedasticity (Cochran) tests, and to the analysis of variance at a probability of 5%. In case significance was presented, Tukey test was carried out (SAS Institute Inc., Cary, NC, USA).
Results
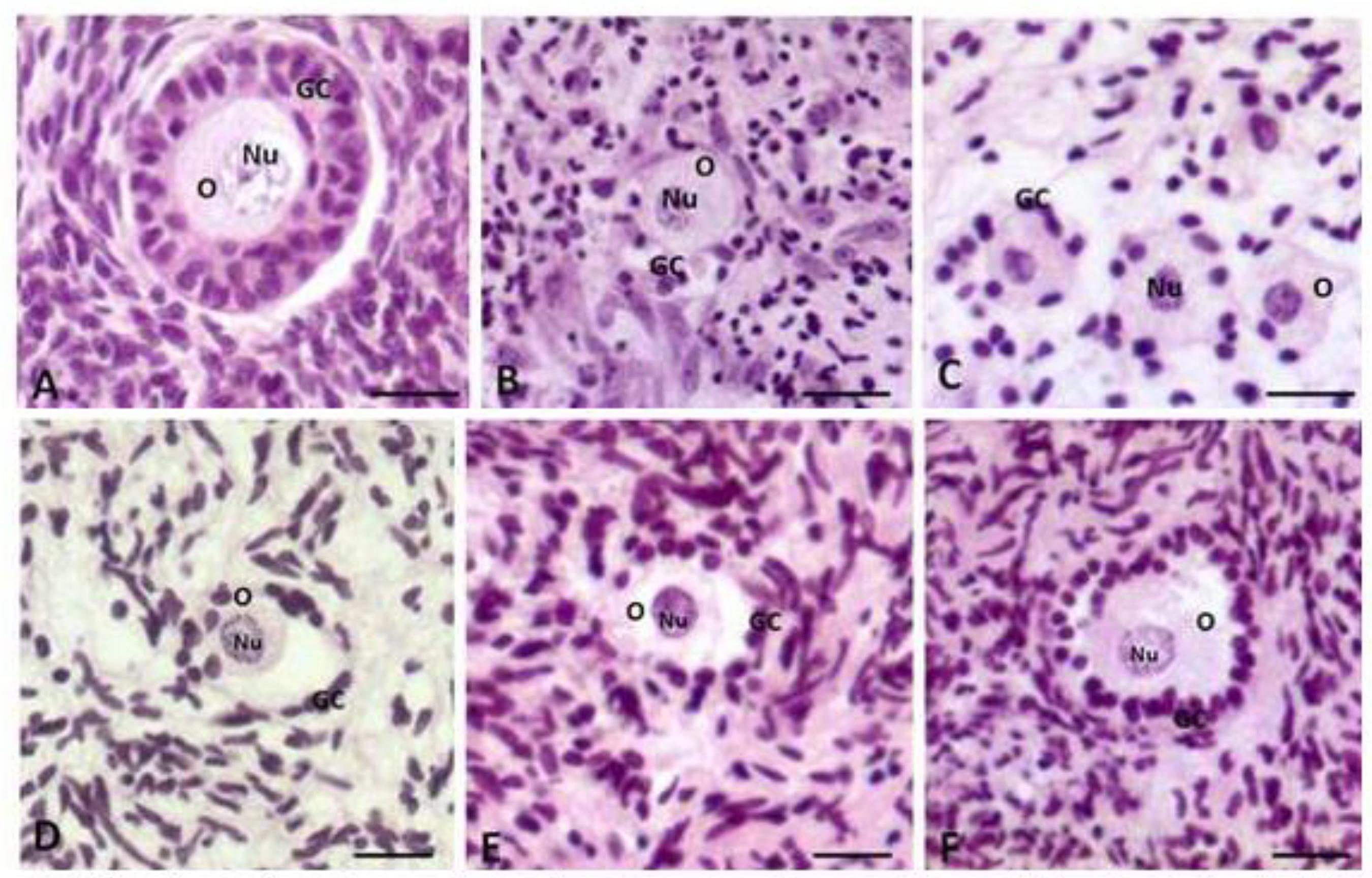
A total of 1,650 preantral follicles were analyzed by classic histology. Figures 1A, B, and C show normal follicles from the fresh control, and after the culture in α-MEM+ supplemented with T4/hFSH (day seven) or T4/hFSH (day 14), respectively. Figures 1D, E and F illustrate degenerated follicles after the culture in α-MEM+ (cultured control, day 28) or in media containing T4/hFSH (day 21) or T4/hFSH (day 28), respectively. In degenerated follicles, retracted oocytes and disorganization of granulosa cells were observed.
Histological sections of ovarian fragments tissue after staining Periodic Acid-Schiff s hematoxylin showing morphologically normal follicles (A: fresh control), (B; T4/hFSH - D7) and (C: T4/hFSH - D14). The degenerate folheies were represented by letters (D: α-MEM+ - D28); (E, T4/hFSH - D21) and (F, T4/hFSH - D28). O: oocyte; Nu: nucleus oocyte; GC: granulosa cells (Ongmal magnification 200×). Scale bars represent 20 µm.
The percentage of morphologically normal preantral follicles in the fresh control and after 1, 7, 14, 21 or 28 days of in vitro culture are shown in Table 2. In relation to the progression of culture from one to 28 days, there was a reduction (P<0.05) in the percentage of morphologically normal follicles in almost all treatments, compared to the fresh control (97.3%). However, with seven days of culture, the medium supplemented with T4/hFSH (20/10ng/mL, P>0.05) showed a rate of normal follicles similar to the non-cultured control.
Percentage (mean ± SEM) of morphologically normal preantral follicles in firesh control (non-cultured) and after in vitro culture for 1, 7, 14, 21 or 28 days in the absence or presence of T4/hFSH (concentrations in ng/mL)
When the α-MEM+ medium (cultured control) and the medium containing T4/hFSH were compared among themselves, for one to 28 days of culture, there was no difference (P>0.05) in the percentage of normal follicles. With the progression of the culture from one to 28 days, all media were able to maintain (P> 0.05) the percentage of normal follicles during 21 days. However, the rate of normal follicles reduced when extending the culture for 28 days (P< 0.05).
The percentages of primordial and developing follicles in fresh ovarian tissue were 71.3% and 31.3%, respectively (Table 3). After 1, 7, 14, 21 and 28 days of culture, there was a reduction in this rate (P<0.05) in all media used, except in the one that contained T4/hFSH (20/10ng/mL, P>0.05) on day 1 of culture compared to the non-cultured control in primordial follicles. However, the rates of developing follicles of all media were similar to the non-cultured control (P>0.05), regardless of the time of culture. When the media were compared among themselves, no difference (P>0.05) was observed in both rates of primordial and developing follicles. With the progression of the culture period from one to 28 days, no differences in the percentages of primordial and developing follicles were observed (P>0.05).
Percentage (mean ± SEM) of primordial follicles and developing (intermediate, primary and secondary) in control (non-cultured) and after in vitro culture for 1, 7, 14, 21 or 28 days in the absence or presence of T4/HFSH (concentrations in ng/mL)
There was a reduction in the oocyte diameter (P<0.05) throughout the culture in all media, except when were cultured for one day with α-MEM+ and T4/hFSH (Table 4). However, the α-MEM+ with day one of culture and the T4/hFSH cultured for one and seven days maintained follicular diameter (P>0.05) when compared to the non-cultured control. When the media were compared among themselves, there was no difference (P>0.05) in oocyte and follicle diameters. With the progression of culture from one to seven days, the α-MEM+ and the media T4/hFSH maintained oocyte and follicular diameter (P>0.05), occurring later a reduction of both, when extending the culture up to 28 days.
Caprine ocyte and follicle diameters (mean ± SEM) in non-cultured tissues (fresh control) and in tissues cultured for 1, 7, 14, 21 or 28 days m the absence or presence of interaction T4/hFSH (concentrations in ng/mL)
The ultrastructural analysis was performed in preantral follicles of the non-cultured control and of follicles cultured for one and seven days in medium with T4/hFSH (20/10ng/mL). This medium with hormones was selected for presenting satisfactory results on the parameters evaluated by the histological analysis.
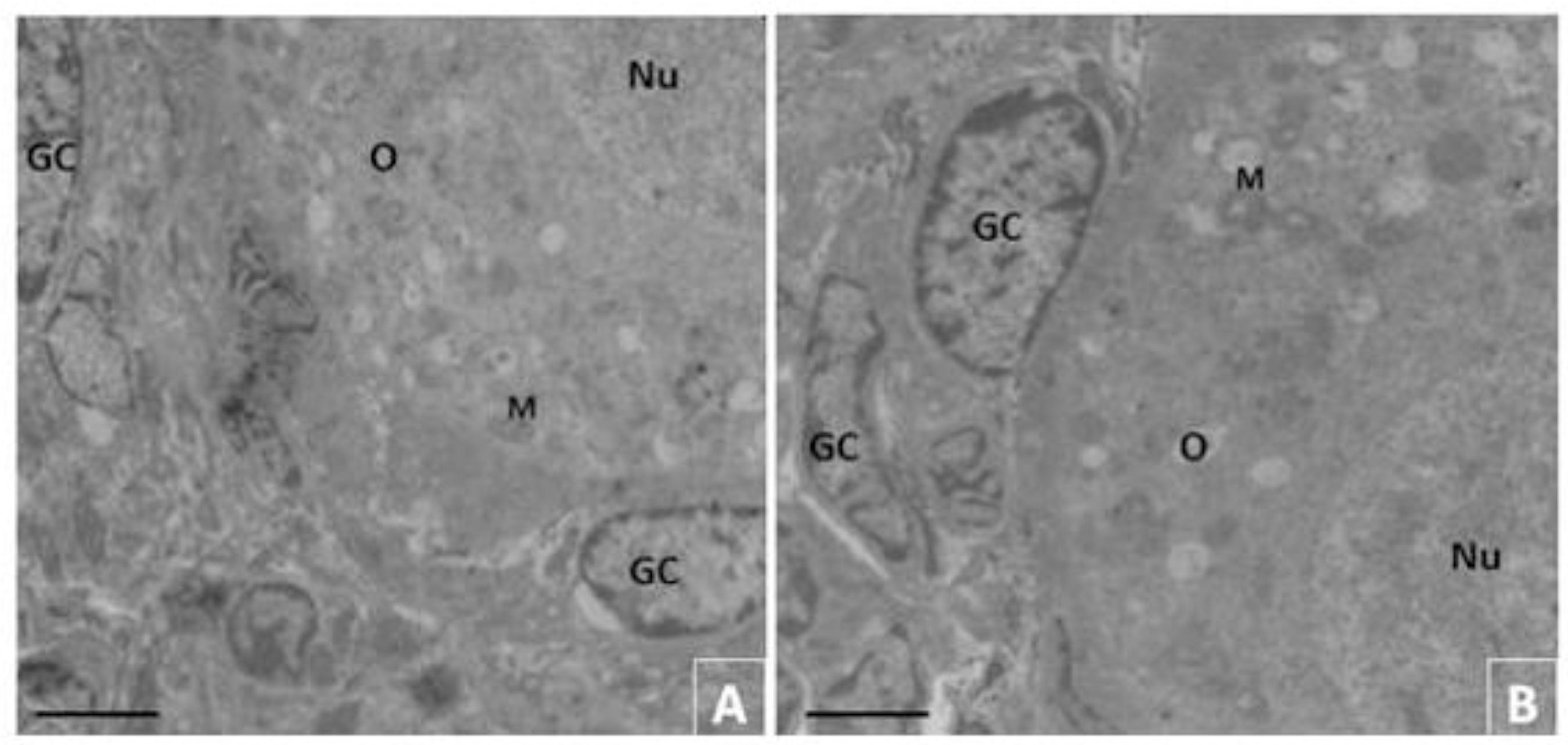
The follicles of the non-cultured control revealed an intact nuclear envelope with a large oocyte nucleus. Moreover, the organelles, particularly the mitochondria and endoplasmic reticulum, were uniformly distributed in the cytoplasm. The granulosa cells were well organized around the oocyte (Fig. 2A). When cultured for seven days in medium with T4/hFSH (20/10ng/mL, Fig 2B), the ultrastructural characteristics of the follicles were similar to the non-cultured control. After that culture, the oocytes showed ultrastructural normality, basal membranes, nuclear envelope, as well as in the large oocyte nucleus (Fig. 2A and 2B, respectively). In addition, the follicles exhibited uniformly distributed organelles in the cytoplasm, particularly the endoplasmic reticulum and the mitochondria. The granulosa cells were normal and well organized around the oocyte, presenting also a large, and elongated nucleus (Fig. 3B).
Ultrastructural analysis of preantral follicles from fresh control (A, 3.000x) cultured for seven days (B. 4.400x) in medium containing T4/hFSH (20/10ng/mL). Note homogeneous cytoplasm, and mitochondria rounded characteristic of the fresh control follicles (A and B). O: oocyte; Nu: oocyte nucleus; GC: granulosa cells; M: mitochondria. (A and B = 5 µm).
Discussion
The addition of hormones and growth factors is essential to the success of in
vitro culture of preantral follicles (PAF) of domestic animals as
demonstrated in the literature (2121 Cortvrindt R., Smitz J. In vitro Follicle Growth:
Achievements in Mammalian Species. Reproduction in Domestic Animals, 2001;
36:3-9.
22 Jewgenow K., Penfold L.M., Meyer H.H.D., Wildt D.E. Viability of small
preantral ovarian follicles from domestic cats after cryoprotectant exposure and
cryopreservation. Journal of Reproduction and Fertility, 1998;
112:39-47.-2323 Wu J., Tian Q. Role of follicle stimulating hormone and epidermal growth
factor in the development of porcine preantral follicle in vitro.
Zygote, 2007; 15(3):233-240.). In this
context, the development of an in vitro culture system is fundamental
for the achievement of a greater number of oocytes included in preantral
follicles(2121 Cortvrindt R., Smitz J. In vitro Follicle Growth:
Achievements in Mammalian Species. Reproduction in Domestic Animals, 2001;
36:3-9.) therefore,
a better comprehension of the different substances that regulate follicular and oocyte
development is fundamentally important for the success of in vitro
culture systems, with the final objective of producing a greater number of viable
embryos.
There are several hormones and growth factors tested in experiments at the present time. It is well known that FSH is outstanding for carrying out an important participation in early folliculogenesis in mammals, in addition to stimulating the survival growth and antrum formation in in vitro culture systems(2424 Cortvrindt R., Smitz J. Van Steirteghem A.C. Assessment of the need for follicle stimulating hormone in early preantral mouse follicle culture in vitro. Human Reproduction, 1997; 12(4):759-768.). Thyroid hormones T3 and T4 demonstrated the capacity to influence cell differentiation and the formation of the follicular antrum in PAF submitted to in vitro culture(2525 Guyton A.C., Hall J.E. Medical Physiology. 11ª ed. Philadelphia: Elsevier Saunders; 2006. 931p.). The association of thyroid hormones with FSH was demonstrated to be effective in in vitro development of preantral follicles of rats(2626 Kobayashi N., Orisaka M., Cao M., Kotsuji F., Leader A., Sakuragi N., Tsang BK. Growth Differentiation Factor-9 Mediates Follicle-Stimulating Hormone-Thyroid Hormone Interaction in the Regulation of Rat Preantral Follicular Development. Endocrinology, 2009; 150(12):5566-5574.), and in antrum formation, and ovulation of sheep PAF cultured in vitro(22 Arunakumari G., Shanmugasundaram N., Rao V.H. Development of morulae from the oocytes of cultured sheep preantral follicles. Theriogenology, 2010; 74:884-894.). This association (hFSH + T4) was also beneficial in the present study because it showed a satisfactory effect by keeping the oocyte diameter after 1 day of culture and promoting the activation of primordial follicles on days 1, 7, 14, 21 or 28 of culture.
When analyzing the survival rate of the cultured preantral follicles in the present work, we noted that the group cultured in the medium T4/hFSH practically maintained the survival rate of the follicles when compared to the α-MEM+ medium without supplementation during the culture for one, seven, 14, 21 or 28 days. These data corroborated those found by Alves(2727 Alves A.M.C.V., Chaves R.N., Rocha R.M.P., Lima L.F., Andrade P.M., Lopes C.A.P., Souza C.E.A., Moura A.A.A., Campello C.C., Báo S.N., Smitz J., and Figueiredo J.R. Dynamic medium containing growth differentiation factor-9 and FSH maintains survival and promotes in vitro growth of caprine preantral follicles after long-term in vitro culture. Reproduction, Fertility and Development, 2012; 25:955-965.), who, using a similar culture medium, maintained the follicular survival for a period of eight days. Apparently, α-MEM+ allows follicular survival considering that it is a compound of amino acids and vitamins, essential for follicular development(2828 Colonna R., Mangia F. Mechanisms of amino acid uptake in cumulusenclosed mouse oocytes. Biology of Reproduction, 1983; 28:797-803.,1111 Rossetto R., Lima-Verd, I.B., Matos M.H.T., Saraiva M.V.A., Martins F.S., Faustino L.R., Araújo V.R., Silva C.M.G., Name K.P.O., Báo S.N., Campello C.C., Figueiredo J.R., Blume H. Interaction between ascorbic acid and follicle-stimulating hormone maintains follicular viability after long-term in vitro culture of caprine preantral follicles. Domestic Animal Endocrinology, 2009; 37:112-123.). Additionally, the enrichment of this medium with the hormones T4 and hFSH may contribute even more to the follicular development and survival, due to the direct effect of these in either the stimulation of granulosa cells and growth factors as in the proliferation of FSH and LH receptors and in follicular maturation(1515 Cecconi S., Rossi G., Coticchio G., Maccharelli G., Borini A., Canipari R. Influence of thyroid hormone on mouse preantral follicle development in vitro. Fertility and Sterility, 2004; 81:919-924.,2929 Rajarajan K., Rao B.S., Vagdevi R., Tamilmani G., Arunakumari G., Sreenu M., Amarnath D., Naik B.R., Rao V.H. Influence of various growth factors on in vitro development of goat preantral follicles. Small Ruminant Research, 2006; 63:204-212.).
During day 7 (D7) of culture, it was possible to observe that T4/hFSH medium was also able to maintain the viability rate of the follicles when compared to the non-cultured control. This situation can be explained due to the latency period of T4 hormone, as it is believed it stays in a latency period of two to three days after its administration, and its action reaches its maximum peak after 10 days of culture(2525 Guyton A.C., Hall J.E. Medical Physiology. 11ª ed. Philadelphia: Elsevier Saunders; 2006. 931p.). Therefore, due to the delay in the activation of this hormone, the positive effect of the association T4/hFSH can be noted only on the 7th day of culture.
However, comparing the culture progression of the media used in our study, the percentage of normal follicles significantly reduced (P<0.05) from D21 to D28, and from D7 to D28 days, in the α-MEM+ and T4/hFSH media, respectively. It should be emphasized that the use of too long culture period has some survival growth limitations, and differentiation of preantral follicles due to the prolonged culture period. However, it has been found that the addition of substances to the medium can contribute to the improvement of the survival rates after the culture. It is believed that, during long culture periods of preantral follicles, the addition of antioxidant substances to the culture medium, such as ascorbic acid, is essential to maintain cellular integrity(1111 Rossetto R., Lima-Verd, I.B., Matos M.H.T., Saraiva M.V.A., Martins F.S., Faustino L.R., Araújo V.R., Silva C.M.G., Name K.P.O., Báo S.N., Campello C.C., Figueiredo J.R., Blume H. Interaction between ascorbic acid and follicle-stimulating hormone maintains follicular viability after long-term in vitro culture of caprine preantral follicles. Domestic Animal Endocrinology, 2009; 37:112-123.). This author also reports that the combination of FSH and ascorbic acid is able to promote a synergetic effect providing a significant increase in oocyte and follicular diameter after seven days of culture. Therefore, the reduction of viable follicles from day 21 to 28 of culture observed in our work could be avoided by adding some substance with a similar effect to the one described.
Moreover, as found in other studies, the low concentration of hFSH used in the present study may have made the follicular survival unviable during a prolonged period of culture. This fact could be previously verified by other researchers, as Silva et al.(1616 Silva J.R.V., Van Den Hurk R., Matos M.H.T., Santos R.R., Pessoa C., Moraes M.O., Figueiredo J.R. Influences of FSH and EGF on primordial follicles during in vitro culture of caprine ovarian cortical tissue. Theriogenology, 2004; 61:1691-1704.), who found no significant effect of FSH on the survival of follicles after 5 days of culture, possibly due to a high concentration of FSH (100ng/mL) used. Conversely, Matos et al.(1414 Matos M.H.T., Lima-Verde I.B., Luque M.C.A., Maia Jr J.E., Silva J.R.V., Celestino J.J.H., Martins F.S., Báo S.N., Lucci C.M., Figueiredo J.R. Essential role of follicle stimulating hormone in the maintenance of caprine preantral follicle viability in vitro. Zygote, 2007; 15:173-182.) maintained follicular survival after seven days of culture with the addition of 50ng/mL of FSH to the culture medium. Therefore, we can evaluate that the concentration of hFSH used in this study was sufficient to maintain the survival until the seventh day of culture; however, it is suggested that after this period this concentration was insufficient, because it was not able to maintain follicular survival in the long duration culture.
Different results obtained using FSH in the culture of preantral follicles can be justified due, especially, to its origin and purity level. Despite the use of FSH from the pituitary extract of domestic species such as pigs (pFSH) and sheep (oFSH), as well as of humans (hFSH) in several studies, it is believed the recombinant FSH (rFSH) produced from the recombinant DNA technique is the purest and most homogeneous form(3030 Magalhães D.M., Fernandes D.D., Araujo V.R., Almeida A.P., Matos M.H.T., Figueiredo J.R. Papel do Hormônio Folículo Estimulante na foliculogênese in vivo e in vitro. Revista Brasileira de Reprodução Animal, 2009; 33(4):171-182.); however, its high cost makes its use in scientific research unviable. Due to the use of hFSH in the present work, the results achieved in the long duration cultures may have been damaged, mainly by its purity level and heterogeneity, that directly interfere in cultures for prolonged periods.
The follicular activation is measured by the ability of differentiation of granulosa cells, resulting in the transition of primordial follicles to intermediate, primary, and secondary follicles(3131 Vitt U.A., Mcgee E.A., Hayashi M., Hsueh A.J.W. In vivo treatment with GDF-9 stimulates primordial and primary follicle progression and theca cell marker CYP17 in ovaries of immature rats. Endocrinology, 2000; 141:3814-3820.); however, the knowledge about the factors and mechanisms involved in the activation of primordial follicles is scarce, mainly because of the difficulty of isolating these follicles(3232 Campbell B.K. The endocrine and local control of ovarian follicle development in the ewe. Animal Reproduction, 2009; 6:159-171.). The evidence of follicular activation in preantral follicles cultured in vitro is noted for the reduction of the number of primordial follicles, and consequent increase of developing follicles along the days of culture(3333 Martins F.S., Celestino J.J.H., Saraiva M.V.A., Chaves R.N., Rossetto R., Silva C.M.G., Lima-Verde I.B., Lopes C.A.P., Campello C.C., Figueiredo J.R. Interaction between growth differentiation factor 9, insulin-like growth factor I and growth hormone on the in vitro development and survival of goat preantral follicles. Brazilian Journal of Medical and Biological Research, 2010; 43:728-736.,33 Saraiva M.V.A., Rossetto R., Brito I.R., Celestino J.J.H., Silva C.M.G., Faustino L.R., Almeida A.P., Bruno J.B., Magalhães D.M., Matos M.H.T., Campello C.C., Figueiredo J.R. Dynamic medium produces caprine embryo from preantral follicles grown in vitro. Reproductive Sciences, 2010; 17:1135-1143.). In all media tested in this study (α-MEM+ and T4/hFSH), there was activation of primordial follicles on days 1, 7, 14, 21 or 28 of culture, except on day 1 of the T4/hFSH medium. This fact can be observed by the reduction of primordial follicles compared to the control; nevertheless, a concomitant increase in developing follicles throughout the days of culture that was expected did not occur, since in all treatment the rates of developing follicles were similar to the non-cultured control. These findings disagree with the ones found by Matos et al.(1414 Matos M.H.T., Lima-Verde I.B., Luque M.C.A., Maia Jr J.E., Silva J.R.V., Celestino J.J.H., Martins F.S., Báo S.N., Lucci C.M., Figueiredo J.R. Essential role of follicle stimulating hormone in the maintenance of caprine preantral follicle viability in vitro. Zygote, 2007; 15:173-182.), who worked with the same concentration of FSH (10 ng/mL), and observed there was not a reduction of primordial follicles and concomitant increase of developing follicles on day 1 of culture; however, at higher concentrations (50 and 100 ng/mL), this authors obtained similar results as ours. The source of FSH used, however, differed between studies, in the work in question porcine FSH was used, different from the one used in our work, human FSH.
In the present study, despite the reduction of primordial follicles during all days of culture, the increase in the developing follicles was not observed on any day of culture, in any researched media; thus, none of the tested media were able to sustain follicular activation initiated by them in primordial follicles on days 1 (α-MEM+ cultured control), 7, 14, 21 or 28 (T4/hFSH and α-MEM+) of culture, mainly by the similarity between the rates of developing follicles to the non-cultured control and the media tested during all days of culture. These results contradict the ones by Matos et al.(1414 Matos M.H.T., Lima-Verde I.B., Luque M.C.A., Maia Jr J.E., Silva J.R.V., Celestino J.J.H., Martins F.S., Báo S.N., Lucci C.M., Figueiredo J.R. Essential role of follicle stimulating hormone in the maintenance of caprine preantral follicle viability in vitro. Zygote, 2007; 15:173-182.), in which, after seven days of culture, follicles maintained their development. Combining our data with follicular survival, herein presented, we suggest that the development of the follicles in question was not continuous after the 1st day of culture due to the rise of their mortality rate.
Despite the considerable anti-apoptotic effect of gonadotropic hormones (LH and FSH(3434 Markström E., Svensson E.C., Shao R., Svanberg B., Billig H. Survival factors regulating ovarian apoptosis - dependence on follicle differentiation. Reproduction, 2002; 123(1):23-30.)) mediated by the action of IGF-I(3535 Chun S.Y., Bilig H., Tilly J.L., Furuta I., Tsafriri A., Hsueh A.J.W. Gonadotropin suppression of apoptosis in cultured preovulatory follicles: mediatory role of endogenous insulin-like growth factor I. Endocrinology, 1994; 135:1845-1853.), in our study, it was not possible to observe their activation and development after day 1 of culture, reflecting in high mortality rates in the tested media, and similarity between the developing follicles to the non-cultured control and α-MEM+ and T4/hFSH media. This may have occurred due to the gaseous atmosphere of the culture kiln by not providing a suitable environment for the culture of the follicles. Furthermore, it is possible the proposed media were not totally sufficient to supplement and sustain the development of the follicles, since the growth factors and hormones are fundamental for the development of the follicle in each stage of its development and occasionally small differences of concentrations or synergism between the two hormones can negatively contribute to follicular survival.
In relation to the diameter of the oocyte and the follicle, none of the tested media were capable of maintaining the growth of the follicles through the culture, except for α-MEM+ on day 1 of culture, and T4/hFSH on days 1 and 7 of culture. In contrast, studies have already proved the addition of FSH to the culture medium was able to promote the increase of preantral follicles cultured in vitro(3636 Itoh T., Kacchi M., Abe H., Sendai Y., Hoshi H. Growth, antrum formation, and estradiol production of bovine preantral follicles cultured in a serum-free medium. Biology of Reproduction, 2002; 67:1099-1105.,1414 Matos M.H.T., Lima-Verde I.B., Luque M.C.A., Maia Jr J.E., Silva J.R.V., Celestino J.J.H., Martins F.S., Báo S.N., Lucci C.M., Figueiredo J.R. Essential role of follicle stimulating hormone in the maintenance of caprine preantral follicle viability in vitro. Zygote, 2007; 15:173-182.,22 Arunakumari G., Shanmugasundaram N., Rao V.H. Development of morulae from the oocytes of cultured sheep preantral follicles. Theriogenology, 2010; 74:884-894.). In the presence of 10 and 50 ng/mL of FSH, Matos et al.(1414 Matos M.H.T., Lima-Verde I.B., Luque M.C.A., Maia Jr J.E., Silva J.R.V., Celestino J.J.H., Martins F.S., Báo S.N., Lucci C.M., Figueiredo J.R. Essential role of follicle stimulating hormone in the maintenance of caprine preantral follicle viability in vitro. Zygote, 2007; 15:173-182.) observed an increase in the diameter of both the oocyte and the caprine follicle on day 7 of culture. Itoh et al.(3636 Itoh T., Kacchi M., Abe H., Sendai Y., Hoshi H. Growth, antrum formation, and estradiol production of bovine preantral follicles cultured in a serum-free medium. Biology of Reproduction, 2002; 67:1099-1105.) also demonstrated that 50ng/mL of FSH increased both the diameter of the oocyte, and of bovine follicles cultured for 13 days. These authors are based on the concept of the existence of FSH receptors during the progressive development of PAF, in both the transition from primordial to primary follicles, and to secondary follicles(3737 Oktay K., Briggs D., Gosden R.G. Ontogeny of follicle-stimulating hormone receptor gene expression in isolated human ovarian follicles. The Journal of Clinical Endocrinology and Metabolism, 1997; 82:3748-3751.); therefore, it is possible that FSH acts on both cell types to promote growth, and follicular development(3838 M'eduri G., Charnaux N., Driancourt M.A., Combettes L., Granet P., Vannier B., Loosfelt H., Migrom E. Follicle-stimulating hormone receptors in oocytes? The Journal of Clinical Endocrinology and Metabolism, 2002; 87:2266-2276.).
In agreement with these authors, Arunakumari et al.(22 Arunakumari G., Shanmugasundaram N., Rao V.H. Development of morulae from the oocytes of cultured sheep preantral follicles. Theriogenology, 2010; 74:884-894.) reported an increase in the diameter in sheep PAF but using other growth inducers as IGF-I and GH and also the combination of FSH + T4. However, it is not always that the T4/FSH association can be considered capable of stimulating the development of caprine PAF. Wongbandue et al.(3939 Wongbandue G., Jewgenow K., Chatdarong K. Effects of thyroxin (T4) and activin A on in vitro growth of preantral follicles in domestic cats. Theriogenology, 2013; 79:824-832.) also did not achieve satisfactory results with the addition of T4, but they used the hormone at the concentrations of 0.5, 1.0 and 2.0 µg/mL added in base culture medium M199 supplemented with 2.13 µg/mL of FSH, besides growth factors and antibiotics. At none of these concentrations there was a difference or gain in the diameter in feline follicles cultured for 0, 3, 7 and 14 days. This study agrees with ours, and the most coherent explanation is that the expression of T4 receptors in granulosa cells possibly develops in stages of follicular development, different from what was used in ours study. Although follicular diameter did not increase in our work through the culture, it did not present a reduction on days 1 and 7 of culture of T4/hFSH medium, if it happened indeed it could indicate a process of atresia. Thus, the observation of the similarity of the diameter in relation to the non-cultured control group greatly showed the viability of the medium tested in the initial period of culture.
Ultimately, we used TEM for a better ultrastructural evaluation of the follicles quality. This tool has been used in several studies to prove the existence of possible ultrastructural damage caused by culture systems that may not be morphologically perceptible(4040 Van Den Hurk R., Spek E.R., Hage W.J., Fair T., Ralph J.H., Schotanus K. Ultrastructure and viability of isolated bovine preantral follicles. Human Reproduction Update, 1998; 4:833-841.,4141 Zhao J., Dorland M., Taverne M.A.M., Van Der Weijden G.C., Bevers M.M., Van Den Hurk R. In vitro culture of rat pre-antral follicles with emphasis on follicular interactions. Molecular Reproduction and Development, 2000; 55:65-74.,4242 Salehnia M., Moghadam E.A., Velojerdi M.R. Ultrastructure of follicles after vitrification of mouse ovarian tissue. Fertility and Sterility, 2002; 78:644-645.). In the present study, the ultrastructural features of the follicles cultured in the presence of hFSH and T4 for seven days were maintained similar to the non-cultured control. The cellular structures, such as endoplasmic reticulum and mitochondria, were evenly distributed in the cytoplasm and the granulosa cells were ultrastructurally normal, and well organized around the oocyte. Other studies also demonstrate that FSH maintains the ultrastructure of caprine PAF after the in vitro culture for seven days(1414 Matos M.H.T., Lima-Verde I.B., Luque M.C.A., Maia Jr J.E., Silva J.R.V., Celestino J.J.H., Martins F.S., Báo S.N., Lucci C.M., Figueiredo J.R. Essential role of follicle stimulating hormone in the maintenance of caprine preantral follicle viability in vitro. Zygote, 2007; 15:173-182.). Matos et al.(1414 Matos M.H.T., Lima-Verde I.B., Luque M.C.A., Maia Jr J.E., Silva J.R.V., Celestino J.J.H., Martins F.S., Báo S.N., Lucci C.M., Figueiredo J.R. Essential role of follicle stimulating hormone in the maintenance of caprine preantral follicle viability in vitro. Zygote, 2007; 15:173-182.) also verified that the ultrastructural integrity of the follicles was maintained after 1 day of culture in α-MEM+ medium, and after 7 days in α-MEM+ + FSH (50ng/mL). On the other hand, Rossetto et al.(1111 Rossetto R., Lima-Verd, I.B., Matos M.H.T., Saraiva M.V.A., Martins F.S., Faustino L.R., Araújo V.R., Silva C.M.G., Name K.P.O., Báo S.N., Campello C.C., Figueiredo J.R., Blume H. Interaction between ascorbic acid and follicle-stimulating hormone maintains follicular viability after long-term in vitro culture of caprine preantral follicles. Domestic Animal Endocrinology, 2009; 37:112-123.) demonstrated the integrity of follicles cultured after 14 days in media containing ascorbic acid and FSH.
In the case of a culture for a prolonged time, the ultrastructural integrity of PAF was also observed by Gutierrez et al.(4343 Gutierrez C.G., Ralph J.H., Telfer E.E., Wilmut I., Webb R. Growth and antrum formation of bovine preantral follicles in long-term culture in vitro. Biology of Reproduction, 2000; 62:1322-1328.), who evaluated culture media for bovine PAF with FSH, EGF, and IGF-I for 28 days. In this system, most follicles maintained their morphology throughout the culture, in the presence of a layer of theca, and basement membrane surrounding the granulosa cells. Moreover, these authors also observed antrum formation, which occurred between the days 10 and 28 of culture. However, because our study was conducted with other species, the chronological comparison of these events is not possible.
Given the previously presented and discussed results, we noted that, although the medium tested in this experiment T4(20)/hFSH(10) has not provided a substantial growth in the follicular and oocyte diameter, it did not impair the activation and viability of caprine preantral follicles cultured in vitro for a period of seven days. The synergistic effect between T4 and FSH can be explained by the action of thyroid hormones in the amplification of FSH effect on the differentiation of granulosa cells, fact demonstrated by Maruo et al.(4444 Maruo T., Hayashi M., Matsuo H., Yamamoto T., Okada H., Mochizuki M. The role of thyroid hormone as a biological amplifier of the actions of follicle-stimulating hormone in the functional differentiation of cultured porcine granulosa cells. Endocrinology, 1987; 121:1233-1241.) and experimentally proved later by Arunakumari et al.(22 Arunakumari G., Shanmugasundaram N., Rao V.H. Development of morulae from the oocytes of cultured sheep preantral follicles. Theriogenology, 2010; 74:884-894.). The action of thyroid hormones on the expression of mRNA of FSH receptors in granulosa cells is still very controversial and needs to be studied and defined more precisely.
Conclusion
The medium tested in this experiment T4(20)/hFSH(10) is able to maintain the survival, follicular activation, and ultrastructural integrity of preantral follicles of goat cultured in vitro for a period of up to seven days, which validates and enables the use of the medium tested in culture systems for this species.
Acknowledgments
To CNPq for the financial support to the research. To CAPES for providing the scholarship to the Doctorate student. To the Nucleous of Microscopy and Microanalysis of UFV for the support in ultrastructural analysis.
References
-
1Telfer E.E., Mclaughlin M., Ding C., Thong K.J. A two-step serum-free culture system supports development of human oocytes from primordial follicles in the presence of activin. Human Reproduction, 2008; 23(5):1151-1158.
-
2Arunakumari G., Shanmugasundaram N., Rao V.H. Development of morulae from the oocytes of cultured sheep preantral follicles. Theriogenology, 2010; 74:884-894.
-
3Saraiva M.V.A., Rossetto R., Brito I.R., Celestino J.J.H., Silva C.M.G., Faustino L.R., Almeida A.P., Bruno J.B., Magalhães D.M., Matos M.H.T., Campello C.C., Figueiredo J.R. Dynamic medium produces caprine embryo from preantral follicles grown in vitro Reproductive Sciences, 2010; 17:1135-1143.
-
4Peng X., Yang M., Wang L., Tong C., Guo Z. In vitro culture of sheep lamb ovarian cortical tissue in a sequential culture medium. The Journal of Assisted Reproduction and Genetics, 2010; 27(5):247-257.
-
5Skinner M.K. Regulation of primordial follicle assembly and development. Human Reproduction Update, 2005; 11:461-471.
-
6Carroll J.D.G., Whittingham M.J., Wood E., Telfer R.G. Gosden. Extraovarian production of mature viable mouse oocytes from frozen primary follicles. Journal Reproduction and Fertility, 1990; 90:321-327.
-
7O'Brien M.J., Pendola J.K., Eppig J.J. A revised protocol for in vitro development of mouse oocyte from primordial follicles dramatically improves their development competence. Biology of Reproduction, 2003; 68:1682-1686.
-
8Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocrine Reviews, 1996; 17:121-154.
-
9Muruvi W., Picton H.M., Rodway R.G., Joyce I.M. In vitro growth of oocytes from primordial follicles isolated from frozen-thawed lamb ovaries. Theriogenology, 2005; 64:1357-1370.
-
10Sadeu J.C., Cortvrindt R., Ron-El R., Kasterstein E., Smitz J. Morphological and ultrastructural evaluation of cultured frozen-thawed human fetal ovarian tissue. Fertility and Sterility, 2006; 85:1130-1141.
-
11Rossetto R., Lima-Verd, I.B., Matos M.H.T., Saraiva M.V.A., Martins F.S., Faustino L.R., Araújo V.R., Silva C.M.G., Name K.P.O., Báo S.N., Campello C.C., Figueiredo J.R., Blume H. Interaction between ascorbic acid and follicle-stimulating hormone maintains follicular viability after long-term in vitro culture of caprine preantral follicles. Domestic Animal Endocrinology, 2009; 37:112-123.
-
12Gutierrez C.G., Campbell B.K., Webb R. Development of a long-term bovine granulosa cell culture system: induction and maintenance of estradiol production, response to follicle stimulating hormone and morphological characteristics. Biology of Reproduction, 1997; 56:608-616.
-
13Hillier S.G. Gonadotropic control of ovarian follicular growth and development. Molecular and Cellular Endocrinology, 2001; 179:39-46.
-
14Matos M.H.T., Lima-Verde I.B., Luque M.C.A., Maia Jr J.E., Silva J.R.V., Celestino J.J.H., Martins F.S., Báo S.N., Lucci C.M., Figueiredo J.R. Essential role of follicle stimulating hormone in the maintenance of caprine preantral follicle viability in vitro Zygote, 2007; 15:173-182.
-
15Cecconi S., Rossi G., Coticchio G., Maccharelli G., Borini A., Canipari R. Influence of thyroid hormone on mouse preantral follicle development in vitro Fertility and Sterility, 2004; 81:919-924.
-
16Silva J.R.V., Van Den Hurk R., Matos M.H.T., Santos R.R., Pessoa C., Moraes M.O., Figueiredo J.R. Influences of FSH and EGF on primordial follicles during in vitro culture of caprine ovarian cortical tissue. Theriogenology, 2004; 61:1691-1704.
-
17Serakides R., Nunes V.A., Nascimento E., Ribeiro A.F.C., Silva C.M. Foliculogênese e esteroidogênese ovarianas em ratas adultas hipertireóideas. Arquivo Brasileiro de Endocrinologia & Metabologia, 2001; 45:258-264.
-
18Asahara S., Sato A., Ali Aljonaid A., Maruo T. Thyroid Hormone Synergizes with Follicle Stimulating Hormone to Inhibit Apoptosis in Porcine Granulosa Cells Selectively from Small Follicles. Kobe Journal of Medical Sciences, 2003; 49:107-116.
-
19Celestino J.J.H., Bruno J.B., Lima-Verde I.B., Matos M.H., Saraiva M.V., Chaves R.N., Martins F.S., Almeida A.P., Cunha R.M., Lima L.F., Name K.P., Campello C.C., Silva J.R., Báo S.N., Figueiredo J.R. Steadystate level of kit ligand mRNA in goat ovaries and the role of kit ligand in preantral follicle survival and growth in vitro Molecular Reproduction and Development, 2010; 77(3):231-40.
-
20Donato M.A.M., Saraiva K.L.A., Silva A.K.S., Wanderley M.I., Peixoto C.A. Follicle development and luteal cell morphology altered by phosphodiesterase-5 inhibitor. Micron, 2009; 40:845-850.
-
21Cortvrindt R., Smitz J. In vitro Follicle Growth: Achievements in Mammalian Species. Reproduction in Domestic Animals, 2001; 36:3-9.
-
22Jewgenow K., Penfold L.M., Meyer H.H.D., Wildt D.E. Viability of small preantral ovarian follicles from domestic cats after cryoprotectant exposure and cryopreservation. Journal of Reproduction and Fertility, 1998; 112:39-47.
-
23Wu J., Tian Q. Role of follicle stimulating hormone and epidermal growth factor in the development of porcine preantral follicle in vitro Zygote, 2007; 15(3):233-240.
-
24Cortvrindt R., Smitz J. Van Steirteghem A.C. Assessment of the need for follicle stimulating hormone in early preantral mouse follicle culture in vitro Human Reproduction, 1997; 12(4):759-768.
-
25Guyton A.C., Hall J.E. Medical Physiology. 11ª ed. Philadelphia: Elsevier Saunders; 2006. 931p.
-
26Kobayashi N., Orisaka M., Cao M., Kotsuji F., Leader A., Sakuragi N., Tsang BK. Growth Differentiation Factor-9 Mediates Follicle-Stimulating Hormone-Thyroid Hormone Interaction in the Regulation of Rat Preantral Follicular Development. Endocrinology, 2009; 150(12):5566-5574.
-
27Alves A.M.C.V., Chaves R.N., Rocha R.M.P., Lima L.F., Andrade P.M., Lopes C.A.P., Souza C.E.A., Moura A.A.A., Campello C.C., Báo S.N., Smitz J., and Figueiredo J.R. Dynamic medium containing growth differentiation factor-9 and FSH maintains survival and promotes in vitro growth of caprine preantral follicles after long-term in vitro culture. Reproduction, Fertility and Development, 2012; 25:955-965.
-
28Colonna R., Mangia F. Mechanisms of amino acid uptake in cumulusenclosed mouse oocytes. Biology of Reproduction, 1983; 28:797-803.
-
29Rajarajan K., Rao B.S., Vagdevi R., Tamilmani G., Arunakumari G., Sreenu M., Amarnath D., Naik B.R., Rao V.H. Influence of various growth factors on in vitro development of goat preantral follicles. Small Ruminant Research, 2006; 63:204-212.
-
30Magalhães D.M., Fernandes D.D., Araujo V.R., Almeida A.P., Matos M.H.T., Figueiredo J.R. Papel do Hormônio Folículo Estimulante na foliculogênese in vivo e in vitro Revista Brasileira de Reprodução Animal, 2009; 33(4):171-182.
-
31Vitt U.A., Mcgee E.A., Hayashi M., Hsueh A.J.W. In vivo treatment with GDF-9 stimulates primordial and primary follicle progression and theca cell marker CYP17 in ovaries of immature rats. Endocrinology, 2000; 141:3814-3820.
-
32Campbell B.K. The endocrine and local control of ovarian follicle development in the ewe. Animal Reproduction, 2009; 6:159-171.
-
33Martins F.S., Celestino J.J.H., Saraiva M.V.A., Chaves R.N., Rossetto R., Silva C.M.G., Lima-Verde I.B., Lopes C.A.P., Campello C.C., Figueiredo J.R. Interaction between growth differentiation factor 9, insulin-like growth factor I and growth hormone on the in vitro development and survival of goat preantral follicles. Brazilian Journal of Medical and Biological Research, 2010; 43:728-736.
-
34Markström E., Svensson E.C., Shao R., Svanberg B., Billig H. Survival factors regulating ovarian apoptosis - dependence on follicle differentiation. Reproduction, 2002; 123(1):23-30.
-
35Chun S.Y., Bilig H., Tilly J.L., Furuta I., Tsafriri A., Hsueh A.J.W. Gonadotropin suppression of apoptosis in cultured preovulatory follicles: mediatory role of endogenous insulin-like growth factor I. Endocrinology, 1994; 135:1845-1853.
-
36Itoh T., Kacchi M., Abe H., Sendai Y., Hoshi H. Growth, antrum formation, and estradiol production of bovine preantral follicles cultured in a serum-free medium. Biology of Reproduction, 2002; 67:1099-1105.
-
37Oktay K., Briggs D., Gosden R.G. Ontogeny of follicle-stimulating hormone receptor gene expression in isolated human ovarian follicles. The Journal of Clinical Endocrinology and Metabolism, 1997; 82:3748-3751.
-
38M'eduri G., Charnaux N., Driancourt M.A., Combettes L., Granet P., Vannier B., Loosfelt H., Migrom E. Follicle-stimulating hormone receptors in oocytes? The Journal of Clinical Endocrinology and Metabolism, 2002; 87:2266-2276.
-
39Wongbandue G., Jewgenow K., Chatdarong K. Effects of thyroxin (T4) and activin A on in vitro growth of preantral follicles in domestic cats. Theriogenology, 2013; 79:824-832.
-
40Van Den Hurk R., Spek E.R., Hage W.J., Fair T., Ralph J.H., Schotanus K. Ultrastructure and viability of isolated bovine preantral follicles. Human Reproduction Update, 1998; 4:833-841.
-
41Zhao J., Dorland M., Taverne M.A.M., Van Der Weijden G.C., Bevers M.M., Van Den Hurk R. In vitro culture of rat pre-antral follicles with emphasis on follicular interactions. Molecular Reproduction and Development, 2000; 55:65-74.
-
42Salehnia M., Moghadam E.A., Velojerdi M.R. Ultrastructure of follicles after vitrification of mouse ovarian tissue. Fertility and Sterility, 2002; 78:644-645.
-
43Gutierrez C.G., Ralph J.H., Telfer E.E., Wilmut I., Webb R. Growth and antrum formation of bovine preantral follicles in long-term culture in vitro Biology of Reproduction, 2000; 62:1322-1328.
-
44Maruo T., Hayashi M., Matsuo H., Yamamoto T., Okada H., Mochizuki M. The role of thyroid hormone as a biological amplifier of the actions of follicle-stimulating hormone in the functional differentiation of cultured porcine granulosa cells. Endocrinology, 1987; 121:1233-1241.
Publication Dates
-
Publication in this collection
Apr-Jun 2015
History
-
Received
14 Aug 2014 -
Accepted
23 Feb 2015