Abstract
The venographic examination allows diagnosing and treating foot diseases. It can be the basis of studies about the administration of medication in the hoof region. The aim of the study was to describe the venographic pattern in the distal region of the forelimb and hindlimbs of healthy sheep and goats, as well as to compare vascularization between them. Ten clinically healthy sheep and goats, five male and five female of different breeds, aged 2 to 4 years and weighing 45 to 63 kg were used. The forelimbs and hindlimbs were subjected to contrast venography of the distal region. The venograms were taken in a digital X -ray machine and analyzed in the EcoView® software. The veins of the metacarpus and metatarsus; and the proximal, middle and distal phalanges of the forelimbs and hindlimbs of sheep and goats were identified 25-30 seconds after contrast injection. The technique error observed during the venography was the contrast perivascular extravasation. The venograms showed the vascularization in the distal region of forelimbs and hindlimbs and the communication of vessels between them. The better radiographic view to evaluate the vessels was the lateral projection. The normal venous pattern of the distal region of forelimbs and hindlimbs of sheep and goats could be observed by venography.
Key words:
digit; venogram; ruminants; X - ray; limbs
Resumo
O exame venográfico permite diagnosticar e tratar afecções da região podal. Pode ser base de estudos sobre a administração de medicação na região do casco. O objetivo do estudo foi descrever o padrão venográfico na região distal dos membros torácicos e pélvicos de ovinos e caprinos saudáveis, bem como comparar a vascularização entre eles. Foram utilizados 10 ovinos e caprinos clinicamente saudáveis, cinco machos e cinco fêmeas de raças diferentes, com idade entre 2 a 4 anos e pesando 45 a 63 kg. Os membros torácicos e pélvicos foram submetidos à venografia contrastada da região distal. Os venogramas foram coletados por um aparelho radiográfico digital e analisados no software EcoView®. As veias da região do metacarpo e metatarso; as falanges proximal, medial e distal dos membros torácicos e pélvicos dos ovinos e caprinos foram identificadas 25-30 segundos após a injeção do contraste. O erro de técnica observado durante a venografia foi o extravasamento perivascular do contraste. Os venogramas revelaram a vascularização na região distal dos membros torácicos e pélvicos e a comunicação dos vasos entre eles. A projeção lateral foi a melhor projeção radiográfica para avaliar os vasos no venograma. O padrão venoso normal da região distal dos membros torácicos e pélvicos de ovinos e caprinos pode ser observado pela venografia.
Palavras-chave:
dígito; venograma; ruminantes; raios-X; membros
Introduction
Sheep and goats had their origin thousands of years ago, and their migratory route followed the human civilization worldwide (11 Egito AA. Programa brasileiro de conservação de recursos genéticos animais. Arq Zool. 2002; 51(50):193-194.). These animals have been adapting and it has resulted in different characteristics in their population (11 Egito AA. Programa brasileiro de conservação de recursos genéticos animais. Arq Zool. 2002; 51(50):193-194.,22 Mariante AS, Cavalcante N. Recursos genéticos animais. Animais do descobrimento, raças domésticas da história do Brasil. Ministério da Agricultura e Abastecimento. Brasília. 2000; 192-204.). They represent an important food source for local families in Northeastern Brazil, as well as play an important role in this region's economy(33 Vasconcelos VR, Vieira LS. A evolução da caprino-ovinocultura brasileira. Embrapa. 2005; 65-69.,44 Sousa WH. O agronegócio da caprinocultura de corte no Brasil. Rev Tecnol Ciên Agropec. 2007; 1(1):51-58.).
Angiography has been used in studies concerning to vascular foot diseases in ruminants (55 Burns J, Cornell C. Angiography of the caprine digit. Vet Radiol Ultrasound. 1981; 22(4):174-176.). This tool can be adopted to treat and to help assessing vascular foot diseases (55 Burns J, Cornell C. Angiography of the caprine digit. Vet Radiol Ultrasound. 1981; 22(4):174-176.). Sheep and goats are often affected by several foot diseases associated with the breed care and with environmental conditions (66 Madruga MS. Qualidade química, sensorial e aromática da carne caprina e ovina: mitos e verdades. Encontro Nacional para o Desenvolvimento da Espécie Caprina. Botucatu. 2004; 215-234.). Studies regarding contrast venography in vivo and in situ, as well anatomical studies concerning the distal region of the limbs in bovine and equine have been conducted (77 Ackerman N, Garner HE, Coffman JR, Clement JW. Angiographic appearance of the normal equine foot and alternations in chronic laminitis. JAVMA. 1975; 66:58-62.
8 Nigam JM, Singh AP. Radiography of bovine foot disorders. Med Vet Prac. 1981; 61:621-624.
9 Boosman R, Nemeth F, Gruys F, Klarenbeek A. Arteriographical and pathological changes in chronic laminitis in dairy cattle. Vet Quart. 1989; 11(3):144-155.
10 Singh SS, Ward WR, Murray RD. An angiographic evaluation of vascular changes in sole lesions in the hooves of cattle. Br Vet J. 1994; 150: 41-52.
11 Rebhun WC, Pearson EG. Clinical management of bovine foot problems. JAVMA. 1995; 180(6):1464-1467.
12 Cripps PJ, Eustace RA. Radiological measurements from the feet of normal horses with relevance to laminitis. Equine Vet J. 1999 ; 31:427-432.
13 Redden RF. Possible therapeutic value of digital venography in two laminitic horses. Equine Vet Educ. 2001; 13:128-134.
14 Arthur EG, Rucker A. The use of digital venography for assessment of perfusion deficits in chronic laminitis. Palm Beach. 2003; 319-320.
15 Rucker A, Redden RF, Arthur EG, Reed SK, Hill BW, et al. How to perform the digital venogram. AAEP. 2006; 52:526-530.
16 Lyle BE.. Venography as a tool for guiding surgery to the foot. In: Floyd AD, Mansmann RA. Equine podiatry. St Louis (MO): Saunders. 2007; 284-293.
17 Brunner CHM, Martins MFM, Bovino EE. Angiografia in vivo para avaliação da vascularização do casco de equinos. Ciênc Rural. 2008; 38(1):116-123.
18 Redde RF. Using venograms in laminitic cases. Redden´s in-deph Podiatry Symposium. Versailles (KY). 2009; 81-83.
19 Rucker A. Clinical applications of digital venography. J Equine Vet Sci. 2010; 30(9):491-503.
20 Hunt RJ; Wharton RE. Clinical presentation, diagnosis, and prognosis of chronic laminitis in North America. Vet Clin North Am. 2010; 26:141-154.
21 Baldwin GI, Pollitt CC. Progression of venographic changes after experimentally induced laminitis. Vet Clin North Am. 2010; 26:135-140.
22 D'Arpe L, Bernardini D. Digital Venography in Horses and Its Clinical Application in Europe. Vet Clin Equine. 2010; 26:349-359.-2323 Rafael LA, Pyles MD, Rodrigues M, Rodrigues CA. Venograma digital para avaliação de laminite crônica em um bovino. VII amostra Científica de Ciências Agrárias. Botucatu. 2011; 23-24.). However, further studies using contrast venography in small ruminants still lack in the literature. The present study aimed to describe the venographic vascular pattern in the distal region of the forelimb and hindlimbs of healthy sheep and goats in order to contribute to further anatomical studies, to the clinical medicine and to compare the vascularization between the limbs and species.
Material and Methods
This study was approved by the Ethics Committee of our Veterinary School (nº. 065/2011-CEUA). The study was carried out between April 2013 and June 2014. Ten clinically healthy sheep and goats, five male and five female of each species, varied breeds, aged 2 to 4 years (mean age 3 years ± 1 SD) weighing 45 to 63 kg (mean weight 54 kg ± 9 SD) were used. The animals were previously dewormed and fed on balanced food (grains and hay). Five animals belonging to each species were housed in 5 x 4 meter stalls. The study was performed in the winter season, between May and August months. The GPS study location was latitude -22.8989º, and longitude -48.4881º.
All the animals were assumed to be healthy after complete physical and hoof examinations. Exclusion criteria included use of medications or lameness. Blood samples (5 mL) were collected from the jugular vein and a complete blood count (CBC) and serum biochemical analyses (blood urea nitrogen, alkaline phosphatase, aspartate aminotransferase, gamma glutamyl transferase) were performed before the venograms.
The venograms were taken at 8 a.m., in a room under controlled temperature (22º C), after 6-hours fasting. The animals' hooves and digits were washed and trimmed; no iodine antiseptic was scrapped from the hooves. The metacarpal and metatarsal regions were clipped and the skin was aseptically prepared using 2% chlorhexidine gluconate (Rioquimica®, Campinas, São Paulo, Brazil). All animals was tranquillized with 0.3 mg/kg (jugular intravenous) of diazepam (Labesfal®, Labesfal, São Paulo, Brazil), and was not used any anesthetic block on the foot.
The sheep and the goats were positioned in lateral recumbency during the venography and the restrain was conducted by two people. Loops were used in the animals' limbs, except for the limb to be subjected to venography. A rubber tourniquet was tied proximal to the metacarpus and the metatarsus regions. The venopunction was performed using a scalp vein 21G (Embramac®, São Paulo, Brazil) in anterograde direction; and were performed on deep branch of radial vein in medial view in the forelimbs of goats and sheeps (2424 Santos IFC, Hussni CA, Rodrigues CA, Watanabe MJ, Alves ALG, Charlier M.. Técnica venográfica contrastada in vivo dos dígitos de ovinos e caprinos. Arq. Bras. Med. Vet. Zootec. 2015; 67(6):1630-1638.). The dorsal pedis vein was used in the sheep's hindlimbs, whereas the deep branch of the dorsal metatarsal III vein was used in the goats' hindlimbs (2424 Santos IFC, Hussni CA, Rodrigues CA, Watanabe MJ, Alves ALG, Charlier M.. Técnica venográfica contrastada in vivo dos dígitos de ovinos e caprinos. Arq. Bras. Med. Vet. Zootec. 2015; 67(6):1630-1638.).
The radiographic images were acquired through digital radiography machine (100 kVp/45 mAs) (Vatech®, Gnatus, Ribeirão Preto, São Paulo, Brazil) and the radiographic exposure settings were conducted using a beam energy of 60 kVp/5 mAs, and 70 cm film focus distance. The distal region of the forelimbs and hindlimbs were centered over a digital X-ray cassette.
The X-rays were performed 25 to 30 seconds after the administration of 10 mL of diatrizoate meglumine (Reliev®, Justesa Image of Brazil, São Paulo, Brazil), according to Santos (2424 Santos IFC, Hussni CA, Rodrigues CA, Watanabe MJ, Alves ALG, Charlier M.. Técnica venográfica contrastada in vivo dos dígitos de ovinos e caprinos. Arq. Bras. Med. Vet. Zootec. 2015; 67(6):1630-1638.), using a 10 mL hand-held leur-lock syringe attached to the distal end of the scalp tubing. The contrast agent was slowly administered in constant speed. The scalp was removed from the vein immediately after the contrast was administered. The X-rays were performed in lateral, dorsopalmar, dorsoplantar, plantodorsal and palmorodorsal projections. The venograms were performed with 2-week intervals for each limb. The animals that observed hematoma at the site of venopunction were excluded from the study for 3-weeks. The venograms which showed full contrast-filled of the distal veins of the metacarpus and metatarsus, and the proximal, middle and distal phalanges were analyzed using the EcoView® software. The veins were identified according to Popesko (2525 Popesko P. Atlas of topographical anatomy of the domestic animals. Philadelphia: W. B. Saunders. 1971; 84-99.), Schummer (2626 Schummer A. Kreislaufsystem Haut und Hautorgane. Anatomie der Haustiere. Band III. Berlin: Verlag Paul Parey. 1976; 211-227.) and Schaller (2727 Schaller O.. Nomenclatura anatômica veterinárira. Zaragoza: Acribia. 1996; 614-615.).
Results
The veins of the metacarpus and metatarsus; and the proximal, middle and distal phalanges of the forelimbs and hindlimbs of sheep and goats were fill-in with contrast in 25-30 seconds after contrast injection. The contrast agent remained in the veins for 30-35 seconds.
All animals showed limb retraction at the time of contrast injection. Hematoma was observed in 20% (2/10) of the sheep´s limbs and in 10% (1/10) of the goats' limbs. No wound breakdown or infection was observed in them.
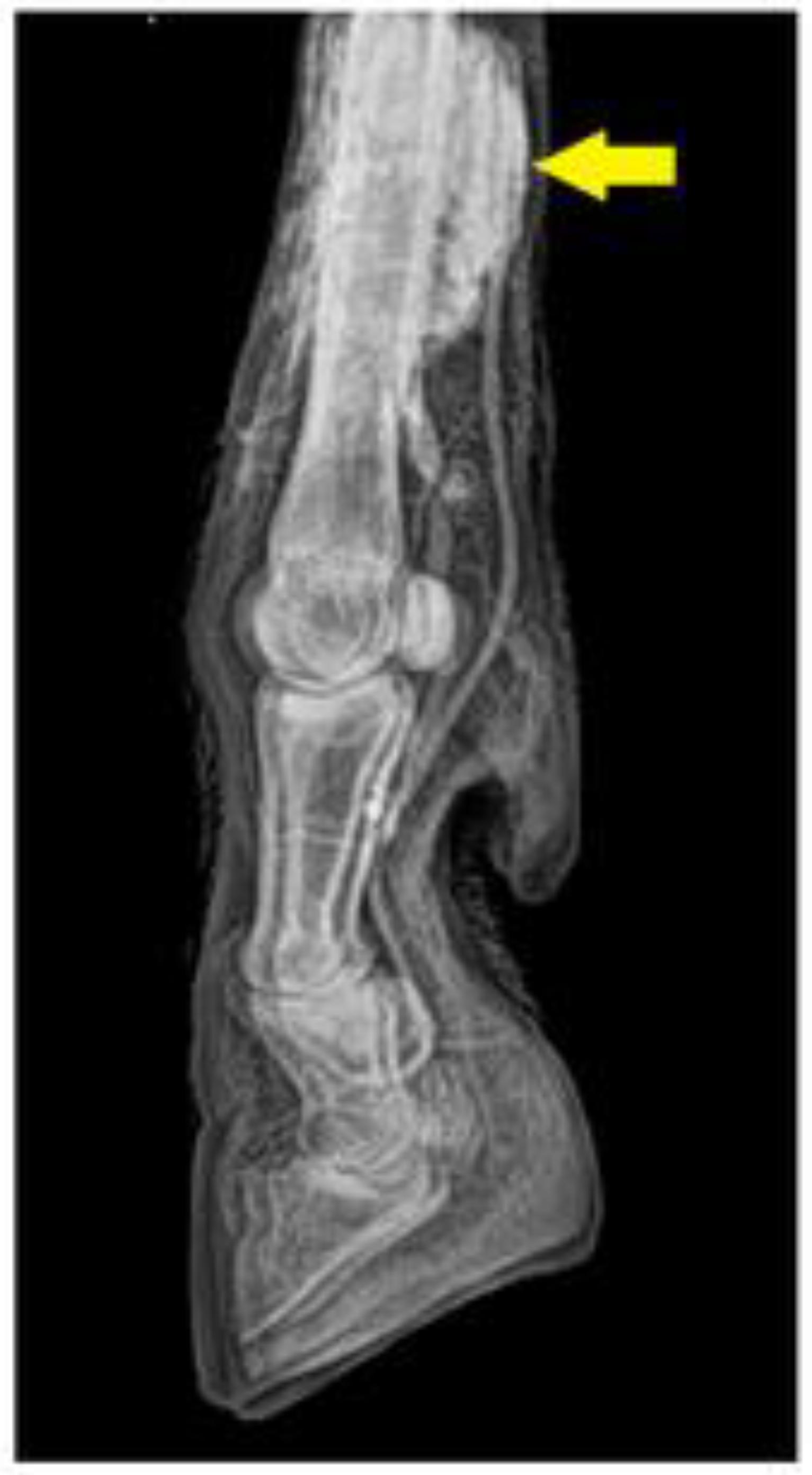
The technique error observed during the venography was contrast perivascular extravasation (Figure 1). The venography of the distal region of the sheep and goats' forelimbs are shown in figures 2 and 3; and the venography from the distal region of the sheep and goats' hindlimbs are shown in figures 4 and 5.
Digital venography of the distal region of left forelimb of sheep (lateral view) showing contrast in the perivascular space (yellow anow).
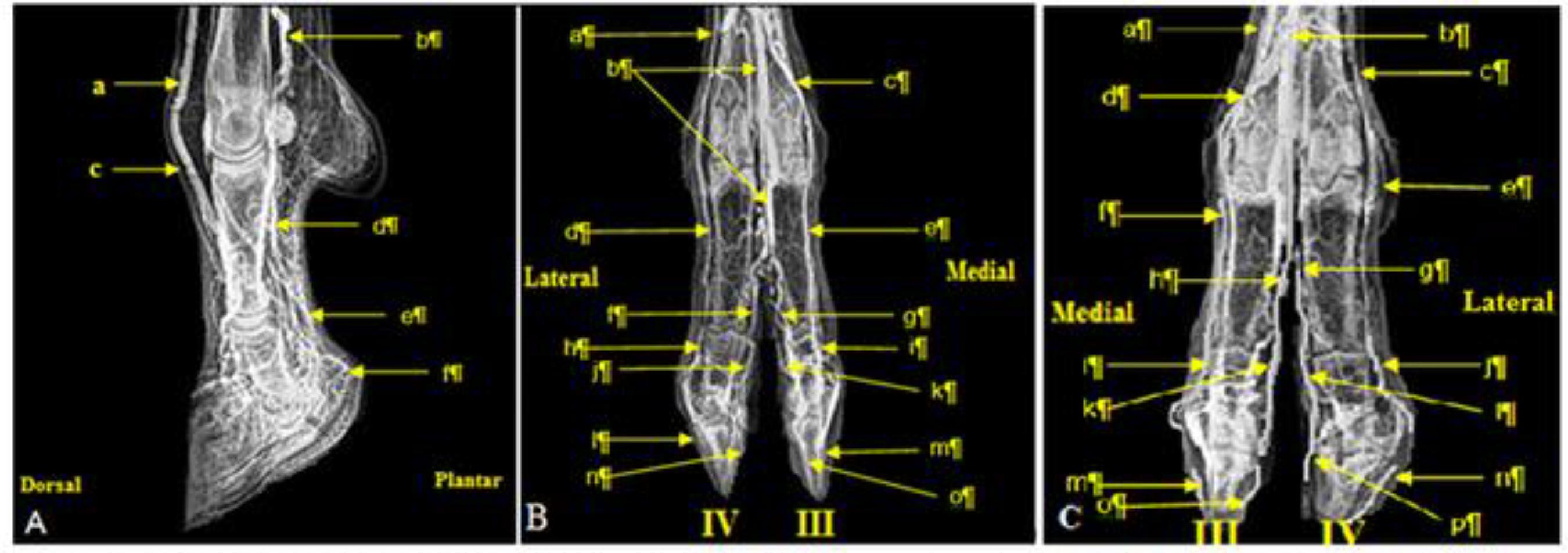
Venograms of the distal region of metacarpus: and proximal, middle and distal phalanges, in right (A and B) and left (C) forelimbs of sheep (Digital X-ray: 60 kVp; 5 mAs).
A - Lateral view: a - Vena digitalis palmaris communis II; a' - Vena digitalis palmaris communis III; a" - Vena digitalis dorsalis lateralis IV; b - Vena digitalis palmaris propria III; b' - Ramus palmaris phalange proximal; b" - Ramus palmaris phalange media; c - Ramus palmaris phalange distal. B - Dorsopalmar view: d - Ramus cutaneus vena interossea cranialis; e - Vena digitalis palmaris communis IV; f - Venas digitales palmaris propria III e IV; h - Ramus dorsalis phalange distal; i - V. digitalis palmaris propria IV (axialis); j - Vena digitalis palmaris propria III (axialis). C - Palmarodorsal view; k - Arcus palmaris superficialis; 1 - Venas digitalis palmares propria II e III; m - Venas digitalis palmares propria III e IV (axialis); n - Ramus dorsalis phalange media.
Venograms of the distal region of metacarpus; and proximal, middle and distal phalanges, in right (A and B) and left (C) forelimbs of goat (Digital X-ray; 60 kVp; 5 mAs).
A - Lateral view; a - Vena digitalis palmaris communis II; b - Vena digitalis palmaris communis III; c - Venas digitalis palmaris propria II and III; d - Ramus palmaris (phalange medial); e - Ramus palmaris (phalange distal). B - Dorsopahnar view; a - Vena digitalis palmaris communs IV; b - Ramus perforans distalis; c - Vena digitalis dorsalis lateralis IV; d - Vena digitalis palmaris commtmis III; e - Vena digitalis dorsalis medialis III; f - Vena digitalis propria dorsalis IV (axialis); g - Vena digitalis propria dorsalis III (axialis); h - Vena digitalis propria dorsalis IV (axialis) (phalange medial); i - Vena digitalis propria dorsalis III (axialis) (phalange medial); j - Ramus dorsalis lateralis (phalange medial); k - Ramus dorsalis medialis (phalange medial); l - Vena digitalis palmaris propria IV (axialis); m - Vena digitalis palmaris propria III (axialis). C - Palmarodorsal view: a - Vena digitalis palmaris communis III; b - Vena digitalis palmaris communis IV; c - Vena digitalis palmaris communis II; d - V. digitalis palmaris communis III (phalange proximal); e- Vena digitalis dorsalis medialis III; f - Ramus palmaris (phalange proximal); g - Vena digitalis lateralis IV; h - Vena digitalis palmaris propria III (axialis); i - Vena digitalis palmaris propria IV (axialis); j - Vena digitalis palmaris propria IV (axialis) (phalange medial); k - Ramus dorsalis medial (phalange medial); 1 - Ramus dorsalis lateral (phalange medial); m - Vena digitalis palmaris propria III (axialis) (phalange medial); n - Vena digitalis palmaris propria IV (axialis) (phalange distal); o - Ramus dorsalis phalange distal medial; p- Ramus dorsalis phalange distal lateral; q - Vena digitalis palmaris propria III (axialis) (phalange distal).
Venograms of distal region of metatarsus, and proximal, middle and distal phalanges, in right (A and B) and left (C) hindlimbs of sheep (Digital X-ray: 60 kVp; 5 mAs).
A - Lateral view; a - Ramus distalis vena plantaris lateralis; b - Ramus perforans distalis vena metatarsea dorsalis III; c - Vena digitalis plantaris communis III; d - Arcus plantaris profundus vena plantaris lateralis; e - Vena digitalis dorsalis communis III; f - Vena digitalis plantaris communis III; g - Ramus plantaris phalange proximal; h - Venas digitales plantares propria II e III; i - Ramus plantaris phalange media; j - Ramus plantaris phalange distal. B - Dorsoplantar view; a - Ramus perforans distalis vena dorsalis pedis; b - Vena digitalis communis dorsalis III; c - Vena digitalis dorsalis lateralis IV; d - Vena digitalis dorsalis medialis III; e - Ramus dorsalis phalange media- lateral; f- Vena digitalis plantaris propria IV; g - Vena digitalis plantaris propria III; h - Ramus dorsalis phalange medial - medial; i - Ramus dorsalis phalange distal - lateral; j - Vena digitalis plantaris propria IV; k - Vena digitalis plantaris propria III; 1 - Ramus dorsalis phalange distal - medial. C - Plantodorsal view; a - Vena digitalis plantaris communis II; b - Arcus plantaris profundus; c - Vena digitalis plantaris communis III; d - Vena digitalis plantaris communis IV; e - Venas digitales plantares propria II and III; f - Vena digitalis communis dorsalis III; g - Venas digitales plantares propria IV e h - Ramus dorsalis phalange media - medial; i - Vena digitalis plantaris propria III; j - Vena digitalis plantaris própria IV; k - Ramus dorsalis phalange media - lateral; 1 - Ramus dorsalis phalange distal - medial; m - Ramus dorsalis phalange distal - lateral.
Venograms of distal region of metatarsus, and proximal, middle and distal phalanges, in right (A and B) and left (C) hindlimbs of goat (Digital X-ray: 60 kVp; 5 mAs).
A - Lateral view: a - Ramus perforans distalis (veia matatarsea dorsalis III); b - Vena digitalis plantaris communis III; c - Vena digitalis dorsalis communis III; d - Vena digitalis plantaris communis II; e - Ramus palmaris phalange medial; f - Ramus palmaris phalange distal. B - Dorsoplantar view: a - Vena digitalis plantaris communis IV; b - Vena digitalis plantaris communis III; c - Vena digitalis plantaris communis III; d - Vena dorsalis lateralis IV; e - Vena digitalis dorsalis medial III; f - Vena digitalis plantaris propria IV (phalange proximal); g - Vena digitalis plantaris propria III (phalange proximal); h - Ramus dorsalis phalange media - lateral; i - Ramus dorsalis phalange media - medial; j - Vena digitalis plantaris propria IV (phalange medial); k - Vena digitalis plantaris propria III (phalange medial); 1 - Ramus dorsalis phalange distal - lateral; m - Ramus dorsalis phalange distal - medial; n - Vena digitalis plantaris propria IV (phalange distal); o - Vena digitalis plantaris propria III (phalange distal). C - Plantodorsal view: a - Vena digitalis plantaris propria III; b - Vena digitalis plantaris communis III; c - Vena digitalis plantaris propria IV (phalangeproximal); d - Vena digitalis plantaris communis III; e - Vetia dorsalis lateralis IV; f - Vena digitalis dorsalis medial III; g - Vena digitalis plantaris propria IV (phalange proximal); h - Vena digitalis plantaris propria III (phalange proximal); i - Ramus dorsalis phalange medial - medial; j - Ramus dorsalis phalange medial - lateral; k - Vena digitalis plantaris propria III (phalange medial); 1 - Vena digitalis plantaris propria IV (phalange medial); m - Ramus dorsalis phalange distal - medial; n - Ramus dorsalis phalange distal - lateral; o - Vena digitalis plantaris propria III (phalange distal); p - Vena digitalis plantaris propria IV (phalange distal).
There was a contrast fill-in variation between the veins of the forelimb and hindlimbs of sheeps and goats. The vena digitalis palmaris propria III derived from the vena mediana. The vena digitalis palmaris communis II and the vena digitalis palmaris communis IV derived from the vena radialis to arcus and the ramus palmaris profundus.
Veins from the distal region of the hindlimbs derived from two main veins, namely: vena metatarsea dorsalis III and vena plantaris medialis. Therefore, the vessels in dorsoplantar view derived from the vena dorsalis pedis and the vena tibialis cranialis. Vena digitalis plantaris communis IV, vena plantaris communis II and vena plantaris communis III derived from the vena plantaris lateralis.
Two veins were observed in lateral view from the dorsal region of the metatarsus and proximal phalanx, namely: ramus distalis vena plantaris lateralis and ramus perforans distalis vena metatarsea dorsalis III. These veins were not observed in the same view in the forelimbs.
There was no difference in number of veins between male and female animals, as well between their right and left forelimbs and hindlimbs.
Discussion
Normal venographic assessment of the vascularization in the distal region of the forelimbs and hindlimbs of sheep and goats can help understanding locomotor disorders and etiology of digital circulatory problems such as the healing of the digit area, sole abscess, sole bruising, foot trauma and lacerations (2828 Nazhvani SD, Sepideh A, Tajali M. Arteriographical evaluation of normal digit and hoof in goat. IJVS. 2007; 2: 43-48.).
Studies regarding cattle lameness diseases are found in the literature (88 Nigam JM, Singh AP. Radiography of bovine foot disorders. Med Vet Prac. 1981; 61:621-624.
9 Boosman R, Nemeth F, Gruys F, Klarenbeek A. Arteriographical and pathological changes in chronic laminitis in dairy cattle. Vet Quart. 1989; 11(3):144-155.-1010 Singh SS, Ward WR, Murray RD. An angiographic evaluation of vascular changes in sole lesions in the hooves of cattle. Br Vet J. 1994; 150: 41-52.,2929 Vermunt JJ, Greenouh PR.. Predisposing factors of laminitis in cattle. Br Vet J. 1994; 150:151-164.,3030 Dehghani SN. Digital vascular pattern variations in normal and laminitis foot in dairy cattle. Lam Ruminants. 2008; 32:320-321.), but documented studies on small ruminant lameness still lack. As long as the authors are aware of, unlike horses (1313 Redden RF. Possible therapeutic value of digital venography in two laminitic horses. Equine Vet Educ. 2001; 13:128-134.
14 Arthur EG, Rucker A. The use of digital venography for assessment of perfusion deficits in chronic laminitis. Palm Beach. 2003; 319-320.
15 Rucker A, Redden RF, Arthur EG, Reed SK, Hill BW, et al. How to perform the digital venogram. AAEP. 2006; 52:526-530.
16 Lyle BE.. Venography as a tool for guiding surgery to the foot. In: Floyd AD, Mansmann RA. Equine podiatry. St Louis (MO): Saunders. 2007; 284-293.
17 Brunner CHM, Martins MFM, Bovino EE. Angiografia in vivo para avaliação da vascularização do casco de equinos. Ciênc Rural. 2008; 38(1):116-123.
18 Redde RF. Using venograms in laminitic cases. Redden´s in-deph Podiatry Symposium. Versailles (KY). 2009; 81-83.
19 Rucker A. Clinical applications of digital venography. J Equine Vet Sci. 2010; 30(9):491-503.
20 Hunt RJ; Wharton RE. Clinical presentation, diagnosis, and prognosis of chronic laminitis in North America. Vet Clin North Am. 2010; 26:141-154.
21 Baldwin GI, Pollitt CC. Progression of venographic changes after experimentally induced laminitis. Vet Clin North Am. 2010; 26:135-140.-2222 D'Arpe L, Bernardini D. Digital Venography in Horses and Its Clinical Application in Europe. Vet Clin Equine. 2010; 26:349-359.) and cattle (99 Boosman R, Nemeth F, Gruys F, Klarenbeek A. Arteriographical and pathological changes in chronic laminitis in dairy cattle. Vet Quart. 1989; 11(3):144-155.
10 Singh SS, Ward WR, Murray RD. An angiographic evaluation of vascular changes in sole lesions in the hooves of cattle. Br Vet J. 1994; 150: 41-52.-1111 Rebhun WC, Pearson EG. Clinical management of bovine foot problems. JAVMA. 1995; 180(6):1464-1467.,2323 Rafael LA, Pyles MD, Rodrigues M, Rodrigues CA. Venograma digital para avaliação de laminite crônica em um bovino. VII amostra Científica de Ciências Agrárias. Botucatu. 2011; 23-24.,3131 Manohar M, Kumar R, Bhargava AK, Tyagi RPS. Angiographic studies of the bovine (Bubalis bubalis) foot. Vet Radiol Ultrasound. 1973; 14:81-86., 3232 Edwards GB, Webbon PM. Angiographic studies of the bovine foot. In: Proceedings of IV International Veterinary Radiology Conference, Cambridge, UK. 1976; 3-4.), there are no reports concerning studies in vivo on the normal vascular pattern of the fore and hindlimb's digit and hoof in small ruminants. However, studies regarding to arteriography in vivo and in situ in goats (55 Burns J, Cornell C. Angiography of the caprine digit. Vet Radiol Ultrasound. 1981; 22(4):174-176.,2828 Nazhvani SD, Sepideh A, Tajali M. Arteriographical evaluation of normal digit and hoof in goat. IJVS. 2007; 2: 43-48.) and venography in situ in sheep (3333 Ozudogru Z.. Morkaraman Koyununun Ön Bacak Venleri Üzerine Makroanatomik Bir Çalışma. Atatürk Üniversitesi Vet. Bil. Derg. 2009; 4(1):39-47.) were conducted. Similar studies involving cattle are expensive; thus, sheep can be used as ruminant model (55 Burns J, Cornell C. Angiography of the caprine digit. Vet Radiol Ultrasound. 1981; 22(4):174-176.,2828 Nazhvani SD, Sepideh A, Tajali M. Arteriographical evaluation of normal digit and hoof in goat. IJVS. 2007; 2: 43-48.,3030 Dehghani SN. Digital vascular pattern variations in normal and laminitis foot in dairy cattle. Lam Ruminants. 2008; 32:320-321.). Therefore, they become an economic alternative due to the foot vascular pattern similarity between small ruminants and cattle.
Nazhvani et al. (2828 Nazhvani SD, Sepideh A, Tajali M. Arteriographical evaluation of normal digit and hoof in goat. IJVS. 2007; 2: 43-48.) have described a goat-hind digit angiography applied to sedated live animals as research model for Fescue toxicity in large-animal lameness studies. The present study raised anatomic references for regional intravenous anesthesia and antibiotic perfusion in small ruminants. The veins in the distal region of the sheep's forelimbs and hindlimbs were described by Popesko (2525 Popesko P. Atlas of topographical anatomy of the domestic animals. Philadelphia: W. B. Saunders. 1971; 84-99.), Schummer (2626 Schummer A. Kreislaufsystem Haut und Hautorgane. Anatomie der Haustiere. Band III. Berlin: Verlag Paul Parey. 1976; 211-227.) through schematic drawings; however, different from the present study, the small and medium veins were not mentioned by him.
Burns & Cornell (55 Burns J, Cornell C. Angiography of the caprine digit. Vet Radiol Ultrasound. 1981; 22(4):174-176.), Nazhvani et al. (2828 Nazhvani SD, Sepideh A, Tajali M. Arteriographical evaluation of normal digit and hoof in goat. IJVS. 2007; 2: 43-48.) used a conventional X-ray machine to describe the normal arteriographic pattern in vivo of goats' hindlimbs and sheep's forelimbs and hindlimbs. A digital X-ray machine was used in the current study which increased the image quality.
Santos et al. (2424 Santos IFC, Hussni CA, Rodrigues CA, Watanabe MJ, Alves ALG, Charlier M.. Técnica venográfica contrastada in vivo dos dígitos de ovinos e caprinos. Arq. Bras. Med. Vet. Zootec. 2015; 67(6):1630-1638.) have found no difference between the number of veins in male and female animals, as well as between the right and left limbs in sheep and goats. However, the hindlimbs showed a higher number of veins than the forelimbs in both species. On the other hand, these authors described differences between the number of veins in sheep and goats' limbs, and these results corroborated the ones in the present study. Santos et al. (2424 Santos IFC, Hussni CA, Rodrigues CA, Watanabe MJ, Alves ALG, Charlier M.. Técnica venográfica contrastada in vivo dos dígitos de ovinos e caprinos. Arq. Bras. Med. Vet. Zootec. 2015; 67(6):1630-1638.) postulated that the distal vascularization in the forelimbs and hindlimbs between sheep and goats are somehow similar, and that the higher number of veins in the hindlimbs can be explained by the efficient vascular anastomoses applied to them. Therefore, it was worth assessing all the views because the high probability to observe all veins, different from the literature that mentions the use of only two views - lateral and dorsopalmar (2828 Nazhvani SD, Sepideh A, Tajali M. Arteriographical evaluation of normal digit and hoof in goat. IJVS. 2007; 2: 43-48.,3434 McEvoy FJ, Webbon PM, Gaffney PJ. An experimental clot model in sheep; generation of a heterologous clot and its detection in vivo using venography and 125I labelled fibrinogen. Res Vet Sci. 2002; 72:217-221.).
The limbs' retraction during contrast injection observed in the present study was associated to heating sensation and low pH of the contrast (1717 Brunner CHM, Martins MFM, Bovino EE. Angiografia in vivo para avaliação da vascularização do casco de equinos. Ciênc Rural. 2008; 38(1):116-123.,2020 Hunt RJ; Wharton RE. Clinical presentation, diagnosis, and prognosis of chronic laminitis in North America. Vet Clin North Am. 2010; 26:141-154.
21 Baldwin GI, Pollitt CC. Progression of venographic changes after experimentally induced laminitis. Vet Clin North Am. 2010; 26:135-140.-2222 D'Arpe L, Bernardini D. Digital Venography in Horses and Its Clinical Application in Europe. Vet Clin Equine. 2010; 26:349-359., 2424 Santos IFC, Hussni CA, Rodrigues CA, Watanabe MJ, Alves ALG, Charlier M.. Técnica venográfica contrastada in vivo dos dígitos de ovinos e caprinos. Arq. Bras. Med. Vet. Zootec. 2015; 67(6):1630-1638.). The venous distention referred to such retraction resulted in hematoma (1717 Brunner CHM, Martins MFM, Bovino EE. Angiografia in vivo para avaliação da vascularização do casco de equinos. Ciênc Rural. 2008; 38(1):116-123.). This retraction could be avoided through anesthetic block, but the aim of the current study was to conduct the venography without increasing the cost of the procedure. The animals which developed hematoma were excluded from the study for 15 days and no adverse reaction or infection was observed.
The venographic technique error observed during the venography was the same reported by Santos et al. (2424 Santos IFC, Hussni CA, Rodrigues CA, Watanabe MJ, Alves ALG, Charlier M.. Técnica venográfica contrastada in vivo dos dígitos de ovinos e caprinos. Arq. Bras. Med. Vet. Zootec. 2015; 67(6):1630-1638.). Santos et al. (2424 Santos IFC, Hussni CA, Rodrigues CA, Watanabe MJ, Alves ALG, Charlier M.. Técnica venográfica contrastada in vivo dos dígitos de ovinos e caprinos. Arq. Bras. Med. Vet. Zootec. 2015; 67(6):1630-1638.) observed contrast perivascular extravasation in 20% of the forelimbs and in 15% of the hindlimbs of sheep, as well 15% in forelimbs and 10% in hindlimbs of goats. Redden (1818 Redde RF. Using venograms in laminitic cases. Redden´s in-deph Podiatry Symposium. Versailles (KY). 2009; 81-83.), Santos et al. (2424 Santos IFC, Hussni CA, Rodrigues CA, Watanabe MJ, Alves ALG, Charlier M.. Técnica venográfica contrastada in vivo dos dígitos de ovinos e caprinos. Arq. Bras. Med. Vet. Zootec. 2015; 67(6):1630-1638.0, McEvoy et al.(3434 McEvoy FJ, Webbon PM, Gaffney PJ. An experimental clot model in sheep; generation of a heterologous clot and its detection in vivo using venography and 125I labelled fibrinogen. Res Vet Sci. 2002; 72:217-221.), Ramos et al. (3535 Ramos CM, Hussni CA; Santos IFC. Estudo anatômico venográfico da porção distal dos membros de ovinos. In: Proceedings of XXIV Congresso de Iniciação Científica da Unesp, Águas de Lindóia, São Paulo. 2012; 3-4.), considered the perivascular contrast extravasation as the most common technique error during venography in horses and small ruminants.
Conclusion
According to the present methodology, it is possible identifying the normal venous pattern in the distal region of forelimbs and hindlimbs of sheep and goats, as well as the communication between the veins in all dimensions and directions of the limbs.
Acknowledgements
The authors are thankful to Coordination for the Improvement of Higher Education Personnel (CAPES - Brazil) for PhD scholarship of Ivan Felismino Charas dos Santos; and Giovanna Cristina Brombini for help during the venograms.
References
-
1Egito AA. Programa brasileiro de conservação de recursos genéticos animais. Arq Zool. 2002; 51(50):193-194.
-
2Mariante AS, Cavalcante N. Recursos genéticos animais. Animais do descobrimento, raças domésticas da história do Brasil. Ministério da Agricultura e Abastecimento. Brasília. 2000; 192-204.
-
3Vasconcelos VR, Vieira LS. A evolução da caprino-ovinocultura brasileira. Embrapa. 2005; 65-69.
-
4Sousa WH. O agronegócio da caprinocultura de corte no Brasil. Rev Tecnol Ciên Agropec. 2007; 1(1):51-58.
-
5Burns J, Cornell C. Angiography of the caprine digit. Vet Radiol Ultrasound. 1981; 22(4):174-176.
-
6Madruga MS. Qualidade química, sensorial e aromática da carne caprina e ovina: mitos e verdades. Encontro Nacional para o Desenvolvimento da Espécie Caprina. Botucatu. 2004; 215-234.
-
7Ackerman N, Garner HE, Coffman JR, Clement JW. Angiographic appearance of the normal equine foot and alternations in chronic laminitis. JAVMA. 1975; 66:58-62.
-
8Nigam JM, Singh AP. Radiography of bovine foot disorders. Med Vet Prac. 1981; 61:621-624.
-
9Boosman R, Nemeth F, Gruys F, Klarenbeek A. Arteriographical and pathological changes in chronic laminitis in dairy cattle. Vet Quart. 1989; 11(3):144-155.
-
10Singh SS, Ward WR, Murray RD. An angiographic evaluation of vascular changes in sole lesions in the hooves of cattle. Br Vet J. 1994; 150: 41-52.
-
11Rebhun WC, Pearson EG. Clinical management of bovine foot problems. JAVMA. 1995; 180(6):1464-1467.
-
12Cripps PJ, Eustace RA. Radiological measurements from the feet of normal horses with relevance to laminitis. Equine Vet J. 1999 ; 31:427-432.
-
13Redden RF. Possible therapeutic value of digital venography in two laminitic horses. Equine Vet Educ. 2001; 13:128-134.
-
14Arthur EG, Rucker A. The use of digital venography for assessment of perfusion deficits in chronic laminitis. Palm Beach. 2003; 319-320.
-
15Rucker A, Redden RF, Arthur EG, Reed SK, Hill BW, et al. How to perform the digital venogram. AAEP. 2006; 52:526-530.
-
16Lyle BE.. Venography as a tool for guiding surgery to the foot. In: Floyd AD, Mansmann RA. Equine podiatry. St Louis (MO): Saunders. 2007; 284-293.
-
17Brunner CHM, Martins MFM, Bovino EE. Angiografia in vivo para avaliação da vascularização do casco de equinos. Ciênc Rural. 2008; 38(1):116-123.
-
18Redde RF. Using venograms in laminitic cases. Redden´s in-deph Podiatry Symposium. Versailles (KY). 2009; 81-83.
-
19Rucker A. Clinical applications of digital venography. J Equine Vet Sci. 2010; 30(9):491-503.
-
20Hunt RJ; Wharton RE. Clinical presentation, diagnosis, and prognosis of chronic laminitis in North America. Vet Clin North Am. 2010; 26:141-154.
-
21Baldwin GI, Pollitt CC. Progression of venographic changes after experimentally induced laminitis. Vet Clin North Am. 2010; 26:135-140.
-
22D'Arpe L, Bernardini D. Digital Venography in Horses and Its Clinical Application in Europe. Vet Clin Equine. 2010; 26:349-359.
-
23Rafael LA, Pyles MD, Rodrigues M, Rodrigues CA. Venograma digital para avaliação de laminite crônica em um bovino. VII amostra Científica de Ciências Agrárias. Botucatu. 2011; 23-24.
-
24Santos IFC, Hussni CA, Rodrigues CA, Watanabe MJ, Alves ALG, Charlier M.. Técnica venográfica contrastada in vivo dos dígitos de ovinos e caprinos. Arq. Bras. Med. Vet. Zootec. 2015; 67(6):1630-1638.
-
25Popesko P. Atlas of topographical anatomy of the domestic animals. Philadelphia: W. B. Saunders. 1971; 84-99.
-
26Schummer A. Kreislaufsystem Haut und Hautorgane. Anatomie der Haustiere. Band III. Berlin: Verlag Paul Parey. 1976; 211-227.
-
27Schaller O.. Nomenclatura anatômica veterinárira. Zaragoza: Acribia. 1996; 614-615.
-
28Nazhvani SD, Sepideh A, Tajali M. Arteriographical evaluation of normal digit and hoof in goat. IJVS. 2007; 2: 43-48.
-
29Vermunt JJ, Greenouh PR.. Predisposing factors of laminitis in cattle. Br Vet J. 1994; 150:151-164.
-
30Dehghani SN. Digital vascular pattern variations in normal and laminitis foot in dairy cattle. Lam Ruminants. 2008; 32:320-321.
-
31Manohar M, Kumar R, Bhargava AK, Tyagi RPS. Angiographic studies of the bovine (Bubalis bubalis) foot. Vet Radiol Ultrasound. 1973; 14:81-86.
-
32Edwards GB, Webbon PM. Angiographic studies of the bovine foot. In: Proceedings of IV International Veterinary Radiology Conference, Cambridge, UK. 1976; 3-4.
-
33Ozudogru Z.. Morkaraman Koyununun Ön Bacak Venleri Üzerine Makroanatomik Bir Çalışma. Atatürk Üniversitesi Vet. Bil. Derg. 2009; 4(1):39-47.
-
34McEvoy FJ, Webbon PM, Gaffney PJ. An experimental clot model in sheep; generation of a heterologous clot and its detection in vivo using venography and 125I labelled fibrinogen. Res Vet Sci. 2002; 72:217-221.
-
35Ramos CM, Hussni CA; Santos IFC. Estudo anatômico venográfico da porção distal dos membros de ovinos. In: Proceedings of XXIV Congresso de Iniciação Científica da Unesp, Águas de Lindóia, São Paulo. 2012; 3-4.
Publication Dates
-
Publication in this collection
2018
History
-
Received
06 Jan 2018 -
Accepted
09 Apr 2018