Abstracts
The aim of this crossover study was to evaluate the effect of a grape concentrate (test drink [TD]) on oxidative stress markers (thiobarbituric acid reactive substances [TBARS], catalase [CAT], superoxide dismutase [SOD], and glutathione [GSH]). Six triathletes had their physical fitness, body fat composition (%BF) and food intake evaluated. Afterwards, the athletes received two doses of 300 mL of the TD (45.8g of polyphenols/kg) or a placebo drink (PL), at breakfast and after a training session (100 km of cycling, 6 km of running and 1.5 km of swimming). Blood samples (5 ml) were collected after an overnight fasting, immediately after exercise, and one hour after exercise. The triathletes presented the following characteristics (mean and standard-deviation): 43.8±10.2 years old, VO2máx 45±5.15 mL/kg/min, %BF 13.6±4.2 %, training 270.8±87.1 km/week, 3.1±1.88 hours/training/day. There was a significant increase in SOD from the 1st to the 2nd (p=0.027) and 3rd (p=0.02) blood tests, in response to exercise, regardless of the drink consumed. One hour after exercise, the increase in glutathione values was greater when the PL was consumed (27.5%) in relation to the TD intake (1.8%). In both tests, exercise increased TBARS values; however, when PL was consumed, subjects' values were higher (PL=2.5±1.1 nmol/ml vs. BT=1.77±1.3 nmol/ml). When PL was consumed, mean CAT values (BT=34.2±6.9 U/mgHb vs. PL=24.6±12.5 U/mgHb) reduced from the 1st to the 2nd blood test (28.6%). TBARS, CAT and GSH values suggest that the TD presents potential to modulate exercise-induced oxidative stress.
Athlete; Grapes; Metabolism; Nutrition; Oxidative stress; Triathletes
O objetivo deste estudo crossover foi avaliar o efeito de um concentrado de uva (bebida teste - BT) sobre biomarcadores do estresse oxidativo (substâncias reativas ao ácido tiobarbitúrico - TBARS, catalase - CAT, superóxido dismutase - SOD e glutationa - GSH). Seis triatletas do sexo masculino foram avaliados quanto à aptidão física, percentual de gordura (%G) e ingestão alimentar. Posteriormente, em duas ocasiões, os atletas receberam duas doses de 300 ml de BT (45,8g de polifenóis/kg) ou bebida placebo (PL) no desjejum e após uma sessão de treinamento (100 km de ciclismo, 6 km de corrida e 1,5 km de natação). Amostras de sangue (5 ml) foram coletadas em jejum, imediatamente após o exercício e 1h após o mesmo. Caracterização da amostra: idade: 43,8±10,2 anos, VO2máx: 45±5,15 ml/kg/min, %G: 13,6±4,2%, volume de treino: 270,8±87,1 km/semana e 3,1±1,88 horas/treino/dia. Houve aumento significativo da atividade de SOD da 1ª para as 2ª (p=0,027) e 3ª coletas (p=0,02) em resposta ao exercício, independente da bebida consumida. Os valores de GSH foram superiores 1 hora após o exercício quando houve consumo do PL (27,5%) em relação ao consumo da BT (1,8%). Ainda, o exercício elevou as concentrações de TBARS, mas no grupo PL os valores médios foram superiores (PL=2,5±1,2 nmol/ml vs. BT=1,77±1,3 nmol/ml). Em relação à atividade da CAT, os valores médios (BT=34,2±6,9 U/mgHb vs. PL=24,6±12,5 U/mgHb) foram menores quando comparadas 1ª e 2ª coletas (28,6%) para os atletas que consumiram PL. Os resultados referentes à concentração de TBARS, atividade de CAT e níveis de GSH sugerem que a BT modulou o estresse oxidativo induzido pelo exercício.
Atletas; Estresse oxidativo; Metabolismo; Nutrição; Triatletas; Uvas
INTRODUCTION
The adoption of balanced diet combined with regular physical activity is considered one of the main strategies to promote health and prevent the onset of diseases. On the other hand, the practice of exhausting exercise for prolonged or unusual time may cause a number of negative effects on the body, including oxidant-antioxidant imbalance (oxidative stress)11. Cruzat VF, Rogero MM, Borges MC, Tirapegui J. Aspectos atuais sobre estresse oxidativo, exercícios físicos e suplementação. Rev Bras Med Esporte 2007;15(5): 336-42. , 22. Ferreira F, Ferreira R, Duarte JR. Stress oxidativo e dano oxidativo muscular esquelético: influência do exercício agudo inabitual e do treino físico. Rev Port Ciênc Desp 2007;7(2):257-75..
Although the production of reactive oxygen species (ROS) is a process of adaptation to physical exercise, when in excess, it can be detrimental to the athlete. ROS can lead to cell damage, such as lipid peroxidation, protein oxidation and DNA changes, which may cause long-term mutations11. Cruzat VF, Rogero MM, Borges MC, Tirapegui J. Aspectos atuais sobre estresse oxidativo, exercícios físicos e suplementação. Rev Bras Med Esporte 2007;15(5): 336-42. , 22. Ferreira F, Ferreira R, Duarte JR. Stress oxidativo e dano oxidativo muscular esquelético: influência do exercício agudo inabitual e do treino físico. Rev Port Ciênc Desp 2007;7(2):257-75.. In this sense, some studies have shown changes in oxidative stress markers in triathletes33. Knez WL, Jenkins DG, Combes JS. Oxidative stress in half and full Ironmen triathletes. Med Sci Sports Exerc 2007;39(2):283-88. , 44. Nieman DC, Henson DA, Mcanulty SR, Mcanulty LS, Morrow JD, Ahmed A, et al. Vitamin E and immunity after the Kona Triathlon World Championship. Med Sci Sports Exerc 2004;36(8):1328-35..
The administration of antioxidants in the form of supplements or foods before, during or after exercise has been investigated in order to verify the effects of this strategy on the modulation of oxidative stress33. Knez WL, Jenkins DG, Combes JS. Oxidative stress in half and full Ironmen triathletes. Med Sci Sports Exerc 2007;39(2):283-88.
4. Nieman DC, Henson DA, Mcanulty SR, Mcanulty LS, Morrow JD, Ahmed A, et al. Vitamin E and immunity after the Kona Triathlon World Championship. Med Sci Sports Exerc 2004;36(8):1328-35.
5. Lafay S, Jan C, Nardon K, Lemaire B, Ibarra A, Roller M, et al. Grape extract improves antioxidant status and physical performance in elite male athletes. J Sport Sci Med 2009;8:468-80.
6. Howatson G, Mchugh MP, Hill JA, Brouner J, Jewell AP, Van Someren KA, et al. Influence of tart cherry juice on indices of recovery following marathon running. Scand J Med Sports 2010;20(6):843-52.
-
77. Morillaz-Ruiz JM, Garcia JAV, López FJ, Vidal-Guevara ML, Zafrilla P. Effects of polyphenolic antioxidants on exercise-induced oxidative stress. Clin Nutr 2006;25:444-53..
Among the investigated foods, grape has attracted attention concerning its content of antioxidant substances such as flavonoids (quercetin), polyphenols (catechins), stilbene derivatives (resveratrol) and anthocyanins, which are present both in its fresh form as processed (juice)88. Nassiri-Asl M, Housseinzadeh H. Review of the pharmacological effects of Vitis vinifera (grape) and its bioactive compounds. Phytother 2009;23:1119-204. , 99. Aguiar Jr O, Gollücke APB, Moraes BB, Pasquini G, Catharino RR Riccio MF, et al. Grape juice concentrate prevents oxidative DNA damage in peripheral blood cells of rats subjected to a high-cholesterol diet. BrJ Nut 2011;105:694-702.. Its consumption has been investigated in clinical88. Nassiri-Asl M, Housseinzadeh H. Review of the pharmacological effects of Vitis vinifera (grape) and its bioactive compounds. Phytother 2009;23:1119-204. and sports areas55. Lafay S, Jan C, Nardon K, Lemaire B, Ibarra A, Roller M, et al. Grape extract improves antioxidant status and physical performance in elite male athletes. J Sport Sci Med 2009;8:468-80. , 1010. Gonçalves MC, Bezerra FF, Eleutherio ECA, Bouskela E, Koury J. Organic grape juice intake improves functional capillary density and postocclusive reactive hyperemia in triathletes. Clinics 2011;66(9):1537-41. due to its potential effect on improving the antioxidant capacity. Thus, the aim of this study was to evaluate the effect of the consumption of grape concentrate on oxidative stress markers after strenuous physical training.
METHODS
Sample
Participants were six male triathletes aged 28-57 years. According to the inclusion criteria, the study included only triathletes who regularly participate in competitive triathlon Ironman, Half Ironman and / or Olympic triathlon training at least four times a week and who provide normal electrocardiogram performed at most three months before the study. The study was approved by the Ethics Research Committee of the Federal University of São Paulo (process No.1266 / 10) and voluntary participation occurred only after signing the Informed Consent Form.
Collection protocol
In the first step, to characterize the sample, anamnesis was applied to identify the training characteristics, the use of supplements and health history. Dietary intake was assessed by 24-hour recall of a normal training day. The Nutwin software (UNIFESP, version 1.5.2.51) was used to determine the consumption of energy, macro and micronutrients (zinc, selenium, vitamin C, E and A). The consumption of other antioxidants (flavonoids and carotenoids) was also evaluated1111. Holden JM, Eldridge AL, Beecher IM, Bhagwat SA, Davis CS, Douglas LW, et al. Carotenoid Content of U.S. Foods: An update of the Database. J Food Comp Anal 1999;12:169-96. , 1212. Bhagwat S, Haytowitz DB, Holden JM. USDA Database for the Flavonoid Content of Selected Foods, Release 3.0. Beltsville: US Department of Agriculture; 2011.. The consumption of micronutrients was compared with the guidelines of the Dietary Reference Intakes1313. Otten JJ, Hellwig JP, Meyers LD (editores). Dietary references intakes: the essential guide to nutrient requirements.Washington: National Academy Press; 2006..
Cardiopulmonary test was also performed in cycle ergometer of the lower limbs (CEFISE(r), São Paulo, Brazil). Warm-up exercise lasted 3 minutes without load, with increment of 35 watts / min until exhaustion. During the test, oxygen consumption (gas analyzer K4B2(r), Cosmed, Pavona di Albano, Italy), heart rate (HR) (12-lead electrocardiogram, Apex 1000(r), TEB, São Paulo, Brazil) and blood pressure (auscultatory technique using sphygmomanometry) were monitored.
Body mass (kg) and height (cm) measures were used to determine the body mass index (BMI, kg/m2). The estimated body fat percentage, seven skinfolds were measured using the equation of Jackson and Pollock1414. Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr 1978;40:497-504..
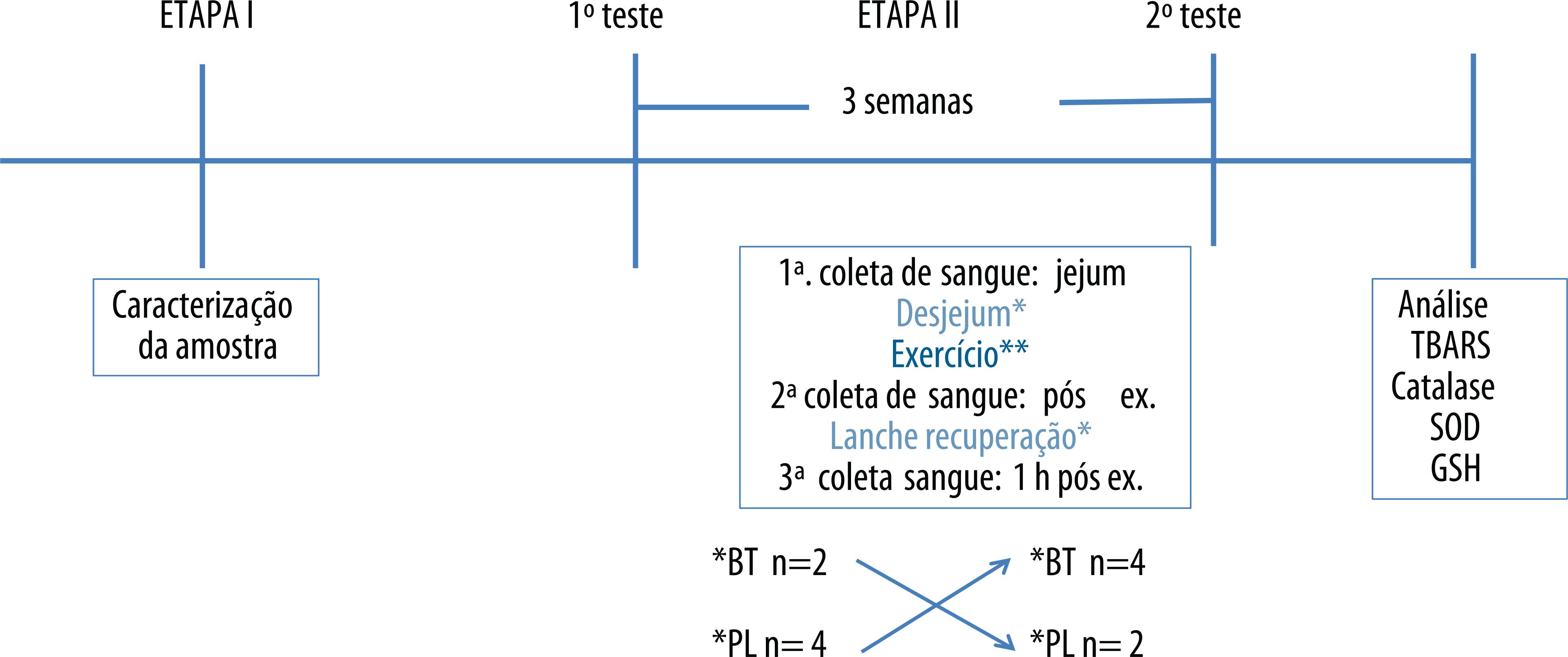
In the second stage, on a crossover design, athletes were randomly assigned into two groups and received the test drink (concentrated grape juice - BT) or placebo (artificial grape juice - PL). The athletes performed two strenuous training protocols scheduled by the coach, performed outdoor on two distinct occasions, with an interval of three weeks (washout), with the same intensity and duration, and simulating the competition conditions. Subjects were instructed to follow the usual lifestyle, especially regarding diet, also avoiding the use of antioxidant supplements for a period of 30 days prior to tests (Figure 1).
Experimental Study Protocol ** 10 km cycling / 6 km running in sand / 1.5 km swimming at sea
In each tests, the following procedures were performed:
-
Collection of fasting blood.
-
Breakfast 45 minutes before test: The meal consisted of foods commonly consumed by athletes (white bread, fruit jelly, banana, rolled oats, cream crackers, cream cheese, white cheese, caramelized rice flakes) as well as BT or PL. Athletes were instructed to consume 1-1.5g carbohydrate / kg.
-
Consumption of carbohydrate during training: water added of 8% maltodextrin or food sources of carbohydrates were offered with guidance for athletes to consume 30-60g carbohydrate / h of exercise.
-
Blood collection immediately after exercise.
-
Offer of fluid and carbohydrate in recovery: water was offered ad libitum and 300 ml of BT or PL and food sources of carbohydrates, with guidance to consume at least 1-1.5g carbohydrate / kg.
-
Blood collection 1 hour after exercise.
-
Application of the Borg scale et al.15 at the end of exercise for identification of perceived exertion.
Although in pre-training, training and post-training periods, standardized snacks with recommended values for exercise lasting more than 60 minutes1616. Burke LM, Hawley JA, Wong SHS, Jeukendrup AE. Carbohydrate for training and competition. J Sports Sci 2011;29(sup1):S17-S27. were offered, for ethical reasons the athletes could adjust their intake to their habits, provided that their choices included only foods provided in the study.
Concentrated grape juice (Vitis labrusca) (G8000(r) Golden Juice, Farroupilha -RS, Brazil) obtained by nanofiltration (membrane with pores of 1 nm) was used as test drink (BT). In this process, there is retention the phenolic contents, reaching concentration five times greater than that of the original juice. The total phenolic content of grape concentrate was 45.8 GAEg / kg (gallic acid equivalents / kg) and antioxidant properties of 27.03g Vitamin C eq / kg (equivalent to vitamin C / kg)99. Aguiar Jr O, Gollücke APB, Moraes BB, Pasquini G, Catharino RR Riccio MF, et al. Grape juice concentrate prevents oxidative DNA damage in peripheral blood cells of rats subjected to a high-cholesterol diet. BrJ Nut 2011;105:694-702..
For each dose of BT, 33g of grape concentrate were used, containing 1.5 g of total polyphenols and sufficient water for a final volume of 300 ml. The 66 g of grape concentrate offered corresponds to the equivalent of polyphenols found in 1 liter of whole grape juice. This concentrate has been shown to have antioxidant effects in recent study with animal models99. Aguiar Jr O, Gollücke APB, Moraes BB, Pasquini G, Catharino RR Riccio MF, et al. Grape juice concentrate prevents oxidative DNA damage in peripheral blood cells of rats subjected to a high-cholesterol diet. BrJ Nut 2011;105:694-702.. Placebo drink (PL) was provided in the form of artificial grape flavor juice powder in the same volume with the same energy content and same sensory characteristics (color, flavor and sweetness). Drinks were offered at breakfast and immediately after exercise.
For the analysis of oxidative stress biomarkers, 5ml of peripheral blood were collected by venipuncture of the antecubital vein, which is immediately transferred to tubes with anticoagulant heparin. Samples were kept on ice until the time of centrifugation to separate plasma, which was used for the measurement of thiobarbituric acid reactive substances (TBARS), erythrocytes were used for the determination of enzymatic and non-enzymatic antioxidants. All samples were stored at -80°C until the time of analyses. The measurement of TBARS levels for the evaluation of lipid peroxidation was based on the spectrophotometric method of Ohkawa et al.1717. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95(2):351-8., whose main product analyzed is malondialdehyde (MDA, nmol / ml). Levels above 2.0 nmol / ml were considered as oxidative stress marker.
For the analysis of antioxidant biomarkers, tubes containing blood hemolysate were thawed in running water and refrozen in a vessel containing dry ice and acetone. This procedure was repeated three times to promote lysis of the plasma membrane of erythrocytes. The tubes were then centrifuged and the supernatant was transferred into 1.5 ml plastic tubes (Eppendorfâ, Germany). These samples were used for the analysis of enzymatic activities of catalase (CAT) and superoxide dismutase (SOD). The determination of erythrocyte CAT activity was based on the spectrophotometric method 1818. Adamo AM, Liesuy LF, Pasquini JM, Boveris A. Brain chemiluminescence and oxidative stress in hyperthyroid rats. Biochem J 1989;263:273-7. and readings were made at wavelength of 230nm (Hitachiâ apparatus, Japan) at 37 ºC. Values from 16.5 to 26.5 U / mgHb were considered as the reference 1919. Vasconcelos SML, Goulart MOF, Moura JBF, Manfredini V, Benfato MS, Kubota LT. Espécies reativas de oxigênio e de nitrogênio, antioxidantes e marcadores de dano oxidativo em sangue humano: principais métodos analíticos para sua determinação. Quim Nova 2007;30(5):1323-38..
The erythrocyte SOD activity was determined by spectrophotometric method 2020. Mccord JM, Fridovich I. Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 1969;244(22):6049-55., in the presence of cyanide at suitable concentration for the inhibition of the cytochrome c oxidase activity: reading was performed at wavelength of 550 nm, at 25ºC. Values from 6.5 to 14.5 U / mgHb were considered as the reference 1919. Vasconcelos SML, Goulart MOF, Moura JBF, Manfredini V, Benfato MS, Kubota LT. Espécies reativas de oxigênio e de nitrogênio, antioxidantes e marcadores de dano oxidativo em sangue humano: principais métodos analíticos para sua determinação. Quim Nova 2007;30(5):1323-38..
The determination of the total erythrocyte glutathione (GSH) (mM) concentration was based on the spectrophotometric method 2121. Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 1969;27:502-22.. Hemoglobin was measured for normalization of enzyme activities and glutathione concentrations among samples. The concentration was expressed as mmol/gHb.
The descriptive analysis of results is presented as mean and standard deviation. To check the behavior of variables, the model of analysis of variance with repeated measures was used. The "R" software statistical for calculations was used significance level of p <0.05 was adopted.
RESULTS
Sample characteristics are described in Table 1.
In relation to the regular intake of substances with antioxidant activity (Table 2), the average intake was in accordance with recommendations, except for the consumption of vitamin E.
Environmental conditions vary between the two collection days, as follows: ambient temperature of 26oC and 27oC, relative humidity of 88% and 65% and winds of 0 and 8 km / h, respectively in the first and second day. Perceived exertion was 16.3 ± 1.0 on day 1 and 14.6 ± 2.3 on day 2, according to the Borg scale et al.1515. Borg G, Hassmen P, Lagerstrom M. Perceived exertion in relation to heart rate and blood lactate during arm and leg exercise. Eur J of Appl Phys 1987;65:679-85..
Table 3 shows the amounts of carbohydrate consumed at breakfast, training and recovery. It was observed that the consumption of carbohydrates was below recommended values during exercise.
Mean levels of TBARS and antioxidants in three collections are described in Table 4.
Although not statistically significant, the TBARS levels in both groups between the first collection (M0) and after exercise (M1), were higher; however, the mean percentage increase (9.9%) in the group that consumed BT was lower than the group who ingested PL (70.7%).
Considering the TBARS levels, which characterize oxidative stress (>2.0 nmol / mL), two athletes began the protocols (with BT and the other with PL) with high values (5.69 nmol / mL and 3, 06 nmol / ml, respectively), and normalization occurred only with the intake of BT. Two athletes who consumed BT showed high TBARS levels immediately after exercise, while three athletes also demonstrated increased values one hour after exercise. On the other hand, four athletes who ingested PL showed increased levels of this important oxidative stress biomarker, both immediately and one hour after the end of exercise.
With respect to CAT activity, mean values were lower in the 2nd collection compared to the 1st collection for athletes who consumed PL (28.6%), while, when BT was consumed, values remained close to baseline. A significant increase in SOD activity from the 1st to the 2nd (p = 0.027) and 3rd collections (p = 0.02) in response to exercise was observed, regardless of drink.
There was an increase in the levels of total GSH immediately after exercise when analyzed both triathletes who received PL (1.5% above baseline) as those who received BT (9.6% above baseline). However, the values of this important oxidative stress biomarker returned to baseline levels 1 hour after the end of exercise.
DISCUSSION
Although the production of free radicals represents a process of adaptation to exercise, when it becomes excessive, it may produce adverse effects11. Cruzat VF, Rogero MM, Borges MC, Tirapegui J. Aspectos atuais sobre estresse oxidativo, exercícios físicos e suplementação. Rev Bras Med Esporte 2007;15(5): 336-42. , 2222. Powers SK, Lennon SL. Analysis of cellular responses to free radicals: focus on exercise and skeletal muscle. Proceed Nutr Soc 1999;58(4):1025-33.. This study shows that athletes have trained under usual environmental conditions such as heat and humidity. It is suggested that the model proposed for this study, as it relates to oxidative stress biomarkers is suitable, considering mainly the TBARS and SOD results2222. Powers SK, Lennon SL. Analysis of cellular responses to free radicals: focus on exercise and skeletal muscle. Proceed Nutr Soc 1999;58(4):1025-33.. However, the results obtained should be analyzed with caution, since there was little climate variation. Another factor to be considered is the limited sample size.
Although the age range was wide, the sample consisted of triathletes competing in events that require high strength such as Ironman, the most challenging test of triathlon. The maximum oxygen uptake values (VO2max), as well as the average load used, indicate that triathletes had amateur level at that time. The type of exercise performed in the study protocol through perceived subjective effort was considered by athletes as tiring. Regarding body composition, body fat percentage of triathletes was higher than that found by other authors1010. Gonçalves MC, Bezerra FF, Eleutherio ECA, Bouskela E, Koury J. Organic grape juice intake improves functional capillary density and postocclusive reactive hyperemia in triathletes. Clinics 2011;66(9):1537-41. , 2323. González-Haro C, Galilea PA, González-de-Suso JM, Drobnic F, Escanero JF. Maximal lipidic power in high competitive level triathletes and cyclists. Br J Sports Med 2007;41(1):3-28. , 2424. Knechtle B, Knechtle P, Andonie JL, Kohler G. Influence of anthropometry on race performance in extreme endurance triathletes: World Challenge Deca Iron Triathlon 2006. Br J Sports Med 2007;41:644-48.; however, similar to values found by Nieman et al.44. Nieman DC, Henson DA, Mcanulty SR, Mcanulty LS, Morrow JD, Ahmed A, et al. Vitamin E and immunity after the Kona Triathlon World Championship. Med Sci Sports Exerc 2004;36(8):1328-35..
The usual intake of antioxidant nutrients was adequate1313. Otten JJ, Hellwig JP, Meyers LD (editores). Dietary references intakes: the essential guide to nutrient requirements.Washington: National Academy Press; 2006., except for vitamin E; the same was observed in the study by Nieman et al.44. Nieman DC, Henson DA, Mcanulty SR, Mcanulty LS, Morrow JD, Ahmed A, et al. Vitamin E and immunity after the Kona Triathlon World Championship. Med Sci Sports Exerc 2004;36(8):1328-35.. The consumption of vitamin E, a and ß-carotenes and lycopene was lower than that found by Rousseau et al.2525. Rousseau A-S, Hininger I, Palazzetti S, Faure H, Roussel A-M, Margaritis I. Antioxidant vitamin status in high exposure to oxidative stress in competitive athletes. Br J Nutr 2004;92:461-8. in athletes of aerobic sports, which can decrease the antioxidant protective action of these substances.
An important point to be highlighted is that athletes ingested initially BT and PL in different conditions, suggesting that the investigated oxidative stress biomarkers may have been hypothetically affected by factors other than exercise, for example, the concentration of endogenous antioxidants, excess training, content of muscle and liver glycogen and also exposure to environment factors11. Cruzat VF, Rogero MM, Borges MC, Tirapegui J. Aspectos atuais sobre estresse oxidativo, exercícios físicos e suplementação. Rev Bras Med Esporte 2007;15(5): 336-42., which could explain the large variability of response to distinct drinks.
Urso and Clarkson2626. Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology 2003;189(1-2):41-54. found increased rest MDA levels in sprinters, marathon runners and swimmers. Moreover, Knez et al.3 3. Knez WL, Jenkins DG, Combes JS. Oxidative stress in half and full Ironmen triathletes. Med Sci Sports Exerc 2007;39(2):283-88.found rest MDA levels lower than those of controls in Ironman triathletes. In our study, only two athletes had high TBARS values and this occurred only in one of the collection days, corroborating the idea that factors other than physical activity have influenced the results. Although not being a common finding in literature33. Knez WL, Jenkins DG, Combes JS. Oxidative stress in half and full Ironmen triathletes. Med Sci Sports Exerc 2007;39(2):283-88. , 2626. Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology 2003;189(1-2):41-54., due to the characteristics of the proposed exercise protocol, which provided more than the usual programmed intensity and to the period of year characterized by high heat and humidity (factors that increase oxidative stress), we expected that physical exercise applied would cause increased TBARS levels in these subjects. Sureda et al.2727. Sureda A, Tauler P, Aguiló A, Cases N, Fuentespina E, Dórdova A, et al. Relation between oxidative stress markers and antioxidant endogenous defences during exhaustive exercise. Free Radic Res 2005;39(12):1317-24. evaluated the MDA levels in professional cyclists in long-term competition and the results showed a 69% increase in plasma MDA levels and 370% in erythrocyte levels. Kanter et al.2828. Kanter MM, Lesmes GR, Kaminsky LA, Ham-Seager JL, Nequin ND. Serum creatine kinase and lactate dehydrogenase changes following an eighty kilometer race. Eur J Appl Physiol 1988;57:60-3. also observed a 70% increase in TBARS levels in elite athletes who participated in a 80km race, which is similar to that observed in the group receiving PL in the present study. Different from the results found here, Knez et al.33. Knez WL, Jenkins DG, Combes JS. Oxidative stress in half and full Ironmen triathletes. Med Sci Sports Exerc 2007;39(2):283-88. observed increased MDA levels in triathletes after half-Ironman (32%) or Ironman (29.7%) when supplemented with antioxidants (without supplements: 13.6% and 12.9%, respectively). The authors suggest that supplementation with vitamin E may have presented a pro-oxidant effect, as also observed by Nieman et al.44. Nieman DC, Henson DA, Mcanulty SR, Mcanulty LS, Morrow JD, Ahmed A, et al. Vitamin E and immunity after the Kona Triathlon World Championship. Med Sci Sports Exerc 2004;36(8):1328-35..
Importantly, the high TBARS values (> 2.0 nmol/mL) found immediately after exercise in four athletes in this study who consumed PL suggest that BT may have attenuated the increase in TBARS. Morillaz- Ruiz et al.77. Morillaz-Ruiz JM, Garcia JAV, López FJ, Vidal-Guevara ML, Zafrilla P. Effects of polyphenolic antioxidants on exercise-induced oxidative stress. Clin Nutr 2006;25:444-53. observed a significant increase in lipid peroxidation (TBARS) in trained cyclists after acute exercise, which was lower among subjects who consumed the drink, providing 2.3 g of polyphenols compared to the test drink. Howatson et al.66. Howatson G, Mchugh MP, Hill JA, Brouner J, Jewell AP, Van Someren KA, et al. Influence of tart cherry juice on indices of recovery following marathon running. Scand J Med Sports 2010;20(6):843-52. observed decrease in the post-competition TBARS production in marathon runners who consumed cherry juice containing about 1.2 g of polyphenols.
Studies on the CAT response to exercise have shown conflicting results, especially in relation to a single exercise session 2222. Powers SK, Lennon SL. Analysis of cellular responses to free radicals: focus on exercise and skeletal muscle. Proceed Nutr Soc 1999;58(4):1025-33.. In our study, CAT activity remained stable in the group that consumed BT, with reduced post-exercise concentration in the group that consumed PL. In the case of an exacerbated production of hydrogen peroxide (H2O2), a result of exercise, in amounts that exceed the capacity of action of glutathione peroxidase, an enzyme with higher affinity to H2O2, an increase in production of CAT was expected2727. Sureda A, Tauler P, Aguiló A, Cases N, Fuentespina E, Dórdova A, et al. Relation between oxidative stress markers and antioxidant endogenous defences during exhaustive exercise. Free Radic Res 2005;39(12):1317-24.. However, Urso and Clarkson 26 in a review on the subject, reported results in which only one subject showed increased CAT concentrations. In other studies, no change or even decreased CAT values were observed33. Knez WL, Jenkins DG, Combes JS. Oxidative stress in half and full Ironmen triathletes. Med Sci Sports Exerc 2007;39(2):283-88. , 2626. Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology 2003;189(1-2):41-54.. Knez et al.33. Knez WL, Jenkins DG, Combes JS. Oxidative stress in half and full Ironmen triathletes. Med Sci Sports Exerc 2007;39(2):283-88. also observed reduction in CAT concentration after acute ultra-endurance exercise, suggesting that this effect may be due to an inhibitory allosteric enzyme reaction, associated with its inactivation by excessive oxidative stress. Thus, although the interpretation of results found for CAT should be made with caution, it could be inferred that BT showed a modulating effect on this reaction, in contrast to that observed when athletes consumed PL.
The basal levels of SOD activity in this study (15.4 ± 3.0 and 18.1 ± 4,0U / mgHb) were lower than those reported by Gonçalves et al.1010. Gonçalves MC, Bezerra FF, Eleutherio ECA, Bouskela E, Koury J. Organic grape juice intake improves functional capillary density and postocclusive reactive hyperemia in triathletes. Clinics 2011;66(9):1537-41., who evaluated triathletes who ingested organic grape for 20 days (300 ml / day) during the training period (27.8 ± 6.3U / mgHb) and found results close to those found by Knez et al.33. Knez WL, Jenkins DG, Combes JS. Oxidative stress in half and full Ironmen triathletes. Med Sci Sports Exerc 2007;39(2):283-88., who also evaluated triathletes before and after half Ironman and Ironman.
In relation to the significant increase of SOD activity in response to exercise, regardless of drink, our results are in agreement with literature, which indicates a wide variation in the increase (20 to 112%) in response to exercise2222. Powers SK, Lennon SL. Analysis of cellular responses to free radicals: focus on exercise and skeletal muscle. Proceed Nutr Soc 1999;58(4):1025-33. , 2626. Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology 2003;189(1-2):41-54., although this finding is not unanimous 33. Knez WL, Jenkins DG, Combes JS. Oxidative stress in half and full Ironmen triathletes. Med Sci Sports Exerc 2007;39(2):283-88.. In relation to the effect of antioxidant substances from grape, unlike our results, Gonçalves et al.1010. Gonçalves MC, Bezerra FF, Eleutherio ECA, Bouskela E, Koury J. Organic grape juice intake improves functional capillary density and postocclusive reactive hyperemia in triathletes. Clinics 2011;66(9):1537-41. identified significantly lower SOD activity in athletes who consumed 300 ml (1.59 g polyphenols) the organic grape juice for 20 days. It is noteworthy that one of the isoforms of SOD is zinc-dependent and a diet rich in this mineral is necessary for this enzyme to act perfectly. The average zinc intake in our study was in agreement with recommended values1414. Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr 1978;40:497-504..
Regarding the response of GSH levels to exercise, some studies found lower values in reduced glutathione (GSH) / oxidized glutathione (GSSG) ratio and also return of GSH and GSSG values to basal concentrations one hour after the end of exercise 2929. Dufaux B, Heine O, Kothe A, Prinz U, Rost R. Blood glutathione status following distance running. Int J of Sports Med 1997;18(2):89-93.. Increased levels of total GSH observed in this study, in both conditions, immediately after exercise, confirms the findings of Ji et al.3030. Ji LL, Katz A, Fu R, Griffiths M, Spencer M. Blood glutathione status during exercise: effect of carbohydrate supplementation. J Appl Physiol 1993;74(2):788-92., who observed increase in total GSH (GSH + GSSG) after exercise; furthermore, these authors also found that carbohydrate (offered in solution containing glucose and fructose polymers at ratio of 1.8: 1 at 7.5% and providing 0.27 g carbohydrate/kg of weight) prevented this increase. The lower increase in BT group may indicate a lower oxidation of GSH to GSSG, or even more efficient resynthesis by glutathione reductase. The fact that carbohydrate consumption during exercise was lower in the two collections can hypothetically have affected these results.
Increases in GSH levels in fasting condition state and after 1 hour of exercise also suggest that BT may have decreased the GSH oxidation, since there was a 1.8% increase in BT condition and 27.5% in PL condition.
Although there are a growing number of studies addressing oxidative stress, its biomarkers and exercise, there are still few investigating foods and their bioactive substances. In addition, differences in the selection of sports, physical activity level (e.g., physically active vs. athlete), exercise protocols (e.g. strength vs. endurance vs. sprint; different intensities, volumes and duration), biomarkers and their methods of analysis have produced conflicting results, which hinder discussion of new findings.
It is necessary to emphasize that the biomarkers evaluated here behaved differently among triathletes. Thus, studies with larger numbers of participants and better control of exercise intensity such as carbohydrate intake should be carried out in order to minimize the occurrence of outlier values.
CONCLUSIONS
The results found for TBARS, CAT and GSH suggest that consumption of grape concentrate containing 45.8g/kg of polyphenols has potential effect on the positive modulation of exercise-induced oxidative stress.
Acknowledgments
To CNPq for funding this scientific work. To Odair Aguiar Junior, Cinthia Castro do Nascimento, Priscila de Medeiros, Ricardo Antoniazzi Peliccioni, Mariana Agnes da S. Alves, Lisandro Lungato, Fábio Montesano e Karla Helene M. Lima.
REFERENCES
-
1Cruzat VF, Rogero MM, Borges MC, Tirapegui J. Aspectos atuais sobre estresse oxidativo, exercícios físicos e suplementação. Rev Bras Med Esporte 2007;15(5): 336-42.
-
2Ferreira F, Ferreira R, Duarte JR. Stress oxidativo e dano oxidativo muscular esquelético: influência do exercício agudo inabitual e do treino físico. Rev Port Ciênc Desp 2007;7(2):257-75.
-
3Knez WL, Jenkins DG, Combes JS. Oxidative stress in half and full Ironmen triathletes. Med Sci Sports Exerc 2007;39(2):283-88.
-
4Nieman DC, Henson DA, Mcanulty SR, Mcanulty LS, Morrow JD, Ahmed A, et al. Vitamin E and immunity after the Kona Triathlon World Championship. Med Sci Sports Exerc 2004;36(8):1328-35.
-
5Lafay S, Jan C, Nardon K, Lemaire B, Ibarra A, Roller M, et al. Grape extract improves antioxidant status and physical performance in elite male athletes. J Sport Sci Med 2009;8:468-80.
-
6Howatson G, Mchugh MP, Hill JA, Brouner J, Jewell AP, Van Someren KA, et al. Influence of tart cherry juice on indices of recovery following marathon running. Scand J Med Sports 2010;20(6):843-52.
-
7Morillaz-Ruiz JM, Garcia JAV, López FJ, Vidal-Guevara ML, Zafrilla P. Effects of polyphenolic antioxidants on exercise-induced oxidative stress. Clin Nutr 2006;25:444-53.
-
8Nassiri-Asl M, Housseinzadeh H. Review of the pharmacological effects of Vitis vinifera (grape) and its bioactive compounds. Phytother 2009;23:1119-204.
-
9Aguiar Jr O, Gollücke APB, Moraes BB, Pasquini G, Catharino RR Riccio MF, et al. Grape juice concentrate prevents oxidative DNA damage in peripheral blood cells of rats subjected to a high-cholesterol diet. BrJ Nut 2011;105:694-702.
-
10Gonçalves MC, Bezerra FF, Eleutherio ECA, Bouskela E, Koury J. Organic grape juice intake improves functional capillary density and postocclusive reactive hyperemia in triathletes. Clinics 2011;66(9):1537-41.
-
11Holden JM, Eldridge AL, Beecher IM, Bhagwat SA, Davis CS, Douglas LW, et al. Carotenoid Content of U.S. Foods: An update of the Database. J Food Comp Anal 1999;12:169-96.
-
12Bhagwat S, Haytowitz DB, Holden JM. USDA Database for the Flavonoid Content of Selected Foods, Release 3.0. Beltsville: US Department of Agriculture; 2011.
-
13Otten JJ, Hellwig JP, Meyers LD (editores). Dietary references intakes: the essential guide to nutrient requirements.Washington: National Academy Press; 2006.
-
14Jackson AS, Pollock ML. Generalized equations for predicting body density of men. Br J Nutr 1978;40:497-504.
-
15Borg G, Hassmen P, Lagerstrom M. Perceived exertion in relation to heart rate and blood lactate during arm and leg exercise. Eur J of Appl Phys 1987;65:679-85.
-
16Burke LM, Hawley JA, Wong SHS, Jeukendrup AE. Carbohydrate for training and competition. J Sports Sci 2011;29(sup1):S17-S27.
-
17Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95(2):351-8.
-
18Adamo AM, Liesuy LF, Pasquini JM, Boveris A. Brain chemiluminescence and oxidative stress in hyperthyroid rats. Biochem J 1989;263:273-7.
-
19Vasconcelos SML, Goulart MOF, Moura JBF, Manfredini V, Benfato MS, Kubota LT. Espécies reativas de oxigênio e de nitrogênio, antioxidantes e marcadores de dano oxidativo em sangue humano: principais métodos analíticos para sua determinação. Quim Nova 2007;30(5):1323-38.
-
20Mccord JM, Fridovich I. Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 1969;244(22):6049-55.
-
21Tietze, F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem 1969;27:502-22.
-
22Powers SK, Lennon SL. Analysis of cellular responses to free radicals: focus on exercise and skeletal muscle. Proceed Nutr Soc 1999;58(4):1025-33.
-
23González-Haro C, Galilea PA, González-de-Suso JM, Drobnic F, Escanero JF. Maximal lipidic power in high competitive level triathletes and cyclists. Br J Sports Med 2007;41(1):3-28.
-
24Knechtle B, Knechtle P, Andonie JL, Kohler G. Influence of anthropometry on race performance in extreme endurance triathletes: World Challenge Deca Iron Triathlon 2006. Br J Sports Med 2007;41:644-48.
-
25Rousseau A-S, Hininger I, Palazzetti S, Faure H, Roussel A-M, Margaritis I. Antioxidant vitamin status in high exposure to oxidative stress in competitive athletes. Br J Nutr 2004;92:461-8.
-
26Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology 2003;189(1-2):41-54.
-
27Sureda A, Tauler P, Aguiló A, Cases N, Fuentespina E, Dórdova A, et al. Relation between oxidative stress markers and antioxidant endogenous defences during exhaustive exercise. Free Radic Res 2005;39(12):1317-24.
-
28Kanter MM, Lesmes GR, Kaminsky LA, Ham-Seager JL, Nequin ND. Serum creatine kinase and lactate dehydrogenase changes following an eighty kilometer race. Eur J Appl Physiol 1988;57:60-3.
-
29Dufaux B, Heine O, Kothe A, Prinz U, Rost R. Blood glutathione status following distance running. Int J of Sports Med 1997;18(2):89-93.
-
30Ji LL, Katz A, Fu R, Griffiths M, Spencer M. Blood glutathione status during exercise: effect of carbohydrate supplementation. J Appl Physiol 1993;74(2):788-92.
Publication Dates
-
Publication in this collection
Sept-Oct 2014
History
-
Received
08 May 2013 -
Accepted
13 Oct 2013