ABSTRACT
The objective of the present study was to evaluate the effect of the irrigation water salinity on the initial growth of Tectona grandis plants. The work was carried out in a greenhouse with 100% solar radiation interception at Goiás State University, Ipameri Campus, Brazil. The Tectona grandis seeds were sown in eight-liter pots containing a mixture of soil, sand and manure at ratio 3:1:0.5, respectively. At 100 days after germination, the plants were irrigated daily with water of different electrical conductivity (0, 2, 4, 6, and 8 dS m-1) for 30 days and then subjected to analysis Tectona grandis to stay alive and reduced water loss by transpiration through efficient stomatal control. Tectona grandis plants in the early stages of development are highly sensitive to salinity stress, significantly slowing down vegetative growth.
Keywords:
abiotic stress; forestry; electrical conductivity
RESUMO
O presente estudo objetivou avaliar o efeito da salinidade da água de irrigação no crescimento inicial de plantas de Tectona grandis. O trabalho foi conduzido em casa de vegetação com interceptação de 100% da radiação solar na Universidade Estadual de Goiás, Campus de Ipameri, Brasil. As sementes de Tectona grandis foram semeadas em vasos de oito litros contendo uma mistura de solo, areia e esterco na proporção de 3:1:0,5, respectivamente. Aos 100 dias após a germinação, as plantas foram irrigadas diariamente com água de diferentes condutividades elétricas (0; 2; 4; 6 e 8 dS m-1) durante 30 dias e, em seguida, submetidas às analises. Para se manterem vivas, as plantas de Tectona grandis reduziram a perda de água por transpiração através de um eficiente controle estomático. As plantas de Tectona grandis na fase inicial de desenvolvimento apresentam elevada sensibilidade ao estresse salino, inclusive com significativa redução do crescimento vegetativo.
Palavras-chave:
estresse abiótico; silvicultura; condutividade elétrica
INTRODUCTION
The competitiveness in the Brazilian forestry sector places the country in an outstanding position in the world scenario (FERREIRA et al., 2012FERREIRA, S. M. et al. Competitividade do Brasil no mercado internacional de madeira serrada. Cerne, Lavras, v. 18, p. 99-104, 2012. doi:10.1590/S0104-77602012000100012.
https://doi.org/10.1590/S0104-7760201200...
). While China, Japan and the United States, the countries with the largest planted forests, have no arable land available, Brazil holds great extensions of land suitable for forest exploitation (CENTRO DE ESTUDOS AVANÇADOS EM ECONOMIA APLICADA, 2015CENTRO DE ESTUDOS AVANÇADOS EM ECONOMIA APLICADA. [Website]. [2015]. Available at: <Available at: http://cepea.esalq.usp.br/pib
>. Access on: 27 apr. 2015.
http://cepea.esalq.usp.br/pib...
). The Brazilian forestry sector accounts for 3.5% of the gross domestic product (ASSOCIAÇÃO BRASILEIRA DE PRODUTORES DE FLORESTAS PLANTADAS, 2013ASSOCIAÇÃO BRASILEIRA DE PRODUTORES DE FLORESTAS PLANTADAS. [Website]. [2013]. Available at: <Available at: www.abraflor.org.br
>. Access on: 16 may 2013.
www.abraflor.org.br...
).
Commercial exploitation of planted forests has increased due to the high demand for timber for various purposes. Currently the explored area exceeds 7.5 million hectares and, consequently, about 90% of the wood produced in Brazil comes from planted forests (CENTRO DE ESTUDOS AVANÇADOS EM ECONOMIA APLICADA, 2015CENTRO DE ESTUDOS AVANÇADOS EM ECONOMIA APLICADA. [Website]. [2015]. Available at: <Available at: http://cepea.esalq.usp.br/pib
>. Access on: 27 apr. 2015.
http://cepea.esalq.usp.br/pib...
). Despite the high growth potential of the Brazilian forestry sector, the use of new areas depends on the species tolerance to abiotic stresses, which are common in northeastern Brazil.
Climate changes have extended water deficit periods in many parts of the world, and abiotic stresses is the leading the cause of low crop productivity worldwide, reducing by over 50% of the average yields of most cultivated plants. In forests, drought is a major limitation to the growth and establishment of seedlings (ZANG et al., 2014ZANG, C. et al. Patterns of drought tolerance in major European temperature forest trees: Climatic drivers and levels of variability. Global Change Biology, Oxford, v. 20, p. 3767-3779, 2014. doi: 10.1111/gcb.12637.
https://doi.org/10.1111/gcb.12637....
). The use of low quality water can expand the agricultural frontier to previously unsuitable areas. Brazil has about 30 million hectares with irrigation potential spread from north to south (BRASIL, 2015BRASIL. Ministério da Integração Nacional. Notícias. [2015]. Available at: <Available at: http://www.integracao.gov.br/noticias
>. Access on: 27 apr. 2015.
http://www.integracao.gov.br/noticias...
). The saline water irrigation is a reality in semi-arid regions that do not have quality water for agriculture (LOPES et al., 2012LOPES, T. C. et al. Desenvolvimento inicial de plantas de Eucalyptus platyphylla submetidas a níveis de salinidade. Irriga, Botucatu, v. 17, p. 494-500, 2012. 10.15809/irriga.2012v17n4p494
https://doi.org/10.15809/irriga.2012v17n...
).
This way, irrigation water salinity causes crop yield loss due to osmotic, toxic and/or nutritional effect (SOUZA et al., 2015SOUZA, B. R. et al. Growth of Eucalyptus plants irrigated with saline water. African Journal of Agricultural Research, Nigeria, v. 10, p. 1091-1096, 2015. doi: 10.5897/AJAR2014.9087.
https://doi.org/10.5897/AJAR2014.9087....
). According to Shannon et al. (1994SHANNON, M. C. et al. Whole plant response to salinity. In: WILKIMAN, R. E. (Ed.). Plant environment interactions. New York: Marcel Dekker, 1994. p. 199-244.), water electric conductivity equal to or greater than 4 dS m-1 dramatically reduces agricultural productivity. However, conductivity lower than 4 dS m-1 may not have an adverse effect on the tree productivity. Souza et al. (2015SOUZA, B. R. et al. Growth of Eucalyptus plants irrigated with saline water. African Journal of Agricultural Research, Nigeria, v. 10, p. 1091-1096, 2015. doi: 10.5897/AJAR2014.9087.
https://doi.org/10.5897/AJAR2014.9087....
) and Lopes et al. (2012LOPES, T. C. et al. Desenvolvimento inicial de plantas de Eucalyptus platyphylla submetidas a níveis de salinidade. Irriga, Botucatu, v. 17, p. 494-500, 2012. 10.15809/irriga.2012v17n4p494
https://doi.org/10.15809/irriga.2012v17n...
), evaluated seedlings of Eucalyptus urophylla and Eucalyptus platyphylla and concluded that the growth of eucalyptus plants has little variation when the plants are irrigated with water conductivity from 0 to 8 dS m-1, but, most of the surveys were carried out with Eucalyptus plants, requiring the development of studies with other forest species such as Tectona grandis.
The commercial use of different species makes the forestry sector less vulnerable to environmental weathering. The cultivation of Tectona grandis irrigated with saline water may represent an important source of income in semi-arid regions. The saline water is used in forest species such as Tectona grandis areas.
Tectona grandis is a commercially valuable tree due to the high quality of the wood, dimensional stability, natural resistance to fungi and insect attacks, design, color and absence of knots. These features make the Tectona grandis wood the most valuable in the world among all hardwood forest. The species is native to Asia and is expanding in mid-western and northern Brazil. The generation of information through research is necessary for business exploration in different regions of Brazil (RECH, 2009RECH, C. Características da teca. Revista da Madeira, Bento Gonçalves, v. 118, p. 55-57, 2009.).
The identification of forest species tolerant to salinity is essential for the expansion of forestry sector in northeastern Brazil. Growth in abiotic stress condition is key to the survival and establishment of forest species in tropical ecosystems. And that resistance can be related to the leaf fall mechanism: deciduous, juicy stem or evergreen (WORBES; BLANCHART; FICHTLER, 2013WORBES, M.; BLANCHART, S.; FICHTLER, E. Relations between water balance, wood traits and phenological behavior of tree species from a tropical dry forest in Costa Rica - a multifactorial study. Tree Physiology, Oxford, v. 33, p. 527-536, 2013.).
Available information regarding the development of Tectona grandis under abiotic stress condition is scarce to support recommendation for commercial exploitation. The development of studies assessing the performance of the species under salt stress condition is necessary for elucidating basic aspects of the plant performance in the field. In order to fill part of the Tectona grandis species physiological knowledge gap, this study was aimed at evaluating the effect of irrigation water salinity on the initial growth of Tectona grandis plants.
MATERIAL AND METHODS
The work was carried out in a covered greenhouse with transparent plastic and side with 50% shade at Goiás State University, Ipameri Campus (17043’19”S, 48009’35”W, Alt. 773m), Ipameri, Goiás state, Brazil. According to the Köppen classification, the region has tropical climate (Aw) with dry winter and wet summer. The experiment was set up using the completely randomized design with five treatments and six replicates. The Tectona grandis seeds were collected from a plant matrix and sown in 8-liter pots (three seeds per pot), containing a mixture of oxisol, sand and cattle manure at ratio 3:1:0.5, respectively. The germination of all seeds occurred 40 days after sowing. The chemical analysis of the mixture showed the following values: pH 6.4; 19 g dm-3 of OM; 2.4 mg dm-3 of P, 109 cmolcdm-3 of K, 1.5 cmolcdm-3 of H+Al, 3.2 cmolcdm-3 of Ca, 1.6 cmolcdm-3 of Mg, 27.7 mg dm-3 of Zn, 77.20% of BS and 6.58 of CEC. The pots were irrigated daily with water volume corresponding to daily evapotranspiration.
The seedlings were irrigated daily with corresponding water volume daily evapotranspiration. The amount of water supplied the plant was estimated by determining the potential evapotranspiration (ETP) and the current crop evapotranspiration (ETA). To determine the potential evaporation, we used the equation:
where in: EVT = water evaporation in the Class A tank in mm day-1; kt = tank coefficient considered in this work equal to 1.2.
To determine the actual evapotranspiration used the following:
where in: kc is the crop coefficient.
Cultivation coefficients (kc) for teak have not been determined, however, these were estimated according to the recommendations of FAO (ALLEN et al., 1998ALLEN, R. G. et al. Crop evapotranspiration: guidelines for computing crop water requirements. Rome: FAO, 1998. 297 p. (FAO. Irrigation and Drainage Paper, 56).) to Eucalyptus plants. The kc varies with the phenological stage of culture, however, we utilize a medium for cultivation of eucalyptus kc equal to 1.0.
At 100 days after germination, when the plants were up uniform, vigorous and at least one pair of fully expanded leaves, the plants were irrigated daily with water of different levels of electrical conductivity (0, 2, 4, 6, and 8 dS m-1, that corresponded to the treatments evaluated) for 30 days. NaCl was added to the local water supply in order to obtain solutions with different electrical conductivity, which quantity (Q) was determined by the equation Q (mg L-1) = 640 x ECw (dS m-1), as per Rhoades, Kandiah e Marshali (2000RHOADES, J. D.; KANDIAH, A. M.; MARSHALI, A. M. Uso de águas salinas para produção agrícola. Estudos da FAO - Irrigação e Drenagem, Campina Grande, v. 48, p. 1-117, 2000.), wherein ECw represents the desired electric conductivity value. Subsequently, the conductivity was checked and confirmed with a digital conductivity. At 30 days after treatment application, all the plants were subjected to the following analysis: leaf number, leaf length and width, relative water content, leaf chlorophyll concentration (a+b) and total carotenoids, leaf mass, stem and root ratios, total biomass, plant height, stem diameter and perspiration.
Growth variables
The number of leaves was measured by counting, and the leaf length and width, plant height and stem diameter were measured using a graduated ruler and a digital pachymeter. The leaves, roots (washed sieves) and stems were extracted and oven-dried at 720C until constant dry matter and then weighed separately weighed on a digital scale with accuracy of 0.01 g. The dry mass data enabled calculation of the leaf mass ratio (LMR), root mass ratio (RMR), stem mass ratio (SMR), and total biomass.
Relative water content and transpiration
In order to obtain the relative water content, ten 12-mm diameter leaf discs were taken, weighed and saturated for four hours in Petri dishes with distilled water. Then the discs were weighed again and oven- dried at 700C for 72 hours. Subsequently, the dry weight was obtained in grams. The daily transpiration was estimated by gravimetry, recording the difference in weight among the pots at one-hour intervals from 07:00 to 18:00 according to Cavatte et al.(2012CAVATTE, P. C. et al. Could shading reduce the negative impacts of drought on coffee? The morpho-physiological analysis. Physiologia Plantarum, Copenhagen, v. 144, p. 111-122, 2012. doi: 10.1111/j.1399-3054.2011.01525.x.
https://doi.org/10.1111/j.1399-3054.2011...
).
Photosynthetic pigments
The chlorophyll and total carotenoids concentrations were obtained, by extracting leaf discs with known area (third pair of fully expanded leaves) and placing them in glasses with dimethyl sulfoxide (DMSO). Subsequently, extraction was made in 650C water bath for three hours. Aliquots were taken for spectrophotometric reading at 480, 649 and 665nm, and the chlorophyll and total carotenoids concentrations were determined according to the equation proposed by Wellburn (1994WELLBURN, A. R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. Journal of Plant Physiology, Oxford, v. 144, p. 307-313, 1994. doi: 10.1016/S0176-1617(11)81192-2.
https://doi.org/10.1016/S0176-1617(11)81...
).
Statistical procedures
The experiment was set up following the completely randomized design with five treatments (plants irrigated with water of electrical conductivity of 0, 2, 4, 6, and 8 dS m-1) and six replicates. Regression analysis was carried out, which Determination Coefficient (R2) was obtained by dividing the sum of the regression squares by the sum of total squares. Statistical analyzes were performed using SISVAR 5.3 software (FERREIRA, 2011FERREIRA, D. F. Sisvar: a computerstatisticalanalysis system. Ciência e Agrotecnologia, Lavras, v. 35, p. 1039-1042, 2011. doi: 10.1590/S1413-70542011000600001.
https://doi.org/10.1590/S1413-7054201100...
).
RESULTS
Analysis of the results showed little variation among treatments as to leaf length, leaf concentration of total carotenoids, and root, stem and leaf mass ratios. These variables did not show significant differences by the F test, and the data did not fit in any significant regression model at 5% probability.
The leaf relative water content and transpiration showed significant variations among treatments (Figure 1). These variables decreased as the electrical conductivity of the irrigation water increased, thus fitting in the decreasing linear regression model.
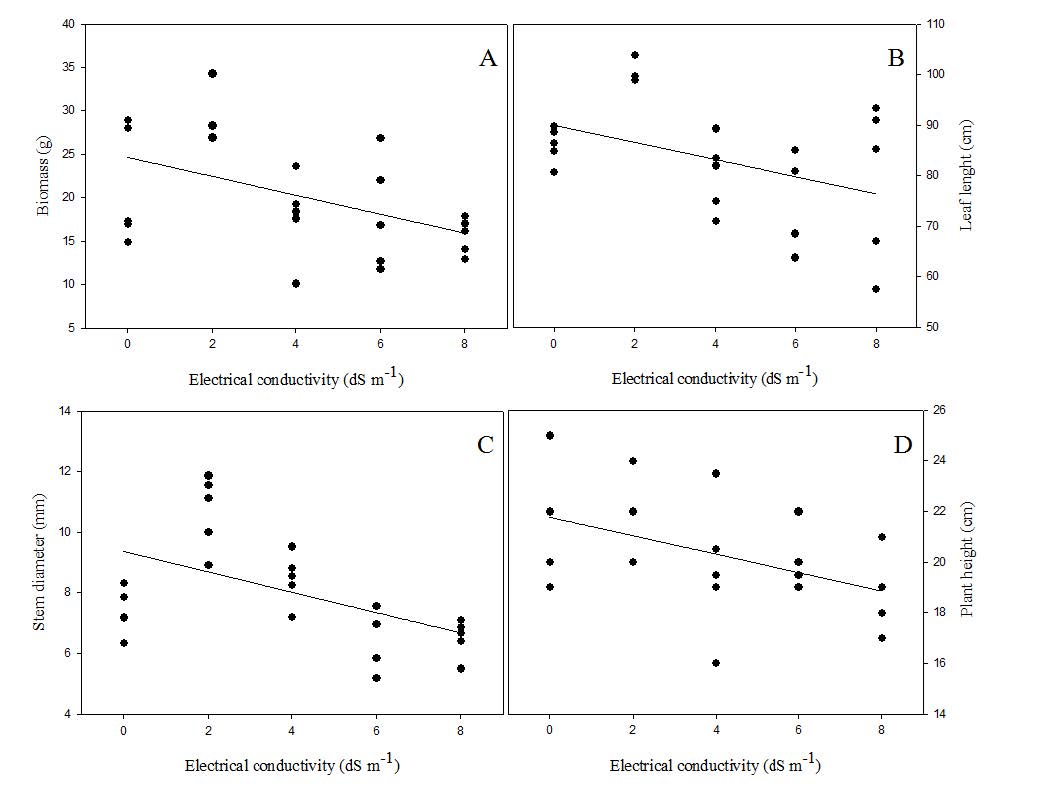
Growth variables: biomass, leaf width, stem diameter and plant height showed significant variations among the treatments (Figure 2). The vegetative growth was inversely proportional to the electrical conductivity values of the irrigation water and, therefore, the variables showed decreasing linear regression.
Equações de regressão para teor relativo de água “A” (Y = 78,89 - 1,85x, R2 = 0,45*) e transpiração “B” (Y= 213.9404 - 6,8426x, R2 = 0,98* de mudas de Tectona grandis irrigadas com água de diferentes condutividades elétricas (0, 2, 4, 6 e 8 dS m-1). *Significativo a 5% de probabilidade.
Figure 1
Regression equations for relative water content “A” (Y= 78.89 - 1.85x, R2 = 0.45*) and transpiration “B” (Y = 213.9404 - 6.8426x, R2 = 0.98*) of Tectona grandis seedlings irrigated with water of different electrical conductivity levels (0, 2, 4, 6, and 8 dS m-1). * Significant at 5% probability.
Equações de regressão para biomassa “A” (Y= 24,6388 - 1,0907x, R2= 0,93*), largura da folha “B” (Y= 89,9549 - 1,6995, R2 = 0,77*), diâmetro do caule “C” (Y= 8,8760 - 0,2337x, R2 = 0,95*) e altura de planta “D” (Y= 21,7632 - 0,3639x, R2 = 0,99*) de mudas de Tectona grandis irrigadas com água de diferentes condutividades elétricas (0, 2, 4, 6 e 8 dS m-1). *Significativo a 5% de probabilidade.
Figure 2
Regression equations for biomass “A” (Y= 24.6388 - 1.0907x, R2 = 0.93*), leaf lenght “B” (Y= 89.9549 - 1.6995, R2 = 0.77*), stem diameter “C” (Y= 8.8760 - 0.2337x, R2 = 0.95*) andplant height “D” (Y= 21.7632 - 0.3639x, R2 = 0.99*) of Tectona grandis seedlings irrigated with water of different electrical conductivity levels (0, 2, 4, 6 and 8 dS m-1). *Significant at 5% probability.
Equações de regressão para área foliar específica “A” (Y= 19,2826 - 0,4291, R2 = 0,98*) e clorofilas totais “B” (Y= 11,7116 - 0,2787x, R2 = 0,80*) de mudas de Tectona grandis irrigadas com água de diferentes condutividades elétricas (0, 2, 4, 6 e 8 dS m-1). *Significativo a 5% de probabilidade.
Figure 3
: Regression equations for specific leaf area “A” (Y= 19.2826 - 0.4291, R2 = 0.98*) and total chlorophyll “B” (Y= 11.7116 - 0.2787x, R2 = 0.80*) of Tectona grandis seedlings irrigated with water of different electrical conductivity levels (0, 2, 4, 6 and 8 dS m-1). *Significant at 5% probability.
Leaf chlorophyll concentrations (a+b) and specific leaf area showed significant differences among the treatments (Figure 3). Overall, both variables showed decreasing values with increasing electrical conductivity of the irrigation water and decreasing linear regression adjustment.
DISCUSSION
Salinity reduces growth by affecting the plant water status due to low osmotic and hydric potential and to the toxic effect of salt that hinders the ionic balance within the cell organelles. The drastic reduction in transpiration and relative water content indicate an alteration in the water status of Tectona grandis. Moreover, it is noted that the plants have high stomatal sensitivity and efficient mechanism for reducing water loss.
The high stomatal control typical of isohydric species has possibly affected the carbon assimilation rate of the treated plants and consequently their growth. The salinity of the irrigation water markedly affected the vegetative growth of Tectona grandis plants. The significant reduction in biomass, leaf width, stem diameter, and plant height is an indication that carbon assimilation was affected, since stomatal closing also limits the inflow of CO2 while limiting the loss of water through evaporation. The results corroborate those found by Souza et al. (2015SOUZA, B. R. et al. Growth of Eucalyptus plants irrigated with saline water. African Journal of Agricultural Research, Nigeria, v. 10, p. 1091-1096, 2015. doi: 10.5897/AJAR2014.9087.
https://doi.org/10.5897/AJAR2014.9087....
) when assessing the growth of Eucalyptus irrigated with saline water in the same greenhouse conditions for this work. The closing of the stomata in response to drought alters water use efficiency and decreases the photosynthetic rate (HOMMEL et al., 2014HOMMEL, R. et al. Drought response of mesophyll conductance in forest understory species - impacts on water-use efficiency and interactions with leaf water movement. Physiologia Plantarum , Copenhagen, v. 152, p. 98-114, 2014. doi: 10.1111/ppl.12160.
https://doi.org/10.1111/ppl.12160...
).
The reduced growth and increased mortality is common in forest species under low water supplies (ALLEN et al., 2010ALLEN, C. et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management, Amsterdam, v. 259, p. 660-684, 2010. doi: 10.1016/j.foreco.2009.09.001.
https://doi.org/10.1016/j.foreco.2009.09...
). Unlike juicy stem plants, which use stored water to supply the leaves and maintain some level of hydration with open stomata (MATOS et al., 2014MATOS, F. S. et al. Estratégia morfofisiológica de tolerância ao déficit hídrico de mudas de pinhão manso. Magistra, Cruz das Almas, v. 26, p. 19-27, 2014.), Tectona grandis deciduous plants reduce leaf transpiration through an efficient stomatal control mechanism. According to Borchert (1999), deciduous trees have low transpiration rate in the dry season and can stand to some extent low water supply due to dehydration. The reduction in the specific leaf area may be related to the increased thickness of the cuticle to minimize perspiration under the low water supply to which plants are subjected.
It is unlikely that the NaCl salt toxic effect is accountable for the decrease in leaf total chlorophyll concentration. The mild reduction in leaf chlorophyll concentration may be associated with the species photoprotection mechanism, because underwater restriction, the formation of free radicals that damage membranes and proteins is common (MATOS et al., 2009MATOS, F. S. et al. Caracterização fisiológica de mudas de Jatropha curcas L. produzidas em diferentes níveis de irradiância. Revista Colombiana de Ciências Hortícolas, Bogotá, v. 3, p. 126-134, 2009.). In these circumstances the reduced light energy absorption due to low chlorophyll concentration is an important morphophysiological adjustment to minimize the deleterious photochemical energy excess effects.
CONCLUSIONS
-
Plants of teak have a high stomatal sensitivity and isohydric control mechanism of transpiration by water loss.
-
At the initial stage of development the plant teak shows sensitivity to salt stress and may even compromise the ones irrigated with saline water, however, further studies are needed to identify toxic salt effects on the metabolism of plant teak and the mortality rate of adult plants in the field.
ACKNOWLEDGMENTS
To Goiás State University (UEG), Coordination for the Improvement of Higher Education (CAPES) and Foundation for Support to Goiás State Research (FAPEG) for the project financing: AUXPE 2370/2014.
REFERENCES
- ASSOCIAÇÃO BRASILEIRA DE PRODUTORES DE FLORESTAS PLANTADAS. [Website]. [2013]. Available at: <Available at: www.abraflor.org.br >. Access on: 16 may 2013.
» www.abraflor.org.br - ALLEN, C. et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology and Management, Amsterdam, v. 259, p. 660-684, 2010. doi: 10.1016/j.foreco.2009.09.001.
» https://doi.org/10.1016/j.foreco.2009.09.001 - ALLEN, R. G. et al. Crop evapotranspiration: guidelines for computing crop water requirements. Rome: FAO, 1998. 297 p. (FAO. Irrigation and Drainage Paper, 56).
- BORCHERT, R. Soil and stem water storage determine phenology and distribution of tropical dry forest Trees. Ecology, Washington, v. 75, p. 1437-1449, 1994. doi: 10.2307/1937467.
» https://doi.org/10.2307/1937467 - BRASIL. Ministério da Integração Nacional. Notícias. [2015]. Available at: <Available at: http://www.integracao.gov.br/noticias >. Access on: 27 apr. 2015.
» http://www.integracao.gov.br/noticias - CAVATTE, P. C. et al. Could shading reduce the negative impacts of drought on coffee? The morpho-physiological analysis. Physiologia Plantarum, Copenhagen, v. 144, p. 111-122, 2012. doi: 10.1111/j.1399-3054.2011.01525.x.
» https://doi.org/10.1111/j.1399-3054.2011.01525.x. - CENTRO DE ESTUDOS AVANÇADOS EM ECONOMIA APLICADA. [Website]. [2015]. Available at: <Available at: http://cepea.esalq.usp.br/pib >. Access on: 27 apr. 2015.
» http://cepea.esalq.usp.br/pib - FERREIRA, D. F. Sisvar: a computerstatisticalanalysis system. Ciência e Agrotecnologia, Lavras, v. 35, p. 1039-1042, 2011. doi: 10.1590/S1413-70542011000600001.
» https://doi.org/10.1590/S1413-70542011000600001 - FERREIRA, S. M. et al. Competitividade do Brasil no mercado internacional de madeira serrada. Cerne, Lavras, v. 18, p. 99-104, 2012. doi:10.1590/S0104-77602012000100012.
» https://doi.org/10.1590/S0104-77602012000100012 - HOMMEL, R. et al. Drought response of mesophyll conductance in forest understory species - impacts on water-use efficiency and interactions with leaf water movement. Physiologia Plantarum , Copenhagen, v. 152, p. 98-114, 2014. doi: 10.1111/ppl.12160.
» https://doi.org/10.1111/ppl.12160 - LOPES, T. C. et al. Desenvolvimento inicial de plantas de Eucalyptus platyphylla submetidas a níveis de salinidade. Irriga, Botucatu, v. 17, p. 494-500, 2012. 10.15809/irriga.2012v17n4p494
» https://doi.org/10.15809/irriga.2012v17n4p494 - MATOS, F. S. et al. Caracterização fisiológica de mudas de Jatropha curcas L. produzidas em diferentes níveis de irradiância. Revista Colombiana de Ciências Hortícolas, Bogotá, v. 3, p. 126-134, 2009.
- MATOS, F. S. et al. Estratégia morfofisiológica de tolerância ao déficit hídrico de mudas de pinhão manso. Magistra, Cruz das Almas, v. 26, p. 19-27, 2014.
- RECH, C. Características da teca. Revista da Madeira, Bento Gonçalves, v. 118, p. 55-57, 2009.
- RHOADES, J. D.; KANDIAH, A. M.; MARSHALI, A. M. Uso de águas salinas para produção agrícola. Estudos da FAO - Irrigação e Drenagem, Campina Grande, v. 48, p. 1-117, 2000.
- SHANNON, M. C. et al. Whole plant response to salinity. In: WILKIMAN, R. E. (Ed.). Plant environment interactions. New York: Marcel Dekker, 1994. p. 199-244.
- SOUZA, B. R. et al. Growth of Eucalyptus plants irrigated with saline water. African Journal of Agricultural Research, Nigeria, v. 10, p. 1091-1096, 2015. doi: 10.5897/AJAR2014.9087.
» https://doi.org/10.5897/AJAR2014.9087. - ZANG, C. et al. Patterns of drought tolerance in major European temperature forest trees: Climatic drivers and levels of variability. Global Change Biology, Oxford, v. 20, p. 3767-3779, 2014. doi: 10.1111/gcb.12637.
» https://doi.org/10.1111/gcb.12637. - WORBES, M.; BLANCHART, S.; FICHTLER, E. Relations between water balance, wood traits and phenological behavior of tree species from a tropical dry forest in Costa Rica - a multifactorial study. Tree Physiology, Oxford, v. 33, p. 527-536, 2013.
- WELLBURN, A. R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. Journal of Plant Physiology, Oxford, v. 144, p. 307-313, 1994. doi: 10.1016/S0176-1617(11)81192-2.
» https://doi.org/10.1016/S0176-1617(11)81192-2.
Publication Dates
-
Publication in this collection
Jul-Sep 2017
History
-
Received
14 Aug 2015 -
Accepted
03 Dec 2015