Abstracts
This paper describes solar heterogeneous photocatalysis using immobilized TiO2 applied in the treatment of agricultural waste resulting from the application of commercial formulations of methyl parathion. The disappearance of the insecticide, as well as the formation of its metabolite, was monitored by high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS), while mineralization efficiency was monitored by measurements of total organic carbon (TOC). Toxicity studies were performed using the microcrustacean Artemia salina. The TOC removal efficiency by photocatalytic process was 48.5%. After 45 minutes of treatment, the removal efficiency of methyl parathion was 90%, being completely mineralized at the end of treatment. The formation and removal of the metabolite methyl paraoxon was observed during the photocatalytic process. The photocatalytic treatment resulted in increased microcrustacean mobility, indicating a reduction of acute toxicity.
Keywords:
methyl parathion; solar photocatalysis; toxicity.
Neste trabalho, fotocatálise heterogênea solar utilizando TiO2 imobilizado foi aplicada no tratamento de efluentes agrícolas decorrentes da aplicação de formulações comercias de metil paration. O desaparecimento do inseticida, bem como a formação de seu metabólito foram monitorados por meio de cromatografia líquída de alta eficiência acoplada ao espectrômetro de massas (LC-MS/MS), enquanto a eficiência de mineralização foi monitorada por medidas de carbono orgânico total (COT). Estudos de toxicidade foram realizados utilizando o microcrustáceo Artemia salina. A eficiência de remoção de COT pelo processo fotocatalítico foi de 48,5%. Após 45 minutos de tratamento, a eficiência de remoção do metil paration foi de 90%, sendo completamente mineralizado ao final do tratamento. Durante o process fotocatalítico foi observada a formação e remoção do metabólito metil paraoxon. O tratamento fotocatalítico resultou num aumento da mobilidade do microcrustáceo, indicando diminuição da toxicidade aguda.
Keywords:
fotocatálise solar; metil paration; toxicidade.
1. INTRODUCTION
Compounds with insecticidal purposes are included in several priority lists of pollutants in many countries due to their worldwide use, high toxicity for biotic systems and probability of being discharged into the aquatic environment (Dai et al., 2009DAI, K.; PING, T.; CHEN, H.; LIU, J.; ZAN, L. Photocatalytic degradation of comercial phoxim over La- Doped TiO2 nanoparticles in aqueous suspension. Environmental Science Technology, v. 43, p. 1540-1545, 2009. http://dx.doi.org/10.1021/es802724q
http://dx.doi.org/10.1021/es802724q...
).
Methyl parathion (MP) is an organophosphorous pesticide (OPP) introduced into the agricultural market in the early 1950s. One of major pollutants designated by the US-Environmental Protection Agency (US-EPA) is 4-nitrophenol (4-NP) (nitroaromatic pollutant), which can be released into soil as a result of the hydrolysis of several organophosphates pesticides such as parathion and methyl parathion. MP is an insecticide characterized by an inhibiting role of cholinesterase, an enzyme which hydrolyzes acetylcholine, a neurotransmitter present in nerve synapses. It can lead to the formation of degradation products which are more toxic than the parent compound, such as methyl paraoxon.
In Brazil, Mato Grosso State is an important producer of soy, cotton and corn. MP is widely used in this state. Farms generate wastewaters that consist of mixtures of several commercial pesticides with relatively high concentrations of the active ingredients (Janin et al., 2013JANIN, T.; GOETZ, V.; BROSILLON, S. Solar photocatalytic mineralization of 2,4-dichlorophenol and mixtures os pesticides: Kinetic model of mineralization. Solar Energy, v. 87, p. 127-135, 2013. http://dx.doi.org/10.1016/j.solener.2012.10.017
http://dx.doi.org/10.1016/j.solener.2012...
). This material is the result of the washing process of the sprayers after the application of the pesticides on the crops. Thus, in order to avoid contamination of the environment, it is necessary to detoxify this wastewater from agricultural farms. In order to do this, it is necessary to develop technologies to treat this type of effluent that are friendly to the field workers.
To degrade OPs, diverse physical-chemical treatments, such as granular-activated carbon, wet air oxidation, photo-Fenton, UV/O3 or UV/H2O2, and electro Fenton have been applied. Beside these, removal of phosphorus from pesticidal effluents can be achieved by combined chemical-biological treatment. Also, among novel techniques, advanced oxidation processes (AOPs) have been widely used (Kukurina et al., 2014KUKURINA, O.; ELEMESOVAA, Z.; SYSKINAA, A. Mineralization of organophosphorous pesticides by electro-generated oxidants. Procedia Chemistry, v .10, p. 209-2016, 2014. http://dx.doi.org/10.1016/j.proche.2014.10.036
http://dx.doi.org/10.1016/j.proche.2014....
)
Advanced oxidation processes (AOPs) have been widely used for environmental remediation applications. Heterogeneous photocatalysis using a solid photocatalyst under UV irradiation produces highly oxidative species (reactive free radicals) (Rammohan and Nadagouda, 2013RAMMOHAN, G.; NADAGOUDA, M. N. Green photocatalytic for degradation of organic contaminants: A review. Current Organic Chemistry, v. 17, p. 2338-2348, 2013.). Combined with the direct use of solar energy, this process is almost energy self-sufficient and permits the design of a water treatment that is simple, robust and inexpensive (Malato et al., 2000MALATO, S.; BLANCO, J.; TICHTER, C.; MALDONATO, M. L. Optimization of pré industrial solar photocatalytic mineralization of commercial pesticide-Application to pesticide container recycling. Applied Catalysis B: Environmental, v. 25, p. 31-38, 2000. http://dx.doi.org/10.1016/S0926-3373(99)00114-9
http://dx.doi.org/10.1016/S0926-3373(99)...
). Among these semiconductor materials, TiO2 is the most extensively and intensively studied.
TiO2-mediated photocatalysis has a number of advantages. It is a sustainable, green process that leads to the complete degradation of organic compounds into innocuous products compared to conventional treatments, which often result in the transformation of the compounds into other persistent and non-biodegradable intermediates (Rammohan and Nadagouda, 2013RAMMOHAN, G.; NADAGOUDA, M. N. Green photocatalytic for degradation of organic contaminants: A review. Current Organic Chemistry, v. 17, p. 2338-2348, 2013.).
Many efforts have been devoted to the design and fabrication of nano-sized or film-state photocatalysts with improved photocatalytic efficiency. Visible-light photocatalysis is extensively considered as sustainable AOP by harnessing solar energy, making it economically feasible and environmentally benign. The key to achieving this goal lies in the fabrication of highly visible-photoactive materials with narrowed but suitable band gap. Wide band gap semiconductor photocatalysts, such as TiO2, can only absorb UV light, which only includes 4% of the whole solar spectra. In order to increase visible light absorption, researchers generally use the above-summarized strategies to fabricate highly visible-responsive materials with narrowed band gap (Xiao et al., 2015XIAO, J.; XU, Y.; CAO, H. Organic pollutants removal in wastewater by heterogeneous photocatalytic ozonation. Chemosphere, v. 121, p.1-17, 2015. http://dx.doi.org/10.1016/j.chemosphere.2014.10.072
http://dx.doi.org/10.1016/j.chemosphere....
).
Examples of the application of TiO2 to treat different pesticides like organophosphate, carbamate, organochlorine, triazine and others are described in the literature (Shawaqfeh and Al Momani, 2010SHAWAQFEH, A. T.; AL MOMANI, F. A. Photocatalytic treatment of water soluble pesticide by advanced oxidation technologies using UV light and solar energy. Solar Energy, v. 84, p. 1157-1165, 2010. http://dx.doi.org/10.1016/j.solener.2010.03.020
http://dx.doi.org/10.1016/j.solener.2010...
; Sud and Kaur, 2012SUD, D.; KAUR, P. Heterogeneous Photocatalytic Degradation of Selected Organophosphate Pesticides: A Review. Critical Reviews in Environmental Science Technology, v. 42, p. 2365-2407, 2012. http://dx.doi.org/10.1080/10643389.2011.574184
http://dx.doi.org/10.1080/10643389.2011....
; Senthilnathan and Phili, 2011SENTHILNATHAN, J.; PHILIP, L. Photodegradation of methyl parathion and diclorvos from drinking water with N-doped TiO2 under solar radiation. Chemical Engineering Journal, v. 172, p. 678-688, 2011. http://dx.doi.org/10.1016/j.cej.2011.06.035
http://dx.doi.org/10.1016/j.cej.2011.06....
).
Senthilnathan and Philip (2011SENTHILNATHAN, J.; PHILIP, L. Photodegradation of methyl parathion and diclorvos from drinking water with N-doped TiO2 under solar radiation. Chemical Engineering Journal, v. 172, p. 678-688, 2011. http://dx.doi.org/10.1016/j.cej.2011.06.035
http://dx.doi.org/10.1016/j.cej.2011.06....
) have carried out studies of the degradation of methyl parathion using TiO2-P25 Degussa and TiO2 doped with nitrogen, both in suspension and in solar radiation. Pesticide concentrations of 50 and 100 µg L-1 have been completely degraded at 180 and 260 minutes, respectively, by TiO2-P25. For the process using TiO2 doped with nitrogen, the pesticide has been mineralized at 45 and 75 minutes of treatment, respectively.
Kukurina et al. (2014KUKURINA, O.; ELEMESOVAA, Z.; SYSKINAA, A. Mineralization of organophosphorous pesticides by electro-generated oxidants. Procedia Chemistry, v .10, p. 209-2016, 2014. http://dx.doi.org/10.1016/j.proche.2014.10.036
http://dx.doi.org/10.1016/j.proche.2014....
) studied the electro-generated oxidizing system for efficient mineralization of toxic OPs, such as glyphosate, malathion, bazudin, chlorophos and methaphos. Aqueous sulfuric acidic polluted solutions have been electrolyzed with lead electrodes in an undivided electrolytic cell. Chemical oxygen demand (COD) was determined and compared to analyses of the phosphate ions accumulation. The pesticides were completely destroyed following pseudo first-order kinetics estimated according to chemical oxygen demand (COD) data and phosphate ions accumulation. The assessment of decay rate constants shows the high degree of mineralization.
This paper reports a study of the solar photocatalytic degradation of commercial organophosphorus pesticide (methyl parathion) using a flat panel photoreactor containing TiO2. The total organic carbon (TOC) composition and inorganic ions generated (NO3- PO4-3 and SO4-2) were evaluated during the degradation process. Mineralization of methyl-parathion and the formation and possible mineralization of its major degradation product (methyl paraoxon) were also evaluated. Tests for acute toxicity were performed with the microcrustacean Artemia salina in order to check the detoxification efficiency. A comparative study of the efficiency of degradation of photolysis and photocatalytic processes was also done.
2. Materials and MethodS
2.1. Chemicals
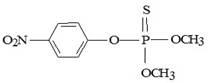
Titanium dioxide (P-25, Degussa), 70:30, anatase, with a surface area of 50 m2 g-1, served as catalyst. A commercial emulsion of parathion-methyl (Folisuper, 600 g L-1) was diluted in water in the laboratory in order to simulate the agricultural wastewater. The structure of the studied pesticide is showed in Figure 1. The analytical standard methyl parathion (dimethyl p-nitrophenyl thiophosphate) used in the chromatographic analysis was acquired from Chem Service (West Chester, USA) with a purity > 98%. Spec Sol (Brazil) supplied the analytical standards traceable to SRM 351a NIST-USA for the determination of inorganic ions (NO3-, PO4-3 e SO4-2). All solvents employed were HPLC grade and the reagents were analytical grade. The ultra-pure water was produced by Millipore Milli-Q Advantage (Millipore, Beldford, USA).
2.2. Analytical determinations
Before chemical analyses, the samples were filtered through membranes with pore diameter of 0.45 µm (PTFE, Millipore Millex(r)). Mineralization was measured through direct injection of the solutions into the total organic carbon analyzer made by Shimadzu, model TOC-VCPH/CPN 5000A, provided with an NDIR detector. Calibration was done with standard solutions of potassium hydrogen phthalate for TC (Total Carbon) and sodium hydrogen carbonate and sodium carbonate for IC (Inorganic Carbon). The inorganic ions (NO3-, PO4-3 e SO4-2) were analyzed by a Metrohm ion chromatography system, Model 861, with detection by ionic conductivity. A Metrosep A, Supp 5 (150 x 4.0 mm) column was used and mobile phase was constituted by a mix of sodium carbonate (3.2 mmol L-1) and sodium hydrogen carbonate (1.0 mmol L-1). The mobile phase flow rate was 0.7 mL min-1. The injection volume was 20 µL.
Concentrations of methyl-parathion and its metabolite were determinated by high performance liquid chromatographic equipment, consisting of a binary pumping system (Varian ProStar 210 model) and Varian ProStar automatic injector (Varian ProStar 410 AutoSampler model). The liquid chromatograph was coupled to an electrospray ionization source (ESI) followed by a Varian 1200L triple quadrupole mass spectrometer (USA). Data acquisition employed Varian MS Workstation software (Version 6). Analytes separation was performed using a Synergi 4µm Fusion RP 80 (150 x 4.60 mm) analytical column (Phenomenex); mobile phases were methanol (A) and 10 mmol L-1 amoniun acetate (B). The total flow rate was 0.4 mL min-1. The initial gradient was 95% (B), maintained for 1 min, then decreased to 70% (B) after 3 minutes and to 5% after 32 min. Multiple reaction monitoring (MRM) data was acquired and processed in positive ESI mode. The optimized analytical parameters had a source temperature of 50°C; capillary voltage was 65 kV for all analytes; and pressure and temperature of drying gas was (N2) 25 psi and 250°C, respectively. The collision gas was argon at 2.0 mTorr pressure. The injection volume was 10 µL. Standard calibration curves of compounds were constructed by plotting analyte concentration against peak areas, at levels of 0.05, 0.1, 0.25, 1.0, 2.5 and 5.0 µg mL-1. The validation parameters were: linearity (correlations coefficients > 0.99) and limit of quantification or LOQ (the lowest concentration that results in RSD ≤ 20%, n=6). Two transitions of higher intensity signal were selected for each analyte in order to quantify and confirm the identity. For methyl-parathion, 263.9<125.0 and 263.9< 232.0, and for methyl-paraoxon, 248 < 202 and 248 < 90 transictions were monitored.
2.3. Agricultural wastewater preparation
The agricultural wastewater was prepared by dilution of commercial methyl parathion solution (Folisuper 600 br) composed of 600 g L-1 of o,o-dimethyl o-4-nitrophenyl phosphorothioate and 528 g L-1 of unknown products. The aqueous suspensions were prepared based on highest application rate recommended by the manufacturer for each product (per hectare) and recommendations described by Chain and Pessoa (2002CHAIN, A.; PESSOA, M. C. P. Y. Método para calibração de pulverizadores utilizados em videiras. Jaguariúna: Embrapa Meio Ambiente, 2002. 5p. (Comunicado Técnico, 9).).
2.4. Photoreactor
The flat panel photo reactor was built at the Laboratory of Residues and Contaminants - Embrapa Meio Ambiente, Jaguariuna-Brazil. The glass plate (dimensions of 40 x 90 cm with 5 mm thickness) was placed on metal supports with the slope angle of 45° (Fig 2). The model and the catalyst immobilization procedure was based on that described by Nogueira and Jardim (1996NOGUEIRA, R.F.P.; JARDIM, W. F. TiO2-Fixed reator for water decontamination using solar light. Solar Energy, v. 5, p. 471-477, 1996. http://dx.doi.org/10.1016/0038-X(96)00036-9 ).
Titanium dioxide adheres strongly to a glass surface and this property has been explored in the degradation of contaminants (Nogueira and Jardim, 1996NOGUEIRA, R.F.P.; JARDIM, W. F. TiO2-Fixed reator for water decontamination using solar light. Solar Energy, v. 5, p. 471-477, 1996. http://dx.doi.org/10.1016/0038-X(96)00036-9 ). TiO2 suspension was passed by gravity flow over the plate and dried with hot air for the immobilization of TiO2 and repeated several times until a homogeneous TiO2 film was attached to the glass. After drying the catalyst, the glass plate was submitted to hot air at 200ºC for 30 minutes for a complete catalyst immobilization. The titanium dioxide film remained stable during the flow of aqueous solutions and no loss of photocatalytic activity was observed during 42 h of use.
Schematic diagram of the TiO2-fixed-bed solar reactor (based on that described by Nogueira and Jardim, 1996NOGUEIRA, R.F.P.; JARDIM, W. F. TiO2-Fixed reator for water decontamination using solar light. Solar Energy, v. 5, p. 471-477, 1996. http://dx.doi.org/10.1016/0038-X(96)00036-9 ).
2.5. Photocatalytic Degradation Experiments
The experiments were performed with a recirculation of the solution from a reservoir of 2 L by means of a peristaltic pump (Ismatec- IPR - 7 channels). The reactor was operated at a flow rate of 4.58 L h-1. Sampling took place at the initial time and after 10, 30, 45, 60 and 90 minutes. The same procedure was applied for the evaluation of degradation via photolysis (absence of catalyst).
2.6. Solar radiation intensity measurement
The experiments were carried out between July 20th and August 19th on days without any noticeable cloud presence, between 9:00 am and 3:00 pm by pyramometer Model SP-LITE-L. The values for intensity of global radiation were utilized and measured at 9:00 am, 12:00 pm and 3:00 pm with the following average values: 318.2; 518.4 and 401.75 Watts m-2, respectively.
2.7. Toxicity
Tests for acute toxicity were performed with nauplii of the microcrustacean Artemia salina, the organisms were exposed during 48 hours to the following concentrations of wastewater: 0 (control), 1, 10 and 100 % (n = 20 per concentration). The acute toxicity of the wastewater prepared in the laboratory before and after applying the photocatalytic process using solar irradiation was evaluated. The test solutions were prepared in reconstituted water for growing Artemia salina, with the organisms remaining at a temperature of 20 ± 2oC and exposed to common light. The organisms were not fed during the bioassay. Statgraphics Plus Version 5 software was used to calculate the median effective concentration that inhibited 50% of organism mobility (EC50 48h) at a confidence interval of 95%. A comparison between the percentage of mobility in the groups exposed to the pesticide solution before and after the catalytic treatment was performed using the t-test. Results were considered statistically significant when p < 0.05 (Vanhaecke et al., 1981VANHAECKE, P.; PERSOONE, G.; CLAUS, C.; SORGELOOS, P. Proposal for a short-term toxicity test with Artemia Nauplii. Ecotoxicology Environmental Safety, v. 5, p.382-387, 1981. http://dx.doi.org/10.1016/0147-6513(81)90012-9
http://dx.doi.org/10.1016/0147-6513(81)9...
).
3. Results and discussion
The simulated agricultural wastewater prepared in the laboratory from commercial insecticide resulted in concentrations of methyl parathion (the active ingredient) of 19.5 mg L-1 and 30.2 mg L-1 of TOC. Some investigations of the photo degradation of pesticides from industrial and agricultural waste have been done using similar concentrations. Sivagami et al. (2014SIVAGAMI, K.; KRISHMA, R. R.; SWAMINATHAN, T. Photocatalytic degradation of pesticides in immobilized bead photo reactor under solar irradiation. Solar Energy, v. 103, p. 488-493, 2014. http://dx.doi.org/10.1016/j.solener.2014.02.001
http://dx.doi.org/10.1016/j.solener.2014...
) studied the solar photocatalytic degradation of pesticide solutions with initial concentrations ranging from 5 to 50 mg L-1, while Dai et al. (2009DAI, K.; PING, T.; CHEN, H.; LIU, J.; ZAN, L. Photocatalytic degradation of comercial phoxim over La- Doped TiO2 nanoparticles in aqueous suspension. Environmental Science Technology, v. 43, p. 1540-1545, 2009. http://dx.doi.org/10.1021/es802724q
http://dx.doi.org/10.1021/es802724q...
) used 20 mg L-1 of commercial phoxim in photocatalytic degradation studies.
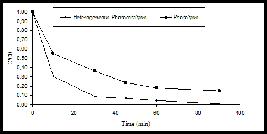
In order to compare the efficiency of the photocatalytic degradation (UV-TiO2) with direct photolysis (UV), experiments were carried out using the same initial concentration of the pesticide, at the original pH of the prepared wastewater (pH 4.6). Figure 3 shows the efficiency of TOC removal by heterogeneous photocatalysis and photolysis in relation to the illumination time. As expected, direct photolysis was less effective than photocatalysis for TOC removal. After 90 minutes of irradiation without TiO2, 39.4% of the initial TOC concentration was degraded while the photocatalytic process achieved 48.5% efficiency.
Despite the incomplete mineralization of the organic load in the wastewaters, a progressive decrease of TOC concentration was observed. Similar results were obtained by Janin et al. (2013JANIN, T.; GOETZ, V.; BROSILLON, S. Solar photocatalytic mineralization of 2,4-dichlorophenol and mixtures os pesticides: Kinetic model of mineralization. Solar Energy, v. 87, p. 127-135, 2013. http://dx.doi.org/10.1016/j.solener.2012.10.017
http://dx.doi.org/10.1016/j.solener.2012...
) in the degradation of commercial formulation pesticide. Several assumptions may explain such behavior, like a low afinity between the by-products and the catalyst and /or a saturation of the surface of the catalyst Janin et al. (2013JANIN, T.; GOETZ, V.; BROSILLON, S. Solar photocatalytic mineralization of 2,4-dichlorophenol and mixtures os pesticides: Kinetic model of mineralization. Solar Energy, v. 87, p. 127-135, 2013. http://dx.doi.org/10.1016/j.solener.2012.10.017
http://dx.doi.org/10.1016/j.solener.2012...
). Bayarri et al. (2005BAYARRI, B.; GIMENEZ, J.; CURCO, D.; ESPLUGAS, S. Photocatalytic degradation 2,4-dichlorophenol byTiO2/UV: kinetics, actinometries and models. Catalysis Today, v. 101, p. 227-236, 2005. http://dx.doi.org/10.1016/j.cattod.2005.03.019
http://dx.doi.org/10.1016/j.cattod.2005....
) have suggested that, at the end of process malic and acetic acids, which are the last compounds detected, develop a low affinity for the TiO2. For the studied wastewater, pH decreased during the degradation process, indicating the formation of acid species, usually carboxylic acids (formic, acetic, and oxalic), which are important intermediate compounds Mahmoodi and Arami (2010)MAHMOOD, N. M.; ARAMI, M. Immobilized titania nanophotocatalysis: Degradation, modeling and toxicity reduction of agricultural pollutants. Journal of Alloys and Compound, v. 506, p. 155-159, 2010. http://dx.doi.org/10.1016/j.jallcom.2010.06.164
http://dx.doi.org/10.1016/j.jallcom.2010...
. These compounds formed during the irradiation period are consistent with other studies that reported the formation of stable organic acids during the photocatalysis. Depending on the pH value, the degradation of pollutant compounds may occur more effectively via hydroxyl radicals or by direct oxidation via h+. Thus, the interpretation of the effect of pH on the degradation efficiency of the photocatalytic process is very complex. Ku and Jung (1998)KU, Y.; JUNG, I. Decomposition of monocrotophos in aqueous solution by UV irradiation in the presence of titanium dioxide. Chemosphere, v. 37, p. 2589-2597, 1998. http://dx.doi.org/10.1016/S0045-6535(98)00158-1
http://dx.doi.org/10.1016/S0045-6535(98)...
have investigated the influence of pH on degradation efficiency of monocrotophos aqueous solutions by TiO2-UV. According to the authors, the pH of the aqueous solution is critical for the decomposition of this pesticide and the process is more effective in acidic solutions.
TOC efficiency removal by heterogeneous photocatalysis and photolysis in relation to illumination time.
The active principle almost disappeared from the solution at the end of the photocatalytic treatment, while the photolysis treatment achieved 86 % of removal efficiency. The results of photocatalytic process (mineralization and degradation efficiency) can be considered very significant, particularly with respect to reduced treatment time (90 min). Janin et al. (2013)JANIN, T.; GOETZ, V.; BROSILLON, S. Solar photocatalytic mineralization of 2,4-dichlorophenol and mixtures os pesticides: Kinetic model of mineralization. Solar Energy, v. 87, p. 127-135, 2013. http://dx.doi.org/10.1016/j.solener.2012.10.017
http://dx.doi.org/10.1016/j.solener.2012...
studied the degradation of the fungicide pyrimethanil from a commercial solution by solar photocatalytic reactor using immobilized TiO2. The commercial solution was treated for 50 h and the mineralization yield reached 60% for an initial TOC concentration of 15 mg L-1. Sivagami et al. (2014)SIVAGAMI, K.; KRISHMA, R. R.; SWAMINATHAN, T. Photocatalytic degradation of pesticides in immobilized bead photo reactor under solar irradiation. Solar Energy, v. 103, p. 488-493, 2014. http://dx.doi.org/10.1016/j.solener.2014.02.001
http://dx.doi.org/10.1016/j.solener.2014...
studied the photo catalytic degradation of three pesticides, widely used in India, monocrotophos (MCP), endosulfan (ES) and chlorpyriphos (CPS), in sunlight using a TiO2 Immobilized Bead Photo Reactor. They observed that nearly 50% of the initial pesticide concentration was removed in the early stage (1h) and the remaining pesticides removal took a longer time due to formation of intermediates Sivagami et al. (2014)SIVAGAMI, K.; KRISHMA, R. R.; SWAMINATHAN, T. Photocatalytic degradation of pesticides in immobilized bead photo reactor under solar irradiation. Solar Energy, v. 103, p. 488-493, 2014. http://dx.doi.org/10.1016/j.solener.2014.02.001
http://dx.doi.org/10.1016/j.solener.2014...
.
Of particular importance in this work was the formation and total degradation of methyl paraoxon using the photocatalytic process, since it is considered even more toxic than the parent compound. During the photolysis process the formation of this compound was observed only after 60 minutes of treatment.
Figure 4 shows the efficiency of MP degradation by the photolysis and TiO2-UV processes.
Methyl parathion formation and degradation during degradation through heterogeneous photocatalysis and photolysis.
For photocatalytic degradation of methyl parathion, ion chromatographic analysis allowed qualitative and quantitative monitoring of mineralization of the compound. It is noticeable that the concentration of nitrate ions is much lower than other monitored anions. This can be explained by the possible formation of other nitrogen ions such as NO2-, NH4+ (which were not monitored in this study) and gaseous compounds such as N2 e NH3 which have been released to the environment during the irradiation period. Mahmmoodi and Arami (2010) reported similar results in the degradation of alachlor with nanoparticles of immobilized TiO2 - P25. It has been found that the concentration of nitrate ions resulting from the process of degradation was lower than expected. According to the authors, species containing nitrogen may have been adsorbed on the catalyst surface or, most likely, resulted in significant amounts of N2 or NH3 transferred to the gas phase. Kim et al. (2006KIM, T. S.; KIM, K.; COI, K.; STENSTROM, M. K.; ZOH, K. D. Degradation mechanism and the toxicity assessment in TiO2 photocatalysis and photolysis of parathion. Chemosphere, v. 62, p. 926-933, 2006. http://dx.doi.org/10.1016/j.chemosphere .2005.05.038
http://dx.doi.org/10.1016/j.chemosphere ...
) reported that 18% of the initial concentration of nitrogen present in the parathion-methyl was converted to NH4+ by the end of the photocatalytic process. A large increase in the concentration of inorganic ions was formed after 50 min of treatment, when the removal of the metabolite paraoxon-methyl was observed, indicating its mineralization.
3.1. Toxicity reduction by photocatalytic process
Little research has been conducted on toxicity reduction using heterogeneous photocatalysis and immobilized TiO2. As the last step of this experimental study, changes in the acute toxicity of agriculture wastewater after solar UV photocatalytic treatment were evaluated considering the microcrustacean Artemia salina.
The solution without the toxic material (control) showed a mobility rate of 92.5%, which was in accordance with the value necessary to validate the test (>90%).
Results of this study showed that the photocatalytic process was efficient enough in reducing toxicity from the wastewater containing methyl-parathion. The EC50-48h value for the prepared wastewater that was not submitted to the photocatalytic treatment was 42.69 (30.12 -63.18) %. This corresponds to 8.11 (5.72 - 12.00) mg L-1 of the active ingredient. The mobility data obtained from the effluent toxicity evaluation after the photocatalytic treatment did not allow plotting a concentration-response relationship necessary to calculate an EC50-48h. Therefore, a comparison between the percentages of mobility in the groups was performed using the t-test. A significant increase was observed (p = 0.0002) in the mobility rate of the group exposed to the treated wastewater (80%) compared to the group exposed to the initial wastewater (5%).
4. Conclusion
The degradation experiments of this study have shown that the solar photocatalytic reactor, using immobilized TiO2 photocatalyst is more efficient than the photolysis process in the degrading of agricultural wastewater with high concentrations of organoposhorous pesticides, with reduced treatment time. The phototocalytic process showed the TOC removal efficiency for commercial pesticide was around 50% and almost total mineralization of the pesticide was achieved at the end treatment (90 minutes). The complete mineralization of its major degradation product was accomplished in the same treatment time. Tests of acute toxicity during the treatment process were performed with the microcrustacean Artemia salina. The comparison between the waste before and after the treatment showed an increase in the mobility rate of the microcrustacean indicating a diminution of acute toxicity. The results showed that immobilized titaniun dioxide solar photocatalysis is a promising and environmentally friendly method for degradation and toxicity reduction of agricultural organophosphorous waste.
5. ACKNOWLEDGEMENTS
The authors gratefully acknowledge the financial support received from the EMBRAPA.
- BAYARRI, B.; GIMENEZ, J.; CURCO, D.; ESPLUGAS, S. Photocatalytic degradation 2,4-dichlorophenol byTiO2/UV: kinetics, actinometries and models. Catalysis Today, v. 101, p. 227-236, 2005. http://dx.doi.org/10.1016/j.cattod.2005.03.019
» http://dx.doi.org/10.1016/j.cattod.2005.03.019 - CHAIN, A.; PESSOA, M. C. P. Y. Método para calibração de pulverizadores utilizados em videiras. Jaguariúna: Embrapa Meio Ambiente, 2002. 5p. (Comunicado Técnico, 9).
- DAI, K.; PING, T.; CHEN, H.; LIU, J.; ZAN, L. Photocatalytic degradation of comercial phoxim over La- Doped TiO2 nanoparticles in aqueous suspension. Environmental Science Technology, v. 43, p. 1540-1545, 2009. http://dx.doi.org/10.1021/es802724q
» http://dx.doi.org/10.1021/es802724q - JANIN, T.; GOETZ, V.; BROSILLON, S. Solar photocatalytic mineralization of 2,4-dichlorophenol and mixtures os pesticides: Kinetic model of mineralization. Solar Energy, v. 87, p. 127-135, 2013. http://dx.doi.org/10.1016/j.solener.2012.10.017
» http://dx.doi.org/10.1016/j.solener.2012.10.017 - KIM, T. S.; KIM, K.; COI, K.; STENSTROM, M. K.; ZOH, K. D. Degradation mechanism and the toxicity assessment in TiO2 photocatalysis and photolysis of parathion. Chemosphere, v. 62, p. 926-933, 2006. http://dx.doi.org/10.1016/j.chemosphere .2005.05.038
» http://dx.doi.org/10.1016/j.chemosphere .2005.05.038 - KU, Y.; JUNG, I. Decomposition of monocrotophos in aqueous solution by UV irradiation in the presence of titanium dioxide. Chemosphere, v. 37, p. 2589-2597, 1998. http://dx.doi.org/10.1016/S0045-6535(98)00158-1
» http://dx.doi.org/10.1016/S0045-6535(98)00158-1 - KUKURINA, O.; ELEMESOVAA, Z.; SYSKINAA, A. Mineralization of organophosphorous pesticides by electro-generated oxidants. Procedia Chemistry, v .10, p. 209-2016, 2014. http://dx.doi.org/10.1016/j.proche.2014.10.036
» http://dx.doi.org/10.1016/j.proche.2014.10.036 - MAHMOOD, N. M.; ARAMI, M. Immobilized titania nanophotocatalysis: Degradation, modeling and toxicity reduction of agricultural pollutants. Journal of Alloys and Compound, v. 506, p. 155-159, 2010. http://dx.doi.org/10.1016/j.jallcom.2010.06.164
» http://dx.doi.org/10.1016/j.jallcom.2010.06.164 - MALATO, S.; BLANCO, J.; TICHTER, C.; MALDONATO, M. L. Optimization of pré industrial solar photocatalytic mineralization of commercial pesticide-Application to pesticide container recycling. Applied Catalysis B: Environmental, v. 25, p. 31-38, 2000. http://dx.doi.org/10.1016/S0926-3373(99)00114-9
» http://dx.doi.org/10.1016/S0926-3373(99)00114-9 - NOGUEIRA, R.F.P.; JARDIM, W. F. TiO2-Fixed reator for water decontamination using solar light. Solar Energy, v. 5, p. 471-477, 1996. http://dx.doi.org/10.1016/0038-X(96)00036-9
- RAMMOHAN, G.; NADAGOUDA, M. N. Green photocatalytic for degradation of organic contaminants: A review. Current Organic Chemistry, v. 17, p. 2338-2348, 2013.
- SHAWAQFEH, A. T.; AL MOMANI, F. A. Photocatalytic treatment of water soluble pesticide by advanced oxidation technologies using UV light and solar energy. Solar Energy, v. 84, p. 1157-1165, 2010. http://dx.doi.org/10.1016/j.solener.2010.03.020
» http://dx.doi.org/10.1016/j.solener.2010.03.020 - SENTHILNATHAN, J.; PHILIP, L. Photodegradation of methyl parathion and diclorvos from drinking water with N-doped TiO2 under solar radiation. Chemical Engineering Journal, v. 172, p. 678-688, 2011. http://dx.doi.org/10.1016/j.cej.2011.06.035
» http://dx.doi.org/10.1016/j.cej.2011.06.035 - SIVAGAMI, K.; KRISHMA, R. R.; SWAMINATHAN, T. Photocatalytic degradation of pesticides in immobilized bead photo reactor under solar irradiation. Solar Energy, v. 103, p. 488-493, 2014. http://dx.doi.org/10.1016/j.solener.2014.02.001
» http://dx.doi.org/10.1016/j.solener.2014.02.001 - SUD, D.; KAUR, P. Heterogeneous Photocatalytic Degradation of Selected Organophosphate Pesticides: A Review. Critical Reviews in Environmental Science Technology, v. 42, p. 2365-2407, 2012. http://dx.doi.org/10.1080/10643389.2011.574184
» http://dx.doi.org/10.1080/10643389.2011.574184 - VANHAECKE, P.; PERSOONE, G.; CLAUS, C.; SORGELOOS, P. Proposal for a short-term toxicity test with Artemia Nauplii. Ecotoxicology Environmental Safety, v. 5, p.382-387, 1981. http://dx.doi.org/10.1016/0147-6513(81)90012-9
» http://dx.doi.org/10.1016/0147-6513(81)90012-9 - XIAO, J.; XU, Y.; CAO, H. Organic pollutants removal in wastewater by heterogeneous photocatalytic ozonation. Chemosphere, v. 121, p.1-17, 2015. http://dx.doi.org/10.1016/j.chemosphere.2014.10.072
» http://dx.doi.org/10.1016/j.chemosphere.2014.10.072
Publication Dates
-
Publication in this collection
Dec 2016
History
-
Received
14 Dec 2015 -
Accepted
20 June 2016