Abstracts
In 2003, symptoms of Pierce Disease (PD) were observed in two vineyards established in two different localities in San Jose province (Santa Ana and La Uruca) and in La Garita, Alajuela province. Twenty four symptomatic plants (15 from Santa Ana, five from La Uruca and four from La Garita) were analyzed by DAS-ELISA using antibodies against Xylella fastidiosa. Sixteen plants (nine from Santa Ana, five from La Uruca, and two from La Garita) were positives for the bacterium. Isolation attempts were made from petioles of these plants, and seven isolates were obtained in liquid PW. Aliquots of liquid media were inoculated on solid PW. Ten days after inoculation, circular and convex colonies, with entire margin, smooth and opalescent, of 0.5-1mm in diameter were observed. From each isolate three colonies were selected and considered as different clones. Every clone was analyzed by DAS-ELISA with positive results. Clones were Gram negative, catalase positive and oxidase negative. The bacteria were rod shaped, and showed a typically rippled cell wall. DNA of each clone was extracted and used as template in PCR with primers 272-1/272-2 and RST31/RST33; products of 500pb and 733pb were obtained, respectively. These results confirmed the presence of X. fastidiosa causing PD in grapevine plants in different vineyards in Costa Rica.
DAS-ELISA; isolation; PCR; electron microscopy; Pierce Disease
Durante el año 2003, se observaron síntomas del mal de Pierce (PD) en dos plantaciones de vid en la provincia de San José (Santa Ana y La Uruca) y en otra en La Garita, provincia de Alajuela. De un total de 24 plantas sintomáticas analizadas mediante DAS-ELISA usando anticuerpos para Xylella fastidiosa, se detectaron 16 plantas positivas (nueve de Santa Ana, cinco de La Uruca y dos de La Garita). A partir de diferentes intentos de aislamiento de los peciolos en medio líquido PW, se obtuvieron siete aislamientos. Alícuotas de medio líquido se inocularon en medio PW sólido. Diez días después de la inoculación, se observaron colonias circulares, cónvexas, lisas y opalescentes, con márgenes enteros, con diámetro entre 0.5-1mm. De cada aislamiento se seleccionaron tres colonias, las cuales fueron consideradas como clones diferentes. Todos los clones presentaron valores positivos mediante DAS-ELISA. Los clones presentaron bacilos Gram negativo, catalasa positivo y oxidasa negativo. Los bacilos al observarse al microscopio electrónico de transmisión presentaron la pared rugosa previamente informada para X. fastidiosa. Al amplificar el ADN extraido de cada clon mediante la técnica de la PCR con los imprimadores 272-1/272-2 y RST31/RST33, se obtuvieron productos de 500pb y 733pb, respectivamente. Estos resultados confirman la presencia de X. fastidiosa causando mal de Pierce en vid en Costa Rica.
DAS-ELISA; aislamiento; PCR; microscopia electrónica; mal de Pierce
SHORT COMMUNICATION COMUNICAÇÃO
Confirmation of Xylella fastidiosa infecting grapes Vitis vinifera in Costa Rica
Confirmación de la presencia de Xylella fastidiosa infectando vid (Vitis vinifera) en Costa Rica
Estela AguilarI; Lisela MoreiraI,II; Carmen RiveraI,III
ICentro de Investigación en Biología Celular y Molecular, Universidad de Costa Rica UCR
IIEscuela de Agronomía, Universidad de Costa Rica
IIIFacultad de Microbiología, Universidad de Costa Rica, 11501x-2060, San José, Costa Rica
ABSTRACT
In 2003, symptoms of Pierce Disease (PD) were observed in two vineyards established in two different localities in San Jose province (Santa Ana and La Uruca) and in La Garita, Alajuela province. Twenty four symptomatic plants (15 from Santa Ana, five from La Uruca and four from La Garita) were analyzed by DAS-ELISA using antibodies against Xylella fastidiosa. Sixteen plants (nine from Santa Ana, five from La Uruca, and two from La Garita) were positives for the bacterium. Isolation attempts were made from petioles of these plants, and seven isolates were obtained in liquid PW. Aliquots of liquid media were inoculated on solid PW. Ten days after inoculation, circular and convex colonies, with entire margin, smooth and opalescent, of 0.5-1mm in diameter were observed. From each isolate three colonies were selected and considered as different clones. Every clone was analyzed by DAS-ELISA with positive results. Clones were Gram negative, catalase positive and oxidase negative. The bacteria were rod shaped, and showed a typically rippled cell wall. DNA of each clone was extracted and used as template in PCR with primers 272-1/272-2 and RST31/RST33; products of 500pb and 733pb were obtained, respectively. These results confirmed the presence of X. fastidiosa causing PD in grapevine plants in different vineyards in Costa Rica.
Keywords: DAS-ELISA, isolation, PCR, electron microscopy, Pierce Disease
RESUMEN
Durante el año 2003, se observaron síntomas del mal de Pierce (PD) en dos plantaciones de vid en la provincia de San José (Santa Ana y La Uruca) y en otra en La Garita, provincia de Alajuela. De un total de 24 plantas sintomáticas analizadas mediante DAS-ELISA usando anticuerpos para Xylella fastidiosa, se detectaron 16 plantas positivas (nueve de Santa Ana, cinco de La Uruca y dos de La Garita). A partir de diferentes intentos de aislamiento de los peciolos en medio líquido PW, se obtuvieron siete aislamientos. Alícuotas de medio líquido se inocularon en medio PW sólido. Diez días después de la inoculación, se observaron colonias circulares, cónvexas, lisas y opalescentes, con márgenes enteros, con diámetro entre 0.5-1mm. De cada aislamiento se seleccionaron tres colonias, las cuales fueron consideradas como clones diferentes. Todos los clones presentaron valores positivos mediante DAS-ELISA. Los clones presentaron bacilos Gram negativo, catalasa positivo y oxidasa negativo. Los bacilos al observarse al microscopio electrónico de transmisión presentaron la pared rugosa previamente informada para X. fastidiosa. Al amplificar el ADN extraido de cada clon mediante la técnica de la PCR con los imprimadores 272-1/272-2 y RST31/RST33, se obtuvieron productos de 500pb y 733pb, respectivamente. Estos resultados confirman la presencia de X. fastidiosa causando mal de Pierce en vid en Costa Rica.
Palabras clave: DAS-ELISA, aislamiento, PCR, microscopia electrónica, mal de Pierce.
Pierce Disease (PD) is a serious problem in most grape growing regions of the United States, mainly in the Gulf Coastal Plains and California (Goheen et al., 1978). The first report of PD,was made by Newton Pierce in 1892 in Santa Ana River Valley in Southern California (Pierce 1892 cited by Goheen et al., 1973). For several decades the etiological agent of PD was consider to be a virus, because it could be transmitted from disease to healthy grapevines by grafting and by several homopterous insects (Hopkins & Mollenhauer, 1973; Davis et al., 1978; Wells et al., 1987). However, the successful suppression of the development of symptoms with tetracycline antibiotics, in 1970, and the observation by electron microscopy of a xylem bacterium in symptomatic plants indicated that the causal agent was not a virus (Hopkins & Mollenhauer, 1973; Mollenhauer & Hopkins, 1974).
In 1978, Davis et al. reported the consistent culture of a Gram negative, catalase-positive bacterium, from petioles of grapevine plants with PD, in special media cultures. The isolate's pathogenicity was tested by the inoculation of green stem cuttings of grapevine varieties. After 2 to 4 months typical PD symptoms developed in 86% of the inoculated cuttings (Davis et al., 1978). After its isolation, researchers referred to this pathogenic bacterium as a fastidious xylem limited bacterium (FXLB) (Hopkins, 1989). In 1984, Wells et al. proposed the name Xylella fastidiosa to establish a new genus with one species to include all FXLBs.
The symptoms of PD include plant vigor decline, marginal necrosis of the leaves, wilting and drying of the fruit (Hopkins et al., 1981). PD was also reported in Mexico and Venezuela, and outside of America in Kosovo, Balkans (Goheen et al., 1979; Hernandez & Corona et al., 1994; 1997; Berisha et al., 1998). Goheen et al. (1979) isolated the bacterium in JD-3 media from leaf petioles showing PD symptoms, collected in Montezuma, Puntarenas province in Costa Rica and reported PD for the first time in Central America. This report was confirmed by Jiménez (1980). Symptomatic plant samples were collected from a vineyard located at Guanacaste, Alajuela, Puntarenas and Cartago provinces The PD bacterium was isolated only from Puntarenas samples, but the presence of the bacterium in other provinces of Costa Rica was not discarded. Between 1982 and 1983 other studies showed more bacterium host plant species and insect vectors in the same four Costa Rica provinces (Mora, 1982; Barrantes, 1983). During the last three years, symptoms similar to PD were observed in vineyards located in Santa Ana and La Uruca in San José and La Garita (Alajuela).
To confirm that X. fastidiosa is the causal agent of these symptoms, symptomatic leaves (Figure 1) were collected from five grapevine plants located at Santa Ana, La Uruca and La Garita. Petioles of these leaves were ground in 1mL of general extraction solution (0.13% sodium sulfite, 2% polyvinylpyrrolid, 0.02% sodium azide, 0.2% powdered chicken-egg albumin, grade II, 2% Tween 20®). The extracts were tested for X. fastidiosa by DAS-ELISA using a PathoScreen kit according to the manufacturer´s instructions (Agdia, Inc., Elkhart, IN). A positive reaction was determined to be greater than the mean of the absorbance at 490 nm of the negative controls plus three times the standard deviation.
For bacterial isolation one, two and four petioles from each sample of Santa Ana, La Uruca and La Garita were used. Each end of the petiole was sealed with paraffin and the surface sterilized in 70% ethanol for 5 to 7 min, then in 1% sodium hypochlorite for 5 to 7 min, followed by three 5-min washes in sterile water. The petioles of Santa Ana and La Uruca were aseptically cut into 3 to 6mL of PW broth, and agitated for 15 min to release the bacteria from xylem. Suspension aliquots of 300µL were inoculated by triplicate in liquid PW and liquid SPW media. Dilutions of the suspension (10-1 -10-4) were done in sterile distilled water; aliquots of 300µL of each dilution were inoculated by triplicate in each medium. In the case of samples from La Uruca, only liquid PW media were used for isolation.
Petioles from La Garita (Alajuela) were cut and ground in 1ml of distilled water and dilutions (10-2 and 10-3) of this suspension were made. Aliquots of 300µL of each dilution were inoculated by triplicate in liquid PW media. All cultures were incubated in the dark at 28ºC under static conditions. After 15 days PW broths were tested by DAS-ELISA and only those positive for X. fastidiosa were used in the isolation procedure. Solid PW broths were inoculated with 100µL aliquots of liquid PW broth. Plates were incubated at 28ºC and observed for bacterial colony growth for 12 days. Colonies were tested by Gram staining, catalase and oxidase. Colony suspensions were also tested by DAS-ELISA. Three single colonies from each ELISA positive culture were selected and subcultured three times on solid PW. The last subculture was considered as a purified clone. Contaminated liquid media showing appreciable degree of turbidity was not tested by DAS-ELISA.
For morphological studies bacterial suspensions were prepared and fixed in Karnovsky solution (Karnovsky, 1965) for 48h and post-fixed in 1% osmium tetroxide for 30min. The cells were dehydrated in an ethanol series, finally embedded in Spurr´s medium and polymerized for three days. Ultrathin sections (60-80nm) were cut with a diamond knife in an ultramicrotome Leica Ultracut (Vienna, Austria). Specimens were stained with uranyl acetate and lead citrate solution and observed with a Hitachi H-7100 electron microscope.
For PCR detection of X. fastidiosa, DNA from each purified clone was extracted using the method for preparing genomic DNA from bacteria (Ausubel et al., 1990). Specific PCR detection of X. fastidiosa was performed by using primers 272-1 int/272-2 int and RST31/RST33 under described conditions (Minsavage, 1994; Pooler & Hartung, 1995). The amplification products were analyzed by electrophoresis in 1% agarose gel and visualized by ethidium bromide staining under UV light.
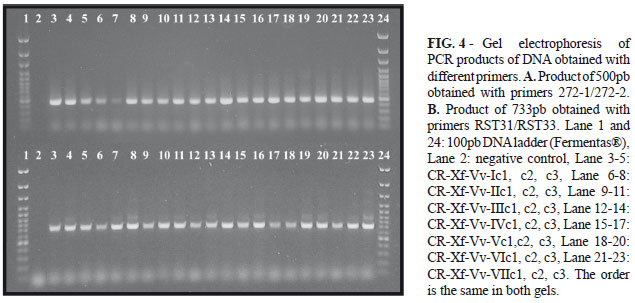
Xylella fastidiosa was confirmed by DAS-ELISA, in sixteen samples collected in different vineyards, located in different regions of Costa Rica (Table 1). The positive samples were used to isolate the bacterium. Bacterial and fungus contaminants were observed in some liquid media isolations. Only one contamination-free tube from Santa Ana, one from La Uruca and five from La Garita were positives for X. fastidiosa by DAS-ELISA. Cell aggregation was visible in liquid PW and medium turned dark red. Ten days after the inoculation on solid PW, characteristic colonies of X. fastidiosa were observed, and six days later the plates turned red. Three clones were obtained from each of the seven isolates and were named as CR-XF-Vv-Ic1 (CR: Costa Rica, Xf: Xylella fastidiosa, Vv: Vitis vinifera, I: number of isolates, c: clone 1). CR-Xf-Vv-Ic1, c2, c3 are from grapevine plants of Santa Ana; CR-Xf-Vv-IIc1, c2, c3 are from La Uruca; and clones CR-Xf-Vv-IIIc1, c2, c3; CR-Xf-Vv-IVc1, c2, c3; CR-Xf-Vv-Vc1, c2, c3; CR-Xf-Vv-VIc1, c2, c3; and CR-Xf-Vv-VIIc1, c2, c3 are from La Garita de Alajuela. After serial subculture of clones colonies grew much more rapidly (6 or 7 days).
Colonies were circular, with entire margin, convex, smooth, opalescent, and reached 0.5-1mm in diameter (Figure 2). The bacterial cells were Gram negative, catalase positive and oxidase negative and showed positive reaction by DAS-ELISA. Transmission electron microscopy of sectioned bacteria (Fig. 3) showed narrow and rod shaped cells with a rippled cell wall, measuring 0.3 µm wide and 3.0 µm long. The method used for preparation of genomic DNA from bacteria (Ausubel et al., 1990) was efficient. All 21 clones yielded products of 500pb and 733pb using primers 272-1/272-2 and RST31/RST33 respectively (Figure 3).
Based on consistent isolation from grapevine plants showing similar symptoms of PD, slow growth rate (ten days), biochemical test (oxidase and catalase), cell morphology, DAS-ELISA and PCR amplification products using two different set of primers, the presence of X. fastidiosa causing PD in new vineyards established in Costa Rica is confirmed. The results showed that in only the 66% of all symptomatic collected samples X. fastidiosa was positive by ELISA (Table 1); they are consistent with previous works that described thet bacterium as not uniformly distributed throughout all tissue of infected plants (Nome et al., 1980; Hopkins, 1989; Nome et al., 1992; Purcell & Hopkins, 1996; He et al., 2000; Yorinori et al., 2003). Bextine & Miller (2004) found also that enzyme-linked immunosorbent assay (ELISA) and polymerase chain reaction (PCR) are limited methods for the detection of X. fastidiosa, due to low titer and patchy distribution of the bacterium within a host plant.
Both PW and PWS media were adequate for primary isolations. In subsequent attempts of primary isolation, PW was preferred because SPW showed higher rates of contamination than PW. Not all inoculated tubes inoculated with extracts from different plants could be evaluated because of the high rates of bacterial and fungal contaminations observed. Some authors (Davis et al., 1981; Purcell & Hopkins, 1996; Buzcan et al., 2005) had already commented on the low growth of the pathogen and the frequent contamination of cultures, a limitation for the studies on X. fastidiosa (Purcell & Hopkins, 1996; Buzcan et al., 2005). The cell aggregation observed at the bottom of the tube after three weeks from inoculation is consistent with the results obtained by Davis et al. (1981). They observed aggregations of cells adhered to the glass, at the surface of the medium, when X. fastidosa was isolated from plum and when grapevine isolates were subcultured in PW liquid. They also observed faint turbidity in the media after a few days of incubation. In our case no turbidity was observed.
Colony pattern (Figure 2) (diameter, color, texture, and margin) did not change after serial transfers, but colonies grew much more rapidly. Hartung et al. (1994) state that after serial transfers, strains of X. fastidiosa adapt to culture and grow faster. Colonies were similar to those reported previously for X. fastidiosa associated with PD (Davis et al., 1978; Wells et al., 1987), and no difference was noticed among different isolates. Electron microscopy showed that cell wall had numerous ridges and furrows (Figure 3), which is consistent with previous descriptions of X. fasitidiosa cells according to Mollenhauer & Hopkins, 1994 and Chagas et al., 1992. Specific primers 272-1/272-2 (Pooler & Hartung 1995) and RST31/RST33 (Minsavage et al., 1994) for X. fastidiosa used in PCR amplified DNA bands of 500pb and 733pb respectively (Figure 4); the size of the bands agrees with reports by Minsavage et al. (1994) and Pooler & Hartung (1995). Our next step is to establish phylogenetic relationships between all isolates of. X. fastidosa obtained from different vineyards and from other hosts plants in Costa Rica.
ACKNOWLEDGMENTS
This research was supported by Costa Rica - USA Foundation (Grant 16-01 CT) and Universidad de Costa Rica. The authors thank Dr. Elliot W. Kitajima for revision of the manuscript.
Received 5 September 2007
Accepted 20 October 2008
Author for correspondence: Lisela Moreira, e-mail: lisela.moreira@ucr.ac.cr
Part of the Magister scientiae Thesis of the first author. Universidad de Costa Rica, Costa Rica. 2007.
TPP 7101
Associated Editor: Reginaldo S. Romeiro
- Ausbel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1990) Current protocols in molecular biology. New York NY. Wiley & Sons. pp. 241-245.
- Barrantes VA (1983) Insectos portadores de la bacteria causante con del mal de Pierce de la vid en Costa Rica. Tesis de licenciatura. Universidad de Costa Rica, Facultad de Agronomía, San José, Costa Rica.
- Berisha B, Chen YD, Zhang GY, Xu BY, Chen TA (1998) Isolation of Pierce's disease bacteria from grapevines in Europe. European Journal of Plant Pathology 104:427-433.
- Bextine BR, Miller TA (2004) Comparison of whole-tissue and xylem fluid collection techniques to detect Xylella fastidiosa in grapevine and oleander. Plant Disease 88:600-604.
- Buzkan N, Kocsis L, Walker LA (2005) Detection of Xylella fastidiosa from resistant and susceptible grapevine by tissue sectioning and membrane entrapment immunoflorescence. Microbiological Research 160:225-231.
- Chagas CM, Rossettti V, Beretta M (1992) Electron microscopy studies of a xylem limited bacterium in sweet orange affected with citrus variegated chlorosis disease in Brazil. Journal of Phytopathology 134:306-312.
- Davis MJ, Purcell AH, Thomson SV (1978) Pierce's disease of grapevines: Isolation of the causal bacterium. Science 199:75-77.
- Davis MJ, French JW, Schaad NW (1981) Axenic culture of the bacteria associated with phony disease of peach and plum leaf scald. Current Microbiology 6:309-314.
- Goheen AC, Nyland G, Lowe, SK (1973) Association of a Rickettsia like organism with Pierce´s disease of grapevine and alfalfa dwarf and heat therapy of the disease in grapevines. Phytopathology 63:341-345.
- Goheen AC, Raju BC, Lowe SK, Nyland G (1978) Pierce disease of grapevines in Central America. Plant Disease Reporter 63:788-792.
- Hartung JS, Beretta J, Brlansky RH, Spisso J, Lee R (1994) Citrus variegated chlorosis bacterium: axenic culture, pathogenicity, and serological relationships with other strains of Xylella fastidiosa Phytopathology 84591-597.
- He CX, Li WB, Ayres AJ, Hartung JS, Miranda VS, Teixeira DC (2000) Distribution of Xylella fastidiosa in citrus rootstocks and transmission of citrus variegated chlorosis between sweet orange plants through natural root grafts. Plant Disease 84:622-626.
- Hernández L, Corona FM (1994) Diagnóstico de Xylella fastidiosa en la vid y malezas asociadas con el cultivo. Manejo-Integrado-de-Plagas 33:7-10.
- Hernández L, Corona FM (1997) Detección de Xylella fastidiosa Wells et al. por Elisa-DAS en vid (Vitis vinifera L) y malezas en viñedos del municipio Mara, estado Zulia, Venezuela. Revista Facultad de Agronomía (Luz) 14:297-306.
- Hopkins DL (1981) Seasonal concentration of the Pierce´s disease bacterium in grapevine stems petioles and leaf veins. Phytopathology 71:415-418.
- Hopkins DL (1989) Xylella fastidiosa xylem-limited bacterial pathogen of plants. Annual Review of Phytopathology 27:271-290.
- Hopkins DL, Mollenhauer HH (1973) Rickettsia-like bacterium associated with Pierce´s Disease of grapes. Science 179:298-300.
- Jiménez LG (1980) El mal de Pierce de la vid su distribución en Costa Rica y características fenotípicas de su agente causal. Tesis de Licenciatura. Universidad de Costa Rica, Facultad de Fitotecnia, San José, Costa Rica.
- Karnovsky MJ (1965) A formaldehyde - glutaraldehyde fixative of high osmolality for use in electro microscopy. Journal Cell Biology 27:137-138.
- Minsavage GV, Thompson CM, Hopkins DL, Leite RMVBC, Stall RE (1994) Development of a Polymerase chain reaction protocol for detection of Xylella fastidiosa in plant tissue. Phytopathology 84:456-461.
- Mora JM (1982) Determinación de los hospedantes alternos del mal de Pierce de vid en Costa Rica, mediante técnicas inmunológicas de adsorción con conjugados enzimáticos. Tesis de licenciatura. Universidad de Costa Rica, Facultad de Fitotecnia, San José, Costa Rica. 1982.
- Mollenhauer HH, Hopkins DL (1974) Ultrastructural study of Pierce´s disease bacterium in grape xylem tissue. Journal of Bacteriology 119:612-618.
- Nome SF, Haekterman RM, Docampo DM, Prataviera AG, Di Feo LV (1992) Escaldadura de las hojas del almendro en Argentina. Fitopatologia Brasileira 17:57-60.
- Nome SF, Raju BC, Goheen AC, Nyland G, Docampo D (1980) Enzyme-linked immunosorbent assay for pierce´s disease bacteria in plant tissues. Phytopathology 70:746-749.
- Pooler M, Hartung J (1995) Specific PCR detection and identification of Xylella fastidiosa strains causing citrus variegated chlorosis. Current Microbiology 31:377-381.
- Purcell HA, Hopkins DL (1996) Fastidious xylem-limited bacterial plant pathogens. Annual Review of Phytopathology 34:131-151.
- Wells JM, Raju BC, Huang HY, Weisburg WG, Mandelco-Paul L, Brenner DJ (1987) Xylella fastidiosa gen. Nov., sp. nov: Gram negative, xylem limited, fastidious plant bacteria related to Xanthomonas spp. International Journal Systematic Bacteriology 37:136-143.
- Yorinori MA, Ribas AF, Ueno B, Massola Jr. NS, Leite Jr RP (2003) Detecção de Xylella fastidiosa em germoplasma de cafeeiro. Fitopatologia Brasileira 28:427-430.
Publication Dates
-
Publication in this collection
19 Feb 2009 -
Date of issue
Dec 2008
History
-
Accepted
20 Oct 2008 -
Received
05 Sept 2007