Abstract
Brazil has a welcoming setting for clinical trials, with national regulations and a well-developed and institutionalized monitoring system. Resolution 466/2012 of the Conselho Nacional de Saúde (Brazilian National Health Council) adopted the principle of justice as a fundamental requirement for ethics in research. The aim of the present study was to investigate the bioethical meanings attributed to this principle in clinical trials with drugs in the country. The study was conducted through a thorough literature review, which was performed in two phases: understanding trial regulations and systematically researching the issue. Discussions regarding the principle of justice vary greatly when addressing the different stages of trials. The authors’ perceptions were organized into three categories, which are interchangeable to a certain degree. Empirical studies and discussions must be conducted in relation to the application of this principle during the ethical analysis of clinical trials, while also addressing the adequacy and effectiveness of this principle in reducing social injustices in the health sector.
Biomedical research; pharmaceutical preparations; social justice; ethics; research
Resumo
O Brasil é campo próspero para ensaios clínicos, possui regulamentação nacional e sistema de monitoramento bem desenvolvido e institucionalizado. A Resolução do Conselho Nacional de Saúde CNS 466/2012 incorpora o princípio da justiça como fundamental para garantir a eticidade das pesquisas. Este estudo teve como objetivo investigar os sentidos bioéticos atribuídos a esse princípio na condução dos ensaios clínicos com medicamentos no país. Trata-se de revisão narrativa da literatura, realizada em duas etapas: compreensão das regulamentações em pesquisa e busca sistemática sobre o tema. Há fragmentação da discussão sobre o princípio da justiça, abordando-se diferentes etapas dos ensaios. As percepções dos autores foram organizadas em três categorias que possuem certo grau de intercambialidade. Devem ser realizados estudos empíricos e discussões sobre a aplicação desse princípio na análise ética dos ensaios clínicos e sobre sua adequação e efetividade com vistas à redução das injustiças em saúde.
Pesquisa biomédica; Preparações farmacêuticas; Justiça social; Ética em pesquisa
Resumen
Brasil es un campo próspero para ensayos clínicos, posee una reglamentación nacional y un sistema de seguimiento bien desarrollado e institucionalizado. La Resolución del Conselho Nacional de Saúde (CNS) (Consejo Nacional de Salud) 466/2012 incorpora el principio de la justicia como fundamental para garantizar la eticidad de las investigaciones. Este estudio tuvo como objetivo investigar los sentidos bioéticos atribuidos a este principio en la realización de ensayos clínicos con medicamentos en el país. Se trata de una revisión narrativa de la literatura, realizada en dos etapas: alcance o ámbito de las regulaciones en investigación y búsqueda sistemática sobre el tema. Hay una fragmentación en la discusión del principio de justicia en las diferentes etapas de los ensayos. Las percepciones de los autores fueron organizadas en tres categorías que, no obstante, tienen cierto grado de intercambiabilidad. Se debe profundizar en estudios empíricos y discusiones sobre la aplicación de este principio en el análisis ético de los ensayos clínicos y sobre la adecuación y efectividad con el fin de reducir las injusticias en la salud.
Investigación biomédica; Preparaciones farmacéuticas; Justicia social; Ética en Investigación
Clinical trials require the participation of humans and involve clinical interventions with experimental drugs, health products and/or therapeutic procedures. These trials represent 80% of all studies registered on an international platform of public records (ClinicalTrials) in 2015. As of March of 2015, Brazil was the south American country with most studies registered on this platform (4323), followed by Argentina (1889) and Chile (1027) 11. United States of America. Trends, charts, and maps. ClinicalTrials.gov. [Internet]. 2016 [acesso 2 jul 2015]. Disponível: http://1.usa.gov/24hpQ0C
http://1.usa.gov/24hpQ0C...
.
The performance of clinical trials can be beneficial for the economy of the participating countries, generating employment and stimulating local scientific and technological development through the scientific data identified and studied in cooperation with research centers. They can also benefit the public health sector in the country, given the potential direct benefits for the participants and the possibility of access to new products for the general population. However, all of these possibilities depend on the involvement of health authorities and effective ethical, social and political monitoring, which should clarify the objectives and activities of research groups and the real perspective of these studies, without neglecting measures to eliminate or reduce the risks to the participants.
In the context of clinical trials, the search for new forms of therapy has received strong social support. In the case of oncology, for example, due to the high morbidity and mortality rates associated with cancer, the appearance of new antineoplastic agents always generates great interest, expectations and pressure from patients, their family members, doctors and even the pharmaceutical industry itself, to incorporate the drug into the Sistema Único de Saúde (Unified Health System) (SUS) 22. Brasil. Agência Nacional de Vigilância Sanitária. Esclarecimento sobre a posição da Anvisa quanto ao registro de medicamentos antineoplásicos novos. [Internet]. 2013 [acesso 5 jan 2013]. Disponível: http://bit.ly/1TOkmuE
http://bit.ly/1TOkmuE...
. The mobilization that surrounds research involving antiretroviral drugs is an example of the activity of individuals with HIV, NGOs, pharmacies, doctors and international organizations, among others, who seek to improve both clinical research and access to drugs in the health assistance network 33. Scheffer M. Coquetel: a incrível história dos antirretrovirais e do tratamento da aids no Brasil. São Paulo: Hucitec; 2012. v. 1. p. 216..
Brazil has become a prosperous region for the performance of clinical trials 44. Lima JS, La Reza D, Teixeira S, Costa C. Pesquisa clínica: fundamentos, aspectos éticos e perspectivas. Revista da Socerj. 2003;16(4):225-233.
5. Glickman SW, McHutchison JG, Peterson ED, Cairns CB, Harrington RA, Califf RM et al. Ethical and scientific implications of the globalization of clinical research. N Engl J Med. 2009;360(8):816-23.-66. Dainesi SM, Goldbaum M. Pesquisa clínica como estratégia de desenvolvimento em saúde. Rev Assoc Med Bras. 2012;58(1):2-6.. However, a number of studies 77. Gomes RP, Pimentel VP, Landim AB, Pieroni JP. Ensaios clínicos no Brasil: competitividade internacional e desafios. Complexo Industrial da Saúde. BNDES Setorial 36. p. 45-84.,88. Lousana G. Pesquisa Clínica na Brasil. Rio de Janeiro: Revinter; 2002.have suggested that these do not always correspond to the needs/priorities of the health of the Brazilian public, as a result of constant conflicts of interest. These conflicts involve researchers and the pharmaceutical industry and are linked to financial incentives to conduct clinical trials, as well as the competition between the participating centers while recruiting participants 99. Morin K, Rakatansky H, Riddick FA Jr, Morse LJ, O’Bannon JM, Goldrich MS et al. Managing conflicts of interest in the conduct of clinical trials. Jama. [Internet]. 2002 [acesso 2 jul 2015];287(1):78-84. Disponível: http://bit.ly/1Y00swX
http://bit.ly/1Y00swX...
, and the marketing strategies of scientific products 1010. Miguelote VRS, Camargo Junior KR. Indústria do conhecimento: uma poderosa engrenagem. Rev Saúde Pública. 2010 [acesso 9 out 2015];44(1):190-6. Disponível: http://bit.ly/1UelOke
http://bit.ly/1UelOke...
. The satisfactory management of conflicts of interests, whether real/potential or financial/personal, is essential in order to ensure the objectivity of studies 1111. American Medical Association. Opinion 8.031 – conflicts of interest: biomedical research. [Internet]. AMA. jun 2001 [acesso 2 jul 2015]. Disponível: http://bit.ly/1XpRr0I
http://bit.ly/1XpRr0I...
and the integrity of their results.
The conflicts between the real needs of the health sector and financial and commercial interests can be exacerbated during trials, given the possibility of obtaining collective benefits 1212. Gava CM, Bermudez JAZ, Pepe VLE, Reis ALA. Novos medicamentos registrados no Brasil: podem ser considerados como avanço terapêutico? Ciênc Saúde Coletiva. 2010;15(3 Suppl):3403-12.. The public health sector needs to expand the availability of safe and effective drugs and therapeutic procedures. To do so, it is important to expand the collection of resources, the performance of research and the professional training of researchers in Brazil 1313. Quental C, Salles Filho S. Ensaios clínicos: capacitação nacional para avaliação de medicamentos e vacinas. Rev Bras Epidemiol. 2006;9(4):408-24.. The performance of clinical trials (studies of efficacy, safety, effectiveness and cost-effectiveness) that generate consistent and important scientific data is essential in order to incorporate the technology in question into the SUS system and to improve the access of the general population to this technology. Thus, there is a need for complex ethical analysis of the studies in question, addressing the principle of justice (among other aspects) in a consistent manner.
Brazil has a well-developed and institutionalized regulatory and control/monitoring system for research involving humans, which aims to protect the rights and duties of researchers, participants and society. The most relevant regulation is Resolution 466/2012 of the National Health Council, which contains directives and rules for research involving humans and guidelines for the ethical analysis of clinical research and practices, as proposed by Tom Beauchamp and James Childress 1414. Beauchamp T, Childress J. Princípios de ética biomédica. São Paulo: Loyola; 2002. in 1979 in their study titled “Principles of biomedical ethics”. The authors indicated that research should assess four ethical principles: 1) respect for autonomy; 2) an absence of maleficence; 3) beneficence and; 4) justice. Brazilian regulations do not exclude the use of other moral principles, although, it does state that the four moral principles are the prima facie responsibilities linked to the ethics of research projects.
Brazilian regulations incorporate different ethical requisites that derived from the principles listed above, including: respect for the participant, their dignity and autonomy (autonomy); weighing risks and benefits (…) and committing to maximum benefits with minimum risks (beneficence); ensuring that predictable harm is avoided (non-maleficence); and the social relevance of the research, which ensures equal consideration for the interests involved, without compromising the meaning of its socio-humanitarian purpose (justice) 1515. Brasil. Conselho Nacional de Saúde. Resolução nº 466, de 12 de dezembro de 2012. Aprova as diretrizes e normas regulamentadoras de pesquisas envolvendo seres humanos. Diário Oficial da União. Brasília; 13 jun 2013..
Concerning the principle of justice (the aim of the present study), Resolution 466/2012 of the National Health Council established this principle as the equal distribution of social burdens and benefits among individuals, and between these individuals and the collective. The greatest challenge lies in the translation of the principle of justice, the distributive meaning of which requires the establishment of a consensus on what should be considered essential in order to satisfy individual and collective wellbeing, as well as the parameters involved in the legitimacy of these distributive criteria and the resolution of ethical conflicts during research.
Therefore, the aim of the present study was to analyze the application of the principle of justice in clinical trials involving drugs in Brazil, while also addressing research ethics.
Methodology
This narrative literature review was conducted in two stages, focusing on the principle of justice in clinical research. Initially, the legislation that governs clinical research in Brazil was analyzed. All of the relevant rules were read and their foundations were organized to highlight relevant questions related to the principle of justice. Subsequently, three electronic databases (the Biblioteca Virtual de Saúde (the Virtual Health Library) (BVS), PubMed and Scopus) were consulted using the terms “justice” and “research ethics”. The period used in the search began with the publication of Resolution 466/2012, 1515. Brasil. Conselho Nacional de Saúde. Resolução nº 466, de 12 de dezembro de 2012. Aprova as diretrizes e normas regulamentadoras de pesquisas envolvendo seres humanos. Diário Oficial da União. Brasília; 13 jun 2013. on June 13 2013, and extended until October of the same year.
The filter “human” and the search keys “justice” or “social justice” were used, together with the keywords research ethics, clinical trials and drugs. The search was limited to articles written in English, Portuguese and Spanish. Articles that were duplicated in the databases were excluded, as were those that were inaccessible or considered inadequate for the aim of this review.
The analysis of ethical regulations, which established the normative context of the discussion of justice, was followed by a thematic analysis of the results in the literature, in accordance with the categories that emerged while reading the texts. The articles were read and the aggregation of links representing significant themes were identified.
This review also considered studies that were not found in the systematic review, but were pertinent to the theme of research ethics and its regulation, which were identified throughout the research. These studies were included to gain a better understanding of the issue and to the develop the bioethical discussion of the principle of justice.
Results and discussion
In total, 48 studies were identified, of which 29 were found in PubMed, 11 were from the BVS and 8 were in Scopus. After reading these texts and applying the exclusion criteria, 22 articles were selected. Table 1 displays a detailed description of the flow of eligibility.
Flow of eligibility for the articles in the systematic search related to justice and research ethics
The reading of the articles selected from the systematic search sought to identify the application and understanding of the authors concerning the principle of justice in the context of clinical research with drugs.
The principle of justice and research ethics
The principle of justice was adopted in research ethics regulations as a fundamental guide to decision making and seeks to recognize (from a general perspective) the right [of each person] to a dignified minimum of health care [and considers that] the justice of social healthcare institutions [aims to] counterbalance the lack of opportunities caused by natural and social lotteries, over which individuals have no substantial control, and make a commitment to fair and effective procedures in the allocation of health resources 1616. Beauchamp T, Childress J. Justiça. In: Beauchamp T, Childress J, organizadores. Princípios de ética biomédica. São Paulo: Loyola; 2002. p. 351-423..
The studies identified indicated that moral issues and conflicts related to research ethics should be treated from the perspective of distributive, rather than commutative 1717. Aristóteles. Ética a Nicômaco. 4ª ed. São Paulo: Martin Claret; 2001., justice and support the idea of “justice as equity” 1818. Rawls J. Uma teoria da justiça. Martins Fontes: São Paulo; 2002.. These ideas are regularly found in public health studies in Brazil, as reported by Escorel 1919. Escorel S. Os dilemas da equidade em saúde: aspectos conceituais. [Internet]. 2013 [acesso 30 maio 2016]. Disponível: http://bit.ly/20QyLp0
http://bit.ly/20QyLp0...
. The concept of equity has been correlated with the coverage of services and the allocation and use of resources, in terms of access and health conditions. This idea has been used as a guide for health policies attempting to reduce inequality, particularly in situations of conflict, thereby operating as an instrument of justice.
In the studies analyzed in this review, the principle of justice in clinical trials mainly refers to distributive justice, and indicates the demands for the equitable distribution of the burdens and benefits of participating in research for the subjects involved and the community in quesiton 2020. World Health Organization. International ethical guidelines for biomedical research involving human subjects. Geneva: WHO; 2002.. However, there are a number of theories related to justice that consider it from utilitarian, liberal, communitarian and egalitarian points of view, with no unified theory that combines these different concepts 1616. Beauchamp T, Childress J. Justiça. In: Beauchamp T, Childress J, organizadores. Princípios de ética biomédica. São Paulo: Loyola; 2002. p. 351-423.. As previously mentioned, the challenge is to identify the relevant and decisive moral aspects for each situation, which should represent the equitable distribution criteria to be adopted, without damaging the egalitarian perspective.
The principle of justice can be applied to different stages of a clinical trial. During the selection of the research participants, levels of justice can be expressed (social and individual) through the absence of unfair distinctions of any kind (color, gender, etc.) and the clear establishment of inclusion criteria that ensure that any eligible member of the community can participate.
Direct benefits of the investigation should also be equally offered to all participants of the trial, and subsequently to the group they represent. However, even when these selection criteria are respected, injustices can occur because certain unfair social standards (prejudices) are institutionalized in our society and can affect the general distribution of the burdens and benefits of the research for the participants 2121. United States of America. Department of Health and Human Services. The Belmont report: ethical principles and guidelines for the protection of human subjects of research. [Internet]. 1979 [acesso 30 maio 2013]. Disponível: http://1.usa.gov/1m4nLEE
http://1.usa.gov/1m4nLEE...
. Thus, additional mitigation measures should be employed by researchers, institutions, the state and society, in order to achieve maximum effective equality in this distribution.
Another aspect of justice in research ethics involves the consideration of the health conditions or needs of the population during assessments, particularly in relation to the vulnerable population to be recruited, since the risks to which they will be submitted are more justifiable when the interventions or procedures that are performed have direct benefits for their health 2020. World Health Organization. International ethical guidelines for biomedical research involving human subjects. Geneva: WHO; 2002.. Under Brazilian legislation, vulnerability is defined as the condition of people or groups who, for any reason, have reduced or impaired self-determination abilities, or who are in any way impeded from resisting, particularly in relation to free and informed consent 1515. Brasil. Conselho Nacional de Saúde. Resolução nº 466, de 12 de dezembro de 2012. Aprova as diretrizes e normas regulamentadoras de pesquisas envolvendo seres humanos. Diário Oficial da União. Brasília; 13 jun 2013..
It is important to stress that the distribution of goods and services based on needs is considered just, as sustained in the principle of necessity 1616. Beauchamp T, Childress J. Justiça. In: Beauchamp T, Childress J, organizadores. Princípios de ética biomédica. São Paulo: Loyola; 2002. p. 351-423.. Health can be considered a fundamental need and its non-fulfilment can lead to harm. The principle of justice, as an ethical reference, is evident in the items of National Health Council resolution 466/2012 (cited below), which formulates demands for research, regardless of the area of expertise.
-
If the participants must be randomly distributed into experimental and control groups, a priori, it is impossible to establish the advantages of one procedure over the other using a literature review, observational methods or methods that do not involve humans (III.2, paragraph f);
-
Whenever possible, ensure that community-based research transmits benefits, the effects of which will continue to be felt after the conclusion of the experiment (III.2, paragraph l);
-
Communicate with competent authorities, as well as the organs responsible for social control, the results and/or findings of the research, provided that they can contribute to an improvement in the living conditions of the collective (III.2, paragraph m);
-
Ensure that the participants of the research will receive the benefits resulting from the project, whether in terms of social return, access to procedures, products or research agents (III.2, paragraph n);
-
Use research conducted abroad or with foreign cooperation to demonstrate the advantages of participating in this type of research for participants and for Brazil (III.2, paragraph p);
-
Studies that are sponsored abroad should also fulfil the knowledge and technology transference requirements of the Brazilian team when applicable. In the case of new drug development, if their safety and effectiveness has been proven, they must be registered in Brazil (III.2, paragraph p);
-
Ensure that all participants have free and undetermined access to the best prophylactic, diagnostic and therapeutic methods at the end of the study1515. Brasil. Conselho Nacional de Saúde. Resolução nº 466, de 12 de dezembro de 2012. Aprova as diretrizes e normas regulamentadoras de pesquisas envolvendo seres humanos. Diário Oficial da União. Brasília; 13 jun 2013.(III.3, paragraph d).
The ethical review and monitoring processes for studies involving humans (ethics committees) should be treated as a question of social justice, in which the interest of all parties involved must be recognized and taken into consideration 2222. Pullman D. Conflicting interests, social justice, and proxy consent to research. J Med Philos. 2002;27(5):523-45..
Although the concepts of autonomy, beneficence and justice have been consecrated in the field of clinical trials through Brazilian resolutions and international research ethics manuals, integrating these ethical pillars into practice is a continuous task 2323. Owonikoko TK. Upholding the principles of autonomy, beneficence, and justice in phase I clinical trials. Oncologist. 2013;18(3):242-4., particularly when considering the principle of justice, which depends on individual and social contextual analysis for its adequate application (due to its range and abstraction).
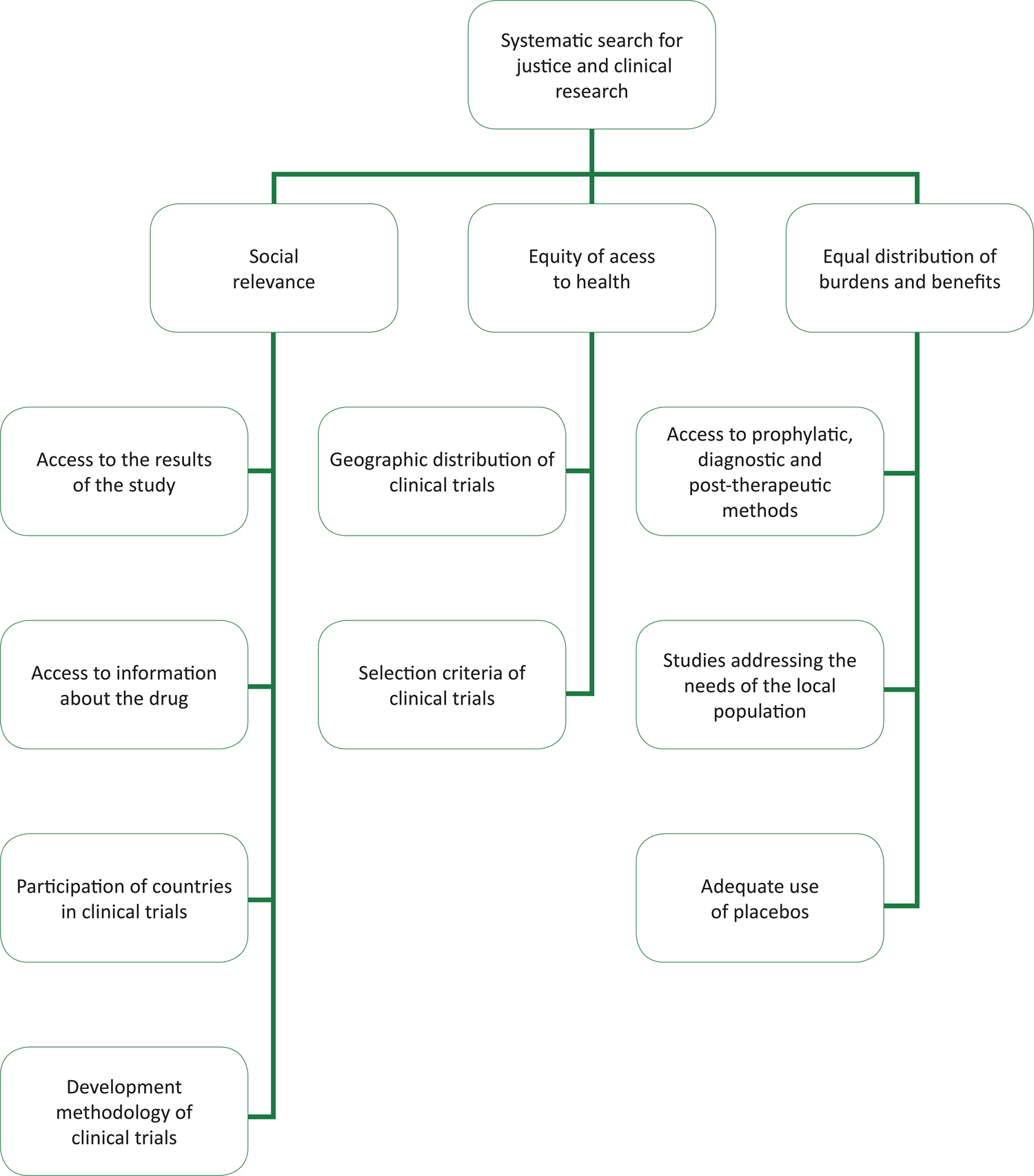
The practical implications and formulations of this principle were one of the aims of this study. The analysis of scientific articles identified three thematic categories, which structured bioethical reflections on this principle in clinical research: 1) social relevance; 2) equal distribution of burdens and benefits for participation in research; and 3) equity in access to health services. Figure 1 displays the categories and their elements or sub-categories.
Organogram containing the analytical categories for justice and ethics in research and their implications
Social relevance
According to Kurihara 2424. Kurihara C. Ethical, legal, and social implications (ELSI) of microdose clinical trials. Adv Drug Deliv Rev. 2011;63(7):503-10., the social relevance of a study involves access to the results, information about the drug in question, the type of participation in clinical trials in a country, and the scientific methodology of the study design. Concerning access to data, publishing results can help improve the living conditions of the population and advise them of the best treatment and/or prophylactic conditions. Thus, the publishing process is also an important element of social relevance. Currently, the non-publication of results after a study is in violation of resolution 466/2012 of the National Health Council 1515. Brasil. Conselho Nacional de Saúde. Resolução nº 466, de 12 de dezembro de 2012. Aprova as diretrizes e normas regulamentadoras de pesquisas envolvendo seres humanos. Diário Oficial da União. Brasília; 13 jun 2013..
Publishing the results of studies is a relatively recent global phenomenon. ClinicalTrials is one of the main recording platforms for clinical trials in the world. Its database of results was released in September 2008, allowing investigators and/or sponsors to send in their results. The results database was developed to fulfil the requirements of the Food and Drug Administration Amendments Act (FDAAA) of 2007 2525. National Institutes of Health. FDAAA - Further Resources for NIH Grantees. NIH. 2011 [acesso 04 jun 2016]. Disponível: http://1.usa.gov/1UVaXM9
http://1.usa.gov/1UVaXM9...
. The practice of publishing results on this platform has grown gradually over the years, and represents a significant gain for society in terms of public access to study data.
Another important aspect of access to data involves registering drugs with regulatory agencies, which is a fundamental stage in the assessment of a drug. These authorities act as mediators between the interests of the drug manufacturers and the needs of the public health sector, primarily seeking to fulfil the duty of health protection 1212. Gava CM, Bermudez JAZ, Pepe VLE, Reis ALA. Novos medicamentos registrados no Brasil: podem ser considerados como avanço terapêutico? Ciênc Saúde Coletiva. 2010;15(3 Suppl):3403-12.. Thus, the public transparency of this data is essential for the legitimacy of the registering process in the country, as well as access for users and the scientific community to data concerning the drugs being studied.
As previously mentioned, the participation of a country in international clinical trials can provide several benefits. However, it is important to assess the potential benefits of these studies in terms of the local context. A number of the aspects discussed below are considered pertinent during this analysis.
Data from the Agência Nacional de Vigilância Sanitária (National Agency of Sanitary Vigilance) (Anvisa) for the year 2011 showed that only 4% of the studies conducted in Brazil were classified as phase I (highly complex), whereas 63% were classified as phase III, followed by 22% for phase II and 11% for phase IV 2626. Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Anvisa divulga perfil de pesquisa clínica de medicamentos no Brasil. [Internet]. 2011 [acesso 31 maio 2013]. Disponível: http://bit.ly/25uGqwD
http://bit.ly/25uGqwD...
. A study of Brazilian participation in clinical trials related to oncology in Brazil found that 88.8% of all studies conducted between 2003 and 2012 were classified as phase III 2727. Silva CF. O princípio da justiça, os ensaios clínicos e o registro de anticorpos monoclonais e biomedicamentos oncológicos no Brasil [dissertação]. Rio de Janeiro: Escola Nacional de Saúde Pública; 2014.. These results suggest that the studies conducted in Brazil involve low levels of technological complexity, which minimizes the possibility of technical improvements among Brazilian teams and/or the transference of technology.
The support of foreign pharmaceutical industries is important for scientific development and research. However, a monopoly in this area could compromise the credibility of the research, if strong (and difficult-to-manage) conflicts of interest exist. Therefore, equal consideration and equity measures are required to achieve the equality levels expected by study participants in relation to the distribution of the burdens and benefits of the research. In an attempt to protect the participants of research projects, as well as the researchers themselves, Resolution 466/2012 of the National Health Council contains information about the transfer of knowledge and technology in cases involving foreign sponsorship 1515. Brasil. Conselho Nacional de Saúde. Resolução nº 466, de 12 de dezembro de 2012. Aprova as diretrizes e normas regulamentadoras de pesquisas envolvendo seres humanos. Diário Oficial da União. Brasília; 13 jun 2013..
Another important aspect when assessing the social relevance of a study is the quality of its design (method). Well-planned scientific studies can provide direct benefits for the participants and the scientific community. The design of clinical trials (diagnostic, therapeutic or preventive) raises ethical and scientific questions related to sponsors, investigators and ethics committees 2020. World Health Organization. International ethical guidelines for biomedical research involving human subjects. Geneva: WHO; 2002..
The randomization of study participants is a significant methodological tool when analyzing justice, since the random attribution of participants to treatment groups promotes equality in the distribution of interventions: predictable benefits and risks are offered equally to all who participate in a study 1515. Brasil. Conselho Nacional de Saúde. Resolução nº 466, de 12 de dezembro de 2012. Aprova as diretrizes e normas regulamentadoras de pesquisas envolvendo seres humanos. Diário Oficial da União. Brasília; 13 jun 2013.. From a scientific viewpoint, this removes the potential for bias (the attribution of patients to one intervention or another). The introduction of unpredictability 2828. Kunz R, Oxman AD. The unpredictability paradox: review of empirical comparisons of randomised and non-randomised clinical trials. BMJ. 1998;317(7167):1185-90. eliminates selection biases linked to the vulnerability of certain participants.
Randomization is also important for the safety of the participants. Non-randomized studies, and those with inadequate allocations, generally overestimate or hide the effects of treatment protocols, 2828. Kunz R, Oxman AD. The unpredictability paradox: review of empirical comparisons of randomised and non-randomised clinical trials. BMJ. 1998;317(7167):1185-90. which has a negative effect on both the participants and the scientific data obtained. According to Kurihara 2424. Kurihara C. Ethical, legal, and social implications (ELSI) of microdose clinical trials. Adv Drug Deliv Rev. 2011;63(7):503-10., a number of new drug development strategies, such as studies with microdoses (microdose clinical trials), can benefit society, provide social relevance and contribute to the field of medicine. However, they generate ethical discussions due to the fact they do not provide a therapeutic effect and involve certain risks and burdens, without any direct benefits for the participants. Blinding is another methodological strategy that seeks to ensure that assessments and medical/clinical decisions are not influenced by the knowledge of the treatment protocol designated to the patient 2929. United States of America. Food and Drug Administration. Guidance for industry: E 10 choice of control group and related issues in clinical trials. [Internet]. maio 2001 [acesso 30 maio 2016]. Disponível: http://1.usa.gov/25wO6BX
http://1.usa.gov/25wO6BX...
.
Assessments of the adequacy of these study design aspects, among others, are important in order to achieve the proposed objectives, while generating data/awareness and expanding the social relevance of the study.
Equal access to health services
Several studies 3030. Bayer A, Fish M. The doctor’s duty to the elderly patient in clinical trials. Drugs Aging. 2003;20(15):1087-97.
31. Booth KM. A magic bullet for the “African” mother? Neo-Imperial reproductive futurism and the pharmaceutical “solution” to the HIV/AIDS crisis. Soc Polit. 2010;17(3):349-78.
32. Botbol-Baum M. The shrinking of human rights: the controversial revision of the Helsinki Declaration. HIV Med. 2000;1(4):238-45.
33. Brunet-Jailly J. The ethics of clinical research in developing countries. IRB. 1999;21(5):8-11.
34. Edwards SJ. Restricted treatments, inducements, and research participation. Bioethics. 2006;20(2):77-91.
35. Kölch M, Ludolph AG, Plener PL, Fangerau H, Vitiello B, Fegert JM. Safeguarding children’s rights in psychopharmacological research: Ethical and legal issues. Curr Pharm Des. 2010;16(22):2398-406.
36. McMillan JR, Conlon C. The ethics of research related to health care in developing countries. J Med Ethics. 2004;30(2):204-6.
37. Oquendo MA, Stanley B, Ellis SP, Mann JJ. Protection of human subjects in intervention research for suicidal behavior. Am J Psychiatry. 2004;161(9):1558-63.
38. Sklar DP. Ethical issues associated with pain research in emergency medicine. Ann Emerg Med. 1996;27(4):418-20.
39. Smart A, Martin P, Parker M. Tailored medicine: whom will it fit? The ethics of patient and disease stratification. Bioethics. 2004;18(4):322-42.
40. Thomas J. Ethical challenges of HIV clinical trials in developing countries. Bioethics. 1998;12(4):320-7.
41. van Delden J, Bolt I, Kalis A, Derijks J, Leufkens H. Tailor-made pharmacotherapy: future developments and ethical challenges in the field of pharmacogenomics. Bioethics. 2004;18(4):303--21.-4242. Zulueta P. Randomised placebo-controlled trials and HIV-infected pregnant women in developing countries: Ethical imperialism or unethical exploitation? Bioethics. 2001;15(4):289-311. have discussed the issue of equality in relation to the opportunity to use health services. In a session of the executive committee for health access, the Organização Pan-Americana da Saúde (the Pan-American Health Organization) (Opas) defined equity in health as the absence of unwarranted differences in health conditions, access to services, financial contributions, access to health centers and service received while attending health service agents 4343. Organização Mundial da Saúde. Estratégia para cobertura universal de saúde. 154ª Sessão do Comitê Executivo. [Internet]. Washington: OMS; 12 maio 2014 [acesso 17 out 2015]. Disponível: http://bit.ly/1Y015qj
http://bit.ly/1Y015qj...
. In the context of clinical research, one of the implications of equal access to health services is the distribution of opportunities to participate in clinical trials, which can be measured through geographic distribution and the eligibility criteria for participation in the study.
The extensive dissemination of studies around the world is advantageous, since different ethnicities, as well as social, cultural and environmental conditions, are exposed to the interventions studied, thereby increasing the chances of the reproducibility of the intervention when extrapolated to the general population. Thus, it would be beneficial if developed countries, who propose most of these research projects, participated in as many studies as host countries (usually developing countries). This could help to avoid double standards and the unfair treatment of local populations.
The homogenous dispersion of studies around the world is something that many authors and researchers have fought for over time. Many authors have debated the performance of clinical trials in developing countries, particularly if the study deals with HIV. Thomas 4040. Thomas J. Ethical challenges of HIV clinical trials in developing countries. Bioethics. 1998;12(4):320-7. addressed these ethical challenges and stated that most research ethics manuals are based on minimum ethical standards (respect for the individual - autonomy, beneficence and distributive justice), rather than the maximalist perspectives of bioethics. The continuous access of participants to advanced treatment protocols and technology, as well as issues of equality and human rights, should be essential components when developing clinical trials.
McMillan and Conlon 3636. McMillan JR, Conlon C. The ethics of research related to health care in developing countries. J Med Ethics. 2004;30(2):204-6. corroborated this idea by reporting that there is no scarcity of general research ethics manuals in literature, although manuals that address the complexities involved in performing research in developing countries are scarce.
Brunet-Jailly 3333. Brunet-Jailly J. The ethics of clinical research in developing countries. IRB. 1999;21(5):8-11., Mayss 4444. Mayss A. Drug-testing on seropositive pregnant women in the developing world: moral and legal implications. Med Law Int. 2000;4(3-4):183-210., Zulueta 4242. Zulueta P. Randomised placebo-controlled trials and HIV-infected pregnant women in developing countries: Ethical imperialism or unethical exploitation? Bioethics. 2001;15(4):289-311., Booth 3131. Booth KM. A magic bullet for the “African” mother? Neo-Imperial reproductive futurism and the pharmaceutical “solution” to the HIV/AIDS crisis. Soc Polit. 2010;17(3):349-78. and Botbol-Baum 3232. Botbol-Baum M. The shrinking of human rights: the controversial revision of the Helsinki Declaration. HIV Med. 2000;1(4):238-45.discussed bioethical problems in international studies of HIV in developing countries. Botbol-Baum 3232. Botbol-Baum M. The shrinking of human rights: the controversial revision of the Helsinki Declaration. HIV Med. 2000;1(4):238-45. criticized the 1999 review of the Helsinki Declaration, which defended research in these countries, for stimulating studies that are aimed at profits and the private interests of companies rather than healthcare, in which the burden of certain people leads to benefits for others. This encourages discrimination against economically vulnerable patients from developing countries and disrespects the concept of equal access. According to the author, the dilemma is not related to the participation or not of these people in scientific studies, but in recognizing the ethical responsibilities of society and not allowing these people to be excluded for purely economic reasons. According to Brunet-Jailly, 3333. Brunet-Jailly J. The ethics of clinical research in developing countries. IRB. 1999;21(5):8-11. who defended the equal access to health services for all of the community, not offering the best therapeutic option to the participants of a study is in violation of the requirements of the principle of justice.
Another factor that leads to flaws in health access is studies that involve clinical indications that are restricted to populations with specific mutations or particular genetic expressions. In the advanced stages of drug testing, there is a common expectation that the participants will respond well to the intervention, based on prior information of their genetic profile. This means that the participants were screened genetically prior to their inclusion in the study 4141. van Delden J, Bolt I, Kalis A, Derijks J, Leufkens H. Tailor-made pharmacotherapy: future developments and ethical challenges in the field of pharmacogenomics. Bioethics. 2004;18(4):303--21.. This strategy is used by pharmaceutical industries to reduce the amount of failures in their drug testing and consequently, to produce cheaper and more effective clinical trials. Van Delden and collaborators discussed the increase in problems related to the fair and equal distribution of drugs/treatment through the provision of drugs for genetic sub-groups on the market.
Smart, Martin and Parker 3939. Smart A, Martin P, Parker M. Tailored medicine: whom will it fit? The ethics of patient and disease stratification. Bioethics. 2004;18(4):322-42. discussed the use of pharmacogenetic drugs, which can lead to individual genetic stratification and consequently, provide flexibility in medical decision-making concerning the treatment protocol to be offered (greater potential to reduce toxic effects and improve efficiency). However, misuse of this method can lead to injustice. It is undeniable that more information about the human genome increases the expectations for a better understanding and classification of illnesses, thereby enabling a more specific selection of drugs for certain illnesses. However, a number of authors 3535. Kölch M, Ludolph AG, Plener PL, Fangerau H, Vitiello B, Fegert JM. Safeguarding children’s rights in psychopharmacological research: Ethical and legal issues. Curr Pharm Des. 2010;16(22):2398-406.,3939. Smart A, Martin P, Parker M. Tailored medicine: whom will it fit? The ethics of patient and disease stratification. Bioethics. 2004;18(4):322-42.,4141. van Delden J, Bolt I, Kalis A, Derijks J, Leufkens H. Tailor-made pharmacotherapy: future developments and ethical challenges in the field of pharmacogenomics. Bioethics. 2004;18(4):303--21. have expressed concern about the use of pharmacogenomics/pharmacogenetics, stating that stratification can lead to discrimination in the access to the potential benefits of the intervention.
Oquendo et al 3737. Oquendo MA, Stanley B, Ellis SP, Mann JJ. Protection of human subjects in intervention research for suicidal behavior. Am J Psychiatry. 2004;161(9):1558-63. highlighted problems of injustice in the application of the selection criteria of several studies of suicidal behavior, including the selection of participants and the exclusion of high-risk individuals, such as those with a history of suicide attempts, comorbidities and severe illnesses. These criteria damage the equity and the results of a study, which consequently, cannot be generalized for the general population.
Unfair participant selection for research has also been addressed by Bayer and Fish, 3030. Bayer A, Fish M. The doctor’s duty to the elderly patient in clinical trials. Drugs Aging. 2003;20(15):1087-97. specifically in relation to age discrimination in clinical studies. The authors demonstrated the unequal representation of the elderly population in clinical trials and stated that, despite the vulnerability of this population and the complexity of certain studies, the inclusion of these individuals should be encouraged in studies in which the existing clinical condition is relevant.
Sklar 3838. Sklar DP. Ethical issues associated with pain research in emergency medicine. Ann Emerg Med. 1996;27(4):418-20. introduced an ethical debate about justice in relation to clinical studies of pain. The authors investigated differentiated treatment protocols that vary in accordance with ethnic characteristics, age groups or gender. A line of argument holds that the culture of the individual affects the gradation and expression of pain. However, according to the authors, the aims and designs of studies should not create limits for the classification of pain based on individual characteristics.
Given the diversity of themes that address the limitations of the eligibility of participants in research, reflections on equity as an aspect of social justice should be initiated upon the confirmation of the exclusion or under-representation of a population group in clinical trials, since these individuals will not benefit directly from the research 3939. Smart A, Martin P, Parker M. Tailored medicine: whom will it fit? The ethics of patient and disease stratification. Bioethics. 2004;18(4):322-42..
Equal distribution of burdens and benefits for participating in a study
The equal distribution of burdens and benefits has been previously addressed by Ballantyne, 4545. Ballantyne A. HIV international clinical research: exploitation and risk. Bioethics. 2005;19(5-6):476-91. Beran 4646. Beran RG. The ethics of excluding women who become pregnant while participating in clinical trials of anti-epileptic medications. Seizure. 2006;15(8):563-70., Clark 4747. Clark PA. Placebo surgery for Parkinson’s disease: Do the benefits outweigh the risks? J Law Med Ethics. 2002;30(1):58-68., Haire 4848. Haire BG. Because we can: clashes of perspective over researcher obligation in the failed PrEP trials. Dev World Bioeth. 2011;11(2):63-74., Hawkins 4949. Hawkins JS. Justice and placebo controls. Soc Theory Pract. 2006;32(3):467-96., Mayss 4444. Mayss A. Drug-testing on seropositive pregnant women in the developing world: moral and legal implications. Med Law Int. 2000;4(3-4):183-210., Resnik 5050. Resnik DB. Exploitation and the ethics of clinical trials. Am J Bioeth. 2002;2(2):28-30. and Varmus & Satcher 5151. Varmus H, Satcher D. Ethical complexities of conducting research in developing countries. N Engl J Med. 1997;337(14):1003-5., who defined it as follows: access to prophylactic methods and available diagnoses; analysis of the indispensability of research in relation to the needs of the population, including the impact of a determined disease on the population to be studied; and the use of a placebo.
When the results of a study recommend an intervention, the subsequent access of the study participants to the therapy in question is considered just, since it represents one of the benefits they can receive. Resolution 466/2012 1515. Brasil. Conselho Nacional de Saúde. Resolução nº 466, de 12 de dezembro de 2012. Aprova as diretrizes e normas regulamentadoras de pesquisas envolvendo seres humanos. Diário Oficial da União. Brasília; 13 jun 2013. defines the benefits of the research as direct or indirect advantages, which can be immediate or delayed, earned by the participant and/or their community as a result of participating in the research (II.4). Varmus & Satcher 5151. Varmus H, Satcher D. Ethical complexities of conducting research in developing countries. N Engl J Med. 1997;337(14):1003-5.argue that the performance of investigational therapeutic protocols that inevitably involve individuals who are unlikely to receive any direct benefits from the research represents a violation of the principle of justice.
Resolution 466/2012 includes the requirement to attempt to ensure that the risks to which individuals will be exposed will be counterbalanced by a guarantee of benefits. Thus, for experimental studies involving humans in the area of biomedicine, the sponsor must ensure that all participants will gain access, for an indeterminate time period, to the best prophylactic, diagnostic and therapeutic methods at the end of the study (III.3, paragraph d). 1515. Brasil. Conselho Nacional de Saúde. Resolução nº 466, de 12 de dezembro de 2012. Aprova as diretrizes e normas regulamentadoras de pesquisas envolvendo seres humanos. Diário Oficial da União. Brasília; 13 jun 2013.
The regulatory process of registering drugs and/or health products, after the required scientific testing (effectiveness and safety) has been completed, is a critical stage in the context of justice. The slowness or speed of this process (delayed access/inadequate assessments/insufficient therapy) can have negative consequences for the population. In Brazil, the process and the deadline for registering drugs after testing is generally considered to be slow, which could potentially prevent the country from participating in international clinical research.
As a result of these issues, in 2013, Anvisa adopted a set of measures to modernize and improve the analysis of new product registrations, using an electronic drug registering system. The main aim of this system is to reduce the final deadline of registering a new drug/product to a maximum of six months for products that are considered important to the SUS or those that provide technological innovations 5252. Escola Nacional de Saúde Pública Sérgio Arouca. Anvisa adota série de medidas para agilizar registro de medicamentos. [Internet]. 25 mar 2013 [acesso 28 fev 2014]. Disponível: http://bit.ly/1OYTTDh
http://bit.ly/1OYTTDh...
.
Furthermore, in order to improve the technical assessment process of clinical trials with drugs by the competent organ, Anvisa (RDC 9 5353. Brasil. Agência Nacional de Vigilância Sanitária. Resolução da Diretoria Colegiada nº 9, de 20 de fevereiro de 2015. Dispõe sobre o regulamento para a realização de ensaios clínicos com medicamentos no Brasil. Diário Oficial da União. Brasília; 3 mar 2015.) stated that they would assess the Dossiê de Desenvolvimento Clínico de Medicamento (Dossier of Clinical Drug Development) (DDCM) within ninety days of receipt. However, if no response is provided within this time period, clinical development can begin, provided it respects the applicable ethical guidelines. This regulation is not valid for clinical development submissions that involve the national production of biological products or studies classified as phase I or II. In these cases, Anvisa will have 180 days to complete the assessment.
However, a speedy process could mask the accuracy of the technological assessment. For a number of authors, 5454. Zuckerman D. Understanding the controversies over a groundbreaking new health care law. Milbank Q. [Internet]. 2015 [acesso 30 maio 2016]. Disponível: http://bit.ly/1snXeas
http://bit.ly/1snXeas...
55. Avorn J, Kesselheim AS. The 21st century cures act: will it take us back in time? N Engl J Med. 2015;372(26):2473-5.-5656. Downing NS, Aminawung JA, Shah ND, Krumholz HM, Ross JS. Clinical trial evidence supporting FDA approval of novel therapeutic agents, 2005-2012. JAMA. 2014;311(4):368-77. a very fast process can lead to a loosening of the safety analysis parameters, thereby compromising the results and offering the public products with a doubtful risk/benefit profile. This extremely important aspect of the safety of technological assessments has been discussed in international literature, due to recent changes in clinical research regulations.
The conditions of use of placebos in clinical trials is another significant aspect in the assessment of the balance between the risks and benefits of participating in the research. Usually, the participants in the control group are subjected to an intervention for which the effectiveness has already been proven, unlike the experimental intervention. However, in some circumstances, the use of an alternative comparison, such as a placebo, can be ethically acceptable. Ballantyne 4545. Ballantyne A. HIV international clinical research: exploitation and risk. Bioethics. 2005;19(5-6):476-91. concluded that, despite the provision of mutual benefits (for researchers and subjects), a study can be considered abusive if the distribution of burdens and benefits is unfair, which is often the case in studies that use a placebo erroneously.
Hawkins 4949. Hawkins JS. Justice and placebo controls. Soc Theory Pract. 2006;32(3):467-96. discussed the moral problems of clinical studies with a placebo, indicating the unjust treatment of participants that used a placebo when the therapy for the condition being studied has already been approved. The author discussed the viability of the execution of these studies and emphatically stressed the moral responsibility of researchers for these subjects. Haire 4848. Haire BG. Because we can: clashes of perspective over researcher obligation in the failed PrEP trials. Dev World Bioeth. 2011;11(2):63-74. corroborated the discussion of the moral obligation of researchers to provide adequate treatment for the study participants, in an attempt to avoid exploitation and fulfil the principle of justice.
Clark 4747. Clark PA. Placebo surgery for Parkinson’s disease: Do the benefits outweigh the risks? J Law Med Ethics. 2002;30(1):58-68.carried out ethical analysis, in light of the principle of justice, for studies that addressed the treatment of Parkinson’s disease. In general, participants in a placebo group should temporarily abstain from other forms of therapy. Despite the fact that the subjects are submitted to constant clinical assessments and examinations, the effects of the placebo are difficult to measure and can be susceptible to assessment bias, which represents a methodological challenge. The author suggested that two questions were intertwined with the principle of justice. He stated that each person must be treated fairly and equally, receiving what they require in accordance with 1) the vulnerability of participants with advanced Parkinson’s disease, and; 2) the equal distribution of resources. According to the author, the potential risks of this type of research are too high for vulnerable individuals, and better investment could be made to adopt more consecrated methodological strategies.
Final Considerations
The present study analyzed the bioethical meanings attributed to the principle of justice during the performance of clinical trials with drugs in Brazil. The limitations of this research include the nature of the search, which was limited to drug trials (due to the greater expected prevalence of this type of clinical trial), as well as the fact that only articles written in Portuguese, Spanish or English were selected, since they would better reflect the scope of the data in a Brazilian context. Furthermore, the fluidity of the categories was probably the most significant issue with this research. Although different thematic categories were used, it was not possible to ensure that the content of the articles was completely limited to those categories. Nevertheless, these limitations did not compromise the analysis.
Based on the full reading of the articles selected in the bibliographical survey, there is evidence of a split in ethical discussions of the principle of justice in different stages of clinical trials, which at times focuses on the recruitment phase and at other times addresses the study design. There is no global discussion of ethics, justice and clinical research.
According to Beauchamp & Childress, 1616. Beauchamp T, Childress J. Justiça. In: Beauchamp T, Childress J, organizadores. Princípios de ética biomédica. São Paulo: Loyola; 2002. p. 351-423. distributive justice theories should specify the different principles, rules and judgements involved in this distribution in a consistent manner. This review confirmed the limitations of the discussion, classifying the application of the principle of justice as insubstantial and difficult to measure. Very few studies have clearly identified the elements and characteristics that are considered morally justifiable for the performance of trials, as well as the distribution of their benefits and burdens.
Since the principle of justice was incorporated into the first Brazilian regulation concerning human research ethics (Resolution 466/2012), as well as its predecessor (Resolution 196/1996), both of which were drawn up by the National Health Council, empirical studies should be conducted and theoretical discussions on the application of these principles in the ethical analysis of clinical trials should be expanded. The effectiveness of these principles in reducing health inequalities should also be assessed.
Referências
-
1United States of America. Trends, charts, and maps. ClinicalTrials.gov. [Internet]. 2016 [acesso 2 jul 2015]. Disponível: http://1.usa.gov/24hpQ0C
» http://1.usa.gov/24hpQ0C -
2Brasil. Agência Nacional de Vigilância Sanitária. Esclarecimento sobre a posição da Anvisa quanto ao registro de medicamentos antineoplásicos novos. [Internet]. 2013 [acesso 5 jan 2013]. Disponível: http://bit.ly/1TOkmuE
» http://bit.ly/1TOkmuE -
3Scheffer M. Coquetel: a incrível história dos antirretrovirais e do tratamento da aids no Brasil. São Paulo: Hucitec; 2012. v. 1. p. 216.
-
4Lima JS, La Reza D, Teixeira S, Costa C. Pesquisa clínica: fundamentos, aspectos éticos e perspectivas. Revista da Socerj. 2003;16(4):225-233.
-
5Glickman SW, McHutchison JG, Peterson ED, Cairns CB, Harrington RA, Califf RM et al. Ethical and scientific implications of the globalization of clinical research. N Engl J Med. 2009;360(8):816-23.
-
6Dainesi SM, Goldbaum M. Pesquisa clínica como estratégia de desenvolvimento em saúde. Rev Assoc Med Bras. 2012;58(1):2-6.
-
7Gomes RP, Pimentel VP, Landim AB, Pieroni JP. Ensaios clínicos no Brasil: competitividade internacional e desafios. Complexo Industrial da Saúde. BNDES Setorial 36. p. 45-84.
-
8Lousana G. Pesquisa Clínica na Brasil. Rio de Janeiro: Revinter; 2002.
-
9Morin K, Rakatansky H, Riddick FA Jr, Morse LJ, O’Bannon JM, Goldrich MS et al. Managing conflicts of interest in the conduct of clinical trials. Jama. [Internet]. 2002 [acesso 2 jul 2015];287(1):78-84. Disponível: http://bit.ly/1Y00swX
» http://bit.ly/1Y00swX -
10Miguelote VRS, Camargo Junior KR. Indústria do conhecimento: uma poderosa engrenagem. Rev Saúde Pública. 2010 [acesso 9 out 2015];44(1):190-6. Disponível: http://bit.ly/1UelOke
» http://bit.ly/1UelOke -
11American Medical Association. Opinion 8.031 – conflicts of interest: biomedical research. [Internet]. AMA. jun 2001 [acesso 2 jul 2015]. Disponível: http://bit.ly/1XpRr0I
» http://bit.ly/1XpRr0I -
12Gava CM, Bermudez JAZ, Pepe VLE, Reis ALA. Novos medicamentos registrados no Brasil: podem ser considerados como avanço terapêutico? Ciênc Saúde Coletiva. 2010;15(3 Suppl):3403-12.
-
13Quental C, Salles Filho S. Ensaios clínicos: capacitação nacional para avaliação de medicamentos e vacinas. Rev Bras Epidemiol. 2006;9(4):408-24.
-
14Beauchamp T, Childress J. Princípios de ética biomédica. São Paulo: Loyola; 2002.
-
15Brasil. Conselho Nacional de Saúde. Resolução nº 466, de 12 de dezembro de 2012. Aprova as diretrizes e normas regulamentadoras de pesquisas envolvendo seres humanos. Diário Oficial da União. Brasília; 13 jun 2013.
-
16Beauchamp T, Childress J. Justiça. In: Beauchamp T, Childress J, organizadores. Princípios de ética biomédica. São Paulo: Loyola; 2002. p. 351-423.
-
17Aristóteles. Ética a Nicômaco. 4ª ed. São Paulo: Martin Claret; 2001.
-
18Rawls J. Uma teoria da justiça. Martins Fontes: São Paulo; 2002.
-
19Escorel S. Os dilemas da equidade em saúde: aspectos conceituais. [Internet]. 2013 [acesso 30 maio 2016]. Disponível: http://bit.ly/20QyLp0
» http://bit.ly/20QyLp0 -
20World Health Organization. International ethical guidelines for biomedical research involving human subjects. Geneva: WHO; 2002.
-
21United States of America. Department of Health and Human Services. The Belmont report: ethical principles and guidelines for the protection of human subjects of research. [Internet]. 1979 [acesso 30 maio 2013]. Disponível: http://1.usa.gov/1m4nLEE
» http://1.usa.gov/1m4nLEE -
22Pullman D. Conflicting interests, social justice, and proxy consent to research. J Med Philos. 2002;27(5):523-45.
-
23Owonikoko TK. Upholding the principles of autonomy, beneficence, and justice in phase I clinical trials. Oncologist. 2013;18(3):242-4.
-
24Kurihara C. Ethical, legal, and social implications (ELSI) of microdose clinical trials. Adv Drug Deliv Rev. 2011;63(7):503-10.
-
25National Institutes of Health. FDAAA - Further Resources for NIH Grantees. NIH. 2011 [acesso 04 jun 2016]. Disponível: http://1.usa.gov/1UVaXM9
» http://1.usa.gov/1UVaXM9 -
26Brasil. Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Anvisa divulga perfil de pesquisa clínica de medicamentos no Brasil. [Internet]. 2011 [acesso 31 maio 2013]. Disponível: http://bit.ly/25uGqwD
» http://bit.ly/25uGqwD -
27Silva CF. O princípio da justiça, os ensaios clínicos e o registro de anticorpos monoclonais e biomedicamentos oncológicos no Brasil [dissertação]. Rio de Janeiro: Escola Nacional de Saúde Pública; 2014.
-
28Kunz R, Oxman AD. The unpredictability paradox: review of empirical comparisons of randomised and non-randomised clinical trials. BMJ. 1998;317(7167):1185-90.
-
29United States of America. Food and Drug Administration. Guidance for industry: E 10 choice of control group and related issues in clinical trials. [Internet]. maio 2001 [acesso 30 maio 2016]. Disponível: http://1.usa.gov/25wO6BX
» http://1.usa.gov/25wO6BX -
30Bayer A, Fish M. The doctor’s duty to the elderly patient in clinical trials. Drugs Aging. 2003;20(15):1087-97.
-
31Booth KM. A magic bullet for the “African” mother? Neo-Imperial reproductive futurism and the pharmaceutical “solution” to the HIV/AIDS crisis. Soc Polit. 2010;17(3):349-78.
-
32Botbol-Baum M. The shrinking of human rights: the controversial revision of the Helsinki Declaration. HIV Med. 2000;1(4):238-45.
-
33Brunet-Jailly J. The ethics of clinical research in developing countries. IRB. 1999;21(5):8-11.
-
34Edwards SJ. Restricted treatments, inducements, and research participation. Bioethics. 2006;20(2):77-91.
-
35Kölch M, Ludolph AG, Plener PL, Fangerau H, Vitiello B, Fegert JM. Safeguarding children’s rights in psychopharmacological research: Ethical and legal issues. Curr Pharm Des. 2010;16(22):2398-406.
-
36McMillan JR, Conlon C. The ethics of research related to health care in developing countries. J Med Ethics. 2004;30(2):204-6.
-
37Oquendo MA, Stanley B, Ellis SP, Mann JJ. Protection of human subjects in intervention research for suicidal behavior. Am J Psychiatry. 2004;161(9):1558-63.
-
38Sklar DP. Ethical issues associated with pain research in emergency medicine. Ann Emerg Med. 1996;27(4):418-20.
-
39Smart A, Martin P, Parker M. Tailored medicine: whom will it fit? The ethics of patient and disease stratification. Bioethics. 2004;18(4):322-42.
-
40Thomas J. Ethical challenges of HIV clinical trials in developing countries. Bioethics. 1998;12(4):320-7.
-
41van Delden J, Bolt I, Kalis A, Derijks J, Leufkens H. Tailor-made pharmacotherapy: future developments and ethical challenges in the field of pharmacogenomics. Bioethics. 2004;18(4):303--21.
-
42Zulueta P. Randomised placebo-controlled trials and HIV-infected pregnant women in developing countries: Ethical imperialism or unethical exploitation? Bioethics. 2001;15(4):289-311.
-
43Organização Mundial da Saúde. Estratégia para cobertura universal de saúde. 154ª Sessão do Comitê Executivo. [Internet]. Washington: OMS; 12 maio 2014 [acesso 17 out 2015]. Disponível: http://bit.ly/1Y015qj
» http://bit.ly/1Y015qj -
44Mayss A. Drug-testing on seropositive pregnant women in the developing world: moral and legal implications. Med Law Int. 2000;4(3-4):183-210.
-
45Ballantyne A. HIV international clinical research: exploitation and risk. Bioethics. 2005;19(5-6):476-91.
-
46Beran RG. The ethics of excluding women who become pregnant while participating in clinical trials of anti-epileptic medications. Seizure. 2006;15(8):563-70.
-
47Clark PA. Placebo surgery for Parkinson’s disease: Do the benefits outweigh the risks? J Law Med Ethics. 2002;30(1):58-68.
-
48Haire BG. Because we can: clashes of perspective over researcher obligation in the failed PrEP trials. Dev World Bioeth. 2011;11(2):63-74.
-
49Hawkins JS. Justice and placebo controls. Soc Theory Pract. 2006;32(3):467-96.
-
50Resnik DB. Exploitation and the ethics of clinical trials. Am J Bioeth. 2002;2(2):28-30.
-
51Varmus H, Satcher D. Ethical complexities of conducting research in developing countries. N Engl J Med. 1997;337(14):1003-5.
-
52Escola Nacional de Saúde Pública Sérgio Arouca. Anvisa adota série de medidas para agilizar registro de medicamentos. [Internet]. 25 mar 2013 [acesso 28 fev 2014]. Disponível: http://bit.ly/1OYTTDh
» http://bit.ly/1OYTTDh -
53Brasil. Agência Nacional de Vigilância Sanitária. Resolução da Diretoria Colegiada nº 9, de 20 de fevereiro de 2015. Dispõe sobre o regulamento para a realização de ensaios clínicos com medicamentos no Brasil. Diário Oficial da União. Brasília; 3 mar 2015.
-
54Zuckerman D. Understanding the controversies over a groundbreaking new health care law. Milbank Q. [Internet]. 2015 [acesso 30 maio 2016]. Disponível: http://bit.ly/1snXeas
» http://bit.ly/1snXeas -
55Avorn J, Kesselheim AS. The 21st century cures act: will it take us back in time? N Engl J Med. 2015;372(26):2473-5.
-
56Downing NS, Aminawung JA, Shah ND, Krumholz HM, Ross JS. Clinical trial evidence supporting FDA approval of novel therapeutic agents, 2005-2012. JAMA. 2014;311(4):368-77.
Publication Dates
-
Publication in this collection
May-Aug 2016
History
-
Received
6 Dec 2015 -
Reviewed
11 May 2016 -
Accepted
24 May 2016