Abstracts
Aspidogastrea are globally-distributed parasites of the class Trematoda, which have been described as pathogens of a range of aquatic organisms, in marine and freshwater environments. The principal morphological characteristic of the group is an adhesive ventral disc, which is responsible for fixing the parasite to the host organism. In this study, 112 specimens ofColomesus psittacus from the municipality of Cametá, in the state of Pará (Brazil), were necropsied. Platyhelminthes of the genus Rohdella attached to the mucous membrane of the fish's intestine by the adhesive disc were observed. Fragments of parasitized tissue were fixed in Davidson solution and then processed and stained with hematoxylin-eosin. Other fragments were fixed in glutaraldehyde, processed and observed under a scanning electron microscope. The prevalence of the parasite was 76.4%, mean intensity of infection was 8.0 and mean abundance was 6.2. The parasitism provoked chronic enteritis with diffused inflammatory infiltration. The adherence of the parasite to the mucous membrane of the intestine resulted in strangulation and hyperplasia of the region, as well as causing hypertrophy of the muscle of the mucous membrane. The present study describes the anatomopathological and ultrastructural aspects of the parasitism of the intestine of C. psittacus byRohdella sp.
Amazonia; parrot puffer fish; intestine; parasite; histopathology; Rohdella sp
Os Aspidogastreas são parasitos da classe Trematoda, distribuídos globalmente e têm sido descritos como patógenos em uma gama de organismos aquáticos de ambientes marinhos e de água doce. A principal característica morfológica do grupo é um disco adesivo na região ventral responsável pela fixação do parasito no organismo hospedeiro. Neste estudo, 112 espécimes de Colomesus psittacus provenientes do município de Cametá, no estado do Pará (Brasil), foram necropsiados. Foram observados platelmintos do gêneroRohdella aderidos à mucosa intestinal através do disco adesivo. Fragmentos de tecido com parasito foram fixados em solução de Davidson e processados e corados em Hematoxilina-Eosina. Outros fragmentos foram fixados em glutaraldeído, processados e observados em microscopia eletrônica de varredura. A prevalência parasitária foi de 76, 4%, intensidade média de infecção de 8,0 e abundância média de 6,2. O parasitismo ocasionou uma enterite crônica com difuso infiltrado inflamatório. A fixação do parasito na mucosa intestinal provocou estrangulamento e hiperplasia da região, bem como hipertrofia da muscular da mucosa. O presente trabalho descreve os aspectos anatomopatológicos e ultra-estruturais da ação parasitária por Rohdella sp. no trato intestinal deC. psittacus.
Amazônia; baiacu papagaio; intestino; parasito; histopatologia; Rohdella sp
Introduction
The genus RohdellaGibson & Chinabut, 1984Gibson DJ, Chinabut S. Rohdella siamensis gen. et sp. nov. (Aspidogastridae: Rohdellinae subfam. nov.) from freshwater fishes in Thailand, with a reorganization of the classification of the subclass Aspidogastrea. Parasitol 1984; 88(3): 383-393. http:∕∕dx.doi.org∕10.1017∕S0031182000054652
http:∕∕dx.doi.org∕10.1017∕S0031182000054...
, belongs to the subclass Aspidogastrea Faust & Tang, 1936 (OLSON; TKACH, 2005Olson PD, Tkach VV. Advances and Trends in the Molecular Systematics of the Parasitic Platyhelminthes. Adv Parasitol 2005; 60: 165-243. http:∕∕dx.doi.org∕10.1016∕S0065-308X(05)60003-6
http:∕∕dx.doi.org∕10.1016∕S0065-308X(05)...
), which contains only four families, 12 genera and approximately 80 species (ROHDE, 2001aRohde K. The Aspidogastrea: an archaic group of Platyhelminthes. In: Littlewood DTJ, Bray RA, editors. Interrelationships of the Platyhelminthes. London: Taylor and Francis; 2001a. p.159-167.; SCHLUDERMANN et al., 2005; MING-XIU et al., 2010Ming-Xiu C, Li-Qianq Z, Chun-Gen W, Jun S, Qian G. Phylogenetic relationship of species in the genus Aspidogaster (Aspidogastridae, Aspidogastrinae) in China as inferred from its rDNA sequences. Acta Hydrobiol Sinica 2010; 34(2): 312-316). Aspidogastreans are distributed worldwide, and have been described as pathogens in different aquatic organisms, such as mollusks, fish, and turtles, including both marine and freshwater species (SNYDER; TKACH, 2007Snyder SD, Tkach VV. Neosychnocotyle maggiae, n. gen., n. sp. (Platyhelminthes: Aspidogastrea) from freshwater turtles in northern Australia. J Parasitol 2007; 93(2): 399-403. PMid:17539425. http:∕∕dx.doi.org∕10.1645∕GE-1001R.1
http:∕∕dx.doi.org∕10.1645∕GE-1001R.1...
; SCHLUDERMANN et al., 2005).
The most marked characteristic of aspidogastreans is the adhesive disc on the ventral surface, which is subdivided into longitudinal lines, or alveoli (GRIZZLE; BRUNNER, 2007; TOLSTENKOV et al., 2010Tolstenkov O, Terenina N, Kreshchenko N, Gustafsson M. The pattern of FMRFamide and serotonin immunoreactive elements in the nervous system of Aspidogaster conchicola K. Baer, 1827 (Aspidogastrea, Aspidogastridae). Belg J Zool 2010; 140: 133-136.). The principal function of this organ is to fix the parasite to its host, but it may also have a sensorial function or play a role in external digestion (GRIZZLE; BRUNNER, 2007). The life cycle of these organisms generally involves a mollusk as intermediate host and a vertebrate definitive host, although in some cases, the mollusk is the definitive host (ROHDE, 2001b; ZAMPARO; BROOKS, 2003). In the case of fish hosts, the parasites are found in the intestinal tract (ABOUL-DAHAB et al., 1993Aboul-Dahab HM, Abd El-Salam FA, El-Damarany ME. An Aspidogastrean parasite, Rohdella anodontiase sp. nov. from the fresh water mussel, Anodonta rubinse. Assiut Vet Med J 1993; 29(57): 81-88.).
The presence of parasites in the host organism, especially in the intestine, may result in both structural and metabolic alterations, which may be reflected in a variety of pathological processes, and may even result in the death of the host in some cases (ROCHA et al., 2010Rocha RM, Coelho RP, Montes CS, Santos SSD, Ferreira MAP. Avaliação histopatológica do fígado de Brachyplatystoma rousseauxii (CASTELNAU, 1855) da baía do Guajará, Belém, Pará. Cienc Anim Bras 2010; 11(1): 101-109.; PEÑA-REHBEIN; RÍOS-ESCALANTE, 2012Peña-Rehbein P, Ríos-Escalante P. Use of negative binomial distribution to describe the presence of Anisakis in Thyrsites atun. Rev Bras Parasitol Vet 2012; 21(1): 78-80. PMid:22534952. http:∕∕dx.doi.org∕10.1590∕S1984-29612012000100017
http:∕∕dx.doi.org∕10.1590∕S1984-29612012...
). The present study describes the anatomopathological and ultra-structural features of parasitic infestation of the intestinal tract of the parrot pufferfish (Colomesus psittacus) by Rohdella sp.
Materials and Methods
1.Hosts
A total of 112 specimens of the euryhaline parrot pufferfish,Colomesus psittacus Bloch and Schneider, 1801, were obtained from the municipality of Cametá (02° 14′ S and 49° 49′ W) in the Brazilian state of Pará, between January 2009, and April 2010. The specimens had a mean body length of 12 ± 3 cm and weight of 5 ± 2 g, and were transported live to the Carlos Azevedo Research Laboratory (UFRA), where they were anesthetized with MS 222 (Sandoz Laboratories), prior to necropsy.
2.Necropsy and optical microscopy
The necropsies on the hosts began with opening the abdominal cavity using a pair of anatomical scissors, in order to gain access to the viscera. The organs were examined under a stereomicroscope. The parasites were found adhering to the intestinal mucous membrane of the hosts, and data on prevalence, mean intensity of infection and mean abundance were calculated as described by Serra-Freire (2002)Serra-Freire NM. Planejamento e análise de pesquisas parasitológicas. Niterói: Ed. Eduff; 2002.. The parasitized intestines were then removed. For examination using optical microscopy, the organ was pre-fixed for 2 hours in order to maintain the cylindrical shape. The intestine was fragmented into 0.5 cm-thick segments. These fragments were fixed in Davidson solution (formaldehyde, acetic acid, 95% ethanol and distilled water) at room temperature for 24 hours, and were then processed and stained with hematoxylin-eosin. The stained sections were documented using a Zeiss Primo Star microscope with a Canon A610∕A620 52 mm adaptor. Adult specimens of the parasites were collected, rinsed in saline solution, fixed in AFA (acetic acid, formaldehyde and 80% ethanol) at room temperature and set between a slide and coverslip, with light pressure. Finally, the specimens were stained in alcoholic carmine and mounted in Canada balsam, as described by Amato (1985)Amato JFR. Manual de Técnicas para a Preparação de Coleções Zoológicas. 8. Platelmintos (Temnocefálidos, Trematódeos, Cestóides, Cestodários) e Acantocéfalos. São Paulo: Sociedade Brasileira de Zoologia; 1985..
3.Electron microscopy
For scanning electron microscopy (SEM), small fragments (1.0 cm) of the intestinal tissue containing parasites were fixed in 5% glutaraldehyde, buffered with sodium cacodylate (pH 7.2), for 12 hours at 4 °C. They were then rinsed overnight in the same buffer and post-fixed in 2% OsO4, buffered in the same solution for 3 hours at the same temperature. The specimens were then dehydrated in an ascending ethanol series. The fragments were dried to the critical point, metalized with a fine (20 nm) layer of gold, and photographed under the LEO 1459 VP SEM, operated at 80 kV.
Results
The fish did not present clinical symptoms of parasitosis, but in the infected specimens, reddish areas could be observed in the interior of the intestine (through the transparent wall), after opening the abdominal cavity (Figure 1a). When the intestine was opened, it was possible to confirm that these reddish areas corresponded to the presence of platyhelminth parasites, of the genus Rohdella, which were adhering to the intestinal wall by means of their ventral region (Figure 1b, c). The parasites were identified through the descriptions of Gibson and Chinabut (1984)Gibson DJ, Chinabut S. Rohdella siamensis gen. et sp. nov. (Aspidogastridae: Rohdellinae subfam. nov.) from freshwater fishes in Thailand, with a reorganization of the classification of the subclass Aspidogastrea. Parasitol 1984; 88(3): 383-393. http:∕∕dx.doi.org∕10.1017∕S0031182000054652
http:∕∕dx.doi.org∕10.1017∕S0031182000054...
. They could be observed either in isolation or in small clumps dispersed throughout the organ.
a) Colomesus psittacus. Intestine.Rohdella sp. Parasitism byRohdella sp. Note the presence of a reddish mark on the intestine (circled); b) C. psittacus. Intestine. Rohdella sp. Fresh parasite (arrows) adhering to the mucous membrane of the intestine (*); c)Rohdella sp. Fresh parasite, with retractable anterior region exposed. Detail - Rohdella sp. Parasite between slide and coverslip stained with alcoholic carmine. Magnification 4×; d) C. psittacus. Intestine. Rohdella sp. Hypertrophy of the muscular mucosa (*) adjacent to the parasite (arrows) in the intestinal tract. H.E. Magnification 4×. Scale bar: 200 µm; e) C. psittacus. Intestine. Rohdella sp. Detail of the insertion point of the projections of the parasite's ventral disc marking the mucous membrane of the intestine (I). H.E. Magnification 40×. Scale bar: 40 µm; f) C. psittacus. Intestine. Rohdella sp. Parasite fixed to the mucous membrane by the adhesive disc (arrow). Note the strangulation (S) and hyperplasia of the tissue, which has become disarranged (arrow head). H.E. Magnification 40×. Scale bar: 40 µm.
The prevalence of Rohdella sp. was 76.4%, with a mean intensity of infection of 8.0 and a mean abundance index of 6.2 (Table 1). Overall, 683 worms were recorded, and these trematodes were observed during all sampling periods (Table 1).
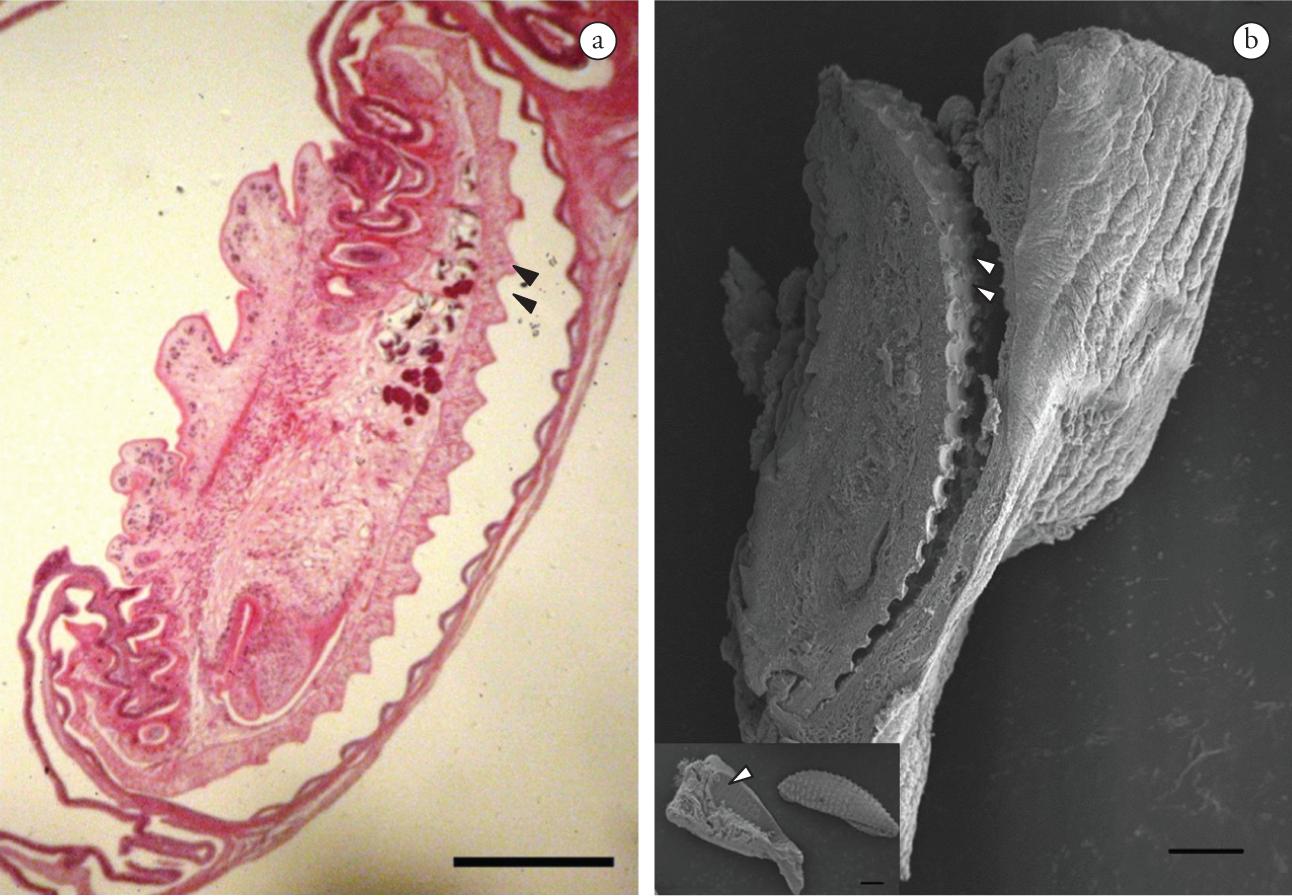
The microscopic lesions corresponded to chronic congested enteritis with discreet and diffuse inflammatory infiltration of the mononuclear cells. Trematodes were observed in the lumen of the viscera, fixed to the mucous membrane by means of adhesive discs. Hypertrophy of the muscle of the mucous membrane adjacent to the parasites could be seen (Figure 1d). The adhesive disk caused compression of the epithelium with its projections (Figure 1e). Strangling and hyperplasia of the intestinal mucous membrane were observed, reflecting the disorganization of the tissue (Figure 1f). The mucous surface of the intestine presented ovoid or discoid imprints corresponding to the shape of the parasite's adhesive disk (Figure 2a, b).
a) Colomesus psittacus. Intestine.Rohdella sp. Longitudinal section showing the parasite's adhesive disc (double arrow) adjacent to the mucous membrane of the intestine. H.E. Magnification 10×. Scale bar: 400 µm; b) Rohdella sp. SEM image ofFigure 2a, showing the adhesive disc (double arrow). Scale bar: 200 µm. Detail: ovoid or discoid indentation of the mucous membrane of the intestine corresponding to the mark left by the parasite's adhesive disc (arrow). Scale bar: 300 µm.
Discussion
Aspidogastreans of the genus Rohdella are reported here for the first time from the Amazonian freshwater parrot pufferfish (Colomesus psittacus). The infection levels ofRohdella sp. were compared with those of other parasites belonging to the subclass Aspidogastrea and parasitizing other hosts. The total prevalence (Table 2) of these parasites (76.4%) was much higher than the values recorded forBarbus barbus from the Fischa River in Australia parasitized byAspidogaster limacoides (SCHLUDERMANN et al., 2005); for Cyprinus carpio and Mylopharyngodon piceus, parasitized by Aspidogaster ijimai and A. conchicola, respectively, in a floodplain lake in the middle Yangtze River region (GAO et al., 2003Gao Q, Nie P, Yao WJ. Scanning electron microscopy of Aspidogaster ijimai Kawamura, 1913 and A. conchicola Baer, 1827 (Aspidogastrea, Aspidogastridae) with reference to their fish definitive-host specificity. Parasitol Res 2003; 91(6): 439-443. PMid:14564509. http:∕∕dx.doi.org∕10.1007∕s00436-003-1002-7
http:∕∕dx.doi.org∕10.1007∕s00436-003-100...
); and for Trachinotus marginatus, parasitized by Lobatostoma hanumanthai and L. kemostoma on the coast of southern Brazil (PEREIRA JUNIOR et al., 2004). However, the latter authors recorded a much higher mean intensity for L. kemostoma than what was recorded in the present study. In this study, higher abundance values were observed, in comparison with the values recorded for other species (Table 2).
Mollusks are known to be intermediate hosts in the life cycle of many aspidogastreans (ROHDE, 2001b), and are an important part of the diet ofC. psittacus (CAMARGO; MAIA, 2008Camargo M, Maia T. Análise populacional do baiacu, Colomesus psittacus (Tetraodontiformes, Tetraodontidae), no estuário do rio Caeté, costa norte do Brasil. Uakari 2008; 4(1): 23-28.). Given this, and the prevalence of Aspidogastrea of the genus Rohdella in the specimens collected in the present study,C. psittacus appears to be a common host of this parasite in the wild.
A high parasite load, such as what was recorded in the present study (which reached its peak in April 2009 – see Table 1), may favor obstruction of the intestinal tract, especially in small fish such as C. psittacus. This may affect the metabolic and reproductive development of the host and, in cases of extreme infection, cause its death (THATCHER, 2006Thatcher VE. Amazon Fish Parasites. 2nd ed. Bulgaria: Pensoft; 2006.; COSTA, CAMARGO, 2009Costa CHA, Camargo M. Procamallanus (Spirocamallanus) sp. (Camallanidae), um endoparasita do trato digestivo de Bivibranchia velox (Eigenmann & Myers, 1927) e B. fowleri (Steindachner, 1908), no setor do médio rio Xingu, Pará, Brasil. Uakari 2009; 5(1): 97-103.).
When fixed to the mucous membrane of the intestine, the adhesive disc of aspidogastreans causes strangulation and hyperplasia of the area, with hypertrophy of the muscle, as described by Grizzle and Brunner (2007) and Tolstenkov et al. (2010)Tolstenkov O, Terenina N, Kreshchenko N, Gustafsson M. The pattern of FMRFamide and serotonin immunoreactive elements in the nervous system of Aspidogaster conchicola K. Baer, 1827 (Aspidogastrea, Aspidogastridae). Belg J Zool 2010; 140: 133-136.. These reactions, according to Korting (1977)Korting W. Las reacciones del hospedador frente a algunos parásitos de los peces. In: Reichenbach-Klinke HH. Trabajos sobre histopatologia de los peces. Zaragoza: Acribia; 1977., are the most frequent manifestations of the process of parasitic invasion.
The anatomopathological features observed in the present study indicate that each parasite tends to provoke relatively small and localized impacts. The most important impact for the host would appear to be nutritional deficiency resulting from alterations caused to the intestinal epithelium. Under intense infestation, however, there is a possibility of intestinal obstruction, especially in hosts of small size, as suggested by Pavanelli et al. (1997)Pavanelli GC, Eiras JC, Guidelli GM. Nota sobre a histopatologia da parasitose de Microrchis oligovitellum LUNASCHI, 1987 (Trematoda - Paramphistomidae) em Parauchenipterus galeatus (LINNAEUS, 1766). UNIMAR 1997; 19(2): 473-478. for a digenetic trematode in a siluriform fish. We were unable to determine the species of parasite: this would require further investigation using electron microscopy and molecular analysis, with possible determination of a new species. This is the first report of parasitism by an aspidogastrean trematode in the intestine of C. psittacus from eastern Amazonia.
We are grateful to CAPES, CNPq, the Edilson Matos Research Laboratory (LPED-UFPA), Prof. Dr. Claudio Lamarão (Electron Microscopy Laboratory, ICG∕UFPA), the “Profa. DraReinalda Marisa Lanfredi” Laboratory for Cellular Biology and Helminthology, Federal University of Pará (LBCH-UFPA) and Prof. Dr. Stephen Ferrari for revising the English translation. The helpful suggestions and comments of the Associate Editor and reviewers were greatly appreciated.
References
- Aboul-Dahab HM, Abd El-Salam FA, El-Damarany ME. An Aspidogastrean parasite, Rohdella anodontiase sp. nov. from the fresh water mussel, Anodonta rubinse. Assiut Vet Med J 1993; 29(57): 81-88.
- Amato JFR. Manual de Técnicas para a Preparação de Coleções Zoológicas. 8. Platelmintos (Temnocefálidos, Trematódeos, Cestóides, Cestodários) e Acantocéfalos. São Paulo: Sociedade Brasileira de Zoologia; 1985.
- Camargo M, Maia T. Análise populacional do baiacu, Colomesus psittacus (Tetraodontiformes, Tetraodontidae), no estuário do rio Caeté, costa norte do Brasil. Uakari 2008; 4(1): 23-28.
- Costa CHA, Camargo M. Procamallanus (Spirocamallanus) sp. (Camallanidae), um endoparasita do trato digestivo de Bivibranchia velox (Eigenmann & Myers, 1927) e B. fowleri (Steindachner, 1908), no setor do médio rio Xingu, Pará, Brasil. Uakari 2009; 5(1): 97-103.
- Gao Q, Nie P, Yao WJ. Scanning electron microscopy of Aspidogaster ijimai Kawamura, 1913 and A. conchicola Baer, 1827 (Aspidogastrea, Aspidogastridae) with reference to their fish definitive-host specificity. Parasitol Res 2003; 91(6): 439-443. PMid:14564509. http:∕∕dx.doi.org∕10.1007∕s00436-003-1002-7
» http:∕∕dx.doi.org∕10.1007∕s00436-003-1002-7 - Gibson DJ, Chinabut S. Rohdella siamensis gen. et sp. nov. (Aspidogastridae: Rohdellinae subfam. nov.) from freshwater fishes in Thailand, with a reorganization of the classification of the subclass Aspidogastrea. Parasitol 1984; 88(3): 383-393. http:∕∕dx.doi.org∕10.1017∕S0031182000054652
» http:∕∕dx.doi.org∕10.1017∕S0031182000054652 - Grizzle JM, Brunner CJ. Assessment of current information available for detection, sampling, necropsy, and diagnosis of diseased mussels. Alabama: Alabama Department of Conservation and Natural Resources Wildlife and Freshwater Fisheries Division Montgomery; 2007. Available from: http:∕∕www.outdooralabama.com
» http:∕∕www.outdooralabama.com - Korting W. Las reacciones del hospedador frente a algunos parásitos de los peces. In: Reichenbach-Klinke HH. Trabajos sobre histopatologia de los peces. Zaragoza: Acribia; 1977.
- Ming-Xiu C, Li-Qianq Z, Chun-Gen W, Jun S, Qian G. Phylogenetic relationship of species in the genus Aspidogaster (Aspidogastridae, Aspidogastrinae) in China as inferred from its rDNA sequences. Acta Hydrobiol Sinica 2010; 34(2): 312-316
- Olson PD, Tkach VV. Advances and Trends in the Molecular Systematics of the Parasitic Platyhelminthes. Adv Parasitol 2005; 60: 165-243. http:∕∕dx.doi.org∕10.1016∕S0065-308X(05)60003-6
» http:∕∕dx.doi.org∕10.1016∕S0065-308X(05)60003-6 - Pavanelli GC, Eiras JC, Guidelli GM. Nota sobre a histopatologia da parasitose de Microrchis oligovitellum LUNASCHI, 1987 (Trematoda - Paramphistomidae) em Parauchenipterus galeatus (LINNAEUS, 1766). UNIMAR 1997; 19(2): 473-478.
- Peña-Rehbein P, Ríos-Escalante P. Use of negative binomial distribution to describe the presence of Anisakis in Thyrsites atun. Rev Bras Parasitol Vet 2012; 21(1): 78-80. PMid:22534952. http:∕∕dx.doi.org∕10.1590∕S1984-29612012000100017
» http:∕∕dx.doi.org∕10.1590∕S1984-29612012000100017 - Pereira Junior J, Velloso AL, Chaves IS, Moraes NCM, Oliveira SS. The relationship between Lobatostoma hanumanthai and L. kemostoma (Trematoda: Aspidogastridae) parasitological indexes and the ontogenetic diet variation of Trachinotus marginatus from the Rio Grande do Sul coast, Brazil. Bol Inst Pesca 2004; 30(2): 155-159.
- Rocha RM, Coelho RP, Montes CS, Santos SSD, Ferreira MAP. Avaliação histopatológica do fígado de Brachyplatystoma rousseauxii (CASTELNAU, 1855) da baía do Guajará, Belém, Pará. Cienc Anim Bras 2010; 11(1): 101-109.
- Rohde K. The Aspidogastrea: an archaic group of Platyhelminthes. In: Littlewood DTJ, Bray RA, editors. Interrelationships of the Platyhelminthes. London: Taylor and Francis; 2001a. p.159-167.
- Rohde K. Platyhelminthes (flat worms). Encyclopedia of Life Sciences; 2001b. Available from: http:∕∕www.els.net
» http:∕∕www.els.net - Schludermann C, Laimgruber S, Konecny R, Schabuss M. Aspidogaster limacoides DIESING, 1835 (Trematoda, Aspidogastridae): A new parasite of Barbus barbus (L.) (Pisces, Cyprinidae) in Austria. Ann Naturhist Mus Wien 2005; 141-144.
- Serra-Freire NM. Planejamento e análise de pesquisas parasitológicas. Niterói: Ed. Eduff; 2002.
- Snyder SD, Tkach VV. Neosychnocotyle maggiae, n. gen., n. sp. (Platyhelminthes: Aspidogastrea) from freshwater turtles in northern Australia. J Parasitol 2007; 93(2): 399-403. PMid:17539425. http:∕∕dx.doi.org∕10.1645∕GE-1001R.1
» http:∕∕dx.doi.org∕10.1645∕GE-1001R.1 - Thatcher VE. Amazon Fish Parasites. 2nd ed. Bulgaria: Pensoft; 2006.
- Tolstenkov O, Terenina N, Kreshchenko N, Gustafsson M. The pattern of FMRFamide and serotonin immunoreactive elements in the nervous system of Aspidogaster conchicola K. Baer, 1827 (Aspidogastrea, Aspidogastridae). Belg J Zool 2010; 140: 133-136.
- Zamparo D, Brooks DR. Phylogenetic systematic assessment of the Aspidobothrea (Platyhelminthes, Neodermata, Trematoda). Zool Scripta 2003; 32(1):83-93. http:∕∕dx.doi.org∕10.1046∕j.1463-6409.2003.00088.x
» http:∕∕dx.doi.org∕10.1046∕j.1463-6409.2003.00088.x
Publication Dates
-
Publication in this collection
Jan-Mar 2013
History
-
Received
17 Feb 2012 -
Accepted
27 June 2012