Abstracts
The objective of the present study was to evaluate the parasite fauna of four species of ornamental fish collected in the Chumucuí River, municipality of Bragança, Pará, Brazil. From June 2006 to December 2007. Fishes (n=307) belonging to four species were collected, including 23 specimens of Moenkhausia sanctaefilomenae (redeye tetra), 37Carnegiella strigata (marbled hatchetfish), 7Chilodus punctatus (spotted headstander), and 240Astyanax bimaculatus (twospot astyanax). The parasites found belonged to three taxa: monogeneans in the gills, nematodes (larvae ofCapillaria sp. and Contracaecum sp.) in the digestive tract and liver and acanthocephalans (Quadrigyrus torquatus, Q. brasiliensis and Q. nickoli) in the stomach and intestine. Astyanax bimaculatus presented higher prevalence of acanthocephalans in the wet season, and lower prevalence of nematodes in the dry season. The possible importance of these parasites in the exportation of ornamental fish is discussed.
Amazon; ornamental fish; parasite; acanthocephalans; monogeneans; nematodes
O objetivo do presente trabalho foi avaliar a fauna parasitária de quatro espécies de peixes ornamentais capturados no rio Chumucuí, no município de Bragança-PA. Foram coletados um total de 307 peixes pertencentes a 4 espécies, sendo elas:Moenkhausia sanctaefilomenae (olho de fogo, n = 23),Carnegiella strigata (borboleta, n = 37),Chilodus punctatus (cabeça-para-baixo, n = 7) e Astyanax bimaculatus (lambari, n = 240) coletados de junho de 2006 a dezembro de 2007. Foram observados 3 taxa parasitando os peixes: monogenéticos nas brânquias, nematóides (larvas deCapillaria sp. e Contracaecum sp.) no trato digestório e fígado e acantocéfalos (Quadrigyrus torquatus, Q. brasiliensis e Q. nickoli) no estômago e intestino. Astyanax bimaculatus apresentou maior prevalência de acantocéfalos na estação chuvosa, menor prevalência de nematóides na estação seca. Discute-se a eventual importância destes parasitas na exportação de peixes ornamentais.
Amazônia; peixes ornamentais; parasita; acantocéfalos; monogenéticos; nematóides
Introduction
The Amazon River basin is a hydrographical complex composed of a large number of rivers, lakes and small tributaries, containing great animal diversity. Within this area, fish are the most important resource for the local economy (MATOS et al., 2004Matos E, Casal G, Matos P, Corral LA, Carlos. Microorganismos Parasitos de Animais Aquáticos da Amazônia. In: Ranzani-Paiva MJT, Takemoto RM, Lizama MAP, editors. Sanidade de organismos aquáticos. Varela; 2004. p.159-178.).
The ornamental fishery is a major activity in that region and constitutes an important economic resource, since these fish are exported to other countries in large quantities (CHAO, 2001Chao NL. The fishery diversity and conservation of ornamental fishes in the Rio Negro Basin, Brasil: A review of Project Piaba (1989-1999). In: Chao NL, Petry P, Prang G, Sonnenschein L, Tlusty MT, editors. Conservation and management of ornamental fish resources of the Rio Negro Basin, Amazonia Brasil - Project Piaba. Manaus: Universidade do Amazonas, Manaus; 2001. p. 161-204.). The state of Pará is the second most important state regarding exportation of ornamental fish in Brazil, and the region of Bragança is an important place for catching fish because of the high number of different ornamental fish species. At least 38 different species have been reported in that region (Silva, unpublished data; Paula, unpublished data). This is especially evident in the Chumucuí River, a tributary of the Caeté River, where Characiform fish (which comprise a large number of ornamental species) represent 49% of the most important fish species (Silva, unpublished data).
It is well known that exportation of ornamental fish may represent a gateway for both ecto and endoparasites to enter a new environment. Their introduction may have serious effects, especially when parasites come into contact with new host fish. Several cases of introduced parasites, sometimes with devastating consequences, can be found in the literature (KIM et al., 2002Kim JH, Hayward CJ, Joh SJ, Heo GJ. Parasitic infections in live freshwater tropical fishes imported to Korea. Dis Aquat Org 2002; 52(2): 169-173. PMid:12542094. http://dx.doi.org/10.3354/dao052169
http://dx.doi.org/10.3354/dao052169...
; MORAVEC et al., 1999Moravec F, Wolter J, Körting W. Some nematodes and acanthocephalans from exotic ornamental freshwater fishes imported into Germany. Folia Parasitol 1999; 46(4): 296-310.; EVANS; LESTER, 2001Evans BB, Lester RJG. Parasites of ornamental fish imported into Australia. EAFP Bul 2001; 21(2): 51-55.), and the pathology of parasites in ornamental fish has been described in a number of studies (FERRAZ; SOMMERVILLE, 1998Ferraz E, Sommerville C. Pathology of Piscinoodinium sp. (Protozoa: Dinoflagellida), parasites of the ornamental freshwater catfishes Corydoras spp. and Brochis splendens (Pisces: Callichthyidae). Dis Aquat Org 1998; 33(1): 43-49. PMid:9653457. http://dx.doi.org/10.3354/dao033043
http://dx.doi.org/10.3354/dao033043...
; PIAZZA et al. 2006Piazza RS, Martins ML, Guiraldelli L, Yamashita M. Parasitic diseases of freshwater ornamental fishes commercialized in Florianópolis, Santa Catarina, Brazil. B Inst Pesca 2006; 32(1): 51-57.; THILAKARATNE et al., 2003Thilakaratne IDSIP, Rajapaksha G, Hewakopara A, Rajapakse RPVJ, Faizal ACM. Parasitic infections in freshwater ornamental fish in Sri Lanka. Dis Aquatic Org 2003; 54(2): 157-162. PMid:12747641. http://dx.doi.org/10.3354/dao054157
http://dx.doi.org/10.3354/dao054157...
; PRANG, 2007Prang G. An industry analysis of the freshwater ornamental fishery with particular reference to the supply of Brazilian freshwater ornamentals to the UK market. Uakari 2007; 3(1): 7-51.; MOUTON et al., 2001Mouton A, Basson L, Impson D. Health status of ornamental freshwater fishes imported to South Africa: a pilot study. Aquar Sci Conserv 2001; 3(4): 313-319. http://dx.doi.org/10.1023/A:1013197928590
http://dx.doi.org/10.1023/A:101319792859...
;GARCIA et al., 2009Garcia F, Fujimoto RY, Martins ML, Moraes FR. Protozoan parasites of Xiphophorus spp. (Poeciliidae) and their relation with water characteristics. Arq Bra Méd Vet Zootec 2009; 61(1): 156-162. http://dx.doi.org/10.1590/S0102-09352009000100022
http://dx.doi.org/10.1590/S0102-09352009...
).
In this paper, ecto and endoparasites of some species of economically important ornamental fish species from the Chumucuí River were studied:Carnegiella strigata (Günther, 1864) (Gasteropelecidae), commonly named “marbled hatchetfish”; Moenkhausia sanctaefilomenae (Steindachner, 1907) (Characidae), “redeye tetra”; Chilodus punctatus (Müller & Troschel, 1844) (Chilodontidae), “spotted headstander”; and Astyanax bimaculatus (Linnaeus, 1758) (Characidae), commonly known as “two-spot lambari” or “matupiri” in the Amazon region, and as “piaba” in the north of the country. It is important to mention that the parasites of these fish species have never previously been studied in this region.
Material and Methods
Fish samples were collected twice a month (at the beginning and end of the month) from the Chumucuí River, a tributary of the Caeté River, in Bragança, Pará (1° 12′ 38.3″ S and 46° 47′ 32″ W). Monthly sampling was done from June 2006 to December 2007. The fish were transported alive in Styrofoam boxes to the laboratory, where they were sacrificed using the anesthetic benzocaine. They were then measured (total and standard lengths) and weighed. Parasites were collected and processed as described by Eiras et al. (2006)Eiras JC, Takemoto RM, Pavanelli GC. Métodos de estudo e técnicas laboratoriais em parasitologia de peixes. 2. ed. Maringá: Eduem; 2006., and were identified at the lower taxonomic level in accordance with Thatcher (2006)Thatcher VE. Amazon Fish Parasites. Manaus: Instituto Nacional de pesquisas da Amazônia; 2006. p. 555. and Travassos et al. (1928)Travassos L, Artigas P, Pereira C. Fauna helmintológica dos peixes de água doce do Brasil. Arch Inst Biol 1928; 1: 5-68.. The parasitological indexes were calculated as recommended byBush et al. (1997)Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis et al revisited. J Parasitol 1997; 83(4): 575-583. PMid:9267395. http://dx.doi.org/10.2307/3284227
http://dx.doi.org/10.2307/3284227...
.
The water pH and dissolved oxygen were recorded at the time of sampling. Environmental data (rainfall and temperature) were obtained from the Meteorological Station of Tracuateua, which belongs to the Brazilian National Institute of Meteorology.
The parasitological indexes were correlated with the weight, total length and standard length of the fish, and with the oxygen, pH, temperature and rainfall, by means of Pearson's correlation test. The variance of the mean values of prevalence and intensity of infection for the dry and wet seasons was analyzed and compared using the t test (P = 0.05). Statistical tests were done using the Biostat 4.0 software.
Results
Four species of ornamental fish were collected from the Chumucuí River: Moenkhausia sanctaefilomenae, Carnegiella strigata, Chilodus punctatus and Astyanax bimaculatus. The number of specimens, number of infected specimens, prevalence, total and standard lengths (cm) and total weight (g) are shown in Table 1. Moenkhausia sanctaefilomenae and A. bimaculatus presented the highest percentage prevalence; C. punctatus showed an intermediate percentage and C. strigata presented the lowest percentage prevalence, compared with the other species (Table 1).
Number of fish collected from Chumucuí River, number of infected fish, prevalence (%) of the infection, total length in cm, standard length in cm, and total weight in g.
The observations on the parasites showed that they belonged to three different taxa: nematodes (larvae of Contracaecum sp. andCapillaria sp.); acanthocephalans (Quadrigyrus torquatus, Van Cleave, 1920; Q. nickoli, Schmidt and Hugghins, 1973; Q. brasiliensis, Machado, 1941); and unidentified monogeneans. The distribution of the parasites according to the hosts is indicated in Table 2.
The infection by monogeneans was not related to the length of the host. However, Moenkhausia sanctaefilomenae showed the highest values for prevalence and mean intensity of infection by monogeneans (17.3% and 5.0 ± 2.2, respectively). Astyanax bimaculatus presented lower prevalence (2.08%) and similar mean intensity of infection (5.3 ± 0.6), compared with M. sanctaefilomenae. On the other hand,C. strigata showed lower prevalence (2.7%) and lower intensity of infection (1.0 ± 0.16). Monogeneans were not observed inC. punctatus.
Nematodes were found only as larval stages in different organs of the fish (Tables 3 and 4). Astyanax bimaculatus was the most parasitized species, and the highest level of nematode prevalence (50%) was in the stomach. Chilodus punctatus showed the highest mean intensity among the four fish species (12 ± 0.3). Larvae ofContracaecum sp. and Capillaria sp. were only found parasitizing the intestine and liver of M. sanctaefilomenae.
Prevalence (%) of nematodes in the organs of the different fish species. Stomach; Anterior intestine; Middle intestine; Posterior intestine; Liver; Pyloric cecae; Total prevalence.
Mean intensity (parasite/fish infected) of infection and standard deviation according to the nematodes in the organs of the different fish species. Stomach; Anterior intestine; Medium intestine; Posterior intestine; Liver; Pyloric cecae.
Concerning acanthocephalans, only cystacanth stages ofQuadrigyrus spp. were found in the stomach and intestine. Only two host species presented larvae of Quadrigyrus sp. in the stomach: C. punctatus (prevalence 25%; mean intensity of infection 1.5 ± 0.74) and A. bimaculatus (10.8%; 1.76 ± 0.63). Q. torquatus and Q. nickoli were observed in A. bimaculatus; Q. brasiliensis,Q. torquatus and Q. nickoli were observed inM. sanctaefilomenae; and unidentified acanthocephalans were seen to be parasitizing C. punctatus.
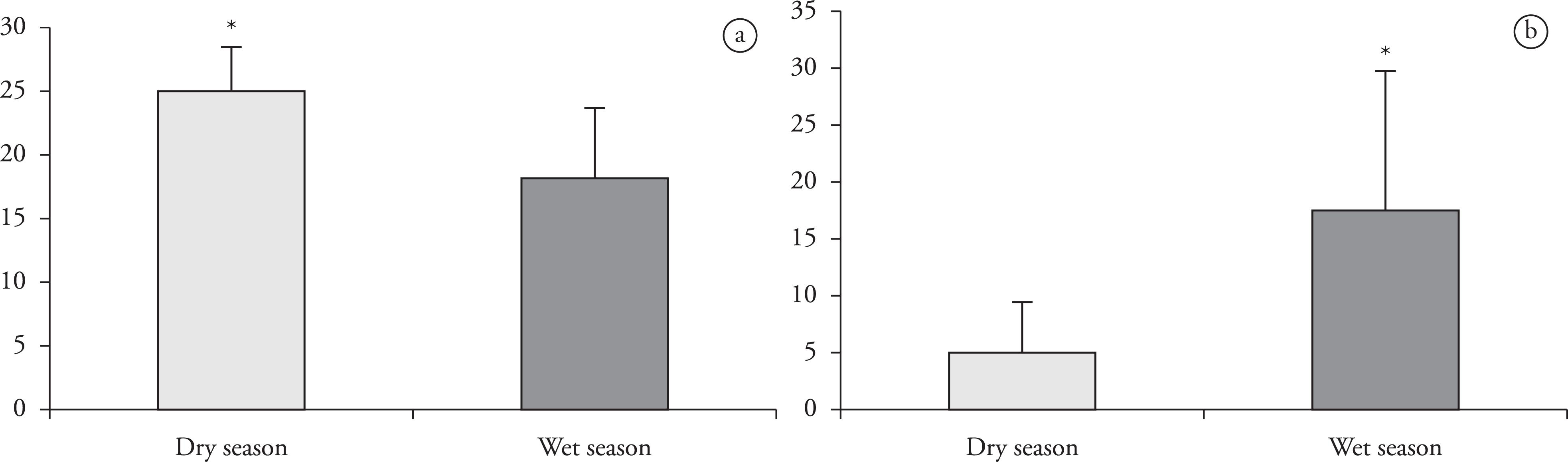
The relationship between abiotic and biotic factors (Figure 1) showed that the only significant correlation with infection due to acanthocephalans was in the anterior intestine of A. bimaculatus, with higher prevalence in the wet season (P < 0.05). In the same host, the prevalence of nematodes in the anterior intestine was also significantly higher in the dry season (P < 0.05).
Prevalence of parasites in the anterior intestine of the fishAstyanax bimaculatus, according to dry and wet seasons. a) nematode; b) acanthocephalan.
Discussion
The results showed that parasites belonging at least to three different taxa were present in the ornamental fish caught from the Chumucuí River. Similar results, sometimes with higher diversity of parasites, have been found in other ornamental fish species (PIAZZA et al., 2006Piazza RS, Martins ML, Guiraldelli L, Yamashita M. Parasitic diseases of freshwater ornamental fishes commercialized in Florianópolis, Santa Catarina, Brazil. B Inst Pesca 2006; 32(1): 51-57.; TAVARES-DIAS et al., 2009Tavares-Dias M, Lemos JRG, Martins ML, Jerônimo GT. Metazoan and protozoan parasites of freshwater ornamental fish from Brazil. In: Tavares-Dias M, editors. Manejo e sanidade de peixes em cultivo. Macapá: Embrapa; 2009. p. 469-494.,2010Tavares-Dias M, Gonzaga Lemos JR, Martins ML. Parasitic fauna of eight species of ornamental freshwater fish species from the middle Negro River in the Brazilian Amazon Region. Rev Bras Parasitol Vet 2010; 19(2): 103-107. PMid:20624347. http://dx.doi.org/10.4322/rbpv.01902007
http://dx.doi.org/10.4322/rbpv.01902007...
). Moenkhausia sanctaefilomenae is a new host for Contracaecum sp.,Capillaria sp., Q. torquatus, Q. nickoli and Q. brasiliensis, and A. bimaculatus is a new host for Q. torquatus andQ. nickoli.
The monogeneans are mostly ectoparasites and are frequently observed in the gills. They are especially important in relation to fish rearing and may cause high mortality rates among farmed fish (FUJIMOTO et al., 2010Fujimoto RY, Castro MP, Martins ML, Moraes FR, Varella JEA, Diniz DG. Effects of Chromium Supplementation on the Infrapopulations of Anacanthorus penilabiatus (Monogenoidea) and Piscinoodinium pillulare (Dinoflagellida) Parasites of Piaractus mesopotamicus (Characidae). Braz Arch Biol Technol 2010; 53(4): 827-833. http://dx.doi.org/10.1590/S1516-89132010000400011
http://dx.doi.org/10.1590/S1516-89132010...
). In these specimens, the intensity of infection was not related to the size of the hosts, contrary to what was observed by Pereira Junior et al. (2002)Pereira Junior J, Da Costa MAS, Vianna RT. Índices parasitológicos de Cucullanidae (Nematoda: Seuratoidea) em Micropogonias furnieri (Desmarest, 1823) no litoral do Rio Grande do Sul, Brasil. Atlântica 2002; 24(2): 97-101. and Gonzáles et al. (2001)Gonzáles MT, Acuña E, Oliva ME. Metazoan parasite fauna of the Bigeye Flounder, Hippoglossina macrops, from Northern Chile. Influence of host age and sex. Mem Inst Oswaldo Cruz 2001; 96(8): 1049-1054. in relation toNeoheterobothrium parasitizing Hippoglossina macrops, which was related to greater surface area of the gills of the older hosts.
Larvae of the nematode species, Contracaecum sp. andCapillaria sp., were observed parasitizing M. sanctaefilomenae. According to Dick and Choudhury (1995)Dick TA, Choudhury A. Phylum nematoda. In: Woo PTK, editors. Fish diseases and Disorders. New York: CABE Publishing; 1995. v. 1. Protozoan and metazoan infections, p. 415-446., the larvae may be more dangerous than the adults given that they can migrate through the fish to several target organs. Astyanax bimaculatus was the most infected fish species, presenting prevalence of 82%. It is interesting to note that for the same host species captured at Lages (state of Rio de Janeiro), Paraguassú and Luque (2007)Paraguassú AR, Luque JL. Metazoários Parasitos de seis espécies de Peixes do Reservatório de Lajes, Estado do Rio de Janeiro, Brasil. Rev Bras Parasitol Vet 2007; 16(3): 121-128. PMid:18078597.http://dx.doi.org/10.1590/S1984-29612007000300002
http://dx.doi.org/10.1590/S1984-29612007...
reported prevalence of 41% for two nematode species, and in A. fasciatus they found prevalence of 39.2% for three nematode species. Also, Martins et al. (2005)Martins ML, Onaka EM, Fenerick Junior J. Larval Contracaecum sp. (Nematoda: Anisakidae) in Hoplias malabaricus and Hoplerythrinus unitaeniatus (Osteichthyes: Erythrinidae) of economic importance in occidental marshlands of Maranhão, Brazil. Vet Parasitol 2005; 127(1): 51-59. PMid:15619375.http://dx.doi.org/10.1016/j.vetpar.2004.09.026
http://dx.doi.org/10.1016/j.vetpar.2004....
found Contracaecum sp. infecting 100% ofHoplias malabaricus and 80% of Hoplyerythrinus unitaeniatus caught in the state of Maranhão, and Barros et al. (2007)Barros LA, Moraes Filho J, Oliveira RL. Larvas de nematóides de importância zoonótica encontradas em traíras (Hoplias malabaricus bloch, 1794) no município de Santo Antonio do Leverger, MT. Arq Bra Med Vet Zootec 2007; 59(2): 533-535. http://dx.doi.org/10.1590/S0102-09352007000200042
http://dx.doi.org/10.1590/S0102-09352007...
observed prevalence of 73% in H. malabaricus caught in the state of Mato Grosso. In the present study, the prevalence of nematodes in the anterior intestine was significantly higher in the dry season. However, the results of Martins et al. (2002)Martins ML, Mello A, Paiva FC, Fujimoto RY, Schalch SHC, Colombano NC. Prevalência, sazonalidade e intensidade de infecção por Diplostomum (Austrodiplostomum) compactum Lutz, 1928 (Digenea, Diplostomidae), em peixes do reservatório de Volta Grande, Estado de Minas Gerais, Brasil. Acta Sci 2002; 24(2): 469-474. showed greater numbers of nematodes in the wet season in the Volta Grande region, state of Minas Gerais. According to Yamamoto et al. (2004)Yamamoto KC, Soares MGM, Freitas CEC. Alimentação de Triportheus angulatus (Spix & Agassiz, 1829) no lago Camaleão, Manaus, AM, Brasil. Acta Amaz 2004; 34(4): 653-659. http://dx.doi.org/10.1590/S0044-59672004000400017
http://dx.doi.org/10.1590/S0044-59672004...
, the alternation of wet and dry seasons and the consequent pronounced variation in the water level of the river could influence the food resources of the fish and indirectly influence the number of nematodes, due to variable predation on their intermediate hosts.
The acanthocephalans (Q. torquatus, Q. nickoli and Q. brasiliensis) were found in A. bimaculatus, M. sanctaefilomenae and C. punctatus hosts in the form of encysted larvae, while they were not observed in C. strigata. The presence in these hosts is probably related to predation on the intermediate hosts of the parasites, in which the feeding habits play an important role in the lifecycle. The related speciesQ. machadoi was reported in Hemisorubim platyrhynchus in the Paraná River (GUIDELLI et al., 2003Guidelli GM, Isaac A, Takemoto RM, Pavanelli GC. Endoparasite infracommunities of Hemisorubim platyrhynchos (Valenciennes, 1840) (Pisces: Pimelodidae) of the Baía River, upper Paraná River floodplain, Brazil: specific composition and ecological aspects. Braz J Biol 2003; 63(2): 261-268. PMid:14509848. http://dx.doi.org/10.1590/S1519-69842003000200011
http://dx.doi.org/10.1590/S1519-69842003...
), and in H. malabaricus in a lagoon at Aguaí, infecting 87.5% of the fish (ROSIM et al., 2005Rosim DF, Cecarelli PS, Silva-Souza AT. Parasitismo de Hoplias malabaricus (Bloch, 1794) (Characiformes, Erythrinidae) por Quadrigyrus machadoi Fábio, 1983 (Eoacanthocephala, Quadrigyridae) de uma Lagoa em Aguaí, Estado De São Paulo, Brasil. Rev Bras Parasitol Vet 2005; 14(4): 147-153. PMid:16445871.). According to Rosim et al. (2005)Rosim DF, Cecarelli PS, Silva-Souza AT. Parasitismo de Hoplias malabaricus (Bloch, 1794) (Characiformes, Erythrinidae) por Quadrigyrus machadoi Fábio, 1983 (Eoacanthocephala, Quadrigyridae) de uma Lagoa em Aguaí, Estado De São Paulo, Brasil. Rev Bras Parasitol Vet 2005; 14(4): 147-153. PMid:16445871., Astyanax altiparanae is considered to be a paratenic host for Q. torquatus, and according to Carvalho et al. (2003)Carvalho S, Guidelli GM, Takemoto RM, Pavanelli GC. Ecological aspects of endoparasite fauna of Acestrorhynchus lacustris (Lütken, 1875) (Characiformes, Acestrorhynchidae) on the Upper Paraná River floodplain, Brazil. Acta Sci Biol Sci 2003; 25(2): 479-483. http://dx.doi.org/10.4025/actascibiolsci.v25i2.2043
http://dx.doi.org/10.4025/actascibiolsci...
,Astyanax spp. are considered both paratenic and intermediate hosts for Q. torquatus.
The results from these authors demonstrated that there was a negative correlation between the mean intensity of infection and the standard length of the host. Our observations do not support such evidence. The acanthocephalans were more prevalent in the anterior intestine of A. bimaculatus during the wet season, than the prevalence in the other hosts. The difference between hosts is probably due to different predation intensities of the intermediate hosts of the parasite, and the higher prevalence in A. bimaculatus during the wet season might be related to more abundant intermediate hosts during the wet season.
The fish of the present report are ornamental fish that are exported in great numbers to other countries. The results showed that these fish can carry several kind of parasites, both ecto and endoparasites, which can infect other fish in different continents and locations. In the literature, there are a number of reports dealing with the parasites of ornamental fish (FERRAZ; SOMMERVILLE, 1998Ferraz E, Sommerville C. Pathology of Piscinoodinium sp. (Protozoa: Dinoflagellida), parasites of the ornamental freshwater catfishes Corydoras spp. and Brochis splendens (Pisces: Callichthyidae). Dis Aquat Org 1998; 33(1): 43-49. PMid:9653457. http://dx.doi.org/10.3354/dao033043
http://dx.doi.org/10.3354/dao033043...
; Piazza et al., 2006Piazza RS, Martins ML, Guiraldelli L, Yamashita M. Parasitic diseases of freshwater ornamental fishes commercialized in Florianópolis, Santa Catarina, Brazil. B Inst Pesca 2006; 32(1): 51-57.; THILAKARATNE et al., 2003Thilakaratne IDSIP, Rajapaksha G, Hewakopara A, Rajapakse RPVJ, Faizal ACM. Parasitic infections in freshwater ornamental fish in Sri Lanka. Dis Aquatic Org 2003; 54(2): 157-162. PMid:12747641. http://dx.doi.org/10.3354/dao054157
http://dx.doi.org/10.3354/dao054157...
; PRANG, 2007Prang G. An industry analysis of the freshwater ornamental fishery with particular reference to the supply of Brazilian freshwater ornamentals to the UK market. Uakari 2007; 3(1): 7-51.; Mouton et al., 2001Mouton A, Basson L, Impson D. Health status of ornamental freshwater fishes imported to South Africa: a pilot study. Aquar Sci Conserv 2001; 3(4): 313-319. http://dx.doi.org/10.1023/A:1013197928590
http://dx.doi.org/10.1023/A:101319792859...
; GARCIA et al., 2009Garcia F, Fujimoto RY, Martins ML, Moraes FR. Protozoan parasites of Xiphophorus spp. (Poeciliidae) and their relation with water characteristics. Arq Bra Méd Vet Zootec 2009; 61(1): 156-162. http://dx.doi.org/10.1590/S0102-09352009000100022
http://dx.doi.org/10.1590/S0102-09352009...
) and with the introduction of parasites by ornamental fish (KIM et al., 2002Kim JH, Hayward CJ, Joh SJ, Heo GJ. Parasitic infections in live freshwater tropical fishes imported to Korea. Dis Aquat Org 2002; 52(2): 169-173. PMid:12542094. http://dx.doi.org/10.3354/dao052169
http://dx.doi.org/10.3354/dao052169...
; MORAVEC et al., 1999Moravec F, Wolter J, Körting W. Some nematodes and acanthocephalans from exotic ornamental freshwater fishes imported into Germany. Folia Parasitol 1999; 46(4): 296-310.; EVANS; LESTER, 2001Evans BB, Lester RJG. Parasites of ornamental fish imported into Australia. EAFP Bul 2001; 21(2): 51-55.). The consequences of parasite introduction can be detrimental to native fish, sometimes causing serious epizootics that may lead to extensive mortality as shown in relation to several species of monogeneans introduced into aquariums (BULLARD et al., 2001Bullard SA, Frasca SJR, Benz GW. Gill lesions associated with Erpocotyle tiburonis (Monogenea: Hexabothriidae) on wild and aquarium-held bonnet head sharks (Sphyrna tiburo). J Parasitol 2001; 87: 972-977. PMid:11695418.), into contact with farmed fish (OGAWA et al., 1995Ogawa K, Bondad-Reantaso MG, Fukudome M, Wakabayashi H. Neobenedenia girellae (Hargis, 1955) Yamaguti, 1963 (Monogenea: Capsalidae) from cultured marine fishes of Japan. J Parasitol 1995; 81(2): 223-227. PMid:7707197.http://dx.doi.org/10.2307/3283923
http://dx.doi.org/10.2307/3283923...
; STERUD et al., 1998Sterud E, Harris PH, Bake TA. The influence of Gyrodactylus salaris Malmberg 1957 (Monogenea) on the epidermis of Atlantic salmon, Salmo salar L., and brook trout, Salvelinus fontinalis (Mitchill): experimental studies. J Fish Dis 1998; 21(4): 257-263. http://dx.doi.org/10.1046/j.1365-2761.1998.00099.x
http://dx.doi.org/10.1046/j.1365-2761.19...
) and also into natural populations with dramatic consequences (WHITTINGTON; CHISHOLM, 2003Whittington ID, Chisholm LA. Biodiversity of marine parasites in Australia; more than just a list of largely invisible creatures. Rec South Aust Mus 2003; 7: 51-60.; BAKKE et al., 2002Bakke TA, Harris PD, Cable J. Host specificity dynamics: observations on gyrodactylid mongeneans. Int J Parasitol 2002; 32(3): 281-308.http://dx.doi.org/10.1016/S0020-7519(01)00331-9
http://dx.doi.org/10.1016/S0020-7519(01)...
).
The parasites observed might constitute a threat to other fish species in the region where they have been introduced. Therefore, it is recommended that prophylactic measures should be taken prior to exportation of fish, and that fish should undergo a period of quarantine at the place of introduction. In the present sample, three groups of endoparasites with heteroxenous life cycles (nematodes and acanthocephalans) were found and one group of ectoparasites with a direct life cycle (monogeneans). Consequently, the prophylactic measures must be especially directed towards monogeneans, for several reasons: they are located in the gills, a particularly fragile and important fish organ; they reproduce very quickly in a closed environment given that they have a direct life cycle; and because they are ectoparasites, use of appropriate drugs is easier and more effective.
The participation of J.C. Eiras on this research was partially supported by the European Regional Development Fund (ERDF) through the COMPETE - Operational Competitiveness Programme and national funds through FCT – Foundation for Science and Technology, under the project “PEst-C/MAR/LA0015/2011
References
- Bakke TA, Harris PD, Cable J. Host specificity dynamics: observations on gyrodactylid mongeneans. Int J Parasitol 2002; 32(3): 281-308.http://dx.doi.org/10.1016/S0020-7519(01)00331-9
» http://dx.doi.org/10.1016/S0020-7519(01)00331-9 - Barros LA, Moraes Filho J, Oliveira RL. Larvas de nematóides de importância zoonótica encontradas em traíras (Hoplias malabaricus bloch, 1794) no município de Santo Antonio do Leverger, MT. Arq Bra Med Vet Zootec 2007; 59(2): 533-535. http://dx.doi.org/10.1590/S0102-09352007000200042
» http://dx.doi.org/10.1590/S0102-09352007000200042 - Bullard SA, Frasca SJR, Benz GW. Gill lesions associated with Erpocotyle tiburonis (Monogenea: Hexabothriidae) on wild and aquarium-held bonnet head sharks (Sphyrna tiburo). J Parasitol 2001; 87: 972-977. PMid:11695418.
- Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology meets ecology on its own terms: Margolis et al revisited. J Parasitol 1997; 83(4): 575-583. PMid:9267395. http://dx.doi.org/10.2307/3284227
» http://dx.doi.org/10.2307/3284227 - Carvalho S, Guidelli GM, Takemoto RM, Pavanelli GC. Ecological aspects of endoparasite fauna of Acestrorhynchus lacustris (Lütken, 1875) (Characiformes, Acestrorhynchidae) on the Upper Paraná River floodplain, Brazil. Acta Sci Biol Sci 2003; 25(2): 479-483. http://dx.doi.org/10.4025/actascibiolsci.v25i2.2043
» http://dx.doi.org/10.4025/actascibiolsci.v25i2.2043 - Chao NL. The fishery diversity and conservation of ornamental fishes in the Rio Negro Basin, Brasil: A review of Project Piaba (1989-1999). In: Chao NL, Petry P, Prang G, Sonnenschein L, Tlusty MT, editors. Conservation and management of ornamental fish resources of the Rio Negro Basin, Amazonia Brasil - Project Piaba. Manaus: Universidade do Amazonas, Manaus; 2001. p. 161-204.
- Dick TA, Choudhury A. Phylum nematoda. In: Woo PTK, editors. Fish diseases and Disorders. New York: CABE Publishing; 1995. v. 1. Protozoan and metazoan infections, p. 415-446.
- Eiras JC, Takemoto RM, Pavanelli GC. Métodos de estudo e técnicas laboratoriais em parasitologia de peixes. 2. ed. Maringá: Eduem; 2006.
- Evans BB, Lester RJG. Parasites of ornamental fish imported into Australia. EAFP Bul 2001; 21(2): 51-55.
- Ferraz E, Sommerville C. Pathology of Piscinoodinium sp. (Protozoa: Dinoflagellida), parasites of the ornamental freshwater catfishes Corydoras spp. and Brochis splendens (Pisces: Callichthyidae). Dis Aquat Org 1998; 33(1): 43-49. PMid:9653457. http://dx.doi.org/10.3354/dao033043
» http://dx.doi.org/10.3354/dao033043 - Fujimoto RY, Castro MP, Martins ML, Moraes FR, Varella JEA, Diniz DG. Effects of Chromium Supplementation on the Infrapopulations of Anacanthorus penilabiatus (Monogenoidea) and Piscinoodinium pillulare (Dinoflagellida) Parasites of Piaractus mesopotamicus (Characidae). Braz Arch Biol Technol 2010; 53(4): 827-833. http://dx.doi.org/10.1590/S1516-89132010000400011
» http://dx.doi.org/10.1590/S1516-89132010000400011 - Garcia F, Fujimoto RY, Martins ML, Moraes FR. Protozoan parasites of Xiphophorus spp. (Poeciliidae) and their relation with water characteristics. Arq Bra Méd Vet Zootec 2009; 61(1): 156-162. http://dx.doi.org/10.1590/S0102-09352009000100022
» http://dx.doi.org/10.1590/S0102-09352009000100022 - Guidelli GM, Isaac A, Takemoto RM, Pavanelli GC. Endoparasite infracommunities of Hemisorubim platyrhynchos (Valenciennes, 1840) (Pisces: Pimelodidae) of the Baía River, upper Paraná River floodplain, Brazil: specific composition and ecological aspects. Braz J Biol 2003; 63(2): 261-268. PMid:14509848. http://dx.doi.org/10.1590/S1519-69842003000200011
» http://dx.doi.org/10.1590/S1519-69842003000200011 - Gonzáles MT, Acuña E, Oliva ME. Metazoan parasite fauna of the Bigeye Flounder, Hippoglossina macrops, from Northern Chile. Influence of host age and sex. Mem Inst Oswaldo Cruz 2001; 96(8): 1049-1054.
- Kim JH, Hayward CJ, Joh SJ, Heo GJ. Parasitic infections in live freshwater tropical fishes imported to Korea. Dis Aquat Org 2002; 52(2): 169-173. PMid:12542094. http://dx.doi.org/10.3354/dao052169
» http://dx.doi.org/10.3354/dao052169 - Martins ML, Mello A, Paiva FC, Fujimoto RY, Schalch SHC, Colombano NC. Prevalência, sazonalidade e intensidade de infecção por Diplostomum (Austrodiplostomum) compactum Lutz, 1928 (Digenea, Diplostomidae), em peixes do reservatório de Volta Grande, Estado de Minas Gerais, Brasil. Acta Sci 2002; 24(2): 469-474.
- Martins ML, Onaka EM, Fenerick Junior J. Larval Contracaecum sp. (Nematoda: Anisakidae) in Hoplias malabaricus and Hoplerythrinus unitaeniatus (Osteichthyes: Erythrinidae) of economic importance in occidental marshlands of Maranhão, Brazil. Vet Parasitol 2005; 127(1): 51-59. PMid:15619375.http://dx.doi.org/10.1016/j.vetpar.2004.09.026
» http://dx.doi.org/10.1016/j.vetpar.2004.09.026 - Matos E, Casal G, Matos P, Corral LA, Carlos. Microorganismos Parasitos de Animais Aquáticos da Amazônia. In: Ranzani-Paiva MJT, Takemoto RM, Lizama MAP, editors. Sanidade de organismos aquáticos. Varela; 2004. p.159-178.
- Moravec F, Wolter J, Körting W. Some nematodes and acanthocephalans from exotic ornamental freshwater fishes imported into Germany. Folia Parasitol 1999; 46(4): 296-310.
- Mouton A, Basson L, Impson D. Health status of ornamental freshwater fishes imported to South Africa: a pilot study. Aquar Sci Conserv 2001; 3(4): 313-319. http://dx.doi.org/10.1023/A:1013197928590
» http://dx.doi.org/10.1023/A:1013197928590 - Ogawa K, Bondad-Reantaso MG, Fukudome M, Wakabayashi H. Neobenedenia girellae (Hargis, 1955) Yamaguti, 1963 (Monogenea: Capsalidae) from cultured marine fishes of Japan. J Parasitol 1995; 81(2): 223-227. PMid:7707197.http://dx.doi.org/10.2307/3283923
» http://dx.doi.org/10.2307/3283923 - Paraguassú AR, Luque JL. Metazoários Parasitos de seis espécies de Peixes do Reservatório de Lajes, Estado do Rio de Janeiro, Brasil. Rev Bras Parasitol Vet 2007; 16(3): 121-128. PMid:18078597.http://dx.doi.org/10.1590/S1984-29612007000300002
» http://dx.doi.org/10.1590/S1984-29612007000300002 - Pereira Junior J, Da Costa MAS, Vianna RT. Índices parasitológicos de Cucullanidae (Nematoda: Seuratoidea) em Micropogonias furnieri (Desmarest, 1823) no litoral do Rio Grande do Sul, Brasil. Atlântica 2002; 24(2): 97-101.
- Piazza RS, Martins ML, Guiraldelli L, Yamashita M. Parasitic diseases of freshwater ornamental fishes commercialized in Florianópolis, Santa Catarina, Brazil. B Inst Pesca 2006; 32(1): 51-57.
- Prang G. An industry analysis of the freshwater ornamental fishery with particular reference to the supply of Brazilian freshwater ornamentals to the UK market. Uakari 2007; 3(1): 7-51.
- Rosim DF, Cecarelli PS, Silva-Souza AT. Parasitismo de Hoplias malabaricus (Bloch, 1794) (Characiformes, Erythrinidae) por Quadrigyrus machadoi Fábio, 1983 (Eoacanthocephala, Quadrigyridae) de uma Lagoa em Aguaí, Estado De São Paulo, Brasil. Rev Bras Parasitol Vet 2005; 14(4): 147-153. PMid:16445871.
- Sterud E, Harris PH, Bake TA. The influence of Gyrodactylus salaris Malmberg 1957 (Monogenea) on the epidermis of Atlantic salmon, Salmo salar L., and brook trout, Salvelinus fontinalis (Mitchill): experimental studies. J Fish Dis 1998; 21(4): 257-263. http://dx.doi.org/10.1046/j.1365-2761.1998.00099.x
» http://dx.doi.org/10.1046/j.1365-2761.1998.00099.x - Tavares-Dias M, Lemos JRG, Martins ML, Jerônimo GT. Metazoan and protozoan parasites of freshwater ornamental fish from Brazil. In: Tavares-Dias M, editors. Manejo e sanidade de peixes em cultivo. Macapá: Embrapa; 2009. p. 469-494.
- Tavares-Dias M, Gonzaga Lemos JR, Martins ML. Parasitic fauna of eight species of ornamental freshwater fish species from the middle Negro River in the Brazilian Amazon Region. Rev Bras Parasitol Vet 2010; 19(2): 103-107. PMid:20624347. http://dx.doi.org/10.4322/rbpv.01902007
» http://dx.doi.org/10.4322/rbpv.01902007 - Thatcher VE. Amazon Fish Parasites. Manaus: Instituto Nacional de pesquisas da Amazônia; 2006. p. 555.
- Thilakaratne IDSIP, Rajapaksha G, Hewakopara A, Rajapakse RPVJ, Faizal ACM. Parasitic infections in freshwater ornamental fish in Sri Lanka. Dis Aquatic Org 2003; 54(2): 157-162. PMid:12747641. http://dx.doi.org/10.3354/dao054157
» http://dx.doi.org/10.3354/dao054157 - Travassos L, Artigas P, Pereira C. Fauna helmintológica dos peixes de água doce do Brasil. Arch Inst Biol 1928; 1: 5-68.
- Whittington ID, Chisholm LA. Biodiversity of marine parasites in Australia; more than just a list of largely invisible creatures. Rec South Aust Mus 2003; 7: 51-60.
- Yamamoto KC, Soares MGM, Freitas CEC. Alimentação de Triportheus angulatus (Spix & Agassiz, 1829) no lago Camaleão, Manaus, AM, Brasil. Acta Amaz 2004; 34(4): 653-659. http://dx.doi.org/10.1590/S0044-59672004000400017
» http://dx.doi.org/10.1590/S0044-59672004000400017
Publication Dates
-
Publication in this collection
26 Mar 2013 -
Date of issue
Jan-Mar 2013
History
-
Received
12 Mar 2012 -
Accepted
29 Oct 2012