Abstracts
Many argasid tick species are known only through their larval descriptions, in which the chaetotaxy, together with other external morphological characteristics, has been used to separate genera and species. However, the illustrations of these features are based on optical microscopy alone and many of these features are not clearly defined. Because of the difficulties in determining the larval and nymph stages of some genera, we have prepared illustrated keys for the immature stages of argasids, including an up-to-date list of the known species of the Neotropical region. We have also included an illustrated key for larvae of the Ornithodoros species from Brazil, based on scanning electron microscopy.
Argasidae; Ornithodoros ; immature; identification; key; Brazil
Muitos carrapatos argasídeos são conhecidos somente por descrições larvais, nas quais a quetotaxia associada a outros caracteres morfológicos tem sido usada para separar gêneros e espécies. No entanto, as ilustrações sobre esses caracteres são baseadas somente em microscopia óptica e muitos deles não estão claramente definidos. Devido às dificuldades em determinar estágios larvais e ninfais de alguns gêneros, elaboramos chaves ilustradas para os estágios imaturos de argasídeos, incluindo uma lista atualizada de espécies conhecidas da região Neotropical. Incluímos também uma chave ilustrada para larvas das espécies de Ornithodoros do Brasil baseada em microscopia eletrônica de varredura.
Argasidae; Ornithodoros ; imaturos; identificação; chave; Brasil
Introduction
The argasid fauna comprises around 200 known species in the world (NAVA et al., 2009Nava S, Guglielmone AA, Mangold AJ. An overview of systematics and
evolution of ticks. Front Biosci 2009; 14(8): 2857-2877.
http://dx.doi.org/10.2741/3418
http://dx.doi.org/10.2741/3418...
; GUGLIELMONE et al., 2010Guglielmone AA, Robins RG, Apanaskevich DA, Petney TN,
Estrada-Peña A, Horak IG, et al. The Argasidae, Ixodidae and
Nuttalliellidae (Acari: Ixodida) of the world: a list of valid species names.
Zootaxa 2010; 2528(6): 1-28.; DANTAS-TORRES et al., 2012Dantas-Torres F, Venzal JM, Bernardi LFO, Ferreira RL, Onofrio VC,
Marcili A, et al. Description of a new species of bat-associated argasid tick
(Acari: Argasidae) from Brazil. J Parasitol 2012; 98(1): 36-45. PMid:21955330.
http://dx.doi.org/10.1645/GE-2840.1
http://dx.doi.org/10.1645/GE-2840.1...
; VENZAL et
al., 2013aVenzal JM, Nava S, González-Acuña D, Mangold AJ,
Muñoz-Leal S, Lado P, et al. A new species of Ornithodoros (Acari:
Argasidae), parasite of Microlophus spp. (Reptilia: Tropiduridae) from northern
Chile. Ticks Tick Borne Dis 2013a; 4(1-2): 128-132. PMid:23219344.
http://dx.doi.org/10.1016/j.ttbdis.2012.10.038
http://dx.doi.org/10.1016/j.ttbdis.2012....
). Of these, 87 are recognized in the Neotropical region,
distributed into five genera: Antricola (17 species),
Argas (12 species), Ornithodoros (55 species),
Nothoaspis (2 species), and Otobius (1
species). In Brazil, 21 species of Argasidae are currently known (DANTAS-TORRES et al., 2012Dantas-Torres F, Venzal JM, Bernardi LFO, Ferreira RL, Onofrio VC,
Marcili A, et al. Description of a new species of bat-associated argasid tick
(Acari: Argasidae) from Brazil. J Parasitol 2012; 98(1): 36-45. PMid:21955330.
http://dx.doi.org/10.1645/GE-2840.1
http://dx.doi.org/10.1645/GE-2840.1...
), as follows: 16 of
Ornithodoros, 3 of Antricola, 1 of
Argas, 1 of Nothoaspis. There is no current
record of Otobius in Brazil, but this genus is represented by two
species around the world; in the Neotropics, only Otobius megnini
(Dugès, 1883) has been recorded.
The adult and nymphal stages of some argasid species are morphologically
very similar, especially within the genus Ornithodoros, which makes
it problematic to critically assess distribution and species relationships based on
previous contributions. Most descriptions of nymphal stages are poor in details,
lacking figures or illustrations of the instars, which hinders morphological
differentiation (ESTRADA-PEÑA et al.,
2010Estrada-Peña A, Mangold AJ, Nava S, Venzal JM, Labruna M,
Guglielmone AA. A review of the systematics of the tick family Argasidae
(Ixodida). Acarologia 2010; 50(3): 317-333.
http://dx.doi.org/10.1051/acarologia/20101975
http://dx.doi.org/10.1051/acarologia/201...
). In the absence of DNA studies on these species, only larval
morphological features have been adequately defined for specific determination
(VENZAL et al., 2008).
Because of the difficulties in determining the larvae and nymphs of some genera and species, we have prepared illustrated keys for these immature stages based on optical and scanning electron microcopy, in order to help in identifying the generic taxa of Argasidae in the Neotropics. Here, we also present a current list of all argasid species in the Neotropical region, including a key to the genera of immature stages in this region and also a key to larvae of Ornithodoros species in Brazil.
Materials and Methods
The specimens illustrated in this study were cleaned by means of ultrasound (40 kHz), using distilled water and commercial detergent in the proportions 8:2. The cleaning process was completed in three separate stages: 3 minutes in water + detergent, 2 minutes in distilled water, and an additional 2 minutes in distilled water. Micrographs were made by using a Zeiss LEO 440 digital scanning microscope. The plates were made using CorelDraw X 5, version 2010. The figures were prepared Photoshop CS 6, version 2012.
The diagnosis and keys for immature ticks of Argasidae in the Neotropics
were based on Cooley and Kohls (1944)Cooley RA, Kohls GM. The Argasidae of North America, Central America
and Cuba. Am. Midland. Nat. Monogr; 1944. PMid:18746793
PMCid:PMC1780695., Kohls and Clifford (1964)Kohls GM, Clifford CM. Ornithodoros (Alectorobius) boliviensis sp.
n. (Acarina: Argasidae) from bats and houses in Bolivia. J Parasitol 1964;
50(6): 792-796. PMid:14244814.
http://dx.doi.org/10.2307/3276204
http://dx.doi.org/10.2307/3276204...
, Kohls et al. (1965Kohls GM, Sonenshine DE, Clifford CM. The systematics of the
subfamily Ornithodorinae (Acarina: Argasidae). II. Identification of the larvae
of the Western Hemisphere and descriptions three news species. Ann Entomol Soc
Am 1965; 58(3): 331-364. PMid:5835857., 1969Kohls GM, Clifford CM, Jones EK. The systematics of the subfamily
Ornithodorinae (Acarina: Argasidae). IV. Eight new species of Ornithodoros from
the Western Hemisphere. Ann Entomol Soc Am 1969; 62(5):
1035-1043., 1970)Kohls GM, Hoogstraal H, Clifford CM, Kaiser MN. The subgenus
Persicargas (Ixodoidea, Argasidae, Argas). 9. Redescription and New World
records of Argas (P.) persicus (Oken), and resurrection, redescription, and
records of A. (P.) radiatus Railliet, A. (P.) sanchezi Dugès, and A. (P.)
miniatus Koch, New World ticks misidentified as A. (P.) persicus. Ann Entomol
Soc Am 1970; 63(2): 590-606., Clifford et al. (1964)Clifford CM, Kohls GM, Sonenshine DE. The systematics of the
subfamily Ornithodorinae (Acarina: Argasidae). I. The genera and subgenera. Ann
Entomol Soc Am 1964; 57(4): 429-437., Keirans et al. (1977)Keirans JE, Clifford CM, Redell JR. Description of the immature
stages of Nothoaspis reddelli (Ixodoidea: Argasidae) from bat caves in Mexico.
Ann Entomol Soc Am 1977; 70(4): 591-595., Klompen
(1992)Klompen JSH. Comparative morphology of argasid larvae (Acari:
Ixodida: Argasidae), with notes on phylogenetic relationships. Ann Ent Soc Am
1992; 85(5): 541-560., Klompen and Oliver (1993)Klompen JSH, Oliver JH Jr. Systematic relationships in the soft
ticks (Acari: Ixodida: Argasidae). Syst Entomol 1993; 18(4): 313-331.
http://dx.doi.org/10.1111/j.1365-3113.1993.tb00669.x
http://dx.doi.org/10.1111/j.1365-3113.19...
,
Venzal et al. (2008), Labruna et al. (2008Labruna MB, Terassini FA, Camargo LMA, Brandão PE, Ribeiro AF,
Estrada-Peña A. New reports of Antricola guglielmonei and Antricola
delacruzi in Brazil, and a description of a new argasid species (Acari). J
Parasitol 2008; 94(4): 788-792. PMid:18576796.
http://dx.doi.org/10.1645/GE-1447.1
http://dx.doi.org/10.1645/GE-1447.1...
,
2011)Labruna MB, Nava S, Terassini FA, Onofrio VC, Barros-Battesti DM,
Camargo LMA, et al. Description of adults and nymph, and redescription of the
larva, of Ornithodoros marinkellei (Acari: Argasidae), with data on its
phylogenetic position. J Parasitol 2011; 97(2): 207-217. PMid:21506769.
http://dx.doi.org/10.1645/GE-2620.1
http://dx.doi.org/10.1645/GE-2620.1...
, Labruna and Venzal (2009)Labruna MB, Venzal JM. Carios fonsecai sp. nov. (Acari, Argasidae),
a bat tick from central-western region of Brazil. Acta Parasitol 2009; 54(4):
355-363. http://dx.doi.org/10.2478/s11686-009-0051-1
http://dx.doi.org/10.2478/s11686-009-005...
, Nava et al.
(2010Nava S, Venzal JM, Terassini FA, Mangold AJ, Camargo LMA, Labruna
MB. Description of a new argasid tick (Acari: Ixodida) from bat caves in
Brazilian Amazon. J Parasitol 2010; 96(6): 1089-1101. PMid:21158616.
http://dx.doi.org/10.1645/GE-2539.1
http://dx.doi.org/10.1645/GE-2539.1...
, 2013)Nava S, Venzal JM, Terassini FA, Mangold AJ, Camargo LMA, Casás G,
et al. Ornithodoros guaporensis (Acari, Ixodida: Argasidae), a new tick species
from the Guaporé River Basin in the Bolivian Amazon. Zootaxa 2013; 3666(4):
579-590. http://dx.doi.org/10.11646/zootaxa.3666.4.10
http://dx.doi.org/10.11646/zootaxa.3666....
, Barros-Battesti et al. (2011Barros-Battesti DM, Landulfo GA, Onofrio VC, Faccini JLH, Marcili A,
Nieri-Bastos FA, et al. Carios mimon (Acari: Argasidae): description of adults
and redescription of larva. Exp Appl Acarol 2011; 54(1): 93-104. PMid:21161720.
http://dx.doi.org/10.1007/s10493-010-9416-2
http://dx.doi.org/10.1007/s10493-010-941...
, 2012)Barros-Battesti DM, Onofrio VC, Nieri-Bastos FA, Soares JF, Marcili
A, Famadas KM, et al. Ornithodoros brasiliensis Aragão (Acari: Argasidae):
description of the larva, redescription of male and female, and neotype
designation. Zootaxa 2012; 3178(31): 22-32., Dantas-Torres et al. (2012)Dantas-Torres F, Venzal JM, Bernardi LFO, Ferreira RL, Onofrio VC,
Marcili A, et al. Description of a new species of bat-associated argasid tick
(Acari: Argasidae) from Brazil. J Parasitol 2012; 98(1): 36-45. PMid:21955330.
http://dx.doi.org/10.1645/GE-2840.1
http://dx.doi.org/10.1645/GE-2840.1...
and Venzal
et al. (2013a)Venzal JM, Nava S, González-Acuña D, Mangold AJ,
Muñoz-Leal S, Lado P, et al. A new species of Ornithodoros (Acari:
Argasidae), parasite of Microlophus spp. (Reptilia: Tropiduridae) from northern
Chile. Ticks Tick Borne Dis 2013a; 4(1-2): 128-132. PMid:23219344.
http://dx.doi.org/10.1016/j.ttbdis.2012.10.038
http://dx.doi.org/10.1016/j.ttbdis.2012....
.
The larval terminology for Antricola,
Argas, and Nothoaspis followed Kohls et al. (1965Kohls GM, Sonenshine DE, Clifford CM. The systematics of the
subfamily Ornithodorinae (Acarina: Argasidae). II. Identification of the larvae
of the Western Hemisphere and descriptions three news species. Ann Entomol Soc
Am 1965; 58(3): 331-364. PMid:5835857., 1969Kohls GM, Clifford CM, Jones EK. The systematics of the subfamily
Ornithodorinae (Acarina: Argasidae). IV. Eight new species of Ornithodoros from
the Western Hemisphere. Ann Entomol Soc Am 1969; 62(5):
1035-1043., 1970)Kohls GM, Hoogstraal H, Clifford CM, Kaiser MN. The subgenus
Persicargas (Ixodoidea, Argasidae, Argas). 9. Redescription and New World
records of Argas (P.) persicus (Oken), and resurrection, redescription, and
records of A. (P.) radiatus Railliet, A. (P.) sanchezi Dugès, and A. (P.)
miniatus Koch, New World ticks misidentified as A. (P.) persicus. Ann Entomol
Soc Am 1970; 63(2): 590-606. and Nava et al. (2010)Nava S, Venzal JM, Terassini FA, Mangold AJ, Camargo LMA, Labruna
MB. Description of a new argasid tick (Acari: Ixodida) from bat caves in
Brazilian Amazon. J Parasitol 2010; 96(6): 1089-1101. PMid:21158616.
http://dx.doi.org/10.1645/GE-2539.1
http://dx.doi.org/10.1645/GE-2539.1...
. Nymphs were determined by
means of the original descriptions as well as from specimens obtained from colonies
maintained at the parasitology laboratory of the Butantan Institute. The key for
larval species of Ornithodoros in Brazil was based on Kohls et al. (1965)Kohls GM, Sonenshine DE, Clifford CM. The systematics of the
subfamily Ornithodorinae (Acarina: Argasidae). II. Identification of the larvae
of the Western Hemisphere and descriptions three news species. Ann Entomol Soc
Am 1965; 58(3): 331-364. PMid:5835857. with modifications proposed
by Venzal et al. (2008) and Labruna and Venzal
(2009)Labruna MB, Venzal JM. Carios fonsecai sp. nov. (Acari, Argasidae),
a bat tick from central-western region of Brazil. Acta Parasitol 2009; 54(4):
355-363. http://dx.doi.org/10.2478/s11686-009-0051-1
http://dx.doi.org/10.2478/s11686-009-005...
.
Results and Discussion
The main morphological characteristics of adults and nymphs of the family Argasidae are as follows: tegument granulated, mammillated, coriaceous or tuberculated; dorsal scutum absent. The spiracular plates are small and they are localized lateroposteriorly, between coxae III and IV. There is a pair of coxal glands in a ventral position that open between coxae I and II. All palpal articles are free, and article IV is not inserted in a depression in article III. Eyes, if present, are located laterally, close to the supracoxal folders. Pulvilli absent or rudimentary in nymphs and adults; however, they can be well developed in Antricola larvae. Sexual dimorphism is generally slight, based mainly on the shape of the genital aperture (VENZAL et al., 2006Venzal JM, Onofrio VC, Barros-Battesti DM, Arzua M. Família Argasidae: características gerais, comentários e chave para gêneros e espécies. In: Barros-Battesti DM, Arzua M, Bechara GH. Carrapatos de Importância Médico-Veterinária da Região Neotropical: Um guia ilustrado para identificação de espécies. São Paulo: Vox/ICTTD-3; Butantan; 2006. p. 223.).
Larvae present a vestigial scutum and the capitulum is placed at the terminal position, while in nymphs and adults the capitulum is inserted in the ventral idiosoma; the camerostome is more accentuated in post-larval stages. Few species present eyes. The generic diagnosis for larvae and nymphs of argasid and lists of species in the Neotropical region are shown below. Species present in Brazil are in bold.
Generic diagnosis
Antricola - Larvae (Figures 1-4): dorsal surface with 14 pairs of setae, typically 14 (11 dorsolateral, 3 central dorsal); dorsal plate, large and elongated with lateral margins parallel, narrowing anteriorly; eyes absent; ventral surface with 11 pairs of setae (3 sternal setae, 3 postcoxal setae, 4 circumanal + 1 on valves), and 1 posteromedial seta; 2 pairs of long post hypostomal setae, hypostome pointed, dentition 3/3 in anterior three-fourths, then 2/2 posteriorly to basis; palpi with 18 setae, number of setae on palpal article 1-4, respectively 0, 4, 5 and 9; pulvilli large, claws absent (except in A. marginatus); dorsal hump absent; Haller's organ with a rounded capsule, open only in a small central portion. Nymphs (Figures 5-9): outline suboval, pointed anteriorly; idiosoma covered by tubercles, most of them bearing short setae, some single, others in groups; hypostome short, broad and rounded apically, with small denticles on anterior and lateral margins; cheeks absent; spiracular plate oval, relatively large, expanded and dorsally visible in some specimens, with numerous minute pores; dorsal humps on tarsus I absent; claws present; Haller's organ similar to the larvae.
Larva of Antricola. 1. Idiosoma dorsal view, showing dorsolateral and central setae (arrow). 2. Idiosoma ventral view, showing pulvilli enlarged (arrow). 3. Dorsal plate. 4. Gnathosoma ventral view, showing hypostome 3/3 (arrow). Scale bars: 1-2, 90 µm; 3, 30 µm; 4, 60 µm.
Nymph of Antricola. 5. Idiosoma dorsal view, showing lateral tubercles elongated (arrow). 5a. Lateral tubercles in detail (arrow). 6. Idiosoma ventral view, showing post-anal groove weakly produced and a wide prominent tubercle posterior to the transverse post-anal groove, without setae (arrow). 7. Capitulum, showing hypostome slightly longer than wide with a few small denticles (arrow). 8. Spiracular plate. 9. Tarsus I, showing Haller's organ with a rounded capsule open only in a small central portion (arrow). Scale bars: 5, 600 µm; 5a, 200 µm; 6, 300 µm; 7, 80 µm; 8, 40 µm; 9, 60 µm.
List of species (Neotropics, N = 17; Brazil, N = 3): A. armasi De La Cruz and Estrada-Peña, 1995; A. centralis De La Cruz and Estrada-Peña, 1995; A. cernyi De La Cruz, 1978; A. coprophilus (McIntosh, 1935); A. delacruzi Estrada-Peña, Barros-Battesti and Venzal, 2004; A. granasi De La Cruz, 1973; A. guglielmonei Estrada-Peña, Barros-Battesti and Venzal, 2004; A. habanensis De La Cruz, 1976; A. hummelincki De La Cruz and Estrada-Peña, 1995; A. inexpectata Estrada-Peña, Barros-Battesti and Venzal, 2004; A. marginatus (Banks, 1910); A. martelorum De La Cruz, 1978; A. mexicanus Hoffmann, 1958; A. naomiae De La Cruz, 1978; A. occidentalis De La Cruz, 1978; A. siboneyi De La Cruz and Estrada-Peña, 1995; and A. silvai Cerný, 1967.
Comments: The diagnosis for larvae of
Antricola was based on Clifford et al. (1964)Clifford CM, Kohls GM, Sonenshine DE. The systematics of the
subfamily Ornithodorinae (Acarina: Argasidae). I. The genera and subgenera. Ann
Entomol Soc Am 1964; 57(4): 429-437., Kohls et al.
(1965)Kohls GM, Sonenshine DE, Clifford CM. The systematics of the
subfamily Ornithodorinae (Acarina: Argasidae). II. Identification of the larvae
of the Western Hemisphere and descriptions three news species. Ann Entomol Soc
Am 1965; 58(3): 331-364. PMid:5835857. and De La Cruz (1976). The nymphal diagnosis followed De La
Cruz (1976). There are at least 2-3 nymphal instars (ESTRADA-PEÑA et al., 2008Estrada-Peña A, Venzal JM, Kocan KM, Tramuta C, Tomassone L,
Fuente J, et al. Observations on Antricola ticks: small nymphs feed on mammalian
hosts and have a salivary gland structure similar to ixodid ticks. J Parasitol
2008; 94(4): 953-955. PMid:18576742.
http://dx.doi.org/10.1645/GE-1371.1
http://dx.doi.org/10.1645/GE-1371.1...
), although the exact
number of instars for this genus is unknown.
Argas - Larvae (Figures 10-14): dorsal surface with around 25-30 pairs of setae (14-16 DL; 12-13 C), dorsal plate oval and elongated; ventral surface with less than 7 pairs of setae + 1 pair on valves; posteromedial seta present or absent; 2 pairs of short post-hypostomal setae; hypostome rounded at apex, dentition 2/2 at basis to 3/3 at apex. Nymphs (Figures 15-18): outline oval, discs present, distributed more or less symmetrically dorsally; idiosoma mammillated, flattened dorsoventrally, with suture and lateral margin demarcating the dorsal and ventral surfaces; Haller's organ with transversely slit-like aperture, placed slightly laterally.
Larva of Argas. 10. Idiosoma dorsal view, showing elongated plate (arrow). 11. Idiosoma ventral view. 12. Gnathosoma ventral view, showing dentition 4/4 close to the apex. 13. Gnathosoma dorsal view. 14. Tarsus I, showing Haller's organ with small capsule aperture transversely slit-like (arrow). Scale bars: 10, 200 µm; 11, 120 µm; 12, 60 µm; 13, 40 µm; 14, 140 µm.
Nymph of Argas. 15. Idiosoma dorsal view, showing suture distinguishing dorsal surface from ventral surface (arrow). 15a. Discs of the tegument in detail. 16. Idiosoma ventral view. 17. Gnathosoma ventral view. 18. Tarsus I, showing Haller's organ with capsule perforated (arrow). Scale bars: 15-16, 300 µm; 15a, 40 µm; 17-18, 60 µm.
List of species (Neotropics, N = 12; Brazil N = 1): A. cucumerinus Neumann, 1901; A. dalei Clifford, Keirans, Hoogstraal and Corwin, 1976; A. dulus Keirans, Clifford and Capriles, 1971; A. keiransi Estrada-Peña, Venzal and González-Acuña, 2003; A. magnus Neumann, 1896; A. miniatus Koch, 1844; A. monachus Keirans, Radovsky and Clifford, 1973; A. moreli Keirans, Hoogstraal and Clifford, 1979; A. neghmei Kohls and Hoogstraal, 1961; A. persicus (Oken, 1818); A. radiatus Railliet, 1893; and A. transversus Banks, 1902.
Comments: Most species are known from all stages. Larval
and nymphal diagnoses were based on Kohls et al.
(1970)Kohls GM, Hoogstraal H, Clifford CM, Kaiser MN. The subgenus
Persicargas (Ixodoidea, Argasidae, Argas). 9. Redescription and New World
records of Argas (P.) persicus (Oken), and resurrection, redescription, and
records of A. (P.) radiatus Railliet, A. (P.) sanchezi Dugès, and A. (P.)
miniatus Koch, New World ticks misidentified as A. (P.) persicus. Ann Entomol
Soc Am 1970; 63(2): 590-606.. This genus presents 2-4 nymphal instars, but usually 3; the
main factor affecting the number of instars is the volume of ingested blood
(SANTOS et al., 2010Santos HA, Angelo IC, Franque MP, Vashist U, Duarte AF, Baldani CD,
et al. The influence of the fasting period on the number of nymphal instars and
the sex ratio of Argas (Persicargas) miniatus (Acari: Argasidae). Rev Bras
Parasitol Vet 2010; 19(3): 164-168. PMid:20943020.
http://dx.doi.org/10.1590/S1984-29612010000300007
http://dx.doi.org/10.1590/S1984-29612010...
).
Nothoaspis - Larva: Dorsal plate with isosceles triangle shape occupying entire length of the dorsum of unfed specimens; dorsal surface with 12-13 pairs of setae; hypostome with apex pointed, dental formula 2/2 with 20 denticles in each row, corona absent. Nymphs: Idiosoma twice longer than wide, anteriorly more abruptly narrowing than posteriorly; false shield (nothoaspis) covered by cells (irregular in shape and size) occupying the anterocentral area of dorsum, most of them at least with 1 seta; setae short, except for posterior margin of idiosoma, where setae are larger. Ventral surface with integument also covered by cells (irregular in shape and size), except for a narrow area located between coxae I and III; anus subcircular, lateral to coxa IV, valves each with 1 pair of setae; spiracular plate small, similar to that of male. Basis capituli subrectangular in outline, with 1 pair of post-hypostomal setae and at least 7 pairs of sublateral setae, bordered posteriorly by integumental fold; postpalpal setae absent; hood large, broadly rounded, not entirely covering capitulum, cheliceral blades, palpal articles II-IV visible dorsally; ventrally, article I forms elongate flaps protecting the pointed hypostome, dental formula 4/4 apically, 5/5 at base.
List of species (Neotropics, N = 2; Brazil, N = 1): N. reddelli Clifford and Keirans, 1975; and N. amazoniensis Nava, Venzal and Labruna, 2010.
Comments: The diagnoses of larvae and nymphs were based
on the original descriptions. Two nymphal instars: the first one does not feed
and has reduced hypostome (NAVA et al.,
2010Nava S, Venzal JM, Terassini FA, Mangold AJ, Camargo LMA, Labruna
MB. Description of a new argasid tick (Acari: Ixodida) from bat caves in
Brazilian Amazon. J Parasitol 2010; 96(6): 1089-1101. PMid:21158616.
http://dx.doi.org/10.1645/GE-2539.1
http://dx.doi.org/10.1645/GE-2539.1...
).
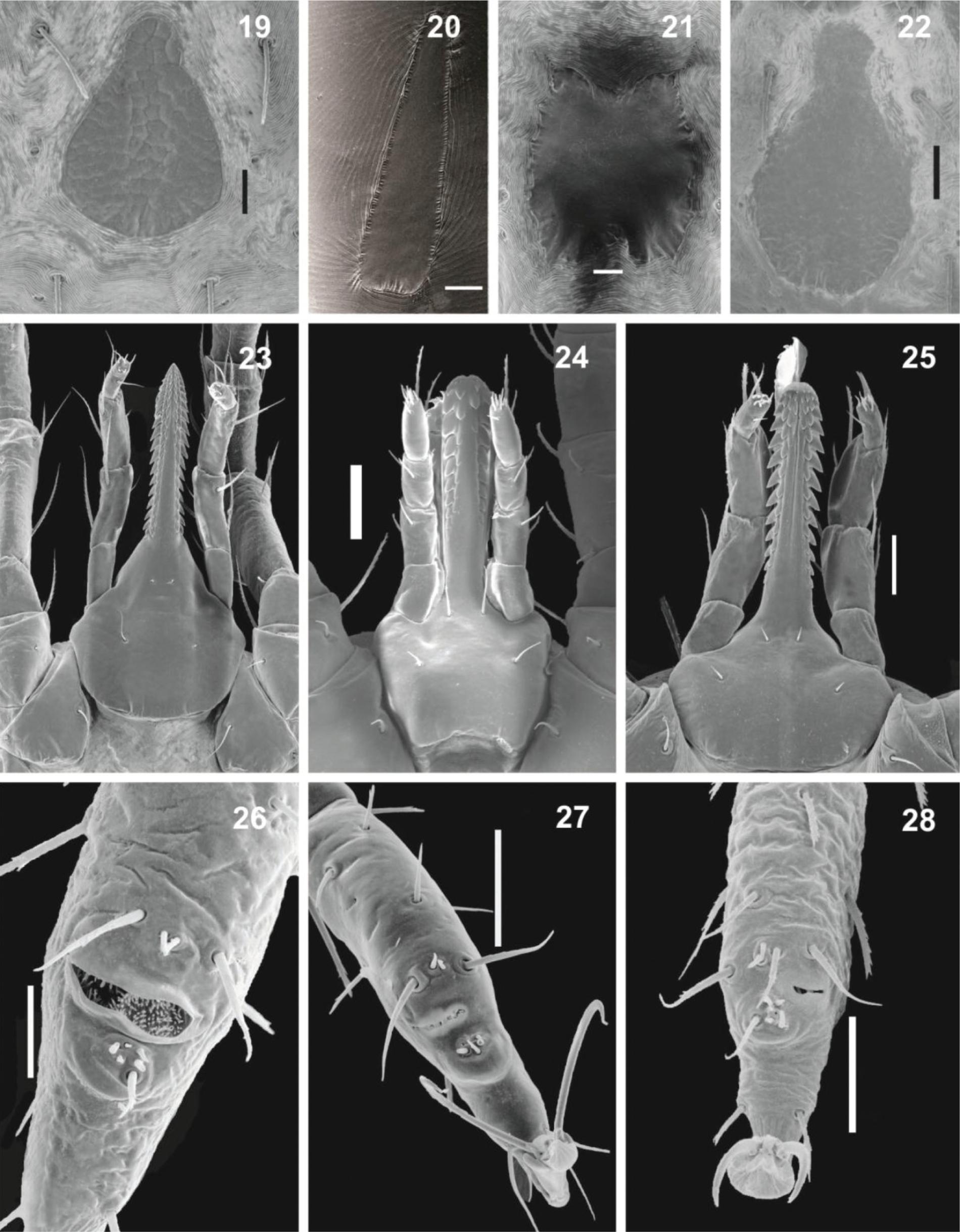
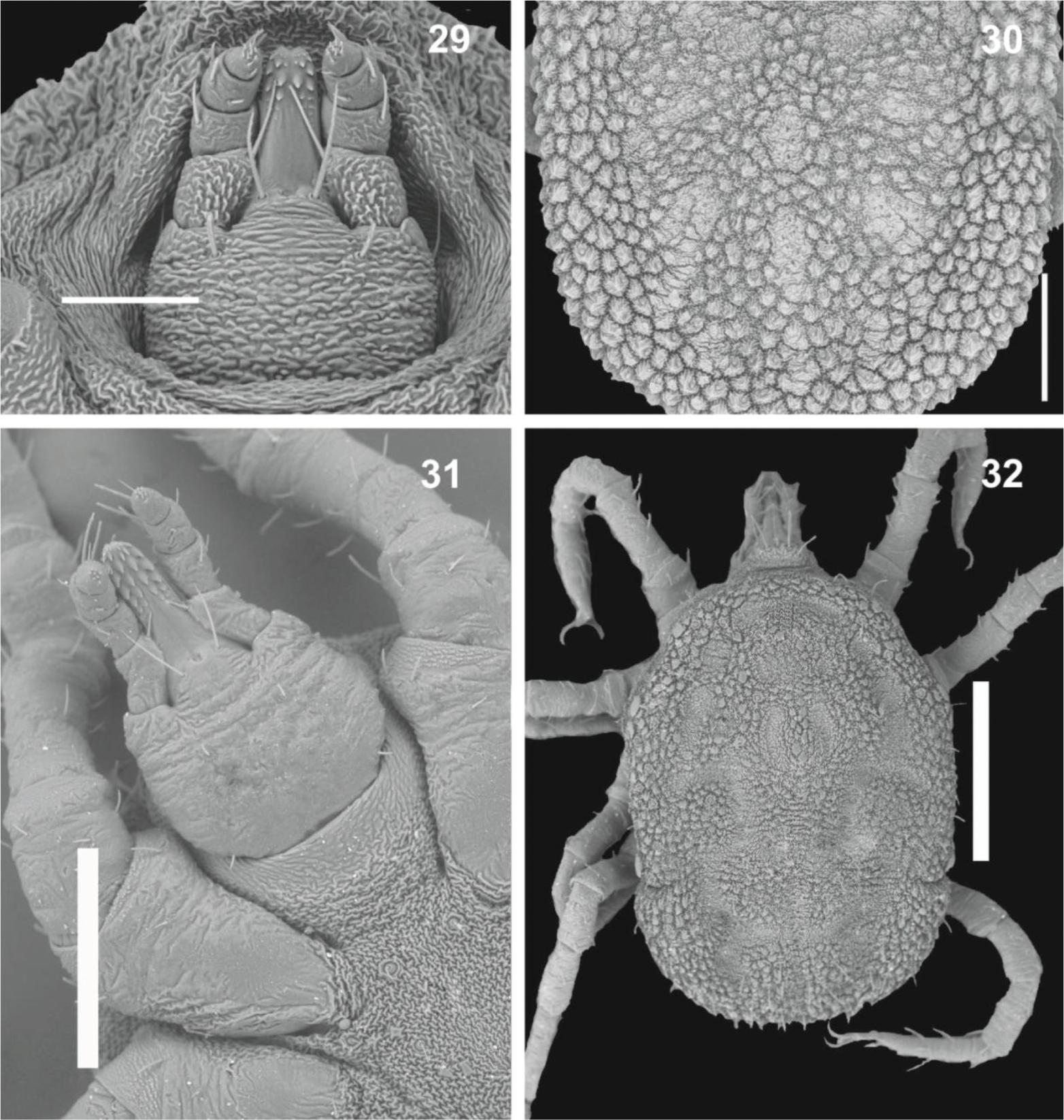
Ornithodoros - Larvae (Figures 19-28): dorsal surface of idiosoma with 13-14 pairs of setae (with some exceptions*), dorsal plate absent in few species, but present in the majority, varying in shape, from triangular to piriform (bat-associated group) to elongated subrectangular with anterior extremity narrowed; ventrally with 7-8 pairs + 1 on valves, and 1 unpaired seta posteromedially (which may be absent). Basis capituli with lateral angles slightly rounded, lateral auriculae present or absent, hypostome with apex rounded or pointed, dental formula: 5/5 to 2/2 at apex, 4/4 to 2/2 in medial portion and 2/2 at basis; Haller's organ with capsule aperture transversely slit-like, large, occupying all of dorsum with many small setae, or small occupying part of the dorsum. Nymphs (Figures 29-32): outline oval, slightly pointed anteriorly, idiosoma covered by tile-like mammillae; presence of 4 pairs of bulging lateral structures resembling large mammillae on supracoxal folds between legs I-IV (soil-living group) or absent (bat-associated group), hypostome rounded on apex; humps present (only in the soil-living group) or absent (bat-associated group), Haller's organ similar to the larvae.
Larva of Ornithodoros. 19. O. mimon, showing dorsal plate with piriform shape. 20. O. marinkellei, showing dorsal plate with elongated subrectangular shape. 21. O. rostratus, showing dorsal plate with subrectangular shape presenting concavity anteriorly and posteriorly. 22. O fonsecai, showing dorsal plate with piriform shape. 23. Capitulum of O. fonsecai, showing pointed hypostome and dentition 3/3 near to the apex. 24. Capitulum of O. rostratus, showing spatulated hypostome and dentition 2/2. 25. Capitulum of O. mimon, showing spatulated hypostome and dentition 4/4 near to the apex. 26. Haller's organ of O. brasiliensis, showing capsule aperture transversely slit-like and large. 27. Haller's organ of O. rostratus, showing capsule aperture transversely slit-like, occupying part of dorsum. 28. Haller's organ of O. mimon, showing small capsule with aperture transversely slit-like, occupying part of dorsum. Scale bars: 19, 30 µm; 20, 60 µm; 21, 20 µm; 22, 60 µm; 23-24, 60 µm; 25, 40 µm; 26, 30 µm; 27, 50 µm; 28, 40 µm.
Nymph of Ornithodoros (first nymphal instar). 29. Gnathosoma of O. mimon. 30. Idiosoma dorsal view of O. mimon. 31. Gnathosoma of O. brasiliensis. 32. Idiosoma dorsal view of O. brasiliensis, with dorsoventral grooves present (arrow). Scale bars: 29, 100 µm; 30, 250 µm; 31, 250 µm. 32, 500 µm.
List of species (Neotropics, N = 55; Brazil, N
= 16): O. amblus Chamberlin, 1920; O.
aragaoi Fonseca, 1960; O. azteci
Matheson, 1935Matheson R. Three new species of ticks, Ornithodoros (Acarina:
Ixodoidea). J Parasitol 1935; 21(5): 347-353.
http://dx.doi.org/10.2307/3271944
http://dx.doi.org/10.2307/3271944...
; O.
brasiliensis Aragão, 1923; O. brodyi
Matheson, 1935Matheson R. Three new species of ticks, Ornithodoros (Acarina:
Ixodoidea). J Parasitol 1935; 21(5): 347-353.
http://dx.doi.org/10.2307/3271944
http://dx.doi.org/10.2307/3271944...
; O.
capensis Neumann 1901; O. casebeeri
Jones and Clifford, 1972; O. cavernicolous
Dantas-Torres, Venzal and Labruna, 2012; O. chironectes Jones
and Clifford, 1972; O. clarki Jones and Clifford, 1972;
O. coriaceus Koch, 1844**; O. cyclurae De
La Cruz, 1984; O. darwini Kohls, Clifford and Hoogstraal, 1969;
O. denmarki
Kohls, Sonenshine and Clifford, 1965Kohls GM, Sonenshine DE, Clifford CM. The systematics of the
subfamily Ornithodorinae (Acarina: Argasidae). II. Identification of the larvae
of the Western Hemisphere and descriptions three news species. Ann Entomol Soc
Am 1965; 58(3): 331-364. PMid:5835857.;
O. dusbabeki Cerný, 1967; O. dyeri
Cooley and Kohls, 1940; O. echimys
Kohls, Clifford and Jones, 1969Kohls GM, Clifford CM, Jones EK. The systematics of the subfamily
Ornithodorinae (Acarina: Argasidae). IV. Eight new species of Ornithodoros from
the Western Hemisphere. Ann Entomol Soc Am 1969; 62(5):
1035-1043.;
O. elongatus
Kohls, Sonenshine and Clifford, 1965Kohls GM, Sonenshine DE, Clifford CM. The systematics of the
subfamily Ornithodorinae (Acarina: Argasidae). II. Identification of the larvae
of the Western Hemisphere and descriptions three news species. Ann Entomol Soc
Am 1965; 58(3): 331-364. PMid:5835857.;
O. epitesicus
Kohls, Clifford and Jones, 1969Kohls GM, Clifford CM, Jones EK. The systematics of the subfamily
Ornithodorinae (Acarina: Argasidae). IV. Eight new species of Ornithodoros from
the Western Hemisphere. Ann Entomol Soc Am 1969; 62(5):
1035-1043.;
O. fonsecai (Labruna and Venzal, 2009Labruna MB, Venzal JM. Carios fonsecai sp. nov. (Acari, Argasidae),
a bat tick from central-western region of Brazil. Acta Parasitol 2009; 54(4):
355-363. http://dx.doi.org/10.2478/s11686-009-0051-1
http://dx.doi.org/10.2478/s11686-009-005...
); O. furcosus
Neumann, 1908; O. galapagensis Kohls, Clifford and Hoogstraal,
1969; O. guaporensis Nava, Venzal and Labruna, 2013; O.
hasei (Schulze, 1935); O.
jul
Schulze, 1940Schulze P. Eine neue Ornithodoros-art (Ixod. Argas) aus Brasilien.
Zool Anz 1940; 130: 131-135.; O.
kelleyi
Cooley and Kohls, 1941Cooley RA, Kohls GM. Three new species of Ornithodoros (Acarina:
Ixodoidea). Pub Health Rept 1941, 56(12): 587-594.
http://dx.doi.org/10.2307/4583666
http://dx.doi.org/10.2307/4583666...
; O.
kohlsi
Guglielmone and Keirans, 2002Guglielmone AA, Keirans JE. Ornithodoros kohlsi Guglielmone and
Keirans (Acari: Argasidae), a new name for Ornithodoros boliviensis Kohls and
Clifford 1964. Proc Entomol Soc Wash 2002; 104(3): 822.; O.
knoxjonesi Jones and Clifford, 1972; O.
marinkellei
Kohls, Clifford and Jones, 1969Kohls GM, Clifford CM, Jones EK. The systematics of the subfamily
Ornithodorinae (Acarina: Argasidae). IV. Eight new species of Ornithodoros from
the Western Hemisphere. Ann Entomol Soc Am 1969; 62(5):
1035-1043.;
O. marmosae Jones and Clifford, 1972; O.
microlophi Venzal, Nava and González-Acuña, 2013;
O. mimon
Kohls, Clifford and Jones, 1969Kohls GM, Clifford CM, Jones EK. The systematics of the subfamily
Ornithodorinae (Acarina: Argasidae). IV. Eight new species of Ornithodoros from
the Western Hemisphere. Ann Entomol Soc Am 1969; 62(5):
1035-1043.;
O. mormoops
Kohls, Clifford and Jones, 1969Kohls GM, Clifford CM, Jones EK. The systematics of the subfamily
Ornithodorinae (Acarina: Argasidae). IV. Eight new species of Ornithodoros from
the Western Hemisphere. Ann Entomol Soc Am 1969; 62(5):
1035-1043.;
O. natalinus Cerný and Dusbábek, 1967;
O. nattereri
Warburton, 1927Warburton C. On five new species of ticks (Arachnida, Ixodoidea),
Ornithodorus (sic) nattereri, Ixodes theodori, Haemaphysalis toxopei, Amblyomma
robinsoni and A. dammermani, with a note on the ornate nymph of A. latum.
Parasitol 1927; 19:405-410.
http://dx.doi.org/10.1017/S0031182000005886
http://dx.doi.org/10.1017/S0031182000005...
; O.
nicollei Mooser, 1932; O. peropteryx
Kohls, Clifford and Jones, 1969Kohls GM, Clifford CM, Jones EK. The systematics of the subfamily
Ornithodorinae (Acarina: Argasidae). IV. Eight new species of Ornithodoros from
the Western Hemisphere. Ann Entomol Soc Am 1969; 62(5):
1035-1043.;
O. peruvianus
Kohls, Clifford and Jones, 1969Kohls GM, Clifford CM, Jones EK. The systematics of the subfamily
Ornithodorinae (Acarina: Argasidae). IV. Eight new species of Ornithodoros from
the Western Hemisphere. Ann Entomol Soc Am 1969; 62(5):
1035-1043.;
O. puertoricensis Fox, 1947; O.
quilinensis Venzal, Nava and Mangold, 2012; O.
rioplatensis Venzal, Estrada-Pena and Mangold, 2008;
O. rondoniensis (Labruna, Terassini, Camargo, Brandão, Ribeiro and
Estrada-Peña, 2008Labruna MB, Terassini FA, Camargo LMA, Brandão PE, Ribeiro AF,
Estrada-Peña A. New reports of Antricola guglielmonei and Antricola
delacruzi in Brazil, and a description of a new argasid species (Acari). J
Parasitol 2008; 94(4): 788-792. PMid:18576796.
http://dx.doi.org/10.1645/GE-1447.1
http://dx.doi.org/10.1645/GE-1447.1...
); O. rossi
Kohls, Sonenshine and Clifford, 1965Kohls GM, Sonenshine DE, Clifford CM. The systematics of the
subfamily Ornithodorinae (Acarina: Argasidae). II. Identification of the larvae
of the Western Hemisphere and descriptions three news species. Ann Entomol Soc
Am 1965; 58(3): 331-364. PMid:5835857.;
O. rostratus
Aragão, 1911Aragão HB. Notas sobre ixódidas brazileiros. Mem Inst Oswaldo Cruz
1911; 3(2): 145-195.
http://dx.doi.org/10.1590/S0074-02761911000200001
http://dx.doi.org/10.1590/S0074-02761911...
; O.
rudis Karsch, 1880; O.
setosus
Kohls, Clifford and Jones, 1969Kohls GM, Clifford CM, Jones EK. The systematics of the subfamily
Ornithodorinae (Acarina: Argasidae). IV. Eight new species of Ornithodoros from
the Western Hemisphere. Ann Entomol Soc Am 1969; 62(5):
1035-1043.;
O. spheniscus Hoogstraal, Wassef, Hays and Keirans, 1985;
O. stageri
Cooley and Kohls, 1941Cooley RA, Kohls GM. Three new species of Ornithodoros (Acarina:
Ixodoidea). Pub Health Rept 1941, 56(12): 587-594.
http://dx.doi.org/10.2307/4583666
http://dx.doi.org/10.2307/4583666...
; O.
tadaridae Cerný and Dusbábek, 1967; O.
talaje (Guérin-Méneville, 1849); O.
tiptoni Jones and Clifford, 1972; O. tuttlei Jones
and Clifford, 1972; O. viguerasi
Cooley and Kohls, 1941Cooley RA, Kohls GM. Three new species of Ornithodoros (Acarina:
Ixodoidea). Pub Health Rept 1941, 56(12): 587-594.
http://dx.doi.org/10.2307/4583666
http://dx.doi.org/10.2307/4583666...
; O.
yumatensis
Cooley and Kohls, 1941Cooley RA, Kohls GM. Three new species of Ornithodoros (Acarina:
Ixodoidea). Pub Health Rept 1941, 56(12): 587-594.
http://dx.doi.org/10.2307/4583666
http://dx.doi.org/10.2307/4583666...
; and O.
yunkeri
Keirans, Clifford and Hoogstraal,
1984Keirans JE, Clifford CM, Hoogstraal. Ornithodoros (Alectorobius)
yunkeri, new species (Acari: Ixodoidea: Argasidae), from seabirds and nesting
sites in the Galapagos Islands. J Med Entomol 1984; 21(3): 344-350.
PMid:6748010..
Comments: *The larvae of the talaje
group species have 17-21 pairs of setae, whereas O. setosus
larvae have 27-29 pairs of setae (KOHLS et al.
1969Kohls GM, Clifford CM, Jones EK. The systematics of the subfamily
Ornithodorinae (Acarina: Argasidae). IV. Eight new species of Ornithodoros from
the Western Hemisphere. Ann Entomol Soc Am 1969; 62(5):
1035-1043.). There are around 2-6 nymphal instars usually, but few species
present 5-6 instars (KLOMPEN; OLIVER,
1993Klompen JSH, Oliver JH Jr. Systematic relationships in the soft
ticks (Acari: Ixodida: Argasidae). Syst Entomol 1993; 18(4): 313-331.
http://dx.doi.org/10.1111/j.1365-3113.1993.tb00669.x
http://dx.doi.org/10.1111/j.1365-3113.19...
). O. peropteryx has a single nymphal instar
(VENZAL et al., 2013bVenzal JM, Nava S, Terrassini FA, Ogrzewalska M, Camargo LMA,
Labruna MB. Ornithodoros peropteryx (Acari: Argasidae) in Bolivia: an argasid
tick with a single nymphal stage. Exp Appl Acarol 2013b; 61(2): 231-241.
http://dx.doi.org/10.1007/s10493-013-9689-3
http://dx.doi.org/10.1007/s10493-013-968...
). The species
O. brasiliensis and O. rostratus are
included among those with 5-6 nymphal instars. **Larvae of O.
coriaceus present two pairs of eyes (KLOMPEN; OLIVER, 1993Klompen JSH, Oliver JH Jr. Systematic relationships in the soft
ticks (Acari: Ixodida: Argasidae). Syst Entomol 1993; 18(4): 313-331.
http://dx.doi.org/10.1111/j.1365-3113.1993.tb00669.x
http://dx.doi.org/10.1111/j.1365-3113.19...
). The diagnoses of larvae and nymphs
were based on Cooley and Kohls (1944)Cooley RA, Kohls GM. The Argasidae of North America, Central America
and Cuba. Am. Midland. Nat. Monogr; 1944. PMid:18746793
PMCid:PMC1780695.;
Clifford et al. (1964Clifford CM, Kohls GM, Sonenshine DE. The systematics of the
subfamily Ornithodorinae (Acarina: Argasidae). I. The genera and subgenera. Ann
Entomol Soc Am 1964; 57(4): 429-437., 1980)Clifford CM, Hoogstraal H, Radovsky FJ, Stiller D, Keirans JE.
Ornithodoros (Alectorobius) amblus (Acarina: Ixodoidea: Argasidae): Identify,
marine bird and human hosts, virus infection, and distribution in Peru. J
Parasitol 1980; 66(2): 312-323. PMid:7391872.
http://dx.doi.org/10.2307/3280825
http://dx.doi.org/10.2307/3280825...
; Kohls et al. (1965Kohls GM, Sonenshine DE, Clifford CM. The systematics of the
subfamily Ornithodorinae (Acarina: Argasidae). II. Identification of the larvae
of the Western Hemisphere and descriptions three news species. Ann Entomol Soc
Am 1965; 58(3): 331-364. PMid:5835857., 1969)Kohls GM, Clifford CM, Jones EK. The systematics of the subfamily
Ornithodorinae (Acarina: Argasidae). IV. Eight new species of Ornithodoros from
the Western Hemisphere. Ann Entomol Soc Am 1969; 62(5):
1035-1043.;
Roberts (1970)Roberts LE. Australian Ticks. Melbourne: CSIRO;
1970.; De La Cruz (1974);
Keirans et al. (1980Keirans JE, Clifford CM, Hoogstraal H. Identify of the nymphs and
adults of the Galapagos iguanid lizard parasites, Ornithodoros (Alectorobius)
darwini and O. (A.) galapagensis (Ixodoidea: Argasidae). J Med Entomol 1980;
17(5): 427-438., 1984)Keirans JE, Clifford CM, Hoogstraal. Ornithodoros (Alectorobius)
yunkeri, new species (Acari: Ixodoidea: Argasidae), from seabirds and nesting
sites in the Galapagos Islands. J Med Entomol 1984; 21(3): 344-350.
PMid:6748010.; Endris et al. (1989)Endris RG, Keirans JE, Robbins RG, Hess WR. Ornithodoros
(Alectorobius) puertoricensis (Acari: Argasidae): Redescription by Scanning
Electron Microscopy. J Med Entomol 1989; 26(3): 146-154.
PMid:2724311.; Venzal et al. (2008, 2012, 2013a, b); Labruna et al. (2008Labruna MB, Terassini FA, Camargo LMA, Brandão PE, Ribeiro AF,
Estrada-Peña A. New reports of Antricola guglielmonei and Antricola
delacruzi in Brazil, and a description of a new argasid species (Acari). J
Parasitol 2008; 94(4): 788-792. PMid:18576796.
http://dx.doi.org/10.1645/GE-1447.1
http://dx.doi.org/10.1645/GE-1447.1...
, 2011)Labruna MB, Nava S, Terassini FA, Onofrio VC, Barros-Battesti DM,
Camargo LMA, et al. Description of adults and nymph, and redescription of the
larva, of Ornithodoros marinkellei (Acari: Argasidae), with data on its
phylogenetic position. J Parasitol 2011; 97(2): 207-217. PMid:21506769.
http://dx.doi.org/10.1645/GE-2620.1
http://dx.doi.org/10.1645/GE-2620.1...
; Labruna and Venzal
(2009)Labruna MB, Venzal JM. Carios fonsecai sp. nov. (Acari, Argasidae),
a bat tick from central-western region of Brazil. Acta Parasitol 2009; 54(4):
355-363. http://dx.doi.org/10.2478/s11686-009-0051-1
http://dx.doi.org/10.2478/s11686-009-005...
; Nava et al. (2010Nava S, Venzal JM, Terassini FA, Mangold AJ, Camargo LMA, Labruna
MB. Description of a new argasid tick (Acari: Ixodida) from bat caves in
Brazilian Amazon. J Parasitol 2010; 96(6): 1089-1101. PMid:21158616.
http://dx.doi.org/10.1645/GE-2539.1
http://dx.doi.org/10.1645/GE-2539.1...
,
2013)Nava S, Venzal JM, Terassini FA, Mangold AJ, Camargo LMA, Casás G,
et al. Ornithodoros guaporensis (Acari, Ixodida: Argasidae), a new tick species
from the Guaporé River Basin in the Bolivian Amazon. Zootaxa 2013; 3666(4):
579-590. http://dx.doi.org/10.11646/zootaxa.3666.4.10
http://dx.doi.org/10.11646/zootaxa.3666....
; Barros-Battesti et al. (2011Barros-Battesti DM, Landulfo GA, Onofrio VC, Faccini JLH, Marcili A,
Nieri-Bastos FA, et al. Carios mimon (Acari: Argasidae): description of adults
and redescription of larva. Exp Appl Acarol 2011; 54(1): 93-104. PMid:21161720.
http://dx.doi.org/10.1007/s10493-010-9416-2
http://dx.doi.org/10.1007/s10493-010-941...
, 2012)Barros-Battesti DM, Onofrio VC, Nieri-Bastos FA, Soares JF, Marcili
A, Famadas KM, et al. Ornithodoros brasiliensis Aragão (Acari: Argasidae):
description of the larva, redescription of male and female, and neotype
designation. Zootaxa 2012; 3178(31): 22-32.; and Dantas-Torres et
al. (2012)Dantas-Torres F, Venzal JM, Bernardi LFO, Ferreira RL, Onofrio VC,
Marcili A, et al. Description of a new species of bat-associated argasid tick
(Acari: Argasidae) from Brazil. J Parasitol 2012; 98(1): 36-45. PMid:21955330.
http://dx.doi.org/10.1645/GE-2840.1
http://dx.doi.org/10.1645/GE-2840.1...
.
Otobius - Larvae (Figures 33-36): integument striated, dorsal surface with 7-10 pairs of setae, dorsal plate large, elongate tapering slightly posteriorly; two pairs of eyes; ventral surface with 5 pairs of setae + 1 pair on valves; pulvilli present on all tarsi, not enlarged, claws present, Haller's organ with capsule aperture large and rounded, with posterior projections; hypostome long without corona, dental formula 2/2. Nymphs (Figures 37-41): camerostome and hood absent; hypostomal dentition 4/4; idiosoma panduriform, integument striated and spinous; spiracular plate cone-shaped; Haller's organ with capsule aperture transversely slit-like, elevated and large, bordered with prolonged pointed projections and with small setae internally.
Larva of Otobius. 33. Idiosoma dorsal view, showing two pairs of eyes (arrow). 34. Haller's organ, showing branch-like posterior projections and very long posthalleral setae (arrow). 35. Gnathosoma dorsal view. 36. Gnathosoma ventral view. Scale bars: 33, 90 µm; 34, 30 µm; 35, 40 µm; 36, 60 µm.
Nymph of Otobius. 37. Idiosoma dorsal view, showing integument with spines. 38. Idiosoma ventral view, showing spines absent in the area surrounding the capitulum. 39. Capitulum, showing hypostomal dentition 4/4. 40. Idiosoma lateral view, showing spiracular plate conical (arrow). 41. Tarsus I, showing Haller's organ with capsule aperture transversely slit-like, elevated and large, bordered superiorly with prolonged pointed projections (arrow). Scale bars: 37, 600 µm; 38, 800 µm; 39, 90 µm; 40, 400 µm; 41, 30 µm.
List of species (N = 1): O. megnini (Dugès, 1883). There are two species but only this one occurs in the Neotropical region.
Comments: There have been isolated reports of O.
megnini in Brazil (FLECHTMANN, 1985; DINIZ et al., 1987Diniz LSM, Belluomini HE, Travassos LP F°, Rocha MB. Presence of
the ear mite Otobius megnini in the external ear canal of lions (Panthera leo).
J Zoo Anim Med 1987; 18(4): 154-155.
http://dx.doi.org/10.2307/20094831
http://dx.doi.org/10.2307/20094831...
); however, there has been no indication
that this species is established in Brazil, even though it is established in
several neighboring countries (GUGLIELMONE et al., 2003).
Argasids from Brazil
Genus Antricola. This occurs in hot and
humid caves inhabited by bats (Chiroptera), from southern United States to
northern Mexico (A. coprophilus), throughout Cuba and the
Caribbean areas, to South America (Colombia, Venezuela and northern and
northeastern Brazil), mainly on the guano. Many species are known only from the
adult stage described in Cuba. Adult ticks have mouthparts incompatible with
blood feeding, and there is no evidence of blood feeding in the late nymphal
instars (ESTRADA-PEÑA et al.,
2008Estrada-Peña A, Venzal JM, Kocan KM, Tramuta C, Tomassone L,
Fuente J, et al. Observations on Antricola ticks: small nymphs feed on mammalian
hosts and have a salivary gland structure similar to ixodid ticks. J Parasitol
2008; 94(4): 953-955. PMid:18576742.
http://dx.doi.org/10.1645/GE-1371.1
http://dx.doi.org/10.1645/GE-1371.1...
). The larvae, in turn, have a long and terminal hypostome and
present well-developed pulvilli that facilitate climbing cave walls. Adults of
three species are known in Brazil: A. inexpectata, described
from a cave at the locality of Brejinho, municipality of Araripe (13°
47′ S, 59° 49′ W), state of Ceará; and A.
delacruzi and A. guglielmonei, both from a cave in
Itabaiana (10° 50′ S, 37° 27′ W), state of Sergipe (ESTRADA-PEÑA et al., 2004Estrada-Peña A, Venzal JM, Barros-Battesti DM, Onofrio VC,
Trajano E, Firmino JVL. Three new species of Antricola (Acari: Argasidae) from
Brazil, with a key to the known species in the genus. J Parasitol 2004; 90(3):
490-498. PMid:15270091. http://dx.doi.org/10.1645/GE-172R
http://dx.doi.org/10.1645/GE-172R...
). The
latter two species were also collected from a cave in the municipality of Porto
Velho (08° 40′ S, 63° 51′ W), state of Rondônia (LABRUNA et al., 2008Labruna MB, Terassini FA, Camargo LMA, Brandão PE, Ribeiro AF,
Estrada-Peña A. New reports of Antricola guglielmonei and Antricola
delacruzi in Brazil, and a description of a new argasid species (Acari). J
Parasitol 2008; 94(4): 788-792. PMid:18576796.
http://dx.doi.org/10.1645/GE-1447.1
http://dx.doi.org/10.1645/GE-1447.1...
).
Genus Argas. Among the 12 species in
the Neotropical region, the genus Argas is represented in
Brazil only by A. miniatus, for which all stages have been
described. The first record of A. miniatus in Brazil was in the
state of Rio de Janeiro (MARCHOUX; SALIMBENI,
1903Marchoux E, Salimbeni A. La spirillose des poules. Annales de
I'Institut Pasteur Lille 1903; 17(1):569-580.). Rohr (1909)Rohr CJ. Estudos sobre ixodideos do Brasil. Rio de Janeiro: Gomes,
Irmão & C.; 1909. referred to
A. miniatus as A. persicus, with
occurrence in the municipality of Campinas (state of São Paulo) and in Rio de
Janeiro (referred to as the "Federal District"). Aragão (1936)Aragão HB. Ixodidas brasileiros e de alguns paizes limitrophes. Mem
Inst Oswaldo Cruz 1936; 31(4): 759-843.
http://dx.doi.org/10.1590/S0074-02761936000400004
http://dx.doi.org/10.1590/S0074-02761936...
considered this tick species to be A.
persicus var. dissimile and mentioned its
distribution in the states of Paraná, Santa Catarina, São Paulo, Rio de Janeiro,
Minas Gerais, Espírito Santo, Mato Grosso, Pernambuco, Paraíba, Maranhão, Ceará,
Pará and Bahia. Cançado et al. (2008)Cançado PH, Piranda EM, Mourão GM, Faccini JL. Spatial distribution
and impact of cattle-raising on ticks in the Pantanal region of Brazil by using
the CO2 tick trap. Parasitol Res 2008; 103(2): 371-377. PMid:18454288.
http://dx.doi.org/10.1007/s00436-008-0982-8
http://dx.doi.org/10.1007/s00436-008-098...
included the Pantanal region of Mato Grosso do Sul in its distribution area.
Besides Brazil, A. miniatus is distributed in Colombia, Guyana,
Panama, Trinidad & Tobago, Cuba, Jamaica, Puerto Rico, Venezuela and the
Nearctic region (GUGLIELMONE et al., 2003). This species occurs mainly on
chickens but may be found on other birds. It is a vector of Borrelia
anserine, the agent of fowl spirochetosis. Although A.
persicus has been recorded in many countries of South America, this
species is originally from the Palearctic region. However, the Neotropical
species A. persicus is probably a sibling species closely
related to a true Palearctic species (GUGLIELMONE et al., 2003).
Genus Nothoaspis. Until recently, this
genus was represented by a single species, N. redelli, in
Mexico. In Brazil, a second species, N. amazoniensis, was
recently found in caves in Rondônia (NAVA
et al., 2010Nava S, Venzal JM, Terassini FA, Mangold AJ, Camargo LMA, Labruna
MB. Description of a new argasid tick (Acari: Ixodida) from bat caves in
Brazilian Amazon. J Parasitol 2010; 96(6): 1089-1101. PMid:21158616.
http://dx.doi.org/10.1645/GE-2539.1
http://dx.doi.org/10.1645/GE-2539.1...
). The descriptions include larvae, nymphal instars and
adults.
Genus Ornithodoros. Most species are
known only from the larval stage, and therefore, the keys for specific
diagnosis, although older, refer to this stage (KOHLS et al., 1965Kohls GM, Sonenshine DE, Clifford CM. The systematics of the
subfamily Ornithodorinae (Acarina: Argasidae). II. Identification of the larvae
of the Western Hemisphere and descriptions three news species. Ann Entomol Soc
Am 1965; 58(3): 331-364. PMid:5835857.; 1969Kohls GM, Clifford CM, Jones EK. The systematics of the subfamily
Ornithodorinae (Acarina: Argasidae). IV. Eight new species of Ornithodoros from
the Western Hemisphere. Ann Entomol Soc Am 1969; 62(5):
1035-1043.).
Currently, the genus comprises around 118 known species around the world (VIAL; CAMICAS, 2009Vial L, Camicas J-L. Description of a new soft tick species of the
genus Ornithodoros Koch, 1844 (Acari: Argasidae). Fauna of Arabia 2009; 24:
135-143.; NAVA et al., 2009Nava S, Guglielmone AA, Mangold AJ. An overview of systematics and
evolution of ticks. Front Biosci 2009; 14(8): 2857-2877.
http://dx.doi.org/10.2741/3418
http://dx.doi.org/10.2741/3418...
; GUGLIELMONE et al., 2010Guglielmone AA, Robins RG, Apanaskevich DA, Petney TN,
Estrada-Peña A, Horak IG, et al. The Argasidae, Ixodidae and
Nuttalliellidae (Acari: Ixodida) of the world: a list of valid species names.
Zootaxa 2010; 2528(6): 1-28.; DANTAS-TORRES et al., 2012Dantas-Torres F, Venzal JM, Bernardi LFO, Ferreira RL, Onofrio VC,
Marcili A, et al. Description of a new species of bat-associated argasid tick
(Acari: Argasidae) from Brazil. J Parasitol 2012; 98(1): 36-45. PMid:21955330.
http://dx.doi.org/10.1645/GE-2840.1
http://dx.doi.org/10.1645/GE-2840.1...
; HEATH,
2012Heath ACG. A new species of soft tick (Ixodoidea: Argasidae) from
the New Zealand lesser short-tailed bat, Mystacina tuberculata Gray. Tuhinga
2012; 23: 29-37.; VENZAL et al., 2013aVenzal JM, Nava S, González-Acuña D, Mangold AJ,
Muñoz-Leal S, Lado P, et al. A new species of Ornithodoros (Acari:
Argasidae), parasite of Microlophus spp. (Reptilia: Tropiduridae) from northern
Chile. Ticks Tick Borne Dis 2013a; 4(1-2): 128-132. PMid:23219344.
http://dx.doi.org/10.1016/j.ttbdis.2012.10.038
http://dx.doi.org/10.1016/j.ttbdis.2012....
,
bVenzal JM, Nava S, Terrassini FA, Ogrzewalska M, Camargo LMA,
Labruna MB. Ornithodoros peropteryx (Acari: Argasidae) in Bolivia: an argasid
tick with a single nymphal stage. Exp Appl Acarol 2013b; 61(2): 231-241.
http://dx.doi.org/10.1007/s10493-013-9689-3
http://dx.doi.org/10.1007/s10493-013-968...
); 55 species occur in the
Neotropical region, and 16 in Brazil. The first species recorded in Brazil was
O. rostratus (ARAGãO, 1911Aragão HB. Notas sobre ixódidas brazileiros. Mem Inst Oswaldo Cruz
1911; 3(2): 145-195.
http://dx.doi.org/10.1590/S0074-02761911000200001
http://dx.doi.org/10.1590/S0074-02761911...
) and the second was O.
brasiliensis (ARAGãO, 1923), followed by
O. nattereri (WARBURTON, 1927Warburton C. On five new species of ticks (Arachnida, Ixodoidea),
Ornithodorus (sic) nattereri, Ixodes theodori, Haemaphysalis toxopei, Amblyomma
robinsoni and A. dammermani, with a note on the ornate nymph of A. latum.
Parasitol 1927; 19:405-410.
http://dx.doi.org/10.1017/S0031182000005886
http://dx.doi.org/10.1017/S0031182000005...
), O. jul
(SCHULZE, 1940Schulze P. Eine neue Ornithodoros-art (Ixod. Argas) aus Brasilien.
Zool Anz 1940; 130: 131-135.), O.
hasei cited as O. dunni
Cooley and Kohls (1944)Cooley RA, Kohls GM. The Argasidae of North America, Central America
and Cuba. Am. Midland. Nat. Monogr; 1944. PMid:18746793
PMCid:PMC1780695., O.
rudis, O. capensis,
O. stageri (from specimens deposited in
the USNTC) (JONES et al., 1972Jones EK, Clifford CM, Keirans JE, Kohls GM. The ticks of Venezuela
(Acarina: Ixodoidea) with a key to the species of Amblyomma in the western
hemisphere. Brigham Young Univ Sci Bull Biol Ser 1972; 17(4):
1-40.),
O. setosus (KOHLS et al., 1969Kohls GM, Clifford CM, Jones EK. The systematics of the subfamily
Ornithodorinae (Acarina: Argasidae). IV. Eight new species of Ornithodoros from
the Western Hemisphere. Ann Entomol Soc Am 1969; 62(5):
1035-1043.), O.
talaje (OBA; BAGGIO,
1977Oba MSP, Baggio D. Ocorrência de Ornithodoros talaje Guérin
Meneville, 1849, (Acari: Argasidae) na localidade de Santo Inácio, Bahia. Arq
Inst Biol 1977; 44(1-2): 107-109.), O. rondoniensis (LABRUNA et al., 2008Labruna MB, Terassini FA, Camargo LMA, Brandão PE, Ribeiro AF,
Estrada-Peña A. New reports of Antricola guglielmonei and Antricola
delacruzi in Brazil, and a description of a new argasid species (Acari). J
Parasitol 2008; 94(4): 788-792. PMid:18576796.
http://dx.doi.org/10.1645/GE-1447.1
http://dx.doi.org/10.1645/GE-1447.1...
), O.
fonsecai (LABRUNA;
VENZAL, 2009Labruna MB, Venzal JM. Carios fonsecai sp. nov. (Acari, Argasidae),
a bat tick from central-western region of Brazil. Acta Parasitol 2009; 54(4):
355-363. http://dx.doi.org/10.2478/s11686-009-0051-1
http://dx.doi.org/10.2478/s11686-009-005...
), O. mimon (BARROS-BATTESTI et al., 2011Barros-Battesti DM, Landulfo GA, Onofrio VC, Faccini JLH, Marcili A,
Nieri-Bastos FA, et al. Carios mimon (Acari: Argasidae): description of adults
and redescription of larva. Exp Appl Acarol 2011; 54(1): 93-104. PMid:21161720.
http://dx.doi.org/10.1007/s10493-010-9416-2
http://dx.doi.org/10.1007/s10493-010-941...
),
O. marinkellei (LABRUNA et al., 2011Labruna MB, Nava S, Terassini FA, Onofrio VC, Barros-Battesti DM,
Camargo LMA, et al. Description of adults and nymph, and redescription of the
larva, of Ornithodoros marinkellei (Acari: Argasidae), with data on its
phylogenetic position. J Parasitol 2011; 97(2): 207-217. PMid:21506769.
http://dx.doi.org/10.1645/GE-2620.1
http://dx.doi.org/10.1645/GE-2620.1...
), O.
cavernicolous (DANTAS-TORRES et al., 2012Dantas-Torres F, Venzal JM, Bernardi LFO, Ferreira RL, Onofrio VC,
Marcili A, et al. Description of a new species of bat-associated argasid tick
(Acari: Argasidae) from Brazil. J Parasitol 2012; 98(1): 36-45. PMid:21955330.
http://dx.doi.org/10.1645/GE-2840.1
http://dx.doi.org/10.1645/GE-2840.1...
) and O.
kohlsi (from larvae deposited in the IBSP collection), Martins et al. (2013)Martins TF, Venzal JM, Terassini FA, Costa FB, Marcili A, Camargo
LMA, et al. New tick records from the state of Rondônia, western Amazon,
Brazil. Exp Appl Acarol 2013; 62(1): 121-128.
http://dx.doi.org/10.1007/s10493-013-9724-4
http://dx.doi.org/10.1007/s10493-013-972...
.
The species O. rostratus described in Brazil also
occurs in Argentina, Paraguay and Bolivia (ARAGãO, 1936Aragão HB. Ixodidas brasileiros e de alguns paizes limitrophes. Mem
Inst Oswaldo Cruz 1936; 31(4): 759-843.
http://dx.doi.org/10.1590/S0074-02761936000400004
http://dx.doi.org/10.1590/S0074-02761936...
; NAVA et al.,
2007Nava S, Lareschi M, Rebollo C, Benítez Usher C, Beati L, Robbins RG,
et al. The ticks (Acari: Ixodida: Argasidae, Ixodidae) of Paraguay. Ann Trop Med
Parasitol 2007; 101(3): 255-270. PMid:17362600.
http://dx.doi.org/10.1179/136485907X176319
http://dx.doi.org/10.1179/136485907X1763...
). Adults and larvae were described (GUGLIELMONE et al., 2003).
It bites humans and several mammal species (ALMEIDA et al., 2012Almeida AP, Marcili A, Leite RC, Nieri-Bastos FA, Domingues LN,
Martins JR, et al. Coxiella symbiont in the tick Ornithodoros rostratus (Acari:
Argasidae). Ticks Tick Borne Dis 2012; 3(4): 203-206. PMid:22480930.
http://dx.doi.org/10.1016/j.ttbdis.2012.02.003
http://dx.doi.org/10.1016/j.ttbdis.2012....
). The larvae feed for few hours. In Brazil
specimens of O. rostratus have been recorded in the states of
São Paulo, Mato Grosso do Sul, Mato Grosso, Goiás and Minas Gerais, generally in
association with domestic animals (ARAGãO,
1936Aragão HB. Ixodidas brasileiros e de alguns paizes limitrophes. Mem
Inst Oswaldo Cruz 1936; 31(4): 759-843.
http://dx.doi.org/10.1590/S0074-02761936000400004
http://dx.doi.org/10.1590/S0074-02761936...
; PARDI; ROCHA, 1954Pardi MC, Rocha UF. Lesões causadas na pele de porcos pelas picadas
de Ornithodoros rostratus Aragão, 1911 (Acari, Argasidae). Importância
econômica. Rev Fac Med Vet S Paulo 1954; 5(1): 35-39.;
CANçADO et al., 2008Cançado PH, Piranda EM, Mourão GM, Faccini JL. Spatial distribution
and impact of cattle-raising on ticks in the Pantanal region of Brazil by using
the CO2 tick trap. Parasitol Res 2008; 103(2): 371-377. PMid:18454288.
http://dx.doi.org/10.1007/s00436-008-0982-8
http://dx.doi.org/10.1007/s00436-008-098...
). The species
O. brasiliensis is known only from the state of Rio Grande
do Sul, where it has been found parasitizing many animals, including humans
(MARTINS et al., 2011Martins JR, Doyle RL, Barros-Battesti DM, Onofrio VC, Guglielmone
AA. Occurrence of Ornithodoros brasiliensis Aragão (Acari: Argasidae) in São
Francisco de Paula, RS, Southern Brazil. Neotrop Entomol 2011; 40(1): 143-144.
PMid:21437496.
http://dx.doi.org/10.1590/S1519-566X2011000100022
http://dx.doi.org/10.1590/S1519-566X2011...
). Adults and
larvae (ARAGãO, 1923; BARROS-BATTESTI et al.,
2012Barros-Battesti DM, Onofrio VC, Nieri-Bastos FA, Soares JF, Marcili
A, Famadas KM, et al. Ornithodoros brasiliensis Aragão (Acari: Argasidae):
description of the larva, redescription of male and female, and neotype
designation. Zootaxa 2012; 3178(31): 22-32.) and all the nymphal instars (LANDULFO et al., 2013Landulfo GA, Pevidor LV, Luz HR, Faccini JLH, Nunes PH,
Barros-Battesti DM. Description of nymphal instars of Ornithodoros mimon Kohls,
Clifford & Jones, 1969 (Acari: Argasidae). Zootaxa 2013; 3710(2): 179-191.
http://dx.doi.org/10.11646/zootaxa.3710.2.4
http://dx.doi.org/10.11646/zootaxa.3710....
) have been described. Some species such as
O. jul and O. nattereri have not been
reported since their description, and only the adult stage is known (GUGLIELMONE
et al., 2003). O. jul was found in a wasp nest used by bats in
the municipality of Nova Teutônia, state of Santa Catarina. The type of
O. jul was reported to be deposited at the Berlin museum
(SCHULZE, 1940Schulze P. Eine neue Ornithodoros-art (Ixod. Argas) aus Brasilien.
Zool Anz 1940; 130: 131-135.); however, it has not
been found in this collection. On the other hand, Warburton (1927)Warburton C. On five new species of ticks (Arachnida, Ixodoidea),
Ornithodorus (sic) nattereri, Ixodes theodori, Haemaphysalis toxopei, Amblyomma
robinsoni and A. dammermani, with a note on the ornate nymph of A. latum.
Parasitol 1927; 19:405-410.
http://dx.doi.org/10.1017/S0031182000005886
http://dx.doi.org/10.1017/S0031182000005...
mentioned that the type of O.
nattereri was deposited at the Vienna Museum (label 86) and that
the 12 specimens were from Brazil, but the host is unknown. This species
resembles O. rostratus.
Although O. capensis had been considered to be among
the Brazilian species of Ornithodoros (DANTAS-TORRES et al., 2012Dantas-Torres F, Venzal JM, Bernardi LFO, Ferreira RL, Onofrio VC,
Marcili A, et al. Description of a new species of bat-associated argasid tick
(Acari: Argasidae) from Brazil. J Parasitol 2012; 98(1): 36-45. PMid:21955330.
http://dx.doi.org/10.1645/GE-2840.1
http://dx.doi.org/10.1645/GE-2840.1...
), it may be confused with
O. denmarki, O. amblus and O.
talaje, among others, which form the "capensis"
group. It has wide among marine birds in Neotropical coastal areas and islands,
and also in the Ethiopian, Nearctic, Oriental and Palearctic regions (KOHLS et al., 1965Kohls GM, Sonenshine DE, Clifford CM. The systematics of the
subfamily Ornithodorinae (Acarina: Argasidae). II. Identification of the larvae
of the Western Hemisphere and descriptions three news species. Ann Entomol Soc
Am 1965; 58(3): 331-364. PMid:5835857.; GUGLIELMONE et al.,
2003). All stages of O. capensis have been described
(GUGLIELMONE et al., 2003).
The species A. hasei (cited as O.
dunni) was originally described in Panama (MATHESON, 1935Matheson R. Three new species of ticks, Ornithodoros (Acarina:
Ixodoidea). J Parasitol 1935; 21(5): 347-353.
http://dx.doi.org/10.2307/3271944
http://dx.doi.org/10.2307/3271944...
), and Cooley
and Kohls (1944)Cooley RA, Kohls GM. The Argasidae of North America, Central America
and Cuba. Am. Midland. Nat. Monogr; 1944. PMid:18746793
PMCid:PMC1780695. cited a female of this species from Marajó Island,
state of Pará, Brazil, collected in 1941, which was found "living in a tree hole
with bats". All stages of O. hasei have been described
(GUGLIELMONE et al., 2003). Cooley and Kohls
(1941)Cooley RA, Kohls GM. Three new species of Ornithodoros (Acarina:
Ixodoidea). Pub Health Rept 1941, 56(12): 587-594.
http://dx.doi.org/10.2307/4583666
http://dx.doi.org/10.2307/4583666...
described O. stageri from adult and immature
specimens collected from bats in California, Arizona, Oklahoma and Texas. This
species was found in Mexico (KOHLS et al.,
1965Kohls GM, Sonenshine DE, Clifford CM. The systematics of the
subfamily Ornithodorinae (Acarina: Argasidae). II. Identification of the larvae
of the Western Hemisphere and descriptions three news species. Ann Entomol Soc
Am 1965; 58(3): 331-364. PMid:5835857.), and Jones et al. (1972)Jones EK, Clifford CM, Keirans JE, Kohls GM. The ticks of Venezuela
(Acarina: Ixodoidea) with a key to the species of Amblyomma in the western
hemisphere. Brigham Young Univ Sci Bull Biol Ser 1972; 17(4):
1-40.
enlarged the distribution of O. stageri to include Venezuela,
Nicaragua and Brazil. Specimens of A. hasei and O.
stageri collected in Brazil are deposited at USNTC (JONES et al., 1972Jones EK, Clifford CM, Keirans JE, Kohls GM. The ticks of Venezuela
(Acarina: Ixodoidea) with a key to the species of Amblyomma in the western
hemisphere. Brigham Young Univ Sci Bull Biol Ser 1972; 17(4):
1-40.).
The larval morphology of O. rudis resembles O. rostratus and O. brasiliensis, mainly because of the dorsal plate, but it lacks spurs in the dorsal region of palpus I. On the other hand, adults of O. rudis may be confounded with other species of the Alectorobius group (BARROS-BATTESTI et al., 2012Barros-Battesti DM, Onofrio VC, Nieri-Bastos FA, Soares JF, Marcili A, Famadas KM, et al. Ornithodoros brasiliensis Aragão (Acari: Argasidae): description of the larva, redescription of male and female, and neotype designation. Zootaxa 2012; 3178(31): 22-32.). All stages have been described (GUGLIELMONE et al., 2003).
The species O. setosus was described from larvae collected from bats in Piedras Negras, state of Rondônia; the holotype and paratypes were deposited under the number RML 49559, according to Kohls et al. (1969)Kohls GM, Clifford CM, Jones EK. The systematics of the subfamily Ornithodorinae (Acarina: Argasidae). IV. Eight new species of Ornithodoros from the Western Hemisphere. Ann Entomol Soc Am 1969; 62(5): 1035-1043.. Larvae have also been collected from bats in Mexico and Venezuela. Only the larval stage is known (GUGLIELMONE et al., 2003).
The species O. talaje forms a species group with
wide distribution from the southern United States to Argentina (HOOGSTRAAL, 1985Hoogstraal H. Argasid and Nuttalliellid ticks as parasites and
vectors. Adv Parasitol 1985; 24: 135-238. PMid:3904345.). According to this
author, most records before 1950 are questionable because they were based
primarily on adult morphology. Venzal et al. (2008) commented that this species
may be restricted to Central America, and that the records from South America
are probably O. rioplatensis known from Uruguay, or O.
puertoricensis, or a yet undescribed closely related species. These
authors also commented that the material from Guatemala that they examined had
been reared from adults collected close to the type locality of O.
talaje. These seem to be the "true" O. talaje
larvae, given that the original description of the species by Guérin-Méneville
was made from adult specimens collected from a nearby locality. All stages have
been described. Two new species were recently described and included in this
group: O. guaporensis (larvae and adults, collected from a
rocky fissure in the Amazon forest, in Bolivia) and O.
microlophi in Chile (larvae collected from lizards of the genus
Microlophus) (NAVA et al.,
2013Nava S, Venzal JM, Terassini FA, Mangold AJ, Camargo LMA, Casás G,
et al. Ornithodoros guaporensis (Acari, Ixodida: Argasidae), a new tick species
from the Guaporé River Basin in the Bolivian Amazon. Zootaxa 2013; 3666(4):
579-590. http://dx.doi.org/10.11646/zootaxa.3666.4.10
http://dx.doi.org/10.11646/zootaxa.3666....
; VENZAL et al.,
2013aVenzal JM, Nava S, González-Acuña D, Mangold AJ,
Muñoz-Leal S, Lado P, et al. A new species of Ornithodoros (Acari:
Argasidae), parasite of Microlophus spp. (Reptilia: Tropiduridae) from northern
Chile. Ticks Tick Borne Dis 2013a; 4(1-2): 128-132. PMid:23219344.
http://dx.doi.org/10.1016/j.ttbdis.2012.10.038
http://dx.doi.org/10.1016/j.ttbdis.2012....
).
The species O. rondoniensis is known from its adult
stage collected from a cave in the municipality of Porto Velho, state of
Rondônia (LABRUNA et al., 2008Labruna MB, Terassini FA, Camargo LMA, Brandão PE, Ribeiro AF,
Estrada-Peña A. New reports of Antricola guglielmonei and Antricola
delacruzi in Brazil, and a description of a new argasid species (Acari). J
Parasitol 2008; 94(4): 788-792. PMid:18576796.
http://dx.doi.org/10.1645/GE-1447.1
http://dx.doi.org/10.1645/GE-1447.1...
) and
from caves in the state of Pará (HENRIQUE-SIMõES
et al., 2012Henrique-Simões M, Bernardi LFO, Ogrzewalska M, Labruna MB, Ferreira
RL. New records of rare Ornithodoros (Acari: Argasidae) species in caves of
Brazilian Amazon. Persian J Acarol 2012; 1(2): 127-135.). The species O. fonsecai and
O. cavernicolous were described from the larvae and adults,
and from all stages, respectively (LABRUNA;
VENZAL, 2009Labruna MB, Venzal JM. Carios fonsecai sp. nov. (Acari, Argasidae),
a bat tick from central-western region of Brazil. Acta Parasitol 2009; 54(4):
355-363. http://dx.doi.org/10.2478/s11686-009-0051-1
http://dx.doi.org/10.2478/s11686-009-005...
; DANTAS-TORRES et al.,
2012Dantas-Torres F, Venzal JM, Bernardi LFO, Ferreira RL, Onofrio VC,
Marcili A, et al. Description of a new species of bat-associated argasid tick
(Acari: Argasidae) from Brazil. J Parasitol 2012; 98(1): 36-45. PMid:21955330.
http://dx.doi.org/10.1645/GE-2840.1
http://dx.doi.org/10.1645/GE-2840.1...
). O. fonsecai is only known from specimens
collected from bats on the inner walls of São Miguel cave, located in the rural
area of Bonito, state of Mato Grosso do Sul. This species has also been found on
bats and on walls of a cave named "Gruta Lagoa Azul", located 80 km from the
municipality of Nobres, state of Mato Grosso (BARROS-BATTESTI personal
communication). On the other hand, the species O. cavernicolous
has wide geographical distribution, with occurrences on bats and in caves in the
states of Pará, Ceará, Rio Grande do Norte, Bahia Goiás and Minas Gerais (DANTAS-TORRES et al., 2012Dantas-Torres F, Venzal JM, Bernardi LFO, Ferreira RL, Onofrio VC,
Marcili A, et al. Description of a new species of bat-associated argasid tick
(Acari: Argasidae) from Brazil. J Parasitol 2012; 98(1): 36-45. PMid:21955330.
http://dx.doi.org/10.1645/GE-2840.1
http://dx.doi.org/10.1645/GE-2840.1...
).
Larvae of O. mimon were originally collected from
bats in Bolivia, with records also from Uruguay and Argentina (VENZAL et al., 2004Venzal JM, Autino AG, Nava S, Guglielmone AA. Ornithodoros mimon
Kohls, Clifford & Jones, 1969 (Acari: Argasidae) on Argentinean bats, and
new records from Uruguay. Syst Appl Acarol 2004; 9: 37-39.). In Brazil, adults and
nymphs of O. mimon were first collected from a household in the
municipality of Araraquara, state of São Paulo. Larvae were reared from females
in a laboratory, and were redescribed along with a description of the adults as
well as the biology of this species under laboratory conditions (BARROS-BATTESTI et al., 2011Barros-Battesti DM, Landulfo GA, Onofrio VC, Faccini JLH, Marcili A,
Nieri-Bastos FA, et al. Carios mimon (Acari: Argasidae): description of adults
and redescription of larva. Exp Appl Acarol 2011; 54(1): 93-104. PMid:21161720.
http://dx.doi.org/10.1007/s10493-010-9416-2
http://dx.doi.org/10.1007/s10493-010-941...
; LANDULFO et al., 2012Landulfo GA, Pevidor LV, Sampaio JS, Luz HR, Onofrio VC, Faccini
JLH, et al. Life cycle of Ornithodoros mimon (Acari: Argasidae) under laboratory
conditions. Exp Appl Acarol 2012; 58(1): 69-80. PMid:22570058.
http://dx.doi.org/10.1007/s10493-012-9567-4
http://dx.doi.org/10.1007/s10493-012-956...
). Nymphal instars of
O. mimon were also described (LANDULFO et al., 2013Landulfo GA, Pevidor LV, Luz HR, Faccini JLH, Nunes PH,
Barros-Battesti DM. Description of nymphal instars of Ornithodoros mimon Kohls,
Clifford & Jones, 1969 (Acari: Argasidae). Zootaxa 2013; 3710(2): 179-191.
http://dx.doi.org/10.11646/zootaxa.3710.2.4
http://dx.doi.org/10.11646/zootaxa.3710....
).
The species O. marinkellei is known from Brazil,
Colombia, Panama and Venezuela (VENZAL et al.,
2006Venzal JM, Onofrio VC, Barros-Battesti DM, Arzua M. Família
Argasidae: características gerais, comentários e chave para gêneros e
espécies. In: Barros-Battesti DM, Arzua M, Bechara GH. Carrapatos de
Importância Médico-Veterinária da Região Neotropical: Um guia ilustrado
para identificação de espécies. São Paulo: Vox/ICTTD-3; Butantan; 2006. p.
223.; LABRUNA et al., 2011Labruna MB, Nava S, Terassini FA, Onofrio VC, Barros-Battesti DM,
Camargo LMA, et al. Description of adults and nymph, and redescription of the
larva, of Ornithodoros marinkellei (Acari: Argasidae), with data on its
phylogenetic position. J Parasitol 2011; 97(2): 207-217. PMid:21506769.
http://dx.doi.org/10.1645/GE-2620.1
http://dx.doi.org/10.1645/GE-2620.1...
).
In Brazil, adults of this species were found in caves in the municipality of
Porto Velho, state of Rondônia (LABRUNA et
al., 2011Labruna MB, Nava S, Terassini FA, Onofrio VC, Barros-Battesti DM,
Camargo LMA, et al. Description of adults and nymph, and redescription of the
larva, of Ornithodoros marinkellei (Acari: Argasidae), with data on its
phylogenetic position. J Parasitol 2011; 97(2): 207-217. PMid:21506769.
http://dx.doi.org/10.1645/GE-2620.1
http://dx.doi.org/10.1645/GE-2620.1...
), and in the state of Pará (HENRIQUE-SIMõES et al., 2012Henrique-Simões M, Bernardi LFO, Ogrzewalska M, Labruna MB, Ferreira
RL. New records of rare Ornithodoros (Acari: Argasidae) species in caves of
Brazilian Amazon. Persian J Acarol 2012; 1(2): 127-135.). In the same cave in Porto Velho,
larvae were collected from bats; few of these larvae molted to nymphs under the
conditions of the cave. Adults and the first nymphal instar were described and
the larva was redescribed by Labruna et al.
(2011)Labruna MB, Nava S, Terassini FA, Onofrio VC, Barros-Battesti DM,
Camargo LMA, et al. Description of adults and nymph, and redescription of the
larva, of Ornithodoros marinkellei (Acari: Argasidae), with data on its
phylogenetic position. J Parasitol 2011; 97(2): 207-217. PMid:21506769.
http://dx.doi.org/10.1645/GE-2620.1
http://dx.doi.org/10.1645/GE-2620.1...
. This species is closely related to O.
viguerasi and O. moormops, and all belong to the
subgenus "Subparmatus".
Larvae of O. kohlsi were collected from bats of the
species Neoplatymops matogrossensis, which were found in a rock
crevice in Monte Negro, state of Rondônia, in 2005. This species was
previously described as O. boliviensis by Kohls and Clifford (1964)Kohls GM, Clifford CM. Ornithodoros (Alectorobius) boliviensis sp.
n. (Acarina: Argasidae) from bats and houses in Bolivia. J Parasitol 1964;
50(6): 792-796. PMid:14244814.
http://dx.doi.org/10.2307/3276204
http://dx.doi.org/10.2307/3276204...
, but the name "boliviensis" had
been preoccuped. Therefore, Guglielmone and
Keirans (2002)Guglielmone AA, Keirans JE. Ornithodoros kohlsi Guglielmone and
Keirans (Acari: Argasidae), a new name for Ornithodoros boliviensis Kohls and
Clifford 1964. Proc Entomol Soc Wash 2002; 104(3): 822. proposed the name O. kohlsi. This
species occurs on bats of the species Myotis nigricans and
Molossus sp. in several localities in Bolivia. According to
Kohls et al. (1965)Kohls GM, Sonenshine DE, Clifford CM. The systematics of the
subfamily Ornithodorinae (Acarina: Argasidae). II. Identification of the larvae
of the Western Hemisphere and descriptions three news species. Ann Entomol Soc
Am 1965; 58(3): 331-364. PMid:5835857., adults and
nymphs were found in bat-infested houses, where the tick was found biting
humans.
Genus Otobius. This genus is
represented by two species in the world, and in the Neotropics only
Otobius megnini (Dugès, 1883) has been recorded.
Although there have been isolated reports from northern and southeastern Brazil
(FLECHTMANN, 1985; DINIZ et al., 1987Diniz LSM, Belluomini HE, Travassos LP F°, Rocha MB. Presence of
the ear mite Otobius megnini in the external ear canal of lions (Panthera leo).
J Zoo Anim Med 1987; 18(4): 154-155.
http://dx.doi.org/10.2307/20094831
http://dx.doi.org/10.2307/20094831...
),
the species is not established in this country. All stages have been
described.
Key to the genera of the larval stage of Argasidae in the Neotropical region
1.Eyes present; Haller's organ with capsule aperture large and rounded, with posterior projections like branches, posthalleral setae very long; dorsal idiosoma striated with 7-10 pairs of setae, dorsal plate large, elongate tapering slightly posteriorly; ventral surface with 5 pairs of setae + 1 pair on valvae; hypostome long without corona, dental formula 2/2 .......................................................... Otobius (Figures 33-36)
-Eyes absent............................................................................ 2
2. Palpal segment 4 as long as, or longer than the other palpal segments. Dorsal idiosoma with 25-30 pairs of setae, dorsal plate oval elongate; ventrally with less than 7 pairs; hypostome rounded on apex, dentition 2/2 from basis to posterior third, then 3/3 to apex; trumpet-shaped sensillum on tarsus I present or absent; if present, extending posteriorly from the capsule of Haller's organ, claws present ..........................................Argas (Figures 10-14)
-Dorsal idiosoma with 13-21 pairs of setae (except for O. setosus with 27-29), hypostome pointed or rounded at apex, claws present or absent on tarsi..................................................................... 3
3.Pulvilli extended, claws absent (except in A. marginatus); with 14-15 pairs of dorsal setae, dorsal plate large with lateral border parallel, narrowing anteriorly; hypostome pointed at apex, dentition 3/3 extending from posterior third to apex and 2/2 at base, 3 pairs of postcoxal setae......................Antricola (Figures 1-4)
-Pulvilli reduced; claws present................................................ 4
4.Dorsal plate with isosceles triangle shape occupying
entire length of the dorsum (in unfed specimens); dorsal surface with 12-13
pairs of setae; hypostome with apex pointed, dental formula 2/2, corona absent
(NAVA et al., 2010Nava S, Venzal JM, Terassini FA, Mangold AJ, Camargo LMA, Labruna
MB. Description of a new argasid tick (Acari: Ixodida) from bat caves in
Brazilian Amazon. J Parasitol 2010; 96(6): 1089-1101. PMid:21158616.
http://dx.doi.org/10.1645/GE-2539.1
http://dx.doi.org/10.1645/GE-2539.1...
)
......................... Nothoaspis
-Dorsal plate elongated and subrectangular, with anterior extremity narrowed, piriform or triangular; hypostome with apex rounded or pointed, hypostomal dentition 2/2 to 4/4; tarsi surface glabrous or rugous; dorsal surface with 13-21 pairs of setae (except for O. setosus, which has 27-29 pairs) .................................................Ornithodoros (Figures 19-28)
Key to the genera of the nymphal stage of Argasidae in the Neotropical region
1.Periphery of the idiosoma flat and structurally different from dorsum, with suture distinguishing dorsal and ventral surfaces ............................................................ Argas (Figures 15-18)*
-Periphery of the idiosoma not flat, without a suture line separating dorsal and ventral surfaces................................................ 2
2.Integument with spines; hypostome developed, dentition 4/4; body panduriform; spiracular plate conical..................Otobius (Figures 37-41)**
-Integument mammillated or tuberculated, lacking spines...... 3
3.False plate occupying the anterocentral area of
dorsum; ventrally palpi I elongate; large flaps shielding the hypostome, which
is pointed; dental formula 4/4 to 5/5 at basis; spiracular plate subcircular and
lateral to coxa IV (NAVA et al., 2010Nava S, Venzal JM, Terassini FA, Mangold AJ, Camargo LMA, Labruna
MB. Description of a new argasid tick (Acari: Ixodida) from bat caves in
Brazilian Amazon. J Parasitol 2010; 96(6): 1089-1101. PMid:21158616.
http://dx.doi.org/10.1645/GE-2539.1
http://dx.doi.org/10.1645/GE-2539.1...
)
...............................................................................
Nothoaspis
-False plate absent; flaps on internal side of palpal article I; if present, they are small and never shielding the hypostome ............................................................................................... 4
4.Hypostome with a few small denticles, not clearly in a definite row; idiosoma tuberculated; most tubercles on dorsum bearing short setae, some single, others in group; spiracular plate oval; humps on tarsi absent, Haller's organ with capsule small, rounded opening.......................................... Antricola (Figures 5-9)***
-Hypostome with distinct denticles in rows; idiosoma mammillated, discs present; Haller's organ with capsule aperture transversely slit-like and large, with many small setae; camerostome present; surface of tarsi mammillated without dorsal humps in bat-associated species, or surface of tarsi flat dorsally, without mammillae and with dorsal humps present ............................................... Ornithodoros (Figures 29-32)*
* Nymph of 1st instar; ** nymph of 2nd instar; *** nymphal instar undetermined
Key to the larvae of species of Ornithodoros in Brazil*
1.Basis capituli ventral with a pair of cornua-like extensions posteriorly and with a pair of auriculae-like extensions laterally2
-Basis capituli ventral without cornua or auriculae-like extensions ..................................................................... 3 (Figures 23-25)
2.Dorsal plate elongated and triangular in shape, with notched posterior margin; dorsum of body with 27-29 pairs of setae .............................................. O. setosus (KOHLS et al., 1965Kohls GM, Sonenshine DE, Clifford CM. The systematics of the subfamily Ornithodorinae (Acarina: Argasidae). II. Identification of the larvae of the Western Hemisphere and descriptions three news species. Ann Entomol Soc Am 1965; 58(3): 331-364. PMid:5835857.)
-Dorsal plate elongated, narrow and triangular in shape, with
posterior margin slightly concave, less than one-third as wide as long, dorsum
with 13 pairs of setae.................. O.
marinkellei (LABRUNA et al.,
2011Labruna MB, Nava S, Terassini FA, Onofrio VC, Barros-Battesti DM,
Camargo LMA, et al. Description of adults and nymph, and redescription of the
larva, of Ornithodoros marinkellei (Acari: Argasidae), with data on its
phylogenetic position. J Parasitol 2011; 97(2): 207-217. PMid:21506769.
http://dx.doi.org/10.1645/GE-2620.1
http://dx.doi.org/10.1645/GE-2620.1...
) (Figure
20)
3. Dorsum with 15 pairs of setae or less (typically 13-14 pairs) ............................................................................................... 4
-Dorsum with 16 pairs of setae or more (talaje group)............ 8
4.Dorsal plate oval, rectangular, elongated or resembling an apple; dorsum with 13-14 pairs of setae (typically 13); ventrally, 8 pairs of setae plus 1 posteromedial seta; presence of short spurs on dorsal surface of palpal article I................................................ 5
-Dorsal plate piriform or triangular; absence of short spurs on dorsal surface of palpal article I................................................ 6
5.Dorsal plate resembling an apple with anterior concavity; tarsus I smooth..................................... O. rostratus (Figures 21, 27)
-Dorsal plate oval and large without anterior concavity; tarsus I rugous.......O. brasiliensis (BARROS-BATTESTI et al., 2012Barros-Battesti DM, Onofrio VC, Nieri-Bastos FA, Soares JF, Marcili A, Famadas KM, et al. Ornithodoros brasiliensis Aragão (Acari: Argasidae): description of the larva, redescription of male and female, and neotype designation. Zootaxa 2012; 3178(31): 22-32.) (Figure 26)
6.Dorsal plate triangular; dorsum of idiosoma with 15
pairs of setae (11 dorsolateral); tarsus I with 24 setae, including 3 basal
pairs; presence of short spurs on dorsal surface of palpal article I
................ O. cavernicolous (DANTAS-TORRES et al., 2012Dantas-Torres F, Venzal JM, Bernardi LFO, Ferreira RL, Onofrio VC,
Marcili A, et al. Description of a new species of bat-associated argasid tick
(Acari: Argasidae) from Brazil. J Parasitol 2012; 98(1): 36-45. PMid:21955330.
http://dx.doi.org/10.1645/GE-2840.1
http://dx.doi.org/10.1645/GE-2840.1...
)
-Dorsal plate piriform or rectangular........... 7 (Figures 19, 22)
7.Dorsal plate piriform; dorsum of body with 13-14 pairs of setae (typically 14) and 3 central pairs; venter with 8 pairs of setae plus 1 PMS (posteromedian seta); hypostome long, pointed apically, dental formula 3/3 in the anterior half, 2/2 posteriorly almost to base; dentition: row 1 with 17-18 denticles, row 2 with 15-17, and row 3 with 9-10; occasionally, an accessory row with a single denticle (4/4 at apex)..................... O. fonsecai (Figures 22-23)
- Dorsal plate piriform; dorsum with 13 pairs of setae (10 dorsolateral and 3 central pairs); 6 dorsal pairs anterolaterally, venter 8-9 pairs of setae plus 1 PMS (posteromedian seta); hypostome long, blunt apically; dental formula 4/4 then 3/3 in the anterior third, 2/2 posteriorly almost to base; dentition: row 1 with 17-18 denticles, row 2 with 14-16, row 3 with 4-5 and row 4 with 3 ................................................O. mimon (Figures 19, 25, 28)
8.Dorsal plate piriform in shape............................................. 9
-Dorsal plate rectangular, about twice as wide as long, with anterior and posterior slightly concave; dorsum with 16-21 pairs of setae; 12-14 dorsolateral pairs (typically 13) and 4-7 central pairs (typically 5); venter of body with 7 pairs of setae plus a posteromedial seta; hypostome dentition 3/3 anteriorly, 2/2 posteriorly to base......................................O. rudis (KOHLS et al., 1965Kohls GM, Sonenshine DE, Clifford CM. The systematics of the subfamily Ornithodorinae (Acarina: Argasidae). II. Identification of the larvae of the Western Hemisphere and descriptions three news species. Ann Entomol Soc Am 1965; 58(3): 331-364. PMid:5835857.)
9.Dorsal plate large and piriform, almost pointed anteriorly; dorsum with 22-25 pairs of setae (18-21 dorsolateral and 4 central pairs); hypostome arises from a small subtriangular median extension and tapers to a blunt apex; dentition 5/5 at the apex, then 4/4 near midlength and 2/2 to the basis ............................................O. capensis (KOHLS et al., 1965Kohls GM, Sonenshine DE, Clifford CM. The systematics of the subfamily Ornithodorinae (Acarina: Argasidae). II. Identification of the larvae of the Western Hemisphere and descriptions three news species. Ann Entomol Soc Am 1965; 58(3): 331-364. PMid:5835857.)
-Dorsum with 20 pairs of setae or less .................................. 10
10.Hypostomal dentition 3/3 ............................................... 11
-Hypostomal dentition 4/4 in the anterior two-thirds, then 3/3 and 2/2 posteriorly to the base; row 1 with 16-18 denticles, row 2 with 15-17, row 3 with 12 or 13 and row 4 with 6-9; hypostome arises from a small, cone-shaped median extension and tapers to a point; dorsal plate moderately large and piriform, widest posteriorly; dorsum with 19 pairs of setae; 15 dorsolateral pairs and 4 central pairs; venter with 8 pairs of setae plus 1 posteromedial seta ........................................ O. stageri (KOHLS et al., 1965Kohls GM, Sonenshine DE, Clifford CM. The systematics of the subfamily Ornithodorinae (Acarina: Argasidae). II. Identification of the larvae of the Western Hemisphere and descriptions three news species. Ann Entomol Soc Am 1965; 58(3): 331-364. PMid:5835857.)
11. Hypostome arises from a small subtriangular median extension ...................................................................................... 12
-Hypostome normal; hypostomal dentition 3/3 in the anterior half, 2/2 posteriorly to base; dorsum with 17-20 pairs of setae; dorsal plate large and piriform; venter of body with 8 pairs of setae plus a posteromedial seta ................................................ O. talaje (KOHLS et al., 1965Kohls GM, Sonenshine DE, Clifford CM. The systematics of the subfamily Ornithodorinae (Acarina: Argasidae). II. Identification of the larvae of the Western Hemisphere and descriptions three news species. Ann Entomol Soc Am 1965; 58(3): 331-364. PMid:5835857.)
12.Dorsum with 17-20 pairs of setae (typically 19); 14 or 15 dorsolateral pairs (typically 15) and 3-5 central pairs (typically 4); dorsal plate moderately large and piriform, widest posteriorly; dentition 3/3 in anterior two-thirds, 2/2 posteriorly to base; row 1 with 16-18 denticles, row 2 with 15-18 and row 3 with 8-12 ................................................. O. hasei (KOHLS et al., 1965Kohls GM, Sonenshine DE, Clifford CM. The systematics of the subfamily Ornithodorinae (Acarina: Argasidae). II. Identification of the larvae of the Western Hemisphere and descriptions three news species. Ann Entomol Soc Am 1965; 58(3): 331-364. PMid:5835857.)
-Dorsum with 17 pairs of setae**, 13 dorsolateral pairs and 4 central pairs; dorsal plate large and piriform; dentition 3/3 in anterior two-thirds and 2/2 in posterior third; row 1 with 20-23, row 2 with 19-21 and row 3 with 13-17 denticles .............................................................................. O. kohlsi **
* For O. jul, O. nattereri and O. rondoniensis, only adults have been described.
** modified by Martins et al.
(2013)Martins TF, Venzal JM, Terassini FA, Costa FB, Marcili A, Camargo
LMA, et al. New tick records from the state of Rondônia, western Amazon,
Brazil. Exp Appl Acarol 2013; 62(1): 121-128.
http://dx.doi.org/10.1007/s10493-013-9724-4
http://dx.doi.org/10.1007/s10493-013-972...
.
This work was supported in part by grants from CNPq (No. 309919/2007-0) and FAPESP (No. 2010/52183-3) to DMBB. We wish to thank Pablo Henrique Nunes (Department of Biology, Institute of Biosciences, UNESP, Rio Claro, Brazil) for preparing the scanning electron micrographs; and Alberto Alejandro Guglielmone (INTA, Rafaela, Argentina), and Romário Cerqueira Leite (Veterinary School, UFMG, Belo Horizonte, Brazil) for sending some tick samples.
References
- Almeida AP, Marcili A, Leite RC, Nieri-Bastos FA, Domingues LN, Martins JR, et al. Coxiella symbiont in the tick Ornithodoros rostratus (Acari: Argasidae). Ticks Tick Borne Dis 2012; 3(4): 203-206. PMid:22480930. http://dx.doi.org/10.1016/j.ttbdis.2012.02.003
» http://dx.doi.org/10.1016/j.ttbdis.2012.02.003 - Aragão HB. Notas sobre ixódidas brazileiros. Mem Inst Oswaldo Cruz 1911; 3(2): 145-195. http://dx.doi.org/10.1590/S0074-02761911000200001
» http://dx.doi.org/10.1590/S0074-02761911000200001 - Aragão HB. Ornithodoros brasiliensis n. sp. Brazil Medico 1923; 37-20.
- Aragão HB. Ixodidas brasileiros e de alguns paizes limitrophes. Mem Inst Oswaldo Cruz 1936; 31(4): 759-843. http://dx.doi.org/10.1590/S0074-02761936000400004
» http://dx.doi.org/10.1590/S0074-02761936000400004 - Barros-Battesti DM, Landulfo GA, Onofrio VC, Faccini JLH, Marcili A, Nieri-Bastos FA, et al. Carios mimon (Acari: Argasidae): description of adults and redescription of larva. Exp Appl Acarol 2011; 54(1): 93-104. PMid:21161720. http://dx.doi.org/10.1007/s10493-010-9416-2
» http://dx.doi.org/10.1007/s10493-010-9416-2 - Barros-Battesti DM, Onofrio VC, Nieri-Bastos FA, Soares JF, Marcili A, Famadas KM, et al. Ornithodoros brasiliensis Aragão (Acari: Argasidae): description of the larva, redescription of male and female, and neotype designation. Zootaxa 2012; 3178(31): 22-32.
- Cançado PH, Piranda EM, Mourão GM, Faccini JL. Spatial distribution and impact of cattle-raising on ticks in the Pantanal region of Brazil by using the CO2 tick trap. Parasitol Res 2008; 103(2): 371-377. PMid:18454288. http://dx.doi.org/10.1007/s00436-008-0982-8
» http://dx.doi.org/10.1007/s00436-008-0982-8 - Clifford CM, Kohls GM, Sonenshine DE. The systematics of the subfamily Ornithodorinae (Acarina: Argasidae). I. The genera and subgenera. Ann Entomol Soc Am 1964; 57(4): 429-437.
- Clifford CM, Hoogstraal H, Radovsky FJ, Stiller D, Keirans JE. Ornithodoros (Alectorobius) amblus (Acarina: Ixodoidea: Argasidae): Identify, marine bird and human hosts, virus infection, and distribution in Peru. J Parasitol 1980; 66(2): 312-323. PMid:7391872. http://dx.doi.org/10.2307/3280825
» http://dx.doi.org/10.2307/3280825 - Cooley RA, Kohls GM. Three new species of Ornithodoros (Acarina: Ixodoidea). Pub Health Rept 1941, 56(12): 587-594. http://dx.doi.org/10.2307/4583666
» http://dx.doi.org/10.2307/4583666 - Cooley RA, Kohls GM. The Argasidae of North America, Central America and Cuba. Am. Midland. Nat. Monogr; 1944. PMid:18746793 PMCid:PMC1780695.
- Dantas-Torres F, Venzal JM, Bernardi LFO, Ferreira RL, Onofrio VC, Marcili A, et al. Description of a new species of bat-associated argasid tick (Acari: Argasidae) from Brazil. J Parasitol 2012; 98(1): 36-45. PMid:21955330. http://dx.doi.org/10.1645/GE-2840.1
» http://dx.doi.org/10.1645/GE-2840.1 - De La Cruz J. Notas Adicionales a la Fauna de Garrapatas (Ixodoidea) de Cuba. III. Redescripción de Ornithodoros tadaridae Cerny y Dusbádek, 1967. Poeyana 1974; 138: 1-5.
- De La Cruz J. Notas adicionales a la fauna de garrapatas (Ixodoidea) de Cuba. V. Uma nueva especie del género Antricola Cooley & Kohls, 1942 (Argasidae). Poeyana 1976; 151: 8.
- Diniz LSM, Belluomini HE, Travassos LP F°, Rocha MB. Presence of the ear mite Otobius megnini in the external ear canal of lions (Panthera leo). J Zoo Anim Med 1987; 18(4): 154-155. http://dx.doi.org/10.2307/20094831
» http://dx.doi.org/10.2307/20094831 - Endris RG, Keirans JE, Robbins RG, Hess WR. Ornithodoros (Alectorobius) puertoricensis (Acari: Argasidae): Redescription by Scanning Electron Microscopy. J Med Entomol 1989; 26(3): 146-154. PMid:2724311.
- Estrada-Peña A, Venzal JM, Barros-Battesti DM, Onofrio VC, Trajano E, Firmino JVL. Three new species of Antricola (Acari: Argasidae) from Brazil, with a key to the known species in the genus. J Parasitol 2004; 90(3): 490-498. PMid:15270091. http://dx.doi.org/10.1645/GE-172R
» http://dx.doi.org/10.1645/GE-172R - Estrada-Peña A, Venzal JM, Kocan KM, Tramuta C, Tomassone L, Fuente J, et al. Observations on Antricola ticks: small nymphs feed on mammalian hosts and have a salivary gland structure similar to ixodid ticks. J Parasitol 2008; 94(4): 953-955. PMid:18576742. http://dx.doi.org/10.1645/GE-1371.1
» http://dx.doi.org/10.1645/GE-1371.1 - Estrada-Peña A, Mangold AJ, Nava S, Venzal JM, Labruna M, Guglielmone AA. A review of the systematics of the tick family Argasidae (Ixodida). Acarologia 2010; 50(3): 317-333. http://dx.doi.org/10.1051/acarologia/20101975
» http://dx.doi.org/10.1051/acarologia/20101975 - Flechtmann CHW. ácaros de importância médico-veterinária. 3. ed. Livraria Nobel AS: São Paulo; 1985. 192 p.
- Guglielmone AA, Keirans JE. Ornithodoros kohlsi Guglielmone and Keirans (Acari: Argasidae), a new name for Ornithodoros boliviensis Kohls and Clifford 1964. Proc Entomol Soc Wash 2002; 104(3): 822.
- Guglielmone AA, Estrada-Peña A, Keirans JE, Robbins RG. Ticks (Acari: Ixodida) of the Neotropical Zoogeographic Region. Atalanta: International Consortium on Ticks and Tick-Borne Diseases; 2003. 173 p.
- Guglielmone AA, Robins RG, Apanaskevich DA, Petney TN, Estrada-Peña A, Horak IG, et al. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the world: a list of valid species names. Zootaxa 2010; 2528(6): 1-28.
- Henrique-Simões M, Bernardi LFO, Ogrzewalska M, Labruna MB, Ferreira RL. New records of rare Ornithodoros (Acari: Argasidae) species in caves of Brazilian Amazon. Persian J Acarol 2012; 1(2): 127-135.
- Heath ACG. A new species of soft tick (Ixodoidea: Argasidae) from the New Zealand lesser short-tailed bat, Mystacina tuberculata Gray. Tuhinga 2012; 23: 29-37.
- Hoogstraal H. Argasid and Nuttalliellid ticks as parasites and vectors. Adv Parasitol 1985; 24: 135-238. PMid:3904345.
- Jones EK, Clifford CM, Keirans JE, Kohls GM. The ticks of Venezuela (Acarina: Ixodoidea) with a key to the species of Amblyomma in the western hemisphere. Brigham Young Univ Sci Bull Biol Ser 1972; 17(4): 1-40.
- Keirans JE, Clifford CM, Redell JR. Description of the immature stages of Nothoaspis reddelli (Ixodoidea: Argasidae) from bat caves in Mexico. Ann Entomol Soc Am 1977; 70(4): 591-595.
- Keirans JE, Clifford CM, Hoogstraal H. Identify of the nymphs and adults of the Galapagos iguanid lizard parasites, Ornithodoros (Alectorobius) darwini and O. (A.) galapagensis (Ixodoidea: Argasidae). J Med Entomol 1980; 17(5): 427-438.
- Keirans JE, Clifford CM, Hoogstraal. Ornithodoros (Alectorobius) yunkeri, new species (Acari: Ixodoidea: Argasidae), from seabirds and nesting sites in the Galapagos Islands. J Med Entomol 1984; 21(3): 344-350. PMid:6748010.
- Klompen JSH. Comparative morphology of argasid larvae (Acari: Ixodida: Argasidae), with notes on phylogenetic relationships. Ann Ent Soc Am 1992; 85(5): 541-560.
- Klompen JSH, Oliver JH Jr. Systematic relationships in the soft ticks (Acari: Ixodida: Argasidae). Syst Entomol 1993; 18(4): 313-331. http://dx.doi.org/10.1111/j.1365-3113.1993.tb00669.x
» http://dx.doi.org/10.1111/j.1365-3113.1993.tb00669.x - Kohls GM, Clifford CM. Ornithodoros (Alectorobius) boliviensis sp. n. (Acarina: Argasidae) from bats and houses in Bolivia. J Parasitol 1964; 50(6): 792-796. PMid:14244814. http://dx.doi.org/10.2307/3276204
» http://dx.doi.org/10.2307/3276204 - Kohls GM, Sonenshine DE, Clifford CM. The systematics of the subfamily Ornithodorinae (Acarina: Argasidae). II. Identification of the larvae of the Western Hemisphere and descriptions three news species. Ann Entomol Soc Am 1965; 58(3): 331-364. PMid:5835857.
- Kohls GM, Clifford CM, Jones EK. The systematics of the subfamily Ornithodorinae (Acarina: Argasidae). IV. Eight new species of Ornithodoros from the Western Hemisphere. Ann Entomol Soc Am 1969; 62(5): 1035-1043.
- Kohls GM, Hoogstraal H, Clifford CM, Kaiser MN. The subgenus Persicargas (Ixodoidea, Argasidae, Argas). 9. Redescription and New World records of Argas (P.) persicus (Oken), and resurrection, redescription, and records of A. (P.) radiatus Railliet, A. (P.) sanchezi Dugès, and A. (P.) miniatus Koch, New World ticks misidentified as A. (P.) persicus. Ann Entomol Soc Am 1970; 63(2): 590-606.
- Labruna MB, Terassini FA, Camargo LMA, Brandão PE, Ribeiro AF, Estrada-Peña A. New reports of Antricola guglielmonei and Antricola delacruzi in Brazil, and a description of a new argasid species (Acari). J Parasitol 2008; 94(4): 788-792. PMid:18576796. http://dx.doi.org/10.1645/GE-1447.1
» http://dx.doi.org/10.1645/GE-1447.1 - Labruna MB, Venzal JM. Carios fonsecai sp. nov. (Acari, Argasidae), a bat tick from central-western region of Brazil. Acta Parasitol 2009; 54(4): 355-363. http://dx.doi.org/10.2478/s11686-009-0051-1
» http://dx.doi.org/10.2478/s11686-009-0051-1 - Labruna MB, Nava S, Terassini FA, Onofrio VC, Barros-Battesti DM, Camargo LMA, et al. Description of adults and nymph, and redescription of the larva, of Ornithodoros marinkellei (Acari: Argasidae), with data on its phylogenetic position. J Parasitol 2011; 97(2): 207-217. PMid:21506769. http://dx.doi.org/10.1645/GE-2620.1
» http://dx.doi.org/10.1645/GE-2620.1 - Landulfo GA, Pevidor LV, Sampaio JS, Luz HR, Onofrio VC, Faccini JLH, et al. Life cycle of Ornithodoros mimon (Acari: Argasidae) under laboratory conditions. Exp Appl Acarol 2012; 58(1): 69-80. PMid:22570058. http://dx.doi.org/10.1007/s10493-012-9567-4
» http://dx.doi.org/10.1007/s10493-012-9567-4 - Landulfo GA, Pevidor LV, Luz HR, Faccini JLH, Nunes PH, Barros-Battesti DM. Description of nymphal instars of Ornithodoros mimon Kohls, Clifford & Jones, 1969 (Acari: Argasidae). Zootaxa 2013; 3710(2): 179-191. http://dx.doi.org/10.11646/zootaxa.3710.2.4
» http://dx.doi.org/10.11646/zootaxa.3710.2.4 - Marchoux E, Salimbeni A. La spirillose des poules. Annales de I'Institut Pasteur Lille 1903; 17(1):569-580.
- Martins JR, Doyle RL, Barros-Battesti DM, Onofrio VC, Guglielmone AA. Occurrence of Ornithodoros brasiliensis Aragão (Acari: Argasidae) in São Francisco de Paula, RS, Southern Brazil. Neotrop Entomol 2011; 40(1): 143-144. PMid:21437496. http://dx.doi.org/10.1590/S1519-566X2011000100022
» http://dx.doi.org/10.1590/S1519-566X2011000100022 - Martins TF, Venzal JM, Terassini FA, Costa FB, Marcili A, Camargo LMA, et al. New tick records from the state of Rondônia, western Amazon, Brazil. Exp Appl Acarol 2013; 62(1): 121-128. http://dx.doi.org/10.1007/s10493-013-9724-4
» http://dx.doi.org/10.1007/s10493-013-9724-4 - Matheson R. Three new species of ticks, Ornithodoros (Acarina: Ixodoidea). J Parasitol 1935; 21(5): 347-353. http://dx.doi.org/10.2307/3271944
» http://dx.doi.org/10.2307/3271944 - Nava S, Lareschi M, Rebollo C, Benítez Usher C, Beati L, Robbins RG, et al. The ticks (Acari: Ixodida: Argasidae, Ixodidae) of Paraguay. Ann Trop Med Parasitol 2007; 101(3): 255-270. PMid:17362600. http://dx.doi.org/10.1179/136485907X176319
» http://dx.doi.org/10.1179/136485907X176319 - Nava S, Guglielmone AA, Mangold AJ. An overview of systematics and evolution of ticks. Front Biosci 2009; 14(8): 2857-2877. http://dx.doi.org/10.2741/3418
» http://dx.doi.org/10.2741/3418 - Nava S, Venzal JM, Terassini FA, Mangold AJ, Camargo LMA, Labruna MB. Description of a new argasid tick (Acari: Ixodida) from bat caves in Brazilian Amazon. J Parasitol 2010; 96(6): 1089-1101. PMid:21158616. http://dx.doi.org/10.1645/GE-2539.1
» http://dx.doi.org/10.1645/GE-2539.1 - Nava S, Venzal JM, Terassini FA, Mangold AJ, Camargo LMA, Casás G, et al. Ornithodoros guaporensis (Acari, Ixodida: Argasidae), a new tick species from the Guaporé River Basin in the Bolivian Amazon. Zootaxa 2013; 3666(4): 579-590. http://dx.doi.org/10.11646/zootaxa.3666.4.10
» http://dx.doi.org/10.11646/zootaxa.3666.4.10 - Oba MSP, Baggio D. Ocorrência de Ornithodoros talaje Guérin Meneville, 1849, (Acari: Argasidae) na localidade de Santo Inácio, Bahia. Arq Inst Biol 1977; 44(1-2): 107-109.
- Pardi MC, Rocha UF. Lesões causadas na pele de porcos pelas picadas de Ornithodoros rostratus Aragão, 1911 (Acari, Argasidae). Importância econômica. Rev Fac Med Vet S Paulo 1954; 5(1): 35-39.
- Roberts LE. Australian Ticks. Melbourne: CSIRO; 1970.
- Rohr CJ. Estudos sobre ixodideos do Brasil. Rio de Janeiro: Gomes, Irmão & C.; 1909.
- Santos HA, Angelo IC, Franque MP, Vashist U, Duarte AF, Baldani CD, et al. The influence of the fasting period on the number of nymphal instars and the sex ratio of Argas (Persicargas) miniatus (Acari: Argasidae). Rev Bras Parasitol Vet 2010; 19(3): 164-168. PMid:20943020. http://dx.doi.org/10.1590/S1984-29612010000300007
» http://dx.doi.org/10.1590/S1984-29612010000300007 - Schulze P. Eine neue Ornithodoros-art (Ixod. Argas) aus Brasilien. Zool Anz 1940; 130: 131-135.
- Venzal JM, Autino AG, Nava S, Guglielmone AA. Ornithodoros mimon Kohls, Clifford & Jones, 1969 (Acari: Argasidae) on Argentinean bats, and new records from Uruguay. Syst Appl Acarol 2004; 9: 37-39.
- Venzal JM, Onofrio VC, Barros-Battesti DM, Arzua M. Família Argasidae: características gerais, comentários e chave para gêneros e espécies. In: Barros-Battesti DM, Arzua M, Bechara GH. Carrapatos de Importância Médico-Veterinária da Região Neotropical: Um guia ilustrado para identificação de espécies. São Paulo: Vox/ICTTD-3; Butantan; 2006. p. 223.
- Venzal JM, Estrada-Peña A, Mangold AJ, González-Acuña D, Guglielmone AA. The Ornithodoros (Alectorobius) talaje species group (Acari: Ixodida: Argasidae): Description of Ornithodoros (Alectorobius) rioplatensis n. sp. from Southern South America. J Med Entomol 2008; 45(5): 832-840. http://dx.doi.org/10.1603/0022-2585(2008)45[832:TOATSG]2.0.CO;2
» http://dx.doi.org/10.1603/0022-2585(2008)45[832:TOATSG]2.0.CO;2 - Venzal JM, Nava S, Mangold AJ, Mastropaolo M, Casás G, Guglielmone AA. Ornithodoros quilinensis sp. nov. (Acari, Argasidae), a new tick species from the Chacoan region in Argentina. Acta Parasitol 2012; 57(3): 329-336. PMid:22875683. http://dx.doi.org/10.2478/s11686-012-0034-5
» http://dx.doi.org/10.2478/s11686-012-0034-5 - Venzal JM, Nava S, González-Acuña D, Mangold AJ, Muñoz-Leal S, Lado P, et al. A new species of Ornithodoros (Acari: Argasidae), parasite of Microlophus spp. (Reptilia: Tropiduridae) from northern Chile. Ticks Tick Borne Dis 2013a; 4(1-2): 128-132. PMid:23219344. http://dx.doi.org/10.1016/j.ttbdis.2012.10.038
» http://dx.doi.org/10.1016/j.ttbdis.2012.10.038 - Venzal JM, Nava S, Terrassini FA, Ogrzewalska M, Camargo LMA, Labruna MB. Ornithodoros peropteryx (Acari: Argasidae) in Bolivia: an argasid tick with a single nymphal stage. Exp Appl Acarol 2013b; 61(2): 231-241. http://dx.doi.org/10.1007/s10493-013-9689-3
» http://dx.doi.org/10.1007/s10493-013-9689-3 - Vial L, Camicas J-L. Description of a new soft tick species of the genus Ornithodoros Koch, 1844 (Acari: Argasidae). Fauna of Arabia 2009; 24: 135-143.
- Warburton C. On five new species of ticks (Arachnida, Ixodoidea), Ornithodorus (sic) nattereri, Ixodes theodori, Haemaphysalis toxopei, Amblyomma robinsoni and A. dammermani, with a note on the ornate nymph of A. latum. Parasitol 1927; 19:405-410. http://dx.doi.org/10.1017/S0031182000005886
» http://dx.doi.org/10.1017/S0031182000005886
Publication Dates
-
Publication in this collection
Oct-Dec 2013
History
-
Received
9 Sept 2013 -
Accepted
29 Nov 2013