Abstracts
The aim of this study was to evaluate parasitism kinetics and tissue lesions in the first week of infection by Neospora caninum in dogs fed Gallus gallus chorioallantoic membranes (CMs) previously infected in ovo. Five two-month-old pups were used. Each dog was given five CMs that were previously infected with N. caninum via the oral route. Four animals were euthanized in the first week of infection. All four dogs had their stools examined one week prior to and up to the day they were euthanized. The stools of the uneuthanized dog were collected for 30 days. After euthanasia, organ sections were utilized for histopathology, immunohistochemistry, indirect immunofluorescent tissue reactions, PCR and real-time PCR to detect parasites. Necropsy revealed that the small and large intestines, spleen, and lungs were affected. No oocysts or N. caninum DNA were identified in the stool samples. Real-time PCR was the most sensitive technique used to detect the protozoa in tissues, which were identified in 41% of the analyzed samples. Our results indicate that an experimental model using previously infected CMs appears to be a useful model for the study of the host-parasite relationship during the infection's acute phase.
Neosporosis; acute phase; infection; real-time PCR; immunohistochemistry
O objetivo deste estudo foi avaliar a cinética de parasitismo e lesões teciduais, na primeira semana de infecção por Neospora caninum, em cães alimentados com membranas corioalantóicas (MCs) de Gallus gallus, previamente infectadas in ovo. Foram utilizados cinco filhotes de dois meses de idade. Cada cão recebeu cinco MCs previamente infectadas com N. caninum, por via oral. Quatro animais foram eutanasiados na primeira semana de infecção. Todos os quatro cães tiveram suas fezes examinadas uma semana antes e até o dia em que foram eutanasiados. O cão que não foi eutanasiado teve suas fezes colhidas durante 30 dias. Depois da eutanasia fragmentos de órgãos foram processados para histopatologia, imuno-histoquímica, reação de imunofluorescência indireta em tecidos, PCR e PCR em tempo real para detecção do parasito. A necropsia revelou que os intestinos delgado e grosso, baço e pulmões foram os órgãos afectados. Oocistos de N. caninum não foram identificados nas amostras de fezes. A PCR em tempo real foi a técnica mais sensível para detectar o protozoário nos tecidos, sendo identificados em 41% das amostras analisadas. Os nossos resultados indicam que o modelo experimental utilizando MCs evidenciou ser um bom modelo para estudar a relação parasito-hospedeiro durante a fase aguda da infecção.
Neosporose; fase aguda; infecção; PCR em tempo real; imonu-histoquímica
Introduction
Since its discovery by Bjerkas et al.
(1984)Bjerkas I, Mohn SF, Presthus J. Unidentified cyst-forming sporozoon
causing encephalomyelitis and myositis in dogs. Z Parasitenkd 1984; 70(2):
271-274. PMid:6426185. http://dx.doi.org/10.1007/BF00942230
http://dx.doi.org/10.1007/BF00942230...
, many studies have been conducted to better understand the
infection of dogs by the parasite Neospora caninum. Most dogs are
symptom-free, irrespective of the antibody titers observed (SICUPIRA et al., 2012Sicupira PM, De Magalhães VCS, Galvão GS, Pereira JMS, Gondim LFP,
Munhoz AD. Factors associated with infection by Neospora caninum in dogs in
Brazil. Vet Parasitol 2012; 185(2-4): 305-308. PMid:22015062.
http://dx.doi.org/10.1016/j.vetpar.2011.09.029
http://dx.doi.org/10.1016/j.vetpar.2011....
), whereas, unlike cattle, horizontal
infection has been characterized as the primary route of parasite dissemination
(DUBEY; SCHARES, 2011Dubey JP, Schares G. Neosporosis in animals - The last five years.
Vet Parasitol 2011; 180(1-2) 90-108. PMid:21704458.
http://dx.doi.org/10.1016/j.vetpar.2011.05.031
http://dx.doi.org/10.1016/j.vetpar.2011....
). In this context,
it has been observed that bradyzoites can infect and induce oocyst production (DIJKSTRA et al., 2001Dijkstra T, Eysker M, Schares G, Conraths FJ, Wouda W, Barkema HW.
Dogs shed Neospora caninum oocysts after ingestion of naturally infected bovine
placenta but not after ingestion of colostrum spiked with Neospora caninum
tachyzoites. Int J Parasitol 2001; 31(8): 747-752.
http://dx.doi.org/10.1016/S0020-7519(01)00230-2
http://dx.doi.org/10.1016/S0020-7519(01)...
; GONDIM et al., 2002Gondim LFP, Gao L, McAllister MM. Improved production of Neospora
caninum oocysts, cyclical oral transmission between dogs and cattle, and in
vitro isolation from oocysts. J Parasitol 2002; 88(6): 1159-1163.
PMid:12537111.) and that the ingestion of oocysts induces
antibody production only (BANDINI et al.,
2011Bandini LA, Alves AF N°, Pena HF, Cavalcante GT, Schares G,
Nishi SM, et al. Experimental infection of dogs (Canis familiaris) with
sporulated oocysts of Neospora caninum. Vet Parasitol 2011; 176(2-3): 151-156.
PMid:21094584. http://dx.doi.org/10.1016/j.vetpar.2010.10.047
http://dx.doi.org/10.1016/j.vetpar.2010....
). Furthermore, whether tissues containing only tachyzoites can be
orally infectious to animals is not known (DUBEY;
SCHARES, 2011Dubey JP, Schares G. Neosporosis in animals - The last five years.
Vet Parasitol 2011; 180(1-2) 90-108. PMid:21704458.
http://dx.doi.org/10.1016/j.vetpar.2011.05.031
http://dx.doi.org/10.1016/j.vetpar.2011....
).
Experimentally, oocyst elimination has been reported in dogs fed infected
bovine and murine tissue (GONDIM et al.,
2002Gondim LFP, Gao L, McAllister MM. Improved production of Neospora
caninum oocysts, cyclical oral transmission between dogs and cattle, and in
vitro isolation from oocysts. J Parasitol 2002; 88(6): 1159-1163.
PMid:12537111.), embryonated egg membranes (FURUTA et al., 2007), bovine placenta
(DIJKSTRA et al., 2001Dijkstra T, Eysker M, Schares G, Conraths FJ, Wouda W, Barkema HW.
Dogs shed Neospora caninum oocysts after ingestion of naturally infected bovine
placenta but not after ingestion of colostrum spiked with Neospora caninum
tachyzoites. Int J Parasitol 2001; 31(8): 747-752.
http://dx.doi.org/10.1016/S0020-7519(01)00230-2
http://dx.doi.org/10.1016/S0020-7519(01)...
) and buffalo
tissue (RODRIGUES et al., 2004Rodrigues AA, Gennari SM, Aguilar DM, Sreekumar C, Hill DE, Miska
KB, et al. Shedding of Neospora caninum oocysts by dogs fed tissues from
naturally infected water buffaloes (Bubalus bubalis) from Brazil. Vet Parasitol
2004; 124(3-4): 139-150. PMid:15381294.
http://dx.doi.org/10.1016/j.vetpar.2004.07.007
http://dx.doi.org/10.1016/j.vetpar.2004....
). The quantity
of eliminated oocysts, however, is directly associated with the intermediate host
(GONDIM et al., 2002Gondim LFP, Gao L, McAllister MM. Improved production of Neospora
caninum oocysts, cyclical oral transmission between dogs and cattle, and in
vitro isolation from oocysts. J Parasitol 2002; 88(6): 1159-1163.
PMid:12537111.), canine age and
reinfection status (GONDIM et al., 2005Gondim LFP, McAllister MM, Gao L. Effects of host maturity and prior
exposure history on the production of Neospora caninum oocysts by dogs. Vet
Parasitol 2005; 134(1-2): 33-39. PMid:16029931.
http://dx.doi.org/10.1016/j.vetpar.2005.06.011
http://dx.doi.org/10.1016/j.vetpar.2005....
), the
parasite's tropism for certain tissues (CAVALCANTE et al., 2011Cavalcante GT, Monteiro RM, Soares RM, Nishi SM, Alves AF N°,
Esmerini PO, et al. Shedding of Neospora caninum oocysts by dogs fed different
tissues from naturally infected cattle. Vet Parasitol 2011; 179(1-3): 220-223.
PMid:21450407. http://dx.doi.org/10.1016/j.vetpar.2011.02.026
http://dx.doi.org/10.1016/j.vetpar.2011....
), and the concentration of parasites in the
ingested tissue.
Because the elimination of oocysts in coccidia precedes the merogonous and
gametogonous phase, attempts have been made to identify these N.
caninum phases in dogs, but without success (CEDILLO et al., 2008Cedillo CJR, Martínez MJJ, Santacruz AM, Banda RVM, Morales SE.
Models for experimental infection of dogs fed with tissue from fetuses and
neonatal cattle naturally infected with Neospora caninum. Vet Parasitol 2008;
154(1-2): 151-155. PMid:18395346.
http://dx.doi.org/10.1016/j.vetpar.2008.02.025
http://dx.doi.org/10.1016/j.vetpar.2008....
). In light of this, the objective of this
study was to evaluate the parasitism kinetics and tissue lesions in the first week
after N. caninum infection in dogs fed Gallus
gallus chorioallantoic membranes previously infected in
ovo.
Materials and Methods
We used N. caninum tachyzoites of isolate NC-1 (DUBEY et al., 1988Dubey JP, Carpenter JL, Speer CA, Topper MJ, Uggla A. Newly recognized fatal protozoan disease of dogs. J Am Vet Med Assoc 1988; 192(9): 1269-1285. PMid:3391851.) cultivated in CV-1 cells (African green monkey kidney fibroblasts) kept according to Furuta et al. (2007). Specific pathogen-free (SPF) embryonated chicken eggs (Hy-Line do Brasil Ltda, Nova Granada, SP, Brazil) were used in these experiments. They were accommodated in a rotary incubator and kept at 37.7±2 °C and 55±5% humidity. Every day from the 7th, the eggs were taken to the egg candler to diagnose embryo viability. On the 10th day, 30 eggs were inoculated with 1 × 106 N. caninum tachyzoites. On the 8th day after infection (DAI), the eggs were assessed and those that had viable embryos were opened. After confirmation of the infective forms in the chorioallantoic membranes by direct microscopy, they were immersed in an antibiotic/antibiotic solution containing penicillin (10000 U), streptomycin (100 µg) and B amphotericin (25 µg) per mL (Invitrogen, USA) and kept under refrigeration at 4 °C, until they were offered to the dogs.
Five two-month-old pups were used. All pups were from the same litter, and the bitch was negative for anti-N. caninum antibodies. The pups were accommodated in individual stalls and vaccinated against canine distemper, parvovirosis, leptospirosis, influenza, infectious hepatitis and coronavirosis. The pups were dewormed (Praziquantel 5 mg/kg and Pyrantel Pamoate 14.4 mg/kg single dose and repeated after 15 days) and treated with giardicid (Metronidazole, 10 mg/kg, twice a day for 5 days). Each dog was submitted to anti-Babesia canis, anti-Ehrlichia canis, anti-Toxoplasma gondii, anti-N. caninum and anti-Leishmania chagasi antibody detection, all of which were found to be seronegative. This treatment was furthered with a diet based on commercial feed, without the possibility of raw meat ingestion or of any other feed to avoid possible coccidian infection. The stalls were cleaned daily and disinfected with a solution of formaldehyde at 1% and sodium hypochlorite at 2%. All animals were submitted to a physical and clinical assessment.
Each dog was given five chorioallantoic membranes previously infected with N. caninum via the oral route. Four animals were euthanized in the first week of infection (3rd, 4th, 5th and 6th DAI). All four dogs had their stools examined for oocysts one week prior to and up to the day they were euthanized. The dog that was not euthanized (the control) had its stools collected and assessed for 30 DAI. This dog was kept as control to verify the possibility of oocysts elimination as well as seroconversion after the 1st week of infection. All dogs received 5 mL of 2% sodium bicarbonate via the oral route 15 minutes prior to ingestion of the membranes. The animals were euthanized following sedation with 0.2% acepromazine (0.04 mg/kg) and then anesthesia with thiopental sodium (12.5 mg/kg), via intravenous infusion of 10% potassium chloride. The study was approved by the Ethics Committee for Animal Use with protocol number CEUA/Unesp-Jaboticabal 025713/2009.
The total volume of feces collected in 24 h was homogenized daily. The centrifugal fluctuation method with sugar solution (density of 1.26 g/cm3) was used for the stool test. Five grams of feces was used, and the protocol used was similar to that of Gondim et al. (2002)Gondim LFP, Gao L, McAllister MM. Improved production of Neospora caninum oocysts, cyclical oral transmission between dogs and cattle, and in vitro isolation from oocysts. J Parasitol 2002; 88(6): 1159-1163. PMid:12537111., with the exception of the final precipitate, which was re-suspended in a 2% potassium dichromate solution regardless of the microscopy result that was kept under aeration for three days and stored at −20 °C until the molecular assay.
Anti-N. caninum antibodies were detected in serum with
an indirect fluorescent antibody test, according to Yamane et al. (1997)Yamane I, Kokuho T, Shimura K, Eto M, Shibahara T, Haritani M, et
al. In vitro isolation and characterisation of a bovine Neospora species in
Japan. Res Vet Sci 1997; 63(1): 77-80.
http://dx.doi.org/10.1016/S0034-5288(97)90162-4
http://dx.doi.org/10.1016/S0034-5288(97)...
, using tachyzoites of the NC-1 strain with a cutoff
point of 1:25. The dogs had their blood sampled at infection day zero and again on
the day of euthanasia. The control dog had its blood taken weekly for 30 days.
Fragments of submandibular, mesenteric and popliteal lymph nodes, tonsils, esophagus, stomach, duodenum, jejunum, ileum, cecum, colon, biliar vesicle, tongue, heart, lungs, liver, spleen, kidney, pancreas, brain and cerebellum were packed in 10% buffered formaldehyde for 24 hours and subsequently transferred to 70% ethanol for histopathology and immunohistochemistry (IHC) according to Furuta et al. (2007) with some modifications. For IHC, the antigen retrieval was performed in buffered citrate solution (pH 6.0) in a water bath at 95 °C for 30 minutes, and endogenous peroxidase was blocked in methyl alcohol solution with hydrogen peroxide at 8% for 20 minutes, after we used polyclonal serum from a naturally infected bovine (MUNHOZ et al., 2011Munhoz AD, Jacintho APP, Machado RZ. Bovine abortion associated with Neospora caninum: Diagnosis and epidemiological aspects of a dairy cattle herd in the Northeast region of São Paulo State, Brazil. Braz J Vet Pathol 2011; 4(2): 112-116.) as the primary antibody at a 1:1000 dilution. The Indirect immunofluorescent reaction in tissues was performed according to Furuta (2008)Furuta PI. Infecção experimental em cães com ovos embrionados de galinha (Gallus gallus domesticus) infectados com taquizoítas de Neospora caninum. [Tese]. Jaboticabal: Universidade Estadual Paulista, Faculdade de Ciências Agrárias e Veterinárias; 2008..
Fragments (20-50 mg) of organs were kept at −70 °C, and DNA was
extracted with a DNeasy kit (Qiagen™) according to the
manufacturer's recommendations. For DNA extraction from dog stool samples, we
used DNAStool kit (Qiagen™). Before adding Proteinase K, we
performed 8 freezing and unfreezing cycles (−70 °C, 96 °C) lasting one
minute each, followed by shaking with 0.5 mm glass beads for 5 minutes. PCR was
conducted using the primers Np6 (5′-CAG TCA ACC TAC GTC TTC T-3′) and
Np21 (5′-GTG CGT CCA ATC CTG TAA C-3′), whose amplicon is approximately
328 base pairs (YAMAGE et al., 1996Yamage M, Flechtner O, Gottstein B. Neospora caninum: specific
oligonucleotide primers for the detection of brain "cyst" DNA of experimentally
infected nude mice by the polymerase chain reaction (PCR). J Parasitol 1996;
82(2): 272-279. PMid:8604096. http://dx.doi.org/10.2307/3284160
http://dx.doi.org/10.2307/3284160...
). The
amplification reactions were performed according to Munhoz et al. (2011)Munhoz AD, Jacintho APP, Machado RZ. Bovine abortion associated with
Neospora caninum: Diagnosis and epidemiological aspects of a dairy cattle herd
in the Northeast region of São Paulo State, Brazil. Braz J Vet Pathol 2011;
4(2): 112-116..
Real-time PCR was performed using System SYBR Green, and the oligonucleotide sequences used were NC5-F1 (5′-AAA CAG GAG GAG AGA ACG GCG ATT-3′) and NC5-R1 (5′-AGT ACG CAA AGA TTG CCG TTG CAG-3′) (FURUTA, 2008Furuta PI. Infecção experimental em cães com ovos embrionados de galinha (Gallus gallus domesticus) infectados com taquizoítas de Neospora caninum. [Tese]. Jaboticabal: Universidade Estadual Paulista, Faculdade de Ciências Agrárias e Veterinárias; 2008.). The amplification reactions were performed with a final volume of 25 µL. A plate with 96 wells was employed, to which we added 12.5 µL of platinum Green™ qPCR SuperMix UDG with ROX (Applied Biosystens™), 0.4 µM of each oligonucleotide initiator and 200 ng of DNA of each the sample, and the final volume was adjusted with sterile double distilled water. All samples were analyzed in triplicate. After pipetting all of the reagents, the plate was sealed and placed in the thermocycler. The cycles were 1x (50 °C for 2 min.), 1x (95 °C for 10 min.) and 40x (95 °C for 15 s, 60 °C for 1 min.). We used NC-1 strain tachyzoite DNA as a positive control in the Real-time PCR and conventional PCR and sterile double distilled water as a negative control.
Results
During the entire experiment, none of the dogs showed any specific or non-specific clinical signs associated with the disease, with the exception of blackened stools. Necropsy revealed that the small intestine (duodenum, jejunum and ileum) and the large intestine (colon and cecum), spleen and lungs were the organs affected (Figure 1). In the lungs, we observed small areas with hemorrhagic points and multifocal emphysema of all lobes. Intense splenomegaly with congestion and white pulp hyperplasia were found in the spleen, whereas in the small intestine, there was intense hemorrhage throughout the segment, especially in the duodenum, with moderate thickening of the mucous membrane associated with hyperplasia of the aggregated lymphoid tissues.
Necroscopic findings in dogs experimentally infected with Neospora caninum. a) Spleen showing white pulp hyperplasia in an animal on the 4th DAI; b) Section of jejunum with thickening of mucous membrane and agglomerated petechia distributed throughout the mucous membrane (5th DAI).
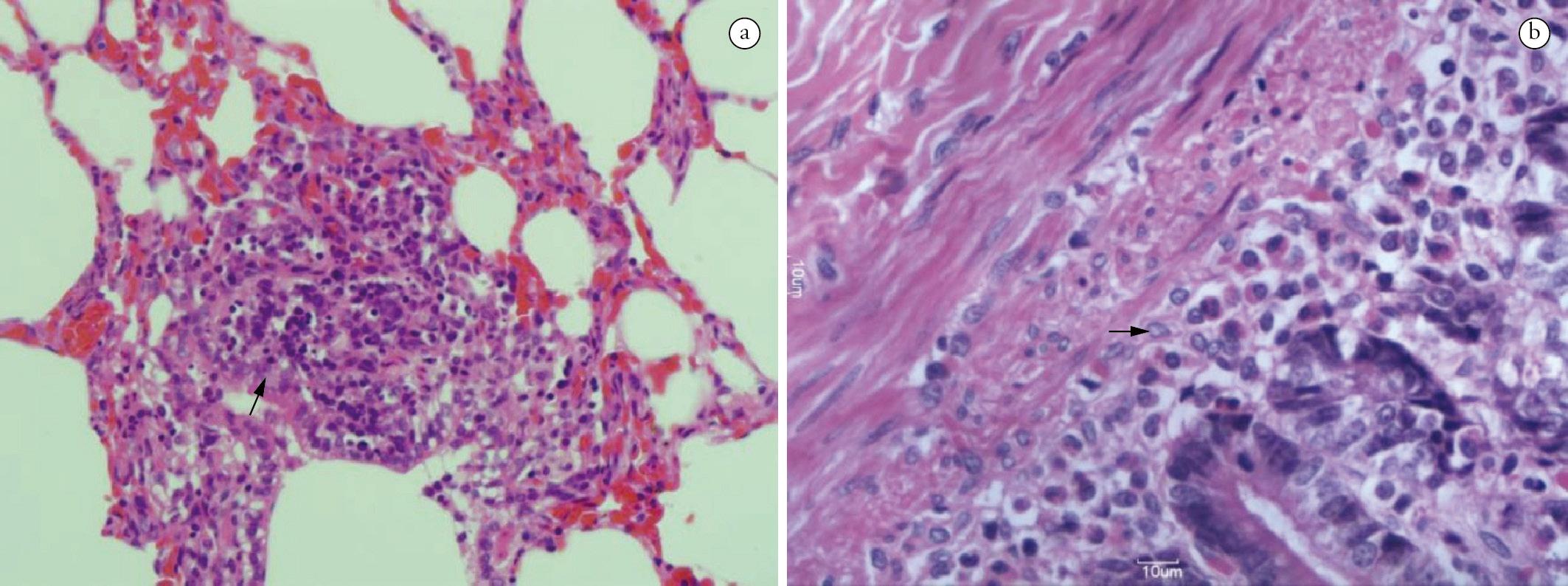
The main microscopy findings were in the small intestine (duodenum): diffuse, moderate infiltrate of mononuclear cells and eosinophils between the mucous membrane and muscularis mucosae, severe degeneration/scaling of the villi and very reactive lymphoid aggregates. The findings in the large intestine revealed a discrete infiltrate of mononuclear cells between the mucous membrane and the muscularis mucosae. In the lungs, we observed the presence of hemoglobin pigments, emphysema, thickening of the septa with congestion and multifocal infiltrate of mononuclear cells (Figure 2) and hepatization and alveolar serous effusion. In the spleen, intense reactivity of the white pulp and congestion were present, whereas in the liver, there was vacuolation characterized by steatosis and hydropic degeneration associated with inflammatory infiltrate that was characterized by neutrophils in the portal and focal centrilobular region.
Histopathological alterations in the organs of dogs experimentally infected with Neospora caninum. a) Parenchymal consolidation associated with congestion and infiltrate inflammatory in the pulmonary interstitium on the 3rd DAI (→, 400X); b) Small intestine with moderate infiltrate characterized by mononuclear cells and eosinophils located in the submucosa and the muscularis mucosae on the 6th DAI (→, 400x), H&E.
In the IHC assay, immunoreactivity was observed in the lungs, duodenum and jejunum on the 3rd DAI; in the lungs and spleens on the 4th and 5th DAI; in the cerebellum, lungs, spleen, jejunum, tonsils, and mesenteric, submandibular and popliteal lymph nodes on the 6th DAI (Table 1, Figure 3). Parasite DNA was not amplified by PCR in the samples used in this analysis. No oocysts or N. caninum DNA were identified in the stool samples analyzed, and no dogs seroconverted to positive during the experiment. Real-time PCR was the most sensitive technique used to detect the protozoa in tissues, and identified them in 41% (38/92) of the samples analyzed. The technique detected N. caninum in all of the euthanized animals. The heart and pancreas were 100% positive in every animal (Table 1). Finally, we observed immunoreactivity of the parasite in indirect immunofluorescence of the liver, lung, spleen, stomach, duodenum and pancreas tissue on the 3rd DAI; lung, spleen, mesenteric and popliteal lymph node tissue on the 4th DAI; heart, kidney and spleen tissue on the 5th DAI; and lung, spleen, pancreas, jejunum and colon tissue on the 6th DAI. With the exception of proliferative forms, no merogonic or gametogonic forms of N. caninum were observed in the epithelial cells of the intestinal mucous membrane.
Immunohistochemical markings in organs of dogs experimentally infected with Neospora caninum. a) Pulmonary interstitium on the 3rd DAI (→, 1000X); b) Small intestine in the muscularis mucosae on the 6th DAI (→, 1000X).
Discussion
The lack of clinical signs in infected dogs demonstrates that the
infection tends to take a subclinical course even when there are macroscopic and
microscopic alterations induced by the parasite. Dijkstra et al. (2001)Dijkstra T, Eysker M, Schares G, Conraths FJ, Wouda W, Barkema HW.
Dogs shed Neospora caninum oocysts after ingestion of naturally infected bovine
placenta but not after ingestion of colostrum spiked with Neospora caninum
tachyzoites. Int J Parasitol 2001; 31(8): 747-752.
http://dx.doi.org/10.1016/S0020-7519(01)00230-2
http://dx.doi.org/10.1016/S0020-7519(01)...
and Cedillo et
al. (2008)Cedillo CJR, Martínez MJJ, Santacruz AM, Banda RVM, Morales SE.
Models for experimental infection of dogs fed with tissue from fetuses and
neonatal cattle naturally infected with Neospora caninum. Vet Parasitol 2008;
154(1-2): 151-155. PMid:18395346.
http://dx.doi.org/10.1016/j.vetpar.2008.02.025
http://dx.doi.org/10.1016/j.vetpar.2008....
did not observe any clinical signs or lesions during necropsy
or histopathology associated with the infection in dogs fed milk containing
tachyzoites or in naturally infected placenta and fetuses, respectively. It is
probable that the post-infection period used in these studies to euthanize the dogs
may have influenced the findings because in the present study, the animals were
euthanized in the first week of infection, which characterizes its acute phase.
Furthermore, Dijkstra et al. (2001)Dijkstra T, Eysker M, Schares G, Conraths FJ, Wouda W, Barkema HW.
Dogs shed Neospora caninum oocysts after ingestion of naturally infected bovine
placenta but not after ingestion of colostrum spiked with Neospora caninum
tachyzoites. Int J Parasitol 2001; 31(8): 747-752.
http://dx.doi.org/10.1016/S0020-7519(01)00230-2
http://dx.doi.org/10.1016/S0020-7519(01)...
believe
that the lack of evidence of infection in dogs fed milk and tachyzoites may have
occurred due to their elimination by stomach pepsin and hydrochloric acid (DUBEY et al., 1998Dubey JP, Lindsay DS, Speer CA. Structures of Toxoplasma gondii
tachyzoites, bradyzoites, and sporozoites and biology of tissue cysts. Clin
Microbiol Rev 1998; 11(2): 267-299. PMid:9564564
PMCid:PMC106833.), a possibility that was
controlled for in the present study by prior ingestion of bicarbonate by the dogs,
as recommended by Furuta et al. (2007).
The PCR results demonstrated that there was insufficient sensitivity to
identify the parasite in dog tissue during the infection's acute phase, even in
tissues with macroscopic and histopathological alterations. Such results may be, in
part, due to the irregular distribution of the parasite (DE MAREZ et al., 1999), low
parasitism (DIJKSTRA et al., 2001Dijkstra T, Eysker M, Schares G, Conraths FJ, Wouda W, Barkema HW.
Dogs shed Neospora caninum oocysts after ingestion of naturally infected bovine
placenta but not after ingestion of colostrum spiked with Neospora caninum
tachyzoites. Int J Parasitol 2001; 31(8): 747-752.
http://dx.doi.org/10.1016/S0020-7519(01)00230-2
http://dx.doi.org/10.1016/S0020-7519(01)...
) or the
location of the parasite in sporadic tissue areas (RODRIGUES et al., 2004Rodrigues AA, Gennari SM, Aguilar DM, Sreekumar C, Hill DE, Miska
KB, et al. Shedding of Neospora caninum oocysts by dogs fed tissues from
naturally infected water buffaloes (Bubalus bubalis) from Brazil. Vet Parasitol
2004; 124(3-4): 139-150. PMid:15381294.
http://dx.doi.org/10.1016/j.vetpar.2004.07.007
http://dx.doi.org/10.1016/j.vetpar.2004....
) that are associated with the quantity of tissue
used for DNA extraction.
Dijkstra et al. (2001)Dijkstra T, Eysker M, Schares G, Conraths FJ, Wouda W, Barkema HW.
Dogs shed Neospora caninum oocysts after ingestion of naturally infected bovine
placenta but not after ingestion of colostrum spiked with Neospora caninum
tachyzoites. Int J Parasitol 2001; 31(8): 747-752.
http://dx.doi.org/10.1016/S0020-7519(01)00230-2
http://dx.doi.org/10.1016/S0020-7519(01)...
suggest that a strict
location of the sexual forms of N. caninum in the intestine would
justify the lack of parasites observed in the tissues; however, as previously
mentioned, it is more likely that low parasitism and irregular distribution are the
best hypotheses because the use of other associated techniques, such as IHC,
indirect immunofluorescent staining of tissues and especially real-time PCR, made it
possible to identify the parasite in a number of tissue samples from animals in this
study. This migration was systemic - we identified the parasite in the brains,
cerebella, lungs, hearts, spleens and pancreases of virtually all animals - and
rapid, as the parasite's DNA was identified in the brain after 72 hours with
the use of real-time PCR.
The elimination of oocysts in dogs fed chicken chorioallantoic membranes
was observed by Furuta et al. (2007). The main advantage of this protocol is that
only the inoculation of N. caninum is guaranteed thus avoiding the
risk of mixed infections with related coccidia (CAVALCANTE et al., 2011Cavalcante GT, Monteiro RM, Soares RM, Nishi SM, Alves AF N°,
Esmerini PO, et al. Shedding of Neospora caninum oocysts by dogs fed different
tissues from naturally infected cattle. Vet Parasitol 2011; 179(1-3): 220-223.
PMid:21450407. http://dx.doi.org/10.1016/j.vetpar.2011.02.026
http://dx.doi.org/10.1016/j.vetpar.2011....
) and providing a good quantity of infected
tissue to the definitive host, enabling more elimination of oocysts (DIJKSTRA et al., 2001Dijkstra T, Eysker M, Schares G, Conraths FJ, Wouda W, Barkema HW.
Dogs shed Neospora caninum oocysts after ingestion of naturally infected bovine
placenta but not after ingestion of colostrum spiked with Neospora caninum
tachyzoites. Int J Parasitol 2001; 31(8): 747-752.
http://dx.doi.org/10.1016/S0020-7519(01)00230-2
http://dx.doi.org/10.1016/S0020-7519(01)...
; GONDIM et al., 2005Gondim LFP, McAllister MM, Gao L. Effects of host maturity and prior
exposure history on the production of Neospora caninum oocysts by dogs. Vet
Parasitol 2005; 134(1-2): 33-39. PMid:16029931.
http://dx.doi.org/10.1016/j.vetpar.2005.06.011
http://dx.doi.org/10.1016/j.vetpar.2005....
).
However, the lack of visualization of oocysts in stools (of both
euthanized animals and the control) and the lack of observation of merogonous and
gametogonous forms are similar to the results found by Cedillo et al. (2008)Cedillo CJR, Martínez MJJ, Santacruz AM, Banda RVM, Morales SE.
Models for experimental infection of dogs fed with tissue from fetuses and
neonatal cattle naturally infected with Neospora caninum. Vet Parasitol 2008;
154(1-2): 151-155. PMid:18395346.
http://dx.doi.org/10.1016/j.vetpar.2008.02.025
http://dx.doi.org/10.1016/j.vetpar.2008....
. These authors associated the possibility
of autolysis or low parasitism with the lack of signs of infection, which differs
from the present study because once the membranes were collected, they were
immediately placed in PBS (pH 7.2), assessed for the viability of the infecting
forms by microscopy, and were then offered to the dogs. Stool PCR was performed with
the purpose of identifying DNA in dog feces in the event that there was little
elimination of the oocysts (less than one oocyst per gram of feces), which was
therefore not detectable by microscopy.
One hypothesis for the lack of seroconversion is the ingestion of a low
parasitic load (CAVALCANTE et al., 2011Cavalcante GT, Monteiro RM, Soares RM, Nishi SM, Alves AF N°,
Esmerini PO, et al. Shedding of Neospora caninum oocysts by dogs fed different
tissues from naturally infected cattle. Vet Parasitol 2011; 179(1-3): 220-223.
PMid:21450407. http://dx.doi.org/10.1016/j.vetpar.2011.02.026
http://dx.doi.org/10.1016/j.vetpar.2011....
).
However, it is likely that the parasite stimulates cell immunity, especially in the
intestine because the reinfection of dogs with tissues containing cysts results in a
lack or reduced number of eliminated oocysts (DIJKSTRA et al., 2001Dijkstra T, Eysker M, Schares G, Conraths FJ, Wouda W, Barkema HW.
Dogs shed Neospora caninum oocysts after ingestion of naturally infected bovine
placenta but not after ingestion of colostrum spiked with Neospora caninum
tachyzoites. Int J Parasitol 2001; 31(8): 747-752.
http://dx.doi.org/10.1016/S0020-7519(01)00230-2
http://dx.doi.org/10.1016/S0020-7519(01)...
, GONDIM et al.,
2005Gondim LFP, McAllister MM, Gao L. Effects of host maturity and prior
exposure history on the production of Neospora caninum oocysts by dogs. Vet
Parasitol 2005; 134(1-2): 33-39. PMid:16029931.
http://dx.doi.org/10.1016/j.vetpar.2005.06.011
http://dx.doi.org/10.1016/j.vetpar.2005....
). This is supported by our study because the uneuthanized dog that
did not seroconvert, as has been observed by Furuta et al. (2007) and Dijkstra et al. (2001)Dijkstra T, Eysker M, Schares G, Conraths FJ, Wouda W, Barkema HW.
Dogs shed Neospora caninum oocysts after ingestion of naturally infected bovine
placenta but not after ingestion of colostrum spiked with Neospora caninum
tachyzoites. Int J Parasitol 2001; 31(8): 747-752.
http://dx.doi.org/10.1016/S0020-7519(01)00230-2
http://dx.doi.org/10.1016/S0020-7519(01)...
.
Our results, therefore, indicate that an experimental model using previously infected chorioallantoic membranes may not be ideal for the production of oocysts or to visualize merogonous and gametogonous forms; however, this model did result in the rapid dissemination of the parasite resulting in important tissue alterations, showing it to be a useful model for the study of the host-parasite relationship during the infection's acute phase. Furthermore, this work demonstrated that in situations where there is no visualization of cysts and a likely low parasitic load, there is need for other diagnostic techniques used in conjunction with PCR or, if possible, other more sensitive methods such as real-time PCR, to minimize the possibility of false negative results.
To Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for funding the Project and to Programa Nacional de Cooperação Acadêmica - Novas Fronteiras n° 1512/2007-NF/CAPES) for the fellowship.
References
- Bandini LA, Alves AF N°, Pena HF, Cavalcante GT, Schares G, Nishi SM, et al. Experimental infection of dogs (Canis familiaris) with sporulated oocysts of Neospora caninum. Vet Parasitol 2011; 176(2-3): 151-156. PMid:21094584. http://dx.doi.org/10.1016/j.vetpar.2010.10.047
» http://dx.doi.org/10.1016/j.vetpar.2010.10.047 - Bjerkas I, Mohn SF, Presthus J. Unidentified cyst-forming sporozoon causing encephalomyelitis and myositis in dogs. Z Parasitenkd 1984; 70(2): 271-274. PMid:6426185. http://dx.doi.org/10.1007/BF00942230
» http://dx.doi.org/10.1007/BF00942230 - Cavalcante GT, Monteiro RM, Soares RM, Nishi SM, Alves AF N°, Esmerini PO, et al. Shedding of Neospora caninum oocysts by dogs fed different tissues from naturally infected cattle. Vet Parasitol 2011; 179(1-3): 220-223. PMid:21450407. http://dx.doi.org/10.1016/j.vetpar.2011.02.026
» http://dx.doi.org/10.1016/j.vetpar.2011.02.026 - Cedillo CJR, Martínez MJJ, Santacruz AM, Banda RVM, Morales SE. Models for experimental infection of dogs fed with tissue from fetuses and neonatal cattle naturally infected with Neospora caninum. Vet Parasitol 2008; 154(1-2): 151-155. PMid:18395346. http://dx.doi.org/10.1016/j.vetpar.2008.02.025
» http://dx.doi.org/10.1016/j.vetpar.2008.02.025 - De Marez T, Liddell S, Dubey JP, Jenkins MC, Gasbarre L. Oral infection of calves with Neospora caninum oocysts from dogs: humoral and cellular immune responses. Int J Parasitol 1999; 29(10): 1647-1657. http://dx.doi.org/10.1016/S0020-7519(99)00154-X
» http://dx.doi.org/10.1016/S0020-7519(99)00154-X - Dijkstra T, Eysker M, Schares G, Conraths FJ, Wouda W, Barkema HW. Dogs shed Neospora caninum oocysts after ingestion of naturally infected bovine placenta but not after ingestion of colostrum spiked with Neospora caninum tachyzoites. Int J Parasitol 2001; 31(8): 747-752. http://dx.doi.org/10.1016/S0020-7519(01)00230-2
» http://dx.doi.org/10.1016/S0020-7519(01)00230-2 - Dubey JP, Schares G. Neosporosis in animals - The last five years. Vet Parasitol 2011; 180(1-2) 90-108. PMid:21704458. http://dx.doi.org/10.1016/j.vetpar.2011.05.031
» http://dx.doi.org/10.1016/j.vetpar.2011.05.031 - Dubey JP, Carpenter JL, Speer CA, Topper MJ, Uggla A. Newly recognized fatal protozoan disease of dogs. J Am Vet Med Assoc 1988; 192(9): 1269-1285. PMid:3391851.
- Dubey JP, Lindsay DS, Speer CA. Structures of Toxoplasma gondii tachyzoites, bradyzoites, and sporozoites and biology of tissue cysts. Clin Microbiol Rev 1998; 11(2): 267-299. PMid:9564564 PMCid:PMC106833.
- Furuta PI, Mineo TWP, Carrasco AOT, Godoy GS, Pinto AA, Machado RZ. Neospora caninum infection in birds: experimental infections in chicken and embryonated eggs. Parasitology 2007; 134(pt.14): 1931-1939. PMid:17686190.
- Furuta PI. Infecção experimental em cães com ovos embrionados de galinha (Gallus gallus domesticus) infectados com taquizoítas de Neospora caninum. [Tese]. Jaboticabal: Universidade Estadual Paulista, Faculdade de Ciências Agrárias e Veterinárias; 2008.
- Gondim LFP, Gao L, McAllister MM. Improved production of Neospora caninum oocysts, cyclical oral transmission between dogs and cattle, and in vitro isolation from oocysts. J Parasitol 2002; 88(6): 1159-1163. PMid:12537111.
- Gondim LFP, McAllister MM, Gao L. Effects of host maturity and prior exposure history on the production of Neospora caninum oocysts by dogs. Vet Parasitol 2005; 134(1-2): 33-39. PMid:16029931. http://dx.doi.org/10.1016/j.vetpar.2005.06.011
» http://dx.doi.org/10.1016/j.vetpar.2005.06.011 - Munhoz AD, Jacintho APP, Machado RZ. Bovine abortion associated with Neospora caninum: Diagnosis and epidemiological aspects of a dairy cattle herd in the Northeast region of São Paulo State, Brazil. Braz J Vet Pathol 2011; 4(2): 112-116.
- Rodrigues AA, Gennari SM, Aguilar DM, Sreekumar C, Hill DE, Miska KB, et al. Shedding of Neospora caninum oocysts by dogs fed tissues from naturally infected water buffaloes (Bubalus bubalis) from Brazil. Vet Parasitol 2004; 124(3-4): 139-150. PMid:15381294. http://dx.doi.org/10.1016/j.vetpar.2004.07.007
» http://dx.doi.org/10.1016/j.vetpar.2004.07.007 - Sicupira PM, De Magalhães VCS, Galvão GS, Pereira JMS, Gondim LFP, Munhoz AD. Factors associated with infection by Neospora caninum in dogs in Brazil. Vet Parasitol 2012; 185(2-4): 305-308. PMid:22015062. http://dx.doi.org/10.1016/j.vetpar.2011.09.029
» http://dx.doi.org/10.1016/j.vetpar.2011.09.029 - Yamane I, Kokuho T, Shimura K, Eto M, Shibahara T, Haritani M, et al. In vitro isolation and characterisation of a bovine Neospora species in Japan. Res Vet Sci 1997; 63(1): 77-80. http://dx.doi.org/10.1016/S0034-5288(97)90162-4
» http://dx.doi.org/10.1016/S0034-5288(97)90162-4 - Yamage M, Flechtner O, Gottstein B. Neospora caninum: specific oligonucleotide primers for the detection of brain "cyst" DNA of experimentally infected nude mice by the polymerase chain reaction (PCR). J Parasitol 1996; 82(2): 272-279. PMid:8604096. http://dx.doi.org/10.2307/3284160
» http://dx.doi.org/10.2307/3284160
Publication Dates
-
Publication in this collection
Oct-Dec 2013
History
-
Received
9 July 2013 -
Accepted
1 Nov 2013