Abstracts
This paper describes the frequency and number of Sphyrion laevigatum in the skin of Genypterus blacodes, an important economic resource in Chile. The analysis of a spatial distribution model indicated that the parasites tended to cluster. Variations in the number of parasites per host could be described by a negative binomial distribution. The maximum number of parasites observed per host was two.
Sphyrion laevigatum ; Genypterus blacodes ; negative binomial distribution
Este artigo descreve a frequência e o número de Sphyrion laevigatum da pele de Genypterus blacodes, o qual é um importante recurso econômico no Chile. A análise baseada em modelos de distribuição espacial, demonstrou que os parasitos tendem a ficar agrupados. A variação numérica de parasitas por hospedeiro pode ser descrita por distribuição binomial negativa. O número máximo observado de parasitas por hospedeiro foi dois.
Sphyrion laevigatum ; Genypterus blacodes ; distribuição binomial negativa
Within the order Ophidiiformes, the genus Genypterus Philippi
1857 contains those species that are most economically important. Three of these species
are found in Chilean waters: Genypterus chilensis (Guichenot, 1881),
Genypterus maculatus (Tschudi, 1846) and Genypterus
blacodes (Forster, 1801), the latter being the most economically
significant of the three (CANALES-AGUIRRE et al.,
2010Canales-Aguirre CB, Ferrada S, Hernandez CE, Galleguillos R.
Population structure and demographic history of Genypterus blacodes using
microsatellite loci. Fish Res 2010; 106: 102-106.
http://dx.doi.org/10.1016/j.fishres.2010.06.010
http://dx.doi.org/10.1016/j.fishres.2010...
). Genypterus blacodes are elongate ell-like fish that
are confined to the continental shelf and slope of the southern hemisphere, including
the coasts of Australia, New Zealand, Argentina, Uruguay and Chile (CANALES-AGUIRRE et al., 2010Canales-Aguirre CB, Ferrada S, Hernandez CE, Galleguillos R.
Population structure and demographic history of Genypterus blacodes using
microsatellite loci. Fish Res 2010; 106: 102-106.
http://dx.doi.org/10.1016/j.fishres.2010.06.010
http://dx.doi.org/10.1016/j.fishres.2010...
; CORDO, 2001Cordo H. Estandarización del esfuerzo de pesca ejercido sobre el
Abadejo (Genypterus blacodes) en aguas Argentinas. Periodo 1986-1996. Rev Invest
Desarr Pesq 2001; 14: 57-78.; FRANCIS et al.,
2002Francis MP, Hurst RJ, McArdle BH, Bagley NW, Anderson OF. New
Zealand demersal fish assemblages. Environ Biol Fish 2002; 65: 215-234.
http://dx.doi.org/10.1023/A:1020046713411
http://dx.doi.org/10.1023/A:102004671341...
; RIFFO, 1994Riffo R. Composición taxonómica y característica cuantitativas de la
fauna de parásitos metazoos del congrio dorado Genypterus blacodes Schneider,
1801. Med Amb 1994; 12: 27-31.; WARD; REILLY, 2001Ward RD, Reilly A. Development of microsatellite loci for population
studies of the pink ling, Genypterus blacodes (Teleostei: Ophidiidae). Mol Ecol
Notes 2001; 1: 173-175.
http://dx.doi.org/10.1046/j.1471-8278.2001.00066.x
http://dx.doi.org/10.1046/j.1471-8278.20...
). G. blacodes
is a tertiary predator characterized by a diet that is dominated by demersal and benthic
fish (NYEGAARD et al., 2004Nyegaard M, Arkhipkin A, Brickle P. Variation in the diet of
Genypterus blacodes (Ophidiidae) around the Falkland Islands. J Fish Biol 2004;
65: 666-682. http://dx.doi.org/10.1111/j.0022-1112.2004.00476.x
http://dx.doi.org/10.1111/j.0022-1112.20...
; RENZI, 1986Renzi MA. Aspectos Biologico-pesqueros del Abadejo (Genypterus
blacodes). Rev Invest Desarr Pesq 1986; 6: 5-19.).
The genus Sphyrion is comprised of three species, Sphyrion laevigatum (Quoy & Gaimard, 1824), Sphyrion lumpi (Krøyer, 1845) and Sphyrion quadricornis Gaevskaya & Kovaleva, 1984 (HO, 1992Ho JS. Does Sphyrion (Kroyer) (Copepoda: Sphyriidae) occur in the Sea of Japan? With a discussion on the origin and dispersal of Sphyrion Cuvier, 1830. Rep Sado Mar Biol Stat, Niigata Univ 1992; 22: 37-48.; WALTER; BOXSHALL, 2012). S. lumpi is a common parasite of deep water pelagic fish in the Atlantic Ocean, New Zealand, and the Beaufort Sea (GORDON, 2009Gordon D. New Zealand Inventory of Biodiversity. Christchurch: Canterbury University Press; 2009.; HO; KIM, 1989Ho JS, Kim IH. Lophoura (Copepoda: Sphyriidae) parasitic on the rattails (Pisces: Macrouridae) in the Pacific, with note on Sphyrion lumpi from the Sea of Japan. Publ Seto Mar Biol Lab 1989; 34: 37-54.; MILLER, 2012Miller R. The museum collection database, Fisheries and Oceans Canada digital collections. Quebec: Maurice Lamontagne Institute; 2012.; WOO, 2006Woo PT. Fish Diseases and Disorders, Volume 1: Protozoan and Metazoan Infections. Cambridge: CABI Publishing; 2006.). Other studies have investigated the ecology of S. laevigatum among populations of G. blacodes in New Zealand, Australia, Chile, and Falkland Islands (BRICKLE et al., 2003), but in general, the ecological characteristics of parasitic copepods have been poorly investigated. To date, copepods have been found to be parasitic in less than 2% of aquatic invertebrates and less than 20% of fish (MORALES-SERNA; GOMEZ, 2012Morales-Serna FN, Gomez S. Generalidades de los copépodos parásitos de peces en aguas profundas y el caso de Lophoura brevicollum (Siphonostomatoida: Sphyriidae). In: Zamorano P, Hendrickx M, Caso M. Biodiversidad y comunidades del talud continental del Pacífico mexicano. Ciudad de México: Secretaría de Medio Ambiente y Recursos Naturales; 2012.).
Damage inflicted by Sphyrion spp. is mainly aesthetic, as the
parasites leave unsightly scars deep in the flesh of affected fish (PAINE, 1986Paine AIL. Observations on some conspicuous parasites of the South
African kingklip Genypterus capensis. S Afr J Mar Sci 1986; 4: 163-168.
http://dx.doi.org/10.2989/025776186784461846
http://dx.doi.org/10.2989/02577618678446...
). Holdfasts that are left behind
following parasite death cause abscesses 2 cm or more in length. In Germany, regulations
prohibit the sale of fillets that are more than 5% affected by the parasite for human
consumption, and therefore heavily infected fish are used for fish meal or pet food
(WOO, 2006Woo PT. Fish Diseases and Disorders, Volume 1: Protozoan and
Metazoan Infections. Cambridge: CABI Publishing; 2006.). The aim of this study is to
determine the frequency distribution of S. laevigatum on G.
blacodes using probabilistic models.
We studied two hundred and nine Genypterus blacodes samples obtained in June and July of 2012 from two marketplaces and one supermarket in Temuco, Chile. Parasites isolated from the skin of the fish were fixed in 96% ethyl alcohol and stored in properly labeled containers until analysis was performed at the School of Veterinary Medicine, Universidad Católica de Temuco, Chile.
The pattern of randomness in the distribution of the number of parasites per
host was investigated (ZAR, 1999Zar JH. Biostatistical analysis. New Yersey: Prentice Hall;
1999.). We used the
variance/mean ratio and the Morisita index to characterize the data as randomly
patterned, uniform or clustered (PEÑA-REHBEIN; DE LOS RIOS-ESCALANTE, 2012).
Furthermore we applied the Poisson distribution, the negative binomial distribution or
the binomial distribution according to the data pattern observed. We used a
χ
2 test to evaluate the fit of the data to the expected distribution (FERNANDES et al., 2003Fernandes MG, Busoli AC, Barbosa JC. Distribuição espacial de
Alabama argillacea (Hübner) (Lepidoptera: Noctuidae) em algodoeiro. Neotrop
Entomol 2003; 32: 107-115.
http://dx.doi.org/10.1590/S1519-566X2003000100016
http://dx.doi.org/10.1590/S1519-566X2003...
). All analyses were
performed with the XLSTAT 5.0 program (Addinsoft, New York, USA).
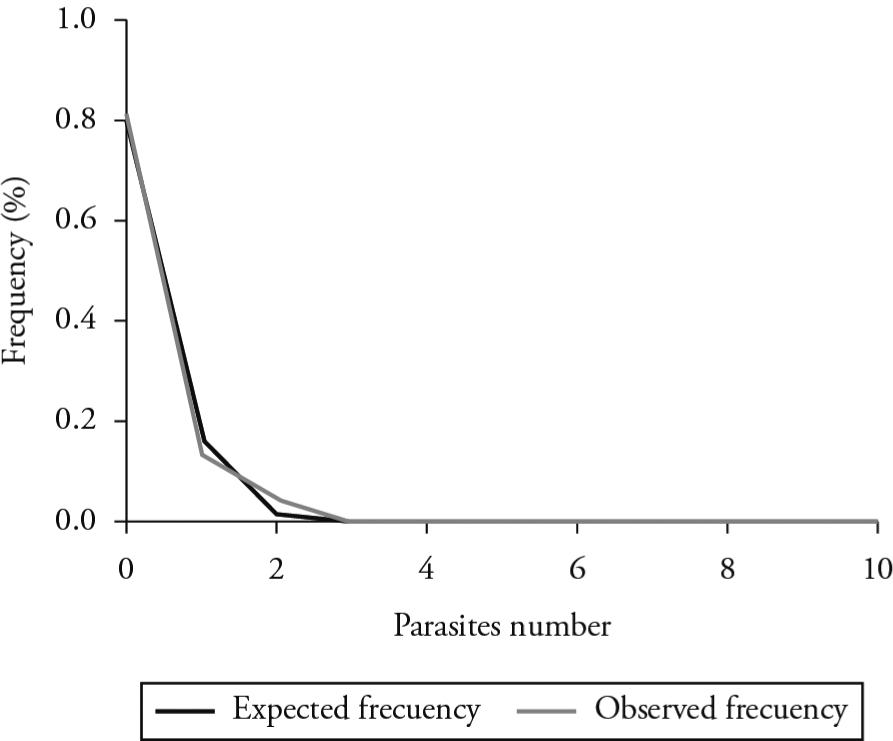
The values of the variance/mean ratio and the Morisita index were 1.16 and 105.72 respectively. These results showed that the data followed an overdispersed frequency distribution in their hosts. We therefore used the negative binomial distribution to model the data. The data fit this distribution (χ 2 Observed = 0.037 < χ 2 table = 28.336; p > 0.05). Many individuals were found not to contain S. laevigatum. The maximum observed number of parasites was two per host (Table 1, Figure 1).
Expected and observed frequencies for the negative binomial distribution model describing the presence of Sphyrion laevigatum in the skin of Genypterus blacodes obtained from the fish market of Temuco (Araucanía region, Chile).
Numbers of Sphyrion laevigatum parasites observed in the skin of Genypterus blacodes samples obtained from the fish market of Temuco (Araucanía region, Chile).
Our findings are similar to the results of Peña-Rehbein and De los
Rios-Escalante (2012) who studied the nematode Anisakis in
Thyrsites atun. Confirmation of a negative binomial distribution
describing the number of parasites per host suggests a robust model that permits an
informative interpretation of parasite distribution patterns (SHAW et al., 1998Shaw DJ, Grenfell BT, Dobson AP. Patterns of macroparasite
aggregation in wildlife host populations. Parasitology 1998; 117: 597-610.
http://dx.doi.org/10.1017/S0031182098003448
http://dx.doi.org/10.1017/S0031182098003...
).
This study was funded by the Veterinary Medicine School and Environmental Sciences School of the Universidad Católica de Temuco, Chile.
References
- Brickle P, Buxton NG, Villalon E. Infection of Sphyrion laevigatum (Copepoda: Sphyriidae) on Genypterus blacodes (Pisces: Ophidiidae) from the Falkland Islands, South Atlantic. J Parasitol 2003; 89: 242-244. http://dx.doi.org/10.1645/0022-3395(2003)089[0242:IOSLCS]2.0.CO;2
» http://dx.doi.org/10.1645/0022-3395(2003)089 - Canales-Aguirre CB, Ferrada S, Hernandez CE, Galleguillos R. Population structure and demographic history of Genypterus blacodes using microsatellite loci. Fish Res 2010; 106: 102-106. http://dx.doi.org/10.1016/j.fishres.2010.06.010
» http://dx.doi.org/10.1016/j.fishres.2010.06.010 - Cordo H. Estandarización del esfuerzo de pesca ejercido sobre el Abadejo (Genypterus blacodes) en aguas Argentinas. Periodo 1986-1996. Rev Invest Desarr Pesq 2001; 14: 57-78.
- Fernandes MG, Busoli AC, Barbosa JC. Distribuição espacial de Alabama argillacea (Hübner) (Lepidoptera: Noctuidae) em algodoeiro. Neotrop Entomol 2003; 32: 107-115. http://dx.doi.org/10.1590/S1519-566X2003000100016
» http://dx.doi.org/10.1590/S1519-566X2003000100016 - Francis MP, Hurst RJ, McArdle BH, Bagley NW, Anderson OF. New Zealand demersal fish assemblages. Environ Biol Fish 2002; 65: 215-234. http://dx.doi.org/10.1023/A:1020046713411
» http://dx.doi.org/10.1023/A:1020046713411 - Gordon D. New Zealand Inventory of Biodiversity. Christchurch: Canterbury University Press; 2009.
- Ho JS. Does Sphyrion (Kroyer) (Copepoda: Sphyriidae) occur in the Sea of Japan? With a discussion on the origin and dispersal of Sphyrion Cuvier, 1830. Rep Sado Mar Biol Stat, Niigata Univ 1992; 22: 37-48.
- Ho JS, Kim IH. Lophoura (Copepoda: Sphyriidae) parasitic on the rattails (Pisces: Macrouridae) in the Pacific, with note on Sphyrion lumpi from the Sea of Japan. Publ Seto Mar Biol Lab 1989; 34: 37-54.
- Miller R. The museum collection database, Fisheries and Oceans Canada digital collections. Quebec: Maurice Lamontagne Institute; 2012.
- Morales-Serna FN, Gomez S. Generalidades de los copépodos parásitos de peces en aguas profundas y el caso de Lophoura brevicollum (Siphonostomatoida: Sphyriidae). In: Zamorano P, Hendrickx M, Caso M. Biodiversidad y comunidades del talud continental del Pacífico mexicano. Ciudad de México: Secretaría de Medio Ambiente y Recursos Naturales; 2012.
- Nyegaard M, Arkhipkin A, Brickle P. Variation in the diet of Genypterus blacodes (Ophidiidae) around the Falkland Islands. J Fish Biol 2004; 65: 666-682. http://dx.doi.org/10.1111/j.0022-1112.2004.00476.x
» http://dx.doi.org/10.1111/j.0022-1112.2004.00476.x - Paine AIL. Observations on some conspicuous parasites of the South African kingklip Genypterus capensis. S Afr J Mar Sci 1986; 4: 163-168. http://dx.doi.org/10.2989/025776186784461846
» http://dx.doi.org/10.2989/025776186784461846 - Peña-Rehbein P, De los Rios-Escalante P. Use of negative binomial distribution to describe the presence of Anisakis in Thyrsites atun. Rev Bras Parasitol Vet 2012; 21: 78-80. http://dx.doi.org/10.1590/S1984-29612012000100017
» http://dx.doi.org/10.1590/S1984-29612012000100017 - Renzi MA. Aspectos Biologico-pesqueros del Abadejo (Genypterus blacodes). Rev Invest Desarr Pesq 1986; 6: 5-19.
- Riffo R. Composición taxonómica y característica cuantitativas de la fauna de parásitos metazoos del congrio dorado Genypterus blacodes Schneider, 1801. Med Amb 1994; 12: 27-31.
- Shaw DJ, Grenfell BT, Dobson AP. Patterns of macroparasite aggregation in wildlife host populations. Parasitology 1998; 117: 597-610. http://dx.doi.org/10.1017/S0031182098003448
» http://dx.doi.org/10.1017/S0031182098003448 - Walter TC, Boxshall G. Sphyrion Cuvier, 1830. World Copepoda database [online]. 2012. [cited 2013 Mar 31]. Available from http://www.marinespecies.org/aphia.php?p=taxdetails&id=135656
» http://www.marinespecies.org/aphia.php?p=taxdetails&id=135656 - Ward RD, Reilly A. Development of microsatellite loci for population studies of the pink ling, Genypterus blacodes (Teleostei: Ophidiidae). Mol Ecol Notes 2001; 1: 173-175. http://dx.doi.org/10.1046/j.1471-8278.2001.00066.x
» http://dx.doi.org/10.1046/j.1471-8278.2001.00066.x - Woo PT. Fish Diseases and Disorders, Volume 1: Protozoan and Metazoan Infections. Cambridge: CABI Publishing; 2006.
- Zar JH. Biostatistical analysis. New Yersey: Prentice Hall; 1999.
Publication Dates
-
Publication in this collection
Oct-Dec 2013
History
-
Received
19 Apr 2013 -
Accepted
1 Oct 2013