Abstracts
Ticks are rich sources of serine protease inhibitors, particularly those that prevent blood clotting and inflammatory responses during blood feeding. The tick Rhipicephalus (Boophlus) annulatusis an important ectoparasite of cattle. The aims of this study were to characterize and purify the serine protease inhibitors present in R. (B.) annulatus larval extract. The inhibitors were characterized by means of one and two-dimensional reverse zymography, and purified using affinity chromatography on a trypsin-Sepharose column. The analysis on one and two-dimensional reverse zymography of the larval extract showed trypsin inhibitory activity at between 13 and 40 kDa. Through non-reducing SDS-PAGE and reverse zymography for proteins purified by trypsin-Sepharose affinity chromatography, some protein bands with molecular weights between 13 and 34 kDa were detected. Western blotting showed that five protein bands at 48, 70, 110, 130 and 250 kDa reacted positively with immune serum, whereas there was no positive reaction in the range of 13-40 kDa. Serine protease inhibitors from R. (B.) annulatus have anti-trypsin activity similar to inhibitors belonging to several other hard tick species, thus suggesting that these proteins may be useful as targets in anti-tick vaccines.
Rhipicephalus (B.) annulatus ; serine protease inhibitors; zymography
Carrapatos são uma rica fonte de inibidores da serina protease, particularmente aqueles que previnem coagulação e respostas inflamatórias durante a alimentação com sangue. O carrapatoRhipicephalus (B.) annulatus é um ectoparasita importante de bovinos. O objetivo deste estudo foi caracterizar e purificar os inibidores da serina protease presentes no extrato de larva doR. (B.) annulatus. Os inibidores foram caracterizados através de zimografia reversa uni e bidimensional e purificados com cromatografia de afinidade em uma coluna de sepharose-tripsina. A análise do extrato de larva pela zimografia reversa uni e bidimensional mostrou atividade inibitória de tripsina entre 13 e 40 kDa. Através de SDS-PAGE e zimografia reversa para proteínas purificadas pela cromatografia por sepharose-tripsina, algumas bandas de proteínas com pesos moleculares entre 13 e 34 kDa foram detectadas. Western blotting mostrou que cinco bandas de proteínas a 48, 70, 110, 130 e 250 kDa reagiram positivamente com o soro imune, enquanto não houve reação positiva nas bandas 13-40 kDa. Inibidores da serina protease do R. (B.) annulatustêm atividade antitripsina semelhante àquelas dos inibidores de outras espécies de carrapatos duros, sugerindo, assim, que essas proteínas podem ser úteis como alvo de vacinas contra carrapatos.
Rhipicephalus (B.) annulatus ; inibidores da serina protease; zimografia
Introduction
Ticks are the most frequent ectoparasites that infest mammals, birds, reptiles and amphibians (SAUER et al., 1995Sauer JR, McSwain JL, Bowman AS, Essenberg RC. Tick salivary gland physiology. Annu Rev Entomol 1995; 40: 245-267. PMid:7810988. http://dx.doi.org/10.1146/annurev.en.40.010195.001333
http://dx.doi.org/10.1146/annurev.en.40....
). In Iran, Rhipicephalus annulatus(formerly Boophilus annulatus) is one of the most common ticks in cattle. This tick has also been recorded in some regions of Europe and the Americas, such as; Portugal, Spain, Italy, Greece, Turkey and Mexico; furthermore, it is common in northern Africa.
Ticks cause serious economic losses among cattle because of their blood-sucking activities and consequent transmission of some tick-borne diseases such as babesiosis and anaplasmosis (ESTRADA-PEÑA et al., 2004Estrada-Peña A, Bouattour A, Camicas JL, Walker AR. Ticks of domestic animals in the Mediterranean region. A guide to identification of species. Zaragoza: University of Zaragoza Press; 2004.). Therefore, controlling them has become a priority in tropical and subtropical regions. Tick control is mainly based on acaricides. Because of the disadvantages of acaricides, which including pollution of the environment and food chain and also acaricide resistance (PREVOT et al., 2007Prevot PP, Couvreur B, Denis V, Brossard M, Vanhamme L, Godfroid E. Protective immunity against Ixodes ricinus induced by a salivary serpin. Vaccine 2007; 25(17): 3284-3292. PMid:17270322. http://dx.doi.org/10.1016/j.vaccine.2007.01.008
http://dx.doi.org/10.1016/j.vaccine.2007...
), alternative control methods such as vaccination are promising. Development of vaccines relies on obtaining more knowledge about tick proteins. Proteases and their inhibitors may be candidates for designing vaccines.
Ticks' success in blood sucking is due to the anti-hemostatic and anti-inflammatory substances present in tick saliva (AROCHA-PIÑANGO et al., 1999Arocha-Piñango CL, Marchi R, Carvajal Z, Guerrero B. Invertebrate compounds acting on the hemostatic mechanism. Blood Coagul Fibrinolysis 1999; 10(2): 43-68. http://dx.doi.org/10.1097/00001721-199903000-00001
http://dx.doi.org/10.1097/00001721-19990...
). These insects are rich source of different serine protease inhibitors, which are distributed in tick tissues over the course of development of ticks' life cycle (VERMEULEN et al., 1988Vermeulen NM, Viljoen GJ, Bezuidenhout JD, Visser L, Neitz AW. Kinetic properties of toxic protease inhibitors isolated from tick eggs. Int J Biochem 1988; 20(6): 621-631. http://dx.doi.org/10.1016/0020-711X(88)90102-4
http://dx.doi.org/10.1016/0020-711X(88)9...
). Proteinase inhibitors have long been recognized in eggs and larvae of R. (B.) microplus, and have been seen to inhibit trypsin, chymotrypsin, elastase and plasma kallikrein (TANAKA et al., 1999Tanaka AS, Andreotti R, Gomes A, Torquato RJ, Sampaio MU, Sampaio CA. A double headed serine proteinase inhibitor--human plasma kallikrein and elastase inhibitor-- from Boophilus microplus larvae. Immunopharmacology 1999; 45(1-3): 171-177. http://dx.doi.org/10.1016/S0162-3109(99)00074-0
http://dx.doi.org/10.1016/S0162-3109(99)...
). Interestingly, some inhibitors have been shown to prolong the activated partial thromboplastin time (APTT) and prothrombin time (PT) for bovine blood (IBRAHIM et al., 2001Ibrahim MA, Ghazy AH, Maharem T, Khalil M. Isolation and properties of two forms of thrombin inhibitor from the nymphs of the camel tick Hyalomma dromedarii (Acari: Ixodidae). Exp Appl Acarol 2001; 25(8): 675-698. PMid:12171275. http://dx.doi.org/10.1023/A:1016136207308
http://dx.doi.org/10.1023/A:101613620730...
). These inhibitors, which are present in different ticks' tissues, can also protect these arthropods from infection by different pathogens (KANOST, 1999Kanost MR. Serine proteinase inhibitors in arthropod immunity. Dev Comp Immunol 1999; 23(4-5): 291-301. http://dx.doi.org/10.1016/S0145-305X(99)00012-9
http://dx.doi.org/10.1016/S0145-305X(99)...
).
In this study, serine protease inhibitors in unfed larvae of R. (B.) annulatus were characterized by means of one and two- dimensional reverse zymography and purified using trypsin-Sepharose affinity chromatography.
Materials and Methods
Ticks
The R. (B.) annulatus ticks were handled as described by Brown et al. (1984)Brown SJ, Shapiro SZ, Askenase PW. Characterization of tick antigens inducing host immune resistance. I. Immunization of guinea pigs with Amblyomma americanum-derived salivary gland extracts and identification of an important salivary gland protein antigen with guinea pig anti-tick antibodies. J Immunol 1984; 133(6): 3319-3325. PMid:6491289.. The tick larvae were obtained by incubating engorged females at 85% relative humidity and 28 °C. Two healthy seronegative four-month-old Holstein calves were provided for tick infestation. Each calf was infested with about 10,000 R. (B.) annulatus larvae. The larvae from the engorged ticks were collected ten days after eclosion and stored at −20 °C.
Protein extraction
Tick larvae (0.1 g) were ground in a mortar on ice with 0.15 M PBS (pH 7.2). The homogenate was centrifuged at 10,000 g for 10 minutes at 4 °C and the supernatant was filtered and collected. The protein assay was performed as described by Bradford (1976)Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Ann Biochem 1976; 72(1-2): 248-254. http://dx.doi.org/10.1016/0003-2697(76)90527-3
http://dx.doi.org/10.1016/0003-2697(76)9...
.
Casein agar plate assay
This assay was done as described by Yuan and Cole (1987)Yuan L, Cole GT. Isolation and characterization of an extracellular proteinase of Coccidioides immitis. Infect Immun 1987; 55(9): 1970-1978. PMid:3305358 PMCid:PMC260642., with some modifications: 0.5 g of agar in 46 ml of 0.5 M Tris buffer (pH 6.5) was boiled and then cooled to approximately 50 °C, and 4 ml of casein (0.5% in 1.0 M NaOH) was added to it. This medium was divided between plates (15 ml) and allowed to harden. Some wells were then constructed in agar and were filled with 50 µl of a mixture of eggs and unfed tick larval extract and trypsin (200 µg/ml). The plates were incubated overnight at room temperature. Bright zones appeared around the wells after adding 3 ml of acetic acid, thereby indicating the proteolytic activity.
Serine protease inhibitor purification using affinity chromatography
The trypsin-Sepharose column was prepared as specified by Hudson and Hay (1989)Hudson L, Hay FC. Practical Immunology. 3. Oxford: Blackwell Scientific Publications; 1989., with some modifications. Sepharose (7 ml) was mixed with cyanogen bromide (250 mg in 2 ml of distilled water) and the pH was adjusted to 11 using NaOH (0.2 M) for 10 min. The Sepharose was then washed with 0.1 M borate saline buffer (pH 8.3). Trypsin (50 mg in 10 ml of borate saline buffer) was added to the activated Sepharose and was kept at 4 °C overnight, then washed with PBS (0.15 M, pH 7.2). The trypsin-Sepharose was mixed with glycine (1 M, pH 8.0) for one hour and was washed with 40 ml of PBS. Then, the trypsin-Sepharose was poured into the column and larval protein (30 mg) was applied to the column, with incubation at room temperature for 45 minutes, followed by washing with PBS. The inhibitors that had bonded were eluted with 0.1 M glycine buffer (pH 2.5) containing 4 M urea and were immediately neutralized with 2 M Tris-HCl buffer (pH 8). The fractions were pooled and concentrated using centrifugal filter tubes (Eppendorf) with a molecular cutoff of 4 kDa.
SDS-PAGE and western blotting
Electrophoresis on 12% SDS polyacrylamide gel in the absence of 2-mercaptoethanol (2-ME) was carried out as described by Laemmli (1970)Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227(5259): 680-685. PMid:5432063. http://dx.doi.org/10.1038/227680a0
http://dx.doi.org/10.1038/227680a0...
, using 10 µg protein. The gels were stained with Coomassie blue and silver. Western blotting was performed as prescribed by Wang and Nuttall (1994). Gel containing resolved proteins was transferred electrophoretically to nitrocellulose membrane. The membrane was blocked for 45 min, by means of PBS containing 2.5% Tween 20, and all the washing steps were carried out with PBS containing 0.05% Tween 20 (3 × 5 min). The membrane was incubated in serum derived from calves that had been experimentally infested with R. (B.) annulatus larvae (1:50 in PBS containing 0.05% Tween 20) for 1 hour at room temperature. It was then washed and incubated with anti-bovine IgG coupled with horseradish peroxidase (1/1000 in PBS) for 30 min at room temperature. After washing, the color development step was performed with diaminobenzidine containing H2O2.
One-dimensional reverse zymography
Serine protease inhibitors were separated using polyacrylamide gel electrophoresis (gel contained 0.1% gelatin) and the Laemmli buffer system. 20 µl of samples containing 1000 µg of protein per ml were mixed with 20 µl of sample buffer without reducing reagents and loaded (10 µl). After electrophoresis, the gel slab was incubated in 0.1 M Tris-HCl buffer (pH 8.0) containing trypsin (10 µg/ml) for 2 h at room temperature. The gels were then stained with Coomassie blue R-250 (PRUETT et al., 2008Pruett JH, Olafson PU, Davey RB. Serologically defined Rhipicephalus (Boophilus) micropolus larval antigens in BmLF3, a partially pure Sephacryl S-300 fraction of crude larval proteins. Vet Parasitol 2008; 155(3-4): 264-272. PMid:18562121. http://dx.doi.org/10.1016/j.vetpar.2008.04.020
http://dx.doi.org/10.1016/j.vetpar.2008....
).
Two-dimensional reverse zymography
Isoelectric focusing (IEF) was performed as described byYatsuda et al. (2003)Yatsuda AP, Krijgsveld J, Cornelissen AW, Heck AJ, De Vries E. Comprehensive analysis of the secreted proteins of the parasite Haemonchus contortus reveals extensive sequence variation and differential immune recognition. J Biol Chem 2003; 278(19): 16941-16951. PMid:12576473. http://dx.doi.org/10.1074/jbc.M212453200
http://dx.doi.org/10.1074/jbc.M212453200...
, using 7-cm immobilized pH gradient (IPG) strips (non-linear pH range 3-10). Samples containing 300 µg of proteins were diluted in rehydration buffer (total volume 120 µl) and used to rehydrate the strips for 18 h at room temperature. IEF was performed in a Protean IEF cell (Bio Rad) under conditions recommended by the manufacturer. The IPG strips were then equilibrated for 20 min at room temperature in equilibration buffer (6 M urea, 30% glycerol, 2% SDS, 50 mM Tris; pH 8.8). The equilibrated IPG strips were transferred onto the gel, and SDS-PAGE for reverse zymography was carried out.
Results
Casein agar plate assay
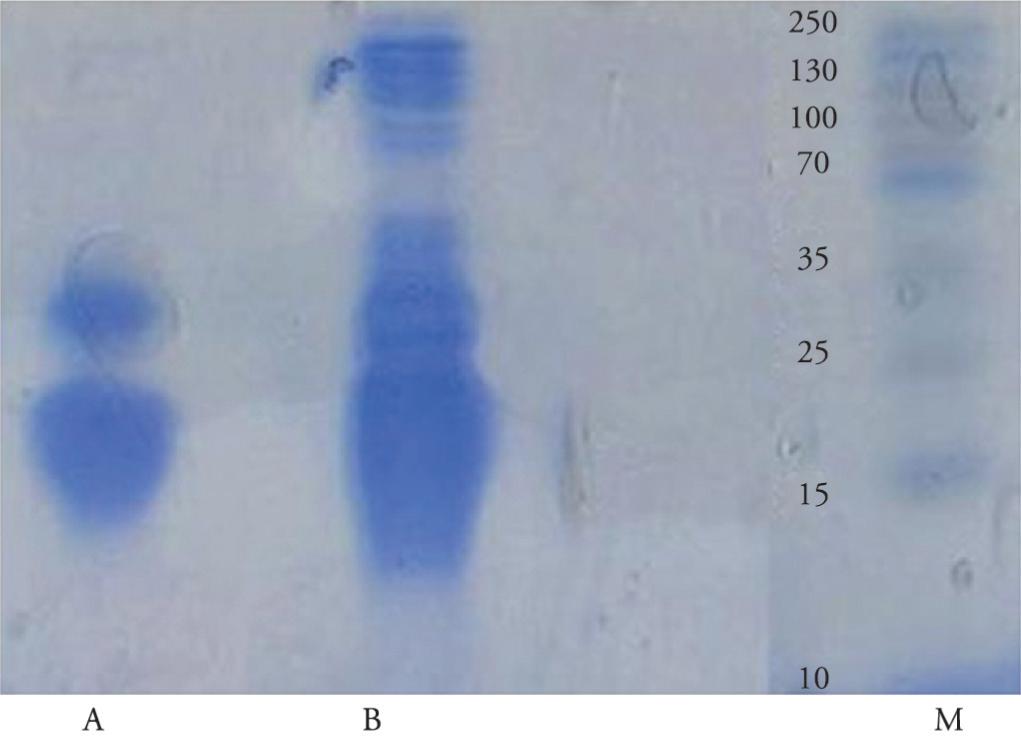
In order to confirm the presence of serine protease inhibitors, the casein agar plate assay method was applied. As is shown in Figure 1, the absence of bright zones around wells 1 and 2 in plate A after adding acetic acid indicated that there was inhibition of proteolytic activity due to presence of trypsin inhibitor in tick egg and larval extracts. The presence of a bright zone around well 2 in plate B was due to trypsin proteolytic activity.
Proteolytic inhibition activity on agar plates containing casein as substrate: well containing mixture of unfed tick larvae extract and trypsin (A-1); well containing mixture of tick egg extract and trypsin (A-2); well containing PBS as negative control (B-1); well containing trypsin as positive control (B-2).
SDS-PAGE
To profile the tick proteins, larval extract was resolved by means of non-reducing SDS-PAGE, and eight sharp bands with molecular weights between 40 and > 250 kDa were detected (Figure 2).
Non-reducing SDS-PAGE (12%) profile of unfedR. (B.) annulatus larval extract (Coomassie blue staining): tick larval extract (lane A); molecular weight standards (lane M).
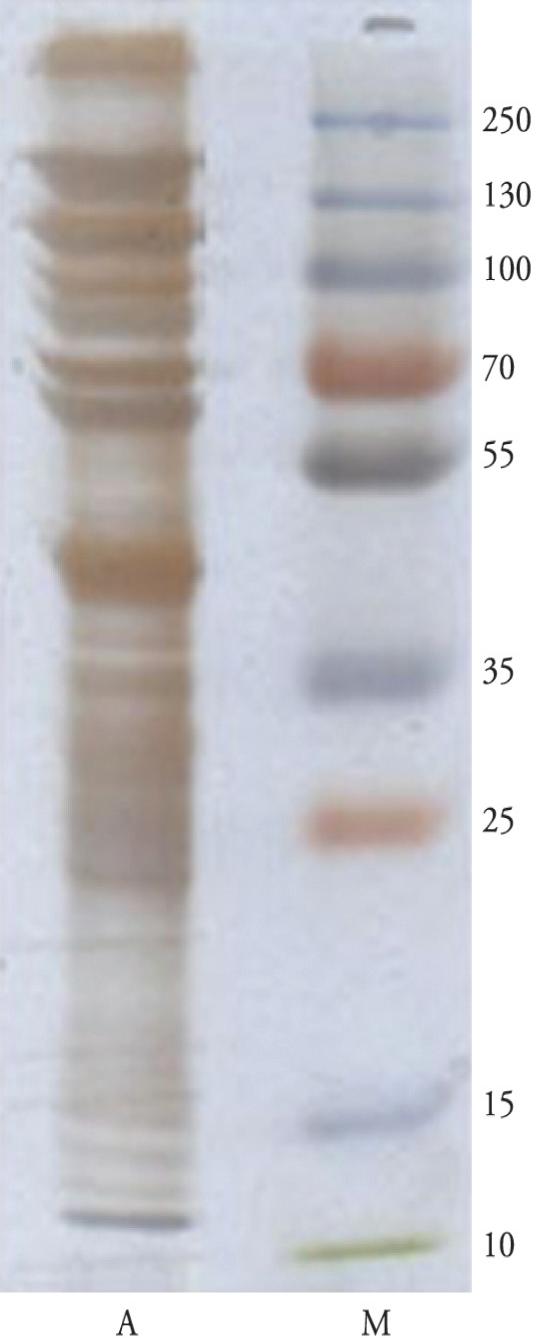
Through the non-reducing SDS-PAGE method for R. (B.) annulatus larval extract using silver staining, several bands with molecular weights between 12 and > 250 kDa were observed (Figure 3).
Non-reducing SDS-PAGE (12% gel) on R. (B.) annulatus larval extract, using silver staining: tick larval extract (lane A); molecular weight standards (lane M).
Western blotting
The serum immunoreactivity of the calves that had been experimentally infested with tick larvae was detected by means of western blotting. Five protein bands at 48, 70, 110, 130 and 250 kDa produced positive reactions with immune serum (Figure 4).
Unfed R. (B.) annulatus larval extract analyzed using western blotting: molecular weight standards (lane M); tick larval extract (lane A).
Reverse zymography
To detect serine protease inhibitors, reverse zymography of larval extracts was applied. Analysis on one-dimensional reverse zymography of larval extracts showed that there was trypsin inhibitory activity between 13 and 40 kDa. The blue zones with a bright background that were seen using Coomassie blue staining were indicative of trypsin inhibitory activity (Figure 5).
One-dimensional reverse zymography analysis on serine protease inhibitors of R. (B.) annulatus larval extracts: molecular weight standards (M). The gel was stained using Coomassie blue R 250.
Analysis on two-dimensional reverse zymography of larval extracts showed that was trypsin inhibitory activity mainly between 13 and 40 kDa, at different isoelectric pH values (3-10). The blue zones with a bright background that were seen using Coomassie blue staining were indicative of trypsin inhibitory activity (Figure 6).
Two-dimensional reverse zymography analysis on serine protease inhibitors of R. (B.) annulatus larval extracts: molecular weight standards (M). The gel was stained using Coomassie blue R 250.
Using non-reducing SDS-PAGE for proteins that were purified by means of trypsin-Sepharose affinity chromatography, some protein bands with molecular weights between 13 and 34 kDa were detected through Coomassie blue and silver staining (Figure 7).
SDS polyacrylamide gel electrophoresis (12%) onR. (B.) annulatus larvae serine protease inhibitors purified by means of trypsin-Sepharose: stained using Coomassie blue (A); stained using silver (B); molecular weight standards (M).
Reverse zymography for proteins that were purified by means of trypsin affinity chromatography showed some blue zones in a bright field, with molecular weights between 13 and 34 kDa, through Coomassie blue staining (Figure 8).
One-dimensional reverse zymography analysis on serine protease inhibitors of R. (B.) annulatus larval extracts purified by means of trypsin-Sepharose affinity chromatography (A) and whole larval extract (B); molecular weight standards (M). The gel was stained using Coomassie blue R250.
Discussion
R. (B.) annulatus is an important tick in meso-Mediterranean climates. In Iran, this tick is distributed in the northern provinces and has an important role in transmission of some pathogens. Novel control methods such as vaccination have been developed recently. Any new biochemical and physiological information about ticks may help in developing new vaccine. Tick larvae have been shown to be important sources of trypsin and chymotrypsin inhibitors (WILLADSEN; RIDING, 1979Willadsen P, Riding GA. Characterization of a proteolytic-enzyme inhibitor with allergenic activity. Multiple functions of a parasite-derived protein. Biochem J 1979; 177(1): 41-47. PMid:426780 PMCid:PMC1186338.; WILLADSEN; MCKENNA, 1983; TANAKA et al., 1999Tanaka AS, Andreotti R, Gomes A, Torquato RJ, Sampaio MU, Sampaio CA. A double headed serine proteinase inhibitor--human plasma kallikrein and elastase inhibitor-- from Boophilus microplus larvae. Immunopharmacology 1999; 45(1-3): 171-177. http://dx.doi.org/10.1016/S0162-3109(99)00074-0
http://dx.doi.org/10.1016/S0162-3109(99)...
; ANDREOTTI et al., 2001Andreotti R, Malavazi-Piza KC, Sasaki SD, Torquato RJ, Gomes A, Tanaka AS. Serine proteinase inhibitors from eggs and larvae of tick Boophilus microplus: purification and biochemical characterization. J Protein Chem 2001; 20(5): 337-343. PMid:11732684. http://dx.doi.org/10.1023/A:1012242817869
http://dx.doi.org/10.1023/A:101224281786...
). There are reports suggesting that serine protease inhibitors may be candidates for use in vaccines and that such vaccines would be effective (MULENG et al., 2001Muleng A, Sugino M, Nakajim M, Sugimoto C, Onuma M. Tick-Encoded serine proteinase inhibitors (serpins); potential target antigens for tick vaccine development. J Vet Med Sci 2001; 63(10): 1063-1069. PMid:11714020. http://dx.doi.org/10.1292/jvms.63.1063
http://dx.doi.org/10.1292/jvms.63.1063...
; IMAMURA et al., 2005Imamura S, Da Silva Vaz Junior I, Sugino M, Ohashi K, Onuma M. A serine protease inhibitor (serpin) from Haemaphysalis longicornis as an anti-tick vaccine. Vaccine 2005; 23(10): 1301-1311. PMid:15652673. http://dx.doi.org/10.1016/j.vaccine.2004.08.041
http://dx.doi.org/10.1016/j.vaccine.2004...
).The inhibitory activities of these proteins relating to blood clotting and inflammatory responses may facilitate ticks' blood sucking process.
In the present study, non-reducing SDS-PAGE was firstly performed onR. (B.) annulatus larval extract and eight sharp bands with molecular weights between 40 and > 250 kDa were obtained using Coomassie staining. Several bands with molecular weights between 12 and > 250 kDa were observed using silver staining. In the second step, we characterized and purified serine protease inhibitors from R. (B.) annulatus larval extracts. Through one and two-dimensional reverse zymography, larval extracts showed trypsin inhibitory activity mainly between 13 and 40 kDa, at different isoelectric pH values (3-10). The purified serine protease inhibitors presented molecular masses between 13 and 34 kDa.
Serine protease inhibitors are classified into at least 18 families based on the primary and three-dimensional structures and inhibition mechanisms (LASKOWSKI; QASIM, 2000Laskowski M, Qasim MA. What can the structures of enzyme-inhibitor complexes tell us about the structures of enzyme substrate complexes? Biochim Biophys Acta 2000; 1477(1-2): 324-337. http://dx.doi.org/10.1016/S0167-4838(99)00284-8
http://dx.doi.org/10.1016/S0167-4838(99)...
). Six of these families, comprising Kazal, BPTI-Kunitz, α-macroglobulin, serpin, pacifastin and Bombyx, are present in invertebrate hemolymph and also in saliva (KANOST, 1999Kanost MR. Serine proteinase inhibitors in arthropod immunity. Dev Comp Immunol 1999; 23(4-5): 291-301. http://dx.doi.org/10.1016/S0145-305X(99)00012-9
http://dx.doi.org/10.1016/S0145-305X(99)...
; PHAM et al., 1996Pham TN, Hayashi K, Takano R, Itoh M, Eguchi M, Shibata H, et al. A new family of serine protease inhibitors (Bombyx family) as established from the unique topological relation between the positions of disulphide bridges and reactive site. J Biochem 1996; 119(3): 428-434. PMid:8830035. http://dx.doi.org/10.1093/oxfordjournals.jbchem.a021259
http://dx.doi.org/10.1093/oxfordjournals...
; SIMONET et al., 2002Simonet G, Claeys I, Broeck JV. Structural and functional properties of a novel serine protease inhibiting peptide family in arthropods. Comp Biochem Physiol B Biochem Mol Biol 2002; 132(1): 247-255. http://dx.doi.org/10.1016/S1096-4959(01)00530-9
http://dx.doi.org/10.1016/S1096-4959(01)...
). Sasaki et al. (2004)Sasaki SD, Azzolini SS, Hirata IY, Andreotti R, Tanaka AS. Boophilus microplus tick larvae, a rich source of Kunitz type serine proteinase inhibitors. Biochimie 2004; 86(9-10): 643-649. PMid:15556274. http://dx.doi.org/10.1016/j.biochi.2004.09.010
http://dx.doi.org/10.1016/j.biochi.2004....
purified serine protease inhibitors with molecular weights between 6 and 18 kDa from R. (B.) micropluslarvae by means of trypsin-Sepharose affinity chromatography. All fractions presented trypsin inhibitory activity, and some of them also inhibited human plasma kallikrein and human neutrophil elastase. Based on amino acid sequence data, these authors classified them as members of the Kunitz-type serine proteinase inhibitor family. In addition, other results have shown that serine proteinase inhibitors fromR. sanguineus with molecular weights between 8 and 18 kDa have inhibitor activity similar to that of R. (B.) microplusinhibitors (SANT'ANNA AZZOLINI et al., 2003). The variations in the molecular weights of serine protease inhibitors in different tick species may be due to the presence of different genes encoding these proteins, which may contain variable numbers of Kunitz domains (CORRAL-RODRÍGUEZ et al., 2009Corral-Rodríguez MA, Macedo-Ribeiro S, Barbosa Pereira PJ, Fuentes-Prior P. Tick-derived Kunitz-type inhibitors as antihemostatic factors. Insect Biochem Mol Biol 2009; 39(9): 579-595. PMid:19631744. http://dx.doi.org/10.1016/j.ibmb.2009.07.003
http://dx.doi.org/10.1016/j.ibmb.2009.07...
). Alternatively, they may be due to proteolytic processing of large Kunitz-type inhibitors during larval development (SASAKI et al., 2004Sasaki SD, Azzolini SS, Hirata IY, Andreotti R, Tanaka AS. Boophilus microplus tick larvae, a rich source of Kunitz type serine proteinase inhibitors. Biochimie 2004; 86(9-10): 643-649. PMid:15556274. http://dx.doi.org/10.1016/j.biochi.2004.09.010
http://dx.doi.org/10.1016/j.biochi.2004....
).
An efficient immune response against serine proteinase inhibitors and neutralization of their enzymatic activity may influence blood feeding and tick survival (PREVOT et al., 2007Prevot PP, Couvreur B, Denis V, Brossard M, Vanhamme L, Godfroid E. Protective immunity against Ixodes ricinus induced by a salivary serpin. Vaccine 2007; 25(17): 3284-3292. PMid:17270322. http://dx.doi.org/10.1016/j.vaccine.2007.01.008
http://dx.doi.org/10.1016/j.vaccine.2007...
). Our western blot results suggested that the calves that had been experimentally infected with ticks did not produce antibodies against serine proteinase inhibitors (in the range of 13-40 kDa). This result was in agreement with Andreotti et al. (2001)Andreotti R, Malavazi-Piza KC, Sasaki SD, Torquato RJ, Gomes A, Tanaka AS. Serine proteinase inhibitors from eggs and larvae of tick Boophilus microplus: purification and biochemical characterization. J Protein Chem 2001; 20(5): 337-343. PMid:11732684. http://dx.doi.org/10.1023/A:1012242817869
http://dx.doi.org/10.1023/A:101224281786...
and Pruett et al. (2008)Pruett JH, Olafson PU, Davey RB. Serologically defined Rhipicephalus (Boophilus) micropolus larval antigens in BmLF3, a partially pure Sephacryl S-300 fraction of crude larval proteins. Vet Parasitol 2008; 155(3-4): 264-272. PMid:18562121. http://dx.doi.org/10.1016/j.vetpar.2008.04.020
http://dx.doi.org/10.1016/j.vetpar.2008....
. Lack of antibody induction against serine protease inhibitors in cattle during tick infestation may be due to the low amounts of these inhibitors introduced for immune response stimulation in the host. It may also be due to bovine unresponsiveness to them as a result of natural exposure via saliva, or due to antibody production at very low concentrations that are undetectable with the western blot method (PRUETT et al., 2008Pruett JH, Olafson PU, Davey RB. Serologically defined Rhipicephalus (Boophilus) micropolus larval antigens in BmLF3, a partially pure Sephacryl S-300 fraction of crude larval proteins. Vet Parasitol 2008; 155(3-4): 264-272. PMid:18562121. http://dx.doi.org/10.1016/j.vetpar.2008.04.020
http://dx.doi.org/10.1016/j.vetpar.2008....
).
Animals have been immunized using purified serine proteinase inhibitors from different tick tissues and larvae in several studies. Experimental challenges performed on animals previously immunized with tick larvae have shown that the animals acquired significant protective immunity against ticks, with reduction of the biological parameters that would favor ticks (ANDREOTTI et al., 2001Andreotti R, Malavazi-Piza KC, Sasaki SD, Torquato RJ, Gomes A, Tanaka AS. Serine proteinase inhibitors from eggs and larvae of tick Boophilus microplus: purification and biochemical characterization. J Protein Chem 2001; 20(5): 337-343. PMid:11732684. http://dx.doi.org/10.1023/A:1012242817869
http://dx.doi.org/10.1023/A:101224281786...
; PREVOT et al., 2007Prevot PP, Couvreur B, Denis V, Brossard M, Vanhamme L, Godfroid E. Protective immunity against Ixodes ricinus induced by a salivary serpin. Vaccine 2007; 25(17): 3284-3292. PMid:17270322. http://dx.doi.org/10.1016/j.vaccine.2007.01.008
http://dx.doi.org/10.1016/j.vaccine.2007...
).
Development of new vaccines relies on characterization of tick proteins. Serine protease inhibitors have fundamental physiological roles in ticks and play an important role in tick-host interactions. Therefore, these proteins can be considered to be potential candidates for use in vaccines. It seems that further studies are needed in order to characterize and identify tick larval proteins.
This study was supported by a grant from Tehran University.
References
- Andreotti R, Malavazi-Piza KC, Sasaki SD, Torquato RJ, Gomes A, Tanaka AS. Serine proteinase inhibitors from eggs and larvae of tick Boophilus microplus: purification and biochemical characterization. J Protein Chem 2001; 20(5): 337-343. PMid:11732684. http://dx.doi.org/10.1023/A:1012242817869
» http://dx.doi.org/10.1023/A:1012242817869 - Arocha-Piñango CL, Marchi R, Carvajal Z, Guerrero B. Invertebrate compounds acting on the hemostatic mechanism. Blood Coagul Fibrinolysis 1999; 10(2): 43-68. http://dx.doi.org/10.1097/00001721-199903000-00001
» http://dx.doi.org/10.1097/00001721-199903000-00001 - Bradford MM. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Ann Biochem 1976; 72(1-2): 248-254. http://dx.doi.org/10.1016/0003-2697(76)90527-3
» http://dx.doi.org/10.1016/0003-2697(76)90527-3 - Brown SJ, Shapiro SZ, Askenase PW. Characterization of tick antigens inducing host immune resistance. I. Immunization of guinea pigs with Amblyomma americanum-derived salivary gland extracts and identification of an important salivary gland protein antigen with guinea pig anti-tick antibodies. J Immunol 1984; 133(6): 3319-3325. PMid:6491289.
- Corral-Rodríguez MA, Macedo-Ribeiro S, Barbosa Pereira PJ, Fuentes-Prior P. Tick-derived Kunitz-type inhibitors as antihemostatic factors. Insect Biochem Mol Biol 2009; 39(9): 579-595. PMid:19631744. http://dx.doi.org/10.1016/j.ibmb.2009.07.003
» http://dx.doi.org/10.1016/j.ibmb.2009.07.003 - Estrada-Peña A, Bouattour A, Camicas JL, Walker AR. Ticks of domestic animals in the Mediterranean region. A guide to identification of species. Zaragoza: University of Zaragoza Press; 2004.
- Hudson L, Hay FC. Practical Immunology. 3. Oxford: Blackwell Scientific Publications; 1989.
- Ibrahim MA, Ghazy AH, Maharem T, Khalil M. Isolation and properties of two forms of thrombin inhibitor from the nymphs of the camel tick Hyalomma dromedarii (Acari: Ixodidae). Exp Appl Acarol 2001; 25(8): 675-698. PMid:12171275. http://dx.doi.org/10.1023/A:1016136207308
» http://dx.doi.org/10.1023/A:1016136207308 - Imamura S, Da Silva Vaz Junior I, Sugino M, Ohashi K, Onuma M. A serine protease inhibitor (serpin) from Haemaphysalis longicornis as an anti-tick vaccine. Vaccine 2005; 23(10): 1301-1311. PMid:15652673. http://dx.doi.org/10.1016/j.vaccine.2004.08.041
» http://dx.doi.org/10.1016/j.vaccine.2004.08.041 - Kanost MR. Serine proteinase inhibitors in arthropod immunity. Dev Comp Immunol 1999; 23(4-5): 291-301. http://dx.doi.org/10.1016/S0145-305X(99)00012-9
» http://dx.doi.org/10.1016/S0145-305X(99)00012-9 - Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970; 227(5259): 680-685. PMid:5432063. http://dx.doi.org/10.1038/227680a0
» http://dx.doi.org/10.1038/227680a0 - Laskowski M, Qasim MA. What can the structures of enzyme-inhibitor complexes tell us about the structures of enzyme substrate complexes? Biochim Biophys Acta 2000; 1477(1-2): 324-337. http://dx.doi.org/10.1016/S0167-4838(99)00284-8
» http://dx.doi.org/10.1016/S0167-4838(99)00284-8 - Muleng A, Sugino M, Nakajim M, Sugimoto C, Onuma M. Tick-Encoded serine proteinase inhibitors (serpins); potential target antigens for tick vaccine development. J Vet Med Sci 2001; 63(10): 1063-1069. PMid:11714020. http://dx.doi.org/10.1292/jvms.63.1063
» http://dx.doi.org/10.1292/jvms.63.1063 - Pham TN, Hayashi K, Takano R, Itoh M, Eguchi M, Shibata H, et al. A new family of serine protease inhibitors (Bombyx family) as established from the unique topological relation between the positions of disulphide bridges and reactive site. J Biochem 1996; 119(3): 428-434. PMid:8830035. http://dx.doi.org/10.1093/oxfordjournals.jbchem.a021259
» http://dx.doi.org/10.1093/oxfordjournals.jbchem.a021259 - Prevot PP, Couvreur B, Denis V, Brossard M, Vanhamme L, Godfroid E. Protective immunity against Ixodes ricinus induced by a salivary serpin. Vaccine 2007; 25(17): 3284-3292. PMid:17270322. http://dx.doi.org/10.1016/j.vaccine.2007.01.008
» http://dx.doi.org/10.1016/j.vaccine.2007.01.008 - Pruett JH, Olafson PU, Davey RB. Serologically defined Rhipicephalus (Boophilus) micropolus larval antigens in BmLF3, a partially pure Sephacryl S-300 fraction of crude larval proteins. Vet Parasitol 2008; 155(3-4): 264-272. PMid:18562121. http://dx.doi.org/10.1016/j.vetpar.2008.04.020
» http://dx.doi.org/10.1016/j.vetpar.2008.04.020 - Sant'Anna Azzolini S, Sasaki SD, Torquato RJ, Andreotti R, Andreotti E, Tanaka AS. Rhipicephalus sanguineus trypsin inhibitors present in the tick larvae: isolation, characterization, and partial primary structure determination. Arch Biochem Biophys 2003; 417(2): 176-182. http://dx.doi.org/10.1016/S0003-9861(03)00344-8
» http://dx.doi.org/10.1016/S0003-9861(03)00344-8 - Sasaki SD, Azzolini SS, Hirata IY, Andreotti R, Tanaka AS. Boophilus microplus tick larvae, a rich source of Kunitz type serine proteinase inhibitors. Biochimie 2004; 86(9-10): 643-649. PMid:15556274. http://dx.doi.org/10.1016/j.biochi.2004.09.010
» http://dx.doi.org/10.1016/j.biochi.2004.09.010 - Sauer JR, McSwain JL, Bowman AS, Essenberg RC. Tick salivary gland physiology. Annu Rev Entomol 1995; 40: 245-267. PMid:7810988. http://dx.doi.org/10.1146/annurev.en.40.010195.001333
» http://dx.doi.org/10.1146/annurev.en.40.010195.001333 - Simonet G, Claeys I, Broeck JV. Structural and functional properties of a novel serine protease inhibiting peptide family in arthropods. Comp Biochem Physiol B Biochem Mol Biol 2002; 132(1): 247-255. http://dx.doi.org/10.1016/S1096-4959(01)00530-9
» http://dx.doi.org/10.1016/S1096-4959(01)00530-9 - Tanaka AS, Andreotti R, Gomes A, Torquato RJ, Sampaio MU, Sampaio CA. A double headed serine proteinase inhibitor--human plasma kallikrein and elastase inhibitor-- from Boophilus microplus larvae. Immunopharmacology 1999; 45(1-3): 171-177. http://dx.doi.org/10.1016/S0162-3109(99)00074-0
» http://dx.doi.org/10.1016/S0162-3109(99)00074-0 - Vermeulen NM, Viljoen GJ, Bezuidenhout JD, Visser L, Neitz AW. Kinetic properties of toxic protease inhibitors isolated from tick eggs. Int J Biochem 1988; 20(6): 621-631. http://dx.doi.org/10.1016/0020-711X(88)90102-4
» http://dx.doi.org/10.1016/0020-711X(88)90102-4 - Wang H, Nuttall PA. Comparison of the proteins in salivary glands, saliva and haemolymph of Rhipicephalus appendiculatus female ticks during feeding. Parasitology 1994; 109(Pt 4): 517-523. PMid:7794318. http://dx.doi.org/10.1017/S003118200008077X
» http://dx.doi.org/10.1017/S003118200008077X - Willadsen P, Riding GA. Characterization of a proteolytic-enzyme inhibitor with allergenic activity. Multiple functions of a parasite-derived protein. Biochem J 1979; 177(1): 41-47. PMid:426780 PMCid:PMC1186338.
- Willadsen P, McKenna RV. Trypsin-chymotrypsin inhibitors from the tick, Boophilus microplus. Aust J Exp Biol Med Sci 1983; 61(Pt 2): 231-238. PMid:6683962. http://dx.doi.org/10.1038/icb.1983.21
» http://dx.doi.org/10.1038/icb.1983.21 - Yatsuda AP, Krijgsveld J, Cornelissen AW, Heck AJ, De Vries E. Comprehensive analysis of the secreted proteins of the parasite Haemonchus contortus reveals extensive sequence variation and differential immune recognition. J Biol Chem 2003; 278(19): 16941-16951. PMid:12576473. http://dx.doi.org/10.1074/jbc.M212453200
» http://dx.doi.org/10.1074/jbc.M212453200 - Yuan L, Cole GT. Isolation and characterization of an extracellular proteinase of Coccidioides immitis. Infect Immun 1987; 55(9): 1970-1978. PMid:3305358 PMCid:PMC260642.
Publication Dates
-
Publication in this collection
Apr-Jun 2014
History
-
Received
18 Dec 2013 -
Accepted
1 Apr 2014