Abstracts
One of the most common problems in breeding of ostriches in captivity is the control of parasitic diseases. This work presents keys for the identification of adult nematodes and infective larvae by morphologic and morphometric characteristics. These keys will allow the scientific community to identify the species that infect the ostriches either based on the characteristics of the posterior end of the infective larvae found through a simple fecal exam or by observing the morphology and morphometry of adult worms recovered during necropsies. These keys will facilitate ecological and systematic studies, as well as increase the understanding of the epidemiology of these parasitosis in ostriches.
Ratites; Nematoda; Strongylida; infective larvae
Um dos problemas mais comuns na criação de avestruzes em cativeiro é o controle das doenças parasitárias. Este trabalho apresenta chaves para a identificação de Nematoda adultos e larvas infectantes através de caracteres morfológicos e morfométricos. Essas chaves de identificação permitirão à comunidade científica o diagnóstico das espécies que infectam as avestruzes com base nas características da extremidade posterior das larvas infectantes encontradas por meio de simples exames fecais ou pela observação da morfologia e morfometria dos espécimes adultos recuperados durante necropsia. Dessa forma, as chaves de identificação facilitarão os estudos ecológicos e sistemáticos, bem como a melhor compreensão da epidemiologia dessas infecções em avestruzes.
Ratitas; Nematoda; Strongylida; larva infectante
The ostriches, Struthio camelus Linnaeus, 1758 are birds belonging to the group of the ratites. These birds originated from Africa, and currently, their commercial breeding has gained economic importance worldwide due to the ability of these birds to adapt to different environments and their lucrative agricultural potential (TULLY; SHANE, 1996Tully TM, Shane SM. Ratite management, medicine, and surgery. Malamar: Krieger Publishing; 1996.). One of the most common problems in breeding ostriches in captivity is the control of parasitic diseases, particularly those caused by parasites that have a direct lifecycle (HUCHZERMEYER, 2005Huchzermeyer FW. Doenças de avestruzes e outras ratitas. Jaboticabal: Funep; 2005.). A diverse helminthic fauna has been reported in ostriches (Table 1), of these, the nematodes are important organisms that limit the development of the ostrich flock.
Main parasites reported parasitizing ratites (ostrich, Struthio camelus; Rhea, Rhea americana; Emu, Dromaius novaehollandiae; Cassowary, Casuarius casuarius; Kiwi, Apterys spp.).
In 1880, small nematodes were collected from an ostrich proventriculum in South Africa by Arthur Douglass and sent to Dr. Cobbold, who identified the worms and named the new species Strongylus douglassii in honor of their collector (COBBOLD, 1882Cobbold TS. New entozoon from the ostriches. J Linn Soc 1882; 16(91): 184-188. http://dx.doi.org/10.1111/j.1096-3642.1882.tb02280.x
http://dx.doi.org/10.1111/j.1096-3642.18...
). Later, Lane (1923)Lane C. Some Strongylata. Parasitology 1923; 15(4): 348-364. http://dx.doi.org/10.1017/S0031182000014876
http://dx.doi.org/10.1017/S0031182000014...
established the genus Libyostrongylus for some African Trichostrongylidae, where L. douglassii is the type species from ostriches and L. hebrenicutus Lane, 1923Lane C. Some Strongylata. Parasitology 1923; 15(4): 348-364. http://dx.doi.org/10.1017/S0031182000014876
http://dx.doi.org/10.1017/S0031182000014...
, as the type species for gorillas. Gilbert (1937)Gilbert LI. New nematode Libyostrongylus magnus, n. sp., parasitic in an African ostrich. In: Skrjabin KJ. Papers on helminthology, 30 year jubilee. Moscow: Lenin Academy of Agricultural Science; 1937. p. 180-182. described a new species L. magnus, being found to-date only in ostriches from the African continent, without recent reports in the world. In this species, unlike others of this genus, the females are smaller than males. Libyostrongylus douglassii and L. hebrenicutus differ in the structure of the terminal bifurcations of the dorsal ray and in the pattern of lateral rays (rays 3 to 5) of the copulatory bursa. Ortlepp (1939)Ortlepp RJ. South African helminthes, Part VI. Some helminthes, chiefly from rodents. Onderstep J Vet Sci An Ind 1939; 12: 75-101. established the genus Paralibyostrongylus; being P. vondwei Ortlepp, 1939Ortlepp RJ. South African helminthes, Part VI. Some helminthes, chiefly from rodents. Onderstep J Vet Sci An Ind 1939; 12: 75-101., the type species and P. hebrenicutus, and P. nigeriae Baylis, 1928 included in this new genus (HOBERG et al., 1995Hoberg EP, Lloyd S, Omar H. Libyostrongylus dentatus n. sp. (Nematoda: Trichostrongylidae) from ostriches in North America, with comments on the genera Libyostrongylus and Paralibyostrongylus. J Parasitol 1995; 81(1): 85-93. PMid:7876985. http://dx.doi.org/10.2307/3284011
http://dx.doi.org/10.2307/3284011...
). Hoberg et al. (1995)Hoberg EP, Lloyd S, Omar H. Libyostrongylus dentatus n. sp. (Nematoda: Trichostrongylidae) from ostriches in North America, with comments on the genera Libyostrongylus and Paralibyostrongylus. J Parasitol 1995; 81(1): 85-93. PMid:7876985. http://dx.doi.org/10.2307/3284011
http://dx.doi.org/10.2307/3284011...
described a third species, named L. dentatus Hoberg, Lloyd and Omar, 1995Hoberg EP, Lloyd S, Omar H. Libyostrongylus dentatus n. sp. (Nematoda: Trichostrongylidae) from ostriches in North America, with comments on the genera Libyostrongylus and Paralibyostrongylus. J Parasitol 1995; 81(1): 85-93. PMid:7876985. http://dx.doi.org/10.2307/3284011
http://dx.doi.org/10.2307/3284011...
, due to the presence of prominent esophageal teeth. Thus, the genus Libyostrongylus is actually comprised of 3 species: L. douglassii, L. magnus and L. dentatus. Of these, the most common species is L. douglassii, which has been reported in South African flocks (FOCKEMA et al., 1985Fockema A, Malan FS, Cooper GG, Visser E. Anthelmintic efficacy of fembendazole against Libyostrongylus douglassii and Houttuynia struthionis in ostriches. J S Afr Vet Assoc 1985; 56: 47-48. PMid:3999107.; MALAN et al., 1988Malan FS, Gruss B, Roper NA, Ashburner AJ, Duplesis CA. Resistence of Libyostrongylus douglassii in ostriches to levamisole. J S Afr Vet Assoc 1988; 59: 202-203. PMid:3210219.; REINECKE, 1983Reinecke RK. Veterinary Helminthology. Durban: Butterworth; 1983.); Australia (BARTON; SEWARD, 1993Barton NJ, Seward DA. Detection of Libyostrongylus douglassii in ostriches in Australia. Aust Vet J 1993; 70(1): 31-32. http://dx.doi.org/10.1111/j.1751-0813.1993.tb00796.x
http://dx.doi.org/10.1111/j.1751-0813.19...
; BUTTON et al., 1993Button C, Barton NJ, Veale PI, Overend DJ. A survey of Libyostrongylus douglassii on ostrich farms in Eastern Victoria. Aust Vet J 1993; 70(2): 70-76. http://dx.doi.org/10.1111/j.1751-0813.1993.tb15152.x
http://dx.doi.org/10.1111/j.1751-0813.19...
; MORE, 1996More SJ. The performance of farmed ostrich hens in eastern Australia. Prev Vet Med 1996; 29(2): 107-120. http://dx.doi.org/10.1016/S0167-5877(96)01063-X
http://dx.doi.org/10.1016/S0167-5877(96)...
); the United States (HOBERG et al., 1995Hoberg EP, Lloyd S, Omar H. Libyostrongylus dentatus n. sp. (Nematoda: Trichostrongylidae) from ostriches in North America, with comments on the genera Libyostrongylus and Paralibyostrongylus. J Parasitol 1995; 81(1): 85-93. PMid:7876985. http://dx.doi.org/10.2307/3284011
http://dx.doi.org/10.2307/3284011...
); Italy (PINTORI et al., 2000Pintori A, Scala A, Giannetto S, Mascia M, De Rosa R. Parasitoses of ostriches (Struthio camelus) in Sardinia (Italy). Acta Parasitol 2000; 45: 164.); Scotland (PENNYCOTT; PATTERSON, 2001Pennycott T, Patterson T. Gastrointestinal parasites of ostriches (Struthio camelus). Vet Rec 2001; 148: 155-156. PMid:11271923.); Spain, Belgium, Portugal and the Netherlands (PONCE GORDO et al., 2002); Sweden (JANSSON et al., 2002Jansson DS, Christensson DA, Christensson BE. Winter survival in Sweden of L3-stage larvae of the ostrich wireworm Libyostrongylus douglassii. Vet Parasitol 2002; 106(1): 69-74. http://dx.doi.org/10.1016/S0304-4017(02)00033-X
http://dx.doi.org/10.1016/S0304-4017(02)...
); New Zealand (MACKERETH, 2004Mackereth GF. Libyostrongylus douglassii in New Zealand ostriches. Surveillance 2004; 31(3): 14-16.; MCKENNA, 2005McKenna PB. Libyostrongylus infections in ostriches: a brief review with particular reference to their detection in New Zealand. N Z Vet J 2005; 53(5): 267-270. PMid:16220116. http://dx.doi.org/10.1080/00480169.2005.36559
http://dx.doi.org/10.1080/00480169.2005....
); Zimbabwe (MUKARATIRWA et al., 2004Mukaratirwa S, Cindzi ZM, Maononga DB. Prevalence of Libyostrongylus douglassii in commercially reared ostriches in the highveld region of Zimbabwe. J Helminthol 2004; 78(4): 333-336. PMid:15575991. http://dx.doi.org/10.1079/JOH2004246
http://dx.doi.org/10.1079/JOH2004246...
) and Brazil (BONADIMAN et al., 2006Bonadiman SF, Ederli NB, Soares AKP, Moraes Neto AHA, Santos CP, DaMatta RA. Occurrence of Libyostrongylus sp. (Nematoda) in ostriches (Struthio camelus Linnaeus, 1758) from the north region of the state of Rio de Janeiro, Brazil. Vet Parasitol 2006; 137(1-2): 175-179. http://dx.doi.org/10.1016/j.vetpar.2005.12.018
http://dx.doi.org/10.1016/j.vetpar.2005....
; EDERLI et al., 2008aEderli NB, Oliveira FCR, Lopes CWG, DaMatta RA, Santos CP, Rodrigues MLA. Morphological diagnosis of infective larvae of Libyostrongylus douglassii (Cobbold, 1882) Lane, 1923 and L. dentatus Hoberg, Lloyd and Omar, 1995 (Nematoda, Trichostrongylidae) of ostriches. Vet Parasitol 2008a; 155(3-4): 323-327. PMid:18565673. http://dx.doi.org/10.1016/j.vetpar.2008.04.023
http://dx.doi.org/10.1016/j.vetpar.2008....
, bEderli NB, Bonadiman SF, Moraes Neto AHA, DaMatta RA, Santos CP. Mixed infection by Libyostrongylus douglassii and L. dentatus (Nematoda: Trichostrongylidae) in Struthio camelus (Ratites: Struthioniformes) from Brazil with further morphological characterization of adults. Vet Parasitol 2008b; 151(2-4): 227-232. PMid:18155840. http://dx.doi.org/10.1016/j.vetpar.2007.11.009
http://dx.doi.org/10.1016/j.vetpar.2007....
). There have been few reports of 2 other species of the genus Libyostrongylus. Libyostrongylus magnus has been reported only in South Africa (GILBERT, 1937Gilbert LI. New nematode Libyostrongylus magnus, n. sp., parasitic in an African ostrich. In: Skrjabin KJ. Papers on helminthology, 30 year jubilee. Moscow: Lenin Academy of Agricultural Science; 1937. p. 180-182.) and L. dentatus only in the United States (HOBERG et al., 1995Hoberg EP, Lloyd S, Omar H. Libyostrongylus dentatus n. sp. (Nematoda: Trichostrongylidae) from ostriches in North America, with comments on the genera Libyostrongylus and Paralibyostrongylus. J Parasitol 1995; 81(1): 85-93. PMid:7876985. http://dx.doi.org/10.2307/3284011
http://dx.doi.org/10.2307/3284011...
) and Brazil (BONADIMAN et al., 2006Bonadiman SF, Ederli NB, Soares AKP, Moraes Neto AHA, Santos CP, DaMatta RA. Occurrence of Libyostrongylus sp. (Nematoda) in ostriches (Struthio camelus Linnaeus, 1758) from the north region of the state of Rio de Janeiro, Brazil. Vet Parasitol 2006; 137(1-2): 175-179. http://dx.doi.org/10.1016/j.vetpar.2005.12.018
http://dx.doi.org/10.1016/j.vetpar.2005....
; EDERLI et al., 2008aEderli NB, Oliveira FCR, Lopes CWG, DaMatta RA, Santos CP, Rodrigues MLA. Morphological diagnosis of infective larvae of Libyostrongylus douglassii (Cobbold, 1882) Lane, 1923 and L. dentatus Hoberg, Lloyd and Omar, 1995 (Nematoda, Trichostrongylidae) of ostriches. Vet Parasitol 2008a; 155(3-4): 323-327. PMid:18565673. http://dx.doi.org/10.1016/j.vetpar.2008.04.023
http://dx.doi.org/10.1016/j.vetpar.2008....
, bEderli NB, Bonadiman SF, Moraes Neto AHA, DaMatta RA, Santos CP. Mixed infection by Libyostrongylus douglassii and L. dentatus (Nematoda: Trichostrongylidae) in Struthio camelus (Ratites: Struthioniformes) from Brazil with further morphological characterization of adults. Vet Parasitol 2008b; 151(2-4): 227-232. PMid:18155840. http://dx.doi.org/10.1016/j.vetpar.2007.11.009
http://dx.doi.org/10.1016/j.vetpar.2007....
). Because most reports are based only on fecal exams, the low reported rate of infections by these 2 species is probably due to errors in diagnosis because of the similarities in the infective larvae.
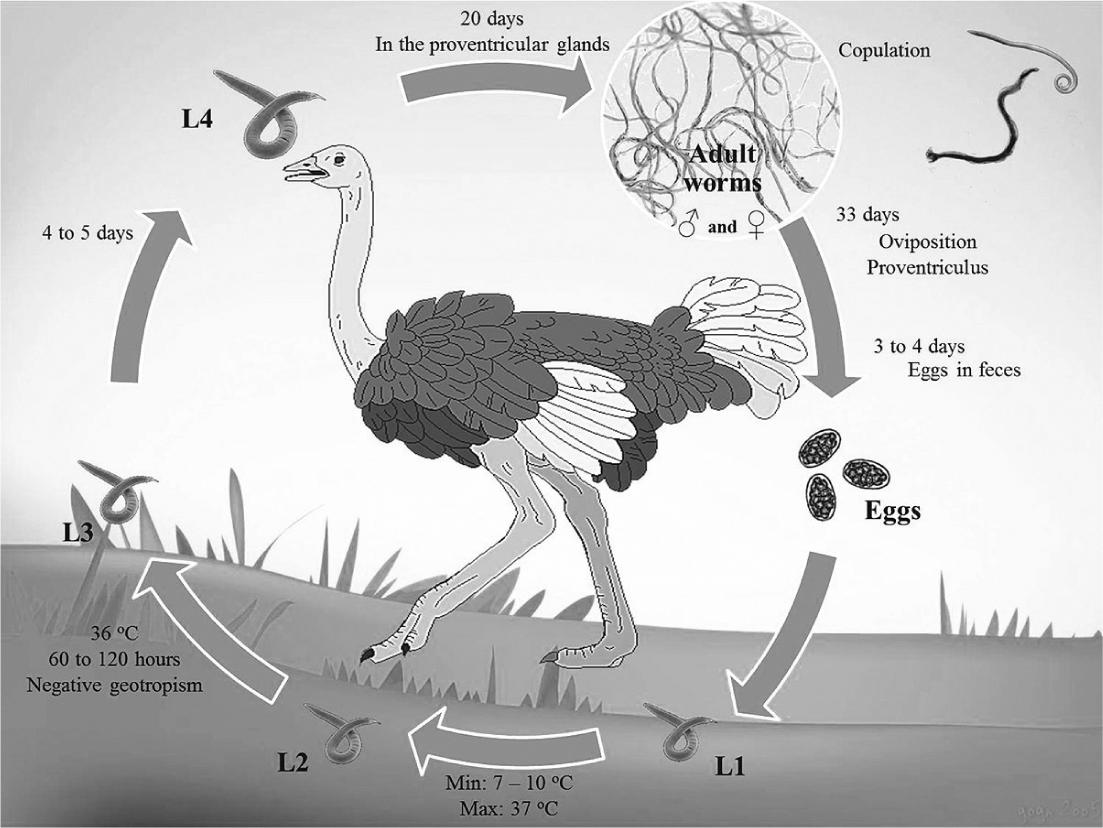
Data about the life cycle of the genus Libyostrongylus refer only to L. douglassii, which has a direct life cycle typical of Trichostrongylidae, with a pre-patent period of approximately 36 days (THEILER; ROBERTSON, 1915) (Figure 1). The eggs are shed in the feces of the infected hosts and develop in the environment into larvae of first (L1), second (L2) and third (L3) stages (infective larvae). This development occurs at a minimum temperature of 7 to 10°C and maximum of 37°C. Infective larvae display negative geotropism, tending to climb to the tip of vegetation through the moisture layer (MCKENNA, 2005McKenna PB. Libyostrongylus infections in ostriches: a brief review with particular reference to their detection in New Zealand. N Z Vet J 2005; 53(5): 267-270. PMid:16220116. http://dx.doi.org/10.1080/00480169.2005.36559
http://dx.doi.org/10.1080/00480169.2005....
). Under optimal temperature conditions (36°C), the development to infective larvae occurs in approximately 60 hours (BARTON; SEWARD, 1993Barton NJ, Seward DA. Detection of Libyostrongylus douglassii in ostriches in Australia. Aust Vet J 1993; 70(1): 31-32. http://dx.doi.org/10.1111/j.1751-0813.1993.tb00796.x
http://dx.doi.org/10.1111/j.1751-0813.19...
). Ostriches become infected by the ingestion of the infective larvae, which develops into the fourth larval stage (L4) within the proventricular glands in a period of 4 to 5 days. After approximately 20 days, these larvae develop into juvenile larvae. After the maturation process, copulation occurs and the females release eggs in the proventriculum approximately 33 days after the infection. It takes about 4 days for eggs appear in the feces of the host (THEILER; ROBERTSON, 1915).
Another species often cited as a parasite from ostriches, although with few reports, is Codiostomum struthionis. It was originally described as Sclerostoma struthionis by Horst in 1885 (POPOVA, 1955). In 1911, Railliet and Henry reclassified this species, and established the genus Codiostomum, being C. struthionis the type species and the only species in the genus (RAILLIET; HENRY, 1911Railliet A, Henry A. Les helminthes du nandou. Bull Soc Nat Acclimatation 1911; 58: 573-582.). Since that time, there have been an extremely small number of studies regarding this nematode. However, this species is cited in several scientific publications describing the similarity of its eggs with that of the genus Libyostrongylus (BARTON; SEWARD, 1993Barton NJ, Seward DA. Detection of Libyostrongylus douglassii in ostriches in Australia. Aust Vet J 1993; 70(1): 31-32. http://dx.doi.org/10.1111/j.1751-0813.1993.tb00796.x
http://dx.doi.org/10.1111/j.1751-0813.19...
; BLACK, 2001Black D. Ostrich flock health. Sem Avian Exotic Pet Med 2001; 10(3): 117-130. http://dx.doi.org/10.1053/saep.2001.24255
http://dx.doi.org/10.1053/saep.2001.2425...
; PENNYCOTT; PATTERSON, 2001Pennycott T, Patterson T. Gastrointestinal parasites of ostriches (Struthio camelus). Vet Rec 2001; 148: 155-156. PMid:11271923.; SOTIRAKI et al., 2001Sotiraki ST, Georgiades G, Antoniadou-Sotiriadou K, Himonas CA. Gastrointestinal parasites in ostriches (Struthio camelus). Vet Rec 2001; 148(3): 84-86. PMid:12503598. http://dx.doi.org/10.1136/vr.148.3.84
http://dx.doi.org/10.1136/vr.148.3.84...
; PONCE GORDO et al., 2002; HUCHZERMEYER, 2002Huchzermeyer FW. Diseases of farmed crocodiles and ostriches. Rev Sci Tech 2002; 21(2): 265-276. PMid:11974614., 2005Huchzermeyer FW. Doenças de avestruzes e outras ratitas. Jaboticabal: Funep; 2005.; MCKENNA, 2005McKenna PB. Libyostrongylus infections in ostriches: a brief review with particular reference to their detection in New Zealand. N Z Vet J 2005; 53(5): 267-270. PMid:16220116. http://dx.doi.org/10.1080/00480169.2005.36559
http://dx.doi.org/10.1080/00480169.2005....
; TIŠLJAR et al., 2007Tišljar M, Beck R, Cooper RG, Marinculić A, Tudja M, Lukač-Novak I, et al. First finding of libyostrongylosis in farm-reared ostriches (Struthio camelus) in Croatia: unusual histopathological finding in the brain of two ostriches, naturally infected with Libyostrongylus douglasi. Vet Parasitol 2007; 147(1-2): 118-124. PMid:17448602. http://dx.doi.org/10.1016/j.vetpar.2007.03.014
http://dx.doi.org/10.1016/j.vetpar.2007....
).
The life cycle of C. struthionis has not been determined. Oliveira et al. (2009)Oliveira FCR, Ederli NB, Lopes CWG, Rodrigues MLA. Pathological findings in the caeca of naturally infected ostriches, Struthio camelus Linnaeus, 1758 (Aves, Struthionidae) parasitized by Codiostomum struthionis (Horst, 1885) Railliet and Henry, 1911 (Nematoda, Strongylidae). Vet Parasitol 2009; 165(1-2): 175-178. PMid:19647369. http://dx.doi.org/10.1016/j.vetpar.2009.06.034
http://dx.doi.org/10.1016/j.vetpar.2009....
believe that these nematodes have a direct life cycle and the larvae migrate into the host tissue, appearing in the nodules found along the cecum of infected birds, however, this fact has not been confirmed.
According to Ponce Gordo et al. (2002), the occurrence of C. struthionis is often overlooked in Europe due to a possible confusion in the diagnosis by the observation of eggs in the feces because the eggs are similar to those of the genus Libyostrongylus, this misdiagnosis is probably happening on other continents.
There are few reports of the occurrence of C. struthionisinfecting ostrich flocks in South Africa (POPOVA, 1955); Europe (PONCE GORDO et al., 2002); Brazil (EDERLI et al., 2008cEderli NB, Oliveira FCR, Lopes CWG, Rodrigues MLA. Further study of Codiostomum struthionis (Horst, 1885) Railliet and Henry, 1911 (Nematoda, Strongylidae) parasite of ostriches (Struthio camelus Linnaeus, 1758) (Aves, Struthioniformes). Vet Parasitol 2008c; 157(3-4): 275-283. PMid:18774651. http://dx.doi.org/10.1016/j.vetpar.2008.07.018
http://dx.doi.org/10.1016/j.vetpar.2008....
) and possibly in Greece, where Sotiraki et al. (2001)Sotiraki ST, Georgiades G, Antoniadou-Sotiriadou K, Himonas CA. Gastrointestinal parasites in ostriches (Struthio camelus). Vet Rec 2001; 148(3): 84-86. PMid:12503598. http://dx.doi.org/10.1136/vr.148.3.84
http://dx.doi.org/10.1136/vr.148.3.84...
found eggs typical of the family Strongylidae in feces of ostriches but did not analyze fecal cultures to confirm the diagnosis. This low incidence of infection is probably due to misdiagnosis because of the similarity of C. struthionis eggs with those from the genus Libyostrongylus. Additionally, there is similarity between the infective larvae of C. struthionis and L. dentatus(EDERLI et al., 2008aEderli NB, Oliveira FCR, Lopes CWG, DaMatta RA, Santos CP, Rodrigues MLA. Morphological diagnosis of infective larvae of Libyostrongylus douglassii (Cobbold, 1882) Lane, 1923 and L. dentatus Hoberg, Lloyd and Omar, 1995 (Nematoda, Trichostrongylidae) of ostriches. Vet Parasitol 2008a; 155(3-4): 323-327. PMid:18565673. http://dx.doi.org/10.1016/j.vetpar.2008.04.023
http://dx.doi.org/10.1016/j.vetpar.2008....
, cEderli NB, Oliveira FCR, Lopes CWG, Rodrigues MLA. Further study of Codiostomum struthionis (Horst, 1885) Railliet and Henry, 1911 (Nematoda, Strongylidae) parasite of ostriches (Struthio camelus Linnaeus, 1758) (Aves, Struthioniformes). Vet Parasitol 2008c; 157(3-4): 275-283. PMid:18774651. http://dx.doi.org/10.1016/j.vetpar.2008.07.018
http://dx.doi.org/10.1016/j.vetpar.2008....
).
Until recently, the specific diagnosis of these nematodes was possible only through the identification of adult worms collected during necropsies. Studies carried out by Ederli et al. (2008a)Ederli NB, Oliveira FCR, Lopes CWG, DaMatta RA, Santos CP, Rodrigues MLA. Morphological diagnosis of infective larvae of Libyostrongylus douglassii (Cobbold, 1882) Lane, 1923 and L. dentatus Hoberg, Lloyd and Omar, 1995 (Nematoda, Trichostrongylidae) of ostriches. Vet Parasitol 2008a; 155(3-4): 323-327. PMid:18565673. http://dx.doi.org/10.1016/j.vetpar.2008.04.023
http://dx.doi.org/10.1016/j.vetpar.2008....
and Ederli et al. (2008c)Ederli NB, Oliveira FCR, Lopes CWG, Rodrigues MLA. Further study of Codiostomum struthionis (Horst, 1885) Railliet and Henry, 1911 (Nematoda, Strongylidae) parasite of ostriches (Struthio camelus Linnaeus, 1758) (Aves, Struthioniformes). Vet Parasitol 2008c; 157(3-4): 275-283. PMid:18774651. http://dx.doi.org/10.1016/j.vetpar.2008.07.018
http://dx.doi.org/10.1016/j.vetpar.2008....
allowed the distinction of C. struthionis, L. douglassii and L. dentatus through the observation of infective larvae recovered from fecal cultures, thus facilitating diagnosis.
Libyostrongylus spp. is considered the most pathogenic nematode, responsible for 50% of the mortality of juvenile birds and occasionally killing adults (REINECKE, 1983Reinecke RK. Veterinary Helminthology. Durban: Butterworth; 1983.). Until recently, C. struthionis was considered a nonpathogenic parasite (HUCHZERMEYER, 2005Huchzermeyer FW. Doenças de avestruzes e outras ratitas. Jaboticabal: Funep; 2005.); however, a recent study by Oliveira et al. (2009)Oliveira FCR, Ederli NB, Lopes CWG, Rodrigues MLA. Pathological findings in the caeca of naturally infected ostriches, Struthio camelus Linnaeus, 1758 (Aves, Struthionidae) parasitized by Codiostomum struthionis (Horst, 1885) Railliet and Henry, 1911 (Nematoda, Strongylidae). Vet Parasitol 2009; 165(1-2): 175-178. PMid:19647369. http://dx.doi.org/10.1016/j.vetpar.2009.06.034
http://dx.doi.org/10.1016/j.vetpar.2009....
verified that C. struthionis is responsible for macroscopic lesions in the ostriches' cecum, characterized as mucosal thickening nodules distributed throughout the cecum, and the presence of hemorrhages caused by small ulcers circumscribed to the mucosal edema. Due to these observations, these authors hypothesize that a high parasite load by C. struthionis could result in loss of fitness.
These parasites appear to be specific to ostriches; however, there was a single report of the occurrence of Libyostrongylus in emu, Dromaius novaehollandiae (Lathan, 1790) in Sweden (JANSSON; CHRISTENSSON, 2000Jansson DS, Christensson D. Gastrointestinala parasiter hos strutsfåglar i Sverige. Svensk Veterinär Tidning 2000; 52: 621-626.; PONCE GORDO et al., 2002), which may have been due an error in diagnosis. The cross-transmission potential of these nematodes to other domestic birds (in particular, other ratites) has not yet been determined. Studies carried out by Zettermann et al. (2005)Zettermann CD, Nascimento JA, Tebaldi JA, Szabó MJP. Observations on helminth infections of free-living and captive rheas (Rhea americana) in Brazil. Vet Parasitol 2005; 129(1-2): 169-172. PMid:15817218. http://dx.doi.org/10.1016/j.vetpar.2004.12.015
http://dx.doi.org/10.1016/j.vetpar.2004....
to detect helminthes in rheas, Rhea americana Linnaeus, 1758, showed that none of the 8 freeliving adult rheas or 12 captive juveniles rheas were infected by Libyostrongylus spp. or C. struthionis. They found 7 other species of parasites in the examined rheas: Sicarius uncinipenis (Molin 1860), Torquatoides crotophaga Williams, 1929, Deletrocephalus dimidiatusDiesing, 1851, D. cesarpintoi Vaz, 1936Vaz Z. Estudos sobre nematóides parasites de emas (Rhea americana). Arch Inst Biol 1936; 7: 253-266., Paradeletrocephalus minor Freitas and Lent, 1947, Capillaria venteli Freitas and Almeida, 1935 and Dicheilonema rheae (Owen, 1843). Gallo et al. (2010)Gallo SSM, Ederli NB, Bôa-Morte MO, Oliveira FCR. Efeitos da infecção experimental em frangos, Gallus gallus, com larvas infectantes de Libyostrongylus douglassii e L. dentatus, nematóides de avestruzes, Struthio camelus. Rev Bras Med Vet 2010; 32(2): 26-32. conducted a study to evaluate the effects of an experimental infection of chickens by Libyostrongylus spp., where they infected normal and immunosuppressed one-day-old chicks with infective larvae of L. douglassii and L. dentatus; nonetheless, the infection was not established. In some birds they observed the elimination of unsheathed larvae in birds' feces from 1 to 5 days following the infection. Moreover, the presence of eggs of Trichostrongylidae was not observed until the end of the experiment (52 days). Because the unsheathed larvae cannot survive in the environment, chickens are not at risk for the transmission or for acting as a paratenic host. However, a possibility of transmission of these parasites to other birds, mainly other ratites, cannot be discounted.
The present article presents keys for the identification of the main genus (infective larvae and adults) in ostriches to facilitate diagnosis by fecal exams and the observation of adults recovered in necropsies.
Adult Nematodes
The parasites of the genus Libyostrongylus are small nematodes, of a reddish color in vivo and found in the proventriculus and gizzard of ostriches. The genus Libyostrongylusis cited as being found under the koilin layer (HOBERG et al., 1995Hoberg EP, Lloyd S, Omar H. Libyostrongylus dentatus n. sp. (Nematoda: Trichostrongylidae) from ostriches in North America, with comments on the genera Libyostrongylus and Paralibyostrongylus. J Parasitol 1995; 81(1): 85-93. PMid:7876985. http://dx.doi.org/10.2307/3284011
http://dx.doi.org/10.2307/3284011...
; HUCHZERMEYER, 2005Huchzermeyer FW. Doenças de avestruzes e outras ratitas. Jaboticabal: Funep; 2005.; EDERLI et al., 2008bEderli NB, Bonadiman SF, Moraes Neto AHA, DaMatta RA, Santos CP. Mixed infection by Libyostrongylus douglassii and L. dentatus (Nematoda: Trichostrongylidae) in Struthio camelus (Ratites: Struthioniformes) from Brazil with further morphological characterization of adults. Vet Parasitol 2008b; 151(2-4): 227-232. PMid:18155840. http://dx.doi.org/10.1016/j.vetpar.2007.11.009
http://dx.doi.org/10.1016/j.vetpar.2007....
); however, a recent study by Ederli and Oliveira (2009)Ederli NB, Oliveira FCR. Differential localization of Libyostrongylus douglassii (Cobbold, 1882) Lane, 1923 and L. dentatus Hoberg, Lloyd, and Omar, 1995 (Nematoda: Trichostrongylidae) in Ostrich (Struthio camelus Linnaeus, 1758) Proventriculi. J Parasitol 2009; 95(3): 757-759. PMid:18990000. http://dx.doi.org/10.1645/GE-1853.1
http://dx.doi.org/10.1645/GE-1853.1...
observed differential localization of L. douglassii and L. dentatus within the proventriculum. Libyostrongylus douglassii is found on the mucosa just under the koilin layer, as described in the literature, while L. dentatusis found inserted into the koilin layer. The distinct localization of these 2 species in the proventriculum may be the reason there are so few reports of the presence of L. dentatus. Until the publication of these observations, L. dentatus was sought along with L. douglassii under the koilin layer, possibly resulting in false negative diagnoses for L. dentatus because the koilin layer is removed and discarded during the collection of the parasites. The description of the differential localization of these 2 species of nematodes in the proventriculum may result in an increase in the number of reports of L. dentatus.
The morphological description of L. magnus is published in Greek (GILBERT, 1937Gilbert LI. New nematode Libyostrongylus magnus, n. sp., parasitic in an African ostrich. In: Skrjabin KJ. Papers on helminthology, 30 year jubilee. Moscow: Lenin Academy of Agricultural Science; 1937. p. 180-182.), which hinders its access. There are no further studies about the morphology of this species. Hoberg et al. (1995)Hoberg EP, Lloyd S, Omar H. Libyostrongylus dentatus n. sp. (Nematoda: Trichostrongylidae) from ostriches in North America, with comments on the genera Libyostrongylus and Paralibyostrongylus. J Parasitol 1995; 81(1): 85-93. PMid:7876985. http://dx.doi.org/10.2307/3284011
http://dx.doi.org/10.2307/3284011...
was able to re-examined some specimens deposited in the International Institute of Parasitology (LSHTM 1317) which was previously identified as L. douglassii in 1933 in Ethiopia. Hoberg et al. (1995)Hoberg EP, Lloyd S, Omar H. Libyostrongylus dentatus n. sp. (Nematoda: Trichostrongylidae) from ostriches in North America, with comments on the genera Libyostrongylus and Paralibyostrongylus. J Parasitol 1995; 81(1): 85-93. PMid:7876985. http://dx.doi.org/10.2307/3284011
http://dx.doi.org/10.2307/3284011...
identified those nematodes as L. magnus. According to Hoberg et al. (1995)Hoberg EP, Lloyd S, Omar H. Libyostrongylus dentatus n. sp. (Nematoda: Trichostrongylidae) from ostriches in North America, with comments on the genera Libyostrongylus and Paralibyostrongylus. J Parasitol 1995; 81(1): 85-93. PMid:7876985. http://dx.doi.org/10.2307/3284011
http://dx.doi.org/10.2307/3284011...
, the measurements of some male and female specimens of L. douglassii described by Durette-Desset and Denke (1978)Durette-Desset MC, Denke MA. Description de noveaux nematodes parasites dun lievre African et complements à letude morphologique de quelques Trichostrongyles. Bull Mus Nat Hist Nat 1978; 354: 331-347. from an ostrich in Somalia should classify them as L. magnus (Table 2). Unlike other species of the genus, the females of L. magnus are smaller than males and have a larger ovejector when compared with L. douglassii and L. dentatus(Table 2). Libyostrongylus douglassii and L. dentatus can be distinguished by the observation of some morphological characteristics. Libyostrongylus dentatus can be distinguished from congeners by the presence of 3 prominent esophageal teeth (Figure 2B). Both males and females of L. dentatusare longer than L. douglassii (HOBERG et al., 1995Hoberg EP, Lloyd S, Omar H. Libyostrongylus dentatus n. sp. (Nematoda: Trichostrongylidae) from ostriches in North America, with comments on the genera Libyostrongylus and Paralibyostrongylus. J Parasitol 1995; 81(1): 85-93. PMid:7876985. http://dx.doi.org/10.2307/3284011
http://dx.doi.org/10.2307/3284011...
; EDERLI et al., 2008bEderli NB, Bonadiman SF, Moraes Neto AHA, DaMatta RA, Santos CP. Mixed infection by Libyostrongylus douglassii and L. dentatus (Nematoda: Trichostrongylidae) in Struthio camelus (Ratites: Struthioniformes) from Brazil with further morphological characterization of adults. Vet Parasitol 2008b; 151(2-4): 227-232. PMid:18155840. http://dx.doi.org/10.1016/j.vetpar.2007.11.009
http://dx.doi.org/10.1016/j.vetpar.2007....
) (Table 2). These 2 species also have differences in the morphology on the cephalic end: L. dentatus has a flat extremity with 3 pairs of rounded cephalic papillae, while L. douglassii has a rounded end with 3 pairs of elongated papillae (EDERLI et al., 2008bEderli NB, Bonadiman SF, Moraes Neto AHA, DaMatta RA, Santos CP. Mixed infection by Libyostrongylus douglassii and L. dentatus (Nematoda: Trichostrongylidae) in Struthio camelus (Ratites: Struthioniformes) from Brazil with further morphological characterization of adults. Vet Parasitol 2008b; 151(2-4): 227-232. PMid:18155840. http://dx.doi.org/10.1016/j.vetpar.2007.11.009
http://dx.doi.org/10.1016/j.vetpar.2007....
). Female L. dentatus can be distinguished from L. douglassii by its longer ovejector (Figures 2A and 3A) and by the presence of a cuticular inflation at the anal level (Figures 2C and 3C). Libyostrongylus magnus was described as having long ovejector, but it can be distinguished from L. dentatus by the larger number of eggs inside (Figure 4A) (SKRJABIN, 1961). Male L. dentatus can be distinguished by an asymmetric dorsal ray (Figure 2D), while other species have a symmetric dorsal ray (Figure 3D and 4D). Moreover, L. dentatushave spicules with the main shaft ending in a rounded point (Figure 2E), while the spicules of L. douglassii have a main shaft ending in a point (Figure 3E) (HOBERG et al., 1995Hoberg EP, Lloyd S, Omar H. Libyostrongylus dentatus n. sp. (Nematoda: Trichostrongylidae) from ostriches in North America, with comments on the genera Libyostrongylus and Paralibyostrongylus. J Parasitol 1995; 81(1): 85-93. PMid:7876985. http://dx.doi.org/10.2307/3284011
http://dx.doi.org/10.2307/3284011...
; EDERLI et al., 2008bEderli NB, Bonadiman SF, Moraes Neto AHA, DaMatta RA, Santos CP. Mixed infection by Libyostrongylus douglassii and L. dentatus (Nematoda: Trichostrongylidae) in Struthio camelus (Ratites: Struthioniformes) from Brazil with further morphological characterization of adults. Vet Parasitol 2008b; 151(2-4): 227-232. PMid:18155840. http://dx.doi.org/10.1016/j.vetpar.2007.11.009
http://dx.doi.org/10.1016/j.vetpar.2007....
).
Libyostrongylus dentatus. (A) Ovejector; (B) Anterior end; (C) Female posterior end; (D) Male copulatory bursa; and (E) Spicules.
Libyostrongylus douglassii. (A) Ovejector; (B) Anterior end; (C) Female posterior end; (D) Male copulatory bursa; and (E) Spicules.
Libyostrongylus magnus (A) Ovejector; (B) Anterior end; (C) Female posterior end; (D) Male copulatory bursa; and (E) Spicules. Re-drawed from Skrjabin (1961).
Comparison of adult males and females from the species of genus Libyostrongylus Cobbold, 1882Cobbold TS. New entozoon from the ostriches. J Linn Soc 1882; 16(91): 184-188. http://dx.doi.org/10.1111/j.1096-3642.1882.tb02280.x
http://dx.doi.org/10.1111/j.1096-3642.18... . Measurements in micrometers.
Codiostomum struthionis are large and robust nematodes, with white coloration in vivo, observed easily in the cecum of ostriches. These nematodes can be easily distinguished from the genus Libyostrongylus because of their greater length. Recently, this species had its morphology and ultrastructure redescribed by Ederli et al. (2008c)Ederli NB, Oliveira FCR, Lopes CWG, Rodrigues MLA. Further study of Codiostomum struthionis (Horst, 1885) Railliet and Henry, 1911 (Nematoda, Strongylidae) parasite of ostriches (Struthio camelus Linnaeus, 1758) (Aves, Struthioniformes). Vet Parasitol 2008c; 157(3-4): 275-283. PMid:18774651. http://dx.doi.org/10.1016/j.vetpar.2008.07.018
http://dx.doi.org/10.1016/j.vetpar.2008....
. Codiostomum struthionis has a well-developed buccal capsule with 2 rows of corona radiata. Males have a strongly curved ventral copulatory bursa, with a large projection of the dorsal lobe and long and slender spicules. Females have prodelphic uteri and a pointed tail tip (EDERLI et al., 2008cEderli NB, Oliveira FCR, Lopes CWG, Rodrigues MLA. Further study of Codiostomum struthionis (Horst, 1885) Railliet and Henry, 1911 (Nematoda, Strongylidae) parasite of ostriches (Struthio camelus Linnaeus, 1758) (Aves, Struthioniformes). Vet Parasitol 2008c; 157(3-4): 275-283. PMid:18774651. http://dx.doi.org/10.1016/j.vetpar.2008.07.018
http://dx.doi.org/10.1016/j.vetpar.2008....
).
To facilitate the differentiation of adult nematodes from the genus Libyostrongylus, an identification key is described below:
Infective Larvae
The infective larvae of the genus Libyostrongylus are characterized by the presence of a knob in the larvae tip tail (BARTON; SEWARD, 1993Barton NJ, Seward DA. Detection of Libyostrongylus douglassii in ostriches in Australia. Aust Vet J 1993; 70(1): 31-32. http://dx.doi.org/10.1111/j.1751-0813.1993.tb00796.x
http://dx.doi.org/10.1111/j.1751-0813.19...
). Bonadiman et al. (2006)Bonadiman SF, Ederli NB, Soares AKP, Moraes Neto AHA, Santos CP, DaMatta RA. Occurrence of Libyostrongylus sp. (Nematoda) in ostriches (Struthio camelus Linnaeus, 1758) from the north region of the state of Rio de Janeiro, Brazil. Vet Parasitol 2006; 137(1-2): 175-179. http://dx.doi.org/10.1016/j.vetpar.2005.12.018
http://dx.doi.org/10.1016/j.vetpar.2005....
reported the presence of L. douglassii and L. dentatus in the northern region of the state of Rio de Janeiro, Brazil, by observing adult worms collected from the proventriculum during necropsies in 3 adult ostriches. They also observed differences in the length and morphology of the infective larvae sheath tail recovered from fecal cultures of samples from the same region. Some larvae had short sheath tail with an acute termination and others had a long and filamentous sheath tail. The authors described these differences as a possible way to distinguish these 2 species, as both were found parasitizing ostrich flocks in the analyzed region (BONADIMAN et al., 2006Bonadiman SF, Ederli NB, Soares AKP, Moraes Neto AHA, Santos CP, DaMatta RA. Occurrence of Libyostrongylus sp. (Nematoda) in ostriches (Struthio camelus Linnaeus, 1758) from the north region of the state of Rio de Janeiro, Brazil. Vet Parasitol 2006; 137(1-2): 175-179. http://dx.doi.org/10.1016/j.vetpar.2005.12.018
http://dx.doi.org/10.1016/j.vetpar.2005....
). Ederli et al. (2008a)Ederli NB, Oliveira FCR, Lopes CWG, DaMatta RA, Santos CP, Rodrigues MLA. Morphological diagnosis of infective larvae of Libyostrongylus douglassii (Cobbold, 1882) Lane, 1923 and L. dentatus Hoberg, Lloyd and Omar, 1995 (Nematoda, Trichostrongylidae) of ostriches. Vet Parasitol 2008a; 155(3-4): 323-327. PMid:18565673. http://dx.doi.org/10.1016/j.vetpar.2008.04.023
http://dx.doi.org/10.1016/j.vetpar.2008....
confirmed this observation, showing the infective larvae of L. douglassii which has a short sheath tail (average of 29.52 µm ± 4.11; ranging from 20.62 to 36.31 µm), while the infective larvae of L. dentatus has a long sheath tail (average of 61.20 µm ± 2.17; ranging from 40.01 to 84.01 µm) (Figures 5A, B). Following this study, it was possible to differentiate these 2 species by the morphology and morphometry of the infective larvae sheath tail recovered from fecal cultures. Because it was no longer necessary to perform a necropsy to collect adult worms, specific diagnosis was facilitated. However, the infective larvae of C. struthionis also have a long and filamentous sheath tail (average of 110.74 µm ± 13.46, ranging from 85.87 to 143.18 µm), but the tail of the larvae inside the cuticle has an acute termination (Figure 5C) (EDERLI et al., 2008cEderli NB, Oliveira FCR, Lopes CWG, Rodrigues MLA. Further study of Codiostomum struthionis (Horst, 1885) Railliet and Henry, 1911 (Nematoda, Strongylidae) parasite of ostriches (Struthio camelus Linnaeus, 1758) (Aves, Struthioniformes). Vet Parasitol 2008c; 157(3-4): 275-283. PMid:18774651. http://dx.doi.org/10.1016/j.vetpar.2008.07.018
http://dx.doi.org/10.1016/j.vetpar.2008....
), which is different from the genus Libyostrongylus, which has a knob on the larvae tip tail (EDERLI et al., 2008aEderli NB, Oliveira FCR, Lopes CWG, DaMatta RA, Santos CP, Rodrigues MLA. Morphological diagnosis of infective larvae of Libyostrongylus douglassii (Cobbold, 1882) Lane, 1923 and L. dentatus Hoberg, Lloyd and Omar, 1995 (Nematoda, Trichostrongylidae) of ostriches. Vet Parasitol 2008a; 155(3-4): 323-327. PMid:18565673. http://dx.doi.org/10.1016/j.vetpar.2008.04.023
http://dx.doi.org/10.1016/j.vetpar.2008....
, bEderli NB, Bonadiman SF, Moraes Neto AHA, DaMatta RA, Santos CP. Mixed infection by Libyostrongylus douglassii and L. dentatus (Nematoda: Trichostrongylidae) in Struthio camelus (Ratites: Struthioniformes) from Brazil with further morphological characterization of adults. Vet Parasitol 2008b; 151(2-4): 227-232. PMid:18155840. http://dx.doi.org/10.1016/j.vetpar.2007.11.009
http://dx.doi.org/10.1016/j.vetpar.2007....
) (Figures 5A, B). The total length of the infective larvae is not a good parameter to distinguish the species due to the similarity between nematodes. Libyostrongylus douglassii has a total length of 874.33 µm ± 33.80, ranging from 784.47 to 957.90 µm; L. dentatus784.47 µm ± 43.63, ranging from 735.84 to 947.01 µm and C. struthionis 598.25 µm ± 25.15, ranging from 511.92 to 642.41 µm (EDERLI et al., 2008aEderli NB, Oliveira FCR, Lopes CWG, DaMatta RA, Santos CP, Rodrigues MLA. Morphological diagnosis of infective larvae of Libyostrongylus douglassii (Cobbold, 1882) Lane, 1923 and L. dentatus Hoberg, Lloyd and Omar, 1995 (Nematoda, Trichostrongylidae) of ostriches. Vet Parasitol 2008a; 155(3-4): 323-327. PMid:18565673. http://dx.doi.org/10.1016/j.vetpar.2008.04.023
http://dx.doi.org/10.1016/j.vetpar.2008....
, cEderli NB, Oliveira FCR, Lopes CWG, Rodrigues MLA. Further study of Codiostomum struthionis (Horst, 1885) Railliet and Henry, 1911 (Nematoda, Strongylidae) parasite of ostriches (Struthio camelus Linnaeus, 1758) (Aves, Struthioniformes). Vet Parasitol 2008c; 157(3-4): 275-283. PMid:18774651. http://dx.doi.org/10.1016/j.vetpar.2008.07.018
http://dx.doi.org/10.1016/j.vetpar.2008....
).
Posterior end of infective larvae. (A) Libyostrongylus douglassii; (B) Libyostrongylus dentatus; and (C) Codiostomum struthionis.
The infective larvae of L. douglassii have a less-rounded cephalic end and became thinner more abruptly than L. dentatus infective larvae (BONADIMAN et al., 2006Bonadiman SF, Ederli NB, Soares AKP, Moraes Neto AHA, Santos CP, DaMatta RA. Occurrence of Libyostrongylus sp. (Nematoda) in ostriches (Struthio camelus Linnaeus, 1758) from the north region of the state of Rio de Janeiro, Brazil. Vet Parasitol 2006; 137(1-2): 175-179. http://dx.doi.org/10.1016/j.vetpar.2005.12.018
http://dx.doi.org/10.1016/j.vetpar.2005....
), while the infective larvae of C. struthionis have a flat, rounded cephalic end (EDERLI et al., 2008cEderli NB, Oliveira FCR, Lopes CWG, Rodrigues MLA. Further study of Codiostomum struthionis (Horst, 1885) Railliet and Henry, 1911 (Nematoda, Strongylidae) parasite of ostriches (Struthio camelus Linnaeus, 1758) (Aves, Struthioniformes). Vet Parasitol 2008c; 157(3-4): 275-283. PMid:18774651. http://dx.doi.org/10.1016/j.vetpar.2008.07.018
http://dx.doi.org/10.1016/j.vetpar.2008....
). These parameters are not reliable in the specific diagnosis of these infective larvae due the similarity between the cephalic ends.
Libyostrongylus douglassii is easily distinguished from L. dentatus and C. struthionis by the infective larvae sheath tail: it is short with an acute termination, differing from the other 2 species, which have a long and filamentous sheath tail (Figure 5). The differential diagnosis between these 2 species is made by the observation of the shape of the larvae tail: L. dentatus has a knob in the tip of the tail (Figure 5B), while C. struthionis has an acute termination in the tip of the tail (Figure 5C) (EDERLI et al., 2008aEderli NB, Oliveira FCR, Lopes CWG, DaMatta RA, Santos CP, Rodrigues MLA. Morphological diagnosis of infective larvae of Libyostrongylus douglassii (Cobbold, 1882) Lane, 1923 and L. dentatus Hoberg, Lloyd and Omar, 1995 (Nematoda, Trichostrongylidae) of ostriches. Vet Parasitol 2008a; 155(3-4): 323-327. PMid:18565673. http://dx.doi.org/10.1016/j.vetpar.2008.04.023
http://dx.doi.org/10.1016/j.vetpar.2008....
, cEderli NB, Oliveira FCR, Lopes CWG, Rodrigues MLA. Further study of Codiostomum struthionis (Horst, 1885) Railliet and Henry, 1911 (Nematoda, Strongylidae) parasite of ostriches (Struthio camelus Linnaeus, 1758) (Aves, Struthioniformes). Vet Parasitol 2008c; 157(3-4): 275-283. PMid:18774651. http://dx.doi.org/10.1016/j.vetpar.2008.07.018
http://dx.doi.org/10.1016/j.vetpar.2008....
). The comparison of the mean lengths of the infective larvae sheaths tail of L. douglassii and L. dentatus was considered extremely significant (EDERLI et al., 2008aEderli NB, Oliveira FCR, Lopes CWG, DaMatta RA, Santos CP, Rodrigues MLA. Morphological diagnosis of infective larvae of Libyostrongylus douglassii (Cobbold, 1882) Lane, 1923 and L. dentatus Hoberg, Lloyd and Omar, 1995 (Nematoda, Trichostrongylidae) of ostriches. Vet Parasitol 2008a; 155(3-4): 323-327. PMid:18565673. http://dx.doi.org/10.1016/j.vetpar.2008.04.023
http://dx.doi.org/10.1016/j.vetpar.2008....
); however, this parameter must be associated with the tail morphology of the larvae, because L. dentatus can be confused with C. struthionis, as mentioned previously. Due to these similarities, the identity of infective larvae can be unclear in fecal examinations, resulting in an error in the diagnosis. This misdiagnosis may be occurring with C. struthionis because there are few reports of this nematode in ostrich flocks (POPOVA, 1955; SOTIRAKI, et al., 2001Sotiraki ST, Georgiades G, Antoniadou-Sotiriadou K, Himonas CA. Gastrointestinal parasites in ostriches (Struthio camelus). Vet Rec 2001; 148(3): 84-86. PMid:12503598. http://dx.doi.org/10.1136/vr.148.3.84
http://dx.doi.org/10.1136/vr.148.3.84...
; PONCE GORDO et al., 2002; EDERLI et al., 2008aEderli NB, Oliveira FCR, Lopes CWG, DaMatta RA, Santos CP, Rodrigues MLA. Morphological diagnosis of infective larvae of Libyostrongylus douglassii (Cobbold, 1882) Lane, 1923 and L. dentatus Hoberg, Lloyd and Omar, 1995 (Nematoda, Trichostrongylidae) of ostriches. Vet Parasitol 2008a; 155(3-4): 323-327. PMid:18565673. http://dx.doi.org/10.1016/j.vetpar.2008.04.023
http://dx.doi.org/10.1016/j.vetpar.2008....
). A recent reported the occurrence of C. struthionis, correlating its presence with dry and rainy seasons. However, the authors demonstrated images of infective larvae of L. dentatus, with a knob on the larvae tip tail and a long and filamentous sheath tail (FAGUNDES et al., 2012Fagundes TF, Soleiro CA, Menezes RCAA. The occurrence of Codiostomum struthionis in ostriches (Struthio camelus) of different ages and during the dry and rainy seasons at two farms in the State of Rio de Janeiro, Brazil. Vet Parasitol 2012; 183(3-4): 269-273. PMid:21856078. http://dx.doi.org/10.1016/j.vetpar.2011.07.041
http://dx.doi.org/10.1016/j.vetpar.2011....
).
Below is a key to the identification of the infective larvae of nematodes of the genera Libyostrongylus and Codiostomum.
Conclusion
It is possible to distinguish between species of ostrich parasites by the morphological and morphometric characteristics of both adult nematodes and infective larvae, thus facilitating the diagnosis, ecology, systematic and epidemiology study of these parasites.
References
- Acomolli J, Ocayo D, Cruz ACS, Milano F, Roux JP. Aspectos morfológicos de Paradeletrocephalus minor (Molin, 1861) Freitas & Lent, 1947, em ñandu (Rhea americana), por medio de microscopio de luz y microscopio electrónico de barrido. Parasitol Latinoam 2006; 61(3-4): 183-187. http://dx.doi.org/10.4067/S0717-77122006000200016
» http://dx.doi.org/10.4067/S0717-77122006000200016 - Barton NJ, Seward DA. Detection of Libyostrongylus douglassii in ostriches in Australia. Aust Vet J 1993; 70(1): 31-32. http://dx.doi.org/10.1111/j.1751-0813.1993.tb00796.x
» http://dx.doi.org/10.1111/j.1751-0813.1993.tb00796.x - Black D. Ostrich flock health. Sem Avian Exotic Pet Med 2001; 10(3): 117-130. http://dx.doi.org/10.1053/saep.2001.24255
» http://dx.doi.org/10.1053/saep.2001.24255 - Bonadiman SF, Ederli NB, Soares AKP, Moraes Neto AHA, Santos CP, DaMatta RA. Occurrence of Libyostrongylus sp. (Nematoda) in ostriches (Struthio camelus Linnaeus, 1758) from the north region of the state of Rio de Janeiro, Brazil. Vet Parasitol 2006; 137(1-2): 175-179. http://dx.doi.org/10.1016/j.vetpar.2005.12.018
» http://dx.doi.org/10.1016/j.vetpar.2005.12.018 - Button C, Barton NJ, Veale PI, Overend DJ. A survey of Libyostrongylus douglassii on ostrich farms in Eastern Victoria. Aust Vet J 1993; 70(2): 70-76. http://dx.doi.org/10.1111/j.1751-0813.1993.tb15152.x
» http://dx.doi.org/10.1111/j.1751-0813.1993.tb15152.x - Cobbold TS. New entozoon from the ostriches. J Linn Soc 1882; 16(91): 184-188. http://dx.doi.org/10.1111/j.1096-3642.1882.tb02280.x
» http://dx.doi.org/10.1111/j.1096-3642.1882.tb02280.x - Dhillon AS. Histomoniasis in a captive great rhea (Rhea Americana). J Wildl Dis 1983; 19(3): 274. PMid:6685779. http://dx.doi.org/10.7589/0090-3558-19.3.274
» http://dx.doi.org/10.7589/0090-3558-19.3.274 - Durette-Desset MC, Denke MA. Description de noveaux nematodes parasites dun lievre African et complements à letude morphologique de quelques Trichostrongyles. Bull Mus Nat Hist Nat 1978; 354: 331-347.
- Ederli NB, Oliveira FCR. Balantidium sp. in ostriches (Struthio camelus Linnaeus, 1758) in the state of Rio de Janeiro, Brazil. Rev Bras Parasitol Vet 2008; 17(S1): 327-330.
- Ederli NB, Oliveira FCR. Differential localization of Libyostrongylus douglassii (Cobbold, 1882) Lane, 1923 and L. dentatus Hoberg, Lloyd, and Omar, 1995 (Nematoda: Trichostrongylidae) in Ostrich (Struthio camelus Linnaeus, 1758) Proventriculi. J Parasitol 2009; 95(3): 757-759. PMid:18990000. http://dx.doi.org/10.1645/GE-1853.1
» http://dx.doi.org/10.1645/GE-1853.1 - Ederli NB, Oliveira FCR, Lopes CWG, DaMatta RA, Santos CP, Rodrigues MLA. Morphological diagnosis of infective larvae of Libyostrongylus douglassii (Cobbold, 1882) Lane, 1923 and L. dentatus Hoberg, Lloyd and Omar, 1995 (Nematoda, Trichostrongylidae) of ostriches. Vet Parasitol 2008a; 155(3-4): 323-327. PMid:18565673. http://dx.doi.org/10.1016/j.vetpar.2008.04.023
» http://dx.doi.org/10.1016/j.vetpar.2008.04.023 - Ederli NB, Bonadiman SF, Moraes Neto AHA, DaMatta RA, Santos CP. Mixed infection by Libyostrongylus douglassii and L. dentatus (Nematoda: Trichostrongylidae) in Struthio camelus (Ratites: Struthioniformes) from Brazil with further morphological characterization of adults. Vet Parasitol 2008b; 151(2-4): 227-232. PMid:18155840. http://dx.doi.org/10.1016/j.vetpar.2007.11.009
» http://dx.doi.org/10.1016/j.vetpar.2007.11.009 - Ederli NB, Oliveira FCR, Lopes CWG, Rodrigues MLA. Further study of Codiostomum struthionis (Horst, 1885) Railliet and Henry, 1911 (Nematoda, Strongylidae) parasite of ostriches (Struthio camelus Linnaeus, 1758) (Aves, Struthioniformes). Vet Parasitol 2008c; 157(3-4): 275-283. PMid:18774651. http://dx.doi.org/10.1016/j.vetpar.2008.07.018
» http://dx.doi.org/10.1016/j.vetpar.2008.07.018 - Fagundes TF, Soleiro CA, Menezes RCAA. The occurrence of Codiostomum struthionis in ostriches (Struthio camelus) of different ages and during the dry and rainy seasons at two farms in the State of Rio de Janeiro, Brazil. Vet Parasitol 2012; 183(3-4): 269-273. PMid:21856078. http://dx.doi.org/10.1016/j.vetpar.2011.07.041
» http://dx.doi.org/10.1016/j.vetpar.2011.07.041 - Fockema A, Malan FS, Cooper GG, Visser E. Anthelmintic efficacy of fembendazole against Libyostrongylus douglassii and Houttuynia struthionis in ostriches. J S Afr Vet Assoc 1985; 56: 47-48. PMid:3999107.
- Foreyt WJ. Parasitas de ratitas. In: Foreyt WJ. Parasitologia veterinária: manual de referência. São Paulo: Roca; 2005. p. 181-184.
- Freitas JFT, Lent H. Revisão da subfamília Deletrocephalinae Railliet, 1916 (Nematoda, Strongyloidea). Rev Bras Biol 1947a; 7: 73-100.
- Freitas JFT, Lent H. “Spiruroidea” parasitos de “Rheiformes” (Nematoda). Mem Inst Osw Cruz 1947b; 45(4): 743-779. http://dx.doi.org/10.1590/S0074-02761947000400002
» http://dx.doi.org/10.1590/S0074-02761947000400002 - Gallo SSM, Ederli NB, Bôa-Morte MO, Oliveira FCR. Efeitos da infecção experimental em frangos, Gallus gallus, com larvas infectantes de Libyostrongylus douglassii e L. dentatus, nematóides de avestruzes, Struthio camelus. Rev Bras Med Vet 2010; 32(2): 26-32.
- Gilbert LI. New nematode Libyostrongylus magnus, n. sp., parasitic in an African ostrich. In: Skrjabin KJ. Papers on helminthology, 30 year jubilee. Moscow: Lenin Academy of Agricultural Science; 1937. p. 180-182.
- Giossa G, Trenchi H, Castro-Ramos M, Morgades D, Douza G, Castro O, et al. Hallazgos bacteriológicos y parasitológicos em una faena de ñandu (Rhea americana). Veterinaria 2004; 39(154): 11-16.
- Hoberg EP, Lloyd S, Omar H. Libyostrongylus dentatus n. sp. (Nematoda: Trichostrongylidae) from ostriches in North America, with comments on the genera Libyostrongylus and Paralibyostrongylus. J Parasitol 1995; 81(1): 85-93. PMid:7876985. http://dx.doi.org/10.2307/3284011
» http://dx.doi.org/10.2307/3284011 - Huchzermeyer FW. Diseases of farmed crocodiles and ostriches. Rev Sci Tech 2002; 21(2): 265-276. PMid:11974614.
- Huchzermeyer FW. Doenças de avestruzes e outras ratitas. Jaboticabal: Funep; 2005.
- Jansson DS, Christensson D. Gastrointestinala parasiter hos strutsfåglar i Sverige. Svensk Veterinär Tidning 2000; 52: 621-626.
- Jansson DS, Christensson DA, Christensson BE. Winter survival in Sweden of L3-stage larvae of the ostrich wireworm Libyostrongylus douglassii. Vet Parasitol 2002; 106(1): 69-74. http://dx.doi.org/10.1016/S0304-4017(02)00033-X
» http://dx.doi.org/10.1016/S0304-4017(02)00033-X - Lane C. Some Strongylata. Parasitology 1923; 15(4): 348-364. http://dx.doi.org/10.1017/S0031182000014876
» http://dx.doi.org/10.1017/S0031182000014876 - Mackereth GF. Libyostrongylus douglassii in New Zealand ostriches. Surveillance 2004; 31(3): 14-16.
- Malan FS, Gruss B, Roper NA, Ashburner AJ, Duplesis CA. Resistence of Libyostrongylus douglassii in ostriches to levamisole. J S Afr Vet Assoc 1988; 59: 202-203. PMid:3210219.
- McKenna PB. Libyostrongylus infections in ostriches: a brief review with particular reference to their detection in New Zealand. N Z Vet J 2005; 53(5): 267-270. PMid:16220116. http://dx.doi.org/10.1080/00480169.2005.36559
» http://dx.doi.org/10.1080/00480169.2005.36559 - Monteiro SG, Flores ML, Segabinazi SD, Lagaggio VRA. Ocorrência de Deletrocephalus dimidiatus (Diesing, 1851) Nematoda em ema (Rhea americana) criada em cativeiro no RS. Rev Fac Zootec Vet Agron 2002; 9(1): 100-103.
- More SJ. The performance of farmed ostrich hens in eastern Australia. Prev Vet Med 1996; 29(2): 107-120. http://dx.doi.org/10.1016/S0167-5877(96)01063-X
» http://dx.doi.org/10.1016/S0167-5877(96)01063-X - Mukaratirwa S, Cindzi ZM, Maononga DB. Prevalence of Libyostrongylus douglassii in commercially reared ostriches in the highveld region of Zimbabwe. J Helminthol 2004; 78(4): 333-336. PMid:15575991. http://dx.doi.org/10.1079/JOH2004246
» http://dx.doi.org/10.1079/JOH2004246 - O’Callaghan MG, Davies M, Andrews RH. Species of Raillietina Fuhrmann, 1920 (Cestoda: Davaineidae) from the emu, Dromaius novaehollandiae. Transac R Soc S Aust 2000; 124(2): 105-116.
- Oliveira FCR, Ederli NB, Ederli BB, Albuquerque MC, Santos MD. Ocorrência de oocistos de Cryptosporidium spp. (Apicomplexa, Cryptosporidiidae) em avestruzes, Struthio camelus L., 1758 (Aves, Struthionidae) criadas nas regiões norte e baixada litorânea do Estado do Rio de Janeiro, Brasil. Rev Bras Parasitol Vet 2008; 17(S1): 322-325. PMid:20059869.
- Oliveira FCR, Ederli NB, Lopes CWG, Rodrigues MLA. Pathological findings in the caeca of naturally infected ostriches, Struthio camelus Linnaeus, 1758 (Aves, Struthionidae) parasitized by Codiostomum struthionis (Horst, 1885) Railliet and Henry, 1911 (Nematoda, Strongylidae). Vet Parasitol 2009; 165(1-2): 175-178. PMid:19647369. http://dx.doi.org/10.1016/j.vetpar.2009.06.034
» http://dx.doi.org/10.1016/j.vetpar.2009.06.034 - Ortlepp RJ. South African helminthes, Part VI. Some helminthes, chiefly from rodents. Onderstep J Vet Sci An Ind 1939; 12: 75-101.
- Pennycott T, Patterson T. Gastrointestinal parasites of ostriches (Struthio camelus). Vet Rec 2001; 148: 155-156. PMid:11271923.
- Pintori A, Scala A, Giannetto S, Mascia M, De Rosa R. Parasitoses of ostriches (Struthio camelus) in Sardinia (Italy). Acta Parasitol 2000; 45: 164.
- Ponce Gordo FP, Herrera S, Castro AT, Durán BG, Díaz RAM. Parasites from farmed ostriches (Struthio camelus) and rheas (Rhea americana) in Europe. Vet Parasitol 2002; 107(1-2): 137-160. http://dx.doi.org/10.1016/S0304-4017(02)00104-8
» http://dx.doi.org/10.1016/S0304-4017(02)00104-8 - Popova TI. Essential of nematodology. Washington: U.S. Department of Agriculture and National Sciences Foundation; 1955. (vol. 5: Strongyloids of animals and man. Strongylidae).
- Railliet A, Henry A. Les helminthes du nandou. Bull Soc Nat Acclimatation 1911; 58: 573-582.
- Reinecke RK. Veterinary Helminthology. Durban: Butterworth; 1983.
- Rickard LG, Steinohrt LA, Black SS. Subclinical cyathostomiasis and unidentified helminthiasis in a juvenile emu (Dromaius novaehollandiae). Avian Dis 1997; 41(4): 993-996. PMid:9454939. http://dx.doi.org/10.2307/1592359
» http://dx.doi.org/10.2307/1592359 - Schuster RK. Philophthalmus aweerensis n. sp. (Trematoda: Philophthalmidae) found in a rhea (Rhea americana) in the United Arab Emirates. Parasitol Res 2011; 109(4): 1029-1033. PMid:21472402. http://dx.doi.org/10.1007/s00436-011-2340-5
» http://dx.doi.org/10.1007/s00436-011-2340-5 - Skrjabin KI. Key to parasitic nematodes. Washington: National Science Foundation: Israel Program for Scientific Translations; 1961. (vol. 3: Strongylata). Translated from Russian.
- Soares MP, Silva SS, Nizoli LQ, Felix SR, Schild AL. Chronic fascioliasis in farmed and wild greater rheas (Rhea americana). Vet Parasitol 2007; 145(1-2): 168-171. PMid:17257760. http://dx.doi.org/10.1016/j.vetpar.2006.12.007
» http://dx.doi.org/10.1016/j.vetpar.2006.12.007 - Sotiraki ST, Georgiades G, Antoniadou-Sotiriadou K, Himonas CA. Gastrointestinal parasites in ostriches (Struthio camelus). Vet Rec 2001; 148(3): 84-86. PMid:12503598. http://dx.doi.org/10.1136/vr.148.3.84
» http://dx.doi.org/10.1136/vr.148.3.84 - Theiler A, Robertson W. Investigations into the life-history of the wire-worm in ostriches. Union of South Africa Department of Agriculture; 1915. p. 293-336. Reports of the Director of Veterinary Research.
- Tišljar M, Beck R, Cooper RG, Marinculić A, Tudja M, Lukač-Novak I, et al. First finding of libyostrongylosis in farm-reared ostriches (Struthio camelus) in Croatia: unusual histopathological finding in the brain of two ostriches, naturally infected with Libyostrongylus douglasi. Vet Parasitol 2007; 147(1-2): 118-124. PMid:17448602. http://dx.doi.org/10.1016/j.vetpar.2007.03.014
» http://dx.doi.org/10.1016/j.vetpar.2007.03.014 - Travassos L. Contribuição ao conhecimento do Deletrocephalus dimidiatus Diesing, 1851 parasitos da Rhea americana. Arch Esc Sup Agr Med Vet 1933; 10: 89-97.
- Tully TM, Shane SM. Ratite management, medicine, and surgery. Malamar: Krieger Publishing; 1996.
- Vakarenko EG, Sharpilo VP. Paratenic parasitism in nematode Dicheilonema rheae (Diplotriaenoidaea, Dicheilonematinae) – parasite of nandu (Rhea Americana). Acta Parasitol 2000; 45: 164.
- Vaughan JL, Charles JA, Boray JC. Fasciola hepatica infection in farmed emus (Dromaius novaehollandiae). Aust Vet J 1997; 75(11): 811-813. PMid:9404615. http://dx.doi.org/10.1111/j.1751-0813.1997.tb15659.x
» http://dx.doi.org/10.1111/j.1751-0813.1997.tb15659.x - Vaz Z. Estudos sobre nematóides parasites de emas (Rhea americana). Arch Inst Biol 1936; 7: 253-266.
- Wehr EE. A new nematode from the rhea. Proc U S Nat Mus 1934; 82(2968): 1-5.
- Zettermann CD, Nascimento JA, Tebaldi JA, Szabó MJP. Observations on helminth infections of free-living and captive rheas (Rhea americana) in Brazil. Vet Parasitol 2005; 129(1-2): 169-172. PMid:15817218. http://dx.doi.org/10.1016/j.vetpar.2004.12.015
» http://dx.doi.org/10.1016/j.vetpar.2004.12.015
Publication Dates
-
Publication in this collection
Jul-Sep 2014
History
-
Received
24 Apr 2014 -
Accepted
7 July 2014