Abstracts
Molecular and morphological methods were evaluated to distinguish between Haemonchus contortus and Haemonchus placei species. A total of 141 H. contortus and 89 H. placei male adult specimens collected from artificially infected lambs were identified individually by PCR analysis, using a species-specific primer pair. These PCR results were used as gold standard for Haemonchus spp. identification. Haemonchus placei presented higher mean spicule and barb lengths than H. contortus (P<0.05). However, some measurements overlapped. For this reason, a discriminate function did not allow the correct identification of 13 H. contortus and one H. placei specimen. The sheath tail length of the third stage larvae (L3), which comprises the distance between the tip of the larval tail and the end of the sheath tail, were measured. Only three of the 485 H. placei larvae (0.619%) had a sheath tail shorter than 85 µm, while only four of the 500 H. contortus larvae (0.8%) presented a sheath tail longer than 85 µm. The results indicated that 6.09% of the male adult specimens would be misclassified based on the discriminate function, while only 0.71% of infective larvae would be misclassified. Therefore, identification of L3 can be used as the first method to indicate the presence of H. placei and/or H. contortus in a population of domestic ruminants.
Diagnosis; Trichostrongyloidea; molecular biology; ruminants; epidemiology
Métodos moleculares e morfológicos foram avaliados para a identificação de Haemonchus contortus e Haemonchus placei. No total, 141 H. contortus e 89 H. placei machos adultos, obtidos de cordeiros artificialmente infectados, foram identificados individualmente por PCR com o emprego de um par de “primers” espécie-específico. Esses resultados da análise por PCR foram considerados como padrão para a identificação das espécies de Haemonchus. Haemonchus placei apresentou valores médios de espículos e ganchos superiores aos de H. contortus (P<0,05). Entretanto, houve sobreposição de alguns valores. Por essa razão, a função discriminante não permitiu a identificação correta de 13 exemplares de H. contortus e de um, de H. placei. Foi medida a cauda da bainha de larvas infectantes (L3), que compreende a distância entre a ponta da cauda da larva e a ponta da cauda da bainha. Apenas três das 485 L3 de H. placei (0,619%) apresentaram a cauda da bainha com medida inferior a 85 µm e somente em quatro das 500 L3 de H. contortus (0,8%) essa medida foi superior a 85 µm. Os resultados demonstraram que 6,09% dos machos adultos seriam identificados erroneamente com base na função discriminante, enquanto a identificação incorreta de L3 seria de apenas 0,71%. Portanto, a identificação de L3 pode ser utilizada como método inicial para indicar a presença de H. placei e/ou H. contortus em uma população de ruminantes domésticos.
Diagnóstico; Trichostrongyloidea; biologia molecular; ruminantes; epidemiologia
Introduction
Haemonchus contortus and Haemonchus placei are among the most important parasites of ruminants in tropical and sub-tropical areas of the world. The prophylaxis of Haemonchus infection is based largely on the use of anthelmintic treatments. However, these treatments have not been very efficient due to the emergence of resistant parasitic populations. Therefore, efforts have focused on the development of vaccines efficient against both parasites. Studies in sheep are more advanced and have demonstrated promising results after immunization with glycoproteins obtained from the intestines of adult parasites (FITZPATRICK, 2013). Such vaccines based on H. contortus antigens have proved to be similarly effective against H. placei infection in cattle (BASSETTO et al., 2011). In areas where cross-infections between sheep and cattle parasites are not significant, grazing strategies using different ruminant species have also been exploited to produce clean pastures (GIUDICI et al., 1999Giudici C, Aumont G, Mahieu M, Saulai M, Cabaret J. Changes in gastro-intestinal helminth species diversity in lambs under mixed grazing on irrigated pastures in the tropics (French West Indies). Vet Res 1999; 30(6): 573-581. PMid:10596405.; ROCHA et al., 2008Rocha RA, Bresciani KDS, Barros TFM, Fernandes LH, Silva MB, Amarante AFT. Sheep and cattle grazing alternately: Nematode parasitism and pasture decontamination. Small Rumin Res 2008; 75(2-3): 135-143. http://dx.doi.org/10.1016/j.smallrumres.2007.09.001.
http://dx.doi.org/10.1016/j.smallrumres....
; BAILEY et al., 2009). In studies where H. contortus and H. placei are sympatric, the proper identification of both species is imperative in order to determine their role in parasitic gastroenteritis and the efficiency of these prophylactic strategies (AMARANTE, 2011).
Haemonchus species have been differentiated by morphological analyses and by the use of molecular techniques. Measurements of male spicules and their barbers are the most common method employed to differentiate both species; H. placei usually present longer spicules and barbs than H. contortus (LICHTENFELS et al., 1994; JACQUIET et al., 1996). Jacquiet et al. (1996) developed a discriminate function combining these measurements to differentiate such species. Differences in the morphology of the infective larvae (L3) have also been reported, with H. placei L3s longer, more robust, and with longer sheath tail than those of H. contortus (SANTIAGO, 1968Santiago MAM. Haemonchus Cobb, 1898 (Nematoda: Trichostrongylidae). Contribuição ao estudo da morfologia, biologia e distribuição geográfica das espécies parasitas de ovinos e bovinos no Rio Grande do Sul [Tese Livre-Docência]. Santa Maria: Universidade Federal de Santa Maria; 1968.; VAN WYK et al., 2004). There are also several approaches using molecular techniques that can be employed do differentiate H. placei from H. contortus, including the use of PCR primers to amplify ribosomal RNA genes (ZARLENGA et al., 1994), and differences in mitochondrial ND4 gene sequences (BLOUIN et al., 1997) and in the ribosomal DNA second internal transcribed spacer sequences (STEVENSON et al., 1995; BRASIL et al., 2012).
In the present study, we evaluated molecular and morphological methods for distinguishing between H. contortus and H. placei species, with emphasis on the morphological identification of third stage larvae.
Materials and Methods
Collection and examination of nematodes
Male specimens were obtained from lambs (Ovis aries) artificially infected with H. placei or H. contortus. Details about the parasite isolates, donor animal management, and infection of experimental sheep have been published by Santos et al. (2014). Briefly, one group of lambs (n=12) was serially infected 12 times (three times a week; on Mondays, Wednesdays and Fridays for four weeks) with 500 infective larvae (L3) of H. placei and then challenged with either H. placei (n=6; Group HpHp) or with H. contortus (n=6; Group HpHc). The lambs of a second group (n=12) were serially infected 12 times with 500 L3 of H. contortus and then challenged with either H. contortus (n=6; Group HcHc) or with H. placei (n=6; Group HcHp). A third group of lambs was single challenged with either H. placei (n=6; Group Hp) or H. contortus (n=6; Group Hc). Before the challenge infection, all the animals received anthelmintic treatment to eliminate worms from the serial infections. All the animals were killed 31 days after the challenge infection and worms were collected and stored in 70% ethanol.
Ten male Haemonchus worms per abomasum sample were randomly chosen for analysis, or all available specimens were analyzed when there were fewer than 10 parasites present. Haemonchus specimens were placed on a glass slide, which was then placed on a ruler to measure their length by stereomicroscopy. The needle of an insulin syringe was used to cut the worms near the copulatory bursa. The anterior region of the worm body was transferred to a 1.5 mL tube for DNA extraction, while its posterior portion was left on the slide and cleared with a drop of phenol-ethanol (80 parts melted phenol crystals and 20 parts absolute ethanol). A cover slip was placed on the specimen and spicules and barbs were measured using an ocular micrometer (Zeiss®). The measurements were used to calculate a discriminate function (DF) using the formula (DF = 0.0016 TL + 0.128 THr + 0.152 THl − 9.97) described by Jacquiet et al. (1996), where TL is the total length of the spicule, THr is the distance from the tip to the barb of the right spicule, and THl is the distance from the tip to the barb of the left spicule. Species identification was established as follows (ACHI et al., 2003):
-
DF <0.63: Haemonchus contortus
-
0.63 <DF <3: Haemonchus placei
Third stage larvae production and measurement
Composite fecal cultures were prepared for each group of sheep with the feces of donor lambs infected with H. contortus and donor calves infected with H. placei. Sheep fecal pellets were crumbled and put in wide-mouthed glass jars of approximately 0.5 L capacity to a depth of about 8 cm. Each glass jar was covered with a Petri dish and incubated in the dark at 26°C for 7 days. In the preparation of cultures with cattle feces, samples were mixed with sterilized horse feces before being placed in the jar in order to reduce the moisture. Larvae were harvested from cultures as described by Ueno & Gonçalves (1998).
A drop of larval suspension and the larvae killed with Lugol's iodine solution were deposited on a glass microscope slide. The sheath tail was measured using an ocular micrometer (Zeiss®). The sheath tail length comprises the distance between the tip of the larval tail and the end of the sheath tail. When possible, 100 larvae per fecal culture were measured.
Molecular analysis
Genomic DNA was extracted from each Haemonchus specimen using a QIAamp® DNA Mini Kit (QIAGEN, Hilden, Germany) according to the manufacturer's instructions. DNA from the cattle and sheep blood samples was also extracted for use as controls in PCR analysis.
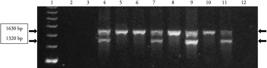
DNA samples were amplified individually by PCR with the primer pair 75 and 86 described by Zarlenga et al. (1994). Haemonchus contortus samples presented two DNA bands (1.63 and 1.32 kb) and H. placei a single one (1.63 kb). The PCR reactions were performed using a GeneAmp® PCR System 9700 thermal cycler (Applied Biosystems, Foster City, California, USA). Amplification was performed in a 10 µL reaction volume containing 10 mM Tris-HCl, 1.5 mM MgCl2, 50 mM KCl (pH 8.0), 100 µM each dNTP, 20-50 ng genomic DNA and 0.5 U Taq polymerase (Invitrogen, Carlsbad, California, USA). The cycling conditions were as follows: 95°C for 5 min, followed by 35 cycles of 95°C for 1 min, 57°C for 1 min and 72°C for 2 min, followed by 10 min at 72°C and 4°C to finalize. The PCR products were electrophoresed on 2% agarose gel in 1% TAE buffer containing ethidium bromide and photographed under UV light using a Sony Cyber-shot DSC-HX1 camera (Sony Electronics, San Diego, California, USA).
Statistical analysis
Data on adult worm and larvae length were analyzed by one-way analysis of variance. Group means were compared by Tukey's test at 5% significance level. The Kappa statistics (coefficient of agreement) was calculated using the Statistical Analysis System, version 9.2 (SAS Institute, Inc., Cary, NC, USA).
Results
A total of 141 H. contortus and 89 H. placei individual male adult specimens were identified by PCR analysis. To illustrate, Figure 1 presents the PCR results of single specimens obtained from each animal group. H. contortus samples presented two bands (1.32 kb and 1.63 kb), while only one band, 1.63 kb, was present in H. placei samples (Figure 1). The PCR reactions confirmed that the animals were infected only with H. placei or H. contortus according to its groups (Table 1).
PCR reactions with the 75/86 primer pair described by Zarlenga et al. (1994) and DNA samples. Lane 1 shows 250 bp molecular markers (GE Healthcare); 2 and 3 –bovine and ovine DNA, respectively (hosts); 4 – H. contortus (control); 5 - H. placei (control); 6 – H. placei from group HpHp; 7 – H. contortus from group HpHc; 8 – H. placei from group Hp; 9 – H. contortus from group HcHc; 10 – H. placei from group HcHp; 11 – H. contortus from group Hc; and 12 – reagents without DNA.
Table 1 describes the morphometrics of the male worms identified by PCR. In all the groups, H. placei presented longer mean spicule and barb lengths than H. contortus (P<0.05), although some measurements overlapped (Table 1). Therefore, the discriminate function did not enable the correct identification of 13 H. contortus specimens (9.78%) whose discriminate function was higher than 0.63 and of a single H. placei specimen whose discriminate function was 0.41, the value of H. contortus. Nevertheless, the agreement between PCR and discriminate function for specific diagnosis was relatively high, with a Kappa coefficient of 0.8748 (Table 2).
There were no consistent differences between species body length (Table 1), although H. placei presented the highest mean length (12.37 mm in group Hp) and H. contortus the lowest (10.90 mm in group HpHc). In contrast, there was a consistent difference in sheath tail length between species, regardless of their source, cattle or sheep (Table 3). Only three of the 485 H. placei larvae (0.619%) had sheath tails shorter than 85 µm, while only four of the 500 H. contortus larvae (0.8%) presented sheath tails longer than 85 µm (Table 3).
Discussion
The body length of H. contortus males ranged from 9 to 15 mm, with a general mean length of 11.4 mm, which is smaller than the mean lengths recorded for H. contortus specimens obtained elsewhere. Haemonchus contortus obtained from sheep and goats in Malaysia and Yemen presented mean values in the range of 14.3 – 18.2 mm, with morphological variations between populations (GHARAMAH et al., 2014). Similarly, higher mean values of males were recorded is specimens recovered from Rhön (13.35 mm) and Merinoland (15.14 mm) lambs 8 weeks after an experimental infection with 5000 H. contortus L3 (GAULY et al., 2002), and in lambs with primary experimental infection with three isolates of H. contortus: from Spain (Aran 99), Moredun Research Institute (MRI), and Merck Sharp and Dohme (MSD). The lowest means was recorded in MSD males (11.94 mm) and the highest in the MRI (13.44 mm) (ANGULO-CUBILLÁN et al., 2010). These differences in body size may be due to genetic differences among populations. In Australia, significant observable genetic and phenotypic divergences were found between isolates of H. contortus, which differed in their apparent infectivity and morphology, as estimated by worm length and vulval appendages in females (HUNT et al., 2008). In addition, worm body length may be influenced by age, with older parasites showing larger sizes (COADWELL & WARD, 1975), making it difficult to compare the results of studies that used different methodologies to obtain worms.
Despite some overlapping of measurements, H. placei presented significantly longer spicules and barbs than H. contortus. Lichtenfels et al. (1994), who analyzed specimens obtained in several American states and in different locations around the world, reported similar values to those described in the present study. They also observed overlapping of measurements between the two species, which is in agreement with the results of this study. For this reason, 14 Haemonchus adult specimens (13 H. contortus and one H. placei) were misdiagnosed by the discriminate function in the present trial. The discriminate function was also not able to accurately identify Haemonchus males at the species level from sheep raised in the Czech Republic (VADLEJCH et al., 2014Vadlejch J, Lukešová D, Vašek J, Vejl P, Sedlák P, Čadková Z, et al. Comparative morphological and molecular identification of Haemonchus species in sheep. Helminthologia 2014; 51(2): 130-140. http://dx.doi.org/10.2478/s11687-014-0220-0.
http://dx.doi.org/10.2478/s11687-014-022...
). Therefore, it is important to highlight that when specimens present intermediate size with a discriminate function between 0.4 and 1.6, using the formula described by Jacquiet et al. (1996) and Achi et al. (2003), another technique must be employed to properly identify individual male specimens.
Despite the laboratorial differences in the methodology used in fecal cultures to produce the third stage larvae, as well as the genetic differences in Haemonchus populations from different parts of the world, very consistent results can be observed with respect to the difference between the length of the sheath tail extension of H. contortus and H. placei. Our findings indicate that the 85 µm could be used as the limit-value to differentiate the two species. In a pioneering study in Australia, Keith (1953)Keith RK. The differentiation of the infective larvae of some common nematode parasites of cattle. Aust J Zool 1953; 1(2): 223-235. http://dx.doi.org/10.1071/ZO9530223.
http://dx.doi.org/10.1071/ZO9530223...
observed that Haemonchus infective larvae from cattle were longer and more robust than those from sheep and had a tail with a much longer whip-like filament. The distance between the tip of the larval tail and the tip of the tail of the sheath was in the range of 87-119 µm in Haemonchus from cattle (KEITH, 1953Keith RK. The differentiation of the infective larvae of some common nematode parasites of cattle. Aust J Zool 1953; 1(2): 223-235. http://dx.doi.org/10.1071/ZO9530223.
http://dx.doi.org/10.1071/ZO9530223...
), which was longer than lengths reported previously in Haemonchus from small ruminants raised in Britain (70-84 µm) (MORGAN, 1930Morgan DO. On the differential diagnosis of the larvae of some helminth parasites of sheep and goats. J Helminthol 1930; 8(4): 223-228. http://dx.doi.org/10.1017/S0022149X00030121.
http://dx.doi.org/10.1017/S0022149X00030...
) or in the USA (65-78 µm) (DIKMANS & ANDREWS, 1933Dikmans G, Andrews JS. A comparative morphological study of the infective larvae of the common nematodes parasitic in the alimentary tract of sheep. Trans Am Microsc Soc 1933; 52(1): 1-25. http://dx.doi.org/10.2307/3222221.
http://dx.doi.org/10.2307/3222221...
). Roberts et al. (1954)Roberts FHS, Newton-Turner H, McKevett M. On the specific distinctness of the ovine and bovine ‘Strains’ of Haemonchus contortus (Rudolphi) Cobb (Nematoda: Trichostronglidae). Aust J Zool 1954; 2(2): 275-295. http://dx.doi.org/10.1071/ZO9540275.
http://dx.doi.org/10.1071/ZO9540275...
also observed that infective larvae obtained from naturally occurring infestations in cattle and sheep could be distinguished visually without any difficulty. Significant differences were found in measurements of total length and tail length. According to Roberts et al. (1954)Roberts FHS, Newton-Turner H, McKevett M. On the specific distinctness of the ovine and bovine ‘Strains’ of Haemonchus contortus (Rudolphi) Cobb (Nematoda: Trichostronglidae). Aust J Zool 1954; 2(2): 275-295. http://dx.doi.org/10.1071/ZO9540275.
http://dx.doi.org/10.1071/ZO9540275...
, the probability of misclassification based on tail length was only 0.03%. Moreover, the larvae of females established in sheep from larvae taken from cattle maintained their cattle-type and vice versa, which is in agreement with our results. In isolates from the state of Rio Grande do Sul, Brazil, Santiago (1968)Santiago MAM. Haemonchus Cobb, 1898 (Nematoda: Trichostrongylidae). Contribuição ao estudo da morfologia, biologia e distribuição geográfica das espécies parasitas de ovinos e bovinos no Rio Grande do Sul [Tese Livre-Docência]. Santa Maria: Universidade Federal de Santa Maria; 1968. reported that the mean sheath tail length of infective larvae was 73.6 µm for H. contortus and 99.2 µm for H. placei. Similarly, van Wyk et al. (2004) reported a mean sheath tail extension length of 102 µm (range of 80-119 µm) for H. placei and of 74 µm (range of 65-82 µm) for H. contortus. These results obtained from populations of H. placei and H. contortus in different parts of the world are in concordance with those observed in the present study, which indicate that the sheath tail of H. placei larvae is usually longer than 85 µm, while that of H. contortus larvae is shorter than 85 µm, with a very small proportion of outliers, usually less than 1%. It is important to emphasize, however, that identification of third stage larvae requires very well trained staff, especially when animals present mixed nematode infections. The differences between Cooperia spp. and Haemonchus larvae, for instance, are subtle and are based on the presence of two refractile spots in the head of Cooperia (VAN WYK et al., 2004).
In conclusion, our results showed that 6.09% of the male adult specimens were misclassified based on the discriminate function, which uses spicule measurements, while only 0.71% of infective larvae were misclassified. Thus, L3 measures can be used as the first method to indicate if H. placei and H. contortus are present in a population of domestic ruminants. Although molecular techniques are the gold standard for specific identification of Haemonchus spp., identification based on the sheath tail length of infective larvae is a simple technique that does not require killing to obtain adult parasites for morphological evaluation, and its cost is lower than that of molecular diagnosis.
Acknowledgments
This study was supported by FAPESP (São Paulo Research Foundation) (grant number 07/07182-6). Michelle Cardoso dos Santos, Maria Regina Lucas da Silva and Alessandro F.T. Amarante gratefully acknowledge the financial support received, respectively, from FAPESP, CAPES (Federal Agency for the Support and Improvement of Higher Education), and CNPq (National Council for Scientific and Technological Development).
References
- Achi YL, Zinsstag J, Yao K, Yeo N, Dorchies P, Jacquiet P. Host specificity of Haemonchus spp. for domestic ruminants in the savanna in northern Ivory Coast. Vet Parasitol 2003; 116(2): 151-158. http://dx.doi.org/10.1016/S0304-4017(03)00258-9 PMid:14519319
» http://dx.doi.org/10.1016/S0304-4017(03)00258-9 - Amarante AF. Why is it important to correctly identify Haemonchus species? Rev Bras Parasitol Vet 2011; 20(4): 263-268. http://dx.doi.org/10.1590/S1984-29612011000400002 PMid:22166378
» http://dx.doi.org/10.1590/S1984-29612011000400002 - Angulo-Cubillán FJ, García-Coiradas L, Alunda JM, Cuquerella M, de la Fuente C. Biological characterization and pathogenicity of three Haemonchus contortus isolates in primary infections in lambs. Vet Parasitol 2010; 171(1-2): 99-105. http://dx.doi.org/10.1016/j.vetpar.2010.03.004 PMid:20363563
» http://dx.doi.org/10.1016/j.vetpar.2010.03.004 - Bailey JN, Walkden-Brown SW, Kahn LP. Comparison of strategies to provide lambing paddocks of low gastro-intestinal nematode infectivity in a summer rainfall region of Australia. Vet Parasitol 2009; 161(3-4): 218-231. http://dx.doi.org/10.1016/j.vetpar.2009.01.016 PMid:19243890
» http://dx.doi.org/10.1016/j.vetpar.2009.01.016 - Bassetto CC, Silva BF, Newlands GFJ, Smith WD, Amarante AFT. Protection of calves against Haemonchus placei and Haemonchus contortus after immunization with gut membrane proteins from H. contortus. Parasite Immunol 2011; 33(7): 377-381. http://dx.doi.org/10.1111/j.1365-3024.2011.01295.x PMid:21535018
» http://dx.doi.org/10.1111/j.1365-3024.2011.01295.x - Blouin MS, Yowell CA, Courtney CH, Dame JB. Haemonchus placei and Haemonchus contortus are distinct species based on mtDNA evidence. Int J Parasitol 1997; 27(11): 1383-1387. http://dx.doi.org/10.1016/S0020-7519(97)00125-2 PMid:9421728
» http://dx.doi.org/10.1016/S0020-7519(97)00125-2 - Brasil BSAF, Nunes RL, Bastianetto E, Drummond MG, Carvalho DC, Leite RC, et al. Genetic diversity patterns of Haemonchus placei and Haemonchus contortus populations isolated from domestic ruminants in Brazil. Int J Parasitol 2012; 42(5): 469-479. http://dx.doi.org/10.1016/j.ijpara.2012.03.003 PMid:22787588
» http://dx.doi.org/10.1016/j.ijpara.2012.03.003 - Coadwell WJ, Ward PFV. Observations on the development of Haemonchus contortus in young sheep given a single infection. Parasitology 1975; 71(3): 505-515. http://dx.doi.org/10.1017/S0031182000047260 PMid:1202415
» http://dx.doi.org/10.1017/S0031182000047260 - Dikmans G, Andrews JS. A comparative morphological study of the infective larvae of the common nematodes parasitic in the alimentary tract of sheep. Trans Am Microsc Soc 1933; 52(1): 1-25. http://dx.doi.org/10.2307/3222221
» http://dx.doi.org/10.2307/3222221 - Fitzpatrick JL. Global food security: the impact of veterinary parasites and parasitologists. Vet Parasitol 2013; 195(3-4): 233-248. http://dx.doi.org/10.1016/j.vetpar.2013.04.005 PMid:23622818
» http://dx.doi.org/10.1016/j.vetpar.2013.04.005 - Gauly M, Kraus M, Vervelde L, van Leeuwen MAW, Erhardt G. Estimating genetic differences in natural resistance in Rhön and Merinoland sheep following experimental Haemonchus contortus infection. Vet Parasitol 2002; 106(1): 55-67. http://dx.doi.org/10.1016/S0304-4017(02)00028-6 PMid:11992711
» http://dx.doi.org/10.1016/S0304-4017(02)00028-6 - Gharamah AA, Rahman WA, Siti Azizah MN. Morphological variation between isolates of the nematode Haemonchus contortus from sheep and goat populations in Malaysia and Yemen. J Helminthol 2014; 88(1): 82-88. http://dx.doi.org/10.1017/S0022149X12000776 PMid:23176779
» http://dx.doi.org/10.1017/S0022149X12000776 - Giudici C, Aumont G, Mahieu M, Saulai M, Cabaret J. Changes in gastro-intestinal helminth species diversity in lambs under mixed grazing on irrigated pastures in the tropics (French West Indies). Vet Res 1999; 30(6): 573-581. PMid:10596405.
- Hunt PW, Knox MR, Le Jambre LF, McNally J, Anderson LJ. Genetic and phenotypic differences between isolates of Haemonchus contortus in Australia. Int J Parasitol 2008; 38(8-9): 885-900. http://dx.doi.org/10.1016/j.ijpara.2007.11.001 PMid:18068173
» http://dx.doi.org/10.1016/j.ijpara.2007.11.001 - Jacquiet P, Cabaret J, Cheikh D, Thiam E. Identification of Haemonchus species in domestic ruminants based on morphometrics of spicules. Parasitol Res 1996; 83(1): 82-86. http://dx.doi.org/10.1007/s004360050213 PMid:9000240
» http://dx.doi.org/10.1007/s004360050213 - Keith RK. The differentiation of the infective larvae of some common nematode parasites of cattle. Aust J Zool 1953; 1(2): 223-235. http://dx.doi.org/10.1071/ZO9530223
» http://dx.doi.org/10.1071/ZO9530223 - Lichtenfels JR, Pilitt PA, Hoberg EP. New morphological characters for identifying individual specimens of Haemonchus spp. (Nematoda: Trichostrongyloidea) and a key to species in ruminants of North America. J Parasitol 1994; 80(1): 107-119. http://dx.doi.org/10.2307/3283353 PMid:8308643
» http://dx.doi.org/10.2307/3283353 - Morgan DO. On the differential diagnosis of the larvae of some helminth parasites of sheep and goats. J Helminthol 1930; 8(4): 223-228. http://dx.doi.org/10.1017/S0022149X00030121
» http://dx.doi.org/10.1017/S0022149X00030121 - Rocha RA, Bresciani KDS, Barros TFM, Fernandes LH, Silva MB, Amarante AFT. Sheep and cattle grazing alternately: Nematode parasitism and pasture decontamination. Small Rumin Res 2008; 75(2-3): 135-143. http://dx.doi.org/10.1016/j.smallrumres.2007.09.001
» http://dx.doi.org/10.1016/j.smallrumres.2007.09.001 - Roberts FHS, Newton-Turner H, McKevett M. On the specific distinctness of the ovine and bovine ‘Strains’ of Haemonchus contortus (Rudolphi) Cobb (Nematoda: Trichostronglidae). Aust J Zool 1954; 2(2): 275-295. http://dx.doi.org/10.1071/ZO9540275
» http://dx.doi.org/10.1071/ZO9540275 - Santiago MAM. Haemonchus Cobb, 1898 (Nematoda: Trichostrongylidae). Contribuição ao estudo da morfologia, biologia e distribuição geográfica das espécies parasitas de ovinos e bovinos no Rio Grande do Sul [Tese Livre-Docência]. Santa Maria: Universidade Federal de Santa Maria; 1968.
- Santos MC, Xavier JK, Amarante MRV, Bassetto CC, Amarante AFT. Immune response to Haemonchus contortus and Haemonchus placei in sheep and its role on parasite specificity. Vet Parasitol 2014; 203(1-2): 127-138. http://dx.doi.org/10.1016/j.vetpar.2014.02.048 PMid:24670867
» http://dx.doi.org/10.1016/j.vetpar.2014.02.048 - Stevenson LA, Chilton NB, Gasser RB. Differentiation of Haemonchus placei from H. contortus (Nematoda: Trichostrongylidae) by the ribosomal DNA second internal transcribed spacer. Int J Parasitol 1995; 25(4): 483-488. http://dx.doi.org/10.1016/0020-7519(94)00156-I PMid:7635624
» http://dx.doi.org/10.1016/0020-7519(94)00156-I - Ueno H, Gonçalves PC. Manual para diagnóstico das helmintoses de ruminantes. 4th ed. Tokyo: Japan International Cooperation Agency; 1998. 149 p.
- Vadlejch J, Lukešová D, Vašek J, Vejl P, Sedlák P, Čadková Z, et al. Comparative morphological and molecular identification of Haemonchus species in sheep. Helminthologia 2014; 51(2): 130-140. http://dx.doi.org/10.2478/s11687-014-0220-0
» http://dx.doi.org/10.2478/s11687-014-0220-0 - van Wyk JA, Cabaret J, Michael LM. Morphological identification of nematode larvae of small ruminants and cattle simplified. Vet Parasitol 2004; 119(4): 277-306. http://dx.doi.org/10.1016/j.vetpar.2003.11.012 PMid:15154594
» http://dx.doi.org/10.1016/j.vetpar.2003.11.012 - Zarlenga DS, Stringfellow F, Nobary M, Lichtenfels JR. Cloning and characterization of ribosomal RNA genes from three species of Haemonchus (Nematoda: Trichostrongyloidea) and identification of PCR primers for rapid differentiation. Exp Parasitol 1994; 78(1): 28-36. http://dx.doi.org/10.1006/expr.1994.1003 PMid:8299758
» http://dx.doi.org/10.1006/expr.1994.1003
Publication Dates
-
Publication in this collection
Dec 2014
History
-
Received
5 June 2014 -
Accepted
11 Aug 2014