Abstract
The aim of this study was to investigate post-immunization apoptotic changes in experimental hydatidosis, using Caspase 3 and p53 immunohistochemical markers. Two groups of rabbits were immunized with a crude antigen (group 1) or a partially purified antigen (group 2) and were compared to an infected non-immunized control group. More effective immune responses were obtained in group 2 than group 1, signified by fewer and smaller cystic lesions and more severe destructive changes. Normal growth of cysts was attained in the control group, with no expression of apoptotic markers. Significantly higher expression of Caspase 3 and p53 were observed in group 1 compared to group 2, as indicated by OD and area percentage, respectively (Group 1 Caspase 3: 0.89±0.21, 93.5%±6.2; Group 1 p53: 0.46±0.18, 53.26%±11.6; Group 2 Caspase 3: 0.52±0.15, 49.23%±11.7; Group 2 p53: 0.19±0.4, 18.17%±7.3). Vaccine-induced immune responses and cellular damage may underlie the expression of apoptotic markers that appeared to result in a degenerative and atrophic course of action upon immunization. The results of the current study emphasize the importance of immunization for the stimulation of protective immune responses and in preventing mechanisms of evasion to ensure normal cell growth. A cost/benefit control program that implements proper vaccine preparations should be further assessed for complete elimination of severe infections in endemic areas.
Keywords:
Experimental hydatidosis; Caspase 3; p53; apoptosis
Resumo
O objetivo do presente estudo foi investigar mudanças de apoptose pós-imunização em hidatidose experimental, usando os marcadores imuno-histoquímicos Caspase 3 e p53. Dois grupos de coelhos foram imunizados com antígeno bruto (grupo 1) e com antígeno parcialmente purificado (grupo 2). Estes grupos foram comparados a um grupo controle infectado e não-imunizado. Respostas imunes mais eficientes foram obtidas do grupo 2, que apresentou lesões císticas menores e menos frequentes, e mudanças destrutivas mais graves. Cistos cresceram normalmente no grupo controle, sem expressão dos marcadores de apoptose. Expressões significativamente mais altas de Caspase 3 e p53 foram observadas no grupo 1 quando comparado ao grupo 2, como indicado por DO e área de percentagem, respectivamente (Grupo 1 Caspase 3: 0,89±0,21, 93,5%±6,2; Grupo 1 p53: 0,46±0,18, 53,26%±11,6; Grupo 2 Caspase 3: 0,52±0,15, 49,23%±11,7; Grupo 2 p53: 0,19±0,4, 18,17%±7,3). Respostas imunológicas induzidas por vacinas e danos celulares podem ser a base para a expressão dos marcadores de apoptose cujos desfechos demonstraram ação degenerativa e atrófica durante imunização. Os resultados do presente estudo enfatizam a importância da imunização para o estímulo de respostas imunes de proteção e para mecanismos de prevenção de evasão para garantir crescimento celular normal. Um programa de controle de custo/benefício que implemente preparações de vacinas adequadas deve ser analisado em mais detalhe para a completa eliminação de infecções graves em áreas endêmicas.
Palavras-chave:
Hidatidose experimental; Caspase 3; p53; apoptose
Introduction
Hydatidosis or cystic Echinococcus is an important zoonosis that affects agricultural countries, causing considerable socio-economic consequences. Transmission to intermediate hosts (IHs), particularly cattle and sheep, predominantly occurs through contact with infected dogs (THOMPSON et al., 1995Thompson RC, Lymbery AJ, Constantine CC. Variation in Echinococcus: towards a taxonomic revision of the genus. Adv Parasitol 1995; 35: 145-176. http://dx.doi.org/10.1016/S0065-308X(08)60071-8. PMid:7709852.
http://dx.doi.org/10.1016/S0065-308X(08)...
). The disease is of a public health concern in both endemic and non-endemic regions due to the migration of infected people and exchange of livestock between these regions. In Egypt, E. granulosus is an endemic zoonotic disease (ELSHAZLY et al., 2007Elshazly AM, Awad SE, Hegazy MA, Mohammad KA, Morsy TA. hydatidosis an endemic zoonotic disease in Egypt. Echinococcosis granulosus/J Egypt Soc Parasitol 2007; 37(2): 609-622. PMid:17985592.). Livestock species play an important role in the transmission of the parasite to domestic dogs and subsequently, to humans (ECKERT & DEPLAZES, 2004Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev 2004; 17(1): 107-135. http://dx.doi.org/10.1128/CMR.17.1.107-135.2004. PMid:14726458.
http://dx.doi.org/10.1128/CMR.17.1.107-1...
), but despite the large amount of data concerning the long-term evaluation of various control strategies for E. granulosus, no commercial vaccine is available for use in livestock. However, research into various control strategies is ongoing.
The selection of a pathogen’s appropriate genetic variants is crucial for a successful vaccine trial. Strains vary in their antigenicity, which may allow the pathogen to escape vaccine-induced immune responses (LIGHTOWLERS et al., 2004Lightowlers MW, Biyikoglu G, Chow C, Cowman AF, Gauci CG, Heath DD, et al. Vaccination against hydatidosis: anticipating the potential for antigenic variation. Southeast Asian J Trop Med Public Health 2004; 35(S1): 206-212.). Several vaccine candidates for IHs have been tested and were shown to elicit variable degrees of protection (LIGHTOWLERS et al., 1996Lightowlers MW, Lawrence SB, Gauci CG, Young J, Ralston MJ, Maas D, et al. Vaccination against hydatidosis using a defined recombinant antigen. Parasite Immunol 1996; 18(9): 457-462. http://dx.doi.org/10.1111/j.1365-3024.1996.tb01029.x. PMid:9226681.
http://dx.doi.org/10.1111/j.1365-3024.19...
, 1999Lightowlers MW, Jensen O, Fernandez E, Iriarte JA, Woollard DJ, Gauci CG, et al. Vaccination trials in Australia and Argentina confirm the effectiveness of the EG95 hydatid vaccine in sheep. Int J Parasitol 1999; 29(4): 531-534. http://dx.doi.org/10.1016/S0020-7519(99)00003-X. PMid:10428628.
http://dx.doi.org/10.1016/S0020-7519(99)...
, 2000Lightowlers MW, Flisser A, Gauci CG, Heath DD, Jensen O, Rolfe R. Vaccination against cysticercosis and hydatid disease. Parasitol Today 2000; 16(5): 191-196. http://dx.doi.org/10.1016/S0169-4758(99)01633-6. PMid:10782077.
http://dx.doi.org/10.1016/S0169-4758(99)...
; WOOLLARD et al., 1998Woollard DJ, Gauci CG, Heath DD, Lightowlers MW. Epitope specificities and antibody responses to the EG95 hydatid vaccine. Parasite Immunol 1998; 20(11): 535-540. http://dx.doi.org/10.1046/j.1365-3024.1998.00176.x. PMid:9988310.
http://dx.doi.org/10.1046/j.1365-3024.19...
). Although there has been extensive research into post-immunization antibody responses, other aspects of the vaccine-induced immune response remain unknown, including those that affect apoptotic changes underlying fertility, suppression and survival of hydatid cysts (SPOTIN et al., 2012Spotin A, Majdi MMA, Sankian M, Varasteh A. The study of apoptotic bifunctional effects in relationship between host and parasite in cystic echinococcosis: a new approach to suppression and survival of hydatid cyst. Parasitol Res 2012; 110(5): 1979-1984. http://dx.doi.org/10.1007/s00436-011-2726-4. PMid:22167369.
http://dx.doi.org/10.1007/s00436-011-272...
). Research into apoptosis has increased substantially since the early 1990s. The apoptosis pathway is induced by specific proteins, such as Caspase 3, and is regulated by several factors, such as p53, a key regulator of cell cycle arrest (SCHULER et al., 2000Schuler M, Bossy-Wetzel E, Goldstein JC, Fitzgerald P, Green DR. p53 induces apoptosis by Caspase activation through mitochondrial cytochrome release. cJ Biol Chem 2000; 275(10): 7337-7342. http://dx.doi.org/10.1074/jbc.275.10.7337. PMid:10702305.
http://dx.doi.org/10.1074/jbc.275.10.733...
). However, data regarding apoptosis in sensitized hydatid hosts is not available. The aim of this study was to investigate post-immunization histopathological and apoptotic changes following immunization by two different hydatid antigens, a partially purified antigen from an imported strain and a crude antigen prepared from a local strain.
Materials and Methods
Antigen preparation
Crude local antigen was prepared according to Dada & Belino (1981)Dada BJ, Belino ED. Immunization of sheep against cystic hydatidosis with homologous and heterologous metacestode antigens. Int J Zoonoses 1981; 8(1): 20-25. PMid:7333782. and Hashemitabar et al. (2006)Hashemitabar GR, Razmi GR, Naghibi A. Protective immunity in mice with whole body of Echinococcus granulosus.Iran Biomed J 2006; 10(1): 51-55.. Hydatid cyst fluid (HCF) was collected from recently slaughtered infected Egyptian camels under strict sterile conditions. Infected or infertile HCF samples were excluded. HCF was subject to three freeze/thaw cycles by freezing in liquid nitrogen and thawing at 42°C. The suspension was then mixed with four volumes of phosphate buffer saline (PBS) at pH 7.4, containing 0.1 mg/ml sodium azide. The sample was then sonicated in a 170 W ultrasonicator for 3x15 sec pulses on ice at a high output setting until no intact protoscolices were visible. The preparation was centrifuged for 30 min at 10,000 g and then filtered (0.22μm) and stored at -20°C until use. The protein concentration of the prepared sample was determined to be 100 µg/ml, using a method previously described by Bradford (1976)Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72(1-2): 248-254. http://dx.doi.org/10.1016/0003-2697(76)90527-3. PMid:942051.
http://dx.doi.org/10.1016/0003-2697(76)9...
. Partially purified antigen B (AgB) was kindly provided by the Department of Microbiology & Biotechnology, National Research Centre, and Cairo, Egypt. The antigen was prepared from an imported strain using column chromatography.
Immunization of infected animals
The work was carried out according to national guidelines for animal welfare and approved by our institutional ethics committee. Animals were kept under normal laboratory conditions, fed with a standard diet and had free access to water. Fifteen New Zealand white rabbits (provided by the Experimental Animal Centre at the National research Center, Cairo, Egypt) of mixed sex (2 months of age, weighing 2.5-3 kg), were randomly allocated to 3 groups of 5 rabbits each: 1) infected animals vaccinated with locally prepared crude antigen, 2) infected animals vaccinated with partially purified imported antigen, and 3) an infected control group receiving no vaccine. Immunizations were administered subcutaneously in the back at a total volume of 1 ml (100 µg dissolved in 1 ml of PBS) and emulsified with an equal volume of complete Freund's adjuvant (CFA), respectively. Animals received 2 booster vaccines 3 weeks apart, with the same antigen preparation emulsified in incomplete Freund's adjuvant (IFA) in place of CFA. Based on a protocol described by Saad & Magzoub (1988)Saad MB, Magzoub M. Experimental transmission of hydatid cysts from camels and cattle to dogs. Ann Trop Med Parasitol 1988; 82(4): 363-365. PMid:3252759., the gravid segments of E. granulosus were collected from laboratory-bred puppies infected by fertile E. granulosus cysts that were derived from the same origin (camel) that was used to prepare the local antigen. As described by Lightowlers et al. (1999)Lightowlers MW, Jensen O, Fernandez E, Iriarte JA, Woollard DJ, Gauci CG, et al. Vaccination trials in Australia and Argentina confirm the effectiveness of the EG95 hydatid vaccine in sheep. Int J Parasitol 1999; 29(4): 531-534. http://dx.doi.org/10.1016/S0020-7519(99)00003-X. PMid:10428628.
http://dx.doi.org/10.1016/S0020-7519(99)...
, 10 days after the last immunization and upon 12 h of starvation, the animals were infected with 10 gravid proglottides through gavage using a syringe, which was washed with approximately 20 ml water with a second syringe to ensure the delivery of the same dose to each animal. Rabbits were sacrificed 4 months after infection and their internal organs were examined for the presence of hydatid cysts.
Light microscopy and immunohistochemical examination of tissue samples
Preparations for structural histological examination were performed according to Prophet et al. (1992)Prophet EB, Mills B, Arrington JB, Sobin LH. Laboratory methods in histotechnology. Washington: Armed Forces Institute of Pathology, American Registry of Pathology; 1992.. After preparing the tissues for immunohistochemistry, primary antibodies for the individual apoptotic markers (Caspase 3 and p53) were applied, followed by staining with secondary antibodies. Biotinylated goat anti-polyvalent was then applied and incubated for 10 min. After each step, the sections were washed four times in PBS. One or two drops of 3,3' diaminobenzidine chromogen DAB was added to 1 ml of DAB substrate, mixed by swirling and applied to the tissues, which were then incubated for 5-15 min, depending on the desired stain intensity (chemicals were purchased from Biological, P.O Box 261, Swampscott, Massachusetts, United States).
Image analysis by real time quantitative morphocytometry
Pathological and morphometric analysis was performed using the Leica Qwin 500 Image Analyzer (LEICA Imaging Systems Ltd, Cambridge, England). Slides were examined at 100x and 200x magnification. Optical density (OD) and area percentage of each marker were automatically measured in 10 fields on real-time images from the microscope, and the values were saved for further analysis. Data were expressed as mean and standard deviation. Comparison between two groups was done using Student t. test. Comparison between different groups (more than 2) was done by (ANOVA) followed by pair wise analysis (Bonferroni test). A p-value ≤ 0.05 was considered significant.
Results
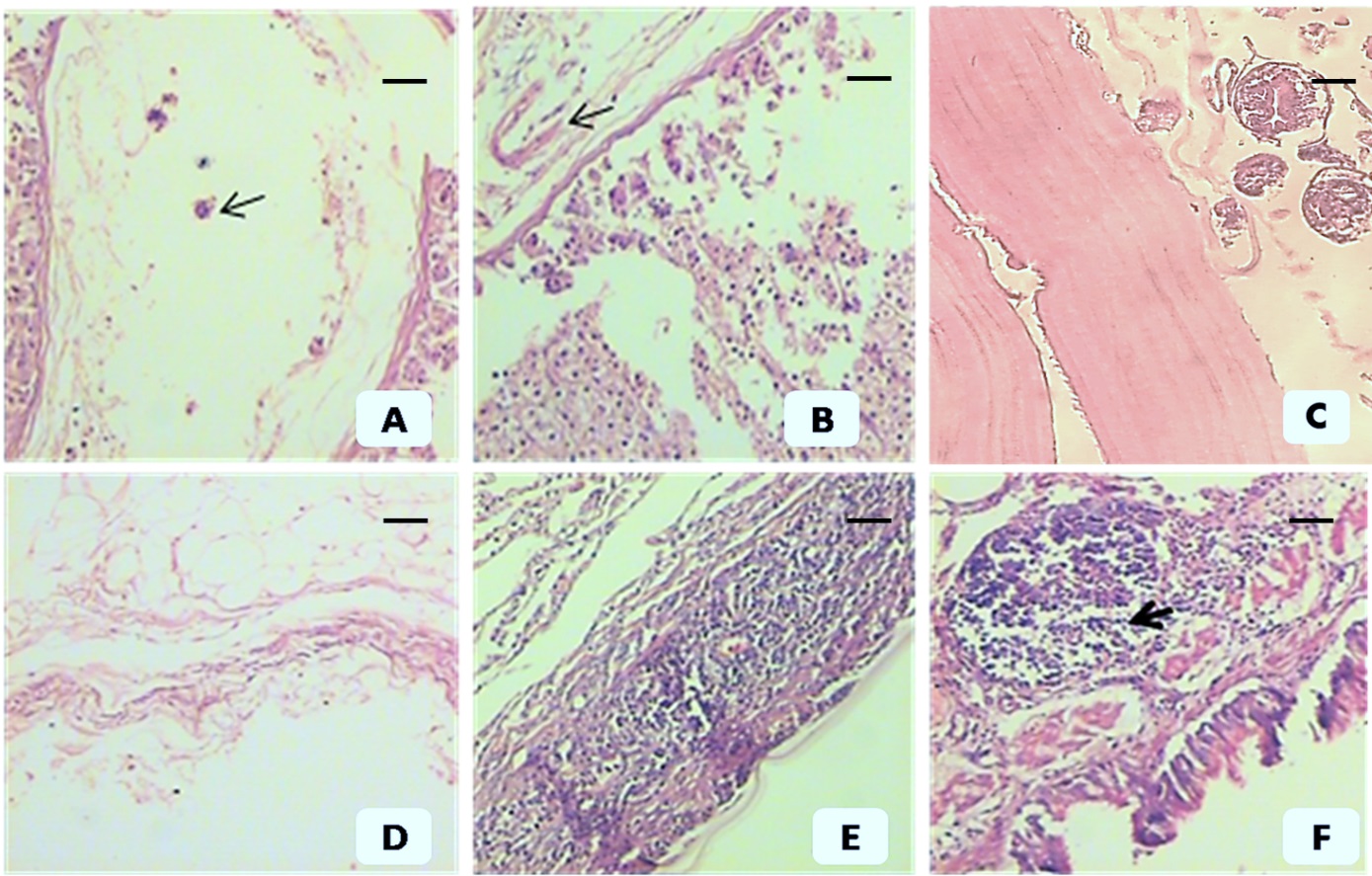
Hydatid cysts were not found in 2 out of the 5 animals included in group 1, while a low number of small cysts were observed in the remaining 3 animals (Table 1). The mean number of hydatid cysts in group 1, group 2 and the group 3 control animals were 2 mm±1.3, 5.7 mm ±2.1, and 18.6mm±6.4, respectively. We observed a reduction in the number of cysts by 90.67% and 68.79% compared to the control group in groups 1 and 2, respectively. The average hydatid cysts size in group 1, group 2 and group 3 control animals were 2.1 mm±1.2, 7.9 mm±2.4 and 23.5 mm±3.7, respectively. There were statistically significant differences (p<0.05) between the two antigens for all parameters, reflecting a more protective immune response in group 1. Histopathological examination revealed that group 1 animals had extensive inflammatory reactions and isolated cysts revealed severe necrotic walls without scolices. Laminated layers were severely necrotic (ghost shape) in some areas or extensively infiltrated by inflammatory cells in other areas. The cystic cavities were nearly obliterated by the extensive infiltration of inflammatory cells. On the other hand, few degenerative scolices, thin laminated layers and degenerated germinal layers were observed in group 2 animals. Variable degrees of inflammatory reactions were reported, ranging from mild to moderate (Figure 1). Immunohistochemistry revealed positive expression of Caspase 3 and p53 in groups 1 and 2, while the expression of both apoptotic markers appeared absent in the control group (Figure 2). Significantly higher expression values for Caspase 3 and p53 (by OD and area percentage, respectively) were observed in group 1 (Caspase 3: 0.89±0.21, 93.5%±6.2; p53: 0.46±0.18, 53.26%±11.6) than group 2 (Caspase 3: 0.52±0.15, 49.23%±11.7) and (p53: 0.19±0.4, 18.17%±7.3), reflecting a higher degree of apoptotic changes in animals sensitized with the crude antigen prepared from a local strain (group 1) than in animals sensitized by the partially purified antigen prepared from an imported strain (group 2) (p<0.05) (Table 2; Figures 3 and 4).
Post-immunization histopathological changes in animals immunized by imported, partially purified antigen (group 2) (A, B, C) and crude local antigen (group 1) (D, E, F). (A) Hydatid cyst wall shows a thin laminated layer & necrotic detached germinal epithelium and degenerated scolices (arrow). (B) Hydatid cyst wall shows a thin laminated layer & necrotic, detached germinal epithelium (arrow). (C) Hydatid cyst wall from the control group shows a well-developed laminated layer, intact germinal epithelium and healthy scolices. (D) Severe necrosis of germinal epithelium and laminated layer (ghost shape). (E) Hydatid cyst wall shows necrosis with severe inflammatory reaction infiltrating the extremely necrotic laminae. (F) Severe necrosis with lymphoid reaction (arrow). A, B and C show moderate inflammatory reactions. Scale bar= 200 µm in all pictures except (C) =50 µm.
Negative expression of Caspase 3 (left) and p53 (right) in group 3 (infected and non-vaccinated control group). The figures show well developed laminated layer (arrows) with v. few inflammatory cells (arrow head) (scale bar=200 µm).
Immunohistochemical expression of Caspase 3 in groups immunized with local strain (group 1) and imported strain (group 2) show disrupted laminae infiltrated by inflammatory cells under different magnifications (scale bar = (A) 400 µm, (B) 200 µm, (C) 100 µm, (D) 50 µm, (E) 400 µm, (F) 200 µm, (G) 100 µm and (H) 50 µm).
Immunohistochemical expression of p53 in groups immunized with the local strain (group 1), and the imported strain (group 2) (scale bar =A&C: 400 µm, B&D: 50 µm).
Discussion
Echinococcus granulosus life cycle is divided on the infection of the two hosts: an IH (human or livestock) and a definitive host (canines). Competent control programs show that preventing its transmission to either host can reduce or even eliminate E. granulosus infections (PAYKARI et al., 2008Paykari H, Heath DD, Dalimi AH, Karimi GR, Motamedi GR. Experimental vaccination of sheep against hydatid cyst using EG95 recombinant vaccine. Arch Razi Inst 2008; 63(2): 29-34.). Unfortunately, hydatid disease remains a serious cause of human morbidity (LIGHTOWLERS et al., 1999Lightowlers MW, Jensen O, Fernandez E, Iriarte JA, Woollard DJ, Gauci CG, et al. Vaccination trials in Australia and Argentina confirm the effectiveness of the EG95 hydatid vaccine in sheep. Int J Parasitol 1999; 29(4): 531-534. http://dx.doi.org/10.1016/S0020-7519(99)00003-X. PMid:10428628.
http://dx.doi.org/10.1016/S0020-7519(99)...
), and it is considered a neglected zoonotic disease in which the vaccination of livestock and effective surveillance have not yet been adequately assessed (WHO, 2011World Health Organization – WHO. Report of the WHO Informal Working Group on cystic and alveolar echinococcosis surveillance, prevention and control, with the participation of the Food and Agriculture Organization of the United Nations and the World Organisation for Animal Health. Geneva: WHO; 2011.).
Immunologically, hydatid cysts secrete and expose numerous immune-modulatory molecules to the host’s immune system. These molecules trigger both the innate and adaptive arms of the immune response and appear to target both humoral and cellular reactions (SIRACUSANO et al., 2008Siracusano A, Rigano R, Ortona E, Profumo E, Margutti P, Buttari B, et al. Immunomodulatory mechanisms during infection. Echinococcus granulosusExp Parasitol 2008; 119(4): 483-489. http://dx.doi.org/10.1016/j.exppara.2008.01.016. PMid:18329023.
http://dx.doi.org/10.1016/j.exppara.2008...
). However, there are no detailed studies describing many aspects of these biological events, especially those associated with sensitization trials. Therefore, the mechanisms underlying protective immunity remain unclear. To the best of our knowledge, post-immunization apoptotic changes in hydatid lesions have never been studied. The aim of this study was to investigate post-immunization histopathological features using two different hydatid antigens and, for the first time, evaluate apoptotic changes in sensitized hosts that confer protection against hydatid disease.
Generally, in our study, the development and growth of hydatid cysts were greatly altered in immunized groups when compared to the normal cyst characteristics observed in the control group. In relation to the control group, we observed a reduction in the number of cysts by 90.67% and 68.79% with crude and partially purified antigen, respectively, indicating that protective immunity was conferred by the vaccines employed in our study. Additionally, in animals that received crude antigen, we observed cysts of smaller size, shrieked cysts or the absence of macroscopic cystic lesions. Microscopic examination revealed extensive inflammatory reactions, obliteration of the cystic cavities by mononuclear inflammatory cells and severely destructed larval tissue remnants infiltrated by numerous inflammatory cells. These findings were more prominent in animals that received the crude-antigen vaccine compared to those that received the partially purified one. In general, there were statistically significant differences between the two antigens for all measured parameters, reflecting more effective immunity in animals vaccinated with the locally prepared crude antigen compared to those vaccinated with the partially purified one. Crude antigens were shown to elicit higher levels of immunity, similar to our results as published by Dempster et al. (1995)Dempster RP, Harrison GBL, Berridge MV. Maternal transfer of protection from Echinococcus granulosus infection in sheep. Res Vet Sci 1995; 58(3): 197-202. http://dx.doi.org/10.1016/0034-5288(95)90101-9. PMid:7659840.
http://dx.doi.org/10.1016/0034-5288(95)9...
who reported higher levels of immunity generated by a crude sonicated oncosphere antigen compared to a partially purified SDS-solubilized oncosphere antigen, which elicited a moderate immune response upon an infection challenge with orally administered E. granulosus. A higher level of E. granulosus immunity was also achieved with a crude oncosphere antigen in sheep (HEATH & LAWRENCE, 1996)Heath DD, Lawrence SB. Antigenic polypeptides of oncospheres and definition of protective molecules. Echinococcus granulosusParasite Immunol 1996; 18(7): 347-357. http://dx.doi.org/10.1046/j.1365-3024.1996.d01-114.x. PMid:9229388.
http://dx.doi.org/10.1046/j.1365-3024.19...
. More recently, using a component of crude sheep hydatid fluid, Al-Qaoud & Abdel-Hafez (2005)Al-Qaoud KM, Abdel-Hafez SK. Humoral and cytokine response during protection of mice against secondary hydatidosis caused by . Echinococcus granulosusParasitol Res 2005; 98(1): 54-60. http://dx.doi.org/10.1007/s00436-005-0004-z. PMid:16261354.
http://dx.doi.org/10.1007/s00436-005-000...
showed a significant level of protection against E. granulosus, as indicated by a 98.3% reduction in cyst load. Furthermore, in a study conducted by Hashemitabar et al. (2006)Hashemitabar GR, Razmi GR, Naghibi A. Protective immunity in mice with whole body of Echinococcus granulosus.Iran Biomed J 2006; 10(1): 51-55., the authors showed that crude extracts of protoscolices provided 100% protective immunity in vaccinated mice. On the other hand, other studies have reported variable levels of protection with purified antigens. The protection rates of a recombinant vaccine (EG95) ranged from 86% to 99.3% in studies conducted by Lightowlers et al. (1999)Lightowlers MW, Jensen O, Fernandez E, Iriarte JA, Woollard DJ, Gauci CG, et al. Vaccination trials in Australia and Argentina confirm the effectiveness of the EG95 hydatid vaccine in sheep. Int J Parasitol 1999; 29(4): 531-534. http://dx.doi.org/10.1016/S0020-7519(99)00003-X. PMid:10428628.
http://dx.doi.org/10.1016/S0020-7519(99)...
and Heath et al. (2003)Heath DD, Jensen O, Lightowlers MW. Progress in control of hydatidosis using vaccination - a review of formulation and delivery of the vaccine and recommendations for practical use in control programmes. Acta Trop 2003; 85(2): 133-143. http://dx.doi.org/10.1016/S0001-706X(02)00219-X. PMid:12606090.
http://dx.doi.org/10.1016/S0001-706X(02)...
, respectively, and Shi et al. (2009)Shi Z, Wang Y, Li Z, Li Z, Bo Y, Ma R, et al. Cloning, expression, and protective immunity in mice of a gene encoding the diagnostic antigen P-29 of Echinococcus granulosus.Acta Biochim Biophys Sin (Shanghai) 2009; 41(1): 79-85. http://dx.doi.org/10.1093/abbs/gmn009. PMid:19129953.
http://dx.doi.org/10.1093/abbs/gmn009...
reported complete immunity persisting for more than a year after vaccination. In another study conducted by Li et al. (2012)Li ZJ, Wang YN, Wang Q, Zhao W. Echinococcus granulosus 14-3-3 protein: a potential vaccine candidate against challenge with in mice. Echinococcus granulosusBiomed Environ Sci 2012; 25(3): 352-358. PMid:22840587., mice vaccinated with rEg14-3-3 and challenged with protoscolices were provided with significant protective immunity at a rate of 84.47%.
Different mechanisms have been proposed to explain the protective effect of these vaccines. Heath et al. (2003)Heath DD, Jensen O, Lightowlers MW. Progress in control of hydatidosis using vaccination - a review of formulation and delivery of the vaccine and recommendations for practical use in control programmes. Acta Trop 2003; 85(2): 133-143. http://dx.doi.org/10.1016/S0001-706X(02)00219-X. PMid:12606090.
http://dx.doi.org/10.1016/S0001-706X(02)...
suggested an antibody and complement mediated lysis mechanism of the parasite oncosphere early in the establishment of a new infection, and a study by Edan & Ardalan (2009)Edan EM, Ardalan NM. Estimation of humural immune response on the rabbits that immunizing with Hydatid cyst antigens by using IHAT and ElISA. J Fac Med Baghdad 2009; 51(3): 332-335. supports a similar mechanism of antibody production. In general, different immunological responses, estimated either serologically or cytopathologically, have been reported. The mechanism of protective immunity depends on the antigenicity, the molecular size and the number of epitopes provided by the different antigens (WATTAL et al., 1986Wattal C, Malla N, Khan I, Agarwal S. Comparative evaluation of enzyme –linked Immunosorbent assay (ELISA) for the diagnosis of pulmonary echinococcosis. J Clin Microbiol 1986; 24(1): 41-46. PMid:3522626.; TABAR et al., 2012Tabar GH, Haghparast A, Borji H. Serodiagnosis of sheep hydatidosis with hydatid fluid, protoscolex, and whole body of Echinococcus granulosus antigens. Comp Clin Pathol 2012; 21(4): 429-432. http://dx.doi.org/10.1007/s00580-010-1112-4.
http://dx.doi.org/10.1007/s00580-010-111...
).
In our study, three immunizations prior to a challenge infection led to sustained immunological responses to promote either successful arrest of cyst growth and cyst degeneration/elimination or extensive necrotic changes in the observed cystic lesions. It is generally accepted that Echinococcus is unaffected by the immune response during the developing stage during primary infection. However, dead, calcified metacestodes or necrotic cysts have been reported in some cystic lesions in naturally infected sheep, indicating that the cysts can be killed during later stages of development (TABAR et al., 2012Tabar GH, Haghparast A, Borji H. Serodiagnosis of sheep hydatidosis with hydatid fluid, protoscolex, and whole body of Echinococcus granulosus antigens. Comp Clin Pathol 2012; 21(4): 429-432. http://dx.doi.org/10.1007/s00580-010-1112-4.
http://dx.doi.org/10.1007/s00580-010-111...
). The authors suggested that the immune response plays a key role in the death of the parasite, which may signify increased immunological stimulation upon cyst progression. This phenomenon may have occurred in our study, regardless of the antigen type. The three immunizations likely intensify the immune response to promote destructive killing of the parasite.
We also observed a laminated layer that was almost absent in some lesions and severely fragmented and extensively infiltrated by inflammatory cells in other lesions. This observation is explained by the phagocytosis of laminated layer fragments by mononuclear inflammatory cells (STEERS et al., 2001Steers NRJ, Rogan MT, Heath S. In-vitro susceptibility of hydatid cysts of Echinococcus granulosus to nitric oxide and the effect of the laminated layer on nitric oxide production. Parasite Immunol 2001; 23(8): 411-417. http://dx.doi.org/10.1046/j.1365-3024.2001.00385.x. PMid:11489164.
http://dx.doi.org/10.1046/j.1365-3024.20...
), which were found extensively in different cystic lesions. Therefore, complete or partial phagocytosis of the laminated layers may occur during the immune response in different hosts.
We also evaluated apoptotic changes in vaccinated animals. We observed the expression of p53 and Caspase 3 in sensitized animals, while no expression of these apoptotic markers was observed in the control group. There are few published in vitro studies that explore the apoptotic changes in hydatid protoscolices exposed to exogenous materials. Kang et al. (2008Kang TH, Hwang EI, Yun BS, Shin CS, Kim SU. Chitin synthase 2 Inhibitory activity of O-Methyl pisiferic acid and 8,20-dihydroxy-9(11),13-abietadien-12-one, isolated from Chamaecyparis pisifera. Biol Pharm Bull 2008; 31(4): 755-759. http://dx.doi.org/10.1248/bpb.31.755. PMid:18379078.
http://dx.doi.org/10.1248/bpb.31.755...
, 2009Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, et al. Amyloid-β dynamics are regulated by orexin and the sleep-wake cycle. Science 2009; 326(5955): 1005-1007. http://dx.doi.org/10.1126/science.1180962. PMid:19779148.
http://dx.doi.org/10.1126/science.118096...
) found a significant increase in Caspase 3 in E. granulosus protoscolices exposed to hydrogen peroxide (H2O2) and adenosine triphosphate (ATP) compared to control values. The first published study on apoptotic changes in hydatidosis reported a higher level of Caspase 3 in infertile cysts than fertile ones (PAREDES et al., 2007Paredes R, Jiménez V, Cabrera G, Iragüen D, Galanti N. Apoptosis as a possible mechanism of infertility in hydatid cysts. Echinococcus granulosusJ Cell Biochem 2007; 100(5): 1200-1209. http://dx.doi.org/10.1002/jcb.21108. PMid:17031852.
http://dx.doi.org/10.1002/jcb.21108...
). Accordingly, we propose that decreased apoptosis may enhance cyst survival. In general, the pathways underlying cyst suppression and survival in humans remain poorly understood, reflecting the importance of such studies.
Findings by Zhou et al. (2010)Zhou BY, Chen YT, Li WG, Yang M. Inhibition on apoptosis of splenocytes in infected mice by immunization with recombinant Bb-Eg95-EgA 31 protein of Echinococcus granulosus.Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2010; 28(1): 26-29. PMid:20411745. may explain the alterations in cyst apoptosis that accompany the extensive inflammatory reactions observed in this study. In contrast to our results, the authors found that conditions of normal hydatid growth lead to the induction of splenocyte apoptosis to prevent protective host immunity. Immunization may thus inhibit splenocyte apoptosis to thereby permit extensive splenocyte expansion and a severe cellular reaction, resulting in protective immunity and destruction of the parasite, as reported in our work. Additionally, in studying mechanisms of cyst survival, Spotin et al. (2012)Spotin A, Majdi MMA, Sankian M, Varasteh A. The study of apoptotic bifunctional effects in relationship between host and parasite in cystic echinococcosis: a new approach to suppression and survival of hydatid cyst. Parasitol Res 2012; 110(5): 1979-1984. http://dx.doi.org/10.1007/s00436-011-2726-4. PMid:22167369.
http://dx.doi.org/10.1007/s00436-011-272...
concluded that apoptosis of the germinal layer is a possible mechanism of suppression in hydatidosis patients, while lymphocyte apoptosis promotes cyst survival. Our results further validate this previous finding, as an increase in apoptotic markers in infertile cysts and decreased apoptosis of inflammatory cells was observed following immunization, leading to the destruction of the larval cestode. Recently, Alam-Eldin & Badawy (2015)Alam-Eldin YH, Badawy AF. Destructive effect of gamma irradiation on Echinococcus granulosus metacestodes. Parasitol Res 2015; 114(8): 3145-3150. PMID: 25982573. http://dx.doi.org/10.1007/s00436-015-4533-9.
http://dx.doi.org/10.1007/s00436-015-453...
reported the apoptosis of hydatid metacestodes following exposure to gamma irradiation, using colorimetric and immunohistochemistry assays of Caspase 3 activity. These results suggest the therapeutic potential of gamma irradiation in CE, and our results highlight a similar potential for disease prophylaxis.
On the other hand, p53 is one of several factors that regulate apoptosis, and its activity is not required for normal cell growth. A major mechanism of p53 stabilization and activation is triggered by DNA damage, which induces protein phosphorylation similar to that caused by immunological killing (O'KEEFE et al., 2003O’Keefe K, Li H, Zhang Y. Nucleocytoplasmic shuttling of p53 is essential for MDM2-mediated cytoplasmic degradation but not ubiquitination. Mol Cell Biol 2003; 23(18): 6396-6405. http://dx.doi.org/10.1128/MCB.23.18.6396-6405.2003. PMid:12944468.
http://dx.doi.org/10.1128/MCB.23.18.6396...
). In our study, both Caspase 3 and p53 were expressed in cysts from vaccinated animals, and these apoptotic markers were nearly absent in the control group. Expression of these apoptotic markers may be required for effective vaccine-induced immune responses, as they may facilitate larval destruction and/or the elimination of damaged immune cells that arise during their successful battle with the parasite. The resultant cellular damage from this immunological struggle may underlie the expression of these apoptotic markers that appeared to give rise to the elimination of this parasite after productive immunization.
In this study, we conclusively show that vaccination with a locally prepared crude antigen resulted in more effective immune responses and apoptotic changes compared to vaccination with a partially purified imported antigen. In general, it is important to use local strains for vaccine preparation to maximize the potential for success of vaccine trials. It is generally accepted that different strains of parasites collected from a variety of geographical locations are likely to produce distinct antigenic components and subsequently may elicit different immunological responses (THOMPSON & McMANUS, 2002Thompson RC, McManus DP. Towards a taxonomic revision of the genus Echinococcus.Trends Parasitol 2002; 18(10): 452-457. http://dx.doi.org/10.1016/S1471-4922(02)02358-9. PMid:12377596.
http://dx.doi.org/10.1016/S1471-4922(02)...
; AL-OLAYAN & HELMY, 2012Al-Olayan EM, Helmy H. Diagnostic value of different antigenic fractions of hydatid cyst fluid from camel and sheep in Kingdom of Saudi Arabia. J Saudi Chem Soc 2012; 16(2): 203-207. http://dx.doi.org/10.1016/j.jscs.2011.01.001.
http://dx.doi.org/10.1016/j.jscs.2011.01...
). Therefore, it must be noted that the positive achievements of successful control programs are significant only when applied on a local level. Unfortunately, in most endemic areas, effective control has not been achieved or even attempted and substantial work is still required (MORO & SCHANTZ, 2009Moro P, Schantz PM. Echinococcosis: a review. Int J Infect Dis 2009; 13(2): 125-133. http://dx.doi.org/10.1016/j.ijid.2008.03.037. PMid:18938096.
http://dx.doi.org/10.1016/j.ijid.2008.03...
). The results of the current study emphasize the importance of immunization for both the induction of protective immune responses and the blockade of evasion mechanisms. A cost/benefit hydatid control plan using local vaccine preparations should be further assessed within IHs, particularly in endemic areas, to achieve complete elimination of this serious disease.
References
- Alam-Eldin YH, Badawy AF. Destructive effect of gamma irradiation on Echinococcus granulosus metacestodes. Parasitol Res 2015; 114(8): 3145-3150. PMID: 25982573. http://dx.doi.org/10.1007/s00436-015-4533-9
» http://dx.doi.org/10.1007/s00436-015-4533-9 - Al-Olayan EM, Helmy H. Diagnostic value of different antigenic fractions of hydatid cyst fluid from camel and sheep in Kingdom of Saudi Arabia. J Saudi Chem Soc 2012; 16(2): 203-207. http://dx.doi.org/10.1016/j.jscs.2011.01.001
» http://dx.doi.org/10.1016/j.jscs.2011.01.001 - Al-Qaoud KM, Abdel-Hafez SK. Humoral and cytokine response during protection of mice against secondary hydatidosis caused by . Echinococcus granulosusParasitol Res 2005; 98(1): 54-60. http://dx.doi.org/10.1007/s00436-005-0004-z PMid:16261354.
» http://dx.doi.org/10.1007/s00436-005-0004-z - Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72(1-2): 248-254. http://dx.doi.org/10.1016/0003-2697(76)90527-3 PMid:942051.
» http://dx.doi.org/10.1016/0003-2697(76)90527-3 - Dada BJ, Belino ED. Immunization of sheep against cystic hydatidosis with homologous and heterologous metacestode antigens. Int J Zoonoses 1981; 8(1): 20-25. PMid:7333782.
- Dempster RP, Harrison GBL, Berridge MV. Maternal transfer of protection from Echinococcus granulosus infection in sheep. Res Vet Sci 1995; 58(3): 197-202. http://dx.doi.org/10.1016/0034-5288(95)90101-9 PMid:7659840.
» http://dx.doi.org/10.1016/0034-5288(95)90101-9 - Eckert J, Deplazes P. Biological, epidemiological, and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev 2004; 17(1): 107-135. http://dx.doi.org/10.1128/CMR.17.1.107-135.2004 PMid:14726458.
» http://dx.doi.org/10.1128/CMR.17.1.107-135.2004 - Edan EM, Ardalan NM. Estimation of humural immune response on the rabbits that immunizing with Hydatid cyst antigens by using IHAT and ElISA. J Fac Med Baghdad 2009; 51(3): 332-335.
- Elshazly AM, Awad SE, Hegazy MA, Mohammad KA, Morsy TA. hydatidosis an endemic zoonotic disease in Egypt. Echinococcosis granulosus/J Egypt Soc Parasitol 2007; 37(2): 609-622. PMid:17985592.
- Hashemitabar GR, Razmi GR, Naghibi A. Protective immunity in mice with whole body of Echinococcus granulosus.Iran Biomed J 2006; 10(1): 51-55.
- Heath DD, Jensen O, Lightowlers MW. Progress in control of hydatidosis using vaccination - a review of formulation and delivery of the vaccine and recommendations for practical use in control programmes. Acta Trop 2003; 85(2): 133-143. http://dx.doi.org/10.1016/S0001-706X(02)00219-X PMid:12606090.
» http://dx.doi.org/10.1016/S0001-706X(02)00219-X - Heath DD, Lawrence SB. Antigenic polypeptides of oncospheres and definition of protective molecules. Echinococcus granulosusParasite Immunol 1996; 18(7): 347-357. http://dx.doi.org/10.1046/j.1365-3024.1996.d01-114.x PMid:9229388.
» http://dx.doi.org/10.1046/j.1365-3024.1996.d01-114.x - Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, et al. Amyloid-β dynamics are regulated by orexin and the sleep-wake cycle. Science 2009; 326(5955): 1005-1007. http://dx.doi.org/10.1126/science.1180962 PMid:19779148.
» http://dx.doi.org/10.1126/science.1180962 - Kang TH, Hwang EI, Yun BS, Shin CS, Kim SU. Chitin synthase 2 Inhibitory activity of O-Methyl pisiferic acid and 8,20-dihydroxy-9(11),13-abietadien-12-one, isolated from Chamaecyparis pisifera. Biol Pharm Bull 2008; 31(4): 755-759. http://dx.doi.org/10.1248/bpb.31.755 PMid:18379078.
» http://dx.doi.org/10.1248/bpb.31.755 - Li ZJ, Wang YN, Wang Q, Zhao W. Echinococcus granulosus 14-3-3 protein: a potential vaccine candidate against challenge with in mice. Echinococcus granulosusBiomed Environ Sci 2012; 25(3): 352-358. PMid:22840587.
- Lightowlers MW, Biyikoglu G, Chow C, Cowman AF, Gauci CG, Heath DD, et al. Vaccination against hydatidosis: anticipating the potential for antigenic variation. Southeast Asian J Trop Med Public Health 2004; 35(S1): 206-212.
- Lightowlers MW, Flisser A, Gauci CG, Heath DD, Jensen O, Rolfe R. Vaccination against cysticercosis and hydatid disease. Parasitol Today 2000; 16(5): 191-196. http://dx.doi.org/10.1016/S0169-4758(99)01633-6 PMid:10782077.
» http://dx.doi.org/10.1016/S0169-4758(99)01633-6 - Lightowlers MW, Jensen O, Fernandez E, Iriarte JA, Woollard DJ, Gauci CG, et al. Vaccination trials in Australia and Argentina confirm the effectiveness of the EG95 hydatid vaccine in sheep. Int J Parasitol 1999; 29(4): 531-534. http://dx.doi.org/10.1016/S0020-7519(99)00003-X PMid:10428628.
» http://dx.doi.org/10.1016/S0020-7519(99)00003-X - Lightowlers MW, Lawrence SB, Gauci CG, Young J, Ralston MJ, Maas D, et al. Vaccination against hydatidosis using a defined recombinant antigen. Parasite Immunol 1996; 18(9): 457-462. http://dx.doi.org/10.1111/j.1365-3024.1996.tb01029.x PMid:9226681.
» http://dx.doi.org/10.1111/j.1365-3024.1996.tb01029.x - Moro P, Schantz PM. Echinococcosis: a review. Int J Infect Dis 2009; 13(2): 125-133. http://dx.doi.org/10.1016/j.ijid.2008.03.037 PMid:18938096.
» http://dx.doi.org/10.1016/j.ijid.2008.03.037 - O’Keefe K, Li H, Zhang Y. Nucleocytoplasmic shuttling of p53 is essential for MDM2-mediated cytoplasmic degradation but not ubiquitination. Mol Cell Biol 2003; 23(18): 6396-6405. http://dx.doi.org/10.1128/MCB.23.18.6396-6405.2003 PMid:12944468.
» http://dx.doi.org/10.1128/MCB.23.18.6396-6405.2003 - Paredes R, Jiménez V, Cabrera G, Iragüen D, Galanti N. Apoptosis as a possible mechanism of infertility in hydatid cysts. Echinococcus granulosusJ Cell Biochem 2007; 100(5): 1200-1209. http://dx.doi.org/10.1002/jcb.21108 PMid:17031852.
» http://dx.doi.org/10.1002/jcb.21108 - Paykari H, Heath DD, Dalimi AH, Karimi GR, Motamedi GR. Experimental vaccination of sheep against hydatid cyst using EG95 recombinant vaccine. Arch Razi Inst 2008; 63(2): 29-34.
- Prophet EB, Mills B, Arrington JB, Sobin LH. Laboratory methods in histotechnology. Washington: Armed Forces Institute of Pathology, American Registry of Pathology; 1992.
- Saad MB, Magzoub M. Experimental transmission of hydatid cysts from camels and cattle to dogs. Ann Trop Med Parasitol 1988; 82(4): 363-365. PMid:3252759.
- Schuler M, Bossy-Wetzel E, Goldstein JC, Fitzgerald P, Green DR. p53 induces apoptosis by Caspase activation through mitochondrial cytochrome release. cJ Biol Chem 2000; 275(10): 7337-7342. http://dx.doi.org/10.1074/jbc.275.10.7337 PMid:10702305.
» http://dx.doi.org/10.1074/jbc.275.10.7337 - Shi Z, Wang Y, Li Z, Li Z, Bo Y, Ma R, et al. Cloning, expression, and protective immunity in mice of a gene encoding the diagnostic antigen P-29 of Echinococcus granulosus.Acta Biochim Biophys Sin (Shanghai) 2009; 41(1): 79-85. http://dx.doi.org/10.1093/abbs/gmn009 PMid:19129953.
» http://dx.doi.org/10.1093/abbs/gmn009 - Siracusano A, Rigano R, Ortona E, Profumo E, Margutti P, Buttari B, et al. Immunomodulatory mechanisms during infection. Echinococcus granulosusExp Parasitol 2008; 119(4): 483-489. http://dx.doi.org/10.1016/j.exppara.2008.01.016 PMid:18329023.
» http://dx.doi.org/10.1016/j.exppara.2008.01.016 - Spotin A, Majdi MMA, Sankian M, Varasteh A. The study of apoptotic bifunctional effects in relationship between host and parasite in cystic echinococcosis: a new approach to suppression and survival of hydatid cyst. Parasitol Res 2012; 110(5): 1979-1984. http://dx.doi.org/10.1007/s00436-011-2726-4 PMid:22167369.
» http://dx.doi.org/10.1007/s00436-011-2726-4 - Steers NRJ, Rogan MT, Heath S. In-vitro susceptibility of hydatid cysts of Echinococcus granulosus to nitric oxide and the effect of the laminated layer on nitric oxide production. Parasite Immunol 2001; 23(8): 411-417. http://dx.doi.org/10.1046/j.1365-3024.2001.00385.x PMid:11489164.
» http://dx.doi.org/10.1046/j.1365-3024.2001.00385.x - Tabar GH, Haghparast A, Borji H. Serodiagnosis of sheep hydatidosis with hydatid fluid, protoscolex, and whole body of Echinococcus granulosus antigens. Comp Clin Pathol 2012; 21(4): 429-432. http://dx.doi.org/10.1007/s00580-010-1112-4
» http://dx.doi.org/10.1007/s00580-010-1112-4 - Thompson RC, Lymbery AJ, Constantine CC. Variation in Echinococcus: towards a taxonomic revision of the genus. Adv Parasitol 1995; 35: 145-176. http://dx.doi.org/10.1016/S0065-308X(08)60071-8 PMid:7709852.
» http://dx.doi.org/10.1016/S0065-308X(08)60071-8 - Thompson RC, McManus DP. Towards a taxonomic revision of the genus Echinococcus.Trends Parasitol 2002; 18(10): 452-457. http://dx.doi.org/10.1016/S1471-4922(02)02358-9 PMid:12377596.
» http://dx.doi.org/10.1016/S1471-4922(02)02358-9 - Wattal C, Malla N, Khan I, Agarwal S. Comparative evaluation of enzyme –linked Immunosorbent assay (ELISA) for the diagnosis of pulmonary echinococcosis. J Clin Microbiol 1986; 24(1): 41-46. PMid:3522626.
- Woollard DJ, Gauci CG, Heath DD, Lightowlers MW. Epitope specificities and antibody responses to the EG95 hydatid vaccine. Parasite Immunol 1998; 20(11): 535-540. http://dx.doi.org/10.1046/j.1365-3024.1998.00176.x PMid:9988310.
» http://dx.doi.org/10.1046/j.1365-3024.1998.00176.x - World Health Organization – WHO. Report of the WHO Informal Working Group on cystic and alveolar echinococcosis surveillance, prevention and control, with the participation of the Food and Agriculture Organization of the United Nations and the World Organisation for Animal Health. Geneva: WHO; 2011.
- Zhou BY, Chen YT, Li WG, Yang M. Inhibition on apoptosis of splenocytes in infected mice by immunization with recombinant Bb-Eg95-EgA 31 protein of Echinococcus granulosus.Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 2010; 28(1): 26-29. PMid:20411745.
Publication Dates
-
Publication in this collection
Sept 2016
History
-
Received
21 May 2016 -
Accepted
04 Aug 2016