Abstract
A total of 41 cestodes were collected during necropsy examination on 2 pumas (Puma concolor) that were found in 2 communities in Canchis province, Cuzco region, Peru, at 4500 meters above sea level (Peruvian Andes). The cestodes were evaluated morphologically and molecularly. A fragment of the mitochondrial cytochrome c oxidase subunit 1 gene (cox1) was used as a genetic marker. All the cestodes were identified as Taenia omissa. In the present report, we give a brief description by molecular and morphological diagnosis of the cestodes and compare nucleotide sequences with previous isolates from GenBank. Upon comparison, the sequences showed a difference in the cox1 gene of 5.1 to 5.3% with other teniids sequences. This finding constitutes the first report of T. omissa in Peru and expands the geographic distribution of this parasite.
Keywords:
Taenia omissa; cestode; taeniid; puma; Puma concolor; cytochrome C oxidase subunit 1 gene
Resumo
Um total de quarenta e um cestóides foram coletados durante a necropsia de duas onça-pardas (Puma concolor) encontradas em duas comunidades na província de Canchis, em Cuzco, a 4500 metros acima do nível do mar, nos Andes peruanos. Os cestóides foram avaliados morfologicamente e molecularmente. Um fragmento do gene citocromo C oxidase subunidade 1 (cox1) foi utilizado como marcador genético. Todos os cestóides foram identificados como Taenia omissa. No presente relato, dá-se uma breve descrição dos cestóides e compara-se sequências de nucleotídeos com isolados anteriores presentes no GenBank. Após a comparação, as sequências mostraram uma diferença de 5,1-5,3% entre o gene cox1 e outras sequências de tênias. Esse achado constitui o primeiro relato de T. omissa no Peru e amplia a informação sobre a distribuição geográfica deste parasita.
Palavras-chave:
Taenia omissa; cestoide; tênia; onça-parda; Puma concolor; gene citocromo C oxidase subunidade 1
The genus Taenia Linnaeus, 1758 (Cestoda: Taeniidae) includes approximately 45 established species (HOBERG, 2006Hoberg EP. Phylogeny of Taenia: Species definitions and origins of human parasites. Parasitol Int 2006; 55(Suppl.): S23-S30. http://dx.doi.org/10.1016/j.parint.2005.11.049. PMid:16371252.
http://dx.doi.org/10.1016/j.parint.2005....
; LAVIKAINEN et al., 2008Lavikainen A, Haukisalmi V, Lehtinen MJ, Henttonen H, Oksanen A, Meri S. A phylogeny of members of the family Taeniidae based on the mitochondrial cox1 and gene data. nad1Parasitology 2008; 135(12): 1457-1467. http://dx.doi.org/10.1017/S003118200800499X. PMid:18937885.
http://dx.doi.org/10.1017/S0031182008004...
; ROSSIN et al., 2010Rossin MA, Timi JT, Hoberg EP. An endemic from South America: validation of . TaeniaT. talicei Dollfus, 1960 (Cestoda: Taeniidae) with characterization of metacestodes and adultsZootaxa 2010; 2636: 49-58.; HAUKISALMI et al., 2011Haukisalmi V, Lavikainen A, Laaksonen S, Meri S. n. sp. (Cestoda: Cyclophyllidea: Taeniidae) from its definitive (brown bear Linnaeus) and intermediate (moose/elk . Taenia arctosUrsus arctosAlces spp.) hostsSyst Parasitol 2011; 80(3): 217-230. http://dx.doi.org/10.1007/s11230-011-9324-9. PMid:22002024.
http://dx.doi.org/10.1007/s11230-011-932...
). Adult stages of these tapeworms develop in the small intestine of carnivorous mammals, and their metacestodes develop in different tissues of herbivorous or omnivorous mammals (ABULADZE, 1964Abuladze KI. Taeniata of animals and man and diseases caused by them. In: Skrjabin KI. Essentials of cestodology. Jerusalem: Israel Program for Scientific Translations; 1964. p. 1-547, vol. 4.). Many species of felids can act as definitive hosts for at least 14 species of Taenia (LOOS-FRANK, 2000Loos-Frank B. An up-date of Verster’s (1969) ‘Taxonomic revision of the genus Linnaeus’ (Cestoda) in table format. TaeniaSyst Parasitol 2000; 45(3): 155-183. http://dx.doi.org/10.1023/A:1006219625792. PMid:10768761.
http://dx.doi.org/10.1023/A:100621962579...
).
The puma (Puma concolor Linnaeus, 1771), also called the mountain lion or cougar, is a large wild felid whose range extends from northern British Columbia in Canada to southern Chile and Argentina. In Peru, pumas are distributed from the rainforest to the Andes mountains, and can be found at altitudes as high as 5800 meters above sea level (LÓPEZ-GONZÁLEZ & GONZÁLEZ-ROMERO, 1998López-González AC, González-Romero A. A synthesis of current literature and knowledge about the ecology of the puma (Linnaeus). Puma concolor Acta Zool Mex 1998; 75: 171-190.). Many studies about parasites in pumas have now been published (RAUSCH et al., 1983Rausch RL, Maser C, Hoberg EP. Gastrointestinal helminths of the cougar, Felis concolor L., in northeastern Oregon. J Wildl Dis 1983; 19(1): 14-19. http://dx.doi.org/10.7589/0090-3558-19.1.14. PMid:6682458.
http://dx.doi.org/10.7589/0090-3558-19.1...
; WAID & PENCE, 1988Waid DD, Pence DB. Helminths of mountain lions (Felis concolorCylicospirura subaequalis) from southwestern Texas, with a redescription of (Molin, 1860) Vevers, 1922. Can J Zool 1988; 66(10): 2110-2117. http://dx.doi.org/10.1139/z88-313.
http://dx.doi.org/10.1139/z88-313...
; RICKARD & FOREYT, 1992Rickard LG, Foreyt WJ. Gastrointestinal parasites of cougars (Felis concolorOllulanus tricuspis) in Washington and the first report of in a sylvatic felid from North America. J Wildl Dis 1992; 28(1): 130-133. http://dx.doi.org/10.7589/0090-3558-28.1.130. PMid:1548792.
http://dx.doi.org/10.7589/0090-3558-28.1...
; FOSTER et al., 2006Foster GW, Cunningham MW, Kinsella JM, McLaughlin G, Forrester DJ. Gastrointestinal helminths of free-ranging Florida panthers (Puma concolor coryi) and the efficacy of the current anthelmintic treatment protocol. J Wildl Dis 2006; 42(2): 402-406.; DARE & WATKINS, 2012Dare OK, Watkins WG. First record of parasites from cougars (Puma concolor) in Manitoba, Canada. Can Field Nat 2012; 126(4): 324-327.). However, most of these studies were conducted in North America (RAUSCH et al., 1983Rausch RL, Maser C, Hoberg EP. Gastrointestinal helminths of the cougar, Felis concolor L., in northeastern Oregon. J Wildl Dis 1983; 19(1): 14-19. http://dx.doi.org/10.7589/0090-3558-19.1.14. PMid:6682458.
http://dx.doi.org/10.7589/0090-3558-19.1...
; WAID & PENCE, 1988Waid DD, Pence DB. Helminths of mountain lions (Felis concolorCylicospirura subaequalis) from southwestern Texas, with a redescription of (Molin, 1860) Vevers, 1922. Can J Zool 1988; 66(10): 2110-2117. http://dx.doi.org/10.1139/z88-313.
http://dx.doi.org/10.1139/z88-313...
; RICKARD & FOREYT, 1992Rickard LG, Foreyt WJ. Gastrointestinal parasites of cougars (Felis concolorOllulanus tricuspis) in Washington and the first report of in a sylvatic felid from North America. J Wildl Dis 1992; 28(1): 130-133. http://dx.doi.org/10.7589/0090-3558-28.1.130. PMid:1548792.
http://dx.doi.org/10.7589/0090-3558-28.1...
; FOSTER et al., 2006Foster GW, Cunningham MW, Kinsella JM, McLaughlin G, Forrester DJ. Gastrointestinal helminths of free-ranging Florida panthers (Puma concolor coryi) and the efficacy of the current anthelmintic treatment protocol. J Wildl Dis 2006; 42(2): 402-406.; DARE & WATKINS, 2012Dare OK, Watkins WG. First record of parasites from cougars (Puma concolor) in Manitoba, Canada. Can Field Nat 2012; 126(4): 324-327.). Approximately 9 species of tapeworms have been found in pumas to date (SCHMIDT & MARTIN, 1978Schmidt GD, Martin RL. Tapeworms of the Chaco Boreal, Paraguay, with two new species. J Helminthol 1978; 52(3): 205-209. http://dx.doi.org/10.1017/S0022149X00005381. PMid:722040.
http://dx.doi.org/10.1017/S0022149X00005...
; D’ALESSANDRO et al., 1981D’Alessandro A, Rausch RL, Morales GA, Collet S, Angel D. infections in Colombian animals. EchinococcusAm J Trop Med Hyg 1981; 30(6): 1263-1276. PMid:7325284.; RAUSCH et al., 1983Rausch RL, Maser C, Hoberg EP. Gastrointestinal helminths of the cougar, Felis concolor L., in northeastern Oregon. J Wildl Dis 1983; 19(1): 14-19. http://dx.doi.org/10.7589/0090-3558-19.1.14. PMid:6682458.
http://dx.doi.org/10.7589/0090-3558-19.1...
; FOSTER et al., 2006Foster GW, Cunningham MW, Kinsella JM, McLaughlin G, Forrester DJ. Gastrointestinal helminths of free-ranging Florida panthers (Puma concolor coryi) and the efficacy of the current anthelmintic treatment protocol. J Wildl Dis 2006; 42(2): 402-406.; VIEIRA et al., 2008Vieira FM, Luque JL, Muniz-Pereira LC. Checklist of helminth parasites in wild carnivore mammals from Brazil. Zootaxa 2008; 1721: 1-23.), of which only four species have been found in South America: Echinococcus oligarthrus and Hydatigera taeniaeformis in Brazil (VIEIRA et al., 2008Vieira FM, Luque JL, Muniz-Pereira LC. Checklist of helminth parasites in wild carnivore mammals from Brazil. Zootaxa 2008; 1721: 1-23.); and Taenia omissa and Spirometra sp. in Brazil and Paraguay (SCHMIDT & MARTIN, 1978Schmidt GD, Martin RL. Tapeworms of the Chaco Boreal, Paraguay, with two new species. J Helminthol 1978; 52(3): 205-209. http://dx.doi.org/10.1017/S0022149X00005381. PMid:722040.
http://dx.doi.org/10.1017/S0022149X00005...
; VIEIRA et al., 2008Vieira FM, Luque JL, Muniz-Pereira LC. Checklist of helminth parasites in wild carnivore mammals from Brazil. Zootaxa 2008; 1721: 1-23.).
The present study confirms morphologically and molecularly the occurrences of T. omissa parasitizing two adult male pumas in Cuzco, Peru. This finding represents the first report of T. omissa in Peru.
In May and September of 2013, 2 adult male pumas were found dead, apparently killed by poachers, on two farms that were operated as alpaca production systems, located in the Pumaconca (14°18ʼ51.73ʼʼS, 71°09ʼ07.41ʼʼW) and Abra La Raya (14°28ʼ43.03ʼʼS, 71°01ʼ41.34ʼʼW) communities, in the province of Canchis, Cuzco region in Peru, at 4500 meters above sea level. The carcasses of the two pumas were donated for necropsy by the Technical Administration for Forestry and Wildlife of Peru to the veterinary research center IVITA-Marangani, at the Universidad Nacional Mayor de San Marcos. During the necropsy, twenty-eight and thirteen complete cestodes were collected from the small intestines, respectively. The parasites were fixed in 4% formaldehyde and then preserved in 70% ethanol. Some gravid proglottids were preserved in absolute ethanol for molecular studies.
Scoleces were mounted in Berlese’s medium to facilitate observation and measurement of rostellar hooks. The rostellar hooks were measured in accordance with the parameters described by Haukisalmi et al. (2011)Haukisalmi V, Lavikainen A, Laaksonen S, Meri S. n. sp. (Cestoda: Cyclophyllidea: Taeniidae) from its definitive (brown bear Linnaeus) and intermediate (moose/elk . Taenia arctosUrsus arctosAlces spp.) hostsSyst Parasitol 2011; 80(3): 217-230. http://dx.doi.org/10.1007/s11230-011-9324-9. PMid:22002024.
http://dx.doi.org/10.1007/s11230-011-932...
. Both mature and gravid proglottids were stained with Semichon’s acetocarmine stain and were dehydrated in an ascending alcohol series up to absolute ethanol. Subsequently, the samples were cleared in clove oil and terpineol, and then mounted in Canada balsam.
Photographs were taken using a Carl Zeiss microscope (Axioskop 40). Measurements were made using image analysis software (Leica IM50, version 4.0 R117). The measurements are reported in micrometers unless otherwise stated. Measured characteristics are given as range, with averages and standard error (SE) values in parentheses. The parasite taxonomic nomenclature used in this study follows Loos-Frank (2000)Loos-Frank B. An up-date of Verster’s (1969) ‘Taxonomic revision of the genus Linnaeus’ (Cestoda) in table format. TaeniaSyst Parasitol 2000; 45(3): 155-183. http://dx.doi.org/10.1023/A:1006219625792. PMid:10768761.
http://dx.doi.org/10.1023/A:100621962579...
and Rausch (1994)Rausch RL. Family Taeniidae. In: Khalil LF, Jones A, Bray RA. Keys to the cestode parasites of vertebrates. Wallingford: CAB International; 1994. p. 665-672.. The host taxonomic nomenclature follows Currier (1983)Currier MJP. Felis concolor. Mamm Species 1983; 200(200): 1-7. http://dx.doi.org/10.2307/3503951.
http://dx.doi.org/10.2307/3503951...
.
Total DNA was extracted from three tapeworms from the puma from Pumaconca (To1, To2 and To3) using Chelex100, in accordance with the methodology described by Gadau (2009)Gadau J. DNA isolation from ants. Cold Spring Harb Protoc 2009(7): 1-3. http://dx.doi.org/10.1101/pdb.prot5245.
http://dx.doi.org/10.1101/pdb.prot5245...
with a minor modification. Tissue samples from gravid proglottids of approximately 1-3 mm3 were put into 0.2 mL plastic vials and were kept at 37 °C for 30 minutes. The tissue was then completely dried, and 100 µl of 5% Chelex solution and 1 µl of proteinase K (20 mg/mL) were added into the vials. The vials were then incubated in a thermocycler using the following program: 1 hour at 57 °C, 10 minutes at 95 °C, 1 minute at 37 °C, 10 minutes at 95 °C, and finally 15 minutes at 15 °C. The DNA samples were then stored at –70 °C until use.
PCR was used to amplify an approximately 400-bp fragment of the mitochondrial cytochrome c oxidase subunit 1 gene (cox1) using the primers JB3 and JB4.5 (BOWLES & MCMANUS, 1994Bowles J, McManus DP. Genetic characterization of the Asian , a newly described taeniid cestode of humans. TaeniaAm J Trop Med Hyg 1994; 50(1): 33-44. PMid:7905720.). The PCR solution was prepared in a 50 µl volume containing 4 µl of template DNA, 0.25 µM of each primer and GoTaq® Green Master Mix, 2X (Promega, Madison, WI, USA). A three-step thermal process consisting of 94 °C for 30 seconds, 55 °C for 30 seconds and 72 °C for 30 seconds was repeated 36 times to amplify the short fragment of cox1 (LIU et al., 2011Liu GH, Lin RQ, Li MW, Liu W, Liu Y, Yuan ZG, et al. The complete mitochondrial genomes of three cestode species of infecting animals and humans. TaeniaMol Biol Rep 2011; 38(4): 2249-2256. http://dx.doi.org/10.1007/s11033-010-0355-0. PMid:20922482.
http://dx.doi.org/10.1007/s11033-010-035...
). The PCR products were analyzed by means of electrophoresis on 1.5% agarose gel with ethidium bromide staining. The PCR products were then sequenced using the Big DyeTM Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Foster City, CA, USA) and an ABI 3100 automated sequencer (Applied Biosystems). The sequences were assembled using the ChromasPro 1.7.6 software (TECHNELYSIUM, 2015Technelysium. ChromasPro 1.7.6 [online]. Brisbane: Technelysium Pty Ltd; 2015 [cited 2016 Feb 21]. Available from: http://www.technelysium.com.au/ChromasPro.html
http://www.technelysium.com.au/ChromasPr...
). All the sequences were compared with a reference sequence in GenBank using ClustalX (CLUSTAL, 2015Clustal. ClustalX [online]. Dublin: 2015 [cited 2016 Feb 21]. Available from: http://www.clustal.org/
http://www.clustal.org/...
). A phylogenetic tree was constructed by means of the neighbor-joining method with the Kimura two-parameter distance, using the MEGA6 software (MEGA, 2015Molecular Evolutionary Genetics Analysis – MEGA. MEGA6 software [online]. 2015 [cited 2016 Feb 21]. Available from: http://www.megasoftware.net/
http://www.megasoftware.net/...
) (TAMURA et al., 2004Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA 2004; 101(30): 11030-11035. http://dx.doi.org/10.1073/pnas.0404206101. PMid:15258291.
http://dx.doi.org/10.1073/pnas.040420610...
; TAMURA et al., 2013Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 2013; 30(12): 2725-2729. http://dx.doi.org/10.1093/molbev/mst197. PMid:24132122.
http://dx.doi.org/10.1093/molbev/mst197...
). Unique nucleotide sequences of the partial cox1 gene of T. omissa from these pumas were deposited in the GenBank database under accession numbers KR095312, KR095313 and KR095314.
All the cestodes studied (41) were identified as T. omissa. The puma from Pumaconca was infected with a total of 28 cestodes (11 mature and 17 immature tapeworms). The puma from Abra La Raya was infected with a total of 13 tapeworms (9 mature and 4 immature tapeworms).
The immature tapeworms were 2.6-7.6 (4.4; SE: 0.3) cm in total length and 2.1-5.0 (2.9; SE: 0.2) mm in maximum width. All immature tapeworms did not show internal organs. The mature tapeworms had a strobila of 19.7-39.5 (29.7; SE: 2.5) cm in length, and 7.5-9.0 (8.2; SE: 0.2) mm in maximum width. The scoleces measured 977-1312 (1106; SE: 77.2) μm in diameter. Each scolex had four muscular suckers measuring 319-581 (467; SE: 43.2) μm in diameter. Each scolex had a rostellum armed with two rows of hooks (21 to 23 hooks in each row). The rostella measured 457-552 (508; SE: 20.1) μm in diameter. Large hooks measured 243-289 (266; SE: 4.4) μm in length and small hooks 186-229 (208; SE: 3.8) μm (Figure 1A-C). Additional morphological characteristics of the hooks are shown in Table 1.
(A) Rostellum of a specimen of Taenia omissa collected from Puma concolor in Peru. Scale bar = 200 μm; (B) Large hook. Scale bar = 100 μm; (C) Small hook. Scale bar = 100 μm; (D) Mature proglottid. Ov = ovarium; T = testes; Cs = cirrus sac; Vi = vitellarium and Mg = Mehlis’ gland. Scale bar = 2.0 mm.
Hook measurements of Taenia omissa (in µm) isolated from pumas in the Andes mountain range of Peru. Selected parameters were based on the system used by Haukisalmi et al. (2011)Haukisalmi V, Lavikainen A, Laaksonen S, Meri S. n. sp. (Cestoda: Cyclophyllidea: Taeniidae) from its definitive (brown bear Linnaeus) and intermediate (moose/elk . Taenia arctosUrsus arctosAlces spp.) hostsSyst Parasitol 2011; 80(3): 217-230. http://dx.doi.org/10.1007/s11230-011-9324-9. PMid:22002024.
http://dx.doi.org/10.1007/s11230-011-932... .
The width of the mature proglottid was greater than its length (Figure 1D). The mature proglottid was 4.8-5.6 (5.3; SE: 60.1) mm long by 1.8-2.8 (2.3; SE 75.9) mm wide. Each mature proglottid had one set of genital organs. The genital pores alternated irregularly, and were positioned in the middle of the lateral margins of the proglottids. The genital atrium measured 210-239 (227; SE: 5.7) μm wide. The number of testes ranged from 315 to 378, with testes distributed anteriorly and laterally to the ovary between longitudinal osmoregulatory canals. The testes were subspherical and measured 43-74 (56; SE: 1.2) μm in diameter. The cirrus sacs were elongate and measured 357-472 (415; SE: 24.3) μm long by 122-155 (142; SE: 7.2) μm wide, extending across the longitudinal ventral canal. The vagina opened posteriorly to the cirrus sac. The ovaries were bilobate and measured 837-1137 (941; SE: 28.1) μm long by 285-640 (410; SE: 33.7) μm wide, and were distributed in the anterior half of the proglottid. The vitellarium was situated posteriorly to the ovary and measured 522-724 (600; SE: 20.0) μm long by 64-104 (83; SE: 4.2) μm wide. Mehlis’ gland was oval or subspherical and measured 89-123 (109; SE: 5.2) μm long by 80-116 (96; SE: 4.8) μm wide.
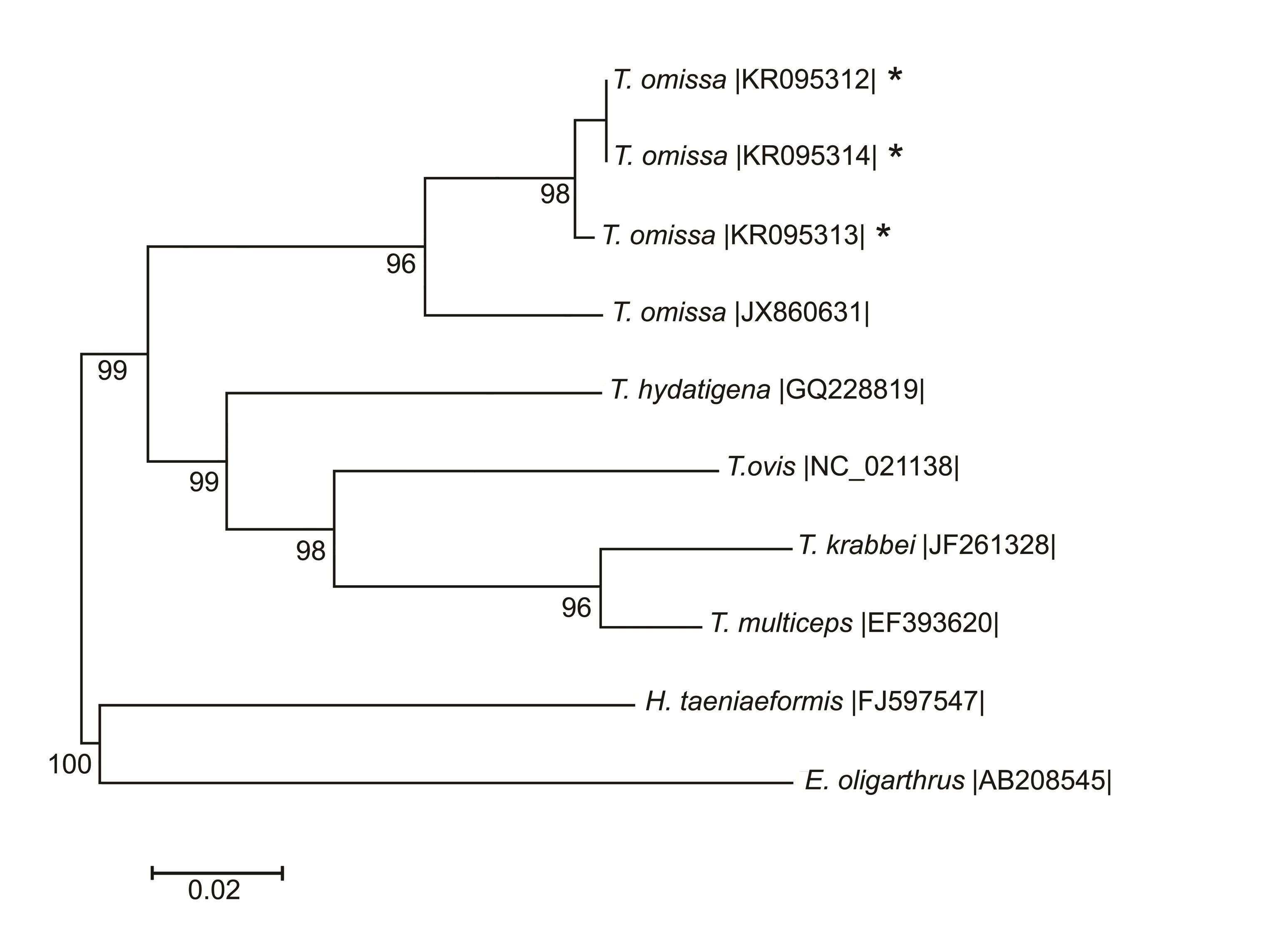
Nucleotide sequences of the cox1 gene were generated for PCR-positive isolates from three T. omissa (To1, To2 and To3). The first two were from the Pumaconca puma, and the third was from the Abra La Raya puma. The genetic identity of T. omissa was calculated from the alignments of the nucleotide sequences of the cox1 gene (Figure 2 and 3). Sequences of T. omissa from this study (GenBank Nos. KR095312 (To1), KR095313 (To2) and KR095314 (To3)) had single nucleotide differences of 0.7% between each other. However, the sequences for the To1 and To3 isolates were identical. All the sequences were compared with a sequence previously published for this species of tapeworm that had been collected from a puma in Canada (GenBank No. JX860631) (LAVIKAINEN et al., 2013Lavikainen A, Haukisalmi V, Deksne G, Holmala K, Lejeune M, Isomursu M, et al. Molecular identification of spp. in the Eurasian lynx (TaeniaLynx lynx) from Finland. Parasitology 2013; 140(5): 653-662. http://dx.doi.org/10.1017/S0031182012002120. PMid:23347590.
http://dx.doi.org/10.1017/S0031182012002...
). The isolate from Canada differed with regard to cox1 by 5.1 – 5.3% from the sequences in this study.
Genetic diversity in Taenia omissa sequences of the cox1 gene in pumas. Dots (.) denote nucleotides identical to those of the T. omissa control (JX860631). Sequences from pumas from Pumaconca (KR095312 and KR095313) and from Abra La Raya (KR095314)
Phylogenetic relationship for Taenia species, based on cox1 gene. Neighbor-joining tree inferred by means of Kimura two-parameter distances from the partial cox1 gene. Sequences from GenBank were used as references. Sequences marked (*) were obtained in this study. The scale bars are proportional to the number of substitutions per site.
Different species of Taenia have been reported in pumas, including Taenia multiceps, Taenia hydatigena, Taenia ovis, Taenia krabbei, T. omissa and H. taeniaeformis (Syn. Taenia taeniaeformis see Nakao et al., 2013Nakao M, Lavikainen A, Iwaki T, Haukisalmi V, Konyaev S, Oku Y, et al. Molecular phylogeny of the genus (Cestoda: Taeniidae): proposals for the resurrection of TaeniaHydatigera Lamarck, 1816 and the creation of a new genus Versteria.Int J Parasitol 2013; 43(6): 427-437. http://dx.doi.org/10.1016/j.ijpara.2012.11.014. PMid:23428901.
http://dx.doi.org/10.1016/j.ijpara.2012....
and Haukisalmi, 2016Haukisalmi V. Global Cestode Database: Hydatigera taeniaeformis (Batsch, 1786) Lamarck, 1816 [online]. Connecticut: University of Connecticut; 2016 [cited 2016 Feb 21]. Available from: http://tapewormdb.uconn.edu/index.php/parasites/species_details/9935/12
http://tapewormdb.uconn.edu/index.php/pa...
) (LUHE, 1910Luhe M. Cystotanien sudamerikanischer Feliden. Zool Jahrb 1910;(Suppl. 12): 687-710.; RAUSCH et al., 1983Rausch RL, Maser C, Hoberg EP. Gastrointestinal helminths of the cougar, Felis concolor L., in northeastern Oregon. J Wildl Dis 1983; 19(1): 14-19. http://dx.doi.org/10.7589/0090-3558-19.1.14. PMid:6682458.
http://dx.doi.org/10.7589/0090-3558-19.1...
; VIEIRA et al., 2008Vieira FM, Luque JL, Muniz-Pereira LC. Checklist of helminth parasites in wild carnivore mammals from Brazil. Zootaxa 2008; 1721: 1-23.; WAID & PENCE, 1988Waid DD, Pence DB. Helminths of mountain lions (Felis concolorCylicospirura subaequalis) from southwestern Texas, with a redescription of (Molin, 1860) Vevers, 1922. Can J Zool 1988; 66(10): 2110-2117. http://dx.doi.org/10.1139/z88-313.
http://dx.doi.org/10.1139/z88-313...
). The main criteria for the morphological diagnosis of taeniid cestodes are measurements and numbers of rostellar hooks (Table 2) (RISER, 1956Riser NW. The hooks of taenioid cestodes from North American felids. Am Midl Nat 1956; 56(1): 133-137. http://dx.doi.org/10.2307/2422449.
http://dx.doi.org/10.2307/2422449...
; ABULADZE, 1964Abuladze KI. Taeniata of animals and man and diseases caused by them. In: Skrjabin KI. Essentials of cestodology. Jerusalem: Israel Program for Scientific Translations; 1964. p. 1-547, vol. 4.; LOOS-FRANK, 2000Loos-Frank B. An up-date of Verster’s (1969) ‘Taxonomic revision of the genus Linnaeus’ (Cestoda) in table format. TaeniaSyst Parasitol 2000; 45(3): 155-183. http://dx.doi.org/10.1023/A:1006219625792. PMid:10768761.
http://dx.doi.org/10.1023/A:100621962579...
). Based on the morphological and molecular characteristics of the specimens in this study, which coincided with data reported by other authors (LUHE, 1910Luhe M. Cystotanien sudamerikanischer Feliden. Zool Jahrb 1910;(Suppl. 12): 687-710.; RISER, 1956Riser NW. The hooks of taenioid cestodes from North American felids. Am Midl Nat 1956; 56(1): 133-137. http://dx.doi.org/10.2307/2422449.
http://dx.doi.org/10.2307/2422449...
; JONG, 1966Jong CGZ. Parasites of the Canada lynx, (Kerr). Felis (lynx) canadensisCan J Zool 1966; 44(4): 499-509. http://dx.doi.org/10.1139/z66-054. PMid:5944283.
http://dx.doi.org/10.1139/z66-054...
; RAUSCH, 1981Rausch RL. Morphological and biological characteristics of Loewen, 1929 (Cestoda: Taeniidae). Taenia rileyiCan J Zool 1981; 59(4): 653-666. http://dx.doi.org/10.1139/z81-095.
http://dx.doi.org/10.1139/z81-095...
; RAUSCH et al., 1983Rausch RL, Maser C, Hoberg EP. Gastrointestinal helminths of the cougar, Felis concolor L., in northeastern Oregon. J Wildl Dis 1983; 19(1): 14-19. http://dx.doi.org/10.7589/0090-3558-19.1.14. PMid:6682458.
http://dx.doi.org/10.7589/0090-3558-19.1...
; LOOS-FRANK, 2000Loos-Frank B. An up-date of Verster’s (1969) ‘Taxonomic revision of the genus Linnaeus’ (Cestoda) in table format. TaeniaSyst Parasitol 2000; 45(3): 155-183. http://dx.doi.org/10.1023/A:1006219625792. PMid:10768761.
http://dx.doi.org/10.1023/A:100621962579...
; DARE & WATKINS, 2012Dare OK, Watkins WG. First record of parasites from cougars (Puma concolor) in Manitoba, Canada. Can Field Nat 2012; 126(4): 324-327.; LAVIKAINEN et al., 2013Lavikainen A, Haukisalmi V, Deksne G, Holmala K, Lejeune M, Isomursu M, et al. Molecular identification of spp. in the Eurasian lynx (TaeniaLynx lynx) from Finland. Parasitology 2013; 140(5): 653-662. http://dx.doi.org/10.1017/S0031182012002120. PMid:23347590.
http://dx.doi.org/10.1017/S0031182012002...
), we conclude that the species isolated in this study corresponded to T. omissa (Table 3). This tapeworm has been reported in pumas in Canada, the USA, Paraguay and Brazil (RISER, 1956Riser NW. The hooks of taenioid cestodes from North American felids. Am Midl Nat 1956; 56(1): 133-137. http://dx.doi.org/10.2307/2422449.
http://dx.doi.org/10.2307/2422449...
; SCHMIDT & MARTIN, 1978Schmidt GD, Martin RL. Tapeworms of the Chaco Boreal, Paraguay, with two new species. J Helminthol 1978; 52(3): 205-209. http://dx.doi.org/10.1017/S0022149X00005381. PMid:722040.
http://dx.doi.org/10.1017/S0022149X00005...
; VIEIRA et al., 2008Vieira FM, Luque JL, Muniz-Pereira LC. Checklist of helminth parasites in wild carnivore mammals from Brazil. Zootaxa 2008; 1721: 1-23.; DARE & WATKINS, 2012Dare OK, Watkins WG. First record of parasites from cougars (Puma concolor) in Manitoba, Canada. Can Field Nat 2012; 126(4): 324-327.). The current study demonstrates additional geographic distribution for this parasite.
Morphologically, T. omissa is characterized by proglottids that are wider than they are long, as was demonstrated in this study (Figure 1D). This is also a morphological characteristic of H. taeniaeformis, a common cestode in felids, including pumas (ABULADZE, 1964Abuladze KI. Taeniata of animals and man and diseases caused by them. In: Skrjabin KI. Essentials of cestodology. Jerusalem: Israel Program for Scientific Translations; 1964. p. 1-547, vol. 4.). However, T. omissa differs from H. taeniaeformis in some morphological characteristics such as the numbers (42-46 vs. 34-36, respectively) and larger rostellar hooks in H. taeniaeformis (Table 2) (RISER, 1956Riser NW. The hooks of taenioid cestodes from North American felids. Am Midl Nat 1956; 56(1): 133-137. http://dx.doi.org/10.2307/2422449.
http://dx.doi.org/10.2307/2422449...
; ABULADZE, 1964Abuladze KI. Taeniata of animals and man and diseases caused by them. In: Skrjabin KI. Essentials of cestodology. Jerusalem: Israel Program for Scientific Translations; 1964. p. 1-547, vol. 4.; LOOS-FRANK, 2000Loos-Frank B. An up-date of Verster’s (1969) ‘Taxonomic revision of the genus Linnaeus’ (Cestoda) in table format. TaeniaSyst Parasitol 2000; 45(3): 155-183. http://dx.doi.org/10.1023/A:1006219625792. PMid:10768761.
http://dx.doi.org/10.1023/A:100621962579...
).
Nucleotide sequences of the cox1 gene showed intraspecific variation of 0.7-5.3% between all the T. omissa isolates, including the isolate from Canada (JX860631). Similar variations have been demonstrated in other taeniid cestodes. Lavikainen et al. (2008)Lavikainen A, Haukisalmi V, Lehtinen MJ, Henttonen H, Oksanen A, Meri S. A phylogeny of members of the family Taeniidae based on the mitochondrial cox1 and gene data. nad1Parasitology 2008; 135(12): 1457-1467. http://dx.doi.org/10.1017/S003118200800499X. PMid:18937885.
http://dx.doi.org/10.1017/S0031182008004...
performed molecular phylogeny on various taeniid cestodes and found an intraspecific variation of 0.0-3.4% in Taenia mustelae, 0.0-6.8% in Taenia polyacantha and 1.3-9.8% in H. taeniaeformis (LAVIKAINEN et al., 2008Lavikainen A, Haukisalmi V, Lehtinen MJ, Henttonen H, Oksanen A, Meri S. A phylogeny of members of the family Taeniidae based on the mitochondrial cox1 and gene data. nad1Parasitology 2008; 135(12): 1457-1467. http://dx.doi.org/10.1017/S003118200800499X. PMid:18937885.
http://dx.doi.org/10.1017/S0031182008004...
).
The life cycle of T. omissa has been described by Forrester & Rausch (1990)Forrester DJ, Rausch RL. Cysticerci (Cestoda: Taeniidae) from white-tailed deer, , in southern Florida. Odocoileus virginianusJ Parasitol 1990; 76(4): 583-585. http://dx.doi.org/10.2307/3282848. PMid:2380871.
http://dx.doi.org/10.2307/3282848...
, they identified metacestodes collected from white-tailed deer (Odocoileus virginianus) in southern Florida, and morphological analysis showed the metacestodes to be T. omissa cysticerci (FORRESTER & RAUSCH, 1990Forrester DJ, Rausch RL. Cysticerci (Cestoda: Taeniidae) from white-tailed deer, , in southern Florida. Odocoileus virginianusJ Parasitol 1990; 76(4): 583-585. http://dx.doi.org/10.2307/3282848. PMid:2380871.
http://dx.doi.org/10.2307/3282848...
). Currently, two species of deer are known to be involved in the life cycle of T. omissa, i.e. Odocoileus hemionus and O. virginianus (JENSEN et al., 1982Jensen LA, Short JA, Andersen FL. Internal parasites of Odocoileus hemionus of central Utah. Proc Helminthol Soc Wash 1982; 49(2): 317-319.; FORRESTER & RAUSCH, 1990Forrester DJ, Rausch RL. Cysticerci (Cestoda: Taeniidae) from white-tailed deer, , in southern Florida. Odocoileus virginianusJ Parasitol 1990; 76(4): 583-585. http://dx.doi.org/10.2307/3282848. PMid:2380871.
http://dx.doi.org/10.2307/3282848...
; PYBUS, 1990Pybus MJ. Survey of hepatic and pulmonary helminths of wild cervids in Alberta, Canada. J Wildl Dis 1990; 26(4): 453-459. http://dx.doi.org/10.7589/0090-3558-26.4.453. PMid:2250321.
http://dx.doi.org/10.7589/0090-3558-26.4...
). In Peru, O. virginianus has wide geographic distribution that includes the Andes mountain range and overlaps with the geographic distribution of the puma (SMITH, 1991Smith WP. Odocoileus virginianus. Mamm Species 1991; 388(388): 1-13. http://dx.doi.org/10.2307/3504281.
http://dx.doi.org/10.2307/3504281...
). Therefore, we predict that O. virginianus is involved in the life cycle of T. omissa in Peru. Futures research will be needed to identify the animal species involved as an intermediate host in the life cycle of T. omissa in the Peruvian highlands.
Acknowledgements
The authors wish to thank Guissela Zans and Maria de la Barra from the Technical Administration for Forestry and Wildlife of Peru (Administracion Tecnica Forestal y de Fauna Silvestre Cusco, Ministerio de Agricultura y Riego) for donating the pumas used in this study.
-
§Cysticercosis Working Group in Peru
References
- Abuladze KI. Taeniata of animals and man and diseases caused by them. In: Skrjabin KI. Essentials of cestodology. Jerusalem: Israel Program for Scientific Translations; 1964. p. 1-547, vol. 4.
- Bowles J, McManus DP. Genetic characterization of the Asian , a newly described taeniid cestode of humans. TaeniaAm J Trop Med Hyg 1994; 50(1): 33-44. PMid:7905720.
- Clustal. ClustalX [online]. Dublin: 2015 [cited 2016 Feb 21]. Available from: http://www.clustal.org/
» http://www.clustal.org/ - Currier MJP. Felis concolor. Mamm Species 1983; 200(200): 1-7. http://dx.doi.org/10.2307/3503951
» http://dx.doi.org/10.2307/3503951 - D’Alessandro A, Rausch RL, Morales GA, Collet S, Angel D. infections in Colombian animals. EchinococcusAm J Trop Med Hyg 1981; 30(6): 1263-1276. PMid:7325284.
- Dare OK, Watkins WG. First record of parasites from cougars (Puma concolor) in Manitoba, Canada. Can Field Nat 2012; 126(4): 324-327.
- Forrester DJ, Rausch RL. Cysticerci (Cestoda: Taeniidae) from white-tailed deer, , in southern Florida. Odocoileus virginianusJ Parasitol 1990; 76(4): 583-585. http://dx.doi.org/10.2307/3282848 PMid:2380871.
» http://dx.doi.org/10.2307/3282848 - Foster GW, Cunningham MW, Kinsella JM, McLaughlin G, Forrester DJ. Gastrointestinal helminths of free-ranging Florida panthers (Puma concolor coryi) and the efficacy of the current anthelmintic treatment protocol. J Wildl Dis 2006; 42(2): 402-406.
- Gadau J. DNA isolation from ants. Cold Spring Harb Protoc 2009(7): 1-3. http://dx.doi.org/10.1101/pdb.prot5245
» http://dx.doi.org/10.1101/pdb.prot5245 - Haukisalmi V, Lavikainen A, Laaksonen S, Meri S. n. sp. (Cestoda: Cyclophyllidea: Taeniidae) from its definitive (brown bear Linnaeus) and intermediate (moose/elk . Taenia arctosUrsus arctosAlces spp.) hostsSyst Parasitol 2011; 80(3): 217-230. http://dx.doi.org/10.1007/s11230-011-9324-9 PMid:22002024.
» http://dx.doi.org/10.1007/s11230-011-9324-9 - Haukisalmi V. Global Cestode Database: Hydatigera taeniaeformis (Batsch, 1786) Lamarck, 1816 [online]. Connecticut: University of Connecticut; 2016 [cited 2016 Feb 21]. Available from: http://tapewormdb.uconn.edu/index.php/parasites/species_details/9935/12
» http://tapewormdb.uconn.edu/index.php/parasites/species_details/9935/12 - Hoberg EP. Phylogeny of Taenia: Species definitions and origins of human parasites. Parasitol Int 2006; 55(Suppl.): S23-S30. http://dx.doi.org/10.1016/j.parint.2005.11.049 PMid:16371252.
» http://dx.doi.org/10.1016/j.parint.2005.11.049 - Jensen LA, Short JA, Andersen FL. Internal parasites of Odocoileus hemionus of central Utah. Proc Helminthol Soc Wash 1982; 49(2): 317-319.
- Jong CGZ. Parasites of the Canada lynx, (Kerr). Felis (lynx) canadensisCan J Zool 1966; 44(4): 499-509. http://dx.doi.org/10.1139/z66-054 PMid:5944283.
» http://dx.doi.org/10.1139/z66-054 - Lavikainen A, Haukisalmi V, Deksne G, Holmala K, Lejeune M, Isomursu M, et al. Molecular identification of spp. in the Eurasian lynx (TaeniaLynx lynx) from Finland. Parasitology 2013; 140(5): 653-662. http://dx.doi.org/10.1017/S0031182012002120 PMid:23347590.
» http://dx.doi.org/10.1017/S0031182012002120 - Lavikainen A, Haukisalmi V, Lehtinen MJ, Henttonen H, Oksanen A, Meri S. A phylogeny of members of the family Taeniidae based on the mitochondrial cox1 and gene data. nad1Parasitology 2008; 135(12): 1457-1467. http://dx.doi.org/10.1017/S003118200800499X PMid:18937885.
» http://dx.doi.org/10.1017/S003118200800499X - Liu GH, Lin RQ, Li MW, Liu W, Liu Y, Yuan ZG, et al. The complete mitochondrial genomes of three cestode species of infecting animals and humans. TaeniaMol Biol Rep 2011; 38(4): 2249-2256. http://dx.doi.org/10.1007/s11033-010-0355-0 PMid:20922482.
» http://dx.doi.org/10.1007/s11033-010-0355-0 - Loos-Frank B. An up-date of Verster’s (1969) ‘Taxonomic revision of the genus Linnaeus’ (Cestoda) in table format. TaeniaSyst Parasitol 2000; 45(3): 155-183. http://dx.doi.org/10.1023/A:1006219625792 PMid:10768761.
» http://dx.doi.org/10.1023/A:1006219625792 - López-González AC, González-Romero A. A synthesis of current literature and knowledge about the ecology of the puma (Linnaeus). Puma concolor Acta Zool Mex 1998; 75: 171-190.
- Luhe M. Cystotanien sudamerikanischer Feliden. Zool Jahrb 1910;(Suppl. 12): 687-710.
- Molecular Evolutionary Genetics Analysis – MEGA. MEGA6 software [online]. 2015 [cited 2016 Feb 21]. Available from: http://www.megasoftware.net/
» http://www.megasoftware.net/ - Nakao M, Lavikainen A, Iwaki T, Haukisalmi V, Konyaev S, Oku Y, et al. Molecular phylogeny of the genus (Cestoda: Taeniidae): proposals for the resurrection of TaeniaHydatigera Lamarck, 1816 and the creation of a new genus Versteria.Int J Parasitol 2013; 43(6): 427-437. http://dx.doi.org/10.1016/j.ijpara.2012.11.014 PMid:23428901.
» http://dx.doi.org/10.1016/j.ijpara.2012.11.014 - Pybus MJ. Survey of hepatic and pulmonary helminths of wild cervids in Alberta, Canada. J Wildl Dis 1990; 26(4): 453-459. http://dx.doi.org/10.7589/0090-3558-26.4.453 PMid:2250321.
» http://dx.doi.org/10.7589/0090-3558-26.4.453 - Rausch RL, Maser C, Hoberg EP. Gastrointestinal helminths of the cougar, Felis concolor L., in northeastern Oregon. J Wildl Dis 1983; 19(1): 14-19. http://dx.doi.org/10.7589/0090-3558-19.1.14 PMid:6682458.
» http://dx.doi.org/10.7589/0090-3558-19.1.14 - Rausch RL. Family Taeniidae. In: Khalil LF, Jones A, Bray RA. Keys to the cestode parasites of vertebrates. Wallingford: CAB International; 1994. p. 665-672.
- Rausch RL. Morphological and biological characteristics of Loewen, 1929 (Cestoda: Taeniidae). Taenia rileyiCan J Zool 1981; 59(4): 653-666. http://dx.doi.org/10.1139/z81-095
» http://dx.doi.org/10.1139/z81-095 - Rickard LG, Foreyt WJ. Gastrointestinal parasites of cougars (Felis concolorOllulanus tricuspis) in Washington and the first report of in a sylvatic felid from North America. J Wildl Dis 1992; 28(1): 130-133. http://dx.doi.org/10.7589/0090-3558-28.1.130 PMid:1548792.
» http://dx.doi.org/10.7589/0090-3558-28.1.130 - Riser NW. The hooks of taenioid cestodes from North American felids. Am Midl Nat 1956; 56(1): 133-137. http://dx.doi.org/10.2307/2422449
» http://dx.doi.org/10.2307/2422449 - Rossin MA, Timi JT, Hoberg EP. An endemic from South America: validation of . TaeniaT. talicei Dollfus, 1960 (Cestoda: Taeniidae) with characterization of metacestodes and adultsZootaxa 2010; 2636: 49-58.
- Schmidt GD, Martin RL. Tapeworms of the Chaco Boreal, Paraguay, with two new species. J Helminthol 1978; 52(3): 205-209. http://dx.doi.org/10.1017/S0022149X00005381 PMid:722040.
» http://dx.doi.org/10.1017/S0022149X00005381 - Smith WP. Odocoileus virginianus. Mamm Species 1991; 388(388): 1-13. http://dx.doi.org/10.2307/3504281
» http://dx.doi.org/10.2307/3504281 - Tamura K, Nei M, Kumar S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA 2004; 101(30): 11030-11035. http://dx.doi.org/10.1073/pnas.0404206101 PMid:15258291.
» http://dx.doi.org/10.1073/pnas.0404206101 - Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 2013; 30(12): 2725-2729. http://dx.doi.org/10.1093/molbev/mst197 PMid:24132122.
» http://dx.doi.org/10.1093/molbev/mst197 - Technelysium. ChromasPro 1.7.6 [online]. Brisbane: Technelysium Pty Ltd; 2015 [cited 2016 Feb 21]. Available from: http://www.technelysium.com.au/ChromasPro.html
» http://www.technelysium.com.au/ChromasPro.html - Vieira FM, Luque JL, Muniz-Pereira LC. Checklist of helminth parasites in wild carnivore mammals from Brazil. Zootaxa 2008; 1721: 1-23.
- Waid DD, Pence DB. Helminths of mountain lions (Felis concolorCylicospirura subaequalis) from southwestern Texas, with a redescription of (Molin, 1860) Vevers, 1922. Can J Zool 1988; 66(10): 2110-2117. http://dx.doi.org/10.1139/z88-313
» http://dx.doi.org/10.1139/z88-313
Publication Dates
-
Publication in this collection
25 Aug 2016 -
Date of issue
Jul-Sep 2016
History
-
Received
04 Jan 2016 -
Accepted
13 June 2016