Abstract
The transplacental transmission is the primary route of Neospora caninum infection in bovine herds around the world. This study aimed to determine the frequency of transplacental transmission of the parasite in dairy cattle of Agreste region of Pernambuco through serological tests (IFAT and ELISA). Three hundred sixteen serum samples from cows and heifers and their offspring were analyzed. The transplacental transmission rate was 72.22% (13/18) for cows and 69.23% (9/13) for heifers by IFAT. ELISA test showed transplacental transmission rate of 43.58% (17/39) for cows and 50% (9/18) for heifers. The transplacental transmission rates were similar, in both groups in test, but a higher seropositivity was found in cows by IFAT. Data were statistically analyzed using the chi-square and Fisher’s exact test. A significant relationship of dependence between seropositivity of mothers and their offspring was found. The more frequent IFAT antibody titers and ELISA levels for N. caninum were, respectively, 200 and between four (cows) and five (heifers and offspring). In the Spearman correlation, no association was found between the magnitude of antibody titers for N. caninum between mothers and their offspring. The kappa test showed an index of 0.35, indicating a mild correlation between the serological tests used. The study suggests that cows and heifers are the main transmitters of N. caninum in the studied region and that vertical transmission is the major form of transmission in dairy herds of the Agreste region of Pernambuco.
Keywords:
Neosporosis; dairy cows; IFAT; ELISA; Northeastern Brazil
Resumo
A transmissão transplacentária é a principal via de infecção do Neospora caninum nos rebanhos bovinos em todo o mundo. O presente estudo teve como objetivo determinar a frequência da transmissão transplacentária do parasita em bovinos leiteiros do Agreste Pernambucano, por meio de testes sorológicos (RIFI e ELISA). Foram analisadas 316 amostras de soro de fêmeas bovinas (vacas e novilhas) e de suas crias. A taxa de transmissão transplacentária pela RIFI foi de 72,22% (13/18) para vacas e 69,23% (9/13) para as novilhas. O ELISA teste mostrou taxa de transmissão transplacentária de 43,58% (17/39) para as vacas e 50% (9/18) para as novilhas. As taxas de transmissão transplacentária foram similares para os dois testes em geral, porém uma maior soropositividade foi encontrada nas vacas pela RIFI. Os dados foram estatisticamente analisados pelo teste de qui-quadrado e teste exato de Fischer. Foi encontrada uma relação significativa de dependência entre a soropositividade das mães e de suas crias. Os títulos de anticorpos anti- N. caninum foi de 200 na RIFI e posicionados entre o nível quatro (vacas) e cinco (novilhas e bezerros) pelo ELISA. Pela correlação de Spearman, não foi observada associação entre a magnitude de títulos de anticorpos anti- N. caninum de fêmeas com o de suas crias. O teste de concordância kappa revelou um índice de 0,35, indicando uma concordância leve entre os testes sorológicos utilizados. O estudo sugere que vacas e novilhas são as principais transmissoras do N. caninum na região estudada, sendo a transmissão vertical, a principal forma de transmissão do agente em rebanhos leiteiros do Agreste de Pernambuco.
Palavras-chave:
Neosporose; vacas de leite; RIFI; ELISA; Nordeste do Brasil
Neosporosis is a disease that shows a global importance, with prevalence ranging from 0.07% to 97.2% (DUBEY & SCHARES, 2011Dubey JP, Schares G. Neosporosis in animals: the last five years. Vet Parasitol 2011; 180(1-2): 90-108. http://dx.doi.org/10.1016/j.vetpar.2011.05.031.
http://dx.doi.org/10.1016/j.vetpar.2011....
). Transplacental transmission is the main form of dissemination of neosporosis through cattle herds, and is considered to be one of the main causes of abortion in livestock. The parasite may cause chronic infection, remaining within the herd across the generations and giving rise to reproduction losses (DUBEY et al., 2007Dubey JP, Schares G, Ortega-Mora LM. Epidemiology and control of neosporosis and Neospora caninum.Clin Microbiol Rev 2007; 20(2): 323-367. http://dx.doi.org/10.1128/CMR.00031-06.
http://dx.doi.org/10.1128/CMR.00031-06...
). When infection occurs during the final third of gestation, fetal deaths and abortions hardly ever occur and offspring appear to be healthy when in fact they are persistently infected (DUBEY & SCHARES, 2011Dubey JP, Schares G. Neosporosis in animals: the last five years. Vet Parasitol 2011; 180(1-2): 90-108. http://dx.doi.org/10.1016/j.vetpar.2011.05.031.
http://dx.doi.org/10.1016/j.vetpar.2011....
). The horizontal infection occurs due to the presence of the main definitive hosts, represented by dogs, coyotes (Canis latrans) and Australian dingos (Canis lupus dingo) (McALLISTER et al., 1998McAllister MM, Dubey JP, Lindsay DS, Jolley WR, Wills RA, Mcguire AM. Dogs are definitive hosts of Neospora caninum.Int J Parasitol 1998; 28(9): 1473-1478. http://dx.doi.org/10.1016/S0020-7519(98)00138-6.
http://dx.doi.org/10.1016/S0020-7519(98)...
; GONDIM et al., 2004Gondim LFP, McAllister MM, Pitt WC, Zemlicka D. Coyotes (Canis latransNeospora caninum.) are definitive hosts of Int J Parasitol 2004; 34(2): 159-161. http://dx.doi.org/10.1016/j.ijpara.2004.01.001.
http://dx.doi.org/10.1016/j.ijpara.2004....
; KING et al., 2010King JS, Slapeta J, Jenkins DJ, Al-Qassab SE, Ellis JT, Windsor PA. Australian dingoes are definitive hosts of Neospora caninum.Int J Parasitol 2010; 40(8): 945-950. http://dx.doi.org/10.1016/j.ijpara.2010.01.008.
http://dx.doi.org/10.1016/j.ijpara.2010....
), which eliminate Neospora caninum oocysts in their faeces and can contaminate food items and water, keeping the infection between generations through of transplacental transmission (DUBEY et al., 2007Dubey JP, Schares G, Ortega-Mora LM. Epidemiology and control of neosporosis and Neospora caninum.Clin Microbiol Rev 2007; 20(2): 323-367. http://dx.doi.org/10.1128/CMR.00031-06.
http://dx.doi.org/10.1128/CMR.00031-06...
).
Transplacental transmission of N. caninum can be estimated from the number of seropositive offspring born from seropositive mothers, soon after their birth and before ingestion of colostrum (PARÉ et al., 1996Paré J, Thurmond MC, Hietala SK. Congenital Neospora caninum infection in dairy cattle and associated calfhood mortality. Can J Vet Res 1996; 60(2): 133-139.).Among the techniques used to show whether neosporosis is involved as a disease with in the reproductive sphere, the Indirect Fluorescente Antibody Test (IFAT) and Enzyme-Linked Immunosorbent Assay (ELISA) are the major serological tests used in the routine (PARÉ et al., 1996Paré J, Thurmond MC, Hietala SK. Congenital Neospora caninum infection in dairy cattle and associated calfhood mortality. Can J Vet Res 1996; 60(2): 133-139.; JENKINS et al., 2002Jenkins M, Baszler T, Bjorkman C, Schares G, Williams D. Diagnosis and seroepidemiology of associated bovine abortion. Neospora caninum-Int J Parasitol 2002; 32(5): 631-636. http://dx.doi.org/10.1016/S0020-7519(01)00363-0.
http://dx.doi.org/10.1016/S0020-7519(01)...
).
Serological studies characterizing the frequency of transplacental transmission among offspring, before ingestion of colostrum, have been reported in Brazil (VIANNA et al., 2008Vianna LC, Sartor IF, Pituco EM, Okuda LH, Camargo CN, Kronka SN. Incidência e transmissão transplacentária de em fêmeas primíparas da raça abatidos em Presidente Prudente, São Paulo, Brasil. Neospora caninumBos indicusSemina: Ciênc Agrá 2008; 29(2): 387-392.; MARQUES et al., 2011Marques FAC, Headley AS, Pereira VF, Taroda A, Barros LD, Cunha IAL, et al. Neospora caninumBos indicus: evaluation of vertical transmission in slaughtered beef cows (). Parasitol Res 2011; 108(4): 1015-1019. http://dx.doi.org/10.1007/s00436-010-2146-x.
http://dx.doi.org/10.1007/s00436-010-214...
; HEIN et al., 2012Hein HE, Machado G, Miranda ICS, Costa EF, Pellegrini DCP, Driemeier D, et al. Neosporose bovina: avaliação da transmissão vertical e fração atribuível de aborto em uma população de bovinos no Estado do Rio Grande do Sul. Pesqui Vet Bras 2012; 32(5): 396-400. http://dx.doi.org/10.1590/S0100-736X2012000500006.
http://dx.doi.org/10.1590/S0100-736X2012...
; SANTOS et al., 2012Santos RRD, Rocha CMBM, Gonçalves TM, Guimarães AM. Quantification of vertical transmission of in dairy cows in Minas Gerais, Brazil. Neospora caninumRev Bras Parasitol Vet 2012; 21(3): 294-297. http://dx.doi.org/10.1590/S1984-29612012000300021.
http://dx.doi.org/10.1590/S1984-29612012...
; MACEDO et al., 2013Macedo CAB, Macedo MFSB, Cardim ST, Paiva MCDC, Tadora A, Barros LD, et al. evaluation of vertical transmission in slaughtered dairy cows (Neospora caninum:Bos taurus). Rev Bras Parasitol Vet 2013; 22(1): 13-17. http://dx.doi.org/10.1590/S1984-29612013000100004.
http://dx.doi.org/10.1590/S1984-29612013...
), but none have been conducted in the state of Pernambuco up to now. In this state, the dairy cattle has been established historically in the Agreste region, counting with approximately 169.581 million dairy cattle (IBGE, 2010Instituto Brasileiro de Geografia e Estatística – IBGE. Produção da Pecuária Municipal. Rio de Janeiro: IBGE; 2010. p. 55-61. vol. 38. Boletim Técnico.). The present study aimed to evaluate the frequency of transplacental transmission of N. caninum between dairy cattle (cows and heifers) and their respective offspring before ingestion of colostrum, in the Agreste region of Pernambuco, by serological techniques.
One hundred fifty eight female cattle (113 cows and 45 heifers), without previous reports of reproductive problems, that were assisted during delivery at the Cattle Clinic, Garanhuns campus, Federal Rural University of Pernambuco (UFRPE), and their respective offspring (n = 158),were evaluated, totalizing 316 animals. Samples were collected at delivery, during the period of 2003-2013. Animal were originated from 25 municipalities of the Agreste region of Pernambuco: Pesqueira (6/316), São João (22/316), Canhotinho (8/316), Garanhuns (68/316), Venturosa (14/316), Correntes (18/316), Paranatama (8/316), Bom Conselho (10/316), Lagoa do Ouro (16/316), Jucatí (14/316), Caetés (24/316), Pedra (32/316), Palmerina (6/316), Alagoinha (2/316), Saloá (2/316), Angelim (8/316), Teresinha (2/316), (2/316), Brejão (6/316), Lajedo (6/316), São Bento do Una (38/316), Iatí (2/316), Quipapá (2/316) and Ibirajuba (2/316).The animals were Holstein, Girolanda and also mixed breed. Positivity in each municipality was calculated by the number of positive animals divided by the total number of animals sampled.
Blood collection was performed during delivery, for cows and heifers, and soon after birth, before ingestion of colostrum, for offspring, totaling 316 samples (158 adults and 158 offspring). Blood samples from females and their respective offspring were collected by venipuncture of the external jugular, using the Vacutainer® system (B.D. Indústria Cirúrgica, Juiz de Fora, MG), in silicon-treated tubes without anticoagulant. Samples were then centrifuged at 3.500 rpm for 10 minutes for serum obtainment, which were liquated in polypropylene microtubes and then kept frozen in ultrafreezer (–80 °C), until the time of use.
For IFAT, tachyzoites of the isolate Nc-1 of N. caninum cultured in Vero cells were used as antigens, which were deposited on previously marked wells (MINEO et al., 2009Mineo TWP, Carrasco AOT, Marciano JA, Werther K, Pinto AA, Machado RZ. Pigeons (Columba livia) are a suitable experimental model for infection in birds. Neospora caninumVet Parasitol 2009; 159(2): 149-153. http://dx.doi.org/10.1016/j.vetpar.2008.10.024.
http://dx.doi.org/10.1016/j.vetpar.2008....
). Ten serum samples were tested on each slide and were compared with negative and positive controls. The positive e negative controls serum samples were obtained from the serum bank of the Immunoparasitology Laboratory, UNESP Jaboticabal. Test serum samples and positive e negative controls were diluted at 1:200. After dilution (1:200) in phosphate-buffered saline solution (PBS) (pH 7.2; 1.3 M NaCl, 27 M KCl, 56 mM Na2HPO4, 10 mM KH2PO4 and 9.2 mM NaH2PO4), 10 μL of diluted serum samples were deposited in each slide well. The slides were then incubated at 37 °C for 30 minutes in humid chamber. After incubation, slides were washed three times with PBS for five minutes, consecutively, and then dried at room temperature. Following this, 10 μL of conjugate (anti-IgG-bovine-SIGMA®, St. Louis, Missouri, United States) diluted in PBS at 1:300 was added to each slide well. Then, slides were incubated in humid chamber again, at 37 °C for 30 minutes, with subsequent washing, as described previously.
After the slides had been dried at room temperature, buffered glycerin was added (glycerin and 0.5 M carbonate-bicarbonate buffer at pH 9.6), and then covered with a cover slips, for observation at a magnification of 400X, under a microscope equipped with fluorescent light (Olympus BX-FLA). Test positivity was determined through observation of total peripheral fluorescence of the tachyzoites, using a cutoff point of 1:200. Positive samples were subjected to sequential dilutions at base 2, to determine the antibody titers (DUBEY et al., 1988Dubey JP, Carpenter JL, Speer CA, Topper MJ, Uggla A. Newly recognized fatal protozoan disease of dogs. J Am Vet Assoc 1988; 192(9): 1269-1285.; MINEO et al., 2009Mineo TWP, Carrasco AOT, Marciano JA, Werther K, Pinto AA, Machado RZ. Pigeons (Columba livia) are a suitable experimental model for infection in birds. Neospora caninumVet Parasitol 2009; 159(2): 149-153. http://dx.doi.org/10.1016/j.vetpar.2008.10.024.
http://dx.doi.org/10.1016/j.vetpar.2008....
).
The indirect ELISA was performed as previously described by Machado et al. (1997)Machado RZ, Montassier HJ, Pinto AA, Lemos EG, Machado MRF, Valadão IFF, et al. An enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies against in cattle. Babesia bovisVet Parasitol 1997; 71(1): 17-26. http://dx.doi.org/10.1016/S0304-4017(97)00003-4.
http://dx.doi.org/10.1016/S0304-4017(97)...
, with some modifications. There was no need for block standardization, since there was a standardized protocol for the antigen under study. To sensitize the ELISA plate (NUNC MaxiSport®), 10 μg/mL of crude soluble antigen of N. caninum was diluted in carbonate-bicarbonate buffer (pH 9.6; 0.5M). One hundred microliters of the diluted antigen was added to each plate well, and then were incubated for 12-14h in a humid chamber at 4 °C. After this period, the excess antigen was discarded and the plate was blocked using 6% powdered skimmed milk (blocker), in carbonate-bicarbonate buffer. For every plate well, 200 μL of the blocker was added, followed by incubation in the humid chamber at 37 °C for one hour. After removal of the blocking solution, the plate was washed three times with PBS Tween-20. One hundred microliters of the serum diluted at 1:400 in PBS Tween-20 were added to each well and, once again, the plate was incubated in the humid chamber at 37 °C for one hour, with subsequent washing, as previously described.
One hundred microliters of anti-IgG-bovine conjugate (SIGMA®, St. Louis, Missouri, United States), bound to alkaline phosphatase, diluted at 1:30.000 in PBS Tween-20, was added to each plate well. Plates were then incubated and washed in the same manner as described previously. The enzyme substrate (p-nitrophenyl), at the concentration of 1mg/mL, was diluted in diethanolamine buffer (pH 9.8), and then 100 μL of the dilution was added per well. Plates were incubated at room temperature protected from the light for 30 minutes. The reaction was read using a microplate reader for ELISA (Microplate Reader MRX TC Plus, Dynex Technology, USA), at the wavelength of 405 nm. The well cavity of the microplate that contained all the reaction elements except for bovine serum was used as reaction “blank”. After the reading, the absorbance values were calculated in accordance with Machado et al. (1997)Machado RZ, Montassier HJ, Pinto AA, Lemos EG, Machado MRF, Valadão IFF, et al. An enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies against in cattle. Babesia bovisVet Parasitol 1997; 71(1): 17-26. http://dx.doi.org/10.1016/S0304-4017(97)00003-4.
http://dx.doi.org/10.1016/S0304-4017(97)...
, with subsequent determination of ELISA levels.
In order to determinate the frequencies of anti-N. caninum antibodies in cows, heifers and their respective offspring, and also in the seroepidemiological survey of infection by this parasite in the municipalities of the Agreste region of Pernambuco, descriptive statistics was performed. The data were expressed as measurements of relative frequency. To evaluate the general transplacental transmission rates among females according to the categories (cow and heifer), as measured by IFAT and ELISA, McNemar’ schi-square test or Fisher’s exact test (when the assumptions used to carry out the chi-square test were not achieved) was performed, in association with descriptive statistics. Spearman’s correlation (r) was used to ascertain the degree of magnitude between the titers observed in the cows and heifers and their respective offspring (SAMPAIO, 2007Sampaio IVB. Testes estatísticos não paramétricos. In: Sampaio IVB. Estatística aplicada à experimentação animal. 3. ed. Belo Horizonte: Fundação de Ensino e Pesquisa em Medicina Veterinária e Zootecnia; 2007. p. 207-223.). A significance level (α) of 5% was used for these tests. The coefficient of concordance between IFAT and ELISA was estimated using the kappa test (LANDIS & KOCH, 1977Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33(1): 159-174. http://dx.doi.org/10.2307/2529310.
http://dx.doi.org/10.2307/2529310...
). The results were analyzed using the Statistical Analysis System Software, version 9.2.
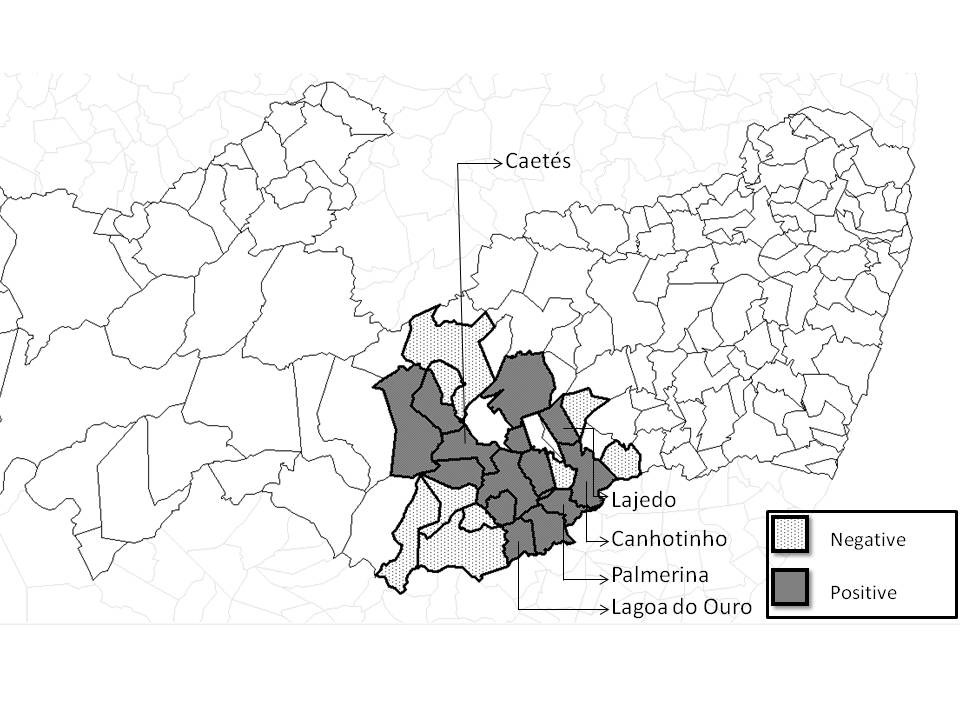
Of the total number of evaluated females (cows and heifers), 19.62% (31/158) and 36.07% (57/158) were seropositive for N. caninum, according to IFAT and ELISA, respectively. Among the 25 evaluated municipalities, seropositivity animals were found in Palmerina (100%), Lagoa do Ouro (100%), Canhotinho (38%), Caetés (33%), Lajedo (33%), Paranatama (25%), São João (23%), Pedra (19%), Brejão (17%), São Bento (16%), Garanhuns (16%), Venturosa (14%), Jucati (14%) and Correntes (11%) (Figure 1).
Map of distribution of seropositive (n = 53) and seronegative (n = 263) animals (cows, heifers and their offspring) for Neospora caninum by IFAT assisted in the Garanhuns cattle Clinic 2003-2013, in the Agreste region of Pernambuco, Brazil. Municipalities with higher seropositivity (Caetes, Lajedo, Canhotinho, Palmerina and Lagoa do Ouro).
By assessing the seropositivity dependent relationship between cows and heifers and their calves using IFAT, it was found that there was dependence between animal categories, shown by Fisher’s exact test for cows and their offspring (p < 0.0001) and heifers and their descendants (p = 0.0106). The ELISA test also showed a significant association of the serological results between cows and their offspring (p = 0.0045), but not among heifers and their descendants by Fisher’s exact test (p = 0.1257) (Table 1).
Serological results for Neospora caninum by IFAT and ELISA, according to the animal category (cows, heifers and their offspring), in Garanhuns, 2003-2013.
The transplacental transmission rate varies according to the category studied. By IFAT, cows and heifers showed rates of 72.22% (13/18) and 69.23% (9/13), respectively. On the other hand, the transplacental transmission rates were 43.58% (17/39) for cows and 50% (9/18) for heifers by ELISA (Table 1).
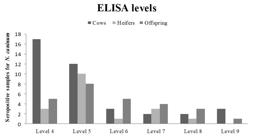
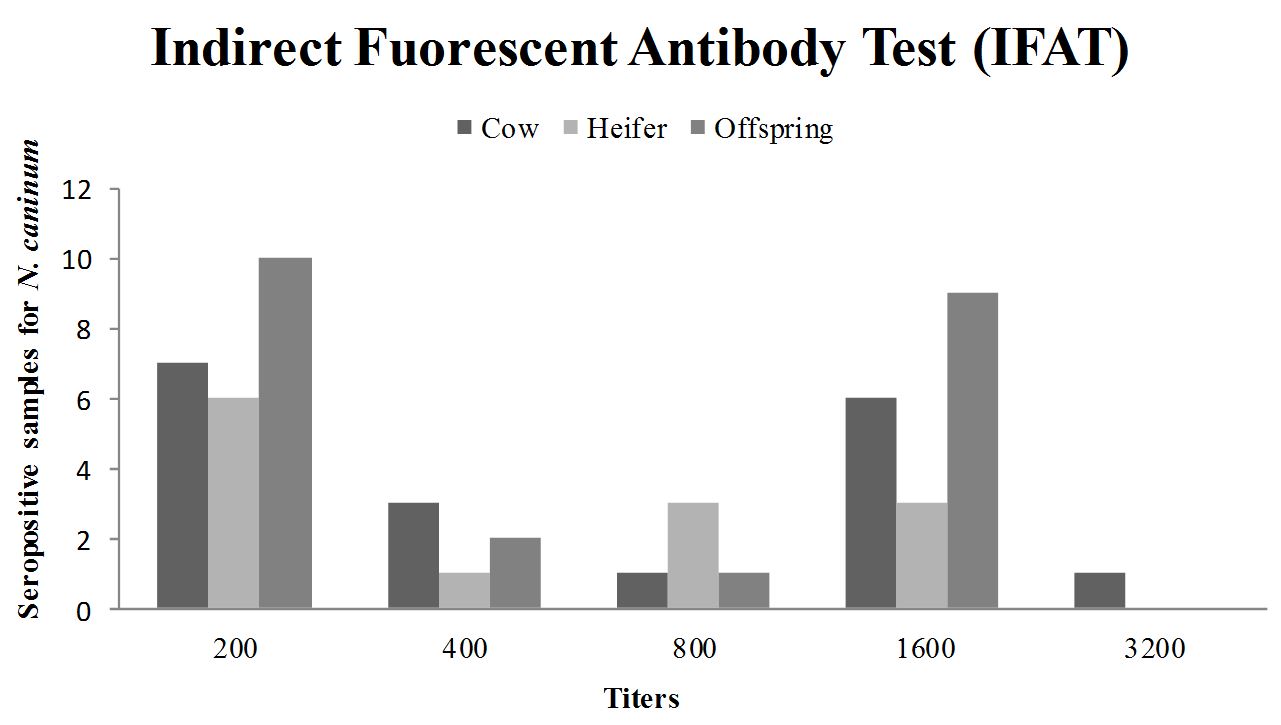
There was no correlation between the magnitudes of titers obtained by IFAT for N. caninum, for cows and their offspring (r = 0.1817; p = 0.4305) and for heifers and their calves (r = –0.138; p = 0.6102) according to the Spearman test. Most seropositive animals (23/316; 7.28%) showed titers of 200 by IFAT (Figure 2). Among the seropositive animals by ELISA, cows were predominant at level four and heifers and offspring at level five (Figure 3). In order to check the degree of agreement between the serological tests employed, considering IFAT as the gold standard, kappa test was performed, showing an index of 0.35 (low correlation).
Distribution of animals according to IFAT titers of antibodies to N.caninum and animal categories (cow, heifer and offspring), in Garanhuns, 2003-2013.
Distribution of animals according to ELISA levels of antibodies to N. caninum and animal categories (cow, heifer and offspring), in Garanhuns, 2003-2013. ELISA level ≥ 4 = Positive; according to the formula previously described by Machado et al. (1997)Machado RZ, Montassier HJ, Pinto AA, Lemos EG, Machado MRF, Valadão IFF, et al. An enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies against in cattle. Babesia bovisVet Parasitol 1997; 71(1): 17-26. http://dx.doi.org/10.1016/S0304-4017(97)00003-4.
http://dx.doi.org/10.1016/S0304-4017(97)... .
The found seropositivity rate ranging from 22.78% and 39.24% by IFAT and ELISA, respectively, was within the values obtained in other parts of the world that ranged from 0.07% to 97.2% (DUBEY & SCHARES, 2011Dubey JP, Schares G. Neosporosis in animals: the last five years. Vet Parasitol 2011; 180(1-2): 90-108. http://dx.doi.org/10.1016/j.vetpar.2011.05.031.
http://dx.doi.org/10.1016/j.vetpar.2011....
). The rate was also consistent with those obtained from animals tested by researchers in others Brazilian states, such as Goiás (MELO et al., 2001Melo CB, Leite RC, Souza GN, Leite RC. Frequency of infection in two different milk production system and predicting factors to infection on cattle herds in the State of Minas Gerais, Brazil. Neospora caninumRev Bras Parasitol Vet 2001; 10(2): 67-74.; OLIVEIRA et al., 2010Oliveira VSF, Álvarez-Garcia G, Ortega-Mora LM, Borges LMF, Silva AC. Abortions in bovines and transmission in an embryo transfer Center. Neospora caninumVet Parasitol 2010; 173(3-4): 206-210. http://dx.doi.org/10.1016/j.vetpar.2010.06.028.
http://dx.doi.org/10.1016/j.vetpar.2010....
), Paraná (OGAWA et al., 2005Ogawa L, Freire RL, Vidotto O, Gondim LFP, Navarro IT. Occurrence of antibodies to Neospora caninum and in dairy cattle from the northern region of the Paraná State, Brazil. Toxoplasma gondiiArq Bras Med Vet Zootec 2005; 57(3): 312-316. http://dx.doi.org/10.1590/S0102-09352005000300006.
http://dx.doi.org/10.1590/S0102-09352005...
; LOCATELLI-DITTRICH et al., 2001Locatelli-Dittrich R, Soccol VT, Richartz RR, Gasino-Joineau ME, Vinne R, Pinckney RD. Serological Diagnosis of Neosporosis in a Herd of Dairy Cattle in Southern Brazil. J Parasitol 2001; 87(6): 1493-1494. http://dx.doi.org/10.1645/0022-3395(2001)087[1493:SDONIA]2.0.CO;2.
http://dx.doi.org/10.1645/0022-3395(2001...
; MARQUES et al., 2011Marques FAC, Headley AS, Pereira VF, Taroda A, Barros LD, Cunha IAL, et al. Neospora caninumBos indicus: evaluation of vertical transmission in slaughtered beef cows (). Parasitol Res 2011; 108(4): 1015-1019. http://dx.doi.org/10.1007/s00436-010-2146-x.
http://dx.doi.org/10.1007/s00436-010-214...
), Minas Gerais (CORBELLINI et al., 2002Corbellini LG, Driemeier D, Cruz CEF, Gondim LFP, Wald V. Neosporosis as a cause of abortion in dairy cattle in Rio Grande do Sul, southern Brazil. Vet Parasitol 2002; 103(3): 195-202. http://dx.doi.org/10.1016/S0304-4017(01)00600-8.
http://dx.doi.org/10.1016/S0304-4017(01)...
; SANTOS et al., 2012Santos RRD, Rocha CMBM, Gonçalves TM, Guimarães AM. Quantification of vertical transmission of in dairy cows in Minas Gerais, Brazil. Neospora caninumRev Bras Parasitol Vet 2012; 21(3): 294-297. http://dx.doi.org/10.1590/S1984-29612012000300021.
http://dx.doi.org/10.1590/S1984-29612012...
), Rio Grande do Sul (RAGOZO et al., 2003Ragozo AMA, Paula VSO, Souza SLP, Bergamaschi DP, Gennari SM. Ocorrência de anticorpos anti- em soros bovinos procedentes de seis estados brasileiros. Neospora caninumRev Bras Parasitol Vet 2003; 12(1): 33-37.; HEIN et al., 2012Hein HE, Machado G, Miranda ICS, Costa EF, Pellegrini DCP, Driemeier D, et al. Neosporose bovina: avaliação da transmissão vertical e fração atribuível de aborto em uma população de bovinos no Estado do Rio Grande do Sul. Pesqui Vet Bras 2012; 32(5): 396-400. http://dx.doi.org/10.1590/S0100-736X2012000500006.
http://dx.doi.org/10.1590/S0100-736X2012...
), Santa Catarina (MACEDO et al., 2013Macedo CAB, Macedo MFSB, Cardim ST, Paiva MCDC, Tadora A, Barros LD, et al. evaluation of vertical transmission in slaughtered dairy cows (Neospora caninum:Bos taurus). Rev Bras Parasitol Vet 2013; 22(1): 13-17. http://dx.doi.org/10.1590/S1984-29612013000100004.
http://dx.doi.org/10.1590/S1984-29612013...
), São Paulo (SARTOR et al., 2003Sartor IF, Hasegawa MY, Canavessi AMO, Pinckney RD. Ocorrência de anticorpos de em vacas leiteiras avaliados pelos métodos de ELISA e IFAT no município de Avaré, SP. Neospora caninumSemina: Ciênc Agrár 2003; 24(1): 3-10.), Tocantins (MARTINS et al., 2011Martins NEX, Freschi CR, Baptista F, Machado RZ, Freitas FLC, Almeida KS. Ocorrência de anticorpos anti- em vacas lactantes do Município de Araguaína, Estado do Tocantins, Brasil. Neospora caninumRev Patol Trop 2011; 40(3): 231-238.), Mato Grosso do Sul, Rio de Janeiro (MUNHOZ et al., 2006Munhoz AD, Flausino W, Silva RT, Almeida CR, Lopes CW. Distribution of anti- antibodies in dairy cows at Municipalities of Resende and Rio Claro in the State of Rio de Janeiro, Brazil. Neospora caninumRev Bras Parasitol Vet 2006; 15(3): 101-104.), Rondônia (AGUIAR et al., 2006Aguiar DM, Cavalcante GT, Rodrigues AAR, Labruna MB, Camargo LMA, Camargo EP, et al. Prevalence of anti- antibodies in cattle and dogs from Western Amazon, Brazil, in association with some possible risk factors. Neospora caninumVet Parasitol 2006; 142(1-2): 71-77. http://dx.doi.org/10.1016/j.vetpar.2006.06.014.
http://dx.doi.org/10.1016/j.vetpar.2006....
; MARQUES et al., 2011Marques FAC, Headley AS, Pereira VF, Taroda A, Barros LD, Cunha IAL, et al. Neospora caninumBos indicus: evaluation of vertical transmission in slaughtered beef cows (). Parasitol Res 2011; 108(4): 1015-1019. http://dx.doi.org/10.1007/s00436-010-2146-x.
http://dx.doi.org/10.1007/s00436-010-214...
; ANDREOTTI et al., 2004Andreotti R, Pinckney RD, Pires PP, Silva EAE. Evidence of in beef cattle and dogs in the state of Mato Grosso do Sul, center-western region, Brazil. Neospora caninumRev Bras Parasitol Vet 2004; 13(3): 129-131.), Alagoas (SOUSA et al., 2012Sousa ME, Porto WJN, Albuquerque PPF, Souza OLS No, Faria EB, Pinheiro JWP Jr, et al. Soroprevalence and risk factors associated with infection by of dairy cattle in the state of Alagoas, Brazil. Neospora caninumPesqui Vet Bras 2012; 32(10): 1009-1013. http://dx.doi.org/10.1590/S0100-736X2012001000011.
http://dx.doi.org/10.1590/S0100-736X2012...
) and Bahia (GONDIM et al., 2004Gondim LFP, McAllister MM, Pitt WC, Zemlicka D. Coyotes (Canis latransNeospora caninum.) are definitive hosts of Int J Parasitol 2004; 34(2): 159-161. http://dx.doi.org/10.1016/j.ijpara.2004.01.001.
http://dx.doi.org/10.1016/j.ijpara.2004....
), where the values ranged from 6% to 58%. In the state of Pernambuco, Silva et al. (2008)Silva MIS, Almeida MAO, Mota RA, Pinheiro JWP Jr, Rabelo SSA. Fatores de riscos associados à infecção por Neospora caninum em Matrizes Bovinas Leiteiras em Pernambuco. Ciênc Anim Bras 2008; 9(2): 455-461. found a seropositive frequency of 31.7% in herds belonging to large milk producers located in Gravesend, by IFAT. Moreover, Amaral et al. (2012)Amaral RLG, Silva LGB, Pinheiro JW Jr, Souza OL No, Leal CAS, Porto WJN, et al. Neospora caninum em bovinos em matadouros de Pernambuco e Alagoas. Pesqui Vet Bras 2012; 32(10): 963-966. http://dx.doi.org/10.1590/S0100-736X2012001000002.
http://dx.doi.org/10.1590/S0100-736X2012...
, evaluating beef cattle from slaughterhouses of the micro-region of Brejo/PE, found a seropositivity rate of 17.3%, which was also consistent with our results.
In this study, some municipalities of the Agreste region of Pernambuco (Lagoa do Ouro, Canhotinho, Caetés, Lajedo and Palmerina), which concentrated a large number of milk producers (SEBRAE, 2013Serviço de Apoio Brasileiro às Micro e Pequenas Empresas – SEBRAE. Cenários para o leite e derivados na Região Nordeste em 2020. Recife: SEBRAE; 2013. 154 p.; DUBEY & SCHARES, 2011Dubey JP, Schares G. Neosporosis in animals: the last five years. Vet Parasitol 2011; 180(1-2): 90-108. http://dx.doi.org/10.1016/j.vetpar.2011.05.031.
http://dx.doi.org/10.1016/j.vetpar.2011....
), showed higher rates of seropositivity than those found among animals in other municipalities.
By serological tests (IFAT or ELISA) revealed that there was significant association (p ≤ 0.05) of seropositivity between mother and offspring, except by ELISA for heifers and offspring. This supports of hypothesis that transplacental route is a major source of transmission and maintenance of neosporosis in dairy cattle to successive generations (ANDERSON et al., 1997Anderson ML, Reynolds JP, Rowe JD, Sverlow KW, Packham AE, Barr BC, et al. Evidence of vertical transmission of Neospora sp. infection in dairy cattle. J Am Vet Med Assoc 1997; 210(8): 1169-1172.). The transplacental transmission rate was similar between the two groups studied, using both serologic techniques, but higher seropositivity was found by IFAT for cows. Higher vertical transmission rate in cows may be related to chronic infections in dairy herds, where animals remain much active time in the production system, spreading the agent over the generations (INNES et al., 2005Innes EA, Wright S, Bartley P, Maley S, Macaldowie C, Esteban-Redondo I, et al. The host-parasite relationship in bovine neosporosis. Vet Immunol Immunopathol 2005; 108(1-2): 29-36. http://dx.doi.org/10.1016/j.vetimm.2005.07.004.
http://dx.doi.org/10.1016/j.vetimm.2005....
; DUBEY & SCHARES, 2011Dubey JP, Schares G. Neosporosis in animals: the last five years. Vet Parasitol 2011; 180(1-2): 90-108. http://dx.doi.org/10.1016/j.vetpar.2011.05.031.
http://dx.doi.org/10.1016/j.vetpar.2011....
). Innes et al. (2000)Innes EA, Buxton D, Maley S, Wright S, Marks J, Esteban I, et al. Neosporosis: aspects of epidemiology and host immune response. Ann N Y Acad Sci 2000; 916(1): 93-101. http://dx.doi.org/10.1111/j.1749-6632.2000.tb05278.x.
http://dx.doi.org/10.1111/j.1749-6632.20...
reported that older animals showed better immune adaptation to the parasite than younger animals, but may have the reactivation of infection in immunosuppressive situations.
It is also important to highlight that the occurrence of birth of seropositive calves from seronegative mothers (cows [3/10] and heifers [3/6]), totalizing six seropositive offspring from sixteen negative females by IFAT, could be due to the infection of mother have happened some time ago and the antibody level is maintained at a level below the detection threshold of serological tests (CONRAD et al., 1993Conrad PA, Barr BC, Sverlow KW, Anderson M, Daft B, Kinde H, et al. isolation and characterization of a sp. from aborted bovine foetuses. In vitroNeosporaParasitology 1993; 106(3): 239-249. http://dx.doi.org/10.1017/S0031182000075065.
http://dx.doi.org/10.1017/S0031182000075...
; FRÖSSLING et al., 2005Frössling J, Uggla A, Björkman C. Prevalence and transmission of Neospora caninum within infected Swedish dairy herds. Vet Parasitol 2005; 128(3-4): 209-218. http://dx.doi.org/10.1016/j.vetpar.2004.12.006.
http://dx.doi.org/10.1016/j.vetpar.2004....
; LÓPEZ-GATIUS et al., 2005López-Gatius F, Santolaria P, Yaniz JL, Garbayo JM, Almería S. The use of beef bull semen reduced the risk of abortion in -seropositive dairy cows. NeosporaJ Vet Med B Infect Dis Vet Public Health 2005; 52(2): 88-92. http://dx.doi.org/10.1111/j.1439-0450.2004.00818.x.
http://dx.doi.org/10.1111/j.1439-0450.20...
; SAGER et al., 2001Sager H, Fischer I, Furrer K, Strasser M, Waldvogel A, Boerlin P, et al. A swiss case-control study to assess Neospora caninum: associated bovine abortions by PCR, histopathology and serology. Vet Parasitol 2001; 102(1-2): 1-15. http://dx.doi.org/10.1016/S0304-4017(01)00524-6.
http://dx.doi.org/10.1016/S0304-4017(01)...
). This condition was also observed by Frössling et al. (2005)Frössling J, Uggla A, Björkman C. Prevalence and transmission of Neospora caninum within infected Swedish dairy herds. Vet Parasitol 2005; 128(3-4): 209-218. http://dx.doi.org/10.1016/j.vetpar.2004.12.006.
http://dx.doi.org/10.1016/j.vetpar.2004....
and López-Gatius et al. (2004)López-Gatius F, López-Béjar M, Murugavel K, Pabón M, Ferrer D, Almería S. Neospora-associated abortion episode over a 1-year period in a dairy herd in north-east Spain. J Vet Med B Infect Dis Vet Public Health 2004; 51(7): 348-352. http://dx.doi.org/10.1111/j.1439-0450.2004.00779.x.
http://dx.doi.org/10.1111/j.1439-0450.20...
in cattle from Sweden.
The presence of antibody titers in calves before colostrum feeding proves transplacental transmission, since maternal antibodies do not cross the placenta in cattle, and can assume that the presence of antibodies in calves immediately after birth, before the intake of colostrum, are generated in uterus resulting N. caninum infection. The frequency of transplacental transmission can be estimated by the number of seropositive calves soon after birth before colostrum intake, born from seropositive mothers (BARTELS et al., 2007Bartels CJ, Huinink I, Beiboer ML, van Schaik G, Wouda W, Dijkstra T, et al. Quantification of vertical and horizontal transmission of infection in Dutch dairy herds. Neospora caninumVet Parasitol 2007; 148(2): 83-92. http://dx.doi.org/10.1016/j.vetpar.2007.06.004.
http://dx.doi.org/10.1016/j.vetpar.2007....
).
In this study, there was no correlation between the magnitude of anti-N. caninum antibodies titers between mothers and their offspring. On the other hand, Dubey et al. (2007)Dubey JP, Schares G, Ortega-Mora LM. Epidemiology and control of neosporosis and Neospora caninum.Clin Microbiol Rev 2007; 20(2): 323-367. http://dx.doi.org/10.1128/CMR.00031-06.
http://dx.doi.org/10.1128/CMR.00031-06...
reported that the magnitude of antibody titers of infected calves showed concordance with the magnitude of those from their mothers. Hietala & Thurmond (1999)Hietala SK, Thurmond MC. Postnatal transmission and transient serologic responses in two dairies. Neospora caninumInt J Parasitol 1999; 29(10): 1669-1676. http://dx.doi.org/10.1016/S0020-7519(99)00102-2.
http://dx.doi.org/10.1016/S0020-7519(99)...
emphasizes the need of special attention of the researchers when interpreting the serological results for N. caninum in cattle aged between 13 and 24 months of age, because of the possibility of false negative results, due to the decline of antibodies in the old congenitally infected animals. Serological studies have suggested that titles ≥ 200 (IFAT) are specific to infection by N. caninum (DUBEY et al., 1996Dubey JP, Lindsay DS, Adams DS, Gay JM, Baszler TV, Blagburn BL, et al. Serologic responses of cattle and other animals infected with Neospora caninum.Am J Vet Res 1996; 57(3): 329-336.), while the highest titers have been associated with occurrence of abortions (TREES et al., 1993Trees AJ, Guy F, Tennant BJ, Balfour AH, Dubey JP. Prevalence of antibodies to in a population of urban dogs in England. Neospora caninumVet Rec 1993; 132(6): 125-126. http://dx.doi.org/10.1136/vr.132.6.125.
http://dx.doi.org/10.1136/vr.132.6.125...
). According to Cox et al. (1998)Cox BT, Reichel MP, Griffiths LM. Serology of a Neospora abortion outbreak on a dairy farm in New Zealand: a case study. N Z Vet J 1998; 46(1): 28-31. http://dx.doi.org/10.1080/00480169.1998.36046.
http://dx.doi.org/10.1080/00480169.1998....
, high titers of anti-N. caninum antibodies quickly decline after the abortion, but low positive titers may persist for a long time.
According to the kappa index, IFAT and ELISA crude antigen presented concordance mild (0.35). IFAT was the first serological test used to demonstrate the presence of antibodies for N.caninum (DUBEY & SCHARES, 2011Dubey JP, Schares G. Neosporosis in animals: the last five years. Vet Parasitol 2011; 180(1-2): 90-108. http://dx.doi.org/10.1016/j.vetpar.2011.05.031.
http://dx.doi.org/10.1016/j.vetpar.2011....
). Cross-reactions with other coccidia are almost non-existent, so it is used as a benchmark for comparison with other serological tests. However, comparing indirect ELISA based on NcSRS2 recombinant antigen for detecting anti-N caninum antibodies in sheep, Andreotti et al. (2009)Andreotti R, Matos MFC, Gonçalves KN, Oshiro LM, Lima MSC Jr, Paiva F, et al. Comparison of indirect ELISA based on recombinant protein NcSRS2 and IFAT for detection of antibodies in sheep. Neospora caninumRev Bras Parasitol Vet 2009; 18(2): 19-22. http://dx.doi.org/10.4322/rbpv.01802004.
http://dx.doi.org/10.4322/rbpv.01802004...
found that these two tests showed similar concordance with an excellent kappa (0,98). Indirect ELISA based on the recombinant protein NcSRS2 was shown to be a highly sensitive and specific tool for serological diagnosis of N. caninum, since it reduces the occurrence of non-specific cross reactions (ANDREOTTI et al., 2009Andreotti R, Matos MFC, Gonçalves KN, Oshiro LM, Lima MSC Jr, Paiva F, et al. Comparison of indirect ELISA based on recombinant protein NcSRS2 and IFAT for detection of antibodies in sheep. Neospora caninumRev Bras Parasitol Vet 2009; 18(2): 19-22. http://dx.doi.org/10.4322/rbpv.01802004.
http://dx.doi.org/10.4322/rbpv.01802004...
).
Conclusion
The high rates of transplacental transmission observed in this study, either by IFAT or ELISA, may indicate that this type of transmission plays an important role in the maintenance and transmission of N. caninum in dairy cattle herds in the Agreste region of Pernambuco.
Acknowledgements
We thank the Foundation for Science and Technology Support (Fundação de Amparo à Ciência e Tecnologia; FACEP/IBPG nº 1297-5. 05/12), for providing the master’s scholarship and PROCAD/CAPES (PROCAD-NF nº 21/2009, convention no. 363/2010), for the exchange between postgraduate programs (UFRPE, Garanhuns campus, and UNESP Jaboticabal).
References
- Aguiar DM, Cavalcante GT, Rodrigues AAR, Labruna MB, Camargo LMA, Camargo EP, et al. Prevalence of anti- antibodies in cattle and dogs from Western Amazon, Brazil, in association with some possible risk factors. Neospora caninumVet Parasitol 2006; 142(1-2): 71-77. http://dx.doi.org/10.1016/j.vetpar.2006.06.014
» http://dx.doi.org/10.1016/j.vetpar.2006.06.014 - Amaral RLG, Silva LGB, Pinheiro JW Jr, Souza OL No, Leal CAS, Porto WJN, et al. Neospora caninum em bovinos em matadouros de Pernambuco e Alagoas. Pesqui Vet Bras 2012; 32(10): 963-966. http://dx.doi.org/10.1590/S0100-736X2012001000002
» http://dx.doi.org/10.1590/S0100-736X2012001000002 - Anderson ML, Reynolds JP, Rowe JD, Sverlow KW, Packham AE, Barr BC, et al. Evidence of vertical transmission of Neospora sp. infection in dairy cattle. J Am Vet Med Assoc 1997; 210(8): 1169-1172.
- Andreotti R, Matos MFC, Gonçalves KN, Oshiro LM, Lima MSC Jr, Paiva F, et al. Comparison of indirect ELISA based on recombinant protein NcSRS2 and IFAT for detection of antibodies in sheep. Neospora caninumRev Bras Parasitol Vet 2009; 18(2): 19-22. http://dx.doi.org/10.4322/rbpv.01802004
» http://dx.doi.org/10.4322/rbpv.01802004 - Andreotti R, Pinckney RD, Pires PP, Silva EAE. Evidence of in beef cattle and dogs in the state of Mato Grosso do Sul, center-western region, Brazil. Neospora caninumRev Bras Parasitol Vet 2004; 13(3): 129-131.
- Bartels CJ, Huinink I, Beiboer ML, van Schaik G, Wouda W, Dijkstra T, et al. Quantification of vertical and horizontal transmission of infection in Dutch dairy herds. Neospora caninumVet Parasitol 2007; 148(2): 83-92. http://dx.doi.org/10.1016/j.vetpar.2007.06.004
» http://dx.doi.org/10.1016/j.vetpar.2007.06.004 - Conrad PA, Barr BC, Sverlow KW, Anderson M, Daft B, Kinde H, et al. isolation and characterization of a sp. from aborted bovine foetuses. In vitroNeosporaParasitology 1993; 106(3): 239-249. http://dx.doi.org/10.1017/S0031182000075065
» http://dx.doi.org/10.1017/S0031182000075065 - Corbellini LG, Driemeier D, Cruz CEF, Gondim LFP, Wald V. Neosporosis as a cause of abortion in dairy cattle in Rio Grande do Sul, southern Brazil. Vet Parasitol 2002; 103(3): 195-202. http://dx.doi.org/10.1016/S0304-4017(01)00600-8
» http://dx.doi.org/10.1016/S0304-4017(01)00600-8 - Cox BT, Reichel MP, Griffiths LM. Serology of a Neospora abortion outbreak on a dairy farm in New Zealand: a case study. N Z Vet J 1998; 46(1): 28-31. http://dx.doi.org/10.1080/00480169.1998.36046
» http://dx.doi.org/10.1080/00480169.1998.36046 - Dubey JP, Carpenter JL, Speer CA, Topper MJ, Uggla A. Newly recognized fatal protozoan disease of dogs. J Am Vet Assoc 1988; 192(9): 1269-1285.
- Dubey JP, Lindsay DS, Adams DS, Gay JM, Baszler TV, Blagburn BL, et al. Serologic responses of cattle and other animals infected with Neospora caninum.Am J Vet Res 1996; 57(3): 329-336.
- Dubey JP, Schares G, Ortega-Mora LM. Epidemiology and control of neosporosis and Neospora caninum.Clin Microbiol Rev 2007; 20(2): 323-367. http://dx.doi.org/10.1128/CMR.00031-06
» http://dx.doi.org/10.1128/CMR.00031-06 - Dubey JP, Schares G. Neosporosis in animals: the last five years. Vet Parasitol 2011; 180(1-2): 90-108. http://dx.doi.org/10.1016/j.vetpar.2011.05.031
» http://dx.doi.org/10.1016/j.vetpar.2011.05.031 - Frössling J, Uggla A, Björkman C. Prevalence and transmission of Neospora caninum within infected Swedish dairy herds. Vet Parasitol 2005; 128(3-4): 209-218. http://dx.doi.org/10.1016/j.vetpar.2004.12.006
» http://dx.doi.org/10.1016/j.vetpar.2004.12.006 - Gondim LFP, McAllister MM, Pitt WC, Zemlicka D. Coyotes (Canis latransNeospora caninum.) are definitive hosts of Int J Parasitol 2004; 34(2): 159-161. http://dx.doi.org/10.1016/j.ijpara.2004.01.001
» http://dx.doi.org/10.1016/j.ijpara.2004.01.001 - Hein HE, Machado G, Miranda ICS, Costa EF, Pellegrini DCP, Driemeier D, et al. Neosporose bovina: avaliação da transmissão vertical e fração atribuível de aborto em uma população de bovinos no Estado do Rio Grande do Sul. Pesqui Vet Bras 2012; 32(5): 396-400. http://dx.doi.org/10.1590/S0100-736X2012000500006
» http://dx.doi.org/10.1590/S0100-736X2012000500006 - Hietala SK, Thurmond MC. Postnatal transmission and transient serologic responses in two dairies. Neospora caninumInt J Parasitol 1999; 29(10): 1669-1676. http://dx.doi.org/10.1016/S0020-7519(99)00102-2
» http://dx.doi.org/10.1016/S0020-7519(99)00102-2 - Innes EA, Buxton D, Maley S, Wright S, Marks J, Esteban I, et al. Neosporosis: aspects of epidemiology and host immune response. Ann N Y Acad Sci 2000; 916(1): 93-101. http://dx.doi.org/10.1111/j.1749-6632.2000.tb05278.x
» http://dx.doi.org/10.1111/j.1749-6632.2000.tb05278.x - Innes EA, Wright S, Bartley P, Maley S, Macaldowie C, Esteban-Redondo I, et al. The host-parasite relationship in bovine neosporosis. Vet Immunol Immunopathol 2005; 108(1-2): 29-36. http://dx.doi.org/10.1016/j.vetimm.2005.07.004
» http://dx.doi.org/10.1016/j.vetimm.2005.07.004 - Instituto Brasileiro de Geografia e Estatística – IBGE. Produção da Pecuária Municipal. Rio de Janeiro: IBGE; 2010. p. 55-61. vol. 38. Boletim Técnico.
- Jenkins M, Baszler T, Bjorkman C, Schares G, Williams D. Diagnosis and seroepidemiology of associated bovine abortion. Neospora caninum-Int J Parasitol 2002; 32(5): 631-636. http://dx.doi.org/10.1016/S0020-7519(01)00363-0
» http://dx.doi.org/10.1016/S0020-7519(01)00363-0 - King JS, Slapeta J, Jenkins DJ, Al-Qassab SE, Ellis JT, Windsor PA. Australian dingoes are definitive hosts of Neospora caninum.Int J Parasitol 2010; 40(8): 945-950. http://dx.doi.org/10.1016/j.ijpara.2010.01.008
» http://dx.doi.org/10.1016/j.ijpara.2010.01.008 - Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33(1): 159-174. http://dx.doi.org/10.2307/2529310
» http://dx.doi.org/10.2307/2529310 - Locatelli-Dittrich R, Soccol VT, Richartz RR, Gasino-Joineau ME, Vinne R, Pinckney RD. Serological Diagnosis of Neosporosis in a Herd of Dairy Cattle in Southern Brazil. J Parasitol 2001; 87(6): 1493-1494. http://dx.doi.org/10.1645/0022-3395(2001)087[1493:SDONIA]2.0.CO;2
» http://dx.doi.org/10.1645/0022-3395(2001)087[1493:SDONIA]2.0.CO;2 - López-Gatius F, López-Béjar M, Murugavel K, Pabón M, Ferrer D, Almería S. Neospora-associated abortion episode over a 1-year period in a dairy herd in north-east Spain. J Vet Med B Infect Dis Vet Public Health 2004; 51(7): 348-352. http://dx.doi.org/10.1111/j.1439-0450.2004.00779.x
» http://dx.doi.org/10.1111/j.1439-0450.2004.00779.x - López-Gatius F, Santolaria P, Yaniz JL, Garbayo JM, Almería S. The use of beef bull semen reduced the risk of abortion in -seropositive dairy cows. NeosporaJ Vet Med B Infect Dis Vet Public Health 2005; 52(2): 88-92. http://dx.doi.org/10.1111/j.1439-0450.2004.00818.x
» http://dx.doi.org/10.1111/j.1439-0450.2004.00818.x - Macedo CAB, Macedo MFSB, Cardim ST, Paiva MCDC, Tadora A, Barros LD, et al. evaluation of vertical transmission in slaughtered dairy cows (Neospora caninum:Bos taurus). Rev Bras Parasitol Vet 2013; 22(1): 13-17. http://dx.doi.org/10.1590/S1984-29612013000100004
» http://dx.doi.org/10.1590/S1984-29612013000100004 - Machado RZ, Montassier HJ, Pinto AA, Lemos EG, Machado MRF, Valadão IFF, et al. An enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies against in cattle. Babesia bovisVet Parasitol 1997; 71(1): 17-26. http://dx.doi.org/10.1016/S0304-4017(97)00003-4
» http://dx.doi.org/10.1016/S0304-4017(97)00003-4 - Marques FAC, Headley AS, Pereira VF, Taroda A, Barros LD, Cunha IAL, et al. Neospora caninumBos indicus: evaluation of vertical transmission in slaughtered beef cows (). Parasitol Res 2011; 108(4): 1015-1019. http://dx.doi.org/10.1007/s00436-010-2146-x
» http://dx.doi.org/10.1007/s00436-010-2146-x - Martins NEX, Freschi CR, Baptista F, Machado RZ, Freitas FLC, Almeida KS. Ocorrência de anticorpos anti- em vacas lactantes do Município de Araguaína, Estado do Tocantins, Brasil. Neospora caninumRev Patol Trop 2011; 40(3): 231-238.
- McAllister MM, Dubey JP, Lindsay DS, Jolley WR, Wills RA, Mcguire AM. Dogs are definitive hosts of Neospora caninum.Int J Parasitol 1998; 28(9): 1473-1478. http://dx.doi.org/10.1016/S0020-7519(98)00138-6
» http://dx.doi.org/10.1016/S0020-7519(98)00138-6 - Melo CB, Leite RC, Souza GN, Leite RC. Frequency of infection in two different milk production system and predicting factors to infection on cattle herds in the State of Minas Gerais, Brazil. Neospora caninumRev Bras Parasitol Vet 2001; 10(2): 67-74.
- Mineo TWP, Carrasco AOT, Marciano JA, Werther K, Pinto AA, Machado RZ. Pigeons (Columba livia) are a suitable experimental model for infection in birds. Neospora caninumVet Parasitol 2009; 159(2): 149-153. http://dx.doi.org/10.1016/j.vetpar.2008.10.024
» http://dx.doi.org/10.1016/j.vetpar.2008.10.024 - Munhoz AD, Flausino W, Silva RT, Almeida CR, Lopes CW. Distribution of anti- antibodies in dairy cows at Municipalities of Resende and Rio Claro in the State of Rio de Janeiro, Brazil. Neospora caninumRev Bras Parasitol Vet 2006; 15(3): 101-104.
- Ogawa L, Freire RL, Vidotto O, Gondim LFP, Navarro IT. Occurrence of antibodies to Neospora caninum and in dairy cattle from the northern region of the Paraná State, Brazil. Toxoplasma gondiiArq Bras Med Vet Zootec 2005; 57(3): 312-316. http://dx.doi.org/10.1590/S0102-09352005000300006
» http://dx.doi.org/10.1590/S0102-09352005000300006 - Oliveira VSF, Álvarez-Garcia G, Ortega-Mora LM, Borges LMF, Silva AC. Abortions in bovines and transmission in an embryo transfer Center. Neospora caninumVet Parasitol 2010; 173(3-4): 206-210. http://dx.doi.org/10.1016/j.vetpar.2010.06.028
» http://dx.doi.org/10.1016/j.vetpar.2010.06.028 - Paré J, Thurmond MC, Hietala SK. Congenital Neospora caninum infection in dairy cattle and associated calfhood mortality. Can J Vet Res 1996; 60(2): 133-139.
- Ragozo AMA, Paula VSO, Souza SLP, Bergamaschi DP, Gennari SM. Ocorrência de anticorpos anti- em soros bovinos procedentes de seis estados brasileiros. Neospora caninumRev Bras Parasitol Vet 2003; 12(1): 33-37.
- Sager H, Fischer I, Furrer K, Strasser M, Waldvogel A, Boerlin P, et al. A swiss case-control study to assess Neospora caninum: associated bovine abortions by PCR, histopathology and serology. Vet Parasitol 2001; 102(1-2): 1-15. http://dx.doi.org/10.1016/S0304-4017(01)00524-6
» http://dx.doi.org/10.1016/S0304-4017(01)00524-6 - Sampaio IVB. Testes estatísticos não paramétricos. In: Sampaio IVB. Estatística aplicada à experimentação animal. 3. ed. Belo Horizonte: Fundação de Ensino e Pesquisa em Medicina Veterinária e Zootecnia; 2007. p. 207-223.
- Santos RRD, Rocha CMBM, Gonçalves TM, Guimarães AM. Quantification of vertical transmission of in dairy cows in Minas Gerais, Brazil. Neospora caninumRev Bras Parasitol Vet 2012; 21(3): 294-297. http://dx.doi.org/10.1590/S1984-29612012000300021
» http://dx.doi.org/10.1590/S1984-29612012000300021 - Sartor IF, Hasegawa MY, Canavessi AMO, Pinckney RD. Ocorrência de anticorpos de em vacas leiteiras avaliados pelos métodos de ELISA e IFAT no município de Avaré, SP. Neospora caninumSemina: Ciênc Agrár 2003; 24(1): 3-10.
- Serviço de Apoio Brasileiro às Micro e Pequenas Empresas – SEBRAE. Cenários para o leite e derivados na Região Nordeste em 2020. Recife: SEBRAE; 2013. 154 p.
- Silva MIS, Almeida MAO, Mota RA, Pinheiro JWP Jr, Rabelo SSA. Fatores de riscos associados à infecção por Neospora caninum em Matrizes Bovinas Leiteiras em Pernambuco. Ciênc Anim Bras 2008; 9(2): 455-461.
- Sousa ME, Porto WJN, Albuquerque PPF, Souza OLS No, Faria EB, Pinheiro JWP Jr, et al. Soroprevalence and risk factors associated with infection by of dairy cattle in the state of Alagoas, Brazil. Neospora caninumPesqui Vet Bras 2012; 32(10): 1009-1013. http://dx.doi.org/10.1590/S0100-736X2012001000011
» http://dx.doi.org/10.1590/S0100-736X2012001000011 - Trees AJ, Guy F, Tennant BJ, Balfour AH, Dubey JP. Prevalence of antibodies to in a population of urban dogs in England. Neospora caninumVet Rec 1993; 132(6): 125-126. http://dx.doi.org/10.1136/vr.132.6.125
» http://dx.doi.org/10.1136/vr.132.6.125 - Vianna LC, Sartor IF, Pituco EM, Okuda LH, Camargo CN, Kronka SN. Incidência e transmissão transplacentária de em fêmeas primíparas da raça abatidos em Presidente Prudente, São Paulo, Brasil. Neospora caninumBos indicusSemina: Ciênc Agrá 2008; 29(2): 387-392.
Publication Dates
-
Publication in this collection
10 Oct 2016 -
Date of issue
Oct-Dec 2016
History
-
Received
20 Apr 2016 -
Accepted
13 June 2016