Abstract
This study describes the occurrence of dogs naturally co-infected with Hepatozoon canis and two Leishmania species: L. infantum or L. braziliensis. Four dogs serologically diagnosed with Visceral Leishmaniasis were euthanized. Liver and spleen samples were collected for histopathological analysis and DNA isolation. H. canis meronts were observed in tissues from all four dogs. H. canis infection was confirmed by PCR followed by sequencing of a fragment of 18S rRNA gene. Leishmania detection and typing was confirmed by ITS1' PCR-RFLP and parasite burden was calculated using ssrRNA quantitative qPCR. A DPP - Dual Path platform test was performed. One out (Dog #2) of four animals was asymptomatic. Dogs #1 and #4 were infected by L. infantum and were DPP test positive. Dogs #2 and #3 were infected by L. braziliensis and were DPP test negative. Furthermore, visceral dissemination was observed in Dogs #2 and #3, since L. braziliensis was detected in liver and spleen samples. The visceral dissemination of L. braziliensis associated with systemic signs suggested that this co-infection could influence the parasite burden and disease progression.
Keywords:
Hepatozoonosis; canine visceral leishmaniasis; Leishmania infantum; Hepatozoon canis; co-infection; Leishmania braziliensis
Resumo
O presente estudo descreve a ocorrência de coinfecção com Hepatozoon canis e duas espécies de Leishmania (L. infantum ou L. braziliensis) em cães. Quatro cães sorologicamente diagnosticados com leishmaniose visceral foram eutanasiados. Amostras do baço e fígado foram submetidas à histopatologia e extração de DNA. Merontes de H. canis foram observados nos quatro cães. A infecção por H. canis foi confirmada por PCR e sequenciamento de um fragmento do gene 18S rRNA. A infecção por Leishmania e tipagem foram realizadas por PCR-RFLP do região intergênica ITS1. A carga parasitária foi calculada pela qPCR quantitativa baseada no gene ssrRNA. O teste DPP - Dual Path platform foi realizado. Apenas o Cão #2 era assintomático. Os cães #1 e #4 estavam infectados com L. infantum e foram positivos no DPP. Os cães #2 e #3 estavam infectados com L. braziliensis e foram negativos no DPP. Além disso, visceralização foi observada nos cães #2 e #3, nos quais L. braziliensis foi detectada em amostras de baço e fígado. A visceralização da L. braziliensis associada a sinais clínicos sistêmicos sugerem que esta coinfecção pode ter influenciado na carga parasitária e progressão da doença.
Palavras-chave:
Hepatozoonose; leishmaniose visceral canina; Leishmania infantum; Hepatozoon canis; coinfecção; Leishmania braziliensis
Introduction
Canine visceral leishmaniasis (CVL) is a zoonotic disease endemic in various countries. In Brazil, visceral leishmaniasis is caused by the protozoa Leishmania infantum. The dog is considered an important urban reservoir and the detection of canine cases may precede the occurrence of human visceral leishmaniasis (BELO et al., 2013Belo VS, Werneck GL, Barbosa DS, Simões TC, Nascimento BWL, Silva ES, et al. Factors associated with visceral leishmaniasis in the americas: a systematic review and meta-analysis. PLoS Negl Trop Dis 2013; 7(4): e2182. PMid:23638203. http://dx.doi.org/10.1371/journal.pntd.0002182.
http://dx.doi.org/10.1371/journal.pntd.0...
). The current public health policy in Brazil demands the culling of infected dogs -based on serologic tests - for VL control (BRASIL, 2006Brasil. Ministério da Saúde. Manual de vigilância e controle da Leishmaniose Visceral. Brasília: MS; 2006.). Cross-reactivity using Leishmania infantum antigen with sera from Leishmania braziliensis-infected dogs is commonly observed (BRASIL, 2006Brasil. Ministério da Saúde. Manual de vigilância e controle da Leishmaniose Visceral. Brasília: MS; 2006.), but dog culling, in this case, is not recommended. Dogs infected with L. braziliensis commonly present localized skin lesions without systemic dissemination (MADEIRA et al., 2005Madeira MF, Schubach AO, Schubach TMP, Serra CMB, Pereira SA, Figueiredo FB, et al. Is preferentially restricted to the cutaneous lesions of naturally infected dogs? Leishmania (Viannia) braziliensisParasitol Res 2005; 97(1): 73-76. PMid:15986254. http://dx.doi.org/10.1007/s00436-005-1374-y.
http://dx.doi.org/10.1007/s00436-005-137...
). The urban reservoir of L. braziliensis is still not known in Brazil.
Canine hepatozoonosis is a systemic disease caused by Apicomplexa protozoa of the genus Hepatozoon, that is transmitted by ingestion of infected ticks belonging to the genus Rhipicephalus (RUBINI et al., 2008Rubini AS, Paduan KS, Lopes VVA, O’Dwyer LH. Molecular and parasitological survey of Hepatozoon canis (Apicomplexa: Hepatozoidae) in dogs from rural area of Sao Paulo state, Brazil. Parasitol Res 2008; 102(5): 895-899. PMid:18188597. http://dx.doi.org/10.1007/s00436-007-0846-7.
http://dx.doi.org/10.1007/s00436-007-084...
; KAUR et al., 2012Kaur P, Deshmukh S, Singh R, Bansal BK, Randhawa CS, Singla LD. Para-clinico-pathological observations of insidious incidence of canine hepatozoonosis from a mongrel dog: a case report. J Parasit Dis 2012; 36(1): 135-138. PMid:23543040. http://dx.doi.org/10.1007/s12639-011-0092-x.
http://dx.doi.org/10.1007/s12639-011-009...
; MIRANDA et al., 2014Miranda RL, O’Dwyer LH, Castro JR, Metzger B, Rubini AS, Mundim AV, et al. Prevalence and molecular characterization of in dogs from urban and rural areas in Southeast Brazil. Hepatozoon canisRes Vet Sci 2014; 97(2): 325-328. PMid:25039064. http://dx.doi.org/10.1016/j.rvsc.2014.06.015.
http://dx.doi.org/10.1016/j.rvsc.2014.06...
). Domestic dogs can be infected by two species: H. canis and H. americanum (O’DWYER, 2011O’Dwyer LH. Brazilian canine hepatozoonosis. Rev Bras Parasitol Vet 2011; 20(3): 181-193. PMid:21961746. http://dx.doi.org/10.1590/S1984-29612011000300002.
http://dx.doi.org/10.1590/S1984-29612011...
). The infected animals usually present low parasitemia (MUNDIM et al., 2008Mundim AV, Morais IA, Tavares M, Cury MC, Mundim MJS. Clinical and hematological signs associated with dogs naturally infected by Hepatozoon sp. and with other hematozoa: a retrospective study in Uberlândia, Minas Gerais, Brazil. Vet Parasitol 2008; 153(1-2): 3-8. PMid:18304739. http://dx.doi.org/10.1016/j.vetpar.2008.01.018.
http://dx.doi.org/10.1016/j.vetpar.2008....
). Clinically, Hepatozoon infected-animals can present anorexia, lack of appetite, fever, pale mucosa, lethargy, diarrhea and vomiting (CHHABRA et al., 2013Chhabra S, Uppal SK, Singla LD. Retrospective study of clinical and hematological aspects associated with dogs naturally infected by in Ludhiana, Punjab, India. Hepatozoon canisAsian Pac J Trop Biomed 2013; 3(6): 483-486. PMid:23730562. http://dx.doi.org/10.1016/S2221-1691(13)60100-8.
http://dx.doi.org/10.1016/S2221-1691(13)...
). Hematological alterations, such as anemia, leucocytosis and neutrophilia or lymphopenia, have also been observed (KAUR et al., 2012Kaur P, Deshmukh S, Singh R, Bansal BK, Randhawa CS, Singla LD. Para-clinico-pathological observations of insidious incidence of canine hepatozoonosis from a mongrel dog: a case report. J Parasit Dis 2012; 36(1): 135-138. PMid:23543040. http://dx.doi.org/10.1007/s12639-011-0092-x.
http://dx.doi.org/10.1007/s12639-011-009...
). Many of these clinical signs described for canine hepatozoonosis are also observed in CVL.
In Brazil, Hepatozoon has been observed since 1979, when Massard studied the infection (O’DWYER, 2011O’Dwyer LH. Brazilian canine hepatozoonosis. Rev Bras Parasitol Vet 2011; 20(3): 181-193. PMid:21961746. http://dx.doi.org/10.1590/S1984-29612011000300002.
http://dx.doi.org/10.1590/S1984-29612011...
). Molecular characterization indicated that Brazilian H. canis was closely related to isolates from Japan, presented 99% nucleotide identity with isolates from Israel and was found to be distantly related to H. americanum (RUBINI et al., 2005Rubini AS, Paduan KS, Cavalcante GG, Ribolla PEM, O’Dwyer LH. Molecular identification and characterization of canine Hepatozoon species from Brazil. Parasitol Res 2005; 97(2): 91-93. PMid:15948009. http://dx.doi.org/10.1007/s00436-005-1383-x.
http://dx.doi.org/10.1007/s00436-005-138...
). In the following years, canine hepatozoonosis has also been described in several Brazilian regions (RUBINI et al., 2008Rubini AS, Paduan KS, Lopes VVA, O’Dwyer LH. Molecular and parasitological survey of Hepatozoon canis (Apicomplexa: Hepatozoidae) in dogs from rural area of Sao Paulo state, Brazil. Parasitol Res 2008; 102(5): 895-899. PMid:18188597. http://dx.doi.org/10.1007/s00436-007-0846-7.
http://dx.doi.org/10.1007/s00436-007-084...
; MIRANDA et al., 2014Miranda RL, O’Dwyer LH, Castro JR, Metzger B, Rubini AS, Mundim AV, et al. Prevalence and molecular characterization of in dogs from urban and rural areas in Southeast Brazil. Hepatozoon canisRes Vet Sci 2014; 97(2): 325-328. PMid:25039064. http://dx.doi.org/10.1016/j.rvsc.2014.06.015.
http://dx.doi.org/10.1016/j.rvsc.2014.06...
; GONÇALVES et al., 2014Gonçalves LR, Filgueira KD, Ahid SMM, Pereira JS, Vale AM, Machado RZ, et al. Study on coinfecting vector-borne pathogens in dogs and ticks in Rio Grande do Norte, Brazil. Rev Bras Parasitol Vet 2014; 23(3): 407-412. PMid:25271465. http://dx.doi.org/10.1590/S1984-29612014071.
http://dx.doi.org/10.1590/S1984-29612014...
; RAMOS et al., 2015Ramos CA, Babo-Terra VJ, Pedroso TC, Souza AF Fo, Araújo FR, Cleveland HP. Molecular identification of in dogs from Campo Grande, Mato Grosso do Sul, Brazil. Hepatozoon canisRev Bras Parasitol Vet 2015; 24(2): 247-250. PMid:26154969. http://dx.doi.org/10.1590/S1984-29612015019.
http://dx.doi.org/10.1590/S1984-29612015...
; DEMONER et al., 2016Demoner LC, Magro NM, Silva MR, Antunes JMP, Calabuig CIP, O’Dwyer LH. spp. infections in wild rodents in an area of endemic canine hepatozoonosis in southeastern Brazil. HepatozoonTicks Tick Borne Dis 2016; 7(5): 859-864. PMid:27091081. http://dx.doi.org/10.1016/j.ttbdis.2016.04.002.
http://dx.doi.org/10.1016/j.ttbdis.2016....
). Many of these areas are also endemic for CVL, and co-infection has already been described in dogs from Rio Grande do Norte based only on molecular analyses (GONÇALVES et al., 2014Gonçalves LR, Filgueira KD, Ahid SMM, Pereira JS, Vale AM, Machado RZ, et al. Study on coinfecting vector-borne pathogens in dogs and ticks in Rio Grande do Norte, Brazil. Rev Bras Parasitol Vet 2014; 23(3): 407-412. PMid:25271465. http://dx.doi.org/10.1590/S1984-29612014071.
http://dx.doi.org/10.1590/S1984-29612014...
). Herein, we described the occurrence of co-infection of dogs with H. canis and L. infantum or L. braziliensis, using molecular and histopathological analyses. In addition, the co-infection of H. canis and L. braziliensis could have lead to visceral dissemination, which may lead to a false CVL diagnosis.
Material and Methods
Animals and samples
Four seropositive dogs (IFAT - Bio-Manguinhos and ELISA - Bio-Manguinhos) for Leishmania, two from Barra do Garças (Latitude: 15° 53’ 24” S; Longitude: 52° 15’ 24” W) and two from Cuiabá (Latitude: 15° 35’ 46” S; Longitude: 56° 05’ 48” W), Mato Grosso state, were included in this study. Clinical evaluations were performed before euthanasia and were performed by veterinarians at the Zoonosis Control Center (Centro de Controle de Zoonoses - CCZ). The procedure included the administration of 1.0 ml/Kg of 1.0% Thiopental intravenously (Thiopentax®, Cristália) for anesthesia. Once the absence of corneal reflex due to deep anesthesia was confirmed, 10 mL of 19.1% potassium chloride (Isofarma) was intravenously administered. During necropsy, fragments from spleen and liver were collected for histopathological analysis and molecular techniques. Samples were also forwarded to the Leishmania Collection of the Oswaldo Cruz Institute (CLIOC, www.clioc.fiocruz.br) for parasite isolation and typing. In addition, serum samples were collected and tested with the rapid Dual Path Platform test (DPP CVL, BioManguinhos, FIOCRUZ), which detects anti-rK26/39 Leishmania antibodies (GRIMALDI et al., 2012Grimaldi G Jr, Teva A, Ferreira AL, Santos CB, Pinto I, de-Azevedo CT, et al. Evaluation of a novel chromatographic immunoassay based on Dual-Path Platform technology (DPP® CVL rapid test) for the serodiagnosis of canine visceral leishmaniasis. Trans R Soc Trop Med Hyg 2012; 106(1): 54-59. PMid:22137538. http://dx.doi.org/10.1016/j.trstmh.2011.10.001.
http://dx.doi.org/10.1016/j.trstmh.2011....
).
Ethics statement
The animals included in this study were naturally infected and destined to euthanasia as recommended by the politics of Brazilian Ministry of Health. All procedures performed in the present study were in accordance with the ethical standards of the institution or practice. The present research uses tissues fragments after euthanasia, so the study did not need to be submitted for The Ethics Committee on Animal Use (CEUA) according to Brazilian’s Law 11794/08.
Leishmania spp. isolation
Spleen samples were cultured in tubes with biphasic medium containing Schneider liquid medium for Drosophyla (Sigma) with 10-20% fetal bovine serum and NNN medium as solid phase. The tubes were maintained at 25 °C in Biological Oxygen Demand (B.O.D.) incubator (Contemp). The cultures were evaluated every 5 days for promastigotes growth and maintained at least for 4 weeks.
DNA extraction
Total DNA was isolated from approximately 10 mg of both spleen and liver samples using QIAmp® DNA Mini Kit (Qiagen, CA, USA), which included a prior phase of digestion with 20 μl of proteinase K (20 mg/mL) for 1 h at 56 °C. DNA was dissolved in 50 µl of Tris EDTA buffer (AE buffer) and quantified via NanoDrop® (Thermo Fisher Scientific, Waltham, MA, USA).
Molecular detection of Leishmania spp.
The molecular detection of Leishmania spp. by PCR targeting the ITS1 intergenic region was employed (GRAÇA et al., 2012Graça GC, Volpini AC, Romero GA, Oliveira-Neto MP, Hueb M, Porrozzi R, et al. Development and validation of PCR-based assays for diagnosis of American cutaneous leishmaniasis and identification of the parasite species. Mem Inst Oswaldo Cruz 2012; 107(5): 664-674. PMid:22850958. http://dx.doi.org/10.1590/S0074-02762012000500014.
http://dx.doi.org/10.1590/S0074-02762012...
). Restriction Fragment Length Polymorphism (RFLP) was performed for Leishmania species identification. The ITS1 PCR product was submitted to digestion using Sau 3AI and loaded on 12.5% polyacrylamide gel (Genephor). Two Leishmania reference strains were used as controls: IOC/L 579 (Li - Leishmania (L.) infantum- MHOM/BR/1974/PP75) and IOC/L 566 (Lb - Leishmania (V.) braziliensis- MHOM/BR/1975/M2903).
Determination of Leishmania spp. burden by quantitative polymerase chain reaction (qPCR)
Leishmania parasite burden in spleen samples was estimated by quantitative real-time PCR (Step One equipment, Applied Biosystems, Molecular Probes, Inc.) using the detection system Power Sybr Green Master Mix (Applied Biosystems, Molecular Probes, Inc.). Hypoxanthine-guanine phosphoribosyltransferase (HPRT) primers were chosen to normalize the concentrations of canine DNA in each sample; the primers described by Prina et al (PRINA et al., 2007Prina E, Roux E, Mattei D, Milon G. Leishmania DNA is rapidly degraded following parasite death: an analysis by microscopy and real-time PCR. Microbes Infect 2007; 9(11): 1307-1315. PMid:17890124. http://dx.doi.org/10.1016/j.micinf.2007.06.005.
http://dx.doi.org/10.1016/j.micinf.2007....
) were used to amplify a product corresponding to the small subunit ribosomal RNA (ssrRNA), a multi-copy gene of Leishmania spp. parasites. DNA (100 ng in 2 μl) was added to a final PCR reaction volume of 20 μl containing Power Sybr Green 1X, 300 nM of each primer for HPRT and 500 nM for ssrRNA PCR assays. Reactions were submitted to a initial denaturation at 95 °C for 10 minutes, followed by 40 cycles of denaturation, annealing and extension (95 °C for 15 seconds, 60°C for 1 minute and 68 °C for 30 seconds) with the option for a melt curve ranging from 60 °C to 95 °C and an incremental temperature of 0.3 °C for second and a Tm 78 ± 2 °C. Reactions were performed in duplicate and both targets were run within the same plate for the same sample.
The number of cells from peripheral blood mononuclear cells (PBMC) of non-infected dogs and cultures of L. infantum promastigotes (MCAN/BR/2007/CG-1) were determined by a cell counter (Z1™ COULTER COUNTER®, Beckman Coulter, Fullerton, CA, USA). The total DNA was isolated from 1.0 × 106 PBMC cells and 1.0 × 107promastigotes. Standard curves for the HPRT (R2=0.966, slope= –2.98, efficiency=1.13, y-intercepto=34.143) and ssrRNA genes (R2=0.943, slope= –2.67, efficiency=1.18, y-intercepto=28.117) were prepared using serial 10-fold dilutions from 10–2 to 107 of total purified DNA. All reactions were performed in duplicate in microtubes (Applied Biosystems). The parameters were according with MIQE guidelines (BUSTIN et al., 2009Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 2009; 55(4): 611-622. PMid:19246619. http://dx.doi.org/10.1373/clinchem.2008.112797.
http://dx.doi.org/10.1373/clinchem.2008....
).
Molecular detection of H. canis
H. canis was detected by PCR based on the amplification of a fragment of 666 pb of 18S rDNA gene, using HepF (5’ – ATA-CAT-GAG-CAA-AAT-CTC-AAC- 3’) and HepR (5’ – CTT-ATT-ATT-CCA-TGC-TGC-AG-3’) primers (INOKUMA et al., 2002Inokuma H, Okuda M, Ohno K, Shimoda K, Onishi T. Analysis of the 18SrRNA gene sequence of a Hepatozoon detected in two Japonese dogs. Vet Parasitol 2002; 106(3): 265-271. PMid:12062514. http://dx.doi.org/10.1016/S0304-4017(02)00065-1.
http://dx.doi.org/10.1016/S0304-4017(02)...
) and the GoTaq® DNA Polymerase kit (Promega, WI, USA). PCR was performed with a denaturation step at 94 °C for 3 minutes, followed by 37 cycles of denaturation, annealing and extension (94°C for 45 seconds, 57 °C for 45 seconds and 72 °C for 1 minute), and a final extension step (72 °C for 7 minutes).
PCR products were purified using ExoSAP® IT (GE Healthcare) accordingly to the manufacturer’s recommendations and sequenced using the BigDye v.3.1 Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Foster City, CA, USA) in an automated Applied Biosystems ABI 3500 DNA genetic analyzer (Applied Biosystems). All sequences obtained were analyzed in the software package Phred/Phrap/Consed Version: 0.020425.c (University of Washington, Seattle, WA, USA) and presented Phred values above 20 Sequences were edited using BioEdit software, version 7.2.5 (HALL, 1999Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp Ser 1999; 41: 95-98.) and compared for similarity using BLAST to the sequences available in GenBank (NCBI, 2016National Center for Biotechnology Information – NCBI. BLAST: Basic Local Alignment Search Tool [online]. Bethesda: NCBI; 2016 [cited 2016 Aug 15]. Available from: http://www.ncbi.nlm.nih.gov/BLAST
http://www.ncbi.nlm.nih.gov/BLAST...
).
Phylogenetic analysis were based on the sequences obtained in the study and sequences retrieved in from GenBank. Multiple alignment analysis was performed using the Clustal X version 2.0 (LARKIN et al., 2007Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and clustal X version 2.0. Bioinformatics 2007; 23(21): 2947-2948. PMid:17846036. http://dx.doi.org/10.1093/bioinformatics/btm404.
http://dx.doi.org/10.1093/bioinformatics...
), and aphylogenetic tree was constructed based on the Neighbor-Joining algorithm andthe Kimura 2-parameter model for generating the distance matrix. As implemented in Molecular Evolutionary Genetics Analysis (MEGA) version 6 (TAMURA et al., 2013Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 2013; 30(12): 2725-2729. PMid:24132122. http://dx.doi.org/10.1093/molbev/mst197.
http://dx.doi.org/10.1093/molbev/mst197...
) Bootstrap of 1000 replicates were performed to estimate the confidence of the branching patterns (FELSENSTEIN, 1985Felsenstein J. Confidence limits of phylogenies: an approach using thebootstrap. Evolution 1985; 39(4): 783-791. http://dx.doi.org/10.2307/2408678.
http://dx.doi.org/10.2307/2408678...
). Histopathology
Spleen and liver fragments were fixed in 10% buffered formalin for one to four weeks, embedded in paraffin and sliced in 5-µm thick sections to be mounted on microscope slides. The sections were stained with hematoxylin and eosin (HE) and examined by light microscopy (Nikon Eclipse E400 – Tokyo, Japan). Structural changes of spleen lymphoid tissue, the cell population in the red pulp and the parasite burden were analyzed.
Results
The sampled dogs presented different clinical signs and Leishmania spp. parasite burdens, but all four were co-infected with Leishmania spp. and Hepatozoon spp. Each case is presented separately and differential diagnosis is summarized in Table 1.
Results obtained by the diagnosis methods used to characterize Hepatozoon and Leishmania species.
Description of cases
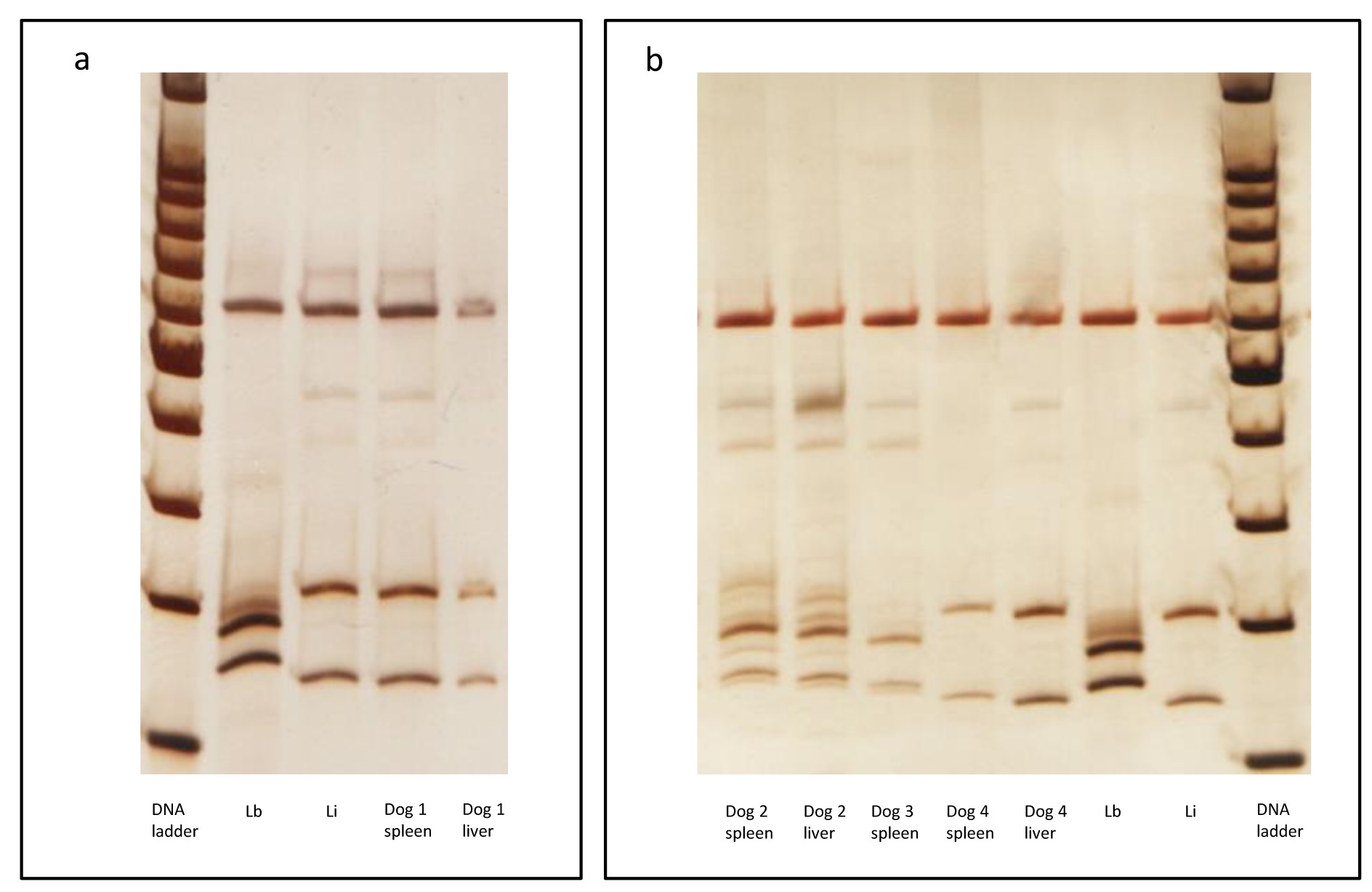
Dog 1: A male dog from Cuiabá, state of Mato Grosso, presenting cachexy and ascites. Parasite isolation was positive and the species identified as L. infantum in the spleen and liver (Figure 1). The splenic histopathological analysis showed an organized white pulp and many mature meronts of H. canis (Figure 2a-e). Histological alterations in the liver were not observed. Molecular analysis of Hepatozoon canis was not performed (Table 1).
Molecular detection of Leishmania sp using ITS1 PCR-RFLP after Sau 3AI digestion. Silver stained polyacrylamide gel (12.5%) (Genephor). (a) Dog #1; and (b) Dogs #2, #3 and #4 analysis. The following Leishmania reference strains were used: Li - IOC/L 579 (Leishmania (L.) infantum- MHOM/BR/1974/PP75) and Lb - IOC/L 566 (Leishmania (V.) braziliensis- MHOM/BR/1975/M2903). DNA Ladder = 100bp from Promega. ITS: internal transcribed spacer; PCR RFLP: Polymerase Chain Reaction Followed by Restriction Fragment Length Polymorphism
Evidence of the parasitic forms of Hepatozoon spp. and Leishmania spp. in the spleen and liver of infected dogs. (a-e) spleen and (f) liver. (a) A monozoic tissue cyst and (b) a mature meront containing four macromerozoites of Hepatozoon canis; (c) a mature meront containing micromerozoites arranged at the periphery presenting a “wheel spoke” pattern of Hepatozoon canis inside a multinucleated giant cell and (d) a meront containing 20-30 micromerozoites; (e) micromerozoites of Hepatozoon canis being released; (f) amastigotes of Leishmania braziliensis.
Dog 2: Asymptomatic male dog from Barra do Garças, state of Mato Grosso. Although parasite isolation was negative, PCR-RFLP confirmed L. braziliensis infection in spleen and liver samples (Figure 1). The histopathological analysis of the spleen showed an organized white pulp and mature meronts of H. canis. The liver presented an intense non-granulomatous inflammation, with vacuolar degeneration, intense fibrosis and colestasis. Amastigotes were observed in the liver (Figure 2f). = Hepatozoon spp. DNA was only detected in spleen and the sequences showed 100% identicalness with H. canis by BLAST analysis (Tables 2, 3, Figure 3).
Neighbor-Joining Tree based on the 18S rDNA gene with 666 bp for Hepatozoon spp. related to sequences obtained with the HepF and HepR oligonucleotides. The Kimura 2-parameter model was used. Numbers at node indicate bootstrap values over 1000 replicates. Scale indicates the evolutionary distance of 0.05 nucleotides per position in the sequence. Cytauxzoon felis, Babesia canis vogeli and Plasmodium falciparum were used as outgroup.
Dog 3: A male dog from Barra do Garças, state of Mato Grosso, presenting cachexy, lymphadenomegaly, onychogryphosis and conjunctivitis. Parasite isolation was negative; species detection was performed confirming L. braziliensis infection in a spleen sample (Figure 1). In the liver sample, the digestion of ITS1 PCR product generated inconclusive results. Histopathological analysis of the spleen showed intense disorganization of white pulp, perisplenitis, absence of granuloma and mature meronts of Hepatozoon canis. The liver presented intense and diffuse non-granulomatous inflammation, with vacuolar degeneration and amastigotes. Hepatozoon spp. DNA was detected in the spleen and liver and the sequences showed 100% identicalness with H. canis by BLAST analysis (Tables 2, 3, Figure 3).
Dog 4: A female dog from Cuiabá, state of Mato Grosso, presenting alopecia, onychogryphosis, dermatitis and conjunctivitis. Parasite isolation was negative, though the infection by L. infantum was confirmed in the spleen and liver (Figure 1) by PCR-RFLP. Histopathological analysis of the spleen showed moderate disorganization of the white pulp, amastigotes and mature meronts of H. canis. The liver presented only a moderate and diffuse non-granulomatous inflammation. Molecular detection of H. canis was not performed.
Discussion
In the present study, H. canis infection and co-infection with Leishmania spp. were confirmed through histopathological and molecular analyses in the spleen for the first time in dogs from Barra do Garças, Mato Grosso state - Brazil. In silico analysis showed that the ssrRNA and 18S rRNA primers used in this work were specific for Leishmania spp. and Hepatozoon spp., respectively (data not shown), and did not generate cross-reaction. We observed 100% identicalness between our sequences with H. canis from Croatia and other regions of Brazil, suggesting either a geographic expansion of this infection or a recent increase in the efficiency in detecting these parasites. Recently, canine hepatozoonosis has been described in several Brazilian regions (RUBINI et al., 2008Rubini AS, Paduan KS, Lopes VVA, O’Dwyer LH. Molecular and parasitological survey of Hepatozoon canis (Apicomplexa: Hepatozoidae) in dogs from rural area of Sao Paulo state, Brazil. Parasitol Res 2008; 102(5): 895-899. PMid:18188597. http://dx.doi.org/10.1007/s00436-007-0846-7.
http://dx.doi.org/10.1007/s00436-007-084...
; MIRANDA et al., 2014Miranda RL, O’Dwyer LH, Castro JR, Metzger B, Rubini AS, Mundim AV, et al. Prevalence and molecular characterization of in dogs from urban and rural areas in Southeast Brazil. Hepatozoon canisRes Vet Sci 2014; 97(2): 325-328. PMid:25039064. http://dx.doi.org/10.1016/j.rvsc.2014.06.015.
http://dx.doi.org/10.1016/j.rvsc.2014.06...
; GONÇALVES et al., 2014Gonçalves LR, Filgueira KD, Ahid SMM, Pereira JS, Vale AM, Machado RZ, et al. Study on coinfecting vector-borne pathogens in dogs and ticks in Rio Grande do Norte, Brazil. Rev Bras Parasitol Vet 2014; 23(3): 407-412. PMid:25271465. http://dx.doi.org/10.1590/S1984-29612014071.
http://dx.doi.org/10.1590/S1984-29612014...
; RAMOS et al., 2015Ramos CA, Babo-Terra VJ, Pedroso TC, Souza AF Fo, Araújo FR, Cleveland HP. Molecular identification of in dogs from Campo Grande, Mato Grosso do Sul, Brazil. Hepatozoon canisRev Bras Parasitol Vet 2015; 24(2): 247-250. PMid:26154969. http://dx.doi.org/10.1590/S1984-29612015019.
http://dx.doi.org/10.1590/S1984-29612015...
; DEMONER et al., 2016Demoner LC, Magro NM, Silva MR, Antunes JMP, Calabuig CIP, O’Dwyer LH. spp. infections in wild rodents in an area of endemic canine hepatozoonosis in southeastern Brazil. HepatozoonTicks Tick Borne Dis 2016; 7(5): 859-864. PMid:27091081. http://dx.doi.org/10.1016/j.ttbdis.2016.04.002.
http://dx.doi.org/10.1016/j.ttbdis.2016....
). Many of these areas are also endemic for CVL and co-infection was indeed described in dogs from Rio Grande do Norte, but only through molecular analysis (GONÇALVES et al., 2014Gonçalves LR, Filgueira KD, Ahid SMM, Pereira JS, Vale AM, Machado RZ, et al. Study on coinfecting vector-borne pathogens in dogs and ticks in Rio Grande do Norte, Brazil. Rev Bras Parasitol Vet 2014; 23(3): 407-412. PMid:25271465. http://dx.doi.org/10.1590/S1984-29612014071.
http://dx.doi.org/10.1590/S1984-29612014...
).
The pathogenesis of H. canis infection is influenced by immunodeficiency conditions, an immature immune system in young pups, or a concurrent infectious agent (BANETH, 2011Baneth G. Hepatozoonosis. In: Greene C. Infectious diseases of the dog and cat. 4th ed. Georgia: Saunders; 2011. p. 750-756.). Conditions that impair the immune responses increase the susceptibility to new infection with H. canis or allow existing infections to reactivate. Co-infections with Toxoplasma, Leishmania, Babesia or Ehrlichia predispose dogs to clinical illness (BANETH, 2011Baneth G. Hepatozoonosis. In: Greene C. Infectious diseases of the dog and cat. 4th ed. Georgia: Saunders; 2011. p. 750-756.). Detection of L. braziliensis parasites DNA in the liver and spleen shows the probability of the visceralization of this species in dogs presenting comorbidities. Although DNA detection may not show the presence of viable parasites, additional results (observation of amastigotes and ELISA and IFAT positivity) confirmed the infection. Currently, L. braziliensis is considered a dermotrophic species in the human leishmaniasis disease complex, with isolation and detection of this species in the viscera only in cases of immunologically compromised patients (SILVA et al., 2002Silva ES, Pacheco RS, Gontijo CMF, Carvalho IR, Brazil RP. Visceral leishmaniasis caused by Leishmania (Viannia) braziliensis in a patient infected with human immunodeficiency virus. Rev Inst Med Trop Sao Paulo 2002; 44(3): 145-149. PMid:12163907. http://dx.doi.org/10.1590/S0036-46652002000300006.
http://dx.doi.org/10.1590/S0036-46652002...
; GONTIJO et al., 2002Gontijo CMF, Pacheco RS, Orefice F, Lasmar E, Silva ES, Melo MN. Concurrent cutaneous, visceral and ocular leishmaniasis caused by in a kidney transplant patient. Leishmania (Viannia) braziliensisMem Inst Oswaldo Cruz 2002; 97(5): 751-753. PMid:12219147. http://dx.doi.org/10.1590/S0074-02762002000500029.
http://dx.doi.org/10.1590/S0074-02762002...
). In wild and synantropic animals (considered potential reservoirs for L. braziliensis), such a distinction regarding tissue tropism does not exist (ROQUE et al., 2010Roque ALR, Cupolillo E, Marchevsky RS, Jansen AM. (Rodentia; Echimyidae) as a putative reservoir of Leishmania infantum and : patterns of experimental infection. Thrichomys laurentiusL. braziliensisPLoS Negl Trop Dis 2010; 4(2): e589. PMid:20126407. http://dx.doi.org/10.1371/journal.pntd.0000589.
http://dx.doi.org/10.1371/journal.pntd.0...
; ROQUE & JANSEN, 2014Roque ALR, Jansen AM. Wild and synanthropic reservoirs of Leishmania species in the Americas. Int J Parasitol Parasites Wildl 2014; 3(3): 251-262. PMid:25426421. http://dx.doi.org/10.1016/j.ijppaw.2014.08.004.
http://dx.doi.org/10.1016/j.ijppaw.2014....
), and the parasite may be detected in many organs. In dogs, this species has only been detected in cutaneous lesions (MADEIRA et al., 2005Madeira MF, Schubach AO, Schubach TMP, Serra CMB, Pereira SA, Figueiredo FB, et al. Is preferentially restricted to the cutaneous lesions of naturally infected dogs? Leishmania (Viannia) braziliensisParasitol Res 2005; 97(1): 73-76. PMid:15986254. http://dx.doi.org/10.1007/s00436-005-1374-y.
http://dx.doi.org/10.1007/s00436-005-137...
; DANTAS-TORRES, 2007Dantas-Torres F. The role of dogs as reservoirs of Leishmania parasites, with emphasis on and Leishmania (Leishmania) infantumLeishmania (Viannia) braziliensis.Vet Parasitol 2007; 149(3-4): 139-146. PMid:17703890. http://dx.doi.org/10.1016/j.vetpar.2007.07.007.
http://dx.doi.org/10.1016/j.vetpar.2007....
). The visceralization detected herein by L. braziliensis may be a consequence of the impaired immune system of the dogs. Such an immunological deficit may be due to either Hepatozoon infection alone or the increased virulence due to co-infection. These hypotheses are difficult to verify, but the first is more likely since the two dogs infected with L. braziliensis showed lower parasite burden than the dog infected by L. infantum, and only one was symptomatic. If we consider that there is a relationship between clinical signs and parasite burden (TEIXEIRA-NETO et al., 2010Teixeira-Neto RG, Giunchetti RC, Carneiro CM, Vitor RW, Coura-Vital W, Quaresma PF, et al. Relationship of -specific IgG levels and IgG avidity with parasite density and clinical signs in canine leishmaniasis. LeishmaniaVet Parasitol 2010; 169(3-4): 248-257. PMid:20188477. http://dx.doi.org/10.1016/j.vetpar.2010.01.023.
http://dx.doi.org/10.1016/j.vetpar.2010....
), the clinical signs described in dog #3 must have been related to the infection by Hepatozoon. Visceralization of L. braziliensis strains was previously reported in dogs (OLIVEIRA et al., 2013Oliveira GM, Madeira MF, Oliveira FS, Pires MQ, Pacheco RS. Canine Cutaneous Leishmaniasis: dissemination and tissue tropism of genetically distinct populations. Leishmania (Viannia) braziliensisVet Med Int 2013; 2013: 982183. http://dx.doi.org/10.1155/2013/982183. PMid:23844317.
http://dx.doi.org/10.1155/2013/982183...
). Although the authors attributed dissemination and tissue tropism of populations to a distinct genetic profile, they did not mention if there were comorbidities in the evaluated dogs.
Therefore, other studies are needed to highlight the potential influence of the immune response that both infections could have on each other. The understanding of this co-infection is crucial since the impaired immune response to leishmaniasis may lead to an increased severity of the disease and, consequently, to a high Leishmania parasite burden and dissemination. This may be - at some point - related to the potential of dogs as reservoirs. The visceralization of L. braziliensis, whether related with the co-infection with Hepatozoon or not, raises a question about its impact on Leishmania parasite burden and consequently on the role of dogs as a source of infection to vectors.
In this study, two animals were infected by L. braziliensis and, for this reason, were unnecessarily submitted to euthanasia. They were DPP negative, which suggests this test may be a good tool for the differential diagnosis and euthanasia. Although cross-reaction could be observed by ELISA based on DPP antigens with sera from dogs infected with Leishmania braziliensis, this cross-reaction is less frequent than that observed when using crude antigens (PORROZZI et al., 2007Porrozzi R, Costa MVS, Teva A, Falqueto A, Ferreira AL, Santos CD, et al. Comparative evaluation of enzyme-linked immunosorbent assays based on crude and recombinant leishmanial antigens for serodiagnosis of symptomatic and asymptomatic visceral infections in dogs. Leishmania infantumClin Vaccine Immunol 2007; 14(5): 544-548. PMid:17314229. http://dx.doi.org/10.1128/CVI.00420-06.
http://dx.doi.org/10.1128/CVI.00420-06...
). Nevertheless, it is not expected that this cross-reaction occurs in immunochromatographic tests. Because only two animals were evaluated in the present study, this hypothesis must be better verified. In spite of that, the data points out the importance in performing an etiological diagnosis at the species level. Since 2012, DPP® (rK39 antigen, Biomanguinhos) has been used as a screening test for CVL followed by confirmation with an ELISA test to identify animals for euthanasia when they are positive by both methods. However, when the samples used herein were collected, DPP® had not been used as a method to diagnose CVL yet. For these two dogs, clinical signs attributed to Leishmania infection could actually be related to Hepatozoon infection, since L. braziliensis species does not lead to such clinical profile. Hepatozoon infection could in turn cause immunosuppression leading to the visceralization of Leishmania braziliensis. In this context, the importance of a complementary diagnosis in the veterinary clinical evaluation, either molecular or serological, is clear. Additionally, the impact of co-infection with Leishmania spp. and Hepatozoon spp. or other pathogens (Ehrlichia canis, Babesia spp., Anaplasma spp., etc.) on clinical manifestations, the immune response and the parasite burden needs to be clarified.
Conclusion
In the present work, Hepatozoon canis infection and co-infection with Leishmania braziliensis were identified for the first time in Barra do Garças, Mato Grosso state, Brazil. From this report, the main points were addressed: i) visceral dissemination of L. braziliensis associated with systemic signs suggested that this co-infection could have influenced the parasite burden, the parasite spread, morbidity, clinical signs and, possibly, the role of co-infected dogs as reservoirs; ii) it is important for veterinarians to promote a differential diagnosis for co-infected dogs, with an emphasis on defining the involved Leishmania species.
References
- Baneth G. Hepatozoonosis. In: Greene C. Infectious diseases of the dog and cat. 4th ed. Georgia: Saunders; 2011. p. 750-756.
- Belo VS, Werneck GL, Barbosa DS, Simões TC, Nascimento BWL, Silva ES, et al. Factors associated with visceral leishmaniasis in the americas: a systematic review and meta-analysis. PLoS Negl Trop Dis 2013; 7(4): e2182. PMid:23638203. http://dx.doi.org/10.1371/journal.pntd.0002182
» http://dx.doi.org/10.1371/journal.pntd.0002182 - Brasil. Ministério da Saúde. Manual de vigilância e controle da Leishmaniose Visceral. Brasília: MS; 2006.
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 2009; 55(4): 611-622. PMid:19246619. http://dx.doi.org/10.1373/clinchem.2008.112797
» http://dx.doi.org/10.1373/clinchem.2008.112797 - Chhabra S, Uppal SK, Singla LD. Retrospective study of clinical and hematological aspects associated with dogs naturally infected by in Ludhiana, Punjab, India. Hepatozoon canisAsian Pac J Trop Biomed 2013; 3(6): 483-486. PMid:23730562. http://dx.doi.org/10.1016/S2221-1691(13)60100-8
» http://dx.doi.org/10.1016/S2221-1691(13)60100-8 - Dantas-Torres F. The role of dogs as reservoirs of Leishmania parasites, with emphasis on and Leishmania (Leishmania) infantumLeishmania (Viannia) braziliensis.Vet Parasitol 2007; 149(3-4): 139-146. PMid:17703890. http://dx.doi.org/10.1016/j.vetpar.2007.07.007
» http://dx.doi.org/10.1016/j.vetpar.2007.07.007 - Demoner LC, Magro NM, Silva MR, Antunes JMP, Calabuig CIP, O’Dwyer LH. spp. infections in wild rodents in an area of endemic canine hepatozoonosis in southeastern Brazil. HepatozoonTicks Tick Borne Dis 2016; 7(5): 859-864. PMid:27091081. http://dx.doi.org/10.1016/j.ttbdis.2016.04.002
» http://dx.doi.org/10.1016/j.ttbdis.2016.04.002 - Felsenstein J. Confidence limits of phylogenies: an approach using thebootstrap. Evolution 1985; 39(4): 783-791. http://dx.doi.org/10.2307/2408678
» http://dx.doi.org/10.2307/2408678 - Gonçalves LR, Filgueira KD, Ahid SMM, Pereira JS, Vale AM, Machado RZ, et al. Study on coinfecting vector-borne pathogens in dogs and ticks in Rio Grande do Norte, Brazil. Rev Bras Parasitol Vet 2014; 23(3): 407-412. PMid:25271465. http://dx.doi.org/10.1590/S1984-29612014071
» http://dx.doi.org/10.1590/S1984-29612014071 - Gontijo CMF, Pacheco RS, Orefice F, Lasmar E, Silva ES, Melo MN. Concurrent cutaneous, visceral and ocular leishmaniasis caused by in a kidney transplant patient. Leishmania (Viannia) braziliensisMem Inst Oswaldo Cruz 2002; 97(5): 751-753. PMid:12219147. http://dx.doi.org/10.1590/S0074-02762002000500029
» http://dx.doi.org/10.1590/S0074-02762002000500029 - Graça GC, Volpini AC, Romero GA, Oliveira-Neto MP, Hueb M, Porrozzi R, et al. Development and validation of PCR-based assays for diagnosis of American cutaneous leishmaniasis and identification of the parasite species. Mem Inst Oswaldo Cruz 2012; 107(5): 664-674. PMid:22850958. http://dx.doi.org/10.1590/S0074-02762012000500014
» http://dx.doi.org/10.1590/S0074-02762012000500014 - Grimaldi G Jr, Teva A, Ferreira AL, Santos CB, Pinto I, de-Azevedo CT, et al. Evaluation of a novel chromatographic immunoassay based on Dual-Path Platform technology (DPP® CVL rapid test) for the serodiagnosis of canine visceral leishmaniasis. Trans R Soc Trop Med Hyg 2012; 106(1): 54-59. PMid:22137538. http://dx.doi.org/10.1016/j.trstmh.2011.10.001
» http://dx.doi.org/10.1016/j.trstmh.2011.10.001 - Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp Ser 1999; 41: 95-98.
- Inokuma H, Okuda M, Ohno K, Shimoda K, Onishi T. Analysis of the 18SrRNA gene sequence of a Hepatozoon detected in two Japonese dogs. Vet Parasitol 2002; 106(3): 265-271. PMid:12062514. http://dx.doi.org/10.1016/S0304-4017(02)00065-1
» http://dx.doi.org/10.1016/S0304-4017(02)00065-1 - Kaur P, Deshmukh S, Singh R, Bansal BK, Randhawa CS, Singla LD. Para-clinico-pathological observations of insidious incidence of canine hepatozoonosis from a mongrel dog: a case report. J Parasit Dis 2012; 36(1): 135-138. PMid:23543040. http://dx.doi.org/10.1007/s12639-011-0092-x
» http://dx.doi.org/10.1007/s12639-011-0092-x - Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and clustal X version 2.0. Bioinformatics 2007; 23(21): 2947-2948. PMid:17846036. http://dx.doi.org/10.1093/bioinformatics/btm404
» http://dx.doi.org/10.1093/bioinformatics/btm404 - Madeira MF, Schubach AO, Schubach TMP, Serra CMB, Pereira SA, Figueiredo FB, et al. Is preferentially restricted to the cutaneous lesions of naturally infected dogs? Leishmania (Viannia) braziliensisParasitol Res 2005; 97(1): 73-76. PMid:15986254. http://dx.doi.org/10.1007/s00436-005-1374-y
» http://dx.doi.org/10.1007/s00436-005-1374-y - Miranda RL, O’Dwyer LH, Castro JR, Metzger B, Rubini AS, Mundim AV, et al. Prevalence and molecular characterization of in dogs from urban and rural areas in Southeast Brazil. Hepatozoon canisRes Vet Sci 2014; 97(2): 325-328. PMid:25039064. http://dx.doi.org/10.1016/j.rvsc.2014.06.015
» http://dx.doi.org/10.1016/j.rvsc.2014.06.015 - Mundim AV, Morais IA, Tavares M, Cury MC, Mundim MJS. Clinical and hematological signs associated with dogs naturally infected by Hepatozoon sp. and with other hematozoa: a retrospective study in Uberlândia, Minas Gerais, Brazil. Vet Parasitol 2008; 153(1-2): 3-8. PMid:18304739. http://dx.doi.org/10.1016/j.vetpar.2008.01.018
» http://dx.doi.org/10.1016/j.vetpar.2008.01.018 - National Center for Biotechnology Information – NCBI. BLAST: Basic Local Alignment Search Tool [online]. Bethesda: NCBI; 2016 [cited 2016 Aug 15]. Available from: http://www.ncbi.nlm.nih.gov/BLAST
» http://www.ncbi.nlm.nih.gov/BLAST - O’Dwyer LH. Brazilian canine hepatozoonosis. Rev Bras Parasitol Vet 2011; 20(3): 181-193. PMid:21961746. http://dx.doi.org/10.1590/S1984-29612011000300002
» http://dx.doi.org/10.1590/S1984-29612011000300002 - Oliveira GM, Madeira MF, Oliveira FS, Pires MQ, Pacheco RS. Canine Cutaneous Leishmaniasis: dissemination and tissue tropism of genetically distinct populations. Leishmania (Viannia) braziliensisVet Med Int 2013; 2013: 982183. http://dx.doi.org/10.1155/2013/982183 PMid:23844317.
» http://dx.doi.org/10.1155/2013/982183 - Porrozzi R, Costa MVS, Teva A, Falqueto A, Ferreira AL, Santos CD, et al. Comparative evaluation of enzyme-linked immunosorbent assays based on crude and recombinant leishmanial antigens for serodiagnosis of symptomatic and asymptomatic visceral infections in dogs. Leishmania infantumClin Vaccine Immunol 2007; 14(5): 544-548. PMid:17314229. http://dx.doi.org/10.1128/CVI.00420-06
» http://dx.doi.org/10.1128/CVI.00420-06 - Prina E, Roux E, Mattei D, Milon G. Leishmania DNA is rapidly degraded following parasite death: an analysis by microscopy and real-time PCR. Microbes Infect 2007; 9(11): 1307-1315. PMid:17890124. http://dx.doi.org/10.1016/j.micinf.2007.06.005
» http://dx.doi.org/10.1016/j.micinf.2007.06.005 - Ramos CA, Babo-Terra VJ, Pedroso TC, Souza AF Fo, Araújo FR, Cleveland HP. Molecular identification of in dogs from Campo Grande, Mato Grosso do Sul, Brazil. Hepatozoon canisRev Bras Parasitol Vet 2015; 24(2): 247-250. PMid:26154969. http://dx.doi.org/10.1590/S1984-29612015019
» http://dx.doi.org/10.1590/S1984-29612015019 - Roque ALR, Cupolillo E, Marchevsky RS, Jansen AM. (Rodentia; Echimyidae) as a putative reservoir of Leishmania infantum and : patterns of experimental infection. Thrichomys laurentiusL. braziliensisPLoS Negl Trop Dis 2010; 4(2): e589. PMid:20126407. http://dx.doi.org/10.1371/journal.pntd.0000589
» http://dx.doi.org/10.1371/journal.pntd.0000589 - Roque ALR, Jansen AM. Wild and synanthropic reservoirs of Leishmania species in the Americas. Int J Parasitol Parasites Wildl 2014; 3(3): 251-262. PMid:25426421. http://dx.doi.org/10.1016/j.ijppaw.2014.08.004
» http://dx.doi.org/10.1016/j.ijppaw.2014.08.004 - Rubini AS, Paduan KS, Cavalcante GG, Ribolla PEM, O’Dwyer LH. Molecular identification and characterization of canine Hepatozoon species from Brazil. Parasitol Res 2005; 97(2): 91-93. PMid:15948009. http://dx.doi.org/10.1007/s00436-005-1383-x
» http://dx.doi.org/10.1007/s00436-005-1383-x - Rubini AS, Paduan KS, Lopes VVA, O’Dwyer LH. Molecular and parasitological survey of Hepatozoon canis (Apicomplexa: Hepatozoidae) in dogs from rural area of Sao Paulo state, Brazil. Parasitol Res 2008; 102(5): 895-899. PMid:18188597. http://dx.doi.org/10.1007/s00436-007-0846-7
» http://dx.doi.org/10.1007/s00436-007-0846-7 - Silva ES, Pacheco RS, Gontijo CMF, Carvalho IR, Brazil RP. Visceral leishmaniasis caused by Leishmania (Viannia) braziliensis in a patient infected with human immunodeficiency virus. Rev Inst Med Trop Sao Paulo 2002; 44(3): 145-149. PMid:12163907. http://dx.doi.org/10.1590/S0036-46652002000300006
» http://dx.doi.org/10.1590/S0036-46652002000300006 - Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 2013; 30(12): 2725-2729. PMid:24132122. http://dx.doi.org/10.1093/molbev/mst197
» http://dx.doi.org/10.1093/molbev/mst197 - Teixeira-Neto RG, Giunchetti RC, Carneiro CM, Vitor RW, Coura-Vital W, Quaresma PF, et al. Relationship of -specific IgG levels and IgG avidity with parasite density and clinical signs in canine leishmaniasis. LeishmaniaVet Parasitol 2010; 169(3-4): 248-257. PMid:20188477. http://dx.doi.org/10.1016/j.vetpar.2010.01.023
» http://dx.doi.org/10.1016/j.vetpar.2010.01.023
Publication Dates
-
Publication in this collection
01 Dec 2016 -
Date of issue
Oct-Dec 2016
History
-
Received
15 Aug 2016 -
Accepted
23 Sept 2016