Abstract
This study evaluated a recombinant aquaporin 1 protein of Rhipicephalus (Boophilus) microplus (RmAQP1) as antigen in a vaccine against R. sanguineus. Five dogs were immunized with RmAQP1 (10 µg) + adjuvant (Montanide) (G1), and five were inoculated with adjuvant only (G2), three times. Twenty-one days after the last immunization, animals of both groups were challenged with R. sanguineus larvae, nymphs and adults, and their biotic potential was compared. Blood samples were collected before each immunization and every 28 days after the last immunization for 10 weeks. Serum antibody titers (IgG) were assessed by ELISA. We observed that: engorgement period of adult females from G1 was 12% shorter than G2; larvae from G1 had 8.7% longer engorgement period than G2 and weighed 7.2% less; nymphs from G1 had 4.5% shorter engorgement period than G2 and weighed 3.6% less; although the antibody titers increased following the second immunization, they rapidly decreased after the third immunization. Results indicated low immunoprotection of RmAQP1 against adult R. sanguineus ticks, and possible efficacy on larvae and nymphs fed on immunized dogs. Further studies should be performed for a full evaluation of the immunoprotection of RmAQP1 against R. sanguineus infestations in dogs.
Keywords:
Rhipicephalus sanguineus; aquaporin; RmAQP1; immunity; domestic dog; ticks
Resumo
Este estudo avaliou a proteína recombinante (aquaporina) do carrapato Rhipicephalus (Boophilus) microplus como antígeno em vacina contra Rhipicephalus sanguineus. Cinco cães foram imunizados com RmAQP1 (10 µg) + adjuvante (G1) e cinco foram inoculados apenas com adjuvante (G2), três vezes. 21 dias após a última imunização todos os animais foram desafiados com larvas, ninfas e adultos de R. sanguineus, e potencial biótico dos carrapatos foi comparado. Amostras de sangue foram coletadas antes de cada imunização e a cada 28 dias após a última imunização, durante 10 semanas. Títulos de anticorpos dos soros dos cães foram avaliados por ELISA. Resultados: o período de ingurgitamento das fêmeas do G1 foi 12% mais curto que o período de ingurgitamento de G2; o período de ingurgitamento das larvas do G1 8,7% foi mais longo e o peso 7,2% menor que no caso de G2; o período de ingurgitamento das ninfas do G1 4,5% foi mais curto e peso 3,6% menor que no caso do G2; aumento dos títulos de anticorpos do G1 após a segunda imunização e declínio após a terceira imunização. Os resultados indicaram baixo potencial de imunoproteção de RmAQP1 contra R. sanguineus adultos, e possível eficácia contra larvas e ninfas, na dose testada. Sugere-se desenvolver novos estudos para melhor avaliação da eficácia de RmAQP1 contra R. sanguineus em cães.
Palavras-chave:
Rhipicephalus sanguineus; aquaporina; RmAQP1; imunidade; cão doméstico; carrapatos
Introduction
Ticks are obligate blood-sucking ectoparasites from the phylum Arthropoda, class Arachnida, subclass Acari and order Ixodida (KRANTZ & WALTER, 2009Krantz GW, Walter DE. A manual of acarology. 3rd ed. Lubbock: Texas Tech University Press; 2009.). Three families of ticks have been described so far: Ixodidae (hard ticks), Argasidae (soft ticks) and the most recently identified family Nuttalliellidae (GUGLIELMONE et al., 2010Guglielmone AA, Robbins RG, Apanaskevich DA, Petney TN, Estrada-Peña A, Horak IG, et al. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the world: a list of valid species names. Zootaxa 2010; 2528(6): 1-28.). The genus Rhipicephalus belongs to the Ixodidae family and includes about 84 tick species, almost all originating from the Afrotropical region, among which are included the brown dog tick, R. sanguineus, and the cattle tick, R. (B.) microplus (APANASKEVICH et al., 2013Apanaskevich DA, Horak IG, Mulumba-Mfumu LK. A new species of (Acari: Ixodidae), a parasite of red river hogs and domestic pigs in the Democratic Republic of Congo. RhipicephalusJ Med Entomol 2013; 50(3): 479-484. PMid:23802441. http://dx.doi.org/10.1603/ME12266.
http://dx.doi.org/10.1603/ME12266...
; GUGLIELMONE et al., 2010Guglielmone AA, Robbins RG, Apanaskevich DA, Petney TN, Estrada-Peña A, Horak IG, et al. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the world: a list of valid species names. Zootaxa 2010; 2528(6): 1-28.; HORAK et al., 2013Horak IG, Apanaskevich DA, Kariuki EK. A new species of (Acari: Ixodidae), a parasite of giraffes in Kenya. RhipicephalusJ Med Entomol 2013; 50(4): 685-690. PMid:23926765. http://dx.doi.org/10.1603/ME12257.
http://dx.doi.org/10.1603/ME12257...
). R. sanguineus can be found parasitizing different domestic and wild animals, including humans, despite having the domestic dog as its main host (DANTAS-TORRES et al., 2006Dantas-Torres F, Figueiredo LA, Brandão-Filho SP. (Acari: Ixodidae), the brown dog tick, parasitizing humans in Brazil. Rhipicephalus sanguineusRev Soc Bras Med Trop 2006; 39(1): 64-67. PMid:16501769. http://dx.doi.org/10.1590/S0037-86822006000100012.
http://dx.doi.org/10.1590/S0037-86822006...
; GUGLIELMONE et al., 2003Guglielmone AA, Estrada-Peña A, Keirans JE, Robins RG. Ticks (Acari: Ixodida) of the Neotropical zoogeographic region. Atlanta: International Consortium on Ticks and Tick-borne Disease; 2003. 173 p.; SZABÓ et al., 2008Szabó MPJ, Pascoli GVT, Marçal OM Jr, Franchin AG, Torga K. Brown dog tick parasitizing the bird . Rhipicephalus sanguineusCoereba flaveola in the Brazilian CerradoCienc Rural 2008; 38(2): 543-545. http://dx.doi.org/10.1590/S0103-84782008000200041.
http://dx.doi.org/10.1590/S0103-84782008...
; RODRIGUEZ-VIVAS et al., 2016Rodríguez-Vivas RI, Apanaskevich DA, Ojeda-Chi MM, Trinidad-Martinez I, Reyes-Novelo E, Esteve-Gassent MD, Perez de León AA. Ticks collected from humans, domestic animals, and wildlife in Yucatan, Mexico. Vet Parasitol 2016; 215: 106-113. PMID: 26790745. http://dx.doi.org/10.1016/j.vetpar.2015.11.010.
http://dx.doi.org/10.1016/j.vetpar.2015....
).
Bearing in mind that an average 95% of the ticks are on the environment and only 5% on the host, an integrated control strategy for eliminating tick population of both animal and environment is required (DANTAS-TORRES, 2008Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol 2008; 152(3-4): 173-185. PMid:18280045. http://dx.doi.org/10.1016/j.vetpar.2007.12.030.
http://dx.doi.org/10.1016/j.vetpar.2007....
). Acaricides are widely used to eliminate and prevent reinfestation for a certain period; however, the excessive use of acaricides has contributed to the development of resistant tick populations as well as environmental and animal products contamination (KUNZ & KEMP, 1994Kunz SE, Kemp DH. Insecticides and acaricides: resistance and environmental impact. Rev Sci Tech Off Int Epiz 1994; 13(4): 1249-1286. PMid:7711312. http://dx.doi.org/10.20506/rst.13.4.816.
http://dx.doi.org/10.20506/rst.13.4.816...
; DE LA FUENTE et al., 2015De La Fuente J, Kocan KM, Contreras M. Prevention and control strategies for ticks and pathogen transmission. Rev Sci Tech Off Int Epiz 2015; 34(1): 249-264. PMid:26470461. http://dx.doi.org/10.20506/rst.34.1.2357.
http://dx.doi.org/10.20506/rst.34.1.2357...
). A sustainable option for tick control is the combination of chemicals with vaccines (GHOSH et al., 2007Ghosh S, Azhahianambi P, Yadav MP. Upcoming and future strategies of tick control: a review. J Vector Borne Dis 2007; 44(2): 79-89. PMid:17722860.; GUERRERO et al., 2012Guerrero FD, Miller RJ, Perez de León AA. Cattle tick vaccines: many candidate antigens, but will a commercially viable product emerge? Int J Parasitol 2012; 42(5): 421-427. PMid:22549026. http://dx.doi.org/10.1016/j.ijpara.2012.04.003.
http://dx.doi.org/10.1016/j.ijpara.2012....
; SPRONG et al., 2014Sprong H, Trentelman J, Seemann I, Grubhoffer L, Rego RO, Hajdušek O, et al. ANTIDotE: anti-tick vaccines to prevent tick-borne diseases in Europe. Parasit Vectors 2014; 7(1): 77. PMid:24559082. http://dx.doi.org/10.1186/1756-3305-7-77.
http://dx.doi.org/10.1186/1756-3305-7-77...
). Thus, as part of a R.(B.) microplus genome study aiming the discovery of transcripts that produce antigens for an effective vaccine against the cattle tick, studies focused on the genome (GUERRERO et al., 2010Guerrero FD, Moolhuijzen P, Peterson DG, Bidwell S, Caler E, Bellgard M, et al. Reassociation kinetics-based approach for partial genome sequencing of the cattle tick, (RhipicephalusBoophilusmicroplus.) BMC Genomics 2010; 11(1): 374. PMid:20540747. http://dx.doi.org/10.1186/1471-2164-11-374.
http://dx.doi.org/10.1186/1471-2164-11-3...
), transcriptome (GUERRERO et al., 2005Guerrero FD, Miller RJ, Rousseau ME, Sunkara S, Quackenbush J, Lee Y, et al. BmiGI: a database of cDNAs expressed in , the tropical/southern cattle tick. Boophilus microplusInsect Biochem Mol Biol 2005; 35(6): 585-595. PMid:15857764. http://dx.doi.org/10.1016/j.ibmb.2005.01.020.
http://dx.doi.org/10.1016/j.ibmb.2005.01...
) and proteome (RACHINSKY et al., 2007Rachinsky A, Guerrero FD, Scoles GA. Differential protein expression in ovaries of uninfected and Babesia-infected southern cattle ticks, Rhipicephalus (Boophilus) microplus.Insect Biochem Mol Biol 2007; 37(12): 1291-1308. PMid:17967348. http://dx.doi.org/10.1016/j.ibmb.2007.08.001.
http://dx.doi.org/10.1016/j.ibmb.2007.08...
, 2008Rachinsky A, Guerrero FD, Scoles GA. Proteomic profiling of Rhipicephalus (Boophilus) microplus midgut responses to infection with Babesia bovis.Vet Parasitol 2008; 152(3-4): 294-313. PMid:18243558. http://dx.doi.org/10.1016/j.vetpar.2007.12.027.
http://dx.doi.org/10.1016/j.vetpar.2007....
) of R.(B.) microplus identified genes and gene coding regions that encode proteins with essential functions in ticks (BELLGARD et al., 2012Bellgard M, Moolhuijzen PM, Guerrero FD, Schibeci D, Rodriguez-Valle M, Peterson DG, et al. Cattle TickBase: An integrated Internet-based bioinformatics resource for Rhipicephalus (Boophilus) microplus.Int J Parasitol 2012; 42(2): 161-169. PMid:22178513. http://dx.doi.org/10.1016/j.ijpara.2011.11.006.
http://dx.doi.org/10.1016/j.ijpara.2011....
). One of these gene coding regions was found to encode proteins whose amino acids were significantly similar to aquaporins.
Aquaporins, also called “water channels”, allow the regulation of water transport through the highly hydrophobic lipid bilayer of cell membranes (GUERRERO et al., 2014Guerrero FD, Andreotti R, Bendele KG, Cunha RC, Miller RJ, Yeater K, et al. Rhipicephalus (Boophilus) microplus aquaporin as an effective vaccine antigen to protect against cattle tick infestations. Parasit Vectors 2014; 7: 475. PMid:25306139.). Members of the aquaporin family are observed from mammal individuals (ROJEK et al., 2008Rojek A, Praetorius J, Frokiaer J, Nielsen S, Fenton RA. A current view of the mammalian aquaglyceroporins. Annu Rev Physiol 2008; 70(1): 301-327. PMid:17961083. http://dx.doi.org/10.1146/annurev.physiol.70.113006.100452.
http://dx.doi.org/10.1146/annurev.physio...
) to bacteria (FU et al., 2000Fu D, Libson A, Miercke LJ, Weitzman C, Nollert P, Krucinski J, et al. Structure of a glycerol-conducting channel and the basis for its selectivity. Science 2000; 290(5491): 481-486. PMid:11039922. http://dx.doi.org/10.1126/science.290.5491.481.
http://dx.doi.org/10.1126/science.290.54...
), being common in certain types of cells such as erythrocytes (DENKER et al., 1988Denker BM, Smith BL, Kuhajda FP, Agre P. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from Erythrocytes and renal tubules. J Biol Chem 1988; 263(30): 15634-15642. PMid:3049610.). Two constrictions on the aquaporin structure act as filters, and the selectivity to water, glycerol, urea, and other small molecules is determined by the size and charge of the constricting pore (BEITZ et al., 2006Beitz E, Wu B, Holm LM, Schultz JE, Zeuthen T. Point mutations in the aromatic/arginine region in aquaporin 1 allow passage of urea, glycerol, ammonia, and protons. Proc Natl Acad Sci USA 2006; 103(2): 269-274. PMid:16407156. http://dx.doi.org/10.1073/pnas.0507225103.
http://dx.doi.org/10.1073/pnas.050722510...
; GUERRERO et al., 2014Guerrero FD, Andreotti R, Bendele KG, Cunha RC, Miller RJ, Yeater K, et al. Rhipicephalus (Boophilus) microplus aquaporin as an effective vaccine antigen to protect against cattle tick infestations. Parasit Vectors 2014; 7: 475. PMid:25306139.).
Due to the fact that cattle ticks ingest large volumes of blood in relation to their size and weight, they are obliged to concentrate blood components and have effective water transport mechanisms for their digestion (MEGAW, 1974Megaw MWJ. Studies on the water balance mechanism of the tick, Canestrini. Boophilus microplusComp Biochem Physiol 1974; 48(1): 115-125. http://dx.doi.org/10.1016/0300-9629(74)90859-7.
http://dx.doi.org/10.1016/0300-9629(74)9...
). Thus, because the ticks’ aquaporins are fundamental in their physiology, they seem to be good candidates for antigens in a vaccine against ticks (GUERRERO et al., 2014Guerrero FD, Andreotti R, Bendele KG, Cunha RC, Miller RJ, Yeater K, et al. Rhipicephalus (Boophilus) microplus aquaporin as an effective vaccine antigen to protect against cattle tick infestations. Parasit Vectors 2014; 7: 475. PMid:25306139.).
In this context, a cDNA that encodes an aquaporin of R. (B.) microplus was expressed as a recombinant protein in Pichia pastoris and the amino acid sequence of the cloned aquaporin protein fragment is representend on Figure 1. The recombinant protein, named RmAQP1, was tested as an anti-cattle tick vaccine in cattle and provided 76% and 73% efficiency in two experiments, being considered a promising antigen in vaccines against infestation with R.(B.) microplus in cattle (GUERRERO et al., 2014Guerrero FD, Andreotti R, Bendele KG, Cunha RC, Miller RJ, Yeater K, et al. Rhipicephalus (Boophilus) microplus aquaporin as an effective vaccine antigen to protect against cattle tick infestations. Parasit Vectors 2014; 7: 475. PMid:25306139.).
Amino acid sequence of the cloned aquaporin protein fragment produced by expression of Pichia pastoris. Amino acids of R. (B.) microplus protein are underlined. Additional amino acids not originating from the tick are not underlined. Image adapted from Guerrero & Pérez-de-Leon (2014)Guerrero FD, Perez De Leon AAVaccination of animals to elicit a protective immune response against tick infestations and tick-borne pathogen transmissiononlinePatent US 8,722,0632014May13cited 2016 Sept 13Available from: http://www.freepatentsonline.com/8722063.html
http://www.freepatentsonline.com/8722063... .
Considering that the brown dog tick and the cattle tick belong to the same genus (Rhipicephalus) as shown by molecular phylogeny studies (BEATI & KEIRANS, 2001Beati L, Keirans JE. Analysis of the systematic relationships among ticks of the genera and (Acari: Ixodidae) based on mitochondrial 12S ribosomal DNA gene sequences and morphological characters. RhipicephalusBoophilusJ Parasitol 2001; 87(1): 32-48. PMid:11227901. http://dx.doi.org/10.1645/0022-3395(2001)087[0032:AOTSRA]2.0.CO;2.
http://dx.doi.org/10.1645/0022-3395(2001...
; MURRELL & BARKER, 2003Murrell A, Barker SC. Synonymy of Curtice, 1891 with Koch, 1844 (Acari: Ixodidae). BoophilusRhipicephalusSyst Parasitol 2003; 56(3): 169-172. PMid:14707501. http://dx.doi.org/10.1023/B:SYPA.0000003802.36517.a0.
http://dx.doi.org/10.1023/B:SYPA.0000003...
; BARROS-BATTESTI et al., 2006Barros-Battesti DM, Arzua M, Bechara GH. Carrapatos de importância médico-veterinária da região Neotropical: um guia lustrado para identificação de espécies. São Paulo: Vox/ICTTD-3/Butantan; 2006.), the present study investigated the immunogenic potential of the recombinant aquaporin protein of R. (B.) microplus, RmAQP1, against different instars of R. sanguineus (aquaporin sequences unkown until now) infesting domestic dogs.
Materials and Methods
Ticks
Rhipicephalus sanguineus ticks were obtained from colonies maintained at the Immunopathology Laboratory of the Department of Veterinary Pathology, School of Agricultural and Veterinary Sciences -FCAV, UNESP, Campus of Jaboticabal.
For colony maintenance, the various instars of R. sanguineus (larvae, nymphs and adults) were fed on rabbits that were provided by the Central Animal Facility of UNESP, Botucatu. The rabbits received food and water “ad libitum” and were kept in individual cages. The ticks were released on the hosts inside specially designed plastic chambers, affixed with synthetic glue (Brascoplast®) to the rabbits’ shaved dorsum. The rabbits had no prior contact with ticks.
To facilitate the metamorphosis of engorged instars as well as their maintenance, once detached from the host, the ticks were placed in clear plastic tubes with a perforated lid, to allow adequate aeration, and placed in an incubator (CD347 model, FANEM) at 27°C, 80% humidity and 12 hours photoperiod.
Hosts and experimental groups
Male and female mongrel dogs (n = 10), 1 to 2 years of age, were used as hosts in the infestation challenge with R. sanguineus larvae, nymphs and adults. The dogs were kept in individual boxes at the Experimental Kennel of the Department of Veterinary Pathology, FCAV-UNESP, where there is strict control of parasites, including ticks. The animals were vaccinated against parvovirus, canine distemper, leptospirosis, hepatitis, parainfluenza and rabies, as well as dewormed and received appropriate food and water “ad libitum”.
The dogs were distributed into two groups with five animals each: G1-immunized with RmAQP1 (10 µg/mL) plus adjuvant (Montanide ISA61VG, SEPPIC, Paris) and G2-control, inoculated only with the adjuvant. All experimental procedures were performed in accordance with the Ethical Principles on Animal Experimentation adopted by the Brazilian College of Experimentation, and approved by the Ethics Committee on Animal Use of FCAV-UNESP, campus of Jaboticabal (Protocol 010221/13, 04/06/2013).
Anti-tick vaccine (R. (B.) microplus recombinant aquaporin)
The RmAQP1 development is detailed elsewhere (GUERRERO et al., 2014Guerrero FD, Andreotti R, Bendele KG, Cunha RC, Miller RJ, Yeater K, et al. Rhipicephalus (Boophilus) microplus aquaporin as an effective vaccine antigen to protect against cattle tick infestations. Parasit Vectors 2014; 7: 475. PMid:25306139.). The vaccine was kindly provided by Dr. Felix D. Guerrero and Dr. Adalberto Pérez de León (USDA-ARS Knipling-Bushland US Livestock Insects Research Laboratory, Kerrville, Texas, USA) as part of a scientific cooperation agreement signed by UNESP and USDA-ARS.
Blood collection
Blood samples (3 mL) were collected from all 10 animals using vials with no anticoagulant immediately before each vaccination (days 0, 21, 42) and every 28 days after the last immunization during 10 weeks (days 70, 98, 126, 154). Subsequently, the blood samples were centrifuged at 3,400 rpm for 15 minutes at 25°C and the serum was collected and stored at -20°C until further analysis by ELISA.
Immunization and challenge infestation
The experiments were developed at the Department of Veterinary Pathology, School of Agricultural and Veterinary Sciences -FCAV, UNESP, Campus of Jaboticabal, São Paulo, Brazil.
Following the methods described by Andreotti (2006)Andreotti R. Performance of two Bm86 antigen vaccine formulation against tick using cross breed bovines in stall test. Rev Bras Parasitol Vet 2006; 15(3): 97-100. PMid:16978472., G1 animals were immunized intramuscularly with 1 mL of a solution containing 10 µg of recombinant protein (RmAQP1) plus adjuvant (Montanide ISA61VG, Seppic, Paris, France) at the beginning of weeks 1, 4 and 7. The animals from G2 received, by the same route and at the same days, an equal volume of adjuvant.
Twenty-one days after the last immunization, each animal from G1 and G2 was challenged with 20 R. sanguineus adult tick couples, 100 R. sanguineus larvae and 100 R. sanguineus nymphs from the Jaboticabal strain. Each tick instar was released in a separate feeding chamber (BECHARA et al., 1995Bechara GH, Szabó MPJ, Ferreira BR, Garcia MV. in Brazil: feeding and reproductive aspects under laboratorial conditions. Rhipicephalus sanguineusRev Bras Parasitol Vet 1995; 4(2): 61-66.) that was affixed with glue (Brascoplast®) to the dogs’ shaved dorsum.
The feeding chambers were opened daily. Engorged females were individually weighed and placed separately in plastic containers in an incubator at 27°C, 80% humidity and 12 hours photoperiod, until complete oviposition. Detached larvae and nymphs were individually counted but weighed and stored into plastic vials in daily batches. Feeding period was assumed as the time elapsed from tick liberation on the host until its detachment, partially or fully engorged; pre-oviposition was the number of days from detachment to the beginning of oviposition; each egg mass was weighed and incubated separately in the BOD under the same conditions previously described; the larval hatching rate for each female offspring was the mean value of visual evaluation performed by three different persons, according to Szabó & Bechara (1995)Szabó MPJ, Bechara GH. An insight into the histopathology caused by the tick (Acarina: ) in the skin of previously infested, vaccinated or tick-bite naive dogs, guinea pigs and hamsters. Rhipicephalus sanguineusIxodidaeBraz J Vet Res Anim Sci 1995; 32(1): 37-42.; finally, molting rates were the proportion of recovered engorged ticks that molted.
Biological parameters of the ticks under laboratory conditions
The effect of the aquaporin antigen on the biotic potential of the different stages of R. sanguineus was assessed by the differences between immunized and control groups regarding: recovery rates of engorged females (% RecA), larvae (% RecL) and nymphs (% RecN); weights of engorged female (EFW), larvae (ELW) and nymphs (ENW); egg mass weight (EMW); engorgement periods of females (FEP), larvae (LEP) and nymphs (NEP); female pre-oviposition period (POP); larval hatchability (% LH), larvae to nymph molting rate (% MoltN) and nymph to adult molting rate (% MoltA). EMW was determined 15 days after tick detachment as there was no significant increase in this parameter after this period (BECHARA et al., 1994Bechara GH, Szabó MPJ, Mukai LS, Rosa PCS. Immunisation of dogs, hamsters and guinea pigs against Rhipicephalus sanguineus using crude unfed adult tick extracts. Vet Parasitol 1994; 52(1-2): 79-90. PMid:8030191. http://dx.doi.org/10.1016/0304-4017(94)90038-8.
http://dx.doi.org/10.1016/0304-4017(94)9...
).
Indirect Enzyme-Linked Immunosorbent Assay - ELISA
Briefly, microtiter plates were coated with antigen (100 µL/well of a solution of 10 µg antigen/mL in coating buffer) and incubated overnight in a humid chamber at 4°C. The wells were washed three times with PBS + 0.05% Tween-20 (PBST) and blocked with 5% skimmed milk in PBST and incubated for 2 hours at 37°C in a humidified chamber. After further washing with PBST, 100 µL of the serum to be tested was added per well, diluted 1:50, following serial dilutions 1:2 from A to H, with subsequent incubation of 1 hour and 30 minutes at 37°C in a humidified chamber. Further washing was performed, as previously described, and 100 µL of anti-dog IgG conjugated to alkaline phosphatase was added to each well diluted 1:10,000. After 1 hour and 30 minutes incubation followed by washing, 100 µL of substrate for alkaline phosphatase, P-nitrophenylphosphate, diluted in diethanolamine buffer (1 mg/mL) was added to each well. After 30 minutes incubation at room temperature, the optical density measurements were performed on a MRX ELISA High Performance reader™ (DYNEX Technologies, Chantilly, VA, USA) at 405 nm. Optimal dogs’ serum dilution was determined after preliminary testing with 1:50, 1:100, 1:200 and 1:400 dilutions.
Results
Biological parameters of adult R. sanguineus ticks after challenge infestation
Biological parameters of engorged females obtained after challenge infestation are shown in Table 1. Only the FEP was statistically different, with the immunized group having 12% shorter engorgement period than the control group.
Biological parameters of R. sanguineus larvae after challenge infestation
Biological parameters of engorged larvae obtained after challenge infestation are shown in Table 2.
Larvae were statistically different in the immunized and control groups regarding their engorgement periods and engorged weights. Although the larvae of the immunized group presented 8.7% longer engorgement period than the control group, they weighed 7.2% less.
Biological parameters of R. sanguineus nymphs after challenge infestation
Biological parameters of engorged nymphs obtained after challenge infestation are shown in Table 3.
The nymphs were statistically different in the immunized and control groups regarding their engorgement periods and engorged weights. Nymphs of the immunized group had 4.5% shorter engorgement period and weighed 3.6% less than the control group.
Indirect Enzyme-Linked Immunosorbent Assay - ELISA
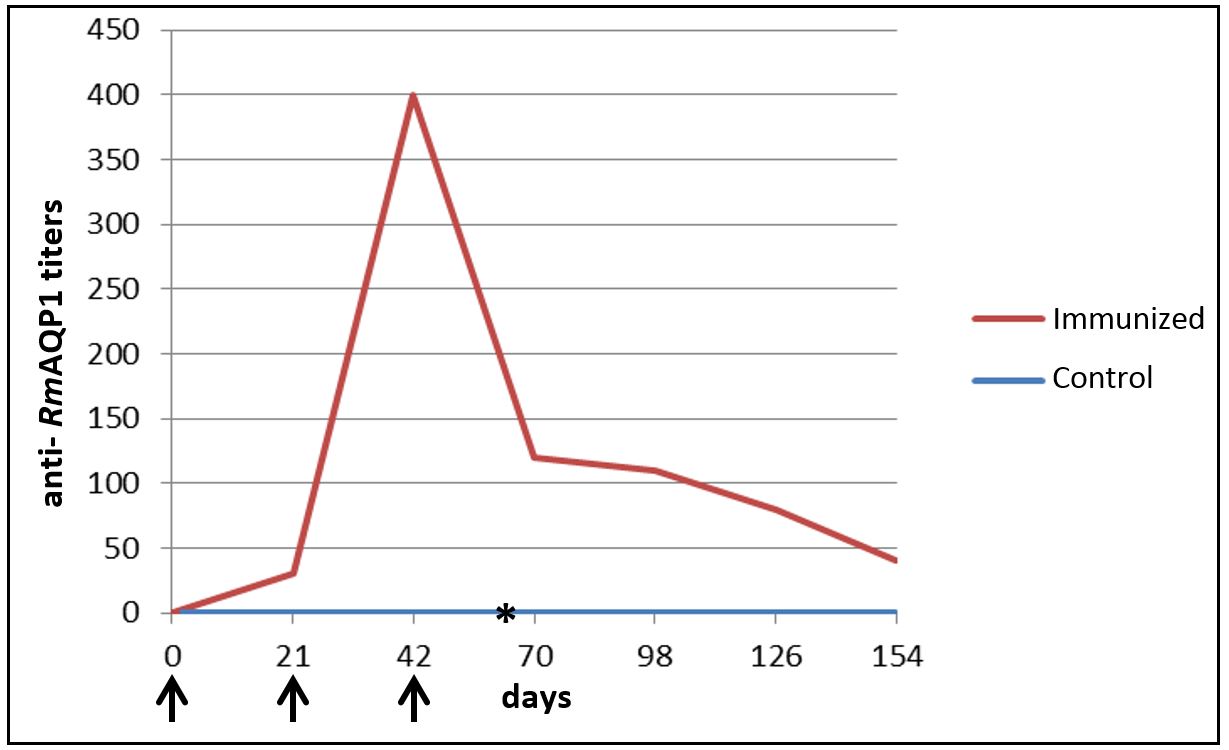
The ELISA showed that the animals of G1 had an increase in anti-RmAQP1 antibody titers post first and second immunization, but these titers began to decrease after the third immunization (Figure 2).
Anti- RmAQP1 antibody titers present in domestic dogs’ serum in different post-immunization times measured by ELISA. Results represent the last serum dilution with a mean optical density greater than three times the mean of the negative control group. ↑ – Represents the days that dogs from G1 were immunized with RmAQP1. *- Represents day 63, date of the challenge infestation with Rhipicephalus sanguineus.
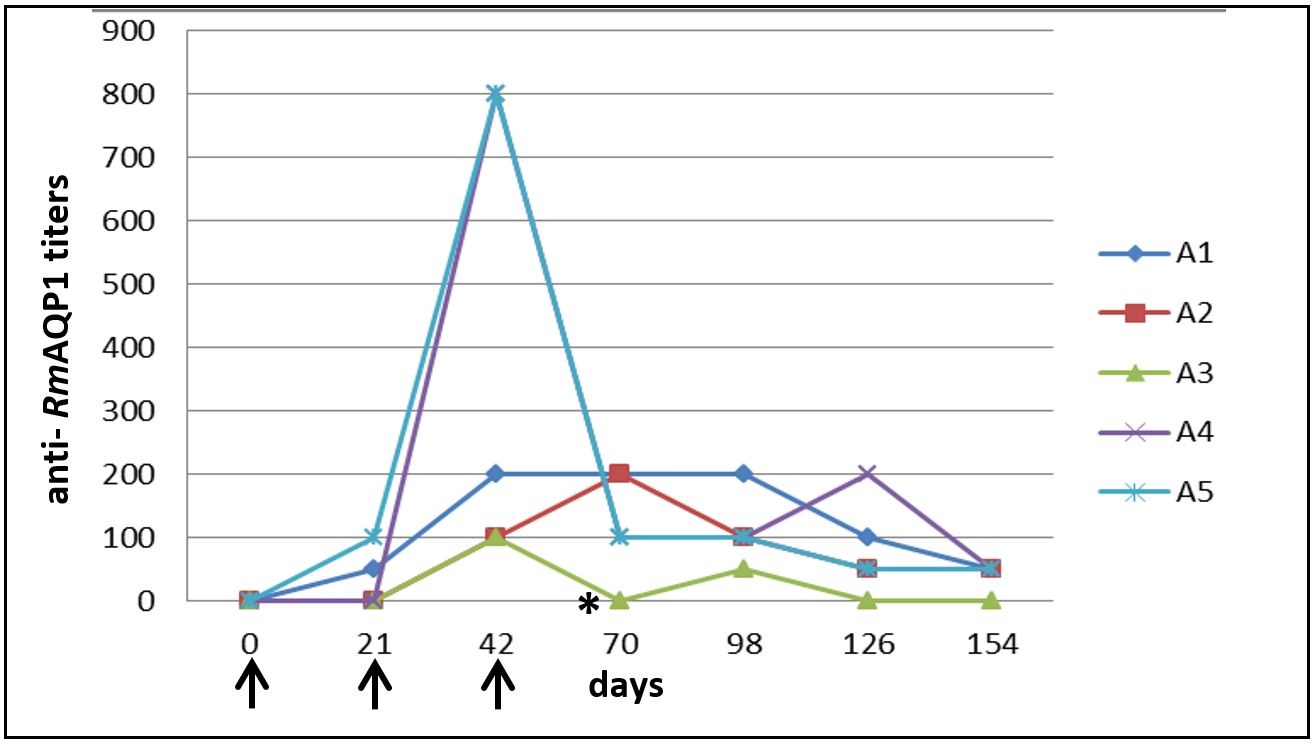
Individual analysis of each animal showed that two dogs of G1 presented longer antibody titers post-second immunization (Figure 3); however, the titers began to decline soon after the third immunization and, like the other animals, decreased until the last analyzed time.
Individual analysis of anti- RmAQP1 antibody titers of dogs immunized with recombinant aquaporin protein at different times after immunization by indirect ELISA. Results represent the last serum dilution with a mean optical density greater than three times the mean of the negative control group. ↑ – Represents the days that dogs from G1 were immunized with RmAQP1. *- Represents day 63, date of the challenge infestation with Rhipicephalus sanguineus.
Discussion
Advances in the biology of ticks may favor vaccines development due to the opening of new opportunities to identify candidates for antigens (GHOSH et al., 2007Ghosh S, Azhahianambi P, Yadav MP. Upcoming and future strategies of tick control: a review. J Vector Borne Dis 2007; 44(2): 79-89. PMid:17722860.). The development of anti-tick vaccine is one of the most promising alternatives to chemical control of ectoparasites with the advantage of being target specific, of easy management and low cost. Moreover, it does not jeopardize human health nor offer environmental risk (WIKEL, 1996Wikel SK. Immunology of the tick-host interface. In: Wikel SK. The immunology of host- ectoparasitic arthropod relationships. Wallingford: Cab International; 1996. p. 204-231.). Finally, with tick infestation reduction, a vaccine would consequently help to lower pathogens transmission by ectoparasites (DE LA FUENTE et al., 2015De La Fuente J, Kocan KM, Contreras M. Prevention and control strategies for ticks and pathogen transmission. Rev Sci Tech Off Int Epiz 2015; 34(1): 249-264. PMid:26470461. http://dx.doi.org/10.20506/rst.34.1.2357.
http://dx.doi.org/10.20506/rst.34.1.2357...
).
Until now, the only commercially available anti-tick vaccine, a Bm86 recombinant protein named Gavac, is triggered against- Rhipicephalus (B.) microplus on bovines (DE LA FUENTE et al., 2007De La Fuente J, Almazán C, Canales M, Pérez de la Lastra JM, Kocan KM, Willadsen P. A ten-year review of commercial vaccine performance for control of tick infestations on cattle. Anim Health Res Rev 2007; 8(1): 23-28. PMid:17692140. http://dx.doi.org/10.1017/S1466252307001193.
http://dx.doi.org/10.1017/S1466252307001...
). However, sequence variations in the Bm86 locus in ticks, among other factors such as cattle genetic variations, could affect the effectiveness of Bm86-containing vaccines (GARCIA-GARCIA et al., 1999Garcia-Garcia JC, Gonzalez IL, Gonzalez DM, Valdes M, Mendez L, Lamberti J, et al. Sequence variantions in the Bm86 locus and implications for immunoprotection in cattle vaccinated with this antigen. Boophilus microplusExp Appl Acarol 1999; 23(11): 883-895. PMid:10668863. http://dx.doi.org/10.1023/A:1006270615158.
http://dx.doi.org/10.1023/A:100627061515...
). Consequently, the development of integrated tick control strategies, including vaccines and acaricides, is important and could lead to the sustainable control of ticks and tick borne diseases (DE LA FUENTE et al., 2015De La Fuente J, Kocan KM, Contreras M. Prevention and control strategies for ticks and pathogen transmission. Rev Sci Tech Off Int Epiz 2015; 34(1): 249-264. PMid:26470461. http://dx.doi.org/10.20506/rst.34.1.2357.
http://dx.doi.org/10.20506/rst.34.1.2357...
).
A more recent study described the ATAQ, a homologous Bm86 protein with primary and secondary structures similarities (AGUIRRE et al., 2016Aguirre AAR, Lobo FP, Cunha RC, Garcia MV, Andreotti R. Design of the ATAQ peptide and its evaluation as an immunogen to develop a vaccine. RhipicephalusVet Parasitol 2016; 221: 30-38. PMid:27084468. http://dx.doi.org/10.1016/j.vetpar.2016.02.032.
http://dx.doi.org/10.1016/j.vetpar.2016....
), as a promising antigen in the development of an anti-tick vaccine. Other types of immunogens that may be effective against ticks are the synthetic peptides, the 64P cementum protein, subolesin/akirin, ferritin 2, P0 protein, SILK antigen and aquaporins (PATARROYO et al., 2002Patarroyo JH, Portela RW, Castro RO, Pimentel JC, Guzman F, Patarroyo ME, et al. Immunization of cattle with synthetic peptides derived from the gut protein (Bm86). Boophilus microplusVet Immunol Immunopathol 2002; 88(3-4): 163-172. PMid:12127414. http://dx.doi.org/10.1016/S0165-2427(02)00154-X.
http://dx.doi.org/10.1016/S0165-2427(02)...
; TRIMNELL et al., 2005Trimnell AR, Davies GM, Lissina O, Hails RS, Nuttall PA. A cross-reactive tick cement antigen is a candidate broad-spectrum tick vaccine. Vaccine 2005; 23(34): 4329-4341. PMid:15913855. http://dx.doi.org/10.1016/j.vaccine.2005.03.041.
http://dx.doi.org/10.1016/j.vaccine.2005...
; ALMAZÁN et al., 2003Almazán C, Kocan KM, Bergman DK, Garcia-Garcia JC, Blouin EF, De La Fuente J. Identification of protective antigens for the control of infestations using cDNA expression library immunization. Ixodes scapularisVaccine 2003; 21(13-14): 1492-1501. PMid:12615446. http://dx.doi.org/10.1016/S0264-410X(02)00683-7.
http://dx.doi.org/10.1016/S0264-410X(02)...
; DE LA FUENTE et al., 2011De La Fuente J, Moreno-Cid JA, Canales M, Villar M, Pérez De La Lastra JM, Kocan KM, et al. Targeting arthropod subolesin/akirin for the development of a universal vaccine for control of vector infestations and pathogen transmission. Vet Parasitol 2011; 181(1): 17-22. PMid:21561715. http://dx.doi.org/10.1016/j.vetpar.2011.04.018.
http://dx.doi.org/10.1016/j.vetpar.2011....
; HAJDUSEK et al., 2010Hajdusek O, Almazán C, Loosova G, Villar M, Canales M, Grubhoffer L, et al. Characterization of ferritin 2 for the control of tick infestations. Vaccine 2010; 28(17): 2993-2998. PMid:20171306. http://dx.doi.org/10.1016/j.vaccine.2010.02.008.
http://dx.doi.org/10.1016/j.vaccine.2010...
; RODRÍGUEZ-MALLON et al., 2012Rodríguez-Mallon A, Fernández E, Encinosa PE, Bello Y, Méndez-Pérez L, Ruiz LC, et al. A novel tick antigen shows high vaccine efficacy against the dog tick, Rhipicephalus sanguineus. Vaccine 2012; 30(10): 1782-1789. PMID: 22245603. http://dx.doi.org/10.1016/j.vaccine.2012.01.011.
http://dx.doi.org/10.1016/j.vaccine.2012...
; MERINO et al., 2013Merino M, Antunes S, Mosqueda J, Moreno-Cid JA, Pérez De La Lastra JM, Rosario-Cruz R, et al. Vaccination with proteins involved in tick-pathogen interactions reduces vector infestations and pathogen infection. Vaccine 2013; 31(49): 5889-5896. PMid:24084474. http://dx.doi.org/10.1016/j.vaccine.2013.09.037.
http://dx.doi.org/10.1016/j.vaccine.2013...
; BELLGARD et al., 2012Bellgard M, Moolhuijzen PM, Guerrero FD, Schibeci D, Rodriguez-Valle M, Peterson DG, et al. Cattle TickBase: An integrated Internet-based bioinformatics resource for Rhipicephalus (Boophilus) microplus.Int J Parasitol 2012; 42(2): 161-169. PMid:22178513. http://dx.doi.org/10.1016/j.ijpara.2011.11.006.
http://dx.doi.org/10.1016/j.ijpara.2011....
; GUERRERO et al., 2014Guerrero FD, Andreotti R, Bendele KG, Cunha RC, Miller RJ, Yeater K, et al. Rhipicephalus (Boophilus) microplus aquaporin as an effective vaccine antigen to protect against cattle tick infestations. Parasit Vectors 2014; 7: 475. PMid:25306139.).
Accordingly, the present study evaluated a recombinant aquaporin 1 protein of R. (B.) microplus as an antigen in a vaccine against the tick R. sanguineus, in domestic dogs. The evaluation included: 1) comparisons between the biotic potential of ticks fed on immunized and control animals and; 2) determination of immunized dogs’ serum antibody titers (IgG) by ELISA test.
Among the biological parameters analyzed in this study, only the engorgement period of adult female ticks was statistically different between immunized and control groups, with the immunized group presenting 12% shorter FEP than the control group. Thus, the results suggest low protective potential of the RmAQP1 antigen against adult R. sanguineus, in the dose used. Different results were observed by Guerrero et al. (2014)Guerrero FD, Andreotti R, Bendele KG, Cunha RC, Miller RJ, Yeater K, et al. Rhipicephalus (Boophilus) microplus aquaporin as an effective vaccine antigen to protect against cattle tick infestations. Parasit Vectors 2014; 7: 475. PMid:25306139. in a study conducted in Campo Grande, MS, Brazil, using the RmAQP1 antigen against R.(B.) microplus on cattle. In that study, authors observed a marked decrease in ticks recovery rate from immunized animals, which was equivalent to only 29% of what was recovered in the control group. The results showed efficacy of 76% and 73% of the vaccine in two trials regardless of the effects on production and hatchability of eggs being insignificant (GUERRERO et al., 2014Guerrero FD, Andreotti R, Bendele KG, Cunha RC, Miller RJ, Yeater K, et al. Rhipicephalus (Boophilus) microplus aquaporin as an effective vaccine antigen to protect against cattle tick infestations. Parasit Vectors 2014; 7: 475. PMid:25306139.). The differences between the two studies with the RmAQP1 can be due to numerous factors, including: a) in the present study, RmAQP1 was used against R. sanguineus in dogs while in the study conducted by Guerrero et al. (2014)Guerrero FD, Andreotti R, Bendele KG, Cunha RC, Miller RJ, Yeater K, et al. Rhipicephalus (Boophilus) microplus aquaporin as an effective vaccine antigen to protect against cattle tick infestations. Parasit Vectors 2014; 7: 475. PMid:25306139. this protein was used in cattle against R. (B.) microplus; and b) the protein dose used in this study may have been insufficient to induce immunity in dogs.
The engorgement period and engorged weight of larvae and nymphs of G1 and G2 was significantly different. Although larvae of the immunized group presented an 8.7% longer engorgement period than the control group, they weighed 7.2% less. Moreover, the nymphs of the immunized group showed 4.5% shorter engorgement period and weighed 3.6% less than the control group. These results suggest a possible effect, though discreet, of the RmAQP1 on larvae and nymphs of R. sanguineus in the dose tested.
To perform the ELISA, the optimum concentration of sera dilution was established by testing sera at 1:50, 1:100, 1:200 and 1:400, with the 1:50 dilution being chosen. The concentration of the tested antigen (10 µg RmAQP1/mL), in turn, was determined by its suppliers.
In the immunized group, the analysis of the antibody titers mean, represented by the last serum dilution that presented a mean optical density three times greater than the mean presented by the negative control group, revealed that although the animals showed an increase in antibody titers post-second immunization, these titers quickly declined after the third immunization. Moreover, individual analysis of the immunized dogs showed that only two of the five animals vaccinated with RmAQP1 showed considerable increase in antibody titers post-second immunization, which decreased after the third immunization. These results point to a low immunogenicity of the antigen in the dose used. In contrast, different results were observed in the ELISA test from the study conducted by Guerrero et al. (2014)Guerrero FD, Andreotti R, Bendele KG, Cunha RC, Miller RJ, Yeater K, et al. Rhipicephalus (Boophilus) microplus aquaporin as an effective vaccine antigen to protect against cattle tick infestations. Parasit Vectors 2014; 7: 475. PMid:25306139. where the cattle immunized with RmAQP1 showed greater increase in antibody titers, and these remained high post third immunization.
Thus, the low immunogenicity of the RmAQP1 in the present study could possibly be related to the fact that here an aquaporin protein of R. (B.) microplus was used against R. sanguineus while in the work of Guerrero et al. (2014)Guerrero FD, Andreotti R, Bendele KG, Cunha RC, Miller RJ, Yeater K, et al. Rhipicephalus (Boophilus) microplus aquaporin as an effective vaccine antigen to protect against cattle tick infestations. Parasit Vectors 2014; 7: 475. PMid:25306139. this protein was used against R. (B.) microplus itself. Furthermore, in the study conducted by Guerrero et al. (2014)Guerrero FD, Andreotti R, Bendele KG, Cunha RC, Miller RJ, Yeater K, et al. Rhipicephalus (Boophilus) microplus aquaporin as an effective vaccine antigen to protect against cattle tick infestations. Parasit Vectors 2014; 7: 475. PMid:25306139. it was administered to each animal 2 mL containing 100 µg of the antigen + Montanide, however, to adjust the antigen dose for a dog´s size, in the present study it was administered to each animal 1 mL containing 10 µg of RmAQP1, which may have been a low dose to induce effective combat against adult ticks. Nonetheless, significant results were observed in larvae and nymphs, indicating possible effectiveness of the antigen against these instars.
Finally, we suggest that further studies should be developed using a higher dose of RmAQP1 for a better evaluation of its immunogenic potential against R. sanguineus in dogs.
Acknowledgements
The authors are grateful to the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for financial support (#2013/09792-7) and GSS (#2013/10394-6) and PME (#2013/09792-7) scholarships, to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for GHB academic career fellowship (#303112/2013-1), to Ms. Mayara Carolina Rosolem, Ms. Leyriane Barbosa Alves de Oliveira and Mrs. Francisca de Assis Ardisson for technical assistance. Thanks to Dr. Luisa N. Domingues, visiting Scientist at the USDA-ARS Knipling-Bushland US Livestock Insects Research Laboratory in Kerrville, Texas, USA for reviewing and providing constructive comments on the manuscript.
References
- Aguirre AAR, Lobo FP, Cunha RC, Garcia MV, Andreotti R. Design of the ATAQ peptide and its evaluation as an immunogen to develop a vaccine. RhipicephalusVet Parasitol 2016; 221: 30-38. PMid:27084468. http://dx.doi.org/10.1016/j.vetpar.2016.02.032
» http://dx.doi.org/10.1016/j.vetpar.2016.02.032 - Almazán C, Kocan KM, Bergman DK, Garcia-Garcia JC, Blouin EF, De La Fuente J. Identification of protective antigens for the control of infestations using cDNA expression library immunization. Ixodes scapularisVaccine 2003; 21(13-14): 1492-1501. PMid:12615446. http://dx.doi.org/10.1016/S0264-410X(02)00683-7
» http://dx.doi.org/10.1016/S0264-410X(02)00683-7 - Andreotti R. Performance of two Bm86 antigen vaccine formulation against tick using cross breed bovines in stall test. Rev Bras Parasitol Vet 2006; 15(3): 97-100. PMid:16978472.
- Apanaskevich DA, Horak IG, Mulumba-Mfumu LK. A new species of (Acari: Ixodidae), a parasite of red river hogs and domestic pigs in the Democratic Republic of Congo. RhipicephalusJ Med Entomol 2013; 50(3): 479-484. PMid:23802441. http://dx.doi.org/10.1603/ME12266
» http://dx.doi.org/10.1603/ME12266 - Barros-Battesti DM, Arzua M, Bechara GH. Carrapatos de importância médico-veterinária da região Neotropical: um guia lustrado para identificação de espécies. São Paulo: Vox/ICTTD-3/Butantan; 2006.
- Beati L, Keirans JE. Analysis of the systematic relationships among ticks of the genera and (Acari: Ixodidae) based on mitochondrial 12S ribosomal DNA gene sequences and morphological characters. RhipicephalusBoophilusJ Parasitol 2001; 87(1): 32-48. PMid:11227901. http://dx.doi.org/10.1645/0022-3395(2001)087[0032:AOTSRA]2.0.CO;2
» http://dx.doi.org/10.1645/0022-3395(2001)087[0032:AOTSRA]2.0.CO;2 - Bechara GH, Szabó MPJ, Ferreira BR, Garcia MV. in Brazil: feeding and reproductive aspects under laboratorial conditions. Rhipicephalus sanguineusRev Bras Parasitol Vet 1995; 4(2): 61-66.
- Bechara GH, Szabó MPJ, Mukai LS, Rosa PCS. Immunisation of dogs, hamsters and guinea pigs against Rhipicephalus sanguineus using crude unfed adult tick extracts. Vet Parasitol 1994; 52(1-2): 79-90. PMid:8030191. http://dx.doi.org/10.1016/0304-4017(94)90038-8
» http://dx.doi.org/10.1016/0304-4017(94)90038-8 - Beitz E, Wu B, Holm LM, Schultz JE, Zeuthen T. Point mutations in the aromatic/arginine region in aquaporin 1 allow passage of urea, glycerol, ammonia, and protons. Proc Natl Acad Sci USA 2006; 103(2): 269-274. PMid:16407156. http://dx.doi.org/10.1073/pnas.0507225103
» http://dx.doi.org/10.1073/pnas.0507225103 - Bellgard M, Moolhuijzen PM, Guerrero FD, Schibeci D, Rodriguez-Valle M, Peterson DG, et al. Cattle TickBase: An integrated Internet-based bioinformatics resource for Rhipicephalus (Boophilus) microplus.Int J Parasitol 2012; 42(2): 161-169. PMid:22178513. http://dx.doi.org/10.1016/j.ijpara.2011.11.006
» http://dx.doi.org/10.1016/j.ijpara.2011.11.006 - Dantas-Torres F, Figueiredo LA, Brandão-Filho SP. (Acari: Ixodidae), the brown dog tick, parasitizing humans in Brazil. Rhipicephalus sanguineusRev Soc Bras Med Trop 2006; 39(1): 64-67. PMid:16501769. http://dx.doi.org/10.1590/S0037-86822006000100012
» http://dx.doi.org/10.1590/S0037-86822006000100012 - Dantas-Torres F. The brown dog tick, Rhipicephalus sanguineus (Latreille, 1806) (Acari: Ixodidae): from taxonomy to control. Vet Parasitol 2008; 152(3-4): 173-185. PMid:18280045. http://dx.doi.org/10.1016/j.vetpar.2007.12.030
» http://dx.doi.org/10.1016/j.vetpar.2007.12.030 - De La Fuente J, Almazán C, Canales M, Pérez de la Lastra JM, Kocan KM, Willadsen P. A ten-year review of commercial vaccine performance for control of tick infestations on cattle. Anim Health Res Rev 2007; 8(1): 23-28. PMid:17692140. http://dx.doi.org/10.1017/S1466252307001193
» http://dx.doi.org/10.1017/S1466252307001193 - De La Fuente J, Kocan KM, Contreras M. Prevention and control strategies for ticks and pathogen transmission. Rev Sci Tech Off Int Epiz 2015; 34(1): 249-264. PMid:26470461. http://dx.doi.org/10.20506/rst.34.1.2357
» http://dx.doi.org/10.20506/rst.34.1.2357 - De La Fuente J, Moreno-Cid JA, Canales M, Villar M, Pérez De La Lastra JM, Kocan KM, et al. Targeting arthropod subolesin/akirin for the development of a universal vaccine for control of vector infestations and pathogen transmission. Vet Parasitol 2011; 181(1): 17-22. PMid:21561715. http://dx.doi.org/10.1016/j.vetpar.2011.04.018
» http://dx.doi.org/10.1016/j.vetpar.2011.04.018 - Denker BM, Smith BL, Kuhajda FP, Agre P. Identification, purification, and partial characterization of a novel Mr 28,000 integral membrane protein from Erythrocytes and renal tubules. J Biol Chem 1988; 263(30): 15634-15642. PMid:3049610.
- Fu D, Libson A, Miercke LJ, Weitzman C, Nollert P, Krucinski J, et al. Structure of a glycerol-conducting channel and the basis for its selectivity. Science 2000; 290(5491): 481-486. PMid:11039922. http://dx.doi.org/10.1126/science.290.5491.481
» http://dx.doi.org/10.1126/science.290.5491.481 - Garcia-Garcia JC, Gonzalez IL, Gonzalez DM, Valdes M, Mendez L, Lamberti J, et al. Sequence variantions in the Bm86 locus and implications for immunoprotection in cattle vaccinated with this antigen. Boophilus microplusExp Appl Acarol 1999; 23(11): 883-895. PMid:10668863. http://dx.doi.org/10.1023/A:1006270615158
» http://dx.doi.org/10.1023/A:1006270615158 - Ghosh S, Azhahianambi P, Yadav MP. Upcoming and future strategies of tick control: a review. J Vector Borne Dis 2007; 44(2): 79-89. PMid:17722860.
- Guerrero FD, Andreotti R, Bendele KG, Cunha RC, Miller RJ, Yeater K, et al. Rhipicephalus (Boophilus) microplus aquaporin as an effective vaccine antigen to protect against cattle tick infestations. Parasit Vectors 2014; 7: 475. PMid:25306139.
- Guerrero FD, Miller RJ, Perez de León AA. Cattle tick vaccines: many candidate antigens, but will a commercially viable product emerge? Int J Parasitol 2012; 42(5): 421-427. PMid:22549026. http://dx.doi.org/10.1016/j.ijpara.2012.04.003
» http://dx.doi.org/10.1016/j.ijpara.2012.04.003 - Guerrero FD, Miller RJ, Rousseau ME, Sunkara S, Quackenbush J, Lee Y, et al. BmiGI: a database of cDNAs expressed in , the tropical/southern cattle tick. Boophilus microplusInsect Biochem Mol Biol 2005; 35(6): 585-595. PMid:15857764. http://dx.doi.org/10.1016/j.ibmb.2005.01.020
» http://dx.doi.org/10.1016/j.ibmb.2005.01.020 - Guerrero FD, Moolhuijzen P, Peterson DG, Bidwell S, Caler E, Bellgard M, et al. Reassociation kinetics-based approach for partial genome sequencing of the cattle tick, (RhipicephalusBoophilusmicroplus.) BMC Genomics 2010; 11(1): 374. PMid:20540747. http://dx.doi.org/10.1186/1471-2164-11-374
» http://dx.doi.org/10.1186/1471-2164-11-374 - Guerrero FD, Perez De Leon AAVaccination of animals to elicit a protective immune response against tick infestations and tick-borne pathogen transmissiononlinePatent US 8,722,0632014May13cited 2016 Sept 13Available from: http://www.freepatentsonline.com/8722063.html
» http://www.freepatentsonline.com/8722063.html - Guglielmone AA, Estrada-Peña A, Keirans JE, Robins RG. Ticks (Acari: Ixodida) of the Neotropical zoogeographic region. Atlanta: International Consortium on Ticks and Tick-borne Disease; 2003. 173 p.
- Guglielmone AA, Robbins RG, Apanaskevich DA, Petney TN, Estrada-Peña A, Horak IG, et al. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the world: a list of valid species names. Zootaxa 2010; 2528(6): 1-28.
- Hajdusek O, Almazán C, Loosova G, Villar M, Canales M, Grubhoffer L, et al. Characterization of ferritin 2 for the control of tick infestations. Vaccine 2010; 28(17): 2993-2998. PMid:20171306. http://dx.doi.org/10.1016/j.vaccine.2010.02.008
» http://dx.doi.org/10.1016/j.vaccine.2010.02.008 - Horak IG, Apanaskevich DA, Kariuki EK. A new species of (Acari: Ixodidae), a parasite of giraffes in Kenya. RhipicephalusJ Med Entomol 2013; 50(4): 685-690. PMid:23926765. http://dx.doi.org/10.1603/ME12257
» http://dx.doi.org/10.1603/ME12257 - Krantz GW, Walter DE. A manual of acarology. 3rd ed. Lubbock: Texas Tech University Press; 2009.
- Kunz SE, Kemp DH. Insecticides and acaricides: resistance and environmental impact. Rev Sci Tech Off Int Epiz 1994; 13(4): 1249-1286. PMid:7711312. http://dx.doi.org/10.20506/rst.13.4.816
» http://dx.doi.org/10.20506/rst.13.4.816 - Megaw MWJ. Studies on the water balance mechanism of the tick, Canestrini. Boophilus microplusComp Biochem Physiol 1974; 48(1): 115-125. http://dx.doi.org/10.1016/0300-9629(74)90859-7
» http://dx.doi.org/10.1016/0300-9629(74)90859-7 - Merino M, Antunes S, Mosqueda J, Moreno-Cid JA, Pérez De La Lastra JM, Rosario-Cruz R, et al. Vaccination with proteins involved in tick-pathogen interactions reduces vector infestations and pathogen infection. Vaccine 2013; 31(49): 5889-5896. PMid:24084474. http://dx.doi.org/10.1016/j.vaccine.2013.09.037
» http://dx.doi.org/10.1016/j.vaccine.2013.09.037 - Murrell A, Barker SC. Synonymy of Curtice, 1891 with Koch, 1844 (Acari: Ixodidae). BoophilusRhipicephalusSyst Parasitol 2003; 56(3): 169-172. PMid:14707501. http://dx.doi.org/10.1023/B:SYPA.0000003802.36517.a0
» http://dx.doi.org/10.1023/B:SYPA.0000003802.36517.a0 - Patarroyo JH, Portela RW, Castro RO, Pimentel JC, Guzman F, Patarroyo ME, et al. Immunization of cattle with synthetic peptides derived from the gut protein (Bm86). Boophilus microplusVet Immunol Immunopathol 2002; 88(3-4): 163-172. PMid:12127414. http://dx.doi.org/10.1016/S0165-2427(02)00154-X
» http://dx.doi.org/10.1016/S0165-2427(02)00154-X - Rachinsky A, Guerrero FD, Scoles GA. Differential protein expression in ovaries of uninfected and Babesia-infected southern cattle ticks, Rhipicephalus (Boophilus) microplus.Insect Biochem Mol Biol 2007; 37(12): 1291-1308. PMid:17967348. http://dx.doi.org/10.1016/j.ibmb.2007.08.001
» http://dx.doi.org/10.1016/j.ibmb.2007.08.001 - Rachinsky A, Guerrero FD, Scoles GA. Proteomic profiling of Rhipicephalus (Boophilus) microplus midgut responses to infection with Babesia bovis.Vet Parasitol 2008; 152(3-4): 294-313. PMid:18243558. http://dx.doi.org/10.1016/j.vetpar.2007.12.027
» http://dx.doi.org/10.1016/j.vetpar.2007.12.027 - Rodríguez-Mallon A, Fernández E, Encinosa PE, Bello Y, Méndez-Pérez L, Ruiz LC, et al. A novel tick antigen shows high vaccine efficacy against the dog tick, Rhipicephalus sanguineus. Vaccine 2012; 30(10): 1782-1789. PMID: 22245603. http://dx.doi.org/10.1016/j.vaccine.2012.01.011
» http://dx.doi.org/10.1016/j.vaccine.2012.01.011 - Rodríguez-Vivas RI, Apanaskevich DA, Ojeda-Chi MM, Trinidad-Martinez I, Reyes-Novelo E, Esteve-Gassent MD, Perez de León AA. Ticks collected from humans, domestic animals, and wildlife in Yucatan, Mexico. Vet Parasitol 2016; 215: 106-113. PMID: 26790745. http://dx.doi.org/10.1016/j.vetpar.2015.11.010
» http://dx.doi.org/10.1016/j.vetpar.2015.11.010 - Rojek A, Praetorius J, Frokiaer J, Nielsen S, Fenton RA. A current view of the mammalian aquaglyceroporins. Annu Rev Physiol 2008; 70(1): 301-327. PMid:17961083. http://dx.doi.org/10.1146/annurev.physiol.70.113006.100452
» http://dx.doi.org/10.1146/annurev.physiol.70.113006.100452 - Sprong H, Trentelman J, Seemann I, Grubhoffer L, Rego RO, Hajdušek O, et al. ANTIDotE: anti-tick vaccines to prevent tick-borne diseases in Europe. Parasit Vectors 2014; 7(1): 77. PMid:24559082. http://dx.doi.org/10.1186/1756-3305-7-77
» http://dx.doi.org/10.1186/1756-3305-7-77 - Szabó MPJ, Bechara GH. An insight into the histopathology caused by the tick (Acarina: ) in the skin of previously infested, vaccinated or tick-bite naive dogs, guinea pigs and hamsters. Rhipicephalus sanguineusIxodidaeBraz J Vet Res Anim Sci 1995; 32(1): 37-42.
- Szabó MPJ, Pascoli GVT, Marçal OM Jr, Franchin AG, Torga K. Brown dog tick parasitizing the bird . Rhipicephalus sanguineusCoereba flaveola in the Brazilian CerradoCienc Rural 2008; 38(2): 543-545. http://dx.doi.org/10.1590/S0103-84782008000200041
» http://dx.doi.org/10.1590/S0103-84782008000200041 - Trimnell AR, Davies GM, Lissina O, Hails RS, Nuttall PA. A cross-reactive tick cement antigen is a candidate broad-spectrum tick vaccine. Vaccine 2005; 23(34): 4329-4341. PMid:15913855. http://dx.doi.org/10.1016/j.vaccine.2005.03.041
» http://dx.doi.org/10.1016/j.vaccine.2005.03.041 - Wikel SK. Immunology of the tick-host interface. In: Wikel SK. The immunology of host- ectoparasitic arthropod relationships. Wallingford: Cab International; 1996. p. 204-231.
Publication Dates
-
Publication in this collection
16 Feb 2017 -
Date of issue
Jan-Mar 2017
History
-
Received
26 Oct 2016 -
Accepted
17 Jan 2017